Diet quality and nutritional status of HIV-exposed children aged between 6 and 18 months in the Greater Accra Region of Ghana
- 1Noguchi Memorial Institute for Medical Research, University of Ghana, Accra, Ghana
- 2Department of Child Health, Korle Bu Teaching Hospital, Accra, Ghana
- 3Department of Medicine, Korle Bu Teaching Hospital, Accra, Ghana
- 4Department of Child Health, University of Ghana Medical School, College of Health Sciences, Accra, Ghana
- 5Princess Marie Louise Children’s Hospital, Accra, Ghana
- 6International Food Policy Research Institute, Washington, DC, United States
Introduction: HIV-exposed children, even when uninfected, have a greater risk of malnutrition than unexposed counterparts. WHO guidelines recommend children aged 6–23 months be fed a variety of foods to meet nutrient requirements. This study aimed to determine infant and young child feeding (IYCF) practices among HIV-exposed children under 2 years old enrolled in a dietary intervention and to explore correlates of the IYCF indicators and associations between IYCF and nutritional status.
Methodology: Six hundred and eighty mother–child pairs were recruited from 19 health facilities from the Greater Accra Region. The sociodemographic data, anthropometry, hemoglobin, and dietary intake were recorded.
Results: Ninety-five percent of HIV-positive mothers breastfed their babies, and 53% initiated breastfeeding in a timely manner. Around one in five mothers (21%) introduced liquids other than breastmilk to their children within the first 2 days of birth, and only around one in four children (26%) aged 12–23 months had received breast milk on the day before assessment. Ninety-three percent of babies between 6 and 8 months had been introduced to solid, semi-solid, or soft foods. Eighteen percent of children reached the threshold for Minimum Dietary Diversity (MDD) by eating from over five of eight food groups. Fifty-four percent received Minimum Meal Frequency (MMF), eating between 2 and 4 meals in a day. Eleven percent received the Minimum Acceptable Diet (MAD). Thirty-two percent were anemic. Underweight and stunting were 12 and 11%, respectively. Children of mothers aged 31–40 years were more likely to meet the MDD and MAD [OR = 2.8, 95%CI (1.185, 6.519), p < 0.05 and OR = 2.8, 95%CI (1.256, 6.279), p < 0.05] compared to children of mothers aged 30 years or less or aged above 40 years. Children from households earning more than GHS 500 were more likely to meet MMF than those from households earning less. No associations were found between IYCF and nutritional status.
Conclusion: Findings highlight the need for nutrition programs to educate HIV-exposed children’s caregivers on optimal feeding practices. The importance of continued breastfeeding and dietary diversity needs to be highlighted. Affordable, iron-rich foods should be promoted. Special attention should be paid to younger, less educated, and lower socioeconomic status mothers.
Introduction
Adequate nutrition is necessary for the growth and development of all children, and every child has the right to good nutrition according to the ‘Convention on the Rights of the Child’ (Verhellen, 2000). The first 1,000 days of life are crucial for growth, and optimum nutrition is required in this window to reduce morbidity and mortality and to foster development (WHO and UNICEF, 2021). The World Health Organization reported in 2021 that globally, undernutrition is associated with 45% of child deaths. In 2020, 149 million children under 5 were estimated to be stunted, 45 million were estimated to be wasted, and 38.9 million were overweight or obese (WHO and UNICEF, 2021). Ghana made significant progress between 2008 and 2018 in reducing stunting and anemia in under-fives from 28 to 18% and 76 to 66%, respectively (Ghana Statistical Service GSS, Ghana Health Service GHS, Macro ICF, 2009; Ghana Statistical Service (GSS), 2015; Aryeetey et al., 2022). Children born to HIV-infected mothers are at higher risk of malnutrition compared to those born to HIV-uninfected mothers, even when they themselves are uninfected (McHenry et al., 2019; Yirga et al., 2019).
Globally, approximately 1.8 million children 0–14 years and 1.8 million adolescents 10–19 years were living with HIV in 2017, of whom more than 85% lived in sub-Saharan Africa (Enane et al., 2018). In Ghana, 42,000 children and adolescents aged 0–19 years were living with HIV in 2018 (UNICEF, 2021). Young children usually get infected with HIV through their mothers during pregnancy, labor, or delivery or through breastfeeding; mother-to-child transmission (MTCT) (World Health Organization, 2004). Maternal factors that may lead to an increased risk of HIV transmission include high maternal viral load, maternal malnutrition, vaginal delivery as opposed to planned cesarean section, and oral disease in the baby during breastfeeding (JICA, 2014). Widely implemented policies to prevent mother-to-child transmission include the provision of HIV-positive mothers with lifelong antiretroviral drugs to reduce the transmission through breast milk and recommendations to exclusively breastfeed for 6 months, breastfeed for at least 12 months, and continue breastfeeding for up to 24 months or longer (similar to the general population) while being fully supported for antiretroviral therapy (ART) adherence. It is recommended that when mothers decide to stop breastfeeding at any time, they should do so gradually within 1 month, not abruptly, and feed safe and appropriate food after breastfeeding stops (World Health Organization, 2016). Mothers or infants who have been receiving ARV drug prophylaxis should continue prophylaxis for 1 week after breastfeeding is fully stopped. Stopping breastfeeding abruptly is not advised as there seems to be no benefit of doing so for HIV-free survival at 24 months (Kuhn et al., 2008) or milk HIV RNA viral load (Phiri et al., 2006; Filteau, 2009). On the contrary, it does not give room for psychological adjustment for mother and child and also puts the mother at increased risk of mastitis. Although challenges still exist to the prevention of mother-to-child transmission program coverage in low- and middle-income countries, there has been considerable success, and there are now globally over 15 million children who are HIV-exposed and uninfected (HEU), 90% of whom live in sub-Saharan Africa (Prendergast and Evans, 2023). The fact remains, however, that HIV-exposed children, regardless of their HIV status, are at greater risk of malnutrition and, consequently, to an extent, slower motor and cognitive development than their HIV-unexposed counterparts (Sint et al., 2013; Abu-Raya et al., 2016).
HIV exposure in utero and early life has been linked to immune abnormalities, which are thought to have a negative effect on the child’s response to infection and T cell-dependent antigens during routine vaccination in early life (Afran et al., 2014). In addition, most antiretroviral drugs cross the placenta (Venhoff and Walker, 2006) and have been linked to biological alterations in babies with varying effects on infant health. Third, HIV-1-infected mothers are at increased risk of co-infections, which can harm the health of their babies in utero or after birth. In resource-poor settings, frequent infections such as malaria and measles, together with malnutrition and poor socioeconomic circumstances, make it harder for HIV-exposed infants to recover from these early insults (Glennie et al., 2010), particularly in HIV-affected households, and may contribute to a vicious cycle of malnutrition and infections. Moreover, HIV-infected mothers are more likely to have low birthweight children compared to their uninfected counterparts (Bailey et al., 1999; Marinda et al., 2007), predisposing them to higher rates of morbidity and mortality (Lawn et al., 2005). Optimal feeding practices are of utmost importance to meet the nutritional needs of this vulnerable group.
Infant and young child feeding (IYCF) practices most importantly affect the nutritional status of children under 2 years of age. Early initiation of breastfeeding (within an hour of birth), exclusive breastfeeding for the first 6 months of life, and introduction of nutritionally adequate and safe complementary foods at 6 months together with continued breastfeeding up to 2 years or beyond is recommended by WHO and UNICEF (Pan American Health Organization, 2003; World Health Organization, 2003). Globally, only about 44% of infants below 6 months were exclusively breastfed over the period of 2015 to 2020 (WHO and UNICEF, 2021). In Ghana, the prevalence of exclusive breastfeeding, as reported by the Ghana Demographic Health Survey (GDHS) in 2015, was 52%, with the median duration of exclusive breastfeeding being about 4 months (Ghana Statistical Service (GSS), 2015).
The WHO recommends that children aged between 6 and 23 months of age should be fed from at least 5 out of a total of 8 food groups (breast milk; grains, roots and tubers; legumes and nuts; dairy products; flesh foods; eggs; Vitamin-A rich fruits and vegetables; and other fruits and vegetables) daily. They also recommend that breastfed infants aged 6–8 months should receive at least two feedings of solid, semi-solid, or soft foods, and those aged 9–23 months should receive at least three of such feeds. Non-breastfed children aged 6–23 months should receive at least four of such feeds or milk feeds, of which at least one must be a solid, semi-solid, or soft feed.
In a joint report, WHO and UNICEF outlined indicators for assessing these IYCF breastfeeding and complementary feeding practices (WHO and UNICEF, 2021). Those of interest to this study are listed and defined next. The infant and young child ever breastfed (EvBF) indicator is defined as the percentage of children born in the last 24 months who were ever breastfed. This indicator is useful for assessing the overall acceptance of breastfeeding. The infant and young child early initiation of breastfeeding (EIBF) is defined as the percentage of children born in the last 24 months who were put to the breast within 1 h of birth. Infant and young children exclusively breastfed for the first 2 days after birth (EBF2D) is defined as the percentage of children born in the last 24 months who were fed exclusively with breast milk for the first 2 days after birth. Continued breastfeeding between 12 and 23 months (CBF) is defined as the percentage of children 12–23 months of age who were fed breast milk during the previous day and replaced previous indicators that assessed breastfeeding at 1 year and 2 years of age.
The infant and young child minimum dietary diversity (MDD) is defined as the percentage of children 6–23 months of age who consumed foods and beverages from at least five out of eight defined food groups during the previous day. MDD is the most widely used maternal and child health (MCH) dietary metric and is routinely collected in studies in low- and middle-income countries (Miller et al., 2020). Infant and young child minimum meal frequency (MMF) is defined as the percentage of children 6–23 months of age who consumed solid, semi-solid, or soft foods (but also including milk feeds for non-breastfed children) at least the minimum number of times recommended during the previous day. For breastfeeding children, the infant and young children’s minimum acceptable diet (MAD) is defined as receiving at least the minimum dietary diversity and minimum meal frequency for their age during the previous day, and for non-breastfed children, receiving at least the minimum dietary diversity and minimum meal frequency for their age during the previous day as well as at least two milk feeds. The Multiple Indicator Cluster Surveys (MICS), 2017/2018 in Ghana, reported that nationally, 41% of children aged 6–23 months met minimum meal frequency (MMF) and 29% met minimum dietary diversity (MDD), while 12% had minimum adequate diet (MAD) (Ghana Statistical Service (GSS), 2018).
