- Key Laboratory of Crop Heterosis and Utilization, Ministry of Education, State Key Laboratory of Agro-Biotechnology, Beijing Key Laboratory of Crop Genetic Improvement, China Agricultural University, Beijing, China
Wheat powdery mildew, caused by Blumeria graminis f. sp. tritici, and wheat leaf rust, caused by Puccinia triticina Eriks, are two important diseases that severely threaten wheat production. Sorento, a hexaploid triticale cultivar from Poland, shows high resistance to the wheat powdery mildew isolate E09 and the leaf rust isolate PHT in Beijing, China. To introduce resistance genes into common wheat, Sorento was crossed with wheat line Xuezao, which is susceptible to both diseases, and the F1 hybrids were then backcrossed with Xuezao as the recurrent male parent. By marker analysis, we demonstrate that the long arm of the 2R (2RL) chromosome confers resistance to both the leaf rust and powdery mildew isolates at adult-plant and seedling stages, while the long arm of 4R (4RL) confers resistance only to powdery mildew at both stages. The chromosomal composition of BC2F3 plants containing 2R or 2RL and 4R or 4RL in the form of substitution and translocation were confirmed by GISH (genomic in situ hybridization) and FISH (fluorescence in situ hybridization). Monosomic and disomic substitutions of a wheat chromosome with chromosome 2R or 4R, as well as one 4RS-4DL/4DS-4RL reciprocal translocation homozigote and one 2RL-1DL translocation hemizigote, were recovered. Such germplasms are of great value in wheat improvement.
Introduction
Wheat, accounting for 25% of total global cereal yield, provides 20% of the calories consumed by humans (FAO, 2014). Breeding wheat with high yield and good quality has been regarded as a pivotal component of efforts to satisfy food demand of the world. However, wheat diseases such as powdery mildew and leaf rust severely threaten the production of common wheat. Since chemical control always brings environmental pollution and health threats, the use of host resistance is considered an environmentally benign and effective way to control these diseases (Murray and Brennan, 2009).
Wheat powdery mildew (PM), caused by the obligate biotrophic fungal pathogen Blumeria graminis f. sp. tritici (Bgt), is a destructive disease of common wheat in areas with cool or maritime climates. The disease can lead to severe yield losses ranging from 13–34% (Griffey et al., 1993; Conner et al., 2003). Another fungus, Puccinia triticina Eriks (Pt), the causal agent of wheat leaf rust (LR), drastically decreases wheat yield across the world and has afflicted wheat for thousands of years (Bolton et al., 2008; Huerta-Espino et al., 2011). Hitherto, approximately 57 Pm (powdery mildew) and 76 Lr (leaf rust) resistance loci have been designated (McIntosh et al., 2014; Hao et al., 2015; Ma et al., 2015; Zhang et al., 2016; Bansal et al., 2017; Liu et al., 2017; Singla et al., 2017). Most of them are race-specific and only a few were characterized as durable resistance such as Lr34, Lr46, and Lr67 (Krattinger et al., 2009; Ellis et al., 2014; Moore et al., 2015). Race-specific resistance genes tend to be overcome within several decades due to rapid evolution of physiological races and strong selection for virulent pathogen mutants (Niewoehner and Leath, 1998; Parks et al., 2008; Huerta-Espino et al., 2011; Zeng et al., 2014). Therefore, continuously exploiting naturally occurring resistance genes remains the most effective measure to improve the disease resistance of bread wheat.
Rye (Secale cereale L.), a relative of wheat, has been utilized intensively in wheat breeding programs due to its excellent stress tolerance and disease resistance (Jiang et al., 1994; Friebe et al., 1996; Rabinovich, 1998; Purnhauser et al., 2010). The short arm of the rye 1R chromosome carries abundant resistance genes, such as Pm8, Pm17, Sr31, Sr50, Yr9, and Lr26, and has been introduced to wheat in the form of T1BL.1RS and T1AL.1RS (Mcintosh et al., 1988; Heun and Friebe, 1990; Singh et al., 1990; Mago et al., 2004, 2015; Hurni et al., 2013). Wheat varieties containing this fragment always display high yield potential, broad adaptation and disease resistance against powdery mildew, stem rust, leaf rust, and stripe rust (Villareal et al., 1995, 1998; Ehdaie et al., 2003; Kim et al., 2004), however, the Sec-1 locus carried on 1RS is detrimental to bread-making quality when it replaces the Glu-3 and Gli-1 genes of wheat (Dhaliwal et al., 1987; Dhaliwal and MacRitchie, 1990; Martin and Stewart, 1990). Besides 1RS, rye chromosomes 2R, 4R, and 6R also possess resistance genes against powdery mildew and leaf rust. Pm20 on chromosome arm 6RL from rye cv. Prolific was translocated onto wheat chromosome 6BS (Friebe et al., 1994). Pm7 was located on 2RL and is present in the form of T4BS.4BL-2RL in the wheat germplasm Transec (Friebe et al., 1996). The long arm of rye chromosome 2R has been reported to carry resistance genes against wheat powdery mildew and leaf rust diseases, and the long arm of chromosome 4R from rye cv. Kustro were also reported to confer resistance to wheat powdery mildew (Friebe et al., 1996; An et al., 2006, 2013; Hysing et al., 2007; Zhuang et al., 2010; Fu et al., 2014). However, due to the coevolution of host and pathogen, the resistance genes (Pm8, Pm17, Sr31, Yr9, and Lr26) on 1RS and Pm7 on 2RL are no longer effective in China and some other parts of the world, and virulence against Pm20 has arisen (Lutz et al., 1992; Zhuang and Li, 1993; Niewoehner and Leath, 1998; Imani et al., 2002; Zhuang, 2003; Zeng et al., 2014; Hubbard et al., 2016).
Hexaploid triticale (× Triticosecale Wittmack, AABBRR, 2n = 6x = 42), synthesized artificially by combining the genomes of Triticum turgidum (AABB, 2n = 4x = 28) and S. cereale (RR, 2n = 2x = 14), possesses outstanding resistance to wheat powdery mildew and leaf rust disease (Oettler, 2005). The rye components in triticale have been adapted to the wheat nucleus and cytoplasm, which renders easier the transfer of rye chromosomes into common wheat (Ma and Gustafson, 2008). By contrast, colchicine treatment and tedious embryo rescue are indispensable for making wheat × rye crosses (Oettler, 1983, 2005). Thus, triticale cultivars can serve as an alternative source in wheat improvement. The R- and D-genome chromosomes in progenies of triticale × wheat crosses were mostly present as univalent in triticale × wheat F1 hybrids (AABBDR) during meiosis (Schlegel et al., 1980; Lukaszewski and Gustafson, 1983). Univalents tend to misdivide at anaphase I followed by the fusion of telecentric chromosomes during interkinesis of meiosis II which results in whole-arm Robertsonian translocations (Friebe et al., 2005). In this way, wheat-rye translocations can be produced within homoeologous or non-homoeologous groups in sufficient numbers (Lukaszewski and Gustafson, 1983). The rye chromosomes in triticale × wheat F1 hybrids can be transmitted through egg cells at a higher frequency than through pollen, and the transmission of individual rye chromosomes in F1 × wheat was around 50% except for 6R, which shows a significant lower frequency than random (Lukaszewski et al., 1982). Triticale cultivar originating from hybridization of several triticale lines varied in the rye genomes represents the diversity of rye. It combines the broad stress tolerance of different triticale lines and can be used to improve the powdery mildew and rust resistance of wheat. The direct application of triticale cultivar × wheat crosses can facilitate the exploration of multiple resistance genes simultaneously in a short time.
Molecular markers facilitate the identification of alien chromosome segments and have been extensively adopted in breeding programs (Shimizu et al., 1997; Song et al., 2008). To date, a large number of PCR-based markers, such as EST, SSR, and EST-SSR markers for marker associated selection (MAS), have been developed and mapped to specific rye chromosomes, and most of them have been employed in the identification of target rye chromosomes (Saal and Wricke, 1999; Hackauf and Wehling, 2002; Khlestkina et al., 2004; Lee et al., 2009; Xu et al., 2012; Martis et al., 2013; Nguyen et al., 2015). These markers enable breeders to identify the specific segments of rye chromosomes present in wheat germplasm at a large scale. Cytological methods such as GISH (genomic in situ hybridization) and FISH (fluorescence in situ hybridization) have been used to detect the rye chromosomal fragments in wheat-rye hybrids (Tsuchida et al., 2008; Zhuang et al., 2010; Fu et al., 2014). S. cereale clone pSc119.2 mainly hybridizes to B-genome chromosomes of wheat and R-genome of rye, and Aegilops squarrosa clone pAs1 produces signals especially on D-genome chromosomes. Multicolor fluorescence in situ hybridization (mc-FISH) using these probes allows the identification of most wheat chromosomes and all rye chromosomes (Schneider et al., 2003; Contento et al., 2005; Tang et al., 2014). By combining molecular marker screening and cytological analysis, chromosomal organization in terms of translocations, substitutions and additions can be characterized in triticale-wheat derivatives easily.
