Morphological characteristics and biological cycle of the hoverfly Eristalinus arvorum (Fabricius, 1787) (Diptera, Syrphidae)
- 1College of Plant Protection, Hunan Agricultural University, Changsha, China
- 2Hunan Agricultural Biotechnology Research Institute, Changsha, China
The hoverfly Eristalinus arvorum (Fabricius, 1787) (Diptera: Syrphidae), which belongs to the tribe Eristalini, is well known as a pollinating agent for crops and flowering plants in agricultural and natural ecosystems. Large quantity, wide distribution and their ecological function of the hoverfly E. arvorum make them an appropriate candidate for use as pollinators and environmental indicator species. However, little information has been known on the morphology and the biological cycle of the hoverfly. In this study, feeding experiments under artificial climate chamber and morphological qualitative and quantitative observations were carried out to study the morphology and the biological cycle of the hoverfly E. arvorum. The morphology of eggs, larvae, pupae, and adults of E. arvorum were described in detail for the first time. A complete generation of E. arvorum lasts about 30.12 ± 0.14 days, and there is no significant difference in body length between males (10.27 ± 0.29 mm) and females (11.16 ± 0.45 mm). The most noteworthy morphological features of E. arvorum are the stripes on the compound eyes, the mesonotum, and the abdomen of adults, the chorionic structure consisting of fusiform units on the egg's surface, and the anterior spiracles and pupal spiracles of the pupae. Thus, the detailed description based on morphology and life history will provide the basis for the identification, biodiversity conservation and artificial breeding of the hoverfly E. arvorum.
Introduction
Hoverflies belong to the family Syrphidae (Insecta: Diptera) and are one of the most diverse groups in Diptera. At present, there are more than 9,600 known species of hoverflies in the world (Montoya et al., 2012; Zhou et al., 2021), with China being home to 800 of those species from 200 different genera, accounting for one-tenth of the world's species. The life habits of hoverfly larvae are complex and can be divided into predatory, phytophagous, and saprophagous hoverflies based on their feeding habits (Heiss, 1938; Reemer, 2013). The larvae of predatory hoverflies are important natural enemies and can prey on aphids, scale insects, and plant hoppers (Sarthou et al., 2005; Rijn et al., 2013). The larvae of saprophagous hoverflies are the decomposers of ecosystems, feeding on decaying animals and plants, animal feces, and other organic material (Hodson, 1932; Ricarte et al., 2011; Mielczarek et al., 2016; Campoy et al., 2020a). Some of the larvae of phytophagous hoverflies are pests and feed on the roots, stems, and leaves of plants (Creager and Spruijt, 1935; Ricarte et al., 2008, 2017; Nunes-Silva et al., 2010; Dumbardon-Martial, 2016).
Most adult hoverflies visit flowers, feeding on pollen and nectar to supplement nutrition and complete sexual development (Grković et al., 2015; Djellab et al., 2019), they are well-known pollinators of crops, ornamentals, and wild angiosperms (Klecka et al., 2018). Hoverflies are second only to bees as pollinators and even better than bees for some plants (Gladis, 1996). Sánchez et al. (2022) found that pollination by hoverflies could improve the yield and fruit quality of mango under protected cultivation (Sánchez et al., 2022). Ollerton et al. (2012) found that hoverflies play a greater role in pollination than western honeybees. For some plants, hoverflies are the only pollinators; for example, only two species of hoverflies have been found to pollinate the Paphiopedidae (Bänziger, 2002).
Not only do the adults of saprophagous hoverflies visit a variety of flowers, but their larvae can also serve as pollution indicators (Burgio and Sommaggio, 2007). The larvae appear in places with a lot of bacteria in the water and can remove decaying organic material that pollutes the environment (Sommaggio and Burgio, 2014; Moquet et al., 2018; Dunn et al., 2020). Saprophagous hoverflies prey on ~45% of all known aphids in China. Eristalinus arvorum belongs to Eristalinus Rondani 1945, the subfamily Eristalinae, and is one of the dominant species of hoverflies in southern China. The adults of this species visit a wide range of flowers (Layek et al., 2022) and are commonly found on flowers of vegetable and cash crops such as Brassicaceae, Asteraceae, Labiatae, Rosaceae, Umbelliferae, Leguminosae, and Liliaceae (Van de Weyer and Dils, 1999; Dousti and Hayat, 2006; Almohamad et al., 2009). However, limited research has been conducted on E. arvorum. The main objective of this research was to study the morphological characteristics and the biological cycle of E. arvorum to improve the artificial rearing and crop pollination applications of this species.
