- Disease Resistant Crops Research Unit, GMO Research Center, National Institute of Agrobiological Sciences, Tsukuba, Japan
Plants are sessile organisms that are continuously exposed to a wide range of environmental stresses. To cope with various stresses using limited resources, plants have evolved diverse mechanisms of “tradeoff” that enable the allocation of resources to address the most life-threatening stress. During our studies on induced disease resistance in rice, we have found some important phenomena relevant to tradeoffs between biotic and abiotic stress responses, and between stress response and plant growth. We characterized these tradeoff phenomena from viewpoints of signaling crosstalks associated with transcriptional regulation. Here, I describe following topics: (1) PTP1-dependent increased disease susceptibility of rice under low temperature and high salinity conditions, (2) OsNPR1-dependent tradeoff between pathogen defense and photosynthesis, (3) tradeoff between pathogen defense and abiotic stress tolerance in WRKY45-overexpressing rice plants, and (4) WRKY62-dependent tradeoff between pathogen defense and hypoxia tolerance. Lastly, I discuss my view regarding the significance of such tradeoffs in agricultural production that should be considered in crop breeding; that is, the tradeoffs, although they benefit plants in nature, can be rather disadvantageous in agricultural production.
Introduction
Plants are sessile organisms that are continuously exposed to a wide range of environmental stresses (Tian et al., 2003; Matyssek et al., 2005). To cope with various stresses using limited resources, plants have evolved diverse mechanisms that enable the allocation of resources to address the most life-threatening stress. Therefore, tradeoffs exist between stress responses and plant growth or between responses to different stresses. These tradeoffs are often regulated by crosstalk between signaling pathways (Fujita et al., 2006; Pieterse et al., 2012; Sharma et al., 2013; Xiao et al., 2013; Takatsuji and Jiang, 2014). Signaling molecules such as plant hormones (Lozano-Duran et al., 2013; Huot et al., 2014; Verma et al., 2016), reactive oxygen and nitrogen species (Considine et al., 2015), and Ca2+ (Mazars et al., 2010) have been implicated in these crosstalks. However, the precise molecular mechanisms involved are yet to be investigated. My laboratory has been studying the salicylic acid (SA) defense signaling pathway in rice with emphasis on signaling crosstalks that mediate the tradeoffs between pathogen defense and abiotic stress responses and/or plant growth. In this review, I provide an overview of these studies, which mainly highlight a negative aspect of tradeoffs that can reduce crop production. I also propose that crop productivity could be improved by regulating tradeoffs through the inhibition of crosstalk between signaling pathways.
Increased Disease Susceptibility of Rice Under Low Temperature and High Salinity Conditions
Rice blast is one of the most serious crop diseases worldwide. Chemical defense inducers affecting the SA signaling pathway have been widely used to protect rice plants from diseases such as rice blast. However, rice plants are more susceptible to blast disease when exposed to specific abiotic stresses, including low temperature, drought, and high salinity (Kahn and Libby, 1958; Bonman et al., 1988; Gill and Bonman, 1988), even in the presence of chemical defense inducers (Ueno et al., 2015). These observations seem to reflect prioritization of abiotic stress responses over blast disease resistance in rice because the abiotic stresses are often more life-threatening than blast disease. This is one of the typical tradeoffs between plant responses to abiotic and biotic stresses. By analyzing the molecular mechanism underlying this phenomenon, we demonstrated that abscisic acid (ABA) signaling, which was activated by cold and high salinity leading to abiotic stress responses, inactivated WRKY45, the central transcription factor in the SA defense signaling pathway in rice (Figure 1A) (Jiang et al., 2010; Yazawa et al., 2012; Ueno et al., 2015). In response to the chemical defense inducer benzothiadiazole (BTH), WRKY45 was activated by a MAP kinase cascade (OsMPKK10-2–OsMPK6)-catalyzed phosphorylation at its carboxyl terminus (Matsushita et al., 2013; Ueno et al., 2013, 2015). This led to an increase in WRKY45 expression through WRKY45 autoregulation of its own transcription (Nakayama et al., 2013). OsMPK6 was activated by a dual phosphorylation of its TEY motif in response to dexamethasone-induced production of a constitutively active form of OsMPKK10-2 (OsMPKK10-2D), which mimics the activation of the SA pathway (Ueno et al., 2015). However, in the presence of ABA, OsMPK6 was dephosphorylated at its tyrosine residue even after the induction of OsMPKK10-2D, which decreased WRKY45 transcript abundance and reduced blast resistance (Ueno et al., 2015).