Triticale cultivar Sorento is highly resistant to wheat powdery mildew and leaf rust diseases as well as triticale diseases. However, the resistance has not been studied and exploited in wheat breeding. In the present study, to incorporate resistance genes against powdery mildew and leaf rust into bread wheat, Xuezao, a wheat line susceptible to powdery mildew and leaf rust, was crossed with the resistant triticale cv. Sorento as the female parent. The F1 hybrids were then backcrossed with Xuezao as the recurrent male parent for several rounds. By means of marker analysis and cytological analysis, it is possible to locate resistance genes on rye chromosomal arms in a wheat background. Here, we report the identification of powdery mildew and leaf rust resistances, both at adult and seedling stage, conferred by chromosome arms 2RL and 4RL of Sorento. Several lines stable genetically were produced, which represent valuable germplasms that can be used in wheat improvement.
Materials and Methods
Materials
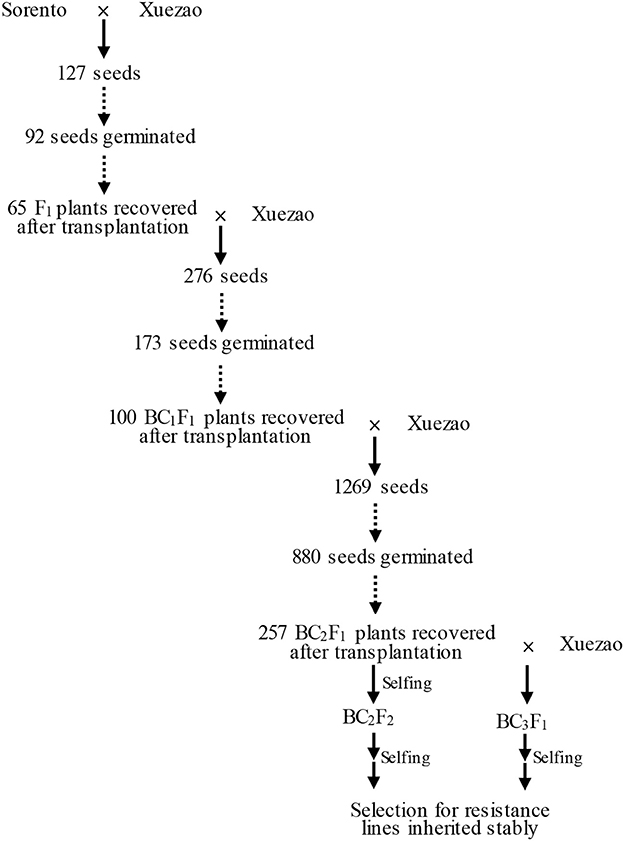
Hexaploid triticale cv. Sorento, highly resistant to wheat powdery mildew and leaf rust disease strains present in Beijing, China, was kindly provided by Danko Hodowla Roślin Sp. z o.o., Choryn, Poland (http://danko.pl/odmiany/sorento/?lang=en). Sorento is a winter triticale cultivar from Poland that yields very well and is remarkably resistant to most triticale diseases. Triticale cv. Sorento derives from a three-way cross of different triticale lines; its pedigree is CT932-89/CHD610-86//Moreno. All those lines in their deep pedigree are based on lines from CIMMYT, Canada and Hungary. The wheat line Xuezao is a winter wheat susceptible to both diseases in Beijing. Triticale cv. Sorento and bread wheat Xuezao were planted in the field at Shang Zhuang Experimental Station in the autumn of 2012. To introduce resistance from Sorento into Xuezao, we designed a backcrossing strategy in which Sorento acts as the donor parent and Xuezao as the recurrent parent (Figure 1). Each autumn during the experiment, both parents and their progenies were evaluated for powdery mildew resistance at seedling stage in the greenhouse at China Agricultural University. The surviving plants were transplanted into the field at Shang Zhuang Experimental Station in Beijing to evaluate their powdery mildew and leaf rust disease symptoms at adult-plant stage. Under our condition, Sorento initiates flowering about 15 days later than Xuezao. In order to minimize the time gap, Sorento and the progenies were covered with plastic film in which a relative high temperature can be maintained. Such condition allows plants to grow faster in spring as well as avoid winterkill.
Field Crosses
Sorento was crossed with Xuezao as the female parent in May in 2013. All the recovered Sorento × Xuezao F1 plants (1–5 spikes per plant) were backcrossed. One to seven spikes of BC1F1 and BC2F1 plants showing resistance to powdery mildew or leaf rust diseases were pollinated with pollen of Xuezao. Field crosses were carried out each May from 2012 to 2016.
Evaluation of Powdery Mildew Symptoms in Field and Greenhouse
The phenotypes of both parents and BC1F1, BC2F1, BC3F1, and BC2F2 progenies were evaluated at the adult-plant stage in the field and at the seedling stage in the greenhouse. For seedling tests, both parents and their progenies were sown into 50-pot trays containing three parts nutrient soil and one part vermiculite. Wheat powdery mildew isolate E09, provided by the Institute of Plant Protection of the Chinese Academy of Agricultural Sciences in Beijing, was maintained and multiplied on susceptible line Xuezao. After about a week, the unfolded first leaf was inoculated with powdery mildew by hand flick. The infection types (IT) of 10–15 days post-inoculation (dpi) plants were scored using the 0–4 scale described by Zhang et al. (2010), in which “0,” “0;”, “1,” “2,” “3,” and “4” indicate “no visible symptoms,” “necrotic flecks without sporulation,” “highly resistant,” “resistant,” “susceptible,” and “highly susceptible,” respectively. All plants were kept under a day/night photoperiod of 18/6 h at 25–30°C in the greenhouse. For field tests, wheat seedlings with well-developed powdery mildew hyphae were used as media for spore transmission and inoculation to the overwintered wheat after being transplanted into the field in April. After 1–2 months, the infection types of the flag leaf and the top second leaf of the progeny were evaluated using the same standard.
Evaluation of Leaf Rust Symptoms in Field and Growth Chamber
Evaluation of leaf rust was carried out in BC1F1, BC2F1, BC3F1, and BC2F2 in the field and in BC2F2 in growth chamber using leaf rust isolate PHT provided by the Institute of Plant Protection of the Chinese Academy of Agricultural Sciences in Beijing. For field tests, each progeny plant that overwintered was inoculated with leaf rust spores by injecting the urediniospore solution containing 0.1% Tween 20 into the stalk in April. After 1–2 months, the infection type of the flag leaf and the top second leaf of each individual was scored on a 0–4 scale, with “0” for immune (no uredinia), “;” for nearly immune (no uredinia, but hypersensitive necrotic or chlorotic flecks present), “1” for very resistant (small uredinia surrounded by necrosis), “2” for moderately resistant (small to medium-sized uredinia often surrounded by chlorosis or necrosis), “3” for moderately susceptible (medium-sized uredinia that may be associated with chlorosis), and “4” for susceptible (large uredinia without chlorosis) (Roelfs et al., 1992). Seedling tests were only performed on self-crossed progenies (BC2F2) of BC2F1 plants showing resistance to leaf rust at the adult-plant stage in the plant growth chamber. Seven- to eight-day-old plants were challenged with leaf rust by spraying urediniospore solution. The inoculated plants were then incubated in a humid growth chamber free from light for 1–2 days. After inoculation, the plants were maintained under a day/night photoperiod of 18/6 h, a temperature of 25–30°C, and a high relative humidity. Xuezao was taken as the susceptible control. After around 10 days, the infection type was scored as described for adult plants.
Markers for Identification of Rye Chromosomes and Resistance Loci
A total of 64 published chromosome-specific rye markers, including REMS (rye expressed microsatellite sites), SCM (Secale cereale microsatellite), GRM, SWES, and a few other types, were chosen to identify the rye chromosomes 1R-7R of triticale (Saal and Wricke, 1999; Hackauf and Wehling, 2002; Khlestkina et al., 2004; Zhuang et al., 2010; Xu et al., 2012; Martis et al., 2013; Nguyen et al., 2015). The number of markers on each rye chromosome ranges from 4 to 25 with an average of 9. All rye markers and their features are listed in the Table S1. All markers were screened for polymorphisms between Xuezao and Sorento by PAGE (polyacrylamide gel electrophoresis) analysis, and the polymorphic markers were then used to genotype the backcrossed individuals.
