- 1Psychopharmacology, Drug Misuse and Novel Psychoactive Substances Research Unit, School of Life and Medical Sciences, University of Hertfordshire, Hatfield, United Kingdom
- 2Neomesia Mental Health, Villa Jolanda Hospital, Jesi, Italy
- 3Polyedra, Teramo, Italy
- 4NHS, Department of Mental Health, Psychiatric Service of Diagnosis and Treatment, Hospital "G. Mazzini", Teramo, Italy
- 5Department of Neuroscience, Imaging and Clinical Science, Chair of Psychiatry, University of "G. D’Annunzio", Chieti, Italy
The critical spread and dissemination of novel psychoactive substances (NPS), particularly among the most vulnerable youngsters, may pose a further concern about the psychotic trajectories related to the intake of new synthetic drugs. The psychopathological pattern of the “new psychoses” appears to be extremely different from the classical presentation. Therefore, clinicians need more data on these new synthetic psychoses and recommendations on how to manage them. The present mini-review aims at deepening both the clinical, psychopathological features of synthetic/chemical NPS-induced psychoses and their therapeutic strategies, according to the different NPS classes implicated, by underlining the main differences with the “classical” psychoses. A comprehensive review was conducted using the PubMed/Medline database by combining the search strategy of free-text terms and exploding a range of MESH headings relating to the topics of novel psychoactive substances and synthetic/chemical psychoses as follows: {(Novel Psychoactive Substances[Title/Abstract]) AND Psychosis[Title/Abstract])} and for each NPS categories as well, focusing on synthetic cannabinoids and cathinones, without time and/or language restrictions. Finally, an overview of the main clinical and psychopathological features between classical versus NPS-induced chemical/synthetic psychoses is provided for clinicians working with dual disorders and addiction psychiatry. Further insight is given here on therapeutic strategies and practical guidelines for managing patients affected with synthetic/chemical NPS-induced psychoses.
Introduction
During the last decade, the “traditional” drug panorama has been gradually “reshaped” and integrated, even though not totally replaced, by the appearance of “new/novel psychoactive substances” (NPS) which are either newly created or existing substances which are now being used in “novel” modalities (1–3). The clinical, toxicological, and psychopathological effects of NPS have not been completely investigated and discovered through modelling; hence, clinical and psychopathological/psychiatric concerns have arisen among clinicians/professionals working in dual diagnosis, drug addiction, and mental health area (1, 2, 4–6). The recent published report from the European Monitoring Centre for Drugs and Drug Addiction (EMCDDA) described more than 730 NPS notified to the agency by the end of 2018, with 55 new compounds reported for the first time in Europe only in 2018 (3). Of these, 51% were synthetic cannabinoids (SC), 24% synthetic cathinones/“bath salts,” 5% benzodiazepines, 2% synthetic opioids, and 18% other substances (i.e., tryptamines, phenethylamines, arylcyclohexylamines, psychostimulants, etc.) (3).
SC/“Spice drugs,” mainly marketed as nail polish remover, deodorizers, incense, and potpourri, labeled as “natural”/“legal” alternatives to cannabis, have been documented to be dramatically associated with extremely more severe adverse health effects compared to "classical" cannabis (1, 7). SC are mainly consumed by inhalation, typically smoked together with a dried herbal material onto which they are previously sprayed during the production phase with chemical additives, or by e-cigarettes, or sold in tablets, etc. (1, 7). SC are mainly sold and purchased in “smart-shops” and on online drug marketplaces (7). Among these adverse effects typically described are: cases of fatalities, cardiac dysrhythmias, seizures, liver toxicity, kidney failure, hypothermia, hypertension, myocardial infarction, cardiac arrest, acute tubular necrosis, interstitial nephritis, rhabdomyolysis, nausea, vomiting, cognitive deficits, memory loss, sedation, catatonia, agitation, irritability, sympathomimetic syndrome, subarachnoid hemorrhage, cerebrovascular accidents, hematuria, bloody noses, bleeding gums, internal hemorrhage, hemoptysis, panic attacks, anxiety, altered mental status, coma, and a peculiar “synthetic” psychosis, designated "Spiceophrenia" (1, 7). Moreover, animal studies demonstrated that the administration of SC to adolescent rodents or non-human primates may determine the onset of a schizophrenia-like phenotype in adulthood (8–11).
Being beta-keto-phenethylamines, synthetic cathinones (i.e., ethylone and methylone) are structurally similar to amphetamines [i.e., 3,4-methyl-enedioxy-methamphetamine (MDMA), methamphetamine, etc.] and catecholamines, with subtle differences which modify their chemical, pharmacokinetic, and pharmacodynamic properties (1). Some cathinones are analogues of pyrovalerone, e.g.,3,4-methylenedioxypyrovalerone (MDPV), naphyrone, 3, 4-methylenedioxy-α-pyrrolidinobutiophenone (MDPBP), and α-pyrrolidinovalerophenone (α-PVP) (12). Different synthetic cathinones may exert different effects and potency levels on the dopaminergic, noradrenergic, and serotoninergic pathways, even though all usually own sympathomimetic and/or amphetamine-like effects (1). They are typically sold as pills, capsules, and powders, commonly insufflated (snorted/sniffed), orally consumed by “bombing” (swallowing the powder wrapped in a cigarette paper), mixed in a drink, or injected intravenously (13). Synthetic cathinones are generally known as “bath salts” in the USA and as “plant food” in Europe (1). Several motivations have been identified in their consumption and “appeal”/attractiveness among NPS and drug consumers, such as attaining feelings of euphoria/stimulation, increased energy, mood improvement, increasing empathy/openness, reaching a more mental clarity, experiencing vivid hallucinations, and increasing libido. Synthetic cathinone users seem to frequently report hyperthermia, rhabdomyolysis, renal failure, seizures, as well cardiac, psychiatric, and other neurological signs, in addition to the onset of several levels of agitation, ranging from mild agitation to severe psychosis (14). Moreover, mood instability and paranoid ideation have been described among chronic users (15–17). Furthermore, most synthetic cathinone consumers described tolerance, dependence, and withdrawal syndrome (1).
The class of synthetic hallucinogens/psychedelics includes all substances able to alter consciousness (i.e., synthetic lysergamides, tryptamines, and phenethylamines) by distorting time/motion/color/sound perceptions of consumers and/or altering the perception of “self” and/or stimulating some sensory/perceptual disturbances (1, 18, 19). Hallucinogens are usually consumed orally, occasionally through small blotter paper portions (i.e., “tabs”) held in the mouth to allow absorption through the oral mucosa. Moreover, it has been reported as route of administration by insufflation, smoking, rectal, and injection (intravenous and intramuscular) (1, 18, 19). The route of administration may influence the effects, their onset, and duration (1, 18, 19). Hypertension, tachycardia, hyperthermia, dizziness, sleeplessness, loss of appetite, xerostomia, sweating, impulsiveness, fast and labile emotional modifications (from fear, anxiety to euphoria), numbness, weakness, and tremors have been reported as frequent short-term effects. While long-term effects may comprise persistent psychosis (i.e., visual disturbances, disorganized thinking, and paranoia), mood fluctuations, and/or onset of a hallucinogen-persisting perception disorder (HPPD) (1, 5, 19).
Synthetic/novel psychostimulants comprise several NPS classes, i.e., piperazines (compounds labeled as “legal” alternatives to ecstasy, mainly identified as cutting agents in some ecstasy pills) and “amphetamine-type stimulants” (ATS) (1, 3). ATS, e.g., PMA (4-methoxyamphetamine/“Dr. Death”), PMMA (4-methoxymeth-amphetamine), 4-MTA (4-methylthioamphetamine/“flatliners”), DMA (2,5-dimethoxyamphetamine), aminorex derivatives (4,4″-DMAR); diclofensine, methiopropamine, etc., have been marketed as para-substituted methoxy drugs pharmacologically and clinically comparable to amphetamines (1, 3, 18). ATS are commonly supplied as tablets/crystals/powder, sometimes mixed with other substances (20). They are usually swallowed/smoked/snorted, and their common “street names” include “crystal meth” (aka “ice”)/“speed"/“crystal,” etc. (20). Obsessive delusions, paranoia, hallucinations, suicidal ideation, anxiety, insomnia, depression, and psychotic symptoms have been commonly reported following intake of ATS (20, 21).
Overall, NPS have been largely associated with several clinical and psychiatric symptomatology which may vary from the occurrence of an acute transient psychotic episode to more complex psychopathological patterns, depending on the substance implicated (and its pharmacological profile), the frequency, intensity and route of administration, and vulnerability and individual characteristics of NPS consumers (1, 4–6, 22, 24) (as shown in Table 1). The psychopathological pattern of NPS-induced “new/synthetic psychoses” appears to be extremely different from the classical presentation, by introducing a new interesting field of research from a psychopathological and phenomenological point of view. More specifically, NPS mainly implicated in the onset of psychotic patterns are represented by SC, synthetic cathinones, psychostimulants, and some psychedelics/hallucinogenic NPS (1, 6, 19). However, as synthetic cannabinoids and cathinones are the most representative NPS groups, the present review mainly focused on these two categories in collecting, analyzing, and critically discussing data so far published about NPS-induced synthetic/chemical psychoses by underlining differences with “classical” psychosis.
Material and Methods
Search Sources and Strategies
A mini-review was conducted by analyzing and collecting clinical data (only human studies) on cases of NPS-induced psychosis, by using PubMed/Medline and focusing on synthetic cannabinoids and cathinones. We combined the search strategy of free-text terms and exploded MESH headings for the topics of “Novel Psychoactive Substances” and “Synthetic/Chemical Psychoses” as follows: {(Novel Psychoactive Substances[Title/Abstract]) AND Psychosis[Title/Abstract])} and for each NPS category (synthetic cannabinoids and synthetic cathinones) as well, without time/language restrictions. All studies published up to 30 June 2019 were included. In addition, secondary searches were performed using the reference listing of all eligible as well as relevant articles and consultation with experts in the field and or manual searches.
Study Selection, Data Extraction, and Management
We considered studies evaluating the relationships between NPS and synthetic psychosis. We examined all titles and abstracts and obtained full texts of potentially relevant papers. Working independently and in duplicate, two reviewers (LO and SC) read the papers and determined whether they met inclusion criteria. Duplicate publications were excluded. All experimental and observational study designs, including case reports, case series and surveys, were retrieved. Narrative and systematic reviews, letters to the editor, and book chapters were excluded, even though they were used for retrieving further secondary searches. To be included in the present review, studies were required to meet the following criteria: a) empirical and peer-reviewed study; b) at least an abstract with estimates and/or full results published in English; c) human studies; and d) provide data on synthetic psychosis induced by NPS. LO and SC independently extracted the data on participant characteristics, intervention details, and outcome measures. Disagreements were resolved by discussion and consensus with a third member of the team (GDP). Data were collected using an ad hoc developed data extraction spreadsheet.
Characteristics of Included Studies
The set of keywords initially generated 402 results. A total of 123 papers were excluded because of duplication; seven papers were excluded through lack of an english abstract. Of the remaining 272 studies, 145 studies were excluded because they did not meet the inclusion criteria or because they were animal studies. Of the remaining 127 papers, 37 papers were excluded because they were reviews, letters to editors, or meta-analyses, while seven papers were not included here due to the lack of an available full text or an abstract useful for collecting relevant data. Finally, a total of 83 papers were included and accounted for in our analysis. Table 2 clearly explains the main characteristics (study design, sample size, main outcomes, and findings) of all studies retrieved.
