- The Last Frontier, Amsterdam, Netherlands
This manuscript provides a glimpse into the future of early human development in space environments, underpinned by interdisciplinary research and technological advancements. The implications of early human development in space are analyzed, exploring potential scenarios that could enhance human biology to adapt effectively to these conditions. It is proposed that the initial phases of human development might serve as an optimal period for fostering biological adaptation to space. Such adaptations could pave the way for the emergence of new evolutionary states, establishing the groundwork for a novel human subspecies. The potential for these emerging subspecies to develop the ability to perceive diverse environmental properties hints at a groundbreaking biological era designated here as Universal Cognition.
1 Introduction
In April 1961, humanity crossed the confines of Earth’s atmosphere and embarked on a journey into space. Yuri Gagarin achieved this historic milestone as the first human to orbit the Earth, completing a full revolution aboard the Vostok 1 spacecraft (Gagarin and Lebedev, 2003). This groundbreaking journey marked the first time that human biology was subjected to the extreme environmental conditions of outer space.
One of the key motivations for establishing a human presence in space is the necessity of ensuring long-term human survival (Hawking, 1996; Sagan, 1997). However, space environments present considerable challenges to human life. Stressors such as microgravity, exposure to a vacuum, ionizing radiation, and variations in circadian rhythms demand substantial biological adaptations (Clément, 2011). Overcoming these challenges is essential for sustaining human life beyond Earth and unlocking the full potential for long-term space exploration.
Space environments are classified as extreme, defined as “environments to which humans are not naturally suited and which demand complex processes of physiological and psychological adaptation” (Kanas and Manzey, 2008:15). As a result, significant resources have been allocated to studying the effects of these environments, particularly in Low Earth Orbit (LEO), on adult human biology.
This research includes understanding the mechanisms of biological adaptation to microgravity, and developing countermeasures designed to sustain life in these challenging conditions (Clément, 2011; Krittanawong et al., 2022).
To date, there is no conclusive evidence that adult humans can adapt to the conditions of space in the long-term. Research has revealed significant challenges with adaptation, for example, in the skeletomuscular and visual systems, where effects of microgravity in LEO have led to issues such as bone and muscle atrophy, as well as ocular damage, even in otherwise healthy adults. Countermeasures have not led to substantive biological adaptation. Although they may alleviate some negative consequences, they also contribute to accelerated aging and a significant decline in nearly all bodily systems (Clément, 2011; Demontis et al., 2017). Moreover, space missions extending beyond 6 months could pose serious risks to astronauts’ health (Mader et al., 2011).
Evolutionary Developmental Biology (Evo-Devo) is a relatively recent scientific field focused on understanding the role of changes in developmental mechanisms in the evolutionary origin of aspects of the phenotype (Hall, 2003). Within the field of Evo-Devo is a subfield known as Eco-Evo-Devo, which emphasizes that biological evolution, or evolutionary change, is influenced by early developmental phases, epigenetic mechanisms, and natural selection. Fundamentally, early development enhances a species’ capacity to adapt to diverse environmental conditions by fostering increased biological plasticity, which is defined as the ability of a given genotype to produce different phenotypes in response to varying environments (Gilbert et al., 2015; Hochberg, 2011; Hochberg et al., 2011; West-Eberhard, 2003; West-Eberhard, 2005).
The morphological and physiological features of organisms shape how they sense, perceive, and construct knowledge about their physical surroundings, as explored in the fields of Embodied Cognition and Physiology (Barsalou, 2003; Barsalou, 2008; Chemero, 2009; Clark, 2008; Gibson, 1966; Gibson, 1979; Thelen and Smith, 1994). Consequently, structural and physiological changes driven by early adaptation to space environments would alter how humans perceive their surroundings.
In this essay, it is postulated that the early stages of human development are particularly conducive to facilitating biological adaptation to space environments, due to a higher degree of biological plasticity compared to later developmental phases, such as adulthood. Furthermore, it is contended that such adaptations could pave the way for the emergence of new evolutionary states, establishing the groundwork for a novel human subspecies. This emerging subspecies would possess the ability to perceive diverse environmental properties, heralding a groundbreaking biological era termed Universal Cognition.
Research in Developmental Space Biology has provided valuable insights into how varying gravitational environments, as well as radiation conditions, affect different animal species from fertilization through early postnatal development. This field has employed a range of model organisms, including fruit flies, sea urchins, fish, amphibians, birds, and rodents.
Key findings indicate that fertilization appears to be unaffected by microgravity in certain non-mammalian species, including sea urchins, fish, frogs, newts, and birds. To date, no instances of successful fertilization in mammals, such as rodents or humans, have been documented during spaceflight. While research has demonstrated that normal gravity is essential for optimal preimplantation development in mammals, recent findings suggest that microgravity and short-term hypergravity might not significantly impede blastocyst formation or embryonic health. Addionally, it has been observed that late stage pregnancy can proceed in microgravity in rodents. The mid- and long-term biological outcomes on animal development in space remain largely unexplored (Amann et al., 1992; Dournon et al., 2001; Horn, 2013; Ijiri, 1995; Jain et al., 2023; Jung et al., 2009; Kojima et al., 2000; Kurotani-Izumi and Kiyomoto, 2003; Lei et al., 2020; Li et al., 2021; Ma et al., 2008; Moody and Golden, 2000; Ning et al., 2015; Ricci and Boschetti, 2003; Ronca, 2003; Ronca et al., 2013; Sapp et al., 1990; Schenker and Forkheim, 1998; Serova and Denisova, 1982). This scenario underscores the necessity for further research to better understand the effects of space stressors on developmental processes, as this area of study is still in its infancy and yields controversial results.
While current studies produce inconclusive results on organisms’ early responses to space environments, the potential for significant biological adaptations during early developmental stages makes a compelling case for actively pursuing this line of investigation. As previously discussed, biological plasticity is particularly pronounced during these early stages, indicating that substantial adaptations to space environments are likely to occur. However, it is essential to approach this process in a safe and controlled manner to mitigate health complications arising from the extreme conditions of space.
To ensure a safe approach to early human adaptation in space, a series of future scenarios leveraging human-enhancing technologies is proposed. These cover a system designated here as Adaptive Enhancement Technology (AET), as well as somatic and germline gene interventions (if deemed safe in the future). By tackling the unique physiological and genetic challenges posed by space environments, these innovations have the potential to help humans not only to adapt, but thrive in the harsh conditions of space.
2 Early development as a catalyst for space adaptations
Galileo envisioned that the gravitational pull governing the orbits of celestial bodies also influences the form and function of animal and plant organs. He posited that objects are drawn toward the Earth with a force proportional to their mass, suggesting that heavier organisms experience a greater gravitational pull and, consequently, must adapt their shape to support themselves effectively (Galilei, 1989).
However, gravitational forces are not the only factors influencing human adaptation. Other natural phenomena within the Earth’s biosphere - environmental stressors - such as atmospheric composition, fluctuations in temperature, and resource availability, also shape the adaptive strategies of organisms.
Environmental stressors exert a powerful influence on organisms, driving anatomical and physiological adaptations that reflect the challenges they face (Lieberman, 2013; Organ et al., 2011; Potts, 2012; Shubin, 2009); for example, temperature and nutrient fluctuations can alter metabolic processes, immune responses, growth rates, and reproductive strategies, thereby influencing reproduction and overall fitness. In hominids, stress stemming from environmental changes has been strongly linked to physical and behavioural shifts, including the evolution of bipedalism in response to food scarcity, which, as a pivotal adaptation, enabled a hunter-gatherer lifestyle characterized by enhanced mobility, longer legs, enlarged hip, knee, and ankle joints, developed arches in the feet and shorter toes, and other structural modifications that supported efficient locomotion, thermoregulation, and balance.
According to Shubin (2013:41–42), “while most of our past lay inside the water, the most recent 300 million years has been defined by our separation from it.” Even our kidneys represent a significant adaptation to terrestrial life, helping to balance water and salts within the body in response to the challenges of living on dry land.
Hunter-gatherer children were actively engaged in group tasks for one to 2 hours daily, including foraging, hunting, fishing, firewood collection, and domestic chores; in addition, they adapted to the nomadic lifestyle of their groups, further shaping their physical and social development. Compared to ape offspring, hunter-gatherer children experienced a longer maturation period, from weaning to full brain development; this extended juvenile phase, lasting approximately three to 6 years, is linked to the development of larger brains and complex neuronal networks, potentially contributing to the doubling of brain size uniquely observed in hunter-gatherers during the Ice Age (Striedter, 2006).
All of these adaptations were crucial for the survival of the genus Homo, contributing to reproductive success, highlighting the strong connection between species adaptation and the challenges presented by novel environments.
Similarly, environmental stressors in space can profoundly affect the development and evolution of the human body, introducing distinctive forms of physical and mental stress that differ from those encountered on Earth. For instance, human bodies on Earth are consistently subjected to a gravitational pull of 1 g, whereas on Mars, this gravitational influence is only 0.38 g, representing a 62% reduction. This decrease in gravitational force can lead to variations in the structural characteristics of developing organisms, particularly during early stages when the most significant changes take place. Consequently, a tendency toward more elongated body shapes as a result of the lower gravitational forces can be observed.
According to Hochberg (2011:1–2), “The ability of a given genotype to produce different phenotypes in response to different environments is termed “plasticity,” and is part of the organism’s ‘adaptability’ to environmental cues.” This concept of plasticity is crucial in understanding how various body systems, including musculoskeletal, physiological, and cognitive systems, can be remodeled in response to new environmental experiences. Plasticity encompasses a range of physiological mechanisms, including transcription, translation, enzyme activity, and hormonal regulation, all of which can lead to localized or systemic adaptations. These physiological responses may manifest as changes in biochemistry, physiological functions, morphology, behavior, or life history traits (Batenson et al., 2004; Belsky and Pluess, 2009; Gilbert, 2001; Mateus et al., 2014; West-Eberhard, 2003; Whitman, 2009).
Genetic variation among individuals is expected to influence not only the extent but also the manner in which they exhibit phenotypic plasticity. While the expression of phenotype primarily arises from differential gene regulation, it is essential to understand that this regulation extends beyond the genetic material within the embryo. External environmental factors, such as temperature, light duration, dietary availability, population density, can also induce specific phenotypic variations by affecting gene expression (Gilbert, 2001; Mateus et al., 2014; West-Eberhard, 2005).