Molecular Marker Analysis
In the present study, PCR-based identification was used to detect the alien chromatin in wheat-triticale derivatives. Total leaf genomic DNA of Sorento, Xuezao, and their progenies was isolated using the CTAB method with some modifications (Allen et al., 2006). PCR amplifications were conducted in a 10 μL mixture containing 10 mM Tris-HCl, 50 mM KCl, 1.5 mM MgCl2, 200 μM dNTPs, 0.75 U Taq DNA polymerase, 0.2 μM of each primer, and 50–100 ng genomic DNA. The PCR program consisted of an initial denaturation at 94°C for 5 min followed by 40 cycles of 94°C for 45 s, 50–60°C for 35 s, and 72°C for 35 s, followed by a final extension at 72°C for 10 min. The PCR products were analyzed on 8% non-denaturing polyacrylamide gels, and the gels were then silver stained and photographed.
Cytological Identification of Rye-Derived Chromosomes
Cytological analysis was carried out using GISH and FISH as described by Han et al. (2006). Triticale-wheat substitution, translocation or addition lines resistant to wheat powdery mildew or leaf rust in BC2F3 were selected for cytological analysis. For GISH analysis, the rye genome was used as a probe to detect the rye-derived chromosomal segments in Sorento. For FISH analysis, the probes pSc119.2 and pAs1, containing highly repetitive sequence of wheat and rye, were used to distinguish the wheat A-, B-, and D-genomes and the rye R-genome. Probes pSc119.2 and pAs1 were labeled with Fluor-488-5-dUTP (Invitrogen) and Texas red-5-dCTP (Invitrogen), respectively as described by Wang et al. (2017). Chromosome preparation and in situ hybridization were carried out according to Han et al. (2006). Images were taken with epifluorescence microscope Olympus BX61.
Results
Introgression of the Powdery Mildew and Leaf Rust Resistance by Backcrossing
To introduce disease resistance from triticale to wheat, the resistant triticale cultivar Sorento was crossed with the susceptible wheat line Xuezao to create the F1 hybrids. The seed setting rate in the cross Sorento × Xuezao was 7.1%. Among 127 seeds sown, 92 (72.4%) germinated. Only 65 (70.6%) of such plants survived after inoculation and transplantation into field (Figure 1). All F1 plants were completely male sterile, as revealed by open glumes and aborted anthers after heading. The rate of seed setting of F1 × Xuezao backcross ranged from 0 to 20% and a total of 276 seeds were obtained with the total germination and recovery rate of 62.7 and 57.8%, respectively (Figure 1). Nearly 90% of the resulting BC1F1 plants remained male sterile. The total germination rate in BC2F1 was 69.3%, whereas the recovery rate was only 29.2% because the plastic film got damaged and most of the transplanted plants were winterkilled (Figure 1). However, the average fertility improved in the BC2F1 and BC3F1 plants.
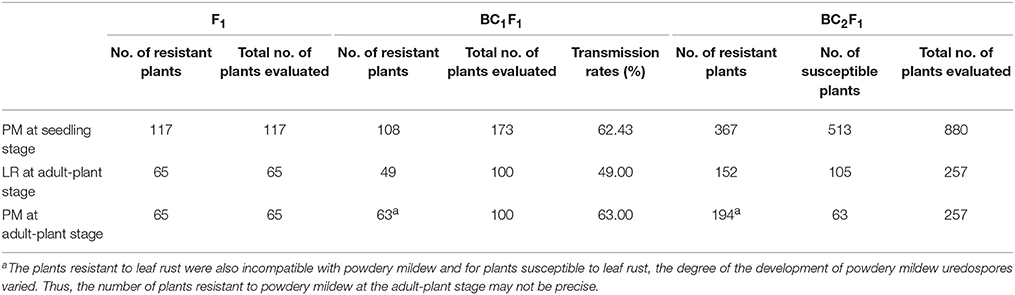
All geminated and recovered F1 plants showed immunity to both powdery mildew isolate E09 and leaf rust isolate PHT at the adult and seedling stage (Table 1). Of 173 BC1F1 individuals challenged with powdery mildew in the greenhouse, 108 plants showed seedling resistance. This indicates that the transmission rate of the powdery mildew resistance at seedling stage through egg cells of Sorento × Xuezao F1 hybrids was 62.4% (Table 1). At adult-plant stage, the occurrence of both powdery mildew and leaf rust was investigated in 100 BC1F1 plants. A total of 49 and 63 adult plants were resistant to leaf rust and powdery mildew respectively, though 73 plants did not survive after transplantation (Table 1). We found that all plants resistant to leaf rust also displayed excellent resistance to wheat powdery mildew in BC1F1 and BC2F1. For plants susceptible to leaf rust, the degree of the development of powdery mildew uredospores varied.
Marker Analysis of Powdery Mildew Resistance at Seedling Stage
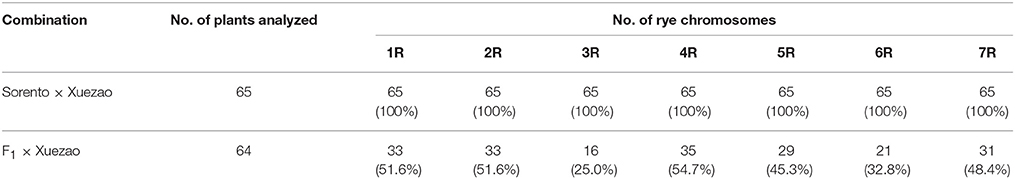
To select rye markers for use in our experiments, 64 markers were first screened for polymorphism between Sorento and Xuezao (Table S1). A total of 56 markers were polymorphic and 51 could be used to unambiguously identify the rye chromosomes present in the BC1F1 backcrossed progenies. The transmissions of different rye chromosomes of Sorento were first investigated on 64 BC1F1 germinated seedlings using these specific markers. The number of the rye chromosomes transmitted through F1 egg cells ranged from 0 to 6 with an average number of 3.09. Most of rye chromosomes showed a transmission rate of about 50% except for 3R and 6R, which showed a much lower frequency (Table 2). The average transmission rate was 44.2%.
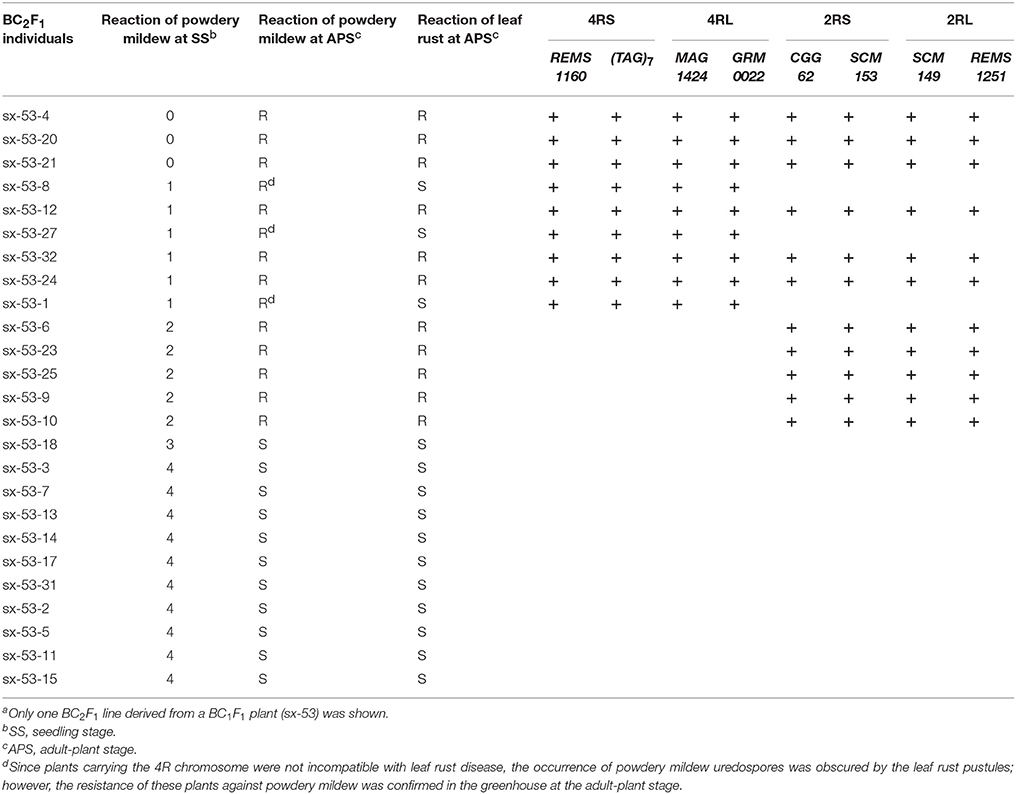
To locate the powdery mildew resistance of Sorento at seedling stage, the polymorphic rye markers on chromosomes 1R-7R were used to analyze the chromosome composition of 284 BC2F1 individuals derived from 9 BC1F1 plants with different rye constitutions (Table S1). The result showed that 125 plants resistant to powdery mildew isolate E09 carried either chromosome 4R or 2R, or both. Specifically, 57 plants carrying the 4R chromosome showed immunity (IT = 0) or high resistance (IT = 0; or 1) to wheat powdery mildew and 33 plants carrying the 2R chromosome only showed moderate resistance (IT = 2) at 10 dpi (Figures S1A,C, Table 4). The remaining 35 BC2F1 plants were immune or highly resistant to powdery mildew and contained both chromosomes 4R and 2R (Table 4). Plants lacking 4R and 2R were highly susceptible (IT = 4) (Figures S1A,C, Table 4). At 16–18 dpi, in contrast to the plants carrying 4R (IT = 0, 0; or 1), all plants only carrying 2R were susceptible (IT = 3). This demonstrated that in these BC2F1 plants, the 2R chromosome from Sorento slowed down the infection progress at the seedling stage.