Materials and methods
Sample collection
Adults of E. arvorum were collected in the field and kept in a bug dorm (30 cm × 30 cm × 30 cm) with tea pollen and 20% honey water for feeding and soaked grains for laying eggs. Each egg was placed separately in a Petri dish with artificial diets for hatching. When the larvae reached their third instar, they were put into a container with a layer of sawdust for pupation. Pupae were isolated in individual Petri dishes and inspected daily until the emergence of adults. Rearing of E. arvorum was performed in a growth chamber at 25 ± 1°C, 70 ± 5% RH, and a constant photoregime of 12L:12D. A total of 120 eggs were selected and raised well into adulthood, and their life histories were observed and recorded daily.
Third-instar larvae were selected as samples for preservation. For permanent preservation, larvae were immersed in cold water to extend them and then heated slowly for about 4 min to kill them. After this, they were preserved in 70% alcohol (Pérez-Bañón et al., 2013; Campoy et al., 2020b). Descriptions were based on preserved specimens, with larval characters checked against living specimens to minimize errors due to preservation (Pérez-Bañón et al., 2013).
Morphological image
Samples of the hoverfly E. arvorum from eggs, the first-instar, second-instar, and third-instar larvae, pupae, and adults were collected during the rearing process and then killed cryogenically. The samples were fixed on the sample stage with the double-sided conductive tape of the scanning electron microscope (SEM) and then sputtered with a thin layer of gold (Pérez-Bañón et al., 2013; Campoy et al., 2020b). Once the samples were prepared for observation, they were transferred to the SEM. Secondary electron images were observed and recorded by an SEM-6380LV scanning electron microscope. Eggs, pupal spiracles, and anterior spiracles with a low water content were studied by SEM and others using a stereomicroscope with an imaging system.
Statistical analysis
Twenty samples of male and female adults were measured using the microscopic image measurement software Digimizer 3.2. SPSS 19.0 software was used for conducting the statistical analysis, and the independent samples t-test was used to analyze the difference in the bodies of male and female adults.
Results
Biological habits
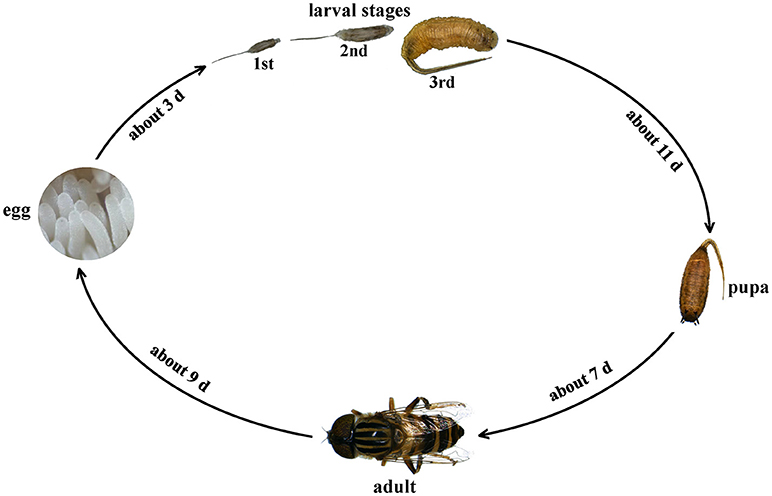
The individual development of E. arvorum goes through four stages: eggs, larvae, pupae, and adults, and the larval stage goes through three instars. E. arvorum can be reared in captivity, and a complete generation lasts about 30.12 ± 0.14 days (Figure 1). The life history varies with temperature. At a temperature of 25°C, the egg stage lasts about 3.24 ± 0.05 days, and the larval stage lasts about 11.38 ± 0.33 days. It takes about 3.53 ± 0.16 days from the first-st to the second-instar larvae, about 4.12 ± 0.02 days from the second- to the third-instar larva, and about 4.28 ± 0.03 days from the third-instar larva to pupal stage. The pupal stage lasts about 7.24 ± 0.13 days. Adult development to sexual maturity takes about 9.38 ± 0.02 days. Larvae can feed on rotten grains, and pupae can pupate on sawdust. Adults feed on nectars and pollens to complete their development and reproduction, enjoy the sun, and live for about 1 to 2 months. The female adult lays 100–150 eggs at a time.