Any factors associated with poor feeding practices hold importance in addressing the issue of malnutrition. It is therefore necessary to obtain information on IYCF feeding indicators in HIV-exposed children and their associated factors to guide the relevant policies and intervention studies. This study therefore aimed to determine infant and young child feeding practices among HIV-exposed children under 2 years old enrolled in a dietary intervention study and to identify the household, maternal, and child factors associated with the IYCF indicators measured. We also explored the relationships between IYCF indicators and nutritional status measured by anthropometry and anemia status.
Materials and methods
Study design and population
This study is a secondary analysis using the baseline data from a randomized controlled trial that aimed to examine the effectiveness of a 6-month soy-based supplementation intervention called KOKOPlus in improving nutritional status and child development among HIV-exposed children aged 6–18 months in Accra. It ran from August 2021 to July 2022. HIV-positive mother–child dyads who attended antiretroviral clinics in the Greater Accra Region were enrolled. The size of the sample was estimated for the larger supplementation trial at 590 with reference to the study by Prendergast et al., (2019), which recorded a 0.26 change in length for age z scores (LAZ) in a randomized control trial given significance level and power risks of 0.05 and 0.80, respectively. A predicted drop-out rate of 10% during follow-up was considered, bringing the final sample size to 649.
Recruitment into this study took place at adult antiretroviral and Child HIV Clinics at Korle-Bu Teaching Hospital, The Greater Accra Regional Hospital, Princess Marie Louise Children’s Hospital, Ledzokuku-Krowor Municipal Assembly (LEKMA) Hospital, Shai-Osudoku District Hospital, Tema General Hospital, Tema Polyclinic, Ashaiman Polyclinic, Manhean Polyclinic, Pentecost Hospital-Madina, Madina Polyclinic Kekele, Weija Gbawe Municipal Hospital, Ga West Municipal Hospital-Amasaman, Ga North Municipal Hospital-Ofankor, Kaneshie Polyclinic, Mamprobi Polyclinic, Maamobi General Hospital, Legon Hospital, and Achimota Hospital. A database of HIV-positive mothers with children between 6 and 18 months of age was compiled from hospital records. Eligible mothers were contacted by phone or in person during clinic attendance and were invited to participate in the study. After mothers had agreed to participate in the study and completed informed consent forms, they were randomized to receive the intervention.
Eligibility for participation in the study included HIV-positive mothers and their children between 6 and 18 months of age attending antiretroviral clinics (ART) and child HIV clinics. Excluded were mothers who declined to participate in the study, children with severe acute malnutrition, children on hospital admission, and children with diagnosed or apparent congenital conditions that negatively affect the child’s ability to eat and/or grow.
Ethical approval was obtained from three institutions: Institutional Review Board of Noguchi Memorial Institute for Medical Research (Federalwide Assurance 00001824, NMIMR-IRB CPN 058–20/21) and Ghana Health Service Ethics Review Committee and Korle-Bu Teaching Hospital Institutional Review Board (KTH-IRB).
Data collection and analyses
Data collection took place at the HIV Clinics at the health facilities enumerated above. Supplementary Table S1 shows the data collected and the time points.
Infant and young child feeding data
The WHO/UNICEF questionnaire to assess infant and young child feeding practices collected information on breastfeeding practices, the timing of initiation of complementary feeding, parental recall of the child’s diet in the previous 24-h period, and meal frequency (WHO and UNICEF, 2021).
The IYCF indicators
Ever breastfed (EvBF): Percentage of children who were ever breastfed. Mothers were asked if their children were ever breastfed.
Early initiation of breastfeeding (EIBF): Percentage of children who were put to the breast within 1 h of birth.
Exclusively breastfed for the first 2 days after birth (EBF2D): Percentage of children who were fed exclusively with breast milk for the first 2 days after birth.
Exclusive breastfeeding is breastfeeding with no other food or drink, not even water. Thus, in this study, we did not include the exclusive breastfeeding rate for infants aged 0 to 6 months because we recruited study participants aged between 6 and 18 months.
Continued breastfeeding 12–23 months (CBF): Percentage of children 12–23 months of age who were fed breast milk during the previous day and night.
Introduction of solid, semi-solid, or soft foods 6–8 months: Percentage of infants 6–8 months of age who consumed solid, semi-solid, or soft foods during the previous day. The indicator was calculated based on a question that asked mothers about foods fed to the infant the day before during the day or at night.
Minimum dietary diversity 6–23 months (MDD): Percentage of children 6–18 months of age who consumed foods and beverages from at least five out of eight defined food groups during the previous day. group 1: breast milk; group 2: grains, roots, and tubers and plantain; group 3: pulses (beans, peas, and lentils), nuts, and seeds; group 4: dairy products (milk, infant formula, yogurt, and cheese); group 5: flesh foods (meat, fish, poultry, and organ meats); group 6: eggs; group 7: vitamin-A rich fruits and vegetables; group 8: other fruits and vegetables. Responses to questions about breastfeeding and other liquid intake were used to account for breast milk intake and other liquids, respectively. Mothers were asked to recall foods using a list-based approach. They were asked about the intake of foods from a list of 16 food groups eaten yesterday during the day or night. To calculate the dietary diversity score, the 16 food groups were categorized into eight food groups (WHO and UNICEF, 2021).
Minimum meal frequency 6–23 months (MMF): Percentage of children who consumed solid, semi-solid, or soft foods (including milk feeds for non-breastfed children) the minimum number of times or more during the previous day. The minimum number of times was calculated as two feeds of solid, semi-solid, or soft foods for breastfed infants aged 6–8 months; three feeds of solid, semi-solid, or soft foods for breastfed children aged 9–18 months; and four feeds of solid, semi-solid, or soft foods or milk feeds for non-breastfed children aged 6–28 months whereby at least one of the four feeds must be a solid, semi-solid, or soft feed. For breastfed children, this indicator was based on questions on the intake of breast milk and solid and semi-solid foods. For non-breastfed infants, the use of milk feeds was taken into account. The numerator for breastfed was children 6–18 months of age recruited into the study who consumed solids, semi-solids, or soft foods the minimum number of times during the previous day. For non-breastfed children, it was children 6–18 months of age recruited into the study who consumed at least four solid, semi-solid, or soft foods feeds or milk feeds during the previous day, with at least one of the four being a solid, semi-solid, or soft food feed.
Minimum acceptable diet 6–23 months (MAD) was computed as the percentage of children 6–18 months of age recruited into the study who consumed a minimum acceptable diet during the previous day and achieved the minimum meal frequency as well.
Sociodemographic data
Data on sociodemographics, household conditions, and mother’s information were collected using a household questionnaire. All questionnaires were administered at the clinic electronically using Computer Assisted Personal Interview (CAPI) technology.
Household food security was assessed with an 8-item food insecurity questionnaire comprising questions that inquire about various aspects of food access, affordability, and availability, providing a comprehensive measure of a household’s vulnerability to food insecurity (Bickel et al., 2000; Coleman-Jensen et al., 2019).
Anthropometry
All anthropometric measurements for children and their mothers were taken twice (and were repeated if they differed by more than 5 mm), and the mean values of the measurements were computed and used in the analysis. Anthropometric measurements were done in accordance with WHO guidelines (WHO and UNICEF, 2019). Recumbent length was measured for all the children using an infantometer since the children were below 2 years of age. The mother’s height was recorded using a height meter. Weight was measured using the Tanita Electronic WB-100A/WB-110A Remote Display Version scale. Tared weighing was done for the children since they were unable to stand on their own. The scale was switched on, and when the reading was 0.0 kg, the mother was asked to mount the scale barefoot, wearing only light clothing. They were asked to stand still in the middle of the scale until their weight was displayed and recorded. After the weight appeared on the display, she remained standing on the scale while it was reset to zero. The child was then given to her, and the child’s weight was recorded.
Nutritional status
For the children, weight-for-age, weight-for-length, and length-for-age were determined using the WHO AnthroPlus Software. Length-for-age < −2 standard deviations from the median of the WHO child growth standards was categorized as stunting. Underweight was categorized as weight-for-age < −2 SD, wasting as weight-for-length < −2 SD, and overweight as weight-for-length/height < −2 SD. Mother’s body mass index (BMI): Body Mass Index (BMI) was used as one of the markers of nutritional status of the mothers and was calculated using the measured mother’s weight in kg and height in meters. BMI was calculated as kg/m2: Underweight BMI is less than 18.5, normal BMI is between 18.5 and less than 25.0, overweight BMI is between 25 and less than 30, and obese BMI is above 30.
Hemoglobin measurement
Children’s hemoglobin levels were tested using a Hemocue® Hb 301 device according to the manufacturer’s user guidelines and WHO guidelines on drawing blood (WHO, 2010).
Anemia status
Children with a hemoglobin concentration of less than or equal to 110 grams per deciliter (g/dl) were classified as anemic (WHO, 2011).
Household food security
A food insecurity experience scale was adopted. This questionnaire asked about the household’s experience in the last 12 months. This is an 8-item tool to assess household food security status comprising questions about various aspects of food access, affordability, and availability, providing a comprehensive measure of a household’s vulnerability to food insecurity. The responses were scored to categorize households into different levels of food security as follows: less than 3: food secure; 4–5: mildly food insecure; and 6–8: severely food insecure. Mild and severe food insecurities were combined as food insecure (Ghana Statistical Service GSS, Ghana Health Service GHS, Macro ICF, 2009).
