Oncological outcomes in minimally invasive vs. open distal pancreatectomy: a systematic review and network meta-analysis
- 1Yong Loo Lin School of Medicine, National University of Singapore, Singapore, Singapore
- 2National University Centre for Organ Transplantation, National University Health System, Singapore, Singapore
- 3Division of Hepatobiliary and Pancreatic Surgery, Department of Surgery, National University Hospital Singapore, Singapore, Singapore
Background: Advancements in surgical techniques have improved outcomes in patients undergoing pancreatic surgery. To date there have been no meta-analyses comparing robotic and laparoscopic approaches for distal pancreatectomies (DP) in patients with pancreatic adenocarcinoma (PDAC). This systematic review and network meta-analysis aims to explore the oncological outcomes of laparoscopic distal pancreatectomy (LDP), robotic distal pancreatectomy (RDP) and open distal pancreatectomy (ODP).
Methods: A systematic search was conducted for studies reporting laparoscopic, robotic or open surgery for DP. Frequentist network meta-analysis of oncological outcomes (overall survival, resection margins, tumor recurrence, examined lymph nodes, administration of adjuvant therapy) were performed.
Results: Fifteen studies totalling 9,301 patients were included in the network meta-analysis. 1,946, 605 and 6,750 patients underwent LDP, RDP and ODP respectively. LDP (HR: 0.761, 95% CI: 0.642–0.901, p = 0.002) and RDP (HR: 0.757, 95% CI: 0.617–0.928, p = 0.008) were associated with overall survival (OS) benefit when compared to ODP. LDP (HR: 1.00, 95% CI: 0.793–1.27, p = 0.968) was not associated with OS benefit when compared to RDP. There were no significant differences between LDP, RDP and ODP for resection margins, tumor recurrence, examined lymph nodes and administration of adjuvant therapy.
Conclusion: This study highlights the longer OS in both LDP and RDP when compared to ODP for patients with PDAC.
Systematic Review Registration: https://www.crd.york.ac.uk/, PROSPERO (CRD42022336417).
Introduction
The introduction of minimally invasive techniques has advanced the field of pancreatic surgery in recent decades (1–3). Despite the increase in procedures performed, LDP and RDP continue to present unique technical challenges for the surgeon (4–7).
Previously published meta-analyses have demonstrated that minimally invasive distal pancreatectomy (MIDP) is associated with lower morbidity and comparable oncological outcomes (overall survival, R0 resection, lymph node yield, use of adjuvant therapy) when compared to ODP (8–15). Whilst RDP appears to be comparable to LDP in terms of safety, to date no studies have compared oncological outcomes between laparoscopic distal pancreatectomy (LDP), robotic distal pancreatectomy (RDP) and open distal pancreatectomy (ODP) (16).
We performed a network meta-analysis on the studies reporting ODP, LDP and RDP in patients with histologically confirmed PDAC with the aim of clarifying if LDP or RDP improve oncological outcomes over ODP.
Methods
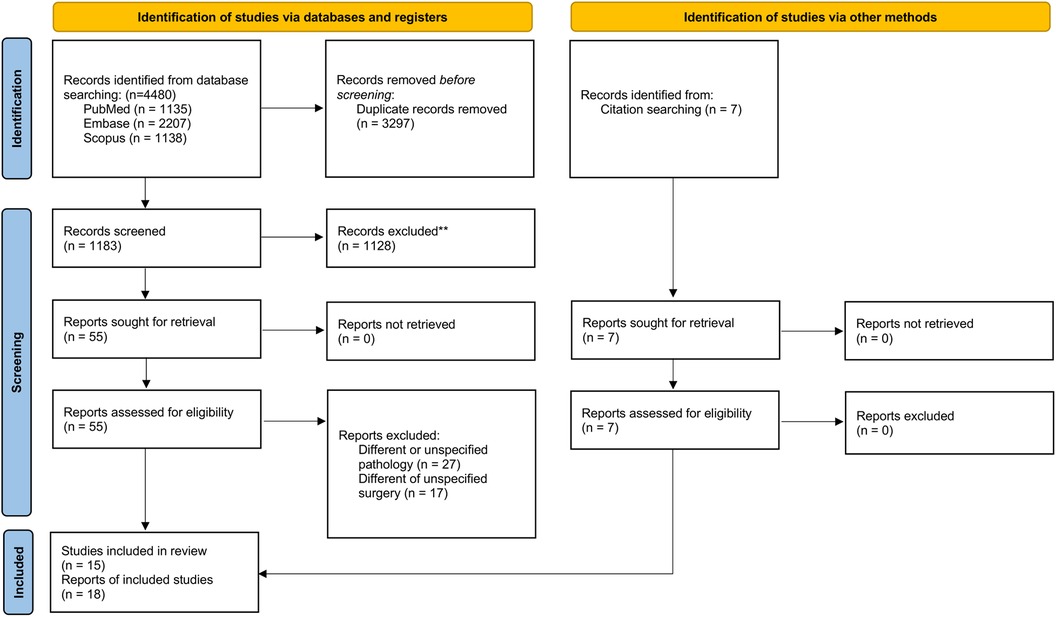
This review is registered on PROSPERO (CRD42022336417) and is reported in accordance with the 2020 Preferred Reporting Items for Systematic Reviews and Meta-Analysis (PRISMA) guideline. The PRISMA checklist is included in Figure 1 (17).
Search strategy
A systematic search of PubMed, EMBASE and the Cochrane Library databases were conducted from inception til 7 July 2022 by two independent investigators (NWZH, SNL). The search terms used were “keyhole” or “robot” or “laparoscopic” or “minimally invasive” or “daVinci”, and “Pancreaticoduodenectomy” or “Whipple” or “pancreatectomy” or “pylorus-preserving pancreaticoduodenectomy” or “duodenopancreatectomy” or “jejunopancreatectomy” individually or in combination. Search terms used for this review are presented in Supplementary Table S1. A thorough manual search of reference lists in eligible studies was also performed.