Adult body data
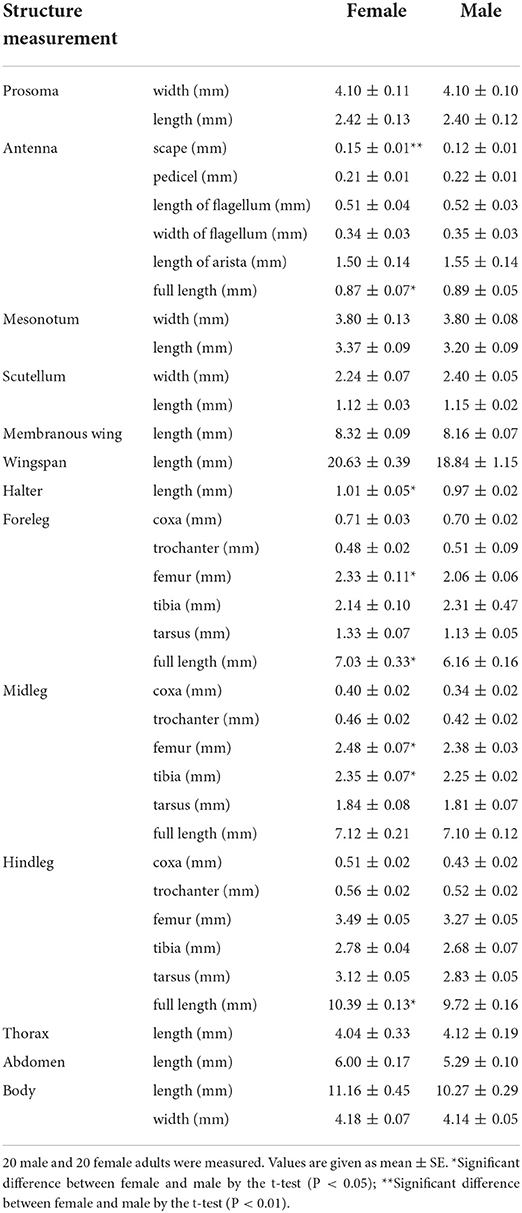
The adult body of E. arvorum is divided into three parts: the prosoma, the mesosoma, and the abdomen. The prosoma bears a pair of antennae, which are divided into the scape, the pedicel, and the flagellum from base to end. The scape in females is significantly longer than that in males (Table 1, P < 0.01), but the overall length of the antenna is shorter in females than in males (Table 1, P < 0.05). The mesosoma bears two pairs of wings and three pairs of legs. The foreleg and hindleg are significantly longer in females than in males (Table 1, P < 0.05). The body length of females is slightly longer than that of males, although there is no significant difference (Table 1).
Description of the morphological characteristics
Egg
The mean lengths of eggs are 1.2 ± 0.12 mm, and the mean maximum widths are 0.35 ± 0.04 mm. Each female adult can lay dozens to hundreds of eggs at a time, depending on the nutritional conditions. Eggs are white when recently laid, become light gray before hatching, and become elongated and rounded at both extremes (Figures 2A,B). The chorionic structure comprises fusiform units, which are branched and deeply hollowed in the middle (Figures 2C,D). The ventral surface is slightly flattened, whereas the dorsal side is convex (Figure 2E). There is a small bulge at the thinner end of the fertilization hole (Figure 2F).

Figure 2. Egg of Eristalinus arvorum: (A) front view of the egg, (B) the thinner end of the egg, (C) egg reticulation, (D) egg reticulation, (E) egg top view, (F) egg hole.
Larva
The larvae are called “rat-tailed maggots” because they have a very long anal segment and a telescopic breathing tube that can extend to the surface of the water. The body is roughly cylindrical with a sub-cylindrical cross-section. The body color is white in the early stage and becomes gray-brown in the later stage. The dorsal and ventral surfaces are slightly flat. The front end is blunt, and the rear end is tapered. The ventral surface is flat and covered with short spicules, while the dorsal side is slightly convex and fully covered with long pubescence backward. The cuticle appears milky-light gray and is slightly translucent when the larva is alive. The first instar larvae (Figures 3A,B) and the second instar larvae (Figures 3C,D) were similar to the third instar larvae (Figures 3E,F) in general morphology. Excluding the posterior trachea, the body lengths of the first instar larvae are 1.51 ± 0.04 to 7.02 ± 0.33 mm (Figures 3A,B), the second instar larvae are 7.02 ± 0.33 to 11.27 ± 0.18 mm (Figures 3C,D) and the third instar larvae are 11.27 ± 0.18 to 23.21 ± 0.07 mm (Figures 3E,F). The body length may vary slightly depending on factors such as environment and food. The body surface has many wrinkled rings, and the somite is not obvious (Figures 3E,F).