Statistical analyses
Data were cleaned, and analysis was conducted using Stata version 16 (StataCorp. 2019. Stata Statistical Software: Release 16. College Station, TX: StataCorp LLC). The Shapiro–Wilk Test was used to test for normality. All values were normally distributed except for the mother’s age. The median for the mother’s age was 33 compared to the mean of 32.7, hence the categorization of the mother’s age for the logistic regression. Descriptive statistics (means and standard deviations) were calculated for continuous variables, and categorical variables were reported as frequencies and percentages. For bivariate analysis, statistical associations between the various IYCF indicators and sociodemographic variables (mother’s age, mother’s level of education, mother’s employment status, household income, mothers’ BMI, and household food insecurity) were tested for statistical significance using the Chi-square test. For multivariate analyses, all predictor variables were initially put in the model, and the goodness-of-fit was assessed using the Likelihood Ratio (LR) test. When the value of p associated with the LR test was less than 0.05, a predictor variable with the largest value of p was dropped from the model. Predictor variables with the highest value of p were dropped until the model recorded an LR test associated with a value of p of less than 0.05. The level of statistical significance was set at p < 0.05. The odds ratios with 95% confidence intervals were derived from the adjusted logistic regression models, which were used to determine the predictors of IYCF indicators among HIV-exposed children.
Results
Sociodemographic characteristics of mothers of HIV-exposed children
Table 1 shows the sociodemographic characteristics of the mothers. The mean age of the mothers was 33 years, with the majority (58%) of them within the ages of 31 to 40 years. Secondary school education was the highest level reached but not necessarily completed for most mothers (70%). Seventy percent of them were employed, and 81% were married or living with their spouses. The Akan ethnic group formed the majority, and almost 90% of mothers were Christians. For the nutritional status of the mothers, their mean weight was 69 kg; 39% of them had normal BMIs, 58.7% were overweight, and 2% were underweight. The average household size was 4, with at least one child under 5 years. The majority of them stayed in compound houses (63%) and used water closets (WC) as their main toilet facility (49%). More than half of the women had a household income greater than GH¢500 ($42, GH¢1.00 is approximately $0.08). The minimum daily wage in 2022 (GH¢13.53) translates to GH¢419 per month, which was rounded to Gh¢500 (Kuhn et al., 2008). More women were from households that were food insecure (57%) than secure (43%).
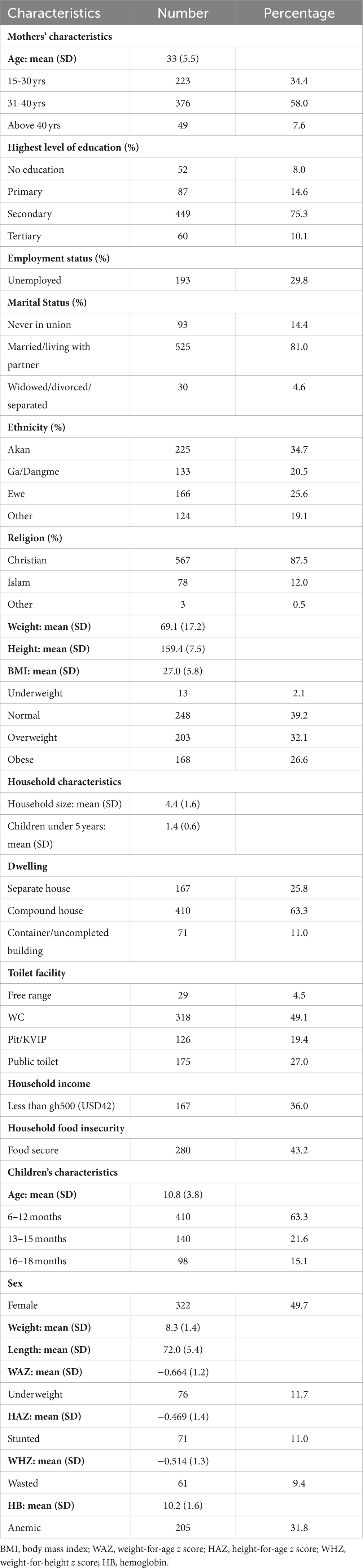
Table 1. Characteristics of mothers, their children, and the households of HIV-exposed children aged 6–18 months from the antiretroviral clinics and HIV clinics in the Greater Accra Region.
Characteristics of HIV-exposed children
The characteristics of the HIV-exposed children are also presented in Table 1. The mean age of the children was 10.8 months, with more than half (63%) of them within the age of 6–12 months. There was a balance between male representation among the children; 12% of the children were underweight, 11% were stunted, 9% were wasted, and 32% were anemic.
Infant and young child feeding practices
A descriptive summary of the IYCF practices of study participants is shown in Table 2. Almost all children (95%) had ever been breastfed, with more than half (53%) initiating breastfeeding within the first hour of delivery. The majority (79.0%) had exclusively breastfed for the first 2 days after birth. Almost all the infants (92%) between 6 and 8 months of age had been introduced to solid, semi-solid, or soft foods. Around one in four (26%) of the participants continued to breastfeed their children beyond 12 months.
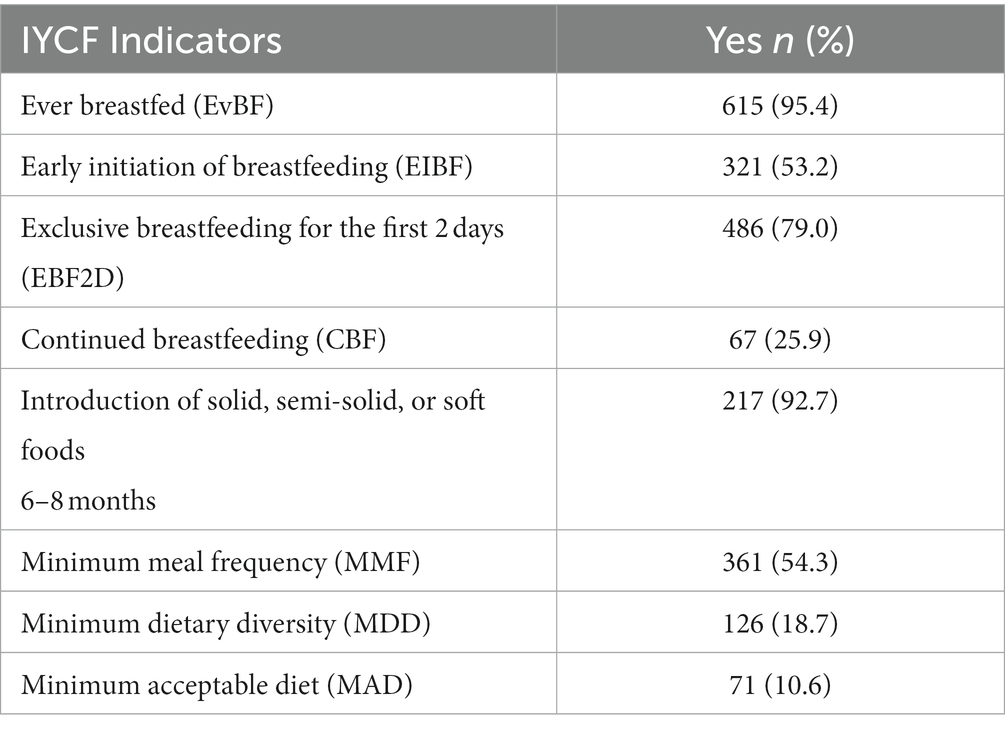
Table 2. Infant and young children feeding practices among HIV-exposed children aged 6–18 months from the antiretroviral clinics and HIV clinics in the Greater Accra Region.
Only 19% of the children attained the minimum dietary diversity, and 11% attained the minimum acceptable diet. However, more than half (54%) of the children met the minimum meal frequency.
Factors associated with IYCF breastfeeding indicators
Factors found to be associated with IYCF breastfeeding indicators after bivariate analyses are presented in Table 3. Mothers who were married or living with a partner had higher rates of ever breastfeeding (p < 0.05) than those who were widowed, divorced, or separated and those who had never been in a union. Mothers’ education was important, and those who had reached secondary education had the highest rates of continued breastfeeding between 12 and 18 months (p < 0.01), followed by primary, tertiary, and no education.
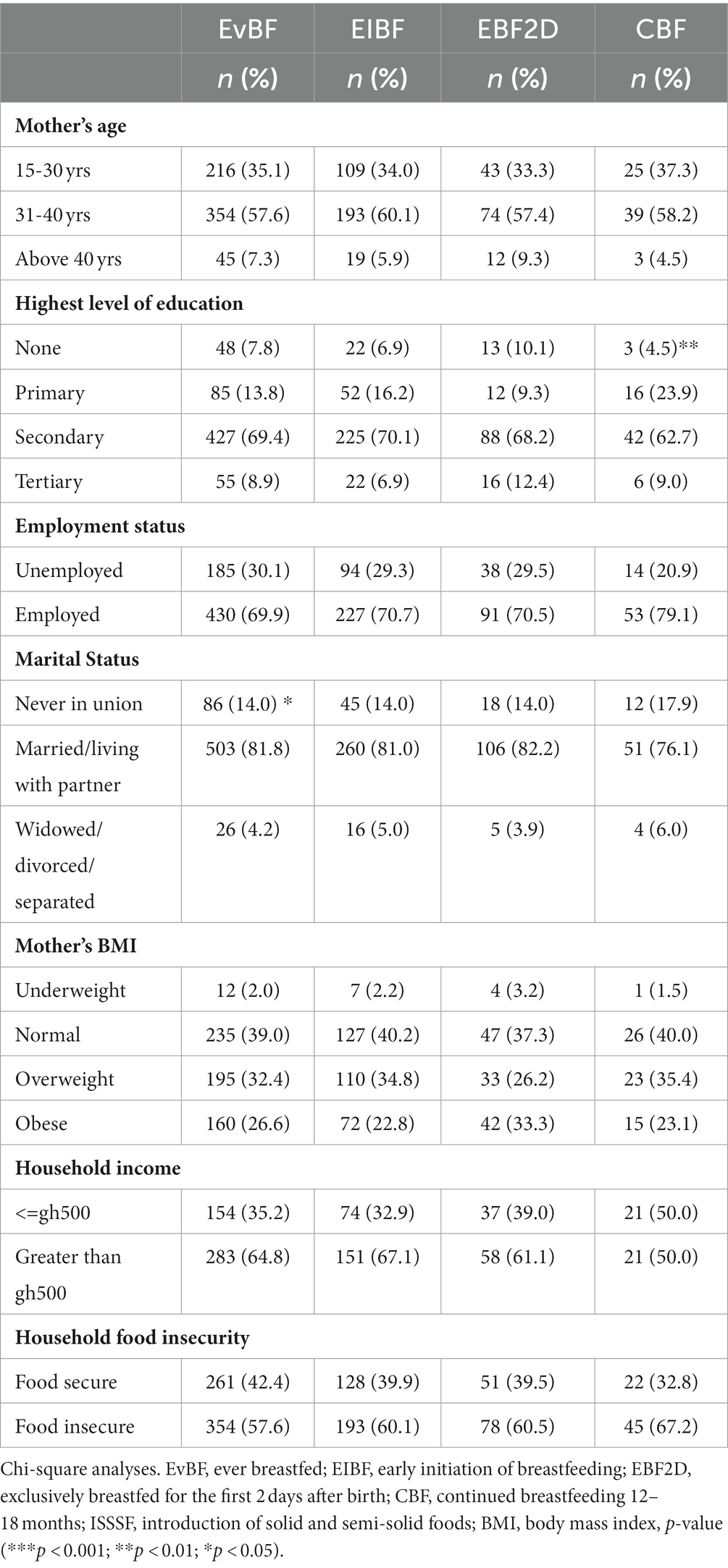
Table 3. Factors associated with IYCF breastfeeding indicators in HIV-exposed children aged 6–18 months from the antiretroviral clinics and HIV clinics in the Greater Accra Region.
