Gas-Phase Reactivity of OH Radicals With Ammonia (NH3) and Methylamine (CH3NH2) at Around 22 K
- 1Departamento de Química Física, Facultad de Ciencias y Tecnologías Químicas, Universidad de Castilla-La Mancha (UCLM), Ciudad Real, Spain
- 2Instituto de Investigación en Combustión y Contaminación Atmosférica, UCLM, Ciudad Real, Spain
- 3Institut de Physique de Rennes-CNRS—UMR 6251, Université de Rennes, Rennes, France
Interstellar molecules containing N atoms, such as ammonia (NH3) and methylamine (CH3NH2), could be potential precursors of amino acids like the simplest one, glycine (NH2CH2COOH). The gas-phase reactivity of these N-bearing species with OH radicals, ubiquitous in the interstellar medium, is not known at temperatures of cold dark molecular clouds. In this work, we present the first kinetic study of these OH-reactions at around 22 K and different gas densities [(3.4–16.7) × 1016 cm−3] in helium. The obtained rate coefficients, with ± 2σ uncertainties, can be included in pure gas-phase or gas-grain astrochemical models to interpret the observed abundances of NH3 and CH3NH2. We observed an increase of k1 and k2 with respect to those previously measured by others at the lowest temperatures for which rate coefficients are presently available: 230 and 299 K, respectively. This increase is about 380 times for NH3 and 20 times for CH3NH2. Although the OH + NH3 reaction is included in astrochemical kinetic databases, the recommended temperature dependence for k1 is based on kinetic studies at temperatures above 200 K. However, the OH + CH3NH2 reaction is not included in astrochemical networks. The observed increase in k1 at ca. 22 K does not significantly change the abundance of NH3 in a typical cold dark interstellar cloud. However, the inclusion of k2 at ca. 22 K, not considered in astrochemical networks, indicates that the contribution of this destruction route for CH3NH2 is not negligible, accounting for 1/3 of the assumed main depletion route (reaction with HCO+) in this IS environment.
Introduction
Unravelling the origin of life on the Earth has been both a challenge and a matter of debate for scientists throughout the history. However, what we can be certain about is that all ingredients essential for life are composed by a few atoms such as H, O, C, N, or S. The combination of these atoms can produce different prebiotic molecules, which are considered the precursors of life on our planet. Principally, two main theories have been proposed for trying to explain how these molecules could have appeared on the globe (Chyba and Sagan, 1992; Bernstein, 2006). The first one states that the organic molecules that serve as the basis of life were formed in the primitive atmosphere of our planet from simpler and smaller molecules (e.g., NH3, CH4, H2O, or H2) (Miller, 1953; Bada and Lazcano, 2002; Cleaves et al., 2008). In fact, this was demonstrated experimentally by Stanley Miller in the middle of the past century when he obtained a considerable number of important compounds from the biological point of view, such as amino acids, from simple molecules like the aforementioned, after exposing them to conditions aiming at mimicking those reigning in the primitive Earth (Miller, 1953). The second one is based on the idea that the prebiotic molecules were firstly synthesized in space and then, they could have been delivered to the Earth by meteorites, comets, asteroids or even interplanetary dust particles (Ehrenfreund et al., 2002; Sandford et al., 2020). This latter hypothesis is now in trend due to the huge and unexpected discovery in the last 70 years of the chemical richness in the interstellar medium (ISM). Currently, about 250 species (including ions and neutrals) have been detected in the ISM or circumstellar shells (Woon, 2021). Interstellar molecules, which are found in ultra-cold environments (∼10–100 K), such as the so-called dense or dark clouds or pre-stellar cores, range from simple diatomic molecules [e.g., CO or the hydroxyl (OH) radical] to more complex systems (e.g., fullerenes). For instance, the OH radical, firstly detected in Cassiopeia A in 1963 (Weinreb et al., 1963), is ubiquitous in the ISM. By definition, carbon-bearing species containing six atoms or more are called complex organic molecules (COMs) (Herbst and van Dishoeck, 2009). Some COMs containing C-O bonds (such as CH3OH) and C-N bonds (such as CH3CN) can be potential precursors of sugars and amino acids in the presence of water, respectively (Balucani, 2009), under the Earth’s conditions. Although ammonia (NH3) is not considered a COM, strictly by definition, this abundant nitrogen-bearing species is very important since it serves as temperature probe in molecular clouds like Sagittarius (Sgr) B2, where it was first detected (Cheung et al., 1968). Ammonia has also been found towards a post-star forming region, W3(OH), with an abundance relative to H2 of 10−8 (Wilson et al., 1993) and in TMC-1, with a column density of ∼ 1015 cm−2 (Freeman and Millar, 1983). Another interesting N-bearing species is methylamine (CH3NH2), which was detected in the 1970s years for the first time towards Sgr B2 and Orion A (Fourikis et al., 1974; Kaifu et al., 1974). Abundances relative to H2 for CH3NH2 have been observed to be 10−8 towards the hot core G10.47 + 0.33 (Ohishi et al., 2019) and 10−9 for Sgr B2 (Halfen et al., 2013). Both NH3 and CH3NH2 have been proposed as precursors of the simplest amino acid, glycine (NH2CH2COOH), through H-atom abstraction reactions forming NH2 (Sorrell, 2001; Garrod, 2013) and CH2NH2 radicals, (Woon, 2002; Garrod, 2013); however interstellar glycine remains undetected in the ISM so far.
Under the ISM conditions, it has been assumed since a long time (Herbst and Klemperer, 1973) that dissociative recombination of NH4+ is the main provider of NH3 in the gas phase. This cation can be generated through a series of hydrogen abstraction reactions starting from N+ + H2 leading step by step to NH+, NH2+, NH3+, and eventually NH4+ (Gerin et al., 2016; Rednyk et al., 2019). Alternatively, NH2+ can be produced by H3+ + N (Scott et al., 1997). Although not included in interstellar chemical networks, another potential source of NH3 was recently claimed by Gianturco et al. (2019) to be the reaction of the NH2− anion with H2. Surface reactions have also been proposed as plausible mechanisms to produce ammonia via a series of atomic hydrogen additions to N-hydrides after NH has been formed through N + H → NH (Jonusas et al., 2020). Regarding CH3NH2, different synthetic routes have been proposed. In the gas-phase, it may be formed via the radiative association between NH3 and the methyl radical cation (CH3+) followed by dissociative recombination (Herbst, 1985). But CH3NH2 can also be formed on grain surfaces by sequential hydrogenation of hydrogen cyanide (HCN), as experimentally observed by Theule et al. (2011). Simulations of the irradiation of CH4 and NH3 ices may also form CH3NH2 (Kim and Kaiser, 2011; Förstel et al., 2017), but also in cold and quiescent molecular clouds (Ioppolo et al., 2021). In the gas-grain chemical model by (Garrod et al., 2008), the CH3+NH2 reaction was also suggested as a source of CH3NH2 during warm-up phases.
It is also important to know how NH3 and CH3NH2 are being destroyed to have a good insight of the chemical evolution of the ISM. Focusing on neutral-neutral reactions, reaction networks that astrochemical models used, such as KIDA and UDfA, include eight depletion routes for NH3. e.g., reactions with H, CH, CN, among other radicals. Concerning CH3NH2, only two depleting reactions by CH and CH3 radicals are included in KIDA database, while UDfA database does not include any. Until now, the reactivity of NH3 with neutral radicals or atoms at ISM temperatures has been investigated experimentally in the presence of CN (Sims et al., 1994), CH (Bocherel et al., 1996), C2H (Nizamov and Leone, 2004) and C(3P) (Bourgalais et al., 2015; Hickson et al., 2015) whereas, only the reactivity of CH3NH2 with CN is documented (Sleiman et al., 2018a; 2018b). Due to the important role of OH radicals as a key intermediate in multiple reactive processes in the ISM (Cazaux et al., 2010; Goicoechea et al., 2011; Acharyya et al., 2015; Linnartz et al., 2015), the kinetic database for OH-molecule reactions has been widely extended in the past years (see e.g., Taylor et al., 2008; Smith and Barnes, 2013; Ocaña et al., 2017, 2019; Potapov et al., 2017; Heard, 2018; Blázquez et al., 2019, 2020). For the OH + NH3 reaction (Eq. R1), of interest in atmospheric and combustion chemistry, its gas-phase kinetics has been extensively studied both experimentally and theoretically.
