Anti-inflammatory effect of tranexamic acid on adult cardiac surgical patients: A PRISMA-compliant systematic review and meta-analysis
- 1Department of Anesthesiology, Fuwai Yunnan Cardiovascular Hospital, Affiliated Cardiovascular Hospital of Kunming Medical University, Kunming, China
- 2Department of Anesthesiology, Fuwai Hospital, National Center for Cardiovascular Diseases, Peking Union Medical College and Chinese Academy of Medical Sciences, Beijing, China
Objective: This study aims to evaluate the anti-inflammatory effect of tranexamic acid (TXA) on adult cardiac surgical patients.
Methods: PubMed, Embase, Ovid, Web of Science, CNKI, VIP, and WANFANG databases were systematically searched using the related keywords for cardiac surgical randomized controlled trials (RCTs) published from their inception to February 1, 2022. The primary outcomes were postoperative inflammatory biomarkers levels. The secondary outcomes were postoperative systemic inflammatory response syndrome and other major postoperative outcomes. The odds ratios and/or the weighted mean difference (WMD) with a 95% confidence interval (CI) were used to pool the data.
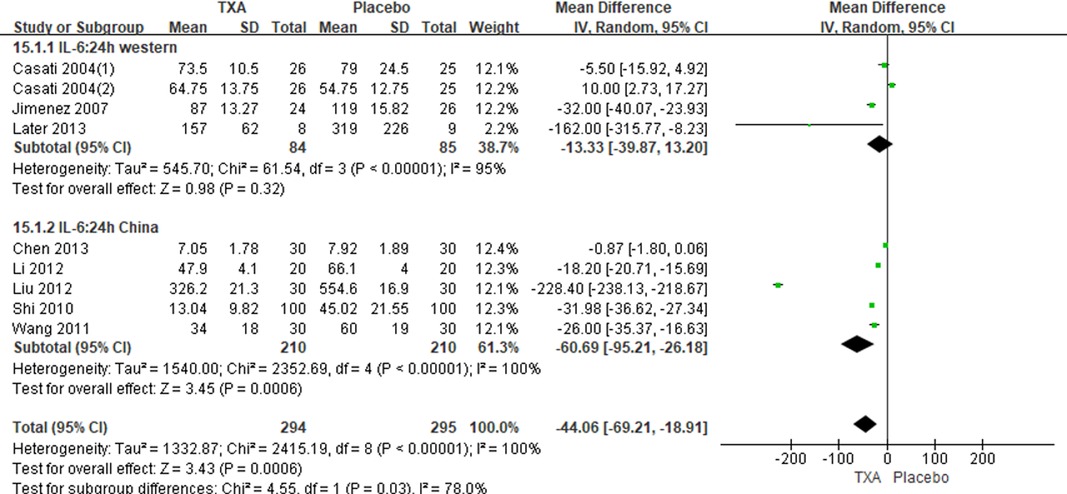
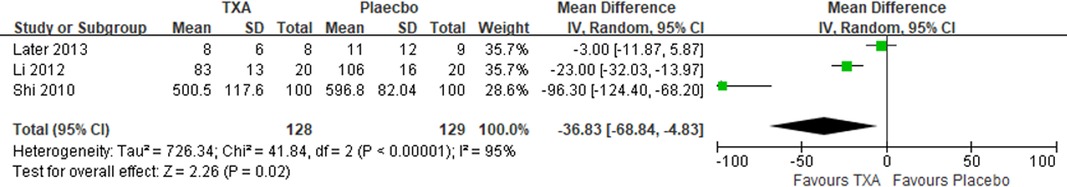
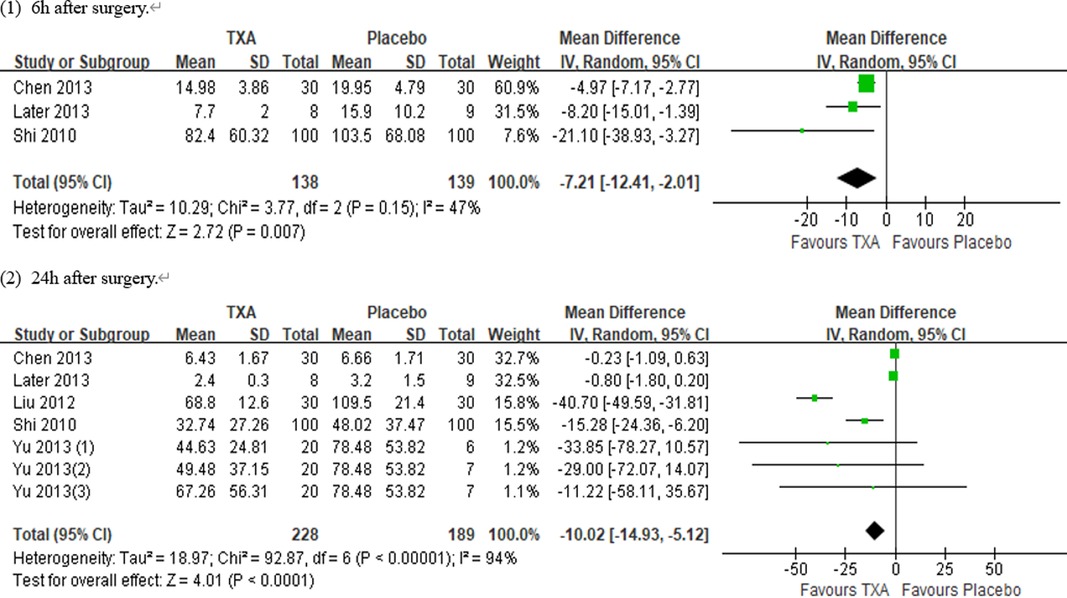
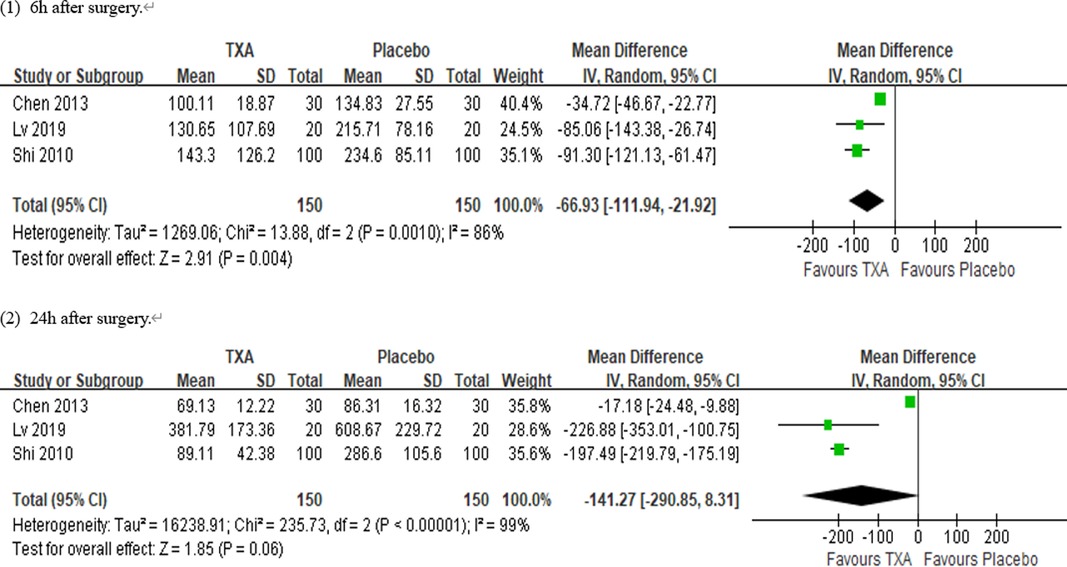
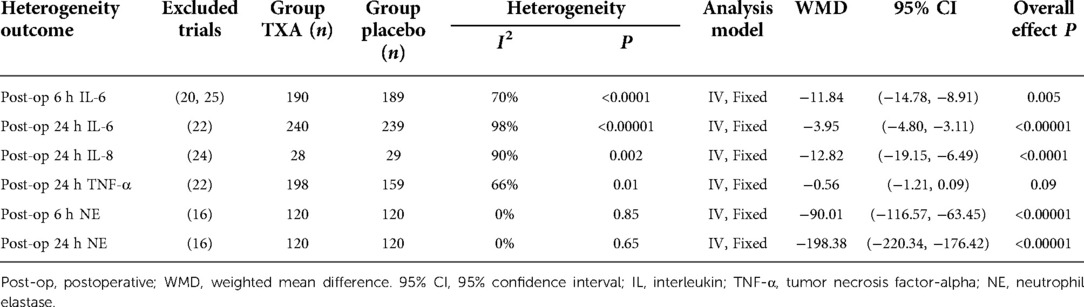
Results: Ten RCTs with 770 adult cardiac surgical patients were included. Compared with placebo, TXA achieved statistically significant inhibition of the postoperative interleukin (IL)-6 level (postoperative 6 h: n = 6 trials; WMD −31.66; 95% CI: −45.90, −17.42; p < 0.0001; I2 = 93%; postoperative 24 h: n = 8 trials; WMD, −44.06; 95% CI: −69.21, −18.91; p = 0.006; I2 = 100%); IL-8 level postoperative 24 h, TNF-α level postoperative 24 h, NE level postoperative 6 h: n = 3 trials; WMD, −36.83; 95% CI: −68.84, −4.83; p = 0.02; I2 = 95%); tissue necrosis factor alpha (TNF-α) level (postoperative 6 h: n = 3 trials; WMD, −7.21; 95% CI: −12.41, −2.01; p = 0.007; I2 = 47%; postoperative 24 h: n = 5 trials; WMD, −10.02; 95% CI: −14.93, −5.12; p < 0.0001; I2 = 94%); and neutrophil elastase (NE) level (postoperative 6 h: n = 3 trials; WMD, −66.93; 95% CI: −111.94, −21.92; p = 0.004; I2 = 86%). However, TXA achieved no statistically significant influence on the postoperative 24 h NE level.
Conclusions: TXA had a significant anti-inflammatory effect in adult cardiac surgical patients, as evidenced by the reduction of multiple postoperative proinflammatory biomarkers levels, but these results should be interpreted carefully and cautiously, as only a limited number of studies were included and there was high heterogeneity between them.
Systematic Review Registration: https://www.crd.york.ac.uk/prospero/#recordDetails, identifier: CRD42022312919.
Introduction
Surgery is known to cause tissue damage, and initiate inflammatory response (1), particularly cardiac surgery with cardiopulmonary bypass (CPB) (2, 3). The inflammatory response characterized by the release of proinflammatory cytokines (4, 5) may cause a hypotension/hypoperfusion state (6). Elevations in IL-6 and IL-8 levels after CPB were associated with an increased risk of organ injury (7–10) and mortality (9, 11). Numerous strategies to reduce inflammatory response and bleeding in cardiac surgical patients exist, among which is the use of tranexamic acid (TXA). TXA is a traditional antifibrinolytic drug, fibrinolysis is a marker for the onset of systemic inflammation (12), and plasmin inhibition can mitigate immunosuppression after certain ischemic events including surgery (13). Simultaneously, Cvachovec et al.'s study (14) summarized the multifaceted role of fibrinogen in tissue injury and inflammation and found that the universal presence of fibrin within inflammatory foci, similarly to the extravascular fibrin deposits, exacerbates inflammation across a spectrum of disease models. Casati et al. reported that TXA significantly reduced bleeding in coronary artery bypass grafting (CABG) and may modulate inflammation in these surgical settings (15). In addition, another study showed that TXA exhibited a minor anti-inflammatory response (16). Inversely, Later et al. reported that aprotinin attenuated the postoperative TNF-α level, whereas TXA did not, and the majority of plasma cytokines (IL-6, IL-8, and IL-10) were not affected by the use of antifibrinolytics when compared with placebo (17). In addition, TXA treatment attenuated the surgery-induced increase in the level of proinflammatory cytokine IL-1β, but it did not significantly alter the levels of TNF-α, IL-6, IL-8, and IL-10 (18).
Therefore, the inflammatory effect of TXA in adult cardiac surgical patients remains controversial. We conducted a systematic review and meta-analysis of randomized controlled trials (RCTs) with the aim of evaluating the anti-inflammatory effect of TXA in adult cardiac surgical patients.
Methods
This study followed the methodology outlined in the Cochrane Handbook for Systematic Reviews of Interventions Version 6.0 (19). We explained it in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses Protocols statement. This protocol has been registered on the International Prospective Systematic Reviews Registry database (PROSPERO: CRD42022312919).
Systematic search
We conducted a comprehensive search of PubMed, Embase, Web of Science, CNKI, WANFANG, VIP, and unpublished sources including ClinicalTrials.gov, ChiCTR, and the Cochrane trial registry from inception to February 1, 2022, for RCTs investigating the role of TXA in adult patients undergoing cardiac surgery. Language was limited to English and Chinese; the related searching words were as follows: (tranexamic acid) OR (TXA) AND [(inflammatory) OR (cytokine)] AND [(cardiac surgery) OR (cardiopulmonary bypass) OR (coronary artery bypass surgery) OR (valve surgery) OR (aortic surgery) OR (congenital heart disease)] AND (randomized controlled trial OR controlled clinical trial OR randomly OR trial) in the title/abstract. In addition, we manually searched the references of the identified studies to identify further relevant studies.
Study selection
The study selection criteria are as follows: (1) Population: Population of interest were adult patients undergoing cardiac surgery. Studies concerning children, infants, or newborns were excluded. (2) Intervention: The intervention group was TXA administration. (3) Comparator: The intervention group was compared with the placebo group. (4) Outcome: The postoperative inflammatory biomarkers levels were included. The inflammatory biomarkers chosen as outcomes were cytokines IL-6, IL-8, TNF-α, and NE. Time points for cytokine measurement were grouped into 6 h postoperatively and 24 h postoperatively. We chose these biomarkers and time points to align with the outcomes most commonly used in identified relevant studies. To limit heterogeneity across sampling periods, we excluded studies that measured inflammatory biomarkers outside our specified time points. (5) Study design: We only included RCTs to ensure that the combined results were of good quality and excluded the studies that could not provide effective analysis data.
After implementing the search strategy, two researchers (C-MX and Y-TY) screened all potentially relevant citations independently and in duplicate. Citations deemed potentially relevant by either screener were advanced to second-stage full-text review. Full texts were subsequently reviewed for eligibility, with disagreements resolved by consensus and third-party adjudication if required. Trials were excluded for not reporting the results of the marker of interest.
Data extraction and quality assessment
Reviewers (C-MX, L-XH, and KY) extracted data independently and in duplicate using prepiloted data abstraction forms. The extracted data are as follows: the first author, published year, demographic data, details of the intervention and placebo, surgical procedure, inflammatory biomarkers levels, modified Jadad score, and risk of bias for each study. Reviewers (C-MX, L-XH, and KY) examined the following risk of bias domains: randomized sequence generation, allocation concealment, blinding, incomplete outcome, selective reporting, and other bias (such as stopping early and funding sources).
Statistical analysis
All data were analyzed by Review Manager 5.4 (Cochrane Collaboration, Oxford, UK). The odds ratios with 95% confidence intervals (CIs) were estimated for dichotomous data, and weighted mean differences (WMDs) with 95% CIs were estimated for continuous data. If fewer than three studies reported a specific outcome and time point, these data were not pooled. Each outcome was tested for heterogeneity, and the randomized-effects model or fixed-effects model was used in the presence or absence of significant heterogeneity, Q-statistical test p < 0.05, and I2 statistics (I2 > 50% was considered as the presence of significant heterogeneity). Sensitivity analyses were performed by examining the influence of the statistical model on estimated treatment effects, and analyses that adopted the fixed-effects model were repeated again by using the randomized-effects model and vice versa. In addition, sensitivity analysis was also performed to evaluate the influence of individual studies on the overall effects. Subgroup analyses were performed to evaluate the possible effects of patient characteristics and control agents on the outcomes, if necessary. Publication bias was explored through visual inspection of funnel plots of the outcomes. All p values were two-sided, and statistical significance was defined as p < 0.05.
Results
Literature search results
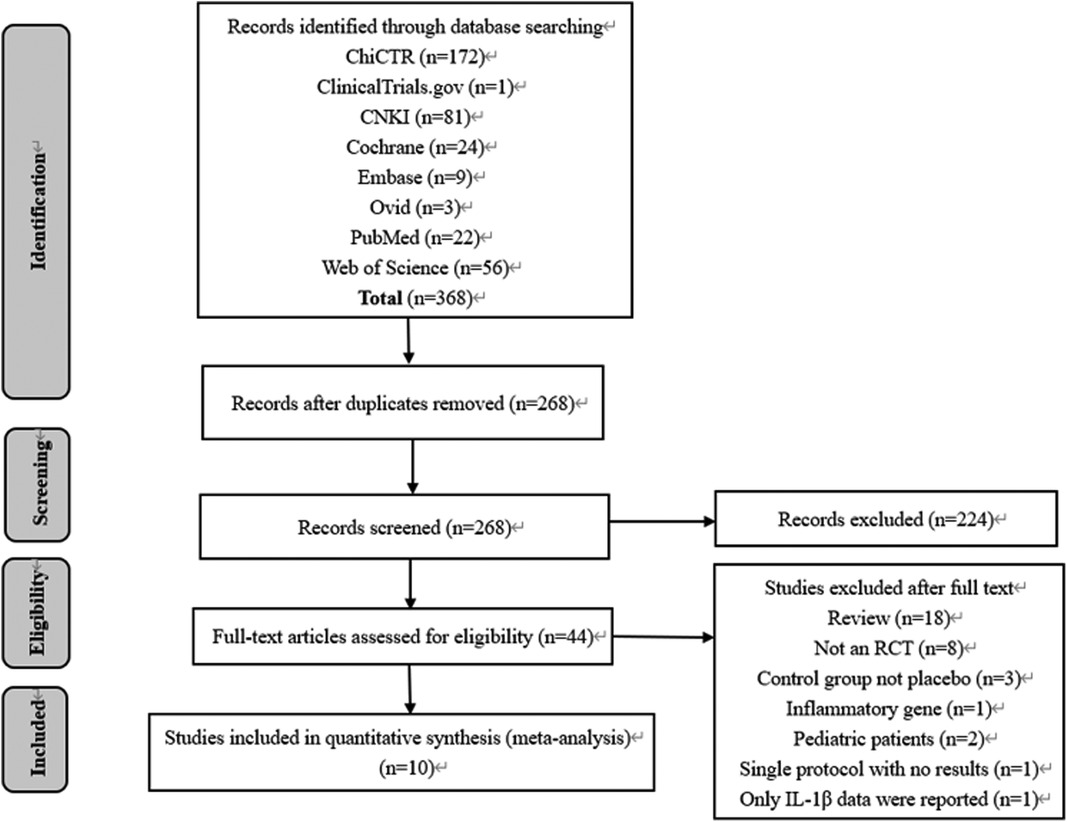
As depicted in the flowchart (Figure 1), our initial search yielded 368 records. A total of 324 trials were excluded by being duplicated and reviewing the titles and abstracts. In total, 44 full texts were assessed, and finally, 10 RCTs with 770 adult cardiac surgical patients were included in this meta-analysis (15–17, 20–26).
Characteristics of included studies
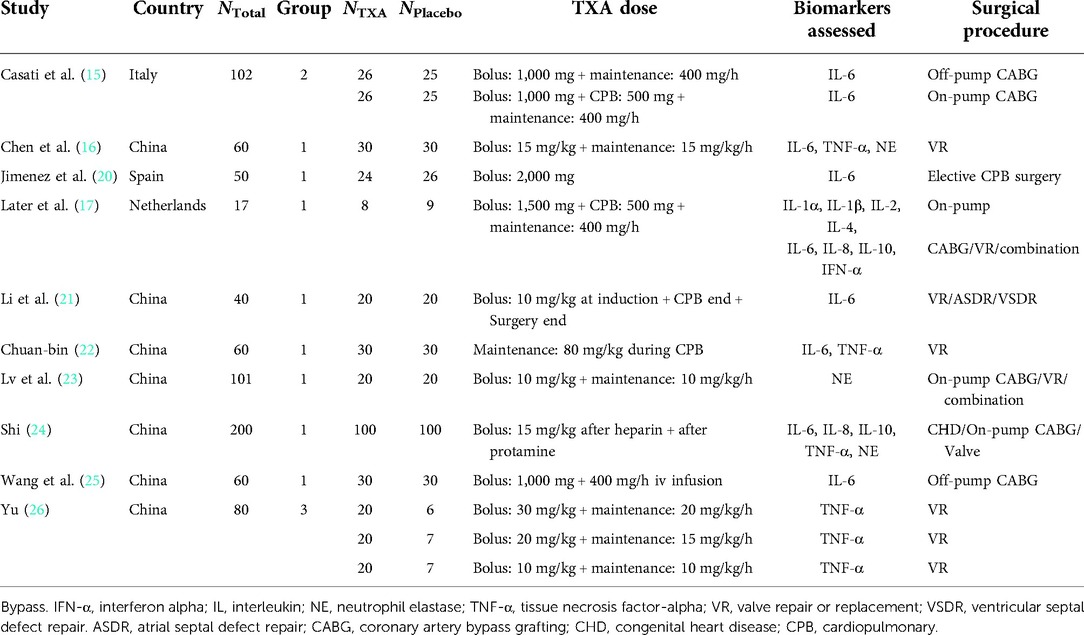
Baseline characteristics of included trials are summarized in Table 1. One RCT was registered in the study (20), and seven studies were conducted in China (16, 21–26). The dosage of TXA was imparity across included trials; similarly, the timing and method of TXA administration varied among studies: six studies selected loading dose and continuous infusion (15–17, 23, 25, 26), while other studies chose a time point for the injection of TXA (20–22, 24).
Risk of bias in included studies
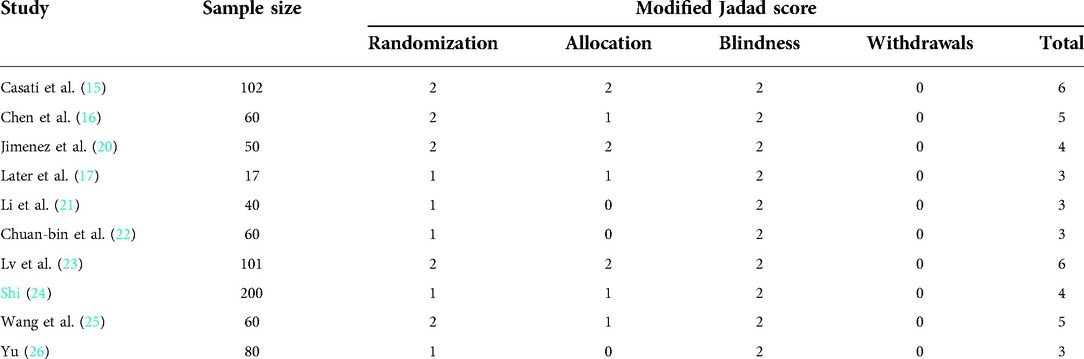
Details regarding the performance of the studies against each domain were presented in the risk of the bias graph (Figure 2). In addition, a visual summary of judgments about each methodological quality item for each included trial is given in Figure 3. Of the 10 included trials, the modified Jadad score for the 3 studies was three points (21, 22, 26), and these studies were considered lowquality studies, as shown in Table 2.
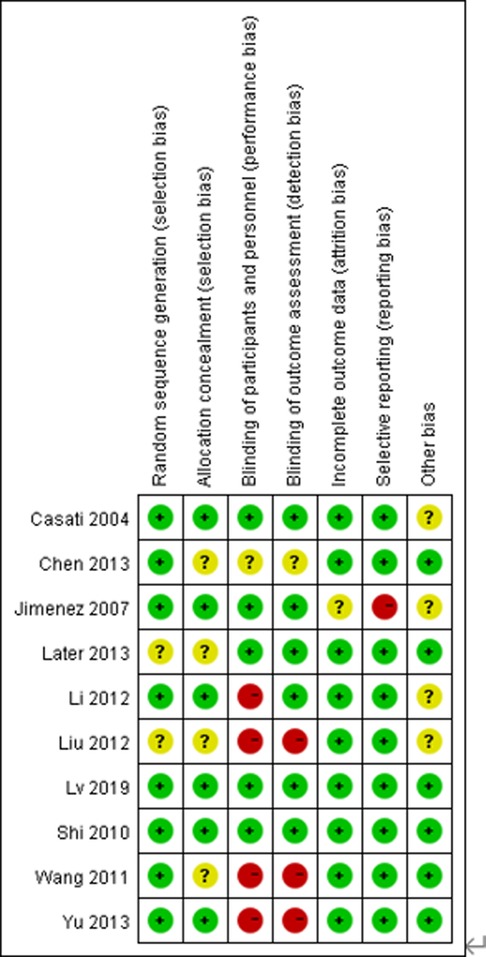
Figure 2. Risk-of-bias graph for each included study. Green (+), red (–), and yellow (?) circles indicate low, high, and unclear risk of bias, respectively.
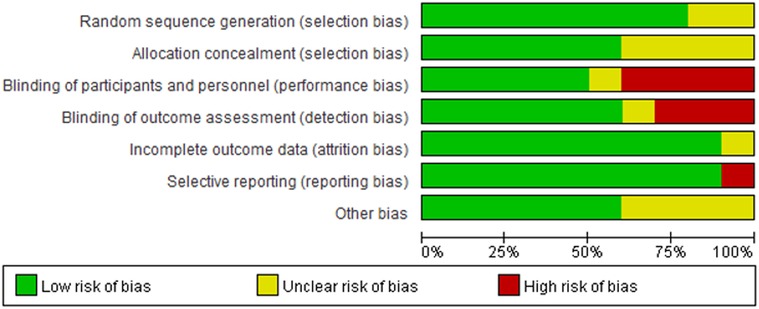
Figure 3. Risk-of-bias summary for each included study. Green (+), red (–), and yellow (?) circles indicate low, high, and unclear risk of bias, respectively.
Primary outcomes
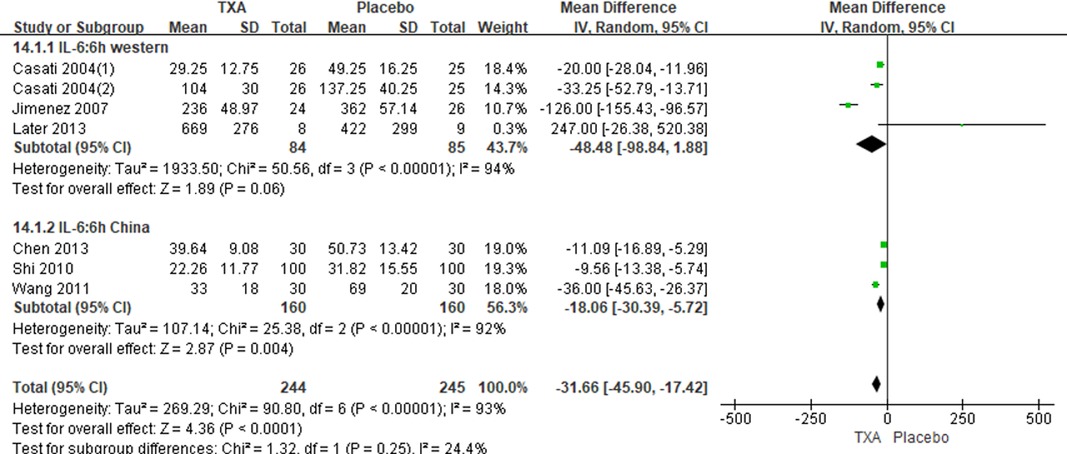
In total, 10 RCTs with 770 cardiac surgical adult patients were included. Compared with placebo, TXA achieved statistically significant inhibition of the postoperative IL-6 level (6 h: n = 6 trials; WMD, −31.66; 95% CI: −45.90, −17.42; p < 0.0001; I2 = 93%; 24 h: n = 8 trials; WMD, −44.06; 95% CI: −69.21, −18.91; p = 0.006; I2 = 100%) (Figures 4, 5), IL-8 level postoperative 24 h, TNF-α level postoperative 24 h, NE level postoperative 6 h: n = 3 trials; WMD, −36.83; 95% CI: −68.84, −4.83; p = 0.02; I2 = 95%) (Figure 6), TNF-α level (6 h: n = 3 trials; WMD, −7.21; 95% CI: −12.41, −2.01; p = 0.007; I2 = 47%; 24 h: n = 5 trials; WMD, −10.02; 95% CI: −14.93, −5.12; p < 0.0001; I2 = 94%) (Figure 7), and NE level (6 h: n = 3 trials; WMD, −66.93; 95% CI: −111.94, −21.92; p = 0.004; I2 = 86%) (Figure 8). However, TXA achieved no statistically significant influence on the postoperative 24 h NE level (Figure 8).
Only two studies reported the postoperative IL-1β level (17, 18), IL-10 level (17, 24), and postoperative 6 h IL-8 level (17, 24); therefore, these time point biomarkers were not included in this study.
In the figures, RCTs were listed in order by the name of the author. The size of each square denoted the weight of each trial's WMD in calculating the summary estimate for the overall effect on IL-6, IL-8, TNF-α, and NE. The diamond represented the summary estimate for the combined WMD at the center; opposing points of the diamond represented the 95% CIs. Three diamonds in each section represented high, low, and overall effects.
Secondary outcomes
Jimenez et al. (20) reported that inflammatory response was found in 26 (33%) of 79 patients who did not receive TXA vs. 8 (9%) of 86 patients who received TXA, and another study (17) shown that systemic inflammatory response syndrome (SIRS) was found in all patients in the placebo group (n = 9) and TXA group (n = 8). Jimenez et al. (20) reported that 20 (12%) of the 165 patients presented vasoplegic shock. In the non-TXA group, 16 (20%) out of 79 patients developed vasoplegic shock. As expected, patients with inflammatory response were more likely to develop vasoplegic shock (58% vs. 0%; p < 0.001).
Sensitivity analysis and publication bias
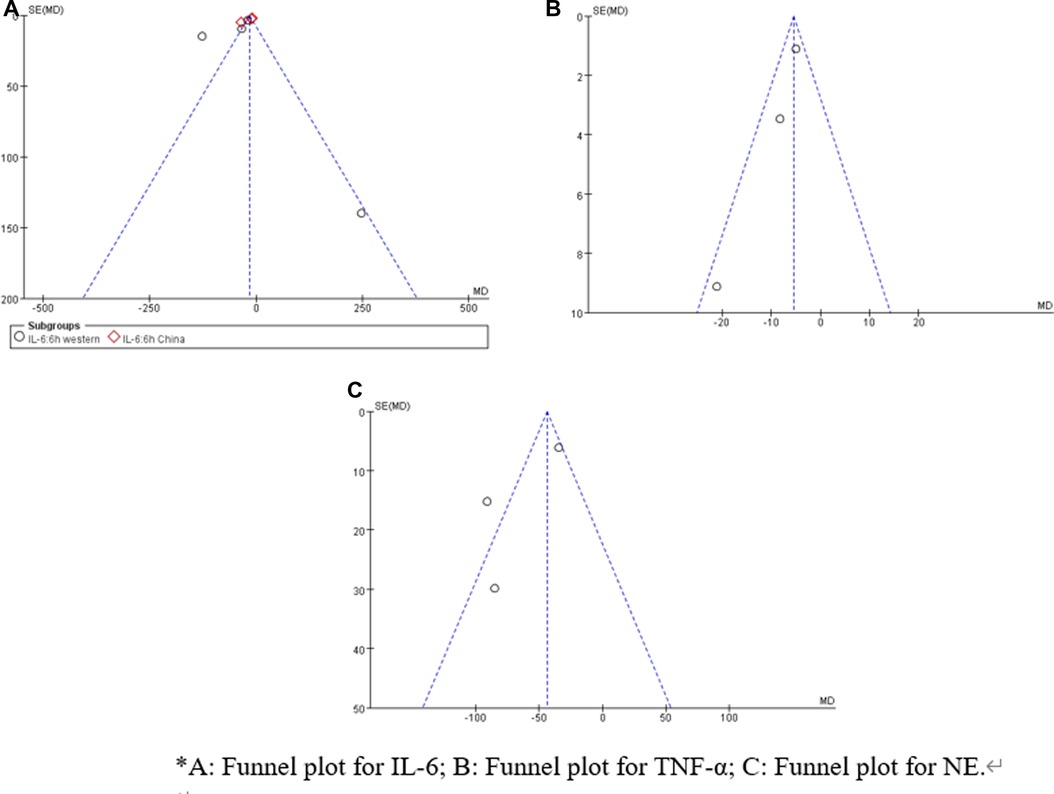
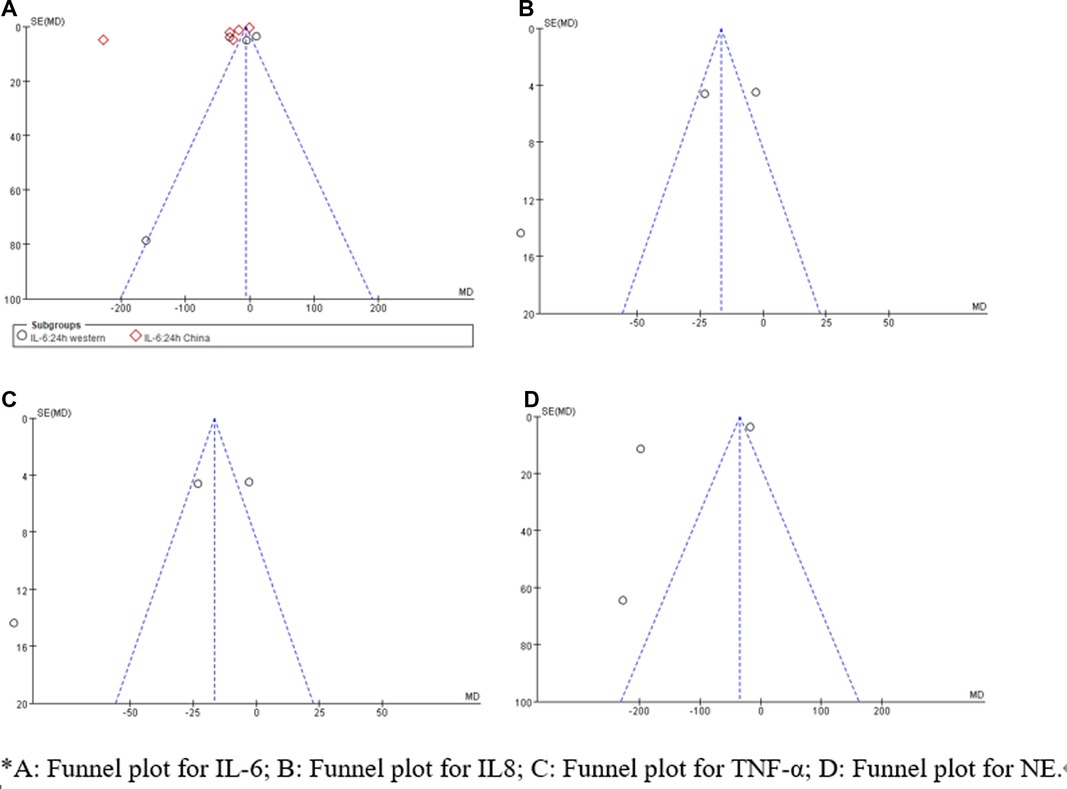
Sensitivity analysis showed that treatment effects on all the outcomes were not affected by choice of the statistical model (Tables 3, 4). Sensitivity tests were also performed by the exclusion of some studies to analyze the influence of the overall treatment effect on high-heterogeneity outcomes (Table 5), but no contradictory results were found. Otherwhile, in Figures 9, 10, we found that there may be little publication bias.
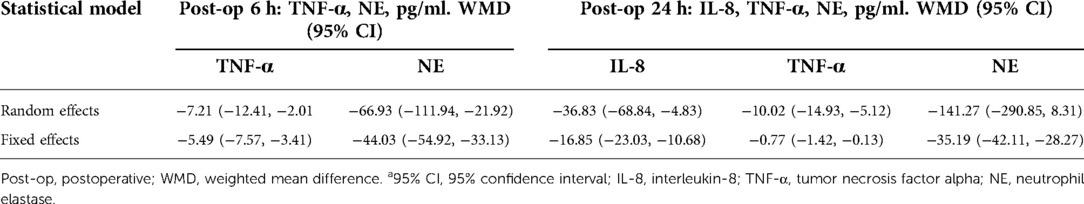
Table 4. Influence of statistical model on TXA efficacy of inflammatory biomarkers IL-8, TNF-α, and NE.
Discussion
Surgery-associated tissue damage stimulates systemic inflammatory cascades to induce a surge in the release of cytokines and stress hormones and leukocyte migration to the injury site. The excessive inflammatory responses not only leave deleterious effects on wound healing but also is thought to cause a series of complications, such as postoperative pain, fatigue, atrial fibrillation, acute kidney injury, and cognitive dysfunction (27–29). In the present meta-analysis, we found a significant decrease in the concentrations of IL-6, IL-8, TNF-a, and NE after TXA administration in adult cardiac surgical patients, which is indicative of the anti-inflammatory potentials of TXA. Together, these data provide evidence that TXA exerts an anti-inflammatory effect and attenuates perioperative inflammation of adult cardiac surgical patients.
Some underlying mechanisms have been discussed. First, TXA is a traditional antifibrinolytic drug. Fibrinolysis is a marker for the onset of systemic inflammation (12), and plasmin inhibition can mitigate immunosuppression after certain ischemic events such as surgery (13). Second, cytokines themselves can cause some typical clinical symptoms such as fever, which involves IL-1, IL-6, TNF-α, IL-1Ra, and IL-10. Third, blood transfusion in surgery has been identified as an independent predictor of increased infection (30). Inflammation influenced the initiation and propagation of blood coagulation (31). TXA had reduced perioperative blood loss and transfusion requirements in cardiac surgical patients (32–37).
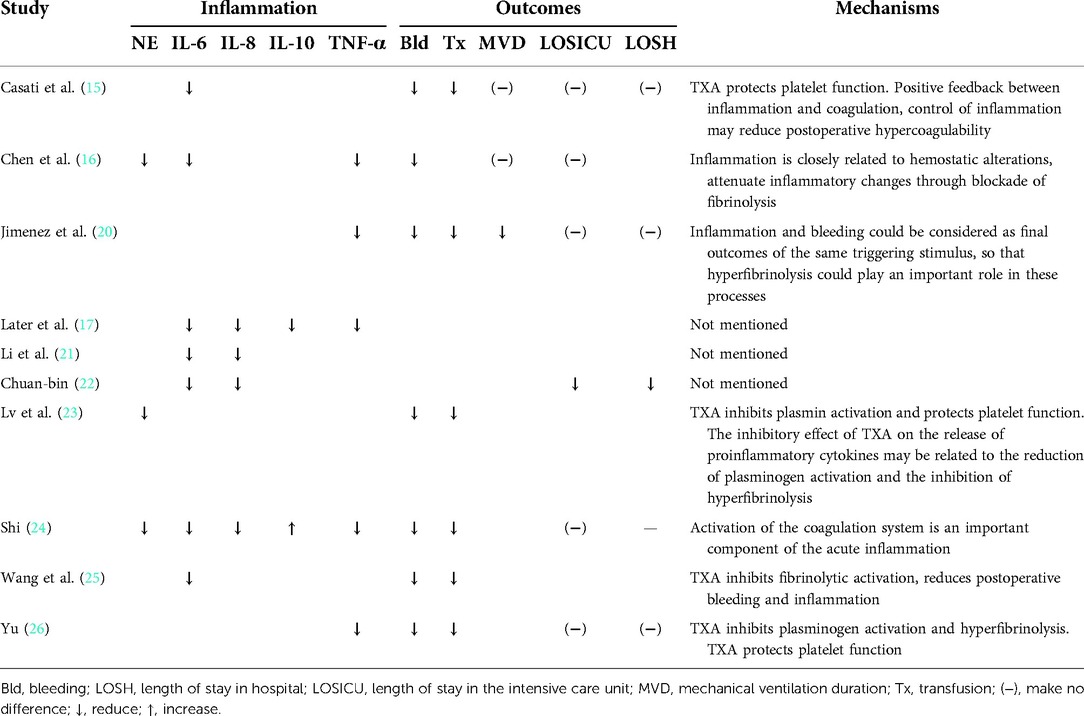
Coagulation, fibrinolysis, and inflammation are closely interconnected. As seen in Table 6, 7 of the 10 RCTs included in this study demonstrated the association between the anti-inflammatory effects of TXA and patients' clinical outcomes (e.g., bleeding, transfusion, and postoperative recovery).
Among this study, IL-6 had been reported in eight studies (15–17, 20–22, 24, 25), so the postoperative IL-6 level was probably the most trustworthy. IL-6 is one of the main proinflammatory cytokines (38) and is widely recognized to play an important role in mediating the systemic inflammatory response to cardiac surgery with CPB (39). The patients aged >70 years undergoing cardiac operations generate more IL-6 during CPB (40). A multicenter cohort study of adults undergoing CABG demonstrated that an elevated level of postoperative IL-6 was associated with a higher risk of readmission and mortality (41). Other studies reported that IL-6 had a good predictive value for 30 days and overall mortality in the cardiac surgery population (42, 43). Meanwhile, numerous studies have demonstrated that elevated IL-6 level was associated with cardiac events, including incidence of heart failure, unstable angina, acute kidney injury, and functional status outcomes for patients after cardiac surgery (44–47). In this study, TXA significantly reduced the expression of postoperative IL-6, which may decrease the incidence of complications associated with an elevated level of postoperative IL-6.
As we all know, meta-analysis could increase the power of analysis by pooling many small, low-quality studies, but there was heterogeneity in the included studies. While these studies were comparable based on their design and outcomes, heterogeneity affected the validity of pooled results. In addition, high heterogeneity has been reported in previous systematic reviews. It indicated that the impact of patient and surgical variables, both measured and unmeasured, on the biomarker response likely far outweighs the impact of agents (48). Race subgroup analysis was performed in this study; however, no exact reason for the observed heterogeneity was determined.
Limitations
The limitations of this study should be acknowledged. First, SIRS is a complex pathophysiology process influenced by multiple factors (49); however, this study only analyzes the concentrations of proinflammatory and anti-inflammatory cytokines to reflect SIRS severity. Second, the sample size of this study might be insufficient (the sample size of most articles is less than 100). Third, included patients of this study are not homogeneous. For example, 7 studies enrolling 601 patients were conducted in China; 3 studies enrolling 169 patients were conducted in western countries; 4 studies enrolling 240 patients undergoing valve replacement/repair or congenital heart disease repair; and 2 studies enrolling 162 patients undergoing CABG, particularly the OPCABG patients could exclude SIR caused by CPB and the return of shed blood into the circulation. Fourth, the most recent RCTs included in our meta-analysis were conducted in 2013, and many perfusion techniques have been adapted since then. For example, average hematocrit during CPB rose from 23% in 2005 to 30% nowadays (hemodilution has been associated with inflammation and bleeding). Fifth, the dosage, time point, and administration of TXA were diverse, and these data were not suitable for subgroup analysis, which may be the source of heterogeneity. Finally, publication bias may exist.
Conclusions
TXA had a significant anti-inflammatory effect in adult cardiac surgical patients, as evidenced by the reduction of multiple postoperative proinflammatory biomarker levels, but these results should be interpreted carefully and cautiously, as only a limited number of studies were included and there was high heterogeneity between them.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author.
Author contributions
C-MX: conceptualization, software, methodology, data collection, data analysis/interpretation, statistics, and writing—original draft. L-XH and KY: conceptualization, data collection, formal analysis, software, critical revision of article. Y-TY: conceptualization, formal analysis, supervision, funding acquisition, critical revision of article. All authors contributed to the article and approved the submitted version.
Funding
This work was partially funded by CAMS Innovation Fund for Medical Sciences (CIFMS)-2021-I2M-C/T-B-038.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Lord JM, Midwinter MJ, Chen YF, Belli A, Brohi K, Kovacs EJ, et al. The systemic immune response to trauma: an overview of pathophysiology and treatment. Lancet. (2014) 384:1455–65. doi: 10.1016/S0140-6736(14)60687-5
2. Kraft F, Schmidt C, Van Aken H, Zarbock A. Inflammatory response and extracorporeal circulation. Best Pract Res Clin Anaesthesiol. (2015) 29(2):113–23. doi: 10.1016/j.bpa.2015.03.001
3. Dvirnik N, Belley-Cote EP, Hanif H, Devereaux PJ, Lamy A, Dieleman JM, et al. Steroids in cardiac surgery: a systematic review and meta-analysis. Br J Anaesth. (2018) 120(4):657–67. doi: 10.1016/j.bja.2017.10.025
4. Mojcik CF, Levy JH. Aprotinin and the systemic inflammatory response after cardiopulmonary bypass. Ann Thorac Surg. (2001) 71(2):745–54. doi: 10.1016/S0003-4975(00)02218-9
5. Massoudy P, Zahler S, Becker BF, Braun SL, Barankay A, Meisner H. Evidence for inflammatory responses of the lungs during coronary artery bypass grafting with cardiopulmonary bypass. Chest. (2001) 119(1):31–6. doi: 10.1378/chest.119.1.31
6. Corral-Velez V, Lopez-Delgado JC, Betancur-Zambrano NL, Lopez-Sune N, Rojas-Lora M, Torrado H, et al. The inflammatory response in cardiac surgery: an overview of the pathophysiology and clinical implications. Inflamm Allergy Drug Targets. (2015) 13(6):367–70. doi: 10.2174/1871528114666150529120801
7. Presta P, Bolignano D, Coppolino G, Serraino F, Mastroroberto P, Andreucci M, et al. Antecedent ACE-inhibition, inflammatory response, and cardiac surgery associated acute kidney injury. Rev Cardiovasc Med. (2021) 22(1):207–13. doi: 10.31083/j.rcm.2021.01.288
8. Gormley SM, McBride WT, Armstrong MA, Young IS, McClean E, MacGowan SW, et al. Plasma and urinary cytokine homeostasis and renal dysfunction during cardiac surgery. Anesthesiology. (2000) 93(5):1210–6. doi: 10.1097/00000542-200011000-00013
9. Squiccimarro E, Labriola C, Malvindi PG, Margari V, Guida P, Visicchio G, et al. Prevalence and clinical impact of systemic inflammatory reaction after cardiac surgery. J Cardiothorac Vasc Anesth. (2019) 33(6):1682–90. doi: 10.1053/j.jvca.2019.01.043
10. Sugita J, Fujiu K. Systemic inflammatory stress response during cardiac surgery. Int Heart J. (2018) 59(3):457–9. doi: 10.1536/ihj.18-210
11. Hauser GJ, Ben-Ari J, Colvin MP, Dalton HJ, Hertzog JH, Bearb M, et al. Interleukin-6 levels in serum and lung lavage fluid of children undergoing open heart surgery correlate with postoperative morbidity. Intensive Care Med. (1998) 24(5):481–6. doi: 10.1007/s001340050600
12. Heissig B, Salama Y, Takahashi S, Osada T, Hattori K. The multifaceted role of plasminogen in inflammation. Cell Signal. (2020) 75:109761. doi: 10.1016/j.cellsig.2020.109761
13. Luyendyk JP, Schoenecker JG, Flick MJ. The multifaceted role of fibrinogen in tissue injury and inflammation. Blood. (2019) 133(6):511–20. doi: 10.1182/blood-2018-07-818211
14. Cvachovec K, Horacek M, Vislocky I. A retrospective survey of fibrinolysis as an indicator of poor outcome after cardiopulmonary bypass and a possible early sign of systemic inflammation syndrome. Eur J Anaesthesiol. (2000) 17(3):173–6. doi: 10.1097/00003643-200003000-00007
15. Casati V, Della Valle P, Benussi S, Franco A, Gerli C, Baili P, et al. Effects of tranexamic acid on postoperative bleeding and related hematochemical variables in coronary surgery: comparison between on-pump and off-pump techniques. J Thorac Cardiovasc Surg. (2004) 128(1):83–91. doi: 10.1016/j.jtcvs.2003.10.034
16. Chen TT, Liu J, Wang G, Jiang SL, Li LB, Gao CQ. Combined treatment of ulinastatin and tranexamic acid provides beneficial effects by inhibiting inflammatory and fibrinolytic response in patients undergoing heart valve replacement surgery. Heart Surg Forum. (2013) 16(1):E38–47. doi: 10.1532/HSF98.20121072
17. Later AF, Bruggemans EF, Romijn FP, Van-Pelt J, Klautz RJ. A comparative study of the immune modulating properties of antifibrinolytics in cardiac surgery. Cytokine. (2013) 61(2):438–44. doi: 10.1016/j.cyto.2012.10.033
18. Draxler DF, Yep K, Hanafi G, Winton A, Daglas M, Ho H, et al. Tranexamic acid modulates the immune response and reduces postsurgical infection rates. Blood Adv. (2019) 3(10):1598–609. doi: 10.1182/bloodadvances.2019000092
19. Higgins JPT, Thomas J, Chandler J, Chandler J, Welch VA, Higgins JP, et al. Cochrane Handbook for Systematic Reviews of Interventions version 6.2. Cochrane. (2021). Available online at: https://training.cochrane.org/handbook/current (accessed October 22, 2021).
20. Jimenez JJ, Iribrren JL, Lorente L. Tranexamic acid attenuates inflammatory response in cardiopulmonary bypass surgery through blockade of fibrinolysis: a case control study followed by a randomized double-blind controlled trial. Crit Care. (2007) 11(6):R117. doi: 10.1186/cc6173
21. Li Y, Tan X-h, Huang H-q. Effects of tranexamic acid on inflammatory factor after cardiopulmonary bypass. J SNAKE. (2012) 24(4):361–2. 374. doi: 10.3969/j.issn.1001-5639.2012.04.010
22. Chuan-bin L. Tranexamic acid during cardiopulmonary bypass the role of the inflammatory response. Anhui Med Univ. (2012) 1(1):1–47.
23. Lv H, Zhang Y, Shi J, Yuan S, Chen F, Shi S, et al. Effect of antifibrinolytic therapy on plasma neutrophil elastase in patients receiving cardiac surgery with cardiopulmonary bypass. Chin Mol Cardiol. (2019) 19(2):2830–4. doi: 10.16563/j.cnki.1671-6272.2019.04.010
24. Shi J. Effects of ulinastatin and tranexamic acid on hemostasis and inflammatory mediators in cardiac surgical procedures. Peking Union Med Coll. (2010) 1(1):1–109.
25. Wang G-y, Wang D, Shi J, Zhang Y, Chen F, Li L-h, et al. Effect of tranexamic acid on inflammatory response in patients undergoing off-pump coronary artery bypass grafting. Chin J Anesthesiol. (2011) 31(7):781–3. doi: 10.3760/cma.j.issn.0254-1416.2011.07.001
26. Yu H. The study on the protective action of high-dose tranexamic acid on the blood and myocardial preservation in the perioperative period of prosthetic heart valve replacement under cardiopulmonary bypass. Luzhou Med Coll. (2013) 1(1):1–58.
27. Li YC, Xi CH, An YF, Dong WH, Zhou M. Perioperative inflammatory response and protein S-100 beta concentrations-relationship with postoperative cognitive dysfunction in elderly patients. Acta Anaesthesiol Scand. (2012) 56(5):595–600. doi: 10.1111/j.1399-6576.2011.02616.x
28. Verwijmeren L, Bosma M, Vernooij LM, Linde EM, Dijkstra IM. Associations between preoperative biomarkers and cardiac surgery-associated acute kidney injury in elderly patients: a cohort study. Anesth Analg. (2021) 133(3):570–7. doi: 10.1213/ANE.0000000000005650
29. Weymann A, Popov AF, Sabashnikov A, Daeter EJ, Ryazanov M, Tse G, et al. Baseline and postoperative levels of C-reactive protein and interleukins as inflammatory predictors of atrial fibrillation following cardiac surgery: a systematic review and meta-analysis. Kardiol Pol. (2018) 76(2):440–51. doi: 10.5603/KP.a2017.0242
30. Sihler KC, Napolitano LM. Complication of massive transfusion. Chest. (2010) 137(1):209–20. doi: 10.1378/chest.09-0252
31. Esmon CT. Crosstalk between inflammation and thrombosis. Maturitas. (2008) 61(1-2):122–31. doi: 10.1016/j.maturitas.2008.11.008
32. Siemens K, Sangaran DP, Hunt BJ, Murdoch IA, Tibby SM. Antifibrinolytic drugs for the prevention of bleeding in pediatric cardiac surgery on cardiopulmonary bypass: a systematic review and meta-analysis. Anesth Analg. (2021) 134:987–1001. doi: 10.1213/ANE.0000000000005760
33. Abd El Baser II, ElBendary HM, ElDerie A. The synergistic effect of tranexamic acid and ethamsylate combination on blood loss in pediatric cardiac surgery. Ann Card Anaesth. (2021) 24(1):17–23. doi: 10.4103/aca.ACA_84_19
34. Wang E, Yuan X, Wang Y, Chen W, Zhou X, Hu S, et al. Blood conservation outcomes and safety of tranexamic acid in coronary artery bypass graft surgery. Int J Cardiol. (2022) 348:50–6. doi: 10.1016/j.ijcard.2021.12.017
35. Habbab LM, Hussain S, Power P, Bashir S, Gao P, Semelhago L, et al. Decreasing postoperative blood loss by topical vs. intravenous tranexamic acid in open cardiac surgery (DEPOSITION) study: results of a pilot study. J Card Surg. (2019) 34(5):305–11. doi: 10.1111/jocs.14027
36. Guo J, Gao X, Ma Y, Lv H, Hu W, Zhang S, et al. Different dose regimes and administration methods of tranexamic acid in cardiac surgery: a meta-analysis of randomized trials. BMC Anesthesiol. (2019) 19(1):129. doi: 10.1186/s12871-019-0772-0
37. Heyns M, Knight P, Steve AK, Yeung JK. A single preoperative dose of tranexamic acid reduces perioperative blood loss: a meta-analysis. Ann Surg. (2021) 273(1):75–81. doi: 10.1097/SLA.0000000000003793
38. Stenvinkel P, Ketteler M, Johnson RJ, Lindholm B, Pecoits-Filho R, Riella M, et al. IL-10, IL-6, and TNF-alpha: central factors in the altered cytokine network of uremia—the good, the bad, and the ugly. Kidney Int. (2005) 67(4):1216–33. doi: 10.1111/j.1523-1755.2005.00200.x
39. Chew MS, Brandslund I, Brix-Christensen V, Ravn HB, Hjortdal VE, Pedersen J, et al. Tissue injury and the inflammatory response to pediatric cardiac surgery with cardiopulmonary bypass: a descriptive study. Anesthesiology. (2001) 94(5):745–53; discussion 745A. doi: 10.1097/00000542-200105000-00010
40. Everett AD, Alam SS, Owens SL, Parker DM, Goodrich C, Likosky DS, et al. The association between cytokines and 365-day readmission or mortality in adult cardiac surgery. J Extra Corpor Technol. (2019) 51(4):201–9. doi: 10.1182/JECT-1900014
41. Howell KW, Cleveland JC, Meng X, Ao L, Su X, Schwartz RS, et al. Interleukin 6 production during cardiac surgery correlates with increasing age. J Surg Res. (2016) 201(1):76–81. doi: 10.1016/j.jss.2015.10.016
42. Brocca A, Virzi GM, de Cal M, Giavarina D, Carta M, Ronco C. Elevated levels of procalcitonin and interleukin-6 are linked with postoperative complications in cardiac surgery. Scand J Surg. (2017) 106(4):318–24. doi: 10.1177/1457496916683096
43. Clementi A, Brocca A, Virzi GM, Hinna-Danesi T, Salvador L, Ronco C, et al. Procalcitonin and interleukin-6 levels: are they useful biomarkers in cardiac surgery patients? Blood Purif. (2017) 43(4):290–7. doi: 10.1159/000454672
44. Askevold ET, Gullestad L, Dahl CP, Yndestad A, Ueland T, Aukrust P. Interleukin-6 signaling, soluble glycoprotein 130, and inflammation in heart failure. Curr Heart Fail Rep. (2014) 11(2):146–55. doi: 10.1007/s11897-014-0185-9
45. DiMaria-Ghalili RA, Sullivan-Marx EM, Compher C. Inflammation, functional status, and weight loss during recovery from cardiac surgery in older adults: a pilot study. Biol Res Nurs. (2014) 16(3):344–52. doi: 10.1177/1099800413503489
46. Gueret G, Lion F, Guriec N, Arvieux J, Dovergne A, Guennegan C, et al. Acute renal dysfunction after cardiac surgery with cardiopulmonary bypass is associated with plasmatic IL6 increase. Cytokine. (2009) 45(2):92–8. doi: 10.1016/j.cyto.2008.11.001
47. Rallidis LS, Zolindaki MG, Pentzeridis PC, Poulopoulos KP, Velissaridou AH, Apostolou TS. Raised concentrations of macrophage colony stimulating factor in severe unstable angina beyond the acute phase are strongly predictive of long-term outcome. Heart. (2004) 90(1):25–9. doi: 10.1136/heart.90.1.25
48. Wang K, Wu M, Xu J, Wu C, Zhang B, Wang G, et al. Effects of dexmedetomidine on perioperative stress, inflammation, and immune function: systematic review and meta-analysis. Br J Anaesth. (2019) 123(6):777–94. doi: 10.1016/j.bja.2019.07.027
Keywords: cardiac surgery, tranexamic acid, inflammatory, meta-analysis, randomized controlled trials
Citation: Xie C, Yao Y, He L and Yang K (2022) Anti-inflammatory effect of tranexamic acid on adult cardiac surgical patients: A PRISMA-compliant systematic review and meta-analysis. Front. Surg. 9:951835. doi: 10.3389/fsurg.2022.951835
Received: 24 May 2022; Accepted: 2 September 2022;
Published: 28 September 2022.
Edited by:
Giuseppe Maria Raffa, Mediterranean Institute for Transplantation and Highly Specialized Therapies (ISMETT), ItalyReviewed by:
Michele Di Mauro, Maastricht University Medical Centre, NetherlandsAntonino S. Rubino, University of Campania Luigi Vanvitelli, Italy
© 2022 Xie, Yao, He, Yang, the Evidence In Cardiovascular Anesthesia (EICA) Group. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yun-Tai Yao yuntaiyao@126.com
Specialty Section: This article was submitted to Thoracic Surgery, a section of the journal Frontiers in Surgery
 Chun-Mei Xie1
Chun-Mei Xie1