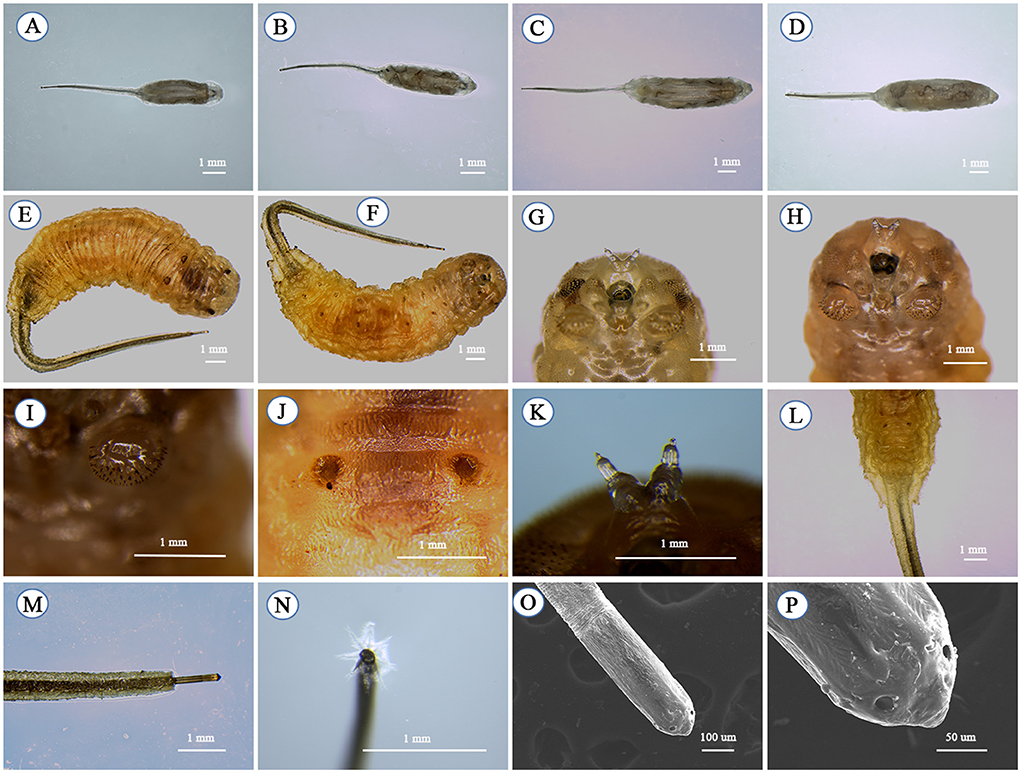
Figure 3. Larva of Eristalinus arvorum; first-instar larvae: (A) dorsal view and (B) ventral view; second-instar larvae: (C) dorsal view and (D) ventral view; third-instar larvae: (E) dorsal view, (F) ventral view, (G) frontal view, (H) frontal view, (I) midthoracic gastropod, (J) pupal respiratory horn foramen, (K) antennomaxilary organs, (L) the anus, (M) posterior tracheal canal, (N) posterior trachea plate tip feathers, (O) posterior valve tip, and (P) fissure.
The third-instar larvae have stout, curved abdominal legs in the mesothorax and 1–6 abdominal segments with toe grooves (Figures 3G–I). Two dots are visible on the back (Figure 3J), where two pupal respiratory horns grow during the pupal stage. The anal process is prominent, and the long digital process retracts into the anus (Figure 3L). A pair of antennomaxilary organs are at the top of the head (Figure 3K). The rear valve is located on the back of the distal segment and attached to a single seat (Figures 3L,M), a telescopic breathing tube extending several times the larva's body length (Figure 3M). Eight feather-like hairs on the end of the rear valve help the rear valve float on the water to breathe while the larva is feeding underwater (Figure 3N). The two plates of the rear valve are closely connected to form a single oval plate with four slits arranged in parallel (Figures 3O,P).
Pupa
The pupa is similar in shape to the larva. The body is pale gray and in the shape of a droplet with a “little tail” at the end of the abdomen that is slightly tan (Figures 4A,B). There is a pair of anterior spiracles at the front of the head and a pair of pupal respiratory horns at the back (Figure 4A). The first to sixth abdominal segments have a pair of abdominal legs (Figure 4B) but they are not prominent. There were no pupal respiratory horns at the early stage of pupation, and the anterior stomata were not obvious. At the later stage of pupation, a pair of pupal spiracles grew (Figures 4C,D) from the foramen of the respiratory horns. The alveolar opening is growing in the front of the pupal spiracles (Figure 4E). There are alveolar openings on the front of the pupal spiracles, varying in number from 4 to 8 (Figures 4F–L). The pupal spiracles folds are covered with villi in a layered distribution (Figure 4M), and there are small irregular bulges at the base with hairs on them (Figures 4N,O). There are breathing openings on the alveolar plates of the anterior spiracles (Figure 4P).
