Environmental chemical exposures and mental health outcomes in children: a narrative review of recent literature
- 1United States Environmental Protection Agency, Office of Children’s Health Protection, Regulatory Support and Science Policy Division, Washington, DC, United States
- 2Oak Ridge Institute for Science Education, Oak Ridge, TN, United States
- 3United States Environmental Protection Agency, Public Health Integrated Toxicology Division, Center for Public Health and Environmental Assessment, Research Triangle Park, NC, United States
Background: Mental health is an important factor for children’s overall wellbeing. National health statistics show that millions of children are diagnosed with mental health disorders every year, and evidence from studies on chemical pollutants like lead and bisphenols indicate that environmental exposures are linked to mental health illnesses in youth. However, the relationship between children’s mental health and the environment is not well understood. This paper aims to review recent literature on prenatal and/or childhood environmental chemical exposures and mental health problems related to mood, anxiety, and behavior. This work also identifies areas of insufficient data and proposes suggestions to fill the data gaps.
Methods: A narrative review was performed by searching Google Scholar and PubMed for literature published in the last 6 years (2017–2022), using search terms related to children, mental health, and environmental chemical exposure. Additional relevant studies were identified by screening the references in these papers.
Results: A total of 29 studies are included in this review and results are summarized by chemical category: heavy metals, endocrine-disrupting chemicals, and pesticides. The majority of studies reported positive and significant associations between chemical exposures and child mental health outcomes including internalizing and externalizing behaviors.
Conclusion: This review demonstrates that there is a growing body of literature that suggests developmental exposure to some environmental chemicals increases a child’s risk of mood, anxiety, and behavior problems. Future research should expand on these findings to understand cumulative impacts, chemical mixtures, neurotoxic mechanisms, sex differences, and windows of vulnerability.
1 Introduction
Children’s mental health is a critical factor of their overall health and wellbeing. Mental health disorders, including mood, anxiety, and behavioral disorders, impact multiple aspects of children’s lives including the development of social skills, academic performance, and physical health. The diagnosis of childhood mental health disorders is also one of the strongest predictors of adult mental health disorders (Fryers and Brugha, 2013). Over the past 2 decades, the prevalence of adverse mental health outcomes among U.S. children has increased (Park-Lee, 2019; Tkacz and Brady, 2021). Based on 2016–2019 surveillance data for children ages 3–17 years living in the United States, 8.9% of children had received a behavior problems diagnosis, 4.4% of children had received a depression diagnosis, and 9.4% of children had received an anxiety diagnosis (Bitsko et al., 2022). Given the inherent gaps in surveillance data, including that many children mental health disorders are undiagnosed or misdiagnosed (Bitsko et al., 2022), these numbers may be underestimates.
The etiology of psychopathology is complex and includes genetic, social, and environmental factors, including exposures to chemicals in the environment. In recent years, the environmental determinants of mental health outcomes have gained increasing attention in multiple scientific disciplines including toxicology, epidemiology, psychology, and psychiatry. For example, there is a growing body of research that indicates air pollution may negatively impact children’s mental health (Brokamp et al., 2019; Roberts et al., 2019; Szyszkowicz et al., 2020; Reuben et al., 2021). Data on anthropogenic climate change and its negative impacts on youth mental health outcomes is also growing (Burke et al., 2018; Van Nieuwenhuizen et al., 2021; Clemens et al., 2022), including the consideration of “climate anxiety,” or anxiety related to the climate crisis and environmental disasters (Wu et al., 2020; Hickman et al., 2021). Further, the emerging discipline of clinical ecopsychology, which seeks to “systematically examine the direct and indirect mental health impacts of climate change, pollution, environmental degradation, and/or destruction of the air, water, and ecosystems,” has shown that environmental crisis and pollution act as stressors and disrupt multiple pathways that can increase a person’s vulnerability to adverse mental health (Thoma et al., 2021). Despite this growing interest, several data gaps related to environmental exposures and child mental health outcomes remain.
Scientists, physicians, and decision-makers need to better understand how environmental pollutants may impact children’s mental health. There are several plausible mechanisms to link environmental chemical exposures to the induction and/or exacerbation of mental health disorders. Chemical pollutants, such as heavy metals and endocrine disruptors, may alter neurotransmitter systems, damage the blood-brain barrier, modify brain gene expression leading to increased vulnerability to mental health disorders (i.e., depression), dysregulate the hypothalamus-pituitary-adrenal axis, reduce neuronal plasticity, and induce oxidative stress and neuroinflammation (Van Den Bosch and Meyer-Lindenberg, 2019; Thoma et al., 2021). The latter three are also consequences of chronic stress, which can have a synergistic effect on mental health outcomes (Van Den Bosch and Meyer-Lindenberg, 2019; Thoma et al., 2021).
Neurodevelopment is a protracted process that occurs from early embryogenesis and continues until at least 21 years of age (Stiles and Jernigan, 2010). Therefore, children are vulnerable to environmental exposures for years, and any perturbation of normal neurodevelopment could hold lifelong consequences (Stiles and Jernigan, 2010). Furthermore, children have limited ability to handle stress due to immature coping mechanisms, and thus have increased vulnerability to life stressors that can impact brain structure and function (Thoma et al., 2021). For example, racial and economic disparities in child mental health outcomes exist. A recent study found that non-Hispanic Black children had higher rates of mental-health related emergency visits than white children (Abrams et al., 2022). Several studies have also demonstrated that children from low-income households have a higher prevalence of mental health disorders than children from middle- and high-income households (Roberts et al., 2007; Melchior et al., 2010; Najman et al., 2010; Acri et al., 2017; Cree et al., 2018). These studies suggest that children living in environmental justice communities may be more at-risk to mental health conditions, given their burden of both chemical and non-chemical stressors. This review aims to assess the landscape of recent literature on prenatal and/or childhood environmental xenobiotic exposures and consequent symptoms related to mood, anxiety, and behavior disorders.
2 Materials and methods
This review focuses on symptoms and behaviors related to mood, anxiety, and behavior disorders such as depression, generalized anxiety disorder, and conduct disorder, respectively. Studies that exclusively focused on neurodevelopmental disorders (i.e., attention deficit hyperactivity disorder and autism spectrum disorder), cognition, intelligence quotient, and/or learning disorders were excluded. Experimental animal and adult population studies were also excluded, unless related to a childhood exposure. This review considered exposures from conception until age 21 years, in accordance with the U.S. Environmental Protection Agency’s Policy on Children’s Health (U.S. EPA, 2021).
To include the most recent research, only original research studies from the last 6 years were included, from January 2017 to December 2022. Google Scholar and PubMed were searched using search terms related to children (i.e., “child” or “infant” or “prenatal” or “adolescent”), mental health (i.e., “mental health” or “stress” or “anxiety” or “behavior” or “mood”), and environmental chemical exposure (i.e., “pollution” or “chemical” or “toxins”). Reference lists were also screened for additional relevant studies. Papers were selected based on title and then abstracts were screened. Full texts were then checked according to the inclusion/exclusion criteria, resulting in 29 final papers selected for review. Given that there is limited data on this emerging research area, we chose a narrative review, which is useful for exploring under researched topics (Sukhera, 2022). The approach was not intended to follow systematic review procedures.