To validate the resistance conferred by 4R and 2R from Sorento at the seedling stage, 234 and 181 BC2F2plants segregating only for 4R and 2R, respectively, were surveyed using chromosomal arm-specific markers (Table 3). In the 4R experiment, a total of 106 resistant individuals were found to carry the 4R chromosome, among which 21 plants contained only the long arm of 4R. The remaining 128 susceptible plants did not contain 4RL, but 16 of these plants contained 4RS (Figure 2). This further suggests that 4RL from Sorento confers excellent resistance to wheat powdery mildew. In the 2R experiment, 70 plants were characterized as resistant to powdery mildew; the resistance was located on the long arm of 2R chromosome, as proven by markers from 2RL (Figure 3). However, plants carrying any of the other five rye chromosomes of Sorento were shown to be completely susceptible to wheat powdery mildew at the seedling stage.
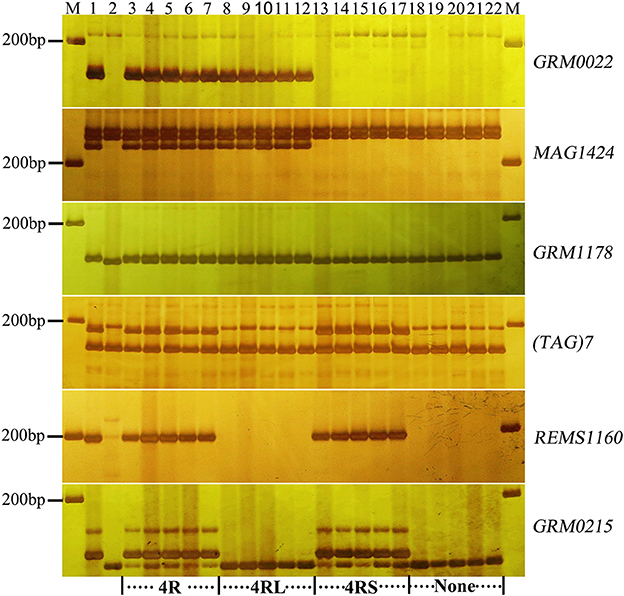
Figure 2. Marker analysis of BC2F2 plants segregating for 4R using rye-specific markers (GRM0022, MAG1424, and GRM1178) on the long arm of 4R chromosome and markers ((TAG)7, REMS1160, and GRM0215) on the short arm of 4R chromosome. Ten plants (lane 3–12) resistant to powdery mildew at the seedling stage and 10 susceptible plants (lane 13–22) were analyzed. Lane 3–7 and 18–22 show plants with entire 4R and without 4R chromosome, respectively. Lane 8–12 and 13–17 signify the presence of 4RL and 4RS alone, respectively. Lane 1 and lane 2 signify the resistant parent (Sorento) and susceptible parent (Xuezao), respectively.
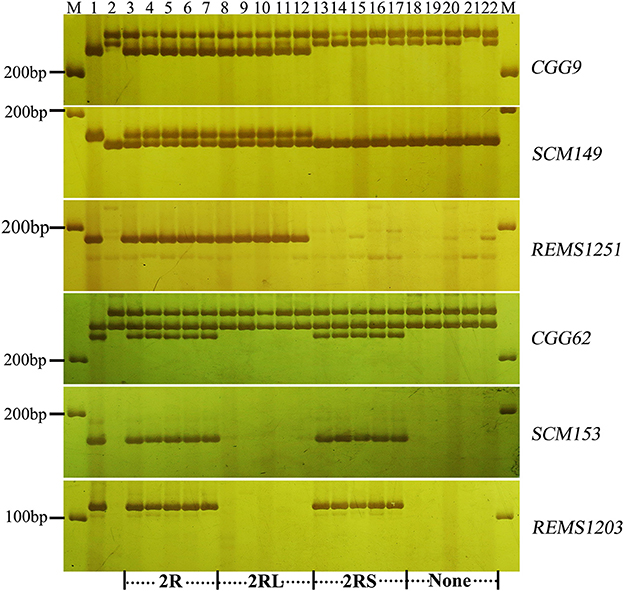
Figure 3. Marker analysis of BC2F2 plants segregating for 2R using rye-specific markers (CGG9, SCM149, and REMS1251) on the long arm of 2R chromosome and markers (CGG62, SCM153, and REMS1203) on the short arm of 2R chromosome. Ten plants (lane 3–12) resistant to powdery mildew at the seedling stage and 10 susceptible plants (lane 13–22) were analyzed. Lane 3–7 and 18–22 show plants with entire 2R and without 2R chromosome, respectively. Lane 8–12 and 13–17 signify the presence of 2RL and 2RS alone, respectively. Lane 1 and lane 2 signify the resistant parent (Sorento) and susceptible parent (Xuezao), respectively.
Marker Analysis of Leaf Rust at Both Stages and Powdery Mildew Resistance at the Adult-Plant Stage
At the adult-plant stage, we analyzed powdery mildew and leaf rust resistance when both diseases had thoroughly developed on Xuezao at around 50 dpi. Among 257 BC2F1 plants, 152 were found to be strongly resistant to both the wheat leaf rust isolate PHT and the powdery mildew isolate E09 (Figure S1D). Our marker analysis showed that all the resistant plants carried the 2R or 2RL chromosome, whereas plants possessing the 1R, 2RS, 3R, 4R, 5R, 6R, and 7R chromosomes appeared to be very susceptible to leaf rust. This indicates that 2RL from Sorento confers strong resistance to wheat powdery mildew and leaf rust diseases at the adult-plant stage (Figure S1D, Table 4). The result was consistent with that from BC1F1, where all plants resistant to leaf rust were also resistant to powdery mildew fungus. In contrast to the moderate resistance conferred by 2RL against powdery mildew at seedling stage, adult plants carrying this chromosome were strongly resistant (Figure S1D). Except for adult plants containing 4R and 2R, plants with other chromosomes of Sorento were moderately or highly susceptible against isolate E09.
Plants carrying the 4R chromosome of Sorento were strongly sensitive to leaf rust, resulting in our inability to distinguish the reaction to powdery mildew at the adult-plant stage in the field. Because the 4RL chromosome conferred high resistance to powdery mildew at the seedling stage, it was necessary to exclude the influence of leaf rust uredinia on the occurrence of powdery mildew at the adult-plant stage. Therefore, the powdery mildew resistance of BC2F2 plants carrying 4RL at the adult-plant stage was investigated in the greenhouse. As expected, plants carrying the 4RL of Sorento chromosome were completely incompatible with powdery mildew, which is consistent with their performance at the seedling stage (Figures S1C,E).
To test the seedling resistance against leaf rust, 50 BC2F2 plants derived from 5 BC2F1 carrying chromosome 2R and one plant carrying chromosome arm 2RL and showing resistance to leaf rust at the adult-plant stage were evaluated in the growth chamber. Eighteen and three plants containing chromosomes 2R and 2RL, respectively, were highly resistant to leaf rust (Figure S1B). The response of both seedling and adult plants carrying the 2R or 2RL chromosome to leaf rust was characterized by a strong hypersensitive reaction (Figures S1B,D). In conclusion, the 2RL chromosome transferred from Sorento confers resistance to leaf rust and powdery mildew, whereas the 4RL chromosome from Sorento contributes resistance only to wheat powdery mildew.
