Eco-friendly and sustainability assessment of technologies for nutrient recovery from human urine—a review
- 1Department of Chemistry, Université de Montréal, Montreal, QC, Canada
- 2Department of Chemical Sciences, Adekunle Ajasin University, Akungba Akoko, Nigeria
Nitrogen (N), phosphorus (P), and potassium (K) represent the primary components of commercial NPK fertilizer and are primarily derived from finite resources through complex and expensive processes. To ensure global food security, the development of sustainable and eco-friendly procedures for fertilizer production has gained attention. Humans generally excrete urine containing 11 g of N/L, 0.3 g of P/L of P and 1.5 g of K/L, which benefit plant growth. The recovery of these essential plant nutrients from human urine has become the focal point of increasing research endeavors. Despite the potential advantages of nutrient recovery from urine, this process is complicated, and the economic implications are substantial. Furthermore, human urine may harbor undesirable contaminants, such as pathogens, pharmaceutical residues, hormones, and elevated salt levels, which could be disseminated into the environment through agriculture. This study appraised various emerging technologies for nutrient recovery from human urine, considering their challenges, environmental impact, economic viability, and the overall sustainability of the processes. This review elucidated that most nutrient recovery technologies demonstrated elevated efficiency in nutrient recovery. Nevertheless, a recurrent oversight involves neglecting the potential transfer of contaminants and pathogens into environmental matrices. The complexity of these processes and their economic feasibility vary, with some proving intricate and economically unviable. Given that no singular technology fully mitigates these challenges, integrating two or more technologies appears imperative to address drawbacks and enhance overall system performance.
1 Introduction
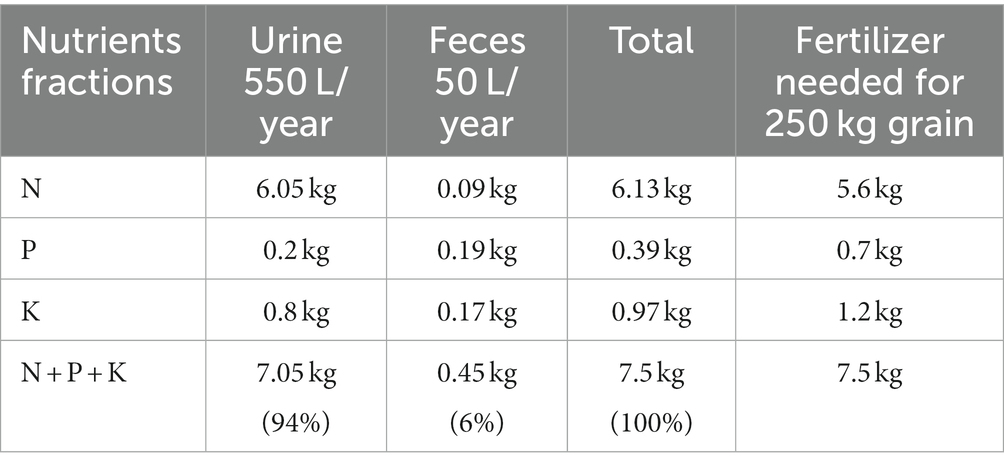
Nutrient recovery from human urine represents one of the approaches developed to address the diminishing global phosphorus reserves and the expensive techniques associated with nitrogen production. This process contributes to environmental health and helps ensure food security for the global population (Merino-Jimenez et al., 2017; Jagtap and Boyer, 2018; Kabdaşlı and Tünay, 2018; Tarpeh et al., 2018; Zuo et al., 2023). The substantial nutrient concentrations, including phosphorus (P) and nitrogen (N), in human urine render it a valuable resource for reuse. An individual excretes about 550 L of urine annually, equivalent to approximately 0.2 kg of P, 6 kg of N, and 0.8 kg of potassium (K) (Patel et al., 2020; Sohn et al., 2023). Reports indicate that while human urine constitutes less than 1% of municipal wastewater, it contributes over 50% phosphorus and more than 75% nitrogen load in wastewater (Larsen et al., 2001; Tarpeh et al., 2018; Saliu and Oladoja, 2021).
Research studies have shown that the nutrient content in human urine is sufficient for maintaining annual cereal cultivation (Heinonen-Tanski and Van Wijk-Sijbesma, 2005; Upreti et al., 2013; Garutti et al., 2022; Table 1) and that the direct application of human urine has been employed to enhance crop production (Ganrot et al., 2007; Upreti et al., 2013; Saliu et al., 2020; Garutti et al., 2022). Nevertheless, several constraints hamper its immediate use in agriculture, encompassing challenges associated with transporting large urine volumes, offensive odor, labor-intensive processes, and ample storage space requirements (Ganrot et al., 2007; Saliu et al., 2020). Additionally, there are significant concerns regarding probable contamination from microorganisms, pharmaceuticals, hormone residues, and other potential micro-pollutants (Bonvin et al., 2015; Amoah et al., 2018; Viskari et al., 2018). It is vital to acknowledge that, particularly during source separation in a “No Mix” toilet system, the potential for fecal cross-contamination of urine exists, and the degree of such contamination correlates with the associated pathogenic health risks.
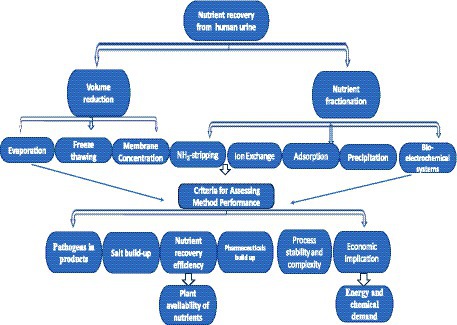
Various nutrient recovery technologies have been developed to address the limitations associated with human urine, various nutrient recovery technologies have been developed (Tong et al., 2017; Freguia et al., 2019; Ray et al., 2019; Osipi et al., 2020; Riechmann et al., 2021). Despite the substantial nutrient recovery efficiencies documented for various technologies, none have resolved all of the identified challenges. The capacity of a specific nutrient recovery technology to mitigate the recognized challenges is often either not thoroughly examined or not reported, rendering the assessment of its sustainability difficult. This review endeavors to evaluate the sustainability and practical viability of using different technologies for nutrient recovery from human urine in real-life applications. To this end, a systematic evaluation of various emerging technologies for nutrient recovery from human urine was made to consider their environmental impact (effect of products on soil and plant health), economic viability (energy and demand for chemical reagents), and overall sustainability of the processes involved. The overview of the approaches and performance criteria used in this study are presented in Figure 1.
2 Nutrient recovery technologies from human urine: the factors of concern
2.1 Urine hygienisation
The utilization of human urine as an agricultural resource faces impediments due to potential contamination with pathogens. While urine from healthy individuals typically lacks pathogens, there is a heightened risk of pathogen presence in the urine of unwell individuals. Numerous studies have evaluated the microbial composition of human urine (Karak and Bhattacharyya, 2011; Weigler et al., 2013; Abejew et al., 2014). For example, diverse pathogens were found in source-separated urine generated in Durban (Bischel et al., 2015). Through polymerase chain reaction (PCR) assays, Bischel et al. (2015) focused on some viral and bacterial human pathogens in source-separated urine. Among the tested pathogens, human adenovirus, rotavirus, and JC polyomavirus exhibited the highest recurrence in 31, 34, and 100% of the tested urine samples. Notably, the predominant gram-negative bacteria identified were Shigella spp. and Aeromonas spp. Present in 61 and 94% of the urine samples, respectively (Bischel et al., 2015). Additionally, the gram-positive bacterium Clostridium perfringens, known for its prolonged persistence in urine, was detected in 72% of the tested urine samples. In a study conducted by Ekwealor et al. (2016), urine samples from sick individuals revealed the isolation of ten distinct pathogens. The identified pathogens encompass Staphylococcus aureus, Escherichia coli, Staphylococcus saprophyticus, Pseudomonas aeruginosa, Proteus spp., Enterococcus faecalis, Klebsiella pneumonia, Streptococcus spp., Neisseria gonorrhoeae, Bacillus spp.
Struvite precipitation from urine has garnered considerable attention, given that the process is relatively simple. However, the sanitary quality of the resulting product requires thorough evaluation in field trials. A study by Decrey et al. (2011) revealed that viruses persisted in struvite derived from urine even after filtration. In the same survey, bigger-sized Helminth eggs were found to accumulate in struvite precipitated from urine. Bischel et al. (2015) recorded the prevalence of Salmonella typhimurium and Enterococcus spp. in struvite precipitated from urine.
Urine storage offers a probable means of diminishing the possible health hazards from fecal microorganisms depending on the storage period and the pH and temperature of the media. Hoglund et al. have concluded from the inactivation curves for rhesus rotavirus, Campylobacter jejuni and Cryptosporidium parvum that if urine is stored at 20°C for a minimum of six months, it may be considered safe to use as a fertilizer for
various crops (Höglund and Stenström, 1999; Höglund et al., 2000). The studies noted that temperature is a crucial point for the inactivation of microorganisms during urine storage. Höglund et al. (2002) observed that 90% of the rhesus rotavirus in the stored urine was rendered inactive after 35 days with a medium temperature of 20°C. However, no substantial microbial count decrease could be achieved if the temperature was maintained at 4°C.
Clostridiales and Lactobacillales dominated bacterial populations in source-separated urine from two sites in the United States after 80 days of storage (Lahr et al., 2016). Some spore-forming gram-positive bacteria resist inactivation even during prolonged storage periods (Höglund and Stenström, 1999).
Through the assessment of relevant studies, it has become evident that extended urine storage may not eliminate the risks associated with pathogens in the urine. Therefore, the requirement lies in reducing or entirely inactivating live pathogens in urine before nutrient recovery. This review evaluates the microorganism inactivation capabilities of various technologies employed for nutrient fraction recovery from human urine. The principal goal is to ensure the safety of the recovered nutrients to promote sustainable agricultural practices.
2.2 Micropollutants in urine
Studies have shown that human urine contains pharmaceuticals, enzymes, trace elements, and hormones (Winker, 2009; Zhang et al., 2014; Bischel et al., 2015). Following metabolism in the human body, medications are excreted in their original form or as metabolites via human urine and feces. Lienert et al. (2007) estimated that approximately 64% of active pharmaceutical components are excreted through urine, with the remaining portion eliminated in fecal matter.
Winker (2009) stated that it was possible to predict the concentration of 124 active pharmaceutical agents in an average German urine. Dascenzo et al. (2003) affirmed that the maximum daily concentration of estrone, 17ß-estradiol and estriol hormone excreted through urine by women between the ages 17 to 64 years were 86, 11 and 86 μg/L.
Bischel et al. (2015) detected trimethoprim and sulfamethoxazole antibiotics, which are often administered as preventive measures for HIV-positive patients with a maximum concentration of 6,800 mg/L and 1,280 mg/L, respectively, in source-separated urine. Emtricitabine, an antiretroviral medication, was identified in 40% of tested urine samples. Additionally, a gene responsible for resistance to sulfonamide antibiotics was found in every urine sample that was examined.
A critical limitation of many treatment technologies is their lack of efficiency in eliminating micropollutants from urine and resulting wastewater (Zhang et al., 2014). This deficiency can release a substantial amount of micropollutants into the environment if proper treatment measures are not employed, contributing to significant adverse effects on land quality and aquatic ecosystems. Of specific concern is the likelihood of micropollutant accumulation in soils and their potential plant uptake when untreated urine is used as fertilizer, posing a risk of entry into the food chain (Winker, 2009).
The investigation of the translocation of pharmaceuticals from water and soils into plants has been reported (Christian et al., 2003; Kumar et al., 2005; Ji et al., 2012; Wu et al., 2012). These studies showed that pharmaceuticals and personal care products (PPCPs) can accumulate in plant tissues via root absorption. Wu et al. (2012) noted that lettuce and spinach could take up some PPCPs from wastewater-irrigated soils. The study detected trimethoprim and sulfamethoxazole in the dried plant biomass of spinach and lettuce. Kumar et al. (2005) evaluated the concentrations of sulfamethazine and chlortetracycline in green onion, cabbage, lettuce, potato, cabbage and corn grown with antibiotic-polluted manure. The absorption of these antibiotics into the plants was noticed in a relatively small range of 2–17 μg/kg; contrary to this study, Dolliver et al. (2007) detected a higher concentration of sulfamethazine in plant tissues ranging from 0.1 to 1.2 mg/kg. Al-Farsi et al. (2018) also observed that radish roots accumulated sulfamethoxazole and amoxicillin when irrigated with wastewater. Li et al. (2020) investigated the fate, uptake and distribution of fifteen frequently used pharmaceuticals in a system involving soil, water, and radishes. They discovered that fourteen out of fifteen pharmaceuticals studied could accumulate in radish tissues with a build-up ranging from 2.1 to 14,000 ng/g. Irrefutably, vegetables and grain products cultivated in micropollutant-contaminated agroecosystems may threaten public health if pharmaceuticals are absorbed and accumulated.
Although fewer data are available on the accumulation of hormones and pharmaceuticals through the application of recovered nutrients from urine, the possible environmental hazards posed by these pollutants in urine should be considered during nutrient recovery processes.
2.3 Soil salinity
Human urine has been observed to contain a high concentration of sodium chloride. The average sodium (Na) concentration in fresh and undiluted human urine ranged between 0.94–0.98 g/L (Kirchmann and Pettersson, 1995). Documented surveys also showed higher Na concentrations of 2.9 to 3.5 g/L (Dickin et al., 2018) and 2.34 g/L (Pradhan et al., 2009) in source-separated urine. Consequently, using human urine to fertilize crops may result in soil sodification (build-up of sodium), which can eventually be detrimental to plant growth. Salts decrease the osmotic potential of water, thereby increasing the energy plants need to extract moisture from soils and reduce the degree to which water infiltrates the soil. Aside from contributing to water stress, salts like sodium chloride are toxic if they accumulate in plant tissues or leaves. Common indicators of sodium toxicity include leaf burn, scorch, and necrosis along the periphery of leaves. Furthermore, it can potentially instigate deficiencies in calcium and potassium within soils. The buildup of chloride in the soil can lead to leaf tip or margin burning and yellowing of plants. Hijikata et al. (2014) investigated salt accumulation in urine-fertilized gardens during the dry season. The salt build-up in the soil is a significant concern when utilizing human urine on agricultural lands. This review assessed developed technologies’ attention to sodium chloride concentration in recovered nutrients from human urine. The challenges associated with Na would probably not be relevant in moderate to high rain climates where excess rain will leach the Na out of the root zone. Still, in more arid areas where rain and water are scarce, monitoring Na levels in soils receiving urine would be necessary to prevent excessive build-up.
2.4 Process stability and nutrient recovery efficiency
Process stability emerges as a crucial parameter in the design of a nutrient recovery method. The various nutrient fractions in human urine respond to changes in pH. In the presence of urease-active bacteria, urea within fresh urine undergoes rapid hydrolysis, forming ammonium ions and ammonia gas (Equation 1).
The concentrations of ammonium ions and ammonia gas in urine depend on the pH and temperature. Through carbonate chemistry, an increase in pH swings the chemical equilibrium to the right, forming ammonia gas. The nitrogen in urine is lost when a high pH value is required for the nutrient recovery process, except when the process is designed to collect the stripped ammonia gas.
The nutrient recovery efficiency of any given technology is an essential factor to consider, as low recovery leaves the system with the discharge of nutrient-laden urine. The efficiency of the process must also consider its cost.
2.5 Process economy
The economic viability of the nutrient recovery process is essential for operational implementation. The costs of the system can be divided into operational and construction costs. The resource demand for different technologies used in nutrient recovery varies. Methods that are energy intensive, capital intensive, or demand the use of chemicals must be scrutinized before they can be accepted as sustainable or eco-friendly, even though the nutrient recovery efficiency is high. The higher the energy demand of a process, the more capital-intensive the process will be.
Nättorp et al. (2017) assessed the cost-effectiveness of various phosphorus recovery methods from wastewater, concluding that the specific costs per kilogram of phosphorus recovered (4 to 10 EUR/kg P) were generally higher than conventional fertilizer production (1.6 EUR/kg P). This underscores the importance of not only considering recovery efficiency but also evaluating the economic sustainability of the process, with a higher cost indicating potential financial impracticality.
