The ongoing debate on NBTs and possible roads for the future
- 1Council for Agricultural Research and Economics - CREA-PB, Rome, Italy
- 2Department of Law and Economic Studies La Sapienza University, Rome, Italy
There are marked differences in the approaches that regulate genetically modified (GM) products and the new breeding techniques (NBTs) in the European Union (EU) and in other areas of the world. Through the review of regulations and ongoing discussions, we show that the world can be divided in two groups based on the discrepancies in the approach of the country's regulations. On the one hand, Europe, with the main countries of Asia and Africa, regulates New Breeding Techniques as a genetically modified organism. On the other, a group of countries mainly located in the American continent, together with Australia, adopted a case-by-case approach, and are generally at a more advanced stage in the implementation of these new techniques. The paper aims to evaluate the possible evolution in the countries' regulations on the use of NBTs in the next years. The division between Western and Eastern countries of the world is confirmed, with some interesting movements in some regions. Greater uniformity among national regulations would be desirable to promote the implementation of biotechnologies in agriculture. The main research findings are that most EU Member States have taken a conservative position, whereas the Eastern group is more advanced and this could be a driving force for some regions toward acceptance of these technologies in the coming years.
1 Introduction
Global agriculture today faces the challenges of climate change and food security. Climate change is rendering the cultivation of traditional crops in some areas more difficult (extreme climatic events and rising temperatures affect production in some areas). At the same time, the global population increase requires an ever-increasing food supply, which could be achieved by increasing crop yield, particularly for cereal crops and oilseeds. In this context, the implementation of new production techniques could represent an opportunity to address these problems, through the development of crops that have higher yields and are more resistant to adverse climatic conditions and pests. As an alternative to traditional genetic selection tools, the development of new varieties can be achieved by genomic techniques like transgenesis (such as GMO), or new breeding techniques (NBTs), such as genome editing tools (e.g., conventional editing, base editing, prime editing, epigenome editing, etc.) (He et al., 2022; Hua et al., 2022).
The artificial engineering of GMOs allows the creation of new cultivars by introducing unrelated DNA sequences into the target species. Conversely, NBTs allow the introduction of new characteristics in a precise manner while also preserving the original genome background. Consequently, the final product is the same to the one produced through traditional breeding methods (EPSO, 2015).
From a regulatory point of view, the international debate is still open since policies are highly differentiated. There are countries where GMO regulations do not impose any restrictions on the use of GMO products and are open to NBTs; vice versa, in other countries, such as those of the EU, the regulations are restrictive, and NBTs are currently regulated as GMOs (Sprink et al., 2016).
The scientific debate is ongoing, and the literature indicates that consumers have less awareness of NBTs and the differences between products delivered therefrom. Consumers' food preferences and purchasing choices are influenced by many factors, including agricultural practices that they may not fully understand (Shew et al., 2018). More consistent opinions and attitudes could come from a huge information about the risks and benefits of purchasing foods containing GM ingredients. Consequently, government decisions to ban or approve GM crop cultivation influences consumer attitudes (Sendhil et al., 2022), as well as the communication strategies used (Marangon et al., 2021; Matsuo and Tachikawa, 2022). Research has found a lack of appropriate communication, and this in turn reinforces consumers' uncertainties. Suitable policies able to guarantee consumer safety could help GM foods spread by decreasing health consumer-perceived risk (Martinez-Poveda et al., 2009; Bawa and Anilakumar, 2012). Consumers can make more informed purchasing decisions with better education on quality information (Wunderlich and Smoller, 2019; Marette et al., 2021; DeMaria and Zezza, 2022).
The different legal frameworks and the current debate clearly show discrepancies across national regulations, and this contributes to slowing down the implementation of new biotechnologies (Menz et al., 2020; Turnbull et al., 2021). Starting from an examination of the nationally applied rules, we provide a possible perspective on the evolution of the regulatory framework regarding NBT use in agriculture over the next 5 years.
