Safety and oncological outcome of early intraoperative intravesicle mitomycin C vs. deferred instillation in patients receiving robot-assisted radical nephroureterectomy
- 1Department of Urology, China Medical University Hospital, Taichung, Taiwan
- 2School of Medicine, China Medical University, Taichung, Taiwan
- 3Department of Radiology, China Medical University Hospital, Taichung, Taiwan
Introduction: Radical nephroureterectomy with concurrent bladder cuff excision (RNUBCE) is the gold standard surgical approach for high-risk primary upper tract urothelial carcinoma (UTUC). Given the notably high incidence of bladder tumor recurrence following this procedure, this study aimed to evaluate the effect and safety of intraoperative mitomycin-C (MMC) instillation vs. deferred instillation on overall oncological outcomes following robot-assisted RNUBCE.
Methods: This is a retrospective chart review study. Patients with non-invasive (N0, not T3/T4) UTUC who underwent robotic RNUBCE combined an intraoperative MMC instillation or a deferred MMC instillation after surgery at a medical center in Taiwan between November 2013 and June 2020 were eligible for inclusion. Patients with prior bladder UC, carcinomas of other origins, received neoadjuvant chemotherapy, and had undergone kidney transplantation were excluded. All surgeries were executed by a single surgical team under the guidance of the same surgeon. The primary outcomes was the risk of bladder tumor recurrence between patients received intraoperative (IO) vs. deferred MMC instillation postoperatively (PO) during one-year follow-up. The secondary outcome was postoperative adverse events assessed by the Clavien–Dindo classification. Univariate and multivariable Cox regression analyses were performed to determine the associations between study variables and the outcomes.
Results: A total of 54 patients were included in the analysis. 12 (22.2%) patients experienced a bladder tumor recurrence during follow-up (IO: 7.7%, PO: 35.7%, p < 0.021). After adjustment in the multivariable, intraoperative MMC instillation was significantly associated with lower risk of bladder recurrence [adjusted hazard ratio (aHR) = 0.15, 95% CI: 0.03–0.81, p = 0.028]. No MMC-related Clavien–Dindo Grade III–IV adverse events were found in either group.
Conclusion: IIntraoperative MMC instillation is safe and associated with a lower bladder tumor recurrence risk in patients undergoing robotic RNUBCE for UTUC than deferred instillation. Future large, prospective studies are still warranted to confirm the findings.
Introduction
Upper tract urothelial carcinoma (UTUC) is a urothelial malignancy involving the renal pelvis or ureter. It is a relatively rare disease and accounts for approximately 5%–10% of all UCs (1). Radical nephroureterectomy with a bladder-cuff excision (RNUBCE) is the gold standard surgical approach for high-risk UTUC across the majority of patients (2). The risk of tumor recurrence in the bladder after the surgery is considerable, estimated at 22%–47% (3). The initial intravesical relapse typically occurs within the first two years following the treatments, and recurrence is regarded as common with a lifelong risk (3).
In superficial bladder cancers, a prospective trial has revealed that the administration of immediate mitomycin-C (MMC) within 24 h after transurethral resection of bladder tumor (TURBT) outperformed the deferred application of MMC over two weeks (4). Additionally, apart from the commonly used MMC, other intravesical chemotherapeutics like epirubicin and pirarubicin (THP) have exhibited effectiveness in reducing the risk of recurrence (5–7). It has been demonstrated that single-dose intravesical chemotherapy can successfully prevent bladder cancer recurrence following NU by lowering the intravesical recurrence rate (8). However, concerns about the risk of adverse events from extravasation of these intravesical agents still exist (6, 9, 10). There is uncertainty regarding the optimal timing for initiating intravesical chemotherapeutic agents, whether in the early stages of immediate postoperative care or after confirming the cystogram, which indicates the absence of extravasation (6). The absence of a specified timing for the planned instillation has left a gap in understanding, and its potential to adversely impact the efficacy of reducing bladder recurrence remains uncertain (8).