3 Assessment of techniques for nutrient recovery from human urine
Human urine, a promising resource for nutrient recovery, has led to the development of various technologies aimed at harnessing its nutrients. These methods range from simple urine concentration to reduced water content to those targeting specific nutrient fractions. The concentration process yields a liquid fertilizer, whereas nutrient fraction recovery typically results in a solid fertilizer. However, these methods are often difficult to sustain and require significant capital investments.
3.1 Volume reduction approach
Approximately 97% of the total volume of human urine is composed of water (Simha and Ganesapillai, 2017). To facilitate more accessible and cost-effective transportation, various methods have been developed to reduce the water content in urine. Different volume reduction methods are enumerated below to evaluate their financial and environmental sustainability.
3.1.1 Evaporation method
The simplest approach for reducing water content in urine is evaporation. Maurer et al. (2006) introduced a thermoelectric integrated membrane evaporation system (TIMES) that involves pre-treating urine with ozone (or UV) and sulfuric acid. The mixture is heated, circulated through hollow fiber membranes, and then subjected to reduced pressure, causing water to evaporate and resulting in a more concentrated urine.
Within air evaporation systems (AES), pre-treated urine undergoes filtration via a particulate filter before being directed to a wick package. Subsequently, applying hot air facilitates water evaporation from the wick, resulting in nutrient-rich solids. This process encounters notable challenges, including losing nitrogen as ammonia gas and high energy consumption. The elevated temperatures in the system lead to urea hydrolysis and subsequent nitrogen loss. Strategies to mitigate ammonia loss involve urine acidification or non-hydrolyzed urine. Energy recovery from the same system can reduce energy usage in the process (Maurer et al., 2006). In a study by Mayer (2002), a pressure set at 200 mbar and 780°C temperature was used to evaporate non-hydrolyzed urine. The study achieved a tenfold urine volume reduction without crystallization and yielded a viscous liquid containing 9.7% nitrogen by weight (Rößler et al., 2015). Antonini et al. (2012) generated heat through a solar thermal technique to concentrate nutrients in urine. Solar radiation generated 360 g of solid fertilizer from 50 L of fresh urine within 26 days.
Employing evaporation for nutrient recovery from urine is practical. However, it requires inhibiting ammonia loss by incorporating additional acid to retain ammonium ions within the solution. Riechmann et al. (2021) showed that virtually all nitrogen (97%) in the urine will be lost during evaporation without urine stabilization. Simha et al. (2020b), in a field test with base-stabilized urine, achieved nitrogen recovery of 30 ± 6% by utilizing hot air (at 60°C) for evaporation, while Dutta and Vinnerås (2016) achieved 74 and 54% nitrogen recovery using air at 35°C and 60°C.
The energy-intensive nature of this technology also makes it a less sustainable method. However, a solar thermal evaporation technique has been investigated to mitigate this problem (Antonini et al., 2012; Bucholtz et al., 2023). Antonini et al. (2012) overcame the energy-intensive challenge faced by the evaporation method. However, the study observed that 32% of the nitrogen fed into the solar evaporated alongside the condensate as dissolved ammonia/ammonium, resulting in a minimal N concentration of 1.84% in the dried matter. When acidified, nitrogen and phosphorus concentrations in the dried urine increased to 8.33 and 17.6%, respectively. Furthermore, the solid matter from this process contained 90% sodium chloride, typical of products recovered from urine through evaporation. In a laboratory scale experiment, 97 ± 2% of the nitrogen in stabilized urine was recovered via the dehydration process with dynamic ventilation on trays within four days (Antonini et al., 2012), and similar results were recorded in a field trial (Riechmann et al., 2021). The study also achieved 91 to 96% phosphate recovery efficiency in the urine stabilization reactor as Ca(OH)2 precipitated P out of the system. The authors also report that the process leads to an inactivation of the pathogens present, making the product safe for use. No helminths or fecal bacteria were detected in the end product. However, one embryonic Ascaris suum egg was noticed in a 500 mL sample of the stabilization reactor. Senecal and Vinnerås (2017) established comprehensive bacteria and virus deactivation within four days of urine stabilization with wood ash at a pH above 10.5 and a temperature of 20°C.
Nutrient recovery through evaporation has also been plagued with micropollutants. Simha and Ganesapillai (2017) noted that therapeutic groups: non-steroidal anti-inflammatory drugs (ibuprofen, naproxen), b-blockers (bisoprolol, metoprolol), analgesics (acetaminophen), CNS stimulants (caffeine), glucocorticosteroids (hydrocortisone), a decongestant (xylometazoline) and two antibiotics (tetracycline, doxycycline) were detected in the dried fertilizer obtained.
A better approach to the urine evaporation process has been developed by adding ash and lime and drying at low temperatures to minimize nutrient loss, reduce energy consumption, and enhance sustainability (Dutta and Vinnerås, 2016; Senecal and Vinnerås, 2017; Simha and Ganesapillai, 2017). Despite the advantages of this approach, a study observed that after alkaline dehydration treatment, pharmaceuticals such as ibuprofen, caffeine, bisoprolol, metoprolol xylometazoline and naproxen were discovered in the recovered dried matter (Simha et al., 2020b). Using this approach, Simha et al. (2020a) treated 35, 62, and 90 L of urine and generated 18.9 kg, 22.8 kg, and 29.7 kg of total solids from the urine in March, April and May, respectively. The fertilizer value of the recovered solids was reported to be 13-2.3-6.0 NPK, respectively, as % of total solids. The system’s total energy consumption was estimated to be 1,913, 2,315, and 1,185 kWh for each of the three months the process was studied. Riechmann et al. (2021) showed that the minimum Ca(OH)2 consumption in urine stabilization at full capacity was 6 g/L, and the electricity demand was 150 Wh/kg of water evaporated from urine. This study reported an operational cost of 0.05 EUR•pers.−1•d−1.
Based on the reviewed studies, nutrients recovered from human urine via evaporation contain high levels of pharmaceuticals, pathogens, and salts. A long-term application of this product can result in the buildup of pharmaceuticals in the soil and the eventual uptake by plants. The high salts in these products can cause soil sodification, which is detrimental to soil and plant health.
3.1.2 Freeze thawing
When ice crystals develop, the water molecules’ structure is re-arranged to a stable and steady tetrahedral network. Ions remain concentrated in the liquid fraction of aqueous salt solutions during the gradual formation of ice and are eliminated from the ice crystals as they grow. As a result of the crystal generation processes, ice is almost salt-free. The faster the ice’s crystallization velocity, the greater the concentrations of ions in the water crystal structure as contaminants (Moriwaki et al., 2020). Because of the density variance between ice and salt, both solids can be easily separated by gravity (Moriwaki et al., 2020). This technology is employed in eutectic freeze crystallization in industrial operations and wastewater purification as a low-energy and effective purification technique (Guessous et al., 2023; Hu et al., 2023).
Lind et al. (2001) observed that by subjecting urine to a temperature of −14°C, it is possible to concentrate around 80% of the nutrients in just 25% of the original volume. This process was applied by Ganrot et al. (2007) to investigate nutrient concentration and urine bulk reduction. The study achieved approximately 80% of nutrient recovery from human urine (Ganrot et al., 2007). In cold climate regions, freeze-thawing is an efficient and economical technique for nutrient recovery from urine (Gay et al., 2003). However, Ganrot et al. (2007) opined that freeze-thawing dramatically increased Daphnia magna survivability. Vinnerås et al. (2008) affirmed that at any temperature below 20°C, human urine poses an elevated risk of carrying live viruses and bacteria. This assertion, therefore, makes freeze-thawing urine to recover nutrients highly susceptible to pathogen contamination.
As stated earlier, in freeze-thawing, all ions in urine stay concentrated at the liquid phase and are omitted from ice crystal development. Consequently, micropollutants, salts, and other unwanted contaminants are concentrated alongside the nutrients, making the recovered nutrient material potentially unsafe for agricultural purposes.
In a recent study, an improved freeze-thawing method was accessed by Courtney and Randall (2023). The study affirmed that the reverse osmosis/eutectic freeze crystallization (RO-EFC) method used could potentially eliminate unwanted salts and excess water, resulting in a pure fertilizer output. The energy requirement of this method (60 kWh m3) was significantly less than that of other urine concentration methods. However, the ion concentration of the solid fertilizer obtained in this study contains 31.2% Na by mass.
Although the method looks attractive, fewer studies have been conducted on its use for nutrient recovery from urine. No data on the pathogen load and pharmaceutical pollution of products generated through this process are available. Given the relatively low-cost potential of this approach in cold areas, this could be used successfully in combination with a second process to reduce pathogen and salt risks.
3.1.3 The membrane concentration method
Under pressure, water permeates membranes with tiny pores and significant parts of salts or organic compounds are retained on the membrane. Recently, a gas-permeable hydrophobic membrane (GPHM) has been used to recover ammonia from various liquid wastes (Kuntke et al., 2016; Vanotti et al., 2017).
The primary membrane techniques for nutrient recovery from wastewater include forward osmosis (FO), reverse osmosis (RO), membrane distillation (MD) and electrodialysis (ED) (Ansari et al., 2017). The effectiveness of these technologies is seen in their ability to reject micro-pollutants, trace elements, pharmaceuticals, and nutrients, resulting in less contaminated water.
3.1.4 Forward osmosis
In FO, ordinary osmotic pressure is used to power the drive of water molecules from the feed side across a water-permeable membrane to the draw side. Thus, nutrient fractions are concentrated at the feed side (Ansari et al., 2017). This process prevents larger molecules from passing through the membrane, decreasing the supply stream’s overall volume, and the draw solution absorbs the water. FO has the advantage of having a lower fouling tendency and a lower energy requirement, especially when there is no need to renew the draw solution (De Vries et al., 2020).
In urine treatment tests, several FO membranes, such as the HTI cellulose triacetate (CTA) or the Porifera TFC-based membrane, as well as a commercially accessible FO system, have been shown to have significant urea rejection rates (Liu et al., 2016). However, the HTI cellulose triacetate (CTA) demanded nearly 48 h to accomplish 80% water retrieval for 1.5 L urine, making the verified technology unrealistic for large-scale nutrient recovery (Borer et al., 2014). Volpin et al. (2018) investigated the concurrent nitrogen and phosphorous reclamation from source-separated urine using the FO process. The study revealed that after the urine was concentrated to 60%, roughly 40% of the P and 50% of the N in the urine were retrieved.
Engelhardt et al. (2020) investigated ammonium and urea rejection using an aquaporin-based hollow fiber membrane based on disparities in variables such as initial feed solution concentration and pH. The study found that ammonium rejection was much higher at highly acidic feed solution (pH of 3), resulting in 90 and 80% ammonium and water recovery, respectively, while at higher pH, ammonium recovery was relatively lower (37%). The study ascribed the upsurge in ammonium recovery at lower pH to the pH sensitivity of the aquaporin-based hollow fiber membrane due to carboxyl functional groups present on the surface of the membrane, containing fewer charges under acidic pH (Liu et al., 2016). The resulting drop in negative surface charge diminished the bi-directional cation exchanges across the membrane, thereby increasing solute rejection. This study concluded that the small molecular size of urea and uncharged structure obscures urea rejection by aquaporin-based FO membranes. It has been posited that the efficiency of N, NH4-N and total organic carbon rejection using the FO process is attained when the urine (feed) pH is maintained below 7.5. Ray et al. (2019) used acetic acid, whereas Volpin et al. (2018) used citric acid to maintain an acidic fresh urine pH for better FO efficiency.
A new study investigated urea hydrolysis with pH modification as a pre-FO approach to enhance urea rejection. The survey showed urea hydrolysis and pH modification before FO can significantly improve total nitrogen recovery. For instance, 89% of total nitrogen was recovered, and 75% water recovery was achieved using a 5 M HCl draw solution (Engelhardt et al., 2020). The major setback of this study is the use of high amounts of HCl for pH modification, making the system unrealistic for real-world applications. Despite the possibilities presented by nutrient recovery through FO, membrane efficiency is hampered by the water permeability – solute selectivity balance, an essential property controlling water solute passage via polymeric membranes. A highly selective membrane filter effectively concentrates ammonium and phosphate, leading to a concentrated stream with high nutrient content in nutrient recovery procedures. Jin et al. (2012) examined the diffusion of four medicines across commercial forward osmosis (FO) membranes composed of cellulose triacetate (CTA). The study found that all medications studied were rejected with a high efficiency (>94%). It suggested that the rejection is mainly influenced by size exclusion and the hydrophobic contact between the chemicals and the membrane, particularly under acidic circumstances (Jin et al., 2012).
This study indicates a high likelihood that recovered nutrients through FO will still contain hormones, pharmaceuticals and sodium chloride as the membrane only allows water passage, thereby retaining the micropollutant in the concentrated urine (Yip and Elimelech, 2011). A long-term application of such products will be detrimental to soil and plant health. Based on our search, the pathogen load in urine concentrated through forward osmosis has not been investigated.
While FO does not require high-pressure instruments and resources compared to RO, the cost of the membrane per unit area is greater than that of RO. According to Osipi et al. (2020) the cost of FO membranes accounts for approximately 30% of the total capital expenditure.
3.1.5 Reverse osmosis
On the other hand, RO operates by utilizing the hydraulic pressure that opposes the osmotic pressure across the inputs and draws solutions. During this procedure, water molecules move from a solution with a low solute concentration to a solution with a high solute concentration. When pressure is exerted, a semi-permeable membrane separates the urine and pure water solutions, causing the water to flow back into the urine. The exerted pressure compels the water to transition from the solution with higher concentration to the less concentrated medium, specifically from urine to clean water. Consequently, the impurities accumulate on one particular surface of the semi-permeable membrane, while the cleaned water is located on the opposite side (Ray et al., 2019).
Ammonium retention in reverse osmosis membranes performs better than in neutral form (ammonia). Hence, the holding performance significantly impacts the pH of the medium (Maurer et al., 2006).
In a study by Dalhammar (1997), the pH of urine was kept at pH 7.1 to avoid ammonia gas loss. When a pressure of 50 bar was applied, a high concentration resulted in recoveries of 73% for phosphate, 71% for potassium and 70% for ammonium in the retentate was achieved. Parallel outcomes were recorded by Thörneby et al. (1999) in decreasing the water content in liquid animal manure with reverse osmosis. The nutrient retention was excellent (98%) but slightly lower for ammonia at 93%. The energy usage of the process hinged on the technical and operational parameters (Thörneby et al., 1999).
Reverse osmosis (RO) has a lower energy consumption than thermal technologies (Osipi et al., 2020). Still, the process requires high-pressure containers and instruments, which can negate its economic benefits when dealing with a higher feed salinity, such as urine (Valladares Linares et al., 2016). The major setback of this process is that RO membranes retain micropollutants alongside the nutrient fraction of interest (Hofman et al., 1997). The process presents no separation between nutrients and other unwanted pollutants such as pathogens, hormones and pharmaceuticals. Hence, the utilization of this product on agricultural lands can potentially result in a negative impact on soil and crop health or would require a second treatment procedure.
Another limiting factor for this process application is the fouling of the RO membrane because of the precipitation of salts (Migliorini and Luzzo, 2004). Fouling of the RO membrane will reduce the water outflow and upsurge the system’s total cost. Fouling of RO membrane is very severe in the nutrient recovery process from urine as the urine dynamics is affected by pH change. While optimizing to regulate fouling, chemical formulations may be introduced using acids, surfactants, or blend reagents for urine pre-treatment (Jaffer, 1994; Al-Rammah, 2000). Kuntke et al. (2016) recovered 95% ammonia from urine using a gas-permeable hydrophobic tubular system within 11–15 days.
FO seems more desirable than the pressure-driven reverse osmosis (RO) separation, as it significantly lowers the operation costs.
3.1.6 Membrane distillation (MD)
Membrane distillation (MD) is a separation technology that relies on a hydrophobic mesoporous membrane and temperature increase to concentrate solutions under low-pressure conditions (Ashoor et al., 2016). In this process, the liquid is incapable of passing through the membrane pores as a result of the hydrophobicity of the mesoporous membrane. The variation in the partial vapor pressure initiates the conveyance of water vapor through the membrane pores. It has been noted that because water is transported exclusively in a gaseous state through the membrane, MD has been shown to provide complete elimination (100% rejection) of all non-volatile components in the solution being treated (Ashoor et al., 2016).
