- 1Graduate School, Wuhan Sports University, Wuhan, China
- 2College of Health Science, Wuhan Sport University, Wuhan, China
- 3Hubei Key Laboratory of Sport Training and Monitoring, Wuhan Sports University, Wuhan, China
While previous studies have suggested that there exists a relationship between obesity and executive function (EF), the mechanisms and causal relationship between them remain unclear. There are important clinical implications of determining whether EF can predict and treat obesity. We conducted a multilevel meta-analysis of randomized controlled trials (RCTs) and longitudinal studies. Specifically, we investigate (a) whether EF interventions have an effect on weight loss, (b) whether baseline EF can be a predictor of future weight loss through obesity intervention, and (c) whether early-life EF can predict future weight loss. Eight RCTs and 17 longitudinal studies with a total of 11,393 participants were identified. We found that (a) EF interventions may not have an effect on weight loss, (b) baseline inhibition (β = 0.259, p = 0.03) and delay discounting (β = −0.17, p = 0.04) significantly predict future weight loss through obesity intervention, (c) age (F = 13.666, p = 0.005) moderates the relationship between working memory and weight loss through intervention, but not weight status, type of intervention, and percentage of female, and (d) early life inhibition (β = 0.185, p = 0.07) is a marginally significant predictor of future weight loss. Our results seem to support the assumption that the relationship between EF and obesity is not direct, and a higher-order factor, such as genes, may link obesity and EF. Building on the preliminary findings, further studies focusing on EF and obesity are needed in the future.
Introduction
Obesity is a worldwide health problem. According to the World Health Organization, 39% of adults were overweight, and 13% were obese in 2016 (World Health Organization, 2018). Being obese is a risk factor for many psychological and physiological diseases, and there is much evidence showing that excess weight is associated with type 2 diabetes (Schwartz and Porte, 2005), cardiovascular diseases (Nakamura et al., 2014), cancer (Calle and Kaaks, 2004), depression (Luppino et al., 2010), and anxiety (Gariepy et al., 2010). Furthermore, obese individuals often experience discrimination (Tomiyama, 2014). Obese individuals may have lower socioeconomic status positions (Wang and Beydoun, 2007), and obese daughters receive less financial support from their parents than their lower body mass index (BMI) sisters (Crandall, 1995). Since obesity has nearly tripled since 1975 (World Health Organization, 2018), the public should pay more attention to obesity and weight loss.
In recent years, a growing number of researchers have linked obesity to executive functions (EFs). A number of studies found that obese and overweight individuals had poor EF performances compared to their counterparts (Hayes et al., 2018; Yang et al., 2018; Favieri et al., 2019; Mamrot and Hanc, 2019). Successful weight loss through intervention has been associated with improvements in EFs (Veronese et al., 2017). Moreover, cognitive training and neuromodulation strategies are seen as promising ways to overcome excess weight (Forcano et al., 2018).
Executive function (EF), also referred to as executive control or cognitive control, is an umbrella term involving interrelated higher-order neurocognitive mental processes that enable goal-directed behaviors (Banich, 2009; Diamond, 2013). There are two types of EF: cool cognitive EF and hot affective EF (Prencipe et al., 2011; Zelazo and Carlson, 2012). Cool EF, such as inhibitory control, working memory, and attentional shifting, refers to cognitive functions that engage in solving abstract and novel problems without the involvement of affect. In contrast, hot EF, such as delay discounting and affective decision making, refers to cognitive functions that involve affect when solving problems. According to Diamond (2013), there are three core EFs: (1) inhibition is the ability to control one's attention, thought, behavior, and emotion, including self-control, behavior control, selective attention, and cognitive inhibition; (2) working memory is the ability to update information and monitor ongoing action and stimuli; and (3) cognitive flexibility is the ability to shift attention, thought, perspective, and mindset in response to environmental conditions. Higher-order EFs such as planning and problem-solving are built up from these three core EFs.
Previous neuroimaging studies found that EF may mainly rely on the prefrontal cortex (Moriguchi and Hiraki, 2013). For instance, inhibition may associate with the inferior frontal cortex, dorsolateral prefrontal cortex, and orbital frontal cortex in the view of the localizationist (Aron et al., 2014). Although neurophysiological studies found that the persistent discharges widespread occurred in cortical and subcortical regions, such as the primary visual and posterior parietal cortices, when participants were engaged in working memory task (Constantinidis and Procyk, 2004; Pasternak and Greenlee, 2005), the prefrontal cortex is still one of the most important regions for working memory (Riley and Christos, 2015). Cognitive flexibility is a relatively advanced cognitive process that involves many brain regions such as basal ganglia, anterior cingulate cortex, and posterior parietal cortex, and undoubtedly, the prefrontal cortex is an important part of it (Leber et al., 2008). A functional magnetic resonance imaging study (Adleman et al., 2002) found a significantly different brain activity in the prefrontal region among children (age 7–11), adolescents (age 12–16), and young adults (age 18–22) when engaged in inhibition on the Stroop color–word task, the result of which was consistent with the idea that EFs continue to develop through childhood and adulthood. However, the developmental trajectories of different EF domains are different (Best and Miller, 2010). Inhibition appears to rapidly develop during the preschool years and shows less change later on, while working memory and cognitive flexibility develop at a relatively stable rate. One interpretation of such differences is that the brain regions supporting EFs are different (O'Connor, 2002).
The Role of EF on Obesity and Weight Loss
EF plays an important role in our lifestyle. Lower EF has been associated with higher levels of unhealthy food consumption (Pieper and Laugero, 2013), more sedentary behavior (Wirt et al., 2015), less physical activity (Riggs et al., 2010), and lower levels of fruit and vegetable consumption (Zhou et al., 2015). EF deficits may lead to obesity through obesogenic habits.
A dual-process model suggested that our eating behaviors are guided by two distinct but associated processes: automatic and reflective processes (Hofmann et al., 2009). Automatic processes associate food-related cues with rewarding effects so that food-related cues can automatically draw one's attention, evoke approach responses, and then influence eating behaviors. Automatic processes occur without cognitive resources and may overwhelm conscious intentions. The reflective processes, also named the controlled processes, are top-down, conscious processes that elicit behaviors as a consequence of decision making and are closely linked to EF. People with a weak reflective system could easily be driven by the automatic system, which would lead them to choose more high-energy-dense food and therefore be obese.
What causes an individual to become obese is that they consume more energy than they expend (Spiegelman and Flier, 2001). Our understanding of the mechanisms of obesity has been improved over decades of research. There are three factors that are related to obesity. The first is homeostasis, which is the maintenance of energy balance by regulating internal physiological processes (Narayanaswami and Dwoskin, 2017). When an individual is objectively hungry or full, the body will spontaneously release signals to maintain energy balance. For example, when your body is hungry, the homeostasis system will make you feel hungry and urge you to eat. The second is reward system. Food itself is a natural reward that refers to a person's natural desire for food (Berridge, 1996). Certain foods, especially those high in sugar and fat, are effective rewards for promoting eating even when there is no energy requirement (Berridge and Robinson, 2003). This is also the reflection process in the dual-process model mentioned earlier. The third is executive function. Homeostasis and reward systems are innate and spontaneous, not controlled by consciousness, while executive function depends on conscious effort. Executive function determines whether to eat and allows us to refuse to eat food in the face of food temptation or hunger. In this way, EF may be able to compete with homeostatic and reward systems, thereby reducing overall energy intake. Therefore, for obese people, better EF may help them regulate their eating behaviors to achieve the goal of weight loss.
Several systematic reviews have investigated the effect of EF on obesity and weight loss (Hayes et al., 2018; Favieri et al., 2019; Mamrot and Hanc, 2019). Hayes et al. (2018) found that compared to normal-weight individuals, obese children showed poorer EF performances. They came to the preliminary conclusion that behavioral weight loss interventions may improve EF performance, which in turn improved the outcomes of interventions. The existence of a relationship between EF and obesity has been confirmed (Favieri et al., 2019). EF and obesity were dependent (Mamrot and Hanc, 2019); however, the mechanisms of them could not be determined (Favieri et al., 2019), and whether EF can be a predictor of obesity remained unknown (Mamrot and Hanc, 2019).
In sum, these researchers found an association between EF and obesity, but the causal relationship could not be determined. All of these studies were not meta-analyses that quantitatively synthesized the data, and they either (a) considered only a specific age stage (Mamrot and Hanc, 2019), (b) aimed only at whether EF influenced obese and overweight individuals (Hayes et al., 2018; Favieri et al., 2019), and (c) did not exclusively focus on the influence of EF (Forcano et al., 2018; Jones et al., 2018). Although many researchers hold the view that EF may have an influence on obesity and weight loss, there is no strong evidence of such a relationship, and the mechanism is not clear. Determining whether EF can predict and treat obesity has important clinical implications.
EF Intervention on Obesity and Weight Loss
Because cognition is important in eating behavior and it is controlled by consciousness, cognitive training has recently gained increasing attention. Previous studies found that repeated cognitive training on EF tasks can improve EF capacity, which makes it possible for EF interventions to become an effective way to treat obesity (Manuel et al., 2013; Berkman et al., 2014). EF can be trained by gradually increasing the difficulty of the EF task, and participants' performances on the EF task are indicators of their EF level. There are also some other cognitive training interventions, such as episodic future thinking (Atance and O'Neill, 2001), approach/avoidance training (Schonberg et al., 2014), attention bias modification (Kemps et al., 2014), and implementation intentions (Armitage, 2004). Several systematic reviews of whether these interventions affect obesity and weight loss have been conducted. However, the results of these systematic reviews were different. Yang et al. (2019) found that, with the exception of inhibition training, other types of cognitive training made no contribution to weight loss. However, Forcano et al. (2018) found that in addition to inhibition training, implementation intentions showed promising results regarding weight loss in people with obesity/overweight, and attention bias modification had an effect in normal-weight participants, while other types of cognitive training were ineffective. The effects of cognitive training need to be further explored.
On the one hand, these cognitive trainings are all related to EF. On the other hand, they involve various cognitive processes in addition to EF, which may make the issue more complicated. Thus, it would be helpful to merely consider the effect of EF intervention. However, randomized controlled trials (RCTs) investigating the effect of EF interventions have shown inconsistent results. While some studies claimed that weight loss intervention through improving EFs significantly reduced participants' BMI (Allom and Mullan, 2015; Raman et al., 2018; Galindo Muñoz et al., 2019), other studies did not find such a pattern (Houben et al., 2016; Dassen et al., 2018b). A meta-analysis using RCTs that may reveal a causal inference is needed to assess these inconsistencies.
EFs as Predictors of Weight Loss
Current weight loss programs focus on reducing energy intake and increasing energy expenditure. The effects of obesity interventions vary from person to person. In bariatric surgery, for example, most patients achieve their weight loss goals after surgery; however, there are still a large number of people who fail to achieve their weight loss goals and even regain the weight a few years after surgery (Karmali et al., 2013; Maleckas et al., 2016). Therefore, it is important to understand the individual factors that influence obesity intervention. The physiological and behavioral factors are the two types most likely to influence obesity intervention outcomes. For example, Wildes et al. (2010) found that children with binge eating lost less weight through family-based obesity intervention. Augustijn et al. (2018) found that balance skills significantly predict weight loss in a 5-month treatment program. Given the close link between EF and obesity, EF is likely to be a strong predictor of obesity intervention outcomes.
EFs can facilitate goal-directed activities and eating behaviors, which may have an impact on obesity interventions (Appelhans et al., 2016). For example, cognitive function is associated with adherence to bariatric postoperative guidelines (Beth Spitznagel et al., 2013), which may be the reason that bariatric patients with poor long-term weight loss have poorer inhibitory control than patients who lose their weight successfully (Hogenkamp et al., 2015). Besides, as the mechanism we discussed above, the impact of EF on eating behavior occurs all the time, it is reasonable to believe that different baseline levels of EF may affect obesity treatment outcomes. Determining the predictive factors of weight loss is important for preventing and treating obesity.
