Oral Vaccination with Heat-Inactivated Mycobacterium bovis Does Not Interfere with the Antemortem Diagnostic Techniques for Tuberculosis in Goats
- 1CZ Veterinaria S.A., Porriño, Pontevedra, Spain
- 2SaBio, Instituto de Investigación en Recursos Cinegéticos IREC (CSIC-UCLM-JCCM), Ciudad Real, Spain
- 3VISAVET Health Surveillance Centre, Complutense University of Madrid, Madrid, Spain
- 4Faculty of Veterinary Medicine, Department of Animal Health, Complutense University of Madrid, Madrid, Spain
- 5Faculty of Veterinary Medicine, Beni-Suef University, Beni-Suef, Egypt
- 6MAEVA SERVET S.L., Madrid, Spain
- 7Servicio Regional de Investigación y Desarrollo Agrario (SERIDA), Villaviciosa, Spain
- 8Animal Health Department, NEIKER-Tecnalia, Derio, Bizkaia, Spain
Vaccination against tuberculosis (TB) is prohibited in cattle or other species subjected to specific TB eradication campaigns, due to the interference that it may cause with the official diagnostic tests. However, immunization with a heat-inactivated (HI) Mycobacterium bovis vaccine via the oral route has been suggested to overcome this issue. In this study, the main goal was to assess the interference of the HI vaccine by different routes of administration using a previous vaccination and re-vaccination (boosting) protocol. TB-free kid goats were divided into three groups: oral (n = 16), intramuscular (IM; n = 16), and control (n = 16). Results showed that there was a significant difference in the percentage of animals positive to the single intradermal test (SIT) and blood based interferon-gamma release assay (IGRA) caused by vaccination when performed in the IM group compared to the oral group (p < 0.001). Nevertheless, no positivity to the SIT or IGRA test was observed in orally vaccinated goats regardless of the different interpretation criteria applied. None of the groups presented positive antibody titers using an in-house ELISA and samples collected 2 months after the boost. These results suggest the potential usefulness of the HI vaccine by the oral route in goats to minimize the interference on diagnostic tests (skin and IGRA tests) and reducing the necessity of defined antigens to replace the traditional purified protein derivatives for diagnosis. Finally, the results pave the way to future efficacy studies in goats using different routes of HI vaccination.
Introduction
Tuberculosis (TB) in goats is mostly caused by Mycobacterium bovis and Mycobacterium caprae, both of which have also been isolated in Spain from other domestic and wildlife species (1–3). According to the Official TB Eradication Program for cattle in Spain, only caprine flocks coexisting, sharing pastures, or having epidemiological links with cattle should be subjected to official tests for the diagnosis of TB. Nevertheless, some regions with a high density of caprine flocks have implemented specific TB control programs for goats (4). These programs are mainly based on the application of skin tests [single intradermal tuberculin test and comparative intradermal tuberculin test, single intradermal test (SIT) and SCIT tests, respectively], while using the blood-based interferon-gamma release assay (IGRA) as an ancillary test under specific circumstances (2). Vaccination against TB is prohibited in cattle in the European Union (Chapter III, Article 13, Council Directive 78/52/EEC). Nevertheless, vaccination has been suggested as a suitable complementary strategy for the control of TB not only in cattle but also in other livestock and wildlife species under certain epidemiological circumstances, and may play a significant role in countries where domestic and wildlife species are not subjected to compulsory TB eradication programs (5). Bacillus Calmette-Guérin (BCG) is the only vaccine approved for use in humans, and it has been assayed in multiple experimental field trials (alone or in combination with others vaccines) in domestic and wild animals, displaying different results in terms of protection conferred (6–12). However, BCG vaccination sensitizes animals to respond to skin and IGRA tests (13), complicating the differentiation between infected and vaccinated animals (14). Therefore, implementation of BCG vaccination as a TB control strategy in livestock would require diagnostic tools able to distinguish vaccinated from infected animals (DIVA) (15, 16) as it was stated in a recent EFSA scientific opinion (17). However, in general terms, diagnostic tools based on DIVA antigens are less sensitive than those using purified protein derivatives (PPDs), increasing the occurrence of false negative reactions (9, 18, 19). Therefore, the development of immunization schemes against TB to reduce the diagnostic interferences and the use of DIVA antigens would be of paramount importance, since they could accelerate the control/eradication process. Such schemes may also be more cost-effective, which is particularly important for other species than cattle.
A heat-inactivated (HI) M. bovis vaccine has been recently developed, conferring similar protection to BCG against TB in wild boar (20) and improving the stability under field conditions (21). Both parenteral and oral vaccinations with IV have shown a significant reduction in TB-compatible lesions in wild boar (20, 22). The efficacy of the HI vaccine against a virulent M. bovis field strain has been also demonstrated in domestic pigs, reducing significantly the lesion and culture scores (23). In these previous studies, the absence of a sensitization effect was suggested, showing neither IGRA nor ELISA positivity in orally immunized wild boar. By contrast, a consistent response to the IGRA and ELISA test was triggered when animals were vaccinated via the IM route (20, 24). Another study confirmed that oral HI vaccine does not sensitize farmed red deer and therefore does not cause false-positive responses in the tuberculin skin test (25). Moreover, a recent study in cattle confirmed these previous findings, demonstrating that the oral vaccination with HI vaccine in calves do not compromise official TB diagnostic tests (26). Nevertheless, the immunization protocol applied in previous studies only considered a single dose and therefore, the effect on the interference of a second boosting re-vaccination, recommended to increase the protection efficacy, was not evaluated. Moreover, evaluation of interference on serological tests may be of interest in goats, since these diagnostic tools are more frequently used in livestock and wildlife not subjected to official eradication programs (27–29).