Eligibility
Key eligibility criteria included: (1) studies reporting the comparison of surgical techniques in human subjects receiving distal pancreatectomy and (2) studies reporting oncological outcomes (overall survival, positive resection margins, number of lymph nodes examined, tumor recurrence) and (3) studies that included pancreatic ductal adenocarcinoma. Exclusion criteria were: (1) Conference abstracts, reviews, case reports; (2) studies where type of MIDP was not specified; (3) studies that included other types of pancreatic surgeries.
Study selection
Two reviewers (NZHW and SNL) independently screened and selected potentially eligible studies based on title and abstract. Full-text evaluation was independently performed by two reviewers (NZHW and SNL). Any conflicts between authors were discussed and resolved by a third independent reviewer (DWTY).
Risk of bias assessment
As all included studies were observational, we used the Newcastle-Ottowa Scale (NOS) to evaluate the risk of bias. The studies were deemed to have high (<5 stars), moderate (5–7 stars) or low (≥8 stars) risk of bias (Supplementary Table S2).
Statistical analysis
A frequentist network meta-analysis was employed to compare ODP, LDP and RDP. The network meta-analysis is a statistical approach that combines both direct and indirect evidence to allow for comparison between 3 or more interventions. Relative effects estimates between pairs within the network are more precise than single direct and indirect estimates (18). Treatments were ranked using the P-score provided by the netmeta package (19–21). A probability of ranking of 0.9 was considered high enough to be confidently reported as the correct ranking position of a surgical approach (22). Funnel plots of treatment estimates were visually inspected. Evidence of asymmetry or points lying outside 95% pseudo-confidence limits was interpreted as publication bias. Network plots of treatments (nodes) and comparisons (lines) were generated (Figure 2). Networks were examined for the inconsistency by the fitting of net splitting models (23). A p value of <0.05 was deemed to represent significant inconsistency between the direct and indirect estimate. A separate meta-analysis with meta-regression was performed by considering the proportion of patients with vascular resection when comparing positive resection margins between ODP and MIDP (RDP or LDP).
Figure 2. Network plot for comparisons amongst ODP, RDP and LDP for (A) overall survival, (B) resection margins, (C) tumor recurrence, (D) examined lymph nodes. The number of studies comparing connected surgical approaches is proportional to the width of the lines in the Network plot.
Hazard ratios (HR) and odds ratios (OR) were reported for categorical outcomes, whilst weighted mean differences (WMD) were reported for continuous data. Mean and standard deviation estimates were derived from studies that reported medians using methods described by Wan et al. and Luo et al. (24, 25). The random effects, restricted maximum likelihood (REML) method was used for the meta-analysis of outcomes. Results were deemed to be statistically significant if the 95% confidence interval did not cross the no-effect line (1 for binary outcomes and 0 for continuous outcomes). A p value of <0.05 was considered to be statistically significant. Data analysis was performed using R Statistical software (R 4.1.3).
Outcomes
The primary outcome was overall survival. Secondary outcomes included positive resection margins, number of lymph nodes examined and tumor recurrence.
Results
Study selection