3 Results
3.1 Literature overview
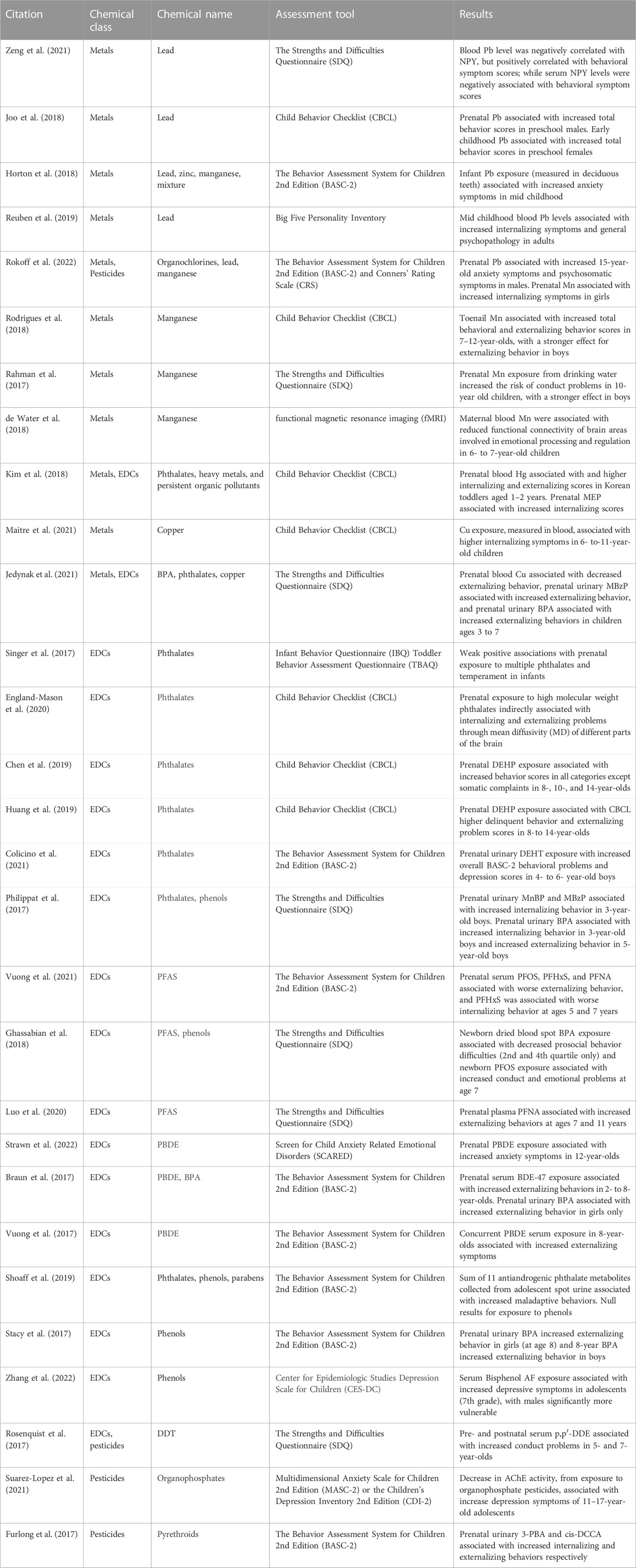
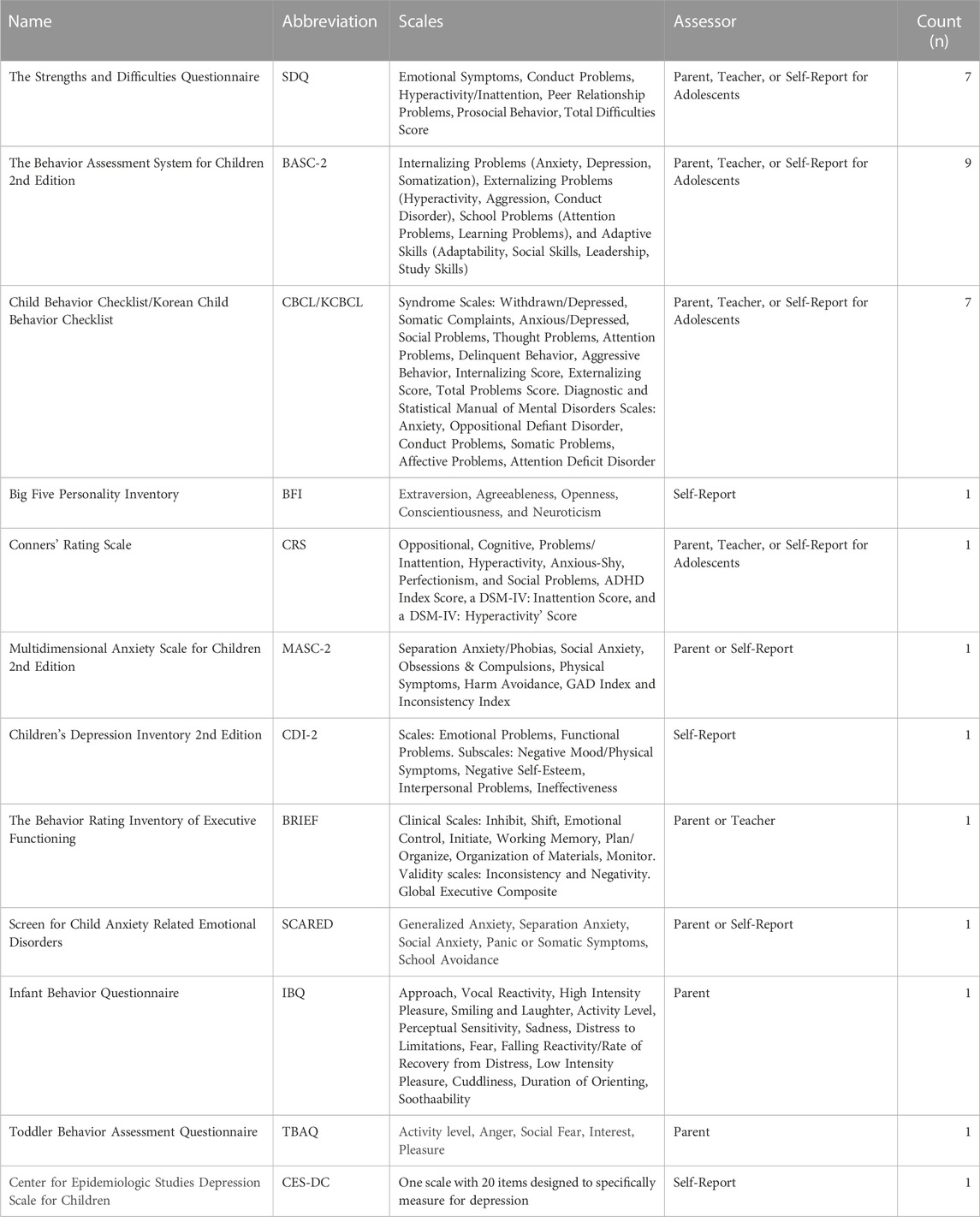
A total of 29 studies were included in this review (Table 1). These results are summarized below by chemical category, with some papers addressing the effects of more than one chemical class. The distribution of papers by chemical class are as follows: 11 papers on heavy metals, 20 on endocrine disrupting chemicals, and 4 on pesticides. Most of the studies (n = 22) used a prospective cohort design, with 20 studies being longitudinal birth cohorts. The majority of studies (n = 20) assessed prenatal exposure and measured outcomes during early childhood, from 0 to 5 years (n = 13), and mid childhood, from 6 to 10 years (n = 14). Each study utilized various covariates like maternal age and education, and this information can be found in the original publications. Eleven of the studies were conducted in North America, 7 in Asia, 5 in Europe, 5 in Latin America, and 1 in Oceania (New Zealand). Internalizing behaviors (i.e., anxiety, depression, somatization, withdrawal) and externalizing behaviors (i.e., aggression, impulsivity, conduct disorder) were the most frequently assessed endpoints. The most common measures used to assess these behaviors were the Behavior Assessment System for Children 2nd Edition (BASC-2) (n = 9), the Strengths and Difficulties Questionnaire (SDQ) (n = 7) and the Child Behavior Checklist (CBCL) (n = 7); information regarding these behavioral metrics can be found in Table 2. All findings summarized in this section are statistically significant, unless stated otherwise.
3.2 Heavy metals
Heavy metals are naturally occurring chemical elements that have a vast range of industrial uses but can be extremely toxic to several organ systems, including the central nervous system. Heavy metal applications include agriculture (e.g., fertilizer), technology (e.g., electronics), home goods (e.g., cookware) and manufacturing (e.g., automobile), which result in multiple possible sources of exposures. Documented exposure routes in humans include inhalation, ingestion, and dermal absorption (Al-Osman et al., 2019).
3.2.1 Lead
Lead (Pb) is one of the most well-established neurodevelopmental toxicants with no known safe level of exposure (U.S. EPA, 2013). Sources of Pb exposure for children include toys, dust, residential lead paint, drinking water, and soil (Njati and Maguta, 2019; Wilson et al., 2022). Pb accumulates in bone, likely due to its similar atomic properties to calcium. During pregnancy, Pb harbored in bone can be released into the bloodstream due to normal maternal bone remodeling, which results in elevated maternal Pb concentrations and consequently increased fetal exposure (reviewed in Goyer, 1996). Hypothesized mechanisms for Pb induced neurotoxicity include oxidative stress and neuronal cell death (Nemsadze et al., 2009; Ramírez Ortega et al., 2021). A study of Chinese pre-school students also found that electronic waste Pb exposure may decrease serum Neuropeptide Y, and that Neuropeptide Y may mediate a positive association between blood Pb level and behavioral deficits. However, this study was cross-sectional and thus could not establish causality (Zeng et al., 2021).
In the U.S., a birth cohort study found a positive association between prenatal Pb exposure (via umbilical cord blood) and BASC-2 anxiety score in 15-year-old adolescents, indicating increased anxiety symptoms (Rokoff et al., 2022). The study found a positive association between prenatal Pb and BASC-2 psychosomatic scores, with a stronger effect in males (Rokoff et al., 2022). A Korean birth cohort study found sex differences as well, with higher prenatal blood Pb levels increasing total CBCL behavior scores more in 5-year-old males, and higher childhood blood Pb levels (measured at ages 2,3, and 5) increasing total CBCL behavior scores more in 5-year-old females (Joo et al., 2018). When analyzing deciduous teeth to estimate developmental Pb exposure, a birth cohort study in Mexico associated increased dentine Pb at 12 months old with increased BASC-2 anxiety symptoms at 8–11 years (Horton et al., 2018).
Only one cohort study, conducted in New Zealand, reported results on early life Pb exposure and adult mental health (up to age 38 years) (Reuben et al., 2019). The study found that higher childhood Pb exposure, as determined by Pb blood concentrations measured at 11 years of age, was associated with increased internalizing symptoms and increased general psychopathology. However, this is a single study conducted in the 1970s, so these results may not be generalizable and should be replicated in other populations. In total, Pb is a recognized neurotoxicant. In addition to established effects like reduced intelligent quotient scores (IQ) (Lanphear et al., 2005; McFarland et al., 2022), these recent epidemiological studies also suggest that increased Pb exposure are correlated to negative mental health consequences in children.