2 The worldwide legal framework for new breeding techniques
From a legal point of view, NBTs are regulated differently from country to country. Some regions do not have regulations regarding the labeling of genome editing (GE) products since the current regulatory systems are not based on the development process but on identified risks of the product.
With a product-based regulation, the regulatory framework and risk assessment only depend on the product characteristics, regardless of the way this product has been developed. With process-triggered regulation, the regulation framework depends on the method used for process innovation.
Although product-based regulation seems more flexible because it can be applied to any technology, while process-triggered regulation must be adjusted each time a new technology is introduced, no studies show a preference between process and product regulation in terms of regulatory efficiency. However, we observed that many regions that implemented NBT regulation use a product-based regulation; conversely, the countries with a process-based regulation are more conservative.
Countries with a product-based approach include Canada and USA; while the EU, China, and Egypt apply a process-based approach. Most countries implement a mixed method, where a process and product-based approach are combined (such as Japan,1 or, it regulates case by case, as the example of Argentina,2 Colombia, Chile,3 Brazil,4 and some other South American countries).
As Figure 1 depicts, countries can be divided in two groups based on the level of progress of discussions on the use of NBTs. The first group includes several Western countries, both from North America (the USA and Canada), and South America (Brazil and Argentina) which have already provided NBT regulation in agricultural production, as well as Australia.5 Many South American countries are advanced as regards NBTs, even if not all have the same regulatory frameworks. South Africa is pro-technology, even though it has not yet regulated NBTs. This is the first African country to regulate GM crops through the Genetically Modified Organisms Act No. 15 of 1997, while other countries started regulating this technology in the 2000s. Some Latin American countries are science-based and flexible with respect to innovation, but regulations have yet to be determined. Others such as Brazil, Colombia, Paraguay, Chile,6 and very recently Honduras and Guatemala have also developed rules, but only Argentina has an official national framework on how to regulate NBT products. Underlying this framework is Resolution 173/15 [SAGyP (Secretaría de Agricultura, Ganadería y Pesca), 2015], which establishes a procedure for determining whether a product derived from NBT can be considered a GMO under Resolution 701/11 [SAGyP (Secretaría de Agricultura, Ganadería y Pesca), 2011a,b]. Therefore, Regulation 173/15 does not change the pre-existing regulations on GM plants, rather it establishes whether an NBT crop or plant is subject to pre-existing GMO rules and regulations (Whelan and Lema, 2015). Brazil has adopted a hybrid system, where the focus is in principle on the characteristics and safety of the final product.