The aim of the present experiment was to assess the interference caused by the HI vaccine using a vaccination and boosting re-vaccination protocol by two different routes of administration (oral and parenteral) on the cellular and humoral TB diagnostic tests in kid goats. These results will be useful for the design of potential immunization protocols applied in goats with the aim of reducing the high prevalence of caprine TB in regions of Spain where caprine production is uncoupled to the cattle industry and where the TB prevalence is high.
Materials and Methods
Animals
Forty-eight female kid goats (2–3 weeks old) were selected from a flock without a previous history of TB. Animals were tested in the farm of origin using a commercial IGRA kit (Bovigam, Thermo Fisher Scientific, USA) and an indirect in-house ELISA that was carried out with a readjusted procedure used by Che-Amat et al. (30) to check their negative status. Afterward, the selected animals were moved to biosafety facilities during the study. Handling of the animals and sampling were performed according to European (Directive 2010/63/EU) and Spanish Legislation (RD 53/2013), and also approved by the Ethics Committee (Complutense University of Madrid) and the Regional Agriculture Authority [Comunidad de Madrid; permit number: PROEX 143/15 (29/06/2015)].
Experimental Design
Kid goats were randomly distributed into three groups. Group 1 (n = 16) non-vaccinated (control); Group 2 (n = 16) intramuscularly vaccinated (IM); and Group 3 (n = 16) orally vaccinated. M. bovis strain Neiker 1403 (spoligotype SB0339) from a naturally infected wild boar was used for the preparation of vaccines as previously described (20). The IM vaccine contained adjuvant Montanide ISA 50 V2 (Seppic, Castres, France), while the oral vaccine consisted of phosphate-buffered saline (PBS) containing the inactivated mycobacteria. Both parenteral and oral inactivated vaccine contained 6 × 107 CFU, and were administered in a 2 mL/dose and 1 mL/dose by oral an IM routes 1 week after arrival, respectively. Groups 2 and 3 received a revaccination 4 weeks after the prime vaccination using the same doses and routes of administration.
Diagnostic Methods
Skin Tests
Both SIT and SCIT tests were carried out 2 months after booster vaccination. SIT and SCIT tests were performed in the neck (cervical region), using PPD of M. bovis (bovine PPD) and M. avium (avian PPD) (CZ Veterinaria, Spain) according to the R.D. 2611/1996 and European Commission Council Directive 64/432/EEC. The skin fold thickness was measured after 72 h. Bovine and avian PPDs were inoculated on the left and right side of the neck, respectively, with a volume of 0.1 mL using a Dermojet® syringe (Akra Dermojet, France). Applying the standard interpretation of the SIT test, animals were considered as positive reactors if presenting a skin fold thickness increase of 4 or more mm or the presence of clinical signs (exudation, edema, or necrosis). Positive animals to the SCIT test were those showing a positive bovine reaction greater than the avian reaction in more than 4 mm, or the presence of clinical signs at the bovine PPD inoculation site.
Interferon-Gamma Release Assay (IGRA)
Blood samples were collected just prior to the vaccination to check the negative status of the goats, and 2 months after re-vaccination. Heparinized whole blood samples were stimulated with bovine and avian PPD (CZ Veterinaria, Spain) at a final concentration of 20 µg/mL and nil antigen PBS as previously described (31–33). Afterward, samples were incubated during 18–20 h at 37° in a humidified atmosphere. The whole blood IGRA was performed following the manufacturer’s instructions (Bovigam® TB Kit, Thermo Fischer Scientific, USA). According to the interpretation criteria prescribed in the Bovine Tuberculosis Spanish Eradication Program, animals were considered positive if the optical density (OD) in the bovine PPD stimulated sample minus the OD of nil antigen sample (PBS) was equal or greater than 0.05 and greater than the avian PPD-stimulated sample. Moreover, a less stringent threshold of 0.1 was also applied for comparison purposes.