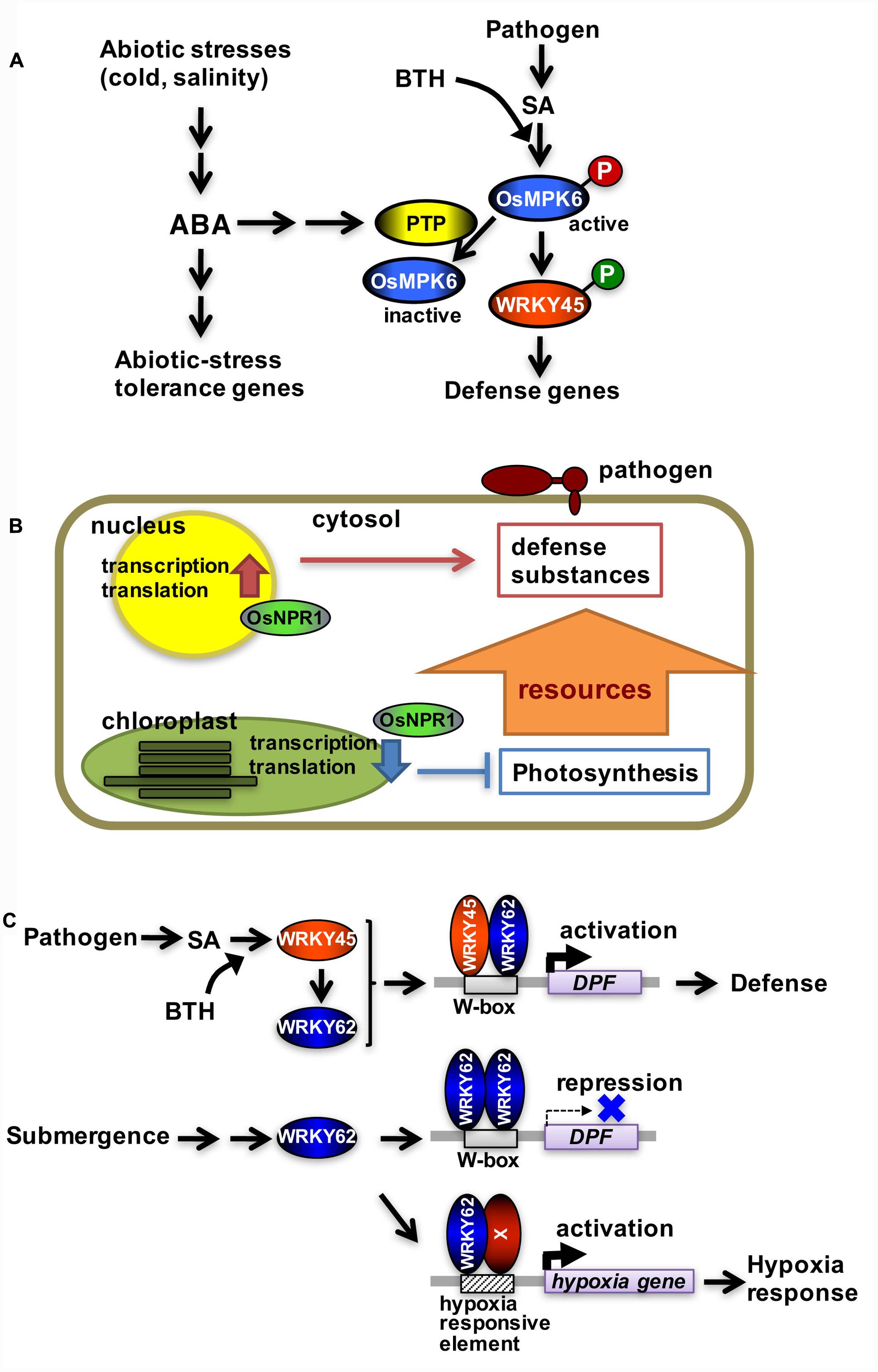
FIGURE 1. Tradeoffs involving the salicylic acid pathway in rice. (A) Tradeoff between pathogen defense and abiotic stress tolerance mediated by protein tyrosine phosphatase. WRKY45 is phosphorylated and activated by OsMPK6 in response to chemical defense inducers. OsMPK6 is inactivated following tyrosine dephosphorylation by protein tyrosine phosphatase, which is mediated by ABA, in response to cold stress. This leads to hypo-phosphorylation and inactivation of WRKY45. (B) Tradeoff between pathogen defense and photosynthesis mediated by OsNPR1. OsNPR1 downregulates chloroplastic activity resulting in a decreased photosynthetic rate, while it upregulates the expression of defense genes. (C) Tradeoff between pathogen defense and submergence tolerance mediated by WRKY62. Following the activation of the salicylic acid pathway, WRKY45 and WRKY62 form heterodimers that activate DPF transcription. Upon submergence, only WRKY62 is produced, resulting in the formation of homodimers that repress DPF expression. Molecule X represents a presumptive transcription factor that binds to a hypoxia-responsive element in the promoter of hypoxia-responsive genes, possibly as a heterodimer with WRKY62.
The rice genome encodes two protein tyrosine phosphatases (PTP1 and PTP2) that dephosphorylate OsMPK6 at its tyrosine residue in vitro (Figure 1A). Knockdown of the two PTP genes by RNA interference in transgenic rice plants increased the abundance of TEY-phosphorylated OsMPK6 following SA treatment