- Department of Microbiology, Jagannath University, Dhaka, Bangladesh
This conceptual analysis elucidates the microbial interaction inside municipal distribution pipes, subsequent deterioration in the quality of the supply water, and its impacts on public health. Literature review involved a total of 21 original reports on microbiological events inside the water distribution system were studied, summarizing the current knowledge about the build-up of microbes in treated municipal water at various points of the distribution system. Next, original reports from the microbiological analysis of supply water from Bangladesh were collected to enlist the types of bacteria found growing actively. A schematic diagram of microbial interaction among the genera was constructed with respect to the physical, chemical, and microbiological quality of the supply water. Finally latest guidelines and expert opinions from public health authorities around the world are reviewed to keep up with using cutting-edge molecular technology to ensure safe and good quality drinking water for municipal supply.
Introduction
Industrial revolution, urbanization, rapid increase in urban population, and increasing demand for expansion of public health infrastructures, increase in the demand for supply of drinking water from the municipality has led to complete change of the quality of lives in urban and sub-urban areas (1). Sustainable supply of safe drinking water is also a target to be achieved in the sustainable development goals 2030 (2). Therefore, maintaining the quality of the supply water is just as important as developing the infra-structures of water supply and conserving the sources of natural waters. The municipal water supply system acquires the surface water or aquifer water, treats the surface water physically (UV radiation or filtration) or chemically (chlorination), if necessary, and then distributes them through a network of pipes and overhead tanks to the points-of-use (homes, industries, public places, health care facilities, etc.). The quality and safety of the water at the receiving ends depend on the quality of the source from which it is acquired, the nature of treatment given in the municipal water treatment plant, the amount of residual disinfectant remaining in the water and the environments in the distribution network (pipes and overhead/underground reservoirs) (3). A good number of intrinsic and extrinsic factors determine the microbiological safety of the municipal (mains) water. Intrinsic factors include the length and duration of the treatment given to the source water, material, and length of the distribution pipes, total carbon, iron, lead, phosphate, and sulfate contents of the water, physical parameters of the water (pH, alkalinity, turbidity, hardness, conductivity), biological oxygen demand (BOD), chemical oxygen demand (COD), dissolved oxygen (DO), loose deposit accumulation inside the pipes, and the overhead tanks and microbes remaining in the supply water (4). All these factors might contribute to the quality deterioration potential (QDP) of the supply water as well (5). External factors also contribute to QDP. The deterioration of the quality of potable waters from distribution network emerged a decade ago when foul-odor or reddish color or incidences of waterborne diseases became a pressing issue (6). Subsequent investigation revealed that organisms from the natural water source, that survive the disinfection process, thrive inside the water distribution system, and interact among themselves as well as with the surface of the distribution pipes to form complex biofilms. This biofilm is able to deteriorate the safety and quality of the supplied water in more than one ways (7). Developed countries with full-scale mains distribution system employ task forces to study and control the in-process change of supply water throughout its passage in the distribution network.
Bangladesh, a nation of 170 million people, has 204 municipalities that supply treated or untreated surface water and groundwater in urban and sub-urban areas [Dhaka Water and Sewage Authority (DWASA) Annual Report 2015–16]1 The capital Dhaka contains the oldest and the largest pipe network for water supply (DWASA Annual Report 2012–13)2 Seasonal epidemics of waterborne diseases are common in Dhaka city (8). Few reports are found referring to the waterborne outbreaks at the beginning of monsoon and the foul quality of water, but the mechanism of water quality deterioration inside the supply network in Bangladesh is yet to find. This conceptual analysis summarizes information from the developed countries and predicts the possible events inside a Bangladesh supply network that poses health hazards to the consumers. The high incidences of morbidity and mortality from waterborne diseases call for re-evaluation of the surveillance, monitoring, and in-process control of the municipal water supply system. According to the annual report of Dhaka Water Supply and Sewage Authority (DWASA 2015–16), the organization supplied 2,450 million liters of water daily and in 2016 from four water treatment plants. There are 3,500 km of water lines connected to 361,938 household supplied from 38 overhead tanks, which also are points of biofouling. In addition, 1,643 hydrants moisturize the streets and highways. The Microbiology and Chemical Division of DWASA measures 50 parameters of the supply water to ensure safety and quality, but the emerging risks in mains water is not assayed (DWASA Annual Report 2012–13). DWASA follows the previous guidelines from WHO (4), which does not include the emerging biological and chemical risk factors known today. Despite the best efforts, risks are mounting from drinking water which common people consider safe to drink. This conceptual analysis brings to light the probable risk factors present in the water supplied by DWASA, so that modern techniques are introduced for water safety. The WHO puts emphasis on chemical residues and microbial interaction in drinking water (9) Risks associated with biofilm formation are enrichment of pathogens in the water, production of toxins, deterioration of the pipe material, release of antibiotic resistance genes, and supporting high-risk parasites such as Cryptosporidium and Naegleria fowlerii feeding off the biofilm (10).
This conceptual analysis attempts to predict the microbial interactions inside the water distribution pipelines, especially development of a biofilm consortium inside the pipes in Bangladesh so that an emerging risk to public health can be dealt with.