Results
Data on NPS-Induced Psychosis in General
A cross-sectional survey carried out as a retrospective review of electronic discharge letters of all patients discharged from general adult psychiatric wards in the Royal Edinburgh Hospital recruited 483 admissions, 86 of which had NPS involvement reported. Among NPS users, the diagnosis of drug-induced psychosis was significantly higher (p < .001, OR = 18.7) compared to non-NPS users (25). The European Drug Emergencies Network (Euro-DEN) collected data on presentations to emergency department (ED) with acute recreational drug and/or NPS intoxication in 16 sentinel centers in 10 European countries, reporting psychosis varying from 3% to 16.3% (26). A retrospective analysis of cases presenting to the ED of the University Hospital of Bern, Switzerland, with symptoms/signs consistent with acute toxicity of recreational and/or NPS use identified two intoxications with NPS: one with methylone and one with 2C-P; 71 cases of psychoses even though the authors do not clearly describe clinical presentation related to the NPS identified (27).
Data on Synthetic Cannabinoids
A case series described some instances of SC-induced reemergence of florid psychotic symptomatology among five forensic patients who took a mixture of SC (like “K2” and JWH-018) contained in “Aroma” blend (28). Psychopathological patterns comprised the onset of psychomotor agitation, disorganized behavior, and ideation, delusional beliefs (grandiose and paranoid) in previously stable patients with a personal history of severe mental illness (SMI) (28). No data are provided about pharmacotherapy and/or specific/suggested treatment (28).
Müller et al. (29) described a 25-year-old man, with a previous history of recurrent psychotic episodes triggered by cannabis consumption and stable on monotherapy with amisulpride (800 mg daily), who presented to hospital with increased levels of anxiety, feelings of manipulation, and psychotic symptoms after smoking “spice” drugs on three different occasions (3 g each). The subject reported the feeling of being controlled and manipulated through a chip which he thought was implanted in his abdomen several years before together with the onset of imperative voices and paranoid hallucinations, symptoms he never had before (29). no data are provided about pharmacotherapy and/or specific/suggested treatment (29).
Another case report described three cases of “Mojo psychosis,” a mixture of SC (30). No further details on psychopathological presentation have been reported by the authors (30).
A study evaluating urine metabolism of SC described three cases of new-onset psychosis following the intake of a mixture blend named “Tropical synergy” containing several SC (31). No further data are provided on psychopathological pattern and/or about pharmacotherapy and/or specific/suggested treatment (31).
Vearrier and Osterhoudt (32) described a case of an adolescent girl who arrived at the ED agitated, violent, and uncontrollable after inhaling a “K2.” She described a previous history of alcohol and cannabis intake. Her psychopathological pattern comprised the onset of visual hallucinations, anxiety, restlessness, tachycardia, higher blood pressure, muscle fasciculation, hypokalemia, and mydriasis. She was given lorazepam 2 mg intravenously, with a good remission (32).
Benford and Caplan (33) reported a case of a 20-year-old honor college student who presented to the ED with severe anxiety/paranoia and auditory/visual hallucinations after smoking “Spice” drugs. Psychopathological pattern comprised the onset of increasing levels of anxiety, paranoia, and both auditory and visual hallucinations. The subject refused voluntary psychiatric admission. No data are provided about pharmacotherapy and/or specific/suggested treatment (33).
A case series described 11 Hispanic adolescents admitted to the South Miami Hospital Addiction Treatment Center in Miami-Dade County, Florida, after smoking SC and developing a new-onset psychosis (34). Subjects reported using Spice drugs more than three times in their lifetime (8 out of 11 subjects), while 4 out of 11 reported smoking SC multiple times per day. Psychopathological patterns comprised mood fluctuations, altered cognition and perception (i.e., visual and auditory), memory difficulty, euphoria, sometimes irritability and anxiety, and paranoid thoughts (34). In addition, subjects reported tachycardia, restlessness, appetite changes, muscle fasciculation, tremors, and weakness. No data are provided about pharmacotherapy and/or specific/suggested treatment (34).
A cohort explorative study recruiting subjects from a Regional Forensic and Rehabilitation service in New Zealand reported a SC-induced psychotic recrudescence in 15 vulnerable individuals previously affected with SMI (35). The psychopathological patterns comprised the onset of psychomotor agitation, disorganized behavior, and ideation, delusions after smoking SC. All subjects reported a previous psychiatric history of psychotic disorder and had been compulsorily treated with therapeutic doses of antipsychotics (unspecified) and, in five cases, together with mood stabilizers (unspecified). No data are provided about pharmacotherapy and/or specific/suggested treatment following SC-induced psychosis (35).
Forrester et al. (36) retrospectively collected data on SC exposures referred to Texas Poison Centers in 2010 by identifying new-onset psychoses following SC intake. Psychopathological patterns comprised the onset of psychomotor agitation and hallucinations. In addition, tachycardia, hypertension, nausea, vomiting, drowsiness, and multiple neurological symptoms were reported. No further information is provided regarding the psychopathology and phenomenology of SC-induced psychoses. No clear data are provided about specific/suggested treatment following SC-induced psychosis, intravenous fluids, benzodiazepines, oxygen, and antiemetics being reported, among the most commonly prescribed medications and, in around 3% of subjects, the use of sedatives and anticonvulsants (36).
Hurst et al. (37) described 10 cases of otherwise healthy men, admitted to the psychiatry ward at the San Diego Naval Medical Center between August and December 2010, who experienced a SC-induced new-onset psychosis. The psychopathological patterns were characterized by auditory and visual hallucinations, disorganized behavior and speech, suicidal ideation, alogia, insomnia, psychomotor agitation and/or retardation, and higher levels of anxiety. No clear data are provided about specific/suggested treatment following SC-induced psychosis, use of antipsychotics (unspecified) being reported in 10 patients (36).
A case report of a 23-year-old high-functioning Caucasian Navy corpsman who developed a SC-induced psychosis was reported by Johnson et al. (38). The psychopathological pattern comprised the onset of nonsensical speech, paranoia of being videotaped, disorganized behavior and speech, tangential thoughts, delusional ideation (i.e., the feeling of own “mind expanded” and the ability to “comprehend infinity,” and so on) without perceptual distortions. The psychotic symptoms of the subject spontaneously resolved after abstinence from SC about 24 h after initial presentation. No data are provided about psychopharmacology and/or specific treatment of SC-induced psychosis (38).
Data collected retrospectively by the Italian database of the Pavia Poison Centre reported 17 cases of SC users who exhibited various psychiatric and clinical patterns, including tachycardia, agitation/anxiety, confusion, hallucinations, mydriasis, paresthesia, palpitations, drowsiness, xerostomia, syncope, seizures, vertigo, tremor, hypertonia, coma, etc. (39). The authors reported symptomatic treatment and benzodiazepines for treating neuroexcitatory effects (39). No further details regarding psychopathological pattern of SC-induced psychosis were reported by Locatelli et al. (39).
McCain et al. (40) retrospectively described six cases of SC-induced psychopathological/clinical pattern referred to the Arkansas Poison and Drug Information Center. Psychopathological patterns comprised agitation, irritability, and hallucinations. Benzodiazepines, intravenous fluids, antiemetics, and potassium supplementation were prescribed, among treatments, without details about dosage(s) and/or treatment duration (40).
Simmons et al. (41) described three cases of young adults referred to an ED after “Spice” intake (JWH-018 and JWH-073) who exhibited anticholinergic and sympathomimetic clinical effects and a new-onset SC-induced psychosis. Benzodiazepines (intravenous lorazepam, 4 mg), antipsychotics (haloperidol, 5 mg), supportive therapy, and observation were among the treatments prescribed (41).
A 17-year-old adolescent boy without significant previous psychiatric and medical history presented to an ED reporting “pounding in his chest,” constant chest pressure, and a new-onset SC-induced psychosis after smoking a SC, called K9 (42). No data are provided about pharmacotherapy and/or specific/suggested treatment (42).
Other case reports of SC-induced new-onset psychoses have been reported by McGuinness and Newell (43). Psychopathological patterns comprised aggressiveness, paranoia, short-term memory deficits, and increasing levels of anxiety. No data are provided about pharmacotherapy and/or specific/suggested treatment (43).
Tung et al. (44) described a 36-year-old male real-estate agent with a previous polysubstance abuse (heroin, codeine, and cannabis) with a family psychiatric history of SMI (unspecified) and substance use disorder (SUD) who presented an episode of acute psychotic disorder characterized by florid persecutory delusion, auditory hallucinations, disorganized behavior, irritability, aggressiveness, and agitation. No further data are provided about pharmacotherapy and/or specific/suggested treatment (44).
Van der Veer and Friday (45) described three patients who presented with severe, persisting psychotic symptoms after regular SC use for 3–4 weeks prior to admission. Psychopathological patterns comprised disorganized speech and behavior, paranoia, bizarre delusions, suicidality, aggressiveness, poverty of thoughts, loosening of associations, poor attention/concentration, and inappropriate affect; onset of Capgras delusions was reported in one case. Two out of three patients did not have a previous psychiatric history, while one reported a post-traumatic stress disorder (PTSD). All three patients required hospitalization and were treated with antipsychotic medications (haloperidol in two cases and risperidone in one case). No further data are provided regarding dosage(s) and/or treatment duration (45).
Bebarta et al. (46) described three cases of “Spice” use in military members followed by onset of psychotic symptomatology. Psychopathological patterns comprised the onset of paranoia, aggressiveness, agitation, and visual hallucinations. Regarding the treatment, intravenous lorazepam 2 mg was reported for managing agitation, with resolution of symptomatology (46).
Cohen et al. (47) reported three cases of adolescents who presented with a new-onset SC-induced psychosis characterized by catatonia, psychomotor agitation, and aggressiveness. No further data are provided about pharmacotherapy and/or specific/suggested treatment (47).
A retrospective series of exposures to SC coming from the US National Poison Data System between January and October 2010 were analyzed by Hoyte et al. (48) by identifying new-onset psychosis induced by SC products. No further data are provided either regarding further specific psychopharmacological treatment beyond benzodiazepines or about psychopathological patterns of SC-induced psychosis (48).
Two adolescents who manifested a new-onset SC-induced psychosis have been reported by Oluwabusi et al. (49). Both patients reported a family psychiatric history for schizophrenia and/or bipolar disorder. Psychopathological pattern comprised disorganized behavior, paranoid delusions, insomnia, hyperactivity, anxiety, musical auditory hallucinations, mood lability, irritability, euphoria, pressure of speech, flights of ideas, paranoia, and grandiose delusions. Regarding treatment, one subject was firstly stabilized on quetiapine and then changed to aripiprazole (20 mg daily) after the onset of an acute dystonic reaction, while the other one was prescribed olanzapine (15 mg daily bedtime) (49).
Another case report has been described of a new-onset SC-induced psychosis in a 59-year-old Latino male with a history of PTSD and polysubstance abuse (heroin, cocaine, cannabis, and alcohol) (50). His psychopathological pattern comprised visual hallucinations and disorganized and bizarre behavior. He was treated with benzodiazepines, gabapentin (400 mg QID), hydroxyzine (25 mg TID), aripiprazole (10 mg qD), benztropine (1 mg BID), and bupropion (150 mg BID) (50).
An Internet-based survey reported the onset of psychotic symptomatology in 28% of a sample (n = 168) of SC users recruited from 13 different countries (51), while an Australian online survey (n = 316) reported paranoia (18%) and psychosis (4%) (52). No further data are provided about pharmacotherapy and/or specific/suggested treatment in both surveys (51, 52).