Factors associated with IYCF complementary feeding indicators
Table 4 presents the results of bivariate analyses and shows associations of other IYCF indicators for complementary feeding with sociodemographic characteristics. MDD and MAD were associated with the mother’s age (p < 0.01), with a greater percentage of children of mothers in the 31–40 years group attaining MDD and MAD compared to those of mothers aged 15–30 years and mothers above 40 years. Children of employed mothers had higher rates of attainment of MDD (p < 0.05), MMF (p < 0.01), and MAD (p < 0.01) than children of unemployed mothers and children from households with household income greater than Ghc500 were more likely to attain MMF than those from households with household income less than Ghc 500 (p < 0.05). In addition, children from food-secure homes were more likely to attain MDD than those from food-insecure homes (p < 0.01).
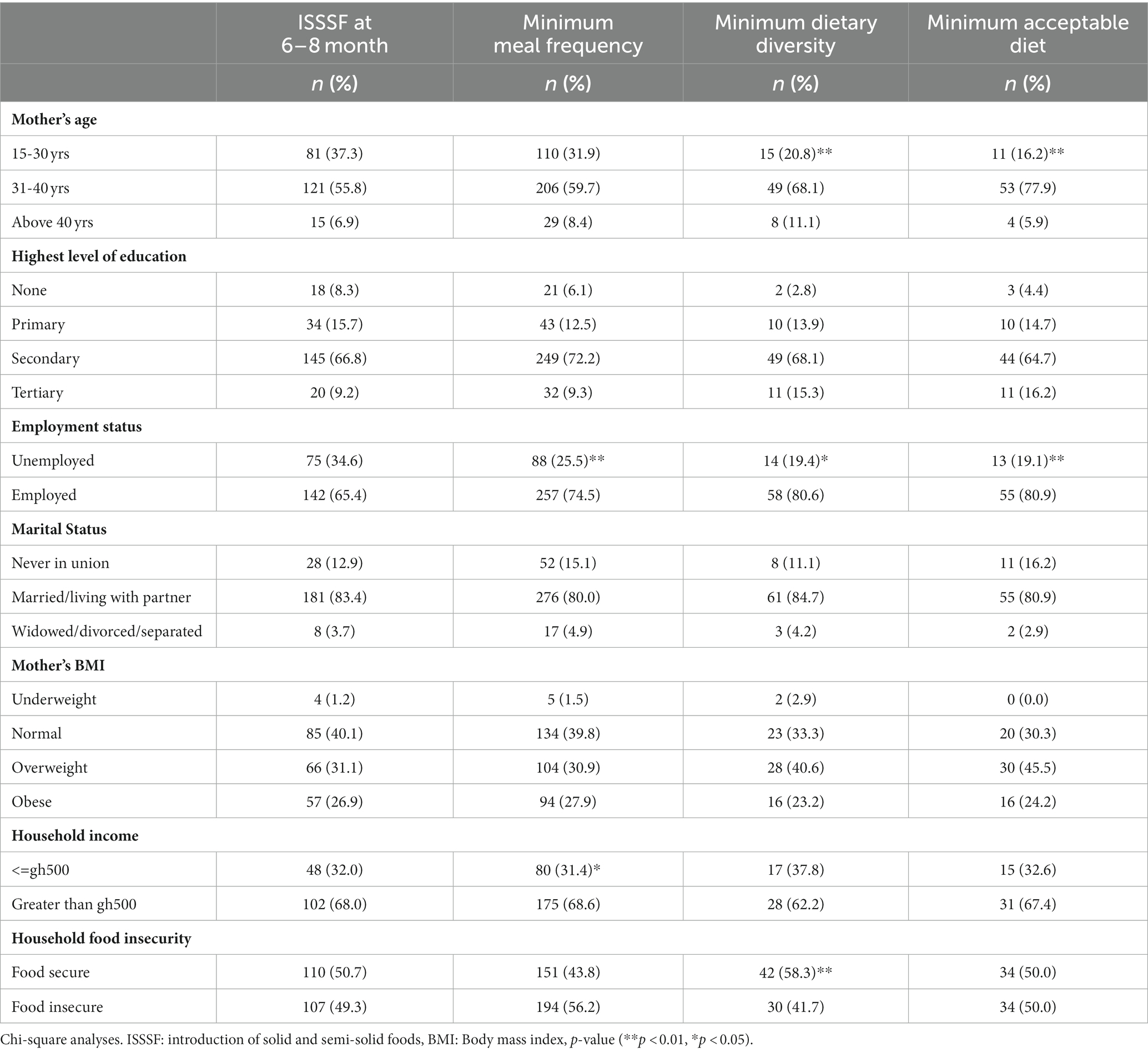
Table 4. Factors associated with IYCF complementary feeding indicators of HIV-exposed children aged 6–18 months from the antiretroviral clinics and HIV clinics in the Greater Accra Region.
Predictors of IYCF breastfeeding and complementary feeding
Logistic regression models were run to find predictors of IYCF practices. None of the breastfeeding indicator models were significant. Table 5 shows the results for complementary IYCF practices. Compared to mothers 15–30 years of age, children born to mothers between the ages of 31 and 40 years were twice as likely to have a diverse diet [AOR 2.1 (1.14, 3.85), p < 0.01] and three times as likely to achieve the minimum acceptable diet [AOR 3.0 (1.52, 5.92), p < 0.001], and those born to mothers above age 40 years had an even greater likelihood of attaining MDD [AOR 2.8 (1.11, 7.2), p < 0.05] compared to mothers 15–30 years. In addition, compared with children of mothers with no education, children of mothers who had some tertiary education were more than 5 times as likely to attain MDD [AOR 5.4 (1.1, 26.1), p < 0.05]. Children of employed mothers were more than one and a half times as likely to attain MMF compared to their counterparts whose mothers were unemployed [AOR 1.6 (1.1, 7.2), p < 0.05]. Coming from a household with income greater than GH¢500 gave a child 60% increased odds of attaining MMF [AOR 1.6 (1.01, 2.33), p < 0.05] compared to households with income less than GH¢500 while children from food insecure homes were 50% less likely to achieve MDD when compared to those from food secure households [AOR 0.5 (0.31, 0.86), p < 0.01].
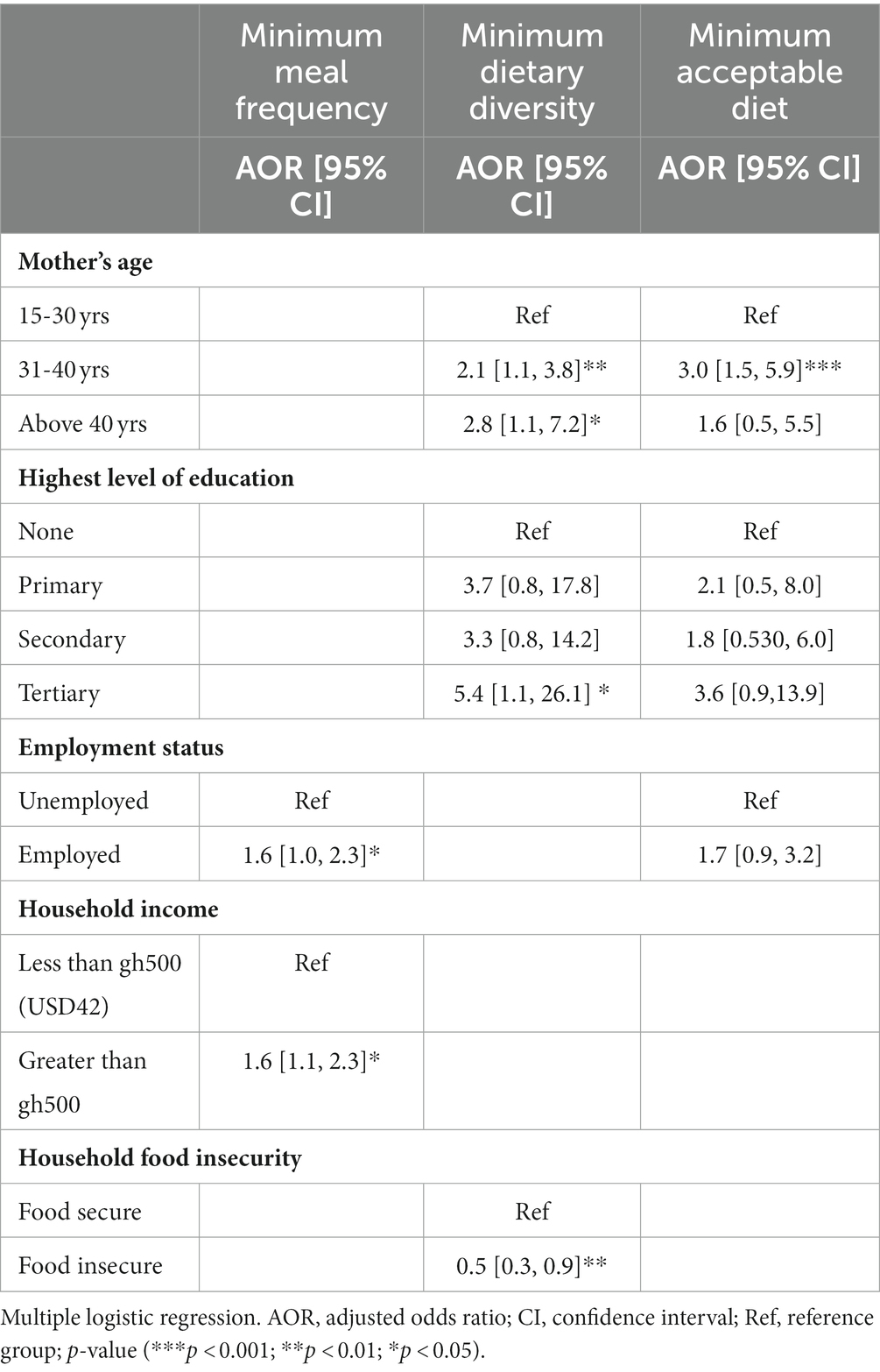
Table 5. Predictors of complementary IYCF indicators among HIV-exposed children aged 6–18 months from the antiretroviral clinics and HIV clinics in the Greater Accra Region.
