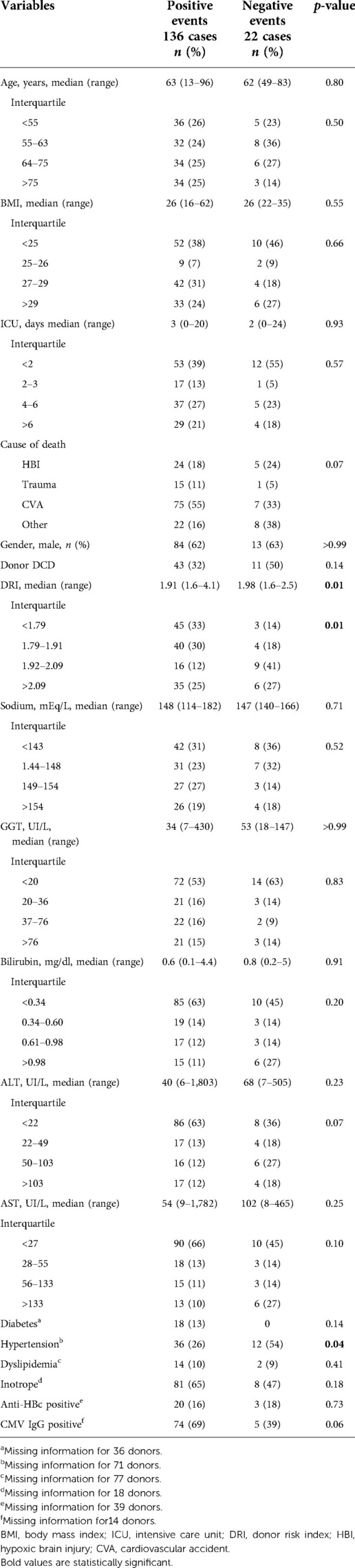
- 1Hepatobiliary and Liver Transplant Unit, Department of Emergency and Organ Transplantation, University Hospital Policlinic of Bari, Bari, Italy
- 2Department of General Surgery and Transplantation, ASST Grande Ospedale Metropolitano Niguarda, Milan, Italy
- 3General Surgery 2U-Liver Transplant Centre, A.O.U. “Città della Salute e della Scienza”, Turin, Italy
- 4Hepatobiliary Surgery and Liver Transplantation Unit, University Hospital of Padua, Padua, Italy
- 5Hepato-Pancreato-Biliary Surgery and Liver Transplant Center, University of Modena and Reggio Emilia, Modena, Italy
- 6Department for the Treatment and the Study of Abdominal Diseases and Abdominal Transplantation, IRCCS-ISMETT, UPMC, Palermo, Italy
- 7Department of Surgery and Medical and Surgical Specialties, University of Catania, Catania, Italy
- 8Liver Transplant Unit, Sapienza University of Rome, Rome, Italy
- 9Department of Medicine and Surgery, University of Milano-Bicocca, Milan, Italy
Machine perfusion (MP) has been shown worldwide to offer many advantages in liver transplantation, but it still has some gray areas. The purpose of the study is to evaluate the donor risk factors of grafts, perfused with any MP, that might predict an ineffective MP setting and those would trigger post-transplant early allograft dysfunction (EAD). Data from donors of all MP-perfused grafts at six liver transplant centers have been analyzed, whether implanted or discarded after perfusion. The first endpoint was the negative events after perfusion (NegE), which is the number of grafts discarded plus those that were implanted but lost after the transplant. A risk factor analysis for NegE was performed and marginal grafts for MP were identified. Finally, the risk of EAD was analyzed, considering only implanted grafts. From 2015 to September 2019, 158 grafts were perfused with MP: 151 grafts were implanted and 7 were discarded after the MP phase because they did not reach viability criteria. Of 151, 15 grafts were lost after transplant, so the NegE group consisted of 22 donors. In univariate analysis, the donor risk index >1.7, the presence of hypertension in the medical history, static cold ischemia time, and the moderate or severe macrovesicular steatosis were the significant factors for NegE. Multivariate analysis confirmed that macrosteatosis >30% was an independent risk factor for NegE (odd ratio 5.643, p = 0.023, 95% confidence interval, 1.27–24.98). Of 151 transplanted patients, 34% experienced EAD and had worse 1- and 3-year-survival, compared with those who did not face EAD (NoEAD), 96% and 96% for EAD vs. 89% and 71% for NoEAD, respectively (p = 0.03). None of the donor/graft characteristics was associated with EAD even if the graft was moderately steatotic or fibrotic or from an aged donor. For the first time, this study shows that macrovesicular steatosis >30% might be a warning factor involved in the risk of graft loss or a cause of graft discard after the MP treatment. On the other hand, the MP seems to be useful in reducing the donor and graft weight in the development of EAD.
Introduction
Extended criteria grafts are increasingly used worldwide (1, 2) to fight the persistent shortage of organs for liver transplantation (LT). However, implantation of these grafts is challenging, being linked to more intraoperative complications, such as post reperfusion syndrome (3, 4), or post-transplant problems like early allograft dysfunction (EAD) (5), renal injury (6), or biliary complications (7). All these complications can cost graft loss or patient's life (8). The duration of static cold ischemia time (S-CIT) plays a significant role in this context, as nonstandard grafts do not tolerate long periods of storage on ice (9). Indeed, S-CIT is a component of several donor risk formulas, such as the donor risk index (DRI) (10), the EuroTransplant donor risk index (11), the Survival Outcomes Following Liver Transplantation (SOFT) score (12), and the Balance of Risk score (13). The pathophysiology of S-CIT is triggered by the lack of oxygen supply during the cold ischemic phase. Cellular energetic processes are slower and many catabolites accumulate to be released in the recipient blood (14). The clinical impact of this process may manifest after the graft implantation with post reperfusion syndrome or even compromise the patient's survival after transplantation (15, 16). In addition, during S-CIT, there is no opportunity to assess graft viability and the transplant surgeon may be discouraged to use extended criteria grafts.
In recent years, the liver transplant community has focused on the administration of oxygen at low- or body-temperature using machine perfusions (MP) ex vivo. The results are positive even with nonstandard grafts (17). The first published study with long-term follow-up showed that hypothermic MP (HMP) treatment of deceased-cardiac donors’ donation after circulatory death (DCD) livers protect grafts and significantly reduce ischemic cholangiopathy and graft loss rates (18). In Italy, since 2015, several liver transplant centers have started to use the MP and its benefits have marked a positive trend to use. Therefore, these devices have slowly ushered in a promising new era for LT.
However, the MP is not immune to drawbacks: costs are still high for the most widely used modalities, namely HMP and normothermic MP (NMP). In Italy, a disposable kit for perfusing a graft costs about 8,000–12,000 euros for the Liver Assist Machine (Organ Assist, Netherlands). The NMP needs packed red cells, drugs, and nutritional solution (17). All devices need qualified personnel on call as well. In addition, the primary nonfunction (PNF) rate for all MPs is low, ranging 1.2%–4%, but is still reported. The frequency of EAD is 10% after NMP treatment and 33% after HMP (9). All these complications increase the overall cost of MP. In addition, not all machine-perfused grafts fulfill viability test criteria to be used and they are obviously discarded after MP treatment. The discard rate for NMP reported in a randomized controlled trial is 12% (9), but there are no data on the risk factors of donor discard with MP. If in the MP era, marginal grafts are used more frequently and successfully, then for MP preserved grafts, the label “marginal” needs to be redefined to understand how far we can push the donor risk boundaries today. Finally, more marginal grafts have been used (1, 2) but there is no clear information on the EAD risk for these grafts after having been oxygenated in MP. Therefore, the aim of this study is to investigate the utility of MP and to delineate the pretransplant risk factors for setting up unnecessary MP.
Materials and methods
Study design
Data of MP grafts, either by HMP or NMP, were collected from six Italian liver transplant centers. The study period was from the first Italian case in 2015 up to September 2019. Both donation after brain death (DBD) and donation after circulatory death (DCD) were considered.
The first endpoint was to identify the risk factors predicting negative events (NegE). NegE were defined as all perfused but discarded livers and implanted grafts lost after LT. Positive events (PosE) included all successfully transplanted perfused grafts (Figure 1).
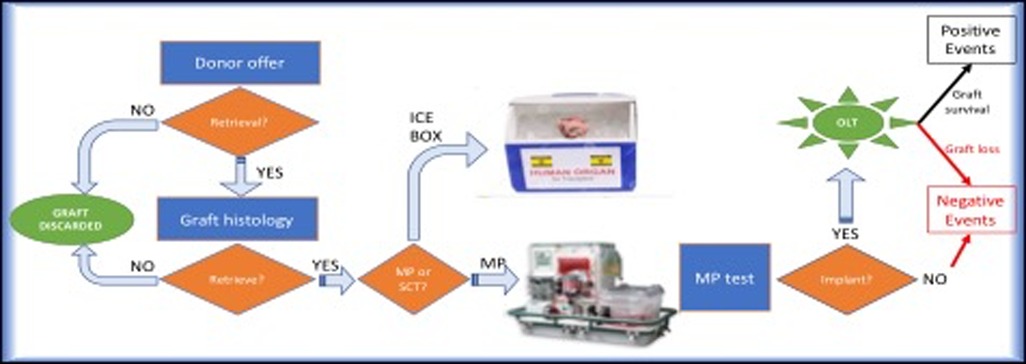
Figure 1. Decision-making process from the donor offer to the transplant and graphic presentation of the two study groups.
Extended criteria grafts were arbitrarily defined as grafts from donors with at least two of the worst interquartile of pretransplant characteristics if the graft was from DBD, or one of them if from DCD. This way was done to better identify the “very extended” criteria in our specific donor population and not considering “extended” criteria from the literature. MP was used mainly for grafts with some very warning characteristics that could rarely be reported in the literature. Therefore, published definitions of marginal grafts would not have fit for this study of “very marginal” donors. This group of grafts was, therefore, analyzed if significant for NegE with the aim of defining a new concept of “marginal graft” in the MP era.
The second endpoint was to analyze the role of MP in terms of post-transplant EAD rates. First, the impact of EAD on patient and graft survival was studied. Finally, donor- and graft-related risk factors of EAD were investigated.
National guidelines for liver transplantation were used for organ donation eligibility and graft allocation. All surgeries were performed using a piggyback technique for caval reconstruction.
Definitions of variables
Histology features were reported according to the grade of macro- and microvesicular steatosis and greater or less than 30%. Ishak fibrosis was cited if greater than grade 1.
The donor risk index was calculated according to the formula published by Feng et al. (10). EAD was defined if the case met Olthoff's criteria (19). PNF was considered if death or retransplantation due to liver failure in the first postoperative week and if the cause was not related to acute rejection or vascular complications (20). Finally, S-CIT was the elapsed time between donor cross-clamp and MP initiation.
Modalities of MP and NRP
For DBD grafts, every center followed its own policy in deciding whether to store the graft in the ice box until the implantation (S-CIT) or to use the MP. For MP grafts, the device was set up at the transplant center after a variable period of S-CIT. All of the grafts were oxygenated ex situ, most in hypothermic mode and a few of them in normothermic setting, for at least 2 h until the recipient hepatectomy was completed. For both modalities, the vessel perfusion was used at manually controlled pressure and flow. The arterial circuit was set up at 40 mmHg with pulsatile perfusion pressure, 60 beats for minute with 1,000 ml/min of flow. Portal flow was set up at 6 mmHg continue perfusion pressure with flow up to 2 L/min. University of Wisconsin MP fluid was used for HMP. The grafts were flushed with saline solution or Celsior fluid and implanted after the HMP treatment.
For DCD grafts, all cases were treated with normothermic regional perfusion (NRP) followed by ex vivo MP. Only grafts with macrosteatosis ≤30% and Ishak grade ≤1 at biopsy were considered available. National criteria for proceeding with liver retrieval on NRP were ALT <1,000 UI/L (or negative transaminase trend) and negative lactate trend (21).
Statistical analysis
Donor and graft characteristics are listed in Table 1. Quantitative variables are described in medians and ranges. Qualitative variables are reported in total numbers and percentages.
The Kolmogorov–Smirnov test was performed to evaluate the normal distribution of continuous variables. Continuous variables with parametric distribution were compared with the nonpaired sample Student’s t test, and variables with skewed distribution with the Mann–Whitney test. Qualitative variables were analyzed with the chi-square or Fischer test, as appropriate. Multivariable logistic regression analysis was performed considering only variables significant to univariate analysis. Odds ratio (OR) and 95% confidence intervals (95% CI) were reported. The Kaplan–Meier modality was used for survival curves, and the log-rank test was used for survival comparison. Results were considered significant if the p-value was <0.05. IBM® SPSS (version 24) was used for statistical analyses
Results
From 2015 to September 2019, 158 grafts were retrieved and preserved with an MP. A total of 151 livers were implanted; therefore, seven grafts were not used, with a discard rate of only 4.4% after MP treatment. After transplant, 15/151 (9.9%) patients lost their grafts (with five cases for PNF, three for vascular complications, three for sepsis, and four for patient-related causes). Therefore, the NegE group consisted of 22/158 (13.9%) grafts. The PosE group consisted of 136/158 (86.1%) patients.
DCD grafts were 53 from Maastricht 3 (33.54%) and 1 from Maastricht 2. Most of the livers were perfused with HMP (n = 144, 91.1%).
Figure 2 graphically illustrates the donor characteristics of all the 158 grafts. The median donor age was 63 years (range = 13–96) and 69 (44%) donors were older than 65 years. The median body mass index (BMI) was 26 (range = 16–62) and 22% of donors were obese. Notably, 9 (5.7%) donors belonged to obesity class III.
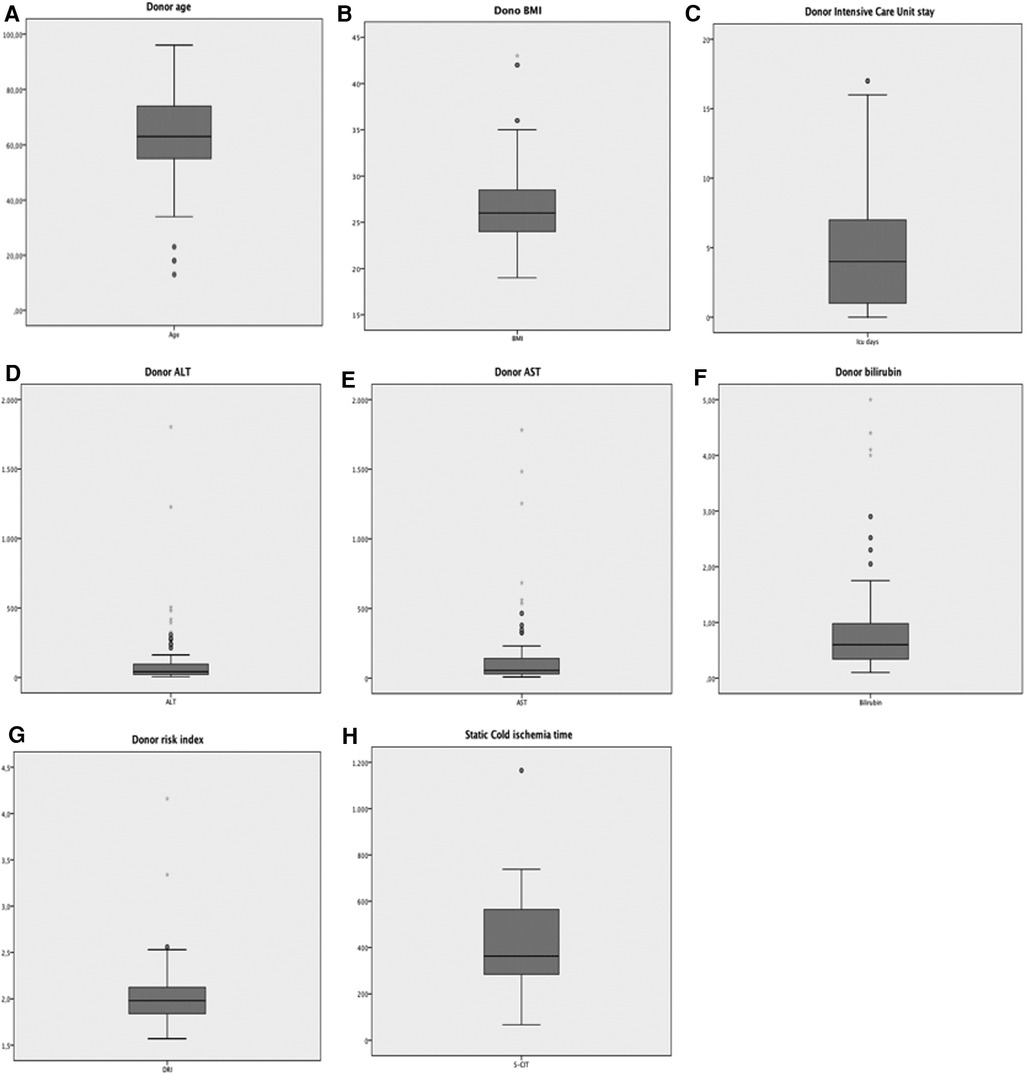
Figure 2. Graphic display of the distribution of the main donor parameters of all 158 donors included in the study. (A) Age; (B) BMI; (C) Intensive Care Unit stay; (D) ALT; (E) AST; (F) bilirubin; (G) donor risk index; (H) S-CIT duration.
The median stay in the intensive care unit (ICU) was 3 days (range = 1–24). Forty-seven (29.7%) donors had deranged transaminases at the time of procurement. The median donor ALT peak value was 49 IU/L (range = 6–1,803), and the median AST peak value was 55 IU/L (range = 8–1,782). Fourteen (8.9%) donors had a peak value of total bilirubin >1.2 mg/dl at the time of procurement, with a median value observed in the whole population of 0.6 mg/dl (range = 0.1–5.0). One hundred forty-three (90.5%) donors had a DRI >1.7 (median 1.9; range = 1.6–4.2). S-CIT lasted longer than 7 h in 41 cases (25.9%). Notably, one graft had up to 20 h of storage in ice before MP (Figure 2).
Table 1 describes the comparison between the NegE and PosE groups in terms of donor characteristics. Donor age, BMI, cause of death, sex, transaminases, GGT, bilirubin, and sodium level were similar in both groups. The median DRI was significantly higher in NegE than PosE (2.0 vs. 1.9; p = 0.01), and the groups had different distribution among the interquartiles. When donor medical history was available, the rates of diabetes, dyslipidemia, cytomegalovirus IgG, and anti-HBc positivity were not statistically different between the groups. There were significantly more hypertensive donors in NegE (Table 1).
A different proportion of MP type was noted: in PosE, 93% of grafts were perfused by HMP, compared with 77% in NegE (p = 0.03). Median S-CIT was similar in both groups, with a different interquartile distribution (p = 0.01) (Table 2). Biopsy results at the time of retrieval showed similar rates of microvesicular steatosis grade and Ishak score of fibrosis. NegE cases had more grafts with moderate-to-severe macrovesicular steatosis (32% vs. 7%, p = 0.02) (Table 2).
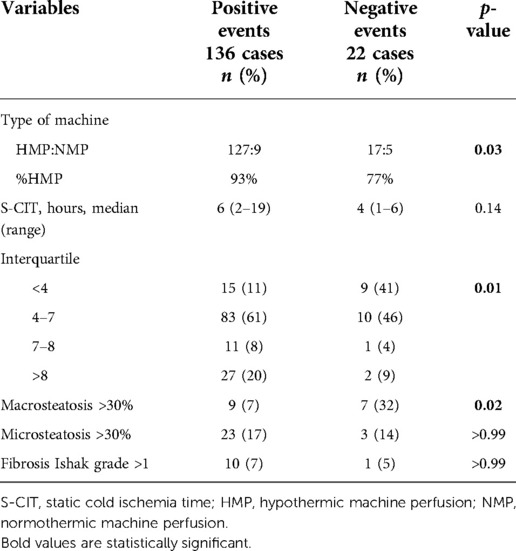
Table 2. Univariate analysis of graft histology features, type of machine perfusion adopted, and S-CIT for the negative events.
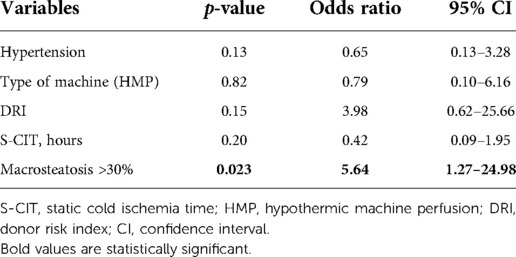
All significant factors at univariate analysis were considered to build a multivariable logistic regression model for the risk of NegE. After introducing five different variables in the model (DRI, presence of hypertension, type of MP, duration of S-CIT expressed in interquartiles, and grade of macrovesicular steatosis), only macrosteatosis >30% was found to be a significant variable, with an odds ratio of 5.643 (95% CI, 1.27–24.98; p = 0.023) (Table 3).
Separate analysis was performed for the two subgroups of NegE. Table 4 shows a comparison of implanted and survived cases vs. discarded grafts and survived vs. lost grafts.
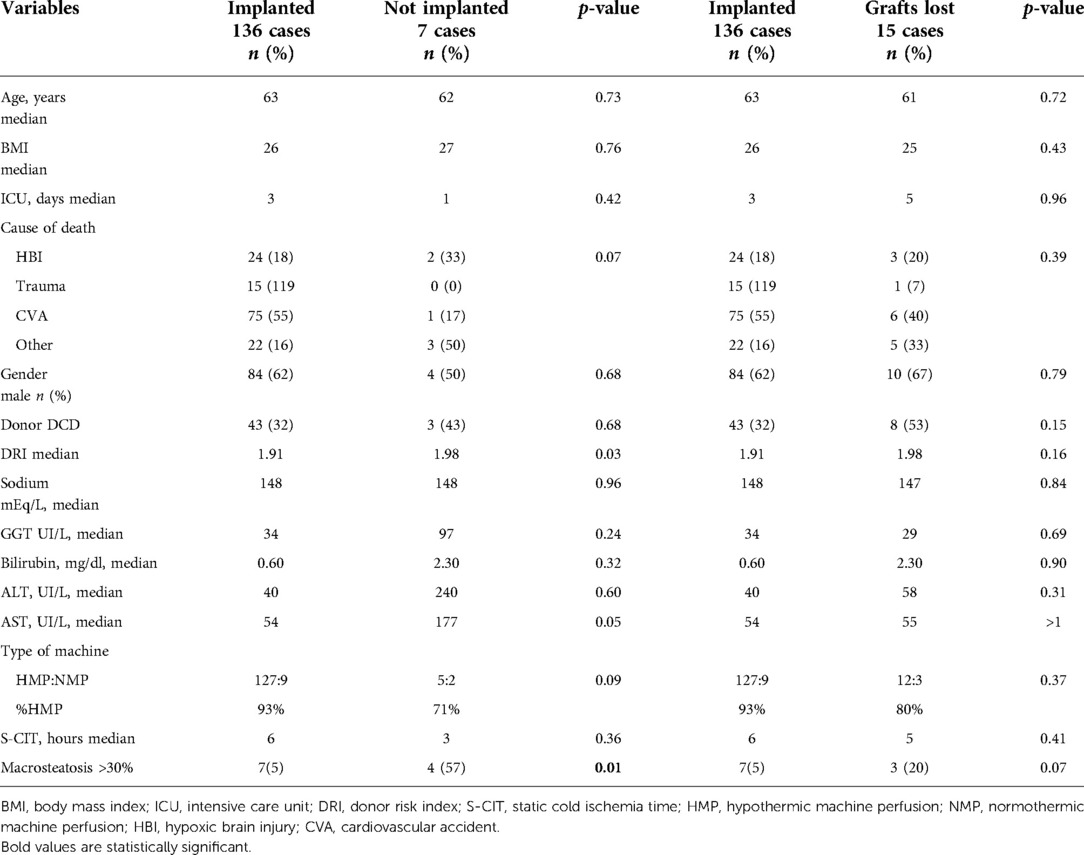
Table 4. Subgroups analysis of the donor and grafts risk factors predicting the discarded grafts and the grafts lost.
Marginal donors
Extended criteria donors (ECDs) were defined as all donors with at least of two of the following characteristics: DCD, age > 65 years, BMI > 30, ICU stay >6 days, DRI > 1.9, ALT > 104 UI, AST > 134 UI, bilirubin > 1.2 mg/dl, S-CIT longer than 8 h, and macrosteatosis > 30% (Table 5). Of the 54 so-called marginal donors, 27% had a negative event, compared with the remaining (9%, p-value of 0.01) defined as standard criteria donors (SCDs).
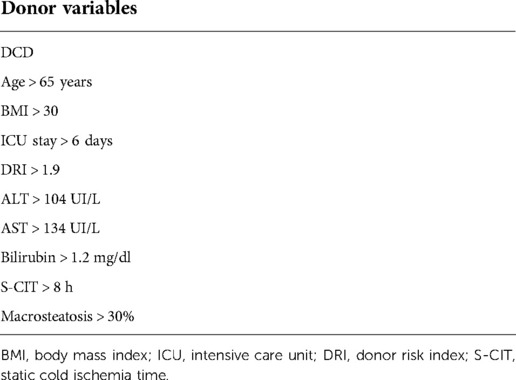
Table 5. List of donor parameters: occurrence of two or more of these factors was considered to define a marginal donor for grafts treated by MP.
Analysis of cases of early allograft dysfunction
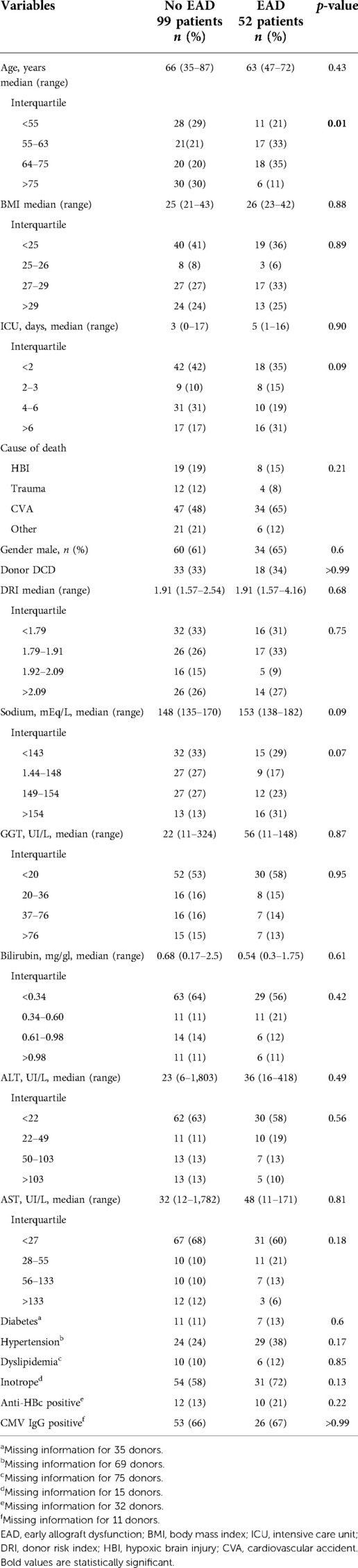
For the analysis of EAD, 151 donors of MP stored and implanted grafts were considered and the rate was 34% (52 patients vs. 99 no EAD). Notably, EAD had a significant impact on patient survival at 1 and 3 years post liver transplant: 96% and 96% for patients who did not develop an EAD (noEAD) vs. 89% and 91% for EAD patients (p = 0.03), respectively. A similar difference was noted between grafts survival curves: at 1 and 3 years 93% and 91% for noEAD and 86% and 79% for EAD patients (p = 0.03) (Figure 3).
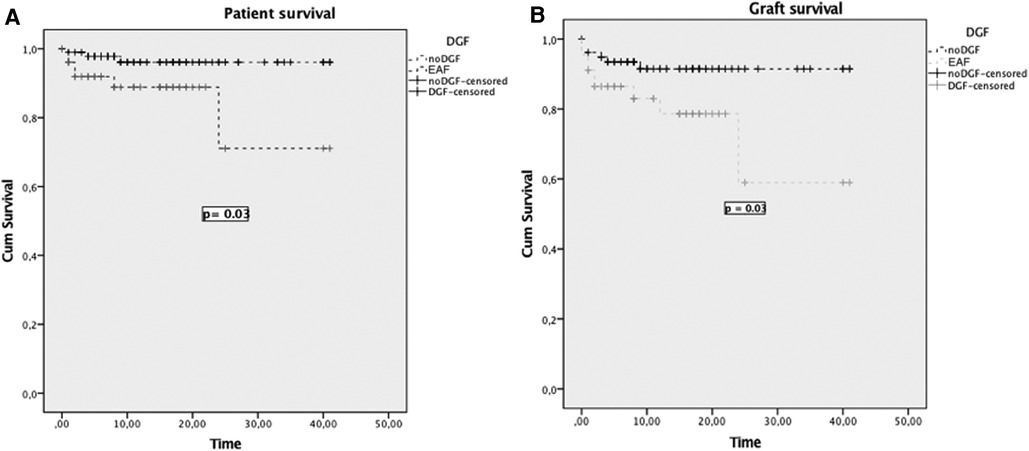
Figure 3. Patient (A) and graft survival (B) curves for patients who developed EAD and patients who did not (NoEAD).
Table 6 summarizes all donor characteristics. In both groups, EAD and noEAD, nearly one-third of grafts were from DCD donors, and more than two-third had DRI > 1.7. Laboratory results, median donor age, BMI, and diseases mentioned in medical history were similar in both groups. The type of MP used, duration of S-CIT, and histology features were also comparable (Table 7).
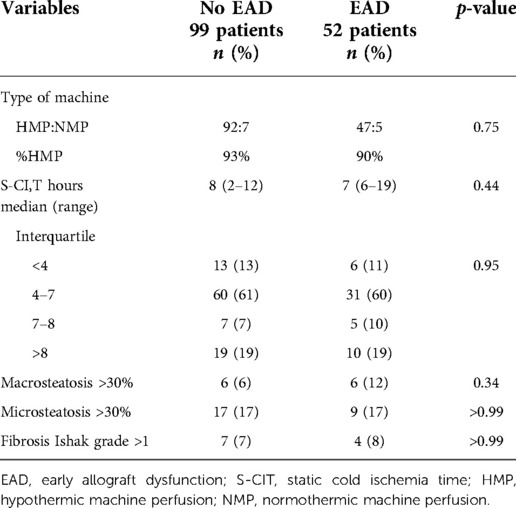
Table 7. Univariate analysis of graft histology features, type of machine perfusion used, and S-CIT for EAD.
Characteristics of the recipients are shown in Table 8: both groups of patients had similar age, BMI, cause of cirrhosis, hepatocellular carcinoma (HCC) rate, and MELD score at the time of transplant.
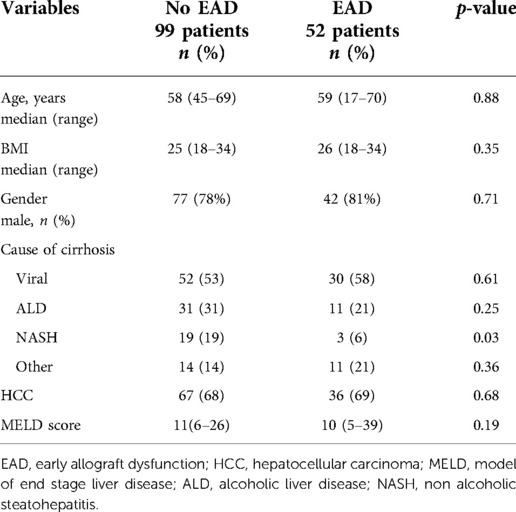
Table 8. Recipient characteristics for patients who developed EAD compared who did not develop (NoEAD).
Discussion
Negative and positive events
This is the first study exploring the possible pretransplant risk factors to discard a liver graft after MP preservation or failure after implantation to help the surgeon determine whether it is worthwhile to set up an MP.
Univariate analysis has confirmed the utility of MP as widely mentioned in the literature (9, 17, 22–24): grafts from elderly donors, or with those with very high BMI, or with deranged laboratory test can be saved by MP (Table 1). In fact, recently a trial has indeed shown that HMP definitely reduces ischemia reperfusion injury in ECD compared with grafts from SCD (17). In addition MP, used after NRP, allowed the safe use of DCD grafts, which would not have been used without this tool due to very long donor warm ischemia time (WIT) (21, 25). Because of the long standoff time (20 min), in Italy, DCD grafts have donor WIT longer than 30 min, which would be an exclusion criteria for the use of these grafts. In this study, 43 DCD grafts were successfully implanted and the percentage of DCD vs. DBD was similar in the NegE (11 vs. 11) and PosE groups (43 vs. 93, p = 0.14). There are no data comparing the sequential of NRP and ex situ preservation vs. NRP alone, but in an evidence-based position paper recently published by the Italian Society of Organ Transplantation (SITO), sequential use in DCD was recommended (26).
Interestingly, this study uncovers a gray area for the use of MP in a clinical setting. Among the significant parameters at univariate analysis, only moderate/severe macrosteatosis was an independent factor to discard the graft after MP preservation or to have graft loss after transplantation. Neither moderate or severe microsteatosis nor Ishak grade >1 limited the usefulness of MP, but macrosteatosis >30% remained a warning factor for grafts even after MP treatment (Table 3). NegE had 32% of moderate/severe steatosis vs. 7% in PosE (p = 0.02). Steatotic livers have been often linked to a worse post-transplant outcome, particularly post reperfusion syndrome, EAD, renal injury, and postoperative mortality (27–29). This new result may dictate greater care in dealing with steatotic grafts, especially before preserving them in an MP. Considered that donor BMI is a surrogate marker of the grade of liver steatosis and is increasing worldwide (1, 2), research needs to be addressed and implemented more in this field. Steatosis is still one the most frequent causes to discard a liver, with rates ranging 13%–28% (30); therefore, there is a tremendous interest in saving these grafts. Separate analysis with the two subgroups of NegE vs. implanted grafts showed that the rate of macrosteatosis was significant in discarded grafts (Table 4) but not in lost grafts subgroup. The numbers are very small, but these data might stress the concept that it needed to treat these very steatotic grafts in the MP to have more functional livers available. If microsteatosis does not scare any surgeon, clinical data on ex vivo perfusion of livers with macrosteatosis are anecdotal, especially for NMP (24, 31, 32). The use of de-fatty cocktail during MP preservation for steatotic grafts has been mentioned in the literature but only with animal models (33, 34). This study presents clinical data based on 158 grafts and the risk of causing an untoward event for livers with moderate or severe steatosis. Finally, steatotic livers might develop HCC, even skipping the cirrhotic stage (35, 36), and the risk of HCC recurrence on steatotic graft used in HCC candidates is unknown.
If the results from marginal grafts implanted after MP preservation are undoubtedly promising at short and long term, the definition of “marginal graft” needs to be reformulated in the era of MP. Different criteria and cut-offs have been used to label an ECD but both HMP and NMP seem to have pushed many donor boundaries (17, 37). The list of parameters in Table 5 might be helpful to track this new classification. Indeed if donors have at least two of the listed characteristics (DCD, donor > 65 years, BMI > 30, DRI > 1.9, ALT > 104, AST > 134, bilirubin > 1.2 mg/dl, S-CIT > 8 h, macrosteatosis > 30%), there is a significant risk of ineffectively perfusing the graft into the MP or implanting it unsuccessfully compared to grafts from donors with less than two of the above characteristics (27% vs. 9%, p = 0.05). As clarified above, NegE includes MP-perfused and discarded plus grafts perfused, implanted, and lost after the transplant. In the literature, graft loss is usually considered as an event to define marginal donors (10, 38); therefore, the listed factors might be not well applicable in this view but they might help the transplant surgeon to match these specific ECD donors, with such potential outcomes and with low disease severity recipients, like patients with HCC.
The study presented the results of using MP routinely, out of trial, without fixed criteria, and in heterogeneous settings. It showed a national overview of the use of ex vivo perfusion of grafts in different centers with their own policy on MP and different availability of this tool.
Early allograft dysfunction for machine-perfused grafts
EAD is a post-transplant complication that can cost the patient's life. Many donor parameters have been shown to be significant in triggering this potentially catastrophic event, such as donor age, BMI, GGT value, duration of S-CIT, and steatosis (5, 39, 40). None of mentioned factors were found to be significant for EAD in this study (Tables 6,7). This again supports the usefulness of MP, as it might indicate that MP clears off all the potential donor risk factors for EAD and any factors before MP treatment, like S-CIT. For MP grafts, EAD could be caused by factors not considered in this analysis, such as implantation time or duration of anhepatic phase. Interestingly, moderate or severe macrosteatosis is also not significant for EAD, despite the fact that EAD group had twice the rate of macrosteatosis >30% compared with noEAD patients (12% vs. 6%, p = 0.34) (Table 7).
EAD had a significant impact on patient and graft survival as shown in Figure 3. Therefore, it urges to explore pretransplant EAD risk factors for machine-perfused grafts to reduce this warning complication.
This study has several limitations. (A) It is a multicenter study; therefore, it includes an heterogeneous graft population. Each center follows its own criteria regarding standard ice storage and MP, which were not considered in this analysis. In some cases, MP was used to manage logistical issues (e.g., multiple donors on the same days), rather than to test marginal grafts, and these data were not traceable in the available database. These grafts might have been stored in the ice box. (B) The analysis included both HMP and NMP and this could cause a bias. In any case, so far there is no solid result that clearly endorses the superiority of one of these tools. (C) Most of the recipients have a low MELD score (11 for noEAD, 10 for EAD) and this could cause a selection bias for EAD analysis. (D) Finally, the samples of the main endpoint are small, so a larger population study is needed.
Conclusions
In conclusion, today MP is an important device to expand the donor pool in the era of very extended marginal grafts. For the first time in the literature, this study showed that a moderate or severe grade of macrosteatosis could be a factor of graft loss post-transplant, even by perfusing these grafts into an MP, or a reason to discard grafts after MP treatment. Macrosteatosis could temper the widespread efficacy of MP, when the rest of the donor bounders have been successfully pushed. Definitely a new donor scoring system is needed in the era of MP grafts.
Finally, MP has been useful in combating the well-known donor and graft risk factors for EAD. This potentially life-threatening complication is still reported; therefore, it is necessary to explore what other factors may trigger it.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author.
Ethics statement
Ethical review and approval was not required for the study on human participants in accordance with the local legislation and institutional requirements. Written informed consent from the patients was not required to participate in this study in accordance with the national legislation and the institutional requirements.
Author contributions
IS participated in performing the research, data analysis, and writing of the paper. RDC, DPt, EC, TO, DPg, MR, SG, FDB, UC, and RR participated in performing the research. QL participated in the writing of the paper and data analysis. LGL and LDC participated in research design. All authors contributed to the article and approved the submitted version.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Kwong A, Kim WR, Lake JR, Smith JM, Schladt DP, Skeans MA, et al. OPTN/SRTR 2018 annual data report: liver. Am J Transplant. (2020) 20(Suppl. 1):193–299. doi: 10.1111/ajt.15674
2. Adam R, Karam V, Cailliez V, Grady JGO, Mirza D, Cherqui D, et al. 2018 annual report of the European liver transplant registry (ELTR)—50-year evolution of liver transplantation. Transpl Int. (2018) 31(12):1293–317. doi: 10.1111/tri.13358
3. Fukazawa K, Yamada Y, Gologorsky E, Arheart KL, Pretto EA Jr. Hemodynamic recovery following postreperfusion syndrome in liver transplantation. J Cardiothorac Vasc Anesth. (2014) 28(4):994–1002. doi: 10.1053/j.jvca.2014.02.017
4. Siniscalchi A, Gamberini L, Laici C, Bardi T, Ercolani G, Lorenzini L, et al. Post reperfusion syndrome during liver transplantation: from pathophysiology to therapy and preventive strategies. World J Gastroenterol. (2016) 22(4):1551–69. doi: 10.3748/wjg.v22.i4.1551
5. Hoyer DP, Paul A, Gallinat A, Molmenti EP, Reinhardt R, Minor T, et al. Donor information based prediction of early allograft dysfunction and outcome in liver transplantation. Liver Int. (2015) 35(1):156–63. doi: 10.1111/liv.12443
6. Umbro I, Tinti F, Scalera I, Evison F, Gunson B, Sharif A, et al. Acute kidney injury and post-reperfusion syndrome in liver transplantation. World J Gastroenterol. (2016) 22(42):9314–23. doi: 10.3748/wjg.v22.i42.9314
7. Welling TH, Heidt DG, Englesbe MJ, Magee JC, Sung RS, Campbell DA, et al. Biliary complications following liver transplantation in the model for end-stage liver disease era: effect of donor, recipient, and technical factors. Liver Transpl. (2008) 14(1):73–80. doi: 10.1002/lt.21354
8. Kalisvaart M, Perera M. Using marginal grafts for liver transplantation: the balance of risk. J Invest Surg. (2020) 33(6):565–7. doi: 10.1080/08941939.2018.1542048
9. Nasralla D, Coussios CC, Mergental H, Akhtar MZ, Butler AJ, Ceresa CDL, et al. A randomized trial of normothermic preservation in liver transplantation. Nature. (2018) 557(7703):50–6. doi: 10.1038/s41586-018-0047-9
10. Feng S, Goodrich NP, Bragg-Gresham JL, Dykstra DM, Punch JD, DebRoy MA, et al. Characteristics associated with liver graft failure: the concept of a donor risk index. Am J Transplant. (2006) 6(4):783–90. doi: 10.1111/j.1600-6143.2006.01242.x
11. Braat AE, Blok JJ, Putter H, Adam R. The Eurotransplant donor risk index in liver transplantation: ET-DRI. Am J Transplant. (2012) 12(10):2789–96. doi: 10.1111/j.1600-6143.2012.04195.x
12. Rana A, Jie T, Porubsky M, Habib S, Rilo H, Kaplan B, et al. Survival Outcomes Following Liver Transplantation (SOFT) score: a novel method to predict patient survival following liver transplantation. Am J Transplant. (2008) 8(12):2537–46. doi: 10.1111/j.1600-6143.2008.02400.x
13. Dutkowski P, Schlegel A, Slankamenac K, Oberkofler CE, Adam R, Burroughs AK, et al. The use of fatty liver grafts in modern allocation systems: risk assessment by the balance of risk (BAR) score. Ann Surg. (2012) 256(5):861–8. doi: 10.1097/SLA.0b013e318272dea2
14. Clavien PA, Harvey PR, Strasberg SM. Preservation and reperfusion injuries in liver allografts. An overview and synthesis of current studies. Transplantation. (1992) 53(5):957–78. doi: 10.1097/00007890-199205000-00001
15. Paugam-Burtz C, Kavafyan J, Merckx P, Dahmani S, Sommacale D, Ramsay M, et al. Postreperfusion syndrome during liver transplantation for cirrhosis: outcome and predictors. Liver Transpl. (2009) 15(5):522–9. doi: 10.1002/lt.21730
16. Bukowicka B, Akar RA, Olszewska A, Smoter P, Krawczyk M. The occurrence of postreperfusion syndrome in orthotopic liver transplantation and its significance in terms of complications and short-term survival. Ann Transplant. (2011) 16(2):26–30. doi: 10.12659/AOT.881861
17. Ravaioli M, De Pace V, Angeletti A, Comai G, Vasuri F, Baldassarre M, et al. Hypothermic oxygenated new machine perfusion system in liver and kidney transplantation of extended criteria donors: first Italian clinical trial. Sci Rep. (2020) 10(1):6063. doi: 10.1038/s41598-020-62979-9
18. Schlegel A, Muller X, Kalisvaart M, Muellhaupt B, Perera M, Isaac JR, et al. Outcomes of DCD liver transplantation using organs treated by hypothermic oxygenated perfusion before implantation. J Hepatol. (2019) 70(1):50–7. doi: 10.1016/j.jhep.2018.10.005
19. Olthoff KM, Kulik L, Samstein B, Kaminski M, Abecassis M, Emond J, et al. Validation of a current definition of early allograft dysfunction in liver transplant recipients and analysis of risk factors. Liver Transpl. (2010) 16(8):943–9. doi: 10.1002/lt.22091
20. Clavien PA, Camargo CA Jr., Croxford R, Langer B, Levy GA, Greig PD. Definition and classification of negative outcomes in solid organ transplantation. Application in liver transplantation. Ann Surg. (1994) 220(2):109–20. doi: 10.1097/00000658-199408000-00002
21. De Carlis R, Di Sandro S, Lauterio A, Botta F, Ferla F, Andorno E, et al. Liver grafts from donors after circulatory death on regional perfusion with extended warm ischemia compared with donors after brain death. Liver Transpl. (2018) 24(11):1523–35. doi: 10.1002/lt.25312
22. Lai Q, Melandro F, Rossi M, Ruberto F, Pugliese F, Mennini G. Role of perfusion machines in the setting of clinical liver transplantation: a qualitative systematic review. Clin Transplant. (2018) 32(8):e13310. doi: 10.1111/ctr.13310
23. Schlegel A, Kron P, Dutkowski P. Hypothermic machine perfusion in liver transplantation. Curr Opin Organ Transplant. (2016) 21(3):308–14. doi: 10.1097/MOT.0000000000000303
24. Patrono D, Lavezzo B, Molinaro L, Rizza G, Catalano G, Gonella F, et al. Hypothermic oxygenated machine perfusion for liver transplantation: an initial experience. Exp Clin Transplant. (2018) 16(2):172–6. doi: 10.6002/ect.2016.0347
25. De Carlis R, Di Sandro S, Lauterio A, Ferla F, Dell’Acqua A, Zanierato M, et al. Successful donation after cardiac death liver transplants with prolonged warm ischemia time using normothermic regional perfusion. Liver Transpl. (2017) 23(2):166–73. doi: 10.1002/lt.24666
26. Ghinolfi D, Lai Q, Dondossola D, De Carlis R, Zanierato M, Patrono D, et al. Machine perfusions in liver transplantation: the evidence-based position paper of the Italian society of organ and tissue transplantation. Liver Transpl. (2020) 26(10):1298–315. doi: 10.1002/lt.25817
27. de Graaf EL, Kench J, Dilworth P, Shackel NA, Strasser SI, Joseph D, et al. Grade of deceased donor liver macrovesicular steatosis impacts graft and recipient outcomes more than the donor risk index. J Gastroenterol Hepatol. (2012) 27(3):540–6. doi: 10.1111/j.1440-1746.2011.06844.x
28. Gehrau RC, Mas VR, Dumur CI, Suh JL, Sharma AK, Cathro HP, et al. Donor hepatic steatosis induce exacerbated ischemia-reperfusion injury through activation of innate immune response molecular pathways. Transplantation. (2015) 99(12):2523–33. doi: 10.1097/TP.0000000000000857
29. Chu MJ, Dare AJ, Phillips AR, Bartlett AS. Donor hepatic steatosis and outcome after liver transplantation: a systematic review. J Gastrointest Surg. (2015) 19(9):1713–24. doi: 10.1007/s11605-015-2832-1
30. Boteon YL, Boteon A, Attard J, Mergental H, Mirza DF, Bhogal RH, et al. Ex situ machine perfusion as a tool to recondition steatotic donor livers: troublesome features of fatty livers and the role of defatting therapies. A systematic review. Am J Transplant. (2018) 18(10):2384–99. doi: 10.1111/ajt.14992
31. He X, Guo Z, Zhao Q, Ju W, Wang D, Wu L, et al. The first case of ischemia-free organ transplantation in humans: a proof of concept. Am J Transplant. (2018) 18(3):737–44. doi: 10.1111/ajt.14583
32. Lai Q, Ruberto F, Pawlik TM, Pugliese F, Rossi M. Use of machine perfusion in livers showing steatosis prior to transplantation: a systematic review. Updates Surg. (2020) 72(3):595–604. doi: 10.1007/s13304-020-00797-4
33. Nagrath D, Xu H, Tanimura Y, Zuo R, Berthiaume F, Avila M, et al. Metabolic preconditioning of donor organs: defatting fatty livers by normothermic perfusion ex vivo. Metab Eng. (2009) 11(4-5):274–83. doi: 10.1016/j.ymben.2009.05.005
34. Doyle A, Adeyi O, Khalili K, Fischer S, Dib M, Goldaracena N, et al. Treatment with Optifast reduces hepatic steatosis and increases candidacy rates for living donor liver transplantation. Liver Transpl. (2016) 22(9):1295–300. doi: 10.1002/lt.24495
35. Perumpail RB, Wong RJ, Ahmed A, Harrison SA. Hepatocellular carcinoma in the setting of non-cirrhotic nonalcoholic fatty liver disease and the metabolic syndrome: US experience. Dig Dis Sci. (2015) 60(10):3142–8. doi: 10.1007/s10620-015-3821-7
36. Mittal S, El-Serag HB, Sada YH, Kanwal F, Duan Z, Temple S, et al. Hepatocellular carcinoma in the absence of cirrhosis in United States veterans is associated with nonalcoholic fatty liver disease. Clin Gastroenterol Hepatol. (2016) 14(1):124–31 e1. doi: 10.1016/j.cgh.2015.07.019
37. De Carlis R, Lauterio A, Ferla F, Di Sandro S, Sguinzi R, De Carlis L. Hypothermic machine perfusion of liver grafts can safely extend cold ischemia for up to 20 hours in cases of necessity. Transplantation. (2017) 101(7):e223–4. doi: 10.1097/TP.0000000000001753
38. Flores A, Asrani SK. The donor risk index: a decade of experience. Liver Transpl. (2017) 23(9):1216–25. doi: 10.1002/lt.24799
39. Pareja E, Cortes M, Hervas D, Mir J, Valdivieso A, Castell JV, et al. A score model for the continuous grading of early allograft dysfunction severity. Liver Transpl. (2015) 21(1):38–46. doi: 10.1002/lt.23990
Keywords: liver donor, machine perfusion, steatosis, early allograft dysfunction, liver transplantation
Citation: Scalera I, De Carlis R, Patrono D, Gringeri E, Olivieri T, Pagano D, Lai Q, Rossi M, Gruttadauria S, Di Benedetto F, Cillo U, Romagnoli R, Lupo LG and De Carlis L (2022) How useful is the machine perfusion in liver transplantation? An answer from a national survey. Front. Surg. 9:975150. doi: 10.3389/fsurg.2022.975150
Received: 21 June 2022; Accepted: 29 August 2022;
Published: 22 September 2022.
Edited by:
Frederik Berrevoet, Ghent University Hospital, BelgiumReviewed by:
Arianeb Mehrabi, University Hospital Heidelberg, GermanyJose M. Ramia, Hospital General Universitario de Alicante, Spain
© 2022 Scalera, De Carlis, Patrono, Gringeri, Olivieri, Pagano, Lai, Rossi, Gruttadauria, Di Benedetto, Cillo, Romagnoli, Lupo and De Carlis. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Irene Scalera SXJlbmVzY2FsZXJhQGdtYWlsLmNvbQ==
Specialty Section: This article was submitted to Visceral Surgery, a section of the journal Frontiers in Surgery
Abbreviations BMI, body mass index; CI, confidence interval; DBD, donation after brain death; DCD, donation after circulatory death; DRI, donor risk index; EAD, early allograft dysfunction; ECD, extended criteria donor; HCC, hepatocellular carcinoma; HMP, hypothermic machine perfusion; ICU, intensive care unit; LT, liver transplantation; MP, machine perfusion; NegE, negative events; NMP, normothermic machine perfusion; NRP, normothermic regional perfusion; OR, odds ratio; PNF, primary nonfunction; PosE, positive events; S-CIT, static cold ischemia time; SCD, standard criteria donor; SITO, Italian Society of Organ Transplantation; SOFT, Survival Outcomes Following Liver Transplantation.
 Irene Scalera
Irene Scalera R. De Carlis2
R. De Carlis2 D. Patrono
D. Patrono E. Gringeri
E. Gringeri D. Pagano
D. Pagano Q. Lai
Q. Lai S. Gruttadauria
S. Gruttadauria U. Cillo
U. Cillo R. Romagnoli
R. Romagnoli