Advances in lung bioengineering: Where we are, where we need to go, and how to get there
- 1Department of Cardiothoracic Surgery, NYU Langone Health, New York, NY, United States
- 2Department of Cardiothoracic Surgery, NYU Transplant Institute, NYU Langone Health, New York, NY, United States
Lung transplantation is the only potentially curative treatment for end-stage lung failure and successfully improves both long-term survival and quality of life. However, lung transplantation is limited by the shortage of suitable donor lungs. This discrepancy in organ supply and demand has prompted researchers to seek alternative therapies for end-stage lung failure. Tissue engineering (bioengineering) organs has become an attractive and promising avenue of research, allowing for the customized production of organs on demand, with potentially perfect biocompatibility. While breakthroughs in tissue engineering have shown feasibility in practice, they have also uncovered challenges in solid organ applications due to the need not only for structural support, but also vascular membrane integrity and gas exchange. This requires a complex engineered interaction of multiple cell types in precise anatomical locations. In this article, we discuss the process of creating bioengineered lungs and the challenges inherent therein. We summarize the relevant literature for selecting appropriate lung scaffolds, creating decellularization protocols, and using bioreactors. The development of completely artificial lung substitutes will also be reviewed. Lastly, we describe the state of current research, as well as future studies required for bioengineered lungs to become a realistic therapeutic modality for end-stage lung disease. Applications of bioengineering may allow for earlier intervention in end-stage lung disease and have the potential to not only halt organ failure, but also significantly reverse disease progression.
1. Introduction
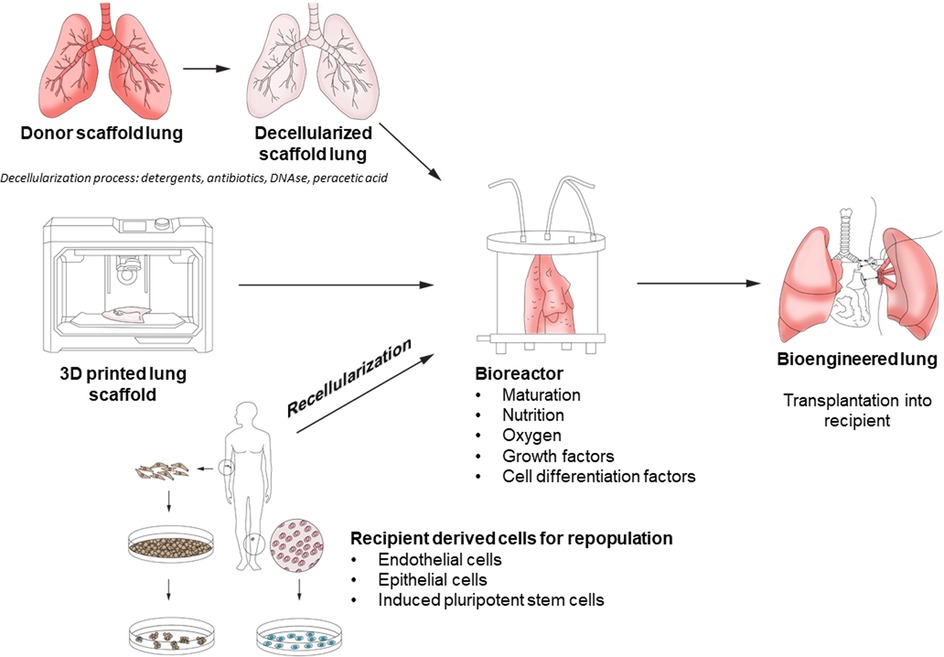
Within the field of end-stage lung disease (ESLD), the conventional treatment of choice is lung transplantation. This therapy is limited by an imbalance between the availability of suitable donor lungs and demand, creating a lengthy waiting list. Therefore, alternatives to conventional transplantation to alleviate donor organ shortage have been investigated, such as use of organs from different species (xenotransplantation) as well as bioengineering or regenerative medicine. The basic concept of bioengineering requires the creation of a form of tissue scaffold or matrix, populated by cells of the desired tissue, to transplant into the patient and allow for function as closely to the native organ as possible (Figure 1) (1). If successful, the promise of this approach lies in the ability to replace diseased and damaged lungs with bio-compatible tissue on demand. Tissue engineering approaches have had successful applications in human skin substitutes, vascular grafts, and bladder tissue (2–4). However, lung tissue engineering is made more challenging given the multiple cell types involved (e.g., over 40 different cell types in the lung), the need for structural and vascular membrane integrity, and in the case of lung tissue, ability to perform adequate gas exchange and the mechanical forces of respiration (5).
2. Selection of the tissue scaffold
The ideal scaffolding for the bioengineered lung has not yet been established. In tissue engineering, the extra-cellular matrix (ECM) is critical for maintaining mechanical support of the regenerating tissue, establishing the optimal microenvironment for tissue repair and regeneration, and incorporating biochemical cues for the modulation of cell behavior (6). Possible sources for such scaffolding include human tissue, animal tissue, or synthetic materials. Use of human or animal tissue requires a process of decellularization to remove antigenic material which may cause an immune reaction in the recipient.
One possible source of human tissue that may be used for scaffolding are donation after circulatory death (DCD) donors whose lungs are unsuitable for allotransplantation (7–11). A significant problem with the standard transplantation of DCD donor lungs is the length of ischemic time. At 18 h of cold ischemic time (CIT), ischemia-reperfusion injury (IRI) can significantly alter phenotypic features of lung cells—epithelial cells more so than endothelial cells (12). It is plausible that DCD lungs deemed to be untransplantable with prolonged ischemic time can be used as scaffolds for bioengineered lungs. There have also been studies on the use of human scaffolds from older donors with pre-existing lung diseases such as chronic obstructive pulmonary disease (COPD). However, these scaffold sources have demonstrated difficulty sustaining prolonged viability after recellularization compared to human lungs without COPD, suggesting that these cohorts of human lungs may not be an ideal choice (8, 13).
The ECM that remains after lung decellularization provides both structure and biophysical cues for whole organ regeneration after recellularization. A study by Gilpin et al. evaluated epithelial stem cells isolated from adult human lung tissue that were cultured on acellular ECM derived from neonatal or adult lung donors (14). There were significantly higher cell proliferation and survival rates for stem cells cultured on neonatal lungs. This may be because the ECM in the neonatal lung is actively undergoing alveologenesis and possesses distinct signals that aid in cell proliferation. The same study also found that treatment of the scaffolds with Fibrillin 2 (FBN-2) and Tenascin C (TN-C) prior to re-epithelization increased epithelial proliferation and tissue remodeling. This finding suggests that FBN-2 and TN-C (identified in neonatal ECM) have an important role in sustaining and maintaining cellular proliferation which is critical for tissue engineering of lung tissue.
