TEVAR and EVAR, the unknown knowns of the cardiovascular hemodynamics; and the immediate and long-term consequences of fabric material on major adverse clinical outcome
- 1Department of Vascular and Endovascular Surgery, Western Vascular Institute, University Hospital Galway, National University of Ireland, Galway, Ireland
- 2Galway Clinic, Doughiska, Royal College of Surgeons in Ireland and the National University of Ireland, Galway Affiliated Hospital, Galway, Ireland
- 3CÚRAM-CORRIB-Vascular Group, National University of Ireland, Galway, Ireland
- 4Department of Vascular Surgery and Biomedical Engineering Department, University of Buenos Aires, Buenos Aires, Argentina
This review discusses the impact of endovascular aneurysm repair on cardiovascular (CV) hemodynamics and the role of stent-graft material, i.e., polytetrafluoroethylene (PTFE) vs. polyester in post-procedural outcomes. Endovascular aneurysm repair has been widely employed in the last decades for thoracic and abdominal aneurysm repair. However, aortic endografts are stiff and alter the native flow hemodynamics. This failure to simulate the native aorta could lead to added strain on the heart, manifesting as increased left ventricular strain, higher pulse pressure, and congestive heart failure later. This could result in adverse CV outcomes. Also, evidence is mounting to support the implication of stent-graft materials, i.e., PTFE vs. polyester, in adverse post-procedural outcomes. However, there is an absence of level one evidence. Therefore, the only way forward is to plan and perform a randomised controlled trial to demonstrate the alterations in the CV hemodynamics in the short and long run and compare the available stent-graft materials regarding procedural and clinical outcomes. We believe the best solution, for now, would be to reduce the stented length of the aorta. At the same time, in the longer term, encourage continuous improvement in stent-graft materials and design.
Introduction
Compared to open surgical repair, endovascular repair of the thoracic and abdominal aorta has been shown to reduce early perioperative morbidity and mortality (1, 2). However, this advantage is not maintained later due to an increment in cardiovascular (CV) complications secondary to arterial stiffening by endograft (3). It is, therefore, essential to be aware of the impact of endograft design, their relative configuration, and stiffness compared to the native aorta (3–5). Also, the role of the endograft composition and structural design (i.e., endograft materials—polyester vs. polytetrafluoroethylene (PTFE), stent wires—nitinol vs. stainless-steel stent vs. cobalt-chromium) on the post-procedural outcomes needs to be acknowledged (3–9).
This review discusses the impact of endograft on CV hemodynamics in the first half and, subsequently, in the second half, the impact of stent-graft material, i.e., PTFE vs. polyester, in post-procedural outcomes, including post-implantation syndrome.
Materials and methods
This study was conducted through a non-structured online literature search (PubMed, Google Scholar and EMBASE) using the keywords—“Cardiovascular Hemodynamics,” “Cardiovascular Complications,” “Cardiovascular Outcomes,” “Abdominal Aortic Aneurysm,” “AAA,” “Endovascular Repair,” “TEVAR,” “Thoracic Endovascular Aneurysm Repair,” “EVAR,” “Endovascular Aneurysm Repair,” “Endograft,” “Stent-graft material,” “PTFE,” “Polytetrafluoroethylene,” “Polyester,” and “Outcome.” No selective restrictions were made on the type of studies, publication year and language. A secondary reference search was used to obtain further studies.
Impact of EVAR and TEVAR on cardiovascular haemodynamics
Aortic endografts are stiffer than the native aorta, and even the best available contemporary endograft design could potentially alter the flow haemodynamics (3–5). Studies have shown that aortic endografts could significantly reduce coronary perfusion by elevating systolic blood and pulse pressure (5–7, 10). These patients suffer on and off chest pain and systolic hypertension from early postoperative days. However, the broader CV community lack insight regarding cardiac remodelling post-aortic stents as interventionalists primarily focus on endo-graft adaptation rather than hemodynamic alterations. Furthermore, our follow-up protocols are based only on close supervision for endograft migration, detecting endoleak and aortic sac regression, for which we are not afraid of further stenting and coiling, thereby creating a stiffer aortic wall, which could further compromise cerebral, cardiac, renal, and mesenteric perfusion (3–7).
Pathophysiology
The aorta receives the left ventricle (LV) stroke volume in systole, which is distributed peripherally through the stored aortic elastic forces gained during diastole. This aortic compliance and blood flow through the aorta is best represented by the “Windkessel effect” (Figure 1) (10).
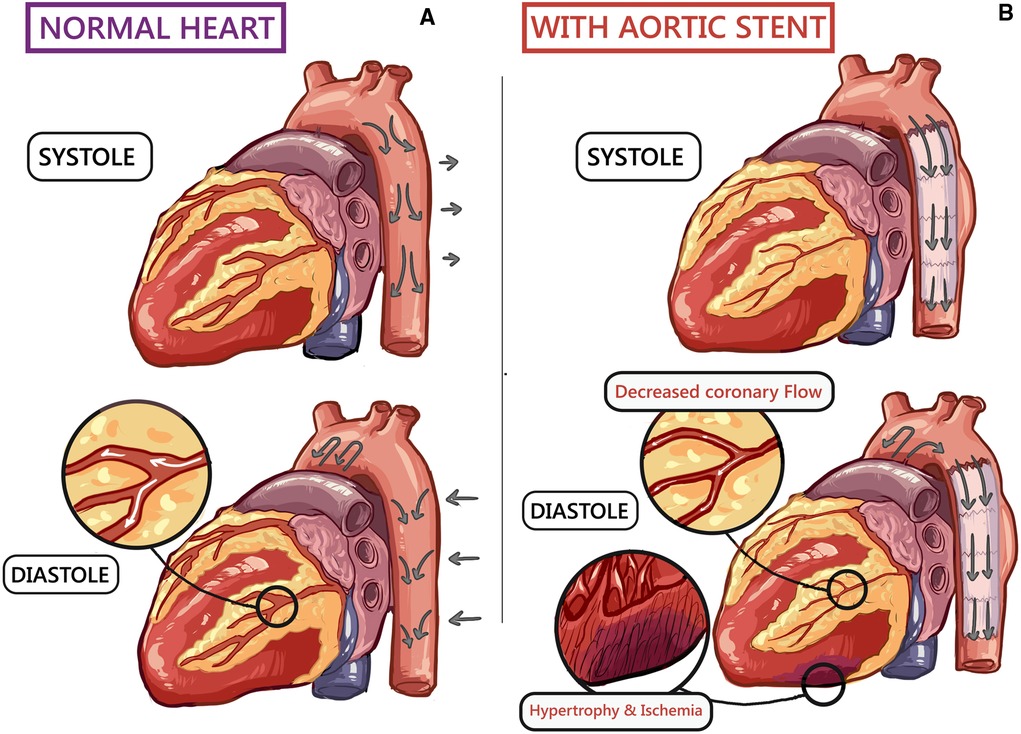
Figure 1. A schematic human heart diagram showing (10): (A) normal cardiovascular hemodynamics during systole and diastole. (B) Endograft in the thoracic aorta increases arterial stiffness, causing left ventricular (LV) strain and subsequent hypertrophy. Furthermore, impaired LV filling decreases coronary blood flow, resulting in non-occlusive ischaemia.
