Seroprevalence and Current Infections of Canine Vector-Borne Diseases in Costa Rica
- 1Centre for Infection Medicine, Institute for Parasitology, University of Veterinary Medicine Hannover, Hanover, Germany
- 2Laboratory of Parasitology, School of Veterinary Medicine, National University of Costa Rica, Campus Benjamín Núñez, Heredia, Costa Rica
- 3IDEXX Laboratories, Ludwigsburg, Germany
Domestic dogs may carry several vector-borne pathogens, including zoonotic agents, especially in tropical regions like Central America. The epidemiology of these pathogens is prone to change due to urbanization, trade and travel as well as climate change, necessitating repeated monitoring. This study aims to present a comprehensive picture of canine vector-borne diseases in Costa Rica, combining data on seroprevalence with molecular species identification of the causative pathogens. In this survey, 294 dogs from all seven provinces of Costa Rica were included. After a clinical examination, diagnostic blood samples were analyzed with regard to packed cell volume (PCV) and presence of microfilaria. Serum samples were tested for antibodies against Ehrlichia spp., Anaplasma spp., Babesia spp., Borrelia burgdorferi sensu lato (s.l.) as well as antigen of Dirofilaria immitis. Seropositive and microfilaremic blood samples were analyzed by PCR to detect current infections and identify the pathogen species. Overall, 45.24% (133/294, 95% CI: 39.45–51.11%) of dogs were seropositive for at least one of the tested pathogens. Seroprevalence was highest for Ehrlichia spp. (39.46%, 116/294, 95% CI: 33.83–45.29%), followed by Babesia spp. (23.13%, 68/294, 95% CI: 18.43–28.38%), Anaplasma spp. (13.27%, 39/294, 95% CI: 9.61–17.69%), and B. burgdorferi s.l. (0.34%, 1/294, 95% CI: 0.01–1.88%). Generalized linear mixed models indicated a significant association of Ehrlichia-, Anaplasma- and Babesia-seropositivity, as well as a significant effect of age and breed on Ehrlichia-seropositivity. Furthermore, a statistically significant negative effect of Ehrlichia-, Anaplasma-, and Babesia-seropositivity on PCV was found. Regarding current infections, Ehrlichia canis DNA was detected in 51.72% (60/116, 95% CI: 42.26–61.10%) of Ehrlichia-seropositive dogs, while Ehrlichia ewingii and Ehrlichia chaffeensis were not detected. Furthermore, 10.26% (4/39, 95% CI: 2.87–24.22%) of Anaplasma-seropositive dogs were coinfected with Anaplasma phagocytophilum and Anaplasma platys, while one animal (2.56%, 95% CI: 0.65–13.48%) was infected with A. phagocytophilum only. Among Babesia-seropositive dogs, Babesia vogeli and Hepatozoon canis were detected in one animal each (1.47%, 1/68, 95% CI: 0.04–7.92%). Dirofilaria immitis antigen was detected in 4.42% (13/294, 95% CI: 2.38–7.44%) of dogs. In microfilaremic animals, D. immitis as well as Acanthocheilonema reconditum infections were identified. This survey demonstrates that canine vector-borne pathogens, including zoonotic agents like A. phagocytophilum and D. immitis, are widespread in Costa Rica. Thus, protection of dogs from disease-transmitting vectors is recommended from an animal welfare as well as public health perspective.
Introduction
Vector-borne diseases, including babesiosis, ehrlichiosis, anaplasmosis and dirofilariosis, may severely compromise canine health. Although often asymptomatic, these infections may lead to life-threatening symptoms such as anemia and thrombocytopenia with increased bleeding tendency, for example, as well as to a variety of unspecific symptoms (1). Furthermore, in the chronic stage of infection, ehrlichiosis, borreliosis, babesiosis and dirofilariosis, among other canine vector-borne diseases, can lead to glomerulopathies with proteinuria in dogs (2). In addition, some of these infections, e.g., dirofilariosis and granulocytic anaplasmosis, represent zoonoses (3). Their presence in dogs may thus indicate a health risk for humans.
Vector-borne diseases are often widespread in tropical regions, including Central America, due to optimal conditions for vectors such as mosquitoes and ticks (4). Tick infestation of dogs is common in this region and mainly involves the brown dog tick, Rhipicephalus sanguineus sensu lato (s.l.) (5–7), which is a competent vector for Ehrlichia canis, Hepatozoon canis, Babesia vogeli, and different Rickettsia species, among others (8). Although different clades of R. sanguineus s.l. may vary in their vector capacity, genetic studies have revealed that R. sanguineus s.l. specimens from Central America belong to the so-called “tropical lineage” with proven vector capacity for E. canis (9). Accordingly, previous surveys of canine vector-borne diseases in Central America have revealed high levels of exposure to Ehrlichia spp. (7, 10), followed by Anaplasma spp. (10, 11). Both Anaplasma platys, the causative agent of canine cyclic thrombocytopenia, and Anaplasma phagocytophilum, causing zoonotic granulocytic anaplasmosis, are present in the region (10, 12, 13). Additionally, B. vogeli as well as Babesia gibsoni have been detected by PCR in dogs from certain locations in Costa Rica and Nicaragua (14–16), but large-scale serological surveys on canine babesiosis in Central America are lacking. In contrast, the region does not appear to be endemic for Borrelia burgdorferi s.l., the causative agent of Lyme borreliosis, as infections have been detected only sporadically (11). Mosquito-borne Dirofilaria immitis infections have so far mainly been found in a regional pattern along the Pacific Coast of Costa Rica (11, 17), in accordance with studies from Mexico demonstrating higher D. immitis prevalences along shorelines (18).
However, the epidemiology of vector-borne diseases is prone to change under the influence of urbanization, changing land use patterns, human trade and travel as well as climate change (4), necessitating repeated monitoring. This study aims to present a comprehensive picture of canine vector-borne diseases in Costa Rica, combining data on the seroprevalence of Ehrlichia spp., Anaplasma spp., Babesia spp., B. burgdorferi s.l., and D. immitis among 294 dogs sampled in 2014 with molecular species identification of the causative pathogens.
Methods
Clinical Examination and Sampling of Dogs
From March to August 2014, 294 dogs were sampled at 21 different locations in Costa Rica, distributed over all seven provinces. The dogs were presented at randomly selected veterinary clinics for varying reasons, e.g., vaccinations, health checks or curative consultations. Dogs from an animal shelter were only included at one location (San Rafael de Heredia, N = 30). Only dogs more than 6 months of age, which had not been treated with ivermectin during the last 6 months nor with doxycycline during the last 12 months, were included in the study, and consent of the owner to use surplus samples for further analyses was obtained. The dogs received a clinical examination and sex, age and breed were noted. Diagnostic blood samples were taken from the cephalic or jugular vein and collected into serum and EDTA tubes. Packed cell volume (PCV) was determined by glass capillary centrifugation of EDTA blood. Remaining EDTA blood and serum was stored at −20°C until shipping to Germany on dry ice for further analyses.