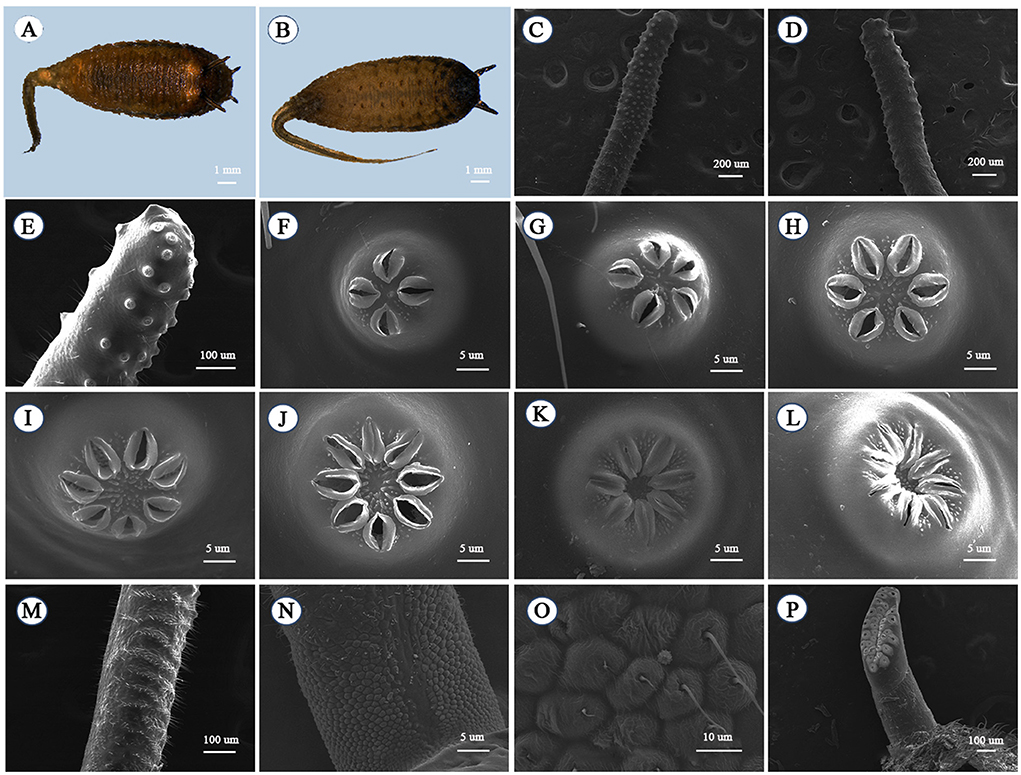
Figure 4. Pupa of Eristalinus arvorum: (A) dorsal view and (B) pupa ventral view; pupal spiracle: (C) frontal view, (D) back view, and (E) partial frontal view; spiracular openings: (F) four-opening, (G) five-opening, (H) six-opening, (I) seven-opening, (J) eight-opening, (K) seven closed-mouth, and (L) eight closed-mouth; (M) pupal spiracle projection base; (N) ornamentation on basal part; (O) ornamentation on basal part; and (P) anterior spiracle.
Adult
The compound eyes with different-sized dark spots are dichoptic in females (Figures 5A,E) and holoptic in males (Figures 5C,D). The upper part of the compound eye has dense dark brown short fluff (Figures 5A,C), and the lower part is nearly naked (Figures 5B,D). The vertical bristle is black (Figure 5E). The front is extremely short and slightly convex, covered with grayish-yellow or yellow fluffs and long black hairs (Figures 5E,G). At the top of the head are three ocelli arranged in a triangle (Figures 5E,G). Adults have licking mouthparts (Figures 5F,H). In males, the length between compound eyes is about 1.5 times the length of the triangle on top of the head (Figure 5G). The orange antennae are composed of three segments; the first and second are short, and the third section is oval (Figure 5I).

Figure 5. Adult of Eristalinus arvorum: female: (A) dorsal view, (B) ventral view, (E) prosoma frontal view, and (F) prosoma dorsal view; male: (C) dorsal view, (D) ventral view, (G) prosoma frontal view, (H) prosoma dorsal view, (I) antennae, (J) wing membrane, (K) halter, (L) foreleg, (M) midleg, (N) hindleg, (O) ovipositor and gonostylus, and (P) gonosaccus and gonostylus.
The mesonotum is bright black with yellow fluff. On the mesonotum, five yellow-gray stripes extend from the front end to the posterior end, with a thin one in the middle and a transverse stripe at the posterior end. The five yellow-gray stripes are thinner in females than in males (Figures 5A,C). The scutellum is pale yellow or bright brown, sometimes with a metallic gloss, with shorter black hair in the middle and long yellow hair on the sides and the ends (Figures 5A,C). A pair of wings are attached to the mesonotum, and the wing membrane of the forewings is transparent (Figure 5J); the hindwings degenerate into halteres (Figure 5K), and the calypteres are black and brown. Three pairs of mesothoracic prolegs are usually brown-yellow or brown-red (Figures 5B,C). The tibia ends of the foreleg and the midleg and the tibiae of the hindleg are black except for the most basal part, and the tarsi ends are brown. Microvilli are on the femora, the tibiae, and the tarsi (Figures 5L–N).
The first abdomere of the abdomen is yellowish to reddish yellow with a shiny skin, and there are red or orange square spots almost over the second abdomere. The abdomen in females is longer than in males. There are yellow lateral stripes on the abdominal segments 2–4 and a pair of yellow lateral spots on segment 5 (Figures 5A,C). At the end of the abdomen is an ovipositor and the gonostylus in females (Figure 5O) and a gonosaccus and the gonostylus in males (Figure 5P).