In this study, we conducted a retrospective evaluation to scrutinize the oncological results and safety profiles associated with the prompt administration of a single dose of MMC into the bladder during the execution of a standardized robot-assisted radical nephroureterectomy (RARNU) technique. Our analysis focused on the bladder tumor recurrence (BTR) for one year and short-term adverse events.
Methods
Patient selection
Patients' electrical medical records at a tertiary referral center in Taiwan were retrospectively reviewed between November 2013 and June 2020. The inclusion criteria were: patients with non-invasive disease (N0, not T3/T4) UTUC who underwent robotic RNUBCE combined early intraoperative MMC instillation or deferred instillation. The exclusion criteria were patients with prior bladder UC, carcinomas of other origins, who received neoadjuvant chemotherapy, and who had undergone kidney transplantation. Patients were categorized into two groups based on the timing of MMC administration: patients received early MMC instillation intraoperatively (i.e., the IO group) and deferred MMC instillation at least 24 h postoperatively (i.e., the PO group).
The study did not employ random allocation; instead, treatment modalities were determined by temporal factors. Specifically, IO treatments were administered after February 2018, whereas prior to this, PO treatments were used. At that time, the decision to use IO instead of PO was based on emerging research findings in the literature suggesting that early instillation could potentially prevent bladder recurrence following transurethral resection of a bladder tumor.
Surgical technique
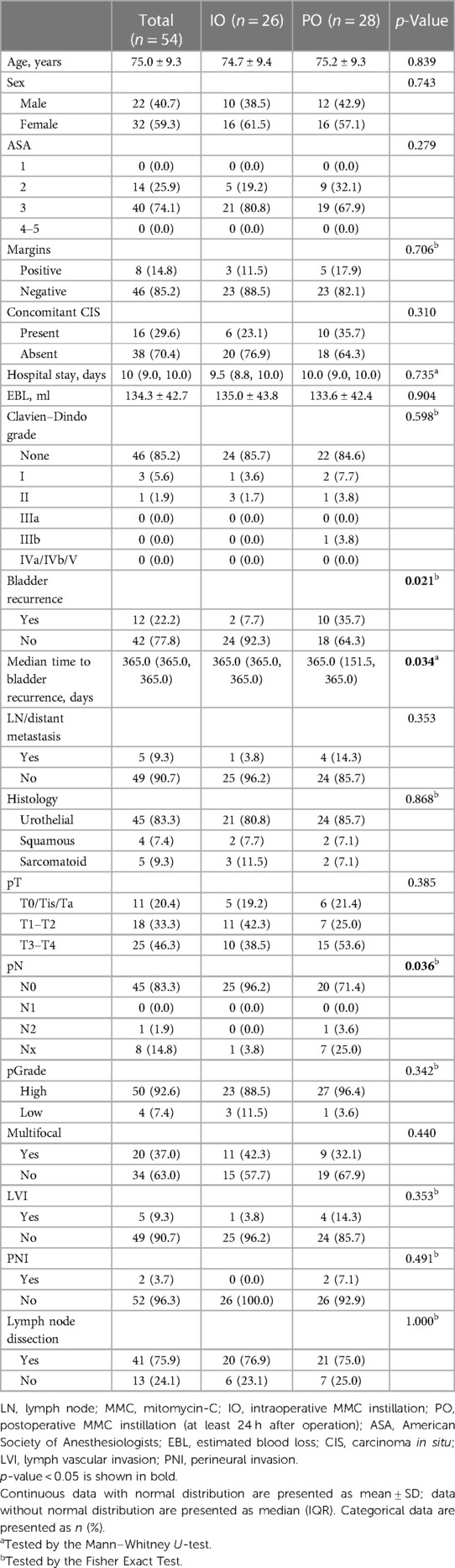
We implemented a meticulous redocking technique for RARNU, marked by precision in the dissection across the entire ureter segment, lymph node dissection based on a defined template, precise bladder cuff excision, and a meticulous assessment of bladder closure's water-tightness. Employing the da Vinci Si robot-assisted platform by Intuitive Surgical Inc. (Sunnyvale, CA, USA), all surgical interventions were conducted under insufflation pressures ranging from 10 to 12 mmHg, utilizing a standard insufflation device. The entire procedure was executed with a minimally invasive approach involving the re-docking of the robot, a technique that preserves the range of motion for each robotic arm. A standardized step-wise transperitoneal approach is used with an extravesical excision of a bladder cuff with the ureter ligated with a Hemlock clip distal to the tumor after renal hilum controlled (Figure 1).
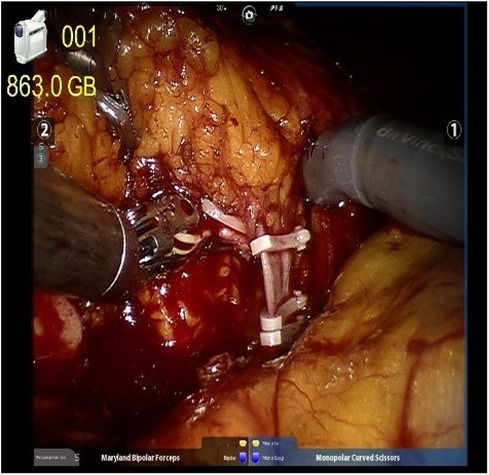
Figure 1. The renal artery and renal vein were double-ligated in the proximal end and single-ligated in the distal end with surgical clips.
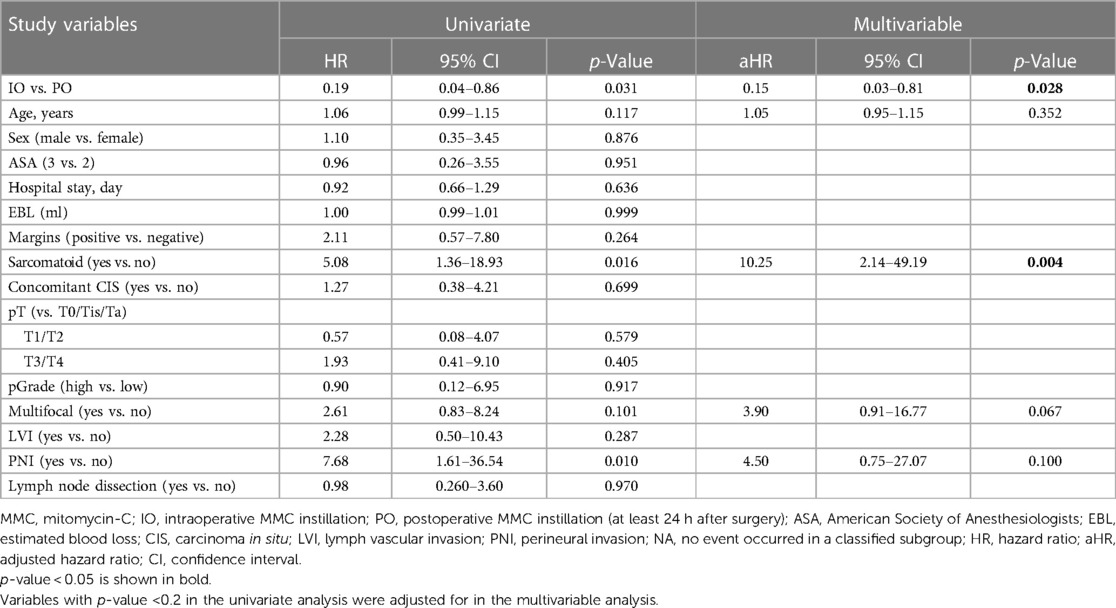
In the intraoperative (IO) MMC group, after the ureter ligated with Hemlock clip (Teleflex Inc., Morrisville, NC, USA), intravesical mitomycin C instillation was followed as MMC 40 mg in 40 ml of sterile water was injected into the bladder through Foley catheter and then clamped. After a certain period, typically 45–60 min, the catheter is unclamped, and the chemotherapeutic agents are drained passively, followed by irrigation. Irrigation of the bladder, before excision of the distal ureter and bladder cuff, with 200–250 ml saline to avoid extravasation from the bladder with chemical toxicity to the surrounding tissue in the pelvis. During the clamping of the Foley catheter, the operation was carried on for excision of the distal ureter and bladder cuff, including the ureteric orifice (Figure 2).
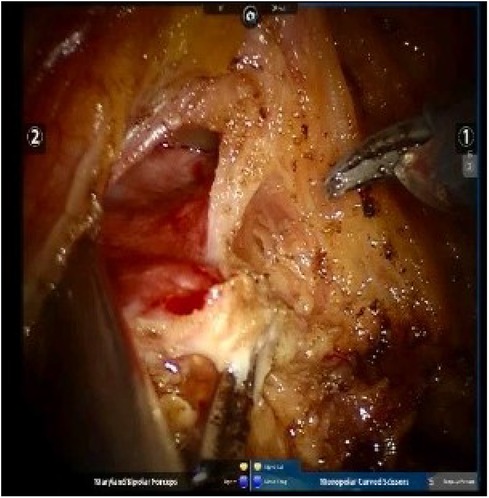
Figure 2. Excision of the distal ureter and bladder cuff, including the ureteric orifice, was done along the ureter.
The cystotomy is closed intracorporeally with an absorbable 2-0 V-Loc (Covidien, Mansfield, MA, USA) or 2-0 Quill (Westwood, MA, USA) (20 cm) continuous suture and bladder leak test is followed (Figure 3). Template-based lymph node dissection is done for every patient. The specimen is placed within an EndoCatchTM bag (Covidien, Mansfield, MA, USA) and removed through an extension of the camera port. All surgeries were executed by a single surgical team under the guidance of the same surgeon.
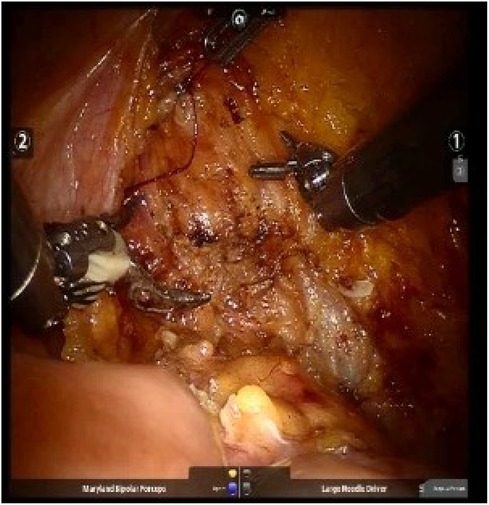
Figure 3. The cystotomy was closed intracorporeally with absorbable continuous sutures, followed by a bladder leak test.
Outcome measures
The primary outcome was the risk of bladder recurrence and after surgery at follow-up. The secondary outcome was the occurrence of postoperative adverse events assessed by the Clavien–Dindo classification (11).
Post RARNU, surveillance for bladder recurrence encompassed cystoscopy and urine cytology conducted at three-month intervals within the initial two years, transitioning to intervals of 6–12 months thereafter. Furthermore, imaging procedures such as contrast-enhanced abdominal/pelvic CT or MRI were performed annually, alongside chest imaging (12).
Statistical analysis
Descriptive statistics of patients' demographic and clinical characteristics are presented as number (n) and percentage (%) and performed by the chi-squared or Fisher Exact test. Continuous data with normal distribution are presented as mean ± standard deviation (SD) using Student's test; continuous data without normal distribution are presented as the median and interquartile range (IQR) and performed by Mann–Whitney U-test. Hazard ratios (HRs) and 95% confidence intervals (CIs) produced by Cox regression analysis were used to evaluate the associations between the study variables and the outcomes. Any variable with a p-value <0.2 in the univariate analysis was input in the multivariable analysis. Kaplan–Meier plot with log-rank test were depicted to demonstrate the survival outcomes between the comparison groups. The results were considered statistically significant at p < 0.05, and all statistical analyses were performed using the statistical package SPSS for Windows (Version 21.0, SPSS Inc., IBM Corp., Armonk, NY, USA).
Result
Characteristics of the study cohort
Data from 97 patients undergoing RARNU for UTUC during the study period were obtained. Of the patients, a total of 54 patients were included in the analysis after applying the inclusion and exclusion criteria. Table 1 shows the baseline demographic and clinical characteristics of 54 patients. Of whom 26 patients received MMC intraoperatively (the IO group), while 28 received deferred MMC at least 24 h postoperatively (the PO group). Patients' mean age was 75.0 ± 9.3 years, and 59.3% were females. No significant differences in presence of concomitant carcinoma-in-situ (CIS), surgical margin status, pathologic T (pT), pathologic grade (pGrade), multifocality, lymph vascular invasion (LVI), perineural invasion (PNI) or lymph node dissection were noted between the groups. We identified positive surgical margins as those confirmed to be margin positive in the ureters. One-year bladder recurrence rate (7.7% vs. 35.7%, p = 0.021) and median time to bladder recurrence (p = 0.034) were significantly different between the two groups.
Risk of bladder recurrence between IO vs. PO
Table 2 summarizes the univariate and multivariable Cox analyses on bladder recurrence. After adjusting for age, sarcomatoid, multifocal and PNI, IO remained significantly associated with a lower risk for bladder recurrence compared to PO [adjusted hazard ratio (aHR) = 0.15, 95% CI = 0.03–0.81, p = 0.028]. The Kaplan-Meier curves of bladder recurrence-free survival among the IO and PO groups are depicted in Figure 4.
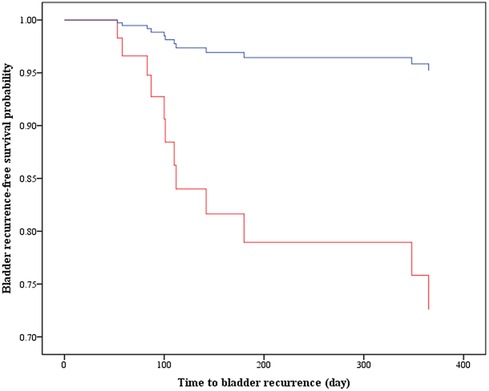
Figure 4. Kaplan–Meier curves display the estimated bladder recurrence-free survival. IO, intraoperative MMC instillation; PO, postoperative MMS instillation (i.e., deferred MMC at least 24 h after surgery); aHR, adjusted hazard ratio; CI, confidence interval.
Additional analysis on the factors associated with lymph node (LN)/distant metastasis showed no significant impact of MMC instillation timing (Supplementary Table S1).
Adverse events
There were seven postoperative Clavien–Dindo Grade I or II complications, including two patients with ileus, one patient with pneumonia, two patients with sepsis, one with a wound infection and one with self-limiting delirium, all managed conservatively (Table 1). Ileus led to a prolonged stay with a median (range) of 6 (4–11) days. Only one Clavien–Dindo Grade III complication was noted as wound infection managed as debridement and delayed suture. Fortunately, there is no adverse event potentially related to MMC instillation was noted, both during the inpatient stay and at first clinic follow-up at 2–3 weeks postoperatively.
Discussion
We retrospectively investigated the adult patients undergoing RARNU for UTUC with intraoperative MMC vs. deferred MMC instillation at our institution. The results indicate that intraoperative MMC is independently associated with a lower risk for bladder recurrence than deferred MMC administration after surgery during a 1-year follow-up. For safety issues, the intraoperative instillation group showed comparative safety profile to the deferred instillation group, with no MMC-related adverse event observed.
The occurrence of bladder recurrence after RNU is highly prevalent, thus emphasizing the necessity of routine cystoscopic surveillance, a recommendation prominently outlined in the NCCN guidelines (12, 13). The high bladder recurrence rate can be attributed to potential factors such as tumor seeding and implantation. This phenomenon involves the dissemination of malignant cells along the urinary tract lining, with these cells infiltrating downstream sites by traversing the urothelium (5, 6). Therefore, treatment strategies have focused on reducing the bladder recurrence rate after RNU, and it has been suggested that the intravesical instillation of chemotherapeutic agents significantly decreased the risk of bladder recurrence in patients with primary UTUC (5, 14–16). In theory, there is a potential for preventive action post-nephroureterectomy, as it could theoretically involve the destruction of viable seeding cells originating from the upper tract or impede the development of metachronous tumors proliferating within the bladder (5).
Adverse events after early MMC instillation still left a big concern, in which primary clinical concern was the potential extravasation of the MMC. Based on the risk of possible extravasation of MMC, the EAU guideline only suggests installing a single postoperative dose of intravesical chemotherapy, such as mitomycin C or pirarubicin, 2–10 days after surgery. The same concern is also mentioned in the ODMIT-C Trial, the first prospective and PCT trial as postoperative IVC following RNU. In that trial, the pragmatic decision was made to administer the chemotherapy when the urologist was as confident as possible that the bladder had healed and was safe to remove the Foley catheter. Thus, the timing of MMC administration showed variation between patients based on the duration of catheterization (6).
Increasing literature support that MMC instillation within the first 24 h is more effective than deferred instillation after two weeks or prolonged adjuvant MMC. This finding is consistent with the bladder tumor research, which reveals that the best time to administer the first instillation is within 24 h to prevent bladder recurrence after transurethral resection of bladder tumor (TURBT) (8). A RCT by Bosschieter J et al. found that the first instillation beginning within 24 h shows lower bladder recurrence than at the deferred group 2 weeks later and no severe adverse effects of the early instillation group (4). In general, our findings are consistent with these prior reports on the potential benefits of early MMC instillation over the deferred administration against bladder recurrence.
The prognostic significance of variant histology has been acknowledged in multiple recent studies. Upper Tract Urothelial Carcinoma (UTUC) featuring histological variants has been significantly linked to a more biologically aggressive disease, higher rates of bladder recurrence, and differing survival outcomes (17). Similarly, another study (18) provided additional evidence supporting these findings. In our current analysis, despite the small case number, we observed that sarcomatoid histology was significantly associated with increased bladder recurrence, even after adjusting for other variables. This aligns with the trends reported in previous research.
On the other hand, a previous study documented that surgical margin's location represents distinct risk factors' patterns in the setting of radical cystectomy. Concomitant CIS was associated with ureteric positive margins, while urethral and soft-tissue PSM showed worse disease-specific survival rates (19). The authors of that study suggest that clinical decision-making paradigms on adjuvant treatment and surveillance might be adapted based on positive margin and their location. In the present study, we identified positive surgical margins as those confirmed to be positive in the ureters. Nevertheless, while there appeared to be a trend, our data did not show that these positive margins were statistically significant in their association with bladder recurrence or overall survival, likely due to the limited number of patients. Further research involving a larger cohort is necessary to validate our conclusions.
Limitation
Several limitations of this study should be noted. Firstly, our study did not employ random allocation; instead, the study's treatment allocation was time-based, as documented previously. This approach could introduce selection bias. Secondly, the study does not take into account financial considerations, and the substantial expense associated with robotic surgery could render it inaccessible to many patients. This could also potentially introduce selection bias into the analytic results. Thirdly, it is understood that IO might increase the length of surgical procedures; however, due to the lack of data collection on operative times, an analysis could not be conducted.
Conclusion
Intraoperative intravesical MMC instillation during RNU is safe and associated with lower bladder recurrence risk compared to deferred instillation postoperatively. Future large, prospective studies are still needed to confirm the current findings.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author.
Ethics statement
The studies involving humans were approved by This study was approved by the ethics committee of China Medical University Hospital. All methods were performed in accordance with the relevant guidelines and regulations. Each patient gave the written informed consent on this study. The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study.
Author contributions
S-FC: Conceptualization, Data curation, Formal Analysis, Project administration, Supervision, Writing – original draft. W-CL: Data curation, Formal Analysis, Writing – review & editing. HC: Data curation, Formal Analysis, Methodology, Writing – review & editing. C-PH: Conceptualization, Data curation, Formal Analysis, Validation, Writing – original draft, Writing – review & editing.
Funding
The author(s) declare that no financial support was received for the research, authorship, and/or publication of this article.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fsurg.2024.1366982/full#supplementary-material
References
1. Siegel RL, Miller KD, Jemal A. Cancer statistics, 2020. CA Cancer J Clin. (2020) 70(1):7–30. doi: 10.3322/caac.21590
2. Rouprêt M, Babjuk M, Burger M, Capoun O, Cohen D, Compérat EM, et al. European association of urology guidelines on upper urinary tract urothelial carcinoma: 2020 update. Eur Urol. (2021) 79(1):62–79. doi: 10.1016/j.eururo.2020.05.042
3. Xylinas E, Rink M, Margulis V, Karakiewicz P, Novara G, Shariat SF. Multifocal carcinoma in situ of the upper tract is associated with high risk of bladder cancer recurrence. Eur Urol. (2012) 61(5):1069–70. doi: 10.1016/j.eururo.2012.02.042
4. Bosschieter J, Nieuwenhuijzen JA, Van Ginkel T, Vis AN, Witte B, Newling D, et al. Value of an immediate intravesical instillation of mitomycin C in patients with non-muscle-invasive bladder cancer: a prospective multicentre randomised study in 2243 patients. Eur Urol. (2018) 73(2):226–32. doi: 10.1016/j.eururo.2017.06.038
5. Wu WJ, Ke HL, Yang YH, Li CC, Chou YH, Huang CH. Should patients with primary upper urinary tract cancer receive prophylactic intravesical chemotherapy after nephroureterectomy? J Urol. (2010) 183(1):56–61. doi: 10.1016/j.juro.2009.08.154
6. O'brien T, Ray E, Singh R, Coker B, Beard R. Prevention of bladder tumours after nephroureterectomy for primary upper urinary tract urothelial carcinoma: a prospective, multicentre, randomised clinical trial of a single postoperative intravesical dose of mitomycin C (the ODMIT-C trial). Eur Urol. (2011) 60(4):703–10. doi: 10.1016/j.eururo.2011.05.064
7. Ito A, Shintaku I, Satoh M, Ioritani N, Aizawa M, Tochigi T, et al. Prospective randomized phase II trial of a single early intravesical instillation of pirarubicin (THP) in the prevention of bladder recurrence after nephroureterectomy for upper urinary tract urothelial carcinoma: the THP monotherapy study group trial. J Clin Oncol. (2013) 31(11):1422–7. doi: 10.1200/JCO.2012.45.2128
8. Hwang EC, Sathianathen NJ, Jung JH, Kim MH, Dahm P, Risk MC. Single-dose intravesical chemotherapy after nephroureterectomy for upper tract urothelial carcinoma. Cochrane Database Syst Rev. (2019) 5(5):Cd013160. doi: 10.1002/14651858.CD013160.pub2
9. Sylvester RJ, Oosterlinck W, Van Der Meijden AP. A single immediate postoperative instillation of chemotherapy decreases the risk of recurrence in patients with stage ta T1 bladder cancer: a meta-analysis of published results of randomized clinical trials. J Urol. (2004) 171(6 Pt 1):2186–90; quiz 2435. doi: 10.1097/01.ju.0000125486.92260.b2
10. Williams SK, Hoenig DM, Ghavamian R, Soloway M. Intravesical therapy for bladder cancer. Expert Opin Pharmacother. (2010) 11(6):947–58. doi: 10.1517/14656561003657145
11. Mitropoulos D, Artibani W, Biyani CS, Bjerggaard Jensen J, Rouprêt M, Truss M. Validation of the Clavien-Dindo grading system in urology by the European association of urology guidelines ad hoc panel. Eur Urol Focus. (2018) 4(4):608–13. doi: 10.1016/j.euf.2017.02.014
12. Flaig TW, Spiess PE, Agarwal N, Bangs R, Boorjian SA, Buyyounouski MK, et al. Bladder cancer, version 6.2021, NCCN clinical practice guidelines in oncology. J Natl Compr Canc Netw. (2021) 2021:329–54. doi: 10.6004/jnccn.2020.0011
13. Mertens LS, Sharma V, Matin SF, Boorjian SA, Houston Thompson R, Van Rhijn BWG, et al. Bladder recurrence following upper tract surgery for urothelial carcinoma: a contemporary review of risk factors and management strategies. Eur Urol Open Sci. (2023) 49:60–6. doi: 10.1016/j.euros.2023.01.004
14. Yoo SH, Jeong CW, Kwak C, Kim HH, Choo MS, Ku JH. Intravesical chemotherapy after radical nephroureterectomy for primary upper tract urothelial carcinoma: a systematic review and network meta-analysis. J Clin Med. (2019) 8(7):1059. doi: 10.3390/jcm8071059
15. Gulamhusein A, Silva P, Cullen D, Tran M, Mumtaz F, Patki P, et al. Safety and feasibility of early single-dose mitomycin C bladder instillation after robot-assisted radical nephroureterectomy. BJU Int. (2020) 126(6):739–44. doi: 10.1111/bju.15162
16. Nadler N, Oedorf K, Jensen JB, Azawi N. Intraoperative mitomycin C bladder instillation during radical nephroureterectomy is feasible and safe. Eur Urol Open Sci. (2021) 34:41–6. doi: 10.1016/j.euros.2021.09.013
17. Takemoto K, Hayashi T, Hsi RS, Kobatake K, Sekino Y, Kitano H, et al. Histological variants and lymphovascular invasion in upper tract urothelial carcinoma can stratify prognosis after radical nephroureterectomy. Urol Oncol. (2022) 40(12):539.e9–e16. doi: 10.1016/j.urolonc.2022.08.010
18. Wang J, Zuo X, Zhang Y, Wang W, Zhou D, Liu W, et al. The impact of histological variants in patients with upper tract urothelial carcinoma treated with radical nephroureterectomy. J Cancer Res Clin Oncol. (2023) 149(11):8279–88. doi: 10.1007/s00432-023-04763-6
Keywords: radical nephroureterectomy (RNU), mitomycin-C (MMC), recurrence, adverse event (AE), upper tract urothelial carcinoma (UTUC)
Citation: Chou S-F, Lin W-C, Chang H and Huang C-P (2024) Safety and oncological outcome of early intraoperative intravesicle mitomycin C vs. deferred instillation in patients receiving robot-assisted radical nephroureterectomy. Front. Surg. 11:1366982. doi: 10.3389/fsurg.2024.1366982
Received: 8 January 2024; Accepted: 10 April 2024;
Published: 25 April 2024.
Edited by:
Sabine Brookman-May, Ludwig Maximilian University of Munich, GermanyReviewed by:
Francesco Claps, The Netherlands Cancer Institute (NKI), NetherlandsFabrizio Di Maida, Careggi University Hospital, Italy
© 2024 Chou, Lin, Chang and Huang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Chi-Ping Huang d17561@mail.cmuh.org.tw
 Sheng-Feng Chou1
Sheng-Feng Chou1  Chi-Ping Huang
Chi-Ping Huang