Since water vapor movement across the MD membrane cannot substantially influence the feed osmotic pressure, the MD process can achieve high water recovery (Xie et al., 2014). In this process, ammonia transfer through the membrane is primarily affected by temperature. Increased temperature will increase ammonia transfer across the membrane. To reduce the exact ammonia movement across the hydrophobic membrane, it is crucial to acidify the source-separated human urine (Tun et al., 2016). In a study conducted by Tun et al. (2016), PTFE (polytetrafluoroethylene)/PP (polypropylene), PTFE/PP and PVDF (polyvinylidene fluoride) membranes were used for nutrient concentration from source-separated urine. The PTFE/PP membrane gave the maximum water flow rate of 60 L m−2 h−1 and > 75% total nitrogen rejection at 70°C. Xu et al. (2019) developed direct contact membrane distillation technology using hollow fiber polypropylene as a membrane. Using a temperature gradient of 20°C, 3.6–5.0 L m−2 h−1 water flux was achieved with percentage TN, P, and K rejection being 31, >97, and > 97%, respectively. Higher rejection of ammonium ions (>90%), ammonia (>95%), sodium (>98%) and potassium (>89%) were achieved by using MD hybrid membrane fabricated from polyvinyl di-fluoride and polytetrafluoroethylene ethylene integrated with methyl functionalized silica nanoparticles (Khumalo et al., 2019). MD has been successfully used for water and reclamation from urine via urine concentration as one solution (Khumalo et al., 2019; Xu et al., 2019).
MD can recover volatile ammonia from hydrolyzed urine. However, the process is hindered by the simultaneous absorption of water vapor, leading to low selectivity for ammonia transport. Pre-treatments for source-separated urine are crucial to prolong the MD membrane’s lifespan as micropollutants stick to the membrane’s surface, invariably block the membrane’s pores and reduce the quality and influx of water (Tun et al., 2016; Khumalo et al., 2019). The fate of organic contaminants is hinged on the membrane selectivity, pollutant properties during membrane separation, and their volatility during vaporization (Alturki et al., 2013; Wijekoon et al., 2014). The process is also plagued with high energy demand, which makes the process less environmentally sustainable.
3.1.7 Electrodialysis (ED)
In the electrodialysis (ED) process, ion-exchange membranes are arranged interchangeably in a direct current field to selectively recover nutrient fractions from wastewater (Li et al., 2020). ED utilizes electrochemical redox processes to produce an electric field and facilitate the movement of ions in solution via ion exchange membranes. In electrodialysis (ED), ion segregation is achieved by employing ion-exchange membranes consisting of cation-selective, anion-selective, and bipolar membranes. Throughout the process, positively charged cationic and negatively charged anionic species move towards the cathode and anode, respectively, under the influence of a direct current acting as the driving force. The selective ion membrane hinders co-ion movement (anions and cations). This is achieved through Donnan’s repulsion (Hu et al., 2018).
Selective phosphate enrichment by ED from urine has been carried out with high recovery efficiency (Escher et al., 2006; Pronk et al., 2007). In a study conducted by Li et al. (2020), an innovative bipolar membrane electrodialysis with membrane contactor (BMED–MC) efficiency for ammonia recovery from synthetic urine with 3,772 mg N L−1 concentration was studied. In the BMED–MC system, electrically induced water dissociation in the bipolar membrane concurrently elevated the pH of the urine stream, providing an acid stream for ammonia stripping. The ammonia gas migrated through the gas-permeable membrane in the membrane reactor as the urine pH increased, and it was collected from the acid stream as ammonium sulphate. The study achieved 90% ammonia recovery with small energy consumption (i.e., 12.5 MJ kg–1 N) using the BMED–MC process.
Nutrient recovered using ED has been observed to be of high purity due to the selective nature of the membrane used in the process. Escher et al. (2006) affirmed that the electrodialysis process was highly efficient concerning toxicity reduction. In their study, urine was spiked with micropollutants containing ethinylestradiol and other pharmaceuticals before ED to ascertain the purity of products recovered from the medium. The study observed that 99.9% of the pharmaceuticals and hormones were removed (Escher et al., 2006).
It can be observed that none of the membrane concentration methods discussed so far is free from challenges. Despite high purity and various products obtained using the ED method, the system is plagued with membrane fouling. Due to permanent fouling, the build-up of fouling deposits in the ED process alters the membranes, increases cell resistance (current drop), and lessens ion migration yield and the ion selectivity of the membranes (Mondor et al., 2008).
Combining different membrane systems to form hybrid systems increases total nutrient recovery efficiency (Xie et al., 2014). For instance, the FO process requires a concentration of diluted wastewater. By coupling forward osmosis (FO) with additional membrane techniques like reverse osmosis (RO) or membrane distillation (MD), it is possible to restore the driving force of FO and simultaneously generate high-quality freshwater8 (Xie et al., 2013, 2014).
Hybrid membrane processes like the FO-RO hybrid system (Coday et al., 2015), FO-MD hybrid system (Xie et al., 2013, 2014), ED-RO hybrid process (Mondor et al., 2008), and ED-MD hybrid process have been used to recover nutrients from various wastewaters (Udert and Wächter, 2012). Recently, FO-MD, as a combined process, has been used to improve nutrient recovery from urine (Volpin et al., 2018). Ray et al. (2019) utilized the FO-MD combination for nutrient recovery from urine. The integrated procedure yielded a product solution with a mean urea concentration of 45–68% while achieving over 90% rejection of TOC. Hybrid membranes such as pressure-driven nanofiltration coupled with reverse osmosis are not often utilized due to inadequate urea and ammonia rejection with high capital and cost of operation of the process (Ray et al., 2019).
3.1.8 NH3-stripping method
The ammonia stripping technique revolves around mass transfer, in which wastewater is exposed to air to strip the ammonia gas present. Ammonia is present in aqueous systems in two states: ammonium ions and ammonia gas. These species’ concentrations depend on the medium pH and temperature (Kinidi et al., 2018). The generation of ammonia gas is enhanced through increased pH, resulting in the movement of the chemical equilibrium to the right, consequently improving the formation of ammonia gas. Elevating the pH of the medium before ammonia stripping is, therefore, critical in enhancing the appearance of molecular ammonia nitrogen for stripping (Figure 2). Lime is often used to raise the pH levels of the medium prior to ammonia stripping (Markou et al., 2017); however, Hidalgo et al. (2016) posited that the extreme rise of pH needed in this process imposes an extra cost for lime and is unprofitable. Hence, it is crucial to maintain an optimal pH level in order to achieve a balance between process efficiency and cost-effectiveness. The study observed that above pH 10.5, the ammonia recovery efficiency was inconsequential as increased pH no longer affects the ionization equilibrium between molecular ammonia and ionic ammonium. Nonetheless, the cost incurred increased due to further lime use to improve the pH value (Hidalgo et al., 2016). Also, Markou et al. (2017) discovered that the type of alkali used (calcium hydroxide, sodium hydroxide and potassium hydroxide) had little or no effect on the ammonia removal efficiency (Markou et al., 2017). Nevertheless, calcium alkali has been found more desirable due to heavy metal concentrations, reduction of solids, and wastewater color after treatment (Guštin and Marinšek-Logar, 2011).
The air-water ratio in the ammonia stripping process has also been observed to be a parameter of interest to achieve better removal efficiency. Airflow into the system affects ammonia concentration in the liquid and air phases (Bonmati and Flotats, 2003). Lei et al. (2007) discovered that a higher ammonia recovery rate was attained after 12 h with an airflow rate of 10 L/min, in contrast with airflow rates of 3 L/min and 5 L/min. However, the study resolved that 5 L/min for 1 L of anaerobic effluent is more economically feasible given that an airflow rate of 10 L/min was found too expensive (Lienert et al., 2007).
Traditional ammonia stripping procedures are usually carried out at room temperature and 50°C, with a pH range of 10–12 (Bonmati and Flotats, 2003; Laureni et al., 2013). A high amount of primary reagent is frequently used to boost the pH value of the medium during ammonia stripping. The pH of the system must be adjusted back to neutral for safe discharge after ammonia removal, making the procedure uneconomical. It has been suggested that increasing the process temperature (high-temperature stripping) will increase ammonia removal efficiency and minimize the amount of reagents necessary to increase the pH (De La Rubia et al., 2010). The higher ammonia removal at higher temperatures is due to the solubility of ammonia in water governed by Henry’s law, which states that the constant of gas depends on solute, solvent, and temperature (Kinidi et al., 2018). Saracco and Genon (1994) discovered that the principal cost of an ammonia stripper at 80°C stripping temperature was half that of a stripping temperature of 40°C. However, increased temperature may result in an impractical rise in the cost of preheating.
The high energy consumption in high-temperature stripping can be minimized by employing wasted heat. For instance, the hydrothermal carbonization (HTC) reaction is performed at 180–260°C (Limoli et al., 2016), and it is possible to raise the temperature of the HTC wastewater to 70–80°C by using HTC heat.
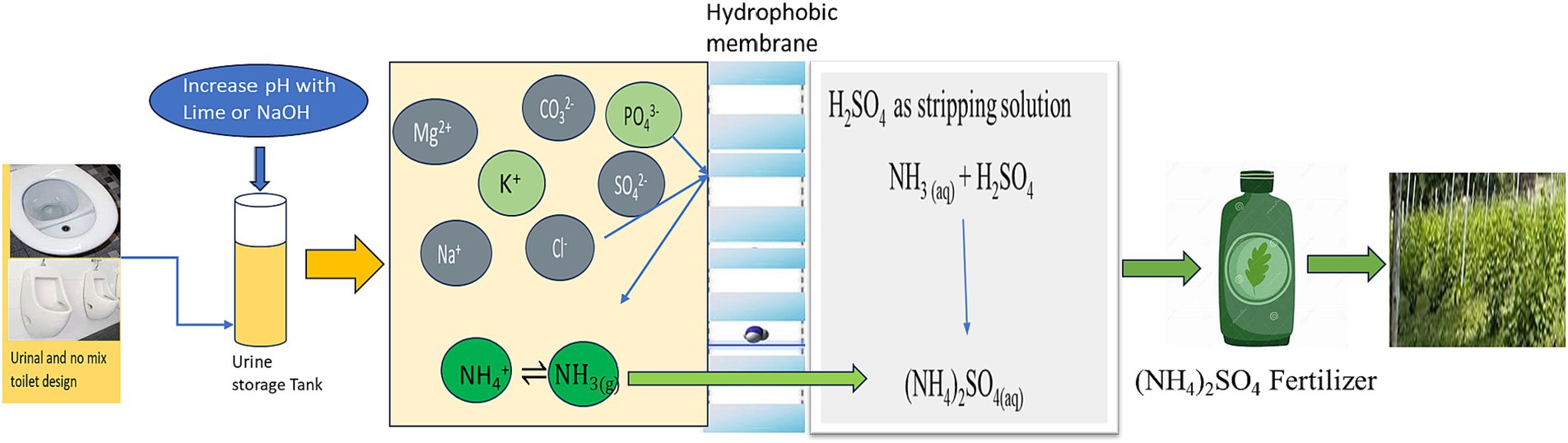
García-González et al. (2015) studied the process of ammonia stripping, which involved raising the pH level of urine by using Ca(OH)2, which causes the precipitation of P and, at the same time, changes NH4+-N into ammonia gas. The gas was transmitted over a hydrophobic membrane that allowed the passage of gases (gas-permeable hydrophobic membrane, GPHM) and underwent a reaction with H2SO4 to generate ammonium sulphate. The recuperated product is a solution of liquid ammonium salt [as described in Equations (2.1, 2.2)]. Both N and P are concurrently retrieved.
Pradhan et al. implemented ammonia stripping and phosphorus precipitation (P) to enhance nutrient reclamation from human urine. The method incorporated Ca(OH)2 to elevate urine pH, facilitating ammonium conversion into ammonia gas and induced phosphorus precipitation as a calcium-phosphate compound. The investigation achieved notable recovery rates for NH4-N, ranging from 85 to 99%, and accomplished a 99% phosphorus recovery from human urine (Saracco and Genon, 1994).
In a subsequent study, Pradhan et al. utilized ammonia stripping to extract nutrients from human urine. Their findings revealed that over 8 h at a temperature of 30°C, more than 98% (w/w) of nitrogen (N) and phosphorus (P) were successfully extracted. The resulting ammonium sulphate (liquid) harvested from the process exhibited a nitrogen (N) content of 19% (w/w), while the sediments (solids) contained phosphorus (P) at levels of 1–2% (w/w) (Christiaens et al., 2019; Pradhan et al., 2019).
Evolving methods challenging traditional ammonia stripping are membrane stripping (Christiaens et al., 2019; Pradhan et al., 2019) and vacuum thermal stripping methods (Tao et al., 2019; Tian et al., 2019). These emerging technologies require no air stream for ammonia transfer, an advantage that simplifies the procedural arrangement. Christiaens et al. (2019) evaluated this new method with conventional stripping. They discovered that direct liquid–liquid passage from a concentrated urine solution across a hydrophobic gas membrane could reduce or halve the energy needed for similar air-stripping outcomes.
While conventional ammonia stripping is chemical and energy-intensive, combining electrodialysis and membrane stripping with electrochemical stripping can be a more viable tactic (Tarpeh et al., 2018). Nevertheless, chlorination by-products such as ClO3−and ClO4− stand as a noteworthy setback of the mediated electro-oxidation of urine because of the high chlorine concentration in the medium (Zöllig et al., 2015).
Pathogens, pharmaceuticals and hormones can be predicted to remain in the urine from which ammonia has been released. This suggests that nutrients obtained via ammonia stripping are generally safe for soil and plant health.
