Inactivation of Salmonella enterica and Surrogate Enterococcus faecium on Whole Black Peppercorns and Cumin Seeds Using Vacuum Steam Pasteurization
- Department of Food Science and Technology, Virginia Tech, Blacksburg, VA, United States
Spices, including black pepper and cumin seeds, have been implicated in outbreaks of salmonellosis and prompted recalls of ready-to-eat products containing contaminated spices. Vacuum-assisted steam pasteurization is performed to improve the safety and quality of many low water activity products, however process parameters associated with inactivation on whole spices are not well described. The objective of this study was to determine the effectiveness of a lab-scale vacuum-assisted steam process for the inactivation of Salmonella enterica and its potential surrogate Enterococcus faecium ATCC 8459 inoculated onto the surface of whole peppercorns and cumin seeds. In addition, the effect of two inoculation preparation methods [growth on tryptic soy agar (TSA) or inclusion within a native microbiota biofilm], on the reduction of S. enterica serovars or E. faecium was compared on steam pasteurized whole black peppercorns. Spices were processed using steam under a vacuum to achieve a mean product temperature of 86.7 ± 2.8°C for different dwell times. Salmonella inoculated using the TSA-grown method, required 83 and 70 s respectively to achieve a 5-log reduction of Salmonella on peppercorns and cumin seeds. Longer time periods were needed to achieve a 5-log reduction of Salmonella when it was present in a native biofilm on whole peppercorns. Survivor estimations were best predicted by the Weibull models. The mean log reductions of E. faecium were 0.9 log CFU/g lower than Salmonella on whole black peppercorns inoculated using the TSA-grown cells (P = 0.0021). The mean log reductions of Salmonella and E. faecium prepared using the biofilm-inclusion method were not significantly different (P = 0.76). E. faecium log CFU/g reductions were not significantly different compared to Salmonella on whole cumin seeds (P = 0.42) indicating that while reductions are comparable the surrogate may not always provide a conservative indication of complete Salmonella elimination for all spices processed using vacuum-assisted steam.
HIGHLIGHTS
- E. faecium is a surrogate for Salmonella on steam processed whole black peppercorn.
- E. faecium is not a conservative surrogate for Salmonella on steam processed >85°C whole cumin.
Introduction
Spices have been used throughout history as a way to enhance the flavor in foods. However, outbreaks of salmonellosis attributed to spices and increased detection of Salmonella in imported spices has prompted increased concern regarding the safety of spices (ASTA, 2011; Van Doren et al., 2013b). Incidence of Salmonella contamination in spices presented for import to the United States is reported at 6.6% (187/2,844 containers) and has also been reported in products available in US grocery stores (Vij et al., 2006; CDC, 2010; Julian et al., 2010; Van Doren et al., 2013a). Salmonella contamination is a major concern because spices may be consumed raw or added to foods after the food is cooked; and in some cases, such as ground black pepper, Salmonella may persist for extended periods of time under typical storage conditions (Keller et al., 2013). The contibued presence of Salmonella in spices will require companies to implement process interventions to be in compliance with the US Food Safety Modernization Act Preventive Controls Rule (FDA, 2016).
Implementing good agricultural and manufacturing practices that comply with the U.S. food code is sometimes a challenge in developing countries, and as a result spices may have Salmonella contamination. Current practices used by the industry to reduce microbial populations in spices include chemical fumigation, irradiation, and steam-based heat treatments (ASTA, 2011). Vacuum-assisted steam pasteurization of spices, sometimes referred to as dry steam, allows for shorter treatment times and lower temperatures compared to dry heat application, resulting in smaller losses of volatile compounds that are integral to the aroma and flavors of spices (Molnár et al., 2018). Use of a vacuum allows the formation of steam at temperatures between 70 and 100°C, and when steam condenses on the spice surface, heat is transferred into the target food, inactivating bacteria associated with the surface (Lilie et al., 2007). In addition, steam has the ability to penetrate small cracks and crevices of spices that could provide protection for surface attached microorganisms (Morgan et al., 1996; James and James, 1997). Various steam treatments have been validated to achieve 4- to 5-log reductions of Salmonella on nuts, seeds, and grains without impacting the quality of the product (Chang et al., 2010; Ban and Kang, 2016; Shah et al., 2017). Vacuum-assisted steam treatments are currently utilized for many types of spices, however the processing parameters vary depending on the type of spice, packaging configurations and other supplier requests. While it is desirable to identify processing parameters resulting in a 5-log CFU reduction of Salmonella on spices, the variability in packaging and processing parameters used by different commercial facilities may result in differences in steam penetration, product temperature and other variables associated with thermal reduction of the target pathogen. Therefore, it is important to identify a surrogate microorganism, whose inactivation is comparable to Salmonella, for commercial facilities to validate their individual, pathogen-inactivation processes.
Enterococcus faecium (NRRL B-2354, ATCC 8459) has been suggested as a surrogate for Salmonella enterica serovars on spices based on its suitability for other thermally processed low water activity foods (ASTA, 2011; Bianchini et al., 2014). E. faecium is more resistant than S. Enteritidis PT30 when exposed to moist-air convection on almonds (Jeong et al., 2011). The log reductions of S. Enteritidis PT30 also exceed that of E. faecium subjected to vacuum steam pasteurization on sunflower kernels, quinoa and flax seed, indicating suitability on these products (Shah et al., 2017). Suitability of E. faecium as a surrogate for Salmonella must however be determined for each product and processing condition. The objective of the study was to evaluate the effectiveness of vacuum-assisted steam pasteurization using a lab scale system (116.5 kPa, mean product temperature of 86.7 ± 2.8°C) for inactivating Salmonella enterica on whole black peppercorns and cumin seeds and determine the amount of time needed to achieve a 5-log reduction. The effect of inoculum preparation on steam inactivation was compared for whole black peppercorns. The validity of E. faecium ATCC 8459 as a surrogate for inactivation on whole black peppercorns and cumin seeds using the lab-scale steam apparatus was also evaluated.
Materials and Methods
Bacterial Strains and Growth Conditions
Four Salmonella enterica serovars were obtained from low water activity (aw) foods (Montevideo, an isolate from 2010 peppercorn associated outbreak, Tennessee, K4643 from a peanut butter outbreak associated in 2007, Ball ARL-SE-085 from black pepper in 2011, and Johannesburg ARL-SE-013 from dried ginger in 2010) and used to inoculate whole black peppercorns and whole cumin seeds. The combination of these strains originating from low aw foods are representative of strains that have demonstrated ability to persist on dried spices. Enterococcus faecium ATCC 8459 was obtained from American Type Culture Collection (Manassas, VA). Individual stocks were maintained at −80°C in glycerol stocks. Strains were resuscitated by streaking onto Tryptic Soy Agar [TSA, Becton Dickinson (BD), Franklin Lakes, NJ] incubated at 37°C for 24 h. One single colony was then transferred onto Xylose-Lysine-Tergitol 4 (XLT4, BD) for each Salmonella strain or Bile Esculin Agar (BEA, BD) for Enterococcus faecium and incubated at 37°C for 24 h. Following incubation, a single colony for each bacterial strain was transferred into 10 mL Tryptic Soy Broth (TSB, BD). The individual cultures were incubated at 180 rpm, 37°C for 24 h.