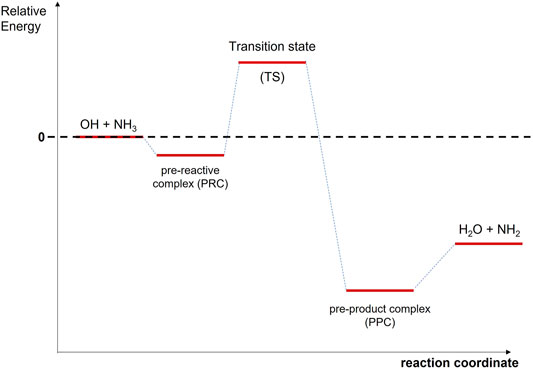
Note that other reaction channels forming H2NO + H2, HNOH + H2, or H2NOH + H are not thermodynamically accessible, since they are endothermic by Gibbs free energies ranging from 19.67 to 31.73 kcal/mol (Vahedpour et al., 2018). Eq. R1 is also of astrochemical interest since it leads to the formation of NH2 radicals, which were also observed towards the same location as ammonia (van Dishoeck et al., 1993). In the laboratory studies, the rate coefficient for R1, k1, has been reported since the 1970’s by many research groups over a wide range of temperature (230–2,360 K) and pressures (1–4,000 mbar) (Stuhl, 1973; Kurylo, 1973; Zellner and Smith, 1974; Hack et al., 1974; Perry et al., 1976; Silver and Kolb, 1980; Fujii et al., 1981, 1986; Salimian et al., 1984; Stephens, 1984; Zabielski and Seery, 1985; Jeffries and Smith, 1986; Diau et al., 1990). A summary of all previous experimental results can be found in Diau et al. (1990). The observed dependence of k1 with temperature is positive, i.e., the rate coefficient increases when temperature increases, and the reported activation energies range from 0.5 to 9 kcal/mol in the 230–2,360 K range (Zellner and Smith, 1974; Hack et al., 1974; Perry et al., 1976; Silver and Kolb, 1980; Fujii et al., 1981, 1986; Salimian et al., 1984; Stephens, 1984; Zabielski and Seery, 1985; Jeffries and Smith, 1986; Diau et al., 1990). The computed energy barriers in the 200–4,000 K range were found to be between 2.0 and 9.05 kcal/mol (Giménez et al., 1992; Corchado et al., 1995; Bowdridge et al., 1996; Nyman, 1996; Lynch et al., 2000; Monge-Palacios et al., 2013b; Nguyen and Stanton, 2017). The reaction mechanism of R1 was also theoretically investigated (Giménez et al., 1992; Espinosa-García and Corchado, 1994; Corchado et al., 1995; Bowdridge et al., 1996; Nyman, 1996; Lynch et al., 2000; Monge-Palacios et al., 2013b; Nguyen and Stanton, 2017). The formation of a pre-reactive complex (PRC) at the entrance channel is accepted, as illustrated in Scheme 1, and quantum mechanical tunneling has been reported to be an important contribution to k1, leading to the observed non-Arrhenius behavior (Espinosa-García and Corchado, 1994; Corchado et al., 1995; Bowdridge et al., 1996; Nyman, 1996; Monge-Palacios et al., 2013b; Nguyen and Stanton, 2017).
Regarding the OH + CH3NH2 reaction (Eq. R2), previous kinetic studies are restricted to temperatures higher than 295 K (Atkinson et al., 1977; Carl and Crowley, 1998; Onel et al., 2013; Butkovskaya and Setser, 2016). From the experimental point of view, Atkinson et al. (1977), Onel et al. (2013) reported the temperature dependence of reaction R2 in the 299–426 K and 298–600 K ranges, respectively. A negative temperature dependence of k2 was observed and this rate coefficient increases when temperature decreases.
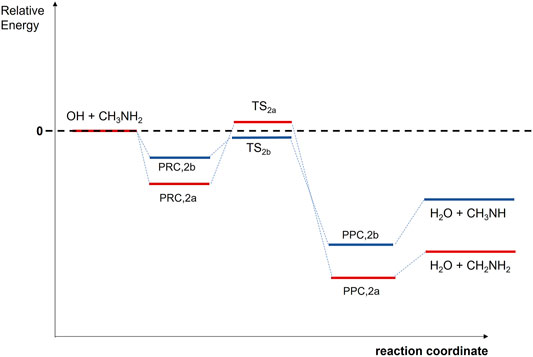
Eq. R2 may proceed by H-abstraction from methyl (−CH3) or amino (−NH2) groups forming CH2NH2 and CH3NH radicals plus water, respectively. The formation of CH2NH2 radicals was measured to be the main reaction channel at room temperature (Nielsen et al., 2011, 2012; Onel et al., 2014; Butkovskaya and Setser, 2016), in agreement with theoretical predictions at 299 K and above (Galano and Alvarez-Idaboy, 2008; Tian et al., 2009). These calculations suggest a stepwise mechanism involving the formation of a PRC at the entrance channels and an energy barrier of a few kcal/mol for the H-abstraction channel from −CH3 group, as depicted in Scheme 2.
As there are no kinetic data of k1 and k2 at temperatures of the cold dark interstellar clouds and since they are necessary to properly model the chemistry of the ISM, we present in this work the first determination of the rate coefficient for the reactions of NH3 and CH3NH2, ki (i = 1 or 2), with OH radicals at ca. 22 K using a combination of a pulsed CRESU (French acronym for Reaction Kinetics in a Uniform Supersonic Flow) reactor with laser techniques. The implications of the reported new rate coefficients will be discussed in terms of their effect on the predicted abundances of NH3 and CH3NH2 in a typical cold dark interstellar cloud at 10 K.
Experimental Methods
CRESU Apparatus Coupled to Pulsed Laser Photolysis-Laser Induced Fluorescence Technique
The experimental system based on the pulsed uniform supersonic expansion of a gas mixture has been already described elsewhere (Jiménez et al., 2015, 2016; Antiñolo et al., 2016; Canosa et al., 2016; Ocaña et al., 2017, 2018, 2019; Blázquez et al., 2019, 2020; Neeman et al., 2021). To carry out the kinetic experiments, three Laval nozzles (He23-HP, He23-IP, and He23-LP) were used with helium as a carrier gas. These nozzles were designed to generate a uniform flow at around 22 K for three different jet pressures (see Table 1) (Jiménez et al., 2015; Canosa et al., 2016; Ocaña et al., 2017), thus allowing us to explore the influence of pressure on the reactivity at a constant temperature. Bath gas and reactants (NH3 or CH3NH2) were introduced in the CRESU chamber through calibrated mass flow controllers, MFCs (Sierra Instruments, Inc., model Smart Trak, Smart-Trak 2, MicroTrak 101, and Smart-Trak 100). CH3NH2 was diluted in He and stored in a 20-L or 50-L bulb. Calibrated mass flow rates of these diluted mixtures ranged from 6.6 to 200 sccm (standard cubic centimeters per minute), depending on the Laval nozzle used. Dilution factor f ranged from 1.78 × 10−2 to 4.54 × 10−2. In contrast, NH3 was flown directly to the pre-expansion chamber (reservoir) from a gas cylinder through a MFC manufactured with anticorrosive materials (Sierra Instruments, Inc., model Smart-Trak 100). The flow rates of pure NH3 ranged from 3 to 15 sccm. The OH-precursor employed was H2O2, since it is a clean source of OH radicals. Gaseous H2O2 was introduced into the reservoir by bubbling the bath gas through an aqueous solution of H2O2, as explained in Jiménez et al. (2005). The flow rate of He through the H2O2 bubbler in different experiments ranged from 35.6 to 265.8 sccm, depending on the Laval nozzle used. Within a kinetic experiment, this flow rate was kept constant to maintain invariable the contribution of the OH loss due to the OH + H2O2 reaction (see Kinetic Analysis). The gas mixture formed by He (main flow), He/H2O2, and the reactant was pulsed by a two holes rotary disk described in Jiménez et al. (2015).
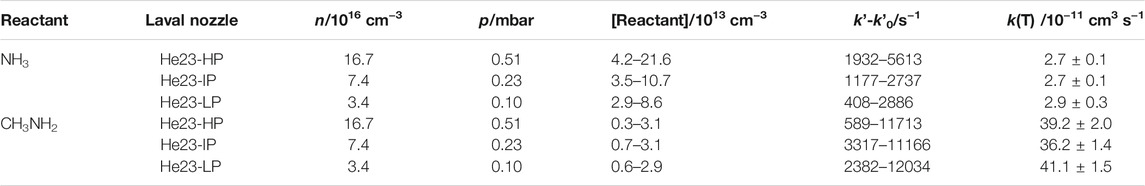
TABLE 1. Summary of the experimental conditions [total gas density (n), jet pressure (p), and reactant concentration in the jet] and corrected pseudo-first order rate coefficient ranges.
After the gas expansion through the Laval nozzle, the jet temperature was measured by a Pitot tube to be (21.7 ± 1.4) K for the He23-HP nozzle, (22.5 ± 0.7) K for the He23-IP nozzle, and (21.1 ± 0.6) K for the He-23LP nozzle, respectively. Within the fluctuation along the flow axis (± σ), the jet temperature in all cases is ca. 22 K. The procedure to determine the jet temperature and gas density has been previously described (Jiménez et al., 2015; Canosa et al., 2016; Ocaña et al., 2017). Pulsed photolysis of H2O2(g) at 248 nm was achieved by the radiation coming from a KrF excimer laser (Coherent, model ExciStar XS 200) with energies at the exit of the nozzle ranging from 0.5 to 0.9 mJ/pulse at 10 Hz, depending on the nozzle used. OH radicals were monitored by collecting the laser induced fluorescence (LIF) at 310 nm, after laser excitation at ca. 282 nm, as a function of the reaction time defined as the time delay between the probe laser pulse and the excimer one.