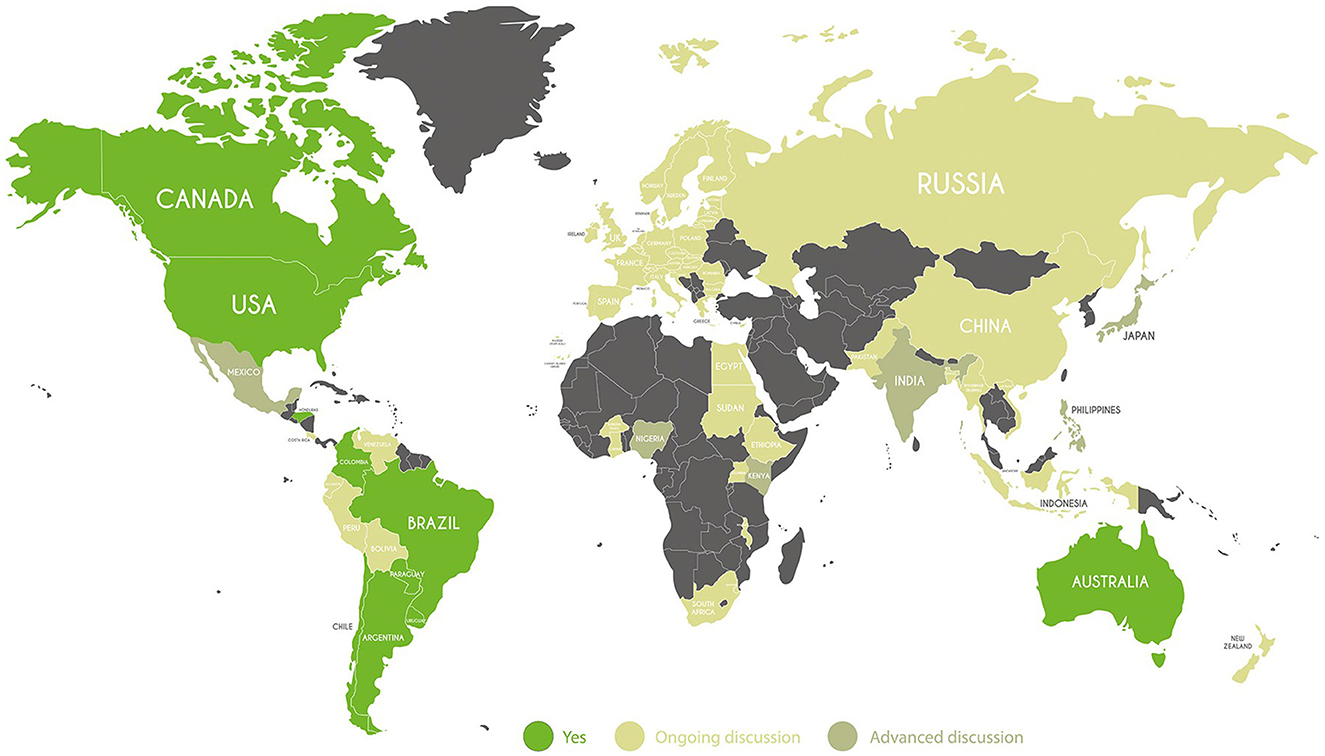
Figure 1. Country categorization by type of NBT legal framework. Source: Authors' categorization based on countries' legal framework.
These regulations are quite diverse, but there are some common elements: if no foreign DNA is found in the final product, then this product will be considered non-GM: instead, if foreign DNA is found, or the product is a GMO, it will go through the typical approval process for a GMO.
The second group includes several Eastern countries, including Russia and China, which have not regulated NBTs. The Russian Federation actually banned GMO crops under amendments to Federal Law No. 358-FZ of July 2016 and again with the recent approval of the new food safety doctrine in January 2020. A similar view concerns some African and Central-South American countries, such as Mexico, Peru, and Venezuela, in which, to date, no specific legislation has been provided on NBTs, and which do not allow commercial cultivation. Ecuador among them, has a more flexible point of view allowing the use of GM seeds for research purposes, through the Organic Law of Agrobiodiversity, Seeds, and Promotion of Sustainable Agriculture.
In this variegate group, there are countries where the possibility of using NBTs has not yet been regulated, but a debate is ongoing. Most of the European countries, in addition to India and Burkina Faso, Kenya, and Nigeria have launched a consultation on future deregulation, suggesting they are moving toward the adoption of NBTs. By a decree-law approved in 2022, Kenya authorized the use of seeds, cultivating, and importing genetically modified crops (GMOs). It also drew up guidelines for regulating products derived from NBTs, but the process is still at an early stage. Kenya is an example of a country that uses a product-based approach. India revised geniting application rules in agriculture in March 2022. The ruling excludes gene editing from the GMO classification. Gene-edited products with classifications SDN1 or SDN2 will not be treated as other transgenic products. In the recent past, there have been some developments that have the potential to allow new breeding techniques to be used for plants. One of these developments is the use of CRISPR. Furthermore, in the UK, a Statutory Instrument was released in the same year that makes it easier to conduct trial research on plants that have undergone gene-editing. The implementation of this measure will allow plant breeders in the UK to maintain their established position as world leaders in research and development.