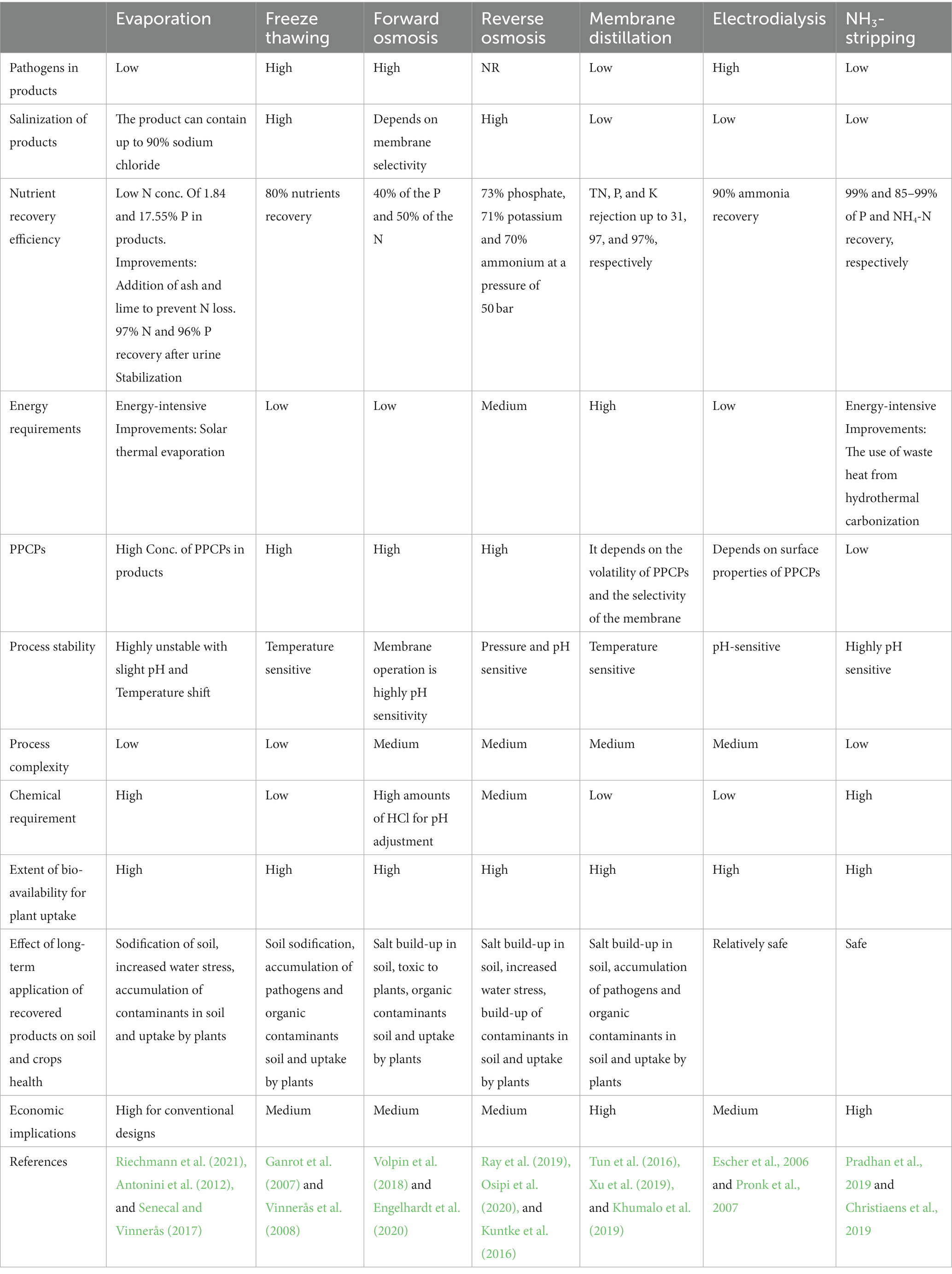
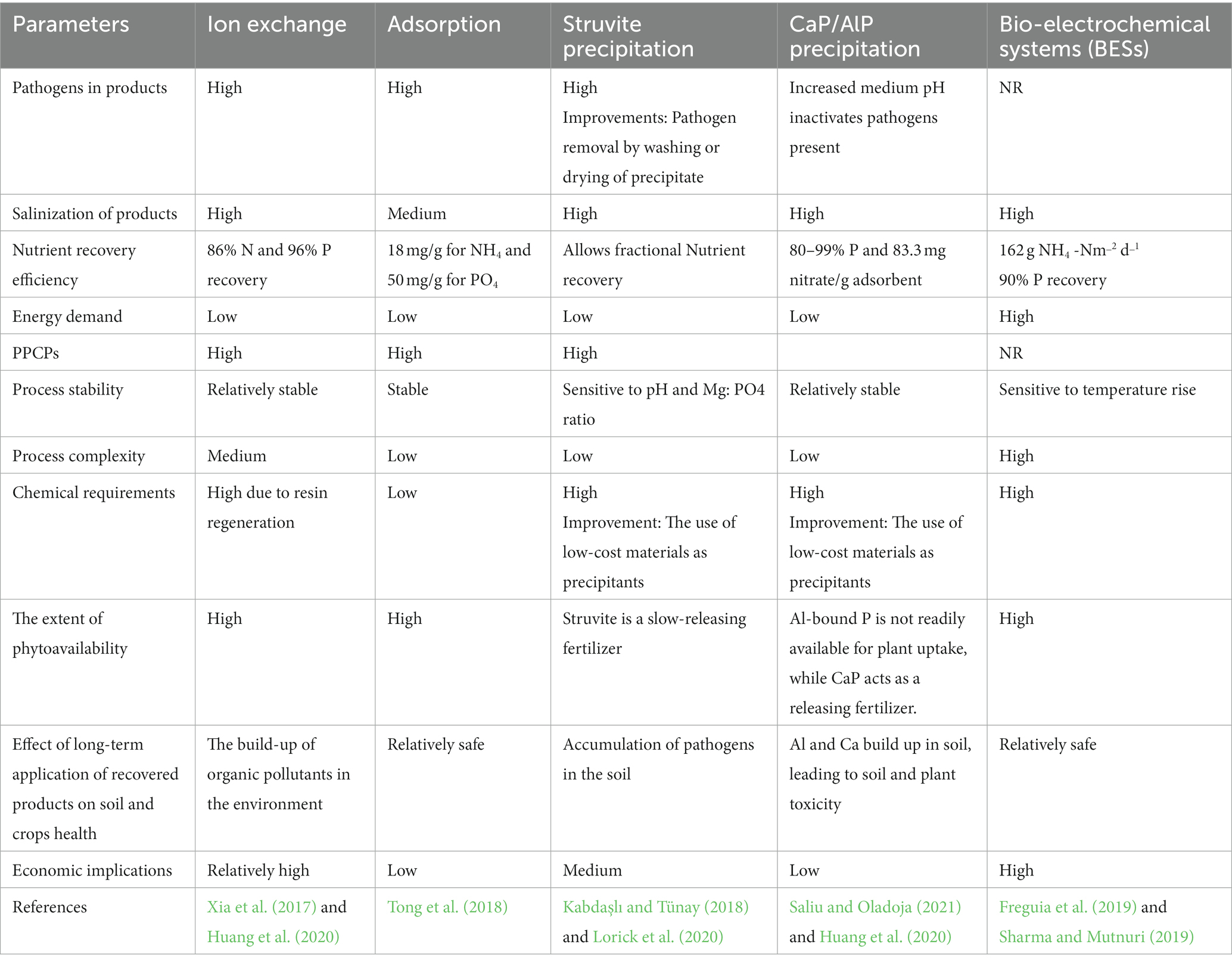
Tables 2, 3 illustrate the assessment of diverse approaches employed for nutrient recovery from human urine.
3.2 The solid recovery approach
3.2.1 Ion exchange and adsorption-based methods
Ion exchange and adsorption are among the first techniques used for nutrient recovery from domestic effluents in tertiary treatment (Yang et al., 2017). Activated charcoal and biochars showed a capacity for PO43− adsorption (Maroušek et al., 2020; Peng et al., 2021) urea adsorption (Kameda et al., 2017), and NH4+ recovery (Cai et al., 2016) due to their microporosity, adsorption capacity, specific surface area and ion exchange capacity. Pyrolysis temperature affects the material’s hydrophobicity, carbon content, microporosity, aromaticity, and surface area. Biochar often contains positively and negatively charged surfaces (zwitterionic) (Windeatt et al., 2014). The anionic functional groups play a crucial role in determining the material’s cation exchange capacity (CEC), which enhances their ability to adsorb ammonium ions from the solution. In contrast, the O-containing functional groups of the biochar contribute to the material’s anion exchange capacity (AEC), which enhances the recovery of nutrient anions such as phosphate.
Ion exchangers are used because of their notable properties, such as fast uptake kinetics and regeneration, high selectivity for NH4+ and high recovery (Malovanyy et al., 2013; Liao et al., 2015). Nutrient recovery through ion exchange is simple and requires less space for operation. This eco-friendly method uses mainly naturally available and modifiable ion-exchanger/adsorbents, like zeolites, and discharges non-hazardous exchangeable cations (Na+, K+, Ca2+, and Mg 2+) (Cai et al., 2016; Yang et al., 2017). Ion exchange processes require regenerant to recover the ion exchange after use. Restoration of ion exchangers is carried out by using highly concentrated brines to enhance the desorption of phosphate and ammonium ions in brine solution, which can be precipitated afterward as nutrient-rich solids appropriate for reuse as fertilizers. The common regenerants used in this process include sodium chloride and potassium chloride, with 68 and 94% regeneration efficiency, respectively (Guida, 2023).
Zeolites, particularly clinoptilolite, have generally been used for ammonia and phosphate removals from human urine (Kavvada et al., 2017; Kabdaşlı and Tünay, 2018).
Numerous studies have been conducted to remove ammonia from human urine through ion exchange techniques. Lind et al. (2001) prepared synthetic urine comprising 0.02–1 M NH4Cl to imitate source-separated human urine to study the adsorption capacity of wollastonite, clinoptilolite, and zeolites. Clinoptilolite uptake of ammonium ions from low-concentration solutions was 70–80 percent, wollastonite achieved 50 percent recovery, and mixed zeolite uptake was 50–60 percent. Ganrot et al. (2007) studied phosphate and ammonia recovery from human urine using a zeolite containing high levels of clinoptilolite. The experiment was carried out by adding MgO, activated carbon and with/without freeze–thaw technique. Results of the experiments showed that phosphorous was largely recovered as struvite, and an enhanced nitrogen recovery was obtained with zeolite addition. In a continuous flow column experiment, Beler-Baykal et al. (2004) employed human urine to appraise ammonium recovery using ion exchange. A concentrated NaCl solution was used to preheat natural clinoptilolite with a 1–2 mm size range. At loadings of up to 3 mg ammonium/g clinoptilolite, removal efficiencies of over 85% were attained.
With diluted urine and clinoptilolite, Kocatürk and Baykal (2012) recovered up to 86% nitrogen, with more significant reductions at higher concentrations. With higher phosphate concentrations, recovery efficiencies rose, reaching 96%. Using struvite precipitation and zeolite adsorption, Bán and Dave (Ban and Dave, 2004) conducted laboratory investigations on the recovery of phosphorous and nitrogen from source-separated urine. With particle sizes of 1.2–2 mm, the zeolite used had a significant clinoptilolite concentration. The reactive material was evaluated at various concentrations ranging from high (0.5–3 g MgO and 30–960 g zeolite per liter of human urine) to low (0.005–0.5 g MgO and 7.5–120 g zeolite per liter of human urine). Phosphorus elimination resulted from struvite precipitation and zeolite adsorption during the low-concentration test.
Zeolite adsorption was the dominating mechanism for pH levels lower than 8.5. In this low-concentration trial, the zeolite was intended to function as a cation exchanger for the residual ammonium ion after the struvite precipitation process.
For phosphate recovery from urine, greywater, and other waste streams, O’Neal and Boyer (2013) used hybrid anion exchange resins (HAIX) comprising hydrous ferric oxide and got excellent recoveries. Fresh urine > hydrolyzed urine > anaerobic digester supernatant > greywater > biological wastewater had the highest phosphorus loading on HAIX resin. HAIX resin had a higher phosphate sorption capacity for source-separated urine than the others. Xia et al. (2017) prepared highly distributed MgO-nanoflake altered diatomite for phosphate adsorption and achieved a high P recovery capacity of 138 mg/g. Kini (2023) removed phosphorus from human urine using granulated blast furnace slag (GGBS). In this investigation, human urine was diluted (50 percent) with tap water to simulate flushing circumstances. The results from the batch experiments demonstrated that a GGBS dosage of 700 g/L, a contact time of 120 min, and a pH range of 6–9 resulted in a phosphorous removal effectiveness above 90%.
While these ion exchange processes appear promising, the considerable challenges impeding their performance in nutrient recovery encompass insufficient media selectivity, bed blockage, and the high cost associated with regenerations.
In the long run, it can be inferred that recurrent regenerations involving fresh chemical solutions and significant expenses for disposing of expended regenerant as hazardous waste are highly uneconomical (Huang et al., 2021). Therefore, the retrieval of regenerants becomes imperative for the preservation of economic viability.
New studies suggest that the appropriate solutions should aim to clean up and reuse the used-up regenerant as well as recover the nutrients simultaneously. Canellas (2023) employed commercially available hollow fiber membrane contactor (HFMC) modules with sulphuric acid to clean up KCl utilized as a regenerant while also recovering ammonia as ammonium sulphate. The phosphorus present in the medium is recovered as calcium phosphate by adding Ca(OH)2 to the wasted sodium hydroxide regenerate, which results in the creation of hydroxyapatite Ca5(PO4)3(OH), which can then be filtered out of the liquid.
A review study by Beckinghausen et al. (2020) compared industrial nitrogen fertilizer production with nitrogen recovery from waste streams using different technologies, suggesting that nutrient recovery from wastewater is currently uneconomical. However, the cost of NH4NO3 recovery through the ion exchange method was observed to be smaller (£1.98 ± 0.23/kg N) compared to other processes, which cost around £2.35/kg NH4-N. Nonetheless, this cost is still relatively high compared to an industrial synthesis method (£0.28/kg N) (Neale et al., 2010; Tong et al., 2017).
Studies have shown that nutrient recovery through ion exchange from wastewater containing micropollutants such as pharmaceuticals and hormones may result in problems (Jiang et al., 2015; Tong et al., 2017, 2018). For example, micropollutants such as ibuprofen, estrone, and sulfadiazine adsorb on polymeric ion exchangers (Neale et al., 2010; Jiang et al., 2015). The ability of micropollutants to adhere to ion exchangers can increase the levels of micropollutants in the regeneration brines. This can contaminate the nutrient-rich precipitate obtained from the brine after regenerating the ion exchanger (Neale et al., 2010; Tong et al., 2017). Tong et al. (2017) investigated the fate of the micropollutants triclosan (neutral and anionic species in aqueous medium at neutral pH), 17 β-estradiol (neutral at neutral pH), and sulfamethoxazole (anionic at neutral pH) during nutrient recovery by ion exchange-precipitation. The study found that triclosan, 17 β-estradiol, and sulfamethoxazole adsorption to the phosphate-selective ion exchange resins LayneRT and DOW-HFO-Cu ranged from 50 to 71% when employing anaerobic effluent but did not adsorb to the ammonium-selective exchanger clinoptilolite. The hydrophobicity of the micropollutant was also reported to increase the interaction between the resins and the micropollutants.
Charcoal and ion exchange resins (natural and synthetic) remove nutrients from aqueous solutions and eliminate some pathogens (Busscher et al., 2006) and organic contaminants (Nam et al., 2014; Tong et al., 2017). Based on these studies, nutrient recovered from urine via ion exchange is susceptible to pathogens, pharmaceuticals and hormone pollution; thus, micropollutant removal from urine before nutrient recovery through ion exchange or adsorption process is needed to mitigate the contaminants’ risks to the environment. Parsons (2000) suggested that there could be a potential accumulation of bacteria on ion exchange resins, implying that the use of ion exchange for nutrient recovery might introduce concerns related to pathogenic microorganisms.
3.2.2 Precipitation method
Chemical precipitation is the most straightforward approach to eliminate excess dissolved phosphorus from wastewater effluent. This is commonly achieved by applying aluminum, calcium, magnesium or iron-rich materials (Tarpeh et al., 2018). The most frequently used reagents for phosphorus precipitation across various wastewater treatment systems are lime (Ca(OH)2), alum (Al2(SO4)3·18H2O), and ferric chloride (FeCl3) (Höglund et al., 2000; Huang et al., 2020).
Nutrients in wastewater have been precipitated in different forms ranging from magnesium ammonium phosphate hexahydrate (MAP) (Lahr et al., 2016; Filho et al., 2017; Zamora et al., 2017), magnesium potassium phosphate (Nakao et al., 2017), aluminum phosphate (Huang et al., 2020), magnesium and sodium phosphates (Huang et al., 2020), calcium phosphate (Saliu et al., 2019) and ferric phosphate (Lin et al., 2017).
The pH and hardness of the medium are the most significant factors often considered in using a chemical precipitation approach for phosphorus recovery. At a pH range of 4.0–6.0, iron phosphate and its oxyhydroxide complexes attain minimum solubility, whereas at a pH range of 5.0–7.0, aluminum phosphate and its oxyhydroxide complexes attain the most negligible solubility. Because this process consumes alkalinity, pH modifications may be necessary, depending on the wastewater influent source. To obtain minimum solubility, calcium apatite (Ca10(PO4)6(OH)2) demands an elevated pH (9.0), which necessitates wastewater softening via the creation of calcium carbonate (CaCO3). The principal component of the sludge produced by the treatment plant is the creation of CaCO3, combined with the desired precipitation of calcium apatite (Okano et al., 2013).