Theoretical Framework for the Conceptual Analysis
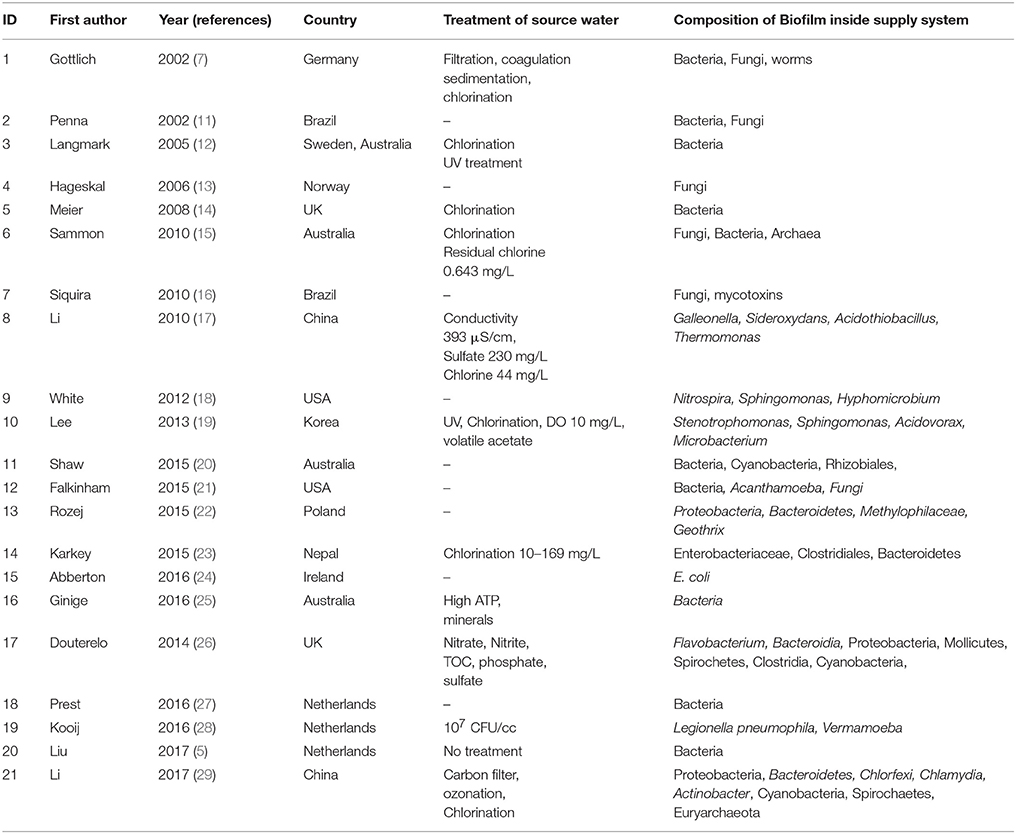
Every natural environment has its own microbial community, that plays characteristic function depending on the interaction between each species in the community. The bacteria present in tap water are likely to represent the species present in the biofilms inside the distribution system. The original reports on bacterial isolates discovered from tap water around the world (Table 1) and in Bangladesh were enlisted (Table 2). The interaction between each pair of species in biofilm were studied from original articles on dual-species biofilm formation experiments. A theoretical diagram was constructed to hypothesize the probable interaction of the reported bacterial species in the biofilm consortium (Figure 1). Interactions between the bacteria and other common members of the biofilm in water distribution system in other countries were studied (Table 1) and most common organisms associated with any given member of the hypothetical biofilm was included in Figure 1 because protozoa, viruses, and worms constitute matured biofilms inside the water supply pipes and pose considerable threat to consumers. The impact of the hypothetical biofilm on corrosion of the water distribution pipes and deterioration of water quality was studied from reports on water quality maintenance from around the world.
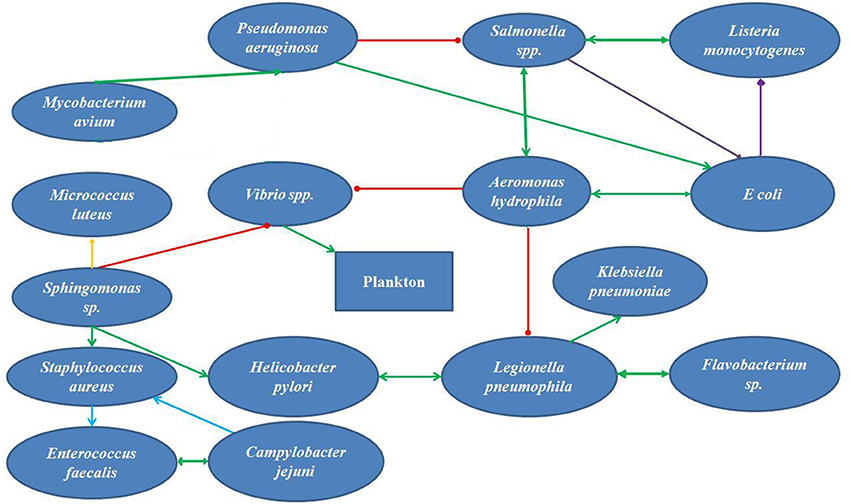
Figure 1. A schematic diagram for interaction among the bacteria found in supplied water in Bangladesh (cooperation  , inhibition
, inhibition  , competition
, competition  , co-aggregation
, co-aggregation  , benefit to one species
, benefit to one species  ).
).
Literature Review and Inclusion-Exclusion Criteria
Literature review was done in three stages. First, Google Scholar, Pubmed, and the Cochrane Library were searched with keywords biofilm, water distribution system, municipality water pipes which returned 100 of results. Original reports that mentioned primary physico-chemical parameters of water and identified microbial species from biofilms developed inside municipal water supply pipes were included in this analytical report. Reports that did not mention the physico-chemical parameters or that did not identify biofilm members upto genus level were omitted. Gray literature was also omitted from literature review because information emerging from experiments done without strict adherence to established protocols might provide inaccurate information about biofilm composition. From the 21 relevant original reports on biofilms inside water distribution pipes listed in Table 1, the patterns of biofilms formed in temperate regions could be outlined. These studies carried out between 2002 and 2017 in Europe, the US, Australia, China, Korea, and Brazil show varied types of microbial populations in biofilms. Secondly, original reports on identification of microbial genera from municipal supply water in Bangladesh were searched in Google Scholar, Pubmed, and the Cochrane Library using search words Bangladesh, WASA supply water, tap water, microorganisms. A total of 11 original reports from Bangladesh satisfied the inclusion-exclusion criteria mentioned above and were included for constructing the schematic diagram of biofilm inside water supply pipes in Bangladesh (Table 2). Thirdly, Google Scholar, Pubmed, and the Cochrane Library were searched using keywords interaction of microbial species in biofilm to retrieve original reports of pair-wise microbial interaction in biofilms. A total of 13 articles that explored metabolic interaction between two bacterial species in an experimental biofilm in the laboratory control environment were included. Articles that could not conclude the specific interaction between dual- species were excluded from this study.
