- 1Collaborative Innovation Center of Henan Grain Crops, College of Agronomy, Henan Agricultural University, Zhengzhou, China
- 2Weinan Vocational and Technical College, Weinan, China
- 3Hainan Key Laboratory for Biotechnology of Salt Tolerant Crops, College of Horticulture, Hainan University, Haikou, China
Soil and freshwater salinization is increasingly becoming a problem worldwide and has adversely affected plant growth. However, most of the related studies have focused on sodium ion (Na+) stress, with relatively little research on chloride ion (Cl–) stress. Here, we found that upland cotton (Gossypium hirsutum) plants accumulated Cl– and exhibited strong growth inhibition under NaCl or KCl treatment. Then, a chloride channel gene (GhCLCg-1) was cloned from upland cotton. Phylogenetic and sequence analyses indicated that GhCLCg-1 was highly homologous to AtCLCg and also have conserved voltage_CLC and CBS domains. The subcellular localization assay showed that GhCLCg-1 was localized on the vacuolar membrane. Gene expression analyses revealed that the expression of GhCLCg-1 increased rapidly in cotton in response to chloride stress (NaCl or KCl), and the transcript levels increased as the chloride stress intensified. The overexpression of GhCLCg-1 in Arabidopsis thaliana changed the uptake of ions with a decrease of the Na+/K+ ratios in the roots, stems, and leaves, and enhanced salt tolerance. In contrast, silencing GhCLCg-1 in cotton plants increased the Cl– contents in the roots, stems, and leaves and the Na+/K+ ratios in the stems and leaves, resulting in compromised salt tolerance. These results provide important insights into the toxicity of chloride to plants and also indicate that GhCLCg-1 can positively regulates salt tolerance by adjusting ion accumulation in upland cotton.
Introduction
Salinization is a serious environmental problem worldwide. During the last few decades, many fresh water regions have been affected by increasing salinity (Kaushal, 2016). The causes of salinization include alterations to freshwater flows, irrigation, wastewater treatment, sea level increases, and the use of salt for deicing roads (Herbert et al., 2015). The application of deicing salts, such as NaCl, CaCl2, and MgCl2, has increased the salinization of water and soil, which has directly and negatively affected natural environments (Buss et al., 2020). Because chloride salts are commonly used, the chloride ion (Cl–) may be useful as a tracer for deicing salts (Thunqvist, 2004). Recent studies proved that the widespread use of road salt has resulted in increased chloride concentrations in lakes and rivers (Thunqvist, 2004; Gutchess et al., 2016; Laceby et al., 2019). Vehicular traffic may be responsible for transferring salt into the adjacent roadside, where the salt may seep into the soil and the underlying water (Fay and Shi, 2012).
Salinization is extremely detrimental to sustainable agricultural production worldwide (Saghir et al., 2002). Sodium chloride (NaCl), which is the most ubiquitous salt, induces cellular osmotic and ionic stresses (Deinlein et al., 2014; Nie et al., 2015). To date, studies on the physiological and molecular mechanisms underlying plant salt tolerance have mostly focused on Na+ toxicity and adaptations, but there has been relatively little research on salt damage caused by Cl– (Teakle and Tyerman, 2010; Li et al., 2016). The previous studies have confirmed that sodium/proton (Na+/H+) antiporters (NHXs) are involved in the regulation and stabilization of Na+ under salt stress in plants and improved the salt tolerance of plants (Ma et al., 2020; Feng et al., 2021).
The chloride ion is one of the essential trace elements in plants. More specifically, it enhances plant growth and development (Broyer et al., 1954; Wei et al., 2019), while also affecting stomatal movement, cell osmotic pressure, electrical charge balance, cell turgor pressure, and intracellular pH (Franco Navarro et al., 2015). Despite its beneficial effects on plants, under salt stress conditions, Cl– is a major toxic element in the cytosol of plant cells, where it accumulates excessively, especially in shoots, thereby adversely affecting growth (Munns and Tester, 2008; Weg et al., 2017). Plants counter these stresses via the elimination of excess ions across the plasma membrane or the intracellular vacuolar compartmentalization to decrease the effective Cl– levels inside cells, particularly in the aerial tissues (Zhang et al., 2011). Earlier research revealed that the vacuolar chloride channel CLCg selectively transports chloride across the vacuolar membrane to enhance the tolerance to chloride salts (Nguyen et al., 2015). A recent study indicated GhCLC4/15 expression is up-regulated in the roots and leaves of cotton seedlings under salt stress (Liu et al., 2020). Additionally, the vacuolar chloride channel CLCc maintains chloride homeostasis. The Arabidopsis thaliana mutant atclc-c reportedly exhibits increased sensitivity to NaCl (Jossier et al., 2010). In tobacco (Nicotiana tabacum), the Cl– content is obviously lower in NtCLC2-silenced plants than in control plants (Zhang et al., 2018). The overexpression of the vacuolar chloride channel gene GmCLC1 in soybean can increase the segregation of Cl– in the roots and decrease the transport of Cl– to the shoots (Wei et al., 2016). These findings suggest that CLCs can take up Cl– to increase salt tolerance at high salt concentrations, and the resulting accumulation of Cl– in plants may minimize the effects of chloride on the environment.
Upland cotton (Gossypium hirsutum) is a widely grown fiber crop and has been used as a pioneer crop in regions with saline and alkaline land (Wang N. et al., 2016; Wang X.G. et al., 2016). In this study, the potential effects of chloride concentrations on cotton plants were analyzed. We treated cotton plants with different chloride salts (NaCl or KCl) and observed the resulting damage. And we also found the vacuolar chloride channel GhCLCg-1 was activated in plants exposed to chloride salt concentrations by quantitative real-time polymerase chain reaction (PCR) (qRT-PCR). Furthermore, overexpression in A. thaliana and virus-induced gene silencing (VIGS) technology in cotton were used to characterize the function of GhCLCg-1 in cotton response to chloride salt stress. The objectives of this study were to determine the toxicity of chloride in cotton plants and prove the important role of GhCLCg-1 in cotton resistance to salinity.
Materials and Methods
Plant Materials and Growth Conditions
Gossypium hirsutum L. acc. TM-1 seeds were soaked in sterile water to promote germination. After a 16-h incubation at 30°C, the germinating seeds were transferred to vermiculite-filled pots in a greenhouse (16-h light/8-h dark photoperiod at 23°C). When the cotyledon expanded, uniformly growing seedlings were selected, rinsed clean with running water, and transferred to a hydroponic tank containing Hoagland solution, which was refreshed weekly. To induce salinity stress, the cotton seedlings were grown to the three-leaf stage and then treated with Hoagland solution containing 50, 100, 150, or 200 mM NaCl or 200 mM KCl (treatment group). The seedlings in the control group were treated with Hoagland solution lacking NaCl or KCl. Root, stem, and leaf samples were collected at 0, 1, 3, and 6 h after treatments. The samples were rinsed with distilled water 3–5 times and then immediately frozen in liquid nitrogen and stored at −80°C for the subsequent total RNA extraction. Each treatment was completed with three biological replicates.
The wild-type (WT) A. thaliana plants used in this study were Columbia (Col-0). After a 2-day vernalization at 4°C, the A. thaliana seeds were surface sterilized by soaking in 75% ethanol for 3–5 min. After they were washed with sterile distilled water six times, the seeds were placed in plates containing Murashige and Skoog (MS) medium supplemented with 3% sucrose and 0.7% agar (pH adjusted to 5.8). The plates were incubated at 21°C with a 16-h light/8-h dark photoperiod. After 2 weeks, the A. thaliana seedlings were transferred to pots filled with vermiculite and nutritive soil (1:1, v/v) and placed in a greenhouse (16-h light/8-h dark photoperiod at 21°C).
Gene Cloning and Sequencing
Total RNA was isolated from TM-1 seedlings using the RNAprep Pure Polysaccharide Polyphenol Plant Total RNA Extraction Kit (Tiangen, Beijing, China). The RNA concentration was determined using the NanoDrop 2000 microvolume spectrophotometer (Thermo Fisher Scientific, Waltham, MA, United States), whereas RNA integrity was assessed by 1% agarose gel electrophoresis. First-strand cDNA was synthesized from 1 μg RNA using the PrimeScriptTM II first Strand cDNA Synthesis Kit (TaKaRa, Dalian, China). To clone the vacuolar chloride channel gene in upland cotton, the BlastP and tBlastN programs were performed against the G. hirsutum genome database1 using the A. thaliana CLCg amino acid sequence as the query. The coding sequences of gene IDs GH_A06G0574 and GH_D06G0541 as the best match were retrieved and gene-specific primers were designed. Full-length cDNAs were amplified using the GhCLCg-1F/R primer pair (Supplementary Table 1) and the KOD-Plus-Neo DNA polymerase (TaKaRa, Dalian, China). The PCR products were purified using the FastPure Gel DNA Extraction Mini Kit (Vazemy Biotech Co., Ltd.) and then cloned using the pEASY®-Blunt Cloning Kit for the subsequent transformation of Trans1-T1 Escherichia coli competent cells (TransGen Biotech). Positive clones cultured at 37°C were analyzed by sequencing to verify they were correctly transformed.
Phylogenetic Tree Construction and Sequence Analysis
Arabidopsis thaliana CLC amino acid sequences were downloaded from TAIR database2. Seven AtCLC sequences along with G. hirsutum GhCLCg-1A and GhCLCg-1D (Supplementary Table 2) were used to construct a phylogenetic tree according to the neighbor-joining method of the MEGA-X software, with pairwise deletion and 1,000 bootstrap replicates. Conserved domains were detected using InterPro3 and gene structures were examined using TBtools (Chen et al., 2020). Amino acid sequences were aligned using the DNAMAN program.
Quantitative Real-Time Polymerase Chain Reaction Analysis
Total RNA was isolated from the roots, stems, and leaves using the RNA Extraction kit (Tiangen). The HiScript® II Q Select RT SuperMix for qPCR (+gDNA wiper) (Vazemy Biotech Co., Ltd.) was used to synthesize cDNA from approximately 1 μg total RNA. Gene-specific primers were designed according to conversed sequences in GhCLCg-1A and GhCLCg-1D (Supplementary Table 1). The qRT-PCR analysis was performed using the LightCycler 480 system (Roche, Switzerland) and the ChamQ Universal SYBR qPCR Master Mix (Vazemy Biotech Co., Ltd.). The GhHIS3 gene served as an internal control (Ma et al., 2020). Three biological replicates were analyzed for each sample. Relative gene expression levels were calculated according to the 2–ΔCT method (Livak and Schmittgen, 2001).