3.2.2 Manganese
Manganese (Mn) is essential for brain function, but neurotoxic in excess. Children can be exposed to Mn prenatally (i.e., maternal blood), and via air pollution, food, and drinking water (Krachler et al., 1999; Rahman et al., 2017; Rodrigues et al., 2018). Inhaled Mn can cross the blood brain barrier, accumulate in the brain, and alter synaptic mechanisms (Davis, 1999; Aschner and Dorman, 2006) Mn has also been shown to impair astrocyte function, disrupt myelination, and disrupt dopamine neurotransmission (Normandin and Hazell, 2002). Using functional magnetic resonance imaging (fMRI), a birth cohort study in Mexico found that higher levels of Mn in maternal blood were associated with reduced functional connectivity of brain areas involved in emotional processing and regulation in 6- to 7-year-old children (de Water et al., 2018).
A cross-sectional study in Brazil assessed behavior in 7- to 12-year-old children residing near a ferro-manganese alloy plant, which resulted in increased exposure to airborne Mn (Rodrigues et al., 2018). The team used toenail clippings to assess long-term (7–12 month) Mn exposure and found significant associations between increased Mn exposure and increased total SDQ behavioral and externalizing behavior scores; this indicates that high Mn correlated to worsening behavioral symptoms. In Bangladesh, Rahman et al. (2017) found increased prenatal Mn exposure from drinking water increased the risk of conduct problems in 10-year-old children, as measure by the SDQ. Both studies saw more pronounced effects for externalizing behavior symptoms in boys (Rahman et al., 2017; Rodrigues et al., 2018). Whereas in the U.S., Rokoff et al. (2022) observed a positive association between prenatal Mn exposure, measured in umbilical cord blood, and internalizing symptoms in girls, at ages 8 years (measured by the Conners’ Rating Scale) and 15 years (measured by BASC-2), but saw null results in boys.
Like Pb, Mn is a well-recognized neurotoxicant. A large portion of the Mn literature focuses on adult neurotoxicity, as Mn excess can cause a Parkinson-like syndrome, including memory and motor deficits (Kwakye et al., 2015). The few studies presented here suggests that Mn excess may also contribute to mental health and behavioral problems in children. Further studies examining these neurological effects during development are warranted.
3.2.3 Copper
Copper (Cu) is an essential mineral for brain development but neurotoxic at high concentrations. Children can be exposed to Cu during gestation, and from sources such as drinking water (e.g., Cu pipes), food cooked on uncoated Cu cookware, Cu rich food, and smoke from burning Cu sulfate (Amorós et al., 2019; Royer and Sharman, 2023). Cu exposure may increase the release of proinflammatory cytokines and damage the structure of brain mitochondria, resulting in neurotoxicity (Kitazawa et al., 2016; Borchard et al., 2018). A cross-sectional European study found increased Cu in blood samples, was associated with higher CBCL internalizing symptoms in 6- to-11-year-old children (Maitre et al., 2021). Another European study found prenatal blood Cu levels to be negatively associated with SDQ externalizing scores in children ages 3–7 years old, suggesting decreased risk (Jedynak et al., 2021). The latter study noted their results should be interpreted with caution because there aren’t enough studies on Cu exposure and externalizing problems in children.
3.2.4 Mercury
Elemental, organic, and inorganic forms of mercury (Hg) are neurotoxic to children and have been linked to neurodevelopmental and neurocognitive disorders (reviewed in Al-Osman et al., 2019). Hg bioaccumulates in the food chain, especially seafood, which is a main source of exposure for children and pregnant people (Bose-O’Reilly et al., 2010). One study explored the effects of Hg on behavioral health and found an association between increased maternal blood Hg levels and higher CBCL internalizing and externalizing scores in Korean toddlers aged 1–2 years (Kim et al., 2018). While this suggests that Hg may be implicated in abnormal internalizing and externalizing behaviors, there is a paucity of data. Other studies are needed to ascertain reproducibility across cohorts.
3.3 Endocrine-disrupting chemicals
Endocrine-disrupting chemicals (EDCs) are xenobiotics that mimic or inhibit hormones, and can lead to abnormal hormone signaling in tissues (Shoaff et al., 2019). EDCs are widely used in a myriad of products including, but not limited to, plastic containers, food packaging, personal care products, medical supplies, and building materials.
3.3.1 Phthalates
High molecular weight phthalates are primarily used as plasticizers to increase flexibility and durability of plastics, while low molecular weight phthalates are used in cosmetics and pharmaceuticals (Chen et al., 2019). Phthalates easily leach into the environment and routes of exposure to children include inhalation, ingestion, and dermal absorption (Braun et al., 2013). Phthalates can also cross the placenta, resulting in prenatal exposure (Qian et al., 2020). Possible neurotoxic mechanisms for phthalates include altering steroid hormone concentrations, lipid metabolism, and disrupting neurotransmitters involved in the release of dopamine (Huang et al., 2019). In Canada, England-Mason et al. (2020) used MRI to examine brain white matter in pre-school children, and also conducted behavioral assessments. The authors found by neuroimaging and statistical modeling that increased prenatal exposure to high molecular weight phthalates was associated with changes in white matter microstructure in children (England-Mason et al., 2020). Specifically, increased mean diffusivity in brain regions that control affective function (i.e., mood) and perceptual processing. Further, the authors found indirect associations with increasing phthalate exposure during pregnancy and increased CBCL internalizing and externalizing scores. Together, these data suggest that the behavioral observations could be mediated by pthalate-induced structural changes in brain white matter (England-Mason et al., 2020).
In addition to the aforementioned study, several observational studies have also identified that increased phthalate exposure is correlated to behavioral outcomes. A U.S. birth cohort study found borderline statistical significance between increased prenatal exposure to multiple phthalates and temperament at 12- and 24-months (Singer et al., 2017). A Korean birth cohort study found a positive association between prenatal monoethyl phthalate (MEP) and CBCL internalizing scores in toddlers (Kim et al., 2018). In Taiwan, a birth cohort study assessed prenatal exposure to di (2-ethylhexyl) phthalate (DEHP) in 8-, 10-, and 14-year-old children (Chen et al., 2019). The authors found significant positive associations between prenatal urinary DEHP concentrations and increased CBCL scores in all of the test’s categories, except for somatic complaints. This indicates that children with higher phthalate exposure had behavioral problems in the following categories: withdrawn, anxious/depressed, social problems, thought problems, attention problems, delinquent behavior, aggressive behavior, internalizing problems, and externalizing problems. Children with higher DEHP exposure also had consistently higher overall CBCL scores (Chen et al., 2019). The authors noted that the median exposure quantified in their study was 4.54 μg/kg bw/day. This, in addition to previous epidemiological studies where DEHP exposure was associated with worsening behavioral symptoms, is well below the reference levels currently recommended by the EU and U.S., which is 50 and 20 μg/kg bw/day, respectively (Chen et al., 2019).
Another Taiwanese birth cohort study found associations between increasing maternal urinary DEHP metabolites and higher CBCL delinquent behavior and externalizing scores and increasing maternal urinary mono-2-ethylhexyl phthalate (MEHP) and higher CBCL internalizing and externalizing scores in children aged 8–14 years (Huang et al., 2019). The researchers also found a positive association between urinary monobenzyl phthalate (MBzP) at ages 2–8 years and CBCL scores for social problems at the age of 8–14 years (Huang et al., 2019). Colicino et al. (2021), studied di-2-ethylhexyl terephthalate (DEHT), a replacement for DEHP marketed as a less toxic alternative. In this Mexican birth cohort study, the authors linked higher maternal urinary DEHT levels with increased overall BASC-2 behavioral and depression scores in 4- to 6-year-old boys.
A French birth cohort study also reported that increased maternal urinary mono-n-butyl phthalate (MnBP) and MBzP during pregnancy were associated with increased SDQ internalizing behaviors in 3-year-old boys, however the incidence rate ratios were close to the null (Philippat et al., 2017). Jedynak et al. (2021) found prenatal urinary MnBP was associated with increased SDQ externalizing problems in 3-to7-year-old European children. In a cross-sectional design, Shoaff et al. (2019) collected spot urine from 15-year-old adolescents living near a Superfund site in the United States and assessed behavior using the BASC-2. The researchers observed a positive association between a sum of 11 antiandrogenic phthalate metabolites and increased maladaptive behaviors, such as externalizing behavior and developmental social disorders (Shoaff et al., 2019). Together, these data suggest that pthalate exposure during pregnancy is associated with a wide range of increased behavior problems in children, with ages ranging from infancy to adolescence.
In all, multiple studies conducted worldwide suggest that developmental phthalate exposure is correlated to behavioral deficits in children. Systematic reviews also suggest that increased developmental exposure to some phthalates is correlated with cognitive and psychomotor impairments, in addition to the behavioral issues reviewed here (Ejaredar et al., 2015; Zhang et al., 2019). While the etiology of these observations is unknown, phthalates are a diverse family of compounds with many metabolites. Additional research should be conducted to test the reproducibility of the epidemiological evidence cited herein.
3.3.2 Polybrominated diphenyl ethers
Polybrominated diphenyl ethers (PBDEs) are flame retardants present in a variety consumer goods and materials, such as furniture, electronics, and car seats (Costa and Giordano, 2007). Children can inhale or ingest PBDEs from contaminated dust, as well as ingest these compounds from food (i.e., fatty fish) and breastmilk (Domingo, 2012; Malliari and Kalantzi, 2017). Data suggests that PBDE’s could negatively impact neurobehavior via several mechanisms including disrupting thyroid hormone action, inducing oxidative stress, altering brain protein expression, increasing neuronal apoptosis, altering cholinergic system responses, and disturbing neurotransmitter function (Costa and Giordano, 2007; Vuong et al., 2018; Strawn et al., 2022). Early life exposure to PBDEs, which can accumulate in tissues and cross the placenta, has been shown to negatively impact cognition and behavior in children (Costa and Giordano, 2007; Lam et al., 2017; Gibson et al., 2018; Strawn et al., 2022).