Discussion
The chorionic structure of the eggs of E. arvorum fits with the descriptions of other Eristalinus species. E. arvorum, Eristalinus taeniops, Eristalinus aeneus, and Eristalinus punctulatus present a chorionic structure with branched fusiform units, but these units, in E. aeneus, are not sunk in the middle (Zalat and Mahmoud, 2009; Campoy et al., 2020b). In the case of E. taeniops, the branches are broader (Zalat and Mahmoud, 2009), although the general pattern is similar and difficult to distinguish from E. arvorum. An adult lays 100 to 150 eggs in masses, which is conducive to collection and feeding.
Despite there being about 75 known species of Eristalinus (Sonet et al., 2019), only five species have had their larval stage described: Eristalinus sepulchralis, Eristalinus megacephalus, E. aeneus, E. taeniops, and E. punctulatus (Pérez-Bañón et al., 2003; Zalat and Mahmoud, 2009; Campoy et al., 2020b). Traditionally, the main diagnostic characters of the larval morphology are found in the transverse band of spicules (number of crochets) and the anterior spiracles (length-width proportion of the spiracle and extension of the spiracular plate relative to the total spiracular length) (Pérez-Bañón et al., 2003; Zalat and Mahmoud, 2009; Campoy et al., 2020b). However, Campoy et al. (2020b) concluded that these characteristics are unreliable for diagnosis by closely examining the morphology of the above species. We found these characteristics of E. arvorum to be similar and difficult to distinguish from those of the above species.
Regarding the pupal stage, Eristalinus species can be separated by the pupal spiracles, following the key provided by Pérez-Bañón et al. (2003). Because there is scant research on the larvae and the pupae of Eristalinus species, Eristalinus species are usually identified as adults.
The identification of E. arvorum mainly focuses on the adult stage. We found that the main diagnostic characteristics of adults are the stripes on their compound eyes, the mesonotum, and the abdomen. Although the pollination efficiency of E. arvorum has not been previously assessed, other Eristalinus species have been found to be good pollinators with high floral constancy and positive pollination perspectives (Huda et al., 2015; Latif et al., 2019; Campoy et al., 2020b). For example, E. punctulatus is an excellent candidate to be artificially reared and tested as a commercial pollinator in Australia, especially under greenhouse conditions (Campoy et al., 2020b). Eristalinus aeneus could be effective pollinators of chickpea and mango trees (Huda et al., 2015; Latif et al., 2019; Sánchez et al., 2022). The morphological photographs of adults in Figures 5A–D show that the chest, the abdomen, and the legs of E. arvorum are covered with dense hair, which enables it to carry a large amount of pollen and have high pollination efficiency. In the preliminary field research, we also found that this species can carry large amounts of pollen. The adults of this species have a long lifespan and can live for 2–3 months in our laboratory with sufficient time for pollination. Even though there are fewer flowers in the winter, this species can still be harvested from November to January in Hunan Province, China, due to its high tolerance for cold and hunger. The large number of eggs this species lays is conducive to large-scale artificial production. We have been able to artificially rear E. arvorum in captivity, although the feeding system still needs to be improved to make it more efficient. In this process, we found that the composition and temperature of the artificial feed are the key factors affecting its growth and development. Further research is needed to determine the best feeding method.
Data availability statement
The original contributions presented in the study are included in the article/supplementary material, further inquiries can be directed to the corresponding author.
Author contributions
LC, QZ, and YZ conceived and designed the project. LC and QZ conducted the experiments and wrote the original draft. LC and QR collected the sample. LC, QZ, and AZ analyzed the data. YZ revised and edited the manuscript. All authors approved the final version of the manuscript.
Funding
This project was supported by the Scientific Research Fund of the Hunan Provincial Education Department (20B295).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Almohamad, R., Verheggen, F., and Haubruge, É. (2009). Searching and oviposition behavior of aphidophagous hoverflies (Diptera: Syrphidae): a review. Biotechnologie, Agronomie, Société et Environnement. 13, 467–481.
Bänziger, H. (2002). “Smart alecks and dumb flies: natural pollination of some wild lady slipper orchids (Paphiopedilum spp., Orchidaceae).” in Proceedings of the 16th World Orchid Conference, Eds J. Clark, W. M. Elliott, G. Tingley, and J. Biro (Vancouver: Vancouver Orchid Society), 165–169.