Biofims Inside Water Supply Pipes
Microbial interactions differ depending on physico-chemical parameters of the natural waters. Temperature, pH, conductivity, turbidity, DO, type, and amounts of minerals, total organic Carbon (TOC), total Nitrogen, and biological/CODs are the non-biologic parameters that set the limits for microbial life (34). Different types of water treatment (desalination, decalcification, sedimentation) improve the physical quality of the source water (35). The distinct pattern of microbial interaction in aquatic environments is determined by the microbial population and the abiotic factors present. The microbes form and thrive in a biofilm through biomass transfer from the organic Carbon and microbial growth on any solid support (36). From the 21 relevant original reports on biofilms inside water distribution pipes listed in Table 1, the patterns of biofilms formed in temperate regions could be outlined. These studies carried out between 2002 and 2017 in Europe, the US, Australia, China, Korea, and Brazil show varied types of microbial populations in biofilms. The summer temperature in Europe and the North America is around 15 to 25°C and the natural population of the surface waters is diverse genera of bacteria, fungi, molds, bacteriophages, aquatic viruses, parasites, and in some cases Archaea. Brazil and Australia have an average summer temperature of 30°C, closer to the summer temperature of Bangladesh. Turbidity of the natural waters in the studies ranged from 0.4 to 58 NTU, closer to the range of the turbidity of surface waters around Dhaka city. The pH of the waters in the studies also falls within the range of Bangladesh river waters. The TOC content was stated to be 8.5 mg/L in one of the reports (31). The water distribution pipes composed of a wide range of materials depending on the soil type, depth, water pressure, flow, and retention pattern, intended life of the pipes etc. The pipe materials include unplansticized polyvinyl chloride (PVCu), chlorinated polyvinylchloride (PVCc), polyethylene-100 (PE-100), steel, polypropylene (PP), latex, polybutyrate (PB), copper, and high-density polyethylene (HDPE), all providing appropriate surface for biofilms to thrive (19). The type and duration of water treatment for disinfection reduces the numbers of organisms present in the natural waters. Most municipalities use multistep water treatments for reduction of unwanted minerals through filtration, flocculation, sedimentation, and disinfection (35). Chlorination and UV radiation are the most widespread methods for supply water disinfection. While UV radiation does not produce residual effect, chlorination is allowed to leave a threshold of 5 mg/L residual free chlorine so that any remaining pathogens are gradually killed on their way to the receiving end (37). However, resistant microbes can survive chlorination and establish complex biofilms inside the supply pipes, deteriorating the quality of supply waters (38). As evident from Table 1, the organisms that establish biofilms successfully inside a municipal distribution system range from pathogens (Aeromonas hydrophila, Salmonella, Klebsiella, Pseudomonas aeruginosa, Legionella pneumophila, Escherichia coli), opportunists (Stenotrophomonas moltophilia, Mycobacterium avis complex), toxin producers (Cyanobacteria) to non-pathogens that destroy the pipe material and cause biofouling (Galleonella, Siderooxydans, Geothrix, Nitrospira). The reports from temperate weather shows enrichment of molds in the biofilm (Penicillium, Alternaria, Fusarium, Aspergillus, Mucor, Geotrichum, Botrytis) (13), whereas reports from Australia and Brazil with higher temperatures show biofilms that are dominated by heterotrophic bacterial species (11, 16, 20, 25).
Microbiological Analysis of Tap Water from Bangladesh
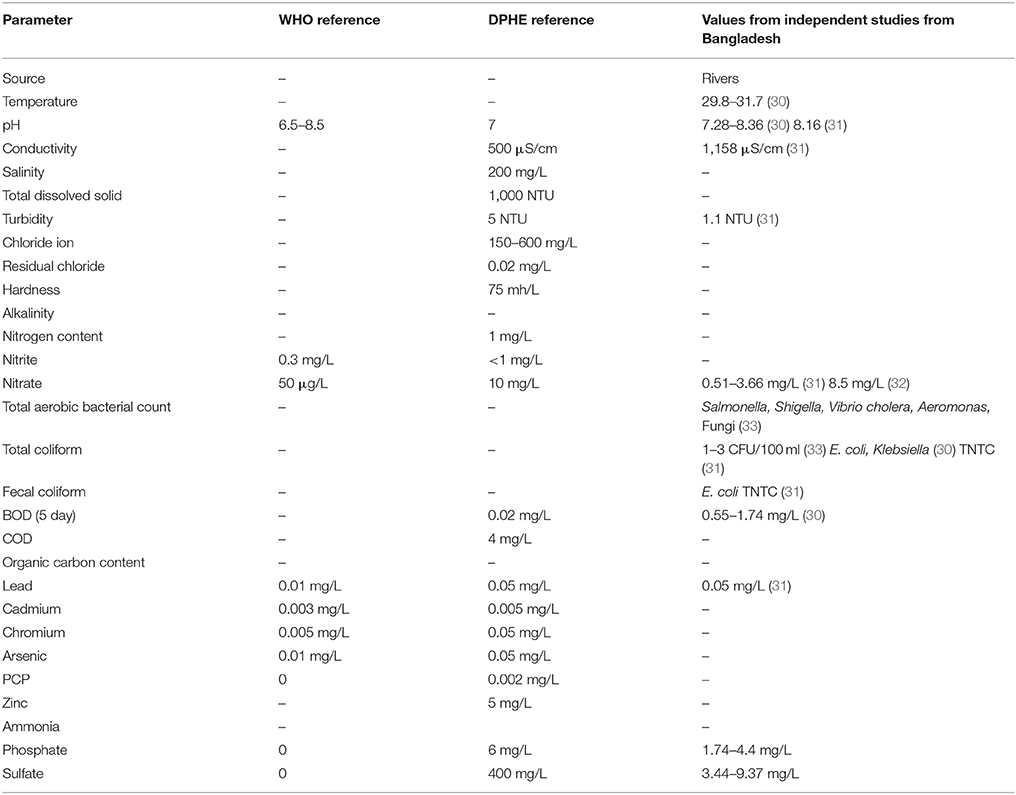
The best-studied water supply system in Bangladesh is in the capital Dhaka, which acquires water from rivers Meghna, Buriganga, Sitalakshya, and Turag, treats them in water treatment plans in Gandharbapur, Saidabad, Rupganj, and Pagla (39). They treat water with sedimentation and chlorination, test the water for safety and drinking quality and supply it through the pipes to overhead tanks, from which water goes into points of use. Mahbub et al. (40) reported finding live bacteria in more than 60% of the sampled tap water in their study, which exceeds the Bangladesh Standards (BDS 1240:2001) for the microbiological quality of water. The Bangladesh Department of Public Health and Engineering (DPHE) has set different set of standards for potable water in Bangladesh, which varies from the universal standards set by the World Health Organization (WHO) in many parameters. Coliforms and E. coli are frequently reported in supply water (31, 33, 41). Acharjee et al. (33, 42) had reported finding E. coli, Klebsiella, Salmonella, Shigella, Vibrio, Aeromonas, and fungi from supply water, indicating that these bacteria and molds survive disinfection procedures. When taken together with the quality of raw water with higher concentration of Iron and Arsenic, we find factors limiting certain kinds of microbes in the distribution system.