Berry-Cabán et al. (53) described a 20-year-old Hispanic male who presented to an ED due to an altered mental status and delirium following SC intake. Initially, the subject was uncommunicative, awake then non-compliant, combative, and aggressive; hence, clinicians applied physical restraint and firstly administered 2 mg/ml parental lorazepam for managing increasing levels of agitation. During hospitalization, he was additionally treated with further 4 mg of lorazepam, 5 mg of haloperidol, and 25 mg of diphenhydramine. His psychopathological pattern comprised agitation, aggressiveness, disorganized speech and behavior, hallucinations, and paranoid ideation. He was discharged with a prescription of risperidone 1 mg daily. No further data on treatment duration has been provided (53).
Chan et al. (54) described a 21-year-old man without a previous psychiatric history who presented to an ED due to the onset of increasing levels of psychomotor agitation and paranoid thoughts after consuming 0.4 g of 6-APB and smoking cannabis over a 2-day period. Psychopathological pattern comprised agitation, paranoia, the belief that others could read his mind, self-harm, and suicidality. He was treated with diazepam (4–10 mg daily) for managing agitation during hospitalization. No further data have been provided regarding further treatments to be prescribed and/or treatment duration (54).
A retrospective cohort study targeting individuals seeking emergency treatment after SC intake and selected from the database of the Freiburg Poisons Information Center between September 2008 and February 2011 reported a plethora of physical symptoms and psychotic presentation (55). Psychopathological patterns included visual and auditory hallucinations, confusion, agitation, delirium, restlessness, and psychotic symptoms. Supportive care, talk down strategies, intravenous fluids, potassium supplementation, and benzodiazepines were prescribed. No further data have been provided regarding specific psychopharmacotherapy and/or treatment duration and/or dosage(s) (55).
A case report of an Italian male who consumed "Bonzai," a mixture of SC, has been presented by Papanti et al. (7). The subject did not have any psychiatric history and his psychopathological pattern was characterized by increasing levels of anxiety, insomnia, ideas of reference, somatic and visual hallucinations, autoscopy, and aggressiveness. He was initially treated with diazepam 5 mg intravenously and was rehydrated, and then he was prescribed olanzapine 5 mg daily and bromazepam 3 mg daily for 4 weeks (7).
Glue et al. (56) retrospectively collected data on 17 patients hospitalized in an acute psychiatric ward, located in New Zealand, following the use of a SC (“K2”) and described their clinical and psychopathological features. Nine out of 17 patients had recurrences of preexisting disorders and four presented with new psychotic symptoms. Psychopathological patterns comprised paranoia, thought disorder, disorganized behavior and speech, anxiety, mood lability, and intense suicidal thinking and/or behavior. Antidepressants and antipsychotics (unspecified) were prescribed, without specifying treatment duration and/or dosage(s) (56).
Leibu et al. (57) described a case of a 36-year-old African-American man with a previous long-lasting psychiatric history of schizophrenia who presented with severe and life-threatening catatonia after consuming SC. Catatonia was successfully treated with electroconvulsive therapy, while psychotic symptoms have been managed with 225 mg BID clozapine (57). No further data are available regarding treatment duration (57).
A Turkish survey carried out on a sample of 158 patients who were admitted to Bakirkoy Research and Training Hospital for Psychiatry, Neurology and Neurosurgery, Alcohol and Drug Research, Treatment and Training Center (AMATEM) revealed a new onset of hallucinations and delusions among SC consumers (58). Psychopathological patterns comprised increasing levels of anxiety (75.6%), irritability (18.6%), insomnia (61.5%), hallucinations (40.4%), and delusions (16%). No data have been provided regarding specific psychopharmacological treatment of SC-induced psychosis (58).
A case series of four paranoid schizophrenic patients who took SC showed a worsening of psychotic symptomatology (59). All subjects were hospitalized and were previously prescribed specific medications for their psychiatric disorder (i.e., haloperidol decanoate, quetiapine, risperidone, diazepam, clozapine, lorazepam, olanzapine, clonazepam, and fluoxetine). Psychopathological patterns comprised cognitive deficits, anhedonia, dysphoria, avolition, blunted affect, incoherent speech, thought blocking, severe agitation, increasing levels of anxiety, and so on. The subjects were furtherly treated by increasing benzodiazepine dosages (lorazepam and/or diazepam) (59).
Haro et al. (60) described a 19-year-old Hispanic woman who developed a psychotic episode after consuming SC. Her psychopathological pattern comprised self-references when eating or walking, visual hallucinations, soliloquy, laughter forfeit, catatonia, depersonalization, and derealization. After 2 months of abstinence from SC and treatment with aripiprazole (15 mg daily), lorazepam, and biperiden, psychotic symptomatology partially resolved (60).
Meijer et al. (61) described a case of bilateral upper-extremity self-mutilation and new-onset SC-induced psychosis in a healthy 26-year-old man presented to an ED, with a previous history of attention deficit hyperactivity disorder (ADHD), treated with lisdexamfetamine dimesylate. His psychopathological pattern comprised the onset of paranoid delusions causing him to feel his hands were going to harm him, then he placed them on the stove and attempted to burn them off to “get the devil out of him.” No further data have been provided regarding the psychopharmacological treatment following the discharge (61).
A 17-year-old male who developed catatonia and new-onset SC-induced psychosis was reported by Smith and Roberts (62). His psychopathological pattern comprised confusion, bizarre behavior, mutism, rigidity, excitatory catatonia, auditory and visual hallucinations, and disorganized thoughts. Lorazepam 2 mg intramuscular was administered for managing agitation, followed by six sessions of electroconvulsive therapy and olanzapine. No further data have been provided regarding dosage(s) and/or treatment duration (62).
Durand et al. (63) described a case of new-onset SC-induced psychosis associated with severe rhabdomyolysis after consuming SC in a 23-year-old man without prior psychiatric history. His psychopathological pattern comprised altered mental status, severe agitation, acute psychosis, irritability, persecutory delusions, and impulsiveness. Intravenous lorazepam every 6 h was prescribed for managing agitation, then haloperidol 10 mg TID, valproic acid 500–1,000 mg, and lorazepam 2 mg TID. No further data on treatment duration has been provided (63).
Schwartz et al. (64) reported seven cases who presented simultaneously at a hospital in Georgia (USA), after smoking a mixture of SC contained in a blend named “Crazy Clown” during a party. Psychopathological patterns comprised a new onset of SC-induced psychosis, aggressiveness, extreme agitation, and increasing levels of anxiety. Regarding the treatment, two patients were admitted to the intensive care unit due to persistent hypertension and tachycardia and mental status alterations and were intubated. One patient presented with cardiac arrest and was resuscitated by paramedics and underwent successful balloon angioplasty; the other subjects were not treated due to non-compliance and/or spontaneous remission of symptomatology. No further data have been provided regarding specific pharmacological treatment (64).
Ustundag et al. (65) described a case of SC-induced mania with psychotic symptoms in an 18-year-old single boy who developed an increasing speech, spending money, a great deal of interest in religion and insomnia, mystic delusions, irritability, euphoria, mood lability, and without hallucinations after consuming SC. Olanzapine (from 10 to 20 mg daily), valproic acid (from 500 to 1,500 mg daily), quetiapine (from 200 to 400 mg daily), and lorazepam (from 0,5 to 1 mg daily) were prescribed. No further data on treatment duration are available (65).
Sönmez and Köşger (66) described a 31-year-old man who developed a new-onset SC-induced psychosis after consuming a mixture of SC called "Bonsai" three times a week for more than 6 months and was treated with olanzapine.
Rahmani et al. (67) described two cases with a strong family psychiatric history who developed a SC-induced psychosis following the intake of “Spice.” Psychopathological pattern comprised the onset of paranoia, bizarre and disorganized behavior and speech, and visual hallucinations. No further data have been provided regarding treatment duration and/or dosage(s) of clozapine prescribed in both cases (67).
An observational case series (n = 35) retrospectively collected laboratory analysis of patients presenting to an ED with a documented suspicion of SC intake and described a new onset of SC-induced psychosis (68). Psychopathological patterns comprised altered mental status (61%), hallucinations (6%), and seizures (40%). Five patients were ventilated and intubated. No further data on specific psychopharmacological treatment has been provided (68).
A cross-sectional study recruiting 81 male patients diagnosed with SC-induced psychotic disorder (n = 50) or with schizophrenia (n = 31) who were concurrently hospitalized described a higher rate of suicidal ideation, involuntary hospitalization, as well as similar clinical picture with schizophrenia by inducing paranoia, disorganized behavior, visual and auditory hallucinations, and suicidal thoughts, not only in vulnerable subjects but also in subjects without a previous history of psychosis (69). Furthermore, verbal learning, short-term memory and working memory, executive functions, abstract ability, and decision-making and attention functions were reported to have been impaired among SC-induced psychotic subjects (69).
Khan et al. (70) described two cases of SC-induced catatonic state associated with psychosis, psychomotor alterations, speech and behavior disorganization, flatted affect, alogia in a 17-year-old male with no psychiatric history who presented to the ED with psychosis after a 2-week Spice binge and in a 21-year-old male who reported a history of childhood ADHD with progressive isolation and negative symptoms after heavy SC consumption. The first case was treated with aripiprazole 7.5 mg daily and lorazepam (2 mg BID), then olanzapine (unspecified dosage) and valproic acid (up to 100 mg qHS) were added while aripiprazole was tapered off, while the second case was treated with lorazepam for managing catatonia and firstly risperidone (1–2 mg BID), then switched to aripiprazole (5 mg daily, titrated up to 25 mg daily) (70).
A retrospective observational case series of patients (n = 22) presenting to an ED with analytically confirmed SC intake were described from a pharmacological, toxicological, and clinical point of view by Hermanns-Clausen et al. (71). Psychopathological patterns comprised restlessness, agitation, and visual hallucinations. Treatment was mainly supportive, benzodiazepines (unspecified), metoprolol, and antiemetics. No further data have been provided regarding dosage(s) and treatment duration (71).
A retrospective chart study (n = 594) evaluating all patients who were admitted to a dual diagnosis psychiatric unit at Mount Sinai Beth Israel in New York City compared SC users vs. cannabis users by reporting more psychotic symptoms (p = .012) and more agitation (p < .001) among SC consumers (72). Among medications, more antipsychotics alone (31.4% vs. 19.6%) or a combination antipsychotic plus mood stabilizers/antidepressants (57.1% vs. 33.5%) were prescribed among SC-only users compared to cannabis-only users (72).
A case report described an 18-year-old antipsychotic-naive African-American male without past psychiatric history admitted after presenting to the psychiatric emergency room with a first-time psychotic episode after a prolonged SC ingestion via inhalation (73). The subject was treated with intermittent doses of lorazepam 2 mg orally and stabilized over 1.5 weeks on oral risperidone (5 mg daily) (73).
An 18-year-old Hispanic male admitted to the ED after 5 days of acute-onset auditory hallucinations, paranoid delusions, and panic attacks with a previous history of cannabis abuse and more recently of SC, purchased from Internet blog websites, has been described by Samaan et al. (74). No further data about specific psychopharmacological treatment of SC-induced psychosis has been provided (74).
Ozer et al. (75) described a case of an adolescent with a new-onset psychosis following inhalation of a mixture of SC called “Bonsai.” Olanzapine (10 mg daily) was prescribed and the subject was followed up 3 months after discharge without a recurrence of psychotic symptoms (75).
A cohort study reported, among SC consumers, higher psychotic levels compared to cannabis users (76). No further data about specific psychopharmacological treatment of SC-induced psychosis has been provided (76).
A retrospective cohort study recruiting data from the UK National Poisons Information Service reported hallucinations and paranoia as consequences of SC intake (77). No further data about specific psychopharmacological treatment of SC-induced psychosis has been provided (77).
