Genetic and Phenotypic Characterization of the Etiological Agent of Canine Orchiepididymitis Smooth Brucella sp. BCCN84.3
- 1Programa de Investigación en Enfermedades Tropicales (PIET), Escuela de Medicina Veterinaria, Universidad Nacional, Heredia, Costa Rica
- 2Facultad de Microbiología, Centro de Investigación en Enfermedades Tropicales, Universidad de Costa Rica, San José, Costa Rica
- 3ISP, INRA, Université François Rabelais de Tours, Nouzilly, France
- 4Centro Nacional de Referencia en Bacteriología, Instituto Costarricense de Investigación y Enseñanza en Nutrición y Salud (INCIENSA), Cartago, Costa Rica
- 5IDISNA and Departamento de Microbiología y Parasitología, Instituto de Salud Tropical, Universidad de Navarra, Pamplona, Spain
- 6Unidad de Producción y Sanidad Animal, Instituto Agroalimentario de Aragón-IA2, CITA-Universidad de Zaragoza, Zaragoza, Spain
- 7Pathogen Genomics, Wellcome Trust Sanger Institute, Hinxton, United Kingdom
- 8Institute for Integrative Biology, University of Liverpool, Liverpool, United Kingdom
Members of the genus Brucella cluster in two phylogenetic groups: classical and non-classical species. The former group is composed of Brucella species that cause disease in mammals, including humans. A Brucella species, labeled as Brucella sp. BCCN84.3, was isolated from the testes of a Saint Bernard dog suffering orchiepididymitis, in Costa Rica. Following standard microbiological methods, the bacterium was first defined as “Brucella melitensis biovar 2.” Further molecular typing, identified the strain as an atypical “Brucella suis.” Distinctive Brucella sp. BCCN84.3 markers, absent in other Brucella species and strains, were revealed by fatty acid methyl ester analysis, high resolution melting PCR and omp25 and omp2a/omp2b gene diversity. Analysis of multiple loci variable number of tandem repeats and whole genome sequencing demonstrated that this isolate was different from the currently described Brucella species. The smooth Brucella sp. BCCN84.3 clusters together with the classical Brucella clade and displays all the genes required for virulence. Brucella sp. BCCN84.3 is a species nova taxonomical entity displaying pathogenicity; therefore, relevant for differential diagnoses in the context of brucellosis. Considering the debate on the Brucella species concept, there is a need to describe the extant taxonomical entities of these pathogens in order to understand the dispersion and evolution.
Introduction
The Brucella genus comprises two phylogenetically related clusters: classical and non-classical (1). The former cluster is a compact group composed of Brucella melitensis, Brucella abortus, Brucella suis, Brucella canis, Brucella neotomae, Brucella ceti, Brucella pinnipedialis, Brucella ovis, Brucella microti, Brucella papionis, and Brucella sp. F5/99. All these species infect and produce disease in mammals, displaying host preference. Members of this cluster are non-motile, devoid of plasmids and their genomes show nucleotide identities of >99% (1, 2). The first six Brucella species of this cluster are zoonotic and can infect humans (3–5).
Non-classical Brucella species, also known as the “BO clade,” cluster in a discrete group that includes the fast-growing Brucella inopinata and BO2 strains isolated in humans as well as Brucella species living in frogs (1). Brucella vulpis, isolated from red foxes in Australia, is more distant to BO clade and contains unique genetic information related to soil bacteria not encoded in classical Brucella organisms (1). Bacteria of the BO clade and B. vulpis display nucleotide identities of 97–98% with those of the classical clade. The species of this cluster also share genes with the soil bacteria Ochrobactrum spp. and show key sequence differences in central genes such as 16S rRNA and recA, as distinctive features (1). With the sole exception of B. inopinata, these Brucella species possess an O-chain lipopolysaccharide (LPS) structure that departs from that of the classical Brucella species (1, 6). This feature hampers the straightforward recognition of non-classical Brucella infections in animals.
Identification of the classical Brucella species and strains by traditional bacteriological and molecular methods is not straightforward. This is due to the high phenotypic and genotypic resemblance among different members of the genus (3, 7). For this reason, many Brucella strains isolated from various animal species have been misclassified or not fully characterized (8, 9). One clear example of clinical relevance has been the discovery of B. neotomae as a human pathogen, which was wrongly classified as an atypical B. abortus strain by classical bacteriological methods (10). With the advent of sophisticated molecular tools and whole genome sequence analysis (WGSA), the correct identification of Brucella species was achieved (1, 4).
Here, we describe the phenotypic and genotypic properties of a new classical pathogenic smooth Brucella sp., isolated from a Saint Bernard dog suffering orchiepididymitis. After its primary isolation in 1984 in Costa Rica (11), the strain was first assigned as an atypical strain of B. melitensis biovar 2 (12).
Materials and Methods
Clinical Case and Bacterial Isolation
A 4-year male domestic Saint Bernard dog from the Central Valley of Costa Rica showing testicular lesions, was brought to the Hospital of the Veterinary School of the National University, in 1984. After hospitalization, the owner was informed of all procedures and clinical studies and gave her written consent. All protocols and actions undertaken to diagnose the disease were under the Veterinary Hospital guidance established in 1980. The protocols used in 1984, were those approved by the “Ley General de Salud” N° 5395, and “Disposiciones sobre Matrícula y Vacunación de Perros” N° 2391.
After anamnesis and clinical examination, the dog was subjected to surgery and both testes removed. Rose Bengal test (13) was used to determine the presence of antibodies against smooth Brucella. Histopathological examination of the testes was performed following previous protocols (14). For bacterial isolation, blood and testicular samples were cultured in blood-agar plates. The plates were incubated at 37°C under the presence or the absence of 10% CO2 atmosphere. The bacterial colonies were identified as Brucella sp. at the Bacteriology Laboratory of INCIENSA, Costa Rica (11). The isolate (code Brucella sp. BCCN84.3) was freeze-dried and submitted for further bacteriological and molecular typing, as described below.