Kinetic Analysis
In Figure 1 an example of the temporal evolution of the LIF intensity (ILIF) is presented. As explained in several papers (Jiménez et al., 2015; Ocaña et al., 2018), the observed rise of the ILIF signal at t > 0 is due to rotational relaxation of OH, coming from H2O2 photodissociation, that occurs in a timescale (t0) of less than 30 μs under the conditions of the experiments, especially, at the concentration levels of the reactant, which is an effective quencher. The fit of the recorded ILIF profiles to an exponential decay (solid lines in Figure 1) confirms that the OH loss follows a pseudo-first order kinetics.
The pseudo-first order rate coefficient, k′, includes all the OH loss processes occurring simultaneously in the cold jet.
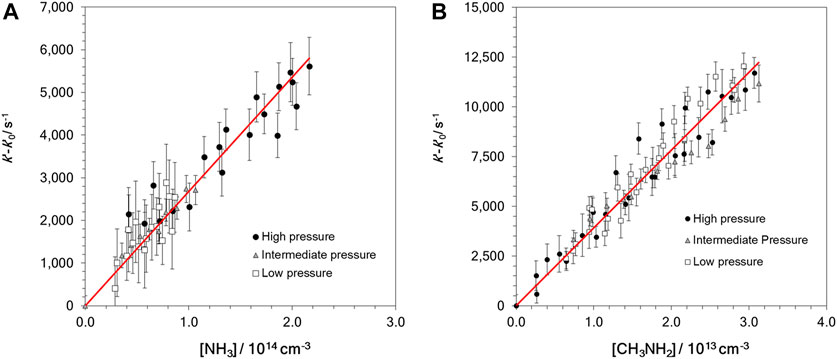
where ki (i = 1 or 2) is the bimolecular rate coefficients for OH-reactions with NH3 (R1) and CH3NH2 (R2). In the absence of reactant, k’0 was measured, and it included the loss of OH radicals by OH-reaction with H2O2 and other OH losses, such as diffusion out of the detection zone. In Table 1, the ranges of the employed reactant concentration, [Reactant], and the determined k′–k′0 values are summarized. According to Eq. E2, the slopes of the k’-k’0 versus [Reactant] plots yield the bimolecular rate coefficients ki. Nevertheless, the linear relationship between k’-k’0 and [Reactant] is not always accomplished, as shown in the example in Figure 2. In this figure, a downward curvature in the plot of k’-k’0 vs. [CH3NH2] was observed at concentrations higher than 3 × 1013 cm−3. As discussed in previous works (Ocaña et al., 2017, 2019; Blázquez et al., 2020; Neeman et al., 2021), this curvature may be the result of the dimerization of the OH-co reactant, CH3NH2 in this case. Considering the onset of dimerization, the red straight line in Figure 2 represents the fit to Eq. E2 at [CH3NH2] below ∼3 × 1013 cm−3. In contrast, for reaction R1 the plot of k’-k’0 vs. [NH3] is linear over the entire concentration range, as displayed in Figure 3A. Since this concentration range is much larger than the one for CH3NH2 and that the reactivity with OH is significantly slower for NH3 than for CH3NH2, it shows that the dimerization of NH3 is much less efficient than that of CH3NH2 at 22 K. In Figure 3B, all kinetic data obtained in the linear part of the k’-k’0 vs. [CH3NH2] plot are depicted. The bimolecular rate coefficient k2 at ca. 22 K is, then, obtained from the slope of such a plot.
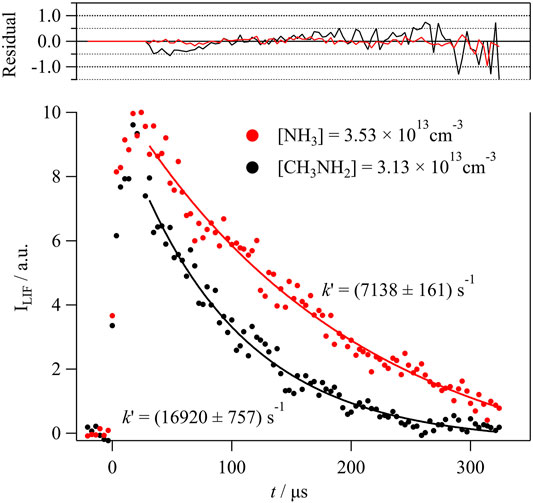
FIGURE 1. Examples of the ILIF temporal profile in the presence of similar concentrations of NH3 (A) and CH3NH2 (B) for a total gas density of 7.4 × 1016 cm−3.
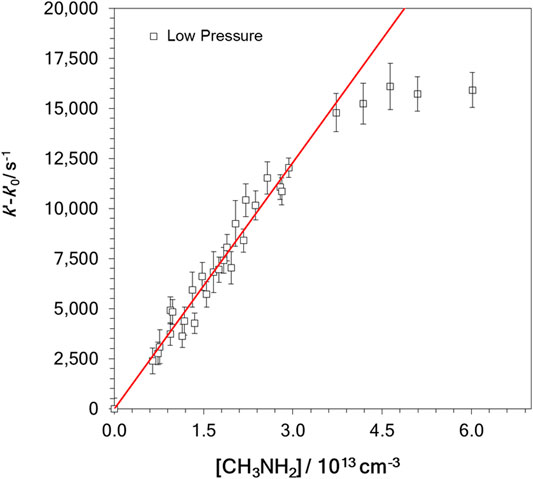
FIGURE 2. Example of the observed curvature in the bimolecular plots for the OH + CH3NH2 reaction at ca. 22 K and 3.4 × 1016 cm−3. Solid line represents k2 obtained from the fit of all data from Figure 3.
Reagents Gases: He (99.999%, Nippon gases), NH3 (≥ 99.95%, Merck) and CH3NH2 (≥ 99.0%, Merck) were used as supplied. Aqueous solution of H2O2 (Sharlab, initially at 50% w/w) was pre-concentrated as explained earlier (Albaladejo et al., 2003).
Results and Discussion
The OH + NH3 Reaction
Temperature Dependence of k1
A summary of the individual rate coefficients k1 obtained at 22 K and different total pressures of the gas jet is presented in Table 1. Within the stated statistical uncertainties (± 2σ), no pressure dependence of k1 was observed in the investigated range (0.10–0.51 mbar). For this reason, we combined all the kinetic results, as shown in Figure 3A The resulting rate coefficient for the OH + NH3 reaction at ca. 22 K is:
where the uncertainty (± 2σ) only includes statistical errors. An additional 10% uncertainty has to be added to account for the systematic errors.
As mentioned in the Introduction section, Eq. R1 has been investigated under extensive experimental conditions of temperature (230–2,360) K and pressure (1–4,000 mbar). A summary of all previous kinetic studies can be found in the most recent investigation from Diau et al. (1990). Focusing on the works carried out as a function of temperature (Zellner and Smith, 1974; Hack et al., 1974; Perry et al., 1976; Silver and Kolb, 1980; Fujii et al., 1981, 1986; Salimian et al., 1984; Stephens, 1984; Zabielski and Seery, 1985; Jeffries and Smith, 1986; Diau et al., 1990), a positive temperature dependence of k1 was observed in all cases, as shown in Figure 4A. For instance, IUPAC recommends a T-expression for k1, based on previous results below 450 K (Zellner and Smith, 1974; Perry et al., 1976; Silver and Kolb, 1980; Stephens, 1984; Diau et al., 1990), with an Ea/R factor of 925 K which yields an activation energy (Ea) of 1.8 kcal/mol (Atkinson et al., 2004):
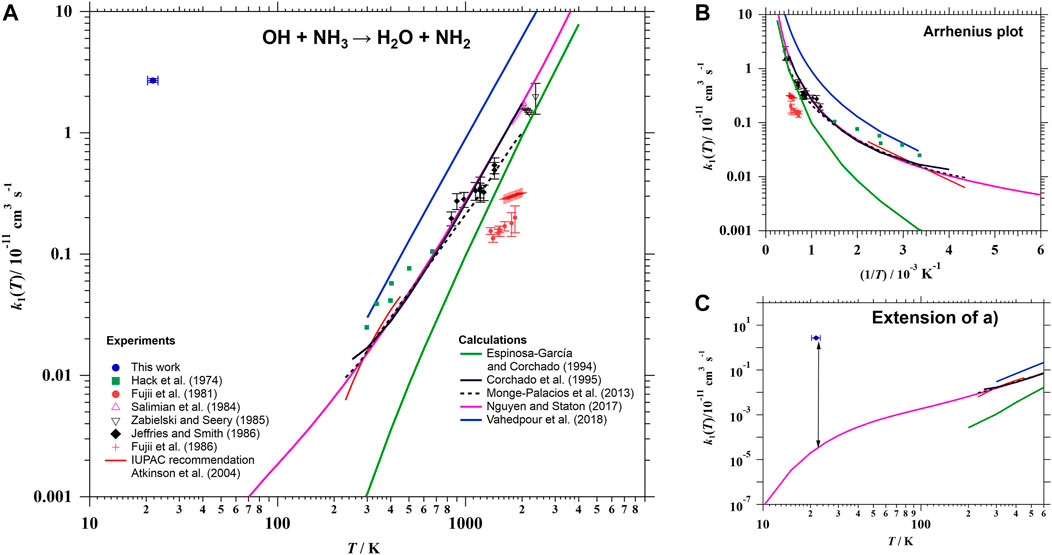
FIGURE 4. (A) Temperature dependence of k1 (log-log plot) in the 22–4,000 K range. Individual rate coefficients at room temperature K (Zellner and Smith, 1974; Perry et al., 1976; Silver and Kolb, 1980; Stephens, 1984; Diau et al., 1990) are not included in the figure, but they are considered in the IUPAC recommendation (red curve). (B) Arrhenius plot for k1 between 170 and 4,000 K to better see the curvature (non-Arrhenius behavior). (C) Extension of the log-log plot shown in (A) to show the great enhancement of k1 with respect to theoretical predictions at 20 K.