Scaffolds need not be of human origin, and decellularized scaffolds from both porcine and non-human primates have been described (15, 16). Primate tissue is the most similar to human in matrix composition, but its use is associated with concerns related to animal welfare (17). Porcine lungs have the advantage of being readily available, and their use is potentially more acceptable in the eyes of the public compared to non-human primates. However, porcine models are susceptible to the formation of blebs and cystic spaces following the decellularization process (9). Furthermore, the composition of the ECM in porcine scaffolds has been shown to be significantly different, possibly inhibiting the proliferation of human endothelial cells (17). Although there is much care taken to decellularize scaffolds to avoid rejection from the recipient, there still exist risks of rejection from other aspects of the ECM. Proteoglycans and collagen in xenogenic ECMs possess antigenic properties and stimulate the production of anti-non-gal antibodies due to differences in protein sequences between human and non-primate ECMs (18, 19). A study by Tao et al. demonstrated evasion of the immune system by coating autologous red blood cell membrane on xenogeneic ECM-based tissue engineering graft surface (19). This may be a promising avenue for further research.
Synthetic scaffolds made of poly lactic-co-glycolic acid (PLGA), poly-L-lactic-acid (PLLA), and poly-DL-lactic acid have been studied as well, although all have shown difficulty in guiding correct cell differentiation (20, 21). The use of Matrigel and laminin are promising in addressing the problems in differentiation in murine embryonic stem cells, but have yet to be applied to human embryonic stem cells (22). Due to the fewer number of cell types present in tracheal tissue, more research has been done into producing synthetic tracheal scaffolds using 3D printable materials such as polyhedral oligomeric silsesquioxane poly (carbonate-urea) urethane (POSS-PCU) and polyglycolic acid, pluronic F-127 (23, 24). While these inks are accurate in printing, they lack integrin binding sites and an environment for the biochemical processes required for lung tissue (11). Interestingly, in an effort to more closely mimic alveolar structure for the purpose of lung cancer drug development, materials such as gelatin mixed with microbial transglutaminase have been preliminarily studied and shown to evenly distribute cells and are able to be efficiently seeded (25).
2.1. Decellularization protocols and decellularized scaffolds
The aim of the decellularization process is to preserve the airway and vascular structures whilst removing all cells and cellular material yet still preserving as much native ECM as possible. Most protocols for decellularization utilize a detergent solution such as Triton X-100 and sodium deoxycholate or 3-[(3-cholamidopropyl)dimethylammonio]-1-propanesulfonate (CHAPS) detergent perfused through the pulmonary artery and trachea, followed by DNAse and peracetic acid to disinfect and dissociate DNA from the extracellular matrix (26–28). The current criteria of complete decellularization from Gilpen et al. includes (i) <50 ng double-stranded DNA per mg of decellularized material; (ii) less than 200 bp length of DNA fragments; (iii) preservation of structural proteins of ECM; and (iv) retention of mechanical properties (29). Confirmation of decellularization can be obtained through DNA quantification techniques (fluorescent nucleic acid stains) and gel electrophoresis to determine DNA fragment size (30). Trace amounts of DNA remain after processing, however, the inflammatory or other responses to small fragments of remnant DNA (<300 base pairs) are unlikely to have clinical effects (31). There is a delicate balance between the goals of removing cellular material and disrupting the mechanical integrity of the ECM with the decellularization process.
Currently, human scaffolds and natural cell-derived ECM matrices are superior to synthetic biomaterials derived hydrogels. However, degradation rates of biomaterials scaffolds are difficult to control, and it is important to choose the right method of decellularization in order to preserve as much of the native ECM as possible. One method of decellularization is through the application of direct pressure to the tissue. Mechanical force is not ideal for many types of tissues which exhibit densely organized ECMs. However, in organs like the lung, mechanical force may be a feasible way to decellularize tissue while maximally preserving the ECM (30). Mechanical methods of decellularization avoid the toxicity of chemical methods and allow surfactant to remain in the decellularized tissue (29).
One of the problems that has been seen during decellularization in whole organs is the loss of the endothelium, resulting in an activation of the coagulation cascade. Consequently, direct contact between the recipient's blood and the ECM of the blood vessels occurs and subsequent intravascular blood coagulation can manifest after implantation and significantly reduces short-term graft survival (32–35). To address this, studies have utilized re-endothelialization of the graft, heparin immobilization on the ECM, end-point immobilization, layer-by-layer techniques, and use of heparin-gelatin to coat the vascular network—all of which have promising results but lack long-term graft survival data (33–37). A recent study from Akinnnola et al. explores endothelial cell specifications necessary to prevent thrombus formation and barrier leaks. The group successfully identified one cell source in a mouse study that was able to accomplish the aforementioned characteristics: pulmonary microvascular endothelial progenitor cells. Fathi et al. has also utilized pre-vascularization methods by axial vascularization and subsequent infusion of either bone marrow or adipose-derived stem cells through the portal vein of the Lewis rat liver scaffold after initial decellularization and implantation. The bone marrow and adipose-derived stem cell groups were functionally superior to the acellular scaffold (38).
One of the ways to overcome the challenge of repopulating the lung with so many cell types is to de-epithelialize instead of fully decellularize the lung. In diseases that primarily affect the conducting airways, de-epithelialization may allow for autologous lung transplantation after specific removal of diseased cell populations (39). Dorrello et al. describes a method of de-epithelization through infusing a CHAPS detergent solution through the airways while using low tidal volume ventilation to optimize distribution of the solution through the airways which was tested and successful in rat lungs (40). Subsequent targeted reseeding of the epithelium using normal epithelial cells or mutation-corrected iPSCs has the potential to allow patients to keep their native lungs which would result in a lower burden on the lung transplant list (41). This is especially viable now that the technology regarding ex-vivo lung perfusion (EVLP) and cross-circulation (XC) has advanced to a level where the possible amount of time on EVLP or XC supersedes the time needed to de-epithelialize and reseed the lung as proven in large animal models on EVLP and both small and large animal models on XC (40, 42–44). Dorrello et al.'s approach to reseeding the epithelium can also be applied to lungs that do not qualify for traditional or extended criteria donation, and would otherwise serve as scaffolds for lung bioengineering. With majority of donor lungs unable to meet the standards for transplantation due to injury located in the alveolar epithelium, de-epithelialization of the donor lung and subsequent reseeding of the lung with recipient stem cells would allow for an increased number of donor lungs to successfully be used as scaffolds. By selectively decellularzing the epithelium, there is the potential to address the problems of recellularization of a wide range of cell types, preservation of the vascular network, and support and delivery of growth factors and signaling molecules vital to the success of lung scaffolds (39).