Theoretical Composition of Biofilm Inside the Wasa Water Supply Pipes in Bangladesh
If we summarize the parameters reported from independent original studies, we can set the parameters of the supply water within the reported ranges. The temperature of the water in summer is around 30°C (30). The pH of the natural water varies between 7 and 8 and the DO ranges between 3 and 5 mg/L. The conductivity of the waters is 1,158 μS/cm, much higher than the reference value for natural waters (30). The turbidity of the water is 1.1 NTU. The nitrate concentrate was 8.5 mg/L, closer to the upper limit of nitrate concentration (33). The Iron concentration was 0.05 mg/L, and phosphate and sulfate concentrations were 4.4 and 9 mg/L, respectively. The tap water for domestic use contained 0.02 mg/L of residual Chlorine (43). Microbes that form biofilm within the water supply pipes must be organisms with their growth optimums within these ranges. According to Li et al. (29) the turbidity, ammonia concentration, nitrate content and TOC content of the water inside the supply system influence the nature and extend of biofilm formation. Pinto et al. (44) reported that the seasonal cycling of the biofilms inside the pipes correlated with seasonal temperature fluctuations. Bacteria that survive and develop biofilms under these conditions would be E. coli, Shigella, Vibrio, Klebsiella, Salmonella, L. pneumophila, Flavobacterium, Sphingomonas, P. aeruginosa, Nitrospira, Actinobacterium, Acidobacterium, Aeromonas, Sphingobacterium, Mycobacterium avium, Bacteroidium, Clostridia, Spirochaetes, Acremonium, Cladosporium, Fusarium, Microbacterium, Stenotrophomonas. Penicillium and Aspergillus are the most abundant molds in the natural waters in the tropics. In addition, Cyanobacteria could survive the parameters of Bangladeshi natural waters and contribute to the toxin production. The biofilm forming bacteria have different aspects regarding their roles on public health. Legionella pneumophila, Salmonella, E. coli, Shigella, Vibrio, Klebsiella, and Clostridia are pathogens that pose considerable threat to consumers. L. pneumophila causes life-threatening pneumonia and respiratory distress (28). Salmonella, Shigella, Vibrio, Klebsiella, and E. coli are waterborne agents of enteric diseases, often causing seasonal outbreaks in Dhaka city (8). Opportunistic waterborne pathogens include M. avium complex, Stenotrophomonas maltophilia, and A. hydrophila that can infect immune-compromised groups such as infants, adults, pregnant women, and people with underlying medical conditions (cancer, HIV/AIDS, etc.). M. avium causes pulmonary, soft tissue and lymph node infections (45, 46). Stenotrophomonas maltophilia causes respiratory infection in cystic fibrosis patients (47). Aeromonas hydrophila is an enteric pathogen infecting children and immunocompromised people (48). The aquatic biofilms have also been implicated in spread of drug-resistance genes. Talukdar et al. (41) had shown presence of extended spectrum beta-lactam (ESBL) E. coli and qnrS elements for quinolone resistance from tap water in Dhaka city. Biofilm microbes also produce metabolites and components that change the quality of drinking water. Aspergillus spores are allergenic (49). The odor in tap water often results from dimethyl polysulfides, produced by Pseudomonas, Flavobacterium, Aeromonas, and Penicillium (50). Sulfur oxidizers like Acidobacterium change the pH of the water and produce foul odor and taste (51). Nitrospira spp. are nitrite-oxidizing autotrophs, colonizing plastic surfaces (52). Metal oxidizing bacteria corrode metal pipes (53). All these information stress on the development of robust and sensitive analytical techniques for evaluation of water quality as well as revised maintenance procedures that would help reduce formation of biofilms inside municipal water distribution pipes. Figure 1 presents a simplified hypothetical diagram of the microbial interactions inside a municipal water supply pipe.
Hypothetical Interaction of Microbial Isolates from Wasa Water in Bangladesh
Over the last decade, original report of microbiological analysis of tap water from Bangladesh mentioned isolating viable cells of coliforms and E. coli (40), Klebsiella, Salmonella, Shigella, Vibrio, Aeromonas, and fungi (33). Literature search on interaction of each pair of these microorganisms in a dual-species interaction helps to construct a hypothetical network of microbes in the water supplied by WASA (Figure 1). Aeromonas hydrophila showed positive interaction Salmonella spp. and Listeria monocytogenes (54, 55) Vibrio spp. (56), but is inhibited by P. aeruginosa at the planktonic phase of biofilm development (57). Salmonella competes with E. coli in biofilm (58). E. coli maintains cooperative interaction with A. hydrophila (59) and P. aeruginosa (60) in a biofilm. P. aeruginosa and M. avium mutually benefit each other (21). Vibrio spp., the major recruiter of plankton in biofilms (61) are supported by A. hydrophila (56) Sphingomonas sp. enhanced sustainance of S. aureus (62) and Helicobacter pylori in biofilm (63). Sphingomonas coaggregates with Micrococus luteus (64). Biofilm-forming potential S. aureus and Enterococcus faecalis are enhanced by the presence of Campylobacter jejuni (65). Aeromonas hydrophila inhibited L. pneumophila (66), but maintained mutually beneficial interactions with H. pylori (63), Klebsiella pneumonia and Flavobacterium (67, 68).