Gene Overexpression Plasmid Construction
The full-length GhCLCg-1A and GhCLCg-1D open reading frames without a stop codon were amplified by PCR using primers with BamHI and SmaI sites (Supplementary Table 1) and then inserted into the BamHI/SmaI-digested pCAMBIA2300-35S-GFP-HA plasmid, which contains the CaMV 35S promoter. The resulting recombinant plasmids were inserted into Trans1-T1 competent cells (TransGen Biotech). The accuracy of the inserted sequences in the transformants was confirmed by sequencing. The recombinant plasmids carrying the GhCLCg-1A-GFP and GhCLCg-1D-GFP constructs were inserted into Agrobacterium tumefaciens strain GV3101 cells via a heat shock protocol. The E. coli and A. tumefaciens strains containing the overexpression plasmids were stored at −80°C.
Subcellular Localization of GhCLCg-1A/D
A transient expression system using A. thaliana mesophyll protoplasts was prepared as previously described (Sheen, 2001; Feng et al., 2021). Protoplasts were co-transfected with 10 μg GhCLCg-1A-GFP/GhCLCg-1D-GFP recombinant plasmids and 10 μg vacuolar marker protein δ-TIP-RFP (Jauh et al., 1998) according to a polyethylene glycol-mediated transfection method (Yoo et al., 2007). After incubating the protoplasts at room temperature for 12–20 h in darkness, the green fluorescent protein (GFP) and red fluorescent protein (RFP) signals were detected using the FV1200 confocal laser scanning microscope (Olympus, Japan).
Virus-Induced GhCLCg-1 Silencing in Upland Cotton
Based on the GhCLCg-1A and GhCLCg-1D conserved sequences, a 430-bp fragment was amplified by PCR from cotton cDNA using the GhCLCg-1-p-F/R primers (Supplementary Table 1) and inserted between the SacI and EcoRI restriction sites in the VIGS vector pTRV2 of the ClonExpress® Ultra One Step Cloning Kit (Vazemy Biotech Co., Ltd.). Plasmids from the positive transformants carrying pTRV1 (helper plasmid) and TRV:GhCLCg-1 were inserted into A. tumefaciens strain GV3101 cells via a heat shock protocol. Similarly, we constructed a visual marker (TRV:CLA, where CLA is cloroplastos alterados) to monitor the silencing efficiency. Additionally, TRV:00 (empty vector) was used as the negative control. The VIGS experiments were performed by the tobacco rattle virus (TRV) system as previously described (Pang et al., 2013).
Two fully expanded cotyledons of cotton seedlings were used for the A. tumefaciens infiltration. At 10 days after the transformation, the true leaves of TRV:CLA-silenced cotton plants exhibited signs of albinism. The second true leaves, stems, and roots were harvested from at least three randomly selected TRV:00 and TRV:GhCLCg-1 cotton plants for an RNA isolation. The efficiency of GhCLCg-1 silencing was evaluated by qRT-PCR, with GhHIS3 used as the internal control (Ma et al., 2020). The TRV:00 and TRV:GhCLCg-1 plants were then treated with 200 mM NaCl for 3-day, the plants were photographed and their roots, stems, and leaves were collected for an analysis of their Cl–, Na+, and K+ contents. The assays were completed with three biological replicates.
GhCLCg-1 Overexpression in Arabidopsis thaliana
Because GhCLCg-1A and GhCLCg-1D have highly similar sequences, we used GhCLCg-1A to generate transgenic A. thaliana plants overexpressing GhCLCg-1. The recombinant plasmid carrying the GhCLCg-1A-GFP fusion construct was inserted into A. thaliana plants using the floral dip transformation method (Clough and Bent, 1998). Homozygous plants were selected on MS medium containing 50 μg/ml kanamycin. The T1 generation plants were examined by PCR to confirm the positive lines were transformed correctly. Homozygous T3 generation progenies were analyzed by reverse transcription PCR using the GhCLCg-1-Q-F/R primers (Supplementary Table 1). Seeds from homozygous T3 plants were placed on MS agar medium (control group) or MS agar medium supplemented with 100 mM NaCl (salt treatment group). The seedlings were photographed after 3 weeks. Additionally, the taproot length was measured using a vernier caliper. Nine plants per line were examined and their tissues were harvested for an analysis of the Cl–, Na+, and K+ contents.
Analysis of Ion Contents
The VIGS plants and transgenic A. thaliana plants overexpressing GhCLCg-1 were collected and incubated at 105°C for 10 min to denature enzymes and then at 75°C to achieve a constant weight. The samples were subsequently ground to a powder. To measure the Cl– content, a 50-ml Erlenmeyer flask was washed with 5% HNO3. After drying, 0.1 g powder was added to the flask and resuspended with 15 ml boiled deionized water. The solution was cooled to room temperature and then filtered. The filtrate was collected in a 25-ml volumetric flask to a constant volume. The solution was analyzed using an ion chromatography system (ICS-5000) (Thermo Fisher Scientific, Waltham, MA, United States) to measure the Cl– content. The experiment was repeated three times.
To measure the Na+ and K+ contents, 0.1 g powder was treated with nitric acid (HNO3), hydrogen peroxide (H2O2), and hydrofluoric acid (HF) and then microwaved (i.e., digested) before being analyzed using an inductively coupled plasma optical emission spectrometry system (ICAP-7400) (Thermo Fisher Scientific, Waltham, MA, United States). Three biological replicates were analyzed for each sample.
Results
Uptake and Effect of Chloride in Upland Cotton Plants
Upland cotton plants were treated with 200 mM NaCl or KCl to assess the effects of chloride. After 10 days, compared with the mock controls, the growth of the plants treated with NaCl or KCl was significantly inhibited. Additionally, the salt-treated plants had shriveled and wilted leaves, some of which had fallen off the plants (Figure 1A).
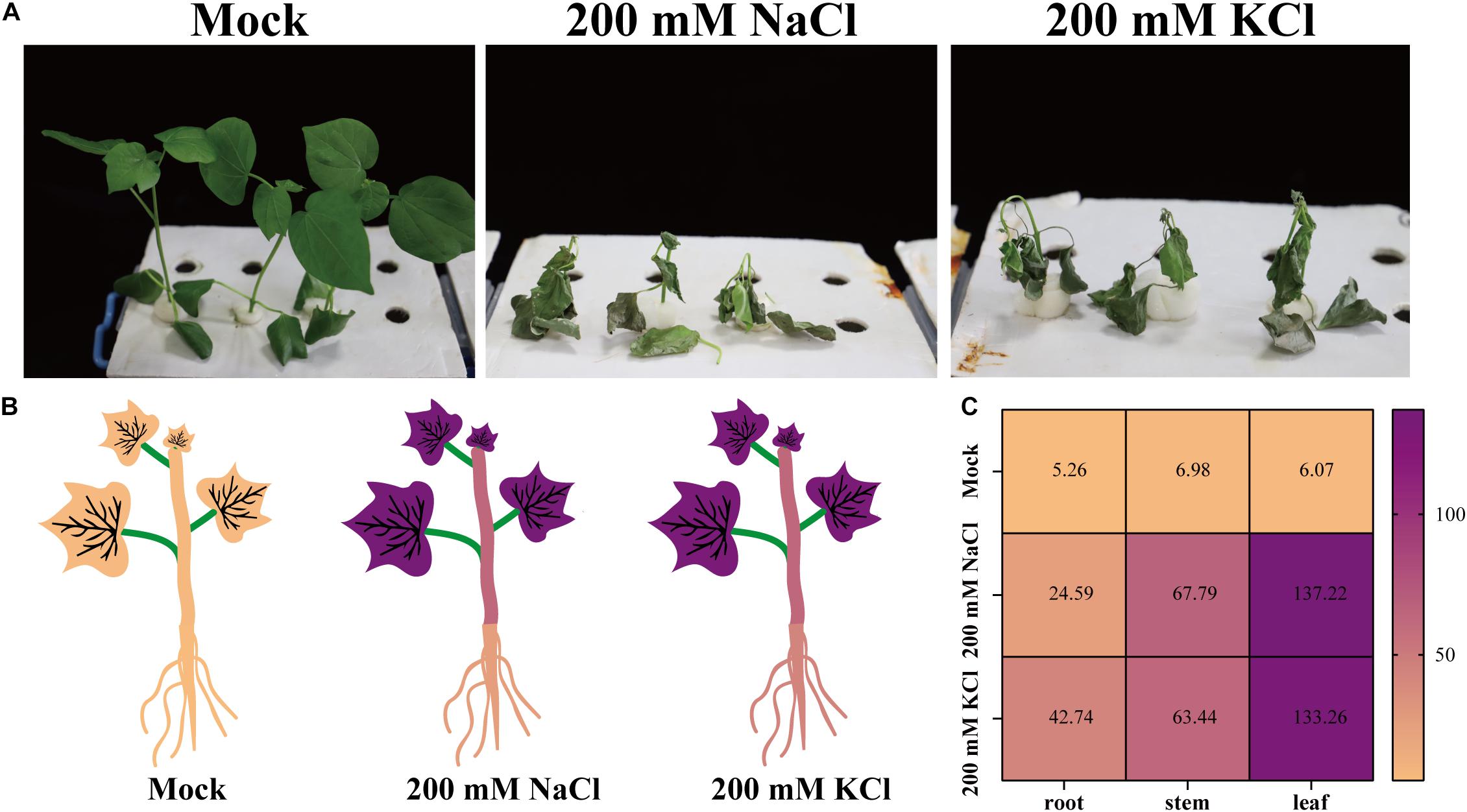
Figure 1. The resulting phenotypes and chloride uptake of upland cotton plants treated with NaCl or KCl. (A) Phenotypes of upland cotton plants treated with no chloride (Mock), 200 mM NaCl, or 200 mM KCl for 10 days. (B) Cl– contents (mg/g DW) of upland cotton plants (roots, stems, and leaves) treated with no chloride (Mock), 200 mM NaCl, or 200 mM KCl for 10 days. (C) Heatmap of the Cl– contents of upland cotton plants treated with no chloride (Mock), 200 mM NaCl, or 200 mM KCl. The Cl– concentrations are indicated by different colors and the colors are consistent in panels (B,C).