West-Eberhard (2005) argues that understanding the origin of species differences is more effectively achieved by focusing on the emergence of novel phenotypic traits rather than solely on reproductive isolation. She proposes that these novel traits arise through “developmental recombination,” which reorganizes existing developmental pathways, followed by “genetic accommodation” that stabilizes and refines the new trait. This process highlights the importance of phenotypic variation, including environmentally induced changes, as the primary substrate for natural selection rather than just genetic mutation. West-Eberhard contends that environmentally induced novelties may hold greater evolutionary potential than genetic mutations because they can simultaneously affect many individuals. If the resulting phenotypic variation has a fitness effect, meaning it correlates with survival or reproductive success, then selection occurs, favoring differential reproduction among individuals with varying phenotypes.
Therefore, both genetic and environmental elements work together to shape the characteristics of organisms.
As previously mentioned, the human body displays remarkable plasticity during early developmental stages. Research demonstrates that environmental factors exert a significant influence on both structural and cognitive development in children, more so than in adults. For example, environmental experiences play a crucial role in various structural transformations in a child’s brain, including processes such as synaptic pruning. This phenomenon is a central element of neuroplasticity, which encompasses long-term changes in neuronal structure and function resulting from alterations in neural activity (Black et al., 1998; Greenough et al., 2002; Huttenlocher and Dabholkar, 1997; Waber et al., 2007). Moreover, growth and body composition show a high degree of plasticity throughout development, further highlighting the adaptability of the human body in response to environmental influences (Hochberg, 2009; Hochberg et al., 2011).
Hochberg (2009:1) asserts that “the window of developmental plasticity extends from conception to early childhood, and even beyond to the transition from juvenility to adolescence, and could be transmitted transgenerationally.” This indicates that these key periods not only facilitate the installation of adaptive traits in response to environmental challenges but may also set the stage for future generations to inherit these adaptations. Therefore, utilizing the mechanisms of developmental plasticity during these critical periods will be essential for optimizing human adaptations for life in space. By analysing the interactions between genetic and environmental factors that influence plasticity, a better preparation for the specific physiological challenges presented by space environments can be achieved. This knowledge will enable us to develop targeted strategies and interventions that enhance resilience and adaptation in future human space explorers.
3 Open questions in developmental space biology
Developmental Space Biology focuses on investigating the effects of space environments on developmental processes, providing valuable insights into how varying gravitational and radiation conditions influence various animal species. Studies have been conducted from fertilization through early postnatal development. This field has employed model organisms such as fruit flies, sea urchins, fish, amphibians, birds, and rodents. Research in this field encompasses a broad range of areas, including cellular, molecular, genetic, morphological, physiological, and central nervous system sensory and vestibular processes (Amann et al., 1992; Dournon et al., 2001; Horn, 2013; Ijiri, 1995; Jain et al., 2023; Jung et al., 2009; Kojima et al., 2000; Kurotani-Izumi and Kiyomoto, 2003; Lei et al., 2020; Li et al., 2021; Ma et al., 2008; Moody and Golden, 2000; Ning et al., 2015; Ricci and Boschetti, 2003; Ronca, 2003; Ronca et al., 2013; Sapp et al., 1990; Schenker and Forkheim, 1998; Serova and Denisova, 1982).
Reproductive experiments have been carried out on sea urchins, fish, frogs, newts, and birds. These studies have demonstrated that fertilization and embryonic development can take place in space in these species; however, minor developmental anomalies have been consistently observed, particularly in the long-term, with the underlying molecular mechanisms largely unclear (Alberts and Ronca, 2005; Dournon et al., 2001). Notably, medaka fish were the first vertebrates to successfully reproduce in space during a 15-day mission aboard the Space Shuttle. This resulted in fertilization rates that were comparable to those of Earth-based controls (Ijiri, 1995).
Next, the effects of space environments on developing mammalian species, which share closer molecular and genetic mechanisms with humans, will be investigated. This similarity makes them vital for understanding the impact of space conditions on developmental dynamics. By focusing on these models, invaluable insights into the potential adaptive responses that are relevant to human space exploration can be gained.
Mammalian fertilization and development in microgravity presents a complex picture, with studies indicating mixed outcomes. Although reproduction is not completely inhibited, research involving rats and mice has demonstrated notable effects.
Studies shown microgravity to reduce rat sperm counts, increase abnormalities, and decrease testicular weight. Postnatal microgravity exposure in rats causes reduced unmyelinated fibers in the cardiovascular system, muscle atrophy, and altered cortical synapse morphology (Amann et al., 1992; Sapp et al., 1990).
To investigate whether mammals can conceive in microgravity, the uncrewed biosatellite Cosmos 1129, launched in 1979, created an environment for rats to mate during flight. Upon returning to Earth, two female rats exhibited signs of early pregnancy with resorption. However, there was no monitoring of rat fertilization in space (Alberts and Ronca, 2005; Wakayama and Wakayama, 2025). Subsequent missions demonstrated that late-stage mammalian pregnancies can occur in microgravity. Notably, Cosmos 1514, launched in 1983, along with the NIH.R1/STS-66 and NIH.R2/STS-70 missions in 1994 and 1995, respectively, resulted in successful parturition upon their return to Earth (Alberts and Ronca, 2005; Ronca, 2003; Ronca et al., 2013). Further missions involving nursing rat mothers and their pups, specifically NIH.R3/STS-72 in 1996 and Neurolab/STS-90 in 1998, encountered significant challenges, including high infant mortality rates and feeding difficulties, which required intervention from the astronauts.
Further studies, such as those conducted during the STS-131 (2010), −133 (2011), and −135 (2011) missions, revealed negative impacts of microgravity on female C57BL/6 and BalbC mice exposed to space from 12 to 15 days. These effects included compromised oocyte health, reduced estrogen receptor levels, and decreased HSPH-1 stress marker expression. Oocyte maturation and production were disrupted, and long-term recovery following spaceflight remains unclear (Ronca et al., 2013). These results highlight the potential detrimental effects of relatively short-term exposure to microgravity on female reproductive function. Additionally, research in cultured cells showed radiation to cause DNA damage to sperm and oocytes (Yatagai et al., 2011).
More recently, Wakayama et al. (2009) investigated the effects of simulated microgravity on mouse fertilization and preimplantation development using a 3D clinostat. In vitro fertilization (IVF) rates were comparable to controls (84% success rate), suggesting that microgravity does not impair gamete function. However, embryo development was affected. Transferring embryos cultured under simulated microgravity to recipient females revealed significantly lower live birth rates (35% vs. 63% for 1G controls) when embryos were transferred at the 2-cell stage. Similarly, blastocyst formation rates were lower (30% vs. 57% for 1 g controls) for embryos cultured under simulated microgravity at the blastocyst stage, although a smaller percentage of these developed into seemingly healthy offspring (16% versus 37% for 1 g controls). While the offspring from microgravity-cultured embryos appeared to have normal weights and fertility, immunostaining showed slower development and reduced trophectoderm cell numbers compared to 1g controls. This study demonstrated, for the first time in mammals, that fertilization can occur normally under simulated microgravity but that subsequent preimplantation development is impaired.
The findings of Wakayama et al. (2009) align with other studies showing that while IVF in mice is possible under simulated microgravity (Kojima et al., 2000), subsequent preimplantation development is compromised (Jung et al., 2009). This negative impact on preimplantation development has also been observed in experiments involving two-cell stage mouse embryos sent into space (Lei et al., 2020; Ma et al., 2008; Schenker and Forkheim, 1998).
Lei et al. (2020) examined mouse preimplantation development during the SJ-10 satellite mission, launched 6 April 2016, and orbiting at 252 km with microgravity levels of 10^-4 to 10^-6 g0 and radiation doses of 0.15 mGy/day. While blastocyst formation occurred, rates and quality were lower due to significant DNA damage and global hypomethylation, primarily caused by radiation, not solely microgravity. This study highlights the radiation risks for mammalian reproduction in space.
Li et al. (2021) investigated the effects of microgravity on mouse embryonic stem cell function and development. Their research indicates that microgravity influences embryonic development through cytoskeletal alterations and the modulation of key signaling pathways, including WNT, Cadherin, and Nitric Oxide. These changes likely trigger stress responses and induce epigenetic modifications that affect developmental outcomes. The study demonstrates that microgravity encourages differentiation into mesoderm and endoderm, which are essential for the formation of various tissues and organs. However, additional research is necessary to fully understand its effects on ectodermal differentiation, which is crucial for the development of the skin and nervous system.
Jain et al. (2023) conducted a review on various studies examining the effects of space conditions, particularly radiation and microgravity, on fetal and neural development in rodents. Their review highlights that radiation exposure during gestation is particularly harmful, potentially leading to structural malformations such as microcephaly and central nervous system defects, resulting in cognitive and mental health impairments. Studies examining embryonic neurons in simulated space conditions indicate changes in neuronal plasticity, morphology, and viability, with significant effects identified after prolonged exposure. While neurons show some adaptation to microgravity, they require extended recovery periods to readjust to Earth’s gravity. Collectively, these findings suggest that normal brain development in rodents altered in space, affecting both structural integrity and functionality.
The findings from the previous studies emphasize the importance of normal gravity for optimal preimplantation development in mice. However, recent research has reported contrasting outcomes, as detailed below.
Wakayama et al. (2023) examined the differentiation of mammalian embryos into the inner cell mass (ICM) and trophectoderm during the 8–16 cell stage to assess normal development in microgravity. Using an innovative device, astronauts thawed and cultured frozen two-cell mouse embryos in the International Space Station (ISS) for 4 days. After a total of 13 days, 9 days of cryopreservation and 4 days of culture, the embryos accumulated a radiation dose of 4.29 mGy (7.15 mSv). Despite fewer blastocysts being analyzed, the study concluded that microgravity and space radiation do not induce DNA damage, as the embryos developed into blastocysts with normal characteristics; two-cell mouse embryos cultured in microgravity aboard the ISS developed into blastocysts at a rate comparable to those in a simulated 1 g environment. However, the distinct effects of radiation exposure during cryopreservation and culturing on development were not separately evaluated.