Recommendations for Risk Alleviation
Public health microbiologists and engineers put much emphasis on preventing formation of complex biofilms inside the municipal water supply network. WHO guidelines suggests that national priorities should be determined while designing the public health infra-structure. A maximum of one water-borne infection per 10,000 consumers per year is an acceptable level for quality drinking water. The first consideration for building a good quality water treatment and supply network starts from identifying the source of water, identifying hazards quantitatively, constructing the pipe network, a cleansing regime should be in practice devised according to the material and the longevity of the pipes. Safe water framework from the WHO constitutes of an iterative method where quantitative microbial risk assessment method outlines the hazard analysis. If the hazards are identified quantitatively, control measures are sought for log removal of microbial hazard and for reducing chemical hazard (carcinogen, irritant, nitrification, and biocorrosion) to an acceptable level. Water pressure inside the pipes and the possibility of leakage along the pipes must be monitored closely. Alternate source of supply water for non-potable purpose could reduce the cost of municipal water treatment. Husna and Rahman (69) re-evaluated the necessity of rainwater harvesting in Dhaka city for industrial purpose. Real-time monitoring with commercial sensors and microchip-based devices should be in place to assess the physical, chemical, and microbiological quality of water. There are critical values of fluoride, nitrate, lead, chromium, arsenic, and pesticide concentrations in drinking water that counter biofilm formation inside the pipe distribution network, but these are detrimental to health at higher concentrations. Silvestry-Rodriguez et al. (70) reported that 100 μg/L of Silver can prevent biofilm formation in PVC and steel pipes but affects of elemental silver on consumer health needs to be studied. Hitzfeld et al. (71) discussed the effectivity of 1.5 mg/L of ozonation for 30 min with a residual concentration of 0.6 mg/L sufficiently maintaining Cyanobacteria under control, saving the water system from toxins. The United States Environment Protection Agency (72) put forward experimental techniques to disrupt chlorine-resistant biofilms by using anti-quorum sensing molecules, such as UW85 from Bacillus cereus. Another approach to getting safe drinking water is the treatment of municipal water at point-of-use, such as microfiltered water dispensation system or reverse osmosis water dispensers installed at hospitals, households, or nurseries (73). Figure 2 summarizes current and proposed preventing measures for biofilm build-up inside the water distribution pipes. National water safety plans should combine total quality management (TQM) and ISO 14001 and ISO9001 to ensure certified standards approved by independent group.
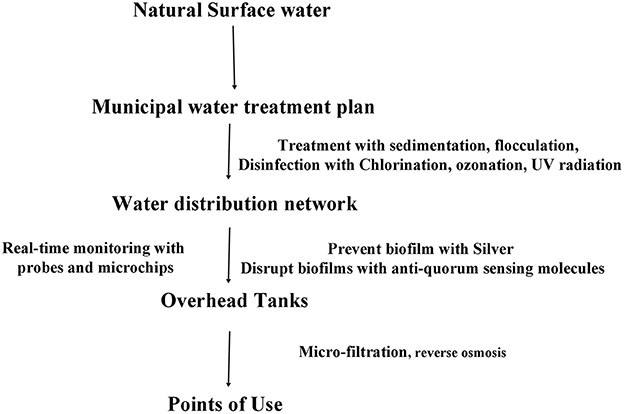
Figure 2. Diagram of preventive and disruptive measures for biofilm control and elimination at different stages of the municipal water supply network.
Conclusion
The multi-faceted problem of development of biofilms inside the municipal water distribution pipe has not been addressed in Bangladesh yet. Microbial interactions get more complicated with virulence genes, especially antimicrobial resistance genes, that subjects consumers to even larger threat of antimicrobial resistance epidemic. The incidences of water-borne infections together with occasional deterioration in the quality of supply waters (odor, discoloration) calls for cutting-edge in-line real-time monitoring facilities with prompt interventions. Biological active Carbon (BAC) filters, granular active carbon (GAC) filter, UV lights, contact chlorine treatment, and an engineered storage and distribution might prove useful in improving water quality and safety.
Author Contributions
This manuscript enlists a single author, who reviewed scientific literature, formulated the study question and developed a theoretical model for the probable kind of microbial interaction inside water supply network in Bangladesh.
Conflict of Interest Statement
The author declares that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Footnotes
1. ^ Dhaka Water and Sewage Authority (DWASA) Annual Report 2015–16. Available online at: http://dwasa.org.bd/annual-reports/2015-16.
2. ^ Dhaka Water and Sewage Authority (DWASA) Annual Report 2012–13. Available online at: http://dwasa.org.bd/annual-reports/2012-13.
References
1. WHO. World Bank documentation of Water safety and Distribution System (2014). Available online at: www.who.int/water_sanitation_health/publications/Water_safety_distribution_systems_2014v1.pdf
2. The United Nations Sustainable Development Goals (2015). Available online at: http://www.un.org/sustainabledevelopment
3. The World Health Organization. Guidelines for Drinking-Water Quality: Surveillance and Control of Community Supplies. 2nd Edn, Vol. 3 (1997), p 83–91. Available online at: http://www.who.int/water_sanitation_health/publications/small-water-supplies-guidelines/en/
4. The World Health Organization. Chapter 8 Guidelines for Drinking-Water Quality. 3rd Edn, Vol 1 (2004), p. 145–192.
5. Liu G, Tao Y, Zhang Y, Lut M, Knibbe WJ, Wielen P, et al. Hotspots for selected metal elements and microbes accumulation and the corresponding water quality deterioration potential in an unchlorinated drinking water distribution system. Water Res. (2017) 124:435–45. doi: 10.1016/j.watres.2017.08.002
6. Volk CJ, LeChevalier M. Impacts of the reduction of nutrient levels on bacterial water quality in distribution systems. Appl Environ Microbiol. (1999) 65:4957–66.