An analysis of the plant ion contents indicated that the chloride (Cl–), sodium (Na+), and potassium (K+) contents were higher in the salt-treated samples than in the mock controls (Figures 1B,C and Supplementary Figure 1). After the NaCl and KCl treatments, cotton plants had extremely high Cl– contents in the leaves, roots, and stems (Figures 1B,C). As expected, the Na+ or K+ contents also increased in the leaves, stems, and roots of plants treated with NaCl or KCl (Supplementary Figure 1). These results suggest that Cl– can accumulate in cotton tissues, thereby affecting plant growth and development.
Phylogenetic Analysis and Conserved Domains of GhCLCg-1
The vacuolar chloride channel (CLCg) can protect plant cells from ion-induced damages (Nguyen et al., 2015). Using a homology-based cloning strategy, we cloned the full-length coding sequences of two CLCg-encoding genes in G. hirsutum, which are homoeologs from D-subgenome and A-subgenome of the allotetraploid upland cotton and named GhCLCg-1A/D (suffixes D and A represented the D and A subgenomes, respectively). The GhCLCg-1A and GhCLCg-1D sequences were 2,325 bp long and encoded 774 amino acids. To further characterize GhCLCg-1A/D, we used the MEGA-X program to construct a phylogenetic tree that included seven AtCLCs. In the constructed tree, the CLC family was divided into two clades, with GhCLCg belonging to class I and highly homologous to AtCLCg (Figure 2A).
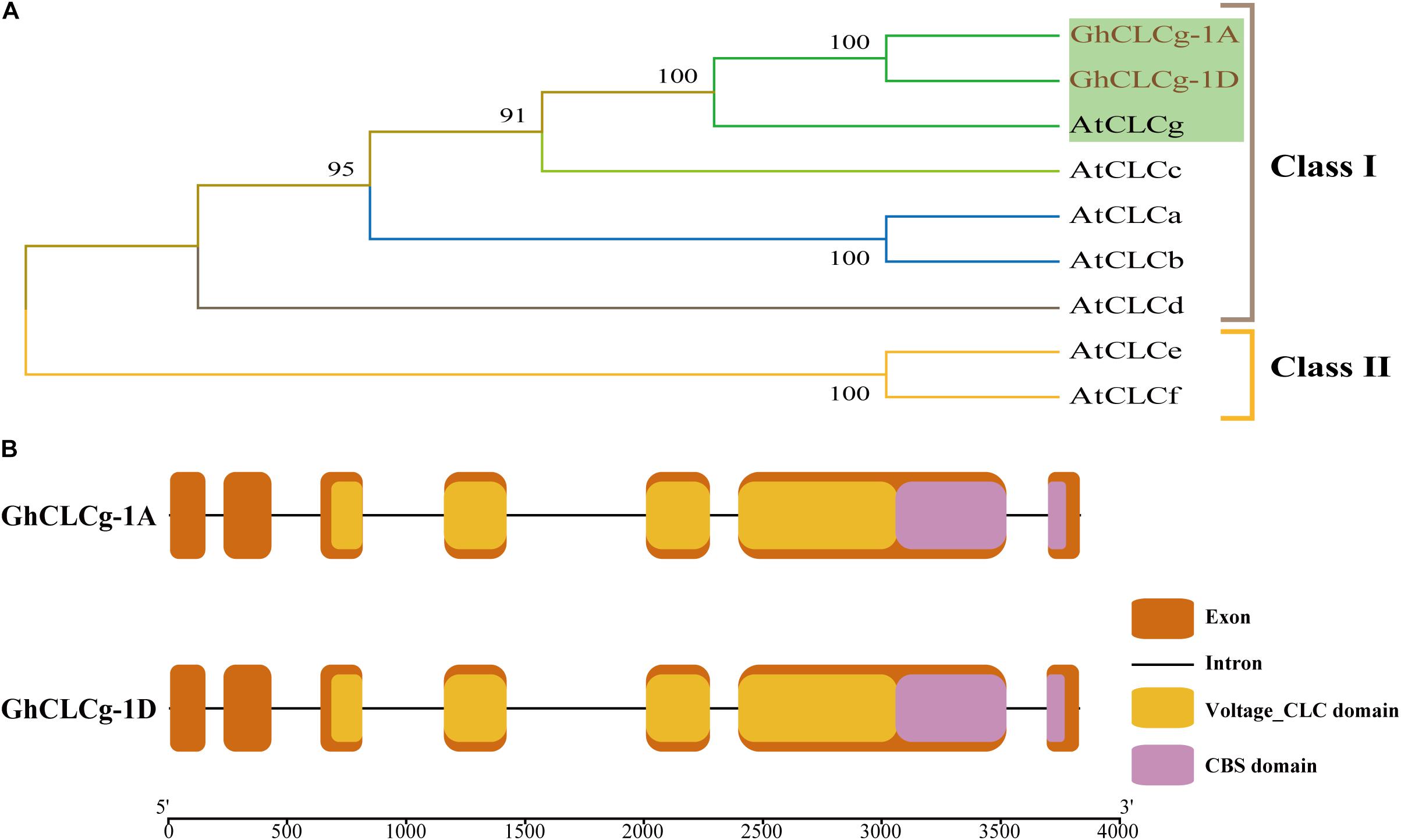
Figure 2. Phylogenetic analysis and gene structures of GhCLCg-1A/D. (A) Phylogenetic tree of GhCLCg-1A/D (Gossypium hirsutum) and AtCLCs (Arabidopsis thaliana). (B) Intron-exon and conserved domains structures of GhCLCg-1A/D. The exons, introns, Voltage_CLC domains and CBS domains are indicated by the orange boxes, black lines, yellow boxes, and purple boxes, respectively. The 5′-3′ scale indicates the size of DNA sequence. The exons/introns and domains sizes are proportional to DNA sequence lengths.
The results of the GhCLCg-1A and GhCLCg-1D gene structural analysis revealed these two genes have a similar intron-exon arrangement (Figure 2B). Specifically, they both comprise seven exons and six introns as well as domains unique to the CLC family (i.e., voltage_CLC and CBS). To further investigate the similarity in the GhCLCg-1A and GhCLCg-1D sequences, their amino acid sequences were aligned to the AtCLCg amino acid sequence (Supplementary Figure 2). The multiple sequence alignment confirmed that GhCLCg-1A and GhCLCg-1D are highly conserved (98.19% identity) and are similar to AtCLCg at the amino acid level (91.13% identity). The multiple sequence alignment also indicated that GhCLCg-1A and GhCLCg-1D have three regions that are conserved in AtCLCg, namely GxGIPE, GKxGPxxH, and PxxGxLF. In the GxGIPE sequence, x was a serine (S), implying GhCLCg-1A/D are specific for Cl– (Supplementary Figure 2) (Barbier-Brygoo et al., 2000; Dutzler et al., 2002; Zifarelli and Pusch, 2010). These results imply that GhCLCg-1A and GhCLCg-1D are functionally similar to AtCLCg.
Subcellular Localization of GhCLCg-1
The localization of proteins provides clues regarding their functions (Long et al., 2020). The subcellular localization of GhCLCg-1A/D was determined using fusion proteins with a C-terminal GFP. The fusion proteins were transiently expressed in A. thaliana leaf protoplasts that also contained the vacuolar marker protein δ-TIP with a C-terminal RFP (Figure 3). In the control protoplasts with GFP alone and δ-TIP-RFP, the GFP signal was detected throughout, whereas the RFP fluorescence was restricted to the tonoplast. The GhCLCg-1A/D-GFP fusion proteins co-localized with δ-TIP-RFP in the protoplast, with red and green fluorescent signals overlapping, which confirmed that GhCLCg-1A/D are localized to the vacuolar membrane.
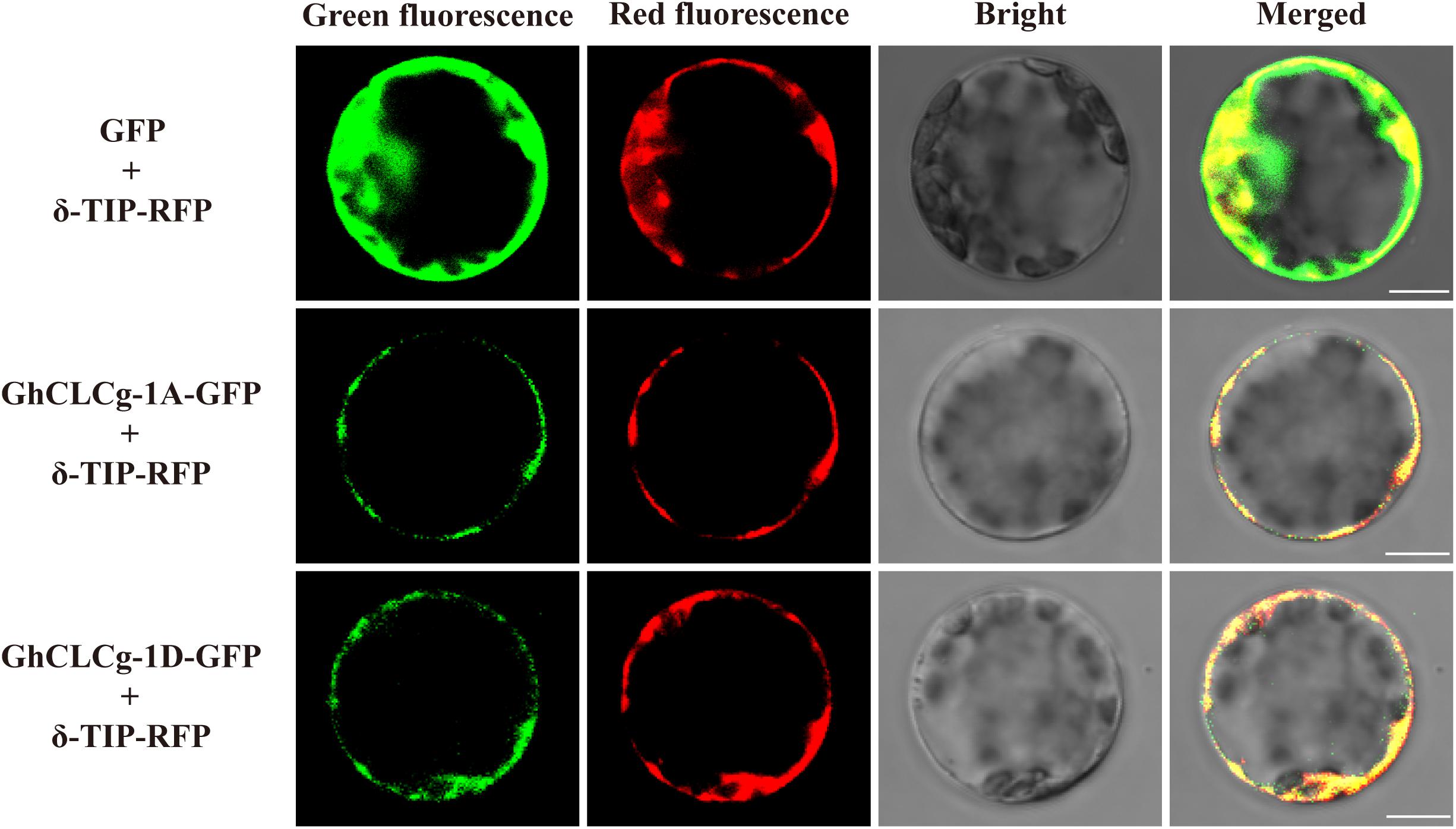
Figure 3. Subcellular localization of GhCLCg-1A/D. GhCLCg-1A-GFP, and GhCLCg-1D-GFP were co-expressed with the vacuolar marker protein δ-TIP-red fluorescent protein (RFP) in leaf protoplasts of Arabidopsis thaliana seedlings. Green fluorescent protein (GFP) co-transformed with δ-TIP-RFP as positive control. Scale bar = 10 μM.