Three papers utilized data obtained from the Health Outcomes and Measures of the Environment (HOME) study, an ongoing U.S.-based birth cohort that assesses the health impact of early childhood exposures to environmental toxicants. Strawn et al. (2022) examined prenatal serum PBDE concentrations and found significant associations with increased anxiety symptoms in 12-year-olds, measured by the Screen for Child Anxiety Related Emotional Disorders. The strongest effects were observed for panic and separation anxiety (Strawn et al., 2022). Braun et al. (2017) found that higher prenatal serum BDE-47 exposure was associated with persistent increases in externalizing behaviors from ages 2 to 8, as quantified by BASC-2 scores. Vuong et al. (2017) assessed serum PBDE concentrations in 8-year-old children and saw a positive association between multiple PBDE congeners (BDE-28, BDE-47, BDE-153, ∑PBDEs) and externalizing symptoms. While these three papers provide great preliminary data, more research from different cohorts can help elucidate the relationship between BPDEs and adverse mental health symptoms.
3.3.3 Bisphenols
A few studies investigated bisphenols, such as bisphenol A (BPA), an estrogenic polymer that is used to produce plastics (Ben-Jonathan and Steinmetz, 1998). In addition to prenatal exposure, children can be exposed to bisphenols from canned and packaged food, plastic baby and beverage bottles, and breast milk (Lakind and Naiman, 2011). Research suggests that BPA may disrupt the brain’s stress system (hypothalamic pituitary adrenal axis) and thus can increase symptoms of stress-related outcomes, such as anxiety and depression (Wiersielis et al., 2020). Several studies from years earlier than 2017 have linked prenatal and childhood BPA exposure to adverse behavior, anxiety, and depression (reviewed in Wiersielis et al., 2020).
In a China-based case-control study, researchers found that serum bisphenol AF exposure, a common BPA replacement, was associated with increased depressive symptoms in 7th grade students, with males being significantly more vulnerable than females (Zhang et al., 2022). A U.S. birth cohort observed a positive association between increased prenatal urinary BPA concentrations and greater BASC-2 externalizing scores in girls ages 2 through 8 years, but not boys (Braun et al., 2017). Using the HOME study cohort, Stacy et al. (2017) collected urinary BPA from pregnant people and children from the 2nd trimester through 8 years of age. The authors found that higher prenatal BPA concentrations were correlated with increased BASC-2 externalizing behavior in girls at 8 years of age. This same effect was not observed in boys. In contrast, higher BPA urinary concentrations was significantly associated with increasing externalizing behavior in boys, when both BPA and behavior were measured at 8-years of age. These studies suggest that BPA may elicit both temporal and sex-specific effects, similar to previous reports (Braun et al., 2009; Perera et al., 2016).
In the U.S., Shoaff et al. (2019) explored the cross-sectional relationship between 7 phenols (including bisphenol A, F, and S) in spot urine samples and adverse behavior in 15-year-old adolescents but produced null results. Another U.S. study observed an inverse association, indicating improved scores, between newborn BPA concentrations measured from dried blood spots and difficulties in SDQ prosocial behavior at 7 years old; however, this association was not significant when BPA concentrations were categorized in quartiles, making interpretation of these data difficult (Ghassabian et al., 2018). Jedynak et al. (2021) determined that increasing prenatal urinary BPA levels were associated with increased SDQ externalizing behaviors in European children ages 3–7 years. Assessing behavior in males with the SDQ in France, Philippat et al. (2017) found prenatal urinary BPA exposure was associated with increased internalizing behavior at 3 years old and increased externalizing behavior at 5 years old, as well as a positive association between triclosan and externalizing behavior at 3 years old.
Collectively, the bisphenol literature reviewed shows that abnormalities in externalizing and internalizing behaviors in children can be correlated to increased bisphenol exposure during pregnancy or childhood. However, the results of epidemiological studies correlate to factors such as the sex of the child and when bisphenols were measured. Additional studies are needed to confirm these observations, ideally with cohorts large enough in scale to permit stratification by sex and with bisphenol exposure measured sequentially at multiple times during development.
3.3.4 Per- and polyfluoroalkyl substances
Per- and polyfluoroalkyl substances (PFAS) are a chemical class with several thousand different congeners, which are widely used in various consumer products from cookware to clothing (Glüge et al., 2020). Many PFAS are persistent in the environment and in humans and have been categorized as persistent organic pollutants (Baker and Knappe, 2022; Starnes et al., 2022). PFAS can be detected in the placenta, amniotic fluid, maternal and neonatal blood, and breastmilk, demonstrating that pregnant people and children have some body burden of PFAS (Vuong et al., 2021). These organohalogens can elicit endocrine disruption in animal models and may be neurotoxic via disruption of the thyroid system (Mariussen, 2012). PFAS may also induce oxidative stress in the brain, and alter dopaminergic signaling pathways (Mariussen, 2012; Salgado et al., 2016).
A U.S. prospective birth cohort study found that prenatal serum perfluorooctane sulfonic acid (PFOS), perfluorohexane sulfonic acid (PFHxS), and perfluorononanoic acid (PFNA) were associated with increased odds of externalizing behaviors, and PFHxS was associated with increased internalizing problems at ages 5 and 7 years (Vuong et al., 2021). Another U.S. prospective birth cohort study found that higher PFOS concentrations in dried blood spot from newborns was associated with increased odds of conduct and emotional problems at 7 years of age (Ghassabian et al., 2018). In a Danish prospective birth cohort, authors observed a positive relationship between prenatal plasma perfluorononanoic acid (PFNA) concentrations and externalizing behaviors at ages 7 and 11 years, as detected by the SDQ (Luo et al., 2020). Overall, the epidemiological studies reviewed here suggests that a developmental PFAS exposure may correlate to later abnormalities in both internalizing and externalizing behaviors. However, there are relatively few studies that examine the mental health consequences of PFAS exposure in children and adolescents. Given that ubiquity of PFAS exposure and their biological persistence, this relationship should be explored in future studies.
3.3.5 Pesticides
Pesticides prevent, repel, and kill pests, and are integral to agriculture. Sources of pesticide exposure to children and pregnant people include contaminated food, water, indoor/outdoor air, household dust, and treated lawns (Roberts et al., 2012). Children who live in households close to agricultural sites, have household members working in agriculture, or who work in agriculture themselves, likely have higher exposure to various pesticides compared to the general population (Fenske et al., 2000; Lu et al., 2000).
Organochlorine pesticides, such as dichlorodiphenyltrichloroethane (DDT), were used heavily worldwide in the 1940s through 1960s. While they have been phased out of use in the U.S., organochlorines are still utilized in South America, Africa, and Asia for vector (i.e., mosquito) control. Nonetheless, these chemicals are persistent in the environment, and bioaccumulate in human tissue due to their lipophilic properties. They also likely cross the placenta, suggesting fetal exposure (Rosenquist et al., 2017). It is hypothesized that organochlorines dysregulate dopamine-mediated functions in animals, which is crucial to the limbic system, or the part of the brain that controls behavior and emotion (Rokoff et al., 2022). In Greenland and the Ukraine, Rosenquist et al. (2017) saw a positive association between a doubling of pre- and postnatal serum exposure to 1,1-dichloro-2,2-bis(p-chlorophenyl)-ethylene (p,p′-DDE), a metabolite of DDT, and odds of conduct problems in 5- and 7-year-olds. Rokoff et al. (2022) also looked at pre- and post-natal p,p′-DDE exposure and the effect on anxiety in 8- and 15-year-olds and found no association.
Organophosphate pesticides are applied widely in agriculture and work by inhibiting acetylcholinesterase (AChE) activity in insects. However, organophosphates also inhibit AChE activity in other species, and the cholinergic system plays a key role in mood regulation (Suarez-Lopez et al., 2021). A prospective cohort study in an Ecuadorian agricultural community saw an increase in adolescent (11–17 years) depression symptoms as AChE activity decreased, a relationship that was strongest at older ages and among female participants (Suarez-Lopez et al., 2021). The researchers did not detect any associations between AChE inhibition and anxiety symptoms (Suarez-Lopez et al., 2021).
Pyrethroid pesticides are widely utilized in homes, gardens, and agriculture; they are also used on pets and clothing to prevent ticks, and as vector control (Richardson et al., 2019). Pyrethroids can cross the blood-brain barrier and may alter various neurotransmitter systems and cause oxidative stress (Nasuti et al., 2007; Richardson et al., 2019). A longitudinal birth cohort study in the United States assessed prenatal exposure to pyrethroids and child behavior at ages at 4, 6, and 7–9 years (Furlong et al., 2017). Even with low detection frequencies, researchers found a positive association between detectable urinary levels of 3-phenoyxbenzoic acid (3-PBA) and cis-(2,2-dichlorovinyl)-2,2-dimethylcyclopropane-1-carboxylic acid (cis-DCCA) with increased BASC-2 internalizing and externalizing behaviors respectively. However, researchers advised interpreting the results with caution because exposure was categorized only as “detect” or “non-detect,” and not quantified. In all, the potential neurotoxicity of pesticides in humans is a known concern. However, there are few studies that specifically investigate if pesticide exposure is related to mental health concerns in children.