The electronic search returned 4,480 publications: 3,297 duplicates were excluded and 1,183 publications were screened. Of those, 1,128 were excluded after reviewing titles and abstracts and forty-four studies were excluded after reviewing full-text articles. An additional six studies were included from searching through reference lists. Nineteeen studies met the eligibility criteria. Two studies were further excluded due to overlapping inclusion periods in the National Cancer Database (NCDB) and one study was excluded due to double reporting of a study (26–28). Fifteen studies were included in the final analysis (Supplementary Table S2) (29–43).
Study characteristics
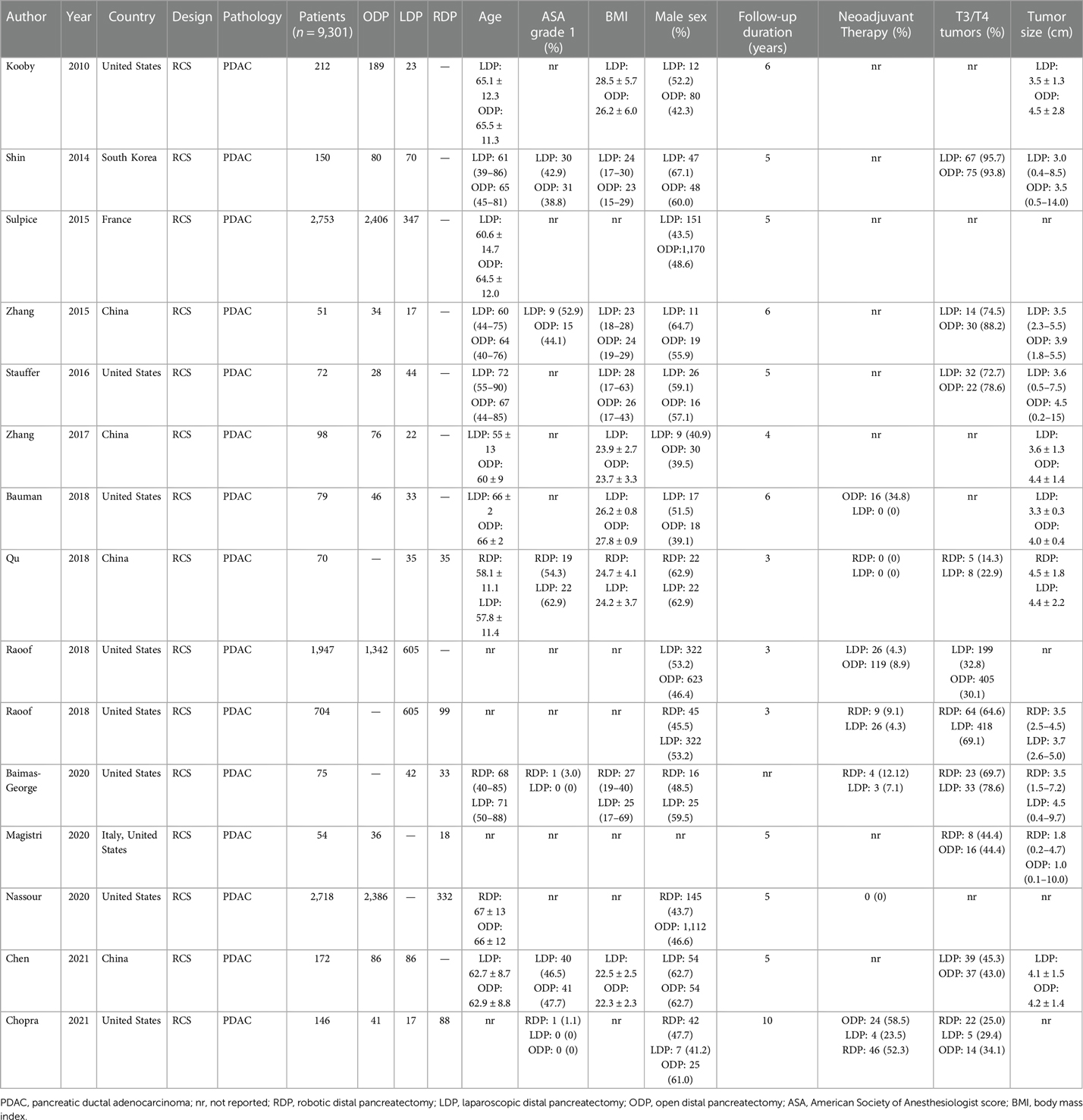
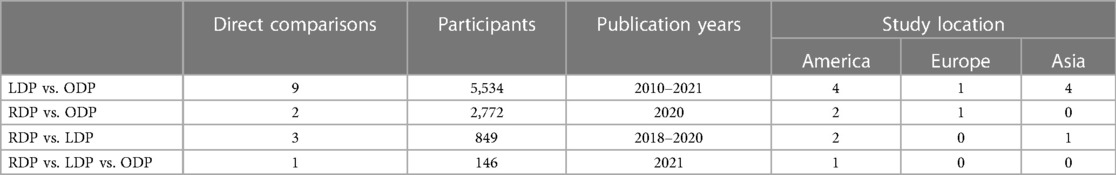
All fifteen studies were retrospective observational studies (Table 1). One study compared all three approaches. Five studies utilised propensity score matching in their analysis (26, 30, 36, 42, 43). ODP and LDP were directly compared in ten studies. RDP and LDP were compared in three studies. ODP and RDP were compared in two studies. A summary of all comparisons made in the network meta-analysis are presented in Table 2.
A total of 9,301 patients were included in this analysis. 1,946 patients underwent LDP, 605 underwent RDP and 6,750 underwent ODP. Baseline characteristics of patients are included in Table 1.
Two studies reported data on Radical Antegrade Modular Pancreatosplenectomy (RAMPS) (36, 37). In both studies, RAMPS were performed more frequently in patients undergoing laparoscopic surgery.
Three studies reported data on vascular resection (37, 41, 42). There was a statistically significant difference in the rates of vascular resection between the three arms in one study with Chopra et al. reporting higher rates of vascular resection in the ODP group (41).
Overall survival
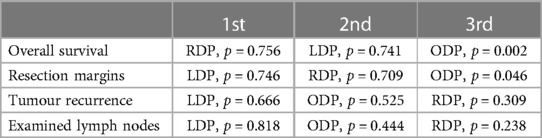
Overall survival was reported in five studies (29–32, 43). Both LDP (HR: 0.761, 95% CI: 0.642–0.901, p = 0.002) and RDP (HR: 0.757, 95% CI: 0.617–0.928, p = 0.008) were associated with better overall survival when compared to ODP. There was no statistically significant difference in overall survival between LDP and RDP (HR: 1.00, 95% CI: 0.793–1.27, p = 0.968) (Figure 3A: Overall survival). RDP and LDP were ranked first and second respectively for overall survival (Table 3). Two studies (29, 32) reported a follow-up duration of five years whilst three studies (30, 31, 43) reported a follow-up duration of three years. In this analysis, only three studies included information on tumor size of the PDAC. Qu et al. reported the median sizes of tumors to be 4.5 cm and 4.4 cm whilst Raoof et al. included tumours of 3.5 cm and 3.7 cm in the RDP and LDP groups respectively (31, 43). In another study by Raoof et al., the majority of tumors were ≥4 cm in both the ODP and LDP groups (30). All studies reported on tumour sizes found them to be comparable in the various arms of comparison.
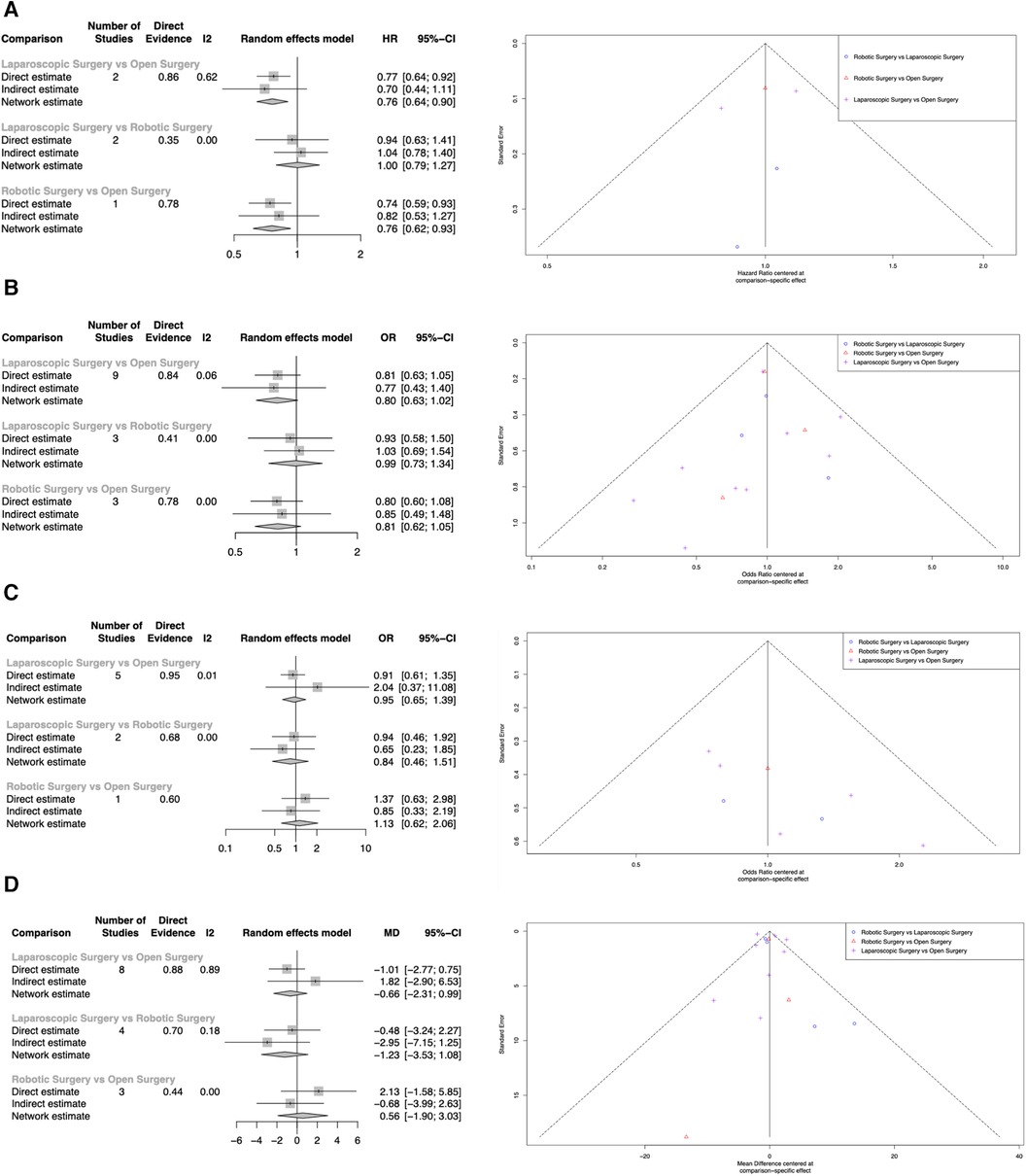
Figure 3. Network meta-analysis of LDP, RDP and ODP. (A) Overall Survival. (B) Resection Margins. (C) Tumor Recurrence. (D) Examined Lymph Nodes.
Resection margins
Resection margins were reported in thirteen studies (29–31, 33–42). Both LDP (OR = 0.803, 95% CI: 0.635–1.02, p = 0.068) and RDP (OR = 0.811, 95% CI: 0.623–1.05, p = 0.115) were not associated with higher rates of positive resection margins. There was no statistically significant difference in positive margins between LDP and RDP (OR = 0.990, 95% CI: 0.730–1.34, p = 0.950) (Figure 3B: Resection Margins). LDP and RDP were ranked first and second respectively for resection margins (Table 3). Positive margins were defined as tumor extension within 1 mm of the margin in 2 studies (34, 41), microscopic evidence of invasion in three studies (33, 39, 42) and microscopic or gross evidence of invasion in two studies (35, 37). Positive margins were not defined in the remaining six studies (29–31, 36, 38, 40).