Activation of GhCLCg-1 by Chloride in Upland Cotton Plants
To determine whether GhCLCg-1A/D are involved in salt stress responses, we analyzed the expression of the corresponding genes following an exposure to chloride salts. The substantial similarity in the GhCLCg-1A and GhCLCg-1D sequences makes it very difficult to study the expression of these two genes by qRT-PCR. In this study, the universal qRT-PCR primers GhCLCg-1-Q-F/R were designed to analyze GhCLCg-1A/D expression in the roots, stems, and leaves at various time-points (Figure 4). Following the 200 mM NaCl treatment, GhCLCg-1 expression was up-regulated at 3 h in the roots (approximately two times higher than expression level of the mock control). In the stems, GhCLCg-1 expression was about 2–3 times higher in the NaCl-treated plants than in the mock controls at 1 and 6 h. Unexpectedly, the expression level of GhCLCg-1 at 3 h in stems of the 200 mM NaCl treated plants was significantly lower than in stems of the mock plants. The GhCLCg-1 transcript levels in stems treated with different salt concentration at 3 h were analyzed. The results showed that the expression of GhCLCg-1 at different salt concentration is lower than the control (0 mM NaCl), but the expression is increased by high salt concentration, and the expression level under 200 mM NaCl is higher than under 50, 100, and 150 mM NaCl (Supplementary Figure 3). In the leaves, GhCLCg-1 expression was up-regulated by the salt treatment at 1, 3, and 6 h. More specifically, the GhCLCg-1 transcript level was highest at 3 h, but the increase (relative to expression level of the mock control) was greatest at 6 h (five times higher) (Figure 4A). To further demonstrate that GhCLCg-1 is involved in plant responses to chloride-mediated salt stress, cotton plants were treated with 200 mM KCl (Figure 4B). The changes in the leaf GhCLCg-1 expression levels under 200 mM KCl were similar to those resulting from the 200 mM NaCl treatment. Given the up-regulated GhCLCg-1 expression induced by 200 mM NaCl, the leaf GhCLCg-1 expression levels following 6 h treatments with various NaCl concentrations (0, 50, 100, 150, and 200 mM) were analyzed. The relative GhCLCg-1 expression level increased as the salt concentration increased, peaking at 200 mM NaCl (Figure 4C). Thus, GhCLCg-1 expression in leaves was induced and regulated by chloride-mediated salt stress. Moreover, this expression was positively related to the chloride concentration.
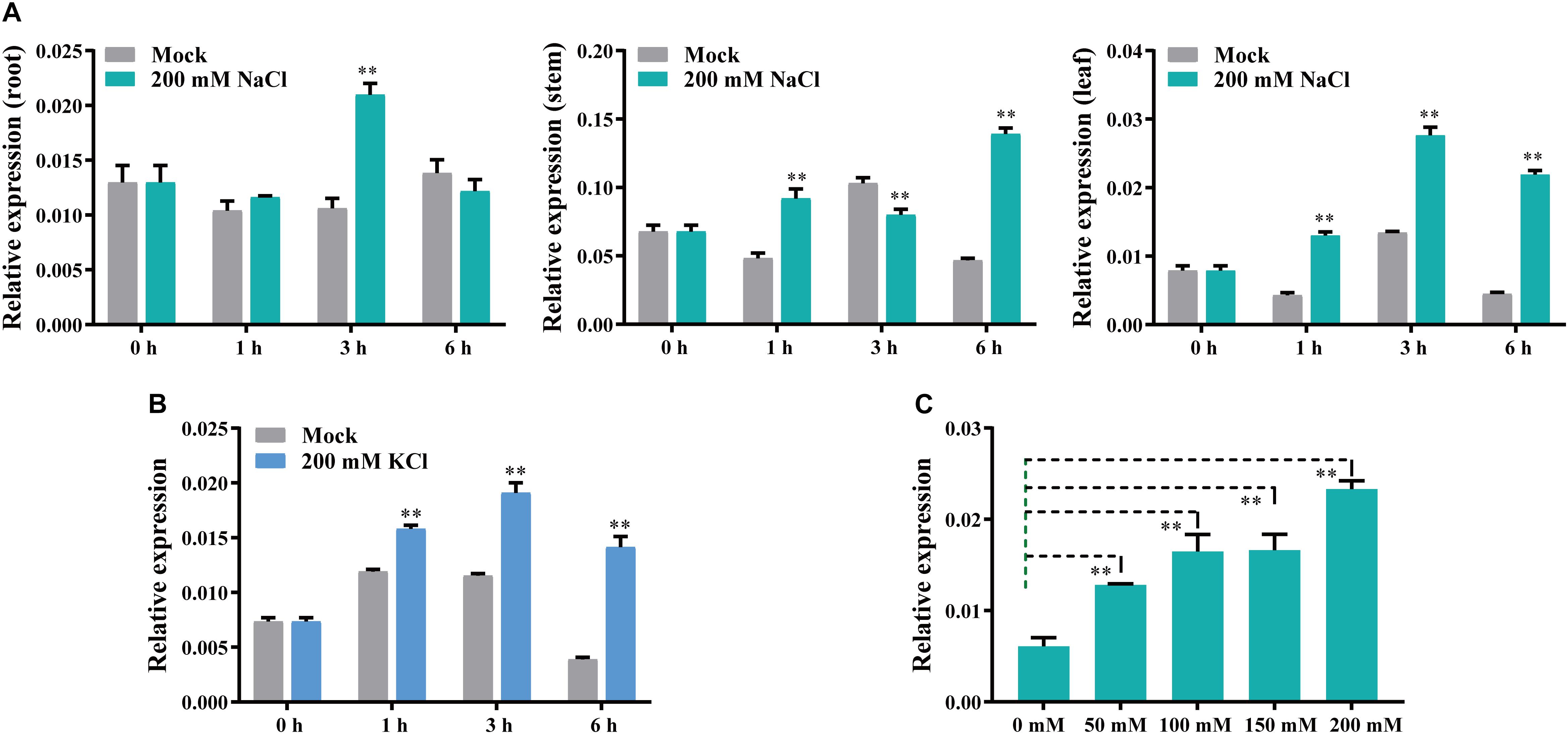
Figure 4. GhCLCg-1 expression profiles in salt-treated upland cotton plants. GhCLCg-1 expression patterns in the roots, stems, and leaves of plants treated with 200 mM NaCl for 0, 1, 3, and 6 h (A) or in the leaves of plants treated with 200 mM KCl for 0, 1, 3, and 6 h (B). (C) GhCLCg-1 expression patterns in the leaves of plants treated for 6 h with different NaCl concentrations (0, 50, 100, 150, and 200 mM). The 2–ΔCT method was used to calculate relative expression levels. Error bars indicate the standard deviation (SD) of three biological replicates (∗∗p < 0.01; t-test).
Overexpression of GhCLCg-1 in Arabidopsis thaliana Enhanced Salt Tolerance
To further clarify the GhCLCg-1 function related to chloride tolerance, GhCLCg-1-overexpressing (OE) transgenic A. thaliana plants were generated and examined (Figure 5). The WT, OE1, OE2, and OE3 A. thaliana plants had similar growth phenotypes on MS medium (Mock), with no significant differences in the root length and shoot growth. However, when treated with 100 mM NaCl, the transgenic A. thaliana plants (OE1, OE2, and OE3) had larger leaves and longer roots than the WT plants (Figure 5A). A quantitative analysis of the root length under salt stress conditions confirmed that the transgenic A. thaliana plants had significantly longer roots than the WT plants (Figure 5C). The GhCLCg-1 expression level increased significantly in the transgenic A. thaliana plants, whereas GhCLCg-1 expression was undetectable in the WT controls (Figure 5B). The changes in the Cl– contents in A. thaliana plants (WT, OE1, OE2, and OE3) were analyzed. Under normal conditions, there were no significant differences in the Cl– contents between the WT and transgenic plants. However, the Cl– contents were significantly higher in the transgenic plants than in the WT controls under salt stress conditions (Figure 5D). These results indicate that GhCLCg-1 confers salt tolerance, while also altering the uptake of Cl– in plants.