Ning et al. (2015) investigated the effects of short-term hypergravity, simulating spacecraft launch conditions, on the development of 2-cell mouse embryos. Embryos in CZB solution, contained in 1.5 mL tubes, were subjected to centrifugation at 1000 rpm (approximately 100 g), 3500 rpm (approximately 1000 g), and 10,500 rpm (approximately 10 Kg) for 20 min. Despite significant hypergravity exposures, no notable differences were observed in blastocyst formation rates or live birth rates after embryo transfer compared to a 1 g control group. Additionally, the actin filament structure within the embryos remained normal, suggesting that short bursts of intense hypergravity during launch may not adversely affect early mouse embryo development.
There have been no documented instances of human conception occurring in spaceflight.
The existing literature on human reproductive health in the context of space exploration is notably sparse. Gimunová et al. (2024) conducted a systematic review to assess the effects of space travel, microgravity, and space radiation on human reproductive functions, following the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines. They searched three databases, PubMed, Web of Science, and Medline Complete, and identified 364 studies, ultimately including 16 relevant studies in their analysis. Their findings indicate that female astronauts may face increased risks such as thromboembolism and hormonal imbalances, while male reproductive health may be affected by decreased testosterone levels and increased sperm DNA fragmentation. At present, the feasibility of human reproduction in space remains uncertain (Balistreri and Umbrello, 2023).
One possible scenario is that if humans are unable to conceive in space due to imbalances and DNA damage in gametes, it may become necessary to remove and preserve healthy gametes from astronauts prior to flight. In such case, assisted reproductive technologies could be employed in space to facilitate fertilization and ensure the possibility of successful reproduction while mitigating potential risks. However, this scenario is just one of many potential pathways, as ongoing research may reveal alternative strategies and solutions for addressing reproductive health challenges in space.
In conclusion, research into mammalian fertilization and early development under altered gravitational conditions reveals an inconclusive picture. Fertilization seems to be unaffected by microgravity in several non-mammalian species, including sea urchins, fish, frogs, newts, and birds. There have been no successful observations of fertilization in mammals, such as rodents or humans, during spaceflight; in fact, no attempts have been made to fertilize humans in space. While research has demonstrated that normal gravity is essential for optimal preimplantation development in mammals, recent findings suggest that microgravity and short-term hypergravity may not significantly harm blastocyst formation or embryonic health. Moreover, the effects of radiation on developing mammalian species are still not fully clarified, particularly given that the maximum time rats and mice have spent in space thus far is 3 months (Wakayama et al., 2021).
Collectively, these findings underscore the complexity of space effects on mammalian fertilization and early development, prompting further research to fully elucidate the implications for future space exploration endeavors involving mammalian life. Efforts are underway to design habitats that can support at least two complete mammalian life cycles in space within and across generations, encompassing mating, fertlilization, pregnancy, embryonic and fetal development, birth, lactation, maternal care, postnatal development through sexual maturity, and the influence of genetic and epigenetic factors.
While current studies produce inconclusive results on organisms’ early responses to space environments, the potential for significant biological adaptations during early developmental stages makes a compelling case for actively pursuing this line of investigation. As previously discussed, biological plasticity is particularly pronounced during these early stages, indicating that substantial adaptations to space environments are likely to occur. However, it is essential to approach this process in a safe and controlled manner to mitigate health complications arising from the extreme conditions of space.
To ensure a safe approach to early human adaptation in space, a series of future scenarios leveraging human-enhancing technologies is proposed: AET, along with possible somatic and germline gene interventions (if deemed safe in the future). By tackling the unique physiological and genetic challenges posed by space environments, these innovations have the potential to help humans not only to adapt, but thrive in the harsh conditions of space. They can foster healthy early human development while ensuring safety and wellbeing in this environment. This topic will be explored further in the next section.
4 Future scenarios on early human adaptation to space environments
While our understanding of the effects of space on developing organisms is still in its early stages, there is substantial potential for innovative solutions to support human development and facilitate adaptation to space.
In this section, potential future scenarios regarding a human-enhancing framework designed to facilitate adaptation to space during the early stages of human development will be explored and speculated upon. These speculations are firmly rooted in current and anticipated scientific and technological advancements, particularly in the field of human enhancement technologies.
Human enhancement involves augmenting human capacities through natural, artificial or technological modifications that exceed typical species standards, with the goal of improving overall biological performance, including physical and cognitive abilities (Daniels, 2000; Raisamo et al., 2019). Human enhancement is centered on modifying individuals to better adapt to varying environmental conditions, rather than solely relying on controlling the surrounding environment (Balistreri and Umbrello, 2023).
While human-enhancing technologies raise numerous ethical questions, they also hold the potential to significantly improve human life if proven safe. A notable example can be seen in the field of microbiology, which initially faced skepticism and opposition due to concerns about its implications for health and safety. Many people were apprehensive about the use of antibiotics, fearing potential side effects and the risk of antibiotic resistance (Opal, 2009). However, as research progressed and safety measures were established, the benefits of microbiology became evident. Today, antibiotics play a crucial role in treating infections, vaccines have eradicated or controlled deadly diseases, and probiotics are widely used to enhance gut health. This illustrates how, despite initial skepticism, advancements in microbiology have led to significant improvements in health outcomes. Similarly, if human-enhancing technologies are rigorously tested and proven safe, they could offer transformative benefits for humanity, warranting careful consideration of their ethical implications.
Enhancements for space missions incorporate a range of promising techniques designed to improve resilience and performance in humans. Among these future possibilities are genetic modifications, for example, using CRISPR-Cas9 technology to enhance traits such as muscle mass and radiation resistance. Additionally, solutions like brain-computer interfaces and corneal replacements have the potential to enhance critical cognitive and visual functions. Innovations in synthetic biology, including synthetic blood, could further improve oxygen transport efficiency, ensuring that humans maintain optimal physiological performance during long-duration missions. Nanotechnology is anticipated to offer advanced materials and targeted therapeutic delivery systems that may repair cellular damage caused by radiation (Almeida and Diogo, 2019; Braddock, 2020; Gouw, 2020; Konrad et al., 2019).
There are two primary approaches to human enhancement. The first focuses on creating new human capacities, enabling individuals to experience improved biological abilities and advantages in various environments. It is linked to interventions that are invasive, irreversible, and heritable (e.g., germline editing) (Konrad et al., 2019). The second approach is primarily therapeutic, aimed at helping individuals survive in harsh or unwelcoming settings, ensuring they can endure and function effectively despite challenging conditions (e.g., somatic gene editing, pharmaceuticals). This approach is mostly centerd in therapy and disease prevention (Almeida and Diogo, 2019; Balistreri and Umbrello, 2023; Szocik and Reiss, 2023; Szocik et al., 2020).
In the next subsection, future scenarios focused on facilitating early human adaptation to space through the use of human-enhancing technologies will be explored. It is important to clarify that the human-enhancing technologies further described are currently speculative and represent long-term visions for the future. These concepts have not yet been realized or tested at practical or deployment levels. While such advancements could become feasible, they are primarily scenarios envisioned for the latter half of the 21st century and are plausible to happen during that time.
4.1 Adaptive enhancement technology and genetic modifications
A groundbreaking framework for human enhancement, specifically aimed at promoting biological adaptation to diverse space environments, from infancy to the end of adolescence, is proposed; a period when biological plasticity is at its peak. This framework centers primarily on therapeutic approaches to enhancement, potentially incorporating invasive procedures when necessary. Its core purpose is to ensure safe biological adaptation to the unique challenges posed by space environments through AET.
AET integrates artificial intelligence (AI), cybernetic systems, and advanced biotechnologies to monitor, predict, and address the biological needs of individuals adapting to space conditions. Operating through real-time closed-loop feedback mechanisms, AET enables seamless adjustments to various environmental stressors such as varying gravitational forces, radiation exposure, changes in atmospheric composition, temperature variations, and pressure changes.
AI in space medicine is an emerging field poised to revolutionize the management of routine medical issues for astronauts during deep-space missions. By detecting, diagnosing, and recommending treatments, AI systems are transforming how health is monitored in space. Additionally, AI possesses the capability to predict potential health concerns through advanced precision space health technologies, such as machine learning predictive models that continuously monitor health metrics, assess biomedical statuses, and anticipate issues for deep-space travelers. This proactive approach allows for health solutions to be provided before complications arise (Cheung et al., 2023; Greatbatch, 2024; Scott et al., 2021; 2023).
The concept of cybernetic systems for humans in space has been explored, particularly by Clynes and Klyne (1960), who introduced the notion of “cyborg” organisms. These cyborgs are envisioned to integrate technology with biological systems to maintain homeostasis and support human functions in the challenging conditions of space. Their framework includes examples such as osmotic pressure pumps for blood pressure regulation, psychic energizers for wakefulness, and pharmacological agents like epinephrine for cardiovascular regulation. These systems are designed to continuously monitor physiological parameters and adapt in real-time, theoretically ensuring that the body’s internal environment remains balanced.
Incorporating advancements in AI within space medicine and aligning with the concepts proposed by Clynes and Klyne, AET functions as an intelligent cybernetic system specifically designed to support homeostatic functions in humans in early developmental stages.
According to Billman (2020), homeostasis is “a self-regulating process by which biological systems maintain stability while adjusting to changing external conditions.” AET not only sustains homeostasis but also enhances the role of environmental stimuli, enabling necessary pressures for biological adaptation through AI predictive models. It allows for controlled exposure to environmental stressors that affect various body systems, thereby minimizing health complications while promoting resilience and adaptability.
To illustrate AET’s principles in action, let’s conceptualize a space biosuit. This suit is equipped with environmental sensors, such as gravitational sensors and devices for monitoring radiation levels, atmospheric composition, temperature fluctuations, and pressure variations. It also features an artificial membrane (AM) designed for selective permeability of environmental stressors, facilitating their interaction with the human body. The integration of invasive biosensing devices internally and/or non-invasive technology on the skin’s surface allows for comprehensive assessment of physiological and biological variables.
Initially, the biosuit maintains an Earth-like atmosphere internally, creating a controlled environment that promotes physiological stability or homeostasis. At designated moments, it facilitates controlled introductions of environmental stressors, encouraging acclimatization and further adaptive responses while ensuring human safety.
The AM, covering the surface of the biosuit, mimics natural biological membranes using a nanoscale architecture for selective permeability, allowing specific molecules and ions to pass through. Advanced materials engineered at the molecular level enable the AM to dynamically respond to changing environmental conditions, regulating the entry of environmental stressors.