A case–control neuroimaging study comparing SC users vs. healthy controls, using magnetic resonance imaging, reported that chronic SC use was associated with dose-dependent downregulation of CB1 receptors, lower fractional anisotropy (FA) values in the left inferior fronto-occipital fasciculus (IFOF), and inferior longitudinal fasciculus (ILF) in the left temporal lobe which might be associated with increased risk of the development of psychosis (78). No further data about specific psychopharmacological treatment of SC-induced psychosis has been provided (78).
A case report described a patient who developed acute psychosis, aggressiveness (e.g., homicidal and violent behavior towards staff), and new-onset refractory status epilepticus necessitating emergent neurological life support and prolonged admission to an intensive care unit following SC consumption (79). The patient was treated with intravenous lorazepam, valproic acid, levetiracetam, and lacosamide. Following a 3-week hospitalization in the intensive care unit, the patient was discharged and followed up at 1 month. No further data on dosage(s) and/or treatment duration are available (79).
A cohort study systematically described the clinical characteristics of 100 SC users in a large sample from an urban public hospital in New York City by reporting a high rate of acute psychotic symptoms, particularly among the already socially vulnerable and psychiatrically ill population of the sample (80). Most SC users (73.7%) were prescribed an antipsychotic medication on discharge. No further data on dosage(s) and treatment duration (80).
Monte et al. (81) collected data from the US ToxIC (2010–2015) and found that among 353 SC consumers, about 40% had SC-induced delirium and toxic psychosis.
A qualitative study of six SC users in Hungary revealed the onset of paranoia and a synthetic psychosis among participants (82). Similarly, an online survey reported the onset of agitation, paranoia, and other psychotic symptomatology following SC intake (83). No further data are available regarding specific psychopharmacological treatment of SC-induced psychosis in both studies (82, 83).
A prospective pilot study recruiting 332 patients with cannabis and/or SC use evaluated the psychosis-inducing potential of cannabis vs. SC, reporting more severe psychotic symptoms among SC vs. cannabis users (84). No further data about specific psychopharmacological treatment of SC-induced psychosis has been provided (84).
A case series described four patients taking SC and presenting to a UK acute psychiatric unit with a SC-induced psychosis by reporting mixed hallucinations, persecutory delusions and thought disorganization, physical and verbal aggressiveness, and sexual disinhibition (85). All subjects recruited were treated with benzodiazepines and antipsychotic drugs, as shown in Table 2.
A 47-year-old African-American man presented for involuntary inpatient psychiatric admission after being brought in by police secondary to bizarre behavior/hallucinations/agitation/delusions following SC intake (86). He was initially treated with olanzapine (10 mg daily), then switched to haloperidol intramuscularly and lorazepam due to aggressiveness and increasing levels of agitation towards clinicians. The patient was observed and he refused any follow-up and/or medication after discharge (86).
A single-center cohort study evaluating hospitalized SC-induced psychotic patients in a Russian hospital identified specific clinical variants of psychoses among SC users (87). No further data about specific psychopharmacological treatment of SC-induced psychosis has been provided (87).
Data on Synthetic Cathinones
Antonowicz et al. (88) described two cases of paranoid synthetic psychosis in a 27-year-old female and in a 32-year-old male who consumed MDPV. Psychopathological patterns comprised the onset of paranoid ideation, disorganized thought process, insomnia, and agitation. The first subject was successfully treated with low doses of risperidone, while the second case refused any medications (88).
Penders and Gestring (90) described the onset of a hallucinatory delirium following use of MDPV in three cases who presented to an ED of a tertiary care hospital in the USA. Two cases were successfully managed with risperidone (0.5 mg orally BID), while the third one was treated with haloperidol (1 mg orally BID) (90).
Striebel and Pierre (91) described a 22-year-old man without any previous psychiatric history who used cannabis regularly to manage his Crohn's disease and who developed a new-onset MDPV-induced psychosis. His psychopathological pattern comprised the onset of hallucinations, altered perceptions, “seeing things move that shouldn't be moving,” feeling an earthquake, and agitation. He was treated with lorazepam and supportive care (91).
Lajoie and Rich (94) described a 50-year-old male with a previous history of methamphetamine dependence who developed a MDPV-induced psychosis characterized by self-mutilation, suicidality, agitation, increasing levels of panic attack, and auditory hallucinations. He required sedation with olanzapine and lorazepam, for managing agitation. No further data on dosage(s) and treatment duration have been provided (94).
Thornton et al. (95) reported a 23-year-old man with a previous psychiatric history who presented to an ED after developing a bizarre and disorganized behavior, suicidality, visual, tactile, and auditory hallucinations, and agitation following the intake of MDPV. He was treated with 6 mg of lorazepam and 2.4 mg of droperidol intravenously over 90 min in order to manage the agitation. He was also taking aripiprazole, valproic acid, lithium, quetiapine, and clonazepam for his psychiatric disorder. No further data on treatment duration and/or dosage(s) are available (95).
Winder et al. (100) described MDPV-induced paranoid psychosis in a 33-year-old male veteran without a previous psychiatric history. He was treated with as-needed doses of quetiapine and lorazepam for paranoid ideation, agitation, and anxiety. Symptomatology resolved within 12 h after admission. He was then started on an antidepressant (unspecified) for residual symptoms of depressed mood, anhedonia, and hopelessness. No further data on treatment duration and/or specific psychopharmacological treatment for MDPV-induced psychosis are available (100).
Bertol et al. (101) described a case of mixed MDPV and benzodiazepine intoxication in a 27-year-old chronic abuser with a previous SUD and a psychiatric history who developed severe agitation, disorganized and bizarre behavior, suicidality, and visual, tactile, and auditory hallucinations. He was managed with diazepam intravenously due to agitation and then he started chlorpromazine due to sleeplessness and aripiprazole for managing psychotic symptoms. No further data on treatment duration and/or dosage(s) are available (101).
Mangewala et al. (98) reported a psychotic onset (i.e., delirium, agitation, paranoia, and hallucinations) in a 16-year-old adolescent man without a previous personal and/or family psychiatric history who abused synthetic cathinones. He was treated with a combination of olanzapine (from 5 to 7.5 mg daily) and lorazepam (0.5 mg BID) (98).
Derungs et al. (89) reported a case of paranoia and psychosis induced by Naphyrone in a 31-year-old man. Psychopathological pattern comprised agitation, restlessness, insomnia, anxiety, and hallucinations. No further details have been provided regarding a specific psychopharmacological treatment of synthetic psychosis (89).
Two cases of severe intoxication delirium with paranoid psychosis and hallucinations following “bath salts” consumption are described by Kasick et al. (93).
Khan et al. (97) described a case of a 19-year-old female, without a family and/or personal psychiatric history, who developed a paranoid psychosis after consuming synthetic cathinones. She was treated with olanzapine and lorazepam. No further data on dosage(s) and/or treatment duration have been provided (97).
Adebamiro and Perazella (92) reported a case of a new-onset psychosis associated with renal and cardiovascular dysfunction following “bath salts” intoxication. The subject was treated with supportive care and hospitalized (92).
Gunderson et al. (96) described a case of paranoid psychosis induced by “bath salts” and diphenhydramine in a subject with a previous psychiatric history of major depressive disorder (MDD).
Stoica and Felthous (99) described a case of acute psychosis in a 30-year-old subject with a psychiatric diagnosis of bipolar affective disorder and schizophrenia following the intake of synthetic cathinones. Psychopathological pattern comprised suicidality, homicidal tendencies, euphoria, alertness, paranoid delusion, and increased levels of energy. The subject was hospitalized and started initially on valproic acid (250 mg orally BID) and trazodone (100 mg daily at night) for managing impulse dyscontrol and insomnia. No further data are available on treatment duration (99).
A case report described a psychotic onset, associated with the attempted murder of his father, in a man with a history of regular recreational use of a wide range of illicit drugs between 14 and 20 years following the intake of 3-methoxyphencyclidine (3-MeO-PCP) and MDPV (102). The patient experienced vivid hallucinations (auditory, visual, and tactile), panoramic visual hallucinations (“full brown eyes closed visuals” and imperative voices saying “kill your father”), and bizarre ideas (e.g., “using house as a base for super heroes”), without other symptoms of schizophrenia such as delusional beliefs or thoughts disorder (102).
Several case reports of alpha-PVP (aka “Flakka”)-induced psychosis have been described (103, 106, 107). Crespi et al. (103) described a 17-year-old who developed a Flakka-induced prolonged psychosis characterized by altered mental status, agitation, auditory hallucinations, disorganized behavior, and psychotic symptoms. She was managed with olanzapine and lorazepam (103). A 20-year-old male who ingested one tablet of Flakka developed a psychosis characterized by agitation, aggressiveness, hyper-religiosity, command auditory and visual hallucinations of serpents, suicidality, homicidal tendencies, and delusions. He was initially managed with 2 mg of lorazepam intramuscularly for agitation, then he was prescribed oral lorazepam 2 mg TID and aripiprazole 10 mg daily for 3 weeks, then titrated lorazepam 3 mg TID and aripiprazole 24 mg daily. After modest improvement, he was switched from lorazepam to clonazepam (2 mg BID) only for 3 days and then restarted on lorazepam, while aripiprazole was modified with quetiapine (800 mg daily), with marked improvement of psychotic symptoms and catatonia (106). Simonato et al. (107) reported a case of “Flakka-induced” psychosis successfully treated with bupropion.
A case of psychosis onset following the use of mephedrone in the context of ChemSex has been reported by Dolengevich-Segal et al. (104), as described in Table 2.
A cohort study reported the major complication of cathinone use as a prolonged psychosis, with a high proportion of cases among MDPV and methylone consumers (107). No further details have been provided regarding a specific psychopharmacological treatment of synthetic psychosis (105).
Discussion
Overall, NPS use seems to exert stronger and more persistent and severe effects among subjects with SMI than in healthy subjects (i.e., without a previous psychiatric history), mostly due to activity in the dopaminergic system, implicated in managing behavior and thought processes and in determining psychosis, or in the serotonergic, noradrenergic, and/or glutamatergic systems (4, 108, 109) (as illustrated in Table 1). NPS may exert severe dissociative states, confusion in previously psychotic patients, relapse or worsening of a preexisting psychosis, persistent worsening of psychotic symptoms course, or the onset of a new severe psychotic symptomatology among healthy subjects (110, 111). In fact, the emergence of a psychotic symptomatology is commonly associated with a plethora of neurotransmission changes, e.g., increased central dopamine levels, cannabinoid CB1 receptor activation, 5-HT2A receptor activation, decreased activity in N‐methyl‐‐aspartate receptors, and k‐opioid receptor activation. NPS may interfere at these neurobiological levels and facilitate the imbalance of several neurotransmitters and receptors (as illustrated in Table 1). For this reason, NPS use may really determine severe psychiatric symptoms, also in individuals not previously affected by a mental disorder. It is still unclear how the frequency of use (continuous vs. discontinued), the intensity of consumption (low, medium, or heavy), and the dosages (low vs. high dosages) may or not influence the development of a specific psychopathological pattern. Therefore, the advent of NPS has posed further clinical concern as not only are their clinical, toxicological and safety profiles often completely unknown (1) but they may also cause the onset of new psychopathological entities or the reemergence of “forgotten” psychopathological patterns, such as HPPD (4, 5).
Furthermore, the subtle gap/bridge between a “classical” vs. a “synthetic” psychosis is still the subject of clinical concern, as it seems the psychopathological, phenomenological, and clinical features of these two entities remain completely undefinable and clear. Moreover, this “gap” may have also meaningful effects in the choice of the best treatment and in defining the best outcome(s) and prognosis.