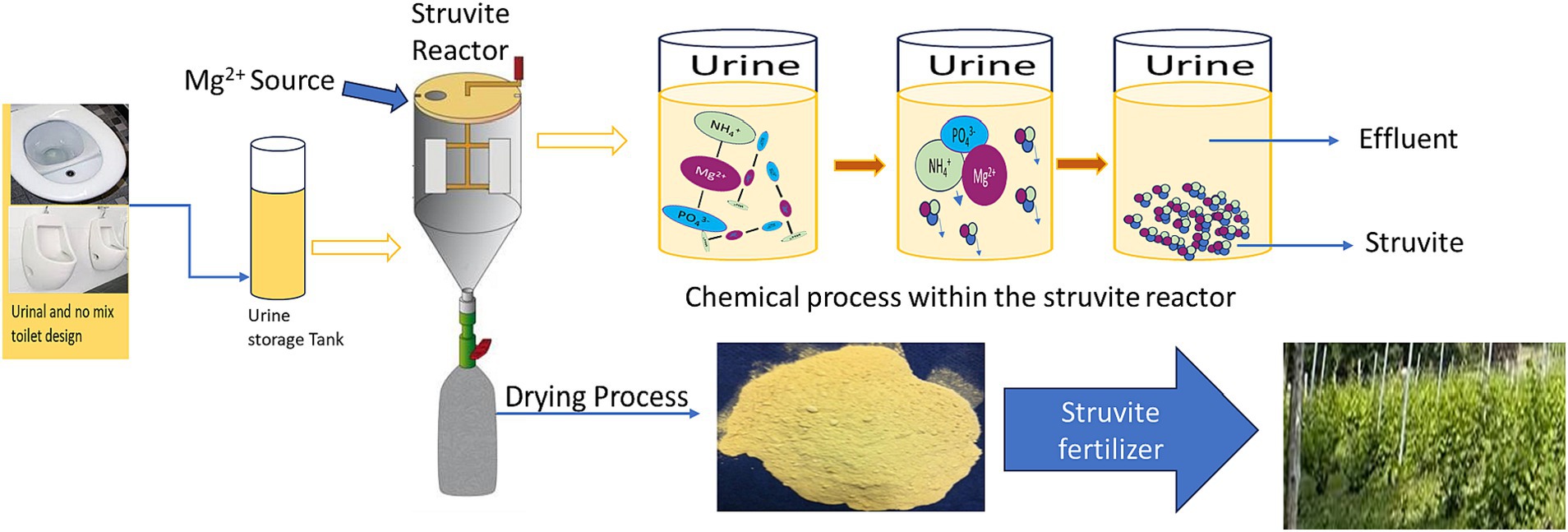
In recent years, the struvite precipitation technique has been effectively employed in industrial, municipal, and agricultural wastewater for nutrient recovery (Lahr et al., 2016; Zamora et al., 2017; Figure 3). Struvite is a white crystalline powder characterized by an orthorhombic crystal structure. It comprises equal molar concentrations of magnesium, ammonium, and phosphate (Mg2+/NH4+/PO43: 1/1/1). Depending on the precipitation media, it can also develop as a yellow, brownish, or light grey precipitate (Filho et al., 2017; Kabdaşlı and Tünay, 2018).
The typical reaction for struvite precipitation is given in Equation (2.3) below (Lahr et al., 2016; Kabdaşlı and Tünay, 2018):
It is hypothesized that the primary form of orthophosphates throughout the reaction is HPO42− or H2PO4− rather than PO43− (Jia et al., 2017; Tansel et al., 2018). This means hydrogen ions are discharged into the solution, lowering the medium’s pH during struvite precipitation (Krähenbühl et al., 2016; Kataki et al., 2016; Lorick et al., 2020).
Struvite is formed in a slightly alkaline medium by the reactions listed as Equations (2.4) and (2.5) below (Kumar et al., 2015):
Consequently, the stability of the struvite precipitation process is highly sensitive to pH change. Struvite precipitation has recently been demonstrated to be viable for recovering nitrogen and phosphorus from human urine (Ganrot et al., 2007; Lahr et al., 2016; Filho et al., 2017; Zamora et al., 2017). Ammonium and phosphate in pre-hydrolyzed or fresh human urine are changed into magnesium ammonium phosphate hexahydrate (MAP) (generally recognized as struvite) under the right operating conditions in the struvite precipitation process. Nitrogen, phosphate, and potassium can be reclaimed from human urine in three ways by struvite precipitation. The primary goal of the first strategy is to recover 100% phosphorus and a small amount of ammonia from newly collected human urine. This approach allows for the recovery of around 5% ammonia and 96–98% phosphate (Liu et al., 2013), resulting in effluents with elevated nitrogen concentration in the form of ammonia and urea, requiring an extra treatment step. The other method relies on comprehensive ammonia and phosphate recovery from human urine. Due to the phosphorous deficit in ammonia, this application asks for urea to ammonia conversion by ureolysis and an outside phosphorous supply (Kabdaşlı and Tünay, 2018). An alternate method was recently proposed, which involves precipitating K-struvite (MgKPO46·H2O) from human urine. To reclaim potassium via K-struvite precipitation, total hydrolysis of urea to ammonia is required (Ray et al., 2019; Patel et al., 2020).
In addition to urea hydrolysis and ammonia removal, dilution of human urine is vital in achieving adequate K-struvite precipitation, particularly in incomplete ammonia recovery (NH4–N > 100 mg N/L) (Xu et al., 2012). Upon meeting these essential conditions and incorporating an external magnesium supply, K-struvite precipitation from human urine, conducted under optimal operational parameters, can recover up to 77% of potassium and nearly all phosphorus. Struvite recovery is feasible exclusively in the presence of Mg2+ ion (Figure 3). Magnesium ions from various sources (e.g., Mg(OH)2, MgCl2, MgO) have been employed for struvite precipitation from wastewater for many years (Barbosa et al., 2016). Using magnesium supplied from low-cost sources significantly lowered the cost of struvite manufacturing (Luo et al., 2018). Compared to other magnesium sources, such as MgCl2 and MgSO4, Hug and Udert (2013) found that using MgO is less expensive. Using MgO or Mg(OH)2 is also advantageous since they tend to keep the solution pH in a desirable range, promoting struvite crystallization (Hutnik et al., 2013).
Using MgO, Morales et al. (2013) successfully recovered more than 95% of P in the stored urine after 30 min of experimental time. Barbosa et al. (2016) used struvite precipitation to extract nutrients from source-separated urine. The precipitating agents MgO, Mg(OH)2, and MgCl2 were evaluated for struvite formation from urine. P recovery efficiency ranged from 82 to 89 percent when MgCl2 was employed, while P recovery efficiency ranged from 79 to 93 percent when Mg(OH)2 was utilized. When MgO was used as the Mg2+ source, the P recovery efficiency ranged from 90 to 100%. The pH of the solution rose from 8.5 to 9 in the first 3 min and stayed at 9.2 during the stirring period (10 min).
Pinatha (2023) studied nutrient recovery from urine using ashes of calcium and magnesium from power plant solid wastes. The findings indicated that the efficiency of P recovery was enhanced when the pH value increased. Precipitation of P into solid precipitates reached maximum levels of 75, 99, and 99% at pH 7, 9, and 11, respectively. The P content in the form of P crystals was approximately 70, 60, and 50%.
Pinatha et al. (2020) investigated nutrient precipitation from urine with sea salts in a study. Results from the experiments conducted within the pH 7 to 11 revealed that the efficiency of phosphorus precipitation increased to 89–97% with the addition of MgCl2 and 72–88% with sea salts. The precipitates obtained from both scenarios exhibited a dry-weight phosphorus content ranging from 10.8 to 17.1%, surpassing numerous commercial fertilizers (8.80% P).
The major setback to this technology is that struvite precipitation only allows fractional nitrogen recovery with a non-optimal NPK ratio for plant growth. The process also involves the addition of expensive magnesium supplements whose accumulation in soil could eventually become detrimental to plant growth.
Studies showed that pathogens may accumulate in the precipitate (Decrey et al., 2011; Udert and Wächter, 2012; Lahr et al., 2016) while pharmaceuticals adhere to the surface of deposits instead of being integrated into the crystal structure, which can be eliminated by washing (Escher et al., 2006; Ronteltap et al., 2007; Schürmann et al., 2012). The choice of reagent type in nutrient precipitation from urine significantly impacts process effectiveness and the economic evaluation of the method. The use of large amounts of precipitant in this process offsets the price of the process. To reduce the financial challenges of the technique, it is crucial to pinpoint and appraise the efficacy of less expensive non-traditional reagents (Pinatha et al., 2020).
3.2.3 Bio-electrochemical systems (BESs)
Microbial fuel cells (MFCs) are bioelectrochemical instruments that degrade organic and inorganic wastes into smaller molecules. Electrons and protons are discharged during the procedure, and electricity is produced. MFCs use microbes or enzymatic catalysis to transform chemical energy directly into electrical energy via bioelectrochemical processes (Deval et al., 2017; Gadkari et al., 2020; Savla et al., 2021).
MFC is a bioelectrochemical device consisting of an anaerobic anode chamber, and an aerobic cathode chamber separated using an anion exchange membrane (AEM). For standard MFC, microorganisms oxidize the substrate in the anode chamber, and the electron generated by the organism is transferred to the cathode through an outer cable. The anode chamber is made up of a microbe (catalyst) and an electrode (anode), nourished with wastewater (anolyte and redox mediator, respectively) (Tamta et al., 2020). Water is formed on the cathode when required protons and electrons are withdrawn during bacterial substrate catabolism and combined with oxygen (Ledezma et al., 2017; Monetti et al., 2019; Jatoi et al., 2021).
Typically, anaerobic bacteria use the anode as the terminal electron acceptor to oxidize organic materials in the wastewater to CO2 (Ye et al., 2019; Matsuda et al., 2020). As demonstrated in Eqs. (2.6 and 2.7) (Matsuda et al., 2020), the oxidation reactions occurring at the anode are illustrated by acetate or glucose degradation:
To move electrons to the solid electron acceptor (i.e., anode) situated outside the cells, bacteria use extracellular electron transport systems (e.g., direct electron or biofilm matrix). Anaerobic bacteria save the energy required for development and conservation by doing so. The electrons flow to the cathode through an outer electrical circuit and a load (resistor) (Matsuda et al., 2020). The electrons are eventually used at the cathode to reduce electron acceptors like oxygen, nitrate, phosphate, and metal ions. MFCs have been used to recover ammonia nitrogen and electricity from human urine (Tice and Kim, 2014; Kuntke et al., 2016; Tremouli et al., 2018).
In previous studies, MFCs have effectively recovered ammonia nitrogen from diluted urine (Ieropoulos et al., 2012). As a result, using source-separated urine, the concept of ammonia recovery was implemented and confirmed a power output of 0.25Wm−2 and an ammonia recovery rate of 3.3 g N m2/d in MFCs (Kuntke et al., 2012). A two-chambered BES with a gas diffusion cathode was used for ammonium nitrogen recovery. The movement of the ammonium ion and circulation of ammonia through the cation exchange membrane were the two main methods for transporting ammonia to the cathode chamber (CEM). Because of the system’s alkaline pH, the NH4+ ion is transformed to NH3 in the cathode chamber (9.5). The catholyte’s ammonia was supposed to be recovered by volatilization and absorption into a dilute sulfuric acid, resulting in liquid ammonium sulphate (Kuntke et al., 2012).
Others have recovered ammonium from urine using the BES in microbial electrolysis cell mode (Kuntke et al., 2014). Ammonium recovery from urine was established at a recovery rate of 162 (±10) g NH4-N m_2 d_1 and a simultaneous H2 generation in microbial electrolysis cells (MEC), with an energy demand of 8.2 MJ kg_1 NH4-N (Kuntke et al., 2014).
Phosphorus removal methods in BES depend on phosphorus solubilization from iron phosphate minerals and soluble phosphorus precipitated as struvite. Moreover, the discharge of iron declines at the cathode, and phosphorus discharge from iron phosphate (Fe-P) crystals improves the electrons obtained from waste organic matter. Direct or electron shuttle within the cathode forces the reduction of iron in the Fe-P. The movement of ammonium ions from the anode to the cathode chamber and alkali generation at the cathode are involved in the precipitation of soluble phosphorus in BES (Zamora et al., 2017; Ye et al., 2019).
In a study conducted by Sharma and Mutnuri (2019), the performance of MFC was assessed by mixing consortia and isolated pure cultures (Firmicutes and Proteobacteria species) from biofilm for nutrient recovery and electricity generation from human urine. The results revealed that microbes only use a fraction of the total phosphorus that is accessible, specifically less than 10%, for their growth, while 90% is recovered as struvite. The study established the concurrent removal of nitrogen and phosphorus from the system as struvite using MFC.
In a recent study, Freguia et al. (2019) studied a self-powered bioelectrochemical nutrient recovery system for nutrient recovery from urine. The investigation demonstrated the world’s first autonomous nutrient recovery mechanism and does not require any chemical or process control. In a microbial fuel cell configuration, a novel air cathode catalyst functional in urine conditions enables in-situ electricity generation and immediate collection of ionic nutrients into a heavy metal-free product stream. Over two months, the system maintained electrical current densities of roughly 3A m–2 while concurrently increasing the concentration of N and K by a factor of 1.5–1.7.
Studies have shown that temperature affects the behavior of the microorganisms used in MFC and the system’s kinetics, activation energy, conductivity of the solution and electrode potentials. Microorganisms have an optimum temperature and pH for growth and metabolism pathways. The pH reduction within the anode impedes the growth of microorganisms that generate electricity. Temperatures between 30–45°C enable biofilm generation and improve the bio-electrocatalytic activity. Heidrich et al. (2014) evaluated the efficiency of MFC at temperatures ranging from 1 to 5°C and discovered that the MFC efficiency (48.7%) was poor and took a long time to attain. However, Jamal et al. (2020) achieved a better result at room temperature.
The financial burden of MFC is tremendously high compared to anaerobic sludge treatment because of the use of expensive electrodes, catalysts, and membranes. Typically, anode and cathode electrodes are costly, and using triggers like Pt increases the inversion cost dramatically, making the process unsuitable for industrial or wastewater treatment (Munoz-Cupa et al., 2021). Despite using carbon-based materials as electrodes, Li et al. (2013) estimated that the cost of MFC operation is roughly 30 times greater than that of conventional wastewater treatment.
4 Conclusion
The various techno-economic assessments, environmental impact and sustainability of different technologies used in nutrient recovery from human urine were reviewed. The evaluation of these nutrient recovery technologies indicated that, although most studies emphasize nutrient recovery efficiency, there has been inadequate and inaccurate consideration of the environmental impact of the final products. Concerns arise regarding the potential high salt content and the contamination of the final product with pathogens and pharmaceuticals. Prolonged application of such products to agricultural land may pose a substantial risk to environmental integrity and human health. Several technologies examined in the review are deemed unsustainable due to their capital and energy-intensive nature. Nonetheless, contemporary studies have sought to reduce the capital costs associated with nutrient recovery from urine by utilizing cost-effective materials, thereby positively influencing overall process costs. The environmental impact assessment of these technologies highlighted a historical oversight, wherein earlier studies paid limited attention to the ecological implications of micropollutants and pathogens present in the recovered nutrients. A recent shift in focus aims to address these environmental concerns more comprehensively. Current methodologies are being designed to eliminate pollutants before engaging in nutrient recovery processes. This involves the development of selective technologies capable of recovering nutrient fractions from urine in a purer form. Innovations are essential to refine existing technologies, enhancing their performance, eco-friendliness, and overall sustainability.
Author contributions
TS: Conceptualization, Writing – review & editing, Data curation, Formal analysis, Investigation, Methodology, Visualization, Writing – original draft. NO: Conceptualization, Writing – review & editing. SS: Conceptualization, Funding acquisition, Supervision, Validation, Writing – review & editing.