Environmental sensors coupled to the AM wirelessly communicate with biosensing devices embedded on the skin and/or with invasive sensors positioned inside the human body. The biosensing devices guide the AM in controlling the selective entry of environmental stressors, allowing for real-time adjustments to manage exposure effectively. For instance, if biosensing devices detect that certain radiation or carbon dioxide levels significantly disrupt the body’s homeostasis, the AM could respond by deploying additional radiation shielding or blocking carbon dioxide entry - prompting structural changes such as increased density or altered molecular arrangements - thereby enhancing protection.
To effectively manage radiation exposure, the AM could integrate advanced materials specifically designed to absorb and deflect harmful radiation. For example, doped silica and high-density polyethylene (HDPE) could be engineered for enhanced absorption and deflection capabilities, while hydrogenated boron nitride nanotubes demonstrate promising radiation shielding properties due to their composition (Montesinos et al., 2021). This is just one potential approach, as emerging materials and innovative techniques may become available in the future, offering even more effective solutions for radiation protection. The AM would serve as a dynamic interface that regulates the exchange of external stimuli, ensuring that the pressures exerted on the body remain within safe thresholds for physiological adaptation.
Safe thresholds refer to specific limits of environmental pressure, beyond which the body’s ability to maintain homeostasis may be compromised, resulting in potential health risks. For example, in the short-term, excessive carbon dioxide levels can lead to symptoms such as headache, dizziness, and impaired cognitive function, while long-term exposure may cause respiratory acidosis, cardiovascular stress, and chronic neurocognitive impairment. Similarly, increased radiation exposure can lead to acute cellular damage and raise the long-term risk of cancer (Clément, 2011).
To determine safe thresholds for each individual in real-time, the biosensing devices could continuously monitor critical physiological parameters, including blood pressure to gauge stress or health conditions, core body temperature to detect thermal imbalances, heart rate as a response to stressors, and respiratory rate to assess oxygen and carbon dioxide management. Additionally, monitoring oxygen saturation would be essential, as low levels can indicate hypoxia, while electrolyte balance is crucial for neuromuscular functions (Libretti and Puckett, 2023). Alongside these parameters, the biosensing devices would gather omics data - genomics, proteomics, and metabolomics - offering insights into genetic and metabolic responses to environmental stressors. Genomic analysis reveals predispositions to specific conditions under stress, while proteomic profiling identifies protein expressions linked to stress responses. Metabolomics focuses on metabolites generated during metabolic processes, providing critical information about cellular responses to environmental challenges (Micheel et al., 2012).
For example, if the biosensing devices detect elevated inflammation markers through physiological data, this could indicate potential cellular damage resulting from radiation exposure. By correlating the elevated inflammation markers with the proteomic data, which identifies changes in protein expressions related to stress responses, the system can gain deeper insights into the biological impact of radiation. In response to the detected increase in inflammation and potential cellular damage, the AM could initiate protective measures, such as enhancing shielding within the suit to mitigate oxidative stress. Conversely, if monitoring reveals no significant inflammation despite increased radiation exposure, the AM may permit controlled exposure, allowing the body to activate natural adaptive mechanisms, such as enhanced DNA repair and upregulation of protective proteins, thereby improving cellular resilience under heightened radiation levels.
The AET model would further optimize individual physiological thresholds through AI predictive models that analyze real-time data from both biosensing and environmental devices, enabling dynamic adjustments tailored to individual conditions and external factors. For instance, nanorobots (Chen et al., 2023) within the body, equipped with biocomputing features and AI capabilities, could function as biosensing devices, continuously monitoring crucial physiological and omics data. These nanorobots would wirelessly receive signals from the environmental sensors embedded in the AM, and collect physiological data using advanced sensors that assess bodily functions and biochemical responses. They would obtain omics data by analyzing biological information at the cellular level. For genomics, they would identify specific biomarkers related to DNA and RNA through selective binding interactions, such as antibodies (Saerens et al., 2008) or DNA probes. In proteomics, nanorobots could employ fluorescent tags (Stawicki et al., 2021) to visualize and quantify protein levels in tissues, providing valuable insights into cellular functions. For metabolomics, they could analyze metabolites and their compositions using miniaturized mass spectrometry (Fan et al., 2025) for real-time data.
By continuously gathering data on physiological, omics, and environmental data, the nanorobots would provide vital feedback to the AI model. The AI would analyze this extensive dataset to identify patterns and trends, predicting safe environmental pressure levels while ensuring physiological effort from the body, promoting acclimatization and further adaptation to environmental conditions.
The AI predictive model could use sophisticated algorithms to analyze correlations among physiological, omics, and environmental data. Regression analysis and pattern recognition (Russo and Lax, 2022) within the AI system would be essential for determining when an individual significantly deviates from homeostasis as environmental conditions change. Upon detecting extreme deviations, the AI could adjust the levels of environmental stressors to mitigate their impact on the human body, thereby facilitating a return to a stable physiological state.
Regression analysis is a statistical technique used to evaluate relationships between variables, allowing AI to identify correlations among physiological parameters, omics data, and environmental stressors. By establishing predictive models, the AI can assess how changes in one variable might influence another. Pattern recognition employs algorithms to detect and classify regularities and anomalies within datasets. This capability enables the AI to identify recurring physiological responses or trends, as well as shifts in omics data that indicate significant deviations from normal conditions. Such insights would facilitate timely interventions to maintain homeostasis.
Should biological adaptation fail to occur through the integrated approach of AET and the body’s natural responses, artificial methods could be utilized to stimulate adaptation in a controlled manner. For instance, if significant health risks from elevated radiation levels are detected, the AI system could activate synthetic biological systems, such as programmed cells designed to express protective proteins or initiate repair mechanisms (Pio-Lopez, 2021). This could involve the synthesis of DNA repair enzymes and antioxidant proteins aimed at mitigating the harmful effects of radiation. In such scenarios, nanorobots embedded with AI capabilities would instruct these synthetic biological systems to respond appropriately, enhancing their effectiveness in managing oxidative stress. The integration of AI would enable ongoing monitoring of the biological response, facilitating real-time adjustments in the delivery of protective compounds as needed. Furthermore, synthetic biology could enhance carbon dioxide metabolism by creating engineered enzymes that assist the body in managing increased concentrations. In addition, if AI detects signs of infection or an imbalance in the immune response, synthetic pathways could be activated to generate specific cytokines - small signaling proteins that modulate immune responses.
The synthetic biological systems would use resources and substrates that are already present in the body or within the cells themselves to synthesize necessary proteins and enzymes for enhancing biological functions and facilitating adaptive responses.
The AET system could offer instant feedback on individual acclimatization and further adaptation via wearable devices; understanding when biological changes move from acute to chronic, and how these could lead to adaptation would be essential.
Important to mention, it is essential that the developing human body has access to artificial gravity at 1 g levels like Earth to guarantee lower developmental risks. Individuals would continuously move from habitats providing this artificial gravity to environments where gravitational conditions change, for example the surface of Mars with its 0.38 g gravitational pull. This transition would allow for environmental pressures to be strategically applied at specific moments, while the artificial gravity habitats provide the necessary counterbalance for healthy development. The idea of creating artificial Earth-like gravity in space was first proposed by Von Braun (1952). Building on von Braun idea, recent designs explore rotating structures - such as large, circular or toroidal habitats - that generate 1 g through centrifugal force, which can be implemented on the surface of planetary bodies for stable living environments, as well as during transit in space where microgravity conditions prevail. The technology should be ready by the second half of the 21st century and will be firstly implemented on the Moon (Human Spaceology Center, 2022).
Additionally, to demonstrate how the AET AI model could be trained to establish physiological thresholds in individuals during early developmental stages, simulations and synthetic data generation could be used, particularly in the absence of direct deep-space biomedical data. Quantum computing could train the AI model to predict safe limits for environmental stressors. Such quantum computing capabilities could also forecast how the human body would develop under specific space environments. Furthermore, rigorous in vitro and animal model testing of the AET system could be conducted to validate findings, ensuring the framework’s robustness before implementation in real-world scenarios.
Furthermore, AET’s ability to support and enhance adaptive processes during early development could increase the likelihood that beneficial adaptations are inherited by future generations. Once these adaptations are established, advantageous traits may be passed on through reproduction, allowing populations within specific space environments to thrive under the same conditions. Over long timescales, and multiple generations, this could lead to the emergence of distinct human subspecies across the universe, each characterized by unique biological traits adapted to their specific environments. This evolutionary trajectory would illustrate humanity’s ongoing adaptation to diverse space conditions, ultimately improving survival and functionality across various environmental settings.
An overview of the AET system is depicted in Figure 1.
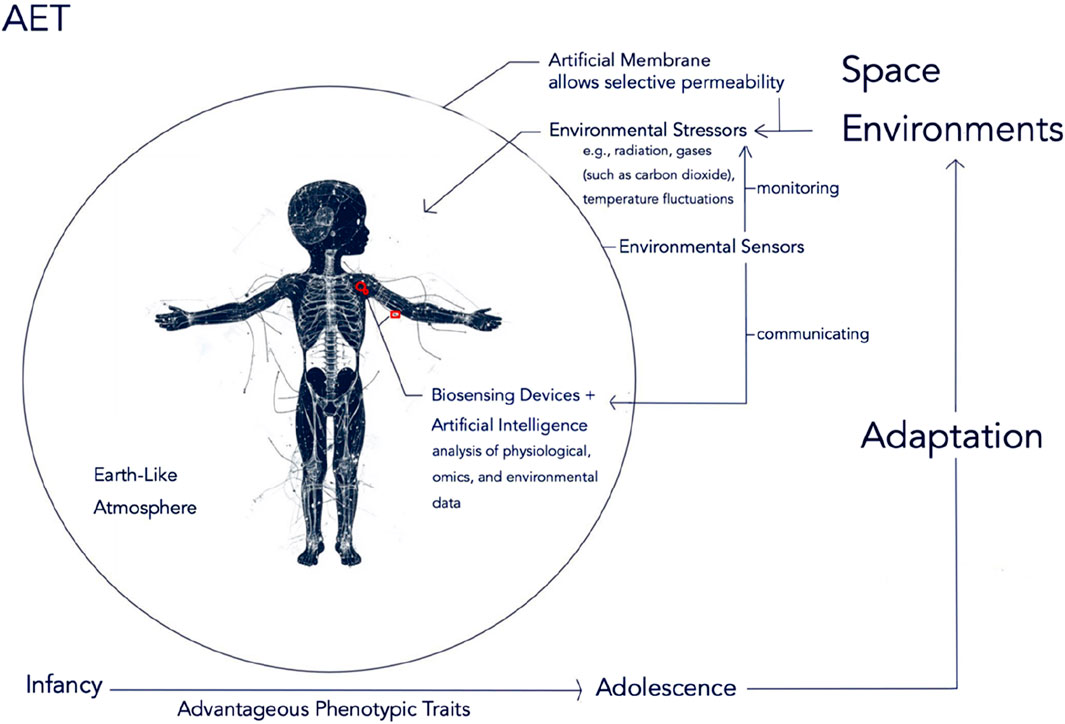
Figure 1. Overview of the AET System. The AET system provides a closed and controlled Earth-like atmosphere that fosters physiological stability and homeostasis. An Artificial Membrane facilitates selective permeability to environmental stressors, including radiation levels, gases (e.g., carbon dioxide), and temperature fluctuations, thereby allowing for disruptions in the human body’s homeostatic states. Biosensing Devices (shown in red; both invasive and/or non-invasive) wirelessly communicate with Environmental Sensors embedded within the Artificial Membrane. Artificial Intelligence analyzes real-time data from these Biosensing Devices - physiological and omics data - as well as data from the Environmental Sensors. This analysis enables dynamic adjustments to the body’s homeostatic levels against Environmental Stressors, facilitating optimal adaptation to space environments. Through adaptation, Advantageous Phenotypic Traits may emerge from infancy to adolescence, enhancing the body’s capacity to respond to the varying conditions of space.