4 Discussion
The purpose of this review was to identify and summarize recent literature on environmental xenobiotic exposures and child mental health outcomes, specifically symptoms related to mood, anxiety, and behavioral disorders. The 29 studies included reveal that there is a growing body of literature demonstrating a potential relationship between increased exposure to pollutants like heavy metals, endocrine disrupting chemicals, and pesticides, with increased adverse mental health outcomes in children.
None of the studies in this review assessed the potential impact of environmental injustice. Low income and/or children of color living in communities disproportionately exposed to environmental pollution, often referred as “environmental justice,” or “overburdened” communities, may have increased vulnerability to the impact of chemical exposures on mental health outcomes. This may be due to increased chemical exposure(s), and/or an increased susceptibility to such environmental exposures due to additional psychological and physical stressors. For example, children in environmental justice communities may be exposed to higher levels of traumatic experiences, such as racial discrimination and environmental disasters (Jones Harden and Slopen, 2022). Furthermore, these communities may have less access to protective factors, such as greenspaces, healthy foods, quality healthcare and other neighborhood amenities (Reuben et al., 2022). It’s essential to understand the degree to which children in environmental justice communities are at increased risk and to what extent chemical exposures contribute to adverse physical health and mental health outcomes.
Potential opportunities to fill data gaps include conducting community based participatory research (CBPR) studies in communities where children and pregnant people have disproportionate exposure to chemically polluted sites (i.e., waste and wastewater facilities, industrial farming, metal working facilities, manufacturers, and oil and gas refineries), and/or xenobiotic exposure from sources like contaminated seafood, lead paint, unsafe water, and certain personal care products.
Along these lines, more research is needed to understand the cumulative impact of prenatal and childhood exposure to chemical and non-chemical stressors (i.e., psychosocial stress) on mental health outcomes, as well as the potential impact of protective factors, For example, Maitre et al. (2021) used an exposome approach to assess chemical and non-chemical environmental stressors and found longer sleep duration, higher family social capital, and a healthy diet during childhood to be protective against behavioral symptoms. Mental health outcomes could also be included in policy and regulatory cumulative impact/risk frameworks, especially given their potential economic burden (Torio et al., 2015; Trautmann et al., 2016; Tkacz and Brady, 2021).
Furthermore, a chemical exposure rarely occurs in isolation, and instead, complex mixtures more accurately represent a human exposure scenario. Two studies included in this review explored chemical mixtures. Horton et al. (2018) found a metal mixture of manganese, zinc and lead was associated with increased anxiety symptoms, with the mixture driven by Mn at 0–8 months and Pb at 8–12 months. When looking at prenatal exposure to a mixture of organochlorines and metals in 8- and 15-year-olds, Rokoff et al. (2022) saw null results. In addition, there have been other recent studies exploring the relationship between chemical mixtures and mental health related outcomes in children (Cowell et al., 2021; de Water et al., 2022). More research to understand chemical interactions in humans is critical, especially during pregnancy and development (Kim et al., 2018).
Hypothesized neurotoxic mechanisms that can translate to adverse mental health outcomes are discussed for each chemical throughout this review. However, only a few studies explored potential mechanisms. There is a need for mechanistic studies to confirm these hypotheses and preliminary results, including additional fMRI and MRI studies showing which regions of the brain may be affected by environmental exposures, as well as hypothesis-driven animal studies. It is difficult for prospective cohort studies to show causation between a chemical exposure and a health effect. As such, if the observations identified in these human studies could be repeated and further explored in an animal model, it would add further weight of evidence linking an exposure to a mental health outcome. In addition, 8 studies in this review saw statistically significant effects, but only in one sex. Future work is also needed to understand sex differences in neurological development, to help explain sex-specific outcomes.
Most studies included in this review are cohorts comprised of mother-child pairs; this is crucial, as it is well established that fetal and early postnatal development is a period of vulnerability to environmental exposures (Bellinger, 2013). However, some of the data reviewed suggest that chemical exposures during later childhood is also linked to mental health outcomes. Therefore, additional longitudinal birth cohort studies would allow for multiple exposure assessments during pregnancy, infancy, and childhood, providing a unique opportunity to explore windows of vulnerability to chemical exposures. Many mental health disorders are not diagnosed until adolescence (Solmi et al., 2022), thus evaluating children in epidemiological studies during this developmental period, instead of infancy and early childhood, may reveal stronger associations between a chemical exposure and neurological health.
Many of the papers in this review controlled for population variables like sociodemographic factors, birth conditions, and family history of mental illness. Future areas of study could explore potential interactions between these risk factors and chemical exposures of interest. And finally, existing national surveys could be expanded to help fill data gaps. For example, the National Survey of Children’s Health currently includes a mental health questionnaire but lacks any environmental exposure data. While an exposure would not be quantified due to the lack of biological sampling, researchers could infer exposure risk based on a participant’s geographical location if this information was made available. In all, recruiting large cohorts of mother-child dyads for extended observational studies, and leveraging existing tools for environmental health research, could further this important area of study.
5 Conclusion
Understanding the impact of environmental chemicals on children’s mental health is critical to promotion of health, wellbeing, and the prevention of disease (Thoma et al., 2021; Reuben et al., 2022). Due to the complex nature of both environmental xenobiotic exposures and mental health outcomes, it is difficult to establish causality with epidemiology studies alone. Unfortunately, the existing data are too limited to formally determine how individual chemicals may influence the development of specific mental health outcomes, though research in this area are steadily growing. With increased studies, this could permit data synthesis through tools like systematic review and metanalysis, and lead to sound scientific conclusions. Not only could this better inform policy and regulations to minimize any potential adverse effects of environmental chemicals, but it could also lead to informed risk management decisions for individuals, communities, and medical professionals. Given the burden of mental health disorders on children’s health, wellbeing, and overall life trajectory, it is essential to identify and take action to address the environmental risks that may increase the development of these disorders.
.
Author contributions
AJ: Conceptualization, Data curation, Investigation, Methodology, Writing–original draft, Writing–review and editing. KO: Funding acquisition, Writing–original draft, Writing–review and editing.
Funding
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. This research was supported by US EPA intramural funding.and in part by an appointment to the U.S. Environmental Protection Agency (EPA) Research Participation Program administered by the Oak Ridge Institute for Science and Education (ORISE) through an interagency agreement between the U.S. Department of Energy (DOE) and the U.S. Environmental Protection Agency. ORISE is managed by ORAU under DOE contract number DE-SC0014664. This document has been subjected to review and approved for publication by the US EPA’s Center for Public Health and Environmental Assessment and the Office of Children’s Health Protection. Approval does not signify that the contents reflect the views of the Agency, nor does mention of trade names or commercial products constitute endorsement or recommendation for use.
Acknowledgments
The authors thank Rebecca Dzubow, Aly Lorenz, Dr. Katherine Cole, Dr. Michael Caudle, and Dr. Emily Eisenhauer for their comments on previous versions of this manuscript.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Author disclaimer
The views expressed are those of the authors and do not necessarily represent the views or policies of the U.S. Environmental Protection Agency, U.S. Department of Energy, or Oak Ridge Institute for Science and Education.