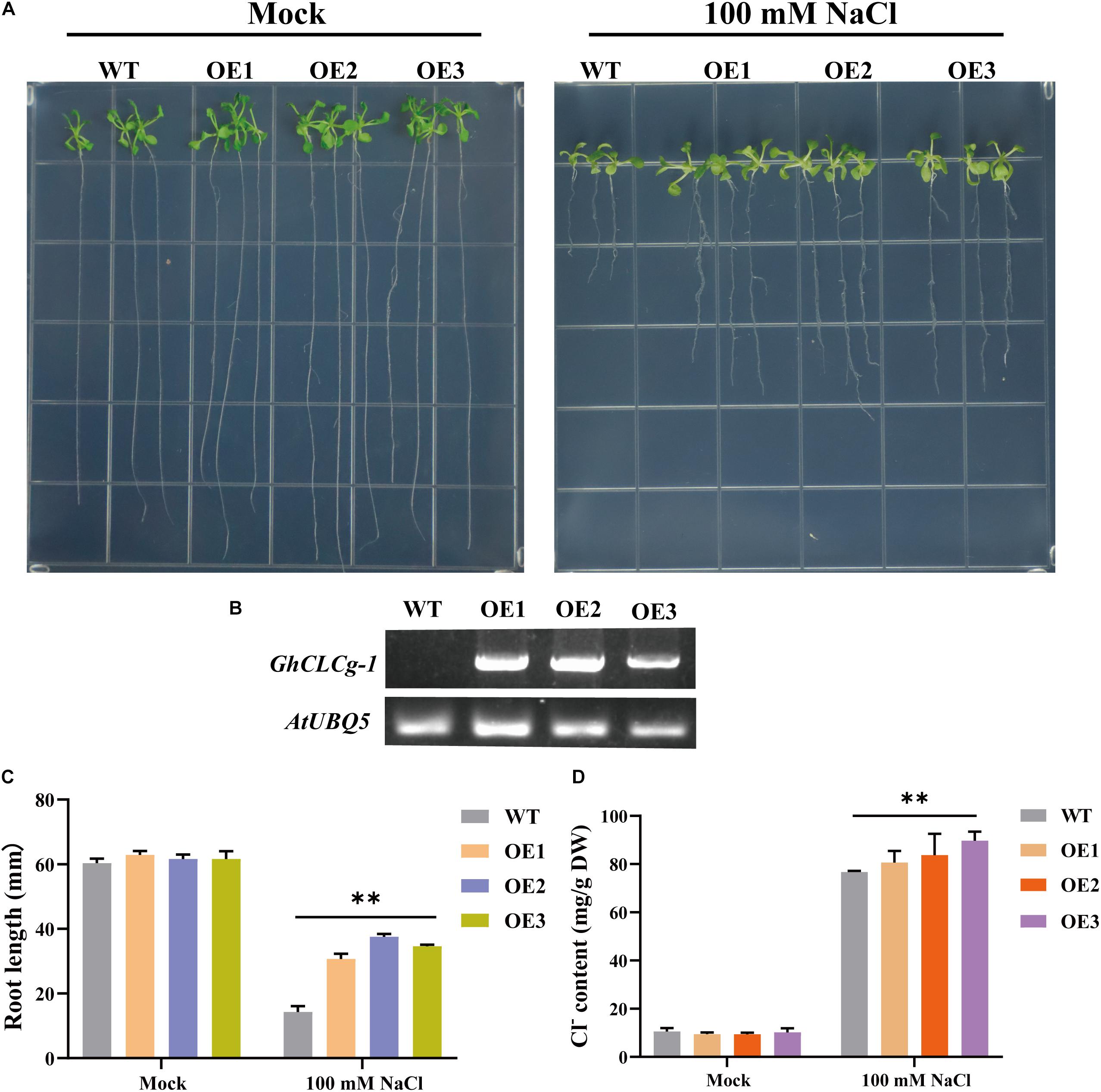
Figure 5. Effects of overexpressing of GhCLCg-1 in Arabidopsis thaliana. (A) Wild-type (WT) and GhCLCg-1-overexpressing transgenic A. thaliana lines (OE1, OE2, and OE3) grown on Murashige and Skoog (MS) medium (Mock) and MS medium containing 100 mM NaCl for 21 days. (B) Reverse transcription PCR analysis of GhCLCg-1 expression levels in WT, OE1, OE2, and OE3 plants, with AtUBQ5 used as an internal control. (C) Comparison of the WT, OE1, OE2, and OE3 root lengths. (D) Comparison of the WT, OE1, OE2, and OE3 chloride contents. Data are presented as the mean ± standard deviation (SD) of three biological replicates (∗∗p < 0.01, t-test).
High environmental Na+ and Cl– levels alter the ion homeostasis of plant cells, resulting in an ionic imbalance and toxicity. Thus, re-establishing cellular ion homeostasis is critical for metabolic functions and growth (Niu et al., 1995). The changes in Na+ and K+ contents as well as the Na+/K+ ratio in WT and transgenic A. thaliana plants (OE1, OE2, and OE3) were analyzed (Figure 6). There were no significant differences in the Na+ and K+ contents or the Na+/K+ ratio among the WT, OE1, OE2, and OE3 plants on MS medium (Mock). An examination of the effects of the 100 mM NaCl treatment revealed that compared with the WT controls, the Na+ and K+ contents were significantly higher in the OE1, OE2, and OE3 plants, whereas the Na+/K+ ratios were significantly lower in the OE1, OE2, and OE3 plants. These findings suggest that overexpression of GhCLCg-1 can affect the accumulation of Na+ and K+, then leads to a decrease of the Na+/K+ ratios under the NaCl treatment, which is beneficial for plant tolerance to salt.
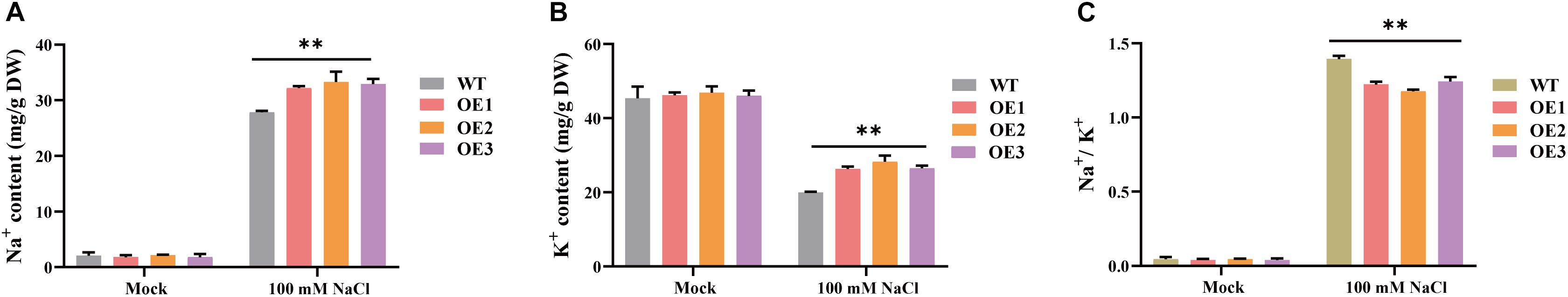
Figure 6. Cation contents of wild-type (WT) and GhCLCg-1-overexpressing transgenic Arabidopsis thaliana lines (OE1, OE2, and OE3). (A) Na+ contents, (B) K+ contents, and (C) Na+/K+ ratio of A. thaliana under 0 mM or 100 mM NaCl treatment for 21 days. Data are presented as the mean ± standard deviation (SD) of three biological replicates (∗∗p < 0.01, t-test).
Silencing of GhCLCg-1 in Upland Cotton Compromised Salt Tolerance
To more thoroughly investigate the roles of GhCLCg-1 related to the salt tolerance of upland cotton plants, we used the TRV-based VIGS system to generate GhCLCg-1 knockdown cotton plants (Figure 7). At 10 days after the A. tumefaciens infiltration, the cotton seedlings transformed with TRV:CLA exhibited an albino phenotype (Figure 7A). The GhCLCg-1 expression levels were determined by qRT-PCR to assess how efficiently the gene was silenced in the cotton plants. The GhCLCg-1 expression levels in the roots, stems, and leaves were obviously lower in the TRV:GhCLCg-1 plants than in the TRV:00 plants (Figure 7B). Accordingly, GhCLCg-1 was efficiently silenced in the TRV:GhCLCg-1 cotton plants.
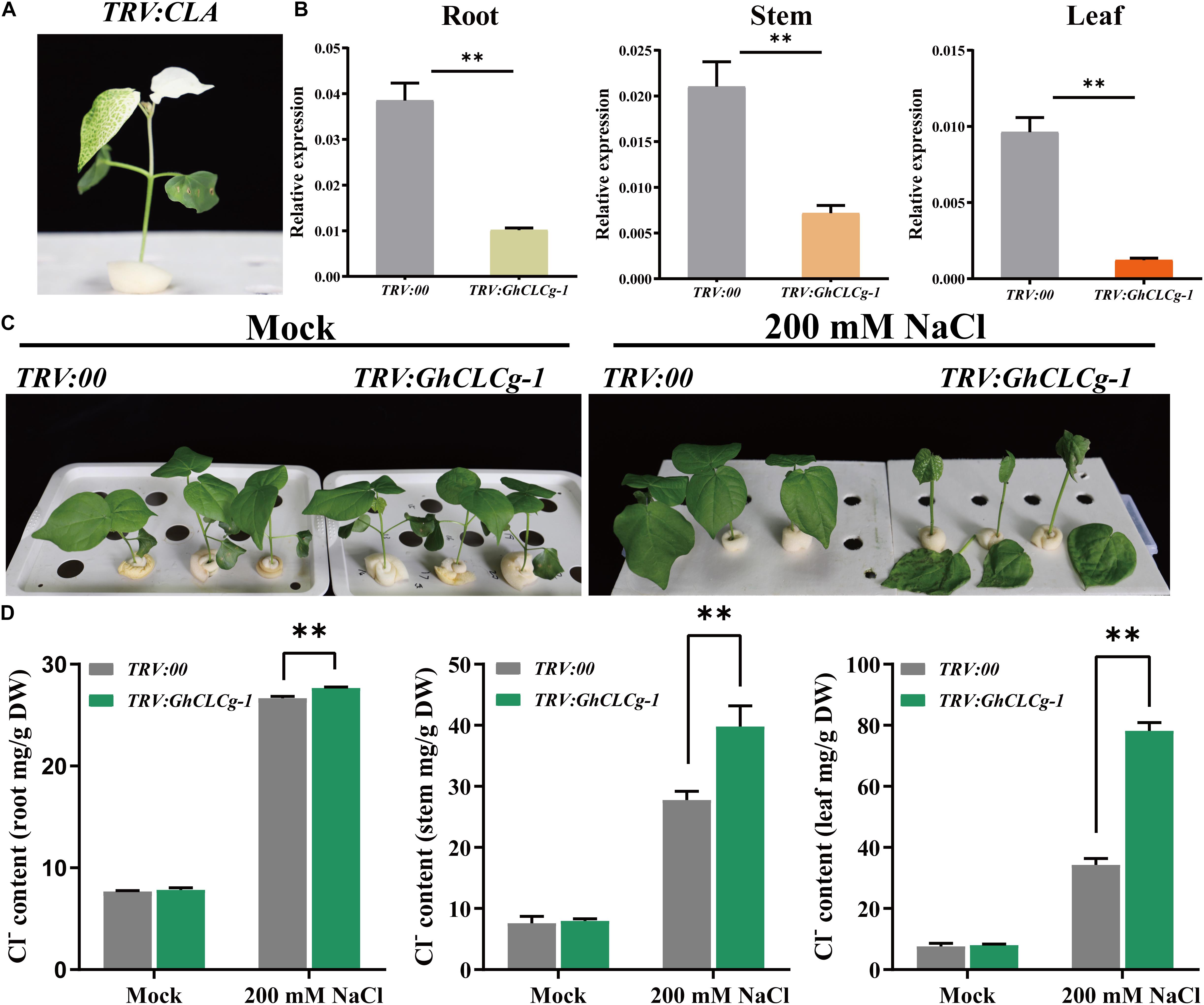
Figure 7. Effects of silencing GhCLCg-1 in upland cotton. (A) The TRV:CLA plants (positive control) exhibited an albino phenotype. (B) Efficiency of the silencing of GhCLCg-1 in TRV:00 and TRV:GhCLCg-1 plants analyzed by qRT-PCR with GhHIS3 used as an internal control. (C) Phenotypes of TRV:00 and TRV:GhCLCg-1 plants treated with 0 mM (Mock) or 200 mM NaCl for 3 days. (D) Cl– contents of TRV:00 and TRV:GhCLCg-1 plants after the salt treatment. Data are presented as the mean ± standard deviation (SD) of three biological replicates (∗∗p < 0.01, t-test).
