- 1Department of Oncology, Third Xiangya Hospital, Central South University, Changsha, China
- 2Department of Breast and Thyroid Surgery, Third Xiangya Hospital, Central South University, Changsha, China
- 3Department of Hepatobiliary and Pancreatic Surgery, Third Xiangya Hospital, Central South University, Changsha, China
- 4Department of Pathology, Third Xiangya Hospital, Central South University, Changsha, China
Background: Primary breast angiosarcoma (PBA) is a rare sarcoma, accounting for only 0.04% of all breast malignancies, with a difficult diagnosis and a poor prognosis. Mastectomy is the standard treatment, and the role of adjuvant treatment (chemotherapy and/or radiotherapy following surgery) remains uncertain with very few studies.
Case Presentation: We report the case of a 17-year-old female patient who presented with a right breast lump that had rapidly increased in size and was hemorrhaging. She was diagnosed with breast angiosarcoma by needle biopsy and pathological evaluation. However, the mass showed a quick tendency to bleed during biopsies. After that, we performed angiography and tumor vascular embolization. The patient underwent a mastectomy followed by adjuvant chemotherapy.
Conclusion: Tumor vascular embolization reduced the surgical risk of PBA with hemorrhage complications. Postoperative therapeutic roles still need further exploration and verification.
Introduction
Breast angiosarcoma is an extremely rare and aggressive malignancy that originates from vascular or lymphatic endothelial cells, accounting for 0.1%–0.2% of all breast neoplasms. It can be divided into primary and secondary angiosarcoma. It has a poor prognosis, with a 5-year survival rate of 40% (1). Primary breast angiosarcoma (PBA) is extremely rare (2). Currently, the most common management approach is surgical excision without involved margins (R0 resection) (3). We report the case of breast angiosarcoma in a patient who appeared with a large lump and bleeding in the right breast.
Case presentation
In February 2021, a 17-year-old female patient presented with a lump on the right breast about 1.5 × 1.5 cm in size in the lower inner quadrant (Figure 1). The lesion gradually increased in size over a period of 1 month without any pain or bleeding. She did not consult any doctor until the right breast underwent rapid enlargement after accidentally falling, reaching 20 × 20 cm. She had skin ulceration, pink-blueish discoloration, and bleeding from the breast lump for 3 days, without heat, pain, or discharge. On physical examination, the lesion appeared firm with a non-mobile, irregular margin. Axillary lymphadenopathy was negative, and there were no palpable supraclavicular nodes. The left breast was normal.
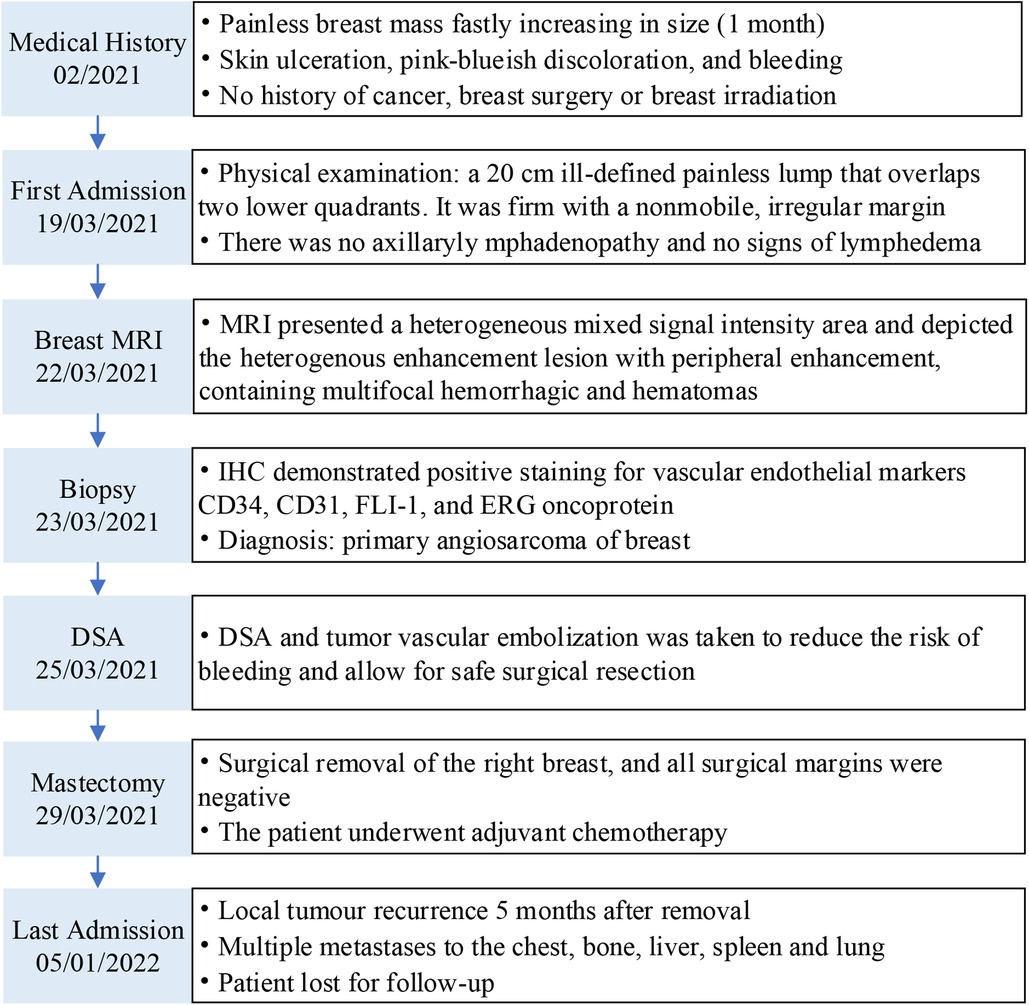
Figure 1. Case report timeline. MRI, magnetic resonance imaging; DSA, digital subtraction angiography; IHC, immunohistochemistry.
There was no other remarkable feature in the patient's medical history. She had no history of chronic illness or radiotherapy and was without a significant medical family history, including breast cancer. During hospitalization, the results of the laboratory tests were as follows: WBCs, 7,760/mm3 (normal: 3,500–9,500/mm3), Hb, 5.1 g/dL (11.5–15.0 g/dL), hematocrit, 18.0% (35.0%–45.0%), platelet, 80,000/mm3 (125,000–350,000/mm3), PT, 17.9 s (9.0–14.0 s), APTT, 39.8 s (20.0–40.0 s), INR, 1.57 (0.8–1.5), fibrinogen, 60 (200–400) mg/dl, D-dimer, 85.27 mg/L (0–0.55 mg/L). An ultrasound examination was performed, demonstrating that the right breast had heterogeneously mixed echogenicity of approximately 254 × 280 × 96 mm with an indistinct margin.
Considering the inconclusive findings on ultrasound, computed tomography (CT) scans of the head and chest were performed and revealed that the right breast had heterogeneously increased in density, with focal skin thickening, and an enhancement of the nipple. There was no obvious axillary lymphadenopathy in both axillae. In addition, the magnetic resonance imaging (MRI) showed a heterogeneous mixed signal intensity area predominantly of isointense signal intensity on T1-weighted images, and high signal intensity on T2-weighted images, containing multifocal hemorrhages and hematomas. Subsequent contrast-enhanced MRI depicted the heterogenous enhancement with peripheral enhancement (Figure 2). An ultrasound of the abdomen and an F-18 bone scan were negative for metastatic disease.
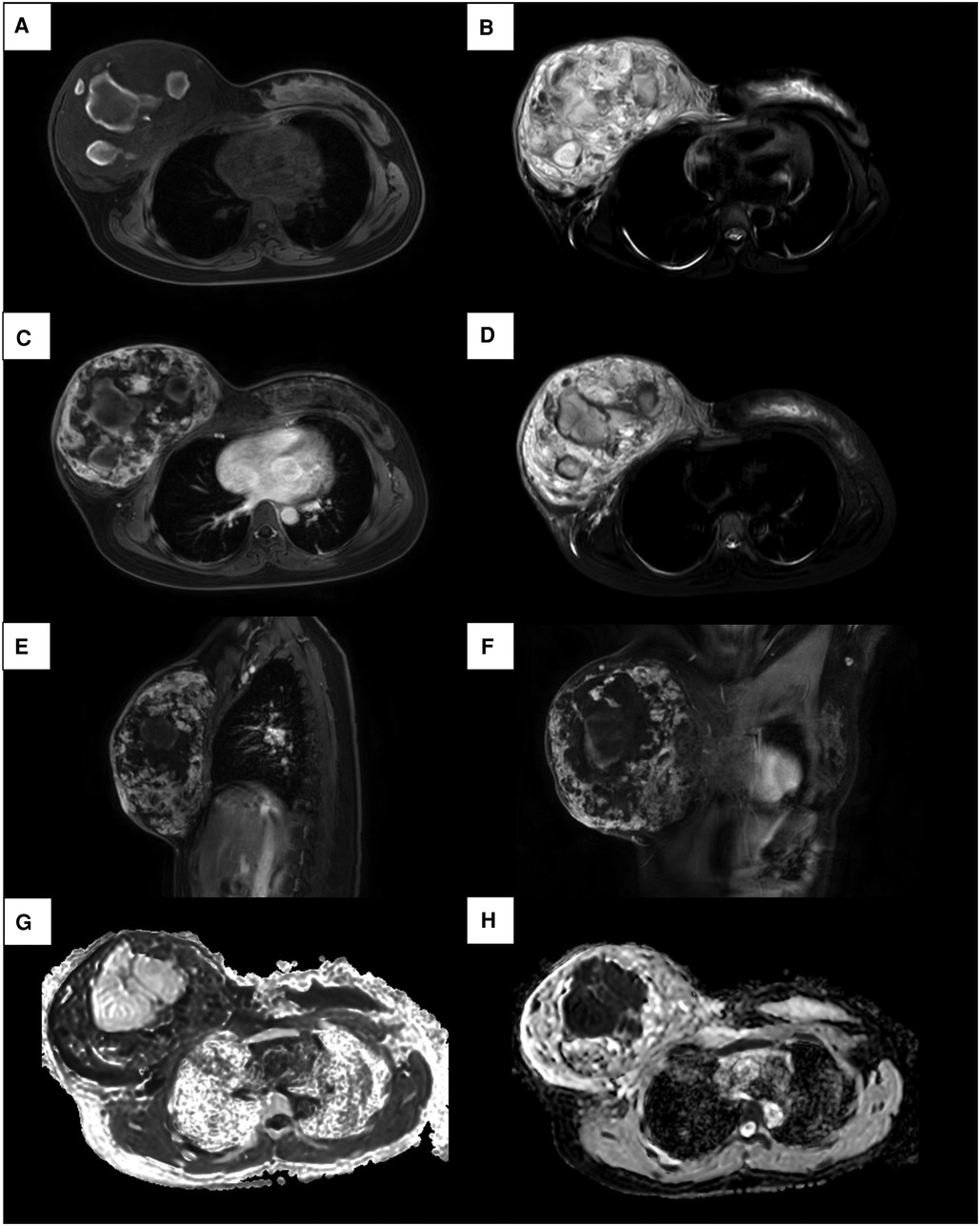
Figure 2. MRI images of a 17-year-old female patient with right primary breast angiosarcoma. (A) MRI images show a mixed signal intensity area predominantly composed of isointense mass on T1. (B) Mixed signal intensity area predominantly of high signal intensity on T2. (C, E, F) Enhancement sequences image showed irregular heterogeneous high signal intensity. (D) On fat-suppressed T2-weighted images, the lesion shows bright (high) signal intensity. (G) High signal intensity on DWI and (H) low signal intensity on ADC images demonstrate restricted diffusion.
Thereafter, a right breast ultrasound-guided needle biopsy was performed. Vascular channels were lined by atypical, plump endothelial cells with hemorrhage and focal mitotic activity. Morphology suggested the possibility of angiosarcoma. Further immunohistochemistry demonstrated positive staining for vascular endothelial markers CD34, CD31, FLI-1, and erythroblast transformation-specific (ETS)-related gene (ERG) oncoprotein but not for cytokeratin (Figures 3A, B, E, F). The proliferation index (Ki-67) was estimated at 40%. So, a diagnosis of primary angiosarcoma of the breast (pT4N0M0) was made.
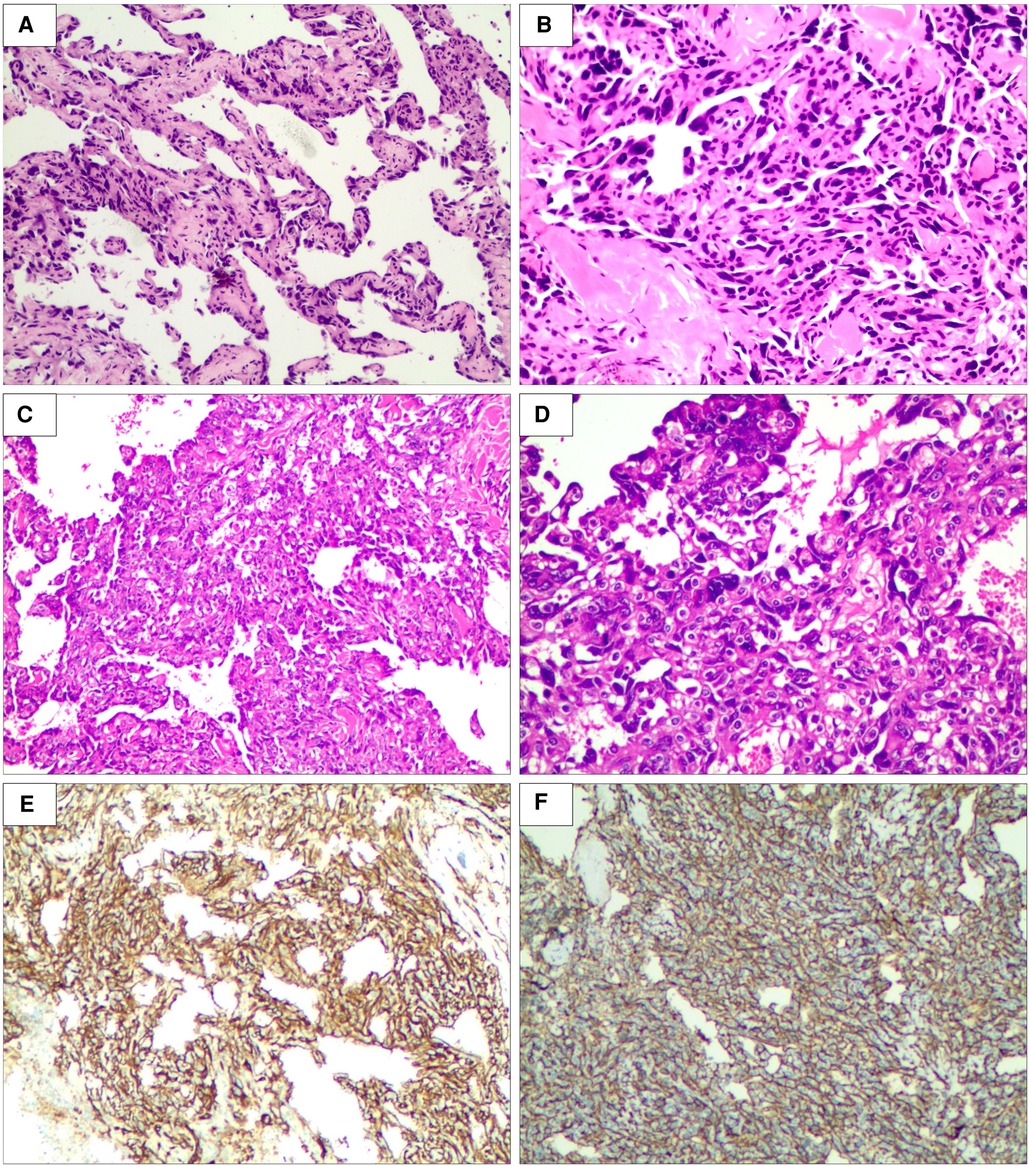
Figure 3. Biopsy of right breast lesion microphotography. The tumor is composed of atypical endothelial cells with different degrees of differentiation, forming irregular vascular cavities that coincide with each other, forming papillary and diffuse infiltration (×200, (A) punch biopsy and (C) excisional biopsy). Tumor cells exhibit fusiform or irregular shapes with little cytoplasm and hyperchromatic nuclei (×400, (B) punch biopsy and (D) excisional biopsy). Special stain CD31 positive (E). Special stain CD34 positive (F) (hematein–eosin staining).
The patient had coagulation dysfunction for tumor-related excessive bleeding, decreased fibrinogen and platelet count, prolonged prothrombin time, and elevated D-dimer levels. She received red cell concentrates, cryoprecipitate, fibrinogen, platelets, and fresh-frozen plasma, but the anemia and coagulation did not improve significantly. A decision to undertake angiography (DSA) and tumor vascular embolization was taken to reduce the risk of bleeding and allow safe surgical resection (Figure 4).
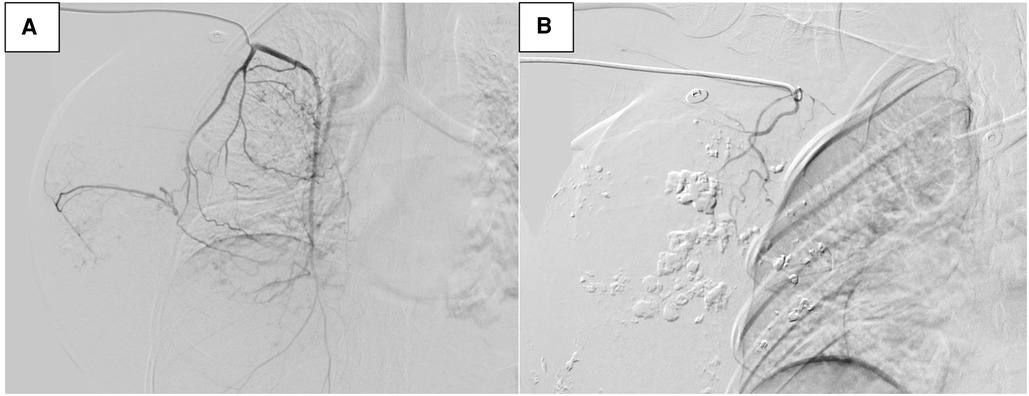
Figure 4. Digital subtraction angiography (DSA) image of tumor vascularization before embolization (A). DSA after embolization (B).
After the risks associated with the surgery were explained to the patient and her family, she accepted them and wanted to be operated on as soon as possible. She underwent a right total mastectomy in March 2021, and all surgical margins were negative. Postoperative pathological examination revealed angiosarcoma with tissue necrosis, hemorrhage and 19.5 × 15.3 × 6.6 cm in size (Figure 5). Histopathologic examination showed the tumor was composed of atypical endothelial cells with different degrees of differentiation, forming irregular vascular cavities that coincide with each other, forming papillary and diffuse infiltration. Tumor cells exhibit fusiform or irregular shapes with little cytoplasm and hyperchromatic nuclei (Figures 3C, D).
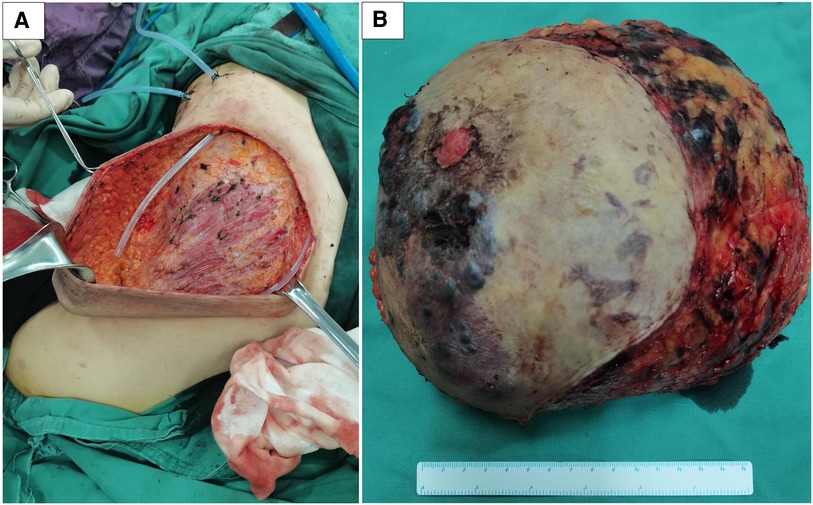
Figure 5. The patient was operated on successfully with less amount of blood loss (A). In the mastectomy specimen section of the right breast, the tumor was ill-defined, with a focal area of necrosis and hemorrhage (B).
After consultation with the patient and her family, she received planned radiotherapy and chemotherapy. The patient received Adriamycin-based chemotherapy every 3 weeks for eight cycles. Medical oncology planned a 50 Gy intensity-modulated radiation therapy to the chest wall (2 Gy per fraction over 5 weeks), but she was unable to complete the radiation therapy due to her poor physical condition.
The patient presented with a 3 cm × 1.5 cm bleeding right chest wall mass 5 months after removal. She was treated with surgical resection with a diagnosis of local tumor recurrence by histological examination. She presented again with a chest wall tumor that was bleeding profusely 1 month after surgery for a recurrent tumor and was admitted to the emergency department. A chest and abdominal CT scan with contrast showed multiple metastatic lesions in the chest, bone, liver, spleen, and lung. She was anemic despite the transfusion. The patient's condition has not yet improved. She left the hospital arbitrarily.
Discussion
Angiosarcoma is an extremely rare and highly malignant mesenchymal vasoformative neoplasm, characterized by rapidly proliferating and extensively infiltrating growth (4), most commonly identified in the skin of the head, neck, and scalp; the breast is an exceedingly rare primary site of occurrence (5). It occurs especially in women 30–40 years of age. The median survival time and 5-year recurrence-free survival rate are 24 months (about 2 years) and 33%, respectively (6). Currently, for the PBA, the underlying cause remains unknown. It represents about 0.05% of all malignant breast tumors (7). Primary lesions affect younger patients, and the median age is 30–50 years (8). Patients usually present with an associated palpable mass in the breast. The prognosis of patients diagnosed with PBA depends on the grade of the tumor. In patients with grade 1 tumors, the corresponding 5-year disease-free survival after the initial treatment is approximately 76%, whereas the probability drops to 15% for grade 3 tumors (9). In comparison, secondary breast angiosarcoma (SBA) is identified as chronic lymphedema resulting from axillary dissection (Stewart-Treves syndrome) or radiation-associated sarcoma (10). Radiotherapy used to treat invasive breast tumors is a well-known risk factor for the development of the so-called radiation-induced angiosarcoma (RIAS). RIAS is a late toxicity that occurs in 0.05%–0.3% of breast cancer patients who undergo breast-conserving surgery and adjuvant radiotherapy. It usually occurs 6–10 years after breast irradiation (11), but RIAS can occur as early as 1–2 years or as late as 41 years after radiation (12). SBA is relatively common in older women, as the median age is 70 years (13). About 5-year survival rates remain low, at 22.5% for secondary angiosarcoma (7). Because the clinical and radiologic findings are not specific (14), the definitive diagnosis can only be achieved by histopathological examination.
PBA originates from breast parenchyma and occasionally affects the skin (15), while SBA often involves the skin, and rarely the breast parenchyma. PBA has similar histologic and morphologic features to SBA. Tumor size ranges between 10 and 160 mm (mean: 59 mm) for post-radiation angiosarcomas and 25–150 mm (mean: 76 mm) for PBA. The pathology of angiosarcoma shows grade 1 (low grade) angiosarcoma with inter-anastomosing vascular channels, subtle endothelial atypia and relatively few mitotic figures. Grade 2 (intermediate grade) tumor cells show moderate nuclear atypia and multilayering of endothelial cells. Grade 3 (high-grade) tumors present with marked nuclear polymorphism, numerous mitoses, and necrosis (16). Most RIAS are high-grade lesions with irregular anastomosing vessels lined by endothelial cells showing nuclear atypia (17). The tumor cells are usually positive for vascular markers (e.g., CD31, CD34, factor VIII-related antigen, FLI1, Ulex europaeus 1 lectin, and ERG) (18). The histological and pathological features are similar between primary and post-radiation angiosarcomas. Therefore, they could not be differentiated on pathological examination (19). Currently, the pathogenesis of angiosarcoma is not completely known.
Several physiopathological mechanisms have been proposed to explain the development of RIAS. Radiotherapy uses ionizing radiation, which either directly affects DNA structure by inducing DNA strand breaks, particularly double strand breaks, or indirectly by generating reactive oxygen species (ROS) that oxidize proteins and lipids and thereby induce additional damage to DNA, like the generation of abasic sites and strand breaks (20). Genome instability and cancer-related gene mutations may drive tumorigenesis (21).
Although radiation-associated angiosarcoma presents a distinct clinical pattern, the difference between radiation-induced vs. sporadic lesions remain unverified. The establishment of genetic differences between sporadic and radiation-induced angiosarcomas will facilitate discrimination between these two entities (22). As of RIAS, several gene mutations have been reported in the literature. All molecular studies carried out on radiation-induced angiosarcomas present amplification of chromosome 8q24 mapping, Myc oncogene inactivation, and the expression of the p53 gene (23).
Oncogenes of the Myc family, including c-Myc, N-Myc, and L-Myc, are master regulators of cell growth, maturation, and death, because of their transcriptional repression function. The fundamental pathogenetic differences between PBA and RIAS are impossible to differentiate morphologically. Some studies have found that Myc amplification frequently presents in secondary angiosarcoma (radiation, chronic lymphedema) and less frequently in primary angiosarcoma, suggesting that it may be utilized to distinguish RIAS of the breast and PBA (17, 19, 24–26). However, Myc overexpression could also be seen in primary angiosarcoma (27, 28).
The morphological profile of atypical vascular lesions (AVL) showing progression to angiosarcoma has not yet been clearly defined. A study identified some tumor protein p53 (TP53) variation from radiotherapy-induced ipsilateral breast carcinoma in 10 out of 12 (83.3%) cases of AVL and in 7 out of 8 (87.5%) cases of angiosarcoma. At the protein level, the elevated expression of p53 and MDM-2 proteins has been found to associate with the increased vascular endothelial growth factor (VEGF) expression that is found in nearly 80% of angiosarcoma. The genetic alterations of the TP53 gene suggest that its mutational inactivation may be implicated in the pathogenesis of vascular proliferations associated with radiotherapy (29, 30).
The VEGF family and VEGF receptors (VEGFR) that control angiogenesis are found to be frequently altered in angiosarcoma. The genetic mutation VEGFR-2 (kinase insert domain receptor = KDR) or amplification of VEGFR-3 (FMS-like tyrosine kinase 4 = FLT4) have been proposed to have a crucial role in the development of angiosarcoma (31, 32). Mutations in KDR and TP53 were mutually exclusive (P = .02, Fisher's exact test), with 8 out of 9 (89%) KDR missense mutations displayed in PBA samples and 9 out of 11 (82%) TP53 missense mutations observed in angiosarcoma samples that were not PBA (33). However, Guo et al. found that the gene amplification of the FLT4 gene encoding the VEGFR3 was found in 25% of secondary angiosarcoma and only associated with Myc amplification. Based on those results, they suggest that FLT4 overexpression may represent a “second hit” in the progression of secondary angiosarcoma and raise a note-worthy possibility of targeting FLT4 as a potential therapeutic option (26). Although activating VEGFR-2 mutations are relatively rare in angiosarcoma, VEGFR-2 is universally overexpressed in angiosarcoma (17, 34, 35). Itakura et al. showed a lower percentage of VEGFR-2 expression was significantly associated with poorer overall survival (31). Uncontrolled VEGF/VEGFR signaling leads to dysregulated angiogenic activity, however, the exact mechanism in angiosarcoma remains to be elucidated.
Previously published case reports have shown that radiation-induced angiosarcoma contained breast cancer-related tumor-suppressor gene BRCA1/BRCA2 mutations (11, 36–38). The defective DNA repair mechanism may also theoretically increase radiosensitivity, increasing susceptibility to carcinogenic effects in surviving cells (39, 40).
The PIK3CA (phosphatidylinositol-4,5-bisphosphate 3-kinase catalytic subunit alpha) gene coding for the p110alpha catalytic subunit of class 1A phosphoinositide-3-kinase (PI3K) is frequently mutated in breast cancer (41). Intriguingly, a study showed that 6 out of the 10 PIK3CA alterations were found in PBA samples (32). In addition, another study showed that 9 out of the 10 PIK3CA alterations were found in PBA samples, whereas PIK3CA mutations were significantly enriched in the angiosarcoma subtype compared with other subtypes (9 out of 18 PBA samples vs. 1 out of 29 angiosarcoma samples that were not PBA; P = .0003, Fisher's exact test). DNA methylation may serve as a marker of breast tumor cell lineage restriction, thereby reflecting the cell type from which cancer originates and, perhaps, explaining the correlations between the histological heterogeneity and prognosis of breast cancers with their DNA methylation profiles. Each type of activating PI3K mutation is derived from a different lineage of breast malignancies, indicating the site the tumor had originated independently of tumor lineage. It may play an important permissive role in PI3K pathway activation and may provide an interaction with the breast microenvironment conducive to new tumor formation. Of clinical importance, these findings suggest that targeting PI3Kα via inhibitors may be useful as a novel therapeutic intervention for patients with PBA (33).
Wei et al. (42) explored the mechanisms of primary and secondary breast angiosarcoma for the discovery of new biomarkers and research into potential therapeutic targets. The study identified 18 differentially expressed genes (DEGs) enriched in the transforming growth factor-β (TGF-β), Wnt, Hippo, and PI3K-Akt signaling pathways. It is possible that genomic testing will help differentiate between the two clinical entities and lay the foundation for the discovery of effective and reliable molecular biomarkers and essential therapeutic targets in the future.
In addition, several other genetic alterations, including POT1, RAS, BRAF, PTPRB, PLCG1, ATM, MSH6, and APC, may be associated with angiosarcoma, although further research is required (43, 44). Table 1 includes clinical, demographic, and genetic variation data for SBA and PBA.

Table 1. Summary of clinical features and genetic variations in various series of patients with breast angiosarcoma.
Our patient, who presented with PBA, is in agreement with a literature review involving younger premenopausal females with no previous history of cancer. The tumor was characterized by rapid growth and bleeding. It was persistent bleeding and hemostasis was difficult to achieve. Ongoing bleeding led to not only coagulation factors, and fibrinogen consumption but also coagulation disorders. Nevertheless, the source of the bleeding in this case could not be identified due to an unclear diagnosis. Furthermore, the clinical and radiologic findings were not specific.
It is frequently difficult to accurately diagnosis a breast lesion as breast angiosarcoma. Differential diagnosis should be made considering both radiological, and histopathology, and immunohistochemistry findings. Differential diagnosis includes benign hemangioma, stromal sarcoma, cystosarcoma phyllodes, metaplastic carcinoma, reactive spindle cell proliferative lesions, fibrosarcoma, myoepithelioma, fibromatosis, liposarcoma, and squamous cell carcinoma with sarcomatoid features (64). This case was initially accepted as a hemangioma. The puncture may result in tumor rupture or great vessel injury, causing hemorrhage and shock. Although a needle biopsy might further aggravate the bleeding risk and can sometimes fail to give definite results, a biopsy is considered the gold standard and an indispensable means of confirming a diagnosis. We performed a biopsy, histological, and immunohistochemical analysis, which revealed the diagnosis of PBA.
Abnormalities in coagulation function make surgery difficult due to the substantial risk of bleeding. Therefore, DSA and tumor vascular embolization were used to reduce the risk of bleeding and allow for safe surgical resection. This treatment successfully stopped tumor bleeding. The assurance of gross tumor resection with tumor-negative resection margins is the primary and preferred treatment modality for localized disease. Lymphatic metastasis is relatively rare, and therefore, axillary node dissection remains controversial in the absence of positive nodes (65). Chemotherapy may help to achieve an improved prognosis in disease control and survival and further reduce the local recurrence rate (9), but the role remains controversial at present (7). A recent study has shown that cytotoxic chemotherapies, in particular anthracycline-based regimens and taxanes can produce significant responses to therapy in a subset of patients (66). Adjuvant radiotherapy could be used to reduce the incidence of locoregional recurrence (67). However, the disease is usually resistant to currently available chemotherapy and radiotherapy, which may not alter the poor prognosis. Sher et al. reported that there was no significant survival difference between patients who had and had not received anthracyclines, taxanes, gemcitabine, and ifosfamide as adjuvant chemotherapy (68). Due to the high-level expression of VEGF in angiosarcoma, therapies targeting VEGF are hopeful to improve prognosis, however, this issue warrants being proven in a properly designed prospective study (69).
Breast angiosarcoma shows hematogenous spread like other sarcomas rather than via lymphogenous route. The most common site of recurrence is local-regional. Distant metastases are frequently observed at an early stage. Breast angiosarcoma has been reported to metastasize usually to the liver, bone, lung, skin, central nervous system, spleen, and subcutaneous soft tissues (65, 68). Unlike other sarcomas and breast cancer, lymph node metastasis is exceedingly rare (70). In this case, chest, bone, liver, spleen, and lung metastases were observed.
Given the rarity of PBA, especially with severe bleeding, and the poor prognosis following the identification, knowledge of this lesion prompts further assessment for diagnosis, therapy, and prognosis is crucial when evaluating a patient. The objective and unified criteria for the diagnosis, staging and treatment are still lacking.
Conclusion
We herein present a case of PBA with severe bleeding in a 17-year-old woman. The definitive diagnosis of PBA is usually difficult, and histopathological examination is the standard approach at present. Mastectomy remains the most favored therapy. Tumor vascular embolization can reduce the risk of bleeding. The role and importance of chemotherapy and radiotherapy in the treatment remains unclear. Furthermore, genetics and genomics will remain powerful approaches to understanding and treating diseases. Therapies targeting VEGFR are hopeful for improving prognosis, but the therapeutic effect still needs further exploration and verification.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author/s.
Ethics statement
Ethical review and approval were not required for the study on human participants in accordance with the local legislation and institutional requirements. Written informed consent from the patient's legal guardian/next of kin was not required to participate in this study in accordance with the national legislation and the institutional requirements. Written informed consent was obtained from the individual for the publication of any potentially identifiable images or data included in this article.
Author contributions
YW, LQ, LC, and YH were the team responsible for the surgical treatment of this patient. YH and PC were responsible for the research idea, study design. The first draft of the manuscript was written by YH. All authors contributed to the article and approved the submitted version.
Funding
This work was supported by the New Xiangya Talent Project of the Third Xiangya Hospital of Central South University (Grant number: 20180301).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Friedrich A, Reisenbichler E, Heller D, LeBlanc J, Park T, Killelea B, et al. Characteristics and long-term risk of breast angiosarcoma. Ann Surg Oncol. (2021) 28(9):5112–8. doi: 10.1245/s10434-021-09689-2
2. Yang O, Lan T, He J, Xu H, Hao L, Shu C, et al. Magnetic resonance imaging and contrast-enhanced ultrasound findings of a recurrent primary breast angiosarcoma: a case report. Medicine (Baltimore). (2021) 100(5):e24625. doi: 10.1097/MD.0000000000024625
3. Abdou Y, Elkhanany A, Attwood K, Ji W, Takabe K, Opyrchal M. Primary and secondary breast angiosarcoma: single center report and a meta-analysis. Breast Cancer Res Treat. (2019) 178(3):523–33. doi: 10.1007/s10549-019-05432-4
4. Yin M, Wang W, Drabick J, Harold H. Prognosis and treatment of non-metastatic primary and secondary breast angiosarcoma: a comparative study. BMC Cancer. (2017) 17(1):295. doi: 10.1186/s12885-017-3292-7
5. Sasahara A, Tanabe M, Hayashi K, Konishi T, Oya M, Sakiyama K, et al. A case of primary breast angiosarcoma with multiple discontinuous small lesions. Surg Case Rep. (2019) 5(1):157. doi: 10.1186/s40792-019-0704-8
6. Mouhoub M, Miry A, Haloui A, Karich N, Kamaoui I, Benkirane S, et al. Primary angiosarcoma of the breast: a case report. Pan Afr Med J. (2019) 33(134):17414. doi: 10.11604/pamj.2019.33.134.17414
7. Gutkin P, Ganjoo K, Lohman M, von Eyben R, Charville G, Nazerali R, et al. Angiosarcoma of the breast: management and outcomes. Am J Clin Oncol. (2020) 43(11):820–5. doi: 10.1097/COC.0000000000000753
8. O'Donnell J, Sugrue R, McLaughlin R, McInerney N. Multidisciplinary approach to chest wall reconstruction in primary breast angiosarcoma resection. BMJ Case Rep. (2020) 13(5):e233156. doi: 10.1136/bcr-2019-233156
9. Abdelhady A, Neamaalla S, Gittens A, Germaine P. Primary angiosarcoma of the breast: case report of a rare vascular tumor. Radiol Case Rep. (2020) 15(4):339–43. doi: 10.1016/j.radcr.2019.12.015
10. Ishizuka Y, Horimoto Y, Onagi H, Arakawa A, Saito M. Microsatellite-stable radiation-induced angiosarcoma after breast-conserving surgery: a case report. Case Rep Oncol. (2020) 13(3):1275–80. doi: 10.1159/000510809
11. Cozzi S, Najafi M, Bardoscia L, Ruggieri M, Giaccherini L, Blandino G, et al. Radiation-induced breast angiosarcoma: report of two patients after accelerated partial breast irradiation (APBI) and review of the literature. Rep Pract Oncol Radiother. (2021) 26(5):827–32. doi: 10.5603/RPOR.a2021.0080
12. Glazebrook K, Magut M, Reynolds C. Angiosarcoma of the breast. AJR Am J Roentgenol. (2008) 190(2):533–8. doi: 10.2214/AJR.07.2909
13. Tomich J, Grove Nigro K, Barr R. Primary angiosarcoma of the breast: a case report and review of the literature. Ultrasound Q. (2017) 33(1):46–8. doi: 10.1097/RUQ.0000000000000274
14. Wang L, Lao I, Yu L, Yang W, Wang J. Primary breast angiosarcoma: a retrospective study of 36 cases from a single Chinese medical institute with clinicopathologic and radiologic correlations. Breast J. (2017) 23(3):282–91. doi: 10.1111/tbj.12731
15. Iacoponi S, Calleja J, Hernandez G, de la Cuesta R S. Primary breast angiosarcoma in a young woman. Int J Surg Case Rep. (2016) 24:101–3. doi: 10.1016/j.ijscr.2016.05.015
16. Kim Y, Kim Y, Yim K, Park W. A case report of primary breast angiosarcoma with fatal pulmonary hemorrhage due to thrombocytopenia. J Korean Surg Soc. (2012) 82(4):251–5. doi: 10.4174/jkss.2012.82.4.251
17. Bonito F, de Almeida Cerejeira D, Dahlstedt-Ferreira C, Oliveira Coelho H, Rosas R. Radiation-induced angiosarcoma of the breast: a review. Breast J. (2020) 26(3):458–63. doi: 10.1111/tbj.13504
18. Esposito E, Avino F, di Giacomo R, Donzelli I, Marone U, Melucci M, et al. Angiosarcoma of the breast, the unknown – a review of the current literature. Transl Cancer Res. (2019) 8:S510–17. doi: 10.21037/tcr.2019.07.38
19. Laé M, Lebel A, Hamel-Viard F, Asselain B, Trassard M, Sastre X, et al. Can c-MYC amplification reliably discriminate postradiation from primary angiosarcoma of the breast? Cancer Radiother. (2015) 19(3):168–74. doi: 10.1016/j.canrad.2015.01.001
20. Wang Q, Xie C, Xi S, Qian F, Peng X, Huang J, et al. Radioprotective effect of flavonoids on ionizing radiation-induced brain damage. Molecules. (2020) 25(23):5719. doi: 10.3390/molecules25235719
21. Javed N, Stowman AM. Educational case: radiation-induced angiosarcoma of the breast. Acad Pathol. (2021) 8:23742895211060529. doi: 10.1177/23742895211060529
22. Torres K, Ravi V, Kin K, Yi M, Guadagnolo B, May C, et al. Long-term outcomes in patients with radiation-associated angiosarcomas of the breast following surgery and radiotherapy for breast cancer. Ann Surg Oncol. (2013) 20(4):1267–74. doi: 10.1245/s10434-012-2755-y
23. Tato-Varela S, Albalat-Fernández R, Pabón-Fernández S, Núñez-García D, Calle-Marcos M. Radiation-induced breast angiosarcoma: a case report. ecancermedicalscience. (2016) 10:697. doi: 10.3332/ecancer.2016.697
24. Retter E, Benn C, Maske C, Rapoport B. A case report of post-radiotherapy c-MYC-positive angiosarcoma of the breast. Case Rep Oncol. (2022) 15(1):62–70. doi: 10.1159/000521069
25. Manner J, Radlwimmer B, Hohenberger P, Mössinger K, Küffer S, Sauer C, et al. MYC high level gene amplification is a distinctive feature of angiosarcomas after irradiation or chronic lymphedema. Am J Pathol. (2010) 176(1):34–9. doi: 10.2353/ajpath.2010.090637
26. Guo T, Zhang L, Chang N, Singer S, Maki R, Antonescu C. Consistent MYC and FLT4 gene amplification in radiation-induced angiosarcoma but not in other radiation-associated atypical vascular lesions. Genes, Chromosomes Cancer. (2011) 50(1):25–33. doi: 10.1002/gcc.20827
27. Shon W, Sukov W, Jenkins S, Folpe A. MYC Amplification and overexpression in primary cutaneous angiosarcoma: a fluorescence in-situ hybridization and immunohistochemical study. Mod Pathol. (2014) 27(4):509–15. doi: 10.1038/modpathol.2013.163
28. Requena C, Rubio L, Lavernia J, Machado I, Llombart B, Sanmartín O, et al. Immunohistochemical and fluorescence in situ hybridization analysis of MYC in a series of 17 cutaneous angiosarcomas: a single-center study. Am J Dermatopathol. (2018) 40(5):349–54. doi: 10.1097/DAD.0000000000001053
29. Santi R, Cetica V, Franchi A, Pepi M, Cesinaro A, Miracco C, et al. Tumour suppressor gene TP53 mutations in atypical vascular lesions of breast skin following radiotherapy. Histopathology. (2011) 58(3):455–66. doi: 10.1111/j.1365-2559.2011.03770.x
30. Zietz C, Rössle M, Haas C, Sendelhofert A, Hirschmann A, Stürzl M, et al. MDM-2 oncoprotein overexpression, p53 gene mutation, and VEGF up-regulation in angiosarcomas. Am J Pathol. (1998) 153(5):1425–33. doi: 10.1016/S0002-9440(10)65729-X
31. Itakura E, Yamamoto H, Oda Y, Tsuneyoshi M. Detection and characterization of vascular endothelial growth factors and their receptors in a series of angiosarcomas. J Surg Oncol. (2008) 97(1):74–81. doi: 10.1002/jso.20766
32. Beca F, Krings G, Chen Y, Hosfield E, Vohra P, Sibley R, et al. Primary mammary angiosarcomas harbor frequent mutations in KDR and PIK3CA and show evidence of distinct pathogenesis. Mod Pathol. (2020) 33(8):1518–26. doi: 10.1038/s41379-020-0511-6
33. Painter C, Jain E, Tomson B, Dunphy M, Stoddard R, Thomas B, et al. The angiosarcoma project: enabling genomic and clinical discoveries in a rare cancer through patient-partnered research. Nat Med. (2020) 26(2):181–7. doi: 10.1038/s41591-019-0749-z
34. Antonescu C, Yoshida A, Guo T, Chang N, Zhang L, Agaram N, et al. KDR Activating mutations in human angiosarcomas are sensitive to specific kinase inhibitors. Cancer Res. (2009) 69(18):7175–9. doi: 10.1158/0008-5472.CAN-09-2068
35. Huang S, Zhang L, Sung Y, Chen C, Kao Y, Agaram N, et al. Recurrent CIC gene abnormalities in angiosarcomas: a molecular study of 120 cases with concurrent investigation of PLCG1, KDR, MYC, and FLT4 gene alterations. Am J Surg Pathol. (2016) 40(5):645–55. doi: 10.1097/PAS.0000000000000582
36. West J, Weitzel J, Tao M, Carpenter M, West J, Fanning C. BRCA mutations and the risk of angiosarcoma after breast cancer treatment. Clin Breast Cancer. (2008) 8(6):533–7. doi: 10.3816/CBC.2008.n.066
37. Parvez E, Popovic S, Elavathil L, Okawara G, Hodgson N. Early occurrence of angiosarcoma in a woman with a BRCA2 gene variation of unknown significance treated with breast-conserving therapy for bilateral ductal carcinoma: a case report. Clin Breast Cancer. (2015) 15(6):536–8. doi: 10.1016/j.clbc.2015.06.011
38. Kadouri L, Sagi M, Goldberg Y, Lerer I, Hamburger T, Peretz T. Genetic predisposition to radiation induced sarcoma: possible role for BRCA and p53 mutations. Breast Cancer Res Treat. (2013) 140(1):207–11. doi: 10.1007/s10549-013-2621-z
39. Sheth G, Cranmer L, Smith B, Grasso-Lebeau L, Lang J. Radiation-induced sarcoma of the breast: a systematic review. Oncologist. (2012) 17(3):405–18. doi: 10.1634/theoncologist.2011-0282
40. Shah S, Rosa M. Radiation-associated angiosarcoma of the breast: clinical and pathologic features. Arch Pathol Lab Med. (2016) 140(5):477–81. doi: 10.5858/arpa.2014-0581-RS
41. Corné J, Le Du F, Quillien V, Godey F, Robert L, Bourien H, et al. Development of multiplex digital PCR assays for the detection of PIK3CA mutations in the plasma of metastatic breast cancer patients. Sci Rep. (2021) 11(1):17316. doi: 10.1038/s41598-021-96644-6
42. Wei Y, Yang X, Gao L, Xu Y, Yi C. Differences in potential key genes and pathways between primary and radiation-associated angiosarcoma of the breast. Transl Oncol. (2022) 19:101385. doi: 10.1016/j.tranon.2022.101385
43. Espejo-Freire AP, Elliott A, Rosenberg A, Costa PA, Barreto-Coelho P, Jonczak E, et al. Genomic landscape of angiosarcoma: a targeted and immunotherapy biomarker analysis. Cancers (Basel). (2021) 13(19):4816. doi: 10.3390/cancers13194816
44. Thibodeau BJ, Lavergne V, Dekhne N, Benitez P, Amin M, Ahmed S, et al. Mutational landscape of radiation-associated angiosarcoma of the breast. Oncotarget. (2018) 9(11):10042–53. doi: 10.18632/oncotarget.24273
45. Chang GV, Xie C, Shahid M, Draine J, Alpers J, Reindl B, et al. Bilateral breast radiation associated angiosarcoma after radiotherapy for bilateral invasive ductal adenocarcinoma. S D Med. (2021) 74(6):260–3. PMID: 3444916534449165
46. Sheu T, Hunt K, Middleton L. MYC and NOTCH1-positive postradiation cutaneous angiosarcoma of the breast. Breast J. (2021) 27(3):264–7. doi: 10.1111/tbj.14171
47. Oliveira L, Pádua Filho A, Medeiros E Melo M, Galvão E, Vieira M, Ibiapina J, et al. Radiation-induced angiosarcoma: case report. Einstein (Sao Paulo, Brazil). (2020) 18:eRC5439. doi: 10.31744/einstein_journal/2020RC5439
48. Webb C, Partain N, Koduru P, Hwang H, Sarode V. Secondary angiosarcoma with C-MYC amplification following prophylactic bilateral mastectomy and autologous breast reconstruction: report of a case and review of the literature. Int J Surg Pathol. (2021) 29(2):205–10. doi: 10.1177/1066896920930100
49. Shiraki E, Kang Y, Shibayama T, Tsuyuki S. Two cases of breast angiosarcoma after breast conserving surgery. Surg Case Rep. (2020) 6(1):81. doi: 10.1186/s40792-020-00841-w
50. Manjee K, Sullivan M. Educational case: radiation-associated angiosarcoma in patients with breast cancer. Acad Pathol. (2020) 7:2374289520912499. doi: 10.1177/2374289520912499
51. Mentzel T, Kiss K. Reduced H3K27me3 expression in radiation-associated angiosarcoma of the breast. Virchows Arch. (2018) 472(3):361–8. doi: 10.1007/s00428-017-2242-8
52. Daniels B, Ko J, Rowe J, Downs-Kelly E, Billings S. Radiation-associated angiosarcoma in the setting of breast cancer mimicking radiation dermatitis: a diagnostic pitfall. J Cutan Pathol. (2017) 44(5):456–61. doi: 10.1111/cup.12917
53. Tidwell W, Haq J, Kozlowski K, Googe P. C-MYC positive angiosarcoma of skin and breast following MammoSite® treatment. Dermatol Online J. (2015) 21(10):13030/qt4t65q9nc. doi: 10.5070/D32110028950
54. Cornejo K, Deng A, Wu H, Cosar E, Khan A, St Cyr M, et al. The utility of MYC and FLT4 in the diagnosis and treatment of postradiation atypical vascular lesion and angiosarcoma of the breast. Hum Pathol. (2015) 46(6):868–75. doi: 10.1016/j.humpath.2015.02.014
55. Silva E, Gatalica Z, Vranic S, Basu G, Reddy S, Voss A. Refractory angiosarcoma of the breast with VEGFR2 upregulation successfully treated with sunitinib. Breast J. (2015) 21(2):205–7. doi: 10.1111/tbj.12380
56. Barbosa O, Reiriz A, Boff R, Oliveira W, Rossi L. Angiosarcoma in previously irradiated breast in patient with Li-Fraumeni syndrome. A case report. Sao Paulo Med J. (2015) 133(2):151–3. doi: 10.1590/1516-3180.2012.6740004
57. Tajima S, Mochizuki R, Sugimura H, Hoshi S. Radiation-induced breast angiosarcoma with a confirmative feature of c-MYC amplification. Jpn J Clin Oncol. (2014) 44(7):702–3. doi: 10.1093/jjco/hyu064
58. Azzariti A, Porcelli L, Mangia A, Saponaro C, Quatrale A, Popescu O, et al. Irradiation-induced angiosarcoma and anti-angiogenic therapy: a therapeutic hope? Exp Cell Res. (2014) 321(2):240–7. doi: 10.1016/j.yexcr.2013.12.018
59. Fernandez A, Sun Y, Tubbs R, Goldblum J, Billings S. FISH for MYC amplification and anti-MYC immunohistochemistry: useful diagnostic tools in the assessment of secondary angiosarcoma and atypical vascular proliferations. J Cutan Pathol. (2012) 39(2):234–42. doi: 10.1111/j.1600-0560.2011.01843.x
60. Mentzel T, Schildhaus H, Palmedo G, Büttner R, Kutzner H. Postradiation cutaneous angiosarcoma after treatment of breast carcinoma is characterized by MYC amplification in contrast to atypical vascular lesions after radiotherapy and control cases: clinicopathological, immunohistochemical and molecular analysis of 66 cases. Mod Pathol. (2012) 25(1):75–85. doi: 10.1038/modpathol.2011.134
61. Teruyama F, Kuno A, Murata Y, Nakagawa T, Shiba-Ishii A, Yuguchi S, et al. Mutational landscape of primary breast angiosarcoma with repeated resection and recurrence over a 15-year period: a case report. Pathol Int. (2022) 72(9):457–63. doi: 10.1111/pin.13257
62. Al-Salam S, Balalaa N, Faour I, Akhter S, Alashari M. HIF-1α, VEGF and WT-1 are protagonists in bilateral primary angiosarcoma of breast: a case report and review of literature. Int J Clin Exp Pathol. (2012) 5(3):247–53. PMID: 22558480; PMCID: PMC334168522558480
63. Fraga-Guedes C, André S, Mastropasqua M, Botteri E, Toesca A, Rocha R, et al. Angiosarcoma and atypical vascular lesions of the breast: diagnostic and prognostic role of MYC gene amplification and protein expression. Breast Cancer Res Treat. (2015) 151(1):131–40. doi: 10.1007/s10549-015-3379-2
64. Ohta M, Tokuda Y, Kuge S, Okumura A, Tanaka M, Kubota M, et al. A case of angiosarcoma of the breast. Jpn J Clin Oncol. (1997) 27(2):91–4. doi: 10.1093/jjco/27.2.91
65. Varghese B, Deshpande P, Dixit S, Koppiker CB, Jalnapurkar N. Primary angiosarcoma of the breast: a case report. J Radiol Case Rep. (2019) 13(2):15–25. doi: 10.3941/jrcr.v13i2.3449
66. Florou V, Wilky B. Current and future directions for angiosarcoma therapy. Curr Treat Options Oncol. (2018) 19(3):14. doi: 10.1007/s11864-018-0531-3
67. Altmışdörtoğlu Ö, Gökgöz M, Yalçınkaya U, Nalca Andrieu M. A case report of primary breast angiosarcoma: clinical presentation and outcome after adjuvant radiotherapy. Eur J Breast Health. (2020) 16(4):290–4. doi: 10.5152/ejbh.2020.4984
68. Sher T, Hennessy BT, Valero V, Broglio K, Woodward WA, Trent J, et al. Primary angiosarcomas of the breast. Cancer. (2007) 110(1):173–8. doi: 10.1002/cncr.22784
69. Cao J, Wang J, He C, Fang M. Angiosarcoma: a review of diagnosis and current treatment. Am J Cancer Res. (2019) 9(11):2303–13. PMID: 31815036; PMCID: PMC689545131815036
Keywords: breast angiosarcoma, chemotherapy, radiotherapy, surgery, DSA (digital subtraction angiography), case report
Citation: He Y, Qian L, Chen L, Liu Y, Wen Y and Cao P (2023) Primary breast angiosarcoma: A case report. Front. Surg. 9:966792. doi: 10.3389/fsurg.2022.966792
Received: 11 June 2022; Accepted: 19 December 2022;
Published: 17 February 2023.
Edited by:
Matteo De Pastena, University of Verona, ItalyReviewed by:
Salvatore Cozzi, IRCCS Local Health Authority of Reggio Emilia, ItalyClaudia Sangalli, National Cancer Institute Foundation (IRCCS), Italy
© 2023 He, Qian, Chen, Liu, Wen and Cao. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Peiguo Cao eHkzY2FvcGdAY3N1LmVkdS5jbg== Yanguang Wen bGFuY2V0OTAyOEAxNjMuY29t
Specialty Section: This article was submitted to Surgical Oncology, a section of the journal Frontiers in Surgery
 Yu He
Yu He Liyuan Qian2
Liyuan Qian2 Lang Chen
Lang Chen Peiguo Cao
Peiguo Cao