Inhibition, delay discounting, working memory, cognitive flexibility, planning, and general EF are considered to be predictors of obesity intervention outcomes. However, inconsistencies also occurred in longitudinal studies investigating whether better baseline EF can predict more weight loss through obesity interventions. Butryn et al. (2019) found that baseline EF measured by the tower task component of the Delis–Kaplan Executive Function System significantly predicted weight loss through 6-month standard behavior treatment. A study of 82 obese adults from an obesity intervention center found that baseline inhibition, shifting, and delay discounting, measured by the stop-signal task, trail-making test, and monetary choice questionnaire, respectively, were not predictors of weight loss, while better working memory and self-reported inhibition measured by the 2-back test and Behavioral Rating Inventory of Executive Functioning-Adult Version, respectively, were predictive of more weight loss across 6-month behavioral treatment.
The above discussion focused on whether EF can predict obesity interventions outcomes. However, whether EF can predict obesity or weight loss under natural conditions is also an important issue. Girls with poorer inhibition at age 7 have higher BMI and more weight gain at ages 9, 11, 13, and 15 (Anzman and Birch, 2009). The ability to delay gratification of preschoolers can even predict their BMI 30 years later (Schlam et al., 2013). Although there is relatively less concern in this area, this is important for understanding the effect of EFs on obesity and weight loss. A meta-analysis is needed to summarize these findings.
The Aim of the Present Study
Although many researchers agree that EF can influence obesity and weight loss, the causal relationship between them could not be determined. If EF does affect obesity, a more effective obesity intervention could be proposed. There are important clinical implications of determining whether EF can treat obesity. However, as discussed above, RCTs investigating the effect of EF interventions have shown inconsistent results (Verbeken et al., 2013; Allom and Mullan, 2015; Houben et al., 2016; Galindo Muñoz et al., 2019). A meta-analysis using RCTs that may reveal a causal inference is needed to assess these inconsistencies. At the same time, it is also important to investigate the predictive effect of EF on obesity and weight loss through obesity interventions and natural conditions. If EF does predict obesity and weight loss, we may take precautions against obesity. To our knowledge, this is the first meta-analysis to investigate whether EFs have an impact on obesity and weight loss. We focused on all ages and all weight statuses (normal weight, overweight, and obesity) to obtain a general conclusion. The aim of this study is to determine (a) whether EF interventions have an effect on weight loss, (b) whether better baseline EF can predict greater weight loss through weight loss intervention, and (c) whether early life EF can predict future weight loss.
In addition, we examined some potential moderating factors. As discussed earlier, the effect of cognitive training on weight loss varies by weight statuses, and the levels of EFs differ by age. Age and weight status may moderate the relationship between EF and obesity. In longitudinal studies, surgical interventions are invasive while non-surgical interventions are not (Puzziferri et al., 2014). There is a large difference between surgical and non-surgical interventions. In some studies, the percentage of female participants was 100%, which may have influenced the results. Therefore, we examined age, weight status, type of intervention, and percentage of female participants as potential moderators of EF and weight loss.
Methods
This systematic review was conducted according to the PRISMA statement (Liberati et al., 2009; Moher et al., 2009). The PROSPERO registration number is CRD42020177212.
Searching Strategies and Study Selection
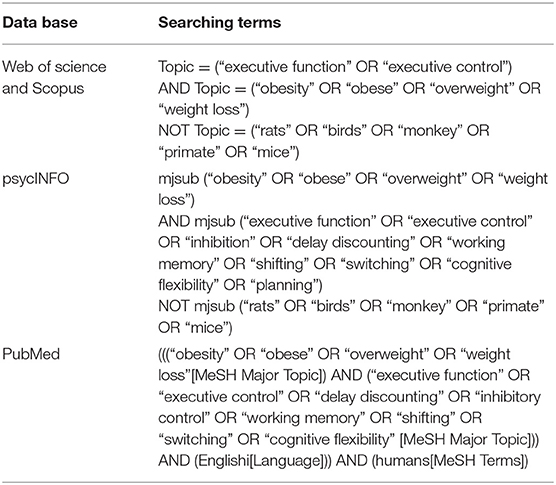
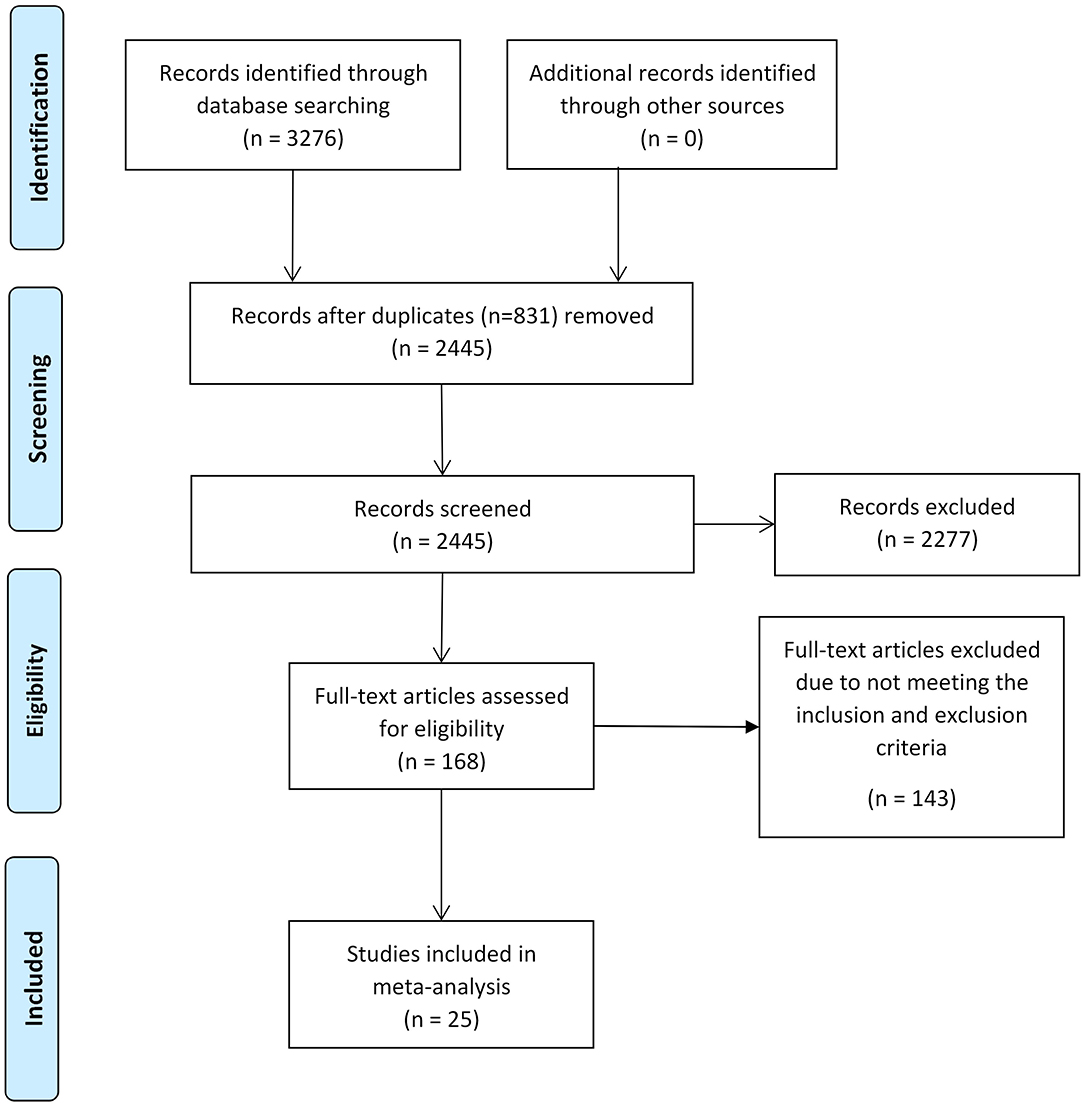
Two investigators (Z.D., J.M.) independently conducted the literature search. Four databases, PubMed, Web of Science, Scopus, and psycINFO, were utilized to obtain the studies for meta-analysis on January 11, 2020. The searching strategies are shown in Table 1. To ensure that we found as many studies as possible, references in relevant studies were also reviewed. The detailed selection procedure is shown in Figure 1.
The following inclusion criteria were used: (a) studies written in English, (b) participants in studies were human, and (c) studies were published in peer-reviewed journals. For the RCTs investigating whether EF interventions were effective, they (a) should include at least one control group, (b) should aim at weight loss through improving EF, and (c) should report participants' baseline and followed-up measures or changes in BMI or other indicators of weight status. For the longitudinal studies investigating whether EF predicts more weight loss through intervention and predicts future weight loss, (a) linear regression should be employed, (b) baseline EF should be used to predict future BMI changes or changes in other indicators of weight status, and (c) standard error of standardized regression coefficient beta should be reported or could be calculated.
We excluded studies if they (a) used secondary data whose first-hand data were included in the meta-analysis, (b) were not empirical studies, (c) lacked important data that were necessary for the meta-analysis, (d) included participants with any significant or chronic condition known to affect cognitive status (e.g., depression, cancer), and (e) included subjects with type 2 diabetes, cardiovascular event antecedents, and other physiological diseases that may influence their weight status.
Quality Assessment
The quality of the study was independently assessed by two reviewers (Z.D., J.M.), and any discrepancies were mediated by the third reviewer (X.X.). For the RCTs, the quality was assessed through Jadad's scale (Jadad et al., 1996), which is one of the most commonly used quality assessment tools for RCTs by researchers with good reliability (Clark et al., 1999). We did not consider blinding as all RCTs were single-blind trials and the same method was used to control the placebo effect; therefore, there was a total of three points in terms of randomization and dropout.
Currently, there are few tools available to evaluate longitudinal observational studies; The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) (von Elm et al., 2008) guidelines were one of the quality assessment tools to assess longitudinal studies, and it has been used in recent systematic reviews (Scales and Dahm, 2008; Ricci-Cabello et al., 2010). STROBE contains a total of 22 items, and 9 of them relevant to the methodology were selected to assess the quality.
Data Extraction
Data extraction was also independently conducted by two reviewers (Z.D., J.M.), and disagreement was mediated by the third reviewer (X.X.). Details of the EF intervention, participant characteristics, baseline weight status, and eligibility criteria of the participants were extracted from the RCTs. For the longitudinal studies, the participant characteristics, baseline BMI, time between baseline, and endpoint, EF of interested and their measurements were identified.
Statistical Analysis
As some studies involved multiple outcomes and the results are not independent of each other, a three-level meta-analytic model followed by Assink and Wibbelink (2016) was conducted. This model takes into account three kinds of variance: (a) sampling variance (level 1), which is the variance in the observed effects, (b) within-study variance (level 2), which is the variance for multiple outcomes in the same studies, and (c) between-study variance (level 3), which is the variance in effects between different studies. Significant variance in within-study or between-study indicates heterogeneity between studies. Restricted maximum likelihood estimation (REML) was used to reduce the bias of variance estimates (Van den Noortgate et al., 2015). As the participants in these studies varied in age, country, and economic status, a random-effect model was employed.
When combining effect sizes, Hedges' g was used to estimate the pooled effect size for the RCTs, as it is a relatively unbiased estimation. For longitudinal studies, the standardized regression coefficient β was used as the effect size (Kim, 2011; Bowman, 2012). One RCT study (Galindo Muñoz et al., 2019) did not provide the posttest BMI standard deviation (SD), and we used baseline SD to estimate it (Higgins and Green, 2011). The standard error of β was calculated by β/t or SE × SX/SY, where t is the result of the significance test, SE is the standard error of the unstandardized regression coefficient, SX is the variance in the independent variable, and SY is the variance in the dependent variable. For studies that did not provide t values, SX or SY, the P-value was used to calculate t, and if the P-value is less than a 0.05 threshold, a conservative estimate of this threshold minus 0.01 was taken as the P-value.