Serological Analyses
Goats were tested before vaccination and 2 months after booster vaccination in order to evaluate the humoral response against different antigens using an in-house ELISA with some modifications to that previously described by Che-Amat et al. (30). Briefly, testing plates were coated with bovine and avian PPDs, as well as paratuberculosis protoplasmic antigen 3 (PPA3; Allied Monitor, Fayette, MO, USA) at 5 µg/ml in carbonate–bicarbonate buffer (Sigma, Barcelona, Spain), and incubated overnight at 4°C. Following one wash with PBS solution containing 0.05% Tween 20 (PBS-T), wells were blocked with 5% skimmed milk powder solution in PBS (SM) for 60 min at room temperature (RT). Sera were added in duplicate at 1:100 dilution in SM, incubated for 60 min at 37°C, and subsequently washed three times with PBS-T. Horseradish peroxidase-conjugated rabbit anti-sheep IgG antibody (SouthernBiotech, Birmingham, AL, USA) at a dilution of 1:2,000 was added, and the plates were incubated for 30 min at RT and then were washed five times with PBS-T. Color was developed by adding o-phenylenediamine dihydrochloride substrate (FAST OPD, Sigma-Aldrich, St Louis, MO, USA) and incubating the plates for 20 min in darkness and RT conditions. The reaction was stopped with H2SO4 (3 N), and the OD was measured at 490 nm with an ELISA reader. Goat negative and positive control sera were included in every plate in quadruplicate. Negative control serum was obtained from a TB-free goat previously described as M. bovis culture negative from a bTB-free area. Positive control serum was obtained from a goat previously infected with M. bovis in an experimental study. Sample results were expressed as an ELISA percentage (E%), according to the following formula: [sample E% = (mean sample OD/(2 × mean of negative control OD)) × 100]. The cutoff value was calculated using a ROC analysis and was defined as the ratio of the mean sample OD to the double of the mean OD of the negative control. Serum samples with E% values greater than 100 were considered as positive.
Statistical Analysis
Wilson’s 95% confidence intervals (95% CI) were calculated for the percentage of reactors to the different techniques. Comparison of proportions of test reactors between groups was performed using Fisher’s exact test. Skin fold thickness, IFN-γ OD, and ELISA percentage (E%) among groups were analyzed by Kruskal–Wallis and Mann–Whitney U tests. Agreement between tests was measured with the kappa statistic (k) and interpreted as follows: <0.00 poor, 0.00–0.20 slight, 0.21–0.40 fair, 0.41–0.60 moderate, 0.61–0.80 substantial, and 0.81–1.00 almost perfect agreement (34). All previous tests were carried out using SPSS Statistics 20 (IBM, New York, NY, USA), and interpreted considering a p-value of 0.05 to determine statistical significance. The normality of the quantitative values was assessed using the Kolmogorov–Smirnov test before further analyses were carried out.
Results
All animals were negative to the IGRA and the ELISA prior to vaccination. Four animals in the oral group [25.00% (95% CI 10.18–49.50%)], three in the IM vaccinated group [18.75% (95% CI 6.59–43.01%)] and two belonging to the control group [12.50% (95% CI 3.50–36.02%)], presented antibody titers against avian PPD above the cutoff point selected (Table 1).
Table 1. Number of positive animals using skin tests, IGRA, and In-house ELISA in the different groups of goats 1 week before vaccination (pre-vaccination analysis) and 8 weeks after booster vaccination (post-vaccination analysis).
Regarding to the SIT and SCIT tests 2 months after booster immunization, 68.75% (95% CI 44.40–85.84%) and 12.50% (95% CI 3.50–36.02%) of the animals were positive reactors to both tests in the IM vaccinated group, respectively. None of the animals in the control and oral vaccinated groups showed a positive reaction to SIT and SICT tests (Table 1). The skin fold thickness increase in the bovine PPD site was significantly higher in the IM vaccinated group [median 5.5 mm, interquartile range (IQR) 0–6 mm] compared to oral vaccinated (median 0 mm, IQR 0–0 mm; Mann–Whitney U test, p < 0.001) and to control goats (median 0 mm, IQR 0–0 mm; Mann–Whitney U test, p < 0.001) (Figure 1). One animal in the IM vaccinated group, presented an increase higher than 4 mm in the avian PPD site. Oral and control groups did not show statistically significant differences in the skin fold thickness increase at the bovine PPD injection site (Figure 1).
Figure 1. Comparison of the skin fold thickness increase (mm) at the bovine purified protein derivative (PPD) site among groups 2 months after booster vaccination. Boxes represent the lower, median, and higher quartile ranges and “outliers” are represented by closed circles.
Similar findings were observed using the IGRA, since no positive animals were detected in the oral vaccinated and control group regardless the cutoff point selected (0.05 and 0.1). However, in the IM vaccinated group, there were 10 [62.50% (95% CI 38.64–81.52%)] and 7 [43.75% (95% CI 23.10–66.82%)] positive reactors out of the 16 animals, employing the two cutoff points 0.05 and 0.1, respectively. The percentage of reactors in the IM vaccinated group was significantly higher compared to the other two groups (Fisher’s exact test, p < 0.001 for both comparisons). According to these results, significant differences (Kruskal–Wallis test, p < 0.001) were found between the optical densities against bovine PPD in the IM vaccinated and the other groups, 2 months after booster vaccination (Figure 2).