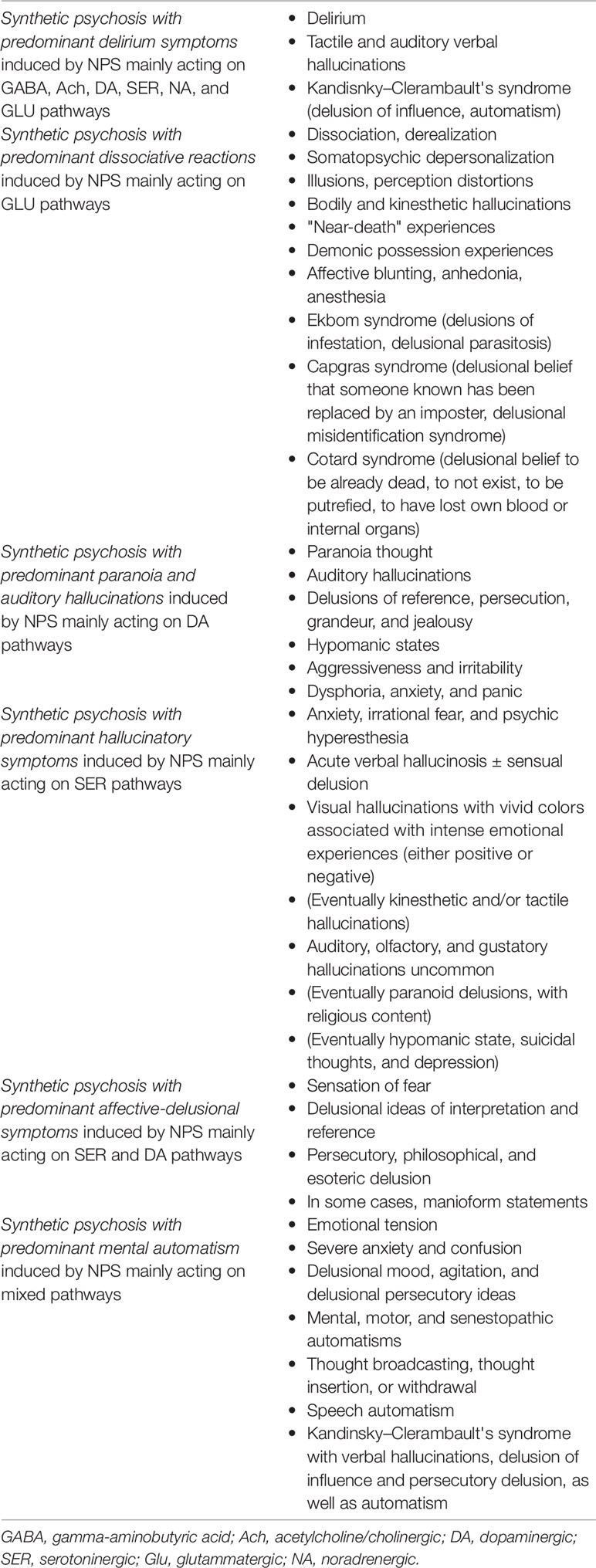
In addition, even though generically classified as NPS, not all NPS possess the same pharmacological and clinical profiles; hence, from a psychopathological point of view, clinicians may observe several types of NPS-induced “synthetic psychoses,” depending on the substance involved. Similarly, a specific NPS may cause different psychopathological effects in vulnerable vs. non-vulnerable individuals (as shown in Table 2). With regard to this, the model of a substance-related exogenous psychosis (SREP) and its toxic subtype (aka “lysergic psychoma”) may be helpful in shedding light to clinicians on differentiating classical/endogenous versus synthetic (NPS-induced) psychoses, from a clinical perspective. Unlike “classical/endogenous” psychosis, SREP is characterized by the following features: a) qualitative and quantitative consciousness alterations (i.e., crepuscular state and onyroid state); b) ego disorders (i.e., somatosensory/allopsychic); c) sensorial–perceptual disorders (i.e., visual, auditory, and coenesthetic); d) egodystonia (i.e., behaviors not coherent with self-image); e) mood swings; f) hyper-presentation of the time/“concrete” psychoses, i.e., alteration of the experienced space; g) modification of body perception; h) anhedonia/apathy/negative symptoms; and i) dyscontrol impulsivity and self-/hetero-aggressiveness (112) (as shown in Table 3). “Lysergic psychoma” is a phenomenological construct characterized by the perception of an “extraneous/foreign” body in one’s own mind, in which the residual critical ego takes position against the intoxicated part of one’s own self (113). It is a syndrome characterized by a clear egodystonic experience in which a subject clearly feels and observes this “foreign entity” as an unusual experience, out of own control, accompanied by hallucinations (mainly visual and kinaesthetic) and delusional perceptions (and thoughts) which are completely resisted by the subject who tries to stem them (114). This psychotic experience is often described as self-limiting, intense, and brief and appears to spontaneously resolve after substance discontinuation, as also observed in several studies examined here (see Table 2). However, chronic, persistent NPS use, mainly at high dosages, may cause a complex, persistent, and long-lasting psychopathological pattern, defined as “synthetic psychosis,” a paraphrenic syndrome due to a NPS-induced mental automatism which causes a psychotic trajectory (114). Furthermore, the pharmacodynamics and pharmacological profile of each NPS seem to be responsible for the psychopathological and clinical manifestation of each NPS-induced synthetic psychosis (as shown in Tables 1, 2, and 4) (1, 19, 115–117). Although there is still a need to clearly discriminate and characterize specific psychopathological and pathognomonic patterns depending on the specific NPS classes involved, as previously stated and shown in Table 2, an attempt had been made here to provide a critical summary of studies in order to describe some NPS-induced psychopathological clusters (Table 4).

Table 3 Classical psychosis vs. synthetic psychosis: psychopathological and phenomenological profile.
Overall, after an acute or repeated consumption of SC, neurological toxidromes have been described, such as mental status changes, panic attacks, agitation, aggressiveness, memory distortions, depersonalization, dissociation, catatonia, recurrent psychotic episodes (e.g., delusional thoughts and paranoia), and auditory and/or visual hallucinations (1, 7, 96, 116–121). Psychosis developing in the context of a SC intake and/or intoxication is often described as endoformic (e.g., verbal hallucinations, kinesthetic automatisms, and delusion of grandeur or influence can be present), long-lasting (from 10–14 days to 4–6 weeks), and gradually self-resolve with the persistence of asthenic-depressive symptomatology and cognitive deterioration for more than 4–8 weeks (7, 38, 44, 87). However, several findings documented here show that SC-induced psychoses may persist even in those subjects without a previous history of mental illness and may induce the development of a schizophrenia-like symptomatology, named “Spiceophrenia” (7, 25, 111, 122–125). In addition, there is evidence of a worsening/recrudescence of a mental health disorder (i.e., mainly an affective and/or a psychotic disorder) in those subjects with a preexisting mental condition (29, 34, 35, 50, 56–59, 61, 67, 69, 80, 84, 85, 111, 122). Some studies reported that SC may influence psychiatric course and prognosis, depending on the first age of SC exposure, psychiatric vulnerability/predisposition, a history of a childhood trauma or other traumatic experiences, and specific genetic factors (67, 69, 124). Furthermore, SC have been supposed to determine a more severe psychosis, accompanied with agitation and significant sympathomimetic effects, compared to “classical” cannabis as SC are more potent full receptor agonists at cannabinoid receptors and do not contain cannabidiol, which possesses anxiolytic and antipsychotic properties (1, 7, 70, 126). Despite the evidence presented here, some authors maintain that it is difficult to clearly prove a causal linkage between SC intake and the onset of an ex novo psychosis in psychosis-prone/vulnerable subjects and/or the exacerbation of a prodromal psychotic syndrome/appearance of basic symptoms (127). From a therapeutic perspective, benzodiazepines are useful for managing anxiety, agitation, and seizure risk, together with a supportive/symptomatic therapy (1, 7, 30, 32, 36, 39, 41, 46, 48, 59–61, 71, 79, 81). Olanzapine, clozapine, quetiapine, and aripiprazole represent the main prescribed antipsychotic treatments in SC-induced psychoses (1, 7, 37, 50, 57, 60, 62, 65–67, 70, 75, 85, 86), while haloperidol, risperidone, and paliperidone have been prescribed/used in isolated cases (45, 53, 63, 73, 85).
In contrast, published findings reported that synthetic cathinones may cause variable effects in patients with SMI, such as acute psychosis, agitation, violent behavior, confusion, disorientation, insomnia, suicidality, mood lability, and instability in patients affected with bipolar disorder and/or substance poly abuse, while tangential thought process, disorganized speech and behavior, paranoid delusions, and auditory and visual hallucinations are demonstrated in patients affected with schizophrenia, associated with amnesia surrounding their psychotic breaks (88, 90, 91, 93, 94, 96, 100–102, 104, 106, 128). Treatment should be single-minded on managing agitation and psychosis and in supporting renal perfusion. Sedation may be required if the patient is markedly agitated and at risk of self-harm or harm other patients or health professionals (1, 92, 95, 97, 98, 103, 104). In particular, benzodiazepines should be preferred to manage physical violence, to decrease tachycardia and blood hypertension, to prevent seizures, and to reduce muscle hyperactivity, rhabdomyolysis, and renal failure (1). Use of antipsychotics alone should be considered as second-line strategy for managing agitation due to the higher risk in lowering seizure threshold and the higher risk in contributing to the acute toxicity of synthetic cathinones (1, 88, 97, 98, 106). Alternatively, anticonvulsants/mood stabilizers have been suggested (99).
The present review has several limitations which may restrict the generalizability of the findings presented here, e.g., most studies considered here have mainly focused on synthetic cannabinoids which represent the main representative NPS group, but do not necessarily represent all NPS-induced synthetic psychoses. In addition, most studies are represented by case reports, which, on the one hand, may represent a good tool/vehicle to provide more detailed psychopathological and clinical features on NPS consumption and NPS-induced psychosis, but it may not be greatly generalizable to a more representative clinical sample. Most studies do not report if NPS intake is acute or chronic and single or repeated, or do not clearly specify NPS dosage, route of administration, comorbid use of other substances, and so on. Furthermore, the present overview does not possess the scientific robustness of a systematic literature review as most studies are poorly comparable with heterogeneous outcomes and measures. Finally, from a therapeutic point of view, not all studies here considered provide data on therapeutic management and/or strategies. Moreover, those studies providing therapeutic management do not always report dosage(s) and/or treatment duration.
Conclusion
The constantly and rapidly developing NPS drug scenario represents a challenge for public health, especially so for the field of addiction and mental health. Indeed, NPS consumption is usually associated with an imbalance of a plethora of neurotransmitter pathways and brain receptors, and, hence, correlated to a huge repertory of psychopathological trajectories. Vulnerable individuals (e.g., children, adolescents, and subjects affected with a psychiatric disorder) may be more prone to develop and/or worsen a psychopathological condition, particularly a psychosis which may have peculiar features, from a psychopathological and phenomenological point of view. Due to a great range of medical and psychopathological disturbances associated with NPS intake, it is detrimental for mental health professionals not to be ignorant about the effects, safety, and toxicity profile of NPS so far known, especially the most widespread ones discussed here, e.g., SC and synthetic cathinones. Indeed, further researches should better investigate their clinical and pharmacological knowledge so that better personalized management and treatment strategies/guidelines can be written and disseminated.
Author Contributions
All authors listed have made a substantial direct and intellectual contribution to the work and approved it for publication. LO and SC collected, analyzed and interpreted data. LO, SC, together with JC and DP drafted the article. DD and FS revised it critically for important intellectual contents.
Funding
This paper was supported in part by grants of the European Commission (Drug Prevention and Information Programme 2014-16; contract no. JUST/2013/DPIP/AG/4823; EU-MADNESS project).