In the EU, the legislative revision process is currently ongoing. In demonstrating the urgency of the issue raised, not only for political actors but also for EU citizens, in 2019, Grow Scientific Progress proposed updating Directive 2001/18/EC based on opinions of European citizens (European Commission, 2001, 2019). The proposal distinguishes between mutagenesis-based NBTs and techniques resulting in conventional GMOs, as they result in heterogeneous products. Novel organisms require safety evaluation and authorization before cultivation. The European Commission public consultation results (September 2022) indicate that legislation needs to be updated for certain NBTs and their products in light of scientific and technological advancements. Legislation should ensure adequate risk assessment and correct the legislative disproportion between products obtained with different techniques but with similar risks (EFSA, 2021, 2022). According to the study, these new techniques pose lower risks in comparison to the conventional ones in altering genetic material. In Europe, the situation is still controversial. England has aligned with Australia, Japan, and the United States in allowing trials of GM plants. The Swiss parliament set up a table to ease restrictions on genetic engineering in agriculture, extending a moratorium on genetically modified organisms (GMOs) until 2025. Norway so far does not allow any production and trade in GM food or feed, even though GM crops are legally permitted under the Genetic Engineering Act.
Similar to EU regions, the Russian Federation is following a comparable approach in prohibiting GMO cultivation and GMO and NBT products use, according to Federal Law No. 358-FZ of July 2016 and the new Doctrine on Food Safety.
In general, one can distinguish the Eastern group, composed of Europe (excluding certain countries), Russia, which has not implemented the use of GM products, along with some countries of South America (Venezuela, Peru, Ecuador) and Africa (Uganda, Egypt, Burkina Faso) and the Western group, which includes countries historically more open to the use of biotechnology in agriculture.
3 A perspective for national regulatory frameworks on NBTs in the coming years
After reviewing the worldwide regulatory framework, in Figure 2 we present a perspective on the possible evolution of NBTs regulations in the coming few years. This perspective is based on the categorization of the country's regulation in light of recent advancements. Here, the several options for the NBT regulatory development have been re-aggregated into three types:
• “Yes, with specific regulation”: areas where specific requirements for NBTs, such as risk assessment, or risk management are in force. These obligations in many cases refer to the specific approval of NBT use by control agencies or safeguard committees.
• “Yes, without a specific regulation”: countries where requirements for NBTs are less stringent than the first group and, in some cases, derive from those on GMOs. In these countries there has been or there is ongoing a deregulation process, often already started with GMO products.