Validation of 2R and 4R Chromosomes by Cytological Analysis
A total of 25 BC2F3 lines derived from BC2F2 plants which had been shown by marker analysis to contain either the 2R or the 4R chromosome were verified by GISH and FISH analysis. After inoculating seedlings of 25 BC2F3 lines with wheat Bgt isolate E09 and Pt isolate PHT, segregant and non-segregant lines for resistance against powdery mildew and leaf rust were identified. By cytological analysis, all plants contain 42 chromosomes. Among those, seven lines homozygous for leaf rust and powdery mildew resistance were presented to carry a pair of 2R chromosomes (Figures 4A,B), and six lines homozygous for powdery mildew resistance were shown to contain a pair of 4R chromosomes (Figures 5A,B). One line, 1,204, appears to contain a 4R-4D reciprocal translocation in which the arms 4RS and 4DL, or 4RL and 4DS, are interchanged (Figures 6A,B). Line 1,887 was shown to be a monosomic 2RL-1DL translocation line in which 2RLand 1DL were interchanged (Figures 7A,B). The rest of the BC2F3 lines segregating for leaf rust and powdery mildew resistance were determined to be monosomic substitution lines. In these lines, 2R chromosomes were substituted by a pair of 2D chromosomes of wheat for all BC2F3 lines homozygous for 2R, which was confirmed by the absence of PCR products of wheat 2D-derived SSR markers. However, the wheat chromosomes substituted by other rye chromosomes still remained unknown and need to be identified later. These results were consistent with the marker analysis and the newly developed lines have been applied in wheat breeding.
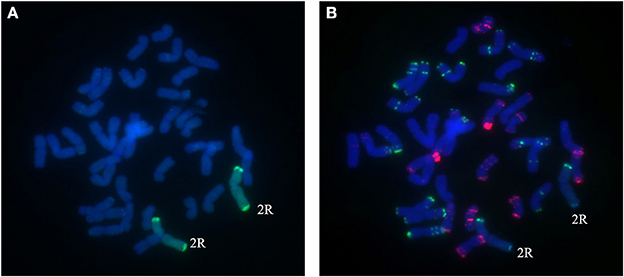
Figure 4. Chromosome composition of BC2F3 line 1289 by FISH (B) and GISH (A) using probe pAs1 (red) and probe pSc119.2 (green). A pair of 2R chromosomes from Sorento have been introduced into wheat.
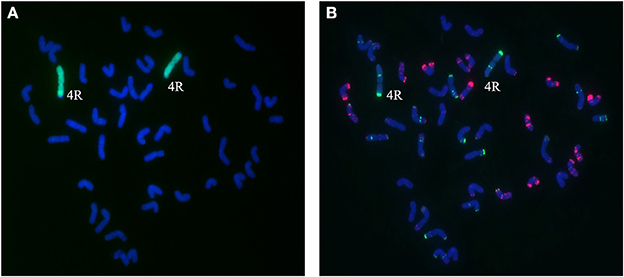
Figure 5. Chromosome composition of BC2F3 line 1419 by FISH (B) and GISH (A) using probe pAs1 (red) and probe pSc119.2 (green). Line 1,419 contains a pair of 4R chromosomes from Sorento.
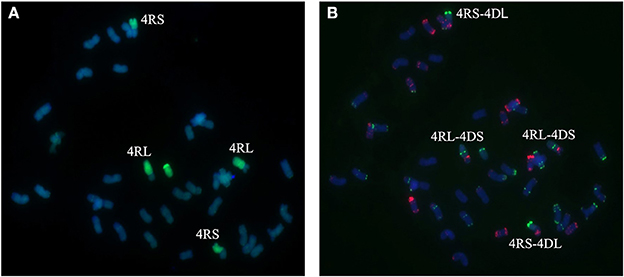
Figure 6. Chromosome composition of BC2F3 line 1204 by FISH (B) and GISH (A) using probe pAs1 (red) and probe pSc119.2 (green). Line 1,204 shows to be a 4R-4D reciprocal translocation in which the short arm of 4R chromosome was translocated to the long arm of 4D chromosome and the long arm of 4R chromosome was translocated to the short arm of 4D chromosome.
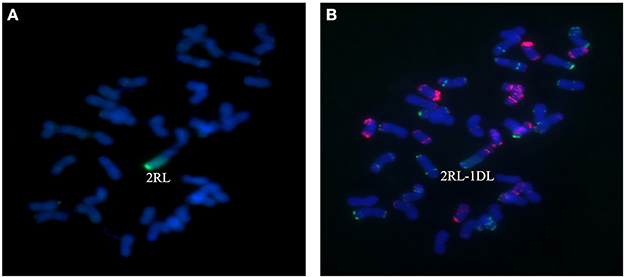
Figure 7. Chromosome composition of BC2F3 line 1,887 by FISH (B) and GISH (A) using probe pAs1 (red) and probe pSc119.2 (green). Line 1,887 was shown to be a monosomic 2RL-1DL translocation line in which the long arm of 2R was translocated to the long arm of 1D chromosome.
Discussion
Triticale cultivar Sorento, which combines the superior stress tolerance of rye and high yield of T. turgidum, integrates the resistance advantages of different triticale lines. Crosses between hexaploid triticale and wheat are easier than those between rye and wheat due to better seed set (Hills et al., 2007), and because colchicine treatment and tedious embryo rescue are not required. The hexaploid feature also assists in the hybridization of triticale and wheat. Triticale cv. Sorento displayed outstanding resistance to wheat powdery mildew and leaf rust diseases as well as triticale diseases. However, the resistance has not been investigated and exploited in wheat breeding. Here, we successfully introduced resistance to wheat powdery mildew and leaf rust derived from hexaploid triticale cv. Sorento into susceptible wheat line Xuezao by backcrossing. The resulting BC1F1, BC2F1, BC2F2, and BC3F1 plants were genotyped with rye chromosome-specific markers and evaluated for resistance against powdery mildew and leaf rust at the seedling and adult-plant stages. We proved that the long arms of the 2R and 4R chromosomes from Sorento carry resistance genes against wheat powdery mildew and leaf rust diseases. The long arm of 4R confers high resistance to wheat powdery mildew at the seedling and adult-plant stages, and the long arm of 2R confers moderate powdery mildew resistance at the seedling stage and strong resistance at the adult-plant stage. Additionally, 2RL confers strong resistance toward wheat leaf rust accompanied by a strong hypersensitive reaction (HR). HR is a notable feature of race-specific response of R genes and is characterized by localized cell death supposed to avoid spread of biotroph pathogens to the healthy tissue of plants (Jones and Dangl, 2006). R genes mainly function throughout whole stage divergent from the adult-plant resistance (APR) which mainly takes place at adult stage and is characterized as non-race-specific (Periyannan et al., 2017). However, race-specific resistance and HR were also reported for APR genes such as Lr12, Lr22, and Lr35 (Ellis et al., 2014), and the characteristics of the resistance of 2RL need to be determined systematically.
Fertility seems to be the main barrier in introducing resistance genes into bread wheat. In our crossing process, most of the early generations such as F1, BC1F1, and BC2F1 displayed inferior crossability with Xuezao and lower self-fertility rate. This may be due to the influences of maternal genotype among plants and meiotic disturbances (Oettler, 2005). The transmission rate of rye chromosomes in the F1 × Xuezao backcross is consistent with that of Lukaszewski et al. (1982). The lower transmission frequency of chromosome 3R and 6R appeared also in the BC2F1 backcross. By contrast, disomic substitutions for chromosomes 2R and 4R showed a high seed setting rate above 90%.
The powdery mildew resistance gene Pm7 located on 2RLin the form of T4BS.4BL-2RL from Transec has been overcome and shows no resistance to E09 (Friebe et al., 1996; Zhuang, 2003; Zhang et al., 2010). Chromosome arm 2RL also carries the leaf rust resistance gene Lr25, however it has not contributed to wheat breeding improvement because the Transec translocation has an additional intercalary segment derived from 5RL (Friebe et al., 1996).