because of suppressed tyrosine dephosphorylation (Ueno et al., 2015). In PTP-knockdown rice lines, the ABA-mediated inhibition of WRKY45 expression was considerably reduced. A blast resistance test indicated that ABA greatly suppressed BTH-induced blast resistance in untransformed (control) rice plants, whereas it did not affect the resistance of PTP-knockdown rice plants (Ueno et al., 2015). Low temperature (i.e., 15°C/8°C, day/night cycle) and high salinity (250 mM NaCl) conditions also suppressed BTH-induced blast resistance, but did not affect the induction of blast resistance in PTP-knockdown rice plants (Ueno et al., 2015). Thus, PTP knockdown eliminates the crosstalk between ABA and SA signaling pathways, which prevents abiotic stresses from suppressing the chemical-induced blast resistance.
One of the concerns regarding the effects of PTP knockdown is whether it affects normal ABA-mediated plant responses to abiotic stresses. In other words, are PTP-knockdown rice plants less tolerant to cold and/or high salinity stresses? It currently appears they are not. The induction of SalT expression, which is a marker gene for ABA responses, is not influenced by PTP knockdown (Ueno et al., 2015). Additionally, we did not observe any differences between PTP-knockdown and control rice plants under our low temperature and high salinity conditions. Therefore, the effects of PTP knockdown appear to be specific to the crosstalk between the ABA and SA pathways, and do not affect normal ABA-mediated abiotic stress tolerance.