Burgio, G., and Sommaggio, D. (2007). Syrphids as landscape bioindicators in Italian agroecosystems. Agric. Ecosyst. Environ. 120, 416–422. doi: 10.1016/j.agee.2006.10.021
Campoy, A., Pérez-Bañón, C., and Aznar, D. (2020b). Description of the preimaginal stages of the golden native dronefly from Australia, Eristalinus punctulatus (Macquart, 1847) (Diptera: Syrphidae). Aust. Entomol. 59, 784–793. doi: 10.1111/aen.12497
Campoy, A., Pérez-Bañón, C., and Rojo, S. (2020a). Intra-puparial development in the hoverflies Eristalinus aeneus and Eristalis tenax (Diptera: Syrphidae). J. Morphol. 281, 1436–1445. doi: 10.1002/jmor.21257
Creager, D. B., and Spruijt, F. J. (1935). The relation of certain fungi to larval development of Eumerus tuberculatus Rond. (Syrphidae, Diptera). Ann. Entomol. Soc. Am. 28, 425–437. doi: 10.1093/aesa/28.4.425
Djellab, S., Mebarkia, N., Neffar, S., and Chenchouni, H. (2019). Diversity and phenology of hoverflies (Diptera: Syrphidae) in pine forests (Pinus halepensis Miller) of Algeria. J. Asia-Pac. Entomol. 22, 766–777. doi: 10.1016/j.aspen.2019.05.012
Dousti, A. F., and Hayat, R. (2006). A catalogue of the syrphidae (insecta: diptera) of Iran. J. Entomol. Res. 8, 5–38.
Dumbardon-Martial, E. (2016). Pollen feeding in the larva of Toxomerus pulchellus (Diptera, Syrphidae). Bull. Soc. entomol. Fr. 121, 413–420. doi: 10.3406/bsef.2016.2778
Dunn, L., Lequerica, M., Reid, C. R., and Latty, T. (2020). Dual ecosystem services of syrphid flies (Diptera: Syrphidae): pollinators and biological control agents. Pest Manag. Sci. 76, 1973–1979. doi: 10.1002/ps.5807
Gladis, T. (1996). Bees versus flies?-rearing methods and effectiveness of pollinators in crop germplasm regeneration. VII International Symposium on Pollination. 437, 235–238. doi: 10.17660/ActaHortic.1997.437.25
Grković, A., Vujić, A., Radenković, S., and Petanidou, T. (2015). Diversity of the genus Eumerus Meigen (Diptera, Syrphidae) on the eastern Mediterranean islands with description of three new species. Ann. Soc. Entomol. Fr. 51, 361–373. doi: 10.1080/00379271.2016.1144483
Heiss, E. M. (1938). A classification of the larvae and puparia of the syrphidae of illinois exclusive of aquatic forms. Ann. Entomol. Soc. Am. 36, 1–142. doi: 10.5962/bhl.title.50277
Hodson, W. E. H. (1932). A comparison of the larvae of Eumerus strigatus, Fln., and Eumerus tuberculatus, Rond. (Syrphidae). Bull. Entomol. Res. 23, 247–249. doi: 10.1017/S0007485300004168
Huda, A. N., Salmah, M. R., and Hassan, A. A. (2015). Pollination services of mango flower pollinators. J. Insect Sci. 15, 113. doi: 10.1093/jisesa/iev090
Klecka, J., Hadrava, J., Biella, P., and Akter, A. (2018). Flower visitation by hoverflies (Diptera: Syrphidae) in a temperate plant-pollinator network. Peer J. 6, 1–23. doi: 10.7717/peerj.6025
Latif, A., Malik, S. A., and Saeed, S. (2019). Diversity of pollinators and their role in the pollination biology of chickpea, Cicer arietinum L. (Fabaceae). J. Asia-Pac. Entomol. 22, 597–601. doi: 10.1016/j.aspen.2019.03.009
Layek, U., Das, A., and Karmakar, P. (2022). Supplemental stingless bee pollination in fennel (Foeniculum vulgare Mill.): an assessment of impacts on native pollinators and crop yield. Front. Sustain. Food Syst. 6, 820264. doi: 10.3389/fsufs.2022.820264
Mielczarek, L. E., Oleksa, A., Meyza, K., and Tofilski, A. (2016). Seasonal polyphenism in Eristalis pertinax (Diptera: Syrphidae). Eur. J. Entomol.113, 489. doi: 10.14411/eje.2016.064
Montoya, A. L., Pérez, S. P., and Wolff, M. (2012). The diversity of flower flies (Diptera: Syrphidae) in Colombia and their neotropical distribution. Neotrop. Entomol. 41, 46–56. doi: 10.1007/s13744-012-0018-z
Moquet, L., Laurent, E., and Bacchetta, R. (2018). Conservation of hoverflies (Diptera, Syrphidae) requires complementary resources at the landscape and local scales. Insect Conserv. Divers. 11, 72–87. doi: 10.1111/icad.12245
Nunes-Silva, P., Cordeiro, G. D., and Obregon, D. (2010). Pollenivory in larval and adult flower flies: pollen availability and visitation rate by Toxomerus politus SAY (Diptera: Syrphidae) on sorghum Sorghum bicolor (L.) MOENCH (Poaceae). Stud. Dipterol. 17, 177–185.