Further financial support was provided by the EU Commission-targeted call on cross border law enforcement cooperation in the field of drug trafficking - DG Justice/DG Migrations and Home Affairs (JUST/2013/ISEC/DRUGS/AG/6429) Project EPS/NPS (Enhancing Police Skills concerning Novel Psychoactive Substances; NPS).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
1. Schifano F, Orsolini L, Papanti D, Corkery JM. Novel psychoactive substances of interest for psychiatry. World Psychiatry (2015) 14:15–26. doi: 10.1002/wps.20174
2. Corkery JM, Orsolini L, Papanti D, Schifano F. From concept(ion) to life after death/the grave: the 'natural' history and life cycle(s) of novel psychoactive substances (NPS). Hum Psychopharmacol (2017) 32:e2566. doi: 10.1002/hup.2566
3. EMCDDA. European Monitoring Centre for Drugs and Drug Addiction.European Drug ReportTrends and Developments. Publications Office of the European Union. Luxembourg. (2019). 2019. Available at: http://www.emcdda.europa.eu/system/files/publications/11364/20191724_TDAT19001ENN_PDF.pdf (Accessed June 6, 2019). doi: 10.2810/191370
4. Martinotti G, Montemitro C, Corbo M, Sarchione F, Cinosi E, Pasquini A. et al. Use of novel psychoactive substances and induced psychiatric symptoms: Outcomes from the Eivissa Project. 25th European Congress of Psychiatry. Eur Psychiatry (2017) 41:S206. doi: 10.1016/j.eurpsy.2017.01.2164
5. Orsolini L, Papanti GD, De Berardis D, Guirguis A, Corkery JM, Schifano F. The "Endless Trip" among the NPS Users: Psychopathology and Psychopharmacology in the Hallucinogen-Persisting Perception Disorder. A Syst Rev Front Psychiatry (2017) 208:240. doi: 10.3389/fpsyt.2017.00240
6. Schifano F, Orsolini L, Papanti D, Corkery J. NPS: Medical Consequences Associated with Their Intake. Curr Top Behav Neurosci (2017) 32:351–80. doi: 10.1007/7854_2016_15
7. Papanti D, Schifano F, Botteon G, Bertossi F, Mannix J, Vidoni D, et al “Spiceophrenia”: a systematic overview of “spice”-related psychopathological issues and a case report. Hum Psychopharmacol (2013) 28(4):379–89. doi: 10.1002/hup.2312
8. Rubino T, Realini N, Braida D, Guidi S, Capurro V, Viganò D, et al. Changes in hippocampal morphology and neuroplasticity induced by adolescent THC treatment are associated with cognitive impairment in adulthood. Hippocampus (2009) 19(8):763–72. doi: 10.1002/hipo.20554
9. Cass DK, Flores-Barrera E, Thomases DR, Vital WF, Caballero A, Tseng KY. CB1 cannabinoid receptor stimulation during adolescence impairs the maturation of GABA function in the adult rat prefrontal cortex. Mol Psychiatry (2014) 19(5):536–43. doi: 10.1038/mp.2014.14
10. Verrico CD, Gu H, Peterson ML, Sampson AR, Lewis DA. Repeated δ9-tetrahydrocannabinol exposure in adolescent monkeys: persistent effects selective for spatial working memory. Am J Psychiatry (2014) 171(4):416–25. doi: 10.1176/appi.ajp.2013.13030335
11. Aguilar DD, Giuffrida A, Lodge DJ. Adolescent synthetic cannabinoid exposure produces enduring changes in dopamine neuron activity in a rodent model of schizophrenia susceptibility. Int J Neuropsychopharmacol (2018) 121(4):393–403. doi: 10.1093/ijnp/pyy003
12. Rickli A, Hoener MC, Liechti ME. Monoamine transporter and receptor interaction profiles of novel psychoactive substances: para-halogenated amphetamines and pyrovalerone cathinones. Eur Neuropsychopharmacol (2015) 25(3):365–76. doi: 10.1016/j.euroneuro.2014.12.012
13. Coppola M, Mondola R.3, 4-Methylenedioxypyrovalerone (MDPV): chemistry, pharmacology and toxicology of a new designer drug of abuse marketed online. Toxicol Lett (2012) 208(1):12–5. doi: 10.1016/j.toxlet.2011.10.002
14. Prosser JM, Nelson LS. The toxicology of bath salts: a review of synthetic cathinones. J Med Toxicol (2012) 8(1):33–42. doi: 10.1007/s13181-011-0193-z
15. Corkery JM, Schifano F, Oyefeso A, Ghodse AH, Tonia T, Naidoo V, et al. 'Bundle of fun' or 'bunch of problems'? Case series of khat-related deaths in the UK. Drugs. Educ Prev Polic (2011) 18(6):408–25. doi: 10.3109/09687637.2010.504200
16. Corkery JM, Schifano F, Ghodse AH. Mephedrone-related fatalities in the United Kingdom: contextual, clinical and practical issues. Ed.Gallelli L. 2012, Pharmacology. Rijeka, Croatia: InTech p.355-80. doi: 10.5772/32935. Available at: http://www.intechopen.com/books/pharmacology/mephedrone-related-fatalities-in-the-united-kingdom-contextual-clinical-and-practical-issues (Accessed June 15, 2019).
17. Loi B, Corkery JM, Claridge H, Goodair C, Chiappini S, Gimeno Clemente C, et al. Deaths of individuals aged 16-24 years in the UK after using mephedrone. Hum Psychopharmacol (2015) 30(4):225–32. doi: 10.1002/hup.2423
18. Schifano F, Papanti GD, Orsolini L, Corkery JM. Novel psychoactive substances: the pharmacology of stimulants and hallucinogens. Expert Rev Clin Pharmacol (2016) 9(7):943–54. doi: 10.1586/17512433.2016.1167597
19. Schifano F, Napoletano F, Chiappini S, Orsolini L, Guirguis A, Corkery JM, et al. New Psychoactive Substances (NPS), psychedelic experiences and dissociation: clinical and clinical pharmacological issue. Curr Addict Rep (2019) 6(2):140–52. doi: 10.1007/s40429-019-00249-z
20. Fulde GW, Forster SL. The impact of amphetamine-type stimulants on emergency services. Curr Opin Psychiatry (2015) 28(4):275–9. doi: 10.1097/YCO.0000000000000171
21. Stojanovska N, Kelly T, Tahtouh M, Beavis A, Fu S. Analysis of amphetamine-type substances and piperazine analogues using desorption electrospray ionisation mass spectrometry. Rapid Commun Mass Spectrom (2014) 1528(7):731–40. doi: 10.1002/rcm.6832
22. Bersani G, Prevete E. Novel psychoactive substances (NPS) use in severe mental illness (SMI) patients: potential changes in the phenomenology of psychiatric diseases. Hum Psychopharmacol (2017) 32:e2591. doi: 10.1002/hup.2591
23. Cohen K, Weinstein AM. Synthetic and non-synthetic cannabinoid drugs and their adverse effects-a review from public health prospective. Front Public Health (2018) 6:162. doi: 10.3389/fpubh.2018.00162
24. Graddy R, Buresh ME, Rastegar DA. New and emerging Illicit psychoactive substances. Med Clin North Am (2018) 102(4):697–714. doi: 10.1016/j.mcna.2018.02.010
25. Stanley JL, Mogford DV, Lawrence RJ, Lawrie SM. Use of novel psychoactive substances by inpatients on general adult psychiatric wards. BMJ Open (2016) May 106(5):e009430. doi: 10.1136/bmjopen-2015-009430
26. Vallersnes OM, Dines AM, Wood DM, Yates C, Heyerdahl F, Hovda KE, et al. Psychosis associated with acute recreational drug toxicity: a European case series. BMC Psychiatry (2016) 1816:293. doi: 10.1186/s12888-016-1002-7. Erratum in: BMC Psychiatry
27. Liakoni E, Müller S, Stoller A, Ricklin M, Liechti ME, Exadaktylos AK. Presentations to an urban emergency department in Bern, Switzerland associated with acute recreational drug toxicity. Scand J Trauma Resusc Emerg Med (2017) 725(1):26. doi: 10.1186/s13049-017-0369-x
28. Every-Palmer S. Warning: legal synthetic cannabinoid-receptor agonists such as JWH-018 may precipitate psychosis in vulnerable individuals. Addiction (2010)(10), 1859–60. doi: 10.1111/j.1360-0443.2010.03119.x
29. Müller H, Sperling W, Köhrmann M, Huttner HB, Kornhuber J, Maler JM. The synthetic cannabinoid Spice as a trigger for an acute exacerbation of cannabis induced recurrent psychotic episodes. Schizophr Res (2010) 118(1–3):309–10. doi: 10.1016/j.schres.2009.12.001
30. Rodgman C, Kinzie E. Bad Mojo: use of the new marijuana substitute leads to more and more ED visits for acute psychosis. Am J Emerg Med (2011) 29(2):232. doi: 10.1016/j.ajem.2010.07.020
31. Sobolevsky T, Prasolov I, Rodchenkov G. Detection of JWH-018 metabolites in smoking mixture post-administration urine. Forensic Sci Int (2010) 200(1–3):141–7. doi: 10.1016/j.forsciint.2010.04.003
32. Vearrier D, Osterhoudt KC. A teenager with agitation: higher than she should have climbed. Pediatr Emerg Care (2010) 26(6):462–5. doi: 10.1097/PEC.0b013e3181e4f416
33. Benford D, Caplan J. Psychiatric Sequelae of Spice, K2, and Synthetic Cannabinoid Receptor Agonists. Psychosomatics. (2011) 52(3):295. doi: 10.1016/j.psym.2011.01.004
34. Castellanos D, Singh S, Thornton G, Avila M, Moreno A. Synthetic cannabinoid use: a case series of adolescents. J Adolesc Health (2011) 49(4):347–9. doi: 10.1016/j.jadohealth.2011.08.002
35. Every-Palmer S. Synthetic cannabinoid JWH-018 and psychosis: an explorative study. Drug Alcohol Depend (2011)(2-3), 152–7. doi: 10.1016/j.drugalcdep.2011.01.012
36. Forrester MB, Kleinschmidt K, Schwarz E, Young A. Synthetic cannabinoid exposures reported to Texas poison centers. J Addict Dis (2011) 30(4):351–8. doi: 10.1080/10550887.2011.609807
37. Hurst D, Loeffler G, McLay R. Psychosis associated with synthetic cannabinoid agonists: a case series. Am J Psychiatry (2011) 168(10):1119. doi: 10.1176/appi.ajp.2011.11010176
38. Johnson LA, Johnson RL, Alfonzo C. Spice: a legal marijuana equivalent. Mil Med (2011) 176(6):718–20. doi: 10.7205/milmed-d-10-00356
39. Locatelli CA, Lonati D, Giampreti A, Petrolini V, Vecchio S, Rognoni C, et al. New synthetic cannabinoids intoxications in Italy: clinical identification and analytical confirmation of cases. JEM (2011) 41(2):220. doi: 10.1016/j.jemermed.2011.06.114
40. McCain KR, Moran JH, James LP, Foster HR. Adverse effects and confirmatory testing of JWH-018 and JWH-073 following synthetic cannabinoid use. Clin Toxicol (Phila.). (2011) 49(6):519.
41. Simmons J, Cookman L, Kang C, Skinner C. Three cases of "spice" exposure. Clin Toxicol (Phila). (2011) 49(5):431–3. doi: 10.3109/15563650.2011.584316
42. Young AC, Schwarz E, Medina G, Obafemi A, Feng SY, Kane C, et al. Cardiotoxicity associated with the synthetic cannabinoid, K9, with laboratory confirmation. Am J Emerg Med (2012) 30(7):1320. doi: 10.1016/j.ajem.2011.05.013
43. McGuinness TM, Newell D. Risky recreation: synthetic cannabinoids have dangerous effects. J Psychosoc Nurs Ment Health Serv. (2012) 50(8):16–8. doi: 10.3928/02793695-20120703-04
44. Tung CK, Chiang TP, Lam M. Acute mental disturbance caused by synthetic cannabinoid: A potential emerging substance of abuse in Hong Kong. East Asian Arch Psychiatry (2012) 22(1):31–3.