In addition to AET, adaptation to space could be further supported through gene modifications.
Genetic modifications have the potential for enhancing human adaptation to space, with two primary approaches: germline and somatic gene modifications. Germline gene modifications involve interventions made to the genetic material of embryos or gametes, leading to changes that can be inherited by future generations. This approach is linked to permanent enhancements, and it is currently invasive and irreversible, raising ethical and safety concerns. In contrast, somatic gene modifications target specific cells within an individual, affecting only the treated person and ensuring that the changes are not passed on to offspring. Somatic modifications are currently regarded as a viable option for fostering adaptation in space environments while germline modifications remain controversial (Konrad et al., 2019; Musunuru, 2019).
Somatic gene therapy utilizes techniques like CRISPR-Cas9 to modify non-reproductive cells, allowing for the insertion, deletion, or alteration of specific genes (Doudna and Charpentier, 2014). Research has shown that certain genes could provide advantages for long-term spaceflight. For example, introducing the Dsup gene from tardigrades into human cells in vitro resulted in a 40% reduction in radiation damage from X-rays (Hashimoto et al., 2016).
Successes in pediatric applications demonstrate the potential of somatic gene therapy for treating inherited disorders. Advances in safer viral vectors, such as lentiviruses and adeno-associated viruses (AAV), have paved the way for clinical success. For instance, AAV-based gene therapy has significantly improved survival and motor function in infants with spinal muscular atrophy (SMA). Similarly, clinical trials for children with Leber’s congenital amaurosis (LCA) have shown significant visual improvement following AAV-mediated gene transfer. Furthermore, lentiviral vector-based therapy has been effective in treating severe combined immunodeficiency (SCID), particularly adenosine deaminase-deficient SCID (ADA-SCID), offering hope for young patients affected by life-threatening conditions (Dunbar et al., 2018; Mohammadian Gol et al., 2024). These applications reflect significant progress in somatic gene therapy at younger ages.
Although somatic changes are transient and do not affect future generations, they have the potential to promote adaptive changes that enhance resilience and overall wellbeing in space environments. For instance, modifications could be specifically designed to enhance immune functions, vital for combating infections. Additionally, optimizing metabolic pathways could improve the body’s ability to process elevated carbon dioxide levels, enhancing respiratory function.
Germline gene modifications represent another avenue for facilitating human adaptation to space, albeit with significant complications. Concerns like pleiotropy, which refers to a single gene impacting multiple unrelated traits, and mosaicism, where genetic changes are not uniform across all cells, pose challenges. Additionally, off-target effects could lead to unintended mutations, potentially causing health issues not anticipated during initial planning (Galis, 2007; Musunuru, 2019).
For these reasons, germline modifications are not currently recommended for improving human adaptation, especially considering their long-term implications for future generations. Despite the challenges, if researchers could develop methods to mitigate these complications, germline modifications could provide transformative advantages. Enhancements like improved resistance to radiation could be ingrained in the genetic code, allowing successive generations to inherit beneficial traits critical for survival in space. For example, a germline modification improving DNA repair mechanisms could lead to humans more adept at managing cellular damage caused by radiation, reducing the risk of radiation-induced illnesses over a lifetime. Over time, such alterations could result in a new subset of the human species specifically adapted to environments with high levels of radiation exposure.
However, and taking into account that germline modifications are permanent, there is another factor to be considered: space environments possess distinct characteristics and are dynamic. For example, the atmosphere on a planet could change, affecting the chemical composition of the environment. Such alteration could require inhabitants to further adapt. As a result, genetic modifications should be implemented with an understanding that while they address current conditions, they must also account for potential future changes. This ensures that adaptations remain beneficial, safeguarding the ability to respond flexibly to new and unforeseen challenges in space.
This approach emphasizes the importance of complementing germline modifications with the inherent biological plasticity of humans, allowing individuals to dynamically adapt to evolving environmental conditions.
It is hypothesized herein that combining somatic and germline modifications with Adaptive AET could significantly enhance biological adaptation to space during early human development and over multiple generations.
Integrating somatic gene modifications would allow for targeted and timely interventions, such as enhancing resistance to radiation and optimizing metabolic functions based on real-time data from AET’s AI model. For instance, if increased oxidative stress is detected due to radiation exposure, AET could signal the introduction of tailored genetic modifications. Nanorobots equipped with biocomputing capabilities would then deliver these modifications directly to the necessary cells, enhancing the precision and efficacy of the interventions. This approach would support real-time adaptability to challenging environments, thereby improving individual health and resilience.
This process could involve using viral vectors or plasmids to transport engineered genes into target tissues (Howarth et al., 2010). For instance, if the goal is to boost antioxidant production, nanorobots could deliver plasmids encoding genes to enhnance the production of glutathione peroxidase (Lubos et al., 2011). As these genes are expressed within target cells, antioxidant levels would increase, contributing to cellular protection against oxidative damage. The AET AI system would then continuously monitor physiological and biological metrics to track the effectiveness of the modifications, leading to adaptation.
In parallel, by combining AET with germline modifications, a comprehensive adaptive framework can be established for contextual long-term evolution. Germline modifications would embed essential traits, such as increased resistance to radiation, enhanced metabolic efficiency, and improved oxygen utilization, in individuals’ genetic codes, creating a baseline level of adaptation that can be inherited by future generations. Meanwhile, AET’s capability for real-time monitoring and adaptive interventions would allow continuous adjustments based on changing environmental conditions. Such a multi-layered strategy would ensure human survival and thriving in challenging extraterrestrial environments, balancing the stability of genetic traits with the flexibility required to cope with the unpredictable nature of space conditions. Ultimately, this comprehensive approach could lead to more effective long-term adaptation, enabling humans to survive and thrive in space.
The integration of germline modifications with AET could emphasize the dual role of artificially directed evolution and natural selection in shaping human adaptation over multiple generations. This strategy could facilitate the emergence of distinct subspecies over longer timescales, enabling populations to thrive under varying environmental conditions by enhancing essential survival traits. Natural selection remains a vital driver of evolution, favoring individuals who exhibit advantageous adaptations to their environments. As articulated by Darwin (1859) natural selection operates on the principle that organisms better adapted to their surroundings have higher survival and reproductive rates. This mechanism underpins the evolution of species, highlighting the critical role of reproduction in transmitting beneficial traits to subsequent generations.
In this context, environmental stressors - such as varying gravitational forces, radiation exposure, and atmospheric changes - would exert selective pressure on human populations, influencing the traits deemed favorable for survival. Here, the environment itself acts as a form of human enhancement. The synergistic effects of germline and somatic modifications, supported by AET, would enable swift and effective adaptation to these challenges, facilitating human resilience in space.
While traditional strategies for space exploration often focus on replicating Earth-like environments through methods like terraforming initiatives (McKay, 1982), recognizing the need for humanity to biologically adapt to diverse extraterrestrial environments is crucial - along with advancing humanity’s biological condition. By adopting a strategy that emphasizes flexible biological adaptations, humanity can enhance resilience and functionality in unfamiliar cosmic contexts, ultimately maximizing potential for long-term survival and success beyond Earth.
In conclusion, the interplay among germline modifications, somatic interventions, and AET could lead to significant evolutionary changes within the human species tailored to specific environmental contexts. As adaptations unfold across multiple generations, distinct traits may solidify within specific populations, potentially resulting in the emergence of diverse human subspecies. This evolutionary trajectory underscores humanity’s response to the unique challenges posed by space environments, reflecting the dynamic nature of evolution shaped by both artificial interventions and the natural processes of adaptation and survival.
The potential for the emergence of subspecies capable of perceiving diverse environmental properties suggests a groundbreaking biological era, the implications of which - designated here as Universal Cognition - are outlined in the next section, as a prelude to a more extensive exploration in future research publications.
5 Universal Cognition: enhancing our perception of the universe
As mentioned previously, biological plasticity is a vital characteristic of biological development, allowing organisms to adjust their phenotypes in response to environmental changes. This capacity for adaptation is crucial for survival and reproductive success, enabling diverse phenotypic expressions depending on the environmental conditions. Such variability, whether physiological, morphological, behavioral, or molecular, plays a fundamental role in the evolutionary processes that shape all living organisms (Gilbert et al., 2015; Hochberg et al., 2011; West-Eberhard, 2003).
The influence of space conditions on human development suggests that significant phenotypic adjustments could occur over generations, potentially enhancing survival in those environments. Should these adjustments take place, structural and functional modifications may impact perception and cognition.