Windkessel effect impact both the heart and the peripheral circulation. Aortic compliance decreases the LV afterload. Furthermore, blood collected within the distended aorta helps to enhance coronary perfusion.
A mismatch between the native aortic to endograft compliance could manifest as adverse CV outcomes. Any change in the Windkessel effect could significantly increase the LV burden, resulting in adaptative LV hypertrophy and loss of ventricular-arterial coupling (11, 12).
As aortic endografts are less compliant than the native aorta, insufficient compliance results in a surge in hemodynamic shifts that impair CV homeostasis (3, 5, 8). Arterial stiffening results in elevated systolic blood pressure but lowers diastolic blood pressure, further exacerbating LV afterload, resulting in mal-perfusion of the coronaries. These changes contribute to LV hypertrophy, coronary ischemia, and arterial wall tissue fatigue, which are independent risk factors for CV morbidity and mortality (10–14).
Rong et al. (15) demonstrated amplification of circumferential strain in the descending thoracic aorta, paralleling distensibility by using intra-operative transoesophageal echocardiography to study the effect of endograft on the haemodynamic alteration. They showed that prosthetic replacement of the ascending aorta could interfere with the propagation of energy to the distal aorta resulting in adverse aortic remodelling. These results explain the development of resistant systolic hypertension post-TEVAR/EVAR with shortness of breath (SOB) and intermittent chest pain.
The impact of aortic flow dynamics on the LV function has been studied in experimental models (16), animals (17) and clinical studies (18). These studies support the Windkessel theory to establish the role of aortic capacitance in resultant ventricular size and function. However, the stented aorta loses its elasticity following simple and/or complex endovascular procedures, like TEVAR, FEVAR, BEVAR, and ChEVAR, failing the Windkessel effect. This failure of the Windkessel effect and change in pulse wave propagation multiplies a substantial workload for the LV putting an extra strain on the aortic valve's functioning. The resultant adaptative LV hypertrophy will manifest as CV complications (10–12).
The negative impedance due to endograft and LV strain will cause a decrease in diastolic systemic BP and reduces coronary blood flow and myocardial ischemia without coronary artery stenosis (10, 13). Sultan et al. (3, 5, 8, 10, 14–18) documented cardiac dysfunction on the postoperative echocardiograms of TEVAR/EVAR cases, with moderate LV hypertrophy and diastolic dysfunction. This is manifested by an increase in proBNP, which supports myocyte stretching and ventricular strain. Moreover, there was a significant troponin rise without coronary artery stenosis. Furthermore, the coronary angiography confirmed the absence of the coronary blockage, which supports the alternative explanation of coronary hypoperfusion following reduced diastolic pressure (Figure 2) (10).
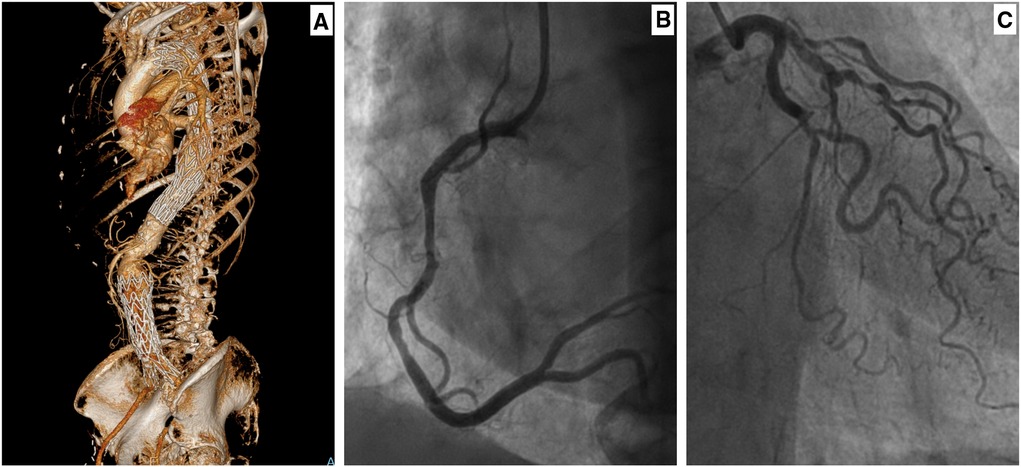
Figure 2. A female in her seventies with a saccular aneurysm in the descending thoracic aorta (10). She had thoracic endovascular aortic repair (TEVAR) in 2015 and subsequently underwent endovascular repair of her infrarenal aortic aneurysm in 2018. Her background history included ex-smoker, hypertension, lipid disorder, and right femoral-popliteal percutaneous transluminal angioplasty. (A) A 3D-CTA reconstruction, showing thoracic endograft. Following the TEVAR, the patient complained of intermittent chest pain and shortness of breath. A coronary angiogram was performed after her symptoms worsened. (B) Coronary angiogram (right main coronary artery) with no evidence of occlusive coronary disease. (C) Coronary angiogram (left main coronary artery) with no evidence of occlusive coronary disease.