Spice Varieties and Sources
Whole black peppercorns and cumin seeds were provided in bulk by a major, national spice processor. Spices were not processed before arrival.
Inoculating Black Peppercorns Using Biofilm Inclusion Method
This inoculation methodology was adapted from a previous method (Aviles et al., 2013) with modification. Briefly, Salmonella strains were incorporated within native microbiota biofilms on the surface of the spice seeds by immersing seeds in inoculum within Tryptic Soy Broth (TSB) and incubating statically for 48 h. Sixty grams of whole peppercorn was transferred into a sterile 2L Erlenmeyer flasks, spread out to cover the bottom in a single layer and submerged in 150 mL TSB (to a depth of about 1 cm) along with 5 mL of 24-h liquid culture (E. faecium or Salmonella cocktail of equally mixed four Salmonella strain cultures) and swirled to mix. The flasks were incubated statically at 37°C for 24 h, then the TSB/liquid culture was decanted. New TSB (150 mL) was added back into flask and then incubated at 37°C for another 24 h. After incubation, the TSB was decanted, and the peppercorns were washed with sterile 0.1% (w/v) peptone (Sigma-Aldrich, Co., MO) with 0.1% Tween 80 (PT, Fisher Scientific, Kansas City, MO) to remove media and any unattached cells and the liquid was decanted.
Wet Inoculation With Salmonella and E. faecium Cells Grown on TSA
The following method was adapted from the method developed for inoculation of almonds for process validation (Almond Board of California, 2014). Briefly, E. faecium and each strain of Salmonella were spread onto individual large Petri plates (150 × 15 mm, BD Falcon, Franklin Lakes, NJ) with TSA and incubated at 37°C for 24 h. After incubation, bacterial cells were harvested by applying PT to the plates and scraping the colonies from the surface using a sterile cotton swab. For Salmonella, the suspensions were combined in comparable volumes of each strain to make an inoculum cocktail. Twenty millimeters of the E. faecium or Salmonella cocktail suspension was then transferred into 27 oz. sterile Whirl-Pak bags containing either 50 g of dry whole peppercorns or 50 g of dry cumin seeds and hand massaged for 1 min.
Water Activity (aw) Equilibrium
After inoculation with either preparation method, the spices were arranged in a single layer on sanitized 13 × 9 in. aluminum foil covered baking sheets and placed in a biological safety cabinet for 24–48 h air drying until the spices reached the pre-inoculation aw at room temperature. Water activity of whole peppercorns (5 g) and cumin seeds (4 g) was determined by using an AquaLab 4TE water activity meter (AquaLab, Pullman, WA). Once the desired water activity was reached, the spices were placed in sealed Whirl-Pak bags and held in a desiccator (RH: ~40–45%) containing Drierite desiccant for 24 h before processing.
Lab Scale Vacuum Steam Pasteurization
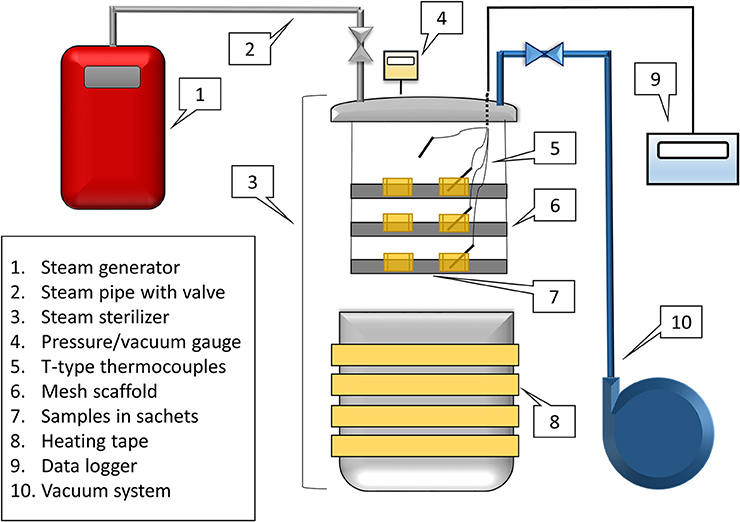
A custom, lab-scale, vacuum-assisted-steam processing apparatus was developed (Figure 1). An aluminum pressure-rated steam sterilizer (All American Model 1925X, Manitowoc, WI) was wrapped in 3 × 120 in. heating tape (BriskHeat, Columbus, OH) and connected to a steam generator (AmeriVap®, Dawsonville, GA) and a vacuum pump (Edwards® RV3, Crawley, Sussex, England). Four wire T-type thermocouples were inserted from the top of the steam sterilizer using a hermetic pass through set (PFT2NPT-4T, Omega Engineering Stanford, CT). A 3-level scaffold system was made out of PVC pipe with mesh racks (304 Stainless Steel Mesh, 20 × 20 mesh, 0.16” wire, McMaster Carr, Atlanta, GA) on each level. This scaffold system was placed inside of the sterilizer to support the spice product during processing. Inoculated spices (50 g peppercorns, 40 g cumin seeds) were placed in muslin drawstring sachets (10 × 15 cm) and filled to a depth of 0.51 ± 0.13 cm for whole black peppercorns, and 0.53 ± 0.10 cm for cumin seeds. Two sachets were placed on each of the three levels of the scaffold.
The heating tape was turned on and set slightly above 177°C. Temperature was measured using the T-type thermocouples and logged using a data logger (Omega, model RDXL6SD, Stamford, CT), while pressure was measured using a pressure gauge (DG-25, Ashcroft, Deer Park, New York). Pre-conditioning prior to the treatment of spices was achieved by applying a vacuum to 32.4 kPa, followed by injecting steam until a measured temperature of the air achieved a temperature of 104°C and 116.5 kPa. A final vacuum was pulled to 32.4 kPa before opening the lid to remove excess moisture that may have formed in the cold chamber during preheating. Once the temperature reached this point, the spice rack holding multiple sachets was inserted. Thermocouples were inserted into the middle of one sachets of spice on each scaffold. Once the lid was closed with the spices inside, the vacuum was pulled to 32.4 kPa and steam was inserted until the product temperature reached 85°C (actual value 86.7 ± 2.8°C for all processes). The dwell time then occurred for a specified amount of time (15 s – 4 min). Once the treatment time was achieved, the vacuum was slowly released with an air inbleed. The lid of the sterilizer was quickly taken off, and the spice rack was removed. The timed treatments were performed separately, because the cycle could not be extended or continued once the lid of the chamber was opened. Product temperature was monitored through the entire process. As a comparison, a dry heating treatment (1 min, without steam injection) was performed separately.