In a separate analysis of three studies reporting both resection margins and vascular resections, MIDP (LDP or RDP) was not associated with a higher rate of positive resection margins (OR = 0.625, 95% CI: 0.078–5.03, p = 0.434). Meta regression considering proportion of patients with vascular resection did not reach a level of statistical significance (p = 0.425) (Supplementary Figure S1).
Tumor recurrence
Tumor recurrences were reported in six studies. Both LDP (OR = 0.948, 95% CI: 0.647–1.39, p = 0.783) and RDP (OR = 1.13, 95% CI: 0.622–2.06, p = 0.684) were not associated with statistically significant higher rates of tumor recurrence. There was no statistically significant difference in tumor recurrence between LDP and RDP (OR = 0.838, 95% CI: 0.463–1.51, p = 0.561) (Figure 3C: Tumor recurrence). LDP and ODP were ranked first and second respectively for tumor recurrence (Table 3).
Examined lymph nodes
The number of examined lymph nodes were reported in thirteen studies. Compared to ODP, LDP (WMD = −0.662, 95% CI: −2.31 to 0.989, p = 0.432) and RDP (WMD = 0.565, 95% CI = −1.90 to 3.03, p = 0.654) did not achieve a statistically significant difference in lymph node examined. Likewise, there was no statistically significant difference in lymph nodes examined between LDP and RDP (WMD = −1.23, 95% CI = −3.53 to 1.08, p = 0.300) (Figure 3D: Examined lymph nodes). LDP and ODP ranked first and second respectively for the number of examined lymph nodes (Table 3).
As some studies included skewed data as described by Shi et al, an additional sensitivity analysis was performed (44). Four studies with significantly skewed data were excluded. There were no statistically significant differences in examined lymph nodes between LDP (WMD = −0.591, 95% CI = −2.64 to 1.46, p = 0.572) and RDP (WMD = 0.526, 95% CI = −2.31 to 3.37, p = 0.719) when compared to ODP. Similarly, there was no statistically significant difference in lymph nodes examined between LDP and RDP (WMD = −1.12, 95% CI = −1.47 to 3.70, p = 0.397).
Discussion and conclusion
Over the past decade, MIDP including the use of LDP and RDP has grown in popularity. With the increasing adoption of MIDP, the Yonsei criteria was described using several pathological factors to determine if minimally invasive approaches were suitable for tumors arising from the pancreatic body and tail (45). Studies from high volume centers have demonstrated that MIDP decreased the risk of complications compared to ODP (9). Despite this, the oncological benefits of LDP, RDP and ODP remain poorly understood. Our network meta-analysis compared oncological outcomes in fifteen cohort studies comparing ODP, LDP and RDP in over 9,000 patients with pancreatic adenocarcinoma. Both LDP and RDP demonstrated longer overall survival when compared to ODP. R0 resections, tumor recurrence and lymph nodes examined were comparable between all three interventions. While there may be potential selection bias in these retrospective studies, where tumor sizes may be different between different intervention groups (e.g., smaller tumours were offered MIDP as compared to ODP), we did not find any significant difference between the tumour size of the comparative groups in this analysis.
There have been limited studies with direct comparisons between RDP and other surgical approaches. However, through the indirect comparisons obtained in this network meta-analysis, we were able to show that oncological outcomes in RDP were comparable to those of ODP and LDP. Network meta-analyses combine direct evidence within studies and indirect evidence across studies to enable indirect comparisons of surgical techniques. The relative effectiveness of different surgical treatments may be assessed even if they have not been previously compared in individual RCTs. A network meta-analysis provides several benefits over a standard pairwise meta-analysis as treatment rankings with probabilities can be accessed. Results are more representative of the available evidence and are more reliable compared to pairwise meta-analysis (46, 47).