Bacterial Phenotypic Characterization
The Brucella sp. BCCN84.3 was subjected to classical bacteriological typing (Table 1) following established protocols (13). Reference Brucella strains were used for comparative purposes (Supplementary Table 1). Total lipids were extracted and analyzed as described elsewhere (15) and resolved on silica gel 60 high-performance TLC plates (Merck Chemicals) using n-propanol/propionic acid/chloroform/water (3:2:2:1) and developed by charring with 15% (v/v) sulfuric acid in ethanol (16). Processing of the fatty acid methyl ester for taxonomical identification and dendrogram assembly were carried out as described before (17). Extraction of LPS by SDS-proteinase K protocol was performed as described previously (18). LPS was analyzed in 12 or 15% polyacrylamide gels and stained by the periodate-alkaline silver method (19). An immune serum obtained from B. melitensis 16M infected rabbits (20), either plain or absorbed with cells from rough Per mutant strain derived from B. abortus 2308W, was used for assessing anti-smooth-LPS reactivity. Immune serum obtained from B. abortus Per immunized rabbit (21) was used for anti-rough-LPS reactivity. Western blots and ELISA with a collection of monoclonal antibodies (Mabs) for the detection of specific Brucella surface antigens were performed as described elsewhere (22, 23). Susceptibility to polymyxin B was determined by estimating the minimal inhibitory concentration on Müller-Hinton agar (Becton Dickinson, Izasa), following the e-test (Liofilchem, Werfen) method (24).
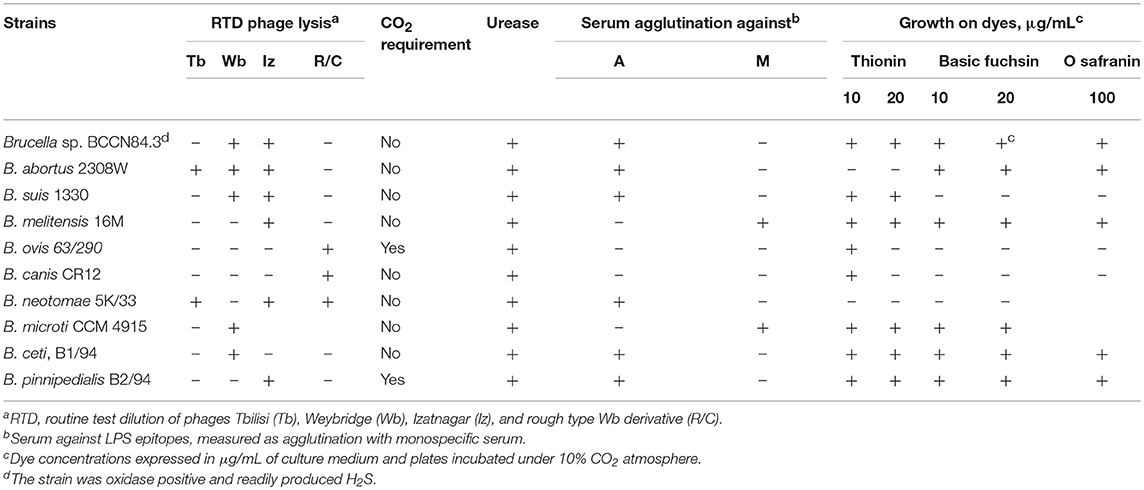
Table 1. Microbiological characterization of Brucella sp. BCCN84.3 and comparison with Brucella reference strains.
Genotypic and Phylogenetic Characterization
Bacterial DNA was extracted with DNeasy Blood & Tissue kit from QIAGEN or Promega Wizard Genomic DNA Purification kit as per manufacturer's instructions. DNA was stored at −70°C until used. Bruce-ladder v2.0 PCR for the differentiation of Brucella species and strains was carried following previous protocols (25). Suis-ladder PCR assay for B. suis biovar typing and the discrimination of B. suis and B. canis was performed as described before (26).
Two different real-time PCRs, for the detection of Brucella genus and B. suis were performed as previously described (27). Additionally, two different high-resolution melting PCR assays (HRM-PCR) for the specific detection and discrimination of B. canis and B. melitensis were performed following previous protocols (27), using a DNA concentration of 1.5 ng/μL and a Type-it HRM-PCR Kit (QIAGEN) in a reaction volume of 25 μL with a Rotor-Gene Q (QIAGEN). Control DNAs from B. canis RM 6/66, B. melitensis 16M, B. suis 1330 and B. neotomae 5K/33 were extracted with DNeasy Blood & Tissue kit from QIAGEN, and stored at −80°C until used. The conditions were one cycle at 50°C for 2 min and one cycle at 95°C for 10 min, followed by 40 cycles at 95°C for 5 s and a cycle at 60°C for 30 s, with data acquired at 60°C in the green channel. After amplification, an HRM-PCR was performed when needed from 73 to 88°C at a rate of 0.03°C per step.
Multiple loci variable number of tandem repeats analysis (MLVA16) and the corresponding cladograms were generated according to described protocols (17, 28) using the MLVA-NET database (29). Values obtained for each MLVA16 marker are in Supplementary Data Sheet 1.
WGSA was performed at the Wellcome Trust Sanger Institute on Illumina platforms according to in-house protocols (30, 31). For WGSA assembly and alignment sequencing reads were de novo assembled using a Velvet Optimiser (32). In order to overcome possible genome deviation through serial cultivation, the strain deposited in 1984 in the Brucella Culture Collection Nouzilly (BCCN) was also sequenced and deposited at DDBJ/ENA/GenBank under the accession NQLX00000000; Accession Brucella sp. BCCN84.3 (NQLX00000000; BioSample SAMN07488835). WGSA from representative Brucella strains used for comparative purposes were obtained from GenBank (Supplementary Data Sheet 1). Low length and N50 scaffold sequences were not included in the analysis. Automatic annotation of the assembly was performed with the Prokka program (33). Genome sequence data was deposited at the European Nucleotide Archive under accession code ERS568777 and at DDBJ/ENA/GenBank under the accession NQLX00000000; BioSample SAMN07488835 (Supplementary Data Sheet 1). The 9 and 21 loci schemes of Multi Locus Sequence typing (MLST) were performed in silico by BLAST comparison with a set of specific primers (34) and the assembled scaffolds as input. The results were confirmed by querying the matched sequences or “amplicons” at the Brucella MLST Database (https://pubmlst.org/brucella/) (Supplementary Data Sheet 1).