Microbiological Enumeration
Spice samples (10 g) were transferred into a sterile filter bag along with 90 mL of sterile PT and bacteria removed from the surface using a lab paddle blender (Interscience BagMixer, Guelph, Ontario) for 60 s. The liquid was vacuum filtered through #4 qualitative filter paper (Whatman, GE Healthcare, Pittsburgh, PA) to remove any spice particles that might continue to release antimicrobial compounds or increase plating error. The filtered supernatant was serial diluted in sterile PT buffer and enumerated by plating onto duplicated TSA. The TSA were incubated for 3 h at 25°C, after which an overlay of 7 ml of XLT4 was applied. This overlay methodology was adapted to improve the recovery of sub lethally injured Salmonella (Kang and Fung, 2000) from spices that have a high background microbial load of spore formers (Pafumi, 1986) preventing the use of non-selective media alone for enumeration. Spices inoculated with E. faecium were enumerated by plating onto selective media BEA in duplicate. All plates were incubated at 37°C for 24 h before enumeration. Spread plating 0.1 ml of the supernatant resulted in a limit of detection of 2.0 log CFU/g. If no cells were recovered on spread plates 24 h after enumeration, 20 mL of filtered supernatant that was incubated in 80 ml of TSB for enrichment at 37°C for 24 h was subsequently streaked (10 uL) onto selective media. Positive and negative streak results after 24 h incubation were recorded as 2.0 log CFU/g or 0 log CFU/g, respectively, for calculation of log reduction.
Experimental Design and Statistical Analysis
Samples were vacuum-steam pasteurized in pairs (one with Salmonella-inoculated spices and the other with E. faecium-inoculated spices) on each shelf of the apparatus per run. Spice varieties were processed separately, with three experimental replicates per dwell time. For each replicate, inoculated samples that were not pasteurized were used as controls. For whole peppercorns, each trial contained both inoculation methodologies for comparison.
Bacterial counts were log transformed prior to statistical analysis. The limit of detection (2.0 log CFU/g) was used as the count for samples where no colonies were detected but yielded a positive enrichment. Log reductions were calculated by subtracting the log CFU/g of the pasteurized sample from the log CFU/g of the non-pasteurized control sample. If no colonies appeared in plates after enrichment, then the log reduction was considered to be comparable to the initial inoculated amount. Statistical analyses were performed using JMP (version 13, SAS, Cary, NC) statistical software. Log reductions of Salmonella and E. faecium were compared within each spice and inoculation method using Matched Pair T-Test. The effect of two different inoculation methods on the log reduction of the target bacteria on whole peppercorns was determined by analysis of variance (ANOVA). P < 0.05 were considered significant.
Survival Model Fitting
The data were fit to several different survival models and survival parameters were estimated using GInaFiT Version 1.7 (Geeraerd et al., 2005). The best fit survival model was determined to be a Weibull model for each bacteria and spice type for the steam pasteurization treatment (TSA-grown cell inoculation method only), but two other models often used to describe pathogen survival were also fit for comparison. Survival parameters of the Weibull model were estimated using the following equation:
where Nt is the concentration of the bacterial population at time t (minutes), N0 is the initial bacterial population concentration at time 0, δ is the time at which the first log reduction occurs, and ρ is the fitting parameter used to determine the shape of the curve.
The Geeraerd-tail model (Geeraerd et al., 2000) was used to estimate the survival parameters using the following equation:
where N is the bacterial population (CFU/g) considering time t (minutes), N0 is the bacterial population (CFU/g) prior to treatment, Nres is the observed population considered to be resistant to the treatment, and kmax is the rate of population inactivation before the tailing effect in minutes.
Additionally, the log-linear model (Bigelow and Esty, 1920) was also used to describe the survival parameters using the following equation:
where N is the bacterial population (CFU/g) considering time t (minutes), N0 is the bacterial population (CFU/g) prior to treatment, and kmax is the rate of population inactivation in minutes.
Results
Whole Black Peppercorns Inoculated Using the Biofilm Inclusion and TSA-Grown Inoculation Preparation Methods
Water activity of the black peppercorns after equilibration before processing was 0.51 ± 0.09 and 0.33 ± 0.09 for biofilm and TSA-grown inoculated whole peppercorns, respectively. There were no significant differences in the water activity for peppercorns inoculated with Salmonella or E. faecium. The initial levels of Salmonella using the biofilm and TSA-grown inoculation preparation methods were 6.9 ± 0.3 and 9.2 ± 0.6 log CFU/g, and the initial levels of E. faecium were 7.5 ± 0.2 and 8.9 ± 0.4 log CFU/g, respectively. Product placed in the pre-heated chamber without the injection of steam did not achieve the target temperatures within 1 min, despite comparable chamber air temperatures; incidentally the reduction of Salmonella on these samples was only 0.8 ± 0.3 log CFU/g. In comparison injection of steam during a 1-min vacuum-assisted steam dwell resulted in larger reductions of Salmonella at 4.6 ± 0.2 log CFU/g on biofilm inoculated whole black peppercorns, and 7.1 ± 0.2 log CFU/g, while using TSA-grown method.
The log reduction of Salmonella after the vacuum steam pasteurization was significantly affected by the time of treatment and inoculation method (two-way ANOVA, P < 0.05). A longer period of time was needed to assure a 5-log reduction of Salmonella inoculated by biofilm inclusion (3 min) compared to TSA-grown cells inoculation (1 min, Figure 2). While overall the product temperature increased with dwell time (Figure S1), there was some variability in the product temperatures between replicates. To account for the differences in product temperature between batches, the log CFU/g reduction was plotted against the total time the product maintained a temperature above 82°C (Figure S2). Examining this information, it was determined that only samples that maintained 82°C for at least 83 s or 202 s for TSA-grown inoculated and biofilm inoculated peppercorns, respectively, achieved a 5-log reduction of Salmonella (Figure S1).
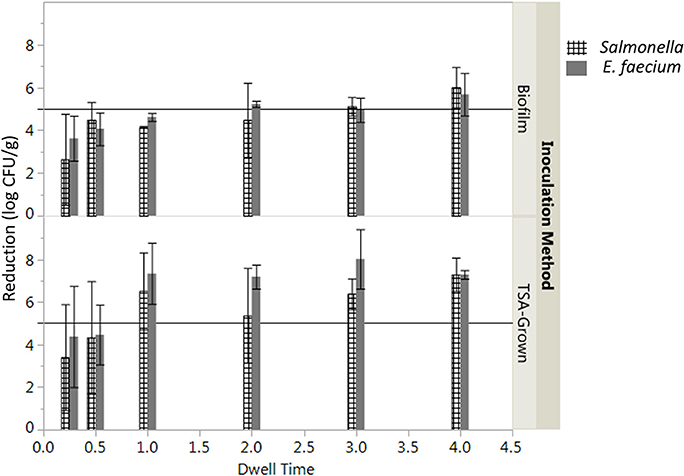
Figure 2. Effect of inoculation preparation on average log reduction in CFU/g of Salmonella enterica on whole black peppercorns processed using a lab scale vacuum steam pasteurizer chamber temperature 88 ± 5°C, 97.9 kPa at different dwell times. Log reductions are the average of three independent runs and three technical replicates within run. Error bars denote standard deviation. Limit of detection was considered.