There were no observable differences in the growth of TRV:00 and TRV:GhCLCg-1 plants under normal conditions (Mock). However, after a 3-day 200 mM NaCl treatment, several symptoms consistent with salt stress were detected on the TRV:GhCLCg-1 plants (Figure 7C). Specifically, the leaves were shrunken and wilted and some of the leaves even fell from the plants. A decrease in the GhCLCg-1 expression level increased the sensitivity of plants to salt stress, suggesting GhCLCg-1 influences cotton salt tolerance. Furthermore, we analyzed the Cl– contents in the roots, stems, and leaves of the TRV:00 and TRV:GhCLCg-1 cotton plants (Figure 7D). As expected, in the absence of chloride stress, there were no significant differences in the Cl– contents of TRV:GhCLCg-1 and TRV:00 plants. However, following the 200 mM NaCl treatment, the root, stem, and leaf Cl– contents were significantly higher in the TRV:GhCLCg-1 plants than in the TRV:00 plants. A comparison of these three tissues in TRV:GhCLCg-1 plants revealed that the Cl– contents were highest in the leaves, followed by the stems and then the roots. Compared with the TRV:00 plants, the GhCLCg-1-silenced plants accumulated more Cl–, especially in the leaves, making them more sensitive to salt stress.
The Na+ and K+ contents as well as the Na+/K+ ratio in TRV:00 and TRV:GhCLCg-1 plants were measured. There were no significant differences in the Na+ and K+ contents or the Na+/K+ ratio between the TRV:00 and TRV:GhCLCg-1 plants in the absence of salt stress (Figure 8). In contrast, after the 200 mM NaCl treatment, the shoot (stems and leaves) Na+ content was higher in the TRV:GhCLCg-1 plants than in the TRV:00 plants, and the Na+ content was highest in the TRV:GhCLCg-1 leaves (Figure 8A). The NaCl treatment did not significantly affect the K+ contents of the TRV:00 and TRV:GhCLCg-1 shoots, but it obviously decreased the K+ contents of the TRV:00 and TRV:GhCLCg-1 roots (Figure 8B). Interestingly, the Na+/K+ ratios sharply and significantly increased in the stems and leaves of TRV:GhCLCg-1 plants in response to the salt treatment, and the Na+/K+ ratio was highest in the TRV:GhCLCg-1 leaves (Figure 8C). These results indicate that silencing GhCLCg-1 causes cotton plants to absorb Na+ and accumulate in shoots, then leads to an increased Na+/K+ ratio in stems and leaves under NaCl treatment, which increases sensitivity to salinity stress.
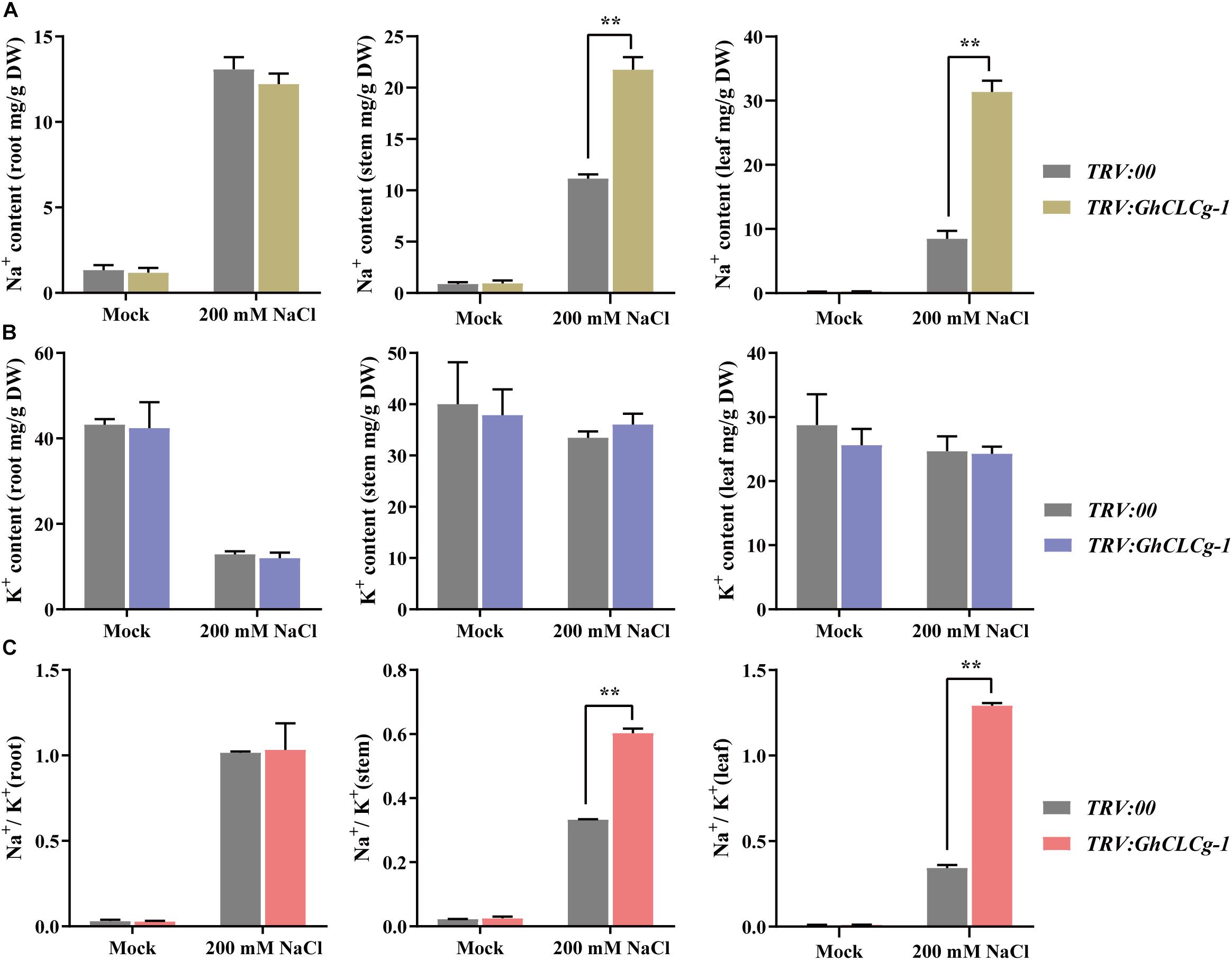
Figure 8. Cation contents of TRV:00 and TRV:GhCLCg-1 plants. (A) Na+ contents, (B) K+ contents, and (C) Na+/K+ ratio of TRV:00 and TRV:GhCLCg-1 plants with salt treated for 3 days. Data are presented as the mean ± standard deviation (SD) of three biological replicates (∗∗p < 0.01, t-test).
Discussion
Many studies showed that salt ions can induce osmotic stress and ion toxicity in plants and restrict their growth (Munns and Tester, 2008). Moreover, soil salinity interferes with water and nutrient uptake by the roots, and salt ions accumulate more in leaves than in roots (Tester and Davenport, 2003). In the current study, we proved that chloride salt stress causes cotton plants to accumulate more salt ions (Cl–, Na+, and K+), especially in the leaves. Cotton plant growth and development were significantly inhibited by high salinity (Figure 1 and Supplementary Figure 1).
Previous studies revealed that AtCLCg in A. thaliana is localized to the tonoplast and modulates chloride homeostasis in response to chloride stress, with shoot chloride levels increasing in atclcg knockout mutants (Nguyen et al., 2015). In the current study, we cloned a chloride channel gene (GhCLCg-1A/D) in upland cotton. Analysis of amino acid sequences revealed GhCLCg-1A/D belong to the CLCg subfamily and contain the conserved amino acid sequence motif (GSGIPE) associated with chloride selectivity (Atsuko et al., 2006; Li et al., 2006; Zifarelli and Pusch, 2010). The qRT-PCR data verified that GhCLCg-1 is responsive to chloride salt stress in leaves. At the transcriptional level, GhCLCg-1 was similarly affected by 200 mM KCl and 200 mM NaCl (Figures 4A,B). The leaf GhCLCg-1 transcription level increased as the treatment duration and chloride concentration increased (Figures 4A,C). These results imply GhCLCg-1 is involved in the chloride-mediated salt stress response in upland cotton plants.