2.2. 3D Printed scaffolds
Recent advances in lung tissue engineering involve production of a synthetic acellular matrix. 3D “bioprinting” is a technology that uses computer-aided models and specific 3D bioprinters with either biomaterials (non-cellular biological material hydrogels such as collagen and gelatin which need to be seeded after printing) or bioinks (which contain biologically active cells that can be processed and printed to produce a biological model) (45). There are challenges with using either. Bioinks must be utilized within temperature and pressure parameters that allow the cells to survive the printing process (45). Scaffolds made with biomaterial inks must be able to sustain cellularization, with the ability to seed on varied cell types. Due to limitations of 3D printers and differences between capabilities in viscosity of materials that can be printed, clogging of the nozzle has often been problematic and limits the number of cell types available to be printed (46, 47).
One promising biomaterial is human recombinant type 1 collagen, which has been produced by crossbreeding tobacco plants to incorporate human genes encoding for procollagen type I, with similar biofunctionality compared to human tissue-derived collagen (48). 3D bioprinting technology has been able to produce models of the alveolus, including endothelial cell, basement membrane, and epithelial cell layers (49). Selection of the most appropriate biomaterial for a synthetic scaffold is challenging. Completely synthetic materials can be altered for varying mechanical properties, an important consideration when supporting respiration, but offer relatively poor cell adhesion and lack biological signals for differentiation and proliferation. Naturally derived hydrogels have the benefit of biocompatibility and biochemical cues for adhesion, although they tend to have poor mechanical properties. Consequently, a mixture of both synthetic materials and naturally derived hydrogels may retain the benefits of each (11, 45).
At the alveolar level, Grigoryan and colleagues developed a model of stereolithographic production of a hydrogel that contained intricate and functional vascular architectures, which subsequently modeled into alveolar structures that could support ventilation cycles for over 6 h (50). This advance proved the ability to produce highly intricate biologically active structures, a previous limitation of 3D printing technologies.
3. Bioreactors
Repopulating the scaffold with a suitable lung cell population is another challenge. Ideally, autologous cells from the transplant recipient would be used to recellularize the scaffold. The advantage of this would be complete biocompatibility without the need for immunosuppressive medications. However, adult cells are difficult to obtain from patients in sufficient numbers. Induced pluripotent stem cells (iPSCs) are a promising cell lineage that offer some benefits in bioengineered lungs. iPSCs are obtained from skin fibroblasts, and differentiation of iPSCs into human fetal lung tissue and population of a human scaffold of alveolar epithelial cells have been reported (51, 52). Development of endothelial cells (also necessary for complete recellularization) through reprogramming has been reported in decellularized vascular scaffolds (53). What remains unknown is whether all cell types necessary for recellularization are present in current models. Interstitial cells make up 30% of the lung (such as fibroblasts) and are vital for supporting epithelial and endothelial cell populations. Mesenchymal stem cells, derived from bone marrow, play a role in lung injury and fibroblasts assist in ECM turnover. Excessive fibroblast activity contributes to the development of pulmonary fibrosis (54). Consequently, it is likely that repopulation with interstitial cells (from mesenchymal stem cells and fibroblasts) are necessary for successful lung tissue engineering (55).
Repopulating either synthetic or decellularized scaffold requires a bioreactor. This allows seeding of the cells, adequate nutrient transfer, and waste removal, and additionally provides the appropriate mechanical and biochemical stimuli for cell differentiation. Current technology for human EVLP utilized in clinical transplantation allows for lung perfusion up to 12 h experimentally and 4 h clinically (56, 57). It is likely that repopulation and maturation of a decellularized scaffold will require more time than this, and therefore further research into the development of appropriate bioreactors for human bioengineered lung is necessary.
One of the adverse events of decellularizing organs is the damage to critical ECM components such as elastic fibers, proteoglycans, and glycosaminoglycans. These elements are crucial for tissues to sustain compressive forces, and thus must be a consideration when evaluating bioreactor systems. While some bioreactor systems can recover some of the lost glycosaminoglycans, there is growing evidence that the recipient's body may serve as a superior bioreactor to restore some of the constructs lost during decellularization. This is especially applicable in partial lung transplants, such as single-lobe or tracheal transplants. Tan et al. implanted a tissue-engineered bronchus in a lung cancer patient, and at four month follow-up confirmed complete revascularization and re-epithelialization of the bronchus (58). While the patient's death was reported 13 months postoperatively, this nevertheless demonstrated the success of using the body as a bioreactor.
It is important to mimic in vivo conditions (mechanical stress, environment) as closely as possible when reseeding the scaffold. This permits the scaffold to maintain the correct signaling and differentiation of cell types in the lung. Due to the variety of cell types that exist in the lung, it may be plausible that synthetic scaffolds be constructed of mixed materials or inks that are optimal in supporting the respective cell types in that area of the lung (59–62).