Perception involves the identification, interpretation, and organization of sensory information to understand and represent the environment. As highlighted by the principles of Embodied Cognition, the way different animal species perceive their surroundings is influenced by their body structures. For instance, a crocodile is likely to perceive a chair differently than a human due to their distinct anatomical features. For most humans, a chair signifies the action of sitting, thanks to their flexible torso and hip joints. In contrast, a crocodile may either overlook the chair entirely or interact with it destructively using its sharp teeth (Barsalou, 2003; Barsalou, 2008; Chemero, 2009; Clark, 2008; Gibson, 1966; Gibson, 1979; Thelen and Smith, 1994). Therefore, our physical form not only defines our interactions with the physical world but also informs our cognitive experiences, illustrating the connection between biology and perception. Our physiological nature also influences how the environment is perceived. For example, the morphology of cortical synapses can influence information processing speed, further shaping how perception emerges in relation to space (Hall, 2011).
Exaptation - the process by which a trait evolves to fulfill a function different from its original purpose - illustrates how species can develop traits better suited for new environments, contributing to new perceptual abilities (Bergman, 2005). For instance, the evolution of jaw bones illustrates how structures initially formed for feeding can adapt to serve new functions, such as transmitting sound in the ear. Kaas (2008) emphasizes that the structural changes in the human brain have fostered advanced cognitive capabilities. His comparative analysis of neuroanatomy and the fossil record indicates that our cognitive functions evolved through a gradual process shaped by environmental pressures. The expansion of specific brain regions, particularly the neocortex, exemplifies adaptations to changing demands, such as improved visual processing helping us navigate new and complex challenges.
Space environments have the potential to profoundly transform our understanding of reality. Conditions beyond Earth may catalyze substantial structural and biological changes in humans, leading to adaptations in sensory systems that enable new modalities of perception. These adaptations could enrich scientific inquiry and broaden the comprehension of the universe, potentially contributing to what is designated here as Universal Cognition.
Shubin (2013) speculates that the gravitational influence of celestial bodies, such as Jupiter, could lead to distinct changes in human body shapes, underscoring the relationship between environmental factors and biological evolution. Space environments could profoundly impact phenotypic expressions throughout human development due to varying forms of physical and mental stress compared to terrestrial conditions. For example, key environmental factors on Mars, including its gravitational pull of 0.38 g, a predominantly carbon dioxide atmosphere, extreme thermal variations ranging from −125 °C at night to 20 °C during the day, and elevated cosmic radiation levels, may impose considerable stress on the human body, leading to critical variations in structural and functional features, particularly during early developmental stages when substantial changes take place. Consequently, structural and functional changes driven by adaptation to space environments would alter the ways in which humans perceive their surroundings.
This shift could herald a new cognitive era, marked by the emergence of novel abilities related to abstract thinking, thereby challenging established axioms that are limited by our current biological state. Such cognitive evolution could facilitate innovative approaches to problem-solving and expand our understanding of the universe.
The connection between axioms and human perception lies in the way humans use fundamental assumptions to make sense of the world. Just as axioms provide the foundational basis in mathematical or logical frameworks, our perceptions often rely on basic, self-evident truths or assumptions to make sense of our experiences (Ashby and Lee, 1993). As our biological adaptations evolve in response to new space environments, these foundational assumptions may also shift. For instance, foundational assumptions we rely on, such as the continuity of space, the nature of physical interactions, and the perception of time, might change. This could lead to a new cognitive framework where previously accepted self-evident truths no longer hold, prompting us to find new ways of reasoning regarding our experiences and the universe around us.
In this context, the ability to process information and make sense of the environment may become more granular and nuanced, reflecting the unique conditions we encounter in space. Ultimately, these changes would not only broaden our perceptual capabilities but also challenge the logical constructs we’ve established, compelling us to rethink our interactions with the universe and adapt our reasoning to align with our transformed reality.
Historically, figures like Galileo may illustrate how human perception, shaped by biological features, can influence the foundational axioms that define our understanding of the universe. His experiments with motion and gravity were rooted in his perceptual experiences of physical objects, enabling him to establish groundbreaking principles that articulated natural laws. These conclusions emerged as revolutionary axioms forged from the interplay between human perception and its inherent biological characteristics. His perceptual biases laid the groundwork for modern science, highlighting how truths about the universe, defined through the lenses of human experience, can arise from experiences informed by our biological realities.
As we move forward, one compelling question arises: How will our evolving adaptations and perceptions in space redefine our understanding of existence and our place within the universe?
Figure 2 illustrates the overall speculated future scenario where AET synergizes with both somatic and germline Gene Modifications to optimize adaptation to space environments, resulting in structural and functional bodily changes that can modify sensory systems, enabling new modalities of perception, ultimately leading to Universal Cognition.
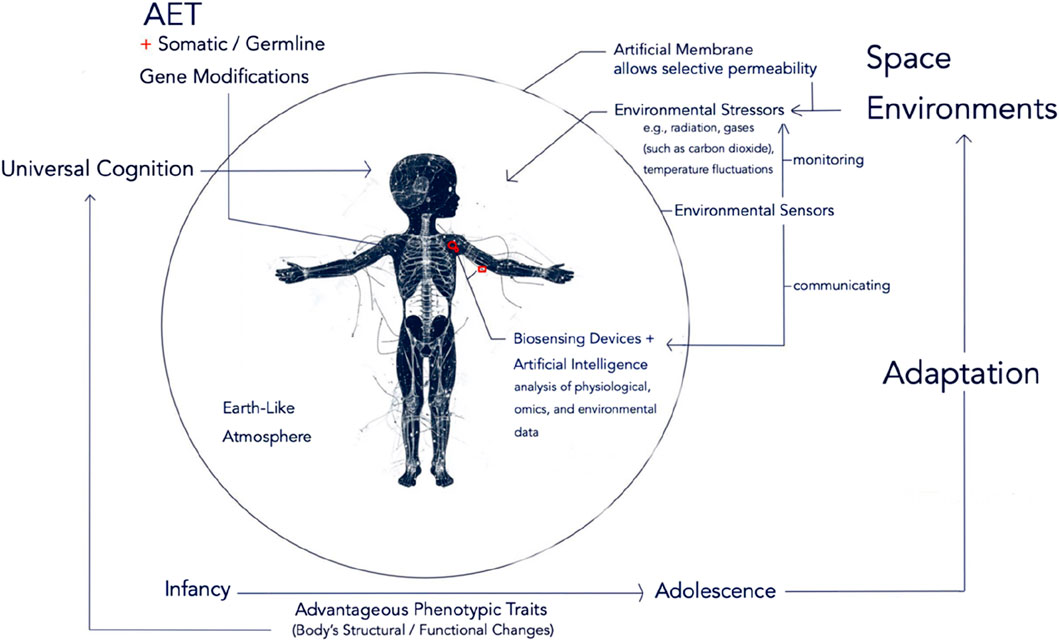
Figure 2. Synergy of AET and Genetic Modifications and Universal Cognition. This figure illustrates the potential synergy between AET and both Somatic and Germline Gene Modifications in optimizing adaptation to space environments. The synergistic effects of genetic modifications, enhanced by AET, facilitate rapid and context-specific adaptations to these challenging conditions. While the synergy between AET and somatic gene modifications allows for targeted and timely interventions - such as enhancing radiation resistance and metabolic functions through real-time data analysis by AET’s AI model - the integration of AET with germline modifications offers a unique advantage by balancing the stability of embedded genetic traits with the flexibility needed to respond to the unpredictable nature of space environments. Ultimately, the structural and functional changes in the body resulting from adaptive biological responses to space can lead to modifications in sensory systems enabling new modalities of perception, thereby expanding our understanding of the universe, contributing to Universal Cognition.
While this paper outlines the potential for technological and genetic interventions to facilitate early human adaptation to space environments, it acknowledges a significant limitation in its current scope: the psychological dimensions of such adaptation. Specifically, a deeper exploration of identity formation and broader psychological challenges is needed to fully understand the complexities of future early human adaptation to space. Therefore, a thorough analysis of these psychological dimensions will be a crucial focus of future research publications.
Data availability statement
The original contributions presented in the study are included in the article/supplementary material, further inquiries can be directed to the corresponding author.
Author contributions
MF: Writing – original draft, Writing – review and editing.
Funding
The author(s) declare that no financial support was received for the research and/or publication of this article.
Acknowledgements
I would like to express my gratitude to the TEDx community for allowing me to share this work through a previous TEDx talk.
Conflict of interest
The author declares that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Alberts, J. R., and Ronca, A. (2005). “Development as adaptation: a paradigm for gravitational and space biology,” in Experimentation with animal models in space. Editor S. Smith (Elsevier B.V), 175–207. doi:10.1016/S1569-2574(05)10007-0
Almeida, M., and Diogo, R. (2019). Human enhancement: genetic engineering and evolution. Evol. Med. Public Health 2019 (1), 183–189. doi:10.1093/emph/eoz026
Amann, R. P., Deaver, D. R., Zirkin, B. R., Grills, G. S., Sapp, J. W., Veeramachaneni, D. N., et al. (1992). Effects of microgravity or simulated launch on testicular function in rats. J. Appl. Physiol. 73, 174S–185S. doi:10.1152/jappl.1992.73.2.S174
Ashby, F. G., and Lee, W. W. (1993). “Perceptual variability as a fundamental axiom of perceptual science,” in Foundations of perceptual theory. Editor S. C. Masin (North-Holland/Elsevier Science Publishers), 369–399. doi:10.1016/S0166-4115(08)62778-8
Balistreri, M., and Umbrello, S. (2023). Modifying the environment or human nature? What is the right choice for space travel and Mars colonization? Nanoethics 17, 5. doi:10.1007/s11569-023-00440-7
Barsalou, L. W. (2003). Situated simulation in the human conceptual system. Lang. Cogn. Process. 18, 513–562. doi:10.1080/01690960344000026
Barsalou, L. W. (2008). Grounded cognition. Annu. Rev. Psychol. 59, 617–645. doi:10.1146/annurev.psych.59.103006.093639
Batenson, P., Barker, D., Clutton-Brock, T., Deb, D., D’Udine, B., Foley, A. R., et al. (2004). Developmental plasticity and human health. Nature 430, 419–421. doi:10.1038/nature02725
Belsky, J., and Pluess, M. (2009). The nature (and nurture?) of plasticity in early human development. Perspect. Psychol. Sci. 4, 345–351. doi:10.1111/j.1745-6924.2009.01136.x
Bergman, J. (2005). Can evolution produce new organs or structures? J. Creat. 19, 76–82. Available online at: https://creation.com/images/pdfs/tj/j19_2/j19_2_76-82.pdf.