Tradeoff Between Pathogen Defense and Photosynthesis
OsNPR1/NH1 is an important transcriptional co-activator acting in the rice SA pathway along with WRKY45 (Sugano et al., 2010). A transcriptome analysis using OsNPR1/NH1-knockdown rice lines with or without BTH treatment revealed an interesting function of OsNPR1/NH1 in the tradeoff between pathogen defense and photosynthesis (Figure 1B). While most of the WRKY45-dependent BTH-responsive genes were upregulated by BTH (Nakayama et al., 2013), more than half of the OsNPR1/NH1-dependent BTH-responsive genes were downregulated by BTH (Sugano et al., 2010). The OsNPR1/NH1-dependent BTH-responsive genes included most of the photosynthetic genes involved in light and dark reactions (Sugano et al., 2010). The majority of genes involved in chloroplastic protein synthesis, such as the 30S and 50S ribosomal genes, also experienced OsNPR1/NH1-dependent downregulation by BTH (Sugano et al., 2010). Additionally, sigma factors involved in chloroplastic transcription were regulated in a similar manner. In contrast, genes associated with cytoplasmic protein synthesis, such as the 40S and 60S ribosomal genes, underwent OsNPR1/NH1-dependent upregulation by BTH (Sugano et al., 2010). Photosynthetic parameter measurements (i.e., Fv/Fm) in rice leaves revealed that photosynthetic activity declined more rapidly in control plants than in OsNPR1/NH1-knockdown plants during BTH treatments (Sugano et al., 2010). These results indicate that BTH-mediated decreases in photosynthetic activity depend on OsNPR1/NH1 (Figure 1B). This regulation probably represents a tradeoff prioritizing pathogen defense over chloroplastic activity unnecessary for pathogen defense. Results from a previous study (Wang et al., 2006) indicated that Arabidopsis NPR1 plays a similar role in the tradeoff between pathogen defense and chloroplastic activity, suggesting this regulation is common in monocots and dicots (Sugano et al., 2010).
Tradeoff Between Pathogen Defense and Abiotic Stress Tolerance in WRKY45-Overexpressing Rice Plants
Tradeoffs between pathogen defense and tolerance to abiotic stresses were observed in WRKY45-overexpressing (WRKY45-ox) rice plants. WRKY45 overexpression conferred rice with strong resistances to blast and leaf-blight diseases (Shimono et al., 2007, 2012). However, the growth of WRKY45-ox rice plants was significantly impaired compared with that of control plants, and varied with environmental conditions (Tao et al., 2011; Goto et al., 2015). Analysis of this phenomenon revealed that low temperature and high salinity conditions severely impaired the growth and viability of WRKY45-ox rice plants, which represents a tradeoff prioritizing pathogen defense over abiotic-stress tolerance. Following exposure to low temperatures (e.g., 8°C) for 7 days and recovery at room temperature for 7 days, 80% of WRKY45-ox rice plants died, while all control plants survived (Goto et al., 2015). Additionally, 75% of WRKY45-ox rice plants died after being irrigated with 250 mM NaCl and then water for 7 days, while 0–13% of control plants survived (Goto et al., 2015). Thus, WRKY45-ox rice plants were more sensitive to low temperature and high salinity conditions. This phenomenon seems to represent a tradeoff in WRKY45-ox rice plants, whereby the plants gained pathogen resistance through WRKY45 overexpression, but became more susceptible to the effects of low temperature and high salinity.
WRKY62-Dependent Tradeoff Between Pathogen Defense and Hypoxia Tolerance
Rice plants are believed to become more susceptible to diseases such as rice blast and bacterial leaf blight after submergence. This is presumably due to a tradeoff mechanism prioritizing hypoxia tolerance over disease resistance. Although the mechanism responsible for this phenomenon has not been characterized, we have identified an important WRKY62 function that is likely involved. WRKY62 is a transcriptional repressor that is regulated downstream of WRKY45. Overexpression of WRKY62 in rice plants leads to increased susceptibility to leaf blight, which suggests WRKY62 is a negative regulator of disease resistance (Peng et al., 2008). However, our analysis of WRKY62-knockdown rice plants revealed that WRKY62 is a positive regulator of diterpenoid phytoalexin biosynthetic genes and other defense genes (Fukushima et al., 2016). We subsequently determined that WRKY62 can activate and repress the expression of defense genes (Fukushima et al., 2016).