• “No”: regions which, according to our perspective, will not endorse regulations on NBTs, at least within the next 5 years.
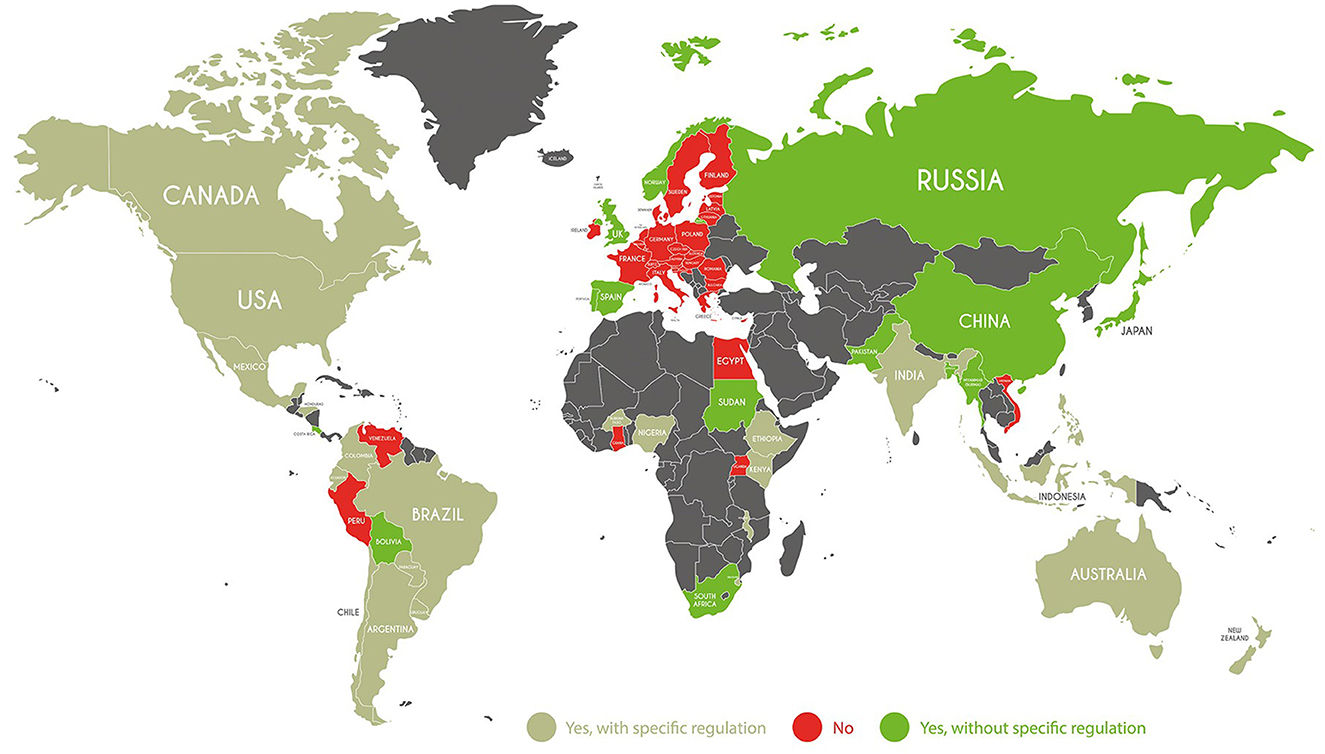
Figure 2. Perspective of the NBTs countries position. Source: Authors' categorization based on countries' legal framework.
Despite the split between Western and Eastern countries, there are some interesting differences.
Comparing current country categorization (Figure 1) with our forecasted NBT regulation development (Figure 2), some regions make advancements in regulation and move into the country group most open to NBTs (“Yes, with specific regulation”). These are regions, such as Mexico, India, some African regions (Kenya, Nigeria, and Ethiopia) where GMO cultivation is authorized and discussions on specific rules for the use of NBTs are ongoing. Also expected to move to this group is New Zealand, where although GMO cultivation is not currently permitted, government agencies are considering the possibility of using NBTs. From a regulatory standpoint, we expect New Zealand to adopt an open view and follow the Australian approach. This is because food safety regulations are supervised by a common Australian and New Zealand authority (FSANZ), suggesting the two countries' regulations will align in the next few years.
Some countries, such as China, South Africa, Sudan, and some European countries, including the UK, Spain and Portugal, are expected to move to the group “yes, without a specific regulation.” We suppose the use of NBTs should be simple and should not lead to more rigid or stringent approval requirements and procedures than those already envisaged for GMOs (ISAAA, 2022). When we look at African countries such as Sudan and South Africa, we can see that they are facing various challenges such as water shortages, extreme climatic events, and pests and diseases. Even though there are some difficulties in using genome editing to address these issues, these countries are now considering modernizing their current legislation to enable the use of genome editing as a potential solution. Russian Federation should follow the same path due to the federal program 2019 which established funding for genome editing.
Among the EU countries, Spain and Portugal are the only ones to allow GMO cultivation, showing a greater openness to NBT use. Norway is also an exception among EU countries, where both stakeholders and consumers seem to be favorable to genome editing (Kjeldaas et al., 2021). In the UK, NBTs are currently regulated under the EU rules (UK Parliament, 2022). However, the UK's exit from the EU with Brexit allowed the UK to adopt a different strategy for the implementation of new NTBs regulation. The UK Government seems to be more open to NBTs, which are considered to have no costs in comparison to GMOs.
Finally, a third group of countries is expected, at least in the few next years, to be less open to the NBTs use (group “No” in Figure 2). Most EU regions are expected to continue maintaining a conservative position.