Billman, G. E. (2020). Homeostasis: the underappreciated and far too often ignored central organizing principle of physiology. Front. Physiol. 11, 200. doi:10.3389/fphys.2020.00200
Black, J. E., Jones, T. A., Nelson, C. A., and Greenough, W. T. (1998). “Neuronal plasticity and the developing brain,” in Handbook of child and adolescent psychiatry: basic psychiatric science and treatment.
Braddock, M. (2020). “Limitations for extraterrestrial colonization and civilization, and the potential for human enhancements,” in Human enhancements for space missions. Editor K. Szocik (Cham: Springer), 1–14. doi:10.1007/978-3-030-42036-9_5
Chen, G., Zhu, F., Gan, A. S. J., Mohan, B., Dey, K. K., Xu, K., et al. (2023). Towards the next generation nanorobots. Next Nanotechnol. 2, 100019. doi:10.1016/j.nxnano.2023.100019
Cheung, H. C., De Louche, C., and Komorowski, M. (2023). Artificial intelligence applications in space medicine. Aerosp. Med. Hum. Perform. 94, 610–622. doi:10.3357/AMHP.6178.2023
Clark, A. (2008). Supersizing the mind: embodiment, action, and cognitive extension. Oxford University Press.
Clynes, M. E., and Klyne, N. S. (1960). Cyborgs and space. New York: Astronautics. Available online at: https://web.mit.edu/digitalapollo/Documents/Chapter1/cyborgs.pdf.
Daniels, N. (2000). Normal functioning and the treatment-enhancement distinction. Camb. Q. Healthc. Ethics 9 (3), 309–322. doi:10.1017/S0963180100903037
Darwin, C. (1859). On the origin of species by means of natural selection, or, the preservation of favoured races in the struggle for life. J. Murray.
Demontis, G. C., Germani, M. M., Gaiani, E. G., Barravecchia, I., Passino, C., and Angeloni, D. (2017). Human pathophysiological adaptations to the space environment. Front. Physiol. 8, 547. doi:10.3389/fphys.2017.00547
Doudna, J. A., and Charpentier, E. (2014). Genome editing: the new frontier of genome engineering with CRISPR-Cas9. Science 346 (6213), 1258096. doi:10.1126/science.1258096
Dournon, C., Durand, D., Tankosic, C., Membre, H., Gualandris-Parisot, L., and Bautz, A. (2001). Effects of microgravity on the larval development, metamorphosis, and reproduction of the urodele amphibian Pleurodeles waltl. Dev. Growth and Differ. 43 (3), 315–326. doi:10.1046/j.1440-169x.2001.00575.x
Dunbar, C. E., High, A. A., Joung, J. K., Kohn, D. B., Ozawa, K., and Sadelain, M. (2018). Gene therapy comes of age. Science 359 (6372), eaan4672. doi:10.1126/science.aan4672
Fan, X., Jiao, B., Zhou, X., Zhang, W., and Ouyang, Z. (2025). Miniaturization of mass spectrometry systems: an overview of recent advancements and a perspective on future directions. Anal. Chem. 97, 9111–9125. doi:10.1021/acs.analchem.5c01223
Gagarin, Y., and Lebedev, V. (2003). Psychology and space. Honolulu, Hawaii: University Press of the Pacific.
Galilei, G. (1989). Sidereus nuncius. A. Van helden, trans. The University of Chicago Press. (Original work published 1610).
Galis, F., and Metz, J. A. J. (2007). Evolutionary novelties: the making and breaking of pleiotropic constraints. Integr. Comp. Biol. 47 (3), 409–419. doi:10.1093/icb/icm081
Gibson, J. J. (1966). The senses considered as perceptual systems. Boston, Massachusetts: Houghton Mifflin Company. doi:10.1080/10407413.2017.1331116
Gibson, J. J. (1979). The ecological approach to visual perception. Lawrence Erlbaum Associates, Inc., Publishers.
Gilbert, S. F. (2001). Ecological developmental biology: developmental biology meets the real world. Dev. Biol. 233 (1), 1–12. doi:10.1006/dbio.2001.0210
Gilbert, S. F., Bosch, T. C. G., and Lédon-Rettig, C. (2015). Eco-evo-devo: developmental symbiosis and developmental plasticity as evolutionary agents. Nat. Rev. Genet. 16, 611–622. doi:10.1038/nrg3982
Gimunová, M., Paludo, A. C., Bernaciková, M., and Bienertova-Vasku, J. (2024). The effect of space travel on human reproductive health: a systematic review. npj Microgravity 10, 10. doi:10.1038/s41526-024-00351-1
Gouw, A. M. (2020). “CRISPR challenges and opportunities for space travel,” in Human enhancements for space missions. Editor K. Szocik (Cham: Springer), 1–14. doi:10.1007/978-3-030-42036-9_2
Greatbatch, C. (2024). The role of artificial intelligence in space medicine. J. Australas. Soc. Aerosp. Med. 13 (1), 1–5. doi:10.2478/asam-2024-0001
Greenough, W. T., Black, J. E., and Wallace, C. S. (2002). Experience and brain development. In M. H. Johnson, Y. Munakata, and R. O. Gilmore (Eds.), Brain development and cognition: A reader (2nd ed). Blackwell Publishing. 186–216.
Hall, B. K. (2003). Evo-devo: evolutionary developmental mechanisms. Int. J. Dev. Biol. 47, 491–495.
Hashimoto, T., Horikawa, D. D., Saito, Y., Kuwahara, H., Kozuka-Hata, H., Shin-I, T., et al. (2016). Extremotolerant tardigrade genome and improved radiotolerance of human cultured cells by tardigrade-unique protein. Nat. Commun. 7, 12808. doi:10.1038/ncomms12808
Hawking, S. (1996). A brief history of time: updated and expanded (tenth anniversary edition). New York City, NY: Bantam Books.
Hochberg, Z. (2009). Evo-devo of child growth II: human life history and transition between its phases. Eur. J. Endocrinol. 160, 135–141. doi:10.1530/EJE-08-0445
Hochberg, Z. (2011). Developmental plasticity in child growth and maturation. Front. Endocrinol. 2, 41. doi:10.3389/fendo.2011.00041
Hochberg, Z., Feil, R., Constancia, M., Fraga, M., Junien, C., Carel, J. C., et al. (2011). Child health, developmental plasticity, and epigenetic programming. Endocr. Rev. 32, 159–224. doi:10.1210/er.2009-0039
Horn, E. R. (2013). The development of gravity sensory systems during periods of altered gravity dependent sensory input. Adv. Space Biol. Med. 9, 133–171. doi:10.1016/S1569-2574(03)09006-3
Howarth, J. L., Lee, Y. B., and Uney, J. B. (2010). Using viral vectors as gene transfer tools (cell biology and toxicology special issue: ETCS-UK 1 day meeting on genetic manipulation of cells). Cell Biol. Toxicol. 26 (1), 1–20. doi:10.1007/s10565-009-9139-5
Human Spaceology Center (2022). Spaceology Newsletter 202201. Available online at: https://space.innovationkyoto.org/wp-content/uploads/2022/03/Spaceology-NewsLetter_202201.pdf.
Huttenlocher, P. R., and Dabholkar, A. S. (1997). Regional differences in synaptogenesis in human cerebral cortex. J. Comp. Neurology 387 (2), 167–178. doi:10.1002/(sici)1096-9861(19971020)387:2<167::aid-cne1>3.0.co;2-z
Ijiri, K. (1995). Medaka fish had the honor to perform the first successful vertebrate mating in space. Fish Biol. J. 7, 1–10.
Jain, V., Chuva de Sousa Lopes, S. M., Benotmane, M. A., Verratti, V., Mitchell, R. T., and Stukenborg, J.-B. (2023). Human development and reproduction in space - a European perspective. npj Microgravity 9, 24. doi:10.1038/s41526-023-00272-5
Jung, S. Y., Bowers, S. D., and Willard, S. T. (2009). Simulated microgravity influences bovine oocyte in vitro fertiization and preimplantation embryo development. J. Animal Veterinary Adv. 8 (9), 1807–1814. doi:10.36478/javaa.2009.1807.1814
Kaas, J. H. (2008). The evolution of the complex sensory and motor systems of the human brain. Brain Res. Bull. 75 (2-4), 384–390. doi:10.1016/j.brainresbull.2007.10.009
Kanas, N., and Manzey, D. (2008). Space psychology and psychiatry. 2nd ed. Springer, Space Technology Library.
Kojima, Y., Sasaki, S., Kubota, Y., Ikeuchi, T., Hayashi, Y., and Kohri, K. (2000). Effects of simulated microgravity on mammalian fertilization and preimplantation embryonic development in vitro. Fertil. Steril. 74 (6), 1142–1147. doi:10.1016/s0015-0282(00)01583-1
Konrad, C., Szocik, K., and Braddock, M. (2019). Why human enhancement is necessary for successful human deep-space missions. New Bioeth. 25 (4), 295–317. doi:10.1080/20502877.2019.1667559
Krittanawong, C., Singh, N. K., Scheuring, R. A., Urquieta, E., Bershad, E. M., Macaulay, T. R., et al. (2022). Human health during space travel: state-of-the-art review. Cells 12 (1), 40. doi:10.3390/cells12010040
Kurotani-Izumi, A., and Kiyomoto, M. (2003). Morphogenesis and gravity in a whole amphibian embryo and in isolated blastomeres of sea urchins. Adv. Space Biol. Med. 9, 83–99. doi:10.1016/S1569-2574(03)09004-X
Lei, X., Cao, Y., Ma, B., Zhang, Y., Ning, L., Qian, J., et al. (2020). Development of mouse preimplantation embryos in space. Nat. Sci. Rev. 7 (9), 1437–1446. doi:10.1093/nsr/nwaa062
Li, F., Ye, Y., Lei, X., and Zhang, W. (2021). Effects of microgravity on early embryonic development and embryonic stem cell differentiation: phenotypic characterization and potential mechanisms. Front. Cell Dev. Biol. 9, 797167. doi:10.3389/fcell.2021.797167
Libretti, S., and Puckett, Y. (2023). Physiology, homeostasis. Treasure Island: StatPearls Publishing LLC.