45. Van Der Veer N, Friday J. Persistent psychosis following the use of Spice. Schizophr Res (2011) 130(1–3):285–6. doi: 10.1016/j.schres.2011.04.022
46. Bebarta VS, Ramirez S, Varney SM. Spice: a new "legal" herbal mixture abused by young active duty military personnel. Subst Abus (2012) 33(2):191–4. doi: 10.1080/08897077.2011.637610
47. Cohen J, Morrison S, Greenberg J, Saidinejad M. Clinical presentation of intoxication due to synthetic cannabinoids. Pediatrics. (2012) 129(4):e1064–7. doi: 10.1542/peds.2011-1797
48. Hoyte CO, Jacob J, Monte AA, Al-Jumaan M, Bronstein AC. Heard KJ. A characterization of synthetic cannabinoid exposures reported to the National Poison Data System in 2010. Ann Emerg Med (2012) 60(4):435–8. doi: 10.1016/j.annemergmed.2012.03.007
49. Oluwabusi OO, Lobach L, Akhtar U, Youngman B, Ambrosini PJ. Synthetic cannabinoid-induced psychosis: two adolescent cases. J Child Adolesc Psychopharmacol (2012) 22(5):393–5. doi: 10.1089/cap.2012.0004
50. Peglow S, Buchner J, Briscoe G. Synthetic cannabinoid induced psychosis in a previously nonpsychotic patient. Am J Addict. (2012) 21(3):287–8. doi: 10.1111/j.1521-0391.2012.00222.x
51. Vandrey R, Dunn KE, Fry JA, Girling ER. A survey study to characterize use of Spice products (synthetic cannabinoids). Drug Alcohol Depend (2012) 120:238–41. doi: 10.1016/j.drugalcdep.2011.07.011
52. Barratt MJ, Cakic V, Lenton S. Patterns of synthetic cannabinoid use in Australia. Drug Alcohol Rev (2013) 32(2):141–6. doi: 10.1111/j.1465-3362.2012.00519.x
53. Berry-Cabán CS, Ingram Ee J, Ingram V, Berry CE, Kim EH. Synthetic cannabinoid overdose in a 20-year-old male US soldier. Subst Abus (2013) 34:70–2. doi: 10.1080/08897077.2012.677754
54. Chan WL, Wood DM, Hudson S, Dargan PI. Acute psychosis associated with recreational use of benzofuran 6-(2-aminopropyl)benzofuran (6-APB) and cannabis. J Med Toxicol (2013) 9(3):278–81. doi: 10.1007/s13181-013-0306-y
55. Hermanns-Clausen M, Kneisel S, Szabo B, Auwärter V. Acute toxicity due to the confirmed consumption of synthetic cannabinoids: clinical and laboratory findings. Addiction. (2013) 108(3):534–44. doi: 10.1111/j.1360-0443.2012.04078.x
56. Glue P, Al-Shaqsi S, Hancock D, Gale C, Strong B, Schep L. Hospitalisation associated with use of the synthetic cannabinoid K2. N Z Med J (2013) 28126(1377):18–23.
57. Leibu E, Garakani A, McGonigle DP, Liebman LS, Loh D, Bryson EO, et al. Electroconvulsive therapy (ECT) for catatonia in a patient with schizophrenia and synthetic cannabinoid abuse: a case report. J ECT. (2013) 29(4):e61–2. doi: 10.1097/YCT.0b013e318290fa36
58. Bozkurt M, Umut G, Evren C, Karabulut V. Clinical characteristics and laboratory test results of patients admitted to outpatient clinic for synthetic cannabinoid usage. Dusunen Adam. (2014) 27:328–34. doi: 10.5350/DAJPN2014270407
59. Celofiga A, Koprivsek J, Klavz J.Use of synthetic cannabinoids in patients with psychotic disorders: Case series. J Dual Diagn. (2014) 10(3):168–73. doi: 10.1080/15504263.2014.929364
60. Haro G, Ripoll C, Ibáñez M, Orengo T, Liaño VM, Meneu E, et al. Could spice drugs induce psychosis with abnormal movements similar to catatonia? Psychiatry. (2014) 77(2):206–8. doi: 10.1521/psyc.2014.77.2.206
61. Meijer KA, Russo RR, Adhvaryu DV. Smoking synthetic marijuana leads to self-mutilation requiring bilateral amputations. Orthopedics (2014) 37(4):e391–4. doi: 10.3928/01477447-20140401-62
62. Smith DL, Roberts C. Synthetic marijuana use and development of catatonia in a 17-year-old male. Minn Med (2014) 97(5):38.
63. Durand D, Delgado LL, de la Parra-Pellot DM, Nichols-Vinueza D. Psychosis and severe rhabdomyolysis associated with synthetic cannabinoid use: a case report. Clin Schizophr Relat Psychoses. (2015) 8(4):205–8. doi: 10.3371/CSRP.DUDE.031513
64. Schwartz MD, Trecki J, Edison LA, Steck AR, Arnold JK, Gerona RR. A common source outbreak of severe delirium associated with exposure to the novel synthetic cannabinoid ADB-PINACA. J Emerg Med (2015) 48(5):573–80. doi: 10.1016/j.jemermed.2014.12.038
65. Ustundag MF, Ozhan Ibis E, Yucel A, Ozcan H. Synthetic cannabis-induced mania. Case Rep Psychiatry (2015) 2015:310930. doi: 10.1155/2015/310930
66. Sönmez İ, Köşger F. [Synthetic Cannabinoid Receptor Agonist-Associated Psychotic Disorder: A Case Report]. Turk Psikiyatri Derg. (2016) 27(1):63–6. Spring.
67. Rahmani M, Paul S, Nguyen ML. Treatment of refractory substance-induced psychosis in adolescent males with a genetic predisposition to mental illness. Int J Adolesc Med Health (2014) 26(2):297–301. doi: 10.1515/ijamh-2013-0505
68. Tyndall J, Gerona R, De Portu G, Trecki J, Elie MC, Lucas J, et al. An outbreak of acute delirium from exposure to the synthetic cannabinoid AB-CHMINACA gas-phase metal oxide reactions view project simulation education view project. Clin Toxicol (Phila) (2015) 53(10):950–6. doi: 10.3109/15563650.2015.1100306
69. Altintas M, Inanc L, Oruc GA, Arpacioglu S, Gulec H. Clinical characteristics of synthetic cannabinoid-induced psychosis in relation to schizophrenia: a single-center cross-sectional analysis of concurrently hospitalized patients. Neuropsychiatr Dis Treat (2016) 212:1893–900. doi: 10.2147/NDT.S107622
70. Khan M, Pace L, Truong A, Gordon M, Moukaddam N. Catatonia secondary to synthetic cannabinoid use in two patients with no previous psychosis. Am J Addict. (2016) 25(1):25–7. doi: 10.1111/ajad.12318
71. Hermanns-Clausen M, Kithinji J, Spehl M, Angerer V, Franz F, Eyer F, et al. Adverse effects after the use of JWH-210 - a case series from the EU Spice II plus project. Drug Test Anal (2016) 8(10):1030–8. doi: 10.1002/dta.1936
72. Bassir Nia A, Medrano B, Perkel C, Galynker I, Hurd YL. Psychiatric comorbidity associated with synthetic cannabinoid use compared to cannabis. J Psychopharmacol (2016) 30(12):1321–30. doi: 10.1177/0269881116658990
73. Roberto AJ, Lorenzo A, Li KJ, Young J, Mohan A, Pinnaka S, et al. First-Episode of Synthetic Cannabinoid-Induced Psychosis in a Young Adult, Successfully Managed with Hospitalization and Risperidone. Case Rep Psychiatry (2016) 2016:7257489. doi: 10.1155/2016/7257489
74. Samaan J, Ferrer GF, Akinyemi B, Junquera P, Oms J, Dumenigo R. Synthetic cannabis overdose and withdrawal in a young adult: a case report, commentary on regulation, and review of the literature. Case Rep Psychiatry (2016) 2016:3640549. doi: 10.1155/2016/3640549
75. Ozer U, Ceri V, Evren C. Capgras syndrome after use of synthetic cannabinoids: an adolescent case. Dusunen. Adam. (2016) 29:374–8. doi: 10.5350/DAJPN2016290410
76. Shalit N, Barzilay R, Shoval G, Shlosberg D, Mor N, Zweigenhaft N, et al. Characteristics of synthetic cannabinoid and cannabis users admitted to a psychiatric hospital: a comparative study. J Clin Psychiatry (2016) 77(8):e989–95. doi: 10.4088/JCP.15m09938
77. Waugh J, Najafi J, Hawkins L, Hill SL, Eddleston M, Vale JA, et al. Epidemiology and clinical features of toxicity following recreational use of synthetic cannabinoid receptor agonists: a report from the United Kingdom National Poisons Information Service. Clin Toxicol (Phila). (2016) 54(6):512–8. doi: 10.3109/15563650.2016.1171329
78. Zorlu N, Angelique Di Biase M, Kalaycı ÇÇ, Zalesky A, Bağcı B, Oğuz N, et al. Abnormal white matter integrity in synthetic cannabinoid users. Eur Neuropsychopharmacol. (2016) Nov26(11):1818–25. doi: 10.1016/j.euroneuro.2016.08.015
79. Babi MA, Robinson CP, Maciel CB. A spicy status: synthetic cannabinoid (spice) use and new-onset refractory status epilepticus-A case report and review of the literature. SAGE Open Med Case Rep (2017) 5;5:2050313X17745206. doi: 10.1177/2050313X17745206
80. Manseau MW, Rajparia A, Joseph A, Azarchi S, Goff D, Satodiya R, et al. Clinical Characteristics of Synthetic Cannabinoid Use in a Large Urban Psychiatric Emergency Setting. Subst Use Misuse (2017) 1252(6):822–5. doi: 10.1080/10826084.2016.1263663
81. Monte AA, Calello DP, Gerona RR, Hamad E, Campleman SL, Brent J, et al. ACMT Toxicology Investigators Consortium (ToxIC). Characteristics and Treatment of Patients with Clinical Illness Due to Synthetic Cannabinoid Inhalation Reported by Medical Toxicologists: A ToxIC Database Study. J Med Toxicol (2017) 13(2):146–52. doi: 10.1007/s13181-017-0605-9
82. Kassai S, Pintér JN, Rácz J, Böröndi B, Tóth-Karikó T, Kerekes K, et al. Assessing the experience of using synthetic cannabinoids by means of interpretative phenomenological analysis. Harm Reduct J (2017) 1014(1):9. doi: 10.1186/s12954-017-0138-1
83. Van Hout MC, Hearne E. New psychoactive substances (NPS) on cryptomarket fora: An exploratory study of characteristics of forum activity between NPS buyers and vendors. Int J Drug Policy (2017) 40:102–10. doi: 10.1016/j.drugpo.2016.11.007
84. Welter S, Lücke C, Lam AP, Custal C, Moeller S, Sörös P, et al. Synthetic cannabinoid use in a psychiatric patient population: a pilot study. Eur Addict Res (2017) 23(4):182–93. doi: 10.1159/000479554
85. Bonaccorso S, Metastasio A, Ricciardi A, Stewart N, Jamal L, Rujully NU, et al. Synthetic cannabinoid use in a case series of patients with psychosis presenting to acute psychiatric settings. Clin Presentation Manage Issues. Brain Sci (2018) 8(7):E133. doi: 10.3390/brainsci8070133
86. Sweet G, Kim S, Martin S, Washington NB, Brahm N. Psychiatric symptoms and synthetic cannabinoid use: Information for clinicians. Ment Health Clin (2018) 267(4):156–9. doi: 10.9740/mhc.2017.07.156
87. Skryabin VY, Vinnikova MA. Clinical characteristics of synthetic cannabinoid-induced psychotic disorders: a single-center analysis of hospitalized patients. J Addict Dis (2019) 4:1–7. doi: 10.1080/10550887.2019.1627635
88. Antonowicz JL, Metzger AK, Ramanujam SL. Paranoid psychosis induced by consumption of methylenedioxypyrovalerone: two cases. Gen Hosp Psychiatry (2011) 33(6):e5–6:640. doi: 10.1016/j.genhosppsych.2011.04.010
89. Derungs A, Schietzel S, Meyer MR, Maurer HH, Krähenbühl S, Liechti ME. Sympathomimetic toxicity in a case of analytically confirmed recreational use of naphyrone (naphthylpyrovalerone). Clin Toxicol (Phila). (2011) 49(7):691–3. doi: 10.3109/15563650.2011.592838
90. Penders TM, Gestring R. Hallucinatory delirium following use of MDPVbath Salts. Gen Hosp Psychiatry (2011) 33(5):525–6. doi: 10.1016/j.genhosppsych.2011.05.014
91. Striebel JM, Pierre JM. Acute psychotic sequelae ofbath salts. Schizophr Res (2011) 133(1–3):259–60. doi: 10.1016/j.schres.2011.09.001
92. Adebamiro A, Perazella MA. Recurrent acute kidney injury following bath salts intoxication. Am J Kidney Dis (2012) 59(2):273–5. doi: 10.1053/j.ajkd.2011.10.012
93. Kasick DP, McKnight CA, Klisovic E. "Bath salt" ingestion leading to severe intoxication delirium: two cases and a brief review of the emergence of mephedrone use. Am J Drug Alcohol Abuse. (2012) 38(2):176–80. doi: 10.3109/00952990.2011.643999
94. Lajoie TM, Rich A. Bath salts: a new drug epidemic – a case report. Am J Addict (2012) 21:572–3. doi: 10.1111/j.1521-0391.2012.00286.x
95. Thornton SL, Lo J, Clark RF, Wu AH, Gerona RR. Simultaneous detection of multiple designer drugs in serum, urine, and CSF in a patient with prolonged psychosis. Clin Toxicol (Phila). (2012) 50(10):1165–8. doi: 10.3109/15563650.2012.744996
96. Gunderson EW, Kirkpatrick MG, Willing LM, Holstege CP. Intranasal substituted cathinone “bath salts” psychosis potentially exacerbated by diphenhydramine. J Addict Med (2013) 7(3):163–8. doi: 10.1097/ADM.0b013e31829084d5
97. Khan S, Shaheen F, Sarwar H, Molina J, Mushtaq S. "Bath salts"-induced psychosis in a young woman. Prim Care Companion CNS Disord (2013) 15(1):PCC.12l01417. doi: 10.4088/PCC.12l01417
98. Mangewala V, Sarwar SR, Shah K, Singh T. Bath salts-induced psychosis: a case report. Innov Clin Neurosci (2013) 10(2):10–1.
