Diagnostic value of procalcitonin in patients with periprosthetic joint infection: a diagnostic meta-analysis
- 1Department of Orthopaedics, Ganzhou Hospital of Traditional Chinese Medicine, Ganzhou, Jiangxi, China
- 2The First Clinical Medical School, Guangzhou University of Chinese Medicine, Guangzhou, Guangdong, China
- 3Department of Cardiology, The First Affiliated Hospital of Nanchang University, Nanchang, China
- 4Academic Affairs Office, Gannan Medical University, Ganzhou, Jiangxi, China
- 5Department of Orthopaedics, The First Affiliated Hospital of Guangzhou University of Chinese Medicine, Guangzhou, Guangdong, China
- 6Longhua Hospital, Shanghai University of Traditional Chinese Medicine, Shanghai, China
Background: The success rate of periprosthetic joint infection (PJI) treatment is still low. Early diagnosis is the key to successful treatment. Therefore, it is necessary to find a biomarker with high sensitivity and specificity. The diagnostic value of serum procalcitonin (PCT) for PJI was systematically evaluated to provide the theoretical basis for clinical diagnosis and treatment in this study.
Methods: We searched the Web of Science, Embase, Cochrane Library, and PubMed for studies that evaluated the diagnostic value of serum PCT for PJI (from the inception of each database until September 2020). Two authors independently screened the literature according to the inclusion and exclusion criteria. The quality of each selected literature was evaluated by using the Quality Assessment of Diagnostic Accuracy Studies tool (QUADAS-2) tool. RevMan 5.3 software was used for the quality evaluation. The sensitivity, specificity, positive likelihood ratio (PLR), negative likelihood ratio (NLR), and diagnostic odds ratio (DOR) were merged by using Meta-DiSc 1.4 software. The area under the curve (AUC) and Q index were calculated after the summary receiver operating characteristic (SROC) was generated. We also performed subgroup analysis.
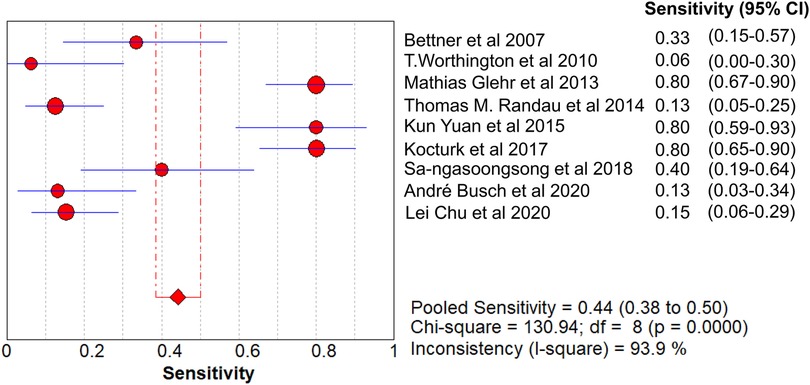
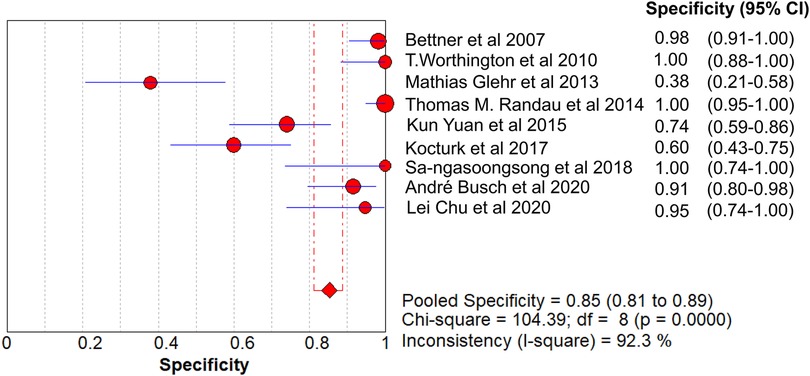
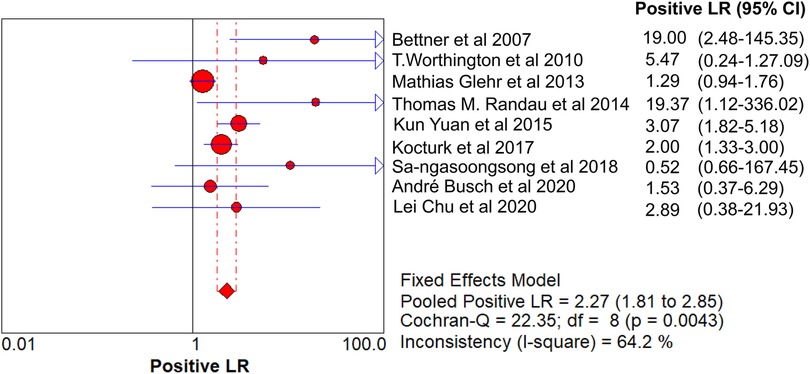
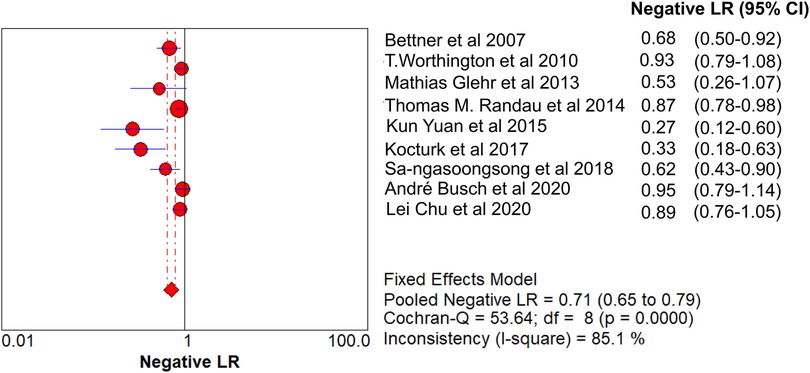
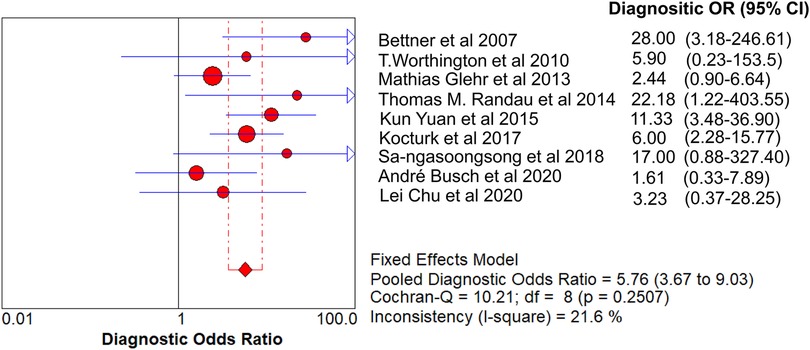
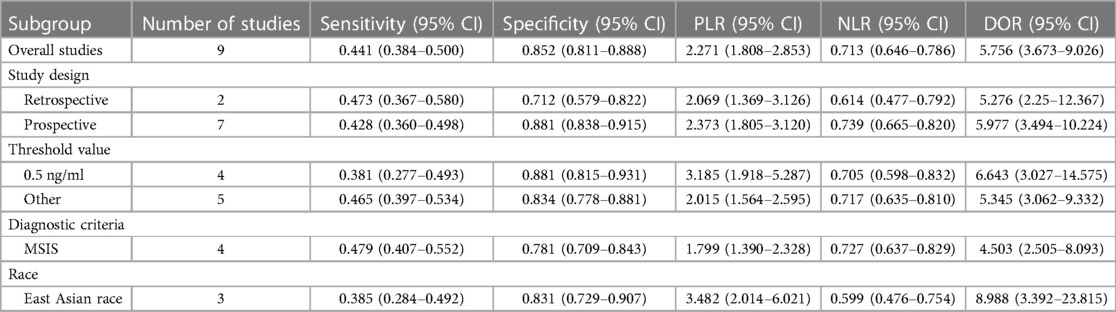
Results: A total of 621 patients were enrolled in the nine studies. The pooled sensitivity of serum PCT for PJI diagnosis was 0.441 [95% confidence interval (CI), 0.384–0.500], the pooled specificity was 0.852 (95% CI, 0.811–0.888), the pooled PLR was 2.271 (95% CI, 1.808–2.853), the pooled NLR was 0.713 (95% CI, 0.646–0.786), and the pooled DOR was 5.756 (95% CI, 3.673–9.026). The area under SROC (the pooled AUC) was 0.76 (0.72–0.79). Q index was 0.6948.
Conclusion: This study showed that PCT detection of PJI had poor diagnostic accuracy. Hence, the serum PCT is not suitable as a serum marker for PJI diagnosis.
Introduction
Periprosthetic joint infection (PJI) is one of the catastrophic complications after total joint arthroplasty (1). The incidence of PJI after total knee arthroplasty (TKA) was 0.7% (2). The total hip arthroplasty (THA) infection incidence was 1.63% within 2 years and 0.59% between 2 and 10 years (3). PJI is the second reason for the early revision of THA (4). It may result in reduced knee or hip function and reduced quality of life for patients, as well as the failure of the implant and the need for revision arthroplasty (5).
Currently, the diagnosis for PJI depends mainly on the guidelines of the American Academy of Orthopedic Surgeon (AAOS)'s guidelines the Infectious Diseases Society of America (IDSA), the Musculoskeletal Infection Society (MSIS) criteria of 2011 and 2018, the European Bone and Joint Infection Society (EBJIS) definition, a large number of biomarkers [white blood cell (WBC), C-reactive protein (CRP), erythrocyte sedimentation rate (ESR), synovial fluid], pathologic examinations and preoperative aspirations (6–10). Unfortunately, the effectiveness of WBC in diagnosing PJI is limited (11, 12). CRP and ESR present high sensitivity and low specificity. We determine the diagnosis according to intraoperative pathologic examination with the lack of typical symptoms. It is difficult to accurately diagnose PJI before surgery, and it is a research hotspot to find specific serum markers such as interleukin-6, procalcitonin (PCT), and α-defensin (13).
PCT is a protein containing 116 amino acids produced by neuroendocrine cells and parafollicular thyroid gland cells (14). Serum PCT levels in uninfected healthy people are low but significantly increase in severe bacterial and fungal infections (15). Aseptic infections such as multiple organ failure and extensive burns are common causes of elevated serum calcitonin. It has been reported that injection of bacterial endotoxin into healthy people can induce the release of systemic PCT. The PCT test has high diagnostic accuracy in identifying systemic infection (14, 16, 17). However, different scholars have drawn entirely different conclusions regarding PCT diagnosis for PJI. Therefore, we performed a systematic review and meta-analysis to evaluate the diagnostic value of PCT in PJI.
Materials and methods
Our study was strictly performed on the basis of the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines (18). All authors participated in formulating the literature retrieval scheme. We finished the work in an orderly fashion.
Data and literature sources
We searched the Web of Science, Embase, Cochrane Library, and PubMed for studies that evaluated the diagnostic value of PCT for PJI (from the inception of each database until September 2020). There was no language limit. The search strategy follows the combination of subject terms and accessible terms. The search subject words and free words are as follows: “prosthesis-related infections” or “Prosthesis Related Infections” or “Infections, Prosthesis-Related” or “Prosthesis-Related Infection” or “Prosthetic joint infection” or “Prosthesis joint infection” or “PJI” stands for disease, “Procalcitonin” or “Calcitonin Precursor Polyprotein” or “Calcitonin-1” or “Calcitonin 1” or “Calcitonin Related Polypeptide Alpha” or “Pro-Calcitonin” or “PCT” represents target index. After electronic research, additional related studies in the literature were added by manual retrieval.
Study selection
The included studies meet the following criteria: (1) Serum PCT was used to diagnose PJI; (2) The type of study is retrospective or prospective analysis; and (3) We can indirectly or directly obtain a 2 × 2 table of PCT diagnosis for PJI; (4) The source of samples for the research is clear; and (5) The reference standard for the diagnosis of PJI is described in the literature. The following studies were excluded: (1) When we could not obtain the necessary 2 × 2 table data through calculating or contacting the author; (2) When patients suffered extra-articular infection of joint; (3) When the review literature, special case reports, animal experiments, and repeated reports were missing; (4) When the detection methods for PCT have not been used in clinical examination or have been eliminated; and (5) When the patients were in a poor nutritional state. Two authors reviewed the title, abstract, and full texts independently. The third author resolved the disagreements between the first two reviewers and made a final decision.
Data extraction and quality assessment
Two authors independently screened the literature according to the inclusion and exclusion criteria and entered the extracted data into a table. The recorded baseline data and outcome indicators were as follows: Author, year, country, study type, gender, median age, and body mass index (BMI), detection method, threshold value, gold standard, operative site, total staff, PJI number, N-PJI number, SN (sensitivity), SP (specificity), TP (true positive), FP (false positive), FN (false negative), and TN (true negative). SN, SP, TP, and FP data were built into a 2 × 2 table.
We used the Quality Assessment of Diagnostic Accuracy Studies tool (QUADAS-2) to evaluate the quality of each selected literature (19). QUADAS-2 was adopted to evaluate the literature along four significant aspects (patient selection, diagnostic test, gold standard, and loss of follow-up). We have assessed the risk of bias in the four aspects and the clinical utility of the first three aspects. RevMan 5.3 software was used for the evaluation of quality.
Data synthesis and analysis
We combined TP, FP, FN, and TN extracted from the literature, and obtained the SN, SP, positive likelihood ratio (PLR), negative likelihood ratio (NLR), and DOR using Meta-DiSc 1.4 software. Whether it is a PLR or an NLR, the further away from 1 it is, the more meaningful it becomes. A PLR greater than 10 means that a positive test is very effective in diagnosing the disease. An NLR less than 0.1 means that a negative test is very effective in ruling out the diagnosis (20). The higher the DOR, the better the diagnostic value (21). In addition, the area under the curve (AUC) and Q index were calculated after the summary receiver operating characteristic (SROC) was generated. An AUC value (greater than 0.8) shows good diagnostic accuracy of PCT.
In diagnostic meta-analysis, both the threshold effect and the non-threshold product can cause heterogeneity. Suppose there is a negative correlation between SN and SP (or a positive correlation between SN and 1 − SP), and there is a “shoulder-arm” point distribution on the receiver operating characteristic (ROC) curve graph. In that case, it can be judged that there is a threshold effect. When the threshold effect causes heterogeneity, Spearman correlation analysis is used. When the heterogeneity is caused by the non-threshold effect, the chi-square test is used to analyze the heterogeneity among the study results, and the inconsistency index (I2) is used as the quantitative judgment for the heterogeneity size. The greater the value of I2 expressed, the greater the value of the heterogeneity, and vice versa. I2 values of 25%, 50%, and 75%, respectively, represent low, moderate, and high heterogeneity. If the heterogeneity of multiple independent studies is low, the fixed-effect model can be selected. If the heterogeneity is high, the random effect model can be selected. Meta-regression and subgroup (Study design, Threshold value, MSIS, East Asian race) analysis are conducted to investigate the source of heterogeneity. When the heterogeneity is still high after heterogeneity analysis and treatment, we use Stata 14.0 software to draw Deeks’ funnel plot and evaluate the publication bias (22).
Results
Identification of studies
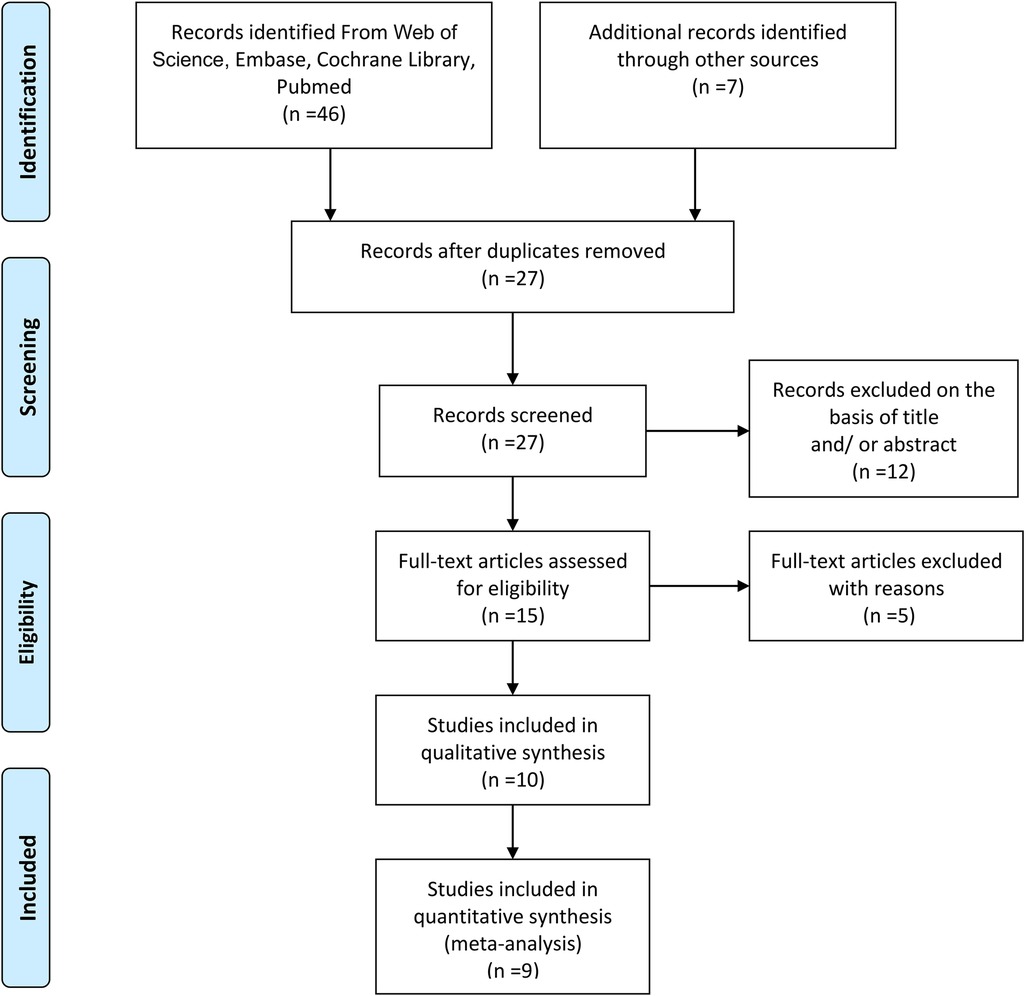
The literature screening flow diagram is detailed in Figure 1. A total of 46 references were obtained in the preliminary search in four online databases (Web of Science, Embase, Cochrane Library, PubMed). Seven additional records were identified through other sources. After removing 26 duplicates, 27 studies remained; of these, 12 articles were excluded based on the title and/or abstract, and five articles were excluded by reading their full text. One literature with qualitative synthesis was excluded. In the end, only nine studies were left for meta-analysis (14, 23–30).
Study characteristics and patient samples
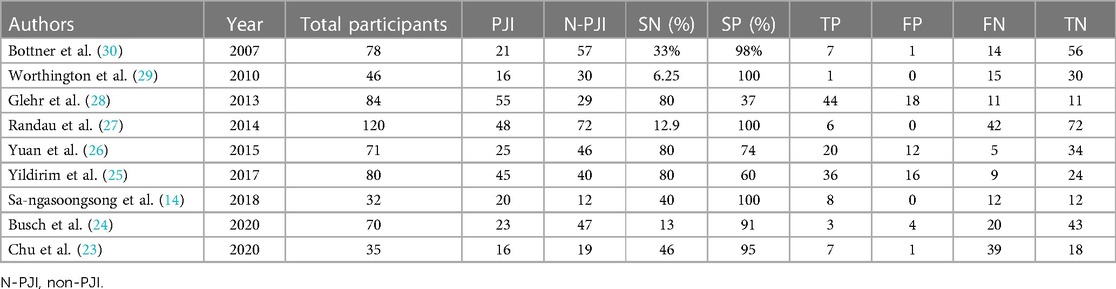
Three studies (14, 23, 26) were from East Asia and six (24, 25, 27–30) from other countries. Two retrospective studies (23, 25) and seven prospective studies (14, 24, 26–30) were included. Totally 621 patients were enrolled in the nine studies (14, 23–30), including 385 women and 236 men. The average age ranged from 62 to 72 years. BMI values were not available in four studies (23, 25, 27, 29), and those that were available in the five other studies (14, 24, 26, 28, 30) ranged from 26.7 to 30 kg/m2. The detection method was not found in the study (23). The threshold is 0.5 ng/ml in four studies (14, 24, 26, 29). MSIS is the “gold standard” for diagnosis in four studies (23, 25, 27, 28). All the studies included patients who underwent hip or knee arthroplasty, and only one study (24) included patients who underwent shoulder arthroplasty. The basic characteristics of the included studies are provided in Table 1. Table 2 summarizes the basic data extracted from each study (2 × 2 table).
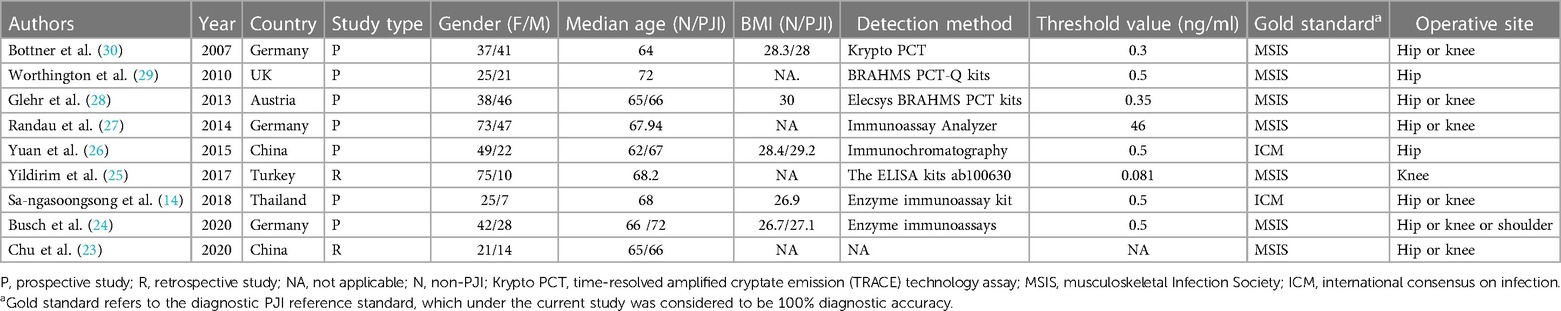
Table 1. Characteristics of the studies in meta-analysis for the diagnosis of PJI applying serum procalcitonin.
Quality and publication biases of the included studies
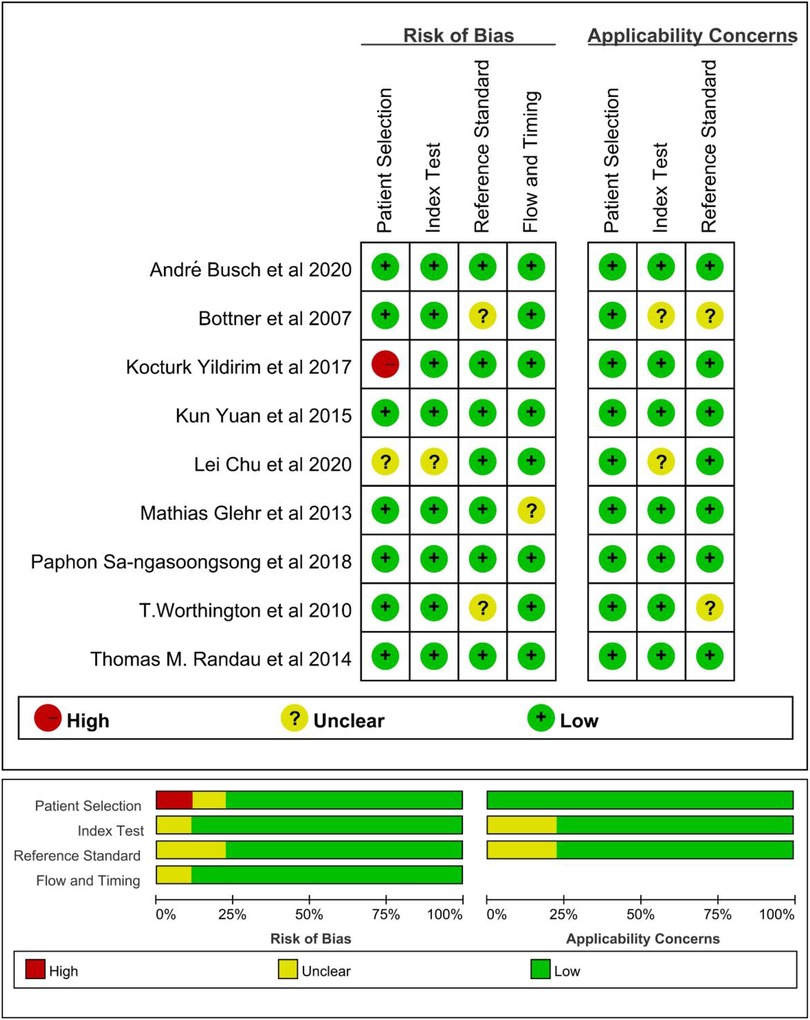
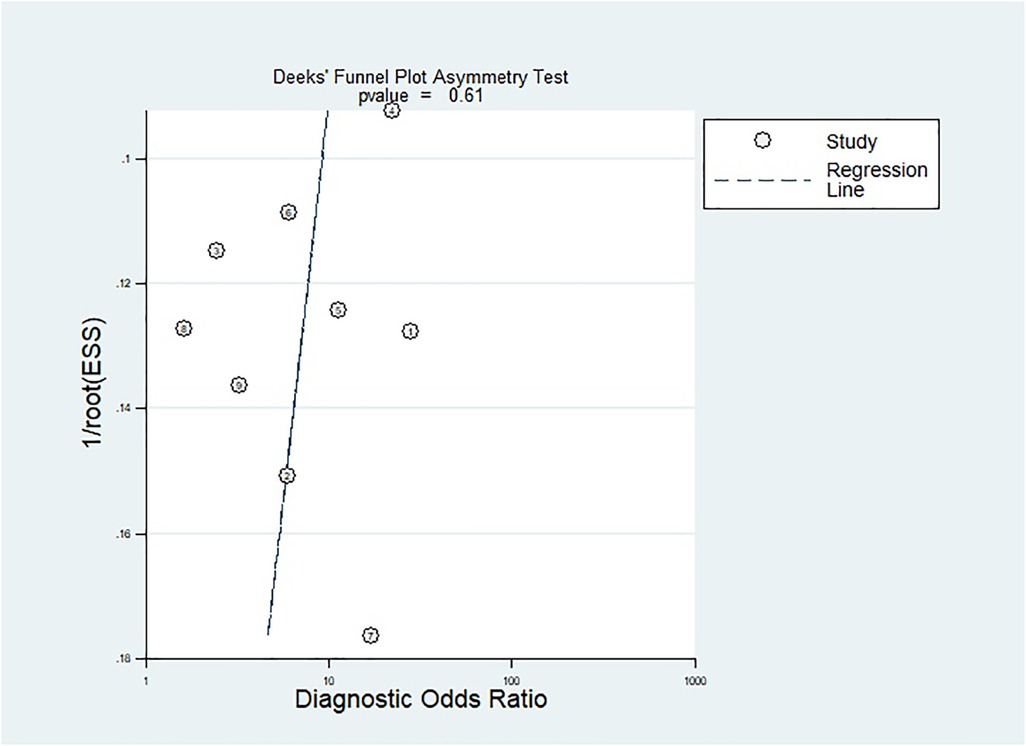
The methodological quality of the studies was assessed by using the QUADAS-2 tool (Figure 2). There is one “high risk” for patient selection, which is shown in the figure (25). Generally speaking, the quality of the included studies was satisfactory. As mentioned previously, Deeks’ test was used to analyze the publication bias of the nine included studies. The Stata command for publication bias in diagnostic meta is “midas tp fp fn tn, pubbias”. As shown in Figure 3, the funnel plot is roughly symmetric (P = 0.61). Therefore, there was no significant bias in publication.
Threshold and diagnostic accuracy of PCT for PJI
The data were imported into the Meta-DiSc 1.4 software for analysis. The Spearman correlation coefficient between the logarithm of SN and 1 − SP was 0.729 (P = 0.026). The result was significant, indicating a threshold effect in this study. Furthermore, a “shoulder-arm” point distribution on the SROC curve graph was drawn symmetrically. It also demonstrated the threshold effects of this study. The heterogeneity might be related to the threshold effects.
The value of Cochran’s Q was 10.21 in the DOR graph (P = 0.250, P > 0.001). In other words, no heterogeneity was caused by a non-threshold effect in this study. Furthermore, the I2 statistics for DOR, SN, SP, PLR, and NLR were 21.6%, 93.9%, 92.3%, 64.2%, and 85.1%, respectively. Therefore, we used the fixed-effect model to combine the aforementioned five effects.
The pooled SN of PCT for PJI diagnosis was 0.441 [95% confidence interval (CI), 0.384–0.500] (Figure 4), the pooled SP was 0.852 (95% CI, 0.811–0.888) (Figure 5), the pooled PLR was 2.271 (95% CI, 1.808–2.853) (Figure 6), the pooled NLR was 0.713 (95% CI, 0.646–0.786) (Figure 7), and the pooled DOR was 5.756 (95% CI, 3.673–9.026) (Figure 8). The area under SROC (the pooled AUC) was 0.76 (0.72–0.79) (Figure 9A). Q index was 0.6948 (Figure 9B).
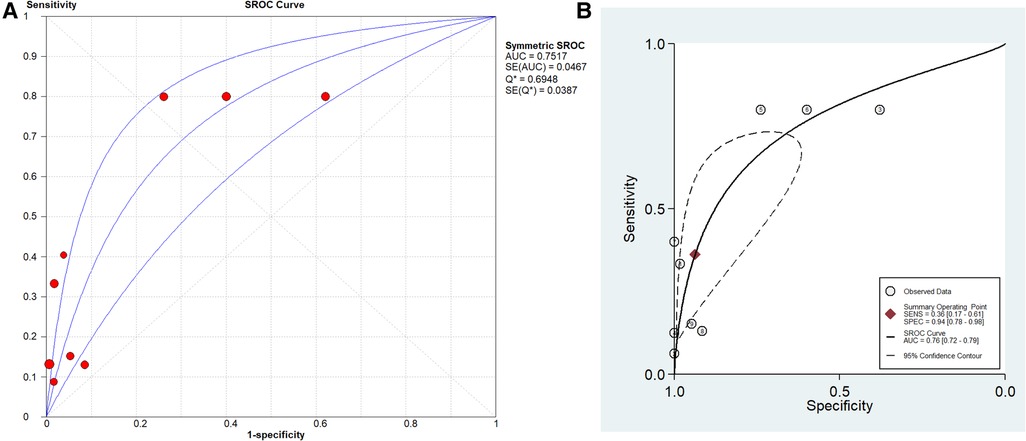
Figure 9. SROC curve of included studies. (A) Stata 14.0 software for analysis and (B) Meta-DiSc 1.4 software for analysis.
The PLR value (2.271 < 5) and NLR value (0.713 > 0.2) indicated inferior diagnostic evidence of PCT for ruling in/ruling out diagnoses. The AUC value (0.76 < 0.8) showed a low diagnostic accuracy of PCT, and the low value of DOR indicated that PCT for PJI diagnosis was an inferior target.
Stata 14.0 was selected for sensitivity analysis of the data in this study. The Stata command for sensitivity analysis of this diagnostic meta-analysis was “midas tp fp fn tn, modchk (all).” One original study (24) caused the sensitivity of results (Figure 10).
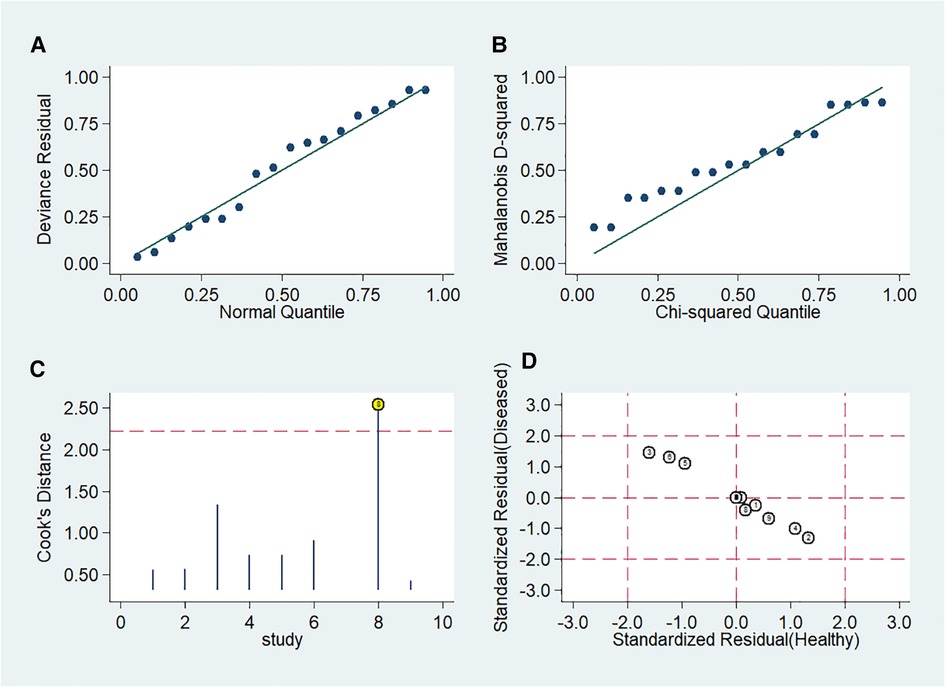
Figure 10. Influence of included studies. (A) Goodness-of-Fit. (B) Bivariate normality. (C) Influence analysis. (D) Outlier detection.
Meta-regression and subgroup analysis
Univariate meta-regression analysis was not conducted because there was no heterogeneity caused by a non-threshold effect in this study. But we performed the following subgroup analysis for variables “Study design, Threshold value, MSIS, East Asian race” (Table 3). The subgroup analysis involves categorizing participants in a study based on certain specific features, which can help researchers explore differences in reactions among different population groups and validate the results of the main analysis.
In the subgroup of retrospective studies (23, 25), the pooled SN, SP, PLR, NLR, and DOR were 0.473 (95% CI, 0.367–0.580), 0.712 (95% CI, 0.579–0.822), 2.069 (95% CI, 1.369–3.126), 0.614 (95% CI, 0.477–0.792), and 5.276 (95% CI, 2.25–12.367), respectively. In the subgroup of prospective studies (14, 24, 26–30), the pooled SN, SP, PLR, NLR, and DOR were 0.428 (95% CI, 0.360–0.498), 0.881 (95% CI, 0.838–0.915), 2.373 (95% CI, 1.805–3.120), 0.739 (95% CI, 0.665–0.820), and 5.977 (95% CI, 3.494–10.224), respectively. In the subgroup of threshold value with 0.5 ng/ml (14, 24, 26, 29), the pooled SN, SP, PLR, NLR, and DOR were 0.381 (95% CI, 0.277–0.493), 0.881 (95% CI, 0.815–0.931), 3.185 (95% CI, 1.918–5.287), 0.705 (95% CI, 0.598–0.832), and 6.643 (95% CI, 3.027–14.575), respectively. In the subgroup of “gold standard” with MSIS (23, 25, 27, 28), the pooled SN, SP, PLR, NLR, and DOR were 0.479 (95% CI, 0.407–0.552), 0.781 (95% CI, 0.709–0.843), 1.799 (95% CI, 1.390–2.328), 0.727 (95% CI, 0.637–0.829), and 4.503 (95% CI, 2.505–8.093), respectively. In the subgroup of East Asian race (14, 23, 26), the pooled SN, SP, PLR, NLR, and DOR were 0.385 (95% CI, 0.284–0.492), 0.831 (95% CI, 0.729–0.907), 3.482 (95% CI, 2.014–6.021), 0.599 (95% CI, 0.476–0.754), and 8.988 (95% CI, 3.392–23.815), respectively (Table 3).
Discussion
Patients suffering from PJI often need long-term antibiotic treatment, secondary revision surgery, joint fusion, and amputation if infection is out of control (31). Early diagnosis of PJI is essential for orthopedic surgeons. It is possible for doctors to perform debridement, antibiotics, and retention surgery without requiring secondary revision surgery for acute PJI that occurs early after surgery (32).
It is difficult to diagnose early postoperative infection only relying on serum biomarkers (such as WBC, CRP, and ESR) as they are physiologically increased due to the healing process in the early postoperative stage (33, 34). ESR and CRP were found to be highly sensitive and lowly specific under all inflammatory conditions (35, 36). CRP often rised continuously within 30–60 days after surgery. ESR and CRP have limitations on the diagnosis of PJI (11). PCT is one of the most popular indicators in the field of infection diagnosis in recent years. It is composed of 116 amino acids and is produced by Thyroid C cells under physiological conditions. During infection, the body produces a large amount of PCT under the action of various cytokines and bacterial endotoxins. PCT is a peptide hormone of calcitonin precursors or a pro-inflammatory cytokine. Bacterial endotoxins cause elevated levels of PCT in serum. In addition, some non-bacterial infections, such as multiple organ failure and large area burn, can also lead to a significant increase in serum calcitonin (37). It is suggested in many studies that PCT is a sensitive marker of bacterial infection (38–40). Therefore, it can be used to accurately distinguish bacterial infection from non-bacterial infections (41). It is increasingly used to detect and guide the use of antibiotics (42). It has also become a sensitive and specific marker for the diagnosis of septic arthritis and acute osteomyelitis (43). Elevated PCT has high specificity for sepsis caused by bacterial infection (15). It can be used as an indicator for the diagnosis of sepsis, the identification of severe bacterial infection, and the determination of the severity of the disease. In the field of joint replacement, there was no consensus on the value of PCT in diagnosing PJI. PCT was not useful in the diagnosis of local infections such as PJI, but it is important in the diagnosis of sepsis and systemic infection (44, 45). Bouaicha et al. showed PCT was not the best indicator for the diagnosis of bone and joint infections (46). Therefore, it could be used as an indicator for inclusion diagnosis instead of exclusion diagnosis because of low sensitivity and high specificity (46).
Glehr et al. found that the serum PCT (Elecsys BRAHMS PCT) threshold was set at 0.35 ng/L with SN (80%) and SP (37%) (28), thus currently PCT could be used as an additional test for infection diagnosis. Drago et al. suggested that PCT could not be used as a diagnostic indicator for PJI (47). Yoon et al. came to the conclusion that PCT with high PLR (12.4) could be used as inclusion criteria for the diagnosis of PJI, but PCT with high NLR (0.44) was not suitable for exclusion of diagnosis (48).
PJI is primarily an advanced infection, and the incidence of early infection is less than 1% (46). PCT responds directly to bacterial endotoxin, so local joint infection does not cause PCT elevation unless the local infection becomes a systemic infection. PJI is a local low-toxicity infection that cannot produce large amounts of PCT in most cases (49). This study summarized the results of different studies on the diagnosis of PJI by PCT, which provided a reference for clinical orthopedic surgeons. The results show that the value of serum PCT in the diagnosis of PJI is extremely limited. The pooled SN of PCT is poor, whereas its SP is high. One possible explanation is that PJI is a low-toxin infection that does not have the virulence to trigger PCT release. Second, transient bacteremia is a common phenomenon in healthy patients, even after toothbrushing, which may induce low-level PCT release. In addition, PCT penetration into the synovial fluid has been rarely studied. The extent of PCT penetration into the synovial fluid may vary among patients. Therefore, caution should be exercised when considering PCT in future clinical practice to avoid potentially misleading results (50). When a patient after THA or TKA has elevated serum PCT, one cannot arbitrarily judge it as PJI and take some radical treatment. While the number of patients in our study may seem limited at 621, it is worth noting that PJI is a rare complication with a low incidence rate. As such, the inclusion of 621 cases in our analysis actually represents a substantial sample size. Moreover, our meta-analysis incorporates data from nine separate studies originating from six distinct countries, rendering our findings remarkably representative. However, there are some limitations in our research. First, there is no gold standard for PJI diagnosis. Second, many studies have shown that the biological biomarker in synovial fluid has a high diagnostic ability for PJI (51–53). We only studied serum PCT. Third, there were few included cases, and the role of PCT for PJI diagnosis could not be completely reflected. Fourth, in the included literature, some patients with acute infection were clearly excluded, and the other part was not clearly stated. Therefore, we cannot effectively represent the proportion of acute and chronic infections. Fifth, the detection method and time are not similar in all studies, which may have a particular impact on the results.
The PLR value (2.271) and NLR value (0.713) indicate inferior diagnostic evidence of PCT for ruling-in/ruling-out diagnoses. The AUC value (0.76 < 0.80) shows a low diagnostic accuracy of PCT, and the low value of DOR indicates that PCT for PJI diagnosis is an inferior target.
Conclusion
This study showed that PCT detection of PJI has poor diagnostic accuracy. PCT is not recommended for use as a rule-out/rule-in diagnostic tool. Consequently, further studies are needed to find markers associated with PCT to improve diagnostic ability. This study examined the current understanding of PCT's diagnostic potential in PJI, providing clinicians with an exclusive reference.
Author contributions
Conceptualization: XS and HZ. Literature search: XS, YuL, HZ, YiL, ZL, and YaL. Software: XS, YiL, and YaL. Writing: XS, YuL, YaL, and HZ. All authors contributed to the article and approved the submitted version.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Postler A, Lützner C, Beyer F, Tille E, Lützner J. Analysis of total knee arthroplasty revision causes. BMC Musculoskelet Disord. (2018) 19(1):55. doi: 10.1186/s12891-018-1977-y
2. Lum ZC, Natsuhara KM, Shelton TJ, Giordani M, Pereira GC, Meehan JP. Mortality during total knee periprosthetic joint infection. J Arthroplasty. (2018) 33(12):3783–8. doi: 10.1016/j.arth.2018.08.021
3. Ong KL, Kurtz SM, Lau E, Bozic KJ, Berry DJ, Parvizi J. Prosthetic joint infection risk after total hip arthroplasty in the Medicare population. J Arthroplasty. (2009) 24(6 Suppl):105–9. doi: 10.1016/j.arth.2009.04.027
4. Dy CJ, Bozic KJ, Pan TJ, Wright TM, Padgett DE, Lyman S. Risk factors for early revision after total hip arthroplasty. Arthritis Care Res (Hoboken). (2014) 66(6):907–15. doi: 10.1002/acr.22240
5. Zhang H, Xie S, Li Y, Li J, Deng P, Zeng H, et al. The potential performance of serum albumin to globulin ratio, albumin and globulin in the diagnosis of periprosthetic joint infection and prediction of reinfection following reimplantation. BMC Musculoskelet Disord. (2022) 23(1):730. doi: 10.1186/s12891-022-05533-0
6. Yu JS, Bornes TD, Youssef M, Tam KW, Nocon AA, Sculco PK, et al. Which combination is best? A comparison of the predictive potential of serum biomarker combinations to diagnose periprosthetic joint infection. J Arthroplasty. (2023) 38(7Suppl 2):S381–8. doi: 10.1016/j.arth.2023.05.025
7. Boelch SP, Rüeckl K, Streck LE, Szewczykowski V, Weißenberger M, Jakuscheit A, et al. Diagnosis of chronic infection at total hip arthroplasty revision is a question of definition. BioMed Res Int. (2021) 2021:8442435. doi: 10.1155/2021/8442435
8. Hutchinson ID, Mathew JI, Taylor SA, Fu MC, Dines DM, Carli AV, et al. How accurate are positive preoperative aspiration culture results in shoulder periprosthetic joint infection? A concordance study. J Shoulder Elbow Surg. (2023) 32(6s):S17–22. doi: 10.1016/j.jse.2023.02.013
9. Parvizi J, Tan TL, Goswami K, Higuera C, Della Valle C, Chen AF, et al. The 2018 definition of periprosthetic hip and knee infection: an evidence-based and validated criteria. J Arthroplasty. (2018) 33(5):1309–4.e2. doi: 10.1016/j.arth.2018.02.078
10. McNally M, Sousa R, Wouthuyzen-Bakker M, Chen AF, Soriano A, Vogely HC, et al. The EBJIS definition of periprosthetic joint infection. Bone Joint J. (2021) 103-b(1):18–25. doi: 10.1302/0301-620X.103B1.BJJ-2020-1381.R1
11. Toossi N, Adeli B, Rasouli MR, Huang R, Parvizi J. Serum white blood cell count and differential do not have a role in the diagnosis of periprosthetic joint infection. J Arthroplasty. (2012) 27(8 Suppl):51–4. doi: 10.1016/j.arth.2012.03.021
12. Berbari E, Mabry T, Tsaras G, Spangehl M, Erwin PJ, Murad MH, et al. Inflammatory blood laboratory levels as markers of prosthetic joint infection: a systematic review and meta-analysis. J Bone Joint Surg Am. (2010) 92(11):2102–9. doi: 10.2106/JBJS.I.01199
13. Bottagisio M, Viganò M, Pellegrini A, Logoluso N, Zagra L, Prina A, et al. Evaluation of synovial calprotectin by using a lateral flow test for the diagnosis of prosthetic joint infections. Diagnostics (Basel). (2023) 13(4):741. doi: 10.3390/diagnostics13040741
14. Sa-Ngasoongsong P, Wongsak S, Jarungvittayakon C, Limsamutpetch K, Channoom T, Kawinwonggowit V. Comparison of synovial fluid and serum procalcitonin for diagnosis of periprosthetic joint infection: a pilot study in 32 patients. BioMed Res Int. (2018) 2018:8351308. doi: 10.1155/2018/8351308
15. Ferrière F. Procalcitonin, a new marker for bacterial infections. Ann Biol Clin. (2000) 58(1):49–59.
16. Simon L, Gauvin F, Amre DK, Saint-Louis P, Lacroix J. Serum procalcitonin and C-reactive protein levels as markers of bacterial infection: a systematic review and meta-analysis. Clin Infect Dis. (2004) 39(2):206–17. doi: 10.1086/421997
17. Morgenthaler NG, Struck J, Fischer-Schulz C, Seidel-Mueller E, Beier W, Bergmann A. Detection of procalcitonin (PCT) in healthy controls and patients with local infection by a sensitive ILMA. Clin Lab. (2002) 48(5–6):263–70.12071576
18. Moher D, Liberati A, Tetzlaff J, Altman DG. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. J Clin Epidemiol. (2009) 62(10):1006–12. doi: 10.1016/j.jclinepi.2009.06.005
19. Whiting PF, Rutjes AW, Westwood ME, Mallett S, Deeks JJ, Reitsma JB, et al. QUADAS-2: a revised tool for the quality assessment of diagnostic accuracy studies. Ann Intern Med. (2011) 155(8):529–36. doi: 10.7326/0003-4819-155-8-201110180-00009
20. Deeks JJ. Systematic reviews in health care: systematic reviews of evaluations of diagnostic and screening tests. Br Med J. (2001) 323(7305):157–62. doi: 10.1136/bmj.323.7305.157
21. Glas AS, Lijmer JG, Prins MH, Bonsel GJ, Bossuyt PM. The diagnostic odds ratio: a single indicator of test performance. J Clin Epidemiol. (2003) 56(11):1129–35. doi: 10.1016/S0895-4356(03)00177-X
22. Deeks JJ, Macaskill P, Irwig L. The performance of tests of publication bias and other sample size effects in systematic reviews of diagnostic test accuracy was assessed. J Clin Epidemiol. (2005) 58(9):882–93. doi: 10.1016/j.jclinepi.2005.01.016
23. Chu L, Ren YL, Yang JS, Yang J, Zhou H, Jiang HT, et al. The combinations of multiple factors to improve the diagnostic sensitivity and specificity after artificial joint infection. J Orthop Surg Res. (2020) 15(1):161. doi: 10.1186/s13018-020-01669-8
24. Busch A, Jäger M, Engler H, Haversath M, Bielefeld C, Landgraeber S, et al. Is procalcitonin (PCT) a reliable biomarker for preoperative diagnosing of low grade periprosthetic joint infection? A prospective study. BMC Musculoskelet Disord. (2020) 21(1):257. doi: 10.1186/s12891-020-03266-6
25. Yildirim K, Misir A, Kizkapan TB, Ozcamdalli M, Duygulu F. Neopterin, interleukin-6, procalcitonin, C-reactive protein and PET-CT staining as markers in infected total knee prosthesis, a retrospective analysis. Acta Orthop Belg. (2017) 83(4):624–30.30423671
26. Yuan K, Li WD, Qiang Y, Cui ZM. Comparison of procalcitonin and C-reactive protein for the diagnosis of periprosthetic joint infection before revision total hip arthroplasty. Surg Infect (Larchmt). (2015) 16(2):146–50. doi: 10.1089/sur.2014.034
27. Randau TM, Friedrich MJ, Wimmer MD, Reichert B, Kuberra D, Stoffel-Wagner B, et al. Interleukin-6 in serum and in synovial fluid enhances the differentiation between periprosthetic joint infection and aseptic loosening. PLoS One. (2014) 9(2):e89045. doi: 10.1371/journal.pone.0089045
28. Glehr M, Friesenbichler J, Hofmann G, Bernhardt GA, Zacherl M, Avian A, et al. Novel biomarkers to detect infection in revision hip and knee arthroplasties. Clin Orthop Relat Res. (2013) 471(8):2621–8. doi: 10.1007/s11999-013-2998-3
29. Worthington T, Dunlop D, Casey A, Lambert R, Luscombe J, Elliott T. Serum procalcitonin, interleukin-6, soluble intercellular adhesin molecule-1 and IgG to short-chain exocellular lipoteichoic acid as predictors of infection in total joint prosthesis revision. Br J Biomed Sci. (2010) 67(2):71–6. doi: 10.1080/09674845.2010.11730294
30. Bottner F, Wegner A, Winkelmann W, Becker K, Erren M, Götze C. Interleukin-6, procalcitonin and TNF-alpha: markers of peri-prosthetic infection following total joint replacement. J Bone Joint Surg Br. (2007) 89(1):94–9. doi: 10.1302/0301-620X.89B1.17485
31. Galat DD, McGovern SC, Larson DR, Harrington JR, Hanssen AD, Clarke HD. Surgical treatment of early wound complications following primary total knee arthroplasty. J Bone Joint Surg Am. (2009) 91(1):48–54. doi: 10.2106/JBJS.G.01371
32. Huotari K, Vuorinen M, Vasara A. Debridement, antimicrobials, and implant retention in the treatment of late acute and early acute Staphylococcus aureus prosthetic joint infections. Infect Dis (Lond Engl). (2023) 55(8):525–32. doi: 10.1080/23744235.2023.2217898
33. Laffey JG, Boylan JF, Cheng DC. The systemic inflammatory response to cardiac surgery: implications for the anesthesiologist. Anesthesiology. (2002) 97(1):215–52. doi: 10.1097/00000542-200207000-00030
34. Dupont C, Rodenbach J, Flachaire E. The value of C-reactive protein for postoperative monitoring of lower limb arthroplasty. Ann Readapt Med Phys. (2008) 51(5):348–57. doi: 10.1016/j.annrmp.2008.01.014
35. Windgassen EB, Funtowicz L, Lunsford TN, Harris LA, Mulvagh SL. C-reactive protein and high-sensitivity C-reactive protein: an update for clinicians. Postgrad Med. (2011) 123(1):114–9. doi: 10.3810/pgm.2011.01.2252
36. Maharajan K, Patro DK, Menon J, Hariharan AP, Parija SC, Poduval M, et al. Serum procalcitonin is a sensitive and specific marker in the diagnosis of septic arthritis and acute osteomyelitis. J Orthop Surg Res. (2013) 8:19. doi: 10.1186/1749-799X-8-19
37. Chen A, Fei J, Deirmegian C. Diagnosis of periprosthetic infection: novel developments. Orthop Clin North Am. (2016) 47(1):1–9. doi: 10.1016/j.ocl.2015.08.003
38. Fuchs A, Gotta V, Decker ML, Szinnai G, Baumann P, Bonhoeffer J, et al. Cytokine kinetic profiles in children with acute lower respiratory tract infection: a post hoc descriptive analysis from a randomized control trial. Clin Microbiol Infect. (2018) 24(12):1341.e1–7. doi: 10.1016/j.cmi.2018.03.016
39. van der Does Y, Limper M, Jie KE, Schuit SCE, Jansen H, Pernot N, et al. Procalcitonin-guided antibiotic therapy in patients with fever in a general emergency department population: a multicentre non-inferiority randomized clinical trial (HiTEMP study). Clin Microbiol Infect. (2018) 24(12):1282–9. doi: 10.1016/j.cmi.2018.05.011
40. van der Does Y, Rood PPM, Ramakers C, Schuit SCE, Patka P, van Gorp ECM, et al. Identifying patients with bacterial infections using a combination of C-reactive protein, procalcitonin, TRAIL, and IP-10 in the emergency department: a prospective observational cohort study. Clin Microbiol Infect. (2018) 24(12):1297–304. doi: 10.1016/j.cmi.2018.09.007
41. Faesch S, Cojocaru B, Hennequin C, Pannier S, Glorion C, Lacour B, et al. Can procalcitonin measurement help the diagnosis of osteomyelitis and septic arthritis? A prospective trial. Ital J Pediatr. (2009) 35(1):33. doi: 10.1186/1824-7288-35-33
42. Maseda E, Suarez-de-la-Rica A, Anillo V, Tamayo E, García-Bernedo CA, Ramasco F, et al. Procalcitonin-guided therapy may reduce length of antibiotic treatment in intensive care unit patients with secondary peritonitis: a multicenter retrospective study. J Crit Care. (2015) 30(3):537–42. doi: 10.1016/j.jcrc.2014.12.014
43. Shen CJ, Wu MS, Lin KH, Lin WL, Chen HC, Wu JY, et al. The use of procalcitonin in the diagnosis of bone and joint infection: a systemic review and meta-analysis. Eur J Clin Microbiol Infect Dis. (2013) 32(6):807–14. doi: 10.1007/s10096-012-1812-6
44. Wang H, Yin F, Shen D-X, Zhang Y-J, Luo Y-P, Liu C-J, et al. Predictive value of procalcitonin for excluding bloodstream infection: results of a retrospective study and utility of a rapid, quantitative test for procalcitonin. J Int Med Res. (2013) 41(5):1671–81. doi: 10.1177/0300060513497558
45. Feng S, Shen X, Hao X, Cao X, Li X, Yao X, et al. Polycyclic and nitro-polycyclic aromatic hydrocarbon pollution characteristics and carcinogenic risk assessment of indoor kitchen air during cooking periods in rural households in north China. Environ Sci Pollut Res Int. (2021) 28(9):11498–508. doi: 10.1007/s11356-020-11316-8
46. Bouaicha S, Blatter S, Moor BK, Spanaus K, Dora C, Werner CML. Early serum procalcitonin level after primary total hip replacement. Mediators Inflamm. (2013) 2013:927636. doi: 10.1155/2013/927636
47. Drago L, Vassena C, Dozio E, Corsi MM, De Vecchi E, Mattina R, et al. Procalcitonin, C-reactive protein, interleukin-6, and soluble intercellular adhesion molecule-1 as markers of postoperative orthopaedic joint prosthesis infections. Int J Immunopathol Pharmacol. (2011) 24(2):433–40. doi: 10.1177/039463201102400216
48. Yoon JR, Yang SH, Shin YA-O. Diagnostic accuracy of interleukin-6 and procalcitonin in patients with periprosthetic joint infection: a systematic review and meta-analysis. Int Orthop. (2018) 42(6):1213–26. doi: 10.1007/s00264-017-3744-3
49. Brunkhorst FM, Wegscheider K, Forycki ZF, Brunkhorst R. Procalcitonin for early diagnosis and differentiation of SIRS, sepsis, severe sepsis, and septic shock. Intensive Care Med. (2000) 26(Suppl 2):S148–52. doi: 10.1007/s001340051134
50. Lucas VS, Gafan G, Dewhurst S, Roberts GJ. Prevalence, intensity and nature of bacteraemia after toothbrushing. J Dent. (2008) 36(7):481–7. doi: 10.1016/j.jdent.2008.03.005
51. Nace J, Siddiqi A, Talmo CT, Chen AF. Diagnosis and management of fungal periprosthetic joint infections. J Am Acad Orthop Surg. (2019) 27(18):e804–18. doi: 10.5435/JAAOS-D-18-00331
52. Xu C, Guo H, Chen JY. Intra-operative diagnosis of periprosthetic joint infection can rely on frozen sections in patients without synovial fluid analyses. Int Orthop. (2019) 43(6):1303–8. doi: 10.1007/s00264-018-4227-x
Keywords: procalcitonin, periprosthetic joint infection, diagnosis, biological markers, meta-analysis
Citation: Sun X, Li Y, Lv Y, Liu Y, Lai Z, Zeng Y and Zhang H (2024) Diagnostic value of procalcitonin in patients with periprosthetic joint infection: a diagnostic meta-analysis. Front. Surg. 11:1211325. doi: 10.3389/fsurg.2024.1211325
Received: 24 April 2023; Accepted: 22 March 2024;
Published: 10 April 2024.
Edited by:
Jaimo Ahn, University of Michigan, United StatesReviewed by:
Roberto Berebichez, Centro Médico ABC, MexicoArunika Mukhopadhaya, Indian Institute of Science Education and Research Mohali, India
© 2024 Sun, Li, Lv, Liu, Lai, Zeng and Zhang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Xiaobo Sun gzsunxiaobo@163.com Haitao Zhang zht1997zht@163.com
†These authors have contributed equally to this work
Abbreviations PJI, periprosthetic joint infection; PCT, procalcitonin; PLR, positive likelihood ratio; NLR, negative likelihood ratio; DOR, diagnostic odds ratio; AUC, the area under the summary receiver operating characteristic curve; MSIS, Musculoskeletal Infection Society; ICM, international consensus meeting; CRP, C-reactive protein; ESR, erythrocyte sedimentation rate; WBC, white blood cell; PRISMA, preferred reporting items for systematic reviews and meta-analyses; AAOS, the American Academy of Orthopedic Surgeon.
 Xiaobo Sun
Xiaobo Sun Yijin Li2,†
Yijin Li2,†  Yirong Zeng
Yirong Zeng