Even so, as hydrogen atom transfer reactions usually show significant dynamical quantum effects (see Reaction mechanism), the kinetics of reaction R1 deviates from the Arrhenius behavior, showing a curvature in the plot of ln k1 versus 1/T. This deviation from Arrhenius behavior has been observed experimentally between 840 and 1,425 K (Jeffries and Smith, 1986) and predicted theoretically between 5 and 4,000 K (Espinosa-García and Corchado, 1994; Corchado et al., 1995; Monge-Palacios et al., 2013b; Nguyen and Stanton, 2017; Vahedpour et al., 2018). Most of the computed k1 have been reported at temperatures higher than 200 K (Espinosa-García and Corchado, 1994; Corchado et al., 1995; Monge-Palacios et al., 2013b; Vahedpour et al., 2018). Only the calculations by Nguyen and Stanton (2017) were performed at lower temperatures and down to 5 K. For ease of presentation, kinetic data between 170 and 4,000 K are plotted in Figure 4B in Arrhenius form to clearly show the curvature. Down to 20 K, the predicted k1 by Nguyen and Stanton (2017), represented by a pink line in Figure 4C, was 2 × 10−16 cm3 s−1. This value is around five orders of magnitude lower than the experimental result reported in this work. Certainly, further kinetic studies between 230 and 22 K (and even below) are needed and are planned in our laboratory in the future.
Reaction Mechanism
As stated in the Introduction section, the reaction mechanism of the OH + NH3 reaction has been widely studied from a theoretical point of view. The effects of vibrational and translational energy of NH3 and OH counter partners have been studied by quasi-classical trajectories (Nyman, 1996; Monge-Palacios and Espinosa-Garcia, 2013; Monge-Palacios et al., 2013a) and by quantum scattering calculations (Nyman, 1996). Besides these dynamical studies, ab initio calculations based on the transition state theory (TST) were reported. In Scheme 1, a simplified illustration of the relative energies to the reactants of the stationary points along the minimum energy pathway (MEP) for the OH + NH3 system is shown. Some studies proposed that product formation in reaction R1 occurs from the transition state and reported energy barriers ranged from 2.03 kcal/mol to 8.94 kcal/mol (Giménez et al., 1992; Bowdridge et al., 1996; Lynch et al., 2000). However, other investigations proposed that reaction R1 occurs through a H-bonded PRC at the entrance channel (Corchado et al., 1995; Bowdridge et al., 1996; Monge-Palacios et al., 2013b; Nguyen and Stanton, 2017). The relative energy of this PRC is positioned a few kcal/mol above the reactants (Nguyen and Stanton, 2017) or −1.8 kcal/mol (Corchado et al., 1995; Monge-Palacios et al., 2013b). In addition to the PRC, a H-bonded complex near the products (PPC, pre-product complex) (Espinosa-García and Corchado, 1994; Corchado et al., 1995; Monge-Palacios et al., 2013b; Nguyen and Stanton, 2017) is proposed at the exit channel, which is stabilized with respect to the reactants by ca. −15 kcal/mol. Table 2 summarizes the relative energies of PRC, TS, and PPC from theoretical calculations reported in the literature.
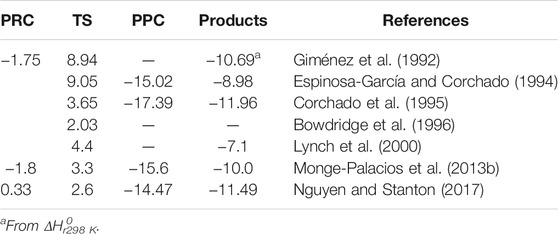
TABLE 2. Summary of the calculated energies relative to reactants (in kcal/mol) of the stationary points in the MEP for the OH + NH3 reaction reported in the literature (see text for more details).
Note that the reaction pathway for the OH + NH3 system is qualitatively similar to the one calculated for the OH + CH3OH reaction (Ocaña et al., 2019), i.e., formation of a H-bonded PRC followed by a transition state with a positive energy barrier. Following that comparison, the observed increase of k1 can be interpreted by the formation of a long-lived PRC at very low temperatures, which surpasses the energy barrier by quantum mechanical tunnelling.
Effect of k1 in the Abundance of Interstellar NH3
Astrochemical networks apply modified Arrhenius expressions to estimate rate coefficients in a certain temperature interval:
For example, KIDA database uses the recommended expression by Atkinson et al. (2004) (Eq. E3, where β = 0) and UDfA database uses the following expression:
Note that these recommended Eqs E3, E5 are valid on the stated temperature range. Using these T-expressions to extrapolate rate coefficients down to 22 K is extremely risky. One gets very low values of k1, on the order of 10−27 cm3 s−1 from KIDA expression Eq. E3 and 10−16 cm3 s−1 from UDfA Eq. E5, compared with the rate coefficient for the OH + NH3 reaction determined in the present work. Using Eqs E3 and E5, the rate coefficient at 230 K, k1(230 K), and extrapolated k1(200 K) are 6.4 and 8.3 × 10−14 cm3 s−1, respectively. At the lowest temperature, the k1(∼22 K)/k1(230 K) ratio is around 380. This value means that the rate of formation of NH2 radicals from the reaction of NH3 with OH radicals is really enhanced by more than two orders of magnitude at the typical temperature of a cold dark cloud. Using k1, obtained in this work, and the rate coefficients from the KIDA and UDfA databases, for a typical cold dark cloud, with H2 molecular density of 1 × 104 cm−3 and a temperature of 10 K (close to the one reproduced in this work) (Agúndez and Wakelam, 2013), the change in modelled abundances of NH3 is negligible for both reaction networks. The main destruction route for NH3 in this cold environment is the reaction with H3O+ cations, being the reaction of NH3 with OH radicals around 1% of that with H3O+ species.
The OH + CH3NH2 Reaction
Temperature Dependence of k2
As shown in Table 1, no pressure dependence of the rate coefficient for the OH + CH3NH2 reaction was observed in the investigated range. The resulting k2 at ca. 22 K from the combination of all kinetic data at different gas densities (see Figure 3B) is:
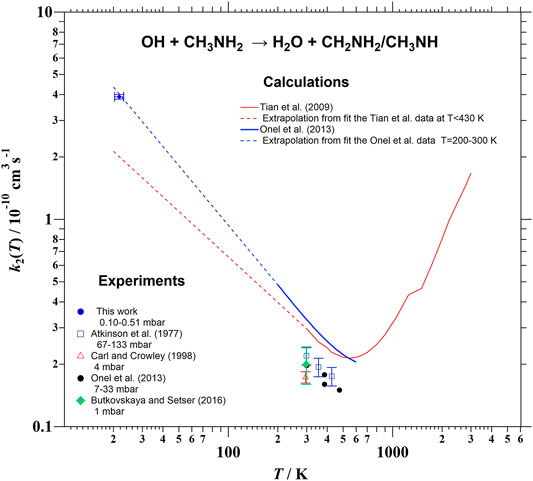
This value together with previously reported k2 over the 298–3,000 K temperature range (Atkinson et al., 1977; Carl and Crowley, 1998; Tian et al., 2009; Onel et al., 2013; Butkovskaya and Setser, 2016) are depicted in Figure 5. The temperature dependence of k2 was first measured by Atkinson et al. (1977) at T > 299 K, who reported the following Arrhenius expression:
The activation energy is slightly negative in this case, −0.45 kcal/mol; however more recently, the negative temperature dependence observed experimentally for Eq. R2 was also reported by Onel et al. (2013) as an expression with no activation energy and a T-dependent pre-exponential factor (black circles in Figure 5):
Using the experimental Onel’s expression, the extrapolated k2(∼22 K) is ca. 8 × 10−11 cm3 s−1 (4.9 times lower than the experimental one reported here), while Eq. E6 provides an extrapolated k2(∼22 K) of 3.4 × 10−7 cm3 s−1, which is a non-realistic value for a neutral-neutral reaction. Onel et al. (2013) also computed k2 between 200 and 500 K (blue line in Figure 5) to be around 60% higher than the experimental values. No experimental kinetic data have been reported at T > 500 K, however Tian et al. (2009) predicted a minimum of k2 around 550 K and a remarkable increase of k2 at higher temperatures, i.e., the rate coefficient for the OH + CH3NH2 reaction is expected to exhibit a non-Arrhenius behavior. As shown by dashed lines in Figure 5, the extrapolated k2 from calculations of Onel et al. (2013) is in excellent agreement with our reported value and the value of k2 obtained by extrapolation of Tian et al. (2009) data is only a factor of ca. 2 lower than the experimental value reported in this work. Additional kinetic studies between 298 and 22 K are clearly needed to confirm the expected trend in the T-dependence of k2.