Plants can minimize the effects of salt stress by eliminating excess salt ions through plasma membranes or by sequestering them in vacuoles (Zelm et al., 2020). The biological roles of plant proteins are closely related to their subcellular localization (Long et al., 2020). Our study confirmed that GhCLCg-1A/D are localized to the tonoplast (Figure 3), suggesting they may contribute to the transport of Cl– into vacuoles to participate in plant response to chloride salt. In a previous study, GmCLC1-overexpressing soybean plants sequestered more Cl– in the roots and transported less Cl– to the shoots than the WT controls, which enhanced the salt tolerance of the transgenic plants (Wei et al., 2016). The overexpression of the vacuolar chloride channel gene GsCLC-c2 influences Cl– homeostasis. More specifically, it improves salt tolerance by increasing the accumulation of Cl– in the roots, thereby decreasing the amount of Cl– transported to the shoots. Using two-electrode voltage clamps and Xenopus laevis oocytes, a previous study proved that GsCLC-c2 mediates the transport of Cl– (Wei et al., 2019). The insertion of the vacuolar chloride channel gene OsCLC-1 into gef1 yeast cells, in which the single putative chloride channel is mutated, decreases the relative sensitivity of yeast to various chloride salts (Atsuko et al., 2006).
In this study, our qRT-PCR analysis suggested that tonoplast-localized GhCLCg-1 is involved in chloride stress responses (Figure 4), which is consistent with the findings of a recent investigation (Liu et al., 2020). The overexpression of GhCLCg-1 in A. thaliana can enhance salt tolerance and alter Cl– accumulation (Figure 5). During an exposure to NaCl stress, most of the Cl– is not in vacuoles, but is in the cytoplasm or the apoplast, where it inhibits enzyme activities or increases water loss, respectively, in the absence of AtCLCg (Flowers et al., 2010; Teakle and Tyerman, 2010). The overexpression of the chloride channel gene CsCLCc in A. thaliana increases the salt tolerance of the transgenic plants. Moreover, the root and shoot Cl– contents are lower in the transgenic plants than in the mutant or WT plants (Wei et al., 2013). In this study, the transgenic A. thaliana plants overexpressing GhCLCg-1 accumulated more Cl– than the WT controls (Figure 5C). This is in contrast to the Cl– accumulation in the CsCLCc-overexpressing A. thaliana plants examined in an earlier investigation (Wei et al., 2013), but it is similar to the reported changes to the Cl– contents in A. thaliana atclcg mutants (Nguyen et al., 2015). It could be deduced that the results might be related to the role of CLCg. In A. thaliana atclcg mutants, loss of function of AtCLCg would result in accumulating more Cl– in the cytoplasm, because Cl– transport to the vacuole was disrupted, which increased Cl– contents and compromised salt tolerance. However, in the A. thaliana plants overexpressing GhCLCg-1, the extra Cl– was transported and accumulated in the vacuole by GhCLCg-1 to reduce its toxic effects in the cytoplasm, which also increased Cl– contents, but enhanced salt tolerance. Similar to the A. thaliana atclcg mutants, silencing GhCLCg-1 expression via VIGS also increased the Cl– contents and the sensitivity of cotton plants to chloride salt (Figure 7). Notably, TRV:GhCLCg-1 plants accumulated more Cl– in the leaves than in the roots under salt stress conditions, which was consistent with previous studies that A. thaliana clc mutants have more Cl– in the shoots than in other tissues (Jossier et al., 2010; Nguyen et al., 2015). The ion homeostasis is crucial for the salt tolerance of higher plants (Li et al., 2006). Furthermore, a low Na+/K+ ratio may maintain cell metabolic activities as well as salt tolerance (Blumwald, 2000). Although more Na+ and K+ were accumulated in the A. thaliana plants overexpressing GhCLCg-1, the Na+/K+ ratio was still lower than that of the WT plants under 100 mM NaCl (Figure 6). By contrary, the stem and leaf accumulated more Na+ in the TRV:GhCLCg-1 plants, leading to a higher Na+/K+ ratios than that in the TRV:00 plants after treatment with 200 mM NaCl (Figure 8). Considered together, these observations reveal that the GhCLCg-1 play a vital role in salt tolerance of upland cotton by regulating ion accumulation.
Conclusion
In conclusion, we revealed upland cotton plants accumulated Cl– and were damaged by chloride salt stress. Gene expression profiles suggested that the expression of GhCLCg-1, which encodes a tonoplast-localized chloride channel, is up-regulated in response to chloride salt stress. The overexpression of GhCLCg-1 in A. thaliana altered the accumulation of ions with a decrease of the Na+/K+ ratios, and enhanced salt tolerance. Moreover, cotton plants, in which GhCLCg-1 was silenced, accumulated more Cl– and increased the Na+/K+ ratios, leading to greater sensitivity to chloride salt. These results indicate that upland cotton plants accumulate Cl– under salt conditions and GhCLCg-1 alleviates damages resulting from excessive amounts of chloride salt.
Data Availability Statement
The datasets presented in this study can be found in online repositories. The names of the repository/repositories and accession number(s) can be found in the article/Supplementary Material.
Author Contributions
WL and ZM conceived and designed the research. WL, JF, and WM performed the experiments and analyzed the data. ZM provided the materials. WL and JF prepared the figures and wrote the manuscript. ZM and YZ revised the manuscript. All authors have read and approved the final manuscript.
Funding
This work was supported by the National Natural Science Foundation of China (32001596) and the National Key Research and Development Program for Crop Breeding (2018YFD0100306).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Acknowledgments
We acknowledge Zhongying Ren and Junjie Zhao (Institute of Cotton Research of the Chinese Academy of Agricultural Sciences, Anyang, China) for technical assistance.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fpls.2021.765173/full#supplementary-material
Supplementary Figure 1 | Cation contents in upland cotton plants. Na+ contents (mg/g DW) of upland cotton plants (roots, stems, and leaves) treated with no chloride (Mock) (A) or 200 mM NaCl (B) for 10 days. K+ contents (mg/g DW) of upland cotton plants (roots, stems, and leaves) treated with no chloride (Mock) (C) or 200 mM KCl (D) for 10 days. (E) Heatmap of the cation contents of upland cotton plants treated with no chloride (Mock), 200 mM NaCl, or 200 mM KCl. The cation concentrations are indicated by different colors and the colors are consistent in panels (A–E).
Supplementary Figure 2 | Sequence alignment of the GhCLCg-1A, GhCLCg-1D, and AtCLCg. Conserved residues GxxGIPE, GKxGPxxH, and PxxGxLF are indicated by the red, blue, and green boxes, respectively.
Supplementary Figure 3 | GhCLCg-1 expression patterns in the stems of plants treated for 3 h with different NaCl concentrations (0, 50, 100, 150, and 200 mM). The 2–ΔCT method was used to calculate relative expression levels. Error bars indicate the standard deviation (SD) of three biological replicates (∗∗p < 0.01; t-test).
Supplementary Table 1 | Primers used in this study.
Supplementary Table 2 | Genes used in this study.
Footnotes
References
Atsuko, N., Atsunori, F., Shingo, S., and Yoshiyuki, T. (2006). Molecular cloning, functional expression and subcellular localization of two putative vacuolar voltage-gated chloride channels in rice (Oryza sativa L.). Plant Cell Physiol. 47, 32–42. doi: 10.1093/pcp/pci220
Barbier-Brygoo, H., Vinauger, M., Colcombet, J., Ephritikhine, G., Frachisse, J. M., and Maurel, C. (2000). Anion channels in higher plants: functional characterization, molecular structure and physiological role. Biochim. Biophys. Acta 1465, 199–218. doi: 10.1016/S0005-2736(00)00139-5
Blumwald, E. (2000). Sodium transport and salt tolerance in plants. Curr. Opin. Cell Biol. 12, 431–434. doi: 10.1016/S0955-0674(00)00112-5
Broyer, T. C., Carlton, A. B., Johnson, C. M., and Stout, P. R. (1954). Chlorine-a micronutrient element for higher plants. Plant Physiol. 29, 526–532. doi: 10.1104/pp.29.6.526
Buss, N., Nelson, K. N., Hua, J., and Relyea, R. A. (2020). Effects of different roadway deicing salts on host-parasite interactions: The importance of salt type. Environ. Pollut. 266:115244. doi: 10.1016/j.envpol.2020.115244
Chen, C. J., Chen, H., Zhang, Y., Thomas, H. R., Frank, M. H., He, Y. H., et al. (2020). TBtools: an integrative toolkit developed for interactive analyses of big biological data. Mol. Plant 13, 1194–1202. doi: 10.1016/j.molp.2020.06.009
Clough, S. J., and Bent, A. F. (1998). Floral dip: a simplified method for Agrobacterium-mediated transformation of Arabidopsis thaliana. Plant J. 16, 735–743. doi: 10.1046/j.1365-313x.1998.00343.x
Deinlein, U., Stephan, A. B., Horie, T., Luo, W., Xu, G., and Schroeder, J. I. (2014). Plant salt-tolerance mechanisms. Trends Plant. Sci. 19, 371–379. doi: 10.1016/j.tplants.2014.02.001