Funding
The author(s) declare that no financial support was received for the research, authorship, and/or publication of this article.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The author(s) declared that they were an editorial board member of Frontiers, at the time of submission. This had no impact on the peer review process and the final decision.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Abejew, A. A., Denboba, A. A., and Mekonnen, A. G. (2014). Prevalence and antibiotic resistance pattern of urinary tract bacterial infections in Dessie area, north-East Ethiopia. BMC. Res. Notes 7:687. doi: 10.1186/1756-0500-7-687
Al-Farsi, R., Ahmed, M., Al-Busaidi, A., and Choudri, B. S. (2018). Assessing the presence of pharmaceuticals in soil and plants irrigated with treated wastewater in Oman. Int. J. Recycl. Org. Waste Agric. 7, 165–172. doi: 10.1007/s40093-018-0202-1
Al-Rammah, A. (2000). The application of acid free Antiscalant to mitigate scaling in reverse osmosis membranes. Desalination 132, 83–87. doi: 10.1016/S0011-9164(00)00174-0
Alturki, A. A., McDonald, J. A., Khan, S. J., Price, W. E., Nghiem, L. D., and Elimelech, M. (2013). Removal of trace organic contaminants by the forward osmosis process. Sep. Purif. Technol. 103, 258–266. doi: 10.1016/j.seppur.2012.10.036
Amoah, I. D., Reddy, P., Seidu, R., and Stenström, T. A. (2018). Concentration of soil-transmitted helminth eggs in sludge from South Africa and Senegal: a probabilistic estimation of infection risks associated with agricultural application. J. Environ. Manag. 206, 1020–1027. doi: 10.1016/j.jenvman.2017.12.003
Ansari, A. J., Hai, F. I., Price, W. E., Drewes, J. E., and Nghiem, L. D. (2017). Forward osmosis as a platform for resource recovery from municipal wastewater – a critical assessment of the literature. J. Membr. Sci. 529, 195–206. doi: 10.1016/j.memsci.2017.01.054
Antonini, S., Nguyen, P. T., Arnold, U., Eichert, T., and Clemens, J. (2012). Solar thermal evaporation of human urine for nitrogen and phosphorus recovery in Vietnam. Sci. Total Environ. 414, 592–599. doi: 10.1016/j.scitotenv.2011.11.055
Ashoor, B. B., Mansour, S., Giwa, A., Dufour, V., and Hasan, S. W. (2016). Principles and applications of direct contact membrane distillation (DCMD): a comprehensive review. Desalination 398, 222–246. doi: 10.1016/j.desal.2016.07.043
Ban, Z. S., and Dave, G. (2004). Laboratory studies on recovery of n and p from human urine through struvite crystallisation and zeolite adsorption. Environ. Technol. 25, 111–121. doi: 10.1080/09593330409355443b
Barbosa, S. G., Peixoto, L., Meulman, B., Alves, M. M., and Pereira, M. A. (2016). A Design of Experiments to assess phosphorous removal and crystal properties in struvite precipitation of source separated urine using different mg sources. Chem. Eng. J. 298, 146–153. doi: 10.1016/j.cej.2016.03.148
Beckinghausen, A., Odlare, M., Thorin, E., and Schwede, S. (2020). From removal to recovery: an evaluation of nitrogen recovery techniques from wastewater. Appl. Energy 263:114616. doi: 10.1016/j.apenergy.2020.114616
Beler-Baykal, B., Bayram, S., Akkaymak, E., and Cinar, S. (2004). Removal of ammonium from human urine through ion exchange with Clinoptilolite and its recovery for further reuse. Water Sci. Technol. 50, 149–156. doi: 10.2166/wst.2004.0371
Bischel, H. N., Özel Duygan, B. D., Strande, L., McArdell, C. S., Udert, K. M., and Kohn, T. (2015). Pathogens and Pharmaceuticals in Source-Separated Urine in eThekwini, South Africa. Water Res. 85, 57–65. doi: 10.1016/j.watres.2015.08.022
Bonmati, A., and Flotats, X. (2003). Air stripping of Ammonia from pig slurry: characterisation and feasibility as a pre- or post-treatment to mesophilic anaerobic digestion. Waste Manag. 23, 261–272. doi: 10.1016/S0956-053X(02)00144-7
Bonvin, C., Etter, B., Udert, K. M., Frossard, E., Nanzer, S., Tamburini, F., et al. (2015). Plant uptake of phosphorus and nitrogen recycled from synthetic source-separated urine. Ambio 44, 217–227. doi: 10.1007/s13280-014-0616-6
Borer, C., Stiles, W., Stevenson, J., and Cabanillas, K. (2014). Assessment of forward osmosis as a possible mitigation strategy for urine management during extended cave exploration. JCKS 76, 26–29. doi: 10.4311/2012LSC0269
Bucholtz, P., Steele, M., Tripathi, V., Graham, C., Crane, L., and Boyer, T. H. (2023). Solar distillation of human urine to recover non-potable water and metal phosphate mineral. Water Sci. Technol. 88, 486–501. doi: 10.2166/wst.2023.218
Busscher, H. J., Dijkstra, R. J. B., Engels, E., Langworthy, D. E., Collias, D. I., Bjorkquist, D. W., et al. (2006). Removal of two waterborne pathogenic bacterial strains by activated carbon particles prior to and after charge modification. Environ. Sci. Technol. 40, 6799–6804. doi: 10.1021/es061282r
Cai, Y., Qi, H., Liu, Y., and He, X. (2016). Sorption/desorption behavior and mechanism of NH 4 + by biochar as a nitrogen fertilizer sustained-release material. J. Agric. Food Chem. 64, 4958–4964. doi: 10.1021/acs.jafc.6b00109
Canellas. (2023). Tertiary ammonium removal with zeolites. Available at: https://dspace.lib.cranfield.ac.uk/handle/1826/18386 (Accessed October 31, 2023)
Christiaens, M. E. R., Udert, K. M., Arends, J. B. A., Huysman, S., Vanhaecke, L., McAdam, E., et al. (2019). Membrane stripping enables effective electrochemical Ammonia recovery from urine while retaining microorganisms and micropollutants. Water Res. 150, 349–357. doi: 10.1016/j.watres.2018.11.072
Christian, T., Schneider, R. J., Färber, H. A., Skutlarek, D., Meyer, M. T., and Goldbach, H. E. (2003). Determination of antibiotic residues in manure, soil, and surface waters. Acta Hydrochim. Hydrobiol. 31, 36–44. doi: 10.1002/aheh.200390014
Coday, B. D., Almaraz, N., and Cath, T. Y. (2015). Forward osmosis desalination of oil and gas wastewater: impacts of membrane selection and operating conditions on process performance. J. Membr. Sci. 488, 40–55. doi: 10.1016/j.memsci.2015.03.059
Courtney, C., and Randall, D. G. (2023). Concentrating stabilized human urine using eutectic freeze crystallization for liquid fertilizer production. Water Res. 233:119760. doi: 10.1016/j.watres.2023.119760
Dalhammar, G. (1997). Behandling Och Koncentrering Av Humanurin (Royal Institute of Technology, Stockholm, Department of Biochemistry and Biochemical Technology), report, personal communication.
Dascenzo, G., Dicorcia, A., Gentili, A., Mancini, R., Mastropasqua, R., Nazzari, M., et al. (2003). Fate of natural estrogen conjugates in municipal sewage transport and treatment facilities. Sci. Total Environ. 302, 199–209. doi: 10.1016/S0048-9697(02)00342-X
De La Rubia, M. Á., Walker, M., Heaven, S., Banks, C. J., and Borja, R. (2010). Preliminary trials of in situ Ammonia stripping from source segregated domestic food waste Digestate using biogas: effect of temperature and flow rate. Bioresour. Technol. 101, 9486–9492. doi: 10.1016/j.biortech.2010.07.096
De Vries, H. J., Stams, A. J. M., and Plugge, C. M. (2020). Biodiversity and ecology of microorganisms in high pressure membrane filtration systems. Water Res. 172:115511. doi: 10.1016/j.watres.2020.115511
Decrey, L., Udert, K. M., Tilley, E., Pecson, B. M., and Kohn, T. (2011). Fate of the pathogen indicators phage ΦX174 and Ascaris Suum eggs during the production of struvite fertilizer from source-separated urine. Water Res. 45, 4960–4972. doi: 10.1016/j.watres.2011.06.042
Deval, A. S., Parikh, H. A., Kadier, A., Chandrasekhar, K., Bhagwat, A. M., and Dikshit, A. K. (2017). Sequential microbial activities mediated bioelectricity production from distillery wastewater using bio-electrochemical system with simultaneous waste remediation. Int. J. Hydrog. Energy 42, 1130–1141. doi: 10.1016/j.ijhydene.2016.11.114
Dickin, S., Dagerskog, L., Jiménez, A., Andersson, K., and Savadogo, K. (2018). Understanding sustained use of ecological sanitation in rural Burkina Faso. Sci. Total Environ. 613-614, 140–148. doi: 10.1016/j.scitotenv.2017.08.251
Dolliver, H., Kumar, K., and Gupta, S. (2007). Sulfamethazine uptake by plants from manure-amended soil. J. Env. Qual. 36, 1224–1230. doi: 10.2134/jeq2006.0266
Dutta, S., and Vinnerås, B. (2016). Fertilizer from dried human urine added to ash and lime – a potential product from eco-sanitation system. Water Sci. Technol. 74, 1436–1445. doi: 10.2166/wst.2016.324
Ekwealor, P. A., Ugwu, M. C., Ezeobi, I., Amalukwe, G., Ugwu, B. C., Okezie, U., et al. (2016). Antimicrobial evaluation of bacterial isolates from urine specimen of patients with complaints of urinary tract infections in Awka, Nigeria. Int. J. Microbiol. 2016, 1–6. doi: 10.1155/2016/9740273
Engelhardt, S., Vogel, J., Duirk, S. E., Moore, F. B., and Barton, H. A. (2020). Assessment of urea hydrolysis as a pretreatment strategy to improve Total nitrogen rejection from urine using aquaporin-based membranes in forward osmosis. J Water Process Eng. 34:101135. doi: 10.1016/j.jwpe.2020.101135
Escher, B. I., Pronk, W., Suter, M. J.-F., and Maurer, M. (2006). Monitoring the removal efficiency of pharmaceuticals and hormones in different treatment processes of source-separated urine with bioassays. Environ. Sci. Technol. 40, 5095–5101. doi: 10.1021/es060598w
Filho, J. L. D. P., Tonetti, A. L., Guimarães, M. T., and Silva, D. (2017). Nutrient recovery from airplane wastewater: composition, treatment and Ecotoxicological assay. Water Sci. Technol. 75, 1952–1960. doi: 10.2166/wst.2017.081
Freguia, S., Logrieco, M., Monetti, J., Ledezma, P., Virdis, B., and Tsujimura, S. (2019). Self-powered bioelectrochemical nutrient recovery for fertilizer generation from human urine. Sustainability 11:5490. doi: 10.3390/su11195490
Gadkari, S., Fontmorin, J.-M., Yu, E., and Sadhukhan, J. (2020). Influence of temperature and other system parameters on microbial fuel cell performance: numerical and experimental investigation. Chem. Eng. J. 388:124176. doi: 10.1016/j.cej.2020.124176
Ganrot, Z., Dave, G., and Nilsson, E. (2007). Recovery of N and P from human urine by freezing, struvite precipitation and adsorption to zeolite and active carbon. Bioresour. Technol. 98, 3112–3121. doi: 10.1016/j.biortech.2006.10.038
García-González, M. C., Vanotti, M. B., and Szogi, A. A. (2015). Recovery of Ammonia from swine manure using gas-permeable membranes: effect of aeration. J. Environ. Manag. 152, 19–26. doi: 10.1016/j.jenvman.2015.01.013
Garutti, M., Nevola, G., Mazzeo, R., Cucciniello, L., Totaro, F., Bertuzzi, C. A., et al. (2022). The impact of cereal grain composition on the health and disease outcomes. Front. Nutr. 9:888974. doi: 10.3389/fnut.2022.888974
Gay, G., Lorain, O., Azouni, A., and Aurelle, Y. (2003). Wastewater treatment by radial freezing with stirring effects. Water Res. 37, 2520–2524. doi: 10.1016/S0043-1354(03)00020-4
Guessous, M., Rich, A., Mountadar, S., Siniti, M., and Mountadar, M. (2023). Treatment of an industrial wastewater for phosphorus and fluoride recovery by a process coupling block freeze concentration and precipitation. J. Cryst. Growth 619:127335. doi: 10.1016/j.jcrysgro.2023.127335
Guida. (2023). Phosphorus and Ammonia Removal and Recovery through Ion Exchange (IEX) Process at Demonstration Scale. Available at: https://www.smart-plant.eu/images/publications/smart-processes/75_IWARR2019_Book-of-Abstracts-2.jpg.pdf (Accessed October 31, 2023)
Guštin, S., and Marinšek-Logar, R. (2011). Effect of pH, temperature and air flow rate on the continuous Ammonia stripping of the anaerobic digestion effluent. Process Saf. Environ. Prot. 89, 61–66. doi: 10.1016/j.psep.2010.11.001
Heidrich, E. S., Edwards, S. R., Dolfing, J., Cotterill, S. E., and Curtis, T. P. (2014). Performance of a pilot scale microbial electrolysis cell fed on domestic wastewater at ambient temperatures for a 12 month period. Bioresour. Technol. 173, 87–95. doi: 10.1016/j.biortech.2014.09.083
Heinonen-Tanski, H., and Van Wijk-Sijbesma, C. (2005). Human excreta for plant production. Bioresour. Technol. 96, 403–411. doi: 10.1016/j.biortech.2003.10.036
Hidalgo, D., Corona, F., Martín-Marroquín, J. M., Del Álamo, J., and Aguado, A. (2016). Resource recovery from anaerobic Digestate: struvite crystallisation versus Ammonia stripping. Desalin. Water Treat. 57, 2626–2632. doi: 10.1080/19443994.2014.1001794
Hijikata, N., Fujii, T., Sangare, D., Sou, M., Ushijima, K., and Funamizu, N. (2014). Salts monitoring and Management for Human Urine Fertilization and Treated Greywater Irrigation in sub-Sahel region. J. Arid. Land Stud. 24, 85–88.