7. Göttlich E, van der Lubbe W, Lange B, Fiedler S, Melchert I, Reifenrath M, et al. Fungal flora in groundwater-derived public drinking water. Int. J. Hyg. Environ. Health (2002) 200:269–79. doi: 10.1078/1438-4639-00158
8. Schwartz BS, Harris JB, Khan AI, Larocque RC, Sack DA, Malek MA, et al. Diarrheal epidemics in Dhaka, Bangladesh, during three consecutive floods: 1988:1998, and 2004. Am J Trop Med Hyg. (2006) 74:1067–73.
9. The World Health Organization. Chapter 11 Guidelines for Drinking-Water Quality. 4th Edn. (2011), p. 231–305.
10. Wang J, Stanford K, McAllister TA, Johnson RP, Chen J, Hou H, et al. Biofilm formation, virulence gene profile and anti-microbial resistance of non-O157 shiga toxin producing E. coli. Foodborne Pathogen Dis. (2016) 13:316–24. doi: 10.1089/fpd.2015.2099
11. Penna VTC, Martinus SAM, Mazzola PG. Identification of bacteria in drinking and purified water during the monitoring of a typical water purification system. BMC Public Health. (2002) 2:13. doi: 10.1186/1471-2458-2-13
12. Langmark J, Storey MV, Ashbolt NJ, Stenstrom TA. Accumulation and fate of microorganisms and microspheres in biofilms formed in a pilot-scale water distribution system. Appl Environ Microbiol. (2005) 71:706–12. doi: 10.1128/AEM.71.2.706-712.2005
13. Hageskal G, Knutsen AK, Gaustad P, Hoog GSD, Skaari I. Diversity and significance of mold species in norwegian drinking water. Appl Environ Microbiol. (2006) 72:7586–93. doi: 10.1128/AEM.01628-06
14. Meier TR, Maute CJ, Cadillac JM, Lee JY, Righter DJ, Hugunin KMS, et al. Quantification, distribution, and possible source of bacterial biofilm in mouse automated watering systems. J Am Assoc Lab Anim Sci. (2008) 47:63–70. doi: 10.1524/9783486843460.1
15. Sammon NB, Harrower KM, Fabbro LD, Reed RH. Incidence and distribution of microfungi in a treated municipal water supply system in sub-tropical Australia. Int J Environ Res Public Health (2010) 7:1597–611. doi: 10.3390/ijerph7041597
16. Siquira VM, Oliviera HMB, Santos C, Paterson RRM, Gusmao NB, Lima N. Filamentous fungi in drinking water, particularly in relation to biofilm formation. Int J Environ Res Public Health (2011) 8:456–69. doi: 10.3390/ijerph8020456
17. Li D, Li Z, Yu J, Cao N, Liu R, Yangi M. Characterization of bacterial community structure in a drinking water distribution system during an occurrence of red water. Appl Environ Microbiol. (2010) 76:7171–80. doi: 10.1128/AEM.00832-10
18. White CP, DeBry RW, Lytles DA. Microbial survey of a full-scale, biologically active filter for treatment of drinking water. Appl Environ Microbiol. (2012) 78:6390–4. doi: 10.1128/AEM.00308-12
19. Lee Y. An evaluation of microbial and chemical contamination sources related to the deterioration of tap water quality in the household water supply system. Int. J. Environ. Res. Public Health (2013) 10:4143–60. doi: 10.3390/ijerph10094143
20. Shaw JLA, Monis P, Weyrich LS, Sawade E, Drikas M, Cooper AJ. Using amplicon sequencing to characterize and monitor bacterial diversity in drinking water distribution systems. Appl Environ Microbiol. (2015) 81:6463–73. doi: 10.1128/AEM.01297-15
21. Falkinham JO, Pruden A, Edwards M. Opportunistic premise plumbing pathogens: increasingly important pathogens in drinking water. Pathogens (2015) 4:373–86. doi: 10.3390/pathogens4020373
22. Rozej A, Cydzik-Kwiatkowska A, Kowalska B, Kowalski D. Structure and microbial diversity of biofilms on different pipe materials of a model drinking water distribution systems. World J Microbiol Biotechnol. (2015) 31:37–47. doi: 10.1007/s11274-014-1761-6
23. Karkey A, Jombart T, Walker AW, Thomsons CN, Torres A, Dondo S, et al. The ecological dynamics of fecal contamination and Salmonella typhi and Salmonella paratyphi a in municipal kathmandu drinking water. PLoS Negl Trop Dis. (2015) 10:e0004346. doi: 10.1371/journal.pntd.0004346
24. Abberton CL, Bereschenko L, van der Wielen PWJJ, Smith CJ. Survival, biofilm formation, and growth potential of environmental and enteric Escherichia coli strains in drinking water microcosms. Appl Environ Microbiol. (2017) 82:5320–32. doi: 10.1128/AEM.01569-16
25. Ginige MP, Garbin S, Wylie J, Krishna KCB. Effectiveness of devices to monitor biofouling and metals deposition on plumbing. Materials exposed to a full-scale drinking water distribution system. PLoS ONE (2017) 12:e0169140. doi: 10.1371/journal.pone.0169140
26. Douterelo I, Sharpe R, Boxal E. Bacterial community dynamics during the early stages of biofilm formation in a chlorinated experimental drinking water distribution system: implications for drinking water discolouration. J Appl Microbiol. (2014) 117:286–301. doi: 10.1111/jam.12516
27. Prest EI, Weissbrodt DG, Hammes F, van Loosdrecht MCM, Vrouwenvelder JS. Long-term bacterial dynamics in a full-scale drinking water distribution system. PLoS ONE (2016) 11:e0164445. doi: 10.1371/journal.pone.0164445