References
Abrams, A. H., Badolato, G. M., Boyle, M. D., McCarter, R., and Goyal, M. K. (2022). Racial and ethnic disparities in pediatric mental health-related emergency department visits. Pediatr. Emerg. Care 38, e214–e218. doi:10.1097/PEC.0000000000002221
Acri, M. C., Bornheimer, L. A., Jessell, L., Heckman Chomancuzuk, A., Adler, J. G., Gopalan, G., et al. (2017). The intersection of extreme poverty and familial mental health in the United States. Soc. Work Ment. Health 15, 677–689. doi:10.1080/15332985.2017.1319893
Al-Osman, M., Yang, F., and Massey, I. Y. (2019). Exposure routes and health effects of heavy metals on children. BioMetals 32, 563–573. doi:10.1007/s10534-019-00193-5
Amorós, R., Murcia, M., González, L., Soler-Blasco, R., Rebagliato, M., Iñiguez, C., et al. (2019). Maternal copper status and neuropsychological development in infants and preschool children. Int. J. Hyg. Environ. Health 222, 503–512. doi:10.1016/j.ijheh.2019.01.007
Aschner, M., and Dorman, D. C. (2006). Manganese: pharmacokinetics and molecular mechanisms of brain uptake. Toxicol. Rev. 25, 147–154. doi:10.2165/00139709-200625030-00002
Baker, E. S., and Knappe, D. R. U. (2022). Per- and polyfluoroalkyl substances (PFAS)—contaminants of emerging concern. Anal. Bioanal. Chem. 414, 1187–1188. doi:10.1007/s00216-021-03811-9
Bellinger, D. C. (2013). Prenatal exposures to environmental chemicals and children’s neurodevelopment: an update. Saf. Health Work 4, 1–11. doi:10.5491/SHAW.2013.4.1.1
Ben-Jonathan, N., and Steinmetz, R. (1998). Xenoestrogens: the emerging story of bisphenol A. Trends Endocrinol. Metabolism 9, 124–128. doi:10.1016/S1043-2760(98)00029-0
Bitsko, R. H., Claussen, A. H., Lichstein, J., Black, L. I., Jones, S. E., Danielson, M. L., et al. (2022). Mental health surveillance among children - United States, 2013-2019. MMWR Suppl. 71, 1–42. doi:10.15585/mmwr.su7102a1
Borchard, S., Bork, F., Rieder, T., Eberhagen, C., Popper, B., Lichtmannegger, J., et al. (2018). The exceptional sensitivity of brain mitochondria to copper. Toxicol. Vitro 51, 11–22. doi:10.1016/j.tiv.2018.04.012
Bose-O’Reilly, S., McCarty, K. M., Steckling, N., and Lettmeier, B. (2010). Mercury exposure and children’s health. Curr. Probl. Pediatr. Adolesc. Health Care 40, 186–215. doi:10.1016/j.cppeds.2010.07.002
Braun, J. M., Sathyanarayana, S., and Hauser, R. (2013). Phthalate exposure and children’s health. Curr. Opin. Pediatr. 25, 247–254. doi:10.1097/MOP.0b013e32835e1eb6
Braun, J. M., Yolton, K., Dietrich, K. N., Hornung, R., Ye, X., Calafat, A. M., et al. (2009). Prenatal bisphenol A exposure and early childhood behavior. Environ. Health Perspect. 117, 1945–1952. doi:10.1289/ehp.0900979
Braun, J. M., Yolton, K., Stacy, S. L., Erar, B., Papandonatos, G. D., Bellinger, D. C., et al. (2017). Prenatal environmental chemical exposures and longitudinal patterns of child neurobehavior. Neurotoxicology 62, 192–199. doi:10.1016/j.neuro.2017.07.027
Brokamp, C., Strawn, J. R., Beck, A. F., and Ryan, P. (2019). Pediatric psychiatric emergency department utilization and fine particulate matter: a case-crossover study. Environ. Health Perspect. 127, 97006. doi:10.1289/EHP4815
Burke, S. E. L., Sanson, A. V., and Van Hoorn, J. (2018). The psychological effects of climate change on children. Curr. Psychiatry Rep. 20, 35. doi:10.1007/s11920-018-0896-9
Chen, C. C., Wang, Y. H., Chen, W. J., Hsiung, C. A., Leon Guo, Y. L., and Julie Wang, S. L. (2019). A benchmark dose study of prenatal exposure to di(2-ethylhexyl) phthalate and behavioral problems in children. Int. J. Hyg. Environ. Health 222, 971–980. doi:10.1016/j.ijheh.2019.06.002
Clemens, V., von Hirschhausen, E., and Fegert, J. M. (2022). Report of the intergovernmental panel on climate change: implications for the mental health policy of children and adolescents in Europe—a scoping review. Eur. Child. Adolesc. Psychiatry 31, 701–713. doi:10.1007/s00787-020-01615-3
Colicino, E., de Water, E., Just, A. C., Navarro, E., Pedretti, N. F., McRae, N., et al. (2021). Prenatal urinary concentrations of phthalate metabolites and behavioral problems in Mexican children: the Programming Research in Obesity, Growth Environment and Social Stress (PROGRESS) study. Environ. Res. 201, 111338. doi:10.1016/j.envres.2021.111338
Costa, L. G., and Giordano, G. (2007). Developmental neurotoxicity of polybrominated diphenyl ether (PBDE) flame retardants. Neurotoxicology 28, 1047–1067. doi:10.1016/j.neuro.2007.08.007
Cowell, W., Colicino, E., Levin-Schwartz, Y., Enlow, M. B., Amarasiriwardena, C., Andra, S. S., et al. (2021). Prenatal metal mixtures and sex-specific infant negative affectivity. Environ. Epidemiol. 5, e147. doi:10.1097/EE9.0000000000000147
Cree, R. A., Bitsko, R. H., Robinson, L. R., Holbrook, J. R., Danielson, M. L., Smith, C., et al. (2018). Health care, family, and community factors associated with mental, behavioral, and developmental disorders and poverty among children aged 2–8 Years — United States, 2016. MMWR Morb. Mortal. Wkly. Rep. 67, 1377–1383. doi:10.15585/mmwr.mm6750a1
Davis, J. M. (1999). Inhalation health risks of manganese: an EPA perspective. Neurotoxicology 20, 511–518.
de Water, E., Curtin, P., Gennings, C., Chelonis, J. J., Paule, M., Bixby, M., et al. (2022). Prenatal metal mixture concentrations and reward motivation in children. Neurotoxicology 88, 124–133. doi:10.1016/j.neuro.2021.11.008
de Water, E., Proal, E., Wang, V., Medina, S. M., Schnaas, L., Téllez-Rojo, M. M., et al. (2018). Prenatal manganese exposure and intrinsic functional connectivity of emotional brain areas in children. Neurotoxicology 64, 85–93. doi:10.1016/j.neuro.2017.06.006
Domingo, J. L. (2012). Polybrominated diphenyl ethers in food and human dietary exposure: a review of the recent scientific literature. Food Chem. Toxicol. 50, 238–249. doi:10.1016/j.fct.2011.11.004
Ejaredar, M., Nyanza, E. C., Ten Eycke, K., and Dewey, D. (2015). Phthalate exposure and childrens neurodevelopment: a systematic review. Environ. Res. 142, 51–60. doi:10.1016/j.envres.2015.06.014
England-Mason, G., Grohs, M. N., Reynolds, J. E., MacDonald, A., Kinniburgh, D., Liu, J., et al. (2020). White matter microstructure mediates the association between prenatal exposure to phthalates and behavior problems in preschool children. Environ. Res. 182, 109093. doi:10.1016/j.envres.2019.109093
Fenske, R. A., Glu, C. S., Simcox, N. J., Loewenherz, C., Touchstone, J., Moate, T. F., et al. (2000). Strategies for assessing children’s organophosphorus pesticide exposures in agricultural communities. J. Expo. Anal. Environ. Epidemiol. 10, 662–671. doi:10.1038/sj.jea.7500116
Fryers, T., and Brugha, T. (2013). Childhood determinants of adult psychiatric disorder. Clin. Pract. Epidemiol. Ment. Health 9, 1–50. doi:10.2174/1745017901309010001
Furlong, M. A., Barr, D. B., Wolff, M. S., and Engel, S. M. (2017). Prenatal exposure to pyrethroid pesticides and childhood behavior and executive functioning. Neurotoxicology 62, 231–238. doi:10.1016/j.neuro.2017.08.005
Ghassabian, A., Bell, E. M., Ma, W. L., Sundaram, R., Kannan, K., Buck Louis, G. M., et al. (2018). Concentrations of perfluoroalkyl substances and bisphenol A in newborn dried blood spots and the association with child behavior. Environ. Pollut. 243, 1629–1636. doi:10.1016/j.envpol.2018.09.107
Gibson, E. A., Siegel, E. L., Eniola, F., Herbstman, J. B., and Factor-Litvak, P. (2018). Effects of polybrominated diphenyl ethers on child cognitive, behavioral, and motor development. Int. J. Environ. Res. Public Health 15, 1636. doi:10.3390/ijerph15081636
Glüge, J., Scheringer, M., Cousins, I. T., Dewitt, J. C., Goldenman, G., Herzke, D., et al. (2020). An overview of the uses of per- and polyfluoroalkyl substances (PFAS). Environ. Sci. Process Impacts 22, 2345–2373. doi:10.1039/d0em00291g
Goyer, R. A. (1996). Results of lead research: prenatal exposure and neurological consequences. Environ. Health Perspect. 104, 1050–1054. doi:10.1289/ehp.961041050
Hickman, C., Marks, E., Pihkala, P., Clayton, S., Lewandowski, R. E., Mayall, E. E., et al. (2021). Climate anxiety in children and young people and their beliefs about government responses to climate change: a global survey. Lancet Planet Health 5, e863–e873. doi:10.1016/S2542-5196(21)00278-3
Horton, M. K., Hsu, L., Henn, B. C., Margolis, A., Austin, C., Svensson, K., et al. (2018). Dentine biomarkers of prenatal and early childhood exposure to manganese, zinc and lead and childhood behavior. Environ. Int. 121, 148–158. doi:10.1016/j.envint.2018.08.045
Huang, H. bin, Kuo, P. H., Su, P. H., Sun, C. W., Chen, W. J., and Wang, S. L. (2019). Prenatal and childhood exposure to phthalate diesters and neurobehavioral development in a 15-year follow-up birth cohort study. Environ. Res. 172, 569–577. doi:10.1016/j.envres.2019.02.029
Jedynak, P., Maitre, L., Guxens, M., Gützkow, K. B., Julvez, J., López-Vicente, M., et al. (2021). Prenatal exposure to a wide range of environmental chemicals and child behaviour between 3 and 7 years of age – an exposome-based approach in 5 European cohorts. Sci. Total Environ. 763, 144115. doi:10.1016/j.scitotenv.2020.144115