Phylogenetic Reconstruction
Two Ochrobactrum species and Brucella isolates were used for phylogenetic reconstruction (Supplementary Data Sheet 1). The 25 WGSA were aligned by bwa and mapped with SMALT v.0.7.4 against B. suis 1330, with an average mapping of 89.41% when excluding Ochrobactrum. Single Nucleotide Polymorphisms (SNPs) were called using SAMtools (35), and 451213 variable sites were extracted using SNP sites (36). The general features of all 25 assemblies annotated by Prokka were used to perform a pangenome analysis (36). Both SNPs and core genome alignments were individually used to each produce a maximum likelihood phylogenetic reconstruction with RAxML v8.2 (37). The phylogenetic trees were rooted using Ochrobactrum anthropi ATCC49188 and Ochrobactrum intermedium LMG3301.
A Specific Search for Regions of Interest
Regions of interest were searched through bwa alignment and SMALT mapping, or BLAST comparison against B. canis ATCC RM6/66 (NC_010103.1-NC_010104.1), B. suis 1330 (NC_004310.3-NC_004311.2), B. abortus 9-941 (NC_006932.1-NC_006933.1), B. abortus 2308W (ERS568782), B. melitensis 16M (NC_003317.1-NC_003318.1), and B. microti CCM 4915 (NC_013119.1-NC_013118.1). The number of SNPs, insertions and deletions in each gene were recorded. BLAST comparisons between Brucella sp. BCCN84.3, B. canis RM6/66 and B. suis 1330 were performed and visualized with the Artemis Comparison Tool (38). The presence of recombination events was analyzed by Genealogies Unbiased By recomBinations In Nucleotide Sequences (39) and visualized by Phandango (40). Southern blot analysis was performed as described previously (41) using the IS elements IS711 and ISBme1 as probes on EcoR1-digested DNA.
For phylogenetic reconstruction, comparisons among omp2a (BAW_10633) and omp2b (BAW_10634) porin gene sequences were assessed through multiple sequence alignments. Characterization of Omp2a and omp2b have been used as molecular tools for the description of Brucella species since 2007 (42), Sanger sequence data from 14 classical Brucella strains were visualized, edited, aligned, and analyzed in MEGA version 7 (43). The resulting alignment of 1,223 positions was used to build a phylogenetic tree by the maximum likelihood method based on the Tamura-Nei model (44). The tree with the highest log likelihood was selected. All the positions containing gaps or missing data were eliminated. Initial trees for the heuristic search were obtained automatically by applying the Neighbor-Join and BioNJ algorithms to a matrix of pairwise distances estimated using the maximum composite likelihood approach and then by selecting the topology with a superior log-likelihood value.
Results
The anamnesis revealed that the Saint Bernard dog was imported from the United States as a puppy to Costa Rica, in 1980. The animal lived in the city of Heredia, Costa Rica and was never in contact with farm animals or mated. Upon arrival to the Veterinary Medicine School, the dog showed unwillingness to walk, general lethargy, refusal to eat, aspermia, fever, enlargement of the scrotum and testicles with local dermatitis and scrotal pain. The animal did not show any rashes, abdominal pain, visceral enlargement or local adenopathy. Platelets and leukocyte counts were normal. Pathological inspection showed bilateral enlargement of the epididymis and inflammation as well mild necrosis of both testes. Histopathological examination of testicular tissue revealed necrotizing foci and granulomatous inflammation. Since the serum of the animal showed positive agglutination in Rose Bengal test for brucellosis, it was not necessary to perform any other serological tests. The presumptive clinical diagnosis was orchiepididymitis due to brucellosis.
Serological diagnosis was confirmed by isolation of smooth Brucella sp. from testicular tissue after 1 week of culture in blood agar. The dog was treated orally with doxycycline (20 mg/Kg), three times a day for 14 days. Then streptomycin (11 mg/Kg) was administrated intramuscular every 12 h during 14 days. After treatment the dog showed improvement; however, the animal was not followed afterward.
Since the isolate displayed an atypical bacteriological profile (11), the strain was sent to the Station de Pathologie de la Reproduction, INRA, Centre de Tours-Nouzilly, France, for typing. The strain presented an atypical oxidative metabolic profile with particularly high levels for L-glutamic acid and L-asparagine utilization. The strain was coded as Brucella sp. BCCN84.3 and identified as an atypical B. melitensis biovar 2 (12). Moreover, Bruce-ladder did not distinguish between B. suis biotype 1 and Brucella sp. BCCN84.3 (Figure 1A). However, the Brucella sp. BCCN84.3 strain displayed a different Suis-ladder profile departing from B. suis and B. canis strains (Figure 1B).
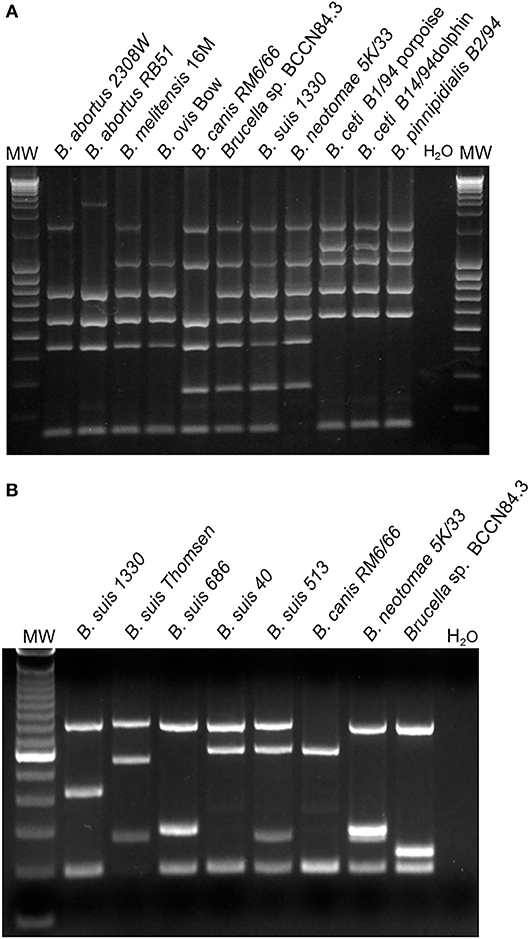
Figure 1. Typification of Brucella sp. BCCN84.3 by Bruce-ladder and Suis-Ladder. (A) Bruce-ladder analysis does not distinguish between B. suis species and BCCN84.3. (B) Suis-Ladder identified a characteristic pattern for Brucella sp. BCCN84.3, different from those of B. suis and B. canis.