WRKY62 forms a homodimer or a heterodimer with WRKY45 depending on environmental conditions (Fukushima et al., 2016). The WRKY62 homodimer functions as a transcriptional repressor of the DPF gene, which encodes a transcription factor that regulates diterpenoid phytoalexin biosynthetic genes (Figure 1C). Conversely, the WRKY45-WRKY62 heterodimer serves as a transcriptional activator of the DPF gene (Figure 1C). The WRKY45 and WRKY62 expression levels are similar following the activation of the SA pathway, which facilitates the formation of the heterodimer (Fukushima et al., 2016). However, under the hypoxic conditions created by submergence, only WRKY62 is expressed, leading to the formation of the homodimer. This selective induction of transcription factor genes most likely explains why DPF is expressed when the SA signaling pathway is activated, but is suppressed in submerged plants. Furthermore, WRKY62 regulates hypoxia-responsive genes, including alcohol dehydrogenase 2, acyl desaturase, and EFR, in a manner opposite from that of DPF. WRKY62 functions as a positive regulator of hypoxia-responsive genes under hypoxic conditions (Fukushima et al., 2016). Thus, WRKY62 acts as a toggle switch between the expression of defense or hypoxia-responsive genes.
Significance of Tradeoffs in Nature and Agricultural Production
We have identified a variety of tradeoffs in rice. The tradeoffs increased susceptibility to diseases, impaired photosynthesis, or decreased tolerance to abiotic stress to prioritize responses to the most damaging stresses in specific conditions. These tradeoffs can be considered beneficial for plants because they increase survival rates, which may explain why they have developed during evolution. However, some tradeoffs may not necessarily be beneficial in agricultural production because prioritizing plant survival often results in decreased crop yields. Resource availability can differ considerably between natural and agricultural settings. Under natural conditions, available resources are often severely limited, which constrains plants to survive by prioritizing the responses to the most life-threatening stresses at the cost of other biological processes. However, in agricultural settings, more resources are usually available because they can be supplied as fertilizers. In such situations, elimination of particular tradeoff mechanisms may allow plants to cope with multiple stresses simultaneously without affecting growth or development. An example is that the elimination of tradeoffs following PTP knockdown prevented any increases in rice blast susceptibility under low-temperature conditions without any adverse effects on growth (Ueno et al., 2015). Unlinking hormone-regulated immunity and plant growth is also discussed in Eichmann and Schafer (2015). As mentioned above, wide variety of tradeoffs and signaling crosstalks exists in plants. Regulating such tradeoffs could be one of directions to be considered upon designing crop improvement strategies. Characterizing the mechanisms mediating various tradeoffs will be necessary to enable their regulation.
Author Contributions
The author confirms being the sole contributor of this work and approved it for publication.
Funding
This work was supported by Japan Society for the Promotion of Science [grant number 25292032] and Japanese Ministry of Agriculture, Forestry and Fisheries, Genomics for Agricultural Innovation [grant number PMI0008, GMA0001, and GMO1006].
Conflict of Interest Statement
The author declares that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgment
I am grateful to my colleagues for contributions to the works described herein.
References
Bonman, J. M., Sanchez, L. M., and Mackill, A. O. (1988). Effects of water deficit on rice blast. II. Disease-development in the field. J. Plant Prot. Trop. 5, 67–73.
Considine, M. J., María Sandalio, L., and Helen Foyer, C. (2015). Unravelling how plants benefit from ROS and NO reactions, while resisting oxidative stress. Ann. Bot. 116, 469–473. doi: 10.1093/aob/mcv153
Eichmann, R., and Schafer, P. (2015). Growth versus immunity–a redirection of the cell cycle? Curr. Opin. Plant Biol. 26, 106–112. doi: 10.1016/j.pbi.2015.06.006
Fujita, M., Fujita, Y., Noutoshi, Y., Takahashi, F., Narusaka, Y., Yamaguchi-Shinozaki, K., et al. (2006). Crosstalk between abiotic and biotic stress responses: a current view from the points of convergence in the stress signaling networks. Curr. Opin. Plant Biol. 9, 436–442. doi: 10.1016/j.pbi.2006.05.014
Fukushima, S., Mori, M., Sugano, S., and Takatsuji, H. (2016). Transcription factor WRKY62 plays a role in pathogen defence and hypoxia responsive gene expression in rice. Plant Cell Physiol. 57, 2541–2551. doi: 10.1093/pcp/pcw185
Gill, M. A., and Bonman, J. M. (1988). Effects of water deficit on rice blast. I. Influence of water deficit on components of resistance. J. Plant Prot. Trop. 5, 61–66.