Reaction Mechanism
The possible exothermic reaction channels for Eq. R2 are the H-abstraction from the methyl group (Eq. R2a) or from the amino group (Eq. R2b):
Theoretically, the mechanism of Eq. R2 has been investigated by several groups (Galano and Alvarez-Idaboy, 2008; Tian et al., 2009; Onel et al., 2013; Borduas et al., 2016). The calculations suggest a stepwise mechanism involving the formation of a PRC in the entrance channels (Tian et al., 2009; Onel et al., 2013; Borduas et al., 2016) and a PPC in the exit channels (Tian et al., 2009; Onel et al., 2013). On the other hand, the OH-addition to N and subsequent concerted C-C cleavage, which produces CH3 radicals and NH2OH, is endothermic with an energy barrier of 37.4 kcal/mol (Borduas et al., 2016). For that reason, in Scheme 2, the energies of the stationary points along the MEP for the OH + CH3NH2 reaction is only depicted for the exothermic channels R2a and R2b. The relative energies of PRC, TS, and PPC for reaction pathways R2a and R2c reported in theoretical calculations are summarized in Table 3 (Galano and Alvarez-Idaboy, 2008; Tian et al., 2009; Onel et al., 2013); Borduas et al., 2016). In the 299–3,000 K temperature range, the dominant exothermic channel is that producing CH2NH2 radicals (Galano and Alvarez-Idaboy, 2008; Tian et al., 2009). The branching ratio for R2a (r) at 298 K was reported to be of 0.797 (Galano and Alvarez-Idaboy, 2008) and 0.74 (Tian et al., 2009). In contrast, Borduas et al. (2016) concluded that channels R2a and R2b are competitive, with energy barriers close to the entrance level energies of the reactants and within 1 kcal mol−1 of each other. Nevertheless, the dominance of R2a channel at room temperature has experimentally been confirmed by Nielsen et al. (2011, 2012), Onel et al. (2014), Butkovskaya and Setser (2016). Nielsen et al. (2011, 2012) performed experiments in EUPHORE atmospheric chamber providing a r of (0.75 ± 0.05) for channel R2a. A similar value (0.79 ± 0.15) was found by Onel et al. (2014) and by Butkovskaya and Setser (2016) (0.74 ± 0.05) No measurements or calculations were found at temperatures below room temperature. Thus, further theoretical and/or experimental studies are needed to know what the branching ratios of channels R2a (forming CH2NH2) and R2b (forming CH3NH) are at interstellar temperatures.
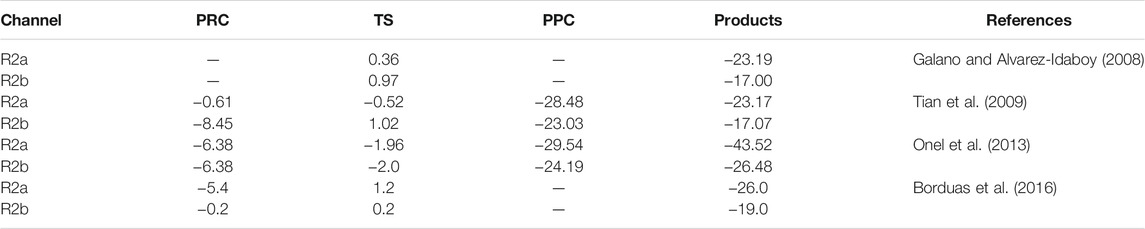
TABLE 3. Summary of the calculated relative (to reactants) energies (in kcal/mol) of the stationary points in the MEP for the OH + CH3NH2 reaction reported in the literature (see text for more details).
Effect of k2 in the Abundance of Interstellar CH3NH2
Neither KIDA database nor UDfA network include this OH-reaction as a potential destruction route for CH3NH2. However, this reaction is extremely fast at ca. 22 K as it is shown by the experimental rate coefficient for the OH + CH3NH2 reaction reported here. Our measured k2(∼22 K) would, then, lead to a quicker depletion of CH3NH2 by reaction with OH in astrochemical models. The k2(∼22 K)/k2(298 K) ratio is around 20, indicating that the use of k2(298 K) in modelling the ISM would underestimate the loss of CH3NH2 by OH by more than one order of magnitude. Using the reported k2(∼22 K) in the pure gas-phase model from Agúndez and Wakelam (2013), the destruction of CH3NH2 by OH radicals in a typical cold dark cloud (H2 molecular density of 1 × 104 cm−3 and temperature 10 K) supposes around 1/3 of that initiated by HCO+ (main depletion route).
Conclusion
The OH-reactivity of NH3 at ca. 22 K is more than two orders of magnitude higher than that observed at the lowest temperature achieved up to now, 230 K. This confirms that the observed curvature in the Arrhenius plot reflects the increase of k1 at low temperatures. Further studies are needed to complete the kinetic behavior between 22 and 230 K and lower temperatures than 22 K. For CH3NH2, the determined rate coefficient at ca. 22 K is almost 20 times higher than the one measured at room temperature. The slightly negative temperature dependence of k2 observed by Atkinson et al. (1977) at T > 298 K, implies that the OH-reactivity increases at temperatures lower than room temperature, as observed in this work. These new experimental data indicate that the inclusion of the rate coefficient for the OH + NH3 reaction at 20 K in gas-phase astrochemical models does not significantly change the abundance of NH3 in a typical cold dark cloud since the main destruction route for NH3 is the reaction with H3O+. However, the inclusion of the rate coefficient for the OH + CH3NH2 reaction at 20 K, not considered in KIDA and UDfA networks, indicated that the contribution of this destruction route is not negligible, accounting for 1/3 of the main assumed depletion route (reaction with HCO+) in this IS environment with temperature close to 10 K.
Data Availability Statement
The raw data supporting the conclusion of this article will be made available by the authors, without undue reservation.
Author Contributions
DG performed the experiments, analyzed the kinetic data, and wrote the draft of the article; BB and EJ contributed to the design and supervision of the experiments; AC and EJ participated in the critical revision of the article; JA and EJ got the funds for carrying out this research and managed the projects. All authors discussed the results and contributed to the final manuscript.
Funding
This work has been supported by the Spanish Ministry of Science and Innovation (MICINN) through the CHEMLIFE project (Ref: PID2020-113936GB-I00), the regional government of Castilla-La Mancha through CINEMOL project (Ref: SBPLY/19/180501/000052) and by the University of Castilla-La Mancha—UCLM (Ayudas para la financiación de actividades de investigación dirigidas a grupos (REF: 2020-GRIN-29016 and 2021-GRIN-31279). DG also acknowledges UCLM (Plan Propio de Investigación) for funding his contract during the performance of this investigation.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Acknowledgments
The authors acknowledge Francisco J. Maigler for his technical assistance during the performance of these experiments and Dr. Marcelino Agúndez for helpful discussion on the effect of current rate coefficients on the abundance of NH3 and CH3NH2 in a typical cold dark cloud.