Ollerton, J., Price, V., and Armbruster, W. S. (2012). Overplaying the role of honey bees as pollinators: a comment on Aebi and Neumann. Trends Ecol. Evol. 27, 141–142. doi: 10.1016/j.tree.2011.12.001
Pérez-Bañón, C., Hurtado, P., García-Gras, E., and Rojo, S. (2013). SEM studies on immature stages of the drone flies (Diptera, Syrphidae): Eristalis similis (Fallen, 1817) and Eristalis tenax (Linnaeus, 1758). Microsc. Res. Tech. 76, 853–861. doi: 10.1002/jemt.22239
Pérez-Bañón, C., Rojo, S., Ståhls, G., and Marcos-García, M. (2003). Taxonomy of European Eristalinus (Diptera: Syrphidae) based on larval morphology and molecular data. Eur. J. Entomol. 100, 417–428. doi: 10.14411/eje.2003.064
Reemer, M. (2013). Taxonomic exploration of neotropical microdontinae (Diptera: Syrphidae) mimicking stingless bees. Zootaxa. 3697, 1–88. doi: 10.11646/zootaxa.3697.1.1
Ricarte, A., Ángeles Marcos-García, M., and Moreno, C. E. (2011). Assessing the effects of vegetation type on hoverfly (Diptera: Syrphidae) diversity in a Mediterranean landscape: implications for conservation. J. Insect Conserv. 15, 865–877. doi: 10.1007/s10841-011-9384-9
Ricarte, A., Marcos-García, M. Á., and Rotheray, G. (2008). The early stages and life histories of three Eumerus and two Merodon species (Diptera: Syrphidae) from the Mediterranean region. Entomol. Fenn. 19, 129–141. doi: 10.33338/ef.84424
Ricarte, A., Souba-Dols, G. J., Hauser, M., and Marcos-García, M. Á. (2017). A review of the early stages and host plants of the genera Eumerus and Merodon (Diptera: Syrphidae), with new data on four species. PLoS ONE. 12, e0189852. doi: 10.1371/journal.pone.0189852
Rijn, P. V., Kooijman, J., and Wäckers, F. L. (2013). The contribution of floral resources and honeydew to the performance of predatory hoverflies (Diptera: Syrphidae). Biol. Control. 67, 32–38. doi: 10.1016/j.biocontrol.2013.06.014
Sánchez, M., Velásquez, Y., González, M., and Cuevas, J. (2022). Hoverfly pollination enhances yield and fruit quality in mango under protected cultivation. Sci. Hortic. 304, 111320. doi: 10.1016/j.scienta.2022.111320
Sarthou, J. P., Ouin, A., Arrignon, F., Barreau, G., and Bouyjou, B. (2005). Landscape parameters explain the distribution and abundance of Episyrphus balteatus (Diptera: Syrphidae). Eur. J. Entomol. 102, 539. doi: 10.14411/eje.2005.077
Sommaggio, D., and Burgio, G. (2014). The use of Syrphidae as functional bioindicator to compare vineyards with different managements. Bull. Insectology. 67, 147–156.
Sonet, G., De Smet, Y., and Tang, M. (2019). First mitochondrial genomes of five hoverfly species of the genus Eristalinus (Diptera: Syrphidae). Genome. 62, 677–687. doi: 10.1139/gen-2019-0009
Van de Weyer, G., and Dils, J. (1999). Contribution to the knowledge of the Syrphidae from Greece (Diptera: Syrphidae). Phegea. 27, 69–77.
Zalat, S. M., and Mahmoud, M. A. M. (2009). Surface ultrastructure of the immature stages of eristalines (Diptera: Syrphidae) from Egypt. Egypt. J. Biol. 11, 99–140.
Keywords: pollinating insect, Eristalinus arvorum, hoverfly, morphology, electron micrograph
Citation: Cao L, Zeng Q, Ren Q, Zeng A and Zhang Y (2022) Morphological characteristics and biological cycle of the hoverfly Eristalinus arvorum (Fabricius, 1787) (Diptera, Syrphidae). Front. Sustain. Food Syst. 6:1052908. doi: 10.3389/fsufs.2022.1052908
Received: 24 September 2022; Accepted: 03 November 2022;
Published: 24 November 2022.
Edited by:
Wen Xie, Institute of Vegetables and Flowers (CASS), ChinaReviewed by:
Yuqian Feng, Chinese Academy of Forestry, ChinaGang Wugang, Huazhong Agricultural University, China
Copyright © 2022 Cao, Zeng, Ren, Zeng and Zhang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yongsheng Zhang, yshzhang@hunau.edu.cn
†These authors have contributed equally to this work
 Liang Cao1†
Liang Cao1†  Yongsheng Zhang
Yongsheng Zhang