Hofman, J. A. M. H., Beerendonk, E. F., Folmer, H. C., and Kruithof, J. C. (1997). Removal of pesticides and other micropollutants with cellulose-acetate, polyamide and ultra-low pressure reverse osmosis membranes. Desalination 113, 209–214. doi: 10.1016/S0011-9164(97)00131-8
Höglund, C., Ashbolt, N., Stenström, T. A., and Svensson, L. (2002). Viral persistence in source-separated human urine. Adv. Environ. Res. 6, 265–275. doi: 10.1016/S1093-0191(01)00057-0
Höglund, C. E., and Stenström, T. A. B. (1999). Survival of Cryptosporidium Parvum oocysts in source separated human urine. Can. J. Microbiol. 45, 740–746. doi: 10.1139/w99-066
Höglund, C., Vinnerås, B., Stenström, T. A., and Jönsson, H. (2000). Variation of chemical and microbial parameters in collection and storage tanks for source separated human urine. J. Environ. Sci. Health A 35, 1463–1475. doi: 10.1080/10934520009377047
Hu, Y., Li, X., Jiang, S., Chen, J., and Yan, B. (2023). Facilitating wastewater purification through progressive thawing by microwave: responses of microbial communities. Water 15:3664. doi: 10.3390/w15203664
Hu, C., Liu, Z., Lu, X., Sun, J., Liu, H., and Qu, J. (2018). Enhancement of the Donnan effect through capacitive ion increase using an Electroconductive rGO-CNT Nanofiltration membrane. J. Mater. Chem. A 6, 4737–4745. doi: 10.1039/C7TA11003K
Huang, X., Guida, S., Jefferson, B., and Soares, A. (2020). Economic evaluation of ion-exchange processes for nutrient removal and recovery from municipal wastewater. NPJ Clean Water 3, 1–10. doi: 10.1038/s41545-020-0054-x
Huang, D., Xiao, R., Du, L., Zhang, G., Yin, L., Deng, R., et al. (2021). Phytoremediation of poly- and Perfluoroalkyl substances: a review on aquatic plants, influencing factors, and Phytotoxicity. J. Hazard. Mater. 418:126314. doi: 10.1016/J.JHAZMAT.2021.126314
Hutnik, N., Kozik, A., Mazienczuk, A., Piotrowski, K., Wierzbowska, B., and Matynia, A. (2013). Phosphates (V) recovery from phosphorus mineral fertilizers industry wastewater by continuous struvite reaction crystallization process. Water Res. 47, 3635–3643. doi: 10.1016/j.watres.2013.04.026
Hug, A., and Udert, K. M. (2013). Struvite precipitation from urine with electrochemical magnesium dosage. Water Res. 47, 289–299. doi: 10.1016/j.watres.2012.09.036
Ieropoulos, I., Greenman, J., and Melhuish, C. (2012). Urine utilisation by microbial fuel cells; energy fuel for the future. Phys. Chem. Chem. Phys. 14, 94–98. doi: 10.1039/C1CP23213D
Jaffer, A. E. (1994). The application of a novel chemical treatment program to mitigate scaling and fouling in reverse osmosis units. Desalination 96, 71–79. doi: 10.1016/0011-9164(94)85158-1
Jagtap, N., and Boyer, T. H. (2018). Integrated, multi-process approach to Total nutrient recovery from stored urine. Environ. Sci. 4, 1639–1650. doi: 10.1039/C8EW00004B
Jamal, M. T., Pugazhendi, A., and Jeyakumar, R. B. (2020). Application of halophiles in air cathode MFC for seafood industrial wastewater treatment and energy production under high saline condition. Environ. Technol. Innovat. 20:101119. doi: 10.1016/j.eti.2020.101119
Jatoi, A. S., Akhter, F., Mazari, S. A., Sabzoi, N., Aziz, S., Soomro, S. A., et al. (2021). Advanced microbial fuel cell for waste water treatment—a review. Environ. Sci. Pollut. Res. 28, 5005–5019. doi: 10.1007/s11356-020-11691-2
Ji, X., Shen, Q., Liu, F., Ma, J., Xu, G., Wang, Y., et al. (2012). Antibiotic resistance gene abundances associated with antibiotics and heavy metals in animal manures and agricultural soils adjacent to feedlots in Shanghai; China. J. Hazard. Mater. 235-236, 178–185. doi: 10.1016/j.jhazmat.2012.07.040
Jia, G., Zhang, H., Krampe, J., Muster, T., Gao, B., Zhu, N., et al. (2017). Applying a chemical equilibrium model for optimizing struvite precipitation for ammonium recovery from anaerobic digester effluent. J. Clean. Prod. 147, 297–305. doi: 10.1016/j.jclepro.2017.01.116
Jiang, M., Yang, W., Zhang, Z., Yang, Z., and Wang, Y. (2015). Adsorption of three pharmaceuticals on two magnetic ion-exchange resins. J. Environ. Sci. 31, 226–234. doi: 10.1016/j.jes.2014.09.035
Jin, X., Shan, J., Wang, C., Wei, J., and Tang, C. Y. (2012). Rejection of pharmaceuticals by forward osmosis membranes. J. Hazard. Mater. 227-228, 55–61. doi: 10.1016/j.jhazmat.2012.04.077
Kabdaşlı, I., and Tünay, O. (2018). Nutrient recovery by struvite precipitation, ion exchange and adsorption from source-separated human urine – a review. Environ. Technol. Rev. 7, 106–138. doi: 10.1080/21622515.2018.1473504
Kameda, T., Ito, S., and Yoshioka, T. (2017). Kinetic and equilibrium studies of urea adsorption onto activated carbon: adsorption mechanism. J. Dispers. Sci. Technol. 38, 1063–1066. doi: 10.1080/01932691.2016.1219953
Karak, T., and Bhattacharyya, P. (2011). Human urine as a source of alternative natural fertilizer in agriculture: a flight of fancy or an achievable reality. Resour. Conserv. Recycl. 55, 400–408. doi: 10.1016/j.resconrec.2010.12.008
Kataki, S., West, H., Clarke, M., and Baruah, D. C. (2016). Phosphorus recovery as struvite from farm, municipal and industrial waste: feedstock suitability, methods and pre-treatments. Waste Manag. 49, 437–454. doi: 10.1016/j.wasman.2016.01.003
Kavvada, O., Tarpeh, W. A., Horvath, A., and Nelson, K. L. (2017). Life-cycle cost and environmental assessment of decentralized nitrogen recovery using ion exchange from source-separated urine through spatial modeling. Environ. Sci. Technol. 51, 12061–12071. doi: 10.1021/acs.est.7b02244
Khumalo, N., Nthunya, L., Derese, S., Motsa, M., Verliefde, A., Kuvarega, A., et al. (2019). Water recovery from hydrolysed human urine samples via direct contact membrane distillation using PVDF/PTFE membrane. Sep. Purif. Technol. 211, 610–617. doi: 10.1016/j.seppur.2018.10.035
Kini. (2023). IAEME. Available at: http://www.iaeme.com/IJCIET/issues.asp?JType=IJCIET&VType=8&IType=3 (Accessed October 31, 2023)
Kinidi, L., Tan, I. A. W., Abdul Wahab, N. B., Tamrin, K. F. B., Hipolito, C. N., and Salleh, S. F. (2018). Recent development in Ammonia stripping process for industrial wastewater treatment. Int. J. Chem. Eng. 2018, 1–14. doi: 10.1155/2018/3181087
Kirchmann, H., and Pettersson, S. (1995). Human urine – chemical composition and fertilizer use efficiency. Fertilizer Res. 40, 149–154. doi: 10.1007/BF00750100
Kocatürk, N. P., and Baykal, B. B. (2012). Recovery of plant nutrients from dilute solutions of human urine and preliminary investigations on pot trials. Clean Soil Air Water 40, 538–544. doi: 10.1002/clen.201100193
Krähenbühl, M., Etter, B., and Udert, K. M. (2016). Pretreated magnesite as a source of low-cost magnesium for producing struvite from urine in Nepal. Sci. Total Environ. 542, 1155–1161. doi: 10.1016/j.scitotenv.2015.08.060
Kumar, K., Gupta, S. C., Baidoo, S. K., Chander, Y., and Rosen, C. J. (2005). Antibiotic uptake by plants from soil fertilized with animal manure. J. Env. Qual. 34, 2082–2085. doi: 10.2134/jeq2005.0026
Kumar, R., and Pal, P. (2015). A novel forward osmosis-nano filtration integrated system for coke-oven wastewater reclamation. Chem. Eng. Res. Design. 100, 542–553. doi: 10.1016/j.cherd.2015.05.012
Kuntke, P., Sleutels, T. H. J. A., Saakes, M., and Buisman, C. J. N. (2014). Hydrogen production and ammonium recovery from urine by a microbial electrolysis cell. Int. J. Hydrog. Energy 39, 4771–4778. doi: 10.1016/j.ijhydene.2013.10.089
Kuntke, P., Śmiech, K. M., Bruning, H., Zeeman, G., Saakes, M., Sleutels, T. H. J. A., et al. (2012). Ammonium recovery and energy production from urine by a microbial fuel cell. Water Res. 46, 2627–2636. doi: 10.1016/j.watres.2012.02.025
Kuntke, P., Zamora, P., Saakes, M., Buisman, C. J. N., and Hamelers, H. V. M. (2016). Gas-permeable hydrophobic tubular membranes for Ammonia recovery in bio-electrochemical systems. Environ. Sci. 2, 261–265. doi: 10.1039/C5EW00299K
Lahr, R. H., Goetsch, H. E., Haig, S. J., Noe-Hays, A., Love, N. G., Aga, D. S., et al. (2016). Urine bacterial community convergence through fertilizer production: storage, pasteurization, and struvite precipitation. Environ. Sci. Technol. 50, 11619–11626. doi: 10.1021/acs.est.6b02094
Larsen, T. A., Peters, I., Alder, A., Eggen, R., Maurer, M., and Muncke, J. (2001). Peer reviewed: re-engineering the toilet for sustainable wastewater management. Environ. Sci. Technol. 35, 192A–197A. doi: 10.1021/es012328d
Laureni, M., Palatsi, J., Llovera, M., and Bonmatí, A. (2013). Influence of pig slurry characteristics on Ammonia stripping efficiencies and quality of the recovered ammonium-sulfate solution. J. Chemical Tech & Biotech 88, 1654–1662. doi: 10.1002/jctb.4016
Ledezma, P., Jermakka, J., Keller, J., and Freguia, S. (2017). Recovering nitrogen as a solid without chemical dosing: bio-electroconcentration for recovery of nutrients from urine. Environ. Sci. Technol. Lett. 4, 119–124. doi: 10.1021/acs.estlett.7b00024
Lei, X., Sugiura, N., Feng, C., and Maekawa, T. (2007). Pretreatment of anaerobic digestion effluent with Ammonia stripping and biogas purification. J. Hazard. Mater. 145, 391–397. doi: 10.1016/j.jhazmat.2006.11.027
Li, X., Xie, Y., Jiang, F., Wang, B., Hu, Q., Tang, Y., et al. (2020). Enhanced phosphate removal from aqueous solution using Resourceable Nano-CaO2/BC composite: behaviors and mechanisms. Sci. Total Environ. 709:136123. doi: 10.1016/j.scitotenv.2019.136123
Li, W.-W., Yu, H.-Q., and He, Z. (2013). Towards sustainable wastewater treatment by using microbial fuel cells-centered technologies. Energy Environ. Sci. 7, 911–924. doi: 10.1039/C3EE43106A
Liao, Z., Chen, H., Zhu, B., and Li, H. (2015). Combination of powdered activated carbon and powdered zeolite for enhancing ammonium removal in Micro-polluted raw water. Chemosphere 134, 127–132. doi: 10.1016/j.chemosphere.2015.03.088
Lienert, J., Bürki, T., and Escher, B. I. (2007). Reducing micropollutants with source control: substance flow analysis of 212 Pharmaceuticals in Faeces and Urine. Water Sci. Technol. 56, 87–96. doi: 10.2166/wst.2007.560
Limoli, A., Langone, M., and Andreottola, G. (2016). Ammonia removal from raw manure Digestate by means of a turbulent mixing stripping process. J. Environ. Manag. 176, 1–10. doi: 10.1016/j.jenvman.2016.03.007
Lin, L., Li, R., Li, Y., Xu, J., and Li, X. (2017). Recovery of organic carbon and phosphorus from wastewater by Fe-enhanced primary sedimentation and sludge fermentation. Process Biochem. 54, 135–139. doi: 10.1016/j.procbio.2016.12.016
Lind, B.-B., Ban, Z., and Bydén, S. (2001). Volume reduction and concentration of nutrients in human urine. Ecol. Eng. 16, 561–566. doi: 10.1016/S0925-8574(00)00107-5
Liu, Y., Kumar, S., Kwag, J., and Ra, C. (2013). Magnesium ammonium phosphate formation, recovery and its application as valuable resources: a review. J. Chem. Tech. Biotech. 88, 181–189. doi: 10.1002/jctb.3936
Liu, Q., Liu, C., Zhao, L., Ma, W., Liu, H., and Ma, J. (2016). Integrated forward osmosis-membrane distillation process for human urine treatment. Water Res. 91, 45–54. doi: 10.1016/j.watres.2015.12.045
Lorick, D., Macura, B., Ahlström, M., Grimvall, A., and Harder, R. (2020). Effectiveness of struvite precipitation and Ammonia stripping for recovery of phosphorus and nitrogen from anaerobic Digestate: a systematic review. Environ. Evid. 9:27. doi: 10.1186/s13750-020-00211-x
Luo, Y., Li, H., Huang, Y.-R., Zhao, T.-L., Yao, Q.-Z., Fu, S.-Q., et al. (2018). Bacterial mineralization of struvite using MgO as magnesium source and its potential for nutrient recovery. Chem. Eng. J. 351, 195–202. doi: 10.1016/j.cej.2018.06.106
Malovanyy, A., Sakalova, H., Yatchyshyn, Y., Plaza, E., and Malovanyy, M. (2013). Concentration of ammonium from municipal wastewater using ion exchange process. Desalination 329, 93–102. doi: 10.1016/j.desal.2013.09.009
Markou, G., Agriomallou, M., and Georgakakis, D. (2017). Forced Ammonia stripping from livestock wastewater: the influence of some Physico-chemical parameters of the wastewater. Water Sci. Technol. 75, 686–692. doi: 10.2166/wst.2016.552
Maroušek, J., Kolář, L., Strunecký, O., Kopecký, M., Bartoš, P., Maroušková, A., et al. (2020). Modified biochars present an economic challenge to phosphate management in Wastewater Treatment Plants. J. Clean. Prod. 272:123015. doi: 10.1016/j.jclepro.2020.123015
Matsuda, Y., Shimizu, T., and Hashimasa, Y. (2020). Effect of carbon monoxide on polymer electrolyte fuel cell performance with a hydrogen circulation system. J. Electrochem. Soc. 167:044509. doi: 10.1149/1945-7111/ab732c
Maurer, M., Pronk, W., and Larsen, T. A. (2006). Treatment processes for source-separated urine. Water Res. 40, 3151–3166. doi: 10.1016/j.watres.2006.07.012
Merino-Jimenez, I., Celorrio, V., Fermin, D. J., Greenman, J., and Ieropoulos, I. (2017). Enhanced MFC power production and struvite recovery by the addition of sea salts to urine. Water Res. 109, 46–53. doi: 10.1016/j.watres.2016.11.017
Migliorini, G., and Luzzo, E. (2004). Seawater reverse osmosis plant using the pressure exchanger for energy recovery: a calculation model. Desalination 165, 289–298. doi: 10.1016/j.desal.2004.06.034
Mondor, M., Masse, L., Ippersiel, D., Lamarche, F., and Massé, D. I. (2008). Use of Electrodialysis and reverse osmosis for the recovery and concentration of Ammonia from swine manure. Bioresour. Technol. 99, 7363–7368. doi: 10.1016/j.biortech.2006.12.039
Monetti, J., Ledezma, P., Virdis, B., and Freguia, S. (2019). Nutrient recovery by bio-Electroconcentration is limited by wastewater conductivity. ACS Omega 4, 2152–2159. doi: 10.1021/acsomega.8b02737
Morales, N., Boehler, M., Buettner, S., Liebi, C., and Siegrist, H. (2013). Recovery of N and P from urine by struvite precipitation followed by combined stripping with digester sludge liquid at full scale. Water 5, 1262–1278. doi: 10.3390/w5031262
Moriwaki, H., Fujii, S., and Oshima, M. (2020). Simple method for removal of pollutants from water using freeze-thaw treatment. Sep. Purif. Technol. 237:116382. doi: 10.1016/j.seppur.2019.116382
Munoz-Cupa, C., Hu, Y., Xu, C., and Bassi, A. (2021). An overview of microbial fuel cell usage in wastewater treatment, resource recovery and energy production. Sci. Total Environ. 754:142429. doi: 10.1016/j.scitotenv.2020.142429
Mayer, M. (2002). Thermische Hygienisierung und Eindampfung von Humanurin (Doctoral dissertation, Diplomarbeit des Institut für Umweltechnik der Fachhochschule beider Basel, Muttenz, Schweiz (Thermal disinfection and evaporation of human urine. Diploma work of the Institute for Environmental Technology, Fachhochschule beider Basel, Muttenz, Switzerland).
Nakao, S., Nishio, T., and Kanjo, Y. (2017). Simultaneous recovery of phosphorus and potassium as magnesium potassium phosphate from synthetic sewage sludge effluent. Environ. Technol. 38, 2416–2426. doi: 10.1080/09593330.2016.1264485
Nam, S.-W., Choi, D.-J., Kim, S.-K., Her, N., and Zoh, K.-D. (2014). Adsorption characteristics of selected hydrophilic and hydrophobic micropollutants in water using activated carbon. J. Hazard. Mater. 270, 144–152. doi: 10.1016/j.jhazmat.2014.01.037
Nättorp, A., Remmen, K., and Remy, C. (2017). Cost assessment of different routes for phosphorus recovery from wastewater using data from pilot and production plants. Water Sci. Technol. 76, 413–424. doi: 10.2166/wst.2017.212
Neale, P. A., Mastrup, M., Borgmann, T., and Schäfer, A. I. (2010). Sorption of micropollutant Estrone to a water treatment ion exchange resin. J. Environ. Monit. 12, 311–317. doi: 10.1039/B913338K
O’Neal, J. A., and Boyer, T. H. (2013). Phosphate recovery using hybrid anion exchange: applications to source-separated urine and combined wastewater streams. Water Res. 47, 5003–5017. doi: 10.1016/j.watres.2013.05.037
Okano, K., Uemoto, M., Kagami, J., Miura, K., Aketo, T., Toda, M., et al. (2013). Novel technique for phosphorus recovery from aqueous solutions using amorphous calcium silicate hydrates (A-CSHs). Water Res. 47, 2251–2259. doi: 10.1016/j.watres.2013.01.052
Osipi, S. R., Secchi, A. R., and Borges, C. P. (2020). “Cost analysis of forward osmosis and reverse osmosis in a case study” in Current trends and future developments on (bio-) Membranes (Amsterdam: Elsevier), 305–324.