Aortic compliance mismatch and hemodynamic alterations will be more evident after increasing the length of the stented aorta, for example, following combined TEVAR and EVAR. As such, endograft tend to adapt to these increments in shear stress. Studies have shown gradual endograft dilation after open surgical repair (3.2% per year post repair) (19–22). This could sometime result in excessive strain on the fabric architecture and the development of new aneurysms (10, 13).
Aortic integrity affects CV outcomes. This is evident in acute aortic syndrome, where CV complications are the main culprit for the late rehospitalisation after discharge (23, 24). Weiss et al. (24) showed nonfatal CV events and heart failure in patients with aortic dissection, intramural hematoma and penetrating aortic ulcer. These outcomes strengthen the need for long-term CV follow-up following endovascular aortic repair.
Pulse wave velocity
One of the ways to measure the impact of endograft stiffness on aortic impedance is to measure PWV.
PWV represents arterial stiffness, as higher arterial stiffness is seen with higher PWV. Subsequent increment in PWV increases the CV morbidity and mortality. Interesting, PWV could increase within a few hours of TEVAR and/or EVAR (21, 22, 25). TEVAR and EVAR increase the PWV by 2–5 and 1–3 m/s; however, a combined TEVAR/EVAR will result in an increment of 3–8 m/s (21, 22, 25). Blacher et al. (21) acknowledged that 1 m/s of PWV increment would double the all-cause mortality.
TEVAR has been shown to increase LV stroke work by 26% (26). Van-Bakel et al. (16) showed that structural stiffness increased from 10.2 to 154.6 MPa/mm post-TEVAR. This is a 15-fold increase in workload for the heart within minutes of deployment of a TEVAR; as the heart was not preconditioned, the CV haemodynamic changes accelerate over time. Furthermore, increment in vascular stiffness with endograft results in ventricular diastolic dysfunction, thereby negatively impairing exercise tolerance amongst patients with lower LV distensibility (11, 12, 27).
We contemplated that PWV could be utilised in risk assessment in the peri-operative period post-TEVAR/EVAR. Risk stratification provides an opportunity to address hemodynamic alterations and modulate the CV risk (21, 22, 25).
Impact of stent-graft materials on post-procedural outcomes and post-implantation syndrome
A 4-dimensional strategy (14) is necessary to manage complex aortic pathologies as altered haemodynamic forces increase wall shear stress and impair blood flow, causing flow turbulence, pressure gradients, and blood viscosity increment. It involves the morphological adjustment and hemodynamic milieu of natural body forces since the resultant flow disturbance affects the management outcome (14).
Sultan et al. (10) documented that patients with combined TEVAR and EVAR can develop adaptive LV hypertrophy and diastolic dysfunction. This could result in a clinical picture like lower limb oedema, SOB, and chest pain with a normal coronary angiogram (Figure 2) (10).
The increase in aortic stiffness post-TEVAR could be seen earlier than EVAR due to proximity to the heart (28). However, the length of the stented aorta also matters. Combined TEVAR and EVAR in this regard have earlier and more pronounced impacts (10). Nonetheless, it is prudent that all the available endograft are less compliant than the native aorta (16, 29).
In terms of the endograft material, the Liapis group (30) showed that endograft made with polyester results in a threefold increment in PWV than PTFE.
We witnessed that TEVAR patients developed the abdominal aortic disease after endograft implantation (10). In these patients, worsening hypertension and late CV complications were potentiated by having a stiff tube in the aorta. This necessitates studies that specifically focus on CV complications post-aortic endograft. Also, it is possible that careful analysis of the endograft based registry could answer that question at present.
Patients with connective tissue disorder, like Marfan's syndrome, have a defect in the aortic wall, which could further complicate the compliance mismatch and result in aneurysmal dilatation.
Suppose this is explained to young trauma patients post aortic transection who underwent emergency TEVAR. This will result in unexplained congestive cardiac failure and dilated cardiomyopathy post-TEVAR in many young patients following aortic trauma.
Modified and complex endovascular techniques (BEVAR, FEVAR, PETTICOAT (31), STABILISE (32), FLIRT (33), Candy Plug (34), Knickerbocker (35), and Kinetic Elephant trunk (36) could provoke additional aortic wall stress. The risk of these CV complications increases more when stents are deployed closer to the heart and aortic valve (7, 36–39).
We acknowledged in our previous publications that the best solution is to reduce the length of the stented aorta through a “Staged hybrid single lumen reconstruction (TIGER)” protocol (3, 4, 26, 40, 41). TIGER protocol combines open abdominal aortic repair with thoracic aortic stenting. For this, we first create a single lumen from supra celiac, infra-diaphragmatic aorta to bilateral common iliac arteries through visceral arteries open surgical patching and subsequently perform TEVAR after that (4). With the reduction in the stented length of the aorta, the TIGER technique has shown that fewer aortic stents and grafts have superior long-term CV outcomes (3, 4, 14, 41).
Cardiac dysfunction following TEVAR/EVAR is a complex challenging scenario for CV interventionalist (10, 29). Therefore, it is essential to contemplate the compliance mismatch and long-term adverse CV outcomes. The nearer to the heart the endograft is deployed, the worse is the effect. The way to the future is to respect the aorta as an active organ, not a mere conduit.