Hysing et al. (2007) reported that SLU translocation lines carrying T2BS.2RL were completely resistant to 17 powdery mildew isolates and 14 leaf rust isolates at the seedling stage and to the same mixture of powdery mildew isolates at the adult plant stage, however, adult-stage resistance conferred by T2BS.2RL against leaf rust was not well-described. The Xiaoyan 6-German White 2R (2D) chromosome substitution lines developed using the nullisomic back-cross procedure were also reported to confer resistance to powdery mildew isolates prevalent in northern China (An et al., 2006). However, the primer pair R1 R2 used for detecting 2R of German White did not amplify the 473 bp product in our resistant lines. Zhuang et al. (2010) demonstrated that the resistance to powdery mildew in H-J DA2RDS1R(1D) was provided by the 2RL from rye cv. Jingzhouheimai. However, markers Xscm32, Xscm33, Xscm75, and Xcinau514 mapped on 2R from Jingzhouheimai amplified no products in Sorento or our resistant progeny. In light of its diverse genetic background and specificity of markers and the unique resistance phenotype of powdery mildew and leaf rust, we infer that the resistance loci on 2RL in Sorento may be novel.
Resistance derived from 4RL has been reported. Fu et al. (2014) showed that monotelosomic or ditelosomic addition lines of the long arm of rye chromosome 4R (4RL) from Kustro displayed immunity to mixed wheat powdery mildew (Bgt) isolates collected from Sichuan, China. The result was confirmed with 4RL-specific marker SCIM808986. To distinguish this segment, we used the same marker to analyze the resistance contributed by 4RL in Sorento and its progenies. Unexpectedly, no product was amplified. An et al. (2013) reported that Xiaoyan 6-German White 4R chromosome translocation line WR41-1 (T4BL.4RL and T7AS.4RS) showed high levels of resistance to powdery mildew. Markers KSUM62 (4RS) and MAG1424 (4RL) associated with powdery mildew resistance of WR41-1 also co-segregated with the resistance of 4R in our plants. However, whether the wheat powdery mildew resistance we identified represents a novel resistance locus on 4RL remains unclear.
Recombination between alien chromosomes and wheat chromosomes are limited and lots of efforts have been made to facilitate recombination between wheat and rye to exclude the disadvantageous chromatin. (Lukaszewski, 2000; Mago et al., 2002; Qi et al., 2007). Lack of recombination hampers the delimitation of resistance genes on a block of rye chromosome arm where multiple resistance genes may be responsible for a particular resistance such as the wheat-Haynaldia villosa translocation line T6VS.6AL (Cao et al., 2011; He et al., 2017). Although both 2RL and 4RL from Sorento confer resistance at seedling and adult stage, it is not clear whether the resistance at both stages was controlled by the same gene or a cluster of genes. Genetic and cytogenetic methods need to be put into practice to promote recombination and minimize the adverse effects brought by the rye chromatin.
Author Contributions
FL and CX: designed the research; FL, YL, LC, PL, MG, QZ, and LQ: did the cross work and phenotype evaluation; FL and LC: completed the molecular marker and cytological analysis; CX and QS: provided the material; FL: wrote the paper.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
This work was financially supported by the National Key Research and Development Project of China (2016YFD0101802), National Natural Science Foundation of China (31271708, 31671676) and the Ministry of Science and Technology of the People's Republic of China (2011YQ08005206). We are grateful to Dr. Fangpu Han for assistance in cytological experiment.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fpls.2018.00085/full#supplementary-material
References
Allen, G. C., Flores-Vergara, M. A., Krasnyanski, S., Kumar, S., and Thompson, W. F. (2006). A modified protocol for rapid DNA isolation from plant tissues using cetyltrimethylammonium bromide. Nat. Protoc. 1, 2320–2325. doi: 10.1038/nprot.2006.384
An, D. G., Li, L. H., Li, J. M., Li, H. J., and Zhu, Y. G. (2006). Introgression of resistance to powdery mildew conferred by chromosome 2R by crossing wheat nullisomic 2D with rye. J. Integr. Plant Biol. 48, 838–847. doi: 10.1111/j.1744-7909.2006.00275.x
An, D. G., Zheng, Q., Zhou, Y. L., Ma, P. T., Lv, Z. L., Li, L. H., et al. (2013). Molecular cytogenetic characterization of a new wheat-rye 4R chromosome translocation line resistant to powdery mildew. Chromosome Res. 21, 419–432. doi: 10.1007/s10577-013-9366-8
Bansal, M., Kaur, S., Dhaliwal, H. S., Bains, N. S., Bariana, H. S., Chhuneja, P., et al. (2017). Mapping of Aegilops umbellulata-derived leaf rust and stripe rust resistance loci in wheat. Plant Pathol. 66, 38–44. doi: 10.1111/ppa.12549
Bolton, M. D., Kolmer, J. A., and Garvin, D. F. (2008). Wheat leaf rust caused by Puccinia triticina. Mol. Plant Pathol. 9, 563–575. doi: 10.1111/j.1364-3703.2008.00487.x
Cao, A., Xing, L., Wang, X., Yang, X., Wang, W., Sun, Y., et al. (2011). Serine/threonine kinase gene stpk-v, a key member of powdery mildew resistance gene Pm21, confers powdery mildew resistance in wheat. Proc. Natl. Acad. Sci. U.S.A. 108, 7727–7732. doi: 10.1073/pnas.1016981108
Conner, R. L., Kuzyk, A. D., and Su, H. (2003). Impact of powdery mildew on the yield of soft white spring wheat cultivars. Can. J. Plant Sci. 83, 725–728. doi: 10.4141/P03-043
Contento, A., Heslop-Harrison, J. S., and Schwarzacher, T. (2005). Diversity of a major repetitive DNA sequence in diploid and polyploid Triticeae. Cytogenet. Genome Res. 109, 34–42. doi: 10.1159/000082379
Dhaliwal, A. S., and MacRitchie, F. (1990). Contributions of protein fractions to dough handling properties of wheat-rye translocation cultivars. J. Cereal Sci. 12, 113–122. doi: 10.1016/S0733-5210(09)80093-3
Dhaliwal, A. S., Mares, D. J., and Marshall, D. R. (1987). Effect of 1B/1R chromosome translocation on milling and quality characteristics of bread wheats. Cereal Chem. 64, 72–76.
Ehdaie, B., Whitkus, R. W., and Waines, J. G. (2003). Root biomass, water-use efficiency, and performance of wheat-rye translocations of chromosomes 1 and 2 in spring bread wheat ‘Pavon’. Crop Sci. 43, 710–717. doi: 10.2135/cropsci2003.0710
Ellis, J. G., Lagudah, E. S., Spielmeyer, W., and Dodds, P. N. (2014). The past, present and future of breeding rust resistant wheat. Front. Plant Sci. 5:641. doi: 10.3389/fpls.2014.00641
Food and Agriculture Organization of the United Nations (FAO) (2014). Available online at: http://www.fao.org/faostat/en/#data
Friebe, B., Heun, M., Tuleen, N., Zeller, F. J., and Gill, B. S. (1994). Cytogenetically monitored transfer of powdery mildew resistance from rye into wheat. Crop Sci. 34, 621–625. doi: 10.2135/cropsci1994.0011183X003400030003x
Friebe, B., Jiang, J., Raupp, W. J., McIntosh, R. A., and Gill, B. S. (1996). Characterization of wheat-alien translocations conferring resistance to diseases and pests: current status. Euphytica 91, 59–87. doi: 10.1007/BF00035277
Friebe, B., Zhang, P., Linc, G., and Gill, B. S. (2005). Robertsonian translocations in wheat arise by centric misdivision of univalents at anaphase I and rejoining of broken centromeres during interkinesis of meiosis II. Cytogenet. Genome Res. 109, 293–297. doi: 10.1159/000082412
Fu, S. L., Ren, Z. L., Chen, X. M., Yan, B. J., Tan, F. Q., Fu, T. H., et al. (2014). New wheat-rye 5DS-4RS.4RL and 4RS-5DS.5DL translocation lines with powdery mildew resistance. J. Plant Res. 127, 743–753. doi: 10.1007/s10265-014-0659-6
Griffey, C. A., Das, M. K., and Stromberg, E. L. (1993). Effectiveness of adult-plant resistance in reducing grain-yield loss to powdery mildew in winter wheat. Plant Dis. 77, 618–622. doi: 10.1094/PD-77-0618
Hackauf, B., and Wehling, P. (2002). Identification of microsatellite polymorphisms in an expressed portion of the rye genome. Plant Breed. 121, 17–25. doi: 10.1046/j.1439-0523.2002.00649.x
Han, F. P., Lamb, J. C., and Birchler, J. A. (2006). High frequency of centromere inactivation resulting in stable dicentric chromosomes of maize. Proc. Natl. Acad. Sci. U.S.A. 103, 3238–3243. doi: 10.1073/pnas.0509650103
Hao, Y. F., Parks, R., Cowger, C., Chen, Z. B., Wang, Y. Y., Bland, D., et al. (2015). Molecular characterization of a new powdery mildew resistance gene Pm54 in soft red winter wheat. Theor. Appl. Genet. 128, 465–476. doi: 10.1007/s00122-014-2445-1
He, H., Ji, Y., Zhu, S., Li, B., Zhao, R., Jiang, Z., et al. (2017). Genetic, physical and comparative mapping of the powdery mildew resistance gene Pm21 originating from Dasypyrum villosum. Front. Plant Sci. 8:1914. doi: 10.3389/fpls.2017.01914
Heun, M., and Friebe, B. (1990). Introgression of powdery mildew resistance from rye into wheat. Phytopathology 80, 242–245. doi: 10.1094/Phyto-80-242
Hills, M. J., Hall, L. M., Messenger, D. F., Graf, R. J., Beres, B. L., and Eudes, F. (2007). Evaluation of crossability between triticale (X Triticosecale Wittmack) and common wheat, durum wheat and rye. Environ. Biosafety Res. 6, 249–257. doi: 10.1051/ebr:2007046
Hubbard, A., Pritchard, L., and Holdgate, S. (2016). United Kingdom Cereal Pathogen Virulence Survey 2016 Annual Report Part 1: Wheat Yellow Rust, Wheat Powdery Mildew and Barley Powdery Mildew. Cambridge: AHDB Cereals & Oilseeds.