Parsons, S. (2000). The effect of domestic ion-exchange water softeners on the microbiological quality of drinking water. Water Res. 34, 2369–2375. doi: 10.1016/S0043-1354(99)00407-8
Patel, A., Mungray, A. A., and Mungray, A. K. (2020). Technologies for the Recovery of nutrients, water and energy from human urine: a review. Chemosphere 259:127372. doi: 10.1016/j.chemosphere.2020.127372
Peng, W., Zhang, H., Lü, F., Shao, L., and He, P. (2021). Char derived from food waste based solid Digestate for phosphate adsorption. J. Clean. Prod. 297:126687. doi: 10.1016/j.jclepro.2021.126687
Pinatha. (2023). Adsorption onto ash particles and common ion effect for phosphorus recovery from urinal wastewater. Available at: https://www.researchgate.net/publication/324013982_Adsorption_onto_ash_particles_and_common_ion_effect_for_phosphorus_recovery_from_urinal_wastewater (Accessed November 1, 2023)
Pinatha, Y., Polprasert, C., and Englande, A. J. (2020). Product and cost perspectives of phosphorus recovery from human urine using solid waste ash and sea salt addition – a case of Thailand. Sci. Total Environ. 713:136514. doi: 10.1016/j.scitotenv.2020.136514
Pradhan, S. K., Holopainen, J. K., and Heinonen-Tanski, H. (2009). Stored human urine supplemented with wood ash as fertilizer in tomato (Solanum Lycopersicum) cultivation and its impacts on fruit yield and quality. J. Agric. Food Chem. 57, 7612–7617. doi: 10.1021/jf9018917
Pradhan, S. K., Mikola, A., Heinonen-Tanski, H., and Vahala, R. (2019). Recovery of nitrogen and phosphorus from human urine using membrane and precipitation process. J. Environ. Manag. 247, 596–602. doi: 10.1016/j.jenvman.2019.06.046
Pronk, W., Zuleeg, S., Lienert, J., Escher, B., Koller, M., Berner, A., et al. (2007). Pilot experiments with Electrodialysis and ozonation for the production of a Fertiliser from urine. Water Sci. Technol. 56, 219–227. doi: 10.2166/wst.2007.575
Ray, H., Perreault, F., and Boyer, T. H. (2019). Urea recovery from fresh human urine by forward osmosis and membrane distillation (FO–MD). Environ. Sci. 5, 1993–2003. doi: 10.1039/C9EW00720B
Riechmann, M. E., Ndwandwe, B., Greenwood, E. E., Reynaert, E., Morgenroth, E., and Udert, K. M. (2021). On-site urine treatment combining ca(OH)2 dissolution and dehydration with ambient air. Water Res. X 13:100124. doi: 10.1016/j.wroa.2021.100124
Ronteltap, M., Maurer, M., and Gujer, W. (2007). The behaviour of pharmaceuticals and heavy metals during struvite precipitation in urine. Water Res. 41, 1859–1868. doi: 10.1016/j.watres.2007.01.026
Rößler, K., Wagner, R., Schatten, R., and Terytze, K. (2015). Verwertung von Urin und Fäkalien und deren Hygienisierung mittels Fermentation und Vermikompostierung. Müll und Abfall No. 6:8. doi: 10.37307/j.1863-9763.2015.06.08
Saliu, T. D., Akinyeye, O. J., Ololade, I. A., Unuabonah, E. I., and Oladoja, N. A. (2019). Expounding the role of interference on the recovery of nutrient fractions from aqua matrix using calcined gastropod Shell. J. Water Process Eng. 27, 152–161. doi: 10.1016/j.jwpe.2018.12.004
Saliu, T. D., Ali, J., Ololade, I. A., and Oladoja, N. A. (2020). Preparation and characterization of a decentralized modular yellow water nutrient recovery system. J. Environ. Manag. 276:111345. doi: 10.1016/j.jenvman.2020.111345
Saliu, T. D., and Oladoja, N. A. (2021). Nutrient recovery from wastewater and reuse in agriculture: a review. Environ. Chem. Lett. 19, 2299–2316. doi: 10.1007/s10311-020-01159-7
Saracco, G., and Genon, G. (1994). High temperature Ammonia stripping and recovery from process liquid wastes. J. Hazard. Mater. 37, 191–206. doi: 10.1016/0304-3894(94)85048-8
Savla, N., Suman,, Pandit, S., Verma, J. P., Awasthi, A. K., Sana, S. S., et al. (2021). Techno-economical evaluation and life cycle assessment of microbial electrochemical systems: a review. Curr. Res. Green Sustain. Chem. 4:100111. doi: 10.1016/j.crgsc.2021.100111
Schürmann, B., Everding, W., Montag, D., and Pinnekamp, J. (2012). Fate of pharmaceuticals and Bacteria in stored urine during precipitation and drying of struvite. Water Sci. Technol. 65, 1774–1780. doi: 10.2166/wst.2012.041
Senecal, J., and Vinnerås, B. (2017). Urea stabilisation and concentration for urine-diverting dry toilets: urine dehydration in ash. Sci. Total Environ. 586, 650–657. doi: 10.1016/j.scitotenv.2017.02.038
Sharma, P., and Mutnuri, S. (2019). Nutrient recovery and microbial diversity in human urine fed microbial fuel cell. Water Sci. Technol. 79, 718–730. doi: 10.2166/wst.2019.089
Simha, P., and Ganesapillai, M. (2017). Ecological sanitation and nutrient recovery from human urine: how far have we come? A review. Sustain. Environ. Res. 27, 107–116. doi: 10.1016/j.serj.2016.12.001
Simha, P., Karlsson, C., Viskari, E.-L., Malila, R., and Vinnerås, B. (2020a). Field testing a pilot-scale system for alkaline dehydration of source-separated human urine: a case study in Finland. Front. Environ. Sci. 8:570637. doi: 10.3389/fenvs.2020.570637
Simha, P., Lalander, C., Nordin, A., and Vinnerås, B. (2020b). Alkaline dehydration of source-separated fresh human urine: preliminary insights into using different dehydration temperature and media. Sci. Total Environ. 733:139313. doi: 10.1016/j.scitotenv.2020.139313
Sohn, W., Jiang, J., Phuntsho, S., Choden, Y., Tran, V. H., and Shon, H. K. (2023). Nutrients in a circular economy: role of urine separation and treatment. Desalination 560:116663. doi: 10.1016/j.desal.2023.116663
Tamta, P., Rani, N., and Yadav, A. K. (2020). Enhanced wastewater treatment and electricity generation using stacked constructed wetland–microbial fuel cells. Environ. Chem. Lett. 18, 871–879. doi: 10.1007/s10311-020-00966-2
Tansel, B., Lunn, G., and Monje, O. (2018). Struvite formation and decomposition characteristics for Ammonia and phosphorus recovery: a review of magnesium-Ammonia-phosphate interactions. Chemosphere 194, 504–514. doi: 10.1016/j.chemosphere.2017.12.004
Tao, W., Bayrakdar, A., Wang, Y., and Agyeman, F. (2019). Three-stage treatment for nitrogen and phosphorus recovery from human urine: hydrolysis, precipitation and vacuum stripping. J. Environ. Manag. 249:109435. doi: 10.1016/j.jenvman.2019.109435
Tarpeh, W. A., Wald, I., Omollo, M. O., Egan, T., and Nelson, K. L. (2018). Evaluating ion exchange for nitrogen recovery from source-separated urine in Nairobi, Kenya. Dev. Eng. 3, 188–195. doi: 10.1016/j.deveng.2018.07.002
Thörneby, L., Persson, K., and Trägårdh, G. (1999). Treatment of liquid effluents from dairy cattle and pigs using reverse osmosis. J. Agric. Eng. Res. 73, 159–170. doi: 10.1006/jaer.1998.0405
Tian, X., Gao, Z., Feng, H., Zhang, Z., Li, J., and Wang, A. (2019). Efficient nutrient recovery/removal from real source-separated urine by coupling vacuum thermal stripping with activated sludge processes. J. Clean. Prod. 220, 965–973. doi: 10.1016/j.jclepro.2019.02.181
Tice, R. C., and Kim, Y. (2014). Energy efficient Reconcentration of diluted human urine using ion exchange membranes in bioelectrochemical systems. Water Res. 64, 61–72. doi: 10.1016/j.watres.2014.06.037
Tong, Y., Kimbell, L., Avila, A., McNamara, P. J., and Mayer, B. K. (2018). Ion exchange for nutrient recovery coupled with biosolids-derived biochar pretreatment to remove micropollutants. Environ. Eng. Sci. 35, 1340–1348. doi: 10.1089/ees.2018.0138
Tong, Y., McNamara, P. J., and Mayer, B. K. (2017). Fate and impacts of Triclosan, sulfamethoxazole, and 17β-estradiol during nutrient recovery via ion exchange and struvite precipitation. Environ. Sci. 3, 1109–1119. doi: 10.1039/C7EW00280G
Tremouli, A., Greenman, J., and Ieropoulos, I. (2018). Investigation of ceramic MFC stacks for urine energy extraction. Bioelectrochemistry 123, 19–25. doi: 10.1016/j.bioelechem.2018.03.010
Tun, L. L., Jeong, D., Jeong, S., Cho, K., Lee, S., and Bae, H. (2016). Dewatering of source-separated human urine for nitrogen recovery by membrane distillation. J. Membr. Sci. 512, 13–20. doi: 10.1016/j.memsci.2016.04.004
Udert, K. M., and Wächter, M. (2012). Complete nutrient recovery from source-separated urine by nitrification and distillation. Water Res. 46, 453–464. doi: 10.1016/j.watres.2011.11.020
Upreti, H., Shrestha, P., and Paudel, P. (2013). Effect of human urine as fertilizer on crop production. Agron. J. Nepal 2, 168–172. doi: 10.3126/ajn.v2i0.7532
Valladares Linares, R., Li, Z., Yangali-Quintanilla, V., Ghaffour, N., Amy, G., Leiknes, T., et al. (2016). Life cycle cost of a hybrid forward osmosis – low pressure reverse osmosis system for seawater desalination and wastewater recovery. Water Res. 88, 225–234. doi: 10.1016/j.watres.2015.10.017
Vanotti, M. B., Dube, P. J., Szogi, A. A., and García-González, M. C. (2017). Recovery of Ammonia and phosphate minerals from swine wastewater using gas-permeable membranes. Water Res. 112, 137–146. doi: 10.1016/j.watres.2017.01.045
Vinnerås, B., Nordin, A., Niwagaba, C., and Nyberg, K. (2008). Inactivation of Bacteria and viruses in human urine depending on temperature and dilution rate. Water Res. 42, 4067–4074. doi: 10.1016/j.watres.2008.06.014
Viskari, E.-L., Grobler, G., Karimäki, K., Gorbatova, A., Vilpas, R., and Lehtoranta, S. (2018). Nitrogen recovery with source separation of human urine—preliminary results of its Fertiliser potential and use in agriculture. Front. Sustain. Food Syst. 2:32. doi: 10.3389/fsufs.2018.00032
Volpin, F., Chekli, L., Phuntsho, S., Cho, J., Ghaffour, N., Vrouwenvelder, J. S., et al. (2018). Simultaneous phosphorous and nitrogen recovery from source-separated urine: a novel application for Fertiliser drawn forward osmosis. Chemosphere 203, 482–489. doi: 10.1016/j.chemosphere.2018.03.193
Weigler, G., Perry, C., Weigler, A., Kim, B., Yangouyian, M., Vicena, J., et al. (2013). The prevalence of unexpected sexually transmitted diseases in women with lower urinary tract complaints suggestive of UTI is high. J. Community Med. Health Educ. 3:240. doi: 10.4172/2161-0711.1000240
Wijekoon, K. C., Hai, F. I., Kang, J., Price, W. E., Cath, T. Y., and Nghiem, L. D. (2014). Rejection and fate of trace organic compounds (TrOCs) during membrane distillation. J. Membr. Sci. 453, 636–642. doi: 10.1016/j.memsci.2013.12.002
Windeatt, J. H., Ross, A. B., Williams, P. T., Forster, P. M., Nahil, M. A., and Singh, S. (2014). Characteristics of biochars from crop residues: potential for carbon sequestration and soil amendment. J. Environ. Manag. 146, 189–197. doi: 10.1016/j.jenvman.2014.08.003
Winker, M. (2009). Pharmaceutical residues in urine and potential risks related to usage as Fertiliser in agriculture. doi: 10.15480/882.481
Wu, C., Spongberg, A. L., Witter, J. D., and Sridhar, B. B. M. (2012). Transfer of wastewater associated pharmaceuticals and personal care products to crop plants from biosolids treated soil. Ecotoxicol. Environ. Saf. 85, 104–109. doi: 10.1016/j.ecoenv.2012.08.007
Xia, P., Wang, X., Wang, X., Zhang, J., Wang, H., Song, J., et al. (2017). Synthesis and characterization of MgO modified diatomite for phosphorus recovery in eutrophic water. J. Chem. Eng. Data 62, 226–235. doi: 10.1021/acs.jced.6b00616
Xie, M., Nghiem, L. D., Price, W. E., and Elimelech, M. (2013). A forward osmosis–membrane distillation hybrid process for direct sewer mining: system performance and limitations. Environ. Sci. Technol. 47, 13486–13493. doi: 10.1021/es404056e
Xie, M., Nghiem, L. D., Price, W. E., and Elimelech, M. (2014). Toward resource recovery from wastewater: extraction of phosphorus from digested sludge using a hybrid forward osmosis–membrane distillation process. Environ. Sci. Technol. Lett. 1, 191–195. doi: 10.1021/ez400189z
Xu, K., Qu, D., Zheng, M., Guo, X., and Wang, C. (2019). Water reduction and nutrient Reconcentration of hydrolyzed urine via direct-contact membrane distillation: Ammonia loss and its control. J. Environ. Eng. 145:04018144. doi: 10.1061/(ASCE)EE.1943-7870.0001496
Xu, K., Wang, C., Wang, X., and Qian, Y. (2012). Laboratory experiments on simultaneous removal of K and P from synthetic and real urine for nutrient recycle by crystallization of magnesium–potassium–phosphate–hexahydrate in a draft tube and baffle reactor. Chemosphere 88, 219–223. doi: 10.1016/j.chemosphere.2012.02.061
Yang, Y., Chen, Z., Wang, X., Zheng, L., and Gu, X. (2017). Partial nitrification performance and mechanism of zeolite biological aerated filter for ammonium wastewater treatment. Bioresour. Technol. 241, 473–481. doi: 10.1016/j.biortech.2017.05.151
Ye, Y., Ngo, H. H., Guo, W., Chang, S. W., Nguyen, D. D., Liu, Y., et al. (2019). Microbial fuel cell for nutrient recovery and electricity generation from municipal wastewater under different ammonium concentrations. Bioresour. Technol. 292:121992. doi: 10.1016/j.biortech.2019.121992
Yip, N. Y., and Elimelech, M. (2011). Performance limiting effects in power generation from salinity gradients by pressure retarded osmosis. Environ. Sci. Technol. 45, 10273–10282. doi: 10.1021/es203197e
Zamora, P., Georgieva, T., Salcedo, I., Elzinga, N., Kuntke, P., and Buisman, C. J. (2017). Long-term operation of a pilot-scale reactor for phosphorus recovery as struvite from source-separated urine: long-term operation of a pilot-scale reactor for phosphorus. J. Chem. Technol. Biotechnol. 92, 1035–1045. doi: 10.1002/jctb.5079
Zhang, J., She, Q., Chang, V. W. C., Tang, C. Y., and Webster, R. D. (2014). Mining nutrients (N, K, P) from urban source-separated urine by forward osmosis dewatering. Environ. Sci. Technol. 48, 3386–3394. doi: 10.1021/es405266d
Zöllig, H., Remmele, A., Fritzsche, C., Morgenroth, E., and Udert, K. M. (2015). Formation of chlorination byproducts and their emission pathways in chlorine mediated electro-oxidation of urine on active and nonactive type anodes. Environ. Sci. Technol. 49, 11062–11069. doi: 10.1021/acs.est.5b01675
Keywords: human urine, nutrient recovery, circular economy, sustainability, agriculture
Citation: Saliu TD, Oladoja NA and Sauvé S (2024) Eco-friendly and sustainability assessment of technologies for nutrient recovery from human urine—a review. Front. Sustain. 4:1338380. doi: 10.3389/frsus.2023.1338380
Edited by:
Gasim Hayder, Tenaga National University, MalaysiaReviewed by:
Mahmud Iwan Solihin, UCSI University, MalaysiaHauwa Mustafa, Independent Researcher, Putrajaya, Malaysia
Copyright © 2024 Saliu, Oladoja and Sauvé. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Sébastien Sauvé, sebastien.sauve@umontreal.ca
 Toyin Dunsin Saliu
Toyin Dunsin Saliu Nurudeen Abiola Oladoja
Nurudeen Abiola Oladoja Sébastien Sauvé
Sébastien Sauvé