Log reductions of Salmonella and E. faecium on whole peppercorns inoculated using the biofilm inclusion method were not significantly different (P = 0.76), whereas when using TSA-grown inoculation, E. faecium was significantly more resistant to the steam treatments (P = 0.0021). One-to-one log reduction comparison plots of the inoculated Salmonella vs. E. faecium are shown in Figures 3, 4 for cells prepared using the TSA-grown method and biofilm method, respectively.
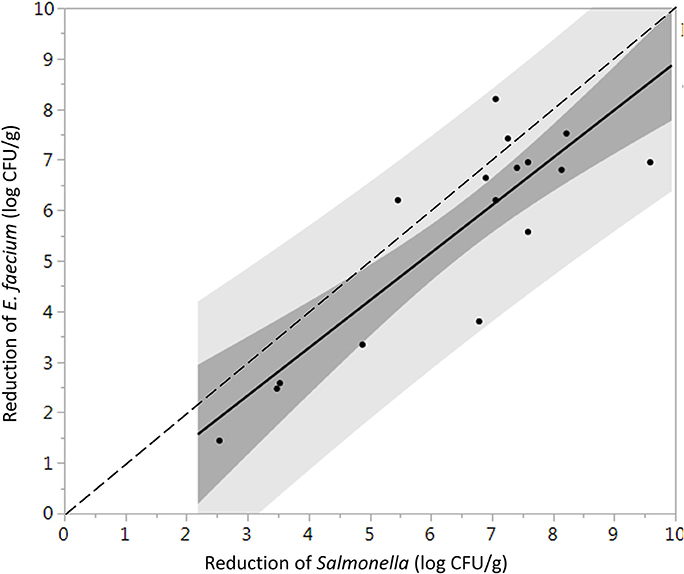
Figure 3. Direct comparison between the average log reduction in CFU/g of Salmonella and E. faecium on whole black peppercorns inoculated using TSA-grown method and pasteurized at 88 ± 5°C, 97.9 kPa for different periods of times (n = 36). Darker and lighter gray range indicate 95% confidence intervals and prediction intervals, respectively. Some data points are overlapping with each other.
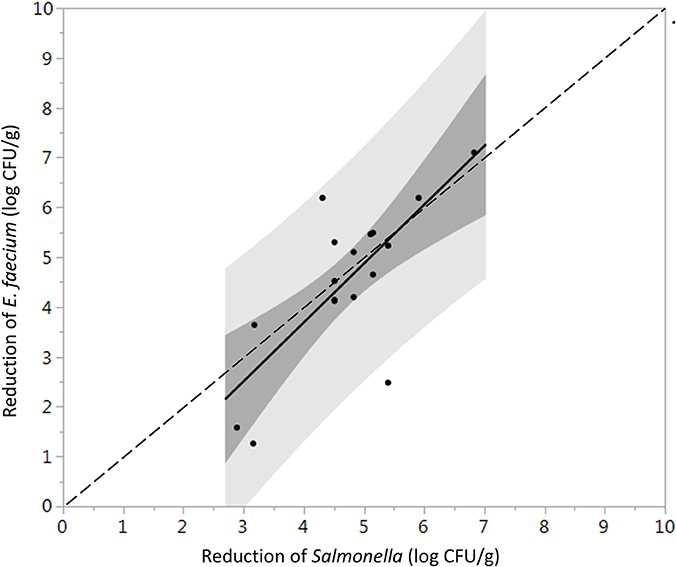
Figure 4. Direct comparison between the average log reduction in CFU/g of Salmonella and E. faecium on whole black peppercorns inoculated using biofilm inclusion method and pasteurized at 88 ± 5°C, 97.9 kPa for different periods of times (n = 36). Darker and lighter gray range indicate 95% confidence intervals and prediction intervals, respectively. Some data points are overlapping with each other.
Cumin Seeds Inoculated Using the TSA-Grown Inoculation Preparation Method
The initial levels of Salmonella and E. faecium inoculated on cumin seeds were 9.2 ± 0.6 and 9.0 ± 0.3 log CFU/g. Water activity of the cumin seeds after equilibration before processing was 0.38 ± 0.12. While overall the product temperature increased with dwell time (Figure S3), there was some variability in the temperatures between runs that contributes to the standard error in reduction. In the absence of vacuum-assisted steam a reduction of 0.3 ± 0.1 log CFU/g of Salmonella on cumin seeds was accomplished after 1 min in the heated chamber, comparing at 7.1 ± 1.6 log CFU/g when vacuum assisted steam was injected. After a 1-min treatment time, there was no statistically significant additional reduction of Salmonella for longer treatments (Figure 5). While overall, an average reduction of 5 log CFU/g of Salmonella was achieved during the 30 s-dwell time, in actuality a temperature of 82°C needed to be maintained for minimum of 66 s for all samples to achieve a 5-log reduction (Figure S4). A matched pairs t-test indicated that E. faecium was not significantly more resistant to the vacuum-assisted steam treatment than Salmonella (P = 0.42); also, there were instances when the reduction of E. faecium exceeded that of Salmonella by up to 2.3 log CFU/g (Figure 6).
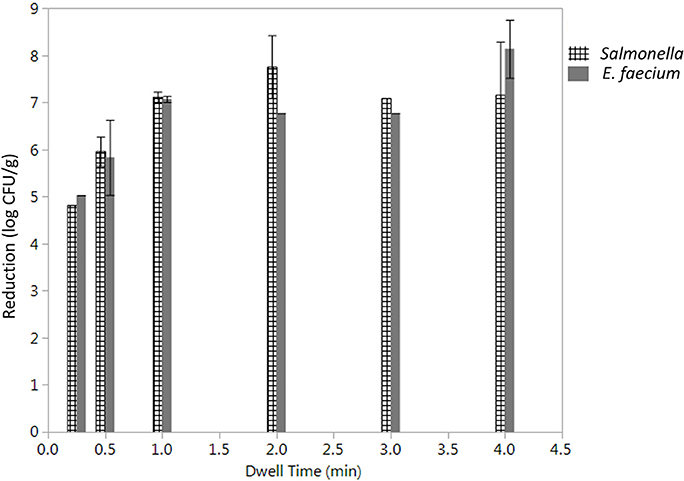
Figure 5. Average log reduction in CFU/g of Salmonella enterica and E. faecium on cumin seeds processed using a lab scale vacuum steam pasteurizer chamber temperature 88 ± 5°C, 97.9 kPa at different dwell times. Log reductions are the average of three independent runs and three technical replicates within run. Error bars denote standard deviation. Limit of detection was considered.