References
Acharyya, K., Herbst, E., Caravan, R. L., Shannon, R. J., Blitz, M. A., and Heard, D. E. (2015). The Importance of OH Radical-Neutral Low Temperature Tunnelling Reactions in Interstellar Clouds Using a New Model. Mol. Phys. 113, 2243–2254. doi:10.1080/00268976.2015.1021729
Agúndez, M., and Wakelam, V. (2013). Chemistry of Dark Clouds: Databases, Networks, and Models. Chem. Rev. 113, 8710–8737. doi:10.1021/cr4001176
Albaladejo, J., Ballesteros, B., Jiménez, E., Díaz de Mera, Y., and Martínez, E. (2003). Gas-phase OH Radical-Initiated Oxidation of the 3-halopropenes Studied by PLP-LIF in the Temperature Range 228-388 K. Atmos. Environ. 37, 2919–2926. doi:10.1016/S1352-2310(03)00297-8
Antiñolo, M., Agúndez, M., Jiménez, E., Ballesteros, B., Canosa, A., Dib, G. E., et al. (2016). Reactivity of OH and CH3OH between 22 and 64 K: Modeling the Gas Phase Production of CH3O in Barnard 1b. ApJ 823, 25. doi:10.3847/0004-637X/823/1/25
A. Silver, J., and E. Kolb, C. (1980). Rate Constant for the Reaction NH3 + OH → NH2 + H2O over a Wide Temperature Range. Chem. Phys. Lett. 75, 191–195. doi:10.1016/0009-2614(80)80492-1
Atkinson, R., Baulch, D. L., Cox, R. A., Crowley, J. N., Hampson, R. F., Hynes, R. G., et al. (2004). Evaluated Kinetic and Photochemical Data for Atmospheric Chemistry: Part 1 – Gas Phase Reactions of Ox, HOx, NOx and SOx Species. Atmos. Chem. Phys. 4, 1461–1738. doi:10.5194/acpd-3-6179-2003
Atkinson, R., Perry, R. A., and Pitts, J. N. (1977). Rate Constants for the Reaction of the OH Radical with CH3SH and CH3NH2 over the Temperature Range 299-426 °K. J. Chem. Phys. 66, 1578–1581. doi:10.1063/1.434076
Bada, J. L., and Lazcano, A. (2002). Some like it Hot, but Not the First Biomolecules. Science 296, 1982–1983. doi:10.1126/science.1069487
Balucani, N. (2009). Elementary Reactions and Their Role in Gas-phase Prebiotic Chemistry. Int. J. Mol. Sci. 10, 2304–2335. doi:10.3390/ijms10052304
Bernstein, M. (2006). Prebiotic Materials from on and off the Early Earth. Phil. Trans. R. Soc. B 361, 1689–1702. doi:10.1098/rstb.2006.1913
Blázquez, S., González, D., García-Sáez, A., Antiñolo, M., Bergeat, A., Caralp, F., et al. (2019). Experimental and Theoretical Investigation on the OH + CH3C(O)CH3 Reaction at Interstellar Temperatures (T = 11.7-64.4 K). ACS Earth Space Chem. 3, 1873–1883. doi:10.1021/acsearthspacechem.9b00144
Blázquez, S., González, D., Neeman, E. M., Ballesteros, B., Agúndez, M., Canosa, A., et al. (2020). Gas-phase Kinetics of CH3CHO with OH Radicals between 11.7 and 177.5 K. Phys. Chem. Chem. Phys. 22, 20562–20572. doi:10.1039/D0CP03203D
Bocherel, P., Herbert, L. B., Rowe, B. R., Sims, I. R., Smith, I. W. M., and Travers, D. (1996). Ultralow-Temperature Kinetics of CH(X2Π) Reactions: Rate Coefficients for Reactions with O2 and NO (T = 13−708 K), and with NH3 (T = 23−295 K). J. Phys. Chem. 100, 3063–3069. doi:10.1021/jp952628f
Borduas, N., Abbatt, J. P. D., Murphy, J. G., So, S., and Da Silva, G. (2016). Gas-Phase Mechanisms of the Reactions of Reduced Organic Nitrogen Compounds with OH Radicals. Environ. Sci. Technol. 50, 11723–11734. doi:10.1021/acs.est.6b03797
Bourgalais, J., Capron, M., Kailasanathan, R. K. A., Osborn, D. L., Hickson, K. M., Loison, J.-C., et al. (2015). The C(3P) + NH3 Reaction in Interstellar Chemistry. I. Investigation of the Product Formation Channels. ApJ 812, 106–140. doi:10.1088/0004-637X/812/2/106
Bowdridge, M., Furue, H., and Pacey, P. D. (1996). Properties of Transition Species in the Reactions of Hydroxyl with Ammonia and with Itself. J. Phys. Chem. 100, 1676–1681. doi:10.1021/jp9522573
Butkovskaya, N. I., and Setser, D. W. (2016). Branching Ratios and Vibrational Distributions in Water-Forming Reactions of OH and OD Radicals with Methylamines. J. Phys. Chem. A. 120, 6698–6711. doi:10.1021/acs.jpca.6b06411
Canosa, A., Ocaña, A. J., Antiñolo, M., Ballesteros, B., Jiménez, E., and Albaladejo, J. (2016). Design and testing of temperature tunable de Laval nozzles for applications in gas-phase reaction kinetics. Exp. Fluids 57. doi:10.1007/s00348-016-2238-1
Carl, S. A., and Crowley, J. N. (1998). Sequential Two (Blue) Photon Absorption by NO2 in the Presence of H2 as a Source of OH in Pulsed Photolysis Kinetic Studies: Rate Constants for Reaction of OH with CH3NH2, (CH3)2NH, (CH3)3N, and C2H5NH2 at 295 K. J. Phys. Chem. A. 102, 8131–8141. doi:10.1021/jp9821937
Cazaux, S., Cobut, V., Marseille, M., Spaans, M., and Caselli, P. (2010). Water Formation on Bare Grains: When the Chemistry on Dust Impacts Interstellar Gas. A&A 522, A74. doi:10.1051/0004-6361/201014026
Cheung, A. C., Rank, D. M., Townes, C. H., Thornton, D. D., and Welch, W. J. (1968). Detection of NH3Molecules in the Interstellar Medium by Their Microwave Emission. Phys. Rev. Lett. 21, 1701–1705. doi:10.1103/PhysRevLett.21.1701
Chyba, C., and Sagan, C. (1992). Endogenous Production, Exogenous Delivery and Impact-Shock Synthesis of Organic Molecules: an Inventory for the Origins of Life. Nature 355, 125–132. doi:10.1038/355125a0
Cleaves, H. J., Chalmers, J. H., Lazcano, A., Miller, S. L., and Bada, J. L. (2008). A Reassessment of Prebiotic Organic Synthesis in Neutral Planetary Atmospheres. Orig. Life Evol. Biosph. 38, 105–115. doi:10.1007/s11084-007-9120-3
Corchado, J. C., Espinosa-Garcia, J., Hu, W.-P., Rossi, I., and Truhlar, D. G. (1995). Dual-Level Reaction-Path Dynamics (The ///Approach to VTST with Semiclassical Tunneling). Application to OH + NH3 → H3O + NH2. J. Phys. Chem. 99, 687–694. doi:10.1021/j100002a037
Diau, E. W. G., Tso, T. L., and Lee, Y. P. (1990). Kinetics of the Reaction Hydroxyl + Ammonia in the Range 273-433 K. J. Phys. Chem. 94, 5261–5265. doi:10.1021/j100376a018
Ehrenfreund, P., Irvine, W., Becker, L., Blank, J., Brucato, J. R., Colangeli, L., et al. (2002). Astrophysical and Astrochemical Insights into the Origin of Life. Rep. Prog. Phys. 65, 1427–1487. doi:10.1088/0034-4885/65/10/202
Espinosa‐García, J., and Corchado, J. C. (1994). Analysis of Certain Factors in the Direct Dynamics Method: Variational Rate Constant of the NH3+OH→NH2+H2O Reaction. J. Chem. Phys. 101, 8700–8708. doi:10.1063/1.468065
Förstel, M., Bergantini, A., Maksyutenko, P., Góbi, S., and Kaiser, R. I. (2017). Formation of Methylamine and Ethylamine in Extraterrestrial Ices and Their Role as Fundamental Building Blocks of Proteinogenicα-Amino Acids. ApJ 845, 83. doi:10.3847/1538-4357/aa7edd
Fourikis, N., Takagi, K., and Morimoto, M. (1974). Detection of Interstellar Methylamine by its 2_{02}->1_{10} A_{a^{-}} State Transition. ApJ 191, L139. doi:10.1086/181570
Freeman, A., and Millar, T. J. (1983). Formation of Complex Molecules in TMC-1. Nature 301, 402–404. doi:10.1038/301402a0
Fujii, N., Chiba, K., Uchida, S., and Miyama, H. (1986). The Rate Constants of the Elementary Reactions of NH3 with O and OH. Chem. Phys. Lett. 127, 141–144. doi:10.1016/S0009-2614(86)80243-3
Fujii, N., Miyama, H., and Asaba, T. (1981). Determination of the Rate Constant for the Reaction NH3 + OH → NH2 + H2O. Chem. Phys. Lett. 80, 355–357. doi:10.1016/0009-2614(81)80125-X
Galano, A., and Alvarez-Idaboy, J. R. (2008). Branching Ratios of Aliphatic Amines + OH Gas-phase Reactions: A Variational Transition-State Theory Study. J. Chem. Theor. Comput. 4, 322–327. doi:10.1021/ct7002786
Garrod, R. T. (2013). A Three-phase Chemical Model of Hot Cores: The Formation of Glycine. ApJ 765, 60. doi:10.1088/0004-637X/765/1/60
Garrod, R. T., Weaver, S. L. W., and Herbst, E. (2008). Complex Chemistry in Star‐forming Regions: An Expanded Gas‐Grain Warm‐up Chemical Model. ApJ 682, 283–302. doi:10.1086/588035
Gerin, M., Neufeld, D. A., and Goicoechea, J. R. (2016). Interstellar Hydrides. Annu. Rev. Astron. Astrophys. 54, 181–225. doi:10.1146/annurev-astro-081915-023409
Gianturco, F. A., Yurtsever, E., Satta, M., and Wester, R. (2019). Modeling Ionic Reactions at Interstellar Temperatures: The Case of NH2- + H2 ⇔ NH3 + H-. J. Phys. Chem. A. 123, 9905–9918. doi:10.1021/acs.jpca.9b07317
Giménez, X., Moreno, M., and Lluch, J. M. (1992). Ab Initio study of the NH3 + OH Reaction. Chem. Phys. 165, 41–46. doi:10.1016/0301-0104(92)80041-S
Goicoechea, J. R., Joblin, C., Contursi, A., Berné, O., Cernicharo, J., Gerin, M., et al. (2011). OH Emission from Warm and Dense Gas in the Orion Bar PDR. A&A 530, L16. doi:10.1051/0004-6361/201116977
Hack, W., Hoyerman, K., and Wagner, H. G. (1974). Reaktionen des Hydroxylradikals mit Ammoniak und Hydrazin in der Gasphase. Ber.Bunsenges. Phys. Chem. Chem. Phys. 78, 386–391.