Goto, S., Sasakura-Shimoda, F., Suetsugu, M., Selvaraj, M. G., Hayashi, N., Yamazaki, M., et al. (2015). Development of disease-resistant rice by optimized expression of WRKY45. Plant Biotechnol. J. 13, 753–765. doi: 10.1111/pbi.12303
Huot, B., Yao, J., Montgomery, B. L., and He, S. Y. (2014). Growth-defense tradeoffs in plants: a balancing act to optimize fitness. Mol. Plant 7, 1267–1287. doi: 10.1093/mp/ssu049
Jiang, C.-J., Shimono, M., Sugano, S., Kojima, M., Yazawa, K., Yoshida, R., et al. (2010). Abscisic acid interacts antagonistically with salicylic acid signaling pathway in rice-Magnaporthe grisea interaction. Mol. Plant Microbe Interact. 23, 791–798. doi: 10.1094/MPMI-23-6-0791
Kahn, R. P., and Libby, J. L. (1958). The effect of environmental factors and plant ages on the infection of rice by the blast fungus, Pyricularia oryzae. Phytopathology 48, 25–30.
Lozano-Duran, R., Macho, A. P., Boutrot, F., Segonzac, C., Somssich, I. E., and Zipfel, C. (2013). The transcriptional regulator BZR1 mediates trade-off between plant innate immunity and growth. eLife 2, e00983–e00983. doi: 10.7554/eLife.00983
Matsushita, A., Inoue, H., Goto, S., Nakayama, A., Sugano, S., Hayashi, N., et al. (2013). Nuclear ubiquitin proteasome degradation affects WRKY45 function in the rice defense program. Plant J. 73, 302–313. doi: 10.1111/tpj.12035
Matyssek, R., Agerer, R., Ernst, D., Munch, J. C., Osswald, W., Pretzsch, H., et al. (2005). The plant’s capacity in regulating resource demand. Plant Biol. (Stuttg) 7, 560–580. doi: 10.1055/s-2005-872981
Mazars, C., Thuleau, P., Lamotte, O., and Bourque, S. (2010). Cross-talk between ROS and calcium in regulation of nuclear activities. Mol. Plant 3, 706–718. doi: 10.1093/mp/ssq024
Nakayama, A., Fukushima, S., Goto, S., Matsushita, A., Shimono, M., Sugano, S., et al. (2013). Genome-wide identification of WRKY45-regulated genes that mediate benzothiadiazole-induced defense responses in rice. BMC Plant Biol. 13:150. doi: 10.1186/1471-2229-13-150
Peng, Y., Bartley, L. E., Chen, X., Dardick, C., Chern, M., Ruan, R., et al. (2008). OsWRKY62 is a negative regulator of basal and Xa21-mediated defense against Xanthomonas oryzae pv. oryzae in rice. Mol. Plant 1, 446–458. doi: 10.1093/mp/ssn024
Pieterse, C. M., Van Der Does, D., Zamioudis, C., Leon-Reyes, A., and Van Wees, S. C. (2012). Hormonal modulation of plant immunity. Annu. Rev. Cell Dev. Biol. 28, 489–521. doi: 10.1146/annurev-cellbio-092910-154055
Sharma, R., De Vleesschauwer, D., Sharma, M. K., and Ronald, P. C. (2013). Recent advances in dissecting stress-regulatory crosstalk in rice. Mol. Plant 6, 250–260. doi: 10.1093/mp/sss147
Shimono, M., Koga, H., Akagi, A., Hayashi, N., Goto, S., Sawada, M., et al. (2012). Rice WRKY45 plays important roles in fungal and bacterial disease resistance. Mol. Plant Pathol. 13, 83–94. doi: 10.1111/j.1364-3703.2011.00732.x
Shimono, M., Sugano, S., Nakayama, A., Jiang, C. J., Ono, K., Toki, S., et al. (2007). Rice WRKY45 plays a crucial role in benzothiadiazole-inducible blast resistance. Plant Cell 19, 2064–2076. doi: 10.1105/tpc.106.046250
Sugano, S., Jiang, C.-J., Miyazawa, S.-I., Masumoto, C., Yazawa, K., Hayashi, N., et al. (2010). Role of OsNPR1 in rice defense program as revealed by genome-wide expression analysis. Plant Mol. Biol. 74, 549–562. doi: 10.1007/s11103-010-9695-3
Takatsuji, H., and Jiang, C.-J. (2014). “Plant hormone crosstalks under biotic stresses,” in Phytohormones: A Window to Metabolism, Signaling and Biotechnological Applications, eds L.-S. P. Tran and S. Pal (New York, NY: Springer), 323–350.