Screening of Blood Samples for Vector-Borne Pathogens
Serum samples were tested for antibodies against Anaplasma spp., Ehrlichia spp. and B. burgdorferi s.l., as well as antigen of D. immitis by use of a commercial rapid ELISA (SNAP®4DXPlus®, IDEXX Laboratories Inc., Westbrook, ME, USA). Sensitivity and specificity of this test system are as follows: 93.2 and 99.2% for A. phagocytophilum, 89.2 and 99.2% for A. platys, 96.7 and 98.8% for B. burgdorferi s.l., 97.8 and 92.3% for E. canis, and for D. immitis 98.9 and 99.3% (19). Regarding Ehrlichia spp., cross-reactivity of the E. canis antigen (peptides from p30 and p30-1 outer membrane proteins) with anti-Ehrlichia chaffeensis has been shown (19); the device additionally detects antibodies to Ehrlichia ewingii (peptide derived from p28 outer surface protein family) Furthermore, cross-reactivity between A. phagocytophilum and A. platys has also been demonstrated (peptide from the major surface protein p44/MSP2) (20). Thus, we refer to Anaplasma spp. and Ehrlichia spp. as results in the present study.
To detect IgG antibodies against Babesia spp., a commercial ELISA test kit was used (Babesia ELISA DOG, afosa GmbH, Blankenfelde-Mahlow, Germany). The reference range of the test score is negative (<14), borderline at 14–19 and positive [>19; for further details see (21)]. According to the manufacturer, sensitivity and specificity of this test for B. canis compared with the indirect immunofluorescence assay are 91.6 and 95.4%, respectively. However, cross-reactions with other Babesia spp. (B. vogeli and B. gibsoni) as well as the related piroplasm Rangelia vitalii occur (22, 23), and we refer to antibodies against Babesia spp. accordingly.
To determine which Anaplasma- and Ehrlichia-seropositive dogs were currently infected (as defined by DNA detection) with E. canis, A. phagocytophilum and A. platys, respectively, species-specific PCRs were carried out as described previously (10). Briefly, DNA was isolated from blood samples using the Nucleospin® 8 Blood Kit (Macherey-Nagel GmbH & Co. KG, Düren, Germany). To detect A. phagocytophilum, a nested PCR targeting a 546 bp fragment of the 16S rRNA was carried out using primers ge3a and ge10r in a first and ge9f and ge2 in a second PCR round (24). For A. platys, a 678 bp fragment of the 16S rRNA gene was targeted by a nested PCR using primer sets 8F and 1448R for a first and EHR16SR and PLATYS for a second PCR round (25). For detection of E. canis, a 389 bp fragment of the 16S rRNA gene was targeted by nested PCR using primer pairs ECC and ECB in a first and ECAN5 and HE3 in a second PCR round (26, 27). PCR products were visualized by gel electrophoresis on 2% agarose gels. Furthermore, all samples seropositive for Ehrlichia spp. were additionally subjected to quantitative real-time PCR for detection of E. canis, E. chaffeensis and E. ewingii DNA as described previously (22).
To determine whether Babesia-seropositive dogs were currently infected, a genus-specific, semi-nested PCR targeting a 350 bp fragment of the 18S rRNA gene was carried out, using primers BJ1 and BN2 (28) in the first round, and BJ1 and PIRO-B (29) in the second round. The 25 μl reaction volume contained 2.5 μl DreamTaq® PCR Mastermix (Thermo Fisher Scientific Inc., Waltham, MA, USA), 0.5 μl of dNTPs (10 mM each), 0.5 μl of each primer (10 μM), 15.5 μl deionized water and 5 μl template DNA. In the second PCR, 1 μl of PCR-product from the first round was included as template, and the amount of water adjusted accordingly. For each round, the following thermoprofile was carried out in a peqSTAR thermocycler (peqlab Biotechnologie GmbH, Erlangen, Germany): initial denaturation at 95°C for 3 min, followed by 40 cycles at 94°C for 30 s, 55°C for 30 s and 72°C for 1 min, and final extension at 72°C for 10 min. Amplicons of the correct size were sequenced with primer BJ1 at a commercial sequencing laboratory (Microsynth Seqlab Sequence Laboratories, Göttingen, Germany). Present infections with B. burgdorferi s.l. were not further investigated due to low seroprevalence.
Additionally, buffy coat of all dogs was investigated microscopically for presence of microfilariae. Samples which contained microfilariae in buffy coat were subjected to a PCR targeting the internal transcribed spacer (ITS) 1-5.8S rDNA-ITS2 complex by use of primers NC2 and NC5 (30) as described previously (10), and amplicons were custom-sequenced in both directions (Microsynth Seqlab Sequence Laboratories, Göttingen, Germany). Obtained sequences were assembled using Clone Manager 9 Professional Edition (Scientific and Educational Software, Denver, CO, USA) and compared with sequences deposited in the GenBank database of the National Centre for Biotechnology Information (NCBI) using the Basic Local Alignment Search Tool (BLAST).
Statistical Analyses
Statistical analyses were conducted in R v. 3.5.0 (31). To assess which factors influenced the likelihood of being seropositive for Ehrlichia spp., Anaplasma spp. and Babesia spp., respectively, generalized linear mixed models (GLMMs) with binomial error structure and logit-link function were constructed [function “glmer,” package “lme4” (32)]. The following predictor variables were included as fixed factors: dog sex, dog age (years), dog breed (dichotomized as with breed/mongrel), and whether the sampling location was a city, a western or eastern coastal area, a rural area at high altitude [defined as ≥1,000 m above sea level (asl)] or low altitude (<1,000 m asl). To examine associations between seropositivity for the different pathogens, test results for Babesia spp. and Anaplasma spp. were included as fixed factors in the model for Ehrlichia spp., and vice versa. The location of sampling was included as a random factor. Multiple comparisons for factors with more than two levels were carried out using the function “glht” [package “multcomp” (33)], with Tukey HSD single-step P-value adjustment.
To examine the relationship between seropositivity and packed cell volume (PCV), we used a linear mixed model (LMM, package “lme4”), including presence of antibodies against Ehrlichia, Anaplasma and Babesia spp. and antigen of D. immitis as fixed factors, and location of sampling as a random factor. Because animal age and sex may affect PCV (34), these variables were included as additional fixed factors. Initially, interactions between all four pathogens were included, and were removed if not significant. LMM fit was assessed by inspecting normality and homogeneity of model residuals. Full models were compared to null models containing only the random factor in a likelihood ratio test (R function “anova,” method = “chisq”).
The ratio of animals displaying clinical symptoms compatible with the investigated vector-borne diseases (i.e., anorexia, apathy, fever, lymphadenopathy, pale mucous membranes, epistaxis, petechia and/or cough) was compared between seronegative animals and animals seropositive for at least one of the tested pathogens using a Chi-square-test.