Huerta-Espino, J., Singh, R. P., Germán, S., McCallum, B. D., Park, R. F., Chen, W. Q., et al. (2011). Global status of wheat leaf rust caused by Puccinia triticina. Euphytica 179, 143–160. doi: 10.1007/s10681-011-0361-x
Hurni, S., Brunner, S., Buchmann, G., Herren, G., Jordan, T., Krukowski, P., et al. (2013). Rye Pm8 and wheat Pm3 are orthologous genes and show evolutionary conservation of resistance function against powdery mildew. Plant J. 76, 957–969. doi: 10.1111/tpj.12345
Hysing, S. C., Hsam, S. L. K., Singh, R. P., Huerta-Espino, J., Boyd, L. A., Koebner, R. M. D., et al. (2007). Agronomic performance and multiple disease resistance in T2BS.2RL wheat-rye translocation lines. Crop Sci. 47, 254–260. doi: 10.2135/cropsci2006.04.0269
Imani, Y., Ouassou, A., and Griffey, C. A. (2002). Virulence of Blumeria graminis f. sp tritici populations in Morocco. Plant Dis. 86, 383–388. doi: 10.1094/PDIS.2002.86.4.383
Jiang, J. M., Friebe, B., and Gill, B. S. (1994). Recent advances in alien gene transfer in wheat. Euphytica 73, 199–212. doi: 10.1007/BF00036700
Jones, J. D. G., and Dangl, J. L. (2006). The plant immune system. Nature 444, 323–329. doi: 10.1038/nature05286
Khlestkina, E. K., Than, M. H., Pestsova, E. G., Roder, M. S., Malyshev, S. V., Korzun, V., et al. (2004). Mapping of 99 new microsatellite-derived loci in rye (Secale cereale L.) including 39 expressed sequence tags. Theor. Appl. Genet. 109, 725–732. doi: 10.1007/s00122-004-1659-z
Kim, W., Johnson, J. W., Baenziger, P. S., Lukaszewski, A. J., and Gaines, C. S. (2004). Agronomic effect of wheat-rye translocation carrying rye chromatin (1R) from different sources. Crop Sci. 44, 1254–1258. doi: 10.2135/cropsci2004.1254
Krattinger, S. G., Lagudah, E. S., Spielmeyer, W., Singh, R. P., Huerta-Espino, J., Mcfadden, H., et al. (2009). A putative ABC transporter confers durable resistance to multiple fungal pathogens in wheat. Science 323, 1360–1363. doi: 10.1126/science.1166453
Lee, T. G., Hong, M. J., Johnson, J. W., Bland, D. E., Kim, D. Y., and Seo, Y. W. (2009). Development and functional assessment of EST-derived 2RL-specific markers for 2BS.2RL translocations. Theor. Appl. Genet. 119, 663–673. doi: 10.1007/s00122-009-1077-3
Liu, W., Koo, D. H., Xia, Q., Li, C., Bai, F., Song, Y., et al. (2017). Homoeologous recombination-based transfer and molecular cytogenetic mapping of powdery mildew-resistant gene Pm57 from Aegilops searsii into wheat. Theor. Appl. Genet. 130, 841–848. doi: 10.1007/s00122-017-2855-y
Lukaszewski, A. J. (2000). Manipulation of the 1RS.1BL translocation in wheat by induced homoeologous recombination. Crop Sci. 40, 216–225. doi: 10.2135/cropsci2000.401216x
Lukaszewski, A. J., and Gustafson, J. P. (1983). Translocations and modifications of chromosomes in triticale × wheat hybrids. Theor. Appl. Genet. 64, 239–248. doi: 10.1007/BF00303771
Lukaszewski, A. J., Gustafson, J. P., and Apolinarska, B. (1982). Transmission of chromosomes through the eggs and pollen of triticale x wheat F1-hybrids. Theor. Appl. Genet. 63, 49–55. doi: 10.1007/BF00303489
Lutz, J., Limpert, E., Bartos, P., and Zeller, F. J. (1992). Identification of powdery mildew resistance genes in common wheat (Triticum Aestivum L). Plant Breed. 108, 33–39. doi: 10.1111/j.1439-0523.1992.tb00097.x
Ma, P. T., Xu, H. X., Xu, Y. F., Li, L. H., Qie, Y. M., Luo, Q. L., et al. (2015). Molecular mapping of a new powdery mildew resistance gene Pm2b in Chinese breeding line KM2939. Theor. Appl. Genet. 128, 613–622. doi: 10.1007/s00122-015-2457-5
Ma, X. F., and Gustafson, J. P. (2008). Allopolyploidization-accommodated genomic sequence changes in triticale. Ann. Bot. 101, 825–832. doi: 10.1093/aob/mcm331
Mago, R., Spielmeyer, W., Lawrence, G. J., Ellis, J. G., and Pryor, A. J. (2004). Resistance genes for rye stem rust (SrR) and barley powdery mildew (Mla) are located in syntenic regions on short arm of chromosome. Genome 47, 112–121. doi: 10.1139/g03-096
Mago, R., Spielmeyer, W., Lawrence, J., Lagudah, S., Ellis, G., and Pryor, A. (2002). Identification and mapping of molecular markers linked to rust resistance genes located on chromosome 1RS of rye using wheat-rye translocation lines. Theor. Appl. Genet. 104, 1317–1324. doi: 10.1007/s00122-002-0879-3
Mago, R., Zhang, P., Vautrin, S., Šimková, H., Bansal, U., Luo, M. C., et al. (2015). The wheat Sr50 gene reveals rich diversity at a cereal disease resistance locus. Nat. Plants 1:15186. doi: 10.1038/nplants.2015.186
Martin, D. J., and Stewart, B. G. (1990). Dough stickiness in rye-derived wheat cultivars. Euphytica 51, 77–86. doi: 10.1007/BF00022895
Martis, M. M., Zhou, R., Haseneyer, G., Schmutzer, T., Vrána, J., Kubaláková, M., et al. (2013). Reticulate evolution of the rye genome. Plant Cell 25, 3685–3698. doi: 10.1105/tpc.113.114553
Mcintosh, R. A., Hart, G. E., and Gale, M. D. (1988). Catalog of gene symbols for wheat - 1988 supplement. Cereal Res. Commun. 16, 121–135.
McIntosh, R. A., Dubcovsky, J., Rogers, W. J., Morris, C., Appels, R., and Xia, X. C. (2014). Catalogue of Gene Symbols for Wheat: 2013–2014 Supplement. Komugi-Wheat Genetic Resources Database. Available online at: http://www.shigen.nig.ac.jp/wheat/komugi/
Moore, J. W., Herrera-Foessel, S., Lan, C., Schnippenkoetter, W., Ayliffe, M., Huerta-Espino, J., et al. (2015). A recently evolved hexose transporter variant confers resistance to multiple pathogens in wheat. Nat. Genet. 47, 1494–1498. doi: 10.1038/ng.3439
Murray, G. M., and Brennan, J. P. (2009). The Current and Potential Costs from Diseases of Wheat in Australia. Barton, KS: Grains Research and Development Corporation.