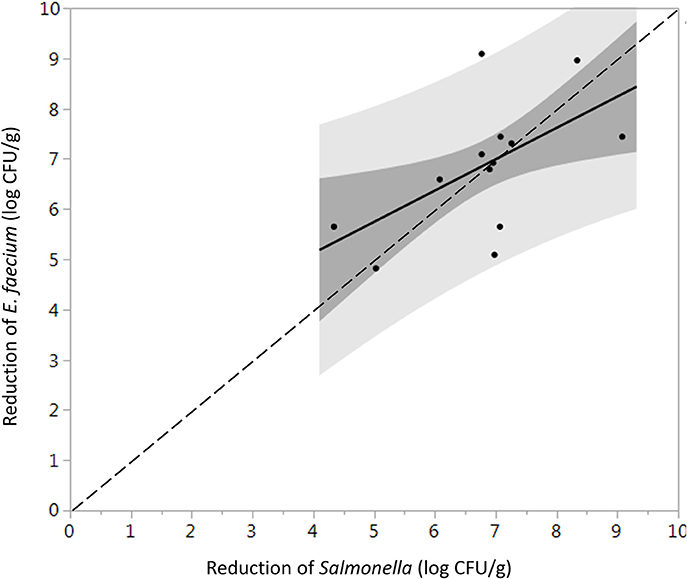
Figure 6. Direct comparison between the average log reduction in CFU/g of Salmonella and E. faecium on whole cumin seeds pasteurized at 88 ± 5°C, 97.9 kPa for different periods of times (n = 34). Darker and lighter gray range indicate 95% confidence intervals and prediction intervals, respectively. Some data points are overlapping with each other.
Model Fitting of Survival Curves
The inactivation of Salmonella and E. faecium on vacuum steam pasteurized whole black peppercorns and cumin seeds were best described by the Weibull model (Table 1). However, E. faecium-inoculated cumin had a relatively high Root Mean Square Error (RMSE) and a low adjusted R2 () compared to the other food and pathogen combinations. Still, the Weibull model provided better survival parameter estimates than log-linear or Geeraerd-tail models.
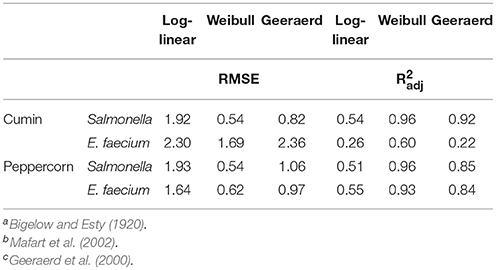
Table 1. Statistical fit by Root Mean Square Error (RMSE) and adjusted R2 of log-linear, Weibull, and Geeraerd-tail models for steam treatment of Salmonella- and E. faecium-inoculated cumin and whole peppercornsa,b,c.
Discussion
The lab-scale steam apparatus designed for this research utilized a vacuum to allow for the production of steam at a temperature of 88°C as opposed 100°C under atmosphere pressure. The use of vacuum in commercial process is used to lower the pasteurization temperature thus to retain the quality of food commodities, and remove excessive moisture after pasteurization (Ivarsson, 2011; Grasso et al., 2014). While superheated steam (when water vapor is at a temperature higher than boiling point 100°C), saturated steam (at 100°C when 100% water is in form of vapor), and vapor-liquid mixture (when percentage of water in the form of vapor is less than 100%) are all being applied in microbial inactivation (Toledo, 1991), this steam apparatus in this study likely produced vapor-liquid mixtures. Multiple cycles of vacuum and steam injections were performed in the chamber before loading the samples to pre-warm the chamber, however, sample loading required the removal of the apparatus lid which may have affected how quickly the spice achieved the target temperature (come up time) and the final temperature at the end of the dwell time for a few of the samples and may explain in part the variability in reduction. Increased variability in reduction of targeted pathogens between replicates with short vacuum steam pasteurizations times comparable to this study was also observed for different grains (Shah et al., 2017). As a result, the time when the sample exceeded a certain temperature, rather than the total dwell time of the sample, may be more indicative to the overall reduction. A temperature threshold of 82°C was chosen to more accurately represent the time needed to result in a 5-log reduction of Salmonella. These differences in product temperature and pathogen inactivation emphasize the importance of understanding the process parameters within the steam pasteurization chamber and within the package.
Different packaging types and configurations are expected to influence the effectiveness of steam processes by affecting the contact of steam with the spices. In the lab scale trials the packaging configuration was designed to be a thin layer, ensuring the access of the steam to the spice particles. In contrast, spices imported into the US are typically packaged in a variety of materials from burlap/polywoven plastic bags to corrugated cardboard containers and rigid plastic containers. The amounts vary from on average 22 to 2,268 kg, depending on the spice and the supplier Howe Personal Communication. Processors should assure that the pressure, temperature, and time of processing are optimized to deliver the necessary amount of steam to the product within the package before performing process validations, otherwise portions of the product may not reach the necessary temperature and may therefore still harbor pathogens.
Inoculum preparation of Salmonella is known to influence the heat resistance and survival in low water activity foods (Kataoka et al., 2014; Bowman et al., 2015; Enache et al., 2015). A high-density inoculum was necessary to demonstrate a 5-log reduction. Salmonella applied as a dry inoculum into a model peanut paste at 0.6 aw was more heat resistant compared to Salmonella grown on TSA plates or TSB; however, E. faecium was more resistant when grown on TSA plates compared to TSB (Enache et al., 2015). In preliminary trials, the necessary dry transfer of 7–9 logs CFU/g of Salmonella to whole peppercorns and cumin seeds could not be achieved using methods adapted for dry inoculation of almonds (Blessington et al., 2013). The TSA-grown cells methodology for preparation was simpler compared to dry inoculation or incorporation within the native biofilm methods. This inoculation method may have resulted in release of antimicrobial compounds (Hildebrandt et al., 2017), however the extent that this would contribute to additional reduction leading to an overestimation of the effectiveness of the vacuum assisted steam pasteurization is unknown. This study compared the inactivation of two surface-associated forms of Salmonella because the nature of Salmonella contamination on whole peppercorns is unknown. Spices have the potential to be contaminated by Salmonella in the form of a biofilm, which can increase protection against stress. The potential for formation of biofilms is possible during peppercorn processing when the peppercorn is soaked overnight in water followed by drying. A biofilm is an extracellular matrix comprised of mostly water and various polymers (Geesey, 1982). When in this form, Salmonella has an increased tolerance to desiccation and can be recovered in greater numbers (Aviles et al., 2013). Encasement of Salmonella within a biofilm of native bacteria on peppercorns has been demonstrated to increase survival within a desiccated lab environment (Bowman, 2015). Inclusion within biofilms has also been shown to increase heat resistance of Salmonella on various surfaces and therefore protect the cells (Ban et al., 2014). Salmonella within biofilms have also been shown to be more resistant to sanitizers compared to planktonic cells (Joseph et al., 2001), however the resistance to antimicrobial compounds of peppercorns have not been studied Encasement within a native microbiota biofilm did increase the resistance of Salmonella to the steam process, creating greater variability in reduction, requiring a longer period of time to achieve an acceptable level of reduction of Salmonella. However, for cumin, it was observed that the biofilm inclusion inoculation process caused the seeds to swell and the shuck to open. Then, once the spices were dried, the shuck of cumin seeds closed. As a result, it is hypothesized that internalization of Salmonella occurred, so that during preliminary steam processes, the steam was unable to reach the internalized Salmonella. It is not reasonable to assume that a liquid immersion scenario would occur for cumin seeds in the real world, thus biofilm inclusion inoculation on cumin seeds was not further studied and discussed.