Tao, Z., Kou, Y., Liu, H., Li, X., Xiao, J., and Wang, S. (2011). OsWRKY45 alleles play different roles in abscisic acid signalling and salt stress tolerance but similar roles in drought and cold tolerance in rice. J. Exp. Bot. 62, 4863–4874. doi: 10.1093/jxb/err144
Tian, D., Traw, M. B., Chen, J. Q., Kreitman, M., and Bergelson, J. (2003). Fitness costs of R-gene-mediated resistance in Arabidopsis thaliana. Nature 423, 74–77. doi: 10.1038/nature01588
Ueno, Y., Yoshida, R., Kishi-Kaboshi, M., Matsushita, A., Jiang, C. J., Goto, S., et al. (2013). MAP kinases phosphorylate rice WRKY45. Plant Signal. Behav. 8:e24510. doi: 10.4161/psb.24510
Ueno, Y., Yoshida, R., Kishi-Kaboshi, M., Matsushita, A., Jiang, C. J., Goto, S., et al. (2015). Abiotic stresses antagonize the rice defence pathway through the tyrosine-dephosphorylation of OsMPK6. PLoS Pathog. 11:e1005231. doi: 10.1371/journal.ppat.1005231
Verma, V., Ravindran, P., and Kumar, P. P. (2016). Plant hormone-mediated regulation of stress responses. BMC Plant Biol. 16:86. doi: 10.1186/s12870-016-0771-y
Wang, D., Amornsiripanitch, N., and Dong, X. (2006). A genomic approach to identify regulatory nodes in the transcriptional network of systemic acquired resistance in plants. PLoS Pathog. 2:e123. doi: 10.1371/journal.ppat.0020123
Xiao, J., Cheng, H., Li, X., Xiao, J., Xu, C., and Wang, S. (2013). Rice WRKY13 regulates cross talk between abiotic and biotic stress signaling pathways by selective binding to different cis-elements. Plant Physiol. 163, 1868–1882. doi: 10.1104/pp.113.226019
Yazawa, K., Jiang, C. J., Kojima, M., Sakakibara, H., and Takatsuji, H. (2012). Reduction of abscisic acid levels or inhibition of abscisic acid signaling in rice during the early phase of Magnaporthe oryzae infection decreases its susceptibility to the fungus. Physiol. Mol. Plant Pathol. 78, 1–7. doi: 10.1016/j.pmpp.2011.12.003
Keywords: rice, tradeoff, WRKY45, WRKY62, OsNPR1, pathogen defense, stress tolerance, photosynthesis
Citation: Takatsuji H (2017) Regulating Tradeoffs to Improve Rice Production. Front. Plant Sci. 8:171. doi: 10.3389/fpls.2017.00171
Received: 31 October 2016; Accepted: 27 January 2017;
Published: 09 February 2017.
Edited by:
Mukesh Jain, Jawaharlal Nehru University, IndiaReviewed by:
Tong Zhu, Syngenta Crop Protection, LLC, USAChengdao Li, Murdoch University, Australia
Copyright © 2017 Takatsuji. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) or licensor are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Hiroshi Takatsuji, takatsuh@affrc.go.jp
 Hiroshi Takatsuji
Hiroshi Takatsuji