28. Kooij DVD, Bakker GL, Italiaander R, Veenendaal HR, Wullings BA. Biofilm composition and threshold concentration for growth of Legionella pneumophila on surfaces exposed to flowing warm tap water without disinfectant. Appl Environ Microbiol. (2016) 83:e02737–16. doi: 10.1128/AEM.02737-16
29. Li Q, Xia PF, Tao ZY, Wang SG. Modeling biofilms in water systems with new variables: a review. Water (2017) 9:462. doi: 10.3390/w9070462
30. Real KH, Khanam N, Mia MY, Nasreen M. Assessment of water quality and microbial load of Dhaleshwari River Tangail, Bangladesh. Adv Microbiol. (2017) 7:523–33. doi: 10.4236/aim.2017.76041
31. Kormoker T, Proshad R, Khan MM. Analysis of water quality in urban water supply system of Bangladesh. J Env Analyt Toxicol. (2017) 7:4. doi: 10.4172/2161-0525.1000492
32. Janzon A, Sjöling A, Lothigius A, Ahmed D, Qadri F, Svennerholm AM. Failure to detect Helicobacter pylori DNA in drinking and environmental water in Dhaka, Bangladesh, using highly sensitive real-time PCR assays. Appl Environ Microbiol. (2009) 75:3039–44. doi: 10.1128/AEM.02779-08
33. Acharjee M, Rahman F, Beauty SA, Feroz F, Rahman MM, Noor R. Microbiological study on supply water and treated water in Dhaka City. Stamford J Microbiol. (2011) 1:42–5 doi: 10.3329/sjm.v1i1.9132
34. Andrew DR, Fitak RR, Munguia-Vega A, Racolta A, Martinson VG, Donstova K. Abiotic factors shape microbial diversity in Sonoran deserts soils. Appl Environ Microbiol. (2012) 78:7527–37. doi: 10.1128/AEM.01459-12
35. CDC. Center for Disease Control and Prevention Documentation on Water Treatment (2015). Available online at: https://www.cdc.gov/healthywater/drinking/public/water_treatment.html
36. Stewart PS. Diffusion in biofilms. J. Bacteriol. (2003) 185:1485–91. Available online at: http://jb.asm.org/content/185/5/1485
37. Kinsey GC, Paterson RR, Kelley J. Methods for the determination of filamentous fungi in treated and untreated waters. J Appl Microbiol SympSuppl. (1999) 85, 214S−24S.
38. Fish KE, Collins R, Green NH, Sharpe RS, Douterelo I, Osborn M.A, Boxall JB. Characterisation of the physical composition and microbial community structure of biofilms within a model full-scale drinking water distribution system. PLoS ONE (2016) 10:e0115824. doi: 10.1371/journal.pone.0115824
39. Khan TA. Dhaka water Supply and Sewerage Authority: Performance and Challenges. (2015). Available online at: http://app.dwasa.org.bd/admin/news/Dhaka%20WASA%20Article-for%20BOOK.pdf
40. Mahbub KR, Nahar A, Ahmed MM, Chakraborty A. Quality analysis of dhaka WASA drinking water: detection and biochemical characterization of the isolates. J Environ Sci Nat Res. (2011) 4:41–49. doi: 10.3329/jesnr.v4i2.10133
41. Talukdar PK, Rahman M, Rahman M, Nabi A, Islam Z, et al. Antimicrobial resistance, virulence factors and genetic diversity of Escherichia coli isolates from household water supply in Dhaka, Bangladesh. (2013) PLoS ONE 8:e61090. doi: 10.1371/journal.pone.0061090
42. Acharjee M, Rahman F, Jahan F, Noor R. Bacterial proliferation in municipal water supplied in mirpur locality of Dhaka City, Bangladesh. Clean Soil Air Water (2014) 42:434–41 doi: 10.1002/clen.201200618
43. Department of Public Health Engineering (DPHE) Water Quality parameters Bangladesh Standards and WHO Guidelines. (2011). Available online at: http://dphe.gov.bd/index.php?option-com_contentandview-articleandid-125&itemid-133
44. Pinto AJ, Schroeder J, Lunn M, Sloan W, Raskin L. Spatial-temporal survey and occupancy-abundance modeling to predict bacterial community dynamics in the drinking water microbiome. mBio (2014) 5:e01135–14. doi: 10.1128/mBio.01135-14
45. September SM, Brozel VS, Venter SN. Diversity of nontuberculoid mycobacterium species in biofilms of urban and semiurban drinking water distribution systems. Appl Environ Microbiol. (2004) 70:7571–3. doi: 10.1128/AEM.70.12.7571-7573.2004
46. Torvinen E, Lehtola MJ, Martikainen PJ, Miettinen IT. Survival of Mycobacterium avium in drinking water biofilms as affected by water flow velocity, availability of phosphorus, and temperature. Appl Environ Microbiol. (2007) 73:6201–7. doi: 10.1128/AEM.00828-07
47. Brooke J. Stenotrophomonas maltophilia: an emerging global opportunistic pathogens. Clin Microbiol Rev. (2012) 25:2–41. doi: 10.1128/CMR.00019-11
48. Igbinosa IH, Igumbor EU, Aghdasi F, Tom M, Okoh AI. Emerging Aeromonas species infections and their significance in public health. Sci W J. (2012) 12:625023. doi: 10.1100/2012/625023
49. Low SY, Dannmiller K, Yao M, Yamamoto N, Peccia J. The allergenicity of Aspergillus fumigatus conidia is influenced by growth temperature. Fungal Biol. (2011) 115:625–32 10.1016/j.funbio.2011.03.006
50. Schultz S, Dickschat JS. Bacterial volatiles: the smell of small organisms. Nat Prod Rep. (2006) 24:814–42. doi: 10.1039/b507392h
51. Luptakova A. Importance of sulphate-reducing bacteria in environment. Nova Biotechnol. (2007) 7:17–22. doi: 10.1080/01490450701672117
52. Yao Q, Peng DC. Nitrite oxidizing bacteria (NOB) dominating in nitrifying community in full-scale biological nutrient removal wastewater treatment plants. AMB Express. (2017) 7:25. doi: 10.1186/s13568-017-0328-y