4. Animal studies
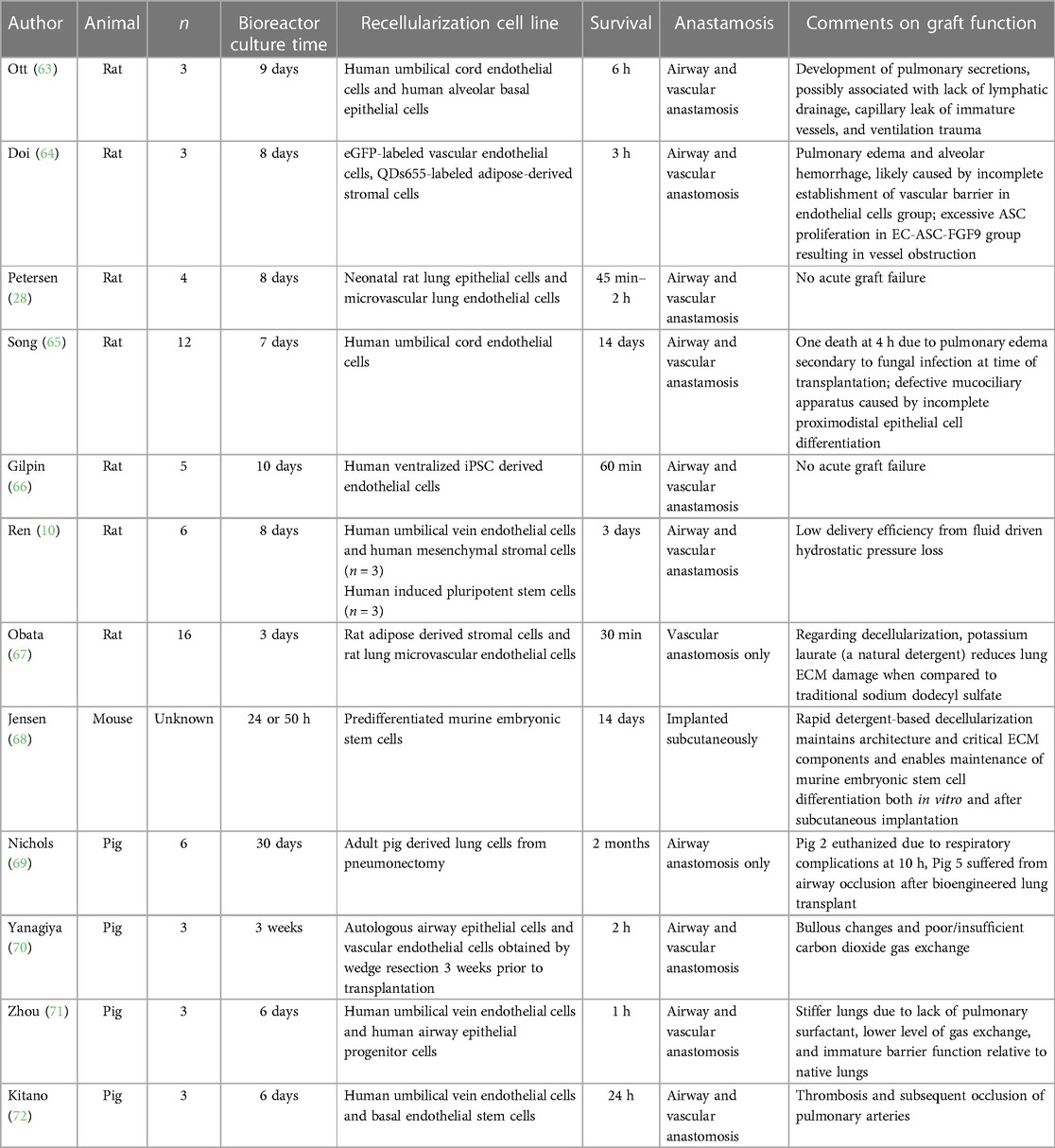
There have been numerous animal transplants performed with bioengineered lungs (Table 1). In large animals comparable to humans, the longest survival report to date is from Nichols and colleagues who performed transplantation of porcine lungs using a decellularized pig scaffold (69). They reported survival of one animal to 2 months. While only the airway was anastomosed in this experiment, vascularization of the airway was observed. Yanagiya et al. reported 3 pigs who received bioengineered lungs, although all developed issues with gas exchange and bullous emphysema (70). This was potentially due to the degradation of elastin during the decelluarization process. A report from Zhou et al. noted reduced lung compliance and gas exchange in the bioengineered lungs, possibly as a result of both the lack of pulmonary surfactant production and immature barrier function (71). Kitano et al. observed thrombus formation in the pulmonary artery and vein in pigs at 24 h, with collapse despite high airway inspiratory pressures of 30 cm H2O in two pig recipients of bioengineered lungs (72). This was likely due to cross-species hyperacute dysfunction and activation of coagulation cascade despite three drug immunosuppression.
Animal transplantation experiments with bioengineered 3D-printed artificial lung scaffolds are ongoing, however, peer reviewed data has yet to be published (73). While these experiments are in the early developmental stages, there is incremental progress in the use of bioengineered lungs utilizing decellularized scaffolds or artificially manufactured scaffolds. Further work is needed to determine the optimum protocol for de- and re-cellularization and bioreactor maturation. If successful, this regenerative technique promises not only to provide a personalized lung organ replacement, but can also likely be applicable to other organs and can shift the transplantation paradigm away from allo- and xenotransplantation.
5. Clinical and human studies
While whole bioengineered lung studies have yet to be done, there have been advances in tracheal and vascular human studies (74, 75). The first successful tissue-engineered vascular graft was a pulmonary artery in 1999 (76). The scaffold was an autologous peripheral vein and showed ability to grow with the patient. In 2008, Macchiarini et al. performed a tracheal transplant using a tracheal scaffold of a human donor that had been first decellularized and then recellularized with the recipient's autologous bronchial epithelial cells from bronchoscopic biopsy. A novel bioreactor was used to mimic the biomechanical cues of shear stress. The recipient did not suffer any complications and had normal lung function tests at two-month follow-up. At one month, “the appearance of the graft was indistinguishable from native trachea, and local mucosal bleeding was elicited when the biopsy sample was taken, indicating successful revascularization” (77). However, subsequent events surrounding this transplant and other tracheal transplants have cast significant doubt into the scientific integrity and success of these experimental procedures.
As mentioned previously, in 2015, Tan et al. addressed the challenge of the extensive (multiweek) revascularization timeframe associated with implanted tissue engineering (58). During the traditional revascularization timeframe, majority of the cells seeded onto the scaffold die, decreasing viability of the implanted scaffold (78). By using an “in-vivo bioreactor” which is bioengineered tissue—or in this case, porcine acellularized dermix matrix—perfused with continuous medium, the group allowed the pre-seeded cells to remain alive during revascularization while simultaneously reseeding the system and allowing for the addition of various growth factors into the perfusate. During the procedure, the left upper lobe, lower superior segment, and left main bronchus were resected and the engineered left bronchus substitute implanted with two PORT-A-CATH (Smiths Medical, London, UK) inserted in between the two matrix layers. Ringer's solution containing gentamicin was continuously pumped into the scaffold for a month while total nucleated cells were injected directly into the matrix twice a week for a month. The group confirmed revascularization and re-epithelialization 4 months postoperatively which showed the efficacy of the patient serving as his own bioreactor for the scaffold. The patient who initially was given a prognosis of 3 months died at 13 months post-op due to a lung cancer relapse.