One of the main problems in changing the current situation is the qualified majority required to adopt future changes in Member States, which should be more than 55% (currently 15 out of 27, without a strong supporter of transgenic crops, such as the UK).7 Indeed, since 2001, a qualified majority for or against approval has never been reached, suggesting that at least in the short term the EU will not move toward NBT use. The most conservative group of countries includes some South American countries (Peru and Venezuela) and some African countries (Egypt, Uganda, and Ghana).
Overall, the results of the country categorization suggest a kind of dynamism in the implementation of biotechnological innovations in the agriculture of some regions. In many areas, the subject of GMOs has always been a hot topic, on which the actors are divided due to environmental, social, and ethical issues. These controversies also have repercussions on new genetic improvement techniques. It follows, therefore, that there is a need for transparency, clarity and dissemination of information on scientific progress.
4 Policy implications and conclusion
The development of new genetic engineering brings opportunities to face critical challenges in different aspects of human life, such as food safety and environmental problems. This paper provides insight into the possible future NBT regulatory framework by classifying countries on the basis of the progress of their regulatory approaches, “Yes, with specific regulation,” and “Yes, without specific regulation.” The results of the paper are in line with empirical evidence which suggests that a universally recognized system does not exist, and there is clearly a problem in terms of harmonization of regulations. Regions such as China, United States, India, and Brazil are also among the top agricultural producers in the world. However, the debate on the deregulation of NBTs is still controversial, especially in the EU. The conservative EU position should be considered an obstacle given its role in international trade. Our work is in line with the spirit of Sprink et al. (2016), which offers considerations on the conservative EU position and the need for some regions to move toward NBT regulation. What has happened in the recent years has meant that some countries have changed their point of view, and this clearly emerges in the category “yes, without specific regulation.” The main question now is what will happen in the EU in the long term. Both stakeholders and the scientific community have noted the need for a common path to promote a process of harmonization leading to the deregulation of the rules in force. This goal could be achieved by a high level of transparency in communication which should be clear, understandable, and addressed to a wide audience.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Author contributions
SR: Conceptualization, Methodology, Writing—original draft. FD: Methodology, Writing—original draft. AQ: Methodology, Writing—original draft. RS: Methodology, Writing—original draft.
Funding
The author(s) declare that no financial support was received for the research, authorship, and/or publication of this article.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Author disclaimer
The views and opinions expressed in this article are those of the authors and do not reflect the views or positions of the institutions.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fpos.2023.1284527/full#supplementary-material
Footnotes
1. ^USDA, 2019.
2. ^USDA FAS, 2021.
3. ^USDA FAS, 2020.
4. ^USDA FAS, 2018.
6. ^SAGyP (Secretaría de Agricultura, Ganadería y Pesca), 2001. Resolution no. 1.523/2001.
7. ^The legislative procedure for changing Directive 2001/18 or any other EU legislative act concerning GMOs also requires a qualified majority among the representatives of the Member States in the Council.
References
Bawa, A. S., and Anilakumar, K. R. (2012). Genetically modified foods: safety, risks and public concerns—a review. J. Food Sci. Technol. 50, 1035–1046. doi: 10.1007/s13197-012-0899-1
DeMaria, F., and Zezza, A. (2022). Scientific information and cognitive bias in the case of New Breeding Techniques: exploring Millennials behaviour in Italy. Ital. Rev. Agric. Econ. 77, 41–60. doi: 10.36253/rea-13676
EFSA (2021). EFSA Annual Activity Report 2021. Parma: European Food Safety Authority. doi: 10.2805/149820
EFSA (2022). EFSA Annual Activity Report 2022. Parma: European Food Safety Authority. doi: 10.2805/226427
EPSO (2015). Opinion on the SAM Explanatory Note on New Techniques in Agricultural Biotechnology. Available online at: https://epsoweb.org/ (accessed December 20, 2022).
European Commission (2001). Directive 2001/18/EC regulation on GMOs. Available online at: https://eur-lex.europa.eu/legal-content/EN/TXT/?uri=celex%3A32001L0018 (accessed December 18, 2022).
