Multiple ossified spinal meningiomas in the thoracic spine: A case report and literature review
- 1Beijing Hospital of Traditional Chinese Medicine, Capital Medical University, Beijing, China
- 2Department of Medical Rehabilitation, Yan Liang Hospital District of Xi’an Honghui Hospital, Xi’an, China
- 3Section III of Internal Medicine Department, Tongzhou District Hospital of Integrated Traditional Chinese Medicine and Western Medicine, Beijing, China
- 4Department of Spine Surgery, China-Japan Friendship Hospital, Beijing, China
- 5Department of Orthopedics, Honghui Hospital, Xi'an Jiaotong University, Xi’an, China
Background: Ossified spinal meningioma (OSM) is a rare form of a spinal tumor. The surgical strategies and pathologic findings related to OSM have been investigated in recent years. However, multiple OSMs are rarely reported. Here, we intend to present a rare case of multiple OSMs and review the relevant published literature.
Case Presentation: A 76-year-old woman experienced a progressive sensorimotor disturbance in her bilateral lower limbs for the past 2 years. She complained of inability to walk, urinary incontinence, and chronic constipation when referred to our hospital. A neurological examination revealed a diminished sensation below the bilateral T7, and her neurological status was Nurick Grade 6. Magnetic resonance imaging (MRI) revealed multiple intradural-extramedullary neoplasms at the T7–T11 level. Computed tomography (CT) scans showed five high-density masses of varying sizes in the spinal canal at the T7–T12 level. The patient underwent tumor resection through T7–T11 laminectomy. A histopathological examination revealed multiple OSMs.
Conclusion: We reported a rare case of multiple OSMs in an elderly patient. After one-stage complete resection, the patient recovered with satisfactory curative effect. Although elderly patients will face various postoperative complications due to their poor physical condition, we still recommend one-stage complete resection of multiple OSMs to reduce recurrence.
Introduction
Meningioma is the second most common of all primary spine tumors, accounting for 25%–45% (1). Multiple meningiomas usually occur inside the cranium and occasionally in both the skull and the spinal canal (2). While intracranial meningiomatosis presents as solid, cystic, or solid-cystic lesions, ranging between WHO Grade I and III, multiple spinal meningiomas are usually homogeneously solid WHO Grade I lesions (3). Ossified spinal meningioma (OSM), a rare form of meningioma, accounts for 0.7%–5.5% of all spinal meningiomas (4–6). However, only one case of multiple OSMs has been reported to date (7). Here, we present the first exclusive case of multiple OSMs with more than two independently situated meningiomas in a 76-year-old woman and a review of the literature.
Case presentation
Medical history
A 76-year-old woman reported a history of progressive sensorimotor disturbance in both lower limbs for the past 2 years. Initially, the patient’s condition was misdiagnosed as “lumbar disc herniation” in the local hospital, and she received conservative treatment. However, her symptoms did not improve and became more serious. When referred to our hospital, she complained of inability to walk and bladder and bowel control loss. A neurological examination revealed a diminished sensation below the T7 level. The strength of both lower limb muscles was 1/5. Her neurological status was diagnosed as Nurick Grade 6. Magnetic resonance imaging (MRI) revealed multiple intradural extramedullary tumors at the T7–T11 level compressing the spinal cord (Figure 1A). Computed tomography (CT) scans showed five high-density masses of varying sizes in the spinal canal at the T7–T12 level, and the dural sac was significantly compressed (Figures 1B,C). Three large high-density masses (13.4 mm*8.4 mm, 21.7 mm*13.0 mm, and 14.9 mm*12.5 mm) were observed in the T7–T9 segment, located in the dorsal left rear spinal cord with clear boundaries and ossification signals (Figures 1D–F). The other two high-density masses (8.7 mm*5.3 mm and 11.1 mm*6.73 mm) of different sizes were found in the T10–T12 segment.
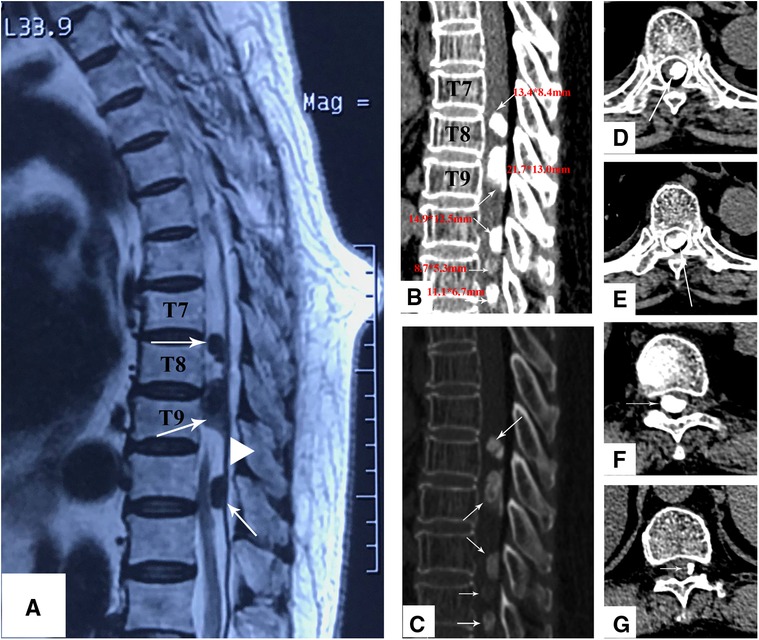
Figure 1. (A) Sagittal T2-weighted MRI shows three large lesions at the T7–T11 level with low signal intensity. The lesions shown with a dural tail sign. (B,C) The sagittal CT scan shows that there are five different sizes of high-density masses at the T7–T8 (D), T8–T9 (E), T10–T11 (F), and T11–T12 (G), levels, and all of them are located at the posterior of the spinal canal.
Surgical management
The patient underwent tumor resection through T7–T11 laminectomy. The tumors occupied >90% of the transverse diameter of the spinal canal and were entirely ossified. As the vertebral fenestration was too small, it was challenging to remove the tumors. Hence, the bilateral facet joints were removed to enlarge the window. To maintain the stability of the spine, we performed T7–T12 long-segment pedicle screw fixation and posterolateral bone graft fusion simultaneously. Bilateral facetectomy provides advantages over simple laminectomy or laminoplasty in terms of width of the operative corridor and long-term preservation of the spinal alignment. As the dura mater had severely adhered, a fine right-angled hook was used to dissect the neural tissue dorsally away from the neoplasms, which were then resected en bloc, together with parts of the dura mater and arachnoid. After a complete separation of multiple OSMs, the dura mater became normal and it was sutured. The drainage was routinely placed, and the wound was closed layer by layer.
Histopathological outcomes
The paraffin sections were stained with hematoxylin and eosin (H/E). A histopathological examination revealed a large number of psammoma bodies in the stroma with significant signs of ossification [World Health Organization (WHO) Grade I]. In addition, there were many mature bone tissues around the tumor cells, including trabecular bone, as well as bone marrow with hematopoiesis (Figures 2A–C). Immunohistochemical findings revealed EMA (+), Vim (+), P53 (−), S-100 (+), and Ki67 (approximately 5%) (Figures 3D–F).
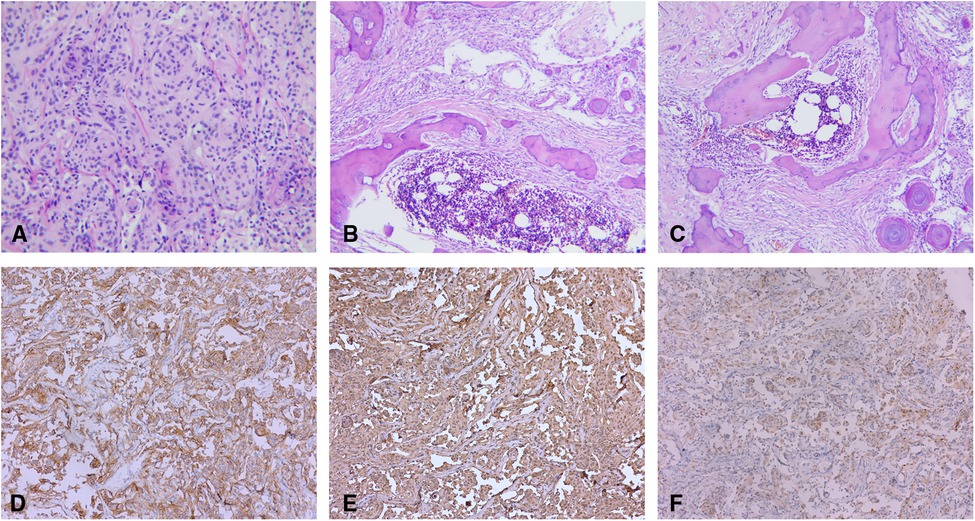
Figure 2. (A) Tumors are demonstrated to be a spinal meningioma on histology (H/E stain, original magnification ×100). (B,C) Psammoma bodies around the tumor cells. Mature bone tissues around the tumor cells, including trabecular bone, as well as bone marrow with hematopoiesis (H/E stain, original magnification ×40). Immunohistochemically, the neoplastic meningothelial cells are immunoreactive for (D) epithelial membrane antigen (EMA) (×10), (E) vimentin (×10), and (F) soluble protein-100 (S-100) (×10).
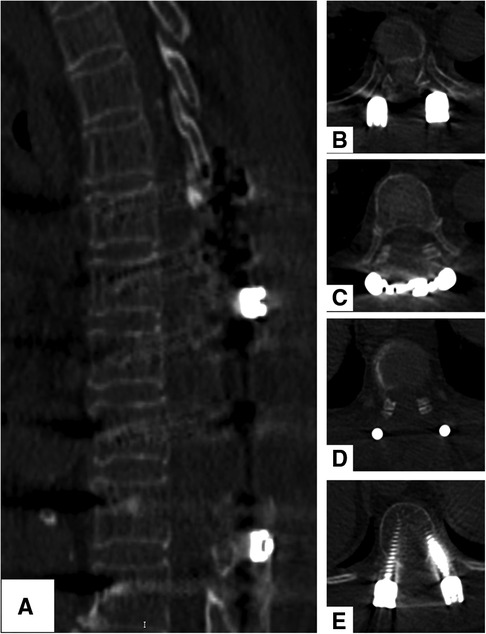
Figure 3. (A–E) CT scans show a satisfactory decompression of the spinal cord, and no recurrence spinal meningioma was found after 2 years.
Postoperative and follow-up results
Postoperative neurological improvement was significant, and no complications were found. The patient was discharged 2 weeks after surgery. After the 2-year follow-up, she was able to walk without assistance. Her neurological status recovered to Nurick Grade 3. CT scans showed a total resection of the tumors, and there was no recurrence 2 years after surgery (Figures 3A–E).
Discussion
Including our current report, 33 articles (4, 7–37) containing 43 cases of OSMs have been published as of this year, according to PubMed. (In Table 1, the search terms are ((((((ossified [Title/Abstract]) OR (osteoblastic [Title/Abstract])) OR (osseous metaplasia [Title/Abstract])) OR (psammomatous [Title/Abstract])) OR (calcified [Title/Abstract])) AND (spinal [Title/Abstract])) AND (meningioma [Title/Abstract]), and 76 potential studies are identified. We also checked the reference lists of all, including articles, to add 10 other articles.) For this condition, female predominance has been clearly noted (female, 38; male, 5), and the average age is 58.4 years, ranging from 15 to 90. Most tumors are located in the thoracic spine, except four in the cervical region and one in the lumbar region. Bone formation and hematopoiesis have been found in 7 cases, and only 2 cases of multiple OSMs have been identified, including our report.
Multiple meningiomas are defined as more than two independently situated meningiomas arising simultaneously or sequentially (2). The pathogenesis of multiple meningiomas remains elusive. In fact, at present, two kinds of hypotheses are proposed by the relevant literature: one supports the theory of monoclonal spreading and the other suggests that of clonally unrelated onset for lesions arising sequentially from two clearly distinct spinal regions (38). A genomic profiling study reveals that multiple meningiomas can be of both mono- and multiclonal origin. Even monoclonal multiple meningiomas can acquire intertumor heterogeneity through branched evolution resulting in pathology, and the landscape of one tumor may not be representative of the others. Thus, multiple meningiomas should be tailored individually if feasible (39).
The concept of ossification of meningioma should be distinguished from calcification. Calcification is more a radiologic description than a histopathological diagnosis and is commonly seen in psammomatous meningioma. Based on the classification by the World Health Organization (WHO), ossification in meningioma is histologically classified as a subtype of metaplastic meningioma and is characterized by the expression of mesenchymal components (40). The occurrence of OSM also remains unclear. One hypothesis is that ossification results from the repeated accumulation of hydroxyapatite crystals in psammoma bodies (41). However, some reports (4, 8, 10, 15, 21) have occasionally found that ossification can occur without psammoma bodies, which may not support the hypothesis. Hence, most studies prefer to believe that ossification is secondary to the metaplasia of arachnoid cells and interstitial cells, which induce the synergistic effect of osteoblast, fibroblast, and angiogenesis components in bone tissue formation (4, 8, 10, 23). Up to now, seven studies have mentioned the formation of hematopoietic tissue in OSM. This unexpected finding seems to confirm the mesenchymal potential of meningiomatous cells, which may undergo bone metaplasia (9, 11–13, 19, 24).
Although OSM was previously reported to grow very slowly and experience a long asymptomatic period, the symptoms may appear at an early stage in intraspinal tumors compared with intracranial tumors due to a smaller space in the intraspinal canal (15). As most commonly in WHO Grade I, multiple spinal meningiomas can be considered benign lesions with a good prognosis, especially if the lesion and affected dura are macroscopically complete removed (Simpson grade I) (42). Mirimanoff et al. (43) found that the incidence rates of secondary operation after a total resection for follow-up periods of 5, 10, and 15 years were 6%, 15%, and 20%, respectively. In contrast, after a subtotal resection, the probability was 25%, 44%, and 84%, respectively.
Compared with the common meningiomas, OSM closely adheres to the dura and arachnoid (7). If the tumor and the dura mater are not separated, they could be removed together and repaired with an artificial dura mater covered with gelatin sponge. Ruggeri et al. (44) proposed that a poor surgical outcome in patients with ossified tumors results from a more “invasive” surgical removal of an ossified mass: an internal debulking is not feasible for a hard tumor. In our case, the ossified tumors occupied the entire transverse diameter of the spinal canal and could be approached more safely with laminectomy by removing the bilateral facet joints from T7 to T12 instead of with hemilaminectomy: it offers a wider operative field for a better manipulation of the neural tissue, especially the thoracic spinal cord, which is more susceptible to damage than the cervical spinal cord and lumbar spinal roots. Therefore, we performed a T7–T12 long-segment pedicle screw fixation and posterolateral bone graft fusion to prevent the occurrence of iatrogenic kyphosis and maintain the stability of the spine. After a 2-year follow-up, no complications such as internal fixation failure and kyphosis occurred. The use of intraoperative ultrasound represents a valuable surgical aid for real-time neuronavigation, allowing the operating team to evaluate the decompression of the spinal cord and rule out subdural blood clots at the time of dural closure/reconstruction (45). For spinal intradural extremedullary lesions, the sensitivity, specificity, and positive and negative predicted values of intraoperative neurophysiological monitoring (IONM) are reported to be 75, 100, 100, and 97%, respectively. This indicates that IONM predicts neurological deficits with high accuracy, although its role in preventing new neurological deficits in spinal meningiomas has yet to be proved (46). The current trends in the use of adjuvant radiotherapy and radiosurgery for spinal meningiomas have increased; nonetheless, this has not led to a significant increase in overall survival rates (47). Larger tumor size and borderline or malignant behavior are reported to be associated with increased radiation use; the introduction of multimodal adjuvant technologies such as radioenhancers has yet to provide evidence for superior outcomes (48). To sum up, surgical en bloc resection of OSM is difficult, especially in multiple OSMs, but it is still the best treatment.
Although ossified meningioma could be identified in a plain radiograph, CT and MRI are complementary methods of diagnosing a calcified spinal meningioma, especially in cases replaced entirely by calcification (49). On MR images, the signal intensities of calcifications within the masses were variable, and the extent of signal intensities suggesting calcification did not concur with that of calcified foci within a mass, as seen on CT images (49).
Conclusion
In the current report, we present a rare case of multiple OSMs in the thoracic spine. Although the mechanism of occurrence is not clear, a total resection is generally required, and a satisfying prognosis and a low recurrence rate can be expected. The surgical strategy for OSMs differs from that for other meningiomas. We suggest that preoperative CT be used to accurately locate ossification. Intraoperative use of a wide surgical corridor with total laminectomy, combined with bilateral facet joint resection, identification of upper and lower poles, and early CSF drainage, is helpful in decreasing neural retraction.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author.
Ethics statement
Ethical review and approval was not required for the study on human participants in accordance with the local legislation and institutional requirements. The patients/participants provided their written informed consent to participate in this study. Written informed consent was obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article.
Author contributions
CD and YL contributed to the conception and design of the study. CD wrote the first draft of the manuscript. YL and HW wrote sections of the manuscript. YZ did the literature review. YM was involved in the concept development, quality control of the data, and interpretation of the manuscript. All authors contributed to the article and approved the submitted version.
Funding
This work was supported by the Natural Science Foundation of Shaanxi Province (Grant no. 2022JM-546) and the General Research Project of Xi’an Health Commission (Grant no. 2022yb09).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Maiti TK, Bir SC, Patra DP, Kalakoti P, Guthikonda B, Nanda A. Spinal meningiomas: clinicoradiological factors predicting recurrence and functional outcome. Neurosurg Focus. (2016) 41:E6. doi: 10.3171/2016.5.FOCUS16163
2. Jain S, Sundar I, Sharma V, Goel R, Prasanna K. Multiple spinal and cranial meningiomas: a case report and review of literature. Asian J Neurosurg. (2015) 10:132. doi: 10.4103/1793-5482.145185
3. Boukobza M, Cebula H, Pop R, Kouakou F, Sadoun A, Coca HA, et al. Cystic meningioma: radiological, histological, and surgical particularities in 43 patients. Acta Neurochir (Wien). (2016) 158:1955–64. doi: 10.1007/s00701-016-2898-x
4. Niijima K, Huang YP, Malis LI, Sachdev VP. Ossified spinal meningioma en plaque. Spine (Phila Pa 1976). (1993) 18:2340–3. doi: 10.1097/00007632-199311000-00036
5. Doita M, Harada T, Nishida K, Marui T, Kurosaka M, Yoshiya S. Recurrent calcified spinal meningioma detected by plain radiograph. Spine (Phila Pa 1976). (2001) 26:e249–52. doi: 10.1097/00007632-200106010-00005
6. Raza SM, Anderson WS, Eberhart CG, Wolinsky J, Gokaslan ZL. The application of surgical cordectomy in the management of an intramedullary-extramedullary atypical meningioma. J Spinal Disord Tech. (2005) 18:449–54. doi: 10.1097/01.bsd.0000155032.69394.23
7. Uchida K, Nakajima H, Yayama T, Sato R, Kobayashi S, Mwaka ES, et al. Immunohistochemical findings of multiple ossified en plaque meningiomas in the thoracic spine. J Clin Neurosci. (2009) 16:1660–2. doi: 10.1016/j.jocn.2009.03.013
8. Tahir M, Usmani N, Ahmad FU, Salmani S, Sharma MS. Spinal meningioma containing bone: a case report and review of literature. BMJ Case Rep. (2009); 2009: bcr11.2008.1186 doi: 10.1136/bcr.11.2008.1186
9. Licci S, Limiti MR, Callovini GM, Bolognini A, Gammone V, Di Stefano D. Ossified spinal tumour in a 58-year-old woman with increasing paraparesis. Neuropathology. (2010) 30:194–6. doi: 10.1111/j.1440-1789.2009.01076.x
10. Ju CI, Hida K, Yamauchi T, Houkin K. Totally ossified metaplastic spinal meningioma. J Korean Neurosurg Soc. (2013) 54:257. doi: 10.3340/jkns.2013.54.3.257
11. Taneoka A, Hayashi T, Matsuo T, Abe K, Kinoshita N, Yasui H, et al. Ossified thoracic spinal meningioma with hematopoiesis: a case report and review of the literature. Case Reports Clin Med. (2013) 02:24–8. doi: 10.4236/crcm.2013.21007
12. Chotai SP, Mrak RE, Mutgi SA, Medhkour A. Ossification in an extra-intradural spinal meningioma—pathologic and surgical vistas. Spine J. (2013) 13:e21–6. doi: 10.1016/j.spinee.2013.06.102
13. Chan TT, Lau VWH, Chau TKF, Lee YL. Ossified thoracic spinal meningioma with lamellar bone formation presented with paraparesis. J Orthop Trauma Rehabil. (2014) 18:106–9. doi: 10.1016/j.jotr.2013.06.001
14. Yamane K, Tanaka M, Sugimoto Y, Ichimura K, Ozaki T. Spinal metaplastic meningioma with osseous differentiation in the ventral thoracic spinal canal. Acta Med Okayama. (2014) 68:313–6. doi: 10.18926/AMO/52901
15. Alafaci C, Grasso G, Granata F, Salpietro FM, Tomasello F. Ossified spinal meningiomas: clinical and surgical features. Clin Neurol Neurosurg. (2016) 142:93–7. doi: 10.1016/j.clineuro.2016.01.026
16. Xia T, Tian JW. Entirely ossified subdural meningioma in thoracic vertebral canal. Spine J. (2016) 16:e11. doi: 10.1016/j.spinee.2015.09.005
17. Demir MK, Yapicier Ö, Toktaş ZO, Akakin A, Yilmaz B, Konya D. Ossified-calcified intradural and extradural thoracic spinal meningioma with neural foraminal extension. Spine J. (2016) 16:e35–7. doi: 10.1016/j.spinee.2015.08.053
18. Rogers L. A spinal meningioma containing bone. Br J Surg. (1928) 15:675–7. doi: 10.1002/bjs.1800156015
19. Cochran EJ, Schlauderaff A, Rand SD, Eckardt GW, Kurpad S. Spinal osteoblastic meningioma with hematopoiesis: radiologic-pathologic correlation and review of the literature. Ann Diagn Pathol. (2016) 24:30–4. doi: 10.1016/j.anndiagpath.2016.07.002
20. Prakash A, Mishra S, Tyagi R, Bhatnagar A, Kansal S, Attri P. Thoracic psammomatous spinal meningioma with osseous metaplasia: a very rare case report. Asian J Neurosurg. (2017) 12:270–2. doi: 10.4103/1793-5482.150222
21. Sakamoto K, Tsutsumi S, Nonaka S, Suzuki T, Ishii H, Ito M, et al. Ossified extradural en-plaque meningioma of the cervical spine. J Clin Neurosci. (2018) 50:124–6. doi: 10.1016/j.jocn.2018.01.058
22. Taha MM, Alawamry A, Abdel-Aziz HR. Ossified spinal meningioma: a case report and a review of the literature. Surg J. (2019) 05:e137–41. doi: 10.1055/s-0039-1697634
23. Murakami T, Tanishima S, Takeda C, Kato S, Nagashima H. Ossified metaplastic spinal meningioma without psammomatous calcification: a case report. Yonago Acta Med. (2019) 62:232–5. doi: 10.33160/yam.2019.06.008
24. Wang C, Chen Y, Zhang L, Ma X, Chen B, Li S. Thoracic psammomatous meningioma with osseous metaplasia: a controversial diagnosis of a case report and literature review. World J Surg Oncol. (2019) 17:150. doi: 10.1186/s12957-019-1694-5
25. Xu F, Tian Z, Qu Z, Yao L, Zou C, Han W, et al. Completely ossified thoracic intradural meningioma in an elderly patient: a case report and literature review. Medicine (Baltimore). (2020) 99:e20814. doi: 10.1097/MD.0000000000020814
26. Buchanan D, Martirosyan NL, Yang W, Buchanan RI. Thoracic meningioma with ossification: case report. Surg Neurol Int. (2021) 12:1–4. doi: 10.25259/SNI_643_2021
27. Wong YP, Tan GC, Mukari SAM, Palaniandy K. Heterotopic ossification in psammomatous spinal meningioma: a diagnostic controversy. Int J Clin Exp Pathol. (2021) 14:627–32.34093948
28. Thakur J, Ulrich CT, Schär RT, Seidel K, Raabe A, Jesse CM. The surgical challenge of ossified ventrolateral spinal meningiomas: tricks and pearls for managing large ossified meningiomas of the thoracic spine. J Neurosurg Spine. (2021) 35:516–26. doi: 10.3171/2020.12.SPINE201526
29. Freidberg SR. Removal of an ossified ventral thoracic meningioma. Case report. J Neurosurg. (1972) 37:728–30. doi: 10.3171/jns.1972.37.6.0728
30. Kandel E, Sungurov E, Morgunov V. Cerebral and two spinal meningiomas removed from the same patient. Neurosurgery. (1989) 25:447–50. doi: 10.1097/00006123-198909000-00021
31. Saito T, Arizono T, Maeda T, Terada K, Iwamoto Y. A novel technique for surgical resection of spinal meningioma. Spine (Phila Pa 1976). (2001) 26:1805–8. doi: 10.1097/00007632-200108150-00017
32. Kitagawa M, Nakamura T, Aida T, Iwasaki Y, Abe H, Nagashima K. [Clinicopathologic analysis of ossification in spinal meningioma]. Noshuyo Byori. (1994) 11:115–9.8162148
33. Nakayama N, Isu T, Asaoka K, Harata T, Hayashi S, Aoki T, et al. [Two cases of ossified spinal meningioma]. No Shinkei Geka. (1996) 24:351–5.8934888
34. Huang TY, Kochi M, Kuratsu J, Ushio Y. Intraspinal osteogenic meningioma: report of a case. J Formos Med Assoc. (1999) 98:218–21.10365544
35. Naderi S, Yilmaz M, Canda T, Acar Ü. Ossified thoracic spinal meningioma in childhood. Clin Neurol Neurosurg. (2001) 103:247–9. doi: 10.1016/S0303-8467(01)00157-3
36. Liu CL, Lai PL, Jung SM, Liao CC. Thoracic ossified meningioma and osteoporotic burst fracture: treatment with combined vertebroplasty and laminectomy without instrumentation—case report. J Neurosurg Spine. (2006) 4:256–9. doi: 10.3171/spi.2006.4.3.256
37. Hirabayashi H, Takahashi J, Kato H, Ebara S, Takahashi H. Surgical resection without dural reconstruction of a lumbar meningioma in an elderly woman. Eur Spine J. (2009) 18:232–5. doi: 10.1007/s00586-009-0895-y
38. Kumar NP, Ravi K. Multiple spinal canal meningiomas. J Evid Based Med Healthc. (2016) 3:4742–5. doi: 10.18410/jebmh/2016/1000
39. Erson-Omay EZ, Vetsa S, Vasandani S, Barak T, Nadar A, Marianayagam NJ, et al. Genomic profiling of sporadic multiple meningiomas. BMC Med Genomics. (2022) 15:112. doi: 10.1186/s12920-022-01258-0
40. Kleihues P, Louis DN, Scheithauer BW, Rorke LB, Reifenberger G, Burger PC, et al. The WHO classification of tumors of the nervous system. J Neuropathol Exp Neurol. (2002) 61:215–25. doi: 10.1093/jnen/61.3.215
41. Kubota T, Sato K, Yamamoto S, Hirano A. Ultrastructural study of the formation of psammoma bodies in fibroblastic meningioma. J Neurosurg. (1984) 60:512–7. doi: 10.3171/jns.1984.60.3.0512
42. Voldřich R, Netuka D, Beneš V. Spinal meningiomas: is Simpson grade II resection radical enough? Acta Neurochir (Wien). (2020) 162:1401–8. doi: 10.1007/s00701-020-04280-2
43. Mirimanoff RO, Dosoretz DE, Linggood RM, Ojemann RG, Martuza RL. Meningioma: analysis of recurrence and progression following neurosurgical resection. J Neurosurg. (1985) 62:18–24. doi: 10.3171/jns.1985.62.1.0018
44. Ruggeri AG, Fazzolari B, Colistra D, Cappelletti M, Marotta N, Delfini R. Calcified spinal meningiomas. World Neurosurg. (2017) 102:406–12. doi: 10.1016/j.wneu.2017.03.045
45. Ganau M, Syrmos N, Martin AR, Jiang F, Fehlings MG. Intraoperative ultrasound in spine surgery: history, current applications, future developments. Quant Imaging Med Surg. (2018) 8:261–7. doi: 10.21037/qims.2018.04.02
46. Harel R, Schleifer D, Appel S, Attia M, Cohen ZR, Knoller N. Spinal intradural extramedullary tumors: the value of intraoperative neurophysiologic monitoring on surgical outcome. Neurosurg Rev. (2017) 40:613–9. doi: 10.1007/s10143-017-0815-2
47. Yolcu YU, Goyal A, Alvi MA, Moinuddin FM, Bydon M. Trends in the utilization of radiotherapy for spinal meningiomas: insights from the 2004-2015 national cancer database. Neurosurg Focus. (2019) 46:E6. doi: 10.3171/2019.3.FOCUS1969
48. Ganau M, Foroni RI, Gerosa M, Ricciardi GK, Longhi M, Nicolato A. Radiosurgical options in neuro-oncology: a review on current tenets and future opportunities. Part II: adjuvant radiobiological tools. Tumori. (2015) 101:57–63. doi: 10.5301/tj.5000215
Keywords: multiple meningiomas, spinal meningioma, ossification, calcification, surgery
Citation: Dong C, Liu Y, Zhu Y, Wei H and Ma Y (2022) Multiple ossified spinal meningiomas in the thoracic spine: A case report and literature review. Front. Surg. 9:965815. doi: 10.3389/fsurg.2022.965815
Received: 10 June 2022; Accepted: 7 September 2022;
Published: 4 October 2022.
Edited by:
Mario Ganau, Oxford University Hospitals NHS Trust, United KingdomReviewed by:
Mahmoud M. Taha Zagazig University, EgyptYin Ping Wong, National University of Malaysia, Malaysia
© 2022 Dong, Liu, Zhu, Wei and Ma. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yuzhuo Ma doctor_mayz@126.com
†These authors have contributed equally to this work and share first authorship
Specialty Section: This article was submitted to Neurosurgery, a section of the journal Frontiers in Surgery
 Chunke Dong
Chunke Dong Yi Liu2,†
Yi Liu2,†