In a meta-analysis by Lyu et al., R0 margins were best achieved by RDP, robotic assisted distal pancreatectomy (RADP), LDP and ODP whilst lymph node harvest was best achieved by RDP followed by RADP, ODP and LDP (48). However the inclusion criteria differed between both studies. In our analysis only studies reporting PDAC were included in the analysis whilst the type of tumor was not defined by Lyu et al. Whilst Lyu et al.'s findings are generalizable to a greater degree of pathologies, our results are more pertinent to PDAC.
Given the lower rates of postoperative complications in MIDP and comparable oncological outcomes in RDP and LDP with ODP, MIDP should be recommended as the treatment of choice in experienced centres (48, 49). The LEOPARD RCT demonstrated that MIDP is associated with better functional recovery and post operative outcomes compared to ODP (50). It must be noted that the adoption and acceptance of minimally invasive techniques are also influenced by tumour characteristics, vascular involvement, logistical issues such as access to robots, and for institutions with lower case-load, prioritizing the education of younger residents in performing traditional open distal pancreatectomy over minimally invasive techniques (51, 52).
Results of this study should be interpreted with due consideration of some limitations. First, there are to date, no randomized controlled trials comparing oncological outcomes in all three surgical approaches for patients with pancreatic adenocarcinoma. Some studies mitigated this through the introduction of propensity matching, which has been shown to be able to adequately match patients to appropriate controls (53, 54). However, randomized controlled trials involving the three surgical approaches are still necessary for direct comparisons between interventions. Furthermore, heterogeneity exists in the majority of studies included in this network meta-analysis due to inherent differences in study populations, tumor factors and surgical experience (55). Although our results were limited to PDAC patients, outcomes continue to be influenced by molecular and metabolic subtypes within PDAC tumors, with basal and glycolytic subtypes demonstrating poorer prognosis (56–58). Lastly, our study was unable to account for other factors that are associated with OS such as nodal positivity, tumor stage, borderline resectable tumours, patient performance status, neoadjuvant and adjuvant therapy as well as pre and postresection tumor markers (59–63).
Resection margins in distal pancreatectomy encompass more than the neck of pancreas. Although R0 was most commonly defined as the absence of microscopic invasion at the surgical resection margins, most studies did not specify the exact definition used. Existing literature also revealed high variability in terms of rates of resections (64, 65). As a result we deemed R1 and R2 resections to be equivalent to positive margins to increase the generalizability of our results. However, the superior, inferior, anterior and posterior margins are all of importance. As pancreatic cancers in the body and tail often are infiltrative and present late, resectable lesions must be removed in a radical resection with clearance of as much surrounding tissues as possible, including the adrenals, parts of the colon or stomach if necessary. With highly skilled minimally invasive hepatobiliary surgical teams, these complex surgical approaches are achievable.
Our study demonstrated that both LDP and RDP was associated with longer OS when compared to ODP. Other oncological outcomes were comparable between all three groups. These results reflect the oncological safety of both minimally invasive approaches for PDAC and pave the way for both LDP and RDP to be recognized as the standard of care for PDAC in experienced centers.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author.
Author contributions
NZ: Conceptualization, Data curation, Formal Analysis, Investigation, Methodology, Project administration, Resources, Software, Supervision, Validation, Visualization, Writing – original draft, Writing – review & editing. DW: Conceptualization, Data curation, Formal Analysis, Investigation, Methodology, Project administration, Resources, Software, Supervision, Validation, Visualization, Writing – original draft, Writing – review & editing. SL: Conceptualization, Data curation, Formal Analysis, Investigation, Methodology, Project administration, Resources, Software, Validation, Visualization, Writing – original draft, Writing – review & editing. JY: Conceptualization, Data curation, Formal Analysis, Investigation, Methodology, Project administration, Software, Validation, Visualization, Writing – original draft, Writing – review & editing. JJ: Conceptualization, Data curation, Formal Analysis, Investigation, Methodology, Project administration, Software, Validation, Visualization, Writing – original draft, Writing – review & editing. AW: Conceptualization, Data curation, Formal Analysis, Funding acquisition, Investigation, Methodology, Project administration, Resources, Software, Supervision, Validation, Visualization, Writing – original draft, Writing – review & editing.