For the RCTs, a positive effect size means that compared to the control condition, the EF intervention was more effective. For the longitudinal studies, a positive effect size means that better EF predicted more weight loss. If the number of studies was > 3, a subgroup analysis was conducted in terms of age, type of intervention, percentage of female participants, and weight status to explore the moderating effects. Q and I-squared statistics were used to measure the study heterogeneity. Publication bias is an important issue in the meta-analysis, which may affect the accuracy of results. Publication bias refers to the fact that studies with insignificant results are less likely to be published than those with significant results (Rosenthal, 1995), which leads to bias in the literature included in the meta-analysis. Funnel plots and Egger's test (Egger et al., 1997) were used to assess publication bias. The funnel plot is a qualitative method to observe whether there is publication bias. If the funnel plot is observed to be symmetrical, there is no publication bias. As the observation of funnel plots is highly subjective, Egger's test can objectively measure whether the funnel plots are symmetrical or not. If the test results are significant, it means that the funnel plot is statistically asymmetric and there is missing data. In the case of significant Egger's test results, the trim-and-fill method (Duval and Tweedie, 2000) was employed to correct the asymmetry of funnel plots caused by publication bias. The multilevel meta-analysis was conducted in R (version 3.6.3) with the metafor package.
Results
Study Characteristics
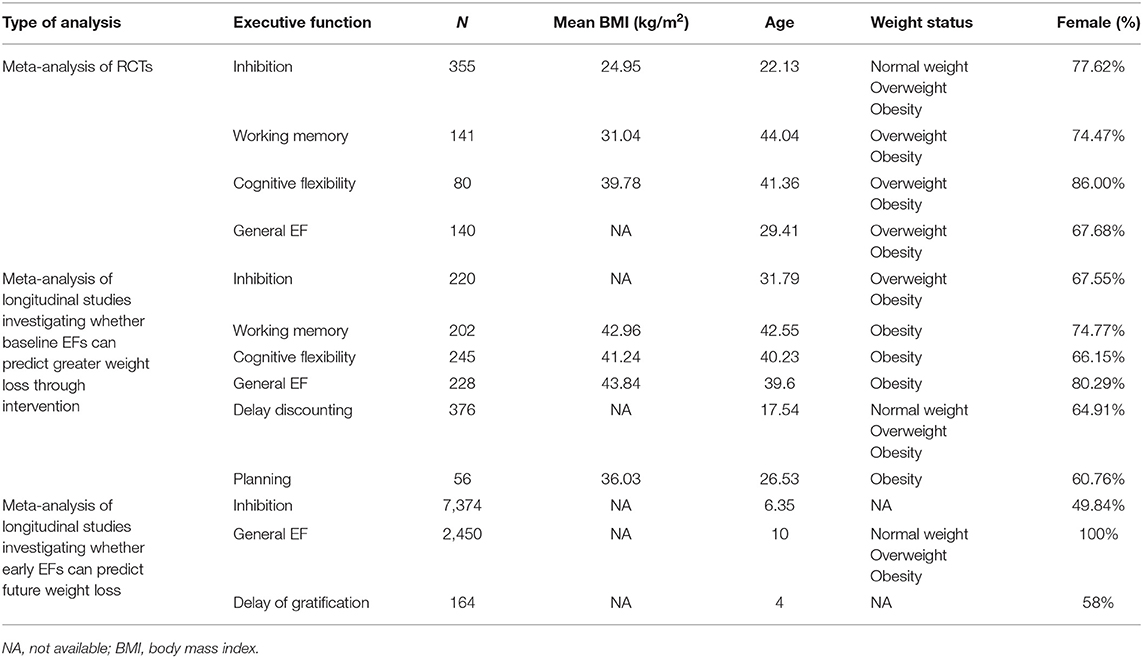
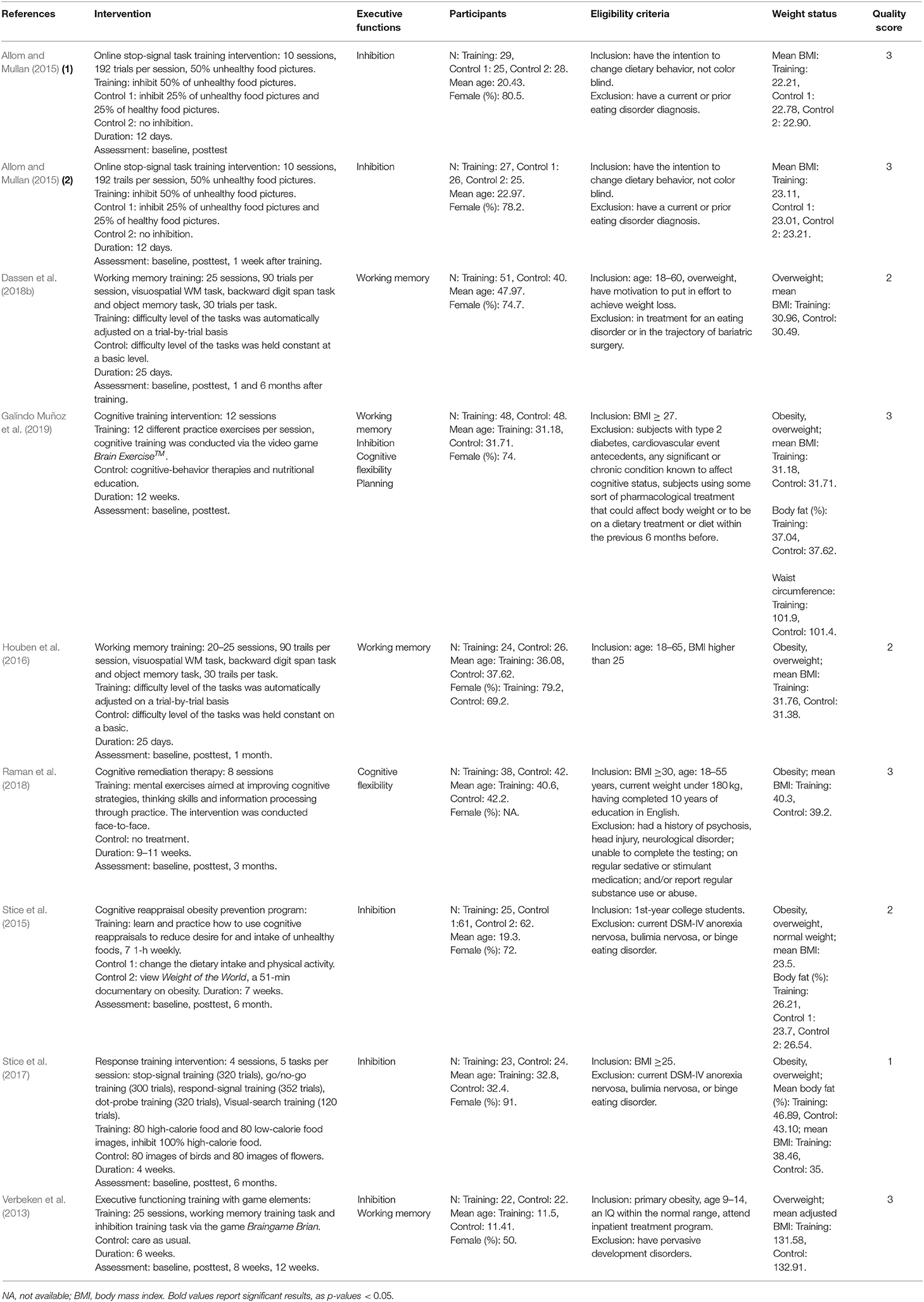
There were totally 11,393 participants included in our meta-analysis. The participants' characteristics are summarized in Table 2. Eight studies (Verbeken et al., 2013; Allom and Mullan, 2015; Stice et al., 2015, 2017; Houben et al., 2016; Dassen et al., 2018b; Raman et al., 2018; Galindo Muñoz et al., 2019) with 716 participants were found, and one study (Allom and Mullan, 2015) included two independent substudies, for a total of 9 RCTs. Seven RCTs solely focused on one specific EF, and in particular, 4 of them focused on inhibition, 2 of them focused on working memory, and 1 focused on cognitive flexibility. Four RCTs (Verbeken et al., 2013; Dassen et al., 2018b; Raman et al., 2018; Galindo Muñoz et al., 2019) employed other weight loss interventions addition to the EF intervention. Regarding quality, 5 RCTs scored 3 points, 2 RCTs scored 2 points, and only 1 RCT scored 1 point. More details about the studies are presented in Table 3.
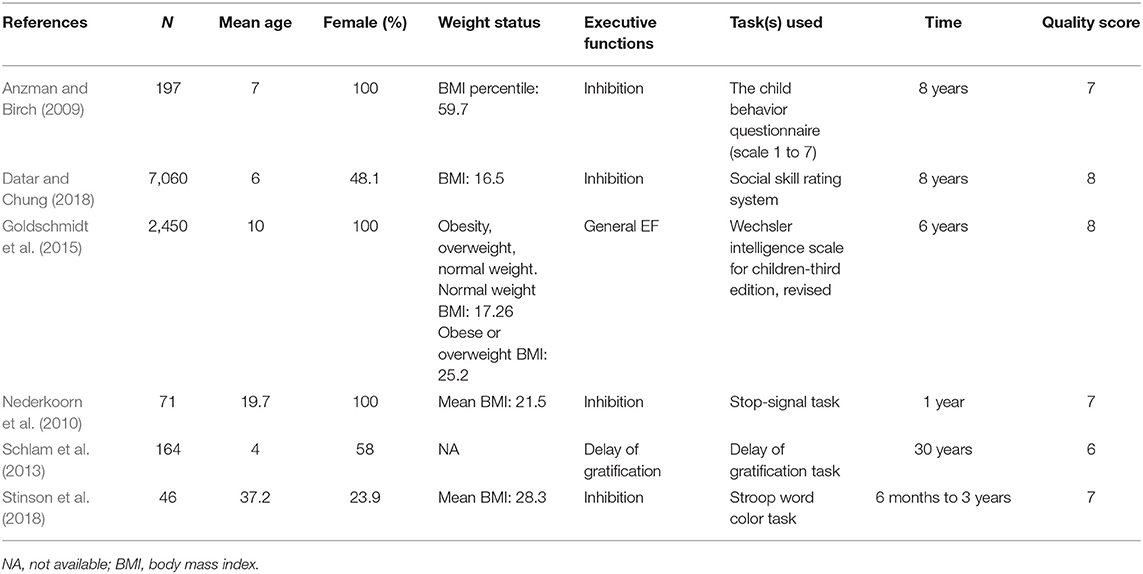
Eleven studies (Nederkoorn et al., 2007; Best et al., 2012; Spitznagel et al., 2013a,b, 2014; Kulendran et al., 2014; Galioto et al., 2016, 2018; Augustijn et al., 2018; Dassen et al., 2018a; Mackey et al., 2018) with 689 participants investigating whether baseline EFs can predict more weight loss in weight loss intervention were found. Several studies used one effect size to estimate more than one EF domain, and we classified these measures as general executive function. One study (Galioto et al., 2016) did not report non-significant results. Thus, 4 studies focused on general EF, 6 studies focused on inhibition, 4 studies focused on working memory, 5 studies focused on cognitive flexibility, 3 studies focused on delay discounting, and 2 studies focused on planning. The quality of studies was moderate to high except for one study that scored 5 points. The characteristics of the studies are summarized in Table 4.