Association between IYCF indicators and nutritional status
Bivariate analyses showed no significant associations between IYCF indicators and nutritional status (Table 6); however, multiple logistic regression with nutritional indices as continuous rather than categorical variables showed some statistically significant associations between IYCF and nutritional status. Attained MDD was associated with 20% less Hb [AOR 0.8 (0.71, 0.99), p < 0.05], and MMF was negatively associated with WHZ [AOR 0.8 (0.72, 0.98), p < 0.05] (Table 7).
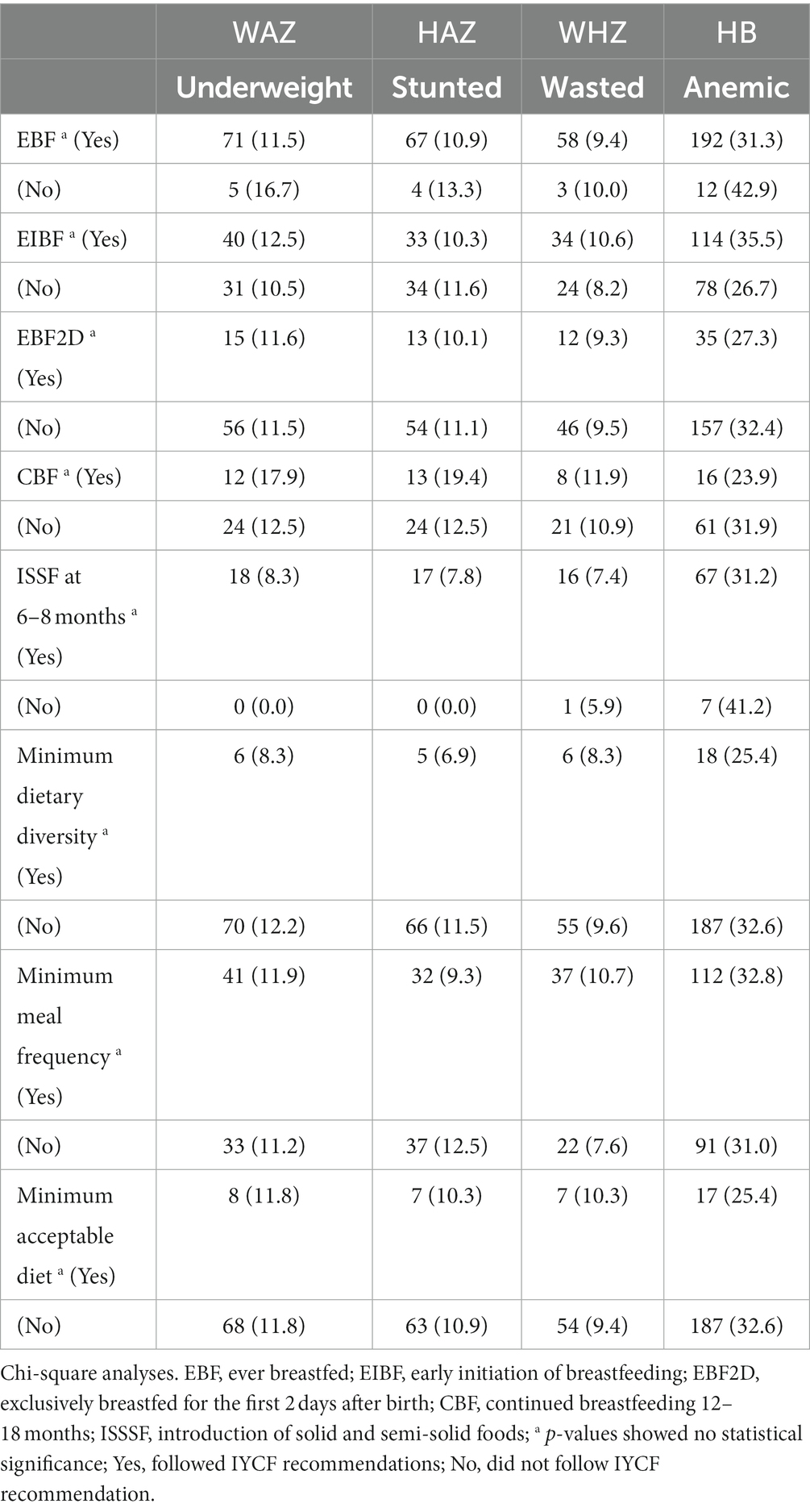
Table 6. Association between IYCF breastfeeding and complementary feeding indicators and nutritional status of HIV-exposed children aged 6–18 months from the antiretroviral clinics and HIV clinics in the Greater Accra Region.
Discussion
This cross-sectional study sought to determine infant and young child feeding practices among HIV-exposed children under 2 years of age enrolled in a dietary intervention study, to explore factors associated with the IYCF indicators and to explore any associations between IYCF indicators and the children’s nutritional status. According to the last GDHS (Ghana Statistical Service (GSS), 2015), the national EvBF rate was 98% of last-born children born in the preceding 2 years, and 56% were breastfed within an hour of birth. Our results reveal comparable breastfeeding practices amongst our population of children born to HIV-positive mothers, with an EvBF rate of 95% and EIBF at 53%.
Although a significant association was lost between marital status and EvBF after multivariate analyses, the bivariate analyses showed a positive association of breastfeeding with marital or relationship stability (p < 0.05). Living with a partner could mean that mothers have more support from a significant other for their own, as well as their child’s care, including breastfeeding (Renata et al., 2022). Having said that, we note that breastfeeding was universal in our population (95%), which is typical of the cultural norm of breastfeeding in Ghana. This is similar to findings in Nigeria (Umeobieri et al., 2018) and Kenya, where the lack of male involvement has been cited as a barrier to breastfeeding (Samburu et al., 2021), and in South Africa, HIV-positive women who attempted breastfeeding had better social support and HIV-related social support (Remmert et al., 2020).
Mother’s education positively influenced continued breastfeeding after 12 months (p < 0.01). Only 5% of non-educated mothers breastfed beyond 12 months. Sociodemographic factors such as the education of HIV-positive mothers have been documented to influence maternal knowledge, attitudes, and practices. Mother’s education has been reported to facilitate understanding and adherence to recommendations (Renata et al., 2022). A retrospective cohort study of sociodemographic factors that enhance breastfeeding uptake in HIV-positive women using DHS surveys from multiple sub-Saharan African countries found that women with the highest levels of education and those without partner support were less likely to breastfeed (Caldwell et al., 2023).
A study in Uganda that looked at the morbidity of HIV-exposed children found that at 6 months, 12 months, and 24 months, the proportion of children breastfeeding was consistently lower for HIV-exposed than for HIV-unexposed children; at 6 months of age, 84.4% vs. 99.7% (p < 0.0001); at 12 months of age, 29% vs. 99% (p < 0.001); and at 24 months of age, 0 vs. 24.4% (p < 0.0001) (Marquez et al., 2014). While mothers living with HIV tend to avoid stigmatization by practicing breastfeeding norms such as initiating breastfeeding (Oguta et al., 2004; Oladokun et al., 2010; Tariq et al., 2016; Umeobieri et al., 2018), their fear of transmitting the virus to their children may cause them to stop breastfeeding earlier than their non-HIV counterparts (Fadnes et al., 2009; Flax et al., 2017; Anderson et al., 2021). HIV-exposed children are less likely to be breastfed after 12 months compared to their unexposed counterparts, as observed in Botswana and Uganda (Muhangi et al., 2013; Chalashika et al., 2017).
For IYCF complementary feeding practices, 2017/2018 MICS reported that nationally, 41% of the children met minimum meal frequency (MMF), 29% met minimum dietary diversity (MDD), and 12% had minimum adequate diet (MAD) (Ghana Statistical Service (GSS), 2018). Intake of MDD was higher among children from wealthier households, with high levels of maternal education, and in urban areas. In addition, mothers within the age bracket of 31 to 40 years were more likely to meet MAD (Ghana Statistical Service (GSS), 2018). Almost all infants in our study between 6 months and 8 months (93%) had been introduced to solid, semi-solid, or soft foods compared to the national rate of 79% (Ghana Statistical Service (GSS), 2018). The higher percentage of mothers who had introduced complementary foods at this stage could be an indication that mothers in our study were more inclined to cease breastfeeding after 6 months, possibly to reduce the chances of MTCT.
While MMF was higher in our study than nationally (54% vs. 41%), MDD was much lower than national rates (19% vs. 29%), and MAD was received by a similar proportion (11% vs. 12%). Although meals may have been frequent enough, they were not diverse enough for the majority of the children. These were children of mothers who were attending ART clinics and who would receive nutrition education from time to time. This could account for more frequent meals than the general population; however, they may have had fewer resources and been less food secure than the general population to provide the necessary diversity in meals for their children, resulting in similar MAD rates. Bivariate analyses showed that MMF was significantly and positively associated with mothers’ employment (p < 0.01) and household income (p < 0.05), and these associations remained strong in the logistic regression, showing that the availability of more financial resources likely translates to greater frequency of feeding (Table 5).