Conventional phenotyping did not allow ascription to any of the currently accepted Brucella nominal species (Tables 1, 2). However, the Brucella sp. BCCN84.3 fatty acid methyl esters profile suggested a different taxonomical rank (Figure 2). Likewise, plus-minus real-time PCR analysis using DNA from Brucella sp. BCCN84.3 was positive for the Brucella genus and B. suis. HRM-PCR analysis using specific primers for B. canis (Figure 3A) or B. melitensis (Figure 3B) showed that the profile of the BCCN84.3 strain was unique as compared to classical Brucella species.
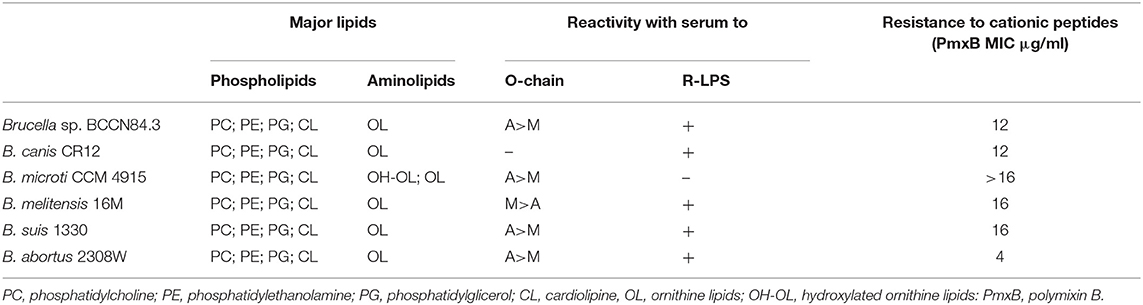
Table 2. Cell envelope characteristics of Brucella sp. BCCN84.3 and comparison with reference Brucella strains.
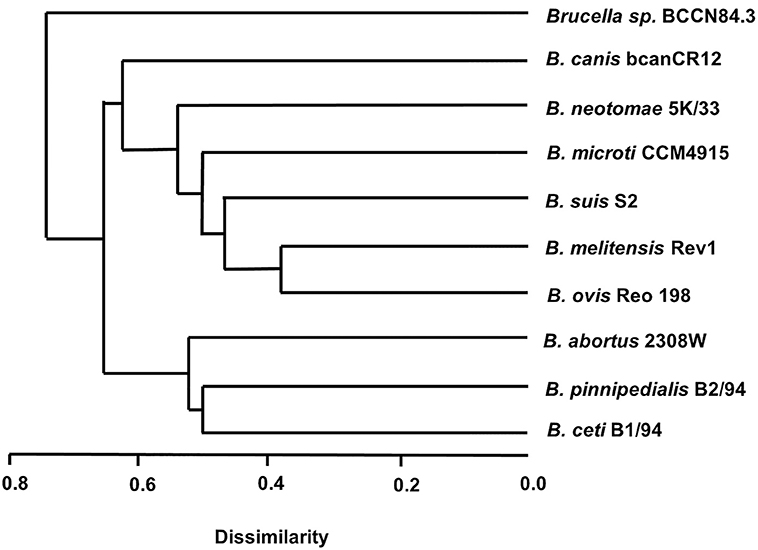
Figure 2. Dendrogram of the fatty acid methyl esters of different Brucella extracts. Notice that the Brucella sp. BCCN84.3 stands alone in relation to the classical species. For values of retention times of fatty acid methyl esters (see Supplementary File 2).
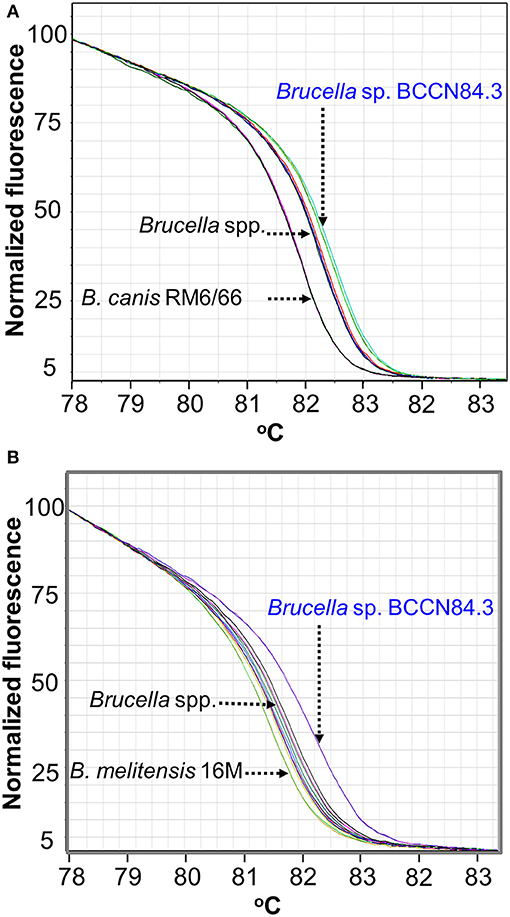
Figure 3. HRM-PCR analysis of Brucella sp. BCCN84.3. (A) HRM-PCR assay using primers designed for B. canis (A) or B. melitensis (B) (27) clearly show that Brucella sp. BCNN84.3 has an HRM profile different from other classical Brucella species.
Following previous experiments (22, 45, 46), no significant differences in bindings against Brucella sp. BCCN84.3 rough-LPS, smooth-LPS, Omp2b, Omp19, and Omp31 were detected by ELISA (Figure 4A). In contrast, when compared with other brucellae (46), a distinct profile against the Brucella sp. BCCN84.3 Omp25 was attained (Figure 4A). Mab A68/04B10/F05 against the Omp25 conformational epitope reacted with Brucella sp. BCCN84.3, the Mab A76/02C12/C11 (also directed against a conformational epitope, 43) reaction was negative. A slightly lower molecular weight of the Omp25 was identified in the Brucella sp. BCCN84.3, as compared to the B. canis and B. abortus counterparts (Figure 4B). This pattern agrees with the length of omp25 (BAW_10696 locus), which is slightly shorter than other omp25 genes of classical Brucella species.