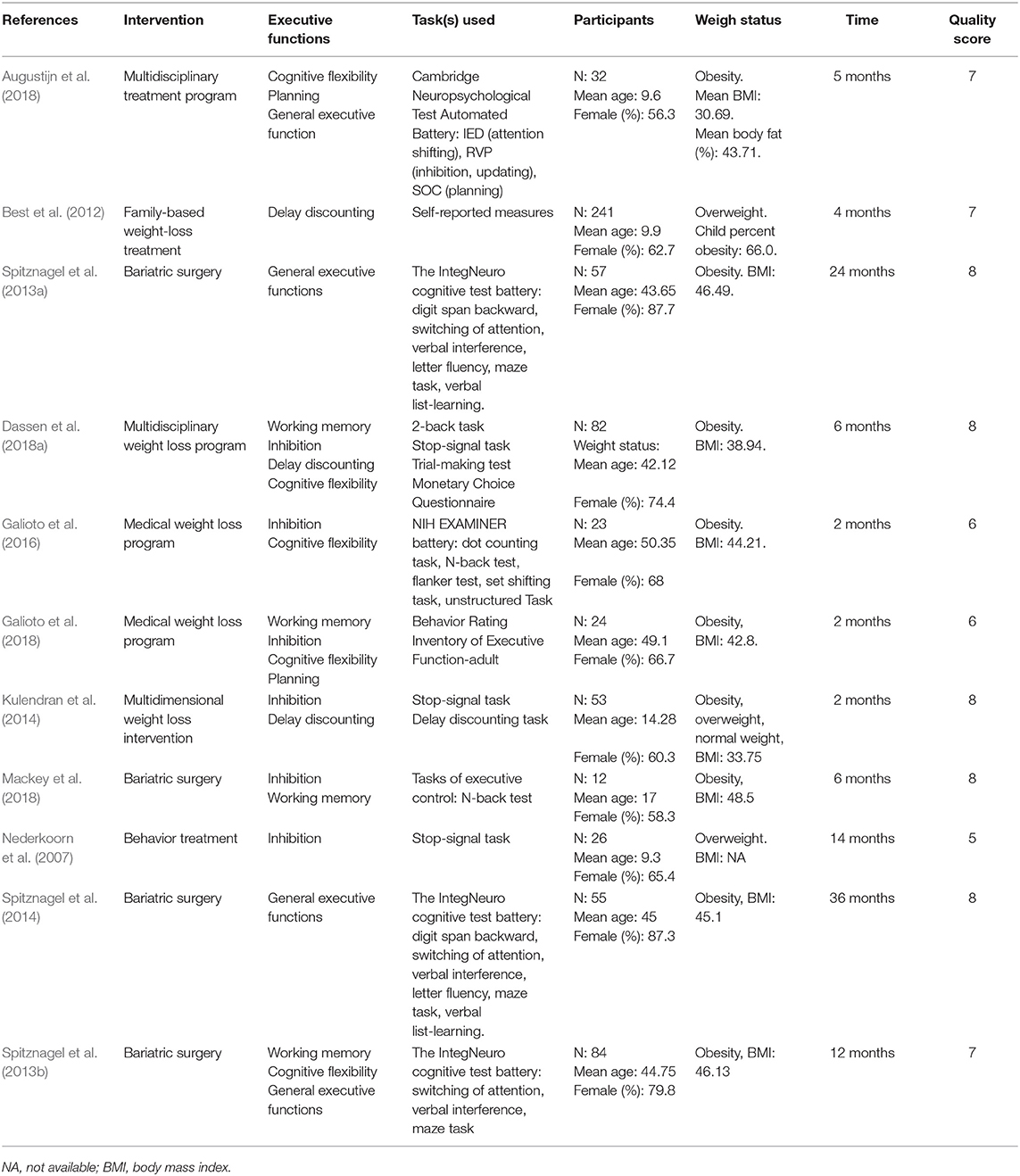
Table 4. Longitudinal studies investigating whether better baseline EFs can predict greater weight loss through intervention.
Six studies (Anzman and Birch, 2009; Nederkoorn et al., 2010; Schlam et al., 2013; Goldschmidt et al., 2015; Datar and Chung, 2018; Stinson et al., 2018) with 9988 participants investigating whether EF early in life can predict future weight loss were found. One study (Goldschmidt et al., 2015) used the Mazes test to measure planning. In fact, this test also involves other EFs, and therefore, we classified the measure as general EF. Four studies focused on inhibition, 1 study focused on general EF and 1 study focused on delay of gratification. One study (Stinson et al., 2018) did not report non-significant results. More information about the studies is shown in Table 5. The quality of the studies was moderate to high. We did not conduct a meta-analysis for general EF and delay gratification, as there was only one effect size for these two EF domains.
Meta-Analysis of RCTs
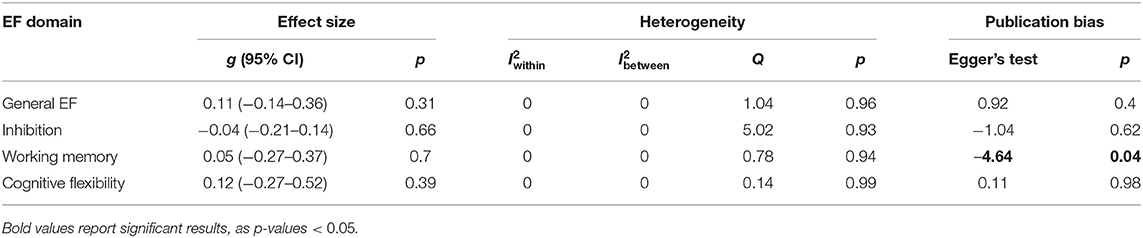
The results showed non-significant effects of general EF, inhibition, working memory, and cognitive flexibility, which indicated that the EF interventions were no more effective than the control conditions. The results are summarized in Table 6. A non-significant result of the Q-statistic for general EF, inhibition, working memory, and cognitive flexibility suggested that there existed a relatively good homogeneity among the studies. For general EF, the distribution of the variance across the three levels was as follows: level 1 was 100% (sampling variance), and level 2 (within-study variance) and level 3 (between-study variance) were 0%. For inhibition, the distribution of the variance across the three levels was as follows: level 1 was 100% (sampling variance), and level 2 (within-study variance) and level 3 (between-study variance) were 0%. The same pattern of the variance distribution was found for working memory and cognitive flexibility.
We divided studies according to participant age (young adults: age 18 to 22; adults: age above 22) and mean baseline BMI (normal weight: BMI < 25; overweight: 25 ≤ BMI ≤ 30; obesity: BMI> 30). For inhibition, subgroup analyses of BMI (normal weight and obesity) and age (young adults and adults) were conducted. A non-significant overall effect size was found for obesity (g = −0.006, p = 0.947) and normal weight (g = 0.287, p = 0.221), and there was no significant difference between these two subgroups (F = 1.703, p = 0.221). The results for age were the same as those for BMI because the studies included in the subgroup analyses were the same. We did not conduct subgroup analysis for general EF, working memory, and cognitive flexibility, as the number of studies was <3.
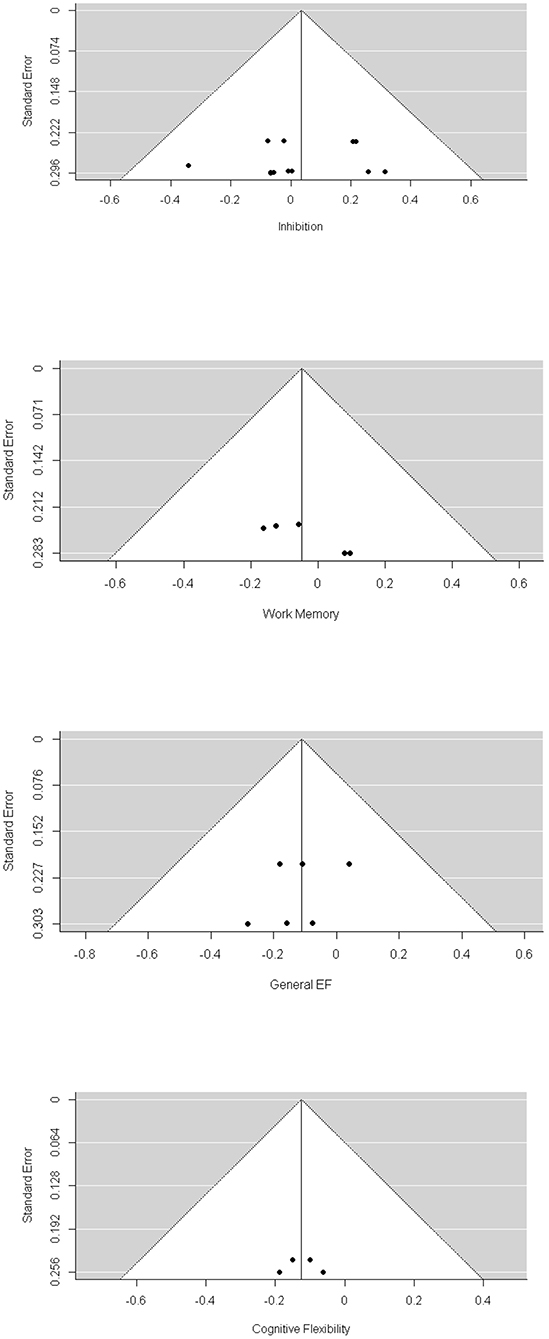
Visual inspection of the funnel plots (Figure 2) did not suggest that publication biases existed for general EF, inhibition, and cognitive flexibility. Egger's test also showed a non-significant result for general EF, inhibition, and cognitive flexibility. We found a significant publication bias for working memory (−4.635, p = 0.039). The trim-and-fill analysis indicated that there was one missing effect size for working memory, and the pooled effect size was still non-significant (g = 0.076, p = 0.47).
Meta-Analysis of Longitudinal Studies Investigating Whether Baseline EFs Can Predict Greater Weight Loss Through Intervention
The results showed that better baseline inhibition significantly predicted more weight loss through weight loss treatment (β = 0.259, p = 0.03, 95% CI: 0.027–0.492), and better baseline delay discounting significantly predicted less weight loss (β= −0.17, p = 0.04, 95% CI: −0.331 to −0.011). The results showed that general EF, working memory, cognitive flexibility, and planning were not significant predictors. The results are summarized in Table 7. In terms of heterogeneity, non-significant Q statistics were found for general EF, cognitive flexibility, and delay discounting. We found significant Q statistics for inhibition (Q = 46.068, p < 0.0001), working memory (Q = 27.928, p = 0.002), and planning (Q = 6.838, p = 0.03). For general EF, the distribution of variance was 76.71% (level 1), 0% (level 2), and 23.29% (level 3). For inhibition, the distribution of variance was 4.89% (level 1), 95.11% (level 2), and 0% (level 3). For working memory, the distribution of variance was 19.94% (level 1), 72.58% (level 2), and 7.48% (level 3). For cognitive flexibility, the distribution of variance was 100% (level 1) and 0% for level 2 and level 3. For delay discounting, the distribution of variance was 100% (level 1) and 0% for level 2 and level 3. For planning, the distribution of variance was 24.93% (level 1), 75.07% (level 2), and 0% (level 3).
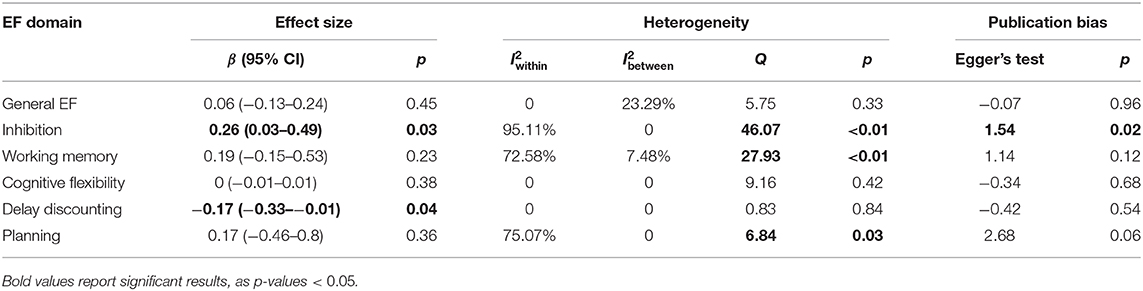
Table 7. Results from longitudinal studies investigating whether baseline EFs can predict greater weight loss through intervention.