Jones Harden, B., and Slopen, N. (2022). Inequitable experiences and outcomes in young children: addressing racial and social-economic disparities in physical and mental health. Annu. Rev. Dev. Psychol. 4, 133–159. doi:10.1146/annurev-devpsych-121020-031515
Joo, H., Choi, J. H., Burm, E., Park, H., Hong, Y. C., Kim, Y., et al. (2018). Gender difference in the effects of lead exposure at different time windows on neurobehavioral development in 5-year-old children. Sci. Total Environ. 615, 1086–1092. doi:10.1016/j.scitotenv.2017.10.007
Kim, S., Eom, S., Kim, H. J., Lee, J. J., Choi, G., Choi, S., et al. (2018). Association between maternal exposure to major phthalates, heavy metals, and persistent organic pollutants, and the neurodevelopmental performances of their children at 1 to 2 years of age- CHECK cohort study. Sci. Total Environ. 624, 377–384. doi:10.1016/j.scitotenv.2017.12.058
Kitazawa, M., Hsu, H. W., and Medeiros, R. (2016). Copper exposure perturbs brain inflammatory responses and impairs clearance of amyloid-beta. Toxicol. Sci. 152, 194–204. doi:10.1093/toxsci/kfw081
Krachler, M., Rossipal, E., and Micetic-Turk, D. (1999). Trace element transfer from the mother to the newborn - investigations on triplets of colostrum, maternal and umbilical cord sera. Eur. J. Clin. Nutr. 53, 486–494. doi:10.1038/sj.ejcn.1600781
Kwakye, G. F., Paoliello, M. M. B., Mukhopadhyay, S., Bowman, A. B., and Aschner, M. (2015). Manganese-induced parkinsonism and Parkinson’s disease: shared and distinguishable features. Int. J. Environ. Res. Public Health 12, 7519–7540. doi:10.3390/ijerph120707519
Lakind, J. S., and Naiman, D. Q. (2011). Daily intake of bisphenol A and potential sources of exposure: 2005-2006 national health and nutrition examination survey. J. Expo. Sci. Environ. Epidemiol. 21, 272–279. doi:10.1038/jes.2010.9
Lam, J., Lanphear, B. P., Bellinger, D., Axelrad, D. A., McPartland, J., Sutton, P., et al. (2017). Developmental pbde exposure and IQ/ADHD in childhood: a systematic review and meta-analysis. Environ. Health Perspect. 125, 086001. doi:10.1289/EHP1632
Lanphear, B. P., Hornung, R., Khoury, J., Yolton, K., Baghurst, P., Bellinger, D. C., et al. (2005). Low-level environmental lead exposure and children’s intellectual function: an international pooled analysis. Environ. Health Perspect. 113, 894–899. doi:10.1289/ehp.7688
Lu, C., Fenske, R. A., Simcox, N. J., and Kalman, D. (2000). Pesticide exposure of children in an agricultural community: evidence of household proximity to farmland and take home exposure pathways. Environ. Res. 84, 290–302. doi:10.1006/enrs.2000.4076
Luo, J., Xiao, J., Gao, Y., Ramlau-Hansen, C. H., Toft, G., Li, J., et al. (2020). Prenatal exposure to perfluoroalkyl substances and behavioral difficulties in childhood at 7 and 11 years. Environ. Res. 191, 110111. doi:10.1016/j.envres.2020.110111
Maitre, L., Julvez, J., López-Vicente, M., Warembourg, C., Tamayo-Uria, I., Philippat, C., et al. (2021). Early-life environmental exposure determinants of child behavior in Europe: a longitudinal, population-based study. Environ. Int. 153, 106523. doi:10.1016/j.envint.2021.106523
Malliari, E., and Kalantzi, O. I. (2017). Children’s exposure to brominated flame retardants in indoor environments - a review. Environ. Int. 108, 146–169. doi:10.1016/j.envint.2017.08.011
Mariussen, E. (2012). Neurotoxic effects of perfluoroalkylated compounds: mechanisms of action and environmental relevance. Arch. Toxicol. 86, 1349–1367. doi:10.1007/s00204-012-0822-6
McFarland, M. J., Hauer, M. E., and Reuben, A. (2022). Half of US population exposed to adverse lead levels in early childhood. Proc. Natl. Acad. Sci. U. S. A. 119, e2118631119. doi:10.1073/pnas.2118631119
Melchior, M., Chastang, J. F., Walburg, V., Arseneault, L., Galéra, C., and Fombonne, E. (2010). Family income and youths’ symptoms of depression and anxiety: a longitudinal study of the French GAZEL Youth cohort. Depress Anxiety 27, 1095–1103. doi:10.1002/da.20761
Najman, J. M., Hayatbakhsh, M. R., Clavarino, A., Bor, W., O’Callaghan, M. J., and Williams, G. M. (2010). Family poverty over the early life course and recurrent adolescent and young adult anxiety and depression: a longitudinal study. Am. J. Public Health 100, 1719–1723. doi:10.2105/AJPH.2009.180943
Nasuti, C., Gabbianelli, R., Falcioni, M. L., Di Stefano, A., Sozio, P., and Cantalamessa, F. (2007). Dopaminergic system modulation, behavioral changes, and oxidative stress after neonatal administration of pyrethroids. Toxicology 229, 194–205. doi:10.1016/j.tox.2006.10.015
Nemsadze, K., Sanikidze, T., Ratiani, L., Gabunia, L., and Sharashenidze, T. (2009). Mechanisms of lead-induced poisoning. Georgian Med. News, 92–96.
Njati, S. Y., and Maguta, M. M. (2019). Lead-based paints and children’s PVC toys are potential sources of domestic lead poisoning – a review. Environ. Pollut. 249, 1091–1105. doi:10.1016/j.envpol.2019.03.062
Normandin, L., and Hazell, A. S. (2002). “Manganese neurotoxicity: an update of pathophysiologic mechanisms,” in Metabolic brain disease. doi:10.1023/A:1021970120965
Park-Lee, R. E. A. (2019). Key substance use and mental health indicators in the United States: results from the 2020 national survey on drug use and health. Rockville, MD, United States HHS Publication No. PEP19-5068. NSDUH Series H-54 170.
Perera, F., Nolte, E. L. R., Wang, Y., Margolis, A. E., Calafat, A. M., Wang, S., et al. (2016). Bisphenol A exposure and symptoms of anxiety and depression among inner city children at 10–12 years of age. Environ. Res. 151, 195–202. doi:10.1016/j.envres.2016.07.028
Philippat, C., Nakiwala, D., Calafat, A. M., Botton, J., de Agostini, M., Heude, B., et al. (2017). Prenatal exposure to nonpersistent endocrine disruptors and behavior in boys at 3 and 5 years. Environ. Health Perspect. 125, 097014. doi:10.1289/EHP1314
Qian, Y., Shao, H., Ying, X., Huang, W., and Hua, Y. (2020). The endocrine disruption of prenatal phthalate exposure in mother and offspring. Front. Public Health 8, 366. doi:10.3389/fpubh.2020.00366
Rahman, S. M., Kippler, M., Tofail, F., Bölte, S., Hamadani, J. D., and Vahter, M. (2017). Manganese in drinking water and cognitive abilities and behavior at 10 years of age: a prospective cohort study. Environ. Health Perspect. 125, 057003. doi:10.1289/EHP631
Ramírez Ortega, D., González Esquivel, D. F., Blanco Ayala, T., Pineda, B., Gómez Manzo, S., Marcial Quino, J., et al. (2021). Cognitive impairment induced by lead exposure during lifespan: mechanisms of lead neurotoxicity. Toxics 9, 23. doi:10.3390/toxics9020023
Reuben, A., Arseneault, L., Beddows, A., Beevers, S. D., Moffitt, T. E., Ambler, A., et al. (2021). Association of air pollution exposure in childhood and adolescence with psychopathology at the transition to adulthood. JAMA Netw. Open 4, e217508. doi:10.1001/jamanetworkopen.2021.7508
Reuben, A., Manczak, E. M., Cabrera, L. Y., Alegria, M., Bucher, M. L., Freeman, E. C., et al. (2022). The interplay of environmental exposures and mental health: setting an agenda. Environ. Health Perspect. 130, 25001. doi:10.1289/EHP9889
Reuben, A., Schaefer, J. D., Moffitt, T. E., Broadbent, J., Harrington, H., Houts, R. M., et al. (2019). Association of childhood lead exposure with adult personality traits and lifelong mental health. JAMA Psychiatry 76, 418–425. doi:10.1001/jamapsychiatry.2018.4192
Richardson, J. R., Fitsanakis, V., Westerink, R. H. S., and Kanthasamy, A. G. (2019). Neurotoxicity of pesticides. Acta Neuropathol. 138, 343–362. doi:10.1007/s00401-019-02033-9
Roberts, J. R., Karr, C. J., Paulson, J. A., Brock-Utne, A. C., Brumberg, H. L., Campbell, C. C., et al. (2012). Pesticide exposure in children. Pediatrics 130, e1757–e1763. doi:10.1542/peds.2012-2757
Roberts, R. E., Roberts, C. R., and Xing, Y. (2007). Rates of DSM-IV psychiatric disorders among adolescents in a large metropolitan area. J. Psychiatr. Res. 41, 959–967. doi:10.1016/j.jpsychires.2006.09.006
Roberts, S., Arseneault, L., Barratt, B., Beevers, S., Danese, A., Odgers, C. L., et al. (2019). Exploration of NO 2 and PM 2.5 air pollution and mental health problems using high-resolution data in London-based children from a UK longitudinal cohort study. Psychiatry Res. 272, 8–17. doi:10.1016/j.psychres.2018.12.050
Rodrigues, J. L. G., Araújo, C. F. S., dos Santos, N. R., Bandeira, M. J., Anjos, A. L. S., Carvalho, C. F., et al. (2018). Airborne manganese exposure and neurobehavior in school-aged children living near a ferro-manganese alloy plant. Environ. Res. 167, 66–77. doi:10.1016/j.envres.2018.07.007
Rokoff, L. B., Shoaff, J. R., Coull, B. A., Enlow, M. B., Bellinger, D. C., and Korrick, S. A. (2022). Prenatal exposure to a mixture of organochlorines and metals and internalizing symptoms in childhood and adolescence. Environ. Res. 208, 112701. doi:10.1016/j.envres.2022.112701
Rosenquist, A. H., Høyer, B. B., Julvez, J., Sunyer, J., Pedersen, H. S., Lenters, V., et al. (2017). Prenatal and postnatal PCB-153 and p,p′ -DDE exposures and behavior scores at 5–9 years of age among children in Greenland and Ukraine. Environ. Health Perspect. 125, 107002. doi:10.1289/EHP553
Royer, A., and Sharman, T. (2023). Copper toxicity. Treasure Island, FL, United States StatPearls Publishing. PMID: 32491388.