Another important consideration for validation protocols is the ability to enumerate all of the surviving target bacteria after an inactivation process. Concentrated antimicrobials from black pepper have been reported to inhibit growth of Salmonella (Tang et al., 2017). However, concentrations typically found in black pepper have minimal effect, as Salmonella has been demonstrated to grow in ground black pepper at higher relative humidity (Keller et al., 2013). Thermal processes may cause sub-lethal injury to cells, which can lead to an underestimation of microbial populations when plating on selective media (Wesche et al., 2009). Selective media contains ingredients that prevent the growth of the native bacteria, but these ingredients may also prevent growth of damaged bacteria. An underestimation of the survival of bacteria could lead to an increase in risk to producers and consumers. In a companion study, Salmonella recovery using the described overlay methodology, where cells are first plated onto non-selective media followed by an overlay of selective media, increased the recovery of damaged Salmonella by 1 log CFU/g for vacuum steam pasteurized peppercorns and 2-log increase in recovery from cumin seeds compared to plating immediately on selective media (Caver, 2016). Based on this study, we recommend that future process validations use an overlay methodology to reduce the risk to producers and consumers created by poor recovery of damaged Salmonella that may later recover and grow. Some process validations first perform an inactivation step to eliminate the native microbiota from the product before inoculation, allowing use of non-selective media to enumerate surviving Salmonella. This is not realistic for whole black peppercorns due to the very large numbers of spore-forming bacteria that are part of the native microbiota and are very difficult to inactivate (Lilie et al., 2007). These microbiota may also interact with Salmonella, influencing its survival during processing.
It has been suggested that E. faecium ATCC 8459 may be used as a surrogate for validation of the reduction of Salmonella enterica on spices (ASTA, 2011). However, the suitability of a surrogate microorganism used for a pathogen must be validated for each spice product and each process. E. faecium ATCC 8459 has been validated as a surrogate for Salmonella Enteritidis PT 30 on the surface of almonds subjected to moist air-heating (Jeong et al., 2011). Reductions of Salmonella were greater than E. faecium on steam treated whole black peppercorns in all instances, considering two inoculation procedures and can therefore be considered an acceptable surrogate. According to matched pairs analysis for TSA-grown cell inoculated black peppercorns (Figure 3), the fact that most data points and the linear regression line lie below the 1:1 line indicates a conservative response of E. faecium to the treatment, making it an appropriate surrogate for validating vacuum steam pasteurization of Salmonella on whole black peppercorns using TSA-grown inoculation method. For cumin, however, the reduction of E. faecium and Salmonella were not significantly different, and there were two instances that the reduction of E. faecium exceeded that of Salmonella by up to 2.3 log (9.1 log CFU/g vs. 6.8 log CFU/g, Figure 6). Combining these two factors, E. faecium is recommended with caution to be used as a surrogate for Salmonella during vacuum-steam processing for cumin.
The survival curves generated from the datasets in the present study highlight the great variability of microorganisms' reactions to thermal treatments in low water activity environments. Typically, thermal inactivation of Salmonella on low water activity foods has been described as non-log-linear (Villa-Rojas et al., 2013; Shah et al., 2017). In this instance the survivor kinetics were different for the two spice types, indicating the kinetics is not based solely on the water activity of the product but also on other properties that may be impacting the transfer of heat and particle interactions. The Weibull model provided appropriate estimations of inactivation for the particular products and treatment parameters. The Root Mean Square Error (RMSE) for the models varied based on both spice type and bacteria, indicating that the models' ability to accurately predict inactivation trends vary between products and inoculations. A similar range of variability for the RMSE was also observed by Shah et al. (2017) in their experiments of vacuum steam pasteurization of Salmonella and E. faecium on low moisture foods. Farakos et al. (2017) also used models to describe the survival kinetics of Salmonella on various types of nuts, but their data suggested the log-linear model only fit populations that were being stored in refrigeration temperatures of 4 and 10°C. The Geeraerd-tail model could provide appropriate estimates of data that exhibits tailing, but the tailing effect observed at the 2 and 4 min dwell times in current study could be due to the limit of detection. Bacterial reduction has often been described as log-linear in the past, but the current study indicated a more complex trend of reduction during steam treatments, as can be seen in the high RMSE and low (Table 1).
In a parallel study from our group, the effects of vacuum steam pasteurization for a 2 min dwell time on the quality of peppercorns and cumin seeds was analyzed (Duncan et al., 2017). No differences in visual appearance for whole black peppercorns were noted based on sensory similarity testing. However, steam pasteurization did result in perceptible odor differences between the control (untreated) and vacuum-assisted steam pasteurized whole black peppercorns detected by a sensory panel, supported by increased concentration of monoterpene volatiles and loss of all sesquiterpenes. Only minimal visual differences, higher L*a*b* values, were noted for cumin seed and no difference in odor was detected by the sensory similarity analysis.
In summary, vacuum assisted steam pasteurization of spices was effective for reduction of Salmonella in a short period of time. E. faecium ATCC 8459 may be considered a surrogate for the inactivation of Salmonella on whole peppercorns and cumin seeds pasteurized using vacuum assisted steam, however its application to other spices, or other processes may result in an unsafe product. These inactivation models apply only to whole black peppercorns and cumin seeds in the same packaging configurations and only with the same stated processes and temperatures, therefore the suitability to a commercial process may not be appropriate; potentially resulting in a hazardous product.
Author Contributions
JN, CC collected data and wrote initial draft of manuscript. JA performed modeling. BW, KM, RW, MP designed apparatus, experiment. JW and MP performed data analysis and wrote the manuscript. All co-authors made contributions to manuscript.