Dutzler, R., Campbell, E. B., Cadene, M., Chait, B. T., and Mackinnon, R. (2002). X-ray structure of a ClC chloride channel at 3.0 A reveals the molecular basis of anion selectivity. Nature 415, 287–294. doi: 10.1038/415287a
Fay, L., and Shi, X. (2012). Environmental impacts of chemicals for snow and ice control: state of the knowledge. Water Air Soil Pollut. 223, 2751–2770. doi: 10.1007/s11270-011-1064-6
Feng, J. P., Ma, W. Y., Ma, Z. B., Ren, Z. Y., Zhou, Y., Zhao, J. J., et al. (2021). GhNHX3D, a vacuolar-localized Na+/H+ antiporter, positively regulates salt response in upland cotton. Intern. J. Mol. Sci. 22:4047. doi: 10.3390/ijms22084047
Flowers, T. J., Haji Ba Gherp, M. A., and Yeo, A. R. (2010). Ion accumulation in the cell walls of rice plants growing under saline conditions: evidence for the Oertli hypothesis. Plant Cell Environ. 14, 319–325. doi: 10.1111/j.1365-3040.1991.tb01507.x
Franco Navarro, J. D., Brumós, J., Rosales, M. A., Cubero Font, P., Talón, M., and Flore, J. M. C. (2015). Chloride regulates leaf cell size and water relations in tobacco plants. J. Exper. Bot. 67, 873–891. doi: 10.1093/jxb/erv502
Gutchess, K., Jin, L., Lautz, L., Shaw, S. B., Zhou, X., and Lu, Z. (2016). Chloride sources in urban and rural headwater catchments, central New York. Sci. Total Environ. 565, 462–472. doi: 10.1016/j.scitotenv.2016.04.181
Herbert, E. R., Boon, P., Burgin, A. J., Neubauer, S. C., Franklin, R. B., Ardón, M., et al. (2015). A global perspective on wetland salinization: ecological consequences of a growing threat to freshwater wetlands. Ecosphere 6, 1–43. doi: 10.1890/es14-00534.1
Jauh, G. Y., Fischer, A. M., Grimes, H. D., Ryan, C. A. Jr., and Rogers, J. C. (1998). δ-Tonoplast intrinsic protein defines unique plant vacuole functions. Proc. Natl. Acad. Sci. U.S.A. 95, 12995–12999. doi: 10.1073/pnas.95.22.12995
Jossier, M., Kroniewicz, L. T., Dalmas, F., Thiec, D. L., Ephritikhine, G., Thomine, S., et al. (2010). The Arabidopsis vacuolar anion transporter, AtCLCc, is involved in the regulation of stomatal movements and contributes to salt tolerance. Plant J. 64, 563–576. doi: 10.1111/j.1365-313X.2010.04352.x
Kaushal, S. S. (2016). Increased salinization decreases safe drinking water. Environ. Sci. Technol. 50, 2765–2766. doi: 10.1021/acs.est.6b00679
Laceby, J. P., Kerr, J. G., Zhu, D., Chung, C., Situ, Q., Abbasi, S., et al. (2019). Chloride inputs to the North Saskatchewan River watershed: the role of road salts as a potential driver of salinization downstream of North America’s northern most major city (Edmonton, Canada). Sci. Total Environ. 688, 1056–1068. doi: 10.1016/j.scitotenv.2019.06.208
Li, B., Qiu, J., Jayakannan, M., Xu, B., Li, Y., Mayo, G. M., et al. (2016). AtNPF2.5 modulates chloride (Cl–) efflux from roots of Arabidopsis thaliana. Front. Plant Sci. 7:2013. doi: 10.3389/fpls.2016.02013
Li, W. Y. F., Wong, F. L., Tsai, S. N., Phang, T. H., Shao, G. H., and Lam, H. M. (2006). Tonoplast-located GmCLC1 and GmNHX1 from soybean enhance NaCl tolerance in transgenic bright yellow (BY)-2 cells. Plant Cell Environ. 29, 1122–1137. doi: 10.1111/j.1365-3040.2005.01487.x
Liu, X., Pi, B. Y., Pu, J. W., Cheng, C., Fang, J. J., and Yu, B. J. (2020). Genome-wide analysis of chloride channel-encoding gene family members and identification of CLC genes that respond to Cl–/salt stress in upland cotton. Mol. Biol. Rep. 47, 9361–9371. doi: 10.1007/s11033-020-06023-z
Livak, K. J., and Schmittgen, T. D. (2001). Analysis of relative gene expression data using real-time quantitative PCR and the 2–ΔΔCT method. Methods 25, 402–408. doi: 10.1006/meth.2001.1262
Long, L., Zhao, J. R., Guo, D. D., Ma, X. N., Xu, F. C., Yang, W. W., et al. (2020). Identification of NHXs in Gossypium species and the positive role of GhNHX1 in salt tolerance. BMC Plant Biol. 20:147. doi: 10.1186/s12870-020-02345-z
Ma, W. Y., Ren, Z. Y., Zhou, Y., Zhao, J. J., Zhang, F., Feng, J. P., et al. (2020). Genome-wide identification of the Gossypium hirsutum NHX genes reveals that the endosomal-type GhNHX4A is critical for the salt tolerance of cotton. Intern. J. Mol. Sci. 21:7712. doi: 10.3390/ijms21207712
Munns, R., and Tester, M. (2008). Mechanisms of salinity tolerance. Annu. Rev. Plant Biol. 59, 651–681. doi: 10.1146/annurev.arplant.59.032607.092911
Nguyen, C. T., Agorio, A., Jossier, M., Depré, S., Thomine, S., and Filleur, S. (2015). Characterization of the chloride channel-like, AtCLCg, involved in chloride tolerance in Arabidopsis thaliana. Plant Cell Physiol. 57, 764–775.
Nie, W. X., Xu, L., and Yu, B. J. (2015). A putative soybean GmsSOS1 confers enhanced salt tolerance to transgenic Arabidopsis sos1-1 mutant. Protoplasma 252, 127–134. doi: 10.1007/s00709-014-0663-7
Niu, X., Bressan, R. A., Hasegawa, P. M., and Pardo, J. M. (1995). Ion homeostasis in NaCl stress environments. Plant Physiol. 109, 735–742. doi: 10.1104/pp.109.3.735
Pang, J. H., Zhu, Y., Li, Q., Liu, J. Z., Tian, Y. C., Liu, Y. L., et al. (2013). Development of Agrobacterium-mediated virus-induced gene silencing and performance evaluation of four marker genes in Gossypium barbadense. PLoS One 8:e73211. doi: 10.1371/journal.pone.0073211
Saghir, A., Khan, N. U. I., Iqbal, M. Z., Altaf, H., and Mahmudul, H. (2002). Salt tolerance of cotton (Gossypium hirsutum L.). Asian J. Plant Sci. 1, 715–719. doi: 10.3923/ajps.2002.715.719
Sheen, J. (2001). Signal transduction in Maize and Arabidopsis mesophyll protoplasts. Plant Physiol. 127, 1466–1475. doi: 10.1104/pp.010820
Teakle, N. L., and Tyerman, S. D. (2010). Mechanisms of Cl– transport contributing to salt tolerance. Plant Cell Environ. 4, 566–589. doi: 10.1111/j.1365-3040.2009.02060.x
Tester, M., and Davenport, R. (2003). Na+ tolerance and Na+ transport in higher plants. Ann. Bot. 91, 503–527. doi: 10.1093/aob/mcg058
Thunqvist, E. L. (2004). Regional increase of mean chloride concentration in water due to the application of deicing salt. Sci. Total Environ. 325, 29–37. doi: 10.1016/j.scitotenv.2003.11.020
Wang, N., Qi, H. K., Su, G. L., Yang, J., Zhou, H., Xu, Q. H., et al. (2016). Genotypic variations in ion homeostasis, photochemical efficiency and antioxidant capacity adjustment to salinity in cotton (Gossypium hirsutum L.). Soil Sci. Plant Nutr. 62, 240–246. doi: 10.1080/00380768.2016.1172022
Wang, X. G., Lu, X. K., Wang, J. J., Wang, D. L., Yin, Z. J., Fan, W. L., et al. (2016). Mining and analysis of SNP in response to salinity stress in upland cotton (Gossypium hirsutum L.). PLoS One 11:e0158142. doi: 10.1371/journal.pone.0158142
Weg, S., Gilliha, M., and Henderso, S. W. (2017). Chloride: not simply a ‘cheap osmoticum’, but a beneficial plant macronutrient. J. Exper. Bot. 68, 3057–3069. doi: 10.1093/jxb/erx050
Wei, P. P., Che, B. N., Shen, L., Cui, Y. Q., Wu, S. Y., Cheng, C., et al. (2019). Identification and functional characterization of the chloride channel gene, GsCLC-c2 from wild soybean. BMC Plant Biol. 19:121. doi: 10.1186/s12870-019-1732-z
Wei, P. P., Wang, L. C., Liu, A. L., Yu, B. J., and Lam, H. M. (2016). GmCLC1 confers enhanced salt tolerance through regulating chloride accumulation in soybean. Front. Plant Sci. 7:1082. doi: 10.3389/fpls.2016.01082
Wei, Q., Liu, Y., Zhou, G., and Li, Q. (2013). Overexpression of CsCLCc, a chloride channel gene from Poncirus trifoliata, enhances salt tolerance in Arabidopsis. Plant Mol. Biol. Rep. 31, 1548–1557. doi: 10.1007/s11105-013-0592-1
Yoo, S. D., Cho, Y. H., and Sheen, J. (2007). Arabidopsis mesophyll protoplasts: a versatile cell system for transient gene expression analysis. Nat. Protoc. 2, 1565–1572. doi: 10.1038/nprot.2007.199
Zelm, E. V., Zhang, Y., and Testerink, C. (2020). Salt tolerance mechanisms of plants. Annu. Rev. Plant Biol. 71, 403–433. doi: 10.1146/annurev-arplant-050718-100005
Zhang, H., Jin, J. J., Jin, L. F., Li, Z. F., Xu, G. Y., Wang, R., et al. (2018). Identification and analysis of the chloride channel gene family members in tobacco (Nicotiana tabacum). Gene 616, 56–64. doi: 10.1016/j.gene.2018.06.073
Zhang, X. K., Zhou, Q. H., Cao, J. H., and Yu, B. J. (2011). Differential Cl–/salt tolerance and NaCl-induced alternations of tissue and cellular ion fluxes in Glycine max, Glycine soja and their hybrid seedlings. J. Agron. Crop Sci. 197, 329–339. doi: 10.1111/j.1439-037X.2011.00467.x
Keywords: chloride channel, Gossypium hirsutum, vacuolar, ion content, salt tolerance
Citation: Liu W, Feng J, Ma W, Zhou Y and Ma Z (2021) GhCLCg-1, a Vacuolar Chloride Channel, Contributes to Salt Tolerance by Regulating Ion Accumulation in Upland Cotton. Front. Plant Sci. 12:765173. doi: 10.3389/fpls.2021.765173
Received: 26 August 2021; Accepted: 27 September 2021;
Published: 15 October 2021.
Edited by:
Fuguang Li, Cotton Research Institute, Chinese Academy of Agricultural Sciences (CAAS), ChinaCopyright © 2021 Liu, Feng, Ma, Zhou and Ma. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Wei Liu, liuwei0205@henau.edu.cn; Zongbin Ma, zongbinma@henau.edu.cn
 Wei Liu
Wei Liu Junping Feng1
Junping Feng1 Yang Zhou
Yang Zhou Zongbin Ma
Zongbin Ma