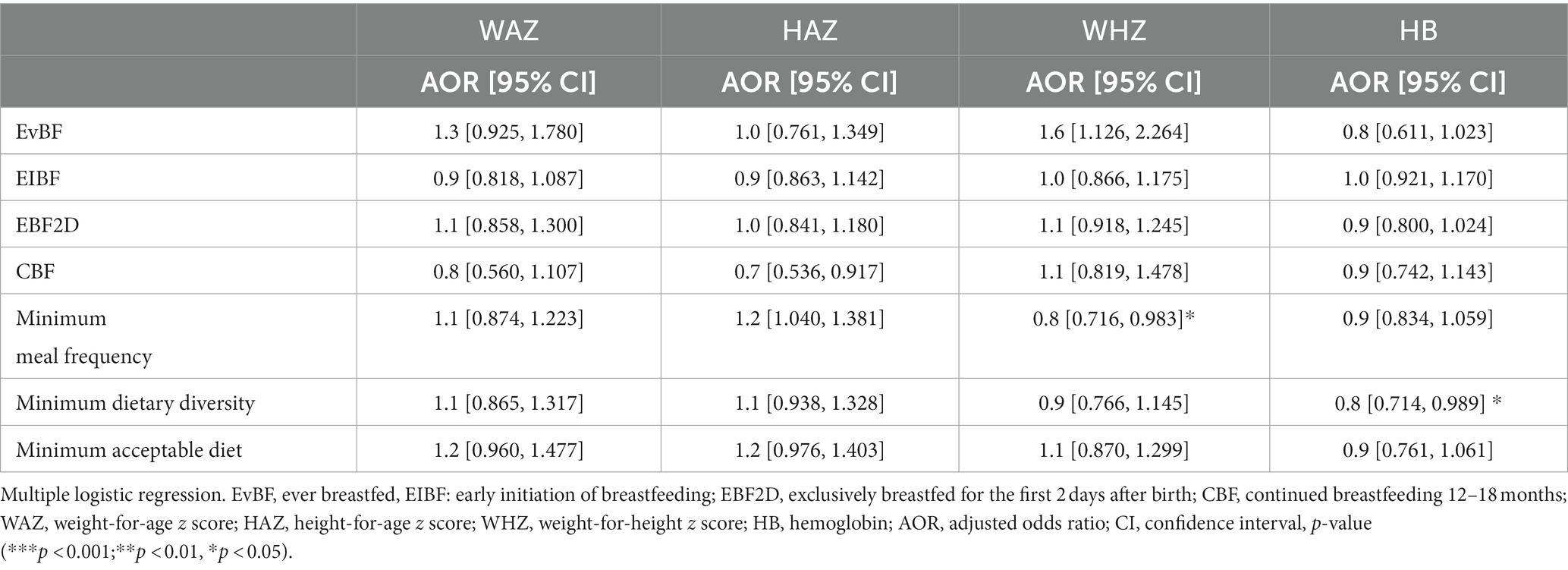
Table 7. Association of IYCF indicators with nutritional status of HIV-exposed children aged 6–18 months from the antiretroviral clinics and HIV clinics in the Greater Accra Region.
MDD showed a significant and positive association with mothers’ age, employment, and household food security in bivariate analyses, and only the association with employment was lost with multivariate analyses. An additional positive association was discovered with tertiary education after logistic regression analyses. Better household food security meant that there were resources available to feed children a more diverse diet. It is also possible that a better understanding of IYCF recommendations on feeding a diverse diet, leading to better practice, comes with age and experience and is facilitated by higher education. In line with our findings, food insecurity has been found to make it difficult for HIV-positive mothers to follow complementary feeding guidelines (Flax et al., 2016).
Bivariate analyses showed that MAD was positively and significantly associated with mothers’ age and employment, but after multiple logistic regression, only the mother’s age was significantly associated with MAD. Mothers between the ages of 31 and 40 years were the most likely to feed their children diverse diets compared to those in the 15–30 age bracket. There could be a number of reasons for this. While the 31–40 years age group may have the benefit of learning more from experience than the youngest age group, they may be more actively and gainfully employed than the above 40 age group and, therefore, have more financial empowerment to access a more diverse diet for their children. This finding is in line with the Ghana 2017/18 MICS report (Ghana Statistical Service (GSS), 2018).
Standard DHS data of Sub-Saharan African countries conducted between 2010 and 2020 were analyzed to determine the minimum acceptable diet (MAD) intake and its associated factors among children aged 6–23 months. Results showed that factors such as achievement of secondary education and higher for the women, being employed, having media exposure, richest household wealth, breastfed child, rural residence, and living in upper middle-income country were significantly associated with MAD (Belay et al., 2022). Studies on IYCF in HIV-exposed children have been conducted in Ethiopia (Haile et al., 2015; Esubalew et al., 2018; Yisak et al., 2020), Malawi (Flax et al., 2016), and Uganda (Kumbakulu et al., 2022), with MAD rates ranging from 40% in Malawi to 34% in Ethiopia, with a myriad of associated factors. These include mothers’ education sometimes acting as a negative or positive predictor of IYCF, inadequate information about infant feeding acting as a barrier to good practice, household wealth and media exposure as positive factors for good IYCF practice. A study conducted among HIV-positive mothers in the Manya-Krobo Municipality of Ghana reported that factors such as being married or in a stable relationship, having a supportive partner, adhering to ART, age of mother, education on IYCF received from the health facilities and influence of the media impacted the IYCF practices (Tofoatsi, 2005).
Ghana has a high burden of child undernutrition (Boah et al., 2019). Nationally, the rates of underweight stunting and wasting in children under 5 years in Ghana are 13, 18, and 7%, respectively (Ghana Statistical Service (GSS), 2018). The prevalence of underweight in this study population, 12%, was similar to the national prevalence, as was the prevalence of wasting (9%). Stunting, however, was lower in our population at 11%. We acknowledge that the national figures are for under-fives while our study population is under 2 years of age. It could be, however, that given a few more years, the stunting rates in our sample may approach the national figure as stunting is an indicator of long-term malnutrition, and malnutrition has been associated with the child’s age in Ghana (Boah et al., 2019). The rates of stunting and wasting observed in this study are of medium public health importance, and the rate of underweight is of high public health importance (De Onis et al., 2019).
Anemia was highly prevalent in our study population (32%) but lower compared to the national prevalence of 66% in under-fives (Ghana Statistical Service (GSS), 2015) which is considered to be a severe public health problem (World Health Organization, 2020). This difference could be due to the fact that the mothers in our study have regular access to healthcare and may have more regular checks for low Hb and iron supplementation than the general population, especially when their children are unwell. In sub-Saharan Africa, risk factors for anemia are age, birth order, sex, comorbidities (such as fever, diarrhea, and acute respiratory infection), malnutrition or stunting, maternal education, maternal age, mother’s anemia status, household wealth, and place of residence (Obasohan et al., 2020). In Ghana, anemia in children is associated with monotonous diets, poor feeding habits during weaning periods, and meals that lack iron, vitamins, and other essential nutrients. Children from poorer and larger households are at higher risk of anemia (Aheto et al., 2023). Lower maternal education, maternal unemployment, and lower household wealth index are the sociodemographic factors most commonly associated with infant undernutrition in sub-Saharan Africa (Chola et al., 2020).
Our study failed to find any meaningful associations between IYCF indicators and nutritional status. Multiple logistic regression with indices of nutritional status treated as continuous variables showed an inverse relationship between MMF and WHZ and between MMD and anemia, which was contrary to expectation. These contrary results may be due to the fact that IYCF indicators were based on a single 24-h recall, which is not representative of habitual food intake. Current nutritional status is a result of habitual food intake, among other factors. The possibility of recall and response bias with regard to IYCF indicators also cannot be ruled out. A study by Saaka et al. in Northern Ghana also found that apart from timely initiation of complementary feeding at 6 months, none of the WHO recommended IYCF indicators, including minimum dietary diversity, minimum meal frequency, and minimum acceptable diet, were associated with HAZ scores of infants 6–23 months of age (Saaka et al., 2015). Some studies, however, have found associations between adequate IYCF practices, especially minimum dietary diversity, and the growth of children. In sub-Saharan Africa, limited MMD has been associated with stunting (Aboagye et al., 2021). A study in 21 countries, including 12 in Africa, found that minimum infant dietary diversity is the IYCF complementary feeding indicator most consistently associated with positive growth patterns (Onyango et al., 2014).
Strengths and limitations of study
This study has contributed to filling the gaps in IYCF of HIV-exposed infants as there are few such studies, especially in sub–Saharan Africa. In addition, we used the most recent WHO/UNICEF IYCF indicators to assess HIV-exposed children based on current feeding recommendations for IYCF in the context of HIV. Our participant age range of 6–18 months, however, was not fully aligned with the target age group for IYCF complementary feeding indicators, which is 6–24 months. Comparisons with national figures for malnutrition, which are usually based on children 0–5 years, were also a limitation. Another limitation of our study is the fact that it failed to identify expected significant associations between IYCF and nutritional status. Measures of habitual intake would have been more useful.
Conclusion
Breastfeeding is nearly universal among HIV-positive women and is associated with mothers’ education and marital status. Continued breastfeeding between 12 and 24 months is not practiced by 75% of HIV-positive mothers. Earlier cessation than recommended could be due to concerns about transmitting the virus. There is a need for more education and support for breastfeeding beyond 12 months, with special attention given to those with low education. Malnutrition rates were of medium (HAZ and WHZ) and high (WAZ) public health significance, and anemia was of moderate public health concern. There is an urgent need for a national focus on improving IYCF and the nutritional status of HIV-exposed children, as this is a vulnerable group. Health workers should be given relevant training in IYCF in the context of HIV infection to assist HIV mothers through regular nutrition education. Affordable iron-rich foods need to be highlighted. In addition, since MDD was significantly associated with food security, market-based interventions that increase the availability and affordability of nutritious foods should be considered. Social support programs for vulnerable households that subsidize nutrient-rich foods, especially iron-rich foods, could improve their IYCF practices. Special attention should be paid to younger, less educated, and lower socioeconomic status mothers.