Funding
Funding for this research was provided in part by the International Life Sciences Institute of North America Committee for Food Microbiology, by the Virginia Agricultural Experiment Station and the Hatch Program of the National Institute of Food and Agriculture, U.S. Department of Agriculture. ILSI North America is a public non-profit foundation that provides a forum to advance understanding of scientific issues related to the nutritional quality and safety of the food supply by sponsoring research programs, educational seminars and workshops, and publications. ILSI North America receives support primarily from its industry membership. In addition, the American Spice Trade Association (ASTA) provided an unrestricted grant to ILSI North America for this research. ASTA is a trade association that represents the U.S. spice industry and works to ensure clean, safe spices. The opinions expressed herein are those of the authors and do not necessarily represent the views of the funding organizations.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
We would like to thank the U.S. Food and Drug Administration, Office of Regulatory Affairs, Arkansas Regional Lab for providing the spice-isolated strains. Special thanks to Kim Waterman, Natalie Pulido, Thomas Saunders, Brett Driver, Robert Lane, and Sandy Janwatin for providing technical assistance.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fsufs.2018.00048/full#supplementary-material
References
Almond Board of California (2014). Guidelines for Using Enterococcus faecium NRRL B-2354 as a Surrogate Microorganism in Almond Process Validation. Available online at: http://www.almonds.com/sites/default/files/content/attachments/guidelines_for_using_enterococcus_faecium_nrrl_b2354_as_a_surrogate_microorganism_in_almond_process_validation.pdf. (Accessed June 26, 2017).
ASTA (2011). Clean, Safe Spices Guidance Document. Available online at: http://www.astaspice.org/food-safety/clean-safe-spices-guidance-document/
Aviles, B., Klotz, C., Eifert, J., Williams, R., and Ponder, M. (2013). Biofilms promote survival and virulence of Salmonella enterica sv. Tennessee during prolonged dry storage and after passage through an in vitro digestion system. Int. J. Food Microbiol. 162, 252–259. doi: 10.1016/j.ijfoodmicro.2013.01.026
Ban, G. H., and Kang, D. H. (2016). Effectiveness of superheated steam for inactivation of Escherichia coli O157:H7, Salmonella typhimurium, Salmonella enteritidis phage type 30, and Listeria monocytogenes on almonds and pistachios. Int. J. Food Microbiol. 220, 19–25. doi: 10.1016/j.ijfoodmicro.2015.12.011
Ban, G. H., Yoon, H., and Kang, D. H. (2014). A comparison of saturated steam and superheated steam for inactivation of Escherichia coli O157:H7, Salmonella typhimurium, and Listeria monocytogenes biofilms on polyvinyl chloride and stainless steel. Food Control 40, 344–350. doi: 10.1016/j.foodcont.2013.12.017
Bianchini, A., Stratton, J., Weier, S., Hartter, T., Plattner, B., Rokey, G., et al. (2014). Use of Enterococcus faecium as a surrogate for Salmonella enterica during extrusion of a balanced carbohydrate-protein meal. J. Food Protect. 77, 75–82. doi: 10.4315/0362-028X.JFP-13-220
Bigelow, W. D., and Esty, J. R. (1920). The thermal death point in relation to typical thermophylic organisms. J. Infect. Dis. 27, 602–617. doi: 10.1093/infdis/27.6.602
Blessington, T., Theofel, C. G., and Harris, L. J. (2013). A dry-inoculation method for nut kernels. Food Microbiol. 33, 292–297. doi: 10.1016/j.fm.2012.09.009
Bowman, L. S. (2015). Impacts of Inoculation Strategy on Survival of Salmonella enterica and Surrogate Enterococcus faecium at Low Water Activity on Dry Peppercorn and Cumin Seeds. Master's Thesis. Blacksburg, VA: Virginia Tech.
Bowman, L. S., Waterman, K. M., Williams, R. C., and Ponder, M. A. (2015). Inoculation preparation affects survival of Salmonella enterica on whole black peppercorns and cumin seeds stored at low water activity. J. Food Prot. 78, 1259–1265. doi: 10.4315/0362-028X.JFP-14-483
Caver, C. B. (2016). Recovery of Salmonella From Steam and Ethylene Oxide Treated Spices Using Supplemented Agar With Overlay. Master's Thesis, Blacksburg, VA: Virginia Tech.
CDC (2010). Investigation Update: Multistate Outbreak of Human Salmonella Montevideo Infections (Final update). Available online at: http://www.cdc.gov/salmonella/montevideo/index.html. (Accessed May 16, 2018).
Chang, S. S., Han, A. R., Reyes-De-Corcuera, J. I., Powers, J. R., and Kang, D. H. (2010). Evaluation of steam pasteurization in controlling Salmonella serotype Enteritidis on raw almond surfaces. Lett. Appl. Microbiol. 50, 393–398. doi: 10.1111/j.1472-765X.2010.02809.x
Duncan, S. E., Moberg, K., Amin, K. N., Wright, M., Newkirk, J. J., Ponder, M. A., et al. (2017). Processes to preserve spice and herb quality and sensory integrity during pathogen inactivation. J. Food Sci. 82, 1208–1215. doi: 10.1111/1750-3841.13702
Enache, E., Kataoka, A., Black, D. G., Napier, C. D., Podolak, R., and Hayman, M. M. (2015). Development of a dry inoculation method for thermal challenge studies in low-moisture foods by using talc as a carrier for Salmonella and a surrogate (Enterococcus faecium). J. Food Prot. 78, 1106–1112. doi: 10.4315/0362-028X.JFP-14-396
FDA (2016). Food Safety Modernization Act Final Rule for Preventive Controls for Human Food. FDA-2011-N-0920. FDA.
Farakos, S. M. S., Pouillot, R., and Keller, S. E. (2017). Salmonella survival kinetics on pecans, hazelnuts, and pine nuts at various water activities and temperatures. J. Food Prot. 80, 879–885. doi: 10.4315/0362-028X.JFP-16-392
Geeraerd, A. H., Herremans, C. H., and Van Impe, J. F. (2000). Structural model requirements to describe microbial inactivation during a mild heat treatment. Int. J. Food Microbiol. 59, 185–209. doi: 10.1016/S0168-1605(00)00362-7
Geeraerd, A. H., Herremans, C. H., and Van Impe, J. F. (2005). GInaFiT, a freeware tool to assess non-log-linear microbial survivor curves. Int. J. Food Microbiol. 102, 185–209. doi: 10.1016/j.ijfoodmicro.2004.11.038
Geesey, G. G. (1982). Microbial exopolymers: ecological and economic considerations. Am. Soc. Microbiol. News 48, 9–14.
Grasso, E. M., Stam, C. N., Anderson, N. M., and Krishnamurthy, K. (2014). “Heat and steam treatments,” in The Microbiological Safety of Low Water Activity Foods and Spices, eds J. B. Gurtler, M. P. Doyle, and J. L. Kornacki (New York, NY: Springer), 403–424.
Hildebrandt, I. M., Hu, C., Grasso-Kelley, E. M., Ye, P., Anderson, N. M., and Keller, S. E. (2017). Dry transfer inoculation of low-moisture spices containing antimicrobial compounds. J. Food Prot. 80, 338–344. doi: 10.4315/0362-028X.JFP-16-279
Ivarsson, C. (2011). Nut pasteurization: minimising impact on appearance, colour and flavor. AgroFOOD Industry Hi-Tech 22, 22–24. https://www.teknoscienze.com/tks_article/nut-pasteurization-minimising-impact-on-appearance-colour-and-flavour/
James, C., and James, S. J. (1997). Past, Present and Future Methods of Meat Decontamination, 1st Edn. Bristol: University of Bristol Press.