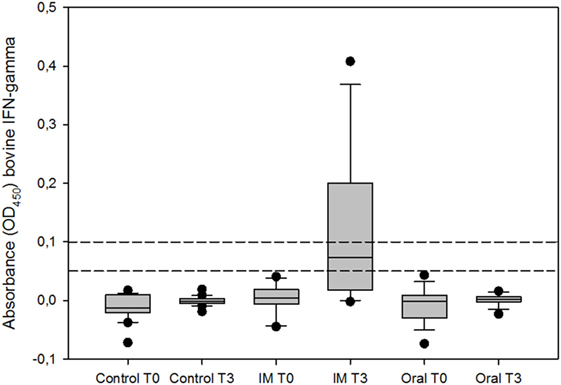
Figure 2. IFN-γ response of whole blood stimulated with bovine purified protein derivative (PPD), expressed as optical density (OD450). T0 represents the day of prime vaccination and T3 3 months later. Boxes represent the lower, median, and higher quartile ranges and “outliers” are represented by closed circles. The dashed lines represent the cutoff values used in this study (0.05 and 0.1).
Humoral response was measured by an indirect in-house ELISA, detecting only one positive animal to the bovine PPD in the IM vaccinated group 2 months after revaccination. Regarding the oral and control groups, all animals were negative to the bovine PPD, avian PPD, and PPA3. No significant differences (Kruskal–Wallis test, p = 0.35) were found in the bovine PPD (E%) values among groups 2 months after booster vaccination (Control: median 35.1, IQR 29.9–37.3; IM: median 44.7, IQR 37.9–58.0; Oral: median 38, IQR 29.9–48.4).
The agreement achieved between the SIT test and the whole blood IGRA (cut off 0.05) in all groups was substantial (k = 0.695). Nevertheless, no agreement was observed between the ELISA and the cell-based diagnostic techniques (k = −0.039, IGRA vs ELISA; k = −0.040, SIT vs ELISA; k = −0.029, SCIT vs ELISA—Table 2).
Table 2. Agreement between tests in all animals 2 months after booster vaccination (kappa index, k).
Discussion
The development of new vaccines may improve control strategies for TB in livestock and wildlife, thereby facilitating the eradication of TB (35, 36). Thus, the application of non-sensitizing vaccines could be a suitable choice, particularly in species not subjected to eradication programs or in regions/countries where the investment in DIVA antigens is not affordable, since the implementation of DIVA tests at a large scale entails high logistical demands and costs.
To the best of our knowledge, this is the first study evaluating the interference on TB cell and antibody-based diagnosis caused by the vaccination with a M. bovis HI vaccine in goats, as well as the effect of booster re-vaccination protocols. The present study shows significant differences in the results of the cell-based diagnostic techniques depending on the route of vaccination used. The interference caused on the tuberculin skin tests in the animals vaccinated via parenteral 2 months after booster vaccination, yielded a 68.75% and a 12.50% of positive reactors using the SIT and SCIT tests, respectively, while no positive skin test reactions were observed in any of the kids orally vaccinated. These results are in agreement with those reported previously in cattle (26) and red deer (25), where oral vaccination did not cause false-positive responses in the tuberculin skin test. Despite this interference on the skin test caused by an HI vaccine has not been previously described using the parenteral route in goats, there are other studies performed with live vaccines which corroborate these findings (37, 38). Using the IGRA, a high proportion of reactors among intramuscularly vaccinated goats (above 40%) were observed 2 months after booster vaccination, regardless the cutoff point used. These findings are similar to those reported in previous studies in wild boar (20) and cattle (26), where the IM route showed a clear and consistent bovine PPD IFN-γ response, but no positive reactors were detected via the oral route. IGRA positive responses to defined antigenic peptide cocktails have been reported following IM vaccination in cattle, whereas no interference was observed via the oral route (26). These peptide cocktails could be considered an extra diagnostic tool combining them with oral vaccination, but nowadays, the implementation of these DIVA reagents in goats is not affordable for widespread use.
The lack of positive reactor animals in all groups after vaccination using our in-house ELISA (only one goat vaccinated via parenteral showed a positive antibody titer) suggests that animals have not developed a humoral response yet. Long-term immunogenicity studies would be required to evaluate the humoral response elicited by the HI vaccine, administered orally and intramuscularly. Goats vaccinated with subcutaneous BCG and boosting with a recombinant adenoviral vaccine 8 weeks later induce a peak in the antibody response 2 weeks after the boost (10). However, the use of an HI vaccine instead of a live-attenuated vaccine in our study might have delayed this peak, being necessary extended serological studies.
This study demonstrates that the use of oral HI vaccine does not produce interference in diagnostic techniques used in TB eradication programs, as previously was observed in wild boar (20), pig (23), red deer (25), and cattle (26). The mechanism for this absence of interference has not been elucidated although it may be related to the different absorption routes or to the adjuvants used. However, in the present study, several key points must be taken into account comparing with previous studies with the M. bovis HI vaccine. First, prime and booster doses of HI vaccine were administered 1 month apart. By contrast, only a single dose was applied in the studies in wild boar, red deer, and cattle (20, 25, 26). Second, animals were only tested 2 months after the booster vaccination, considering sufficient time to induce cell-mediated immunity. Hence, further studies at extended time points are necessary to evaluate the cell mediated immunity induced by the oral HI vaccine throughout time, which could be delayed as described previously in orally BCG vaccinated cattle (39). This limited sampling time schedule was due to these animals entering a second experimental phase where they were subsequently exposed to a group of TB-infected animals in order to continue with an efficacy study. Third, the age of vaccination with the HI vaccine differed among previous studies: red deer (adults), cattle (5–7 months), wild boar (3–4 months), and pigs (3–4 months); sampling schedule after vaccination was also different in these studies: red deer (months 7 and 12), cattle (weekly up to 8 weeks after vaccination), wild boar (day 60), and pigs (day 57) (20, 23–26). Few studies in humans have focused on the role of vaccine administration-related parameters on vaccine efficacy, since factors as the number and interval between immunizations could trigger different immunological responses (40). Moreover, many clinical studies in humans demonstrated that longer intervals between two immunizations may help to achieve better immune responses (41–43). In this sense, we chose an earlier age of vaccination for this study, in concordance with that demonstrated in epidemiological studies in which the beneficial effects of BCG vaccination are greater in very young individuals (44, 45). Moreover, different researchers have highlighted the protection against TB in pigs and wild boar vaccinated orally with HI vaccine suggesting the stimulation of the mucosal immune system (20, 23) and that permeability of the gastrointestinal tract decreases with age (46, 47).
A further advantage of using an inactivated vaccine is that the environmental risks, production costs, and restrictions on storage conditions could be reduced. Therefore, this oral HI vaccine is suitable for its use in wild animals where it is difficult to implement control strategies based on slaughter positive animals to the diagnostic tests (22, 25). Data obtained in goats as experimental models can lay the ground for further studies in large ruminants or humans (48). These considerations might encourage the use of vaccination as an alternative strategy in developing countries, where control strategies based on diagnosis and cull of positive animals are not feasible, or in countries where prevalence of bovine TB is very high (37, 49). The absence of interference caused by the oral HI vaccine in the diagnostic TB tests allows the possibility of dispensing with the implementation of DIVA tests, thereby reducing implementation costs.
In summary, oral vaccination with HI vaccine in kid goats proves to be a suitable route, not causing any interference on TB cell and antibody-based diagnostic techniques, even if applying a booster protocol which could trigger a stronger immune response than a single dose. In addition, this study paves the way for vaccine efficacy studies of HI vaccine in goats. These future efficacy studies could demonstrate the efficacy previously suggested in orally vaccinated wild boars and pigs.
Ethics Statement
Handling of the animals and sampling were performed according to European (Council Directive 2010/63/EU) and Spanish Legislation (RD 53/2013), and also approved by the Ethics Committee (Complutense University of Madrid) and the Regional Agriculture Authority [Comunidad de Madrid; permit number: PROEX 143/15 (29/06/2015)].
Author Contributions
Participated in experimental design: AR, MR, CC, BR, LJ, AD-G, RJ, CG, LD, and JB. Prepared the inactivated vaccines: JG and IS. Conducted field and laboratory work: AR, MR, CC, ASM, AD-G, and JB. Performed data analysis: AR, LD, CG, and JB. Drafted the manuscript: AR and JB. Revised the manuscript: MR, RJ, LD, CG, and JB.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
AR is recipient of an Industrial Doctorate contract (DI-15-08110) funded by the Spanish Ministry of Economy, Industry and Competitiveness and the European Social Fund. The authors thank the Mycobacteria Unit of VISAVET and Ana Alonso (MAEVA-SERVET S.L.) for their technical support.
References
1. Aranaz A, Liebana E, Gomez-Mampaso E, Galan JC, Cousins D, Ortega A, et al. Mycobacterium tuberculosis subsp. caprae subsp. nov.: a taxonomic study of a new member of the Mycobacterium tuberculosis complex isolated from goats in Spain. Int J Syst Bacteriol (1999) 49(Pt 3):1263–73. doi:10.1099/00207713-49-3-1263
2. Bezos J, Alvarez J, Romero B, Aranaz A, Juan L. Tuberculosis in goats: assessment of current in vivo cell-mediated and antibody-based diagnostic assays. Vet J (2012) 191(2):161–5. doi:10.1016/j.tvjl.2011.02.010
3. Buendia AJ, Navarro JA, Salinas J, McNair J, de Juan L, Ortega N, et al. Ante-mortem diagnosis of caprine tuberculosis in persistently infected herds: influence of lesion type on the sensitivity of diagnostic tests. Res Vet Sci (2013) 95(3):1107–13. doi:10.1016/j.rvsc.2013.10.003
4. Bezos J, Marques S, Alvarez J, Casal C, Romero B, Grau A, et al. Evaluation of single and comparative intradermal tuberculin tests for tuberculosis eradication in caprine flocks in Castilla y Leon (Spain). Res Vet Sci (2014) 96(1):39–46. doi:10.1016/j.rvsc.2013.10.007
5. Vordermeier HM, Perez de Val B, Buddle BM, Villarreal-Ramos B, Jones GJ, Hewinson RG, et al. Vaccination of domestic animals against tuberculosis: review of progress and contributions to the field of the TBSTEP project. Res Vet Sci (2014) 97(Suppl):S53–60. doi:10.1016/j.rvsc.2014.04.015
6. Corner LA, Costello E, O’Meara D, Lesellier S, Aldwell FE, Singh M, et al. Oral vaccination of badgers (Meles meles) with BCG and protective immunity against endobronchial challenge with Mycobacterium bovis. Vaccine (2010) 28(38):6265–72. doi:10.1016/j.vaccine.2010.06.120
7. Corner LA, Norton S, Buddle BM, Morris RS. The efficacy of bacille Calmette-Guerin vaccine in wild brushtail possums (Trichosurus vulpecula). Res Vet Sci (2002) 73(2):145–52. doi:10.1016/S0034-5288(02)00038-3
8. Lesellier S, Palmer S, Gowtage-Sequiera S, Ashford R, Dalley D, Dave D, et al. Protection of Eurasian badgers (Meles meles) from tuberculosis after intra-muscular vaccination with different doses of BCG. Vaccine (2011) 29(21):3782–90. doi:10.1016/j.vaccine.2011.03.028
9. Buddle BM, Parlane NA, Wedlock DN, Heiser A. Overview of vaccination trials for control of tuberculosis in cattle, wildlife and humans. Transbound Emerg Dis (2013) 60(Suppl 1):136–46. doi:10.1111/tbed.12092
10. Perez de Val B, Vidal E, Villarreal-Ramos B, Gilbert SC, Andaluz A, Moll X, et al. A multi-antigenic adenoviral-vectored vaccine improves BCG-induced protection of goats against pulmonary tuberculosis infection and prevents disease progression. PLoS One (2013) 8(11):e81317. doi:10.1371/journal.pone.0081317
11. Palmer MV, Thacker TC, Waters WR, Robbe-Austerman S. Oral vaccination of white-tailed deer (Odocoileus virginianus) with Mycobacterium bovis bacillus Calmette-Guerin (BCG). PLoS One (2014) 9(5):e97031. doi:10.1371/journal.pone.0097031
12. Dean GS, Clifford D, Whelan AO, Tchilian EZ, Beverley PC, Salguero FJ, et al. Protection induced by simultaneous subcutaneous and endobronchial vaccination with BCG/BCG and BCG/adenovirus expressing antigen 85A against Mycobacterium bovis in cattle. PLoS One (2015) 10(11):e0142270. doi:10.1371/journal.pone.0142270
13. Whelan AO, Coad M, Upadhyay BL, Clifford DJ, Hewinson RG, Vordermeier HM. Lack of correlation between BCG-induced tuberculin skin test sensitisation and protective immunity in cattle. Vaccine (2011) 29(33):5453–8. doi:10.1016/j.vaccine.2011.05.057
14. Vordermeier HM, Jones GJ, Buddle BM, Hewinson RG. Development of immune-diagnostic reagents to diagnose bovine tuberculosis in cattle. Vet Immunol Immunopathol (2016) 181:10–4. doi:10.1016/j.vetimm.2016.02.003
15. Conlan AJ, Brooks Pollock E, McKinley TJ, Mitchell AP, Jones GJ, Vordermeier M, et al. Potential benefits of cattle vaccination as a supplementary control for bovine tuberculosis. PLoS Comput Biol (2015) 11(2):e1004038. doi:10.1371/journal.pcbi.1004038
16. Perez de Val B, Vidal E, Lopez-Soria S, Marco A, Cervera Z, Martin M, et al. Assessment of safety and interferon gamma responses of Mycobacterium bovis BCG vaccine in goat kids and milking goats. Vaccine (2016) 34(7):881–6. doi:10.1016/j.vaccine.2016.01.004
17. EFSA. Scientific opinion on field trials for bovine tuberculosis vaccination. EFSA J (2013) 11(12):1–35. doi:10.2903/j.efsa.2013.3475
18. Casal C, Bezos J, Diez-Guerrier A, Alvarez J, Romero B, de Juan L, et al. Evaluation of two cocktails containing ESAT-6, CFP-10 and Rv-3615c in the intradermal test and the interferon-gamma assay for diagnosis of bovine tuberculosis. Prev Vet Med (2012) 105(1–2):149–54. doi:10.1016/j.prevetmed.2012.02.007
19. Vordermeier M, Gordon SV, Hewinson RG. Mycobacterium bovis antigens for the differential diagnosis of vaccinated and infected cattle. Vet Microbiol (2011) 151(1–2):8–13. doi:10.1016/j.vetmic.2011.02.020
20. Garrido JM, Sevilla IA, Beltran-Beck B, Minguijon E, Ballesteros C, Galindo RC, et al. Protection against tuberculosis in Eurasian wild boar vaccinated with heat-inactivated Mycobacterium bovis. PLoS One (2011) 6(9):e24905. doi:10.1371/journal.pone.0024905
21. Beltran-Beck B, Romero B, Sevilla IA, Barasona JA, Garrido JM, Gonzalez-Barrio D, et al. Assessment of an oral Mycobacterium bovis BCG vaccine and an inactivated M. bovis preparation for wild boar in terms of adverse reactions, vaccine strain survival, and uptake by nontarget species. Clin Vaccine Immunol (2014) 21(1):12–20. doi:10.1128/cvi.00488-13
22. Diez-Delgado I, Rodriguez O, Boadella M, Garrido JM, Sevilla IA, Bezos J, et al. Parenteral vaccination with heat-inactivated Mycobacterium bovis reduces the prevalence of tuberculosis-compatible lesions in farmed wild boar. Transbound Emerg Dis (2016). doi:10.1111/tbed.12526
23. Beltran-Beck B, Romero B, Boadella M, Casal C, Bezos J, Mazariegos M, et al. Tonsils of the soft palate do not mediate the response of pigs to oral vaccination with heat-inactivated Mycobacterium bovis. Clin Vaccine Immunol (2014) 21(8):1128–36. doi:10.1128/cvi.00221-14
24. Beltran-Beck B, de la Fuente J, Garrido JM, Aranaz A, Sevilla I, Villar M, et al. Oral vaccination with heat inactivated Mycobacterium bovis activates the complement system to protect against tuberculosis. PLoS One (2014) 9(5):e98048. doi:10.1371/journal.pone.0098048
25. Lopez V, Gonzalez-Barrio D, Lima-Barbero JF, Ortiz JA, Dominguez L, Juste R, et al. Oral administration of heat-inactivated Mycobacterium bovis reduces the response of farmed red deer to avian and bovine tuberculin. Vet Immunol Immunopathol (2016) 172:21–5. doi:10.1016/j.vetimm.2016.03.003
26. Jones GJ, Steinbach S, Sevilla IA, Garrido JM, Juste R, Vordermeier HM. Oral vaccination of cattle with heat inactivated Mycobacterium bovis does not compromise bovine TB diagnostic tests. Vet Immunol Immunopathol (2016) 182:85–8. doi:10.1016/j.vetimm.2016.10.010
27. Busch F, Bannerman F, Liggett S, Griffin F, Clarke J, Lyashchenko KP, et al. Control of bovine tuberculosis in a farmed red deer herd in England. Vet Rec (2017) 180(3):68. doi:10.1136/vr.103930
28. Boadella M, Lyashchenko K, Greenwald R, Esfandiari J, Jaroso R, Carta T, et al. Serologic tests for detecting antibodies against Mycobacterium bovis and Mycobacterium avium subspecies paratuberculosis in Eurasian wild boar (Sus scrofa). J Vet Diagn Invest (2011) 23(1):77–83. doi:10.1177/104063871102300111
29. Lyashchenko KP, Greenwald R, Esfandiari J, Chambers MA, Vicente J, Gortazar C, et al. Animal-side serologic assay for rapid detection of Mycobacterium bovis infection in multiple species of free-ranging wildlife. Vet Microbiol (2008) 132(3–4):283–92. doi:10.1016/j.vetmic.2008.05.029
30. Che-Amat A, Risalde MA, Gonzalez-Barrio D, Ortiz JA, Gortazar C. Effects of repeated comparative intradermal tuberculin testing on test results: a longitudinal study in TB-free red deer. BMC Vet Res (2016) 12(1):184. doi:10.1186/s12917-016-0825-2
31. Wood PR, Corner LA, Rothel JS, Baldock C, Jones SL, Cousins DB, et al. Field comparison of the interferon-gamma assay and the intradermal tuberculin test for the diagnosis of bovine tuberculosis. Aust Vet J (1991) 68(9):286–90. doi:10.1111/j.1751-0813.1991.tb03254.x
32. Wood PR, Rothel JS. In vitro immunodiagnostic assays for bovine tuberculosis. Vet Microbiol (1994) 40(1–2):125–35. doi:10.1016/0378-1135(94)90051-5
33. Bezos J, Alvarez J, Juan L, Romero B, Rodriguez S, Castellanos E, et al. Factors influencing the performance of an interferon-gamma assay for the diagnosis of tuberculosis in goats. Vet J (2011) 190(1):131–5. doi:10.1016/j.tvjl.2010.09.026
34. Landis JR, Koch GG. The measurement of observer agreement for categorical data. Biometrics (1977) 33(1):159–74. doi:10.2307/2529310
35. Vordermeier HM, de Val BP, Buddle BM, Villarreal-Ramos B, Jones GJ, Hewinson RG, et al. Vaccination of domestic animals against tuberculosis: review of progress and contributions to the field of the TBSTEP project. Res Vet Sci (2014) 97:S53–60. doi:10.1016/j.rvsc.2014.04.015
36. Vordermeier HM, Jones GJ, Buddle BM, Hewinson RG, Villarreal-Ramos B. Bovine tuberculosis in cattle: vaccines, DIVA tests, and host biomarker discovery. Annu Rev Anim Biosci (2016) 4:87–109. doi:10.1146/annurev-animal-021815-111311
37. Bezos J, Casal C, Puentes E, Diez-Guerrier A, Romero B, Aguilo N, et al. Evaluation of the immunogenicity and diagnostic interference caused by M. tuberculosis SO2 vaccination against tuberculosis in goats. Res Vet Sci (2015) 103:73–9. doi:10.1016/j.rvsc.2015.09.017
38. Perez de Val B, Villarreal-Ramos B, Nofrarias M, Lopez-Soria S, Romera N, Singh M, et al. Goats primed with Mycobacterium bovis BCG and boosted with a recombinant adenovirus expressing Ag85A show enhanced protection against tuberculosis. Clin Vaccine Immunol (2012) 19(9):1339–47. doi:10.1128/cvi.00275-12
39. Buddle BM, Aldwell FE, Skinner MA, de Lisle GW, Denis M, Vordermeier HM, et al. Effect of oral vaccination of cattle with lipid-formulated BCG on immune responses and protection against bovine tuberculosis. Vaccine (2005) 23(27):3581–9. doi:10.1016/j.vaccine.2005.01.150
40. Zhang L, Wang W, Wang S. Effect of vaccine administration modality on immunogenicity and efficacy. Expert Rev Vaccines (2015) 14(11):1509–23. doi:10.1586/14760584.2015.1081067
41. Rumke HC, Loch HP, Hoppenbrouwers K, Vandermeulen C, Malfroot A, Helm K, et al. Immunogenicity and safety of a measles-mumps-rubella-varicella vaccine following a 4-week or a 12-month interval between two doses. Vaccine (2011) 29(22):3842–9. doi:10.1016/j.vaccine.2011.02.067
42. Ledgerwood JE, Zephir K, Hu Z, Wei C-J, Chang L, Enama ME, et al. Prime-boost interval matters: a randomized phase 1 study to identify the minimum interval necessary to observe the H5 DNA influenza vaccine priming effect. J Infect Dis (2013) 208(3):418–22. doi:10.1093/infdis/jit180
43. Park DE, Johnson TS, Nonyane BAS, Chandir S, Conklin L, Fleming-Dutra KE, et al. The differential impact of coadministered vaccines, geographic region, vaccine product and other covariates on pneumococcal conjugate vaccine immunogenicity. Pediatr Infect Dis J (2014) 33:S130–9. doi:10.1097/INF.0000000000000081
44. Aaby P, Martins CL, Garly ML, Bale C, Andersen A, Rodrigues A, et al. Non-specific effects of standard measles vaccine at 4.5 and 9 months of age on childhood mortality: randomised controlled trial. BMJ (2010) 341:c6495. doi:10.1136/bmj.c6495
45. Aaby P, Roth A, Ravn H, Napirna BM, Rodrigues A, Lisse IM, et al. Randomized trial of BCG vaccination at birth to low-birth-weight children: beneficial nonspecific effects in the neonatal period? J Infect Dis (2011) 204(2):245–52. doi:10.1093/infdis/jir240
46. Kalach N, Rocchiccioli F, de Boissieu D, Benhamou PH, Dupont C. Intestinal permeability in children: variation with age and reliability in the diagnosis of cow’s milk allergy. Acta Paediatr (2001) 90(5):499–504. doi:10.1080/08035250116842
47. Wood KM, Palmer SI, Steele MA, Metcalf JA, Penner GB. The influence of age and weaning on permeability of the gastrointestinal tract in Holstein bull calves. J Dairy Sci (2015) 98(10):7226–37. doi:10.3168/jds.2015-9393
48. de Val Perez B, Lopez-Soria S, Nofrarias M, Martin M, Vordermeier HM, Villarreal-Ramos B, et al. Experimental model of tuberculosis in the domestic goat after endobronchial infection with Mycobacterium caprae. Clin Vaccine Immunol (2011) 18(11):1872–81. doi:10.1128/cvi.05323-11
Keywords: tuberculosis, goat, oral vaccination, heat-inactivated vaccine, diagnosis, interference
Citation: Roy A, Risalde MA, Casal C, Romero B, de Juan L, Menshawy AM, Díez-Guerrier A, Juste RA, Garrido JM, Sevilla IA, Gortázar C, Domínguez L and Bezos J (2017) Oral Vaccination with Heat-Inactivated Mycobacterium bovis Does Not Interfere with the Antemortem Diagnostic Techniques for Tuberculosis in Goats. Front. Vet. Sci. 4:124. doi: 10.3389/fvets.2017.00124
Received: 06 March 2017; Accepted: 20 July 2017;
Published: 07 August 2017
Edited by:
Alejandra Victoria Capozzo, Consejo Nacional de Investigaciones Científicas y Técnicas (CONICET), ArgentinaReviewed by:
Mairead Lesley Bermingham, University of Edinburgh, United KingdomAndrew William Byrne, Agri Food and Biosciences Institute, United Kingdom
Copyright: © 2017 Roy, Risalde, Casal, Romero, de Juan, Menshawy, Díez-Guerrier, Juste, Garrido, Sevilla, Gortázar, Domínguez and Bezos. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) or licensor are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Javier Bezos, jbezosga@visavet.ucm.es
 Alvaro Roy
Alvaro Roy María A. Risalde
María A. Risalde Carmen Casal
Carmen Casal Beatriz Romero
Beatriz Romero Lucía de Juan3,4
Lucía de Juan3,4
 Ahmed M. Menshawy
Ahmed M. Menshawy Ramon A. Juste
Ramon A. Juste Joseba M. Garrido
Joseba M. Garrido Iker A. Sevilla
Iker A. Sevilla Christian Gortázar
Christian Gortázar Lucas Domínguez
Lucas Domínguez Javier Bezos
Javier Bezos