HIV infection coupled with ART exposure affects the maternal gestational environment and can also affect the immune system of the exposed children, making them vulnerable to poor nutritional status right from birth. There is a paucity of information on the dietary intake of HIV-exposed children. This study aimed to determine infant and young children's feeding practices among HIV-exposed children under 2 years by measuring indicators outlined in a recent joint report by WHO and UNICEF (2021). The indicators of interest in this study were the introduction to solids, semi-solids, and soft foods, minimum dietary diversity (MDD), minimum meal frequency (MMF), and minimum acceptable diet (MAD). Our study also determined the correlates of the IYCF indicators measured.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
This study involving human subjects was approved by the Institutional Review Board of Noguchi Memorial Institute for Medical Research, University of Ghana (Federalwide Assurance 00001824, NMIMR-IRB CPN 058–20/21), Ghana Health Service Ethics Review Committee and Korle-Bu Teaching Hospital Institutional Review Board (KTH-IRB). The studies were conducted in accordance with the local legislation and institutional requirements. Written informed consent for participation in this study was provided by the participants’ legal guardians/next of kin.
Author contributions
GF, FY, GT, GA, and BB designed the research. GF, BB, GT, GA, VA, PP, JD, and MN conducted the research. BB analyzed the data. GF and BB interpreted the data. MA, BB, and GF wrote the article. GF and FY provided the critical revision of the manuscript for important intellectual content. All authors have read and approved the final manuscript.
Funding
This study was a part of the 2019 Government of Japan Supplementary Budget-funded project entitled “Distributing Japanese Nutrition Supplements to Young Children of Refugee Families in Ghana” at the International Food Policy Research Institute. This study was supported by the Economic and Social Research Council [grant number ES/T003871/1] under the ARUA-GCRF UKRI Partnership Program as part of the Capacity Building in Food Security (CaBFoodS-Africa) project.
Acknowledgments
The authors thank the government of Japan for their kind contributions to improving the livelihoods and food security of mothers and children living with HIV in Ghana. The authors would like to thank the International Food Policy Research Institute (IFPRI) Washington and Ghana office staff and the Ghana AIDS Commission for supporting this research. They are also grateful to the Ghana Health Service, heads of health facilities, doctors and nurses in charge of ART clinics, and their counterparts at all 19 hospitals for their support, guidance, feedback, and faith in the research process over the period. Additionally, this project would not have been possible without the hardworking field staff and staff of the Noguchi Memorial Institute of Medical Research. Finally, they would like to extend our sincere thanks to all the participants in our study, who generously shared their time, experiences, and insights with them. The participant’s willingness to engage with this research was essential to the success of this project, and the authors are deeply grateful for their participation.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fsufs.2024.1251611/full#supplementary-material
References
Aboagye, R. G., Seidu, A.-A., Ahinkorah, B. O., Arthur-Holmes, F., Cadri, A., Dadzie, L. K., et al. (2021). Dietary diversity and undernutrition in children aged 6–23 months in sub-Saharan Africa. Nutrients 13:3431. doi: 10.3390/nu13103431
Abu-Raya, B., Kollmann, T. R., Marchant, A., and MacGillivray, D. M. (2016). The immune system of HIV-exposed uninfected infants. Front. Immunol. 7:383. doi: 10.3389/fimmu.2016.00383
Afran, L., Garcia Knight, M., Nduati, E., Urban, B. C., Heyderman, R. S., and Rowland-Jones, S. L. (2014). HIV-exposed uninfected children: a growing population with a vulnerable immune system? Clin. Exp. Immunol. 176, 11–22. doi: 10.1111/cei.12251
Aheto, J. M. K., Alhassan, Y., Puplampu, A. E., Boglo, J. K., and Sedzro, K. M. (2023). Anemia prevalence and its predictors among children under-five years in Ghana. A multilevel analysis of the cross-sectional 2019 Ghana malaria Indicator survey. Health. Sci. Rep. 6:e1643. doi: 10.1002/hsr2.1643
Anderson, K., Kalk, E., Madlala, H. P., Nyemba, D. C., Kassanjee, R., Jacob, N., et al. (2021). Increased infectious-cause hospitalization among infants who are HIV-exposed uninfected compared with HIV-unexposed. AIDS 35, 2327–2339. doi: 10.1097/QAD.0000000000003039
Aryeetey, R., Atuobi-Yeboah, A., Billings, L., Nisbett, N., van den Bold, M., and Toure, M. (2022). Stories of change in nutrition in Ghana: a focus on stunting and anemia among children under-five years (2009–2018). Food Secur. 14, 355–379. doi: 10.1007/s12571-021-01232-1
Bailey, R. C., Kamenga, M. C., Nsuami, M. J., Nieburg, P., and St Louis, M. E. (1999). Growth of children according to maternal and child HIV, immunological and disease characteristics: a prospective cohort study in Kinshasa, Democratic Republic of Congo. Int. J. Epidemiol. 28, 532–540. doi: 10.1093/ije/28.3.532
Belay, D. G., Taddese, A. A., and Gelaye, K. A. (2022). Minimum acceptable diet intake and its associated factors among children age at 6–23 months in sub-Saharan Africa: a multilevel analysis of the sub-Saharan Africa demographic and health survey. BMC Public Health 22:684. doi: 10.1186/s12889-022-12966-8
Bickel, G., Nord, M., Price, C., Hamilton, W., and Cook, J. (2000). Guide to measuring household food security, Revised. Measuring Food Security in the United States: Reports of the Federal Interagency Food Security Measurement Project. Alexandria VA: U.S. Department of Agriculture Food and Nutrition Service.
Boah, M., Azupogo, F., Amporfro, D. A., and Abada, L. A. (2019). The epidemiology of undernutrition and its determinants in children under five years in Ghana. PLoS One 14:e0219665. doi: 10.1371/journal.pone.0219665
Caldwell, C., Salihu, H. M., Dongarwar, D., Mercado-Evans, V., Batiste, A., Beal, T., et al. (2023). Breastfeeding practices of women with HIV in sub-Saharan Africa. Nursing for. Womens Health 27, 354–361. doi: 10.1016/j.nwh.2023.03.006
Chalashika, P., Essex, C., Mellor, D., Swift, J., and Langley-Evans, S. (2017). Birthweight, HIV exposure and infant feeding as predictors of malnutrition in Botswanan infants. J. Hum. Nutr. Diet. 30, 779–790. doi: 10.1111/jhn.12517
Chola, L., Magan, A., Momberg, D., Ngandu, C. B., Norris, S. A., and Said-Mohamed, R. (2020). The association between household socio-economic status, maternal socio-demographic characteristics and adverse birth and infant growth outcomes in sub-Saharan Africa: a systematic review. J. Dev. Orig. Health Dis. 11, 317–334. doi: 10.1017/S2040174419000680
Coleman-Jensen, A., Rabbitt, M. P., Gregory, C. A., and Singh, A. (2019). Household food security in the United States in 2018, ERR-270. US Dep. Agricul. Econom. Res. Serv. 47, 1–47.
De Onis, M., Borghi, E., Arimond, M., Webb, P., Croft, T., Saha, K., et al. (2019). Prevalence thresholds for wasting, overweight and stunting in children under 5 years. Public Health Nutr. 22, 175–179. doi: 10.1017/S1368980018002434
Enane, L. A., Davies, M.-A., Leroy, V., Edmonds, A., Apondi, E., Adedimeji, A., et al. (2018). Traversing the cascade: urgent research priorities for implementing the ‘treat all’ strategy for children and adolescents living with HIV in sub-Saharan Africa. J. Virus Erad. 4, 40–46. doi: 10.1016/S2055-6640(20)30344-7
Esubalew, F., Atenafu, A., and Abebe, Z. (2018). Feeding practices according to the WHO- recommendations for HIV exposed children in Northwest Ethiopia: a cross-sectional study. Clin Nutr. ESPEN. 28, 114–120. doi: 10.1016/j.clnesp.2018.08.019
Fadnes, L. T., Engebretsen, I. M. S., Wamani, H., Semiyaga, N. B., Tylleskär, T., and Tumwine, J. K. (2009). Infant feeding among HIV-positive mothers and the general population mothers: comparison of two cross-sectional surveys in eastern Uganda. BMC Public Health 9:124. doi: 10.1186/1471-2458-9-124
Filteau, S. (2009). The HIV-exposed, uninfected african child. Trop. Med. Int. Health 14, 276–287. doi: 10.1111/j.1365-3156.2009.02220.x
Flax, V. L., Hamela, G., Mofolo, I., Hosseinipour, M. C., Hoffman, I., and Maman, S. (2016). Infant and Young child feeding counseling, decision-making, and practices among HIV-infected women in Malawi’s option B+ prevention of mother-to-child transmission program: a mixed methods study. AIDS Behav. 20, 2612–2623. doi: 10.1007/s10461-016-1378-x
Flax, V. L., Hamela, G., Mofolo, I., Hosseinipour, M. C., Hoffman, I. F., and Maman, S. (2017). Factors influencing postnatal option B+ participation and breastfeeding duration among HIV-positive women in Lilongwe District, Malawi: a qualitative study. PLoS One 12:e0175590. doi: 10.1371/journal.pone.0175590
Ghana Statistical Service (GSS) (2015). Ghana health service (GHS), ICF international. Ghana statistical service, Ghana demographic and health survey 2014. Rockville, Maryland, USA: Ghana Statistical Service.
Ghana Statistical Service (GSS) (2018). Multiple Indicator cluster survey (MICS2017/18), survey findings report. Accra, Ghana: Ghana Statistical Service.
Ghana Statistical Service GSS, Ghana Health Service GHS, Macro ICF (2009). Ghana demographic and health survey 2008. Accra, Ghana: GSS, GHS, and ICF Macro.
Glennie, S. J., Williams, N. A., and Heyderman, R. S. (2010). Mucosal immunity in resource-limited setting: is the battle ground different? Trends Microbiol. 18, 487–493. doi: 10.1016/j.tim.2010.08.002
Haile, D., Belachew, T., Berhanu, G., Setegn, T., and Biadgilign, S. (2015). Complementary feeding practices and associated factors among HIV positive mothers in southern Ethiopia. J. Health Popul. Nutr. 34, 1–9. doi: 10.1186/s41043-015-0006-0
Kuhn, L., Aldrovandi, G. M., Sinkala, M., Kankasa, C., Semrau, K., Mwiya, M., et al. (2008). Effects of early, abrupt weaning on HIV-free survival of children in Zambia. N. Engl. J. Med. 359, 130–141. doi: 10.1056/NEJMoa073788
Kumbakulu, PK, Ndeezi, G, Egesa, WI, Nakalema, G, Odoch, S, Kambele, RL, et al. Prevalence, feeding practices, and factors associated with undernutrition among HIV-exposed uninfected children aged 6 to 18 months in Bushenyi district, western Uganda: a cross-sectional study. (2022).
Lawn, J. E., Cousens, S., and Zupan, J. (2005). 4 million neonatal deaths: when? Where? Why? Lancet 365, 891–900. doi: 10.1016/S0140-6736(05)71048-5
Marinda, E., Humphrey, J. H., Iliff, P. J., Mutasa, K., Nathoo, K. J., Piwoz, E. G., et al. (2007). Child mortality according to maternal and infant HIV status in Zimbabwe. Pediatr. Infect. Dis. J. 26, 519–526. doi: 10.1097/01.inf.0000264527.69954.4c
Marquez, C., Okiring, J., Chamie, G., Ruel, T. D., Achan, J., Kakuru, A., et al. (2014). Increased morbidity in early childhood among HIV-exposed uninfected children in Uganda is associated with breastfeeding duration. J. Trop. Pediatr. 60, 434–441. doi: 10.1093/tropej/fmu045
McHenry, M. S., Apondi, E., Ayaya, S. O., Yang, Z., Li, W., Tu, W., et al. (2019). Growth of young HIV-infected and HIV-exposed children in western Kenya: a retrospective chart review. PLoS One 14:e0224295. doi: 10.1371/journal.pone.0224295
Miller, V., Webb, P., Micha, R., and Mozaffarian, D. (2020). Defining diet quality: a synthesis of dietary quality metrics and their validity for the double burden of malnutrition. Lancet Planet Health 4, e352–e370. doi: 10.1016/S2542-5196(20)30162-5
Muhangi, L., Lule, S. A., Mpairwe, H., Ndibazza, J., Kizza, M., Nampijja, M., et al. (2013). Maternal HIV infection and other factors associated with growth outcomes of HIV-uninfected infants in Entebbe, Uganda. Pub Health Nutr 16, 1548–1557. doi: 10.1017/S1368980013000499
Obasohan, P. E., Walters, S. J., Jacques, R., and Khatab, K. (2020). A scoping review of the risk factors associated with Anaemia among children under five years in sub-Saharan African countries. Int. J. Environ. Res. Pub. Health 17:8829. doi: 10.3390/ijerph17238829
Oguta, T., Omwega, A., and Sehmi, J. (2004). Infant feeding alternatives for HIV positive mothers in Kenya. Field Exchange 22. 25.
Oladokun, R. E., Brown, B. J., and Osinusi, K. (2010). Infant-feeding pattern of HIV-positive women in a prevention of mother-to-child transmission (PMTCT) programme. AIDS Care 22, 1108–1114. doi: 10.1080/09540120903511008
Onyango, A. W., Borghi, E., de Onis, M., del Carmen, C. M., and Garza, C. (2014). Complementary feeding and attained linear growth among 6–23-month-old children. Pub. Health Nutr. 17, 1975–1983. doi: 10.1017/S1368980013002401
Pan American Health Organization. Guiding principles for complementary feeding of the breastfed child. World Health Organisation, Washington DC (2003).
Phiri, W., Kasonka, L., Collin, S., Makasa, M., Sinkala, M., Chintu, C., et al. (2006). Factors influencing breast milk HIV RNA viral load among Zambian women. AIDS Res Human Retrovirus 22, 607–614. doi: 10.1089/aid.2006.22.607
Prendergast, A. J., Chasekwa, B., Evans, C., Mutasa, K., Mbuya, M. N., Stoltzfus, R. J., et al. (2019). Independent and combined effects of improved water, sanitation, and hygiene, and improved complementary feeding, on stunting and anaemia among HIV-exposed children in rural Zimbabwe: a cluster-randomised controlled trial. Lancet Child Adolescent Health 3, 77–90. doi: 10.1016/S2352-4642(18)30340-7
Prendergast, A. J., and Evans, C. (2023). Children who are HIV-exposed and uninfected: evidence for action. AIDS 37, 205–215. doi: 10.1097/QAD.0000000000003409
Remmert, J. E., Mosery, N., Goodman, G., Bangsberg, D. R., Safren, S. A., Smit, J. A., et al. (2020). Breastfeeding practices among women living with HIV in KwaZulu-Natal, South Africa: an observational study. Matern. Child Health J. 24, 127–134. doi: 10.1007/s10995-019-02848-8
Renata, Eccles, Maria, Toitdu, Grethe, Jonghde, and Esedra, K. Breastfeeding outcomes and associated risks in HIV-infected and HIV-exposed infants: a systematic review. Breastfeed. Med. (2022). 17: 112–130, doi: 10.1089/bfm.2021.0107
Saaka, M., Wemakor, A., Abizari, A.-R., and Aryee, P. (2015). How well do WHO complementary feeding indicators relate to nutritional status of children aged 6–23 months in rural northern Ghana? BMC Pub. Health 15:1157. doi: 10.1186/s12889-015-2494-7
Samburu, B. M., Kimiywe, J., Young, S. L., Wekesah, F. M., Wanjohi, M. N., Muriuki, P., et al. (2021). Realities and challenges of breastfeeding policy in the context of HIV: a qualitative study on community perspectives on facilitators and barriers related to breastfeeding among HIV positive mothers in Baringo County, Kenya. Int. Breastfeed. J. 16:39. doi: 10.1186/s13006-021-00385-1
Sint, T. T., Lovich, R., Hammond, W., Kim, M., Melillo, S., Lu, L., et al. (2013). Challenges in infant and young child nutrition in the context of HIV. AIDS 27, S169–S177. doi: 10.1097/QAD.0000000000000089
Tariq, S., Elford, J., Tookey, P., Anderson, J., de Ruiter, A., O'Connell, R., et al. (2016). “It pains me because as a woman you have to breastfeed your baby”: decision-making about infant feeding among African women living with HIV in the UK. Sex. Transm. Infect. 92, 331–336. doi: 10.1136/sextrans-2015-052224
Tofoatsi, EK-A. Infant and young child feeding practices among HIV positive mothers in Manya Krobo District, eastern region, Ghana: University of Ghana; (2005).
Umeobieri, A.-K., Mbachu, C., Uzochukwu, B. S. C., Elias, A., Omotowo, B., Agunwa, C., et al. (2018). Perception and practice of breastfeeding among HIV positive mothers receiving care for prevention of mother to child transmission in south-east, Nigeria. Int. Breastfeed. J. 13:50. doi: 10.1186/s13006-018-0191-8
UNICEF. HIV/global and regional trends. Available at: https://data.unicef.org/topic/hivaids/global-regional-trends/ University of Ghana, Groundwork, University of Wisconsin-Madison, KEMRI-WellcomeTrust. (2021).
Venhoff, N., and Walker, U. A. (2006). Mitochondrial disease in the offspring as a result of antiretroviral therapy. Expert Opin. Drug Saf. 5, 373–381. doi: 10.1517/14740338.5.3.373
Verhellen, E. (2000). Convention on the rights of the child: background, motivation, strategies, main themes. Gent, Belgium: ERIC.
WHO. (2010) WHO guidelines on drawing blood: best practices in phlebotomy. Geneva, Switzerland: WHO Document Production Services.
WHO (2011). “Haemoglobin concentrations for the diagnosis of anaemia and assessment of severity” in Vitamin and mineral nutrition information system (Geneva: World Health Organization)
World Health Organization (2003). UNICEF. Global strategy for infant and young child feeding. Geneva: World Health Organization.
World Health Organization (2004). HIV and infant feeding data analysis: Workshop report. Available at: https://www.who.int/publications/i/item/WHO_FCH_CAH_04.9.
World Health Organization (2016). Guideline: Updates on HIV and infant feeding: The duration of breastfeeding, and support from health services to improve feeding practices among mothers living with HIV.
World Health Organization (2020). WHO guideline on use of ferritin concentrations to assess iron status in individuals and populations. WHO guideline on use of ferritin concentrations to assess iron status in individuals and populations, 82.
WHO and UNICEF (2021). Indicators for assessing infant and young child feeding practices: Definitions and measurement methods. Geneva: WHO UNICEF.
WHO and UNICEF (2019). Recommendations for data collection, analysis and reporting on anthropometric indicators in children under 5 years old. Geneva: WHO, UNICEF.
Yirga, A. A., Mwambi, H. G., Ayele, D. G., and Melesse, S. F. (2019). Factors affecting child malnutrition in Ethiopia. Afr. Health Sci. 19, 1897–1909. doi: 10.4314/ahs.v19i2.13
Keywords: diet quality, HIV-exposed children, IYCF, dietary intake, sub-Saharan Africa
Citation: Folson GK, Bannerman B, Asante M, Tokor GS, Ador G, Atadze V, Puplampu P, Dame JA, Neizer M and Yamauchi F (2024) Diet quality and nutritional status of HIV-exposed children aged between 6 and 18 months in the Greater Accra Region of Ghana. Front. Sustain. Food Syst. 8:1251611. doi: 10.3389/fsufs.2024.1251611
Edited by:
Yunyun Gong, University of Leeds, United KingdomReviewed by:
Pui Yee Tan, University of Leeds, United KingdomJulia De Bruyn, AVRDC - The World Vegetable Center, Tanzania
Copyright © 2024 Folson, Bannerman, Asante, Tokor, Ador, Atadze, Puplampu, Dame, Neizer and Yamauchi. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Gloria K. Folson, gfolson@noguchi.ug.edu.gh
 Gloria K. Folson
Gloria K. Folson Boateng Bannerman
Boateng Bannerman Millicent Asante
Millicent Asante Grace Siba Tokor
Grace Siba Tokor Gabriel Ador1
Gabriel Ador1  Joycelyn Assimeng Dame
Joycelyn Assimeng Dame Margaret Neizer
Margaret Neizer Futoshi Yamauchi
Futoshi Yamauchi