The ideal design of the aortic endograft should resemble the native aorta in terms of its flexibility and hemodynamic impedance. The stent-graft polymers should be lightweight but strong and resilient and capable of withstanding the impact of normal pulsatile high flow arterial blood pressure. However, ePTFE and polyester are synthetic polymers that are relatively stiff and rigid compared to the native aorta (42–47).
There are no RCTs or CCTs to validate post-procedural outcomes following EVAR/TEVAR with specific stent-graft materials. Although not powered to demonstrate the difference in outcomes based on endografts, the EVAR I trial showed reduced major adverse clinical events (MACEs) with the PTFE based GORE Excluder graft (48, 49). Furthermore, direct comparisons are further complicated by the heterogeneity of individual manufacturers' differences in endograft design and procedural deployment techniques (50–53).
Consequently, it is difficult to accurately predict the impact of the stent-graft materials on hemodynamic alteration. PWV is a surrogate marker that demonstrates changes in stiffness following EVAR. Liapis et al. (30) showed that post-EVAR with polyester endografts, there could be a threefold increase in PWV compared to PTFE. PTFE endografts have been reported to offer significantly stronger resistance to dilatation than polyester-based endografts initially, albeit this advantage is lost over time (54). Similarly, there were lower complications with PTFE grafts (55). However, there are no reports of apparent long-term advantages.
PTFE-based endografts, compared to polyester, are associated with a lower incidence of post-implantation syndrome (PIS). PIS has been reported in up to two-thirds of the patients following TEVAR/EVAR (56), resulting in acute liver and/or multiple-organ failure (57–62). Ito et al. (56), Voûte et al. (63), and Sartipy et al. (64) implicated polyester-based endografts in the development of postoperative pyrexia, PIS, and more extended hospital stay post-EVAR compared to the PTFE-based endografts.
Ferreira et al. (65) suggested a probable interlink between PIS and increased CV mortality as polyester-based endografts increased inflammatory responses that caused endothelial damage. Higher serum IL-8 levels support this as IL-8 has pro-inflammatory and pro-tumoural functions. Also, IL-8 implicates the potential of polyester-based endografts; however, it is yet to be established (66–68).
Similarly, the use of polymers in EVAR within PTFE fabric has been controversial, and the polymer-based endografts, like Nellix (Endologix Inc., Irvine, CA, USA) and Ovation iX (Endologix Inc., Irvine, CA, USA) abdominal stent graft system device, were subsequently removed from the market (69, 70). They failed in short and mid-term follow-ups because of an inadequate proximal fixation with continuous pressure necrosis on the aortic sac for Nellix and aortic neck wall for the Ovation (69, 70). Any technology that uses embedded high inflation rings (Ovation iX) or balloons/endobags (Nellix) must be contraindicated, as the aorta is an organ that must be respected. Any attempt to manage it as a mere conduit is destined to fail.
The Alto device is a newer generation of the Ovation Xi platform, which combines PTFE limbs with the main body with polymer-filled rings to assist with sealing the proximal aortic neck (69). The technology is evolving, and there is limited long-term data on performance.
There have been studies looking at the effect of Ovation on PWV, which found no increment, but they did not compare it to other devices (71). However, PIS with polymer-based EVAR has the equivalent outcome as PTFE-based endografts with the added complications of aggravated PIS due to activation of TNF and monocytes at the site of high inflation balloons and/or rings (63–65).
The future
We must innovate in creating intelligent, compliant, durable endoprostheses that do not require any maintenance or follow up. It will be manufactured by a “Bio-inspired Smart Self-Healing Material with Autonomous and Non-Autonomous Nanoparticles” as a nano-carrier for self-healing, self-repairing and self-assembly systems. These elements are vital components for durable smart endoprostheses.
The intelligent endoprosthesis will adapt itself to prevent tissue ingrowth into its' microstructure, preventing rigidity and maintaining distensibility. Therefore, the Smart endoprosthesis will retain the ability to expand in systole and collapse in diastole. After implantation, it gives back the elastic recoil to the heart, creating an almost standard aortic flow curve.
Bio-active-bio-inspired scaffolds will allow the smart endoprosthesis to be more robust and fault-tolerant. Transverse and longitudinal crimping that expands in systole and contracts in diastole will mimic the elastic recoil of the aorta. Hence it will abolish CV hemodynamic consequences of adaptive LV hypertrophy, the wide pulse pressure, the congestive heart failure and the renal impairment.
This paradigm shift towards utilising bio-inspired smart self-healing materials to build smart endoprosthesis capable of advanced self-healing during the functional lifetime of the endograft is a disruptive technology and will augment bio-convergence (72).
Intelligent bio-inspired endoprosthesis will lengthen product lifetime and abolish the need for follow-up or re-interventions. It is an intelligent green environmental friendly endoprosthesis that requires no service—a “TESLA like scenario”.
Conclusion
There is increasing evidence of adverse hemodynamic alteration post-TEVAR/EVAR. Furthermore, evidence to support the implication of specific stent-graft materials, i.e., PTFE vs. polyester, in adverse post-procedural outcomes following endovascular repair of AAA is mounting. Interventionalists must respect the aorta as an active organ, not a mere conduit. The best solution in the short term could be to reduce the stented length of the aorta while in the longer-term encouraging continuous improvement in stent-graft materials and design. In the absence of level one evidence, the only way forward is to plan and perform an RCT or CCT to compare the available stent-graft materials regarding procedural and clinical outcomes.
Author contributions
Concept and design: SS, YA, OS, JCP, NH. Data collection: N/A. Analysis and interpretation: SS, YA, OS, JCP, NH. Writing the article: SS, YA, OS, JCP, NH. Critical revision of the article: SS, YA, OS, JCP, NH. Final approval of the article: SS, YA, OS, JCP, NH. Overall responsibility: SS, YA, OS, JCP, NH. All authors contributed to the article and approved the submitted version.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Dangas G, O'Connor D, Firwana B, Brar S, Ellozy S, Vouyouka A, et al. Open versus endovascular stent graft repair of abdominal aortic aneurysms: a meta-analysis of randomized trials. JACC Cardiovasc Interv. (2012) 5(10):1071–80. doi: 10.1016/j.jcin.2012.06.015
2. Paravastu SC, Jayarajasingam R, Cottam R, Palfreyman SJ, Michaels JA, Thomas SM. Endovascular repair of abdominal aortic aneurysm. Cochrane Database Syst Rev. (2014) (1):CD004178. doi: 10.1002/14651858.CD004178.pub2
3. Sultan S, Barrett N, Tawfick W, Parodi JC, Hynes N. Contemporary abdominal aortic aneurysm devices, three decades of research and development with big data. Why has the best graft not been produced yet? A missed opportunity. Ital J Vasc Endovasc Surg. (2019) 26:121–34. doi: 10.23736/S1824-4777.19.01417-7
4. Sultan S, Barrett N, Kamal MH, Tawfick W, Atteia EM, Clarkson K, et al. Hybrid single lumen reconstruction (TIGER) in management of chronic symptomatic complex type B aortic dissection, techniques, and literature review. Ann Vasc Surg. (2020) 65:261–70. doi: 10.1016/j.avsg.2019.12.028
5. Morris L, Stefanov F, Hynes N, Diethrich EB, Sultan S. An experimental evaluation of device/arterial wall compliance mismatch for four stent-graft devices and a multi-layer flow modulator device for the treatment of abdominal aortic aneurysms. Eur J Vasc Endovasc Surg. (2016) 51:44–55. doi: 10.1016/j.ejvs.2015.07.041
6. Hynes N, Sultan S, Elhelali A, Diethrich EB, Kavanagh EP, Sultan M, et al. Systematic review and patient-level meta-analysis of the streamliner multilayer flow modulator in the management of complex thoracoabdominal aortic pathology. J Endovasc Ther. (2016) 23(3):501–12. doi: 10.1177/1526602816636891
7. Stefanov F, Sultan S, Morris L, Elhelali A, Kavanagh EP, Lundon V, et al. Computational fluid analysis of symptomatic chronic type B aortic dissections managed with the streamliner multilayer flow modulator. J Vasc Surg. (2017) 65(4):951–63. doi: 10.1016/j.jvs.2016.07.135
8. Lejay A, Geny B, Kolh P, Chakfé N. Effects of aortic graft implantation on heart and downstream vessels: an artery is not a rigid pipe. Eur J Vasc Endovasc Surg. (2019) 58:477–8. doi: 10.1016/j.ejvs.2019.08.002
9. Tai NR, Salcinski HJ, Edwards A, Hamilton G, Seifalian AM. Compliance properties of conduits used in vascular reconstruction. Br J Surg. (2000) 87(11):1516–24. doi: 10.1046/j.1365-2168.2000.01566.x
10. Sultan S, Acharya Y, Hazima M, Salahat H, Parodi JC, Hynes N. Combined thoracic endovascular aortic repair and endovascular aneurysm repair and the long-term consequences of altered cardiovascular haemodynamics on morbidity and mortality: case series and literature review. Eur Heart J Case Rep. (2021) 5(10):ytab339. doi: 10.1093/ehjcr/ytab339
11. Kolh P, D'Orioo V, Lambermont B, Gerard P, Gommes C, Limet R. Increased aortic compliance maintains left ventricular performance at lower energetic cost. Eur J Cardiothorac Surg. (2000) 17:272–8. doi: 10.1016/S1010-7940(00)00341-9
12. Ikonomidis I, Aboyans V, Blacher J, Brodmann M, Brutsaert DL, Chirinos JA, et al. The role of ventricular-arterial coupling in cardiac disease and heart failure: assessment, clinical implications and therapeutic interventions. A consensus document of the European society of cardiology working group on aorta & peripheral vascular diseases, European association of cardiovascular imaging, and heart failure association. Eur J Heart Fail. (2019) 21:402–24. doi: 10.1002/ejhf.1436
13. Vlachopoulos C, Aznaouridis K, Stefanadis C. Prediction of cardiovascular events and all-cause mortality with arterial stiffness: a systematic review and meta-analysis. J Am Coll Cardiol. (2010) 55:1318–27. doi: 10.1016/j.jacc.2009.10.061
14. Sultan S, Acharya Y, Hynes N. The 4-D in management of complex aortic pathology. Ital J Vasc Endovasc Surg. (2020) 27(3):105–7. doi: 10.23736/S1824-4777.20.01483-7
15. Rong LQ, Palumbo MC, Rahouma M, Meineri M, Arguelles GR, Kim J, et al. Immediate impact of prosthetic graft replacement of the ascending aorta on circumferential strain in the descending aorta. Eur J Vasc Endovasc Surg. (2019) 58:521–8. doi: 10.1016/j.ejvs.2019.05.003
16. van Bakel TMJ, Arthurs CJ, Nauta FJH, Eagle KA, van Herwaarden JA, Moll FL, et al. Cardiac remodelling following thoracic endovascular aortic repair for descending aortic aneurysms. Eur J Cardiothorac Surg. (2019) 55:1061–70. doi: 10.1093/ejcts/ezy399
17. Wang JJ, O'Brienn AB, Shrive NG, Parker KH, Tyberg JV. Time-domain representation of ventricular-arterial coupling as a windkessel and wave system. Am J Physiol Heart Circ Physiol. (2003) 284:H1358–68. doi: 10.1152/ajpheart.00175.2002
18. Pucci G, Hametner B, Battista F, Wassertheurer S, Schillaci G. Pressure-independent relationship of aortic characteristic impedance with left ventricular mass and geometry in untreated hypertension. J Hypertens. (2015) 33:153–60. doi: 10.1097/HJH.0000000000000354
19. Laurent S, Boutouyrie P. Arterial stiffness: a new surrogate end point for cardiovascular disease? J Nephrol. (2007) 20:S45–50.18050143
20. Takami Y, Tajima K, Kato W, Fujii K, Hibino M, Munakata H, et al. Long-term size follow-up of knitted dacron grafts (Gelseal™) used in the ascending aorta. Interact Cardiovasc Thorac Surg. (2012) 14:529–31. doi: 10.1093/icvts/ivr086
21. Blacher J, Guerin AP, Pannier B, Marchais SJ, Safar ME, London GM. Impact of aortic stiffness on survival in end-stage renal disease. Circulation. (1999) 99:2434–9. doi: 10.1161/01.CIR.99.18.2434
22. Cavalcante JL, Lima JA, Redheuil A, Al-Mallah MH. Aortic stiffness: current understanding and future directions. J Am Coll Cardiol. (2011) 57:1511–22. doi: 10.1016/j.jacc.2010.12.017
23. D'Oriaa M, Sen I, Day CN, Mandrekar J, Weiss S, Bower TC, et al. Burden and causes of readmissions following initial discharge after aortic syndromes. J Vasc Surg. (2021) 73:836.e3–43.e3. doi: 10.1016/j.jvs.2020.05.080
24. Weiss S, Sen I, Huang Y, Killian JM, Harmsen WS, Mandrekar J, et al. Cardiovascular morbidity and mortality after aortic dissection, intramural hematoma, and penetrating aortic ulcer. J Vasc Surg. (2019) 70:724.e1–31.e1. doi: 10.1016/j.jvs.2018.12.031
25. Ben-Shlomo Y, Spears M, Boustred C, May M, Anderson SG, Benjamin EJ, et al. Aortic pulse wave velocity improves cardiovascular event prediction: an individual participant meta-analysis of prospective observational data from 17,635 subjects. J Am Coll Cardiol. (2014) 63:636–46. doi: 10.1016/j.jacc.2013.09.063
26. Takeda Y, Sakata Y, Ohtani T, Tamaki S, Omori Y, Tsukamoto Y, et al. Endovascular aortic repair increases vascular stiffness and alters cardiac structure and function. Circ J. (2014) 78:322–28. doi: 10.1253/circj.CJ-13-0877
27. Spadaccio C, Nappi F, Al-Attar N, Sutherland FW, Acar C, Nenna A, et al. Old myths, new concerns: the long-term effects of ascending aorta replacement with dacron grafts. Not all that glitters is gold. J Cardiovasc Transl Res. (2016) 9:334–42. doi: 10.1007/s12265-016-9699-8
28. Marketou M, Papadopoulos G, Kontopodis N, Patrianakos A, Nakou E, Maragkoudakis S, et al. Early left ventricular global longitudinal strain deterioration after aortic aneurysm repair: impact of aortic stiffness. J Endovasc Ther. (2021) 28(2):352–59. doi: 10.1177/1526602820976636
29. Goto T, Ohte N, Fukuta H, Wakami K, Tani T, Kimura G. Relationship between effective arterial elastance, total vascular resistance, and augmentation index at the ascending aorta and left ventricular diastolic function in older women. Circ J. (2013) 77:123–9. doi: 10.1253/circj.CJ-12-0733
30. Kadoglou NP, Moulakakis KG, Papadakis I, Ikonomidis I, Alepaki M, Spathis A, et al. Differential effects of stent-graft fabrics on arterial stiffness in patients undergoing endovascular aneurysm repair. J Endovasc Ther. (2014) 21:850–8. doi: 10.1583/14-4772MR.1
31. Molinari AC, Leo E, Ferraresi M, Ferrari SA, Terzi A, Sommaruga S, et al. Distal extended endovascular aortic repair PETTICOAT: a modified technique to improve false lumen remodeling in acute type B aortic dissection. Ann Vasc Surg. (2019) 59:300–5. doi: 10.1016/j.avsg.2019.02.053
32. Melissano G, Bertoglio L, Rinaldi E, Mascia D, Kahlberg A, Loschi D, et al. Satisfactory short-term outcomes of the STABILISE technique for type B aortic dissection. J Vasc Surg. (2018) 68(4):966–75. doi: 10.1016/j.jvs.2018.01.029
33. Yuan X, Mitsis A, Semple T, Castro Verdes M, Cambronero-Cortinas E, Tang Y, et al. False lumen intervention to promote remodelling and thrombosis-the FLIRT concept in aortic dissection. Catheter Cardiovasc Interv. (2018) 92(4):732–40. doi: 10.1002/ccd.27599
34. Kölbel T, Lohrenz C, Kieback A, Diener H, Debus ES, Larena-Avellaneda A. Distal false lumen occlusion in aortic dissection with a homemade extra-large vascular plug: the candy-plug technique. J Endovasc Ther. (2013) 20(4):484–9. doi: 10.1583/13-4318.1
35. Kölbel T, Carpenter SW, Lohrenz C, Tsilimparis N, Larena-Avellaneda A, Debus ES. Addressing persistent false lumen flow in chronic aortic dissection: the knickerbocker technique. J Endovasc Ther. (2014) 21(1):117–22. doi: 10.1583/13-4463MR-R.1
36. Sultan S, Kavanagh EP, Veerasingam D, Costache V, Elhelali A, Fitzgibbon B, et al. Kinetic elephant trunk technique: early results in chronic symptomatic aortic dissection management. Ann Vasc Surg. (2019) 57:244–52. doi: 10.1016/j.avsg.2018.08.083
37. Sultan S, Kavanagh EP, Diethrich E, Costache V, Sultan M, Jordan F, et al. A clinical review of early outcomes from contemporary flow modulation versus open, fenestrated and branch technologies in the management of thoracoabdominal aortic aneurysm. Vascular. (2018) 26(2):209–15. doi: 10.1177/1708538117724933
38. Sultan S, Kavanagh EP, Stefanov F, Sultan M, Elhelali A, Costache V, et al., Global MFM collaborators, Endovascular management of chronic symptomatic aortic dissection with the streamliner multilayer flow modulator: twelve-month outcomes from the global registry. J Vasc Surg. (2017) 65(4):940–50. doi: 10.1016/j.jvs.2016.09.059
39. Sultan S, Elsherif M, Tawfick W, Hynes N. Endovascular scissoring in the management of complicated acute aortic dissection involving the infradiaphragmatic aorta. J Vasc Surg Cases Innov Tech. (2018) 4(4):320–3. doi: 10.1016/j.jvscit.2018.07.007
40. Moulakakis KG, Mylonas SN, Kakisis J, Kadoglou NP, Papadakis I, Sfyroeras GS, et al. Arterial stiffness alterations and inflammatory response following endovascular aortic repair: based on a presentation at the 2013 VEITH symposium, November 19-23, 2013 (New York, NY, USA). Aorta (Stamford). (2015) 3(2):75–80. doi: 10.12945/j.aorta.2015.14-071
41. Sultan S, Acharya Y, Salahat H, Hynes N. Staged hybrid single lumen reconstruction (TIGER) with bilateral subclavian transposition coupled with thoracic endovascular aneurysm repair in the management of acute symptomatic complex type B aortic dissection in a patient with arteria lusoria. BMJ Case Rep. (2021) 14(8):e244137. doi: 10.1136/bcr-2021-244137
42. Parodi JC, Palmaz JC, Barone HD. Transfemoral intraluminal graft implantation for abdominal aortic aneurysms. Ann Vasc Surg. (1991) 5:491–9. doi: 10.1007/BF02015271
43. Volodos’ NL, Shekhanin VE, Karpovich IP, Troian VI, Gur’ev I. A self-fixing synthetic blood vessel endoprosthesis. Vestn Khir Im I I Grek. (1986) 137(11):123–5.
44. Sultan S, O’Donohoe M, Colgan MP, Moore D, Molloy M, Shanik G. Critical ischaemia; transfemoral endoluminal aortic management; a minimally invasive option in aortic intervention. Vasc Surg. (1999) 33(2):179–84. doi: 10.1177/153857449903300213
45. Moulakakis KG, Kadoglou NPE, Antonopoulos CN, Mylonas SN, Kakisis J, Papadakis I, et al. Changes in arterial stiffness and N-terminal pro-brain natriuretic peptide levels after endovascular repair of descending thoracic aorta. Ann Vasc Surg. (2017) 38:220–26. doi: 10.1016/j.avsg.2016.04.025
46. Kleinstreuer C, Li Z, Basciano CA, Seelecke S, Farber MA. Computational mechanics of nitinol stent grafts. J Biomech. (2008) 41:2370–8. doi: 10.1016/j.jbiomech.2008.05.032
47. Roccabianca S, Figueroa CA, Tellides G, Humphrey JD. Quantification of regional differences in aortic stiffness in the aging human. J Mech Behav Biomed Mater. (2014) 29:618–34. doi: 10.1016/j.jmbbm.2013.01.026
48. United Kingdom EVAR Trial Investigators, Greenhalgh RM, Brown LC, Powell JT, Thompson SG, Epstein D, Sculpher MJ. Endovascular versus open repair of abdominal aortic aneurysm. N Engl J Med. (2010) 362:1863–71. doi: 10.1056/NEJMoa0909305
49. United Kingdom EVAR Trial Investigators, Greenhalgh RM, Brown LC, Powell JT, Thompson SG, Epstein D. Endovascular repair of aortic aneurysm in patients physically ineligible for open repair. N Engl J Med. (2010) 362:1872–80. doi: 10.1056/NEJMoa0911056
50. Duffy JM, Rolph R, Waltham M. Stent graft types for endovascular repair of abdominal aortic aneurysms. Cochrane Database Syst Rev. (2015) 2015:CD008447. doi: 10.1002/14651858.CD008447.pub3
51. Wirthlin DJ, Alcocer F, Whitley D, Jordan WD. Use of hybrid aortic stent grafts for endovascular repair of abdominal aortic aneurysms: indications and outcomes. J Surg Res. (2002) 108(1):14–9. doi: 10.1006/jsre.2002.6509
52. Malas MB, Freischlag JA. Interpretation of the results of OVER in the context of EVAR trial, DREAM, and the EUROSTAR registry. Semin Vasc Surg. (2010) 23:165–9. doi: 10.1053/j.semvascsurg.2010.05.009
53. Kim HO, Yim NY, Kim JK, Kang YJ, Lee BC. Endovascular aneurysm repair for abdominal aortic aneurysm: a comprehensive review. Korean J Radiol. (2019) 20:1247–65. doi: 10.3348/kjr.2018.0927
54. Stollwerck PL, Kozlowski B, Sandmann W, Grabitz K, Pfeiffer T. Long-term dilatation of polyester and expanded polytetrafluoroethylene tube grafts after open repair of infrarenal abdominal aortic aneurysms. J Vasc Surg. (2011) 53:1506–13. doi: 10.1016/j.jvs.2011.02.028
55. Patel R, Powell JT, Sweeting MJ, Epstein DM, Barrett JK, Greenhalgh RM. The UK endovascular aneurysm repair (EVAR) randomised controlled trials: long-term follow-up and cost-effectiveness analysis. Health Technol Assess. (2018) 22:1–132. doi: 10.3310/hta22050
56. Ito E, Toya N, Fukushima S, Nishie R, Akiba T, Ohki T. Polyester grafts are a risk factor for postimplantation syndrome after abdominal endovascular aneurysm repair: retrospective analysis for polyester graft, Excluder®, and Endologix Powerlink®/AFX®. Ann Vasc Dis. (2018) 11:520–4. doi: 10.3400/avd.oa.18-00058
57. Arnaoutoglou E, Kouvelos G, Papa N, Kallinteri A, Milionis H, Koulouras V, et al. Prospective evaluation of post-implantation inflammatory response after EVAR for AAA: influence on patients’ 30 day outcome. Eur J Vasc Endovasc Surg. (2015) 49:175–83. doi: 10.1016/j.ejvs.2014.12.006
58. Syk I, Brunkwall J, Ivancev K, Lindblad B, Montgomery A, Wellander E, et al. Postoperative fever, bowel ischaemia and cytokine response to abdominal aortic aneurysm repair–a comparison between endovascular and open surgery. Eur J Vasc Endovasc Surg. (1998) 15:398–405. doi: 10.1016/S1078-5884(98)80200-1
59. Arnaoutoglou E, Papas N, Milionis H, Kouvelos G, Koulouras V, Matsagkas MI. Post-implantation syndrome after endovascular repair of aortic aneurysms: need for postdischarge surveillance. Interact Cardiovasc Thorac Surg. (2010) 11:449–54. doi: 10.1510/icvts.2010.242628
60. Moulakakis KG, Alepaki M, Sfyroeras GS, Antonopoulos CN, Giannakopoulos TG, Kakisis J, et al. The impact of endograft type on inflammatory response after endovascular treatment of abdominal aortic aneurysm. J Vasc Surg. (2013) 57:668–77. doi: 10.1016/j.jvs.2012.09.034
61. Ohara N, Miyata T, Oshiro H, Shigematsu H, Ohki T. Adverse outcome following transfemoral endovascular stent-graft repair of an abdominal aortic aneurysm in a patient with severe liver dysfunction: report of a case. Surg Today. (2000) 30:764–7. doi: 10.1007/s005950070094
62. Blum U, Voshage G, Lammer J, Beyersdorf F, Töllner D, Kretschmer G, et al. Endoluminal stent-grafts for infrarenal abdominal aortic aneurysms. N Engl J Med. (1997) 336:13–20. doi: 10.1056/NEJM199701023360103
63. Voûte MT, Bastos Gonçalves FM, van de Luijtgaarden KM, Klein Nulent CG, Hoeks SE, Stolker RJ, et al. Stent graft composition plays a material role in the postimplantation syndrome. J Vasc Surg. (2012) 56:1503–9. doi: 10.1016/j.jvs.2012.06.072
64. Sartipy F, Lindström D, Gillgren P, Ternhag A. The impact of stent graft material on the inflammatory response after EVAR. Vasc Endovascular Surg. (2015) 49:79–83. doi: 10.1177/1538574415595209
65. Ferreira RS, Oliveira-Pinto J, Ultee K, Vôute M, Oliveira NFG, Hoeks S, et al. Post implant syndrome influences long-term cardiovascular prognosis after EVAR. Eur J Vasc Endovasc Surg. (2019) 58(Suppl 3):e808. doi: 10.1016/j.ejvs.2019.09.401
66. Yuan A, Chen JJ, Yao PL, Yang PC. The role of interleukin-8 in cancer cells and microenvironment interaction. Front Biosci. (2005) 10:853–65. doi: 10.2741/1579
67. Alfaro C, Sanmamed MF, Rodríguez-Ruiz ME, Teijeira Á, Oñate C, González Á, et al. Interleukin-8 in cancer pathogenesis, treatment and follow-up. Cancer Treat Rev. (2017) 60:24–31. doi: 10.1016/j.ctrv.2017.08.004
68. Sultan S, Mustafa M, Bennani F, Atteia E, Acharya Y, Hynes N. Challenges in diagnosing aortic leiomyosarcoma post endovascular repair of abdominal aortic aneurysm. J Vasc Surg Cases Innov Tech. (2020) 6(4):666–70. doi: 10.1016/j.jvscit.2020.08.013
69. de Donato G, Pasqui E, Panzano C, Brancaccio B, Grottola G, Galzerano G, et al. The polymer-based technology in the endovascular treatment of abdominal aortic aneurysms. Polymers (Basel). (2021) 13(8):1196. doi: 10.3390/polym13081196
70. Verhoeven ELG, Mani K. New technology failures: who to blame or time to be cautious? Eur J Vasc Endovasc Surg. (2018) 56(3):318–9. doi: 10.1016/j.ejvs.2018.07.009
71. Georgakarakos E, Argyriou C, Georgiadis GS, Lazarides MK. Pulse wave analysis after treatment of abdominal aortic aneurysms with the ovation device. Ann Vasc Surg. (2017) 40:146–53. doi: 10.1016/j.avsg.2016.07.080
Keywords: abdominal aortic aneurysm (AAA), endovascular aneurysm repair (EVAR), thoracic endovascular aneurysm repair (TEVAR), stent-Graft material, cardiovascular outcome
Citation: Sultan S, Acharya Y, Soliman O, Parodi JC and Hynes N (2022) TEVAR and EVAR, the unknown knowns of the cardiovascular hemodynamics; and the immediate and long-term consequences of fabric material on major adverse clinical outcome. Front. Surg. 9:940304. doi: 10.3389/fsurg.2022.940304
Received: 10 May 2022; Accepted: 11 August 2022;
Published: 30 August 2022.
Edited by:
Pasqualino Sirignano, Sapienza University of Rome, ItalyReviewed by:
Edoardo Pasqui, University of Siena, ItalyGiovanni Nano, IRCCS San Donato Polyclinic, Italy
© 2022 Sultan, Acharya, Soliman, Parodi and Hynes. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Sherif Sultan sherif.sultan@hse.ie, sherif.sultan@nuigalway.ie
†ORCID Sherif Sultan orcid.org/0000-0001-8767-4929 Yogesh Acharyaorcid.org/0000-0003-1829-5911 Niamh Hynesorcid.org/0000-0002-6133-3322
Specialty Section: This article was submitted to Vascular Surgery, a section of the journal Frontiers in Surgery
 Sherif Sultan
Sherif Sultan Yogesh Acharya
Yogesh Acharya Osama Soliman
Osama Soliman Juan Carlos Parodi4
Juan Carlos Parodi4