99. Stoica MV, Felthous AR. Acute psychosis induced by bath salts: a case report with clinical and forensic implications. J Forensic Sci (2013) 58(2):530–3. doi: 10.1111/1556-4029.12038
100. Winder GS, Stern N, Hosanagar A. Are “bath salts” the next generation of stimulant abuse? J Subst Abuse Treat (2013) 44(1):42–5. doi: 10.1016/j.jsat.2012.02.003
101. Bertol E, Mari F, Boscolo Berto R, Mannaioni G, Vaiano F, Favretto D. A mixed MDPV and benzodiazepine intoxication in a chronic drug abuser: determination of MDPV metabolites by LC-HRMS and discussion of the case. Forensic Sci Int (2014) 243:149–55. doi: 10.1016/j.forsciint.2014.08.002
102. Stevenson R, Tuddenham L. Novel psychoactive substance intoxication resulting in attempted murder. J Forensic Leg Med (2014) 25:60–1. doi: 10.1016/j.jflm.2014.04.007
103. Crespi C. Flakka-Induced Prolonged Psychosis. Case Rep Psychiatry (2016) 2016:3460849. doi: 10.1155/2016/3460849
104. Dolengevich-Segal H, Rodríguez-Salgado B, Gómez-Arnau J, Sánchez-Mateos D. Severe psychosis, drug dependence, and hepatitis C related to slamming mephedrone. Case Rep Psychiatry (2016) 2016:8379562. doi: 10.1155/2016/8379562
105. Romanek K, Stenzel J, Schmoll S, Schrettl V, Geith S, Eyer F, et al. Synthetic cathinones in Southern Germany - characteristics of users, substance-patterns, co-ingestions, and complications. Clin Toxicol (Phila). (2017) 55(6):573–8. doi: 10.1080/15563650.2017.1301463
106. Richman EE, Skoller NJ, Fokum B, Burke BA, Hickerson CA, Cotes RO. α-Pyrrolidinopentiophenone (“Flakka”) Catalyzing Catatonia: a case report and literature review. J Addict Med (2018) 12(4):336–8. doi: 10.1097/ADM.0000000000000407
107. Simonato P, Bulsis L, Negri A, Bansal Gurjeet K, Pessa G, Mioni D, et al. “Marvin, the Paranoid Android”: the case of an Alpha-PVP user in the expanding galaxy of NPS. J Psychoactive Drugs (2018) 50(4):306–13. doi: 10.1080/02791072.2018.1447172
108. Cools R. Role of dopamine in the motivational and cognitive control of behavior. Neuroscientist. (2008) 14(4):381–95. doi: 10.1177/1073858408317009
109. Howes OD, Kapur S. The dopamine hypothesis of schizophrenia: version III—the final common pathway. Schizophr Bull (2009) 35(3):549–62. doi: 10.1093/schbul/sbp006
110. Deng H, Verrico CD, Kosten TR, Nielsen DA. Psychosis and synthetic cannabinoids. Psychiatry Res (2018) 268:400–12. doi: 10.1016/j.psychres.2018.08.012
111. Fantegrossi WE, Wilson CD, Berquist MD. Pro-psychotic effects of synthetic cannabinoids: interactions with central dopamine, serotonin, and glutamate systems. Drug Metab Rev (2018) 50(1):65–73. doi: 10.1080/03602532.2018.1428343
112. Martinotti G, Di Nicola M, Quattrone D, Santacroce R, Schifano F, Murray R, et al. Novel psychoactive substances and induced phenomena in psychopathology: the lysergic psychoma. J Psychopathology. (2015) 21:400–5.
113. Callieri B, Felici F. La depersonalizzazione. Psicologia e clinica. (1968) 93. Riv. sperim. Freniat.
115. Baumann MH, Solis E Jr, Watterson LR, Marusich JA, Fantegrossi WE, Wiley JL. Baths salts, spice, and related designer drugs: the science behind the headlines. J Neurosci (2014) 34(46):15150–8. doi: 10.1523/JNEUROSCI.3223-14.2014
116. Liechti M. Novel psychoactive substances (designer drugs): overview and pharmacology of modulators of monoamine signaling. Swiss Med Wkly (2015) 145:w14043. doi: 10.4414/smw.2015.14043
117. Weinstein AM, Rosca P, Fattore L, London ED. Synthetic cathinone and cannabinoid designer drugs pose a major risk for public health. Front Psychiatry (2017) 8:156. doi: 10.3389/fpsyt.2017.00156
118. McGrath J, Welham J, Scott J, Varghese D, Degenhardt L, Hayatbakhsh MR, et al. Association between cannabis use and psychosis-related outcomes using sibling pair analysis in a cohort of young adults. Arch Gen Psychiatry (2010) 67(5):440–7. doi: 10.1001/archgenpsychiatry.2010.6
119. Winstock AR, Barratt MJ. Synthetic cannabis: a comparison of patterns of use and effect profile with natural cannabis in a large global sample. Drug Alcohol Depend. (2013) 131(1-2):106–11. doi: 10.1016/j.drugalcdep.2012.12.011
120. Van Amsterdam J, Brunt T, van den Brink W. The adverse health effects of synthetic cannabinoids with emphasis on psychosis-like effects. J Psychopharmacol (2015) 29(3):254–63. doi: 10.1177/0269881114565142
121. Davidson C, Opacka-Juffry J, Arevalo-Martin A, Garcia-Ovejero D, Molina-Holgado E, Molina-Holgado F. Spicing Up Pharmacology: A Review of Synthetic Cannabinoids From Structure to Adverse Events. Adv Pharmacol (2017) 80:135–68. doi: 10.1016/bs.apha.2017.05.001
122. Fattore L. Synthetic cannabinoids-further evidence supporting the relationship between cannabinoids and psychosis. Biol Psychiatry (2016) 79(7):539–48. doi: 10.1016/j.biopsych.2016.02.001
123. Akram H, Mokrysz C, Curran HV. What are the psychological effects of using synthetic cannabinoids? A systematic review. J Psychopharmacol (2019) 33(3):271–83. doi: 10.1177/0269881119826592
124. Yeruva RR, Mekala HM, Sidhu M, Lippmann S. Synthetic cannabinoids-"spice" can induce a psychosis: a brief review. Innov Clin Neurosci (2019) 16(1-2):31–2.
125. Alipour A, Patel PB, Shabbir Z, Gabrielson S. Review of the many faces of synthetic cannabinoid toxicities. Ment Health Clin (2019) Mar 19(2):93–9. doi: 10.9740/mhc.2019.03.093
126. Castaneto MS, Gorelick DA, Desrosiers NA, Hartman RL, Pirard S, Huestis MA. Synthetic cannabinoids: epidemiology, pharmacodynamics, and clinical implications. Drug Alcohol Depend (2014) 144:12–41. doi: 10.1016/j.drugalcdep.2014.08.005
127. Bennett KH, Hare HM, Waller RM, Alderson HL, Lawrie S. Characteristics of NPS use in patients admitted to acute psychiatric services in Southeast Scotland: a retrospective cross-sectional analysis following public health interventions. BMJ Open (2017) 7(12):e015716. doi: 10.1136/bmjopen-2016-015716
Keywords: psychosis, synthetic psychosis, chemical psychosis, novel psychoactive substances, NPS
Citation: Orsolini L, Chiappini S, Papanti D, De Berardis D, Corkery JM and Schifano F (2019) The Bridge Between Classical and “Synthetic”/Chemical Psychoses: Towards a Clinical, Psychopathological, and Therapeutic Perspective. Front. Psychiatry 10:851. doi: 10.3389/fpsyt.2019.00851
Received: 23 July 2019; Accepted: 30 October 2019;
Published: 20 November 2019.
Edited by:
Liana Fattore, Italian National Research Council (CNR), ItalyReviewed by:
Gabriel Rubio, University Hospital October 12, SpainMercedes Lovrecic, National Institute for Public Health, Slovenia
Copyright © 2019 Orsolini, Chiappini, Papanti, De Berardis, Corkery and Schifano. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Laura Orsolini, laura.orsolini@hotmail.it
 Laura Orsolini
Laura Orsolini Stefania Chiappini
Stefania Chiappini Duccio Papanti1
Duccio Papanti1 Domenico De Berardis
Domenico De Berardis John M. Corkery
John M. Corkery Fabrizio Schifano
Fabrizio Schifano