We divided studies according to participant mean ages (children: age 7–11; adolescents age 12–17; young adults: age 18–22; adults: age above 22) and type of weight loss intervention (surgery and non-surgery). For general EF, subgroup analysis was conducted for age (children and adults) and intervention. Because the studies in subgroups in terms of age and intervention were the same, the results were also the same. We did not find moderating effects of age and intervention (F = 0.104, p = 0.76). For the children and non-surgery subgroups, the overall effect size was β = 0.09, p = 0.53; for the adults and surgery subgroups, the overall effect size was β = 0.04, p = 0.76. For inhibition, subgroup analysis was conducted for age (children, adolescents, and adults) and intervention (surgery and non-surgery). We found that age was not a moderating factor (F = 0.815, p = 0.47), and the overall effect size with children (β = 0.039, p = 0.51), adolescents (β = 0.459, p = 0.43), and adults (β = 0.241, p = 0.15) were non-significant. Intervention type (F = 2.39, p = 0.15) was also not a moderating factor. The overall effect size with non-surgery was marginally significant (β = 0.198, p = 0.07), while that with surgery was non-significant (β = 0.648, p = 0.15). For working memory, subgroup analysis was conducted for age (adolescents and adults) and intervention. We found a significant moderating effect of age (F = 13.666, p = 0.005) and significant overall effect size with both adolescents (β = 0.661, p = 0.002) and adults (β = 0.04, p = 0.005). The intervention type was not a moderating factor (F = 0.197, p = 0.67), and the overall effect sizes with surgery (β = 0.278, p = 0.67) and non-surgery (β = 0.116, p = 0.66) were not significant. We also found that the Q statistic for residual heterogeneity was not significant (Q = 12.417, p = 0.19). For cognitive flexibility, subgroup analysis was conducted for age (children and adults) and intervention. We did not find moderating effects of age (F = 0.185, p = 0.68) or intervention (F = 0.49, p = 0.5). The overall effect sizes with children (β = 0.004, p = 0.93), adults (β = −0.025, p = 0.68), surgery (β = 0.004, p = 0.37), and non-surgery (β = −0.046, p = 0.5) were not significant. There were only 3 studies focused on delay discounting; however, the mean participants' age of these studies varied from children, adolescents, and adults. Thus, we divided them into two subgroups: adults and non-adults. The result revealed a non-significant moderating effect (F = 0.42, p = 0.58). The overall effect size with non-adults was marginally significant (β = 0.186, p = 0.08), and the overall effect size with adults was non-significant (β = 0.1, p = 0.58). We did not conduct subgroup analysis for planning, as there were only two studies focused on this EF domain.
Through visual inspection of the funnel plot (Figure 3) and Egger's test, we found that general EF, working memory, cognitive flexibility, delay discounting, and planning were non-significant in terms of publication bias. However, we found a significant publication bias for inhibition (1.537, p = 0.02). The trim-and-fill analysis indicated that there were two missing effect sizes for inhibition, and the overall effect size of inhibition was still significant (β = 0.104, p = 0.03).
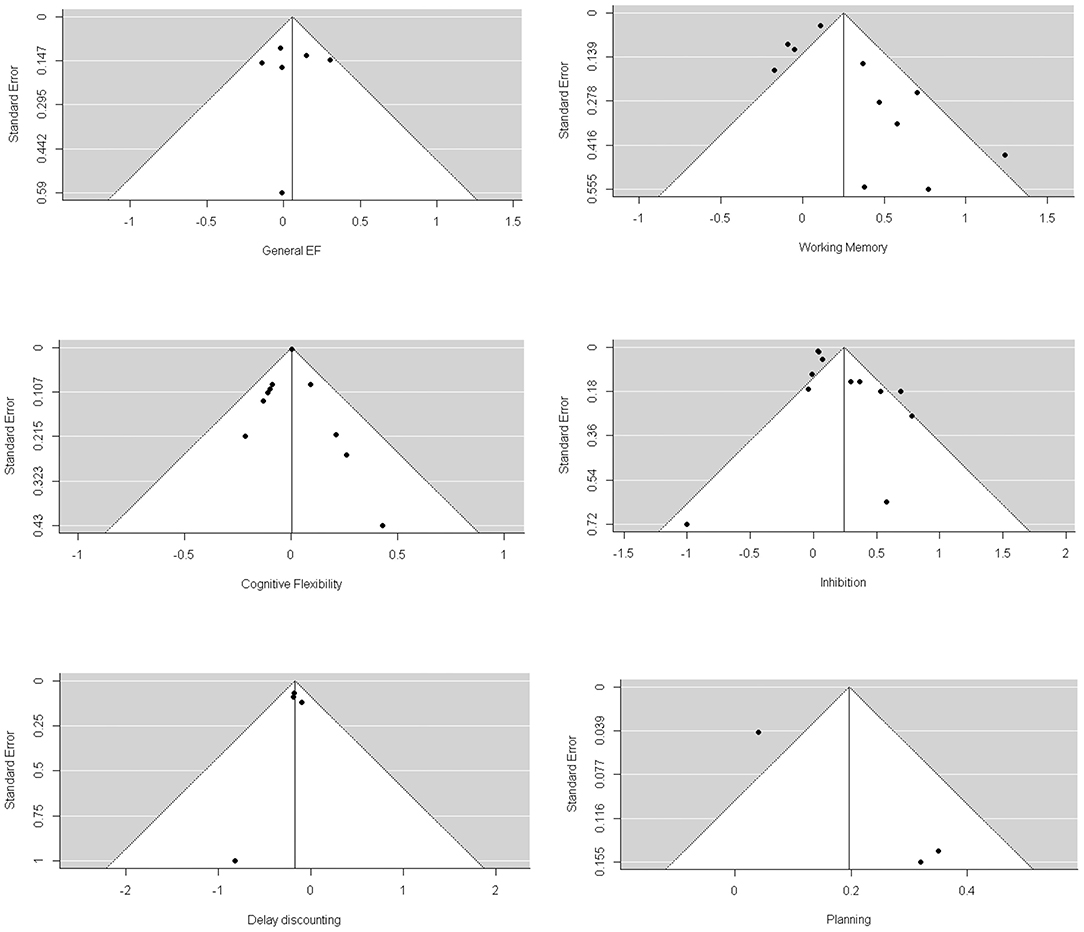
Figure 3. Funnel plots of longitudinal studies investigating whether baseline EFs can predict greater weight loss through intervention.
Meta-Analysis of Longitudinal Studies Investigating Whether Early EFs Can Predict Future Weight Loss
We performed a multilevel meta-analysis only for inhibition, as there was only one study focused on delay of gratification and one on general EF. For delay of gratification, the effect size was 0.21; for general EF, the effect size was 0.05. The overall effect of inhibition was marginally significant (β = 0.185, p = 0.07, 95% CI: −0.021–0.391). A significant Q statistic result (Q = 25.364, p = 0.0001) indicated heterogeneity between studies. The distribution of variance was 12.74% (sampling variance), 80.93% (within-study variance), and 6.33% (between-study variance).
We divided studies according to the percentage of females in the studies (100% and not 100%) and age (children: age under 7; young adults: age 18–22; adults age above 22). We did not find a significant moderating effect of age (F = 0.027, p = 0.97) or gender (F = 0.005, p = 0.95). The overall effects with children (β = 0.179, p = 0.83), young adults (β = 0.19, p = 0.88), adults (β = 0.248, p = 0.38), 100% females (β = 0.194, p = 0.15), and non-100% females (β = 0.187, p = 0.95) were not significant.
We found a significant publication bias by Egger's test (3.129, p = 0.002) and by visual inspection of the funnel plot (Figure 4). Because the result of Egger's test was significant, we conducted the trill-and-fill analysis. The trim-and-fill analysis indicated that there were 3 missing effect sizes, and the overall effect size was not significant (β = 0.066, p = 0.19).
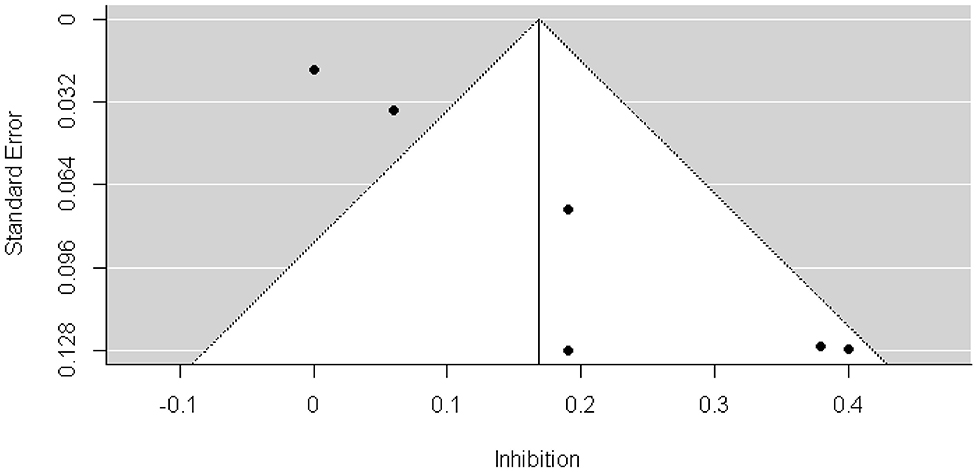
Figure 4. Funnel plots of longitudinal investigating whether early EFs can predict future weight status change.
Discussion
This is the first meta-analysis to investigate whether EF interventions have an effect on weight loss and whether EF can predict obesity intervention outcomes or future weight loss. Generally speaking, we found that (a) EF interventions have no effect on weight loss; (b) several baseline EF, such as inhibition and delay discounting, can predict obesity intervention outcomes; and (c) early-life inhibition can predict future weight loss. Our result may support the view that a higher-order factor, such as gene, may link obesity and EF together.
We found that EF interventions showed non-significant results on weight loss, even when publication bias was taken into consideration. This surprising result may suggest that EF has no direct effect on obesity or weight loss. The RCTs included in our meta-analysis mainly focused on changing participants' attitudes or behaviors toward foods by improving their EF. Researchers have reached a consensus that cognitive training has a significant effect on eat behaviors (Yang et al., 2019). Several studies included in our meta-analysis also reported that participants significantly changed their attitude and behavior toward food, while their BMI did not change (Stice et al., 2015; Dassen et al., 2018b). It is apparent that people with healthier eating behaviors have a greater possibility of weight loss. However, a non-significant result with the RCT studies seems to not support the assumption that EF can lead to obesity. This is a puzzling result. Favieri et al. (2019) argued that it is the bidirectional relationship between EF and obesity that causes the failure of the intervention. If this is true, obesity may hinder improvements in EF. However, most RCTs showed significant improvement in EF, which seems to not support his argument.
Consider that individual weight loss depends on differences in energy intake and expenditure; it is possible that participants in these RCTs reduced their energy intake as well as their energy expenditure, ultimately rendering EF interventions ineffective. As we discussed earlier, homeostasis, reward system, and EF are related to obesity. After EF training, obese individuals can resist food temptations effectively. However, they cannot alter their homeostasis system, and the homeostasis system begins to function so that the individual's energy expenditure decreases. The link between EF and obesity may be more complex than we thought.
One plausible explanation is that the relationship between EF and obesity may not be direct. The relationships between EF or eating behavior and weight status may not be as close as we think. There is no causal relationship between EF and obesity. A higher-order factor, such as gene, may link obesity and EF together, although such an assumption needs further research. Previous reviews of twins studies showed that 50–90% of BMI variance can be attributed to genes (Maes et al., 1997; Schousboe et al., 2003). Several genes were found to be the common genetic backgrounds of BMI and EF (Bressler et al., 2013; Zhao et al., 2013; Marioni et al., 2016). This hypothesis of the relationship between EF and obesity increases our understanding of the mechanisms of obesity. Since obesity and EF have a common genetic background, obesity could not lead to poor EF, and poor EF could not result in obesity, it is likely that they are accompanying phenomena. An individual's obesity and poor EF may occur together. Poor EF and obesity may interact in a variety of ways, creating a vicious cycle (Tomiyama, 2019).
Another explanation of such a result is that the indicator of weight status is not suitable. All the included studies used BMI as the indicator, and only a few studies employed other indicators, such as body fat and waist circumference, in addition to BMI. As many researchers have argued, BMI cannot distinguish the centralized distribution of visceral fat or central obesity and may not explain obesity-related health risk (Micozzi and Albanes, 1987; Janssen et al., 2004). Notably, a non-significant result does not mean that there was no effect, especially when the number of studies included in the meta-analysis was too small.
We found that baseline inhibition and delay discounting were significant predictors of weight loss through intervention, which was in agreement with other empirical studies (Weller et al., 2008; Epstein et al., 2010). This result could also be interpreted by the assumption that the relationship between EF and obesity is not direct. People with better EF may be indicative of a genetic background that allows them to lose more weight. The result of delay discounting is consistent with the result that the ability to delay gratification at age 4 can predict lower BMI 30 years later (Schlam et al., 2013), as delay discounting is reverse of delay of gratification (Anokhin et al., 2011). Despite the significant predictive effect of delay discounting, obesity intervention through reductions in delay discounting, which may be a promising treatment, has not yet been proposed.