Jeong, S., Marks, B. P., and Ryser, E. T. (2011). Quantifying the performance of Pediococcus sp. (NRRL b-2354: Enterococcus faecium) as a nonpathogenic surrogate for Salmonella enteritidis PT 30 during moist-air convection heating of almonds. J. Food Prot. 74, 603–609. doi: 10.4315/0362-028X.JFP-10-416
Joseph, B., Otta, S. K., Karunasagar, I., and Karunasagar, I. (2001). Biofilm formation by Salmonella spp. on food contact surfaces and their sensitivity to sanitizers. Int. J. Food Microbiol. 64, 367–372. doi: 10.1016/S0168-1605(00)00466-9
Julian, E., Macdonald, K., Marsden-Haug, N., Bonavolante, R., Otero, S., Nosari, J., et al. (2010). Salmonella Montevideo infections associated with salami products made with contaminated imported black and red pepper-United States, July 2009-April 2010. Morb. Mortal. Wkly. Rep. 59, 1647–1650. https://www.cdc.gov/mmwr/preview/mmwrhtml/mm5950a3.htm
Kang, D. H., and Fung, D. Y. C. (2000). Application of thin agar layer method for recovery of injured Salmonella typhimurium. Int. J. Food Microbiol. 54, 127–132. doi: 10.1016/S0168-1605(99)00174-9
Kataoka, A., Enache, E., Black, D. G., Elliott, P. H., Napier, C. D., Podolak, R., et al. (2014). Survival of Salmonella Tennessee, Salmonella typhimurium DT104, and Enterococcus faecium in peanut paste formulations at two different levels of water activity and fat. J. Food Prot. 77, 1252–1259. doi: 10.4315/0362-028X.JFP-13-553
Keller, S. E., VanDoren, J. M., Grasso, E. M., and Halik, L. A. (2013). Growth and survival of Salmonella in ground black pepper (Piper nigrum). Food Microbiol. 34, 182–188. doi: 10.1016/j.fm.2012.12.002
Lilie, M., Hein, S., Wilhelm, P., and Mueller, U. (2007). Decontamination of spices by combining mechanical and thermal effects–an alternative approach for quality retention. Int. J. Food Sci. Tech. 42, 190–193. doi: 10.1111/j.1365-2621.2006.01204.x
Mafart, P., Couvert, O., Gaillard, S., and Leguerinel, I. (2002). On calculating sterility in thermal preservation methods: application of the Weibull frequency distribution model. Int. J. Food Microbiol. 72, 107–113. doi: 10.1016/S0168-1605(01)00624-9
Molnár, H., Bata-Vidács, I., Baka, E., Cserhalmi, Z., Ferenczi, S., Tömösközi-Farkas, R., et al. (2018). The effect of different decontamination methods on the microbial load, bioactive components, aroma and colour of spice paprika. Food Control 83, 131–140. doi: 10.1016/j.foodcont.2017.04.032
Morgan, A. I., Goldberg, N., Radewonuk, E. R., and Scullen, O. J. (1996). Surface pasteurization of raw poultry meat by steam. LWT Food Sci. Technol. 29, 447–451. doi: 10.1006/fstl.1996.0068
Pafumi, J. (1986). Assessment of the microbiological quality of spices and herbs. J. Food Prot. 49, 958–963. doi: 10.4315/0362-028X-49.12.958
Shah, M. K., Asa, G., Sherwood, J., Graber, K., and Bergholz, T. M. (2017). Efficacy of vacuum steam pasteurization for inactivation of Salmonella PT 30, Escherichia coli O157:H7 and Enterococcus faecium on low moisture foods. Int. J. Food Microbiol. 244, 111–118. doi: 10.1016/j.ijfoodmicro.2017.01.003
Tang, H., Chen, W., Dou, Z. M., Chen, R., Hu, Y., Chen, W., et al. (2017). Antimicrobial effect of black pepper petroleum ether extract for the morphology of Listeria monocytogenes and Salmonella typhimurium. J. Food Sci. Technol. 54, 2067–2076. doi: 10.1007/s13197-017-2644-2
Toledo, R. T. (1991). Fundamentals of Food Process Engineering, 2nd Edn. New York, NY: Van Nostrand Reinhold.
Van Doren, J. M., Kleinmeier, D., Hammack, T. S., and Westerman, A. (2013a). Prevalence, serotype diversity, and antimicrobial resistance of Salmonella in imported shipments of spice offered for entry to the United States, FY2007-FY2009. Food Microbiol. 34, 239–251. doi: 10.1016/j.fm.2012.10.002
Van Doren, J. M., Neil, K. P., Parish, M., Gieraltowski, L., Gould, L. H., and Gombas, K. L. (2013b). Foodborne illness outbreaks from microbial contaminants in spices, 1973–2010. Food Microbiol. 36, 456–464. doi: 10.1016/j.fm.2013.04.014
Vij, V., Ailes, E., Wolyniak, C., Angulo, F. J., and Klontz, K. C. (2006). Recalls of spices due to bacterial contamination monitored by the US Food and drug administration: the predominance of Salmonellae. J. Food Prot. 69, 233–237. doi: 10.4315/0362-028X-69.1.233
Villa-Rojas, R., Tang, J., Wang, S., Gao, M., Kang, D. H., Mah, J. H., et al. (2013). Thermal inactivation of Salmonella enteritidis PT 30 in almond kernels as influenced by water activity. J. Food Prot. 76, 26–32. doi: 10.4315/0362-028X.JFP-11-509
Keywords: Salmonella, Enterococcus faecium, surrogate, steam, peppercorns, cumin
Citation: Newkirk JJ, Wu J, Acuff JC, Caver CB, Mallikarjunan K, Wiersema BD, Williams RC and Ponder MA (2018) Inactivation of Salmonella enterica and Surrogate Enterococcus faecium on Whole Black Peppercorns and Cumin Seeds Using Vacuum Steam Pasteurization. Front. Sustain. Food Syst. 2:48. doi: 10.3389/fsufs.2018.00048
Received: 18 May 2018; Accepted: 18 July 2018;
Published: 07 August 2018.
Edited by:
Joshua B. Gurtler, Agricultural Research Service (USDA), United StatesReviewed by:
Tam L. Mai, IEH Laboratories and Consultant Group, United StatesMieke Uyttendaele, Ghent University, Belgium
Elizabeth Grasso-Kelley, Illinois Institute of Technology, United States
Copyright © 2018 Newkirk, Wu, Acuff, Caver, Mallikarjunan, Wiersema, Williams and Ponder. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Monica A. Ponder, mponder@vt.edu
 Jordan J. Newkirk
Jordan J. Newkirk  Jian Wu
Jian Wu Jennifer C. Acuff
Jennifer C. Acuff Monica A. Ponder
Monica A. Ponder