Funding
The author(s) declare that no financial support was received for the research, authorship, and/or publication of this article.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fsurg.2024.1369169/full#supplementary-material
References
1. Esposito A, Balduzzi A, De Pastena M, Fontana M, Casetti L, Ramera M, et al. Minimally invasive surgery for pancreatic cancer. Expert Rev Anticancer Ther. (2019) 19(11):947–58. doi: 10.1080/14737140.2019.1685878
2. Kendrick ML, van Hilst J, Boggi U, de Rooij T, Walsh RM, Zeh HJ, et al. Minimally invasive pancreatoduodenectomy. HPB (Oxford). (2017) 19(3):215–24. doi: 10.1016/j.hpb.2017.01.023
3. Nickel F, Haney CM, Kowalewski KF, Probst P, Limen EF, Kalkum E, et al. Laparoscopic versus open pancreaticoduodenectomy: a systematic review and meta-analysis of randomized controlled trials. Ann Surg. (2020) 271(1):54–66. doi: 10.1097/SLA.0000000000003309
4. Guerra F, Pesi B, Amore Bonapasta S, Di Marino M, Perna F, Annecchiarico M, et al. Challenges in robotic distal pancreatectomy: systematic review of current practice. Minerva Chir. (2015) 70(4):241–7.25916194
5. Melotti G, Butturini G, Piccoli M, Casetti L, Bassi C, Mullineris B, et al. Laparoscopic distal pancreatectomy: results on a consecutive series of 58 patients. Ann Surg. (2007) 246(1):77–82. doi: 10.1097/01.sla.0000258607.17194.2b
6. Iacobone M, Citton M, Nitti D. Laparoscopic distal pancreatectomy: up-to-date and literature review. World J Gastroenterol. (2012) 18(38):5329–37. doi: 10.3748/wjg.v18.i38.5329
7. Rutz DR, Squires MH, Maithel SK, Sarmiento JM, Etra JW, Perez SD, et al. Cost comparison analysis of open versus laparoscopic distal pancreatectomy. HPB (Oxford). (2014) 16(10):907–14. doi: 10.1111/hpb.12288
8. Venkat R, Edil BH, Schulick RD, Lidor AO, Makary MA, Wolfgang CL. Laparoscopic distal pancreatectomy is associated with significantly less overall morbidity compared to the open technique: a systematic review and meta-analysis. Ann Surg. (2012) 255(6):1048–59. doi: 10.1097/SLA.0b013e318251ee09
9. de Rooij T, Klompmaker S, Abu Hilal M, Kendrick ML, Busch OR, Besselink MG. Laparoscopic pancreatic surgery for benign and malignant disease. Nat Rev Gastroenterol Hepatol. (2016) 13(4):227–38. doi: 10.1038/nrgastro.2016.17
10. Nigri GR, Rosman AS, Petrucciani N, Fancellu A, Pisano M, Zorcolo L, et al. Metaanalysis of trials comparing minimally invasive and open distal pancreatectomies. Surg Endosc. (2011) 25(5):1642–51. doi: 10.1007/s00464-010-1456-5
11. Jin T, Altaf K, Xiong JJ, Huang W, Javed MA, Mai G, et al. A systematic review and meta-analysis of studies comparing laparoscopic and open distal pancreatectomy. HPB (Oxford). (2012) 14(11):711–24. doi: 10.1111/j.1477-2574.2012.00531.x
12. Drymousis P, Raptis DA, Spalding D, Fernandez-Cruz L, Menon D, Breitenstein S, et al. Laparoscopic versus open pancreas resection for pancreatic neuroendocrine tumours: a systematic review and meta-analysis. HPB (Oxford). (2014) 16(5):397–406. doi: 10.1111/hpb.12162
13. Riviere D, Gurusamy KS, Kooby DA, Vollmer CM, Besselink MG, Davidson BR, et al. Laparoscopic versus open distal pancreatectomy for pancreatic cancer. Cochrane Database Syst Rev. (2016) 4(4):Cd011391. doi: 10.1002/14651858.CD011391.pub2
14. Ricci C, Casadei R, Taffurelli G, Toscano F, Pacilio CA, Bogoni S, et al. Laparoscopic versus open distal pancreatectomy for ductal adenocarcinoma: a systematic review and meta-analysis. J Gastrointest Surg. (2015) 19(4):770–81. doi: 10.1007/s11605-014-2721-z
15. van Hilst J, Korrel M, de Rooij T, Lof S, Busch OR, Groot Koerkamp B, et al. Oncologic outcomes of minimally invasive versus open distal pancreatectomy for pancreatic ductal adenocarcinoma: a systematic review and meta-analysis. Eur J Surg Oncol. (2019) 45(5):719–27. doi: 10.1016/j.ejso.2018.12.003
16. Chen C, Hu J, Yang H, Zhuo X, Ren Q, Feng Q, et al. Is robotic distal pancreatectomy better than laparoscopic distal pancreatectomy after the learning curve? A systematic review and meta-analysis. Front Oncol. (2022) 12:4–8. doi: 10.3389/fonc.2022.954227
17. Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. Br Med J. (2021) 372:n71. doi: 10.1136/bmj.n71
18. Chaimani A, Caldwell DM, Li T, Higgins JPT, Salanti G. Additional considerations are required when preparing a protocol for a systematic review with multiple interventions. J Clin Epidemiol. (2017) 83:65–74. doi: 10.1016/j.jclinepi.2016.11.015
19. Schwarzer GRaUKaJKaOEaADaTPaG. netmeta: Network Meta-Analysis using Frequentist Methods (2022). Available online at: https://CRAN.R-project.org/package=netmeta (accessed July 15, 2022).
20. Neupane B, Richer D, Bonner AJ, Kibret T, Beyene J. Network meta-analysis using R: a review of currently available automated packages. PLoS One. (2014) 9(12):e115065. doi: 10.1371/journal.pone.0115065
21. Rücker G, Schwarzer G. Ranking treatments in frequentist network meta-analysis works without resampling methods. BMC Med Res Methodol. (2015) 15(1):58. doi: 10.1186/s12874-015-0060-8
22. Dias S, Welton NJ, Sutton AJ, Caldwell DM, Lu G, Ades AE. NICE Decision Support Unit Technical Support Documents. NICE DSU Technical Support Document 4: Inconsistency in Networks of Evidence Based on Randomised Controlled Trials. London: National Institute for Health and Care Excellence (NICE) (2014).