European Commission (2019). EC Study on New Genomic Techniques. Available online at: https://ec.europa.eu/food/plant/gmo/modern_biotech/new-genomic-techniques_en (accessed November 16, 2022).
FSANZ (2018). Consultation Paper: Food Derived Using New Breeding Techniques. Available online at: http://www.foodstandards.gov.au/consumer/gmfood/Documents/Consultation%20paper%20-%20Food%20derived%20using%20new%20breeding%20techniques.pdf (accessed December 2, 2022).
FSANZ (2019a). FSANZ Application Handbook. Available online at: https://www.foodstandards.gov.au/food-standards-code/consultation/applicationshandbook (accessed December 2, 2022).
FSANZ (2019b). Final Report—Review of Food Derived Using New Breeding Techniques. Available online at: https://www.foodstandards.gov.au/consumer/gmfood/new-breeding-techniques-nbts (accessed December 19, 2022).
He, Y., Mudgett, M., and Zhao, Y. (2022). Advances in gene editing without residual transgenes in plants. Plant Physiol. 188, 1757–1768. doi: 10.1093/plphys/kiab574
Hua, K., Han, P., and Zhu, J. K. (2022). Improvement of base editors and prime editors advances precision genome engineering in plants. Plant Physiol. 188, 1795–1810. doi: 10.1093/plphys/kiab591
ISAAA (2022). To Regulate, or Not to Regulate, the Answer to the Question: The NCBP Policy on Plant Breeding Innovations or New Breeding Techniques (policy brief) Ms. Ma. Lorelie U. Agbagala, DOST Assistant Scientist and NCBP Head Secretariat. Ithaca, NY: ISAAA.
Kjeldaas, S., Antonsen, T., Hartley, S., and Myhr, A. I. (2021). Public consultation on proposed revisions to Norway's gene technology act: an analysis of the consultation framing, stakeholder concerns, and the integration of non-safety considerations. Sustainability 13, 7643. doi: 10.3390/su13147643
Marangon, F., Troiano, S., Carzedda, M., and Nassivera, F. (2021). Consumers' acceptance of genome edited food and the role of information. Ital. Rev. Agric. Econ. 76, 5–21. doi: 10.36253/rea-13115
Marette, S., Disdier, A.-C., and Beghin, J. C. (2021). A comparison of EU and US consumers' willingness to pay for gene-edited food: evidence from apples. Appetite 159, 105064. doi: 10.1016/j.appet.2020.105064
Martinez-Poveda, A., Brugarolas Molla-Bauza, M., del Campo Gomis, F. J., and Martinez-Carrasco Martinez, L. (2009). Consumer-perceived risk model for the introduction of genetically modified food in Spain. Food Policy 34, 519–528. doi: 10.1016/j.foodpol.2009.08.001
Matsuo, M., and Tachikawa, M. (2022). Implications and lessons from the introduction of genome-edited food products in Japan. Front. Genome Ed. 4, 899154. doi: 10.3389/fgeed.2022.899154
Menz, J., Modrzejewski, D., Hartung, F., Wilhelm, R., and Sprink, T. (2020). Genome edited crops touch the market: a view on the global development and regulatory environment. Front. Plant Sci. 11, 586027. doi: 10.3389/fpls.2020.586027
SAGyP (Secretaría de Agricultura, Ganadería y Pesca) (2011b). Resolución 701/2011, de 27 de octubre de 2011, Argentina. Available online at: https://www.argentina.gob.ar/normativa/nacional/resoluci%C3%B3n-701-2011-189067/texto (accessed December 20, 2022).
SAGyP (Secretaría de Agricultura, Ganadería y Pesca) (2015). Resolución 173/2015, 12 de mayo de 2015, Argentina. Available online at: https://www.argentina.gob.ar/normativa/nacional/resoluci%C3%B3n-173-2015-246978/texto (accessed December 20, 2022).
SAGyP (Secretaría de Agricultura, Ganadería y Pesca) (2011a). Resolución 763/2011, de 17 de Agosto de 2011, Argentina. Available online at: https://www.argentina.gob.ar/normativa/nacional/resoluci%C3%B3n-763-2011-185806/texto (accessed Decemeber 18, 2022).