Lubos, E., Loscalzo, J., and Handy, D. E. (2011). Glutathione peroxidase-1 in health and disease: from molecular mechanisms to therapeutic opportunities. Antioxidants and Redox Signal. 15 (7), 1957–1997. doi:10.1089/ars.2010.3586
Ma, B. H., Cao, Y. J., Zheng, W. B., Lu, J.-R., Kuang, H.-b., Leu, X.-H., et al. (2008). Real-time micrography of mouse preimplantation embryos in an orbit module on SJ-8 satellite. Microgravity Sci. Technol. 20, 127–136. doi:10.1007/s12217-008-9013-8
Mader, T. H., Gibson, C. R., Pass, A. F., Stren, C., Kuyumjian, R., Polk, J. D., et al. (2011). Optic disc edema, globe flattening, choroidal folds, and hyperopic shifts observed in astronauts after long-duration space flight. Ophthalmology 118, 2058–2069. doi:10.1016/j.ophtha.2011.06.021
Mateus, A. R., Marques-pita, M., Oostra, V., Lafuente, E., Brakefield, P. M., Zwaan, B. J., et al. (2014). Adaptive developmental plasticity: compartmentalized responses to environmental cues and to corresponding internal signals provide phenotypic flexibility. BMC Biol. 12, 97. doi:10.1186/s12915-014-0097-x
McKay, C. P. (1982). On terraforming Mars. Extrapolation 23 (4), 309–314. doi:10.3828/extr.1982.23.4.309
Micheel, C. M., Nass, S. J., and Omenn, G. S.Institute of Medicine (2012). “Committee on the review of omics-based tests for predicting patient outcomes in clinical trials, board on health care services, board on health sciences policy,” in Evolution of translational omics: lessons learned and the path forward (Washington, DC: National Academies Press US). doi:10.17226/13297
Mohammadian Gol, T., Zahedipour, F., Trosien, P., Ureña-Bailén, G., Kim, M., Antony, J. S., et al. (2024). Gene therapy in pediatrics - clinical studies and approved drugs (as of 2023). Life Sci. 348, 122685. doi:10.1016/j.lfs.2024.122685
Montesinos, C. A., Khalid, R., Cristea, O., Greenberger, J. S., Epperly, M. W., Lemon, J. A., et al. (2021). Space radiation protection countermeasures in microgravity and planetary exploration. Life 11 (8), 829. doi:10.3390/life11080829
Moody, S. A., and Golden, G. (2000). “Developmental biology research in space: issues and directions in the era of the international space station: notes from the September 1999 meeting of the international space life sciences working group in woods hole, Massachusetts,” in Developmental biology. Elsevier Inc, 1–5.
Musunuru, K. (2019). The CRISPR generation: the story of the world's first gene-edited babies. New Jersey, US: BookBaby.
Ning, L. N., Lei, X. H., Cao, Y. J., Zhang, Y.-F., Cao, Z.-H., Chen, Q., et al. (2015). Effect of short-term hypergravity treatment on mouse 2-cell embryo development. Microgravity Sci. Technol. 27, 465–471. doi:10.1007/s12217-015-9446-9
Opal, S. M. (2009). “A brief history of microbiology and immunology,” in Vaccines: a biography, 31–56. doi:10.1007/978-1-4419-1108-7_3
Organ, C., Nunn, C. L., Machanda, Z., and Wrangham, R. W. (2011). Phylogenetic rate shifts in feeding time during the evolution of homo. Proc. Natl. Acad. Sci. 108 (35), 14555–14559. doi:10.1073/pnas.1107806108
Pio-Lopez, L. (2021). The rise of the biocyborg: synthetic biology, artificial chimerism and human enhancement. New Genet. Soc. 40, 599–619. doi:10.1080/14636778.2021.2007064
Potts, R. (2012). Evolution and environmental change in early human prehistory. Annu. Rev. Anthropol. 41, 151–167. doi:10.1146/annurev-anthro-092611-145754
Raisamo, R., Rakkolainen, I., Majaranta, P., Salmien, K., Rantala, J., and Farooq, A. (2019). Human augmentation: past, present, and future. Int. J. Human-Computer Stud. 131, 131–143. doi:10.1016/j.ijhcs.2019.05.008
Ricci, C., and Boschetti, C. (2003). Bdelloid rotifers as model system to study developmental biology in space. Adv. Space Biol. Med. 9, 25–39. doi:10.1016/S1569-2574(03)09002-6
Ronca, A. (2003). Mammalian development in space. Adv. Space Biol. Med. 9, 217–251. doi:10.1016/S1569-2574(03)09009-9
Ronca, A., Alwood, J. S., Globus, R. K., and Souza, K. A. (2013). Mammalian reproduction and development on the international space station (ISS): proceedings of the rodent mark III habitat workshop. Gravitational Space Res. 1 (1), 107–123. doi:10.2478/gsr-2013-0009
Russo, A., and Lax, G. (2022). Using artificial intelligence for space challenges: a survey. Appl. Sci. 12, 5106. doi:10.3390/app12105106
Saerens, D., Huang, L., Bonroy, K., and Muyldermans, S. (2008). Antibody fragments as probe in biosensor development. Sensors 8 (8), 4669–4686. doi:10.3390/s8084669
Sagan, C. (1997). Pale blue dot: a vision of the human future in space. New York, NY: Ballantine Books.
Sapp, W. J., Philpott, D. E., Williams, C. S., Kato, K., Stevenson, J., Vasquez, M., et al. (1990). Effects of spaceflight on the spermatogonial population of rat seminiferous epithelium. FASEB J. 4 (1), 101–104. official publication of the Federation of American Societies for Experimental Biology. doi:10.1096/fasebj.4.1.2295370
Schenker, E., and Forkheim, K. (1998). Mammalian mice embryo early development in weightlessness environment on STS 80 space flight. Isr. Aerosp. Med. Inst. Rep. 5.
Scott, R. T., Antonsen, E. L., Sanders, L. M., Hastings, J. J. A., Park, S.-m., MacKintosh, G., et al. (2021). Beyond low Earth orbit: biomonitoring, artificial intelligence, and precision space health. doi:10.48550/arXiv.2112.12554
Scott, R. T., Sanders, L. M., Antonsen, E. L., Hastings, J. J. A., Park, S.-m., MacKintosh, G., et al. (2023). Biomonitoring and precision health in deep space supported by artificial intelligence. Nat. Mach. Intell. 5, 196–207. doi:10.1038/s42256-023-00617-5
Serova, L. V., and Denisova, L. A. (1982). The effect of weightlessness on the reproductive function of mammals. Physiologist 25, S9–S12.
Shubin, N. (2009). Your inner fish: a journey into the 3.5 billion-year history of the human body. New York: Pantheon Books.
Shubin, N. (2013). The universe within: discovering the common story of rocks, planets, and people. Pantheon Books.
Stawicki, C. M., Rinker, T. E., Burns, M., Tonapi, S. S., Galimidi, R. P., Anumala, D., et al. (2021). Modular fluorescent nanoparticle DNA probes for detection of peptides and proteins. Sci. Rep. 11, 19921. doi:10.1038/s41598-021-99084-4
Striedter, G. F. (2006). Précis of principles of brain evolution. Behav. Brain Sci. 29 (1), 1–36. doi:10.1017/S0140525X06009010
Szocik, K., and Reiss, M. J. (2023). The final frontier: what is distinctive about the bioethics of space missions? The cases of human enhancement and human reproduction. Monash Bioeth. Rev. 41, 87–102. doi:10.1007/s40592-022-00164-6
Szocik, K., Wojtowicz, T., Rappaport, M. B., and Corbally, J. (2020). Ethical issues of human enhancements for space missions to Mars and beyond. Futures 115, 102489. doi:10.1016/j.futures.2019.102489
Thelen, E., and Smith, L. B. (1994). A dynamical systems approach to the development of cognition and action. MIT Press.
Waber, D. P., De Moor, C., Forbes, P. W., Almli, C. R., Botteron, K. N., Leonard, G., et al. (2007). The NIH MRI study of normal brain development: performance of a population based sample of healthy children aged 6 to 18 years on a neuropsychological battery. J. Int. Neuropsychological Soc. JINS 13 (5), 729–746. doi:10.1017/S1355617707070841
Wakayama, S., and Wakayama, T. (2025). “Mammalian reproduction in spaceflight,” in In precision medicine for long and safe permanence of humans in space (Elsevier Inc), 397–407.
Wakayama, S., Kawahara, Y., Li, C., Yamagata, K., Yuge, L., and Wakayama, T. (2009). Detrimental effects of microgravity on mouse preimplantation development in vitro. PloS One 4 (8), e6753. doi:10.1371/journal.pone.0006753
Wakayama, S., Ito, D., Kamada, Y., Shimazu, T., Suzuki, T., Nagamatsu, A., et al. (2021). Evaluating the long-term effect of space radiation on the reproductive normality of Mammalian sperm preserved on the international space station. Sci. Adv. 7 (24), eabg5554. doi:10.1126/sciadv.abg5554
Wakayama, S., Kikuchi, Y., Soejima, M., Hayashi, E., Ushigome, N., Yamazaki, C., et al. (2023). Effect of microgravity on mammalian embryo development evaluated at the international space station. iScience 26 (11), 108177. doi:10.1016/j.isci.2023.108177
West-Eberhard, M. J. (2005). Developmental plasticity and the origin of species differences. Proc. Natl. Acad. Sci. 102 (Suppl. 1), 6543–6549. doi:10.1073/pnas.0501844102
Whitman, D. (2009). Phenotypic plasticity of insects: mechanisms and consequences. The University of Chicago Press. doi:10.1086/648172
Keywords: human space exploration, reproduction and development in space, developmental space biology, Evo-Devo, human enhancements, Universal Cognition, futures
Citation: Ferraz M (2025) The role of early human development and multidimensional enhancements in driving space adaptation and Universal Cognition. Front. Space Technol. 6:1705650. doi: 10.3389/frspt.2025.1705650
Received: 15 September 2025; Accepted: 24 October 2025;
Published: 07 November 2025.
Edited by:
Ilaria Cinelli, Aerospace Medical Association, United StatesReviewed by:
Peter J. Marshall, Temple University, United StatesJoão Ereiras Vedor, Jungian Clinical Institute, Portugal
Copyright © 2025 Ferraz. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Marta Ferraz, ZmVycmF6QGZhcmZ1dHVyZXMub3Jn
 Marta Ferraz
Marta Ferraz