23. Dias S, Welton NJ, Caldwell DM, Ades AE. Checking consistency in mixed treatment comparison meta-analysis. Stat Med. (2010) 29(7–8):932–44. doi: 10.1002/sim.3767
24. Wan X, Wang W, Liu J, Tong T. Estimating the sample mean and standard deviation from the sample size, median, range and/or interquartile range. BMC Med Res Methodol. (2014) 14(1):135. doi: 10.1186/1471-2288-14-135
25. Luo D, Wan X, Liu J, Tong T. Optimally estimating the sample mean from the sample size, median, mid-range, and/or mid-quartile range. Stat Methods Med Res. (2018) 27(6):1785–805. doi: 10.1177/0962280216669183
26. Chen K, Tong Q, Yan J-F, Huang C-J, Pan Y, Zhang R-C, et al. Laparoscopic versus open distal pancreatectomy for pancreatic ductal adenocarcinoma: a single-center propensity score matching study. Updates Surg. (2020) 72(2):387–97. doi: 10.1007/s13304-020-00742-5
27. Kantor O, Bryan DS, Talamonti MS, Lutfi W, Sharpe S, Winchester DJ, et al. Laparoscopic distal pancreatectomy for cancer provides oncologic outcomes and overall survival identical to open distal pancreatectomy. J Gastrointest Surg. (2017) 21(10):1620–5. doi: 10.1007/s11605-017-3506-y
28. Sharpe SM, Talamonti MS, Wang E, Bentrem DJ, Roggin KK, Prinz RA, et al. The laparoscopic approach to distal pancreatectomy for ductal adenocarcinoma results in shorter lengths of stay without compromising oncologic outcomes. Am J Surg. (2015) 209(3):557–63. doi: 10.1016/j.amjsurg.2014.11.001
29. Nassour I, Winters SB, Hoehn R, Tohme S, Adam MA, Bartlett DL, et al. Long-term oncologic outcomes of robotic and open pancreatectomy in a national cohort of pancreatic adenocarcinoma. J Surg Oncol. (2020) 122(2):234–42. doi: 10.1002/jso.25958
30. Raoof M, Ituarte PHG, Woo Y, Warner SG, Singh G, Fong Y, et al. Propensity score-matched comparison of oncological outcomes between laparoscopic and open distal pancreatic resection. Br J Surg. (2018) 105(5):578–86. doi: 10.1002/bjs.10747
31. Raoof M, Nota CLMA, Melstrom LG, Warner SG, Woo Y, Singh G, et al. Oncologic outcomes after robot-assisted versus laparoscopic distal pancreatectomy: analysis of the national cancer database. J Surg Oncol. (2018) 118(4):651–6. doi: 10.1002/jso.25170
32. Sulpice L, Farges O, Goutte N, Bendersky N, Dokmak S, Sauvanet A, et al. Laparoscopic distal pancreatectomy for pancreatic ductal adenocarcinoma: time for a randomized controlled trial? Results of an all-inclusive national observational study. Ann Surg. (2015) 262(5):868–74. doi: 10.1097/SLA.0000000000001479
33. Zhang AB, Wang Y, Hu C, Shen Y, Zheng SS. Laparoscopic versus open distal pancreatectomy for pancreatic ductal adenocarcinoma: a single-center experience. J Zhejiang Univ Sci B. (2017) 18(6):532–8. doi: 10.1631/jzus.B1600541
34. Zhang M, Fang R, Mou Y, Chen R, Xu X, Zhang R, et al. LDP vs ODP for pancreatic adenocarcinoma: a case matched study from a single-institution. BMC Gastroenterol. (2015) 15(1):182. doi: 10.1186/s12876-015-0411-2
35. Kooby DA, Hawkins WG, Schmidt CM, Weber SM, Bentrem DJ, Gillespie TW, et al. A multicenter analysis of distal pancreatectomy for adenocarcinoma: is laparoscopic resection appropriate? J Am Coll Surg. (2010) 210(5):779–85. doi: 10.1016/j.jamcollsurg.2009.12.033
36. Shin SH, Kim SC, Song KB, Hwang DW, Lee JH, Lee D, et al. A comparative study of laparoscopic vs open distal pancreatectomy for left-sided ductal adenocarcinoma: a propensity score-matched analysis. J Am Coll Surg. (2015) 220(2):177–85. doi: 10.1016/j.jamcollsurg.2014.10.014
37. Stauffer JA, Coppola A, Mody K, Asbun HJ. Laparoscopic versus open distal pancreatectomy for pancreatic adenocarcinoma. World J Surg. (2016) 40(6):1477–84. doi: 10.1007/s00268-016-3412-6
38. Bauman MD, Becerra DG, Kilbane EM, Zyromski NJ, Schmidt CM, Pitt HA, et al. Laparoscopic distal pancreatectomy for pancreatic cancer is safe and effective. Surg Endosc. (2018) 32(1):53–61. doi: 10.1007/s00464-017-5633-7
39. Chen K, Pan Y, Huang C-J, Chen Q-L, Zhang R-C, Zhang M-Z, et al. Laparoscopic versus open pancreatic resection for ductal adenocarcinoma: separate propensity score matching analyses of distal pancreatectomy and pancreaticoduodenectomy. BMC Cancer. (2021) 21(1):382. doi: 10.1186/s12885-021-08117-8
40. Baimas-George M, Watson M, Salibi P, Tschuor C, Murphy KJ, Iannitti D, et al. Oncologic outcomes of robotic left pancreatectomy for pancreatic adenocarcinoma: a single-center comparison to laparoscopic resection. Am Surg. (2021) 87(1):45–9. doi: 10.1177/0003134820949524
41. Chopra A, Nassour I, Zureikat A, Paniccia A. Perioperative and oncologic outcomes of open, laparoscopic, and robotic distal pancreatectomy for pancreatic adenocarcinoma. Updates Surg. (2021) 73(3):947–53. doi: 10.1007/s13304-020-00927-y
42. Magistri P, Boggi U, Esposito A, Carrano FM, Pesi B, Ballarin R, et al. Robotic vs open distal pancreatectomy: a multi-institutional matched comparison analysis. J Hepatobiliary Pancreat Sci. (2021) 28(12):1098–106. doi: 10.1002/jhbp.881
43. Qu L, Zhiming Z, Xianglong T, Yuanxing G, Yong X, Rong L, et al. Short- and mid-term outcomes of robotic versus laparoscopic distal pancreatosplenectomy for pancreatic ductal adenocarcinoma: a retrospective propensity score-matched study. Int J Surg. (2018) 55:81–6. doi: 10.1016/j.ijsu.2018.05.024
44. Shi J, Luo D, Wan X, Liu Y, Liu J, Bian Z, et al. Detecting the skewness of data from the sample size and the five-number summary. arxiv [Preprint] (2020).