6. Towards the development of a bioartificial lung
There has been much research regarding prolonging the direction of mechanical circulation and gas exchange in the context of lung injury and bridge to transplant. Along with the increasing use of extracorporeal membrane oxygenation (ECMO) as a bridge to lung transplant, it is possible to envision a device that goes beyond a bridge and serves as the lung itself. In lung transplant patients, longer term use of ECMO as a bridge to transplantation has been associated with worse post-transplant survival rates (79, 80). There are also many complications associated with long term use of ECMO that would also be concerns for an ECMO-type bioartificial lung, such as vascular complications, organ dysfunction (such as kidney injury) due to alteration in blood flow characteristics, oxygenator degradation and malfunction, major bleeding, and significant infection (81). The requirement for battery power (for pump systems) and a continuous oxygen supply are also limitations. Now more than ever, it is crucial to find an accessible and suitable alternative to orthotopic lung transplantation to fulfill the gap between available organs and patients on the waitlist. In 2020, the lung transplant waitlist mortality was 16.1 deaths per 100 waitlist years in the United States, with even more occurring in other solid organ transplantation waitlists (82). Over 2,600 new candidates were added to the lung transplant waiting list that year. Waitlist mortality in other countries with differing policies and availability of donor organs is even higher (83, 84). The field of bioengineering offers unparalleled potential to drastically extend life expectancy in patients with ESLD who may otherwise have no options for salvage therapy.
Author contributions
TH and LJ: wrote sections of the manuscript. SC, TG and LA: contributed to the design and provided critical review and manuscript revision. JC: designed the review article, wrote the first draft and provided critical reviews and revision. All authors contributed to the article and approved the submitted version.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Langer R, Vacanti JP. Tissue engineering. Science. (1993);260(5110):920–6. doi: 10.1126/science.8493529
2. Atala A, Bauer SB, Soker S, Yoo JJ, Retik AB. Tissue-engineered autologous bladders for patients needing cystoplasty. Lancet. 2006;367(9518):1241–6. doi: 10.1016/S0140-6736(06)68438-9
3. Ghio SC, Larouche D, Doucet EJ, Germain L. The role of cultured autologous bilayered skin substitutes as epithelial stem cell niches after grafting: a systematic review of clinical studies. Burns Open. 2021;5(2):56–66. doi: 10.1016/j.burnso.2021.02.002
4. Matsuzaki Y, John K, Shoji T, Shinoka T. The evolution of tissue engineered vascular graft technologies: from preclinical trials to advancing patient care. Appl Sci. (2019) 9(7):1274. doi: doi: 10.3390/app9071274.31890320
5. Franks TJ, Colby TV, Travis WD, Tuder RM, Reynolds HY, Brody AR, et al. Resident cellular components of the human lung. Proc Am Thorac Soc. 2008;5(7):763–6. doi: 10.1513/pats.200803-025HR
6. Yi S, Ding F, Gong L, Gu X. Extracellular matrix scaffolds for tissue engineering and regenerative medicine. Curr Stem Cell Res Ther. 2017;12(3):233–46. doi: 10.2174/1574888X11666160905092513
7. Ohata K, Ott HC. Human-scale lung regeneration based on decellularized matrix scaffolds as a biologic platform. Surg Today. 2020;50(7):633–43. doi: 10.1007/s00595-020-02000-y
8. Wagner DE, Bonenfant NR, Parsons CS, Sokocevic D, Brooks EM, Borg ZD, et al. Comparative decellularization and recellularization of normal versus emphysematous human lungs. Biomaterials. 2014;35(10):3281–97. doi: 10.1016/j.biomaterials.2013.12.103
9. Wagner DE, Bonenfant NR, Sokocevic D, DeSarno MJ, Borg ZD, Parsons CS, et al. Three-dimensional scaffolds of acellular human and porcine lungs for high throughput studies of lung disease and regeneration. Biomaterials. 2014;35(9):2664–79. doi: 10.1016/j.biomaterials.2013.11.078
10. Ren X, Moser PT, Gilpin SE, Okamoto T, Wu T, Tapias LF, et al. Engineering pulmonary vasculature in decellularized rat and human lungs. Nat Biotechnol. 2015;33(10):1097–102. doi: 10.1038/nbt.3354
11. De Santis MM, Bölükbas DA, Lindstedt S, Wagner DE. How to build a lung: latest advances and emerging themes in lung bioengineering. Eur Respir J. 2018;52(1):1601355. doi: 10.1183/13993003.01355-2016
12. Saren G, Wong A, Lu Y-B, Baciu C, Zhou W, Zamel R, et al. Ischemia-reperfusion injury in a simulated lung transplant setting differentially regulates transcriptomic profiles between human lung endothelial and epithelial cells. Cells. 2021;10(10):2713. doi: 10.3390/cells10102713
13. Godin LM, Sandri BJ, Wagner DE, Meyer CM, Price AP, Akinnola I, et al. Decreased laminin expression by human lung epithelial cells and fibroblasts cultured in acellular lung scaffolds from aged mice. PLoS One. 2016;11(3):e0150966. doi: 10.1371/journal.pone.0150966
14. Gilpin SE, Li Q, Evangelista-Leite D, Ren X, Reinhardt DP, Frey BL, et al. Fibrillin-2 and tenascin-C bridge the age gap in lung epithelial regeneration. Biomaterials. 2017;140:212–9. doi: 10.1016/j.biomaterials.2017.06.027
15. Platz J, Bonenfant NR, Uhl FE, Coffey AL, McKnight T, Parsons C, et al. Comparative decellularization and recellularization of wild-type and alpha 1,3 galactosyltransferase knockout pig lungs: a model for ex vivo xenogeneic lung bioengineering and transplantation. Tissue Eng Part C Methods. 2016;22(8):725–39. doi: 10.1089/ten.tec.2016.0109
16. Bonvillain RW, Danchuk S, Sullivan DE, Betancourt AM, Semon JA, Eagle ME, et al. A nonhuman primate model of lung regeneration: detergent-mediated decellularization and initial in vitro recellularization with mesenchymal stem cells. Tissue Eng Part A. 2012;18(23-24):2437–52. doi: 10.1089/ten.tea.2011.0594
17. Balestrini JL, Gard AL, Gerhold KA, Wilcox EC, Liu A, Schwan J, et al. Comparative biology of decellularized lung matrix: implications of species mismatch in regenerative medicine. Biomaterials. 2016;102:220–30. doi: 10.1016/j.biomaterials.2016.06.025