Results
Clinical Presentation of Dogs
In total, 294 dogs were included in the study (21–73 per province, Table 1), comprising 215 mongrels and 79 dogs with a breed. In the clinical examination, 25.51% (75/294) of dogs were infested with ticks, while 25.17% (74/294) showed flea infestation. Pale mucous membranes were noted in 22.11% (65/294) of dogs, two of these (0.68%) additionally showed petechial bleeding. Three further dogs (1.02%) showed epistaxis. Furthermore, 3.74% (11/294) of dogs presented with fever, 3.40% (10/294) with apathy, 2.04% (6/294) with anorexia, and 1.02% (3/294) each with lymphadenopathy and muscle weakness. Overall, 26.53% of dogs (79/294) showed at least one symptom compatible with the investigated vector-borne diseases, i.e., ehrlichiosis, anaplasmosis, babesiosis, borreliosis, and dirofilariosis. Further clinical findings included alopecia (9.18%, 27/294), nail overgrowth (2.43%, 7/294), purulent eye discharge (1.36%, 4/294), lameness (0.68%, 2/294), and cough (0.34%, 1/294).
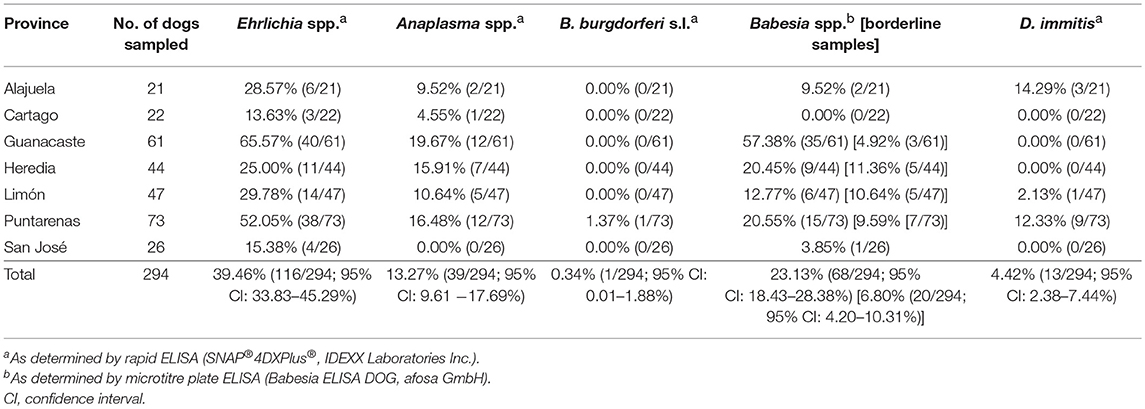
Table 1. Seroprevalence of Anaplasma spp., Ehrlichia spp., Borrelia burgdorferi s.l., Babesia spp. and prevalence of Dirofilaria immitis antigen in dogs from Costa Rica.
Seroprevalence of Rickettsiales and Babesia spp. and Effect on PCV
Overall, 45.24% (133/294, 95% CI: 39.45–51.11%) of dogs were seropositive for at least one of the tested vector-borne pathogens. Seroprevalence was highest for Ehrlichia spp. (39.46%, 116/294, 95% CI: 33.83–45.29%), followed by Babesia spp. (23.13%, 68/294, 95% CI: 18.43–28.38%). An additional 6.80% (20/294, 95% CI: 4.20–10.31%) of dogs showed a borderline Babesia ELISA test result. Seroprevalence of Anaplasma spp. as indicated by the rapid ELISA was 13.27% (39/294, 95% CI: 9.61–17.69%), and Borrelia burgdorferi s.l. antibodies were found in a single dog (0.34%, 95% CI: 0.01–1.88%). Seropositivity for more than one pathogen was observed in 23.13% of all dogs (68/294, 95% CI: 18.43–28.38%). Rates of co-exposure for the different pathogens are displayed in Table 2.
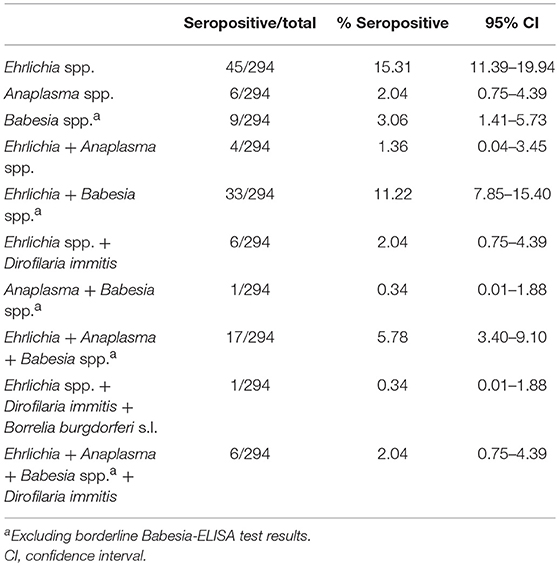
Table 2. Single and multiple exposure to vector-borne pathogens among 294 Costa Rican dogs as assessed by rapid ELISA (Ehrlichia spp., Anaplasma spp., Borrelia burgdorferi s.l. and Dirofilaria immitis), and microtitre plate ELISA (Babesia spp.a).
Because retesting of dogs with a borderline Babesia spp. ELISA result after 4–6 weeks, as recommended by the test manufacturer, was not possible in this study, sera with borderline test results were excluded from further analyses. In addition, no information on breed was available for one dog, resulting in a final sample size of N = 273 for statistical analyses. Generalized linear mixed models indicated that seroprevalence for Ehrlichia, Anaplasma, and Babesia spp. was significantly associated (Table 3). Specifically, the models estimated that Babesia-seropositive dogs had 13.69 times higher odds of also being Ehrlichia-seropositive and 7.91 times higher odds of also being Anaplasma-seropositive (Table 3, P < 0.001). In addition, age and breed were significant predictors of Ehrlichia-seropositivity, with older dogs and mongrels having a higher probability of being seropositive (GLMM, Table 3, P = 0.043 and P = 0.018, respectively). Regarding Anaplasma- and Babesia-seropositivity, neither a significant effect of age nor of breed was observed.
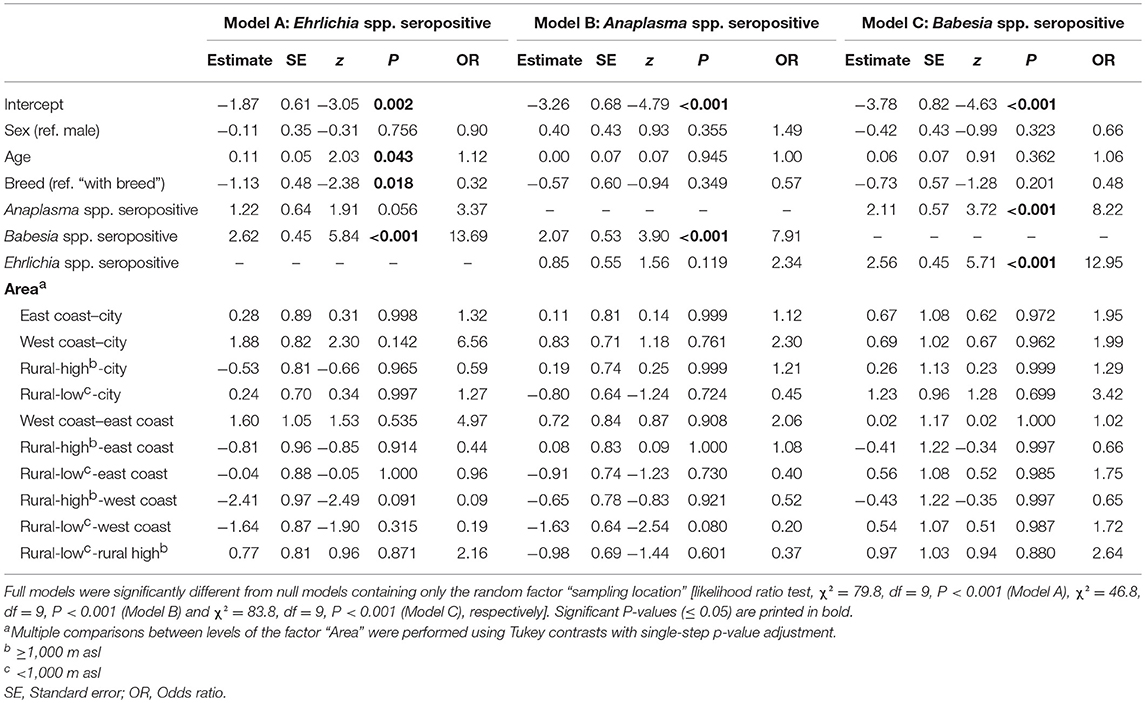
Table 3. Results of binomial GLMMs testing the influence of different predictor variables on the probability of testing seropositive for Ehrlichia spp. (Model A), Anaplasma spp. (Model B) and Babesia spp. (Model C), amongst 273 dogs from Costa Rica.
On province level, seroprevalence rates were highest in Guanacaste (Ehrlichia spp.: 65.57%, Babesia spp.: 57.38%, Anaplasma spp.: 19.67%) and lowest in Cartago (Ehrlichia spp.: 13.63%, Babesia spp.: 0.00%, Anaplasma spp.: 4.55%) (Table 1, Figure 1). However, no statistically significant differences in seroprevalence were found between dogs sampled in rural areas of high or low altitude, eastern or western coastal areas or cities (GLMMs, Table 3).
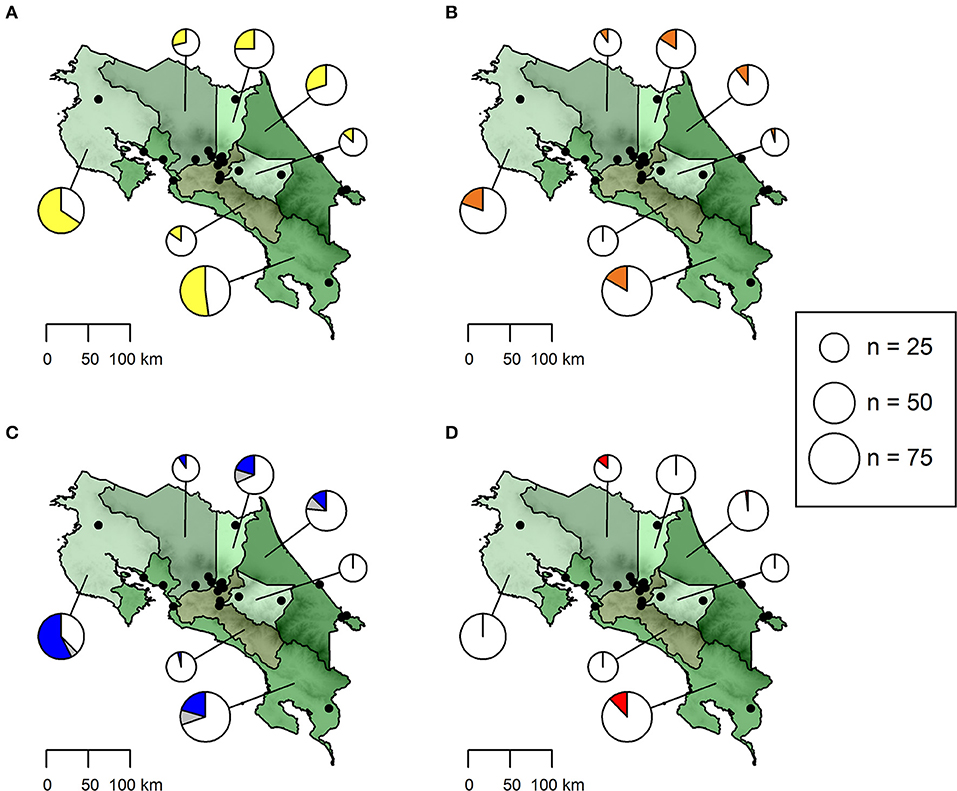
Figure 1. Seroprevalence of antibodies against (A) Ehrlichia spp. (yellow), (B) Anaplasma spp. (orange) and (C) Babesia spp. (blue) as well as (D) Dirofilaria immitis antigen (red) in dogs from Costa Rica. The size of pie charts corresponds to the number of dogs sampled per province. Sampling locations are shown as black dots. The proportion of samples with a doubtful Babesia spp. test result is indicated in gray.
No significant difference was observed regarding the proportion of animals showing clinical signs compatible with vector-borne disease when comparing animals seropositive for at least one of the tested vector-borne pathogens to seronegative animals (χ2 = 2.76, df = 1, P = 0.097). However, an effect of seropositivity on PCV was found for Babesia spp., Ehrlichia spp., and Anaplasma spp., and the interaction between Babesia spp. and Ehrlichia spp. was also statistically significant (LMM, Table 4). Babesia-seropositive dogs showed on average 6.3% lower PCV values than seronegative dogs (P = 0.003). The effect was less pronounced for Ehrlichia- and Anaplasma-seropositive dogs, which showed approximately 2.9% lower PCV values on average than seronegative animals (P = 0.016 and P = 0.034, respectively). Being seropositive for both Babesia spp. and Ehrlichia spp. led to a less pronounced reduction of PCV than expected if the effect had been additive, namely a 4.1% reduction in PCV on average, compared to seronegative dogs. PCV values of seronegative and seropositive dogs are displayed in Figure 2.
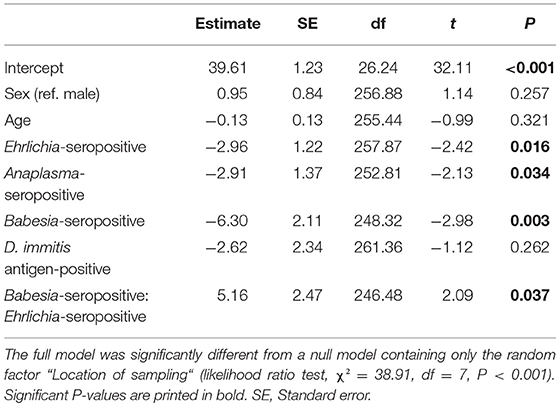
Table 4. Results of LMM testing the influence of animal sex, age, and seropositivity for Ehrlichia spp., Anaplasma spp. and Babesia spp. antibodies as well as D. immitis antigen on packed cell volume of 273 dogs from Costa Rica.
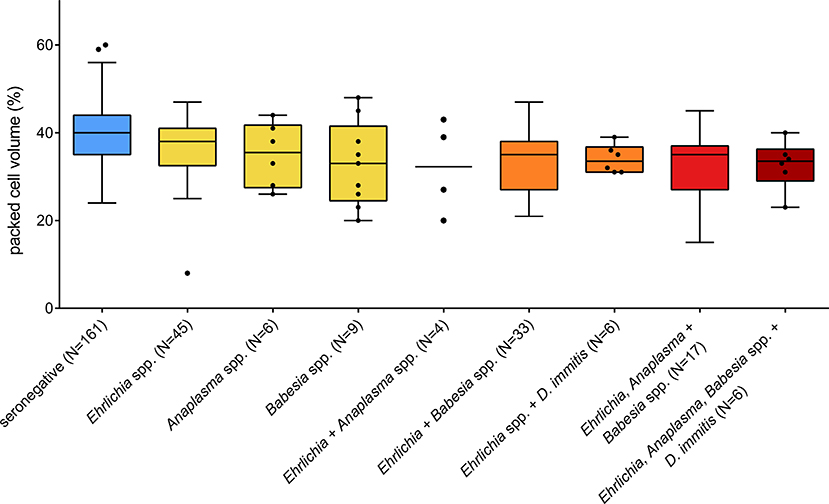
Figure 2. Packed cell volume of dogs seropositive for different vector-borne pathogens in Costa Rica. Only one animal was seropositive for Anaplasma and Babesia spp. and was not plotted. Boxes extend from the 25th to the 75th percentile, with a line at the median and whiskers extending to 1.5 the interquartile range or up to the maximum/minimum value. Individual data points are shown for N < 10.
Current Infections
In the blood samples of 51.72% (60/116, 95% CI: 42.26–61.10%) of Ehrlichia-seropositive dogs, E. canis DNA was detected by conventional PCR and/or qPCR. In contrast, neither E. ewingii nor E. chaffeensis were detected in any sample. Only 33.33% (20/60, 95% CI: 21.69–46.69%) of E. canis-positive dogs showed clinical symptoms, namely pale mucous membranes, apathy, fever, epistaxis or a combination of these.
Furthermore, 12.82% (5/39, 95% CI: 4.30–27.43%) of Anaplasma-seropositive dogs were also PCR-positive. Four of these five animals were co-infected with A. platys and A. phagocytophilum, while one animal was infected with A. phagocytophilum only. Among the 32 animals tested by PCR for Anaplasma spp. as well as E. canis based on a positive serological result, 15 (46.88%, 95% CI: 29.09–65.26%) were mono-infected with E. canis, two (6.25%, 95% CI: 0.77–20.81%) were co-infected with A. platys and A. phagocytophilum and one dog (3.13%, 95% CI: 0.08–16.22%) was infected with all three pathogens. Only the triple-infected dog showed pale mucous membranes in the clinical examination, while no symptoms were noted in the remaining Anaplasma-infected animals.
Of the 68 Babesia-seropositive dogs, only one (1.47%, 95% CI: 0.04–7.92%) was positive for B. vogeli in the PCR (100% sequence identity [ID], 99% query cover [QC]), but did not show any clinical symptoms of babesiosis, whereas Hepatozoon canis DNA was amplified from a second, asymptomatic dog (99% ID, 98% QC). The H. canis-infected dog was also seropositive for Ehrlichia spp., but negative in the Ehrlichia PCR. The B. vogeli-infected dog was seronegative for all other pathogens tested, thus, no further PCRs were carried out.
Dirofilaria immitis antigen was detected only in dogs from the provinces Alajuela, Limón and Puntarenas (Table 1, Figure 1), with an overall prevalence of 4.42% (13/294, 95% CI: 2.38–7.44%). Microfilariae were detected in buffy coat of 11 dogs, three of which were also positive for D. immitis antigen in the rapid ELISA and yielded a positive D. immitis PCR result (96% ID, 98% QC). Acanthocheilonema reconditum DNA (99% ID, 97% QC) was amplified from the blood samples of three further microfilaremic dogs, which were tested negative for D. immitis antigen in the rapid ELISA. For the remaining five dogs, the filarial species could not be identified, as no amplicon resulted from the PCR.
Discussion
Canine vector-borne diseases, including important zoonoses, are widespread in Central America. In the present study, exposure to at least one of five tested pathogens was detected in 45.24% of the 294 tested dogs, while multiple exposure was demonstrated in 23.13%. A significant association between seropositivity for Ehrlichia, Anaplasma and Babesia spp. was shown. This may be due to the fact that E. canis and A. platys as well as B. vogeli and B. gibsoni share a common vector, namely the brown dog tick, R. sanguineus s.l., which is the most common tick species parasitizing dogs in Central America (5–7). Furthermore, experimental infections have shown that concurrent Ehrlichia-infection intensifies the humoral immune response to A. platys in dogs, resulting in a more persistent A. platys infection (35). Similar immune-mediated interactions could apply to Babesia/Ehrlichia or Babesia/Anaplasma co-infections, however, no experimental data on these combinations are available to date.
Seroprevalence of Ehrlichia spp. was almost 40%, which is comparable to previous studies conducted in Costa Rica (7, 11) and Mexico (36), whereas a considerably higher seroprevalence of more than 60% was detected in the neighboring country of Nicaragua (10). Ehrlichia-seropositive dogs were found in all seven Costa Rican provinces, and no statistically significant differences between different sampling locations (urban areas, high/low elevation rural areas or coastal areas) were found. Nevertheless, the highest prevalences were detected in the provinces of Guanacaste and Puntarenas, bordering the Pacific Coast, similar to the pattern reported by Montenegro et al. (11). Older dogs as well as mongrels had a higher probability of being Ehrlichia-seropositive, which also confirms previous findings (7, 11).
Current E. canis infections, as defined by amplification of E. canis DNA by PCR, were detected in 51.72% of Ehrlichia-seropositive dogs. The high rate of current E. canis infections in asymptomatic dogs is concerning, if the pathogen might also infect humans. Recently, anti-Ehrlichia spp. antibodies have been found in 35% of 280 human blood donor samples examined in Costa Rica, with 3.5% of samples containing DNA of a novel E. canis genotype (37). In contrast, E. chaffeensis and E. ewingii, which possess higher zoonotic potential, were not detected in the present study, nor in the mentioned blood donor study (37). E. chaffeensis DNA was isolated from symptomatic human patients in Costa Rica (38), but the pathogen has neither been found in dogs nor in ticks in Central America so far.
Regarding Anaplasma spp., the present study may indicate an increasing seroprevalence of this genus in Costa Rica. In 2011, Bonilla et al. (13) detected 2.7% Anaplasma-seropositive animals among 408 sampled dogs, with regional prevalences up to 6.5%. A similar study from 2011 to 2012, which tested 314 Costa Rican dogs with the same method as in the present study, detected a country-wide Anaplasma spp. seroprevalence of 6.5%, with the highest value in the province of Guanacaste (16.2%) (11). In the present study, based on a comparable sample size, Anaplasma-seropositive dogs were detected in six of the seven Costa Rican provinces, overall Anaplasma spp. seroprevalence was 13.27%, and reached 19.27% in Guanacaste. Thus, canine anaplasmosis may constitute an emerging infection in Costa Rica. Nevertheless, the detected differences might also be due to methodological reasons, since the 2011/12 survey by Bonilla et al. (13) used a different serological test. Furthermore, although all three surveys covered the seven provinces of Costa Rica, actual sampling locations differed. For example, the present survey covered more coastal regions, while the 2011/12 survey mainly focused on the Greater Metropolitan area (as described in 7). Thus, local variation in seroprevalence may also underlie the detected differences, as well as further factors such as the age or breed composition of the study populations.
Among Anaplasma-seropositive dogs, A. platys as well as A. phagocytophilum were detected at almost the same frequency, and predominantly as co-infections. A similar infection pattern was found in dogs from Nicaragua (10). In Central America, ixodid ticks, which are the usual vectors for A. phagocytophilum, are rather rare as canine parasites (5, 39). However, a low prevalence of A. phagocytophilum has been found in R. sanguineus s.l. collected from dogs in Costa Rica (12). Nevertheless, it remains unknown whether R. sanguineus s.l. might be implicated in the transmission of zoonotic A. phagyocytophilum in Central America.
The present study contains the first large-scale serological survey of canine babesiosis in Central America, and demonstrates the presence of the pathogen in six of seven Costa Rican provinces, with an overall seroprevalence of more than 20%. Previous studies on Babesia spp. in dogs from Costa Rica used a PCR-based approach, thus detecting only current infections. Wei et al. (15) demonstrated current Babesia-infections by quantitative real-time PCR (qPCR) in 10 of 40 dogs sampled in northwestern Costa Rica. In Nicaragua, a similar infection rate of 26% was found in a sample of 39 dogs (16). Both B. vogeli- and B. gibsoni-infections were detected in these studies. In contrast, a lower prevalence of 8% was found among 146 Costa Rican dogs tested by conventional PCR (14). In the present study, B. vogeli DNA was only amplified from one seropositive dog, while B. gibsoni DNA was not detected. The discrepancies in infection rates between these studies might be due to geographical differences in Babesia prevalence within Costa Rica, as well as to a higher sensitivity of qPCRs compared to conventional PCRs, or to sensitivity differences related to the different target genes. Chronic infections with B. vogeli and B. gibsoni are commonly associated with very low parasitemia, and it is additionally recommended to use capillary rather than venous blood or buffy coat preparations for diagnosis (23, 40). Low parasitemias and the fact that venous blood was used in this study may have negatively affected PCR sensitivity. Babesia-seropositive dogs, as well as Ehrlichia- and Anaplasma-seropositive dogs, had a significantly lower PCV as compared to seronegative dogs, as shown in previous studies [e.g., (10, 14)].This also indicates that the rate of current infections among Babesia-seropositive dogs may have actually been higher than detected. Alternatively, the lower PCV values in Babesia-seropositive dogs may be a sign of immune-mediated hemolytic anemia, a complication of canine babesiosis due to the production of anti-erythrocyte antibodies, which may persist even after the infection has been cleared (41). Furthermore, only blood of seropositive dogs was tested for current infections, thus, we may have missed current infections in dogs which had not seroconverted yet.
Although not specifically targeted in this study, H. canis DNA was detected in one dog, confirming previous reports from Costa Rica (14). Both R. sanguineus s.l. and Amblyomma ovale may act as vectors for this apicomplexan parasite and both tick species occur in Central America (5, 6). Hepatozoon canis mostly causes moderate or asymptomatic infections with low parasitemia in dogs and is not considered a zoonotic pathogen (42). However, severe clinical signs may occur in cases of canine hepatozoonosis characterized by a high level of parasitemia, and co-infections with other pathogens are common, complicating the clinical picture (42).
Dirofilaria immitis infections in Costa Rican dogs have so far mainly been found in the provinces of Guanacaste and Puntarenas, bordering the Pacific Coast (11, 15, 17). The present study confirms these results, as the majority of D. immitis-positive dogs (9/13 infected animals) were from the province of Puntarenas. The three D. immitis-positive dogs in the central Costa Rican province of Alajuela may have been translocated from a coastal region. Alternatively, this might indicate a geographic spread of the parasite to central regions of Costa Rica, which needs to be confirmed in future studies.
In addition to D. immitis, A. reconditum, which is transmitted by fleas, was identified in three microfilaremic dogs from the eastern parts of Costa Rica (provinces Heredia and Limón). Although flea infestation is common in dogs all over Costa Rica, the regional presence of A. reconditum confirms findings by Rojas et al. (17), who detected a high prevalence of A. reconditum in the province of Limón. Thus, A. reconditum needs to be considered as a differential diagnosis to dirofilariosis if microfilaria are observed in these areas. Acanthocheilonema reconditum is considered as less pathogenic than D. immitis, and is also of less zoonotic importance (43).
As in previous studies from Central America and Mexico (10, 11, 36), B. burgdorferi s.l. seroprevalence was very low. Here, only one seropositive dog was found, and it cannot be excluded that this dog had a travel history and got infected outside of Central America. Furthermore, it should be borne in mind that the positive predictive value of a diagnostic test, i.e., the number of true positives among all positive test results, is influenced by the prevalence of the pathogen as well as by the test's sensitivity and specificity. Since the prevalence of B. burgdorferi s.l. was very low, the resulting positive predictive value is also low (21.6%), thus, positive test results for B. burgdorferi s.l. in this region should be treated with caution, as the probability of false-positive results is high.
Clinical symptoms compatible with the investigated vector-borne diseases were noted in more than 25% of the studied dogs. However, apart from a lower PCV in Ehrlichia-, Anaplasma-, and Babesia-seropositive animals, no statistically significant association between seropositivity and clinical signs was found. Many of these symptoms, such as anorexia and apathy, are rather unspecific. They occurred equally often in seronegative animals, possibly due to other infectious or non-infectious causes, whereas severe symptoms of vector-borne diseases, e.g., petechial bleeding, were only noted in very few dogs. Furthermore, the incubation period following a tick bite for anaplasmosis and babesiosis is shorter (~1 week) than the time to seroconversion (~2 weeks) (23) and may thus further explain the missing association.
Conclusions
This study demonstrated high seroprevalences of several canine vector-borne pathogens in Costa Rica, with a possible rise of Anaplasma spp. infections as compared to previous surveys. In addition, Babesia-seroprevalence was assessed for the first time in Costa Rican dogs, revealing exposure of more than 20% of dogs. Although most animals were asymptomatic, a significant effect of Ehrlichia, Anaplasma and Babesia seropositivity on PCV was found. In addition, chronically infected dogs may constitute a reservoir of human infection in the case of zoonotic pathogens, such as A. phagocytophilum and D. immitis. Thus, protection of dogs from disease-transmitting vectors is recommended from an animal welfare as well as public health perspective.
Data Availability
All datasets supporting the conclusions of the study are included in the manuscript.
Ethics Statement
Analyzed blood samples represent surplus from diagnostic blood samples of dogs that were presented at veterinary clinics for varying reasons. Consent of the dog owners to use surplus samples for further analyses was obtained.
Author Contributions
CS and VM designed and coordinated the study. VM collected the blood samples. VM, SS, MG, NP, JB and AS performed laboratory analyses. AS performed the statistical analyses and drafted the manuscript. All authors participated in data analysis and interpretation. All authors read and approved the final version of the manuscript.
Funding
This project was partially funded by IDEXX Laboratories. Study data interpretation is completely independent from the company's opinion and there is no conflict with commercial interests. VM received funding from the German Academic Exchange Service (DAAD) for a research stay in Germany. This publication was supported by Deutsche Forschungsgemeinschaft and University of Veterinary Medicine Hannover, Foundation within the funding programme Open Access Publishing.
Conflict of Interest Statement
MG, NP, and JB are currently employed by IDEXX Laboratories. Study data collection and interpretation is completely independent from the company's opinion.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
1. Otranto D, Dantas-Torres F, Breitschwerdt EB. Managing canine vector-borne diseases of zoonotic concern: part two. Trends Parasitol. (2009) 25:228–35. doi: 10.1016/j.pt.2009.02.005
3. Otranto D, Dantas-Torres F, Breitschwerdt EB. Managing canine vector-borne diseases of zoonotic concern: part one. Trends Parasitol. (2009) 25:157–63. doi: 10.1016/j.pt.2009.01.003
4. Kilpatrick AM, Randolph SE. Drivers, dynamics, and control of emerging vector-borne zoonotic diseases. The Lancet. (2012) 380:1946–55. doi: 10.1016/S0140-6736(12)61151-9
5. Springer A, Montenegro VM, Schicht S, Wölfel S, Schaper SR, Chitimia-Dobler L, et al. Detection of Rickettsia monacensis and Rickettsia amblyommatis in ticks collected from dogs in Costa Rica and Nicaragua. Ticks Tick Borne Dis. (2018) 9:1565–72. doi: 10.1016/j.ttbdis.2018.08.002
6. Ferrell AM, Brinkerhoff RJ, Bernal J, Bermúdez SE. Ticks and tick-borne pathogens of dogs along an elevational and land-use gradient in Chiriquí province, Panamá. Exp Appl Acarol. (2017) 71:371–85. doi: 10.1007/s10493-017-0116-z
7. Barrantes-González AV, Jiménez-Rocha AE, Romero-Zuñiga JJ, Dolz G. Serology, molecular detection and risk factors of Ehrlichia canis infection in dogs in Costa Rica. Ticks Tick Borne Dis. (2016) 7:1245–51. doi: 10.1016/j.ttbdis.2016.07.006
8. Dantas-Torres F. The brown dog tick, Rhipicephalus sanguineus (Latreille, 1806) (Acari: Ixodidae): from taxonomy to control. Vet Parasitol. (2008) 152:173–85. doi: 10.1016/j.vetpar.2007.12.030
9. Moraes-Filho J, Krawczak FS, Costa FB, Soares JF, Labruna MB. Comparative evaluation of the vector competence of four South American populations of the Rhipicephalus sanguineus group for the bacterium Ehrlichia canis, the agent of canine monocytic ehrlichiosis. PLoS ONE. (2015) 10:e0139386. doi: 10.1371/journal.pone.0139386
10. Springer A, Montenegro VM, Schicht S, Pantchev N, Strube C. Seroprevalence and current infections of canine vector-borne diseases in Nicaragua. Parasit Vectors. (2018) 11:585. doi: 10.1186/s13071-018-3173-1
11. Montenegro VM, Bonilla MC, Kaminsky D, Romero-Zúñiga JJ, Siebert S, Krämer F. Serological detection of antibodies to Anaplasma spp., Borrelia burgdorferi sensu lato and Ehrlichia canis and of Dirofilaria immitis antigen in dogs from Costa Rica. Vet Parasitol. (2017) 236:97–107. doi: 10.1016/j.vetpar.2017.02.009
12. Campos-Calderón L, Ábrego-Sánchez L, Solórzano-Morales A, Alberti A, Tore G, Zobba R, et al. Molecular detection and identification of Rickettsiales pathogens in dog ticks from Costa Rica. Ticks Tick Borne Dis. (2016) 7:1198–202. doi: 10.1016/j.ttbdis.2016.07.015
13. Bonilla MC, Campos-Calderón L, Jiménez-Rocha AE, Romero-Zúñiga JJ, Alberti A, Zobba R, et al. Characterization of Anaplasma spp. infection in dogs from Costa Rica. Vet Parasitol Reg Stud Reports. (2017) 8:60–5. doi: 10.1016/j.vprsr.2017.02.003
14. Rojas A, Rojas D, Montenegro V, Gutiérrez R, Yasur-Landau D, Baneth G. Vector-borne pathogens in dogs from Costa Rica: First molecular description of Babesia vogeli and Hepatozoon canis infections with a high prevalence of monocytic ehrlichiosis and the manifestations of co-infection. Vet Parasitol. (2014) 199:121–8. doi: 10.1016/j.vetpar.2013.10.027
15. Wei L, Kelly P, Ackerson K, El-Mahallawy Heba S, Kaltenboeck B, Wang C. Molecular detection of Dirofilaria immitis, Hepatozoon canis, Babesia spp., Anaplasma platys and Ehrlichia canis in dogs on Costa Rica. Acta Parasitol. (2015) 60:21. doi: 10.1515/ap-2015-0003
16. Wei L, Kelly P, Ackerson K, Zhang J, El-Mahallawy HS, Kaltenboeck B, et al. First report of Babesia gibsoni in Central America and survey for vector-borne infections in dogs from Nicaragua. Parasit Vectors. (2014) 7:126. doi: 10.1186/1756-3305-7-126
17. Rojas A, Rojas D, Montenegro VM, Baneth G. Detection of Dirofilaria immitis and other arthropod-borne filarioids by an HRM real-time qPCR, blood-concentrating techniques and a serological assay in dogs from Costa Rica. Parasit Vectors. (2015) 8:170. doi: 10.1186/s13071-015-0783-8
18. Labarthe N, Guerrero J. Epidemiology of heartworm: what is happening in South America and Mexico? Vet Parasitol. (2005) 133:149–56. doi: 10.1016/j.vetpar.2005.04.006
19. Stillman BA, Monn M, Liu J, Thatcher B, Foster P, Andrews B, et al. Performance of a commercially available in-clinic ELISA for detection of antibodies against Anaplasma phagocytophilum, Anaplasma platys, Borrelia burgdorferi, Ehrlichia canis, and Ehrlichia ewingii and Dirofilaria immitis antigen in dogs. J Am Vet Med Assoc. (2014) 245:80–6. doi: 10.2460/javma.245.1.80
20. Chandrashekar R, Mainville CA, Beall MJ, O'Connor T, Eberts MD, Alleman AR, et al. Performance of a commercially available in-clinic ELISA for the detection of antibodies against Anaplasma phagocytophilum, Ehrlichia canis, and Borrelia burgdorferi and Dirofilaria immitis antigen in dogs. Am J Vet Res. (2010) 71:1443–50. doi: 10.2460/ajvr.71.12.1443
21. Pantchev N, Schnyder M, Vrhovec MG, Schaper R, Tsachev I. Current surveys of the seroprevalence of Borrelia burgdorferi, Ehrlichia canis, Anaplasma phagocytophilum, Leishmania infantum, Babesia canis, Angiostrongylus vasorum and Dirofilaria immitis in dogs in Bulgaria. Parasitol Res. (2015) 114(Suppl. 1):S117–30. doi: 10.1007/s00436-015-4518-8
22. Dyachenko V, Pantchev N, Balzer H-J, Meyersen A, Straubinger RK. First case of Anaplasma platys infection in a dog from Croatia. Parasit Vectors. (2012) 5:49. doi: 10.1186/1756-3305-5-49
23. Pantchev N, Pluta S, Huisinga E, Nather S, Scheufelen M, Vrhovec MG, et al. Tick-borne diseases (borreliosis, anaplasmosis, babesiosis) in German and Austrian dogs: status quo and review of distribution, transmission, clinical findings, diagnostics and prophylaxis. Parasitol Res. (2015) 114:19–54. doi: 10.1007/s00436-015-4513-0
24. Massung RF, Slater K, Owens JH, Nicholson WL, Mather TN, Solberg VB, et al. Nested PCR assay for detection of granulocytic ehrlichiae. J Clin Microbiol. (1998) 36:1090–5.
25. Martin AR, Brown GK, Hugh Dunstan R, Roberts TK. Anaplasma platys: an improved PCR for its detection in dogs. Exp Parasitol. (2005) 109:176–80. doi: 10.1016/j.exppara.2004.11.007
26. Murphy GL, Ewing SA, Whitworth LC, Fox JC, Kocan AA. A molecular and serologic survey of Ehrlichia canis, E. chaffeensis, and E. ewingii in dogs and ticks from Oklahoma. Vet Parasitol. (1998) 79:325–39. doi: 10.1016/S0304-4017(98)00179-4
27. Dawson JE, Stallknecht DE, Howerth EW, Warner C, Biggie K, Davidson WR, et al. Susceptibility of white-tailed deer (Odocoileus virginianus) to infection with Ehrlichia chaffeensis, the etiologic agent of human ehrlichiosis. J Clin Microbiol. (1994) 32:2725–8.
28. Casati S, Sager H, Gern L, Piffaretti JC. Presence of potentially pathogenic Babesia sp. for human in Ixodes ricinus in Switzerland. Ann Agric Environ Med. (2006) 13:65–70.
29. Olmeda AS, Armstrong PM, Rosenthal BM, Valladares B, del Castillo A, de Armas F, et al. A subtropical case of human babesiosis. Acta Trop. (1997) 67:229–34. doi: 10.1016/S0001-706X(97)00045-4
30. Newton LA, Chilton NB, Beveridge I, Hoste H, Nansen P, Gasser RB. Genetic markers for strongylid nematodes of livestock defined by PCR-based restriction analysis of spacer rDNA. Acta Trop. (1998) 69:1–15. doi: 10.1016/S0001-706X(97)00105-8
31. R Core Team. R: A language and environment for statistical computing. 3.5.0 ed. Vienna: : R Foundation for Statistical Computing (2018).
32. Bates D, Mächler M, Bolker B, Walker S. Fitting linear mixed-effects models using lme4. J Stat Softw. (2015) 67:1. doi: 10.18637/jss.v067.i01
33. Hothorn T, Bretz F, Westfall P. Simultaneous inference in general parametric models. Biom J. (2008) 50:346–63. doi: 10.1002/bimj.200810425
34. Latimer KS editor. Duncan and Prasse's Veterinary Laboratory Medicine: Clinical Pathology. 5th ed. Ames, IA: Wiley-Blackwell (2011).
35. Gaunt S, Beall M, Stillman B, Lorentzen L, Diniz P, Chandrashekar R, et al. Experimental infection and co-infection of dogs with Anaplasma platys and Ehrlichia canis: hematologic, serologic and molecular findings. Parasit Vectors. (2010) 3:33. doi: 10.1186/1756-3305-3-33
36. Movilla R, García C, Siebert S, Roura X. Countrywide serological evaluation of canine prevalence for Anaplasma spp., Borrelia burgdorferi (sensu lato), Dirofilaria immitis and Ehrlichia canis in Mexico. Parasit Vectors. (2016) 9:421. doi: 10.1186/s13071-016-1686-z
37. Bouza-Mora L, Dolz G, Solórzano-Morales A, Romero-Zuñiga JJ, Salazar-Sánchez L, Labruna MB, et al. Novel genotype of Ehrlichia canis detected in samples of human blood bank donors in Costa Rica. Ticks Tick Borne Dis. (2017) 8:36–40. doi: 10.1016/j.ttbdis.2016.09.012
38. Rojas N, Castillo D, Marin P. Molecular detection of Ehrlichia chaffeensis in humans, Costa Rica. Emerg Infect Dis. (2015) 21:532. doi: 10.3201/eid2103.131759
39. Bermúdez C SE, Mejía B L, Hernández L, Apanaskevich DA. First records of Ixodes boliviensis Neumann, 1904 and Dermacentor dissimilis Cooley, 1947 (Ixodida: Ixodidae) as parasites of domestic mammals in Nicaragua. Syst Appl Acarol. (2015) 20:462–4. doi: 10.11158/saa.20.4.12
40. Schoeman JP. Canine babesiosis. Onderstepoort J Vet Res. (2009) 76:59–66. doi: 10.4102/ojvr.v76i1.66
41. Ayoob AL, Hackner SG, Prittie J. Clinical management of canine babesiosis. J Vet Emerg Crit Care. (2010) 20:77–89. doi: 10.1111/j.1476-4431.2009.00489.x
42. Baneth G. Perspectives on canine and feline hepatozoonosis. Vet Parasitol. (2011) 181:3–11. doi: 10.1016/j.vetpar.2011.04.015
Keywords: vector-borne diseases, tick-borne diseases, zoonoses, Rickettsia spp., Ehrlichia spp., Anaplasma spp., Babesia spp., Central America
Citation: Springer A, Montenegro VM, Schicht S, Globokar Vrohvec M, Pantchev N, Balzer J and Strube C (2019) Seroprevalence and Current Infections of Canine Vector-Borne Diseases in Costa Rica. Front. Vet. Sci. 6:164. doi: 10.3389/fvets.2019.00164
Received: 04 April 2019; Accepted: 13 May 2019;
Published: 04 June 2019.
Edited by:
David Modrý, University of Veterinary and Pharmaceutical Sciences Brno, CzechiaReviewed by:
Jan Slapeta, University of Sydney, AustraliaXiangye Liu, Xuzhou Medical University, China
Copyright © 2019 Springer, Montenegro, Schicht, Globokar Vrohvec, Pantchev, Balzer and Strube. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Christina Strube, christina.strube@tiho-hannover.de
 Andrea Springer1
Andrea Springer1  Christina Strube
Christina Strube