53. Kip N, Veen JAV. The dual role of microbes in corrosion. ISME J. (2014) 9:542–51. doi: 10.1038/ismej.2014.169
54. Kostaki M, Chorianopoulos N, Braxou E, Nychas G-J, Giaouris E. Differential biofilm formation and chemical disinfection resistance of sessile cells of listeria monocytogenes strains under monospecies and dual-species (with Salmonella enterica) conditions. Appl Environ Microbiol. (2012) 78:2586–95. doi: 10.1128/AEM.07099-11
55. Smith JN, Dyszel JL, Soares JA, Ellermeier CD, Altier C, et al. SdiA, an N-acylhomoserine lactone receptor, becomes active during the transit of Salmonella enterica through the gastrointestinal tract of turtles. PLoS ONE (2008) 3:e2826. doi: 10.1371/journal.pone.0002826
56. Shrout JD, Nerenberg R. Monitoring bacterial twitter: does quorum sensing determine the behavior of water and wastewater treatment biofilms? Environ Sci Technol. (2012) 46:1995–2005. doi: 10.1021/es203933h
57. Pang XY, Yang YS, Yuk HG. Biofilm formation and disinfectant resistance of Salmonella sp. in mono- and dual-species with Pseudomonas aeruginosa. J Appl Microbiol. (2017). 123:651–60. doi: 10.1111/jam.13521
58. Wang R, Kalchayanand N, King DA, Luedtke BE, Bosilevac JM, Arthur TM. Biofilm formation and sanitizer resistance of Escherichia coli O157:H7 strains isolated from “High Event Period” meat contamination. J Food Prot. (2014) 77:1982–7. doi: 10.4315/0362-028X
59. Banning N, Toze S, Mee BJ. Persistence of biofilm-associated Escherichia coli and Pseudomonas aeruginosa in groundwater and treated effluent in a laboratory model system. Microbiology (2003) 149(Pt 1):47–55. doi: 10.1099/mic.0.25938-0
60. Culotti A, Packman AI. Pseudomonas aeruginosa promotes Escherichia coli biofilm formation in nutrient-limited medium. PLoS ONE (2014) 9:e107186. doi: 10.1371/journal.pone.0107186
61. Haugo AJ, Watnick PI. Vibrio cholerae CytR is a repressor of biofilm development. Mol Microbiol. (2002) 45:471–83. doi: 10.1046/j.1365-2958.2002.03023.x
62. Simões LC, Simões M, Vieira MJ. Biofilm interactions between distinct bacterial genera isolated from drinking water. Appl Environ Microbiol. (2007) 73:6192–200. doi: 10.1128/AEM.00837-07
63. Gião MS, Azevedo NF, Wilks SA, Vieira MJ, Keevil CW. Interaction of Legionella pneumophila and Helicobacter pylori with bacterial species isolated from drinking water biofilms. BMC Microbiol. (2011). 11:57. doi: 10.1186/1471-2180-11-57
64. Min KR, Rickard AH. Coaggregation by the freshwater bacterium Sphingomonas natatoria alters dual-species biofilm formation. Appl Environ Microbiol. (2009) 75:3987–97. doi: 10.1128/AEM.02843-08
65. Teh KH, Flint S, French N. Biofilm formation by Campylobacter jejuni in controlled mixed-microbial populations. Int J Food Microbiol. (2010) 143:118–24. doi: 10.1016/j.ijfoodmicro.2010.07.037
66. Sulami AA, Al-Taee AMR, Juma's MG. Isolation and identification of Helicobacter pylori from drinking water in Basra governorate. Iraq Gastroenterol Res Pract. (2010) 16:920–5.
67. Stewart CR, Muthye V, Cianciotto NP. Legionella pneumophila persists within biofilms formed by Klebsiella pneumoniae, Flavobacterium sp., and Pseudomonas fluorescens under dynamic flow conditions. PloS ONE (2012) 7:e50560. doi: 10.1371/journal.pone.0050560
68. Whiley H, Keegan A, Fallowfield H, Bentham R. Detection of Legionella pneumophila and Mycobacterium avium Complex (MAC) along potable water distribution pipelines. Int J Environ Res Public Health. (2014) 11:7393–405. doi: 10.3390/ijerph110707393
69. Husna AU, Rahman MS. Potentials of rainwater harvesting as an alternative water supply system in dhaka city. J Sci Res Rep. (2017) 13:1–7. doi: 10.9734/JSRR/2017/31866
70. Silvestry-Rodriguez N, Bright KR, Slack DC, Uhlmann DR, Gerbia CP. Silver as a residual disinfectant to prevent biofilm formation in water distribution systems. Appl Environ Microbiol. (2008) 74:1639–41. doi: 10.1128/AEM.02237-07
71. Hitzfeld BC, Hoger SJ, Dietrich DR. Cyanobacterial toxins: removal during drinking water treatment, and human risk assessment.environ health Perspect (2000) 1:113–22 doi: 10.1289/ehp.00108s1113
72. United States Environmental Protection Agency Documents on Parameters of Water Quality: Interpretations Standards. (2011). Available online at: https://www.epa.ie/pubs/advice/water/quality/Water_Quality.pdf
Keywords: municipal water distribution, microbial biofilm, waterborne diseases, deterioration of water quality, biofouling
Citation: Towhid ST (2018) Microbial Interaction as a Determinant of the Quality of Supply Drinking Water: A Conceptual Analysis. Front. Public Health 6:184. doi: 10.3389/fpubh.2018.00184
Received: 31 January 2018; Accepted: 08 June 2018;
Published: 26 June 2018.
Edited by:
Md. Asaduzzaman Shishir, University of Dhaka, BangladeshReviewed by:
Gulnihal Ozbay, Delaware State University, United StatesMohd. Raeed Jamiruddin, BRAC University, Bangladesh
Copyright © 2018 Towhid. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Syeda T. Towhid, towhidst@mib.jnu.ac.bd
 Syeda T. Towhid
Syeda T. Towhid