18. Stone KR, Walgenbach A, Galili U. Induced remodeling of porcine tendons to human anterior cruciate ligaments by α-GAL epitope removal and partial cross-linking. Tissue Eng Part B Rev. 2017;23(4):412–9. doi: 10.1089/ten.teb.2016.0332
19. Tao C, Nie X, Zhu W, Iqbal J, Xu C, Wang D-A. Autologous cell membrane coatings on tissue engineering xenografts for suppression and alleviation of acute host immune responses. Biomaterials. 2020;258:120310. doi: 10.1016/j.biomaterials.2020.120310
20. Lin YM, Boccaccini AR, Polak JM, Bishop AE, Maquet V. Biocompatibility of poly-DL-lactic acid (PDLLA) for lung tissue engineering. J Biomater Appl. 2006;21(2):109–18. doi: 10.1177/0885328206057952
21. Mondrinos MJ, Koutzaki S, Jiwanmall E, Li M, Dechadarevian JP, Lelkes PI, et al. Engineering three-dimensional pulmonary tissue constructs. Tissue Eng. 2006;12(4):717–28. doi: 10.1089/ten.2006.12.717
22. Lin YM, Zhang A, Rippon HJ, Bismarck A, Bishop AE. Tissue engineering of lung: the effect of extracellular matrix on the differentiation of embryonic stem cells to pneumocytes. Tissue Eng Part A. 2010;16(5):1515–26. doi: 10.1089/ten.tea.2009.0232
23. Crowley C, Klanrit P, Butler CR, Varanou A, Platé M, Hynds RE, et al. Surface modification of a POSS-nanocomposite material to enhance cellular integration of a synthetic bioscaffold. Biomaterials. 2016;83:283–93. doi: 10.1016/j.biomaterials.2016.01.005
24. Cortiella J, Nichols JE, Kojima K, Bonassar LJ, Dargon P, Roy AK, et al. Tissue-engineered lung: an in vivo and in vitro comparison of polyglycolic acid and pluronic F-127 hydrogel/somatic lung progenitor cell constructs to support tissue growth. Tissue Eng. 2006;12(5):1213–25. doi: 10.1089/ten.2006.12.1213
25. Sun Y-J, Hsu C-H, Ling T-Y, Liu L, Lin T-C, Jakfar S, et al. The preparation of cell-containing microbubble scaffolds to mimic alveoli structure as a 3D drug-screening system for lung cancer. Biofabrication. 2020;12(2):025031. doi: 10.1088/1758-5090/ab78ee
26. Keane TJ, Swinehart IT, Badylak SF. Methods of tissue decellularization used for preparation of biologic scaffolds and in vivo relevance. Methods. 2015;84:25–34. doi: 10.1016/j.ymeth.2015.03.005
27. Uhl FE, Wagner DE, Weiss DJ. Preparation of decellularized lung matrices for cell culture and protein analysis. Methods Mol Biol. 2017;1627:253–83. doi: 10.1007/978-1-4939-7113-8_18
28. Petersen TH, Calle EA, Zhao L, Lee EJ, Gui L, Raredon MB, et al. Tissue-engineered lungs for in vivo implantation. Science. 2010;329(5991):538–41. doi: 10.1126/science.1189345
29. Gilpin A, Yang Y. Decellularization strategies for regenerative medicine: from processing techniques to applications. BioMed Res Int. 2017;2017:9831534. doi: 10.1155/2017/9831534
30. Gilbert TW, Freund JM, Badylak SF. Quantification of DNA in biologic scaffold materials. J Surg Res. 2009;152(1):135–9. doi: 10.1016/j.jss.2008.02.013
31. Keane TJ, Londono R, Turner NJ, Badylak SF. Consequences of ineffective decellularization of biologic scaffolds on the host response. Biomaterials. 2012;33(6):1771–81. doi: 10.1016/j.biomaterials.2011.10.054
32. Uygun BE, Soto-Gutierrez A, Yagi H, Izamis ML, Guzzardi MA, Shulman C, et al. Organ reengineering through development of a transplantable recellularized liver graft using decellularized liver matrix. Nat Med. 2010;16(7):814–20. doi: 10.1038/nm.2170
33. Ko IK, Peng L, Peloso A, Smith CJ, Dhal A, Deegan DB, et al. Bioengineered transplantable porcine livers with re-endothelialized vasculature. Biomaterials. 2015;40:72–9. doi: 10.1016/j.biomaterials.2014.11.027
34. Bao J, Wu Q, Sun J, Zhou Y, Wang Y, Jiang X, et al. Hemocompatibility improvement of perfusion-decellularized clinical-scale liver scaffold through heparin immobilization. Sci Rep. 2015;5(1):10756. doi: 10.1038/srep10756
35. Bruinsma BG, Kim Y, Berendsen TA, Ozer S, Yarmush ML, Uygun BE. Layer-by-layer heparinization of decellularized liver matrices to reduce thrombogenicity of tissue engineered grafts. J Clin Transl Res. 2015;1(1):48–56. doi: 10.18053/jctres.201501.004
36. Kojima H, Yasuchika K, Fukumitsu K, Ishii T, Ogiso S, Miyauchi Y, et al. Establishment of practical recellularized liver graft for blood perfusion using primary rat hepatocytes and liver sinusoidal endothelial cells. Am J Transplant. 2018;18(6):1351–9. doi: 10.1111/ajt.14666
37. Hussein KH, Park KM, Kang KS, Woo HM. Heparin-gelatin mixture improves vascular reconstruction efficiency and hepatic function in bioengineered livers. Acta Biomater. 2016;38:82–93. doi: 10.1016/j.actbio.2016.04.042
38. Fathi I, Imura T, Inagaki A, Nakamura Y, Nabawi A, Goto M. Decellularized whole-organ pre-vascularization: a novel approach for organogenesis. Front Bioeng Biotechnol. (2019) 9:756755. doi: 10.3389/fbioe.2021.756755
39. Dorrello NV, Vunjak-Novakovic G. Bioengineering of pulmonary epithelium with preservation of the vascular niche. Front Bioeng Biotechnol. 2020;8:269. doi: 10.3389/fbioe.2020.00269
40. Dorrello NV, Guenthart BA, O’Neill JD, Kim J, Cunningham K, Chen Y-W, et al. Functional vascularized lung grafts for lung bioengineering. Sci Adv. 2017;3(8):e1700521. doi: 10.1126/sciadv.1700521
41. Berical A, Lee RE, Randell SH, Hawkins F. Challenges facing airway epithelial cell-based therapy for cystic fibrosis. Front Pharmacol. (2019);10:00074. doi: 10.3389/fphar.2019.00074
42. Hozain AE, Tipograf Y, Pinezich MR, Cunningham KM, Donocoff R, Queen D, et al. Multiday maintenance of extracorporeal lungs using cross-circulation with conscious swine. J Thorac Cardiovasc Surg. 2020;159(4):1640–53.e18. doi: 10.1016/j.jtcvs.2019.09.121
43. Kim J, Guenthart B, O’Neill JD, Dorrello NV, Bacchetta M, Vunjak-Novakovic G. Controlled delivery and minimally invasive imaging of stem cells in the lung. Sci Rep. 2017;7(1):13082. doi: 10.1038/s41598-017-13280-9
44. O’Neill JD, Guenthart BA, Kim J, Chicotka S, Queen D, Fung K, et al. Cross-circulation for extracorporeal support and recovery of the lung. Nat Biomed Eng. 2017;1(3):0037. doi: 10.1038/s41551-017-0037
45. Barreiro Carpio M, Dabaghi M, Ungureanu J, Kolb MR, Hirota JA, Moran-Mirabal JM. 3D bioprinting strategies, challenges, and opportunities to model the lung tissue microenvironment and its function. Front Bioeng Biotechnol. 2021;9:773511. doi: 10.3389/fbioe.2021.773511