Accordingly, we found that early-life inhibition significantly predicted future weight loss. There was significant heterogeneity between studies. When taking publication bias into consideration, we obtained a non-significant result. However, this approach may not be suitable for testing publication bias because one assumption of publication tests is homogeneity of the data. If this assumption is violated, publication bias tests cannot distinguish heterogeneity and publication bias and might lead to uninterpretable results (Ioannidis, 2005). The significant predictive effect of inhibition and delay discounting on weight loss through both obesity interventions and natural conditions indicates that such a relationship is relatively stable. Therefore, EF is a good predictor of weight loss and obesity, and EF training in early life may be helpful to prevent obesity. Limited by the number of studies, we did not investigate whether other types of EFs can predict future weight loss.
We found that although baseline working memory was not a significant predictor of weight loss through intervention, the subgroup analysis showed that age played a moderating role, and the results of the two subgroups (adolescents and adults) were significant which indicated that baseline working memory did have an effect on obesity intervention outcomes. Except for age, we did not find any other moderating factors. A non-significant moderating effect of age in other EF domains may be attributed to the different developmental trajectories of EFs. However, there exists a conflict regarding age as a moderator in other meta-analyses investigating the relationship between EF and obesity. Yang et al. (2018) found that age was not a moderator of the relationship between obesity and EF, while Wu et al. (2015) showed that age was a moderator. One explanation of these conflicting data is, as Yang et al. (2018) argued, due to the different analytic strategies. This may also be due to the type of study. In our study, we wanted to investigate whether EF can influence obesity, while Yang et al. (2018) and Wu et al. (2015) investigated whether there were differences in EF performances between obese and normal-weight individuals. Empirical studies have also reported these conflicting results. Gunstad et al. (2007) found that the relationship between obesity and executive dysfunction was not associated with age. In contrast, the brain activation measured by functional near-infrared spectroscopy technology (fNIRS) due to Stroop interference did not increase with age in obese subjects (Huang et al., 2019), while activation in normal-weight individuals continued to increase (Adleman et al., 2002). The role of age needs further study.
Limitations and Future Directions
There are several limitations in the current study. First, as the number of studies included in the meta-analysis was small, the results we obtained may be biased. Thus, more RCTs investigating whether EF can impact obesity and more longitudinal studies investigating whether EF can predict weight loss through obesity intervention or in natural conditions are needed. Second, we did not investigate the role of the weight status indicator. As mentioned above, BMI may not be the appropriate indicator; it is possible that using different obesity indicators for the same population could lead different results. In fact, one RCT included in our meta-analysis found that after EF intervention, participants showed a significant body fat reduction while their BMI was not changed (Stice et al., 2017). To avoid the bias caused by obesity indicators, multiple indicators should be used in future studies. Third, although we investigated the role of gender, this was based on the proportion of females. We know that there are differences between males and females in obesity (Schousboe et al., 2003). The prevalence of obesity is different in men and women (Flegal et al., 2012). Moreover, obese men and women are different in brain activation (Mueller et al., 2011) and brain functional connection (Atalayer et al., 2014) when dealing with food cues. Gender plays an important role in obesity, and the role of gender in the relationship between obesity and EF needs to be further explored. Finally, the tools used to measure EF may not have been adequate. The EF tasks we used may not be pure. Although it has been stated that the tasks measure a specific EF, it is difficult to rule out the possibility that there are no other EFs involves in the tasks. In addition, the relationship between EF and frontal lobe activity may not be one-to-one (Alvarez and Emory, 2006). People with frontal lobe lesions may perform as well as normal individuals in a particular EF task (Ahola et al., 1996), and healthy people may perform as poor as individuals with frontal lobe lesions (Axelrod et al., 1996). With the development of neuroimaging technology, an increasing number of studies have used brain imaging data as indicator of EF (Kishinevsky et al., 2012; Hege et al., 2013), which is a promising area.
Conclusion
This is a meta-analysis to investigate whether EFs have an impact on obesity and weight loss. Specifically, we investigate whether EF interventions have an effect on obesity and weight loss and whether baseline EF can predict future weight loss through obesity interventions and in natural conditions. We also examined some potential moderating factors, such as age, weight status, type of intervention, and percentage of female participants. First, the results showed that EF interventions may not have an effect on weight loss, which indicates that the relationship between EF and obesity may not be direct. A higher-order factor such as genes may link obesity and EF. However, as the number of studies included in the meta-analysis was small, the results we obtained may be biased, and more interventional studies are needed. Second, we found that baseline inhibition and delay discounting can significantly predict weight loss through obesity interventions and natural conditions, which shows that EF is a good predictor for weight loss and obesity. Therefore, EF training in early life may be an effective way to prevent obesity. Since EF can predict the outcome of obesity interventions, we may be able to provide more personalized treatment based on subjects' baseline EF. Despite the significant predictive effect of delay discounting, obesity intervention through reductions in delay discounting, which may be a promising treatment, has not yet been proposed. Finally, age moderates the relationship between working memory and weight loss through intervention, but not weight status, type of intervention, and percentage of female. Although we investigated several potential moderators, we cannot draw a strong conclusion based on the existing results. The roles of age, gender, and obesity indicators remain unclear and need to be further explored. In addition, neuroimaging technology may overcome the shortcomings of behavioral researches, which is a promising area.
Data Availability Statement
The original contributions presented in the study are included in the article/supplementary material, further inquiries can be directed to the corresponding author/s.
Author Contributions
ZD conceived and designed the study, collected and interpreted the data, and wrote the first draft of the manuscript. JL reviewed the manuscript. JH modified the manuscript. XiaoX and JM collected and interpreted the data. RZ interpreted the data. XiaX conceived, designed the study and modified, and reviewed the manuscript. All authors critically revised the manuscript and approved the final version.
Funding
This research was supported by the Science Fund for Hubei Superior Discipline Groups of Physical Education and Health Promotion, National Natural Science Foundation of China (Grant No. 81971661), and Natural Science Foundation of Hubei Province (Grant No. 2016CFA098).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
The authors thank Scientific Sports and Health Promotion Hubei Collaborative Innovation Center and all the participants.
References
Adleman, N. E., Menon, V., Blasey, C. M., White, C. D., Warsofsky, I. S., Glover, G. H., et al. (2002). A developmental fMRI study of the stroop color-word task. Neuroimage 16, 61–75. doi: 10.1006/nimg.2001.1046
Ahola, K., Vilkki, J., and Servo, A. (1996). Frontal tests do not detect frontal infarctions after ruptured intracranial aneurysm. Brain Cogn. 31, 1–16. doi: 10.1006/brcg.1996.0021
Allom, V., and Mullan, B. (2015). Two inhibitory control training interventions designed to improve eating behaviour and determine mechanisms of change. Appetite 89, 282–290. doi: 10.1016/j.appet.2015.02.022
Alvarez, J. A., and Emory, E. (2006). Executive function and the frontal lobes: a meta-analytic review. Neuropsychol. Rev. 16, 17–42. doi: 10.1007/s11065-006-9002-x
Anokhin, A. P., Golosheykin, S., Grant, J. D., and Heath, A. C. (2011). Heritability of delay discounting in adolescence: a longitudinal twin study. Behav. Genet. 41, 175–183. doi: 10.1007/s10519-010-9384-7
Anzman, S. L., and Birch, L. L. (2009). Low inhibitory control and restrictive feeding practices predict weight outcomes. J. Pediatr. 155, 651–656. doi: 10.1016/j.jpeds.2009.04.052
Appelhans, B. M., French, S. A., Pagoto, S. L., and Sherwood, N. E. (2016). Managing temptation in obesity treatment: a neurobehavioral model of intervention strategies. Appetite 96, 268–279. doi: 10.1016/j.appet.2015.09.035
Armitage, C. J. (2004). Evidence that implementation intentions reduce dietary fat intake: a randomized trial. Health Psychol. 23:319. doi: 10.1037/0278-6133.23.3.319
Aron, A. R., Robbins, T. W., and Poldrack, R. A. (2014). Inhibition and the right inferior frontal cortex: one decade on. Trends Cogn Sci. 18, 177–185. doi: 10.1016/j.tics.2013.12.003
Assink, M., and Wibbelink, C. J. (2016). Fitting three-level meta-analytic models in R: a step-by-step tutorial. Quant. Methods Psychol. 12, 154–174. doi: 10.20982/tqmp.12.3.p154