Halfen, D. T., Ilyushin, V. V., and Ziurys, L. M. (2013). Insights into Surface Hydrogenation in the Interstellar Medium: Observations of Methanimine and Methyl Amine in Sgr B2(N). ApJ 767, 66. doi:10.1088/0004-637X/767/1/66
Heard, D. E. (2018). Rapid Acceleration of Hydrogen Atom Abstraction Reactions of OH at Very Low Temperatures through Weakly Bound Complexes and Tunneling. Acc. Chem. Res. 51, 2620–2627. doi:10.1021/acs.accounts.8b00304
Herbst, E., and Klemperer, W. (1973). The Formation and Depletion of Molecules in Dense Interstellar Clouds. ApJ 185, 505–533. doi:10.1086/152436
Herbst, E. (1985). The Rate of the Radiative Association Reaction between CH3(+) and NH3 and its Implications for Interstellar Chemistry. ApJ 292, 484–486. doi:10.1086/163179
Herbst, E., and van Dishoeck, E. F. (2009). Complex Organic Interstellar Molecules. Annu. Rev. Astron. Astrophys. 47, 427–480. doi:10.1146/annurev-astro-082708-101654
Hickson, K. M., Loison, J.-C., Bourgalais, J., Capron, M., Picard, S. D. L., Goulay, F., et al. (2015). The C(3P) + NH3 Reaction in Interstellar Chemistry. Ii. Low Temperature Rate Constants and Modeling of Nh, Nh2, and Nh3Abundances in Dense Interstellar Clouds. ApJ 812, 107–125. doi:10.1088/0004-637X/812/2/107
Ioppolo, S., Fedoseev, G., Chuang, K.-J., Cuppen, H. M., Clements, A. R., Jin, M., et al. (2021). A Non-energetic Mechanism for glycine Formation in the Interstellar Medium. Nat. Astron. 5, 197–205. doi:10.1038/s41550-020-01249-0
Jeffries, J. B., and Smith, G. P. (1986). Kinetics of the Reaction Hydroxyl + Ammonia. J. Phys. Chem. 90, 487–491. doi:10.1021/j100275a027
Jiménez, E., Antiñolo, M., Ballesteros, B., Canosa, A., and Albaladejo, J. (2016). First Evidence of the Dramatic Enhancement of the Reactivity of Methyl Formate (HC(O)OCH3) with OH at Temperatures of the Interstellar Medium: a Gas-phase Kinetic Study between 22 K and 64 K. Phys. Chem. Chem. Phys. 18, 2183–2191. doi:10.1039/C5CP06369H
Jiménez, E., Ballesteros, B., Canosa, A., Townsend, T. M., Maigler, F. J., Napal, V., et al. (2015). Development of a Pulsed Uniform Supersonic Gas Expansion System Based on an Aerodynamic Chopper for Gas Phase Reaction Kinetic Studies at Ultra-low Temperatures. Rev. Scientific Instr. 86, 045108. doi:10.1063/1.4918529
Jiménez, E., Lanza, B., Garzón, A., Ballesteros, B., and Albaladejo, J. (2005). Atmospheric Degradation of 2-butanol, 2-Methyl-2-Butanol, and 2,3-Dimethyl-2-Butanol: OH Kinetics and UV Absorption Cross Sections. J. Phys. Chem. A. 109, 10903–10909. doi:10.1021/jp054094g
Jonusas, M., Leroux, K., and Krim, L. (2020). N + H Surface Reaction under Interstellar Conditions: Does the NH/NH2/NH3 Distribution Depend on N/H Ratio? J. Mol. Struct. 1220, 128736. doi:10.1016/j.molstruc.2020.128736
Kaifu, N., Morimoto, M., Nagane, K., Akabane, K., Iguchi, T., and Takagi, K. (1974). Detection of Interstellar Methylamine. ApJ 191, L135. doi:10.1086/181569
Kim, Y. S., and Kaiser, R. I. (2011). On the Formation of Amines (RNH2) and the Cyanide Anion (Cn−) in Electron-Irradiated Ammonia-Hydrocarbon Interstellar Model Ices. ApJ 729, 68. doi:10.1088/0004-637X/729/1/68
Kurylo, M. J. (1973). Kinetics of the Reactions OH (V = O) + NH3 →; H2O + NH2 and OH(v = O) + O3 → HO2 + O2 at 298°K. Chem. Phys. Lett. 23, 467–471. doi:10.1016/0009-2614(73)89003-7
Linnartz, H., Ioppolo, S., and Fedoseev, G. (2015). Atom Addition Reactions in Interstellar Ice Analogues. Int. Rev. Phys. Chem. 34, 205–237. doi:10.1080/0144235X.2015.1046679
Lynch, B. J., Fast, P. L., Harris, M., and Truhlar, D. G. (2000). Adiabatic Connection for Kinetics. J. Phys. Chem. A. 104, 4811–4815. doi:10.1021/jp000497z
Miller, S. L. (1953). A Production of Amino Acids under Possible Primitive Earth Conditions. Science 117, 528–529. doi:10.1126/science.117.3046.528
Monge-Palacios, M., Corchado, J. C., and Espinosa-Garcia, J. (2013a). Dynamics Study of the OH + NH3 Hydrogen Abstraction Reaction Using QCT Calculations Based on an Analytical Potential Energy Surface. J. Chem. Phys. 138, 214306–214312. doi:10.1063/1.4808109
Monge-Palacios, M., and Espinosa-Garcia, J. (2013). Role of Vibrational and Translational Energy in the OH + NH3 Reaction: A Quasi-Classical Trajectory Study. J. Phys. Chem. A. 117, 5042–5051. doi:10.1021/jp403571y
Monge-Palacios, M., Rangel, C., and Espinosa-Garcia, J. (2013b). Ab Initiobased Potential Energy Surface and Kinetics Study of the OH + NH3 hydrogen Abstraction Reaction. J. Chem. Phys. 138, 084305. doi:10.1063/1.4792719
Neeman, E. M., González, D., Blázquez, S., Ballesteros, B., Canosa, A., Antiñolo, M., et al. (2021). The Impact of Water Vapor on the OH Reactivity toward CH3CHO at Ultra-low Temperatures (21.7-135.0 K): Experiments and Theory. J. Chem. Phys. 155, 034306. doi:10.1063/5.0054859
Nguyen, T. L., and Stanton, J. F. (2017). High-level Theoretical Study of the Reaction between Hydroxyl and Ammonia: Accurate Rate Constants from 200 to 2500 K. J. Chem. Phys. 147, 152704. doi:10.1063/1.4986151
Nielsen, C., D’Anna, B., Aursnes, M., and Boreave, A. (2012). Summary Report from Atmospheric Chemistry Studies of Amines, Nitrosamines, Nitramines and Amides; Climit Project No. 208122. Oslo: NILU, University of Oslo.
Nielsen, C. J., D’Anna, B., Karl, M., Aursnes, M., Boreave, A., Bossi, R., et al. (2011). Summary Report: Photo-Oxidation of Methylamine, Dimethylamine and Trimetahylamine. Climit Project No. 201604NILU 2. Oslo: University of Oslo.