Salgado, R., López-Doval, S., Pereiro, N., and Lafuente, A. (2016). Perfluorooctane sulfonate (PFOS) exposure could modify the dopaminergic system in several limbic brain regions. Toxicol. Lett. 240, 226–235. doi:10.1016/j.toxlet.2015.10.023
Shoaff, J. R., Calafat, A. M., Schantz, S. L., and Korrick, S. A. (2019). Endocrine disrupting chemical exposure and maladaptive behavior during adolescence. Environ. Res. 172, 231–241. doi:10.1016/j.envres.2018.12.053
Singer, A. B., Wolff, M. S., Silva, M. J., Calafat, A. M., and Engel, S. M. (2017). Prenatal phthalate exposures and child temperament at 12 and 24 months. Neurotoxicology 62, 248–257. doi:10.1016/j.neuro.2017.08.002
Solmi, M., Radua, J., Olivola, M., Croce, E., Soardo, L., Salazar de Pablo, G., et al. (2022). Age at onset of mental disorders worldwide: large-scale meta-analysis of 192 epidemiological studies. Mol. Psychiatry 27, 281–295. doi:10.1038/s41380-021-01161-7
Stacy, S. L., Papandonatos, G. D., Calafat, A. M., Chen, A., Yolton, K., Lanphear, B. P., et al. (2017). Early life bisphenol A exposure and neurobehavior at 8 years of age: identifying windows of heightened vulnerability. Environ. Int. 107, 258–265. doi:10.1016/j.envint.2017.07.021
Starnes, H. M., Rock, K. D., Jackson, T. W., and Belcher, S. M. (2022). A critical review and meta-analysis of impacts of per- and polyfluorinated substances on the brain and behavior. Front. Toxicol. 4, 881584. doi:10.3389/ftox.2022.881584
Stiles, J., and Jernigan, T. L. (2010). The basics of brain development. Neuropsychol. Rev. 20, 327–348. doi:10.1007/s11065-010-9148-4
Strawn, J. R., Xu, Y., Cecil, K. M., Khoury, J., Altaye, M., Braun, J. M., et al. (2022). Early exposure to flame retardants is prospectively associated with anxiety symptoms in adolescents: a prospective birth cohort study. Depress Anxiety 39, 780–793. doi:10.1002/da.23284
Suarez-Lopez, J. R., Nguyen, A., Klas, J., Gahagan, S., Checkoway, H., Lopez-Paredes, D., et al. (2021). Associations of acetylcholinesterase inhibition between pesticide spray seasons with depression and anxiety symptoms in adolescents, and the role of sex and adrenal hormones on gender moderation. Expo. Health 13, 51–64. doi:10.1007/s12403-020-00361-w
Sukhera, J. (2022). Narrative reviews: flexible, rigorous, and practical. J. Grad. Med. Educ. 14, 414–417. doi:10.4300/JGME-D-22-00480.1
Szyszkowicz, M., Zemek, R., Colman, I., Gardner, W., Kousha, T., and Smith-Doiron, M. (2020). Air pollution and emergency department visits for mental disorders among youth. Int. J. Environ. Res. Public Health 17, 4190–4211. doi:10.3390/ijerph17124190
Thoma, M. V., Rohleder, N., and Rohner, S. L. (2021). Clinical ecopsychology: the mental health impacts and underlying pathways of the climate and environmental crisis. Front. Psychiatry 12, 675936. doi:10.3389/fpsyt.2021.675936
Tkacz, J., and Brady, B. L. (2021). Increasing rate of diagnosed childhood mental illness in the United States: incidence, prevalence and costs. Public Health Pract. 2, 100204. doi:10.1016/j.puhip.2021.100204
Torio, C. M., Encinosa, W., Berdahl, T., McCormick, M. C., and Simpson, L. A. (2015). Annual report on health care for children and youth in the United States: national estimates of cost, utilization and expenditures for children with mental health conditions. Acad. Pediatr. 15, 19–35. doi:10.1016/j.acap.2014.07.007
Trautmann, S., Rehm, J., and Wittchen, H. (2016). The economic costs of mental disorders. EMBO Rep. 17, 1245–1249. doi:10.15252/embr.201642951
Tsai, T. L., Hsieh, C. J., Wu, M. T., Chen, M. L., Kuo, P. H., and Wang, S. L. (2023). Co-exposure to toxic metals and phthalates in pregnant women and their children’s mental health problems aged four years — taiwan Maternal and Infant Cohort Study (TMICS). Environ. Int. 173, 107804. doi:10.1016/j.envint.2023.107804
U.S. EPA (2013). Integrated science assessment (ISA) for lead (final report, jul 2013). Washington, DC, United States U.S. Environmental Protection Agency.
U.S. EPA (2021). 2021 policy on children’s health. Washington, DC, United States Office of the Administrator. U.S. Environmental Protection Agency. Available at: https://www.epa.gov/system/files/documents/2021-10/2021-policy-on-childrens-health.pdf.
Van Den Bosch, M., and Meyer-Lindenberg, A. (2019). Environmental exposures and depression: biological mechanisms and epidemiological evidence. Annu. Rev. Public Health 40, 239–259. doi:10.1146/annurev-publhealth-040218-044106
Van Nieuwenhuizen, A., Hudson, K., Chen, X., and Hwong, A. R. (2021). The effects of climate change on child and adolescent mental health: clinical considerations. Curr. Psychiatry Rep. 23, 88. doi:10.1007/s11920-021-01296-y
Vuong, A. M., Webster, G. M., Yolton, K., Calafat, A. M., Muckle, G., Lanphear, B. P., et al. (2021). Prenatal exposure to per- and polyfluoroalkyl substances (PFAS) and neurobehavior in US children through 8 years of age: the HOME study. Environ. Res. 195, 110825. doi:10.1016/j.envres.2021.110825
Vuong, A. M., Yolton, K., Dietrich, K. N., Braun, J. M., Lanphear, B. P., and Chen, A. (2018). Exposure to polybrominated diphenyl ethers (PBDEs) and child behavior: current findings and future directions. Horm. Behav. 101, 94–104. doi:10.1016/j.yhbeh.2017.11.008
Vuong, A. M., Yolton, K., Xie, C., Webster, G. M., Sjödin, A., Braun, J. M., et al. (2017). Childhood polybrominated diphenyl ether (PBDE) exposure and neurobehavior in children at 8 years. Environ. Res. 158, 677–684. doi:10.1016/j.envres.2017.07.028
Wiersielis, K. R., Samuels, B. A., and Roepke, T. A. (2020). Perinatal exposure to bisphenol A at the intersection of stress, anxiety, and depression. Neurotoxicol Teratol. 79, 106884. doi:10.1016/j.ntt.2020.106884
Wilson, J., Dixon, S. L., Wisinski, C., Speidel, C., Breysse, J., Jacobson, M., et al. (2022). Pathways and sources of lead exposure: Michigan Children’s Lead Determination (the MI CHILD study). Environ. Res. 215, 114204. doi:10.1016/j.envres.2022.114204
Wu, J., Snell, G., and Samji, H. (2020). Climate anxiety in young people: a call to action. Lancet Planet Health 4, e435–e436. doi:10.1016/S2542-5196(20)30223-0
Zeng, X., Xu, C., Xu, X., Zhang, Y., Huang, Y., and Huo, X. (2021). Elevated lead levels in relation to low serum neuropeptide Y and adverse behavioral effects in preschool children with e-waste exposure. Chemosphere 269, 129380. doi:10.1016/j.chemosphere.2020.129380
Zhang, C., Zhou, L., Wu, X. chang, Guan, T. yue, Zou, X. min, Chen, C., et al. (2022). Association of serum bisphenol AF concentration with depressive symptoms in adolescents: a nested case–control study in China. Ecotoxicol. Environ. Saf. 241, 113734. doi:10.1016/j.ecoenv.2022.113734
Keywords: children’s mental health, behavioral health, environmental justice, environmental exposure, toxicity, heavy metals, endocrine disrupting chemicals, pesticides
Citation: James AA and OShaughnessy KL (2023) Environmental chemical exposures and mental health outcomes in children: a narrative review of recent literature. Front. Toxicol. 5:1290119. doi: 10.3389/ftox.2023.1290119
Received: 07 September 2023; Accepted: 13 November 2023;
Published: 30 November 2023.
Edited by:
Aina Olubukola Adeogun, University of Ibadan, NigeriaReviewed by:
Francheska Merced-Nieves, Icahn School of Medicine at Mount Sinai, United StatesAdesola Ogunniyi, University of Ibadan, Nigeria
Copyright © 2023 James and OShaughnessy. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Ashley A. James, james.ashley@epa.gov
 Ashley A. James
Ashley A. James Katherine L. OShaughnessy
Katherine L. OShaughnessy