Nguyen, V., Fleury, D., Timmins, A., Laga, H., Hayden, M., Mather, D., et al. (2015). Addition of rye chromosome 4R to wheat increases anther length and pollen grain number. Theor. Appl. Genet. 128, 953–964. doi: 10.1007/s00122-015-2482-4
Niewoehner, A. S., and Leath, S. (1998). Virulence of Blumeria graminis f. sp. tritici on winter wheat in the eastern United States. Plant Dis. 82, 64–68. doi: 10.1094/PDIS.1998.82.1.64
Oettler, G. (1983). Crossability and embryo development in wheat-rye hybrids. Euphytica 32, 593–600. doi: 10.1007/BF00021472
Oettler, G. (2005). The fortune of a botanical curiosity – triticale: past, present and future. J. Agr. Sci. 143, 329–346. doi: 10.1017/S0021859605005290
Parks, R., Carbone, I., Murphy, J. P., Marshall, D., and Cowger, C. (2008). Virulence structure of the eastern U.S. wheat powdery mildew population. Plant Dis. 92, 1074–1082. doi: 10.1094/PDIS-92-7-1074
Periyannan, S., Milne, R. J., Figueroa, M., Lagudah, E. S., and Dodds, P. N. (2017). An overview of genetic rust resistance: from broad to specific mechanisms. PLoS Pathog. 13:e1006380. doi: 10.1371/journal.ppat.1006380
Purnhauser, L., Bóna, L., and Láng, L. (2010). Occurrence of 1BL.1RS wheat-rye chromosome translocation and of Sr36/Pm6 resistance gene cluster in wheat cultivars registered in Hungary. Euphytica 179, 287–295. doi: 10.1007/s10681-010-0312-y
Qi, L., Friebe, B., Zhang, P., and Gill, B. S. (2007). Homoeologous recombination, chromosome engineering and crop improvement. Chromosome Res. 15, 3–19. doi: 10.1007/s10577-006-1108-8
Rabinovich, S. V. (1998). Importance of wheat-rye translocations for breeding modern cultivars of Triticum aestivum L. (reprinted from wheat: prospects for global improvement, 1998). Euphytica 100, 323–340. doi: 10.1023/A:1018361819215
Roelfs, A. P., Singh, R. P., and Saari, E. E. (1992). Rust Diseases of Wheat: Concepts and Methods of Disease Management. Mexico: CIMMYT.
Saal, B., and Wricke, G. (1999). Development of simple sequence repeat markers in rye (Secale cereale L.). Genome 42, 964–972. doi: 10.1139/g99-052
Schlegel, R., Zaripova, Z., and Shchapova, A. I. (1980). Further evidence on wheat-rye chromosome pairing in F1 triticale X wheat hybrids. Biol. Zent. 585–590.
Schneider, A., Linc, G., and Molnar-Lang, M. (2003). Fluorescence in situ hybridization polymorphism using two repetitive DNA clones in different cultivars of wheat. Plant Breed. 122, 396–400. doi: 10.1046/j.1439-0523.2003.00891.x
Shimizu, Y., Nasuda, S., and Endo, T. R. (1997). Detection of the Sec-1 locus of rye by a PCR-based method. Genes Genet. Syst. 72, 197–203. doi: 10.1266/ggs.72.197
Singh, N. K., Shepherd, K. W., and Mcintosh, R. A. (1990). Linkage mapping of genes for resistance to leaf, stem and stripe rusts and omega-secalins on the short arm of rye chromosome 1R. Theor. Appl. Genet. 80, 609–616. doi: 10.1007/BF00224219
Singla, J., Luthi, L., Wicker, T., Bansal, U., Krattinger, S. G., and Keller, B. (2017). Characterization of Lr75: a partial, broad-spectrum leaf rust resistance gene in wheat. Theor. Appl. Genet. 130, 1–12. doi: 10.1007/s00122-016-2784-1
Song, W., Xie, C. J., Du, J. K., Xie, H., Liu, Q., Ni, Z. F., et al. (2008). A “one-marker-for-two-genes” approach for efficient molecular discrimination of Pm12 and Pm21 conferring resistance to powdery mildew in wheat. Mol. Breed. 23, 357–363. doi: 10.1007/s11032-008-9235-x
Tang, Z. X., Yang, Z. J., and Fu, S. L. (2014). Oligonucleotides replacing the roles of repetitive sequences pAs1, pSc119.2, pTa-535, pTa71, CCS1, and pAWRC.1 for FISH analysis. J. Appl. Genet. 55, 313–318. doi: 10.1007/s13353-014-0215-z
Tsuchida, M., Fukushima, T., Nasuda, S., Masoudi-Nejad, A., Ishikawa, G., Nakamura, T., et al. (2008). Dissection of rye chromosome 1R in common wheat. Genes Genet. Syst. 83, 43–53. doi: 10.1266/ggs.83.43
Villareal, R. L., Banuelos, O., Mujeeb-Kazi, A., and Rajaram, S. (1998). Agronomic performance of chromosomes 1B and T1BL.1RS near-isolines in the spring bread wheat Seri M82. Euphytica 103, 195–202. doi: 10.1023/A:1018392002909
Villareal, R., Toro, E. D., Mujeeb-Kazi, A., and Rajaram, S. (1995). The 1BL/1RS chromosome translocation effect on yield characteristics in a Triticum aestivum L. cross. Plant Breed. 114, 497–500. doi: 10.1111/j.1439-0523.1995.tb00843.x
Wang, J., Liu, Y. L., Su, H. D., Guo, X. R., and Han, F. P. (2017). Centromere structure and function analysis in wheat-rye translocation lines. Plant J. 91, 199–207. doi: 10.1111/tpj.13554
Xu, H., Yin, D., Li, L., Wang, Q., Li, X., Yang, X., et al. (2012). Development and application of EST-based markers specific for chromosome arms of rye (Secale cereale L.). Cytogenet. Genome Res. 136, 220–228. doi: 10.1159/000336478
Zeng, F. S., Yang, L. J., Gong, S. J., Shi, W. Q., Zhang, X. J., Wang, H., et al. (2014). Virulence and diversity of Blumeria graminis f. sp. tritici populations in China. J. Integr. Agric. 13, 2424–2437. doi: 10.1016/S2095-3119(13)60669-3
Zhang, H. T., Guan, H. Y., Li, J. T., Zhu, J., Xie, C. J., Zhou, Y. L., et al. (2010). Genetic and comparative genomics mapping reveals that a powdery mildew resistance gene Ml3D232 originating from wild emmer co-segregates with an NBS-LRR analog in common wheat (Triticum aestivum L.). Theor. Appl. Genet. 121, 1613–1621. doi: 10.1007/s00122-010-1414-6
Zhang, R. Q., Sun, B. X., Chen, J., Cao, A. Z., Xing, L. P., Feng, Y. G., et al. (2016). Pm55, a developmental-stage and tissue-specific powdery mildew resistance gene introgressed from Dasypyrum villosum into common wheat. Theor. Appl. Genet. 129, 1975–1984. doi: 10.1007/s00122-016-2753-8
Zhuang, L. F., Sun, L., Li, A. X., Chen, T. T., and Qi, Z. J. (2010). Identification and development of diagnostic markers for a powdery mildew resistance gene on chromosome 2R of Chinese rye cultivar Jingzhouheimai. Mol. Breed. 27, 455–465. doi: 10.1007/s11032-010-9443-z
Zhuang, Q. S. (2003). Chinese Wheat Improvement and Pedigree Analysis. Beijing: Chinese Agriculture Press.
Keywords: hexaploid triticale, powdery mildew, leaf rust, resistance, marker analysis, GISH, FISH
Citation: Li F, Li Y, Cao L, Liu P, Geng M, Zhang Q, Qiu L, Sun Q and Xie C (2018) Simultaneous Transfer of Leaf Rust and Powdery Mildew Resistance Genes from Hexaploid Triticale Cultivar Sorento into Bread Wheat. Front. Plant Sci. 9:85. doi: 10.3389/fpls.2018.00085
Received: 29 November 2017; Accepted: 15 January 2018;
Published: 05 February 2018.
Edited by:
Thomas Miedaner, University of Hohenheim, GermanyReviewed by:
Volker Mohler, Bavarian State Research Center for Agriculture, GermanyTomás Naranjo, Complutense University of Madrid, Spain
Copyright © 2018 Li, Li, Cao, Liu, Geng, Zhang, Qiu, Sun and Xie. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Chaojie Xie, xiecj127@126.com
 Feng Li
Feng Li Yinghui Li
Yinghui Li Lirong Cao
Lirong Cao Peiyuan Liu
Peiyuan Liu Qiang Zhang
Qiang Zhang Chaojie Xie
Chaojie Xie