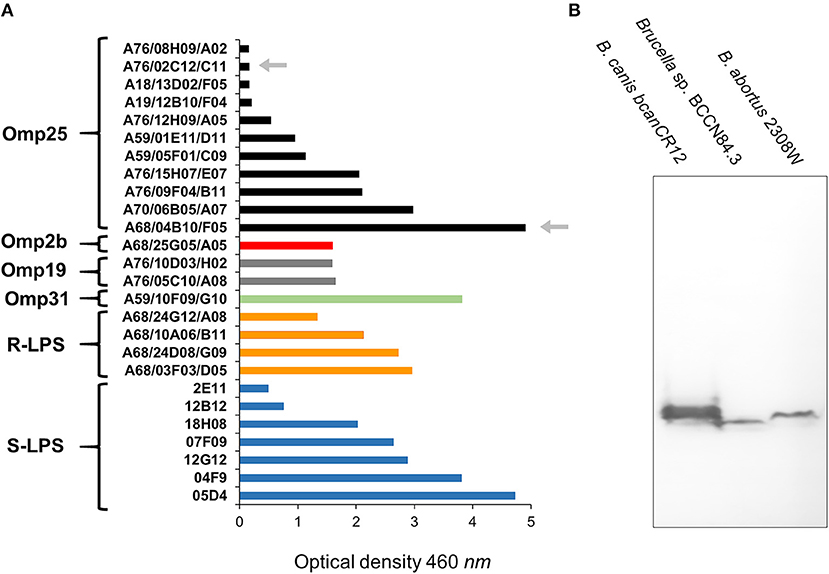
Figure 4. The binding intensity of a collection of Mabs against Brucella sp. BCCN84.3. (A) A collection Mabs against Brucella Omp31, Omp35, Omp2b, Omp19, smooth LPS (S-LPS), and rough-LPS (R-LPS) were tested by ELISA against sonicated Brucella sp. BCCN84.3 sonicated cells and the binding compared with B. abortus or B. melitensis cells. The arrows indicate differential reactivity of both Mabs against a conformational epitope of the Omp25 in comparison to B. abortus or B. melitensis. For details of the ELISA assay see Cloeckaert et al. (22) (B) WB with Mabs 68/04B10/F05 against Omp25, identifies a slightly lower molecular weight protein in Brucella sp. BCCN84.3 strain in relation to other classical Brucella species.
Phylogenetic analysis of the Brucella sp. BCCN84.3 porin sequences showed a separation in the omp2a and omp2b corresponding clusters (Figure 5). However, the Brucella sp. BCCN84.3 omp2a was somewhat closer to the omp2b cluster, due to a putative recombination event in a region close to the 5′, which is identical to the porin sequence of the latter (47).
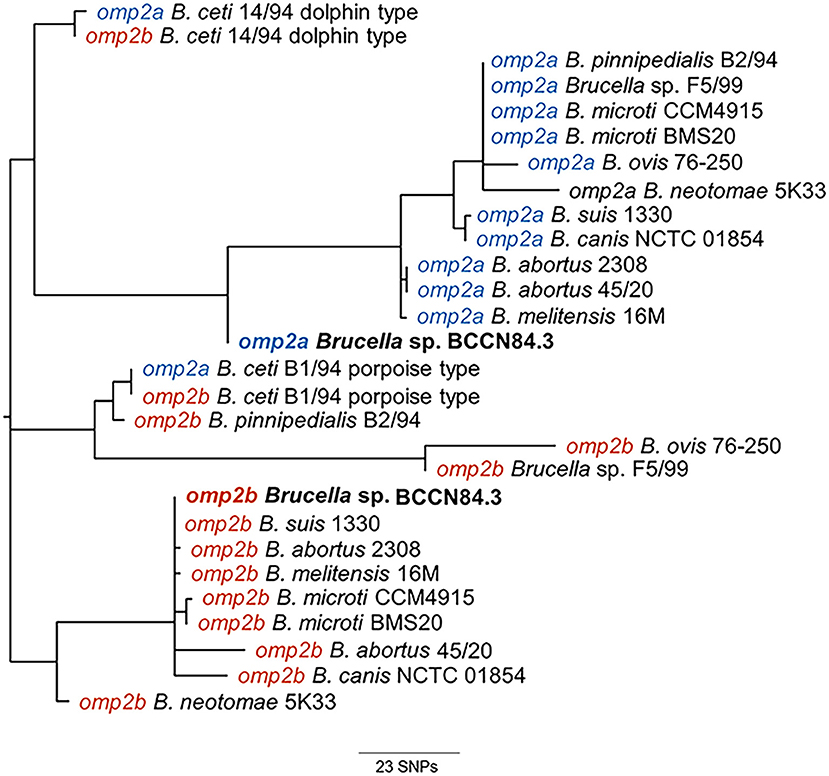
Figure 5. Phylogenetic tree from CLUSTAL Wallis aligned omp2a and omp2b nucleotide sequences of BCCN84.3 and other Brucella strains. The analysis reveals the separation in BCCN84.3 omp2a and omp2b clusters. The Brucella sp. BCCN84.3 omp2a is somewhat closer to the omp2b cluster; probably due to a recombination event in the 5′region, which is identical to the latter porin sequence.
The Brucella sp. BCCN84.3 formed a distinct branch in relation to other species, as revealed by the MLVA16 analysis (Figure 6). This result is in agreement with a previous analysis, using a somewhat different MLVA strategy (48). WGSA demonstrated that the overall genomic structure of the Brucella sp. BCCN84.3 isolate corresponds to a new species of classical brucellae, with a size of 3.26 Mb. Parallel sequencing of the strain conserved in the BCCN collection (named Brucella sp. BCCN84.3) confirmed the stability of the genome. When both WGS were compared, no deletions or insertions were found between the strains and, only three SNPs were detected at intergenic regions.