45. Chong JU, Kim SH, Hwang HK, Kang CM, Lee WJ. Yonsei criteria: a clinical reflection of stage I left-sided pancreatic cancer. Oncotarget. (2017) 8(67):110830–6. doi: 10.18632/oncotarget.22734
46. Mills EJ, Ioannidis JP, Thorlund K, Schünemann HJ, Puhan MA, Guyatt GH. How to use an article reporting a multiple treatment comparison meta-analysis. JAMA. (2012) 308(12):1246–53. doi: 10.1001/2012.jama.11228
47. Ter Veer E, van Oijen MGH, van Laarhoven HWM. The use of (network) meta-analysis in clinical oncology. Front Oncol. (2019) 9:822. doi: 10.3389/fonc.2019.00822
48. Lyu Y, Cheng Y, Wang B, Zhao S, Chen L. Comparison of 3 minimally invasive methods versus open distal pancreatectomy: a systematic review and network meta-analysis. Surg Laparosc Endosc Percutan Tech. (2021) 31(1):105–8. doi: 10.1097/SLE.0000000000000846
49. Hayashi H, Baba H. Current statement and safe implementation of minimally invasive surgery in the pancreas. Ann Gastroenterol Surg. (2020) 4(5):505–13. doi: 10.1002/ags3.12366
50. de Rooij T, van Hilst J, van Santvoort H, Boerma D, van den Boezem P, Daams F, et al. Minimally invasive versus open distal pancreatectomy (LEOPARD): a multicenter patient-blinded randomized controlled trial. Ann Surg. (2019) 269(1):2–9. doi: 10.1097/SLA.0000000000002979
51. Ho CK, Kleeff J, Friess H, Büchler MW. Complications of pancreatic surgery. HPB (Oxford). (2005) 7(2):99–108. doi: 10.1080/13651820510028936
52. Krautz C, Denz A, Weber GF, Grützmann R. Influence of hospital volume effects and minimum caseload requirements on quality of care in pancreatic surgery in Germany. Visc Med. (2017) 33(2):131–4. doi: 10.1159/000456042
53. Ali MS, Prieto-Alhambra D, Lopes LC, Ramos D, Bispo N, Ichihara MY, et al. Propensity score methods in health technology assessment: principles, extended applications, and recent advances. Front Pharmacol. (2019) 10:4–7. doi: 10.3389/fphar.2019.00973
54. Austin PC. An introduction to propensity score methods for reducing the effects of confounding in observational studies. Multivariate Behav Res. (2011) 46(3):399–424. doi: 10.1080/00273171.2011.568786
55. Boone BA, Zenati M, Hogg ME, Steve J, Moser AJ, Bartlett DL, et al. Assessment of quality outcomes for robotic pancreaticoduodenectomy: identification of the learning curve. JAMA Surg. (2015) 150(5):416–22. doi: 10.1001/jamasurg.2015.17
56. Karasinska JM, Topham JT, Kalloger SE, Jang GH, Denroche RE, Culibrk L, et al. Altered gene expression along the glycolysis–cholesterol synthesis axis is associated with outcome in pancreatic cancer. Clin Cancer Res. (2020) 26(1):135–46. doi: 10.1158/1078-0432.CCR-19-1543
57. Nicolle R, Blum Y, Duconseil P, Vanbrugghe C, Brandone N, Poizat F, et al. Establishment of a pancreatic adenocarcinoma molecular gradient (PAMG) that predicts the clinical outcome of pancreatic cancer. EBioMedicine. (2020) 57:102858. doi: 10.1016/j.ebiom.2020.102858
58. Moffitt RA, Marayati R, Flate EL, Volmar KE, Loeza SG, Hoadley KA, et al. Virtual microdissection identifies distinct tumor- and stroma-specific subtypes of pancreatic ductal adenocarcinoma. Nat Genet. (2015) 47(10):1168–78. doi: 10.1038/ng.3398
59. Ferrone CR, Finkelstein DM, Thayer SP, Muzikansky A, Castillo C-d, Warshaw AL. Perioperative CA19-9 levels can predict stage and survival in patients with resectable pancreatic adenocarcinoma. J Clin Oncol. (2006) 24(18):2897–902. doi: 10.1200/JCO.2005.05.3934
60. Elshaer M, Gravante G, Kosmin M, Riaz A, Al-Bahrani A. A systematic review of the prognostic value of lymph node ratio, number of positive nodes and total nodes examined in pancreatic ductal adenocarcinoma. Ann R Coll Surg Engl. (2017) 99(2):101–6. doi: 10.1308/rcsann.2016.0340
61. Wentz SC, Zhao ZG, Shyr Y, Shi CJ, Merchant NB, Washington K, et al. Lymph node ratio and preoperative CA 19-9 levels predict overall survival and recurrence-free survival in patients with resected pancreatic adenocarcinoma. World J Gastrointest Oncol. (2012) 4(10):207–15. doi: 10.4251/wjgo.v4.i10.207
62. Tas F, Sen F, Odabas H, Kılıc L, Keskın S, Yıldız I. Performance status of patients is the major prognostic factor at all stages of pancreatic cancer. Int J Clin Oncol. (2013) 18(5):839–46. doi: 10.1007/s10147-012-0474-9
63. Neoptolemos JP, Stocken DD, Friess H, Bassi C, Dunn JA, Hickey H, et al. A randomized trial of chemoradiotherapy and chemotherapy after resection of pancreatic cancer. N Engl J Med. (2004) 350(12):1200–10. doi: 10.1056/NEJMoa032295
64. Strobel O, Hank T, Hinz U, Bergmann F, Schneider L, Springfeld C, et al. Pancreatic cancer surgery: the new R-status counts. Ann Surg. (2017) 265(3):565–73. doi: 10.1097/SLA.0000000000001731
Keywords: pancreatectomy outcomes, pancreatic ductal adenocarcinoma (PDAC), open distal pancreatectomy, laparoscopic surgery, robotic surgery
Citation: Zhun Hong Wong N, Wei Ting Yap D, Lei Ng S, Yu Ning Ng J, James JJ and Wei Chieh Kow A (2024) Oncological outcomes in minimally invasive vs. open distal pancreatectomy: a systematic review and network meta-analysis. Front. Surg. 11:1369169. doi: 10.3389/fsurg.2024.1369169
Received: 11 January 2024; Accepted: 8 May 2024;
Published: 11 June 2024.
Edited by:
Airazat M. Kazaryan, Østfold Hospital, NorwayReviewed by:
Igor Khatkov, A.S. Loginov Moscow Clinical Scientific Centre, RussiaJose M. Ramia, Hospital General Universitario de Alicante, Spain
© 2024 Zhun Hong Wong, Wei Ting Yap, Lei Ng, Yu Ning Ng, James and Wei Chieh Kow. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Alfred Wei Chieh Kow, surkwc@nus.edu.sg
 Nicky Zhun Hong Wong
Nicky Zhun Hong Wong Dominic Wei Ting Yap1
Dominic Wei Ting Yap1  Juanita Jaslin James
Juanita Jaslin James