Atalayer, D., Pantazatos, S. P., Gibson, C. D., McOuatt, H., Puma, L., Astbury, N. M., et al. (2014). Sexually dimorphic functional connectivity in response to high vs. low energy-dense food cues in obese humans: an fMRI study. Neuroimage 100, 405–413. doi: 10.1016/j.neuroimage.2014.05.054
Atance, C. M., and O'Neill, D. K. (2001). Episodic future thinking. Trends Cogn. Sci. 5, 533–539. doi: 10.1016/S1364-6613(00)01804-0
Augustijn, M. J. C. M., D'Hondt, E., Van Acker, L., De Guchtenaere, A., Lenoir, M., Caeyenberghs, K., et al. (2018). Role of motor competence and executive functioning in weight loss: a study in children with obesity. J. Dev. Behav. Pediatr. 39, 642–651. doi: 10.1097/DBP.0000000000000589
Axelrod, B. N., Goldman, R. S., Heaton, R. K., Curtiss, G., Thompson, L. L., Chelune, G. J., et al. (1996). Discriminability of the Wisconsin card sortingtest using the standardization sample. J. Clin. Exp. Neuropsychol. 18, 338–342. doi: 10.1080/01688639608408991
Banich, M. T. (2009). Executive function: the search for an integrated account. Curr. Dir. Psychol. 18, 89–94. doi: 10.1111/j.1467-8721.2009.01615.x
Berkman, E. T., Kahn, L. E., and Merchant, J. S. (2014). Training-induced changes in inhibitory control network activity. J. Neurosci. 34, 149–157. doi: 10.1523/JNEUROSCI.3564-13.2014
Berridge, K. C. (1996). Food reward: brain substrates of wanting and liking. Neurosci. Biobehav. Rev. 20, 1–25. doi: 10.1016/0149-7634(95)00033-B
Berridge, K. C., and Robinson, T. E. (2003). Parsing reward. Trends Neurosci. 26, 507–513. doi: 10.1016/S0166-2236(03)00233-9
Best, J. R., and Miller, P. H. (2010). A developmental perspective on executive function. Child Dev. 81, 1641–1660. doi: 10.1111/j.1467-8624.2010.01499.x
Best, J. R., Theim, K. R., Gredysa, D. M., Stein, R. I., Welch, R. R., Saelens, B. E., et al. (2012). Behavioral economic predictors of overweight childr. J. Consult. Clin. Psychol. 80, 1086–1096. doi: 10.1037/a0029827
Beth Spitznagel, M., Galioto, R., Limbach, K., John Gunstad, P. D., and Leslie Heinberg, P. D. (2013). Cognitive function is linked to adherence to bariatric postoperative guidelines. Surg. Obes. Relat. Dis. 9, 580–585. doi: 10.1016/j.soard.2013.04.007
Bowman, N. A. (2012). Effect sizes and statistical methods for meta-analysis in higher education. Res. High. Educ. 53, 375–382. doi: 10.1007/s11162-011-9232-5
Bressler, J., Fornage, M., Demerath, E. W., Knopman, D. S., Monda, K. L., North, K. E., et al. (2013). Fat mass and obesity gene and cognitive decline. Neurology 80:92. doi: 10.1212/WNL.0b013e3182768910
Butryn, M. L., Martinelli, M. K., Remmert, J. E., Roberts, S. R., Zhang, F., Forman, E. M., et al. (2019). Executive functioning as a predictor of weight loss and physical activity outcomes. Ann. Behav. Med. 53, 909–917. doi: 10.1093/abm/kaz001
Calle, E. E., and Kaaks, R. (2004). Overweight, obesity and cancer: epidemiological evidence and proposed mechanisms. Nat. Rev. Cancer 4, 579–591. doi: 10.1038/nrc1408
Clark, H. D., Wells, G. A., Huët, C., McAlister, F. A., Salmi, L. R., Fergusson, D., et al. (1999). Assessing the quality of randomized trials: reliability of the Jadad scale. Controlled Clin. Trials 20, 448–452. doi: 10.1016/S0197-2456(99)00026-4
Constantinidis, C., and Procyk, E. (2004). The primate working memory networks. Cogn. Affect. Behav. Neurosci. 4, 444–465. doi: 10.3758/CABN.4.4.444
Crandall, C. S. (1995). Do parents discriminate against their heavyweight daughters? Pers. Soc. Psychol. Bull. 21, 724–735. doi: 10.1177/0146167295217007
Dassen, F. C. M., Houben, K., Allom, V., and Jansen, A. (2018a). Self-regulation and obesity: the role of executive function and delay discounting in the prediction of weight loss. J. Behav. Med. 41, 806–818. doi: 10.1007/s10865-018-9940-9
Dassen, F. C. M., Houben, K., Van Breukelen, G. J. P., and Jansen, A. (2018b). Gamified working memory training in overweight individuals reduces food intake but not body weight. Appetite 124, 89–98. doi: 10.1016/j.appet.2017.05.009
Datar, A., and Chung, P. J. (2018). Childhood self-control and adolescent obesity: evidence from longitudinal data on a national cohort. Child Obes. 14, 238–247. doi: 10.1089/chi.2017.0217
Diamond, A. (2013). Executive functions. Annu. Rev. Psychol 64, 135–168. doi: 10.1146/annurev-psych-113011-143750
Duval, S., and Tweedie, R. (2000). Trim and fill: a simple funnel-plot–based method of testing and adjusting for publication bias in meta-analysis. Biometrics 56, 455–463. doi: 10.1111/j.0006-341X.2000.00455.x
Egger, M., Smith, G. D., Schneider, M., and Minder, C. (1997). Bias in meta-analysis detected by a simple, graphical test. BMJ 315, 629–634. doi: 10.1136/bmj.315.7109.629
Epstein, L. H., Salvy, S. J., Carr, K. A., Dearing, K. K., and Bickel, W. K. (2010). Food reinforcement, delay discounting and obesity. Physiol. Behav. 100, 438–445. doi: 10.1016/j.physbeh.2010.04.029
Favieri, F., Forte, G., and Casagrande, M. (2019). The executive functions in overweight and obesity: a systematic review of neuropsychological cross-sectional and longitudinal studies. Front. Psychol. 10:2126. doi: 10.3389/fpsyg.2019.02126
Flegal, K. M., Carroll, M. D., Kit, B. K., and Ogden, C. L. (2012). Prevalence of obesity and trends in the distribution of body mass index among US adults, 1999-2010. JAMA 307, 491–497. doi: 10.1001/jama.2012.39
Forcano, L., Mata, F., de la Torre, R., and Verdejo-Garcia, A. (2018). Cognitive and neuromodulation strategies for unhealthy eating and obesity: Systematic review and discussion of neurocognitive mechanisms. Neurosci. Biobehav. Rev. 87, 161–191. doi: 10.1016/j.neubiorev.2018.02.003
Galindo Muñoz, J. S., Morillas-Ruiz, J. M., Gómez Gallego, M., Díaz Soler, I., Barberá Ortega, M. D. C., Martínez, C. M., et al. (2019). Cognitive training therapy improves the effect of hypocaloric treatment on subjects with overweight/obesity: a randomised clinical trial. Nutrients 11:925. doi: 10.3390/nu11040925
Galioto, R., Bond, D., Gunstad, J., Pera, V., Rathier, L., and Tremont, G. (2016). Executive functions predict weight loss in a medically supervised weight loss programme. Obes. Sci. Prac. 2, 334–340. doi: 10.1002/osp4.70
Galioto, R., Britton, K., Bond, D. S., Gunstad, J., Pera, V., Rathier, L., et al. (2018). Executive functions are associated with weight loss during participation in a medically supervised weight loss program. Obes. Med. 9, 18–20. doi: 10.1016/j.obmed.2017.12.002
Gariepy, G., Nitka, D., and Schmitz, N. (2010). The association between obesity and anxiety disorders in the population: a systematic review and meta-analysis. Int. J. Obes. 34, 407–419. doi: 10.1038/ijo.2009.252
Goldschmidt, A. B., Hipwell, A. E., Stepp, S. D., McTigue, K. M., and Keenan, K. (2015). Weight gain, executive functioning, and eating behaviors among girls. Pediatrics 136:e856. doi: 10.1542/peds.2015-0622
Gunstad, J., Paul, R. H., Cohen, R. A., Tate, D. F., Spitznagel, M. B., and Gordon, E. (2007). Elevated body mass index is associated with executive dysfunction in otherwise healthy adults. Compr. Psychiat. 48, 57–61. doi: 10.1016/j.comppsych.2006.05.001
Hayes, J. F., Eichen, D. M., Barch, D. M., and Wilfley, D. E. (2018). Executive function in childhood obesity: promising intervention strategies to optimize treatment outcomes. Appetite 124, 10–23. doi: 10.1016/j.appet.2017.05.040
Hege, M. A., Stingl, K. T., Ketterer, C., Häring, H. U., Heni, M., Fritsche, A., et al. (2013). Working memory-related brain activity is associated with outcome of lifestyle intervention. Obesity 21, 2488–2494. doi: 10.1002/oby.20442
Higgins, J. P., and Green, S. (2011). Cochrane Handbook for Systematic Reviews of Interventions. Chichester: John Wiley & Sons.
Hofmann, W., Friese, M., and Strack, F. (2009). Impulse and self-control from a dual-systems perspective. Perspect. Psychol. Sci. 4, 162–176. doi: 10.1111/j.1745-6924.2009.01116.x
Hogenkamp, P. S., Sundbom, M., Nilsson, V. C., Benedict, C., and Schiöth, H. B. (2015). Patients lacking sustainable long-term weight loss after gastric bypass surgery show signs of decreased inhibitory control of prepotent responses. PLoS ONE 10:e0119896. doi: 10.1371/journal.pone.0119896
Houben, K., Dassen, F. C. M., and Jansen, A. (2016). Taking control: Working memory training in overweight individuals increases self-regulation of food intake. Appetite 105, 567–574. doi: 10.1016/j.appet.2016.06.029
Huang, J., Xiong, M., Xiao, X., Xu, X., and Hong, X. (2019). fNIRS correlates of the development of inhibitory control in young obese subjects. J. Integr. Neurosci. 18, 253–259. doi: 10.31083/j.jin.2019.03.183
Ioannidis, J. (2005). “Differentiating biases from genuine heterogeneity: Distinguishing artifactual from substantive effects,” in Publication Bias in Meta-Analysis: Prevention, Assessment and Adjustments, eds H. R. Rothstein, A. J. Sutton, and M. Borenstein (New York, NY: John Wiley & Sons, Ltd.), 287–302. doi: 10.1002/0470870168.ch15
Jadad, A. R., Moore, R. A., Carroll, D., Jenkinson, C., Reynolds, D. J. M., Gavaghan, D. J., et al. (1996). Assessing the quality of reports of randomized clinical trials: is blinding necessary? Contr. Clin. Trials 17, 1–12. doi: 10.1016/0197-2456(95)00134-4
Janssen, I., Katzmarzyk, P. T., and Ross, R. (2004). Waist circumference and not body mass index explains obesity-related health risk. Amer. J. Clin. Nutr. 79, 379–384. doi: 10.1093/ajcn/79.3.379
Jones, A., Hardman, C. A., Lawrence, N., and Field, M. (2018). Cognitive training as a potential treatment for overweight and obesity: a critical review of the evidence. Appetite 124, 50–67. doi: 10.1016/j.appet.2017.05.032
Karmali, S., Brar, B., Shi, X., Sharma, A. M., de Gara, C., and Birch, D. W. (2013). Weight recidivism post-bariatric surgery: a systematic review. Obes. Surg. 23, 1922–1933. doi: 10.1007/s11695-013-1070-4
Kemps, E., Tiggemann, M., Orr, J., and Grear, J. (2014). Attentional retraining can reduce chocolate consumption. J. Exp. Psychol. Appl. 20:94. doi: 10.1037/xap0000005
Kim, R. S. (2011). Standardized Regression Coefficients as Indices of Effect Sizes in Meta-Analysis. PhD diss. Florida State University, Tallahassee, FL, United States.
Kishinevsky, F. I., Cox, J. E., Murdaugh, D. L., Stoeckel, L. E., Cook, E. W., and Weller, R. E. (2012). fMRI reactivity on a delay discounting task predicts weight gain in obese women. Appetite 58, 582–592. doi: 10.1016/j.appet.2011.11.029
Kulendran, M., Vlaev, I., Sugden, C., King, D., Ashrafian, H., Gately, P., et al. (2014). Neuropsychological assessment as a predictor of weight loss in obese adolescents. Int. J. Obes. 38, 507–512. doi: 10.1038/ijo.2013.198
Leber, A. B., Turk-Browne, N. B., and Chun, M. M. (2008). Neural predictors of moment-to-moment fluctuations in cognitive flexibility. Proc. Natl. Acad. Sci. U.S.A. 105, 13592–13597. doi: 10.1073/pnas.0805423105
Liberati, A., Altman, D. G., Tetzlaff, J., Mulrow, C., Gøtzsche, P. C., Ioannidis, J. P. A., et al. (2009). The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: explanation and elaboration. J. Clin. Epidemiol. 62, e1–e34. doi: 10.1016/j.jclinepi.2009.06.006
Luppino, F. S., de Wit, L. M., Bouvy, P. F., Stijnen, T., Cuijpers, P., Penninx, B. W. J. H., et al. (2010). Overweight, obesity, and depression: A systematic review and meta-analysis of longitudinal studies. Arch. Gen. Psychiatry 67, 220–229. doi: 10.1001/archgenpsychiatry.2010.2
Mackey, E. R., Jacobs, M., Nadler, E. P., Olson, A., Pearce, A., Cherry, J. B. C., et al. (2018). Cognitive performance as predictor and outcome of adolescent bariatric surgery: a nonrandomized pilot study. J. Pediatr. Psychol. 43, 916–927. doi: 10.1093/jpepsy/jsy028
Maes, H. H. M., Neale, M. C., and Eaves, L. J. (1997). Genetic andenvironmental factors in relative body weight and human adiposity. Behav. Genet. 27, 325–351. doi: 10.1023/A:1025635913927
Maleckas, A., Gudaityte, R., Petereit, R., Venclauskas, L., and Veličkiene, D. (2016). Weight regain after gastric bypass: etiology and treatment options. Gland Surg. 5, 617–624. doi: 10.21037/gs.2016.12.02
Mamrot, P., and Hanc, T. (2019). The association of the executive functions with overweight and obesity indicators in children and adolescents: a literature review. Neurosci. Biobehav. Rev. 107, 59–68. doi: 10.1016/j.neubiorev.2019.08.021
Manuel, A. L., Bernasconi, F., and Spierer, L. (2013). Plastic modifications within inhibitory control networks induced by practicing a stop-signal task: an electrical neuroimaging study. Cortex 49, 1141–1147. doi: 10.1016/j.cortex.2012.12.009
Marioni, R. E., Yang, J., Dykiert, D., Mõttus, R., Campbell, A., Ibrahim-Verbaas, C. A., et al. (2016). Assessing the genetic overlap between BMI and cognitive function. Mol. Psychiatr. 21, 1477–1482. doi: 10.1038/mp.2015.205
Micozzi, M., and Albanes, D. (1987). Three limitations of the body mass index. Amer. J. clin. Nutri. 46, 376–377. doi: 10.1093/ajcn/46.2.376
Moher, D., Liberati, A., Tetzlaff, J., Altman, D. G., and The, P. G. (2009). Preferred reporting items for systematic reviews and meta-analyses: the PRISMA Statement. PLoS Med. 6:e1000097. doi: 10.1371/journal.pmed.1000097
Moriguchi, Y., and Hiraki, K. (2013). Prefrontal cortex and executive function in young children: a review of NIRS studies. Front. Hum. Neurosci. 7:867. doi: 10.3389/fnhum.2013.00867
Mueller, K., Anwander, A., Möller, H. E., Horstmann, A., Lepsien, J., Busse, F., et al. (2011). Sex-dependent influences of obesity on cerebral white matter investigated by diffusion-tensor imaging. PLoS ONE 6:e18544. doi: 10.1371/journal.pone.0018544
Nakamura, K., Fuster, J. J., and Walsh, K. (2014). Adipokines: A link between obesity and cardiovascular disease. J. Cardiol. 63, 250–259. doi: 10.1016/j.jjcc.2013.11.006
Narayanaswami, V., and Dwoskin, L. P. (2017). Obesity: current and potential pharmacotherapeutics and targets. Pharmacol. Ther. 170, 116–147. doi: 10.1016/j.pharmthera.2016.10.015
Nederkoorn, C., Houben, K., Hofmann, W., Roefs, A., and Jansen, A. (2010). Control yourself or just eat what you like? Weight gain over a year is predicted by an interactive effect of response inhibition and implicit preference for snack foods. Health Psychol. 29, 389–393. doi: 10.1037/a0019921
Nederkoorn, C., Jansen, E., Mulkens, S., and Jansen, A. (2007). Impulsivity predicts treatment outcome in obese children. Behav. Res. Ther. 45, 1071–1075. doi: 10.1016/j.brat.2006.05.009
O'Connor, T. G. (2002). Handbook of developmental cognitive neuroscience. Attachment Hum Dev. 4, 388–389. doi: 10.1080/146167302762042865
Pasternak, T., and Greenlee, M. W. (2005). Working memory in primate sensorysystems. Nat. Rev. Neurosci. 6, 97–107. doi: 10.1038/nrn1603
Pieper, J. R., and Laugero, K. D. (2013). Preschool children with lower executive function may be more vulnerable to emotional-based eating in the absence of hunger. Appetite 62, 103–109. doi: 10.1016/j.appet.2012.11.020
Prencipe, A., Kesek, A., Cohen, J., Lamm, C., Lewis, M. D., and Zelazo, P. D. (2011). Development of hot and cool executive function during the transition to adolescence. J. Exp. Child Psychol. 108, 621–637. doi: 10.1016/j.jecp.2010.09.008
Puzziferri, N., Roshek, T. B., Mayo, H. G., Gallagher, R., Belle, S. H., and Livingston, E. H. (2014). Long-term follow-up after bariatric surgery: a systematic review. JAMA 312, 934–942. doi: 10.1001/jama.2014.10706
Raman, J., Hay, P., Tchanturia, K., and Smith, E. (2018). A randomised controlled trial of manualized cognitive remediation therapy in adult obesity. Appetite 123, 269–279. doi: 10.1016/j.appet.2017.12.023
Ricci-Cabello, I., Ruiz-Pérez, I., Olry de Labry-Lima, A., and Márquez-Calderón, S. (2010). Do social inequalities exist in terms of the prevention, diagnosis, treatment, control and monitoring of diabetes? A systematic review. Health Soc. Care Commun. 18, 572–587. doi: 10.1111/j.1365-2524.2010.00960.x
Riggs, N., Chou, C.-P., Spruijt-Metz, D., and Pentz, M. A. (2010). Executive cognitive function as a correlate and predictor of child food intake and physical activity. Child Neuropsychol. 16, 279–292. doi: 10.1080/09297041003601488
Riley, M. R., and Christos, C. (2015). Role of prefrontal persistent activity in working memory. Front. Syst. Neuro. 9:181. doi: 10.3389/fnsys.2015.00181
Rosenthal, R. (1995). Writing meta-analytic reviews. Psychol. Bull, 118, 183–192. doi: 10.1037/0033-2909.118.2.183
Scales, C. D., and Dahm, P. (2008). The critical use of population-based medical databases for prostate cancer research. Curr. Opin. Urol. 18, 320–325. doi: 10.1097/MOU.0b013e3282fb781b
Schlam, T. R., Wilson, N. L., Shoda, Y., Mischel, W., and Ayduk, O. (2013). Preschoolers' delay of gratification predicts their body mass 30 years later. J. Pediatr. 162, 90–93. doi: 10.1016/j.jpeds.2012.06.049
Schonberg, T., Bakkour, A., Hover, A. M., Mumford, J. A., Nagar, L., Perez, J., et al. (2014). Changing value through cued approach: an automatic mechanism of behavior change. Nat. Neurosci. 17, 625–630. doi: 10.1038/nn.3673
Schousboe, K., Willemsen, G., Kyvik, K. O., Mortensen, J., Boomsma, D. I., Cornes, B. K., et al. (2003). Sex differences in heritability of BMI: a comparative study of results from twin studies in eight countries. Twin Res. 6, 409–421. doi: 10.1375/twin.6.5.409
Schwartz, M. W., and Porte, D. (2005). Diabetes, obesity, and the brain. Science 307:375. doi: 10.1126/science.1104344
Spiegelman, B. M., and Flier, J. S. (2001). Obesity and the regulation of energy balance. Cell 104, 531–543. doi: 10.1016/S0092-8674(01)00240-9
Spitznagel, M. B., Alosco, M., Galioto, R., Strain, G., Devlin, M., Sysko, R., et al. (2014). The role of cognitive function in postoperative weight loss outcomes: 36-month follow-up. Obes. Surg. 24, 1078–1084. doi: 10.1007/s11695-014-1205-2
Spitznagel, M. B., Alosco, M., Strain, G., Devlin, M., Cohen, R., Paul, R., et al. (2013a). Cognitive function predicts 24-month weight loss success after bariatric surgery. Surg. Obes. Relat. Dis. 9, 765–770. doi: 10.1016/j.soard.2013.04.011
Spitznagel, M. B., Garcia, S., Miller, L. A., Strain, G., Devlin, M., Wing, R., et al. (2013b). Cognitive function predicts weight loss after bariatric surgery. Surg. Obes. Relat. Dis 9, 453–459. doi: 10.1016/j.soard.2011.10.008
Stice, E., Yokum, S., Burger, K., Rohde, P., Shaw, H., and Gau, J. M. (2015). A pilot randomized trial of a cognitive reappraisal obesity prevention program. Physiol. Behav. 138, 124–132. doi: 10.1016/j.physbeh.2014.10.022
Stice, E., Yokum, S., Veling, H., Kemps, E., and Lawrence, N. S. (2017). Pilot test of a novel food response and attention training treatment for obesity: brain imaging data suggest actions shape valuation. Behav. Res. Ther. 94, 60–70. doi: 10.1016/j.brat.2017.04.007
Stinson, E. J., Krakoff, J., and Gluck, M. E. (2018). Depressive symptoms and poorer performance on the stroop task are associated with weight gain. Physiol. Behav. 186, 25–30. doi: 10.1016/j.physbeh.2018.01.005
Tomiyama, A. J. (2014). Weight stigma is stressful. A review of evidence for the cyclic obesity/weight-based stigma model. Appetite 82, 8–15. doi: 10.1016/j.appet.2014.06.108
Tomiyama, A. J. (2019). Stress and obesity. Annu. Rev. Psychol. 70, 703–718. doi: 10.1146/annurev-psych-010418-102936
Van den Noortgate, W., López-López, J. A., Marín-Martínez, F., and Sánchez-Meca, J. (2015). Meta-analysis of multiple outcomes: a multilevel approach. Behav. Res. Methods 47, 1274–1294. doi: 10.3758/s13428-014-0527-2
Verbeken, S., Braet, C., Goossens, L., and van der Oord, S. (2013). Executive function training with game elements for obese children: a novel treatment to enhance self-regulatory abilities for weight-control. Behav. Res. Ther. 51, 290–299. doi: 10.1016/j.brat.2013.02.006
Veronese, N., Facchini, S., Stubbs, B., Luchini, C., Solmi, M., Manzato, E., et al. (2017). Weight loss is associated with improvements in cognitive function among overweight and obese people: a systematic review and meta-analysis. Neurosci. Biobehav. Rev. 72, 87–94. doi: 10.1016/j.neubiorev.2016.11.017
von Elm, E., Altman, D. G., Egger, M., Pocock, S. J., Gøtzsche, P. C., and Vandenbroucke, J. P. (2008). The strengthening the reporting of observational studies in epidemiology (STROBE) statement: guidelines for reporting observational studies. J. Clin. Epidemiol. 61, 344–349. doi: 10.1016/j.jclinepi.2007.11.008
Wang, Y., and Beydoun, M. A. (2007). The obesity epidemic in the United States—gender, age, socioeconomic, racial/ethnic, and geographic characteristics: a systematic review and meta-regression analysis. Epidemiol. Rev. 29, 6–28. doi: 10.1093/epirev/mxm007
Weller, R. E., Cook, E. W., Avsar, K. B., and Cox, J. E. (2008). Obese women show greater delay discounting than healthy-weight women. Appetite 51, 563–569. doi: 10.1016/j.appet.2008.04.010
Wildes, J. E., Marcus, M. D., Kalarchian, M. A., Levine, M. D., Houck, P. R., and Cheng, Y. (2010). Self-reported binge eating in severe pediatric obesity: impact on weight change in a randomized controlled trial of family-based treatment. Int. J. Obes. 34, 1143–1148. doi: 10.1038/ijo.2010.35
Wirt, T., Schreiber, A., Kesztyüs, D., and Steinacker, J. M. (2015). Early life cognitive abilities and body weight: cross-sectional study of the association of inhibitory control, cognitive flexibility, and sustained attention with BMI percentiles in primary school children. J. Obes. 2015:534651. doi: 10.1155/2015/534651
Wu, M., Brockmeyer, T., Hartmann, M., Skunde, M., Herzog, W., and Friederich, H.-C. (2015). Reward-related decision making in eating and weight disorders: A systematic review and meta-analysis of the evidence from neuropsychological studies. Neurosci. Biobehav. Rev. 61, 177–196. doi: 10.1016/j.neubiorev.2015.11.017
Yang, Y., Shields, G. S., Guo, C., and Liu, Y. (2018). Executive function performance in obesity and overweight individuals: a meta-analysis and review. Neurosci. Biobehav. Rev. 84, 225–244. doi: 10.1016/j.neubiorev.2017.11.020
Yang, Y., Shields, G. S., Wu, Q., Liu, Y., Chen, H., and Guo, C. (2019). Cognitive training on eating behaviour and weight loss: a meta-analysis and systematic review. Obes. Rev. 20, 1628–1641. doi: 10.1111/obr.12916
Zelazo, P. D., and Carlson, S. M. (2012). Hot and cool executive function in childhood and adolescence: development and plasticity. Child Develop. Perspect. 6, 354–360. doi: 10.1111/j.1750-8606.2012.00246.x
Zhao, J., Goldberg, J., and Vaccarino, V. (2013). Promoter methylation of serotonin transporter gene is associated with obesity measures: a monozygotic twin study. Int. J. Obes. 37, 140–145. doi: 10.1038/ijo.2012.8
Keywords: executive function, obesity, weight loss, overweight, cognitive function, cognitive training
Citation: Du Z, Li J, Huang J, Ma J, Xu X, Zou R and Xu X (2021) Executive Functions in Predicting Weight Loss and Obesity Indicators: A Meta-Analysis. Front. Psychol. 11:604113. doi: 10.3389/fpsyg.2020.604113
Received: 08 September 2020; Accepted: 21 December 2020;
Published: 28 January 2021.
Edited by:
Chun-Qing Zhang, Sun Yat-sen University, ChinaReviewed by:
Yu-Qin Deng, Nantong University, ChinaMing-Yang Cheng, Shanghai University of Sport, China
Copyright © 2021 Du, Li, Huang, Ma, Xu, Zou and Xu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Xia Xu, xuxia@whsu.edu.cn
 Zhongquan Du
Zhongquan Du Jingjing Li
Jingjing Li Jiaai Huang
Jiaai Huang Jing Ma1
Jing Ma1 Xiaoyu Xu
Xiaoyu Xu Rong Zou
Rong Zou Xia Xu
Xia Xu