46. Moon S, Hasan SK, Song YS, Xu F, Keles HO, Manzur F, et al. Layer by layer three-dimensional tissue epitaxy by cell-laden hydrogel droplets. Tissue Eng Part C Methods. (2010) 16(1):157–66. doi: 10.1089/ten.tec.2009.0179.19586367
47. Gudapati H, Dey M, Ozbolat I. A comprehensive review on droplet-based bioprinting: past, present and future. Biomaterials. 2016;102:20–42. doi: 10.1016/j.biomaterials.2016.06.012
48. Shoseyov O, Posen Y, Grynspan F. Human recombinant type I collagen produced in plants. Tissue Eng Part A. 2013;19(13-14):1527–33. doi: 10.1089/ten.tea.2012.0347
49. Horváth L, Umehara Y, Jud C, Blank F, Petri-Fink A, Rothen-Rutishauser B. Engineering an in vitro air-blood barrier by 3D bioprinting. Sci Rep. 2015;5:7974. doi: 10.1038/srep07974
50. Grigoryan B, Paulsen SJ, Corbett DC, Sazer DW, Fortin CL, Zaita AJ, et al. Multivascular networks and functional intravascular topologies within biocompatible hydrogels. Science. 2019;364(6439):458–64. doi: 10.1126/science.aav9750
51. Ghaedi M, Calle EA, Mendez JJ, Gard AL, Balestrini J, Booth A, et al. Human iPS cell-derived alveolar epithelium repopulates lung extracellular matrix. J Clin Invest. 2013;123(11):4950–62. doi: 10.1172/JCI68793
52. Huang SXL, Islam MN, O’Neill J, Hu Z, Yang Y-G, Chen Y-W, et al. Efficient generation of lung and airway epithelial cells from human pluripotent stem cells. Nat Biotechnol. 2014;32(1):84–91. doi: 10.1038/nbt.2754
53. Margariti A, Winkler B, Karamariti E, Zampetaki A, Tsai TN, Baban D, et al. Direct reprogramming of fibroblasts into endothelial cells capable of angiogenesis and reendothelialization in tissue-engineered vessels. Proc Natl Acad Sci U S A. 2012;109(34):13793–8. doi: 10.1073/pnas.1205526109
54. Boyd DF, Allen EK, Randolph AG, Guo XJ, Weng Y, Sanders CJ, et al. Exuberant fibroblast activity compromises lung function via ADAMTS4. Nature. 2020;587(7834):466–71. doi: 10.1038/s41586-020-2877-5
55. Calle EA, Ghaedi M, Sundaram S, Sivarapatna A, Tseng MK, Niklason LE. Strategies for whole lung tissue engineering. IEEE Trans Biomed Eng. 2014;61(5):1482–96. doi: 10.1109/TBME.2014.2314261
56. Cypel M, Yeung JC, Hirayama S, Rubacha M, Fischer S, Anraku M, et al. Technique for prolonged normothermic ex vivo lung perfusion. J Heart Lung Transplant. 2008;27(12):1319–25. doi: 10.1016/j.healun.2008.09.003
57. Cypel M, Yeung JC, Liu M, Anraku M, Chen F, Karolak W, et al. Normothermic ex vivo lung perfusion in clinical lung transplantation. N Engl J Med. 2011;364(15):1431–40. doi: 10.1056/NEJMoa1014597
58. Tan Q, Liu R, Chen X, Wu J, Pan Y, Lu S, et al. Clinic application of tissue engineered bronchus for lung cancer treatment. J Thorac Dis. 2017;9(1):22–9. doi: 10.21037/jtd.2017.01.50
59. Foss C, Merzari E, Migliaresi C, Motta A. Silk fibroin/hyaluronic acid 3D matrices for cartilage tissue engineering. Biomacromolecules. 2013;14(1):38–47. doi: 10.1021/bm301174x
60. Cui X, Li J, Hartanto Y, Durham M, Tang J, Zhang H, et al. Advances in extrusion 3D bioprinting: a focus on multicomponent hydrogel-based bioinks. Adv Healthc Mater. 2020;9(15):e1901648. doi: 10.1002/adhm.201901648
61. Aljohani W, Ullah MW, Li W, Shi L, Zhang X, Yang G. Three-dimensional printing of alginate-gelatin-agar scaffolds using free-form motor assisted microsyringe extrusion system. J Polym Res. 2018;25(3):62. doi: 10.1007/s10965-018-1455-0
62. Mancha Sánchez E, Gómez-Blanco JC, López Nieto E, Casado JG, Macías-García A, Díaz Díez MA, et al. Hydrogels for bioprinting: a systematic review of hydrogels synthesis, bioprinting parameters, and bioprinted structures behavior. Front Bioeng Biotechnol. 2020;8:776. doi: 10.3389/fbioe.2020.00776
63. Ott HC, Clippinger B, Conrad C, Schuetz C, Pomerantseva I, Ikonomou L, et al. Regeneration and orthotopic transplantation of a bioartificial lung. Nat Med. 2010;16(8):927–33. doi: 10.1038/nm.2193
64. Doi R, Tsuchiya T, Mitsutake N, Nishimura S, Matsuu-Matsuyama M, Nakazawa Y, et al. Transplantation of bioengineered rat lungs recellularized with endothelial and adipose-derived stromal cells. Sci Rep. 2017;7(1):8447. doi: 10.1038/s41598-017-09115-2
65. Song JJ, Kim SS, Liu Z, Madsen JC, Mathisen DJ, Vacanti JP, et al. Enhanced in vivo function of bioartificial lungs in rats. Ann Thorac Surg. 2011;92(3):998–1005; discussion -6. doi: 10.1016/j.athoracsur.2011.05.018
66. Gilpin SE, Ren X, Okamoto T, Guyette JP, Mou H, Rajagopal J, et al. Enhanced lung epithelial specification of human induced pluripotent stem cells on decellularized lung matrix. Ann Thorac Surg. 2014;98(5):1721–9; discussion 9. doi: 10.1016/j.athoracsur.2014.05.080
67. Obata T, Tsuchiya T, Akita S, Kawahara T, Matsumoto K, Miyazaki T, et al. Utilization of natural detergent potassium laurate for decellularization in lung bioengineering. Tissue Eng Part C Methods. 2019;25(8):459–71. doi: 10.1089/ten.tec.2019.0016
68. Jensen T, Roszell B, Zang F, Girard E, Matson A, Thrall R, et al. A rapid lung de-cellularization protocol supports embryonic stem cell differentiation in vitro and following implantation. Tissue Eng Part C Methods. 2012;18(8):632–46. doi: 10.1089/ten.tec.2011.0584
69. Nichols JE, Francesca SL, Niles JA, Vega SP, Argueta LB, Frank L, et al. Production and transplantation of bioengineered lung into a large-animal model. Sci Transl Med. 2018;10(452):eaao3926. doi: 10.1126/scitranslmed.aao3926
70. Yanagiya M, Kitano K, Yotsumoto T, Asahina H, Nagayama K, Nakajima J. Transplantation of bioengineered lungs created from recipient-derived cells into a large animal model. Semin Thorac Cardiovasc Surg. 2021;33(1):263–71. doi: 10.1053/j.semtcvs.2020.03.005
71. Zhou H, Kitano K, Ren X, Rajab TK, Wu M, Gilpin SE, et al. Bioengineering human lung grafts on porcine matrix. Ann Surg. 2018;267(3):590–8. doi: 10.1097/SLA.0000000000002129
72. Kitano K, Ohata K, Economopoulos KP, Gorman DE, Gilpin SE, Becerra DC, et al. Orthotopic transplantation of human bioartificial lung grafts in a porcine model: a feasibility study. Semin Thorac Cardiovasc Surg. 2022;34(2):752–9. doi: 10.1053/j.semtcvs.2021.03.006
73. United Therapeutics, 3D Systems Corporation. United Therapeutics Provides an Update on Its Organ Printing Programs [press release] (2022).
74. Fux T, Österholm C, Themudo R, Simonson O, Grinnemo K-H, Corbascio M. Synthetic tracheal grafts seeded with bone marrow cells fail to generate functional tracheae: First long-term follow-up study. J Thorac Cardiovasc Surg. (2020) 159(6):2525–37.e23. doi: 10.1016/j.jtcvs.2019.09.185
75. Adamo D, Galaverni G, Genna VG, Lococo F, Pellegrini G. The growing medical need for tracheal replacement: reconstructive strategies should overcome their limits. Front Bioeng Biotechnol. 2022;10:846632. doi: 10.3389/fbioe.2022.846632
76. Shin'oka T, Imai Y, Ikada Y. Transplantation of a tissue-engineered pulmonary artery. N Engl J Med. 2001;344(7):532–3. doi: 10.1056/NEJM200102153440717
77. Macchiarini P, Jungebluth P, Go T, Asnaghi MA, Rees LE, Cogan TA, et al. Clinical transplantation of a tissue-engineered airway. Lancet. 2008;372(9655):2023–30. doi: 10.1016/S0140-6736(08)61598-6
78. Jungebluth P, Haag JC, Sjöqvist S, Gustafsson Y, Rodríguez AB, Del Gaudio C, et al. Tracheal tissue engineering in rats. Nat Protoc. 2014;9(9):2164–79. doi: 10.1038/nprot.2014.149
79. Oh DK, Hong S-B, Shim TS, Kim DK, Choi S, Lee GD, et al. Effects of the duration of bridge to lung transplantation with extracorporeal membrane oxygenation. PLoS One. 2021;16(7):e0253520. doi: 10.1371/journal.pone.0253520
80. Langer F, Aliyev P, Schäfers H-J, Trudzinski FC, Seiler F, Bals R, et al. Improving outcomes in bridge-to-transplant: extended extracorporeal membrane oxygenation support to obtain optimal donor lungs for marginal recipients. ASAIO J. 2019;65(5):516–21. doi: 10.1097/MAT.0000000000000843
81. Conway RG, Douglas T, Bartley PG, Zhongjun JW. Extracorporeal membrane oxygenation (ECMO) for long-term support: recent advances. In: Michael SF, editor. Advances in extra-corporeal perfusion therapies. Rijeka: IntechOpen; 2018. p. Ch. 14.
82. Valapour M, Lehr CJ, Skeans MA, Smith JM, Miller E, Goff R, et al. OPTN/SRTR 2020 annual data report: lung. Am J Transplant. 2022;22(S2):438–518. doi: 10.1111/ajt.16991
83. Yeo HJ, Oh DK, Yu WS, Choi SM, Jeon K, Ha M, et al. Outcomes of patients on the lung transplantation waitlist in Korea: a Korean network for organ sharing data analysis. J Korean Med Sci. 2022;37(41):e294. doi: 10.3346/jkms.2022.37.e294
Keywords: lung transplant, tissue engineering, ex vivo lung perfusion, bioreactor, tissue scaffolds, extracorporeal membrane oxygenation
Citation: Hsiung T, James L, Chang SH, Geraci TC, Angel LF and Chan JCY (2023) Advances in lung bioengineering: Where we are, where we need to go, and how to get there. Front. Transplant. 2:1147595. doi: 10.3389/frtra.2023.1147595
Received: 18 January 2023; Accepted: 27 March 2023;
Published: 17 April 2023.
Edited by:
Varun Puri, Washington University in St. Louis, United StatesReviewed by:
Ibrahim Fathi, Nihon University, Japan© 2023 Hsiung, James, Chang, Geraci, Angel and Chan. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Justin C. Y. Chan justin.chan2@nyulangone.org
Specialty Section: This article was submitted to Thoracic Transplantation, a section of the journal Frontiers in Transplantation
Abbreviations CIT, cold ischemic time; CHAPS, sodium deoxycholate or 3-[(3-cholamidopropyl)dimethylammonio]-1-propanesulfonate; COPD, chronic obstructive pulmonary disease; DCD, donation after circulatory death; ECM, extracellular matrix; ECMO, extracorporeal membrane oxygenation; ESLD, end-stage lung disease; EVLP, ex-vivo lung perfusion; FBN-2, fibrillin 2; iPSC, induced pluripotent stem cell; IRI, ischemia reperfusion injury; PGLA, poly lactic-co-glycolic acid; PLLA, poly-L-lactic acid; POSS-PCA, polyhedral oligomeric silsesquioxane poly(carbonate-urea) urethane; TN-C, tenascin C; XC, cross circulation
 Tiffany Hsiung
Tiffany Hsiung Les James
Les James Stephanie H. Chang
Stephanie H. Chang Travis C. Geraci1,2
Travis C. Geraci1,2  Luis F. Angel
Luis F. Angel Justin C. Y. Chan
Justin C. Y. Chan