SAGyP (Secretaría de Agricultura, Ganadería y Pesca) (2001). Resolución N° 1.523/2001, de 6 de julho de 2001, Chile. Available online at: http://www.sag.cl/sites/default/files/RES_1523_2001.pdf (accessed December 19, 2022).
Sendhil, R., Nyikab, J., Yadavc, S., Mackolild, J., Prashat Ge, R., and Workief, E. (2022), Genetically modified foods: bibliometric analysis on consumer perception preference. GM Crops Food 13, 65–85. doi: 10.1080/21645698.2022.2038525.
Shew, A. M., Nalley, L. L., Snell, H. A., Nayga, R. M., and Dixon, B. L. (2018). CRISPR versus GMOs: public acceptance and valuation. Glob. Food Secur. 19, 71–80. doi: 10.1016/j.gfs.2018.10.005
Sprink, T., Eriksson, D., Schiemann, J., and Hartung, F. (2016). Regulatory hurdles for genome editing: process- vs. product-based approaches in different regulatory contexts. Plant Cell Rep. 35, 1493–1506. doi: 10.1007/s00299-016-1990-2
Turnbull, C., Lillemo, M., and Hvoslef-Eide, T. A. K. (2021). Global regulation of genetically modified crops amid the gene edited crop boom – a review. Front. Plant Sci. 12, 630396. doi: 10.3389/fpls.2021.630396
UK Parliament (2022). Genome Edited Food Crops. London: The Parliamentary Office of Science and Technology.
USDA (2019). Japan Modifies Handling Procedures for Genome Edited Foods. Available online at: https://apps.fas.usda.gov/newgainapi/api/Report/DownloadReportByFileName?fileName=Japan%20Modifies%20Handling%20Proceduers%20for%20Genome%20Edited%20Foods_Tokyo_Japan_09-24-2019 (accessed December 17, 2022).
USDA FAS (2018). GAIN Report: Agricultural Biotechnology Annual- Brazil. Available Online at: https://apps.fas.usda.gov/newgainapi/api/Report/DownloadReportByFileName?fileName=Agricultural%20Biotechnology%20Annual_Brasilia_Brazil_12-26-2018 (accessed December 17, 2022).
USDA FAS (2020). GAIN Report: Agricultural Biotechnology Annual - Chile. Available online at: https://apps.fas.usda.gov (accessed December 21, 2022).
USDA FAS (2021). GAIN Report: Agricultural Biotechnology Annual- Argentina. Available online at: https://apps.fas.usda.gov/newgainapi/api/Report/DownloadReportByFileName?fileName=Agricultural%20Biotechnology%20Annual_Buenos%20Aires_Argentina_10-20-2020 (accessed December 21, 2022).
Whelan, A. I., and Lema, M. A. (2015). Regulatory framework for gene editing and other new breeding techniques (NBTs) in Argentina. GM Crops Food 6, 253–265. doi: 10.1080/21645698.2015.1114698
Keywords: new breeding techniques (NBTs), international frameworks, biotechnology regulations, countries' analysis, perspectives
Citation: Romeo Lironcurti S, Demaria F, Quarto A and Solazzo R (2024) The ongoing debate on NBTs and possible roads for the future. Front. Polit. Sci. 5:1284527. doi: 10.3389/fpos.2023.1284527
Received: 28 August 2023; Accepted: 15 December 2023;
Published: 09 January 2024.
Edited by:
Micah Altman, Massachusetts Institute of Technology, United StatesReviewed by:
Hayley Hesseln, University of Saskatchewan, CanadaCopyright © 2024 Romeo Lironcurti, Demaria, Quarto and Solazzo. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Simona Romeo Lironcurti, simona.romeo@crea.gov.it
 Simona Romeo Lironcurti
Simona Romeo Lironcurti Federica Demaria
Federica Demaria Angelo Quarto
Angelo Quarto Roberto Solazzo
Roberto Solazzo