Nizamov, B., and Leone, S. R. (2004). Rate Coefficients and Kinetic Isotope Effect for the C2H Reactions with NH3 and ND3 in the 104−294 K Temperature Range. J. Phys. Chem. A. 108, 3766–3771. doi:10.1021/jp031361e
Nyman, G. (1996). Quantum Scattering Calculations on the NH3+OH→NH2+H2O Reaction. J. Chem. Phys. 104, 6154–6167. doi:10.1063/1.471281
Ocaña, A. J., Blázquez, S., Ballesteros, B., Canosa, A., Antiñolo, M., Albaladejo, J., et al. (2018). Gas Phase Kinetics of the OH + CH3CH2OH Reaction at Temperatures of the Interstellar Medium (T = 21-107 K). Phys. Chem. Chem. Phys. 20, 5865–5873. doi:10.1039/C7CP07868D
Ocaña, A. J., Blázquez, S., Potapov, A., Ballesteros, B., Canosa, A., Antiñolo, M., et al. (2019). Gas-phase Reactivity of CH3OH toward OH at Interstellar Temperatures (11.7-177.5 K): Experimental and Theoretical Study. Phys. Chem. Chem. Phys. 21, 6942–6957. doi:10.1039/c9cp00439d
Ocaña, A. J., Jiménez, E., Ballesteros, B., Canosa, A., Antiñolo, M., Albaladejo, J., et al. (2017). Is the Gas-phase OH+H2CO Reaction a Source of HCO in Interstellar Cold Dark Clouds? A Kinetic, Dynamic, and Modeling Study. ApJ 850, 28. doi:10.3847/1538-4357/aa93d9
Ohishi, M., Suzuki, T., Hirota, T., Saito, M., and Kaifu, N. (2019). Detection of a New Methylamine (CH3NH2) Source: Candidate for Future glycine Surveys. Publ. Astron. Soc. Jpn. 71, 1–11. doi:10.1093/pasj/psz068
Onel, L., Blitz, M., Dryden, M., Thonger, L., and Seakins, P. (2014). Branching Ratios in Reactions of OH Radicals with Methylamine, Dimethylamine, and Ethylamine. Environ. Sci. Technol. 48, 9935–9942. doi:10.1021/es502398r
Onel, L., Thonger, L., Blitz, M. A., Seakins, P. W., Bunkan, A. J. C., Solimannejad, M., et al. (2013). Gas-phase Reactions of OH with Methyl Amines in the Presence or Absence of Molecular Oxygen. An Experimental and Theoretical Study. J. Phys. Chem. A. 117, 10736–10745. doi:10.1021/jp406522z
Perry, R. A., Atkinson, R., and Pitts, j. N. (1976). Rate Constants for the Reactions OH+H2S→H2O+SH and OH+NH3→H2O+NH2 over the Temperature Range 297-427 °K. J. Chem. Phys. 64, 3237. doi:10.1063/1.432663
Potapov, A., Canosa, A., Jiménez, E., and Rowe, B. (2017). Uniform Supersonic Chemical Reactors: 30 Years of Astrochemical History and Future Challenges. Angew. Chem. Int. Ed. 56, 8618–8640. doi:10.1002/anie.201611240
Rednyk, S., RoučkaKovalenko, Š., Kovalenko, A., Tran, T. D., Dohnal, P., Plašil, R., et al. (2019). Reaction of NH+, NH2+, and NH3+ Ions with H2 at Low Temperatures. A&A 625, A74–A78. doi:10.1051/0004-6361/201834149
Salimian, S., Hanson, R. K., and Kruger, C. H. (1984). High Temperature Study of the Reactions of O and OH with NH3. Int. J. Chem. Kinet. 16, 725–739. doi:10.1002/kin.550160609
Sandford, S. A., Nuevo, M., Bera, P. P., and Lee, T. J. (2020). Prebiotic Astrochemistry and the Formation of Molecules of Astrobiological Interest in Interstellar Clouds and Protostellar Disks. Chem. Rev. 120, 4616–4659. doi:10.1021/acs.chemrev.9b00560
Scott, G. B. I., Freeman, C. G., and McEwan, M. J. (1997). The Interstellar Synthesis of Ammonia. Monthly Notices R. Astronomical Soc. 290, 636–638. doi:10.1093/mnras/290.4.636
Sims, I. R., Queffelec, J. L., Defrance, A., Rebrion‐Rowe, C., Travers, D., Bocherel, P., et al. (1994). Ultralow Temperature Kinetics of Neutral-Neutral Reactions. The Technique and Results for the Reactions CN+O2down to 13 K and CN+NH3down to 25 K. J. Chem. Phys. 100, 4229–4241. doi:10.1063/1.467227
Sleiman, C., El Dib, G., Rosi, M., Skouteris, D., Balucani, N., and Canosa, A. (2018a). Low Temperature Kinetics and Theoretical Studies of the Reaction CN + CH3NH2: a Potential Source of Cyanamide and Methyl Cyanamide in the Interstellar Medium. Phys. Chem. Chem. Phys. 20, 5478–5489. doi:10.1039/c7cp05746f
Sleiman, C., El Dib, G., Talbi, D., and Canosa, A. (2018b). Gas Phase Reactivity of the CN Radical with Methyl Amines at Low Temperatures (23-297 K): A Combined Experimental and Theoretical Investigation. ACS Earth Space Chem. 2, 1047–1057. doi:10.1021/acsearthspacechem.8b00098
Smith, I. W. M., and Barnes, P. W. (2013). Advances in Low Temperature Gas-phase Kinetics. Annu. Rep. Prog. Chem. Sect. C: Phys. Chem. 109, 140–166. doi:10.1039/c3pc90011h
Sorrell, W. H. (2001). Origin of Amino Acids and Organic Sugars in Interstellar Clouds. Astrophys. J. 555, L129–L132. doi:10.1086/322525
Stephens, R. D. (1984). Absolute Rate Constants for the Reaction of Hydroxyl Radicals with Ammonia from 297 to 364 K. J. Phys. Chem. 88, 3308–3313. doi:10.1021/j150659a034
Stuhl, F. (1973). Absolute Rate Constant for the Reaction OH+NH3→NH2+H2O. J. Chem. Phys. 59, 635–637. doi:10.1063/1.1680069
Taylor, S. E., Goddard, A., Blitz, M. A., Cleary, P. A., and Heard, D. E. (2008). Pulsed Laval Nozzle Study of the Kinetics of OH with Unsaturated Hydrocarbons at Very Low Temperatures. Phys. Chem. Chem. Phys. 10, 422–437. doi:10.1039/B711411G
Theule, P., Borget, F., Mispelaer, F., Danger, G., Duvernay, F., Guillemin, J. C., et al. (2011). Hydrogenation of Solid Hydrogen Cyanide HCN and Methanimine CH2NH at Low Temperature. Astron. Astrophys. 534, A64. doi:10.1051/0004-6361/201117494
Tian, W., Wang, W., Zhang, Y., and Wang, W. (2009). Direct Dynamics Study on the Mechanism and the Kinetics of the Reaction of CH3NH2 with OH. Int. J. Quan. Chem. 109, 1566–1575. doi:10.1002/qua.22000
Vahedpour, M., Douroudgari, H., Afshar, S., and Asgharzade, S. (2018). Comparison of Atmospheric Reactions of NH3 and NH2 with Hydroxyl Radical on the Singlet, Doublet and Triplet Potential Energy Surfaces, Kinetic and Mechanistic Study. Chem. Phys. 507, 51–69. doi:10.1016/j.chemphys.2018.03.022
van Dishoeck, E. F., Jansen, D. J., Schilke, P., and Phillips, T. G. (1993). Detection of the Interstellar NH2 Radical. Astrophys. J. 416, L83. doi:10.1086/187076
Weinreb, S., Barret, A. H., Meeks, M. L., and Henry, J. C. (1963). Radio Observations of OH in the Interstellar Medium. Nature 200, 829–831. doi:10.1038/200829a0
Wilson, T. L., Gaume, R. A., and Johnston, K. J. (1993). Ammonia in the W3(OH) Region. Astrophys. J. 402, 230. doi:10.1086/172126
Woon, D. E. (2021). A Continously Updated List of the Observed Molecules Is. available online: http://www.astrochymist.org.
Woon, D. E. (2002). Pathways to Glycine and Other Amino Acids in Ultraviolet-Irradiated Astrophysical Ices Determined via Quantum Chemical Modeling. Astrophys. J. 571, L177–L180. doi:10.1086/341227
Zabielski, M. F., and Seery, D. J. (1985). High Temperature Measurements of the Rate of the Reaction of OH with NH3. Int. J. Chem. Kinet. 17, 1191–1199. doi:10.1002/kin.550171105
Keywords: ISM, prebiotic molecules, OH radicals, CRESU technique, reaction kinetics, ultralow temperatures
Citation: González D, Ballesteros B, Canosa A, Albaladejo J and Jiménez E (2022) Gas-Phase Reactivity of OH Radicals With Ammonia (NH3) and Methylamine (CH3NH2) at Around 22 K. Front. Astron. Space Sci. 8:802297. doi: 10.3389/fspas.2021.802297
Received: 26 October 2021; Accepted: 02 December 2021;
Published: 20 January 2022.
Edited by:
Majdi Hochlaf, Université Paris Est Marne la Vallée, FranceReviewed by:
Marzio Rosi, University of Perugia, ItalyChristian Alcaraz, Centre National de la Recherche Scientifique (CNRS), France
Copyright © 2022 González, Ballesteros, Canosa, Albaladejo and Jiménez. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Elena Jiménez, Elena.Jimenez@uclm.es
 Daniel González
Daniel González Bernabé Ballesteros
Bernabé Ballesteros André Canosa
André Canosa José Albaladejo1,2
José Albaladejo1,2  Elena Jiménez
Elena Jiménez