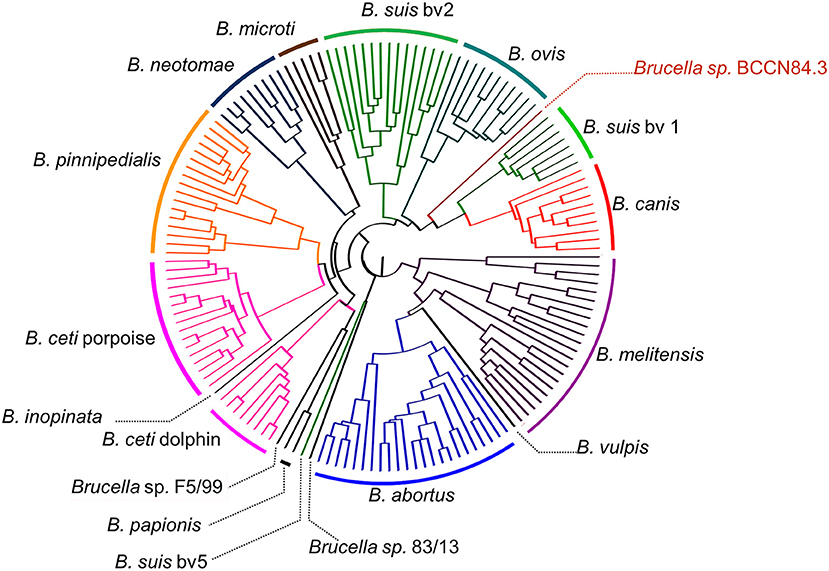
Figure 6. Dendrogram based on MLVA16 analysis of Brucella species and strains representatives. The Brucella sp. BCCN84.3 showed a MLVA16 profile different from that of the classical smooth Brucella species; consistent with a previous report using a different MLVA strategy (48). MLVA-NET for Brucella. MLVA web service, CNRS. http://microbesgenotyping.i2bc.paris-saclay.fr/ (accessed 21 December, 2017).
As other classical brucellae, Brucella sp. BCCN84.3 presents two chromosomes with no plasmids, no major recent recombination events (Figure 7) and a similar number of anomalous regions (Figure 8). The genes encoding for virulence factors such as smooth type LPS, VirB operon, Bac, cyclic glucans, flagellum-like, and BvrR/BvrS system are conserved (Supplementary Data Sheet 1). The B. canis genomic island GIFeGSH coding for iron uptake enzymes and parts of the glutathione pathway (49) is not present in the Brucella sp. BCCN84.3. Putative genes in loci BAW_10265 coding for the TIR domain-containing protein BtpA claimed to be a VirB effector of the type IV secretion system and to modulate microtube dynamics (50), and for putative integrases (BAW_10237; BAW_10274) are also absent. The manBOAg (BAW_10538) putatively involved in the synthesis of mannose of the LPS core (51) was 48 bp shorter than the B. melitensis (BMEI1396) and about the same size as B. ovis (BOV_0540) and B. abortus 2308W (BAW_10538) counterparts. The number of IS711 elements identified by southern blot ranged from 6 to 7. Due to the repetitive nature of the IS elements, determination of the exact number by WGSA on Illumina platforms was not possible.
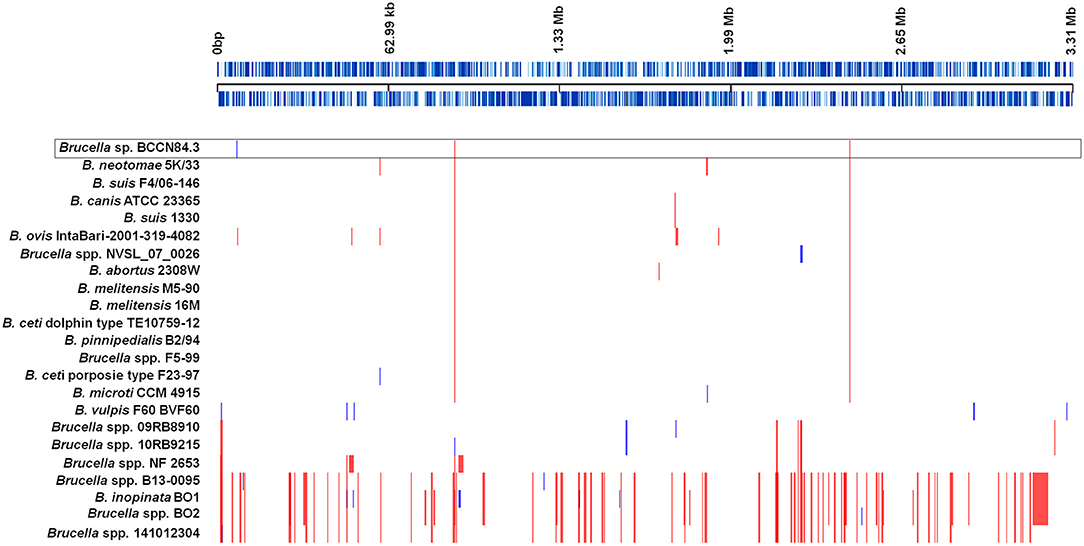
Figure 7. Recombination events in representative Brucella species. Each event is shown by a vertical block ordered along the genome. Upper black line represents the approximate coordinates in base pairs according to the reference B. suis 1330; each blue line represents a coding sequence in the reference. Red blocks are recombination events shared by more than two genomes included; blue blocks are unique. Classic Brucella species show few recombination regions; however, a higher number was detected in the non-classical clades. The Brucella sp. BCCN84.3 is highlighted by a box.
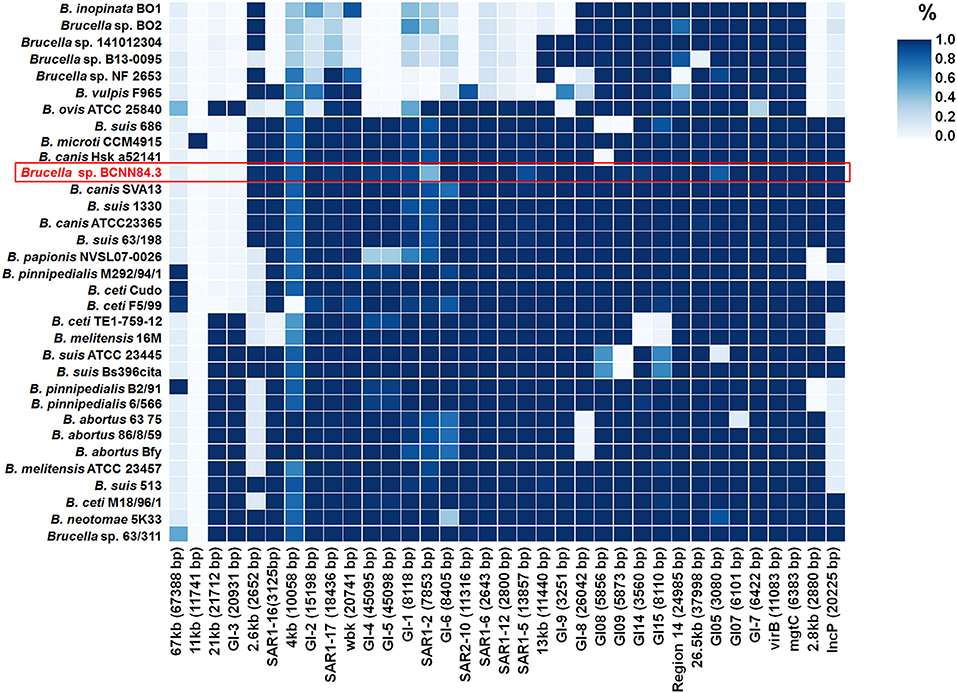
Figure 8. The presence and absence of anomalous regions or genomic islands in the Brucella genomes. The upper right color scale represents the percentage of the island present in each genome, where the darker blue color means that the whole region is present. Brucella sp. BCCN84.3 is highlighted by a red box.
A total of 205,055 SNPs were found among the Brucella genomes (Supplementary Data Sheet 1) and were used for phylogenetic analysis using O. anthropi and O. intermedium cluster as an outgroup. The general topology of the SNPs based tree was consistent with previous studies (1). Brucella sp. BCCN84.3 showed 7,281 polymorphic sites as compared to B. suis 1330, of those 5,911 were located in coding regions with a dN/dS ratio of 0.54 (p-value = 0.00). This shows a compact cluster harboring classical Brucella species and a more dispersed clade harboring the BO group (Figure 9). Within the classical cluster, Brucella sp. BCCN84.3 branches alone (Figure 9). This branching order does not fully agree with the classical MLVA16 dispersion. In silico identical matches of the 9 loci included in the MLST-9 profile were not able to classify the B. abortus sp. BCCN84.3 into a sequence type. The-21 loci MLST profile did not provide more information, 20 loci showed identical match, except for the ddlA locus, that partially matched to the allele 26, so no further typing was achieved by this scheme.
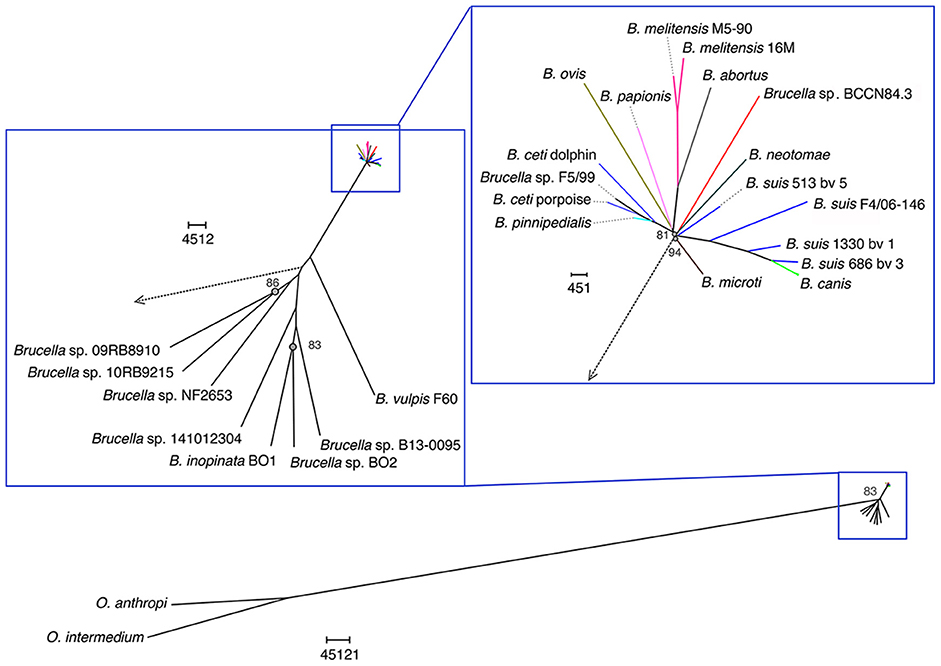
Figure 9. Phylogenetic relationship of Brucella sp. BCCN84.3 with other Brucella species and Ochrobactrum sp. Brucella BCCN84.3 results are colored in red. Branching points with bootstrap values lower than 100 are indicated by a gray dot and small gray font next to it. Segments of the tree were magnified by the use of Dendroscope version 3.5.8 in order to increase resolution; the adapted scale is indicated next to each magnified region. A blue square highlights all Brucella species; the classic species are within the upright square, which includes the Brucella sp. BCCN84.3.
Discussion
Canine brucellosis, caused by B. canis, is difficult to diagnose by serological assays due to the extensive cross-reaction of antigens with smooth brucellae (52, 53). The unambiguous diagnosis of B. canis infections is just carried out after the isolation and identification of the bacterium (14) or molecular typing (26, 54). In contrast, when positive serological reactions against smooth brucellae arise in dogs presenting clinical signs of brucellosis, the presumptive diagnosis seems straightforward and commonly attributed to B. melitensis, B. abortus or B. suis (55–59). However, a detailed identification of the smooth Brucella strains isolated from dogs is seldom performed.
We were unable to trace the source of the Saint Bernard dog infection. The dog was imported as a puppy from the United States to Costa Rica. Whether the infection remained latent or it was acquired de novo in Costa Rica, is unknown. This is not trivial since there are several reports describing “atypical B. suis strains” isolated from dogs in different countries, including in the United States. For instance, in the same year as the Brucella sp. BCCN84.3 was isolated, a collection of “atypical B. suis” strains, which were also unusually resistant to fuchsin, were described in various countries (60). A new B. suis biovar was suggested for these atypical strains, some of them isolated from dogs and humans. Likewise, in the same year, an “atypical B. suis biotype 1” was also isolated in Brazil, from the testes of a dog suffering orchitis (61). In a survey carried out in 674 dogs in Georgia, United States, it was established that nine dogs presented positive serological reactions against smooth Brucella antigens (58). Brucella organisms were isolated from the canine testes displaying necrotizing, suppurative epididymitis and orchitis. After conventional biochemical assays and 16SrRNA sequencing, the bacterial strains were assigned to the “B. suis” group. Unfortunately, these latter isolates were destroyed, precluding any further detailed characterization. More recently, several dogs were reported to be infected with “B. suis” in Australia; even though not all dogs were in contact with wild boars (62). In all these studies the bacterial strains were identified by conventional methods or rRNA PCR analysis; though, none of these methods are capable to unambiguously discern among the various Brucella classical species (63). The initial bacteriological characterization of the Brucella sp. BCCN84.3 was also misleading. It was only after genomic analyses that it became clear that the strain belonged to a new taxonomic entity.
From the genomic perspective, the Brucella sp. BCCN84.3 is a new taxonomical entity, since it departs phylogenetically from other strains, being the closest relative B. neotomae but distinct from this species. The total number of SNPs between Brucella sp. BCCN84.3 and B. suis 1330 (7281 SNPs) is bigger than the number between B. suis and B. abortus (6790 SNPs), two well-recognized species. It is also closer to the number that separates B. ovis st. IntaBari-2001-319-4082 from B. suis st. 1330 (7499 SNPs). Considering the zoonotic potential of Brucella species, a correct identification by molecular methods is becoming mandatory. Moreover, in the light of distinct host preferences (64) and differences in WGSA (1), the various B. suis strains need to be taxonomically reevaluated, since they seem to represent a collection of different Brucella species. In particular B. suis biovar 5 isolated from rodents which branches closer to B. microti (4) and the two clusters composed, on one hand by B. suis biovars 2 and 3, and on the other hand by B. suis biovars 1 and 4 (4). The problem with this latter cluster is the close phylogenetic relationship of B. canis with B. suis biovar 4 (4), which requires an idiosyncratic solution. The correct classification of Brucella species is particularly relevant in countries like Costa Rica, in which B. melitensis and B. suis are absent (65), or in countries in which bovine, caprine, and swine brucellosis have been eradicated from livestock, but that still have pathogenic Brucella infecting wildlife (66). In this regard, the differential diagnosis of the various Brucella species and strains is a requirement for taking the infection source.
Brucella sp. BCCN84.3 is a species nova. More isolates of this bacterium are necessary and additional epidemiological and biological information needs to be collected before assigning the corresponding taxonomical species name. In spite of this, and taking into account the difficulties surrounding the debate on the Brucella species concept (5, 7), it is mandatory to describe the extant taxonomical entities in order to understand the dispersion and evolution of these important pathogens.
The fact that Brucella sp. BCCN84.3. has the ability to invade the reproductive tract of dogs, may favor the venereal transmission of this bacterium, as it the case of B. canis which rapidly disperse in kennel facilities. We do not know the zoonotic potential of Brucella sp. BCCN84.3. However, it is a smooth strain that possesses all the virulent machinery for being pathogenic for humans and other animals. Moreover, the fact that it was isolated from a domestic dog increases the zoonotic risk.
Data Availability
Publicly available datasets were analyzed in this study. This data can be found here: https://www.ebi.ac.uk/ena/data/view/ERS568777.
Ethics Statement
This is a clinical case. The dog was brought by its owner for therapy. Following the regular arrangements for hospitalization, the owner was informed for all procedures and clinical studies and gave her written consent. All protocols and actions undertaken to diagnose the disease were under the Veterinary Hospital guidance established in 1980. The protocols used in 1984, were those approved by the Ley General de Salud N° 5395, and Disposiciones sobre Matrícula y Vacunación de Perros N° 2391.
Author Contributions
EM, CG-V, and AC conceived the study. EM, CG-V, IM, NT, and JB obtained funding. EC and EM performed the isolation of the bacterium. CC-D performed fatty acid analysis. CG-V, MS-E, KB, AC, NR-V, MZ, EV-R, and MG performed genomics analyses. RC-Á, BA-A, and IM performed the LPS and lipid characterization. JB, EC, PM, and IM performed the bacteriological analysis. EM, CG-V, MS-E, AC, NR-V, NT, MG, and CC-D performed data interpretation. EM and CG-V wrote the paper. All authors read and approved the manuscript content.
Funding
This work was supported by FEES-CONARE, Costa Rica; Fondo Institucional de Desarrollo Académico (FIDA), Universidad Nacional; Wellcome Trust; CITA-INIA, Spain (project Bru-Epidia 291815-FP7/ERANET/ANIHWA); MINECO (AGL2014-58795-CA), and Aragon Government (Consolidated Group A14). Authors from the Sanger Institute were supported by Wellcome Trust (098051). NR-V was partially sponsored by a scholarship from the University of Costa Rica. KB was founded by a Wellcome Trust Postdoctoral Training Fellowship for Clinicians (106690/Z/14/Z). MS-E was granted with a fellowship from SEP, Universidad de Costa Rica.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
We would like to thank Gordon Dougan (Sanger Institute, UK) and Esteban Chaves (CIET, Universidad de Costa Rica) for their helpful discussions. The genetic resources mentioned in this paper were accessed according to the Biodiversity Law #7788 and the Convention on Biological Diversity of Costa Rica, under the terms of respect to an equal and fair distribution of benefits among those who provided such resources under CONAGEBIO Costa Rica permit # R-028-203-OT.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fvets.2019.00175/full#supplementary-material
Supplementary Table S1. Control and reference Brucella species and strains used for typing.
Supplementary Data Sheet 1. (i) Excel files displaying the Brucella strains used in MLVA16; (ii) the Brