Transversus Abdominis Plane Block With Liposomal Bupivacaine vs. Regular Anesthetics for Pain Control After Surgery: A Systematic Review and Meta-Analysis
- Department of Anesthesiology, Hunan Children's Hospital, Changsha, China
Background: Transverse abdominal plane (TAP) blocks are used to provide pain relief after abdominopelvic surgeries. The role of liposomal bupivacaine (LB) for TAP blocks is unclear. Therefore, this study aimed to synthesize evidence on the efficacy of LB vs. regular anesthetics in improving outcomes of TAP block.
Methods: PubMed, Science Direct, Embase, Springer, and CENTRAL databases were searched up to July 24, 2020. Studies comparing LB with any regular anesthetic for TAP block for any surgical procedure and reporting total analgesic consumption (TAC) or pain scores were included.
Results: Seven studies including five randomized controlled trials (RCTs) were reviewed. LB was compared with regular bupivacaine (RB) in all studies. A descriptive analysis was conducted for TAC due to heterogeneity in data presentation. There were variations in the outcomes of studies reporting TAC. Meta-analysis of pain scores indicated statistically significant reduction of pain with the use of LB at 12 h (MD: −0.89 95% CI: −1.44, −0.34 I2 = 0% p = 0.01), 24 h (MD: −0.64 95% CI: −1.21, −0.06 I2 = 0% p = 0.03), 48 h (MD: −0.40 95% CI: −0.77, 0.04 I2 = 0% p = 0.03) but not at 72 h (MD: −0.37 95% CI: −1.31, 0.56 I2 = 57% p = 0.43). Pooled analysis indicated no difference in the duration of hospital stay between LB and RB (MD: −0.18 95% CI: −0.49, 0.14 I2 = 61% p = 0.27). LB significantly reduced the number of days to first ambulation postsurgery (MD: −0.28 95% CI: −0.50, −0.06 I2 = 0% p = 0.01).
Conclusions: Current evidence on the role of LB for providing prolonged analgesia with TAP blocks is unclear. Conflicting results have been reported for TAC. LB may result in a small reduction in pain scores up to 48 h but not at 72 h. Further, high-quality homogenous RCTs are needed to establish high-quality evidence.
Introduction
Postsurgical pain can significantly impact patient satisfaction and overall quality of life (1). Literature suggests that optimal management of acute pain may also influence the development of chronic pain after the surgical procedure (2). Opioids are one of the most predominant drugs used for pain relief worldwide. However, side effects like nausea, vomiting, constipation, respiratory depression, etc. are commonly associated with their use. These adverse effects can increase patient morbidity while also adding to the overall healthcare costs (3). A multimodal approach for pain control is therefore recommended by balancing the benefits and adverse events of every drug or intervention.
The transverse abdominal plane (TAP) block was first described by Rafi et al. (4) in 2001 for pain relief after abdominopelvic surgeries. The technique involves infiltration of local anesthetic into the plane between the internal oblique and transversus abdominus muscles thereby blocking the neural afferents of the thoracoabdominal nerves originating from T6 to L1 spinal roots (5). Since then, TAP blocks have been used to provide postoperative pain relief after several surgical procedures like colorectal surgery, hernia repairs, prostatectomy, hysterectomy, cesarean sections, and sleeve gastrectomy (6–8). Cai et al. (6), in a recent systematic review and meta-analysis of 15 studies, have demonstrated that TAP provides more effective and steady analgesia compared to infiltration with local anesthetics in adult patients in the early postoperative period.
Regular anesthetics like bupivacaine are commonly used for TAP blocks to provide prolonged analgesia after surgery (9). Since regular bupivacaine (RB) provides pain relief for only up to 10 h after surgery, it is not surprising for trials to report reduced opioid consumption and lower pain scores only on the first postoperative day with the use of RB (10, 11). The use of infusion pumps or catheters may provide longer analgesia but are associated with patient discomfort and other complications (6). Liposomal bupivacaine (LB) is a multivesicular liposomal formulation of 1.3% bupivacaine capable of providing pain relief for up to 72 h (9). Several studies have assessed their efficacy for joint arthroplasties and other soft tissue surgeries (12). However, evidence of its prolonged efficacy has not been coherent. While some meta-analysis studies comparing LB vs. RB for pain relief after hip arthroplasty have indicated improved outcomes with LB (13), others have reported no difference in outcomes compared to regular anesthetics after shoulder surgeries (14). LB has been used by several clinicians for administering the TAP block. However, to the best of our knowledge, no review has attempted to synthesize evidence on its efficacy vs. regular anesthetics. Therefore, this review aimed to conduct a systematic literature search and pool data to answer the following clinical question: Does the use of LB vs. regular anesthetics for the TAP block improve clinical outcomes in adults undergoing abdominopelvic surgeries?
Materials and Methods
Inclusion Criteria
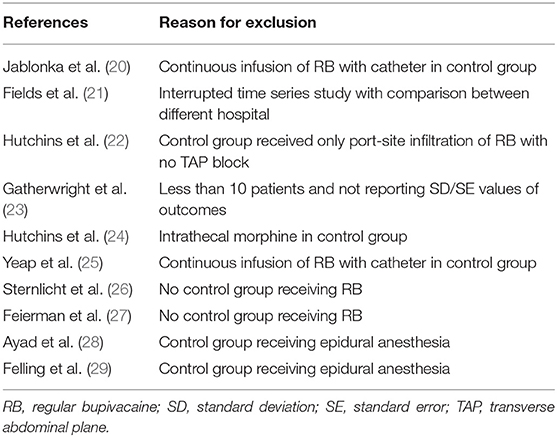
We framed our inclusion criteria based on the Population, Intervention, Comparison, Outcome, and Study design (PICOS) outline. Studies conducted on patients undergoing any type of surgical procedure and receiving transverse abdominal pain (TAP) blocks for pain control were to be included (Population). The study intervention was to be the use of LB compared to any regular anesthetic. Outcomes measured were to be total analgesic consumption or pain scores. We included only randomized controlled trials (RCTs), prospective nonrandomized, and retrospective single-center case-control studies. The following studies were excluded from the review: (1) studies not reporting outcomes, relevant outcomes, or not mentioning the distribution of data using standard deviations (SD), standard errors (SE), range or 95% confidence intervals (CI); (2) studies including <10 patients per group; (3) studies utilizing continuous anesthetic infiltration using catheters; (4) studies using other anesthesia regimens in the control group like epidural anesthesia; (5) interrupted time series design studies and studies comparing outcomes among different healthcare setups; and (6) single-arm studies, case series, case reports, review articles were also excluded.
Search Strategy
Two reviewers independently conducted electronic searches of PubMed, ScienceDirect, Embase, Springer, and CENTRAL databases from inception up to July 24, 2020 using the following search terms: “transverse abdominal block,” “transverse abdominal plane,” “bupivacaine,” “liposomal bupivacaine,” “anesthesia,” “analgesia,” and “block.” The literature search was restricted to English language publications. The search strategy and results of the PubMed database are presented in Supplementary Table S1. After evaluating the studies at the title and abstract level, full texts of selected articles were scanned for inclusion in the review. References of included studies were hand searched for identification of any missed out studies. Any disagreements were resolved by discussion between the two reviewers. Guidelines of the PRISMA statement (Preferred Reporting Items for Systematic Reviews and Meta-analyses) (15) and Cochrane Handbook for Systematic Reviews of Intervention (16) were followed during the conduct of this review.
Data Extraction and Outcomes
Data were extracted from the included studies by two reviewers independently. The following details were obtained using a pre-prepared data collection form: authors, publication year, study location, surgery type, sample size, mean age, intervention and control protocol, use of other analgesics, and study outcomes. The primary outcome of interest of this review was the total analgesic consumption and pain scores. The secondary outcomes were the length of hospital stay and time for ambulation postsurgery.
Risk of Bias
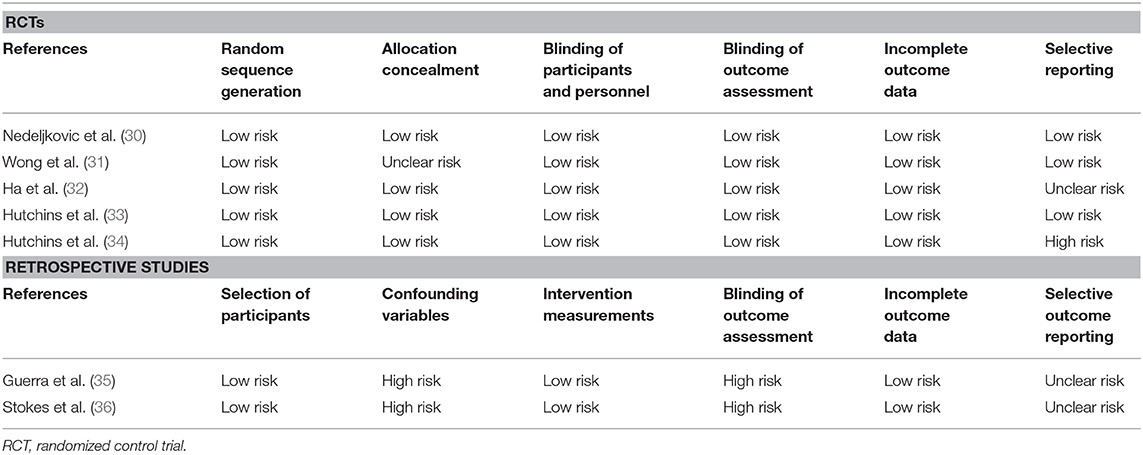
All included RCTs were assessed for bias using the Cochrane Collaboration risk assessment tool (17). Studies were rated as low risk, high risk, or unclear risk of bias for each of the following variables: random sequence generation, allocation concealment, blinding of participants and personnel, blinding of outcome assessment, incomplete outcome data, and selective reporting. For non-RCTs, the risk of a bias assessment tool for nonrandomized studies (RoBANS) was used (18). Studies were assessed for the selection of participants, confounding variables, intervention measurements, blinding of outcome assessment, incomplete outcome data, and selective outcome reporting.
Statistical Analysis
As all outcomes of the review were continuous variables, they were summarized using the mean difference (MD) with 95% confidence intervals (CI), if the data was measured on the same scale. In case different scales were used, standardized mean difference (SMD) was to be calculated with 95% CI. We used a random-effects model to calculate the pooled effect size for all our analyses. Heterogeneity was assessed using the I2 statistic. I2 values of 25–50% represented low values of 50–75% medium, and more than 75% represented substantial heterogeneity. For studies not reporting continuous variables as mean and standard deviation scores, they were calculated using methods reported by Wan et al. (19). We used the software Engauge Digitizer to extract numerical data if outcomes were reported only graphically. Due to the inclusion of fewer than 10 studies in the review, funnel plots were not used to assess publication bias. We used the software “Review Manager” (RevMan, version 5.3; Nordic Cochrane Centre [Cochrane Collaboration], Copenhagen, Denmark; 2014) for the meta-analysis.
Results
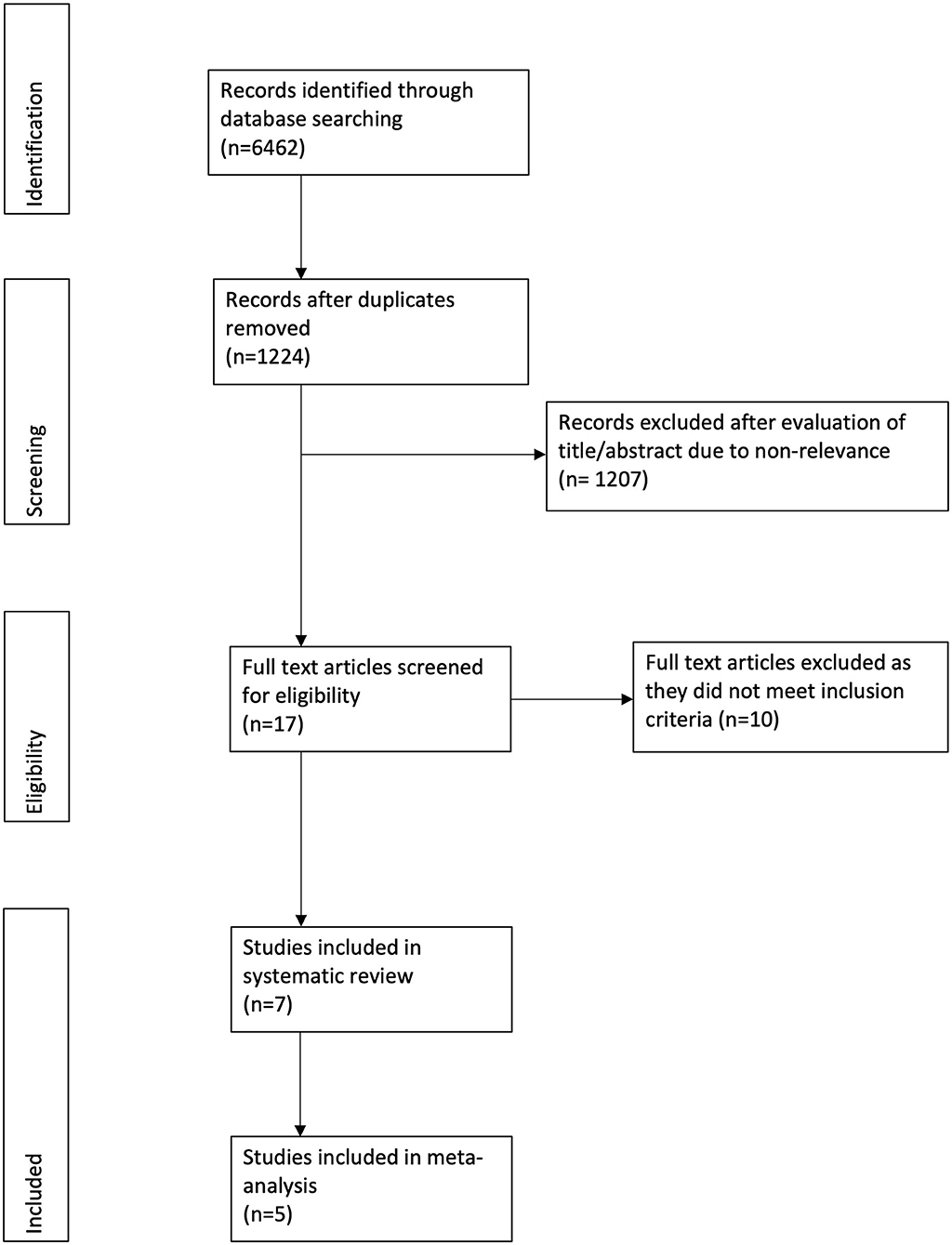
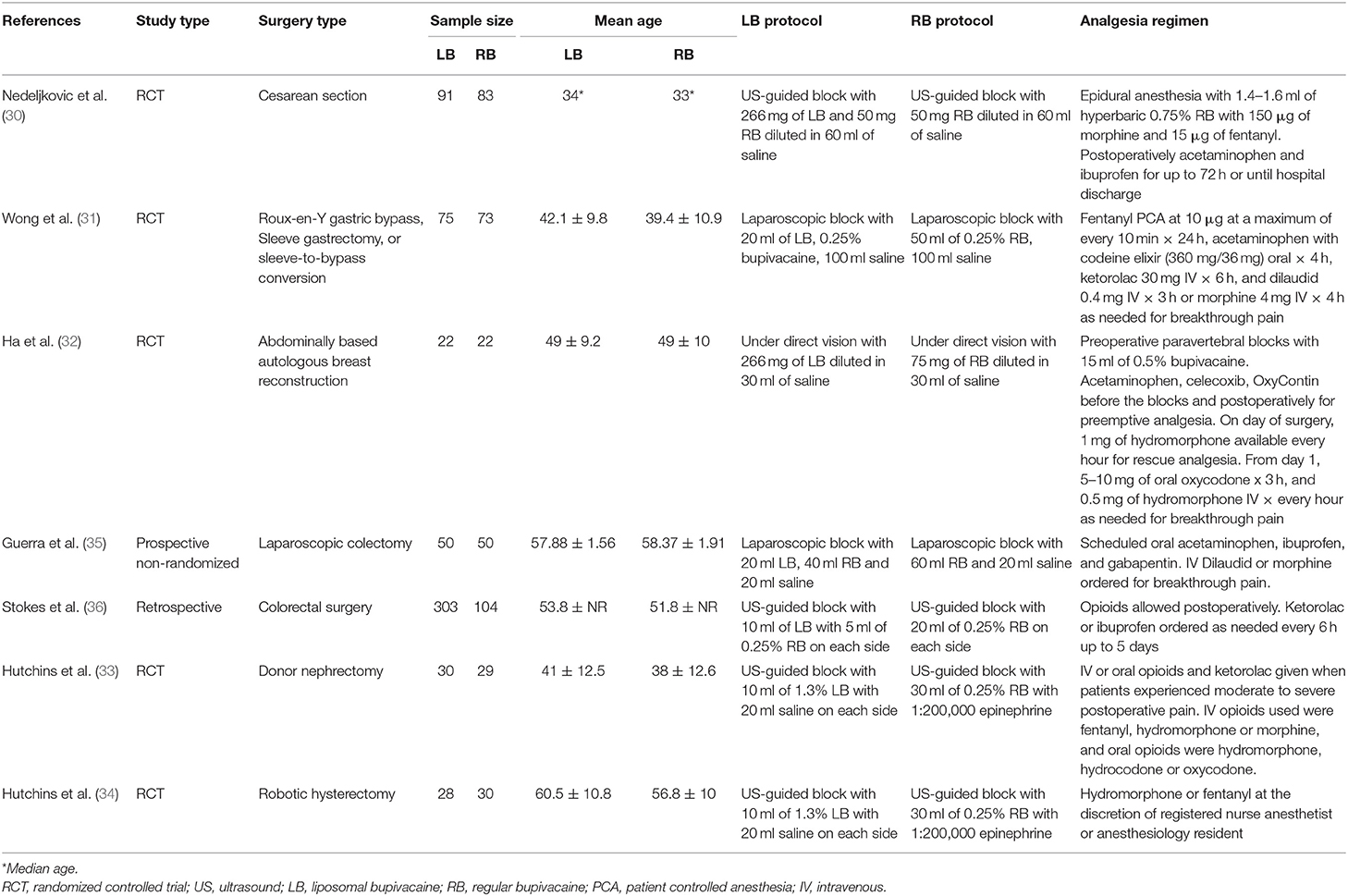
Figure 1 depicts the study flow chart. Seventeen studies were assessed by their full texts. Ten were excluded as per the reasons mentioned in Table 1 (20–29). A total of seven studies were included in this systematic review and meta-analysis (30–36). Details of the included studies are presented in Table 2. All studies were conducted in the United States. Five studies were RCTs (30–34), one was a prospective (35), while another was a retrospective study of a prospectively collected database (36). Ultrasound (US) guidance was used for the TAP blocks in four studies (30, 33, 34, 36). The amount of LB and RB varied across studies along with variations in the analgesic regimen.
Primary Outcomes
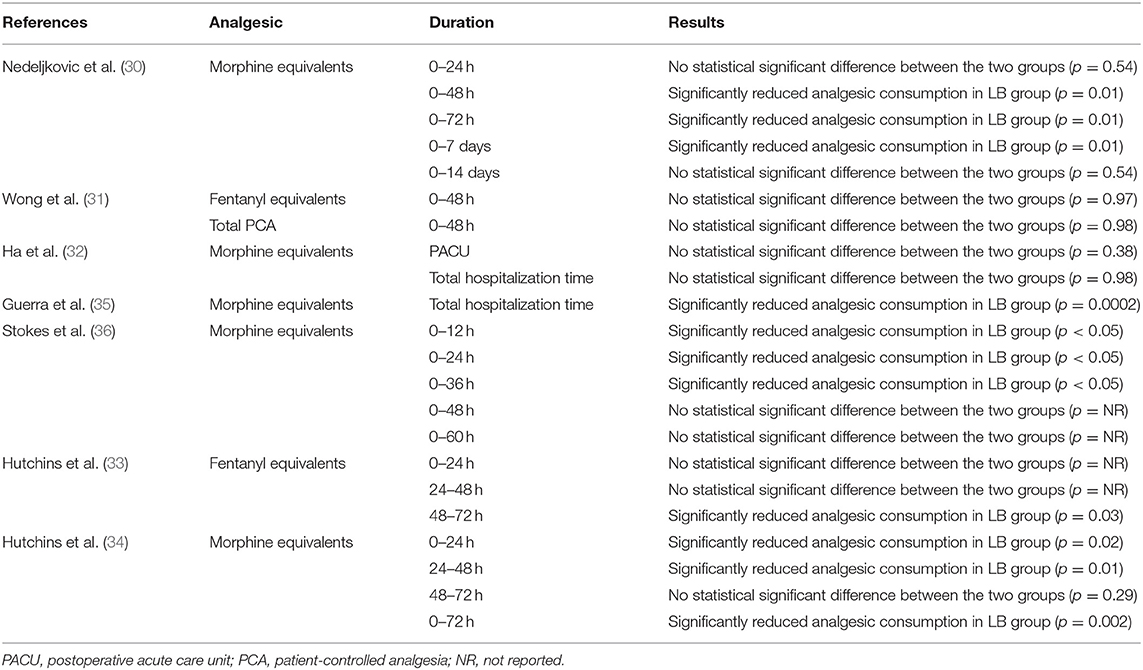
The total analgesic consumption postsurgery was reported by all included studies. However, there was significant heterogeneity in the presentation of data (least-square means, median, and means) with the outcome being measured for different time intervals. Hence, only a descriptive analysis was carried out. Results reported by the included studies for total analgesic consumption are presented in Table 3. Except for the studies of Wong et al. (31) and Ha et al. (32), which did not report any statistically significant difference in total analgesic consumption between the two groups, all studies reported significantly lower consumption of analgesics in the LB group at some time interval.
Except for the study of Guerra et al. (35), all studies included pain scores as one of their outcomes. Hutchins et al. (34) did not report the numerical or graphical data on pain scores in their article, while Nedeljkovic et al. (30) did not report data as mean and SD. Data from the remaining four studies were pooled for a meta-analysis. Our results indicated statistically significant reduction of pain with the use of LB for TAP block at 12 h (MD: −0.89, 95% CI: −1.44, −0.34 I2 = 0% p = 0.01), 24 h (MD: −0.64, 95% CI: −1.21, −0.06 I2 = 0% p = 0.03), 48 h (MD: −0.40, 95% CI: −0.77, −0.04 I2 = 0% p = 0.03) but not at 72 h (MD: −0.37, 95% CI: −1.31, 0.56 I2 = 57% p = 0.43) (Figure 2). Nedeljkovic et al. (30) in their study reported a statistically significant difference in pain scores between LB and RB at all time intervals up to 72 h. Hutchins et al. (34) reported significantly lower maximal pain scores in the LB group at 24-, 48-, and 72-h time points compared to the RB group.
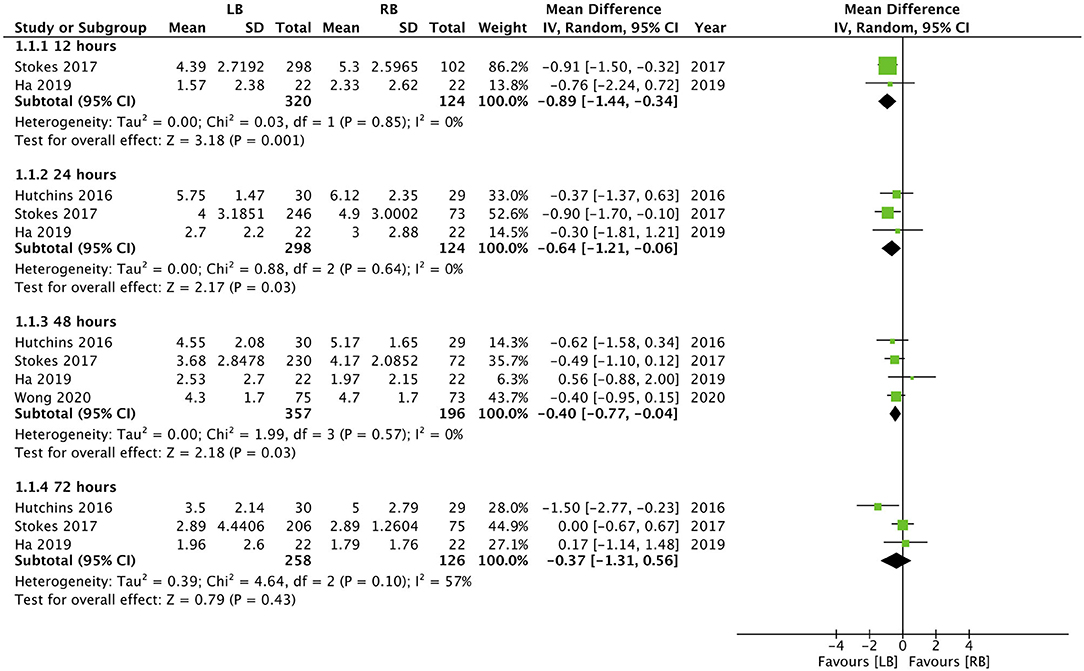
Figure 2. Forest plot of pain scores for liposomal bupivacaine (LB) vs. regular bupivacaine (RB) for transverse abdominal plane (TAP) block at different time intervals.
Secondary Outcomes
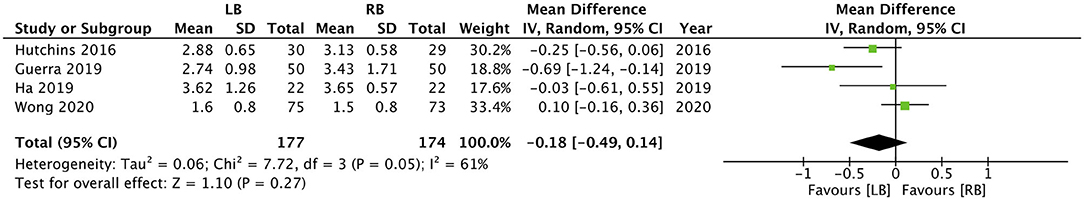
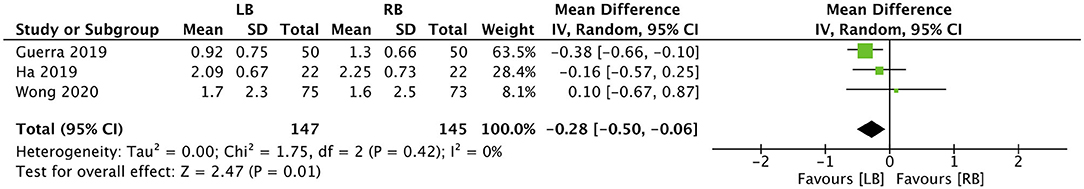
Four studies reported data on the length of hospital stay. Our pooled analysis indicated that the use of LB does not reduce the duration of hospital stay in days (MD: −0.18, 95% CI: −0.49, 0.14 I2 = 61% p = 0.27) (Figure 3). However, when data from three studies were analyzed, our results indicated that LB may significantly reduce the number of days to first ambulation postsurgery (MD: −0.28, 95% CI: −0.50, −0.06 I2 = 0% p = 0.01) (Figure 4).
Risk of Bias Analysis
Our judgment on the risk of bias in the included studies is presented in Table 4. The included RCTs were of moderate-high quality with low risk of bias in majority domains. The non-RCTs had a high risk of bias in the domains of confounding factors and blinding of outcome assessment.
Discussion
RB has one of the longest half-lives among commonly used local anesthetics in clinical practice. However, even with a terminal half-life of 3.5 h pain control by RB may not extend beyond the first postoperative day (12). In this context, LB was developed to prolong the analgesic effect of an already long-acting anesthetic agent. LB is based on the DepoFoam technology, which encapsulates the drug in a liposomal platform and releases them over a period of 1–30 days. In LB, the active drug lies in microscopic spherical liposomes, which are composed of biodegradable cholesterol, triglycerides, and phospholipids. Gradually, these microscopic vesicles reorganize and break open thereby releasing the drug at the injected site over a longer duration. The liposomes do not alter the chemical composition of the drug and also reduce the systemic toxicity by avoiding high drug plasma levels (12, 37). In 2011, the USA Food and drug administration (FDA) first approved the use of LB in wound infiltration for hemorrhoidectomies and bunionectomies. Since then, LB has also been approved for local infiltration in total knee arthroplasty, mammoplasty, inguinal hernia repair, and also as a field block agent in TAP blocks (37). However, whether the pharmacological properties of LB translate into clinically relevant prolonged analgesia with the TAP block is not known and was the subject of this study.
The effect of TAP blocks with regular anesthetic agents is known to last anywhere between 6 and 24 h (34). Ma et al. (38), in their systematic review and meta-analysis of 56 RCTs, have demonstrated that postoperative pain in the first 24 h is significantly reduced by the TAP block administered for various surgical procedures. They also reported that the TAP block significantly reduced morphine consumption and increased the time for the first analgesic request. While several RCTs have assessed the efficacy of regular anesthetics for the TAP block, only limited studies have compared single infiltration of TAP blocks with LB and regular anesthetics for various abdominopelvic surgical procedures. For the first primary outcome of our study i.e., total analgesic consumption, a meta-analysis could not be performed owing to significant differences in data collection and presentation among the included studies. Examining the results by specific periods, out of three studies (30, 31, 36) evaluating analgesic consumption for 0–48 h, only Nedeljkovic et al. (30) reported statistically significant results. Hutchins et al. (33, 34) in their two trials reported analgesic consumption for 24–48 and 48–72 h but with opposite results. For 0–72 h, data were reported only by Nedeljkovic et al. (30) and Hutchins et al. (34) with both reporting reduced analgesic consumption in the LB group. On the other hand, Ha et al. (32) and Guerra et al. (35) reported analgesic consumption for the entire duration of hospitalization but with contrasting results.
The overall incoherent results among the studies of our review can be attributed to many factors. First, TAP blocks were a part of multimodal analgesia plans in the included studies and not singularly used for pain control. The use of additional pain control measures like paravertebral blocks in the enhanced recovery protocol of Ha et al. (32) could have contributed to nonsignificant results. Second, accurate placement of the block is essential for optimal pain relief (30). In the trial of Nedeljkovic et al. (30), around 6% of patients received incorrect TAP blocks and were excluded from the analysis. Since none of the other studies assessed the accuracy of the TAP blocks, its exact influence on the review outcomes cannot be determined. Third, there was interstudy heterogeneity in the technique of TAP (ultrasound vs. laparoscopic) and the dosage of LB and RB. Despite the different techniques, RCTs have demonstrated that laparoscopic and ultrasound-guided TAP blocks have similar efficacy (39, 40). The precise role of variable dosage of RB in the control group and different combinations of LB and RB in the study group is also difficult to ascertain. As for RB, Ng et al. (41) in a meta-analysis of 14 studies were unable to delineate any difference in analgesic efficacy with high dose (>50 mg) or low dose (≤50 mg) RB for TAP blocks when used for cesarean sections. However, no study has measured the relationship of different doses of LB with the duration of pain relief after nerve or field blocks.
For the second primary outcome of our review, our analysis indicated a statistically significant reduction in pain scores with the use of LB at 12, 24, and 48 h. However, the pooled analysis failed to demonstrate any significant differences at 72 h. Further, the effect size for the results was not large with the upper end of 95% CI very close to zero (−0.06 for 24 h and −0.04 for 48 h). The concept of minimum clinically important difference (MCID) for patient-reported measures describes "the smallest difference in score in the domain of interest which participants perceive as beneficial” (42). While the MCID for pain scores after abdominopelvic surgery is not known (43), considering the very small effect size, its clinical relevance may not be high. The results of our study concur with other meta-analysis studies assessing the role of LB. Kolade et al. (14), in a review of LB for shoulder surgeries, have failed to elucidate any significant benefit of LB vs. regular anesthetics for reducing total opioid consumption or pain scores up to 48 h after the procedure. Hamilton et al. (44), in a Cochrane review of 2016, concluded that there is a lack of sufficient data to support or refute the use of LB for peripheral nerve blocks for the management of postoperative pain. Yayac et al. (45), in a recent review of 42 studies on total knee arthroplasty, have reported that despite significantly lower pain scores with LB, better pain control may not be clinically significant, and the use of LB may not reduce total opioid consumption.
One important variable that may limit a more liberal use of LB is its high cost. While some studies report that the better and prolonged pain control offered by LB reduces hospital stay and in turn compensates for the cost of the drug (33, 35), the same has not been duplicated by other clinicians (46, 47). Beachler et al. (47) have indicated that given the minimal clinical benefits of LB and the substantial cost of the drug, its use is not justifiable in total hip arthroplasty patients. In our analysis, despite reduced time to ambulation, the length of hospital stay was similar in both groups. Owing to the lack of monetary data, we cannot comment on the cost effectiveness of the drug for TAP blocks.
The results of our study should be interpreted with the following limitations. Foremost, only a limited number of studies were available for analysis of which only two were non-RCTs. The incorporation of nonrandomized studies may have skewed the results of our review. Second, as discussed earlier, there was significant methodological heterogeneity in the included studies. Further, the outcomes of interest were nonconsistently reported by all included studies. There were variations in time intervals, data presentation (least-square means, medians, or means) with some presenting data only pictorially. Data conversion and graphical extraction of data were undertaken to ensure maximum studies are included in the review. However, this may have introduced bias in our results. This may limit the generalization of our review results in wider clinical settings.
To conclude, current evidence on the role of LB vs. RB for providing prolonged analgesia with TAP blocks is unclear. There is conflicting evidence on the role of LB in reducing total analgesic consumption beyond 24 h. LB may result in a small reduction in pain scores up to 48 h but not at 72 h. Further, high-quality homogenous RCTs are needed to establish high-quality evidence.
Data Availability Statement
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author/s.
Author Contributions
YZ conceived and designed the study. TX, ZC, ZD, and JW were involved in the literature search and data collection. YZ and SQ analyzed the data. YZ wrote the paper. SQ reviewed and edited the manuscript. All authors read and approved the final manuscript.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fsurg.2020.596653/full#supplementary-material
References
1. Weiser TG, Regenbogen SE, Thompson KD, Haynes AB, Lipsitz SR, Berry WR, et al. An estimation of the global volume of surgery: a modelling strategy based on available data. Lancet. (2008) 372:139–44. doi: 10.1016/S0140-6736(08)60878-8
2. Katz J, Seltzer Z. Transition from acute to chronic postsurgical pain: risk factors and protective fa. Exp Rev Neurother. (2009) 9:723–44. doi: 10.1586/ern.09.20
3. Oderda GM, Said Q, Evans RS, Stoddard GJ, Lloyd J, Jackson K, et al. Opioid-related adverse drug events in surgical hospitalizations: impact on costs and length of stay. Ann Pharm. (2007) 41:400–6. doi: 10.1345/aph.1H386
4. Rafi AN. Abdominal field block: a new approach via the lumbar triangle. Anaesthesia. (2001) 56:1024–6. doi: 10.1046/j.1365-2044.2001.02279-40.x
5. Tsai HC, Yoshida T, Chuang TY, Yang SF, Chang CC, Yao HY, et al. Transversus abdominis plane block: an updated review of anatomy and techniques. BioMed Res Int. (2017) 2017:8284363. doi: 10.1155/2017/8284363
6. Cai Q, Gao ML, Chen GY, Pan LH. Transversus abdominis plane block versus wound infiltration with conventional local anesthetics in adult patients underwent surgery: a systematic review and meta-analysis of randomized controlled trials. BioMed Res Int. (2020) 2020:8914953. doi: 10.1155/2020/8914953
7. Hamid HK, Emile SH, Saber AA, Ruiz-Tovar J, Minas V, Cataldo TE. Laparoscopic-guided transversus abdominis plane block for postoperative pain management in minimally invasive surgery: systematic review and meta-analysis. J Am Coll Surg. (2020) 231:376–386.e15. doi: 10.1016/j.jamcollsurg.2020.05.020
8. Zhang D, Zhou C, Wei D, Ge L, Li Q. Dexamethasone added to local anesthetics in ultrasound-guided transversus abdominis plain (TAP) block for analgesia after abdominal surgery: a systematic review and meta-analysis of randomized controlled trials. PLoS ONE. (2019) 14:e0209646. doi: 10.1371/journal.pone.0209646
9. Dasta J, Ramamoorthy S, Patou G, Sinatra R. Bupivacaine liposome injectable suspension compared with bupivacaine HCl for the reduction of opioid burden in the postsurgical setting. Curr Med Res Opin. (2012) 28:1609–15. doi: 10.1185/03007995.2012.721760
10. Zhong T, Ojha M, Bagher S, Butler K, Srinivas C, McCluskey SA, et al. Transversus abdominis plane block reduces morphine consumption in the early postoperative period following microsurgical abdominal tissue breast reconstruction: A double-blind, placebo-controlled, randomized trial. Plast Reconstr Surg. (2014) 134:870–8. doi: 10.1097/PRS.0000000000000613
11. Hosgood SA, Thiyagarajan UM, Nicholson HFL, Jeyapalan I, Nicholson ML. Randomized clinical trial of transversus abdominis plane block versus placebo control in live-donor nephrectomy. Transplantation. (2012) 94:520–5. doi: 10.1097/TP.0b013e31825c1697
12. Tong YCI, Kaye AD, Urman RD. Liposomal bupivacaine and clinical outcomes. Best Pract Res. (2014) 28:15–27. doi: 10.1016/j.bpa.2014.02.001
13. Zhang X, Yang Q, Zhang Z. The efficiency and safety of local liposomal bupivacaine infiltration for pain control in total hip arthroplasty: a systematic review and meta-analysis. Medicine. (2017) 96:e8433. doi: 10.1097/MD.0000000000008433
14. Kolade O, Patel K, Ihejirika R, Press D, Friedlander S, Roberts T, et al. Efficacy of liposomal bupivacaine in shoulder surgery: a systematic review and meta-analysis. J Shoulder Elbow Surg. (2019) 28:1824–34. doi: 10.1016/j.jse.2019.04.054
15. Moher D, Liberati A, Tetzlaff J, Altman DG, PRISMA Group. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med. (2009) 6:e1000097. doi: 10.1371/journal.pmed.1000097
16. Higgins J, Thomas J, Chandler J, Cumpston M, Li T, Page M, et al. Cochrane Handbook for Systematic Reviews of Interventions. Version 6. Cochrane. (2019) Chichester, UK: John Wiley & Sons doi: 10.1002/9781119536604
17. Higgins J, Altman D, Sterne J. Cochrane statistical methods group and the cochrane bias methods group. Chapter 8: assessing risk of bias in included studies. In: Cochrane Handbook for Systemic Reviews of Interventions (The Cochrane Collaboration). Available online at: https://handbook-5-1.cochrane.org (accessed June 1, 2020).
18. Kim SY, Park JE, Lee YJ, Seo H-J, Sheen S-S, Hahn S, et al. Testing a tool for assessing the risk of bias for nonrandomized studies showed moderate reliability and promising validity. J Clin Epidemiol. (2013) 66:408–14. doi: 10.1016/j.jclinepi.2012.09.016
19. Wan X, Wang W, Liu J, Tong T. Estimating the sample mean and standard deviation from the sample size, median, range and/or interquartile range. (2014).
20. Jablonka EM, Lamelas AM, Kim JN, Molina B, Molina N, Okwali M, et al. Transversus abdominis plane blocks with single-dose liposomal bupivacaine in conjunction with a nonnarcotic pain regimen help reduce length of stay following abdominally based microsurgical breast reconstruction. Plast Reconstr Surg. (2017) 140:240–51. doi: 10.1097/PRS.0000000000003508
21. Fields AC, Weiner SG, Maldonado LJ, Cavallaro PM, Melnitchouk N, Goldberg J, et al. Implementation of liposomal bupivacaine transversus abdominis plane blocks into the colorectal enhanced recovery after surgery protocol: a natural experiment. Int J Colorect Dis. (2020) 35:133–8. doi: 10.1007/s00384-019-03457-1
22. Hutchins J, Argenta P, Berg A, Habeck J, Kaizer A, Geller MA. Ultrasound-guided subcostal transversus abdominis plane block with liposomal bupivacaine compared to bupivacaine infiltration for patients undergoing robotic-assisted and laparoscopic hysterectomy: a prospective randomized study. J Pain Res. (2019) 12:2087–94. doi: 10.2147/JPR.S193872
23. Gatherwright J, Knackstedt RW, Ghaznavi AM, Bernard S, Schwarz G, Moreira A, et al. Prospective, randomized, controlled comparison of bupivacaine versus liposomal bupivacaine for pain management after unilateral delayed deep inferior epigastric perforator free flap reconstruction. Plast Reconstr Surg. (2018) 141:1327–30. doi: 10.1097/PRS.0000000000004360
24. Hutchins JL, Renfro L, Orza F, Honl C, Navare S, Berg AA. The addition of intrathecal morphine to a transversus abdominis plane block with liposome bupivacaine provides more effective analgesia than transversus abdominis plane block with liposome bupivacaine alone: A retrospective study. Local Reg Anesth. (2019) 12:7–13. doi: 10.2147/LRA.S190225
25. Yeap YL, Fridell JA, Wu D, Mangus RS, Kroepfl E, Wolfe J, et al. Comparison of methods of providing analgesia after pancreas transplant: IV opioid analgesia versus transversus abdominis plane block with liposomal bupivacaine or continuous catheter infusion. Clin Transpl. (2019) 33:e13581. doi: 10.1111/ctr.13581
26. Sternlicht A, Shapiro M, Robelen G, Vellayappan U, Tuerk IA. Infiltration of liposome bupivacaine into the transversus abdominis plane for postsurgical analgesia in robotic laparoscopic prostatectomy: A pilot study. Local Reg Anesth. (2014) 7:69–74. doi: 10.2147/LRA.S64515
27. Feierman DE, Kronenfeld M, Gupta PM, Younger N, Logvinskiy E. Liposomal bupivacaine infiltration into the transversus abdominis plane for postsurgical analgesia in open abdominal umbilical hernia repair: Results from a cohort of 13 patients. J Pain Res. (2014) 7:477–82. doi: 10.2147/JPR.S65151
28. Ayad S, Babazade R, Elsharkawy H, Nadar V, Lokhande C, Makarova N, et al. Comparison of transversus abdominis plane infiltration with liposomal bupivacaine versus continuous epidural analgesia versus intravenous opioid analgesia. PLoS ONE. (2016) 11:e0153675. doi: 10.1371/journal.pone.0153675
29. Felling DR, Jackson MW, Ferraro J, Battaglia MA, Albright JJ, Wu J, et al. Liposomal bupivacaine transversus abdominis plane block versus epidural analgesia in a colon and rectal surgery enhanced recovery pathway: A randomized clinical trial. Dis Colon Rectum. (2018) 61:1196–204. doi: 10.1097/DCR.0000000000001211
30. Nedeljkovic SS, Kett A, Vallejo MC, Horn J-L, Carvalho B, Bao X, et al. Transversus abdominis plane block with liposomal bupivacaine for pain after cesarean delivery in a multicenter, randomized, double-blind, controlled trial. Anesth Analg. (2020) doi: 10.1213/ANE.0000000000005075
31. Wong KA, Cabrera AG, Argiroff AL, Pechman DM, Parides MK, Vazzana JT, et al. Transversus abdominis plane block with liposomal bupivacaine and its effect on opiate use after weight loss surgery: a randomized controlled trial. Surg Obesity Relat Dis. (2020) 16:886–93. doi: 10.1016/j.soard.2020.03.031
32. Ha AY, Keane G, Parikh R, Odom E, Tao Y, Zhang L, et al. The analgesic effects of liposomal bupivacaine versus bupivacaine hydrochloride administered as a transversus abdominis plane block after abdominally based autologous microvascular breast reconstruction: a prospective, single-blind, randomized, controlled. Plastic Reconstruc Surg. (2019) 144:35–44. doi: 10.1097/PRS.0000000000005698
33. Hutchins JL, Kesha R, Blanco F, Dunn T, Hochhalter R. Ultrasound-guided subcostal transversus abdominis plane blocks with liposomal bupivacaine vs. Non-liposomal bupivacaine for postoperative pain control after laparoscopic hand-assisted donor nephrectomy: a prospective randomised observer-blinded study. Anaesthesia. (2016) 71:930–7. doi: 10.1111/anae.13502
34. Hutchins J, Delaney D, Vogel RI, Ghebre RG, Downs LS, Carson L, et al. Ultrasound guided subcostal transversus abdominis plane (TAP) infiltration with liposomal bupivacaine for patients undergoing robotic assisted hysterectomy: A prospective randomized controlled study. Gynecol Oncol. (2015) 138:609–13. doi: 10.1016/j.ygyno.2015.06.008
35. Guerra L, Philip S, Lax EA, Smithson L, Pearlman R, Damadi A. Transversus abdominis plane blocks in laparoscopic colorectal surgery: better pain control and patient outcomes with liposomal bupivacaine than bupivacaine. Am Surg. (2019) 85:1013–6. doi: 10.1177/000313481908500945
36. Stokes AL, Adhikary SD, Quintili A, Puleo FJ, Choi CS, Hollenbeak CS, et al. Liposomal bupivacaine use in transversus abdominis plane blocks reduces pain and postoperative intravenous opioid requirement after colorectal surgery. Dis Colon Rectum. (2017) 60:170–7. doi: 10.1097/DCR.0000000000000747
37. Malik O, Kaye AD, Kaye A, Belani K, Urman RD. Emerging roles of liposomal bupivacaine in anesthesia practice. J Anaesthesiol Clin Pharmacol. (2017) 33:151–6. doi: 10.4103/joacp.JOACP_375_15
38. Ma N, Duncan JK, Scarfe AJ, Schuhmann S, Cameron AL. Clinical safety and effectiveness of transversus abdominis plane (TAP) block in post-operative analgesia: a systematic review and meta-analysis. J Anesth. (2017) 31:432–52. doi: 10.1007/s00540-017-2323-5
39. Park SY, Park JS, Choi GS, Kim HJ, Moon S, Yeo J. Comparison of analgesic efficacy of laparoscope-assisted and ultrasound-guided transversus abdominis plane block after laparoscopic colorectal operation: a randomized, single-blind, non-inferiority trial. J Am Coll Surg. (2017) 225:403–10. doi: 10.1016/j.jamcollsurg.2017.05.017
40. Wong DJ, Curran T, Poylin VY, Cataldo TE. Surgeon-delivered laparoscopic transversus abdominis plane blocks are non-inferior to anesthesia-delivered ultrasound-guided transversus abdominis plane blocks: a blinded, randomized non-inferiority trial. Surg Endosc. (2020) 34:3011–9. doi: 10.1007/s00464-019-07097-y
41. Ng SC, Habib AS, Sodha S, Carvalho B, Sultan P. High-dose versus low-dose local anaesthetic for transversus abdominis plane block post-Caesarean delivery analgesia: a meta-analysis. Br J Anaesth. (2018) 120:252–63. doi: 10.1016/j.bja.2017.11.084
42. Jaeschke R, Singer J, Guyatt GH. Measurement of health status. Ascertaining the minimal clinically important difference. Controll Clin Trials. (1989) 10:407–15. doi: 10.1016/0197-2456(89)90005-6
43. Olsen MF, Bjerre E, Hansen MD, Hilden J, Landler NE, Tendal B, et al. Pain relief that matters to patients: Systematic review of empirical studies assessing the minimum clinically important difference in acute pain. BMC Med. (2017) 15:35. doi: 10.1186/s12916-016-0775-3
44. Hamilton TW, Athanassoglou V, Trivella M, Strickland LH, Mellon S, Murray D, et al. Liposomal bupivacaine peripheral nerve block for the management of postoperative pain. Cochr Datab System Rev. (2016) 2016:CD011476. doi: 10.1002/14651858.CD011476.pub2
45. Yayac M, Li WT, Ong AC, Courtney PM, Saxena A. The efficacy of liposomal bupivacaine over traditional local anesthetics in periarticular infiltration and regional anesthesia during total knee arthroplasty: a systematic review and meta-analysis. J Arthroplasty. (2019) 34:2166–83. doi: 10.1016/j.arth.2019.04.046
46. Schumer G, Mann JW, Stover MD, Sloboda JF, Cdebaca CS, Woods GM. Liposomal bupivacaine utilization in total knee replacement does not decrease length of hospital Stay. J Knee Surg. (2019) 32:934–9. doi: 10.1055/s-0038-1673617
Keywords: extended analgesia, local anesthetic, pain, opioids, surgery
Citation: Zhu Y, Xiao T, Qu S, Chen Z, Du Z and Wang J (2020) Transversus Abdominis Plane Block With Liposomal Bupivacaine vs. Regular Anesthetics for Pain Control After Surgery: A Systematic Review and Meta-Analysis. Front. Surg. 7:596653. doi: 10.3389/fsurg.2020.596653
Received: 20 August 2020; Accepted: 05 October 2020;
Published: 05 November 2020.
Edited by:
René H. Fortelny, Wilhelminenspital, AustriaReviewed by:
Andrew Gumbs, Centre Hospitalier Intercommunal de Poissy, FranceAtil Çakmak, Ankara University, Turkey
Copyright © 2020 Zhu, Xiao, Qu, Chen, Du and Wang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Shuangquan Qu, qusq_dr@163.com
 Yi Zhu
Yi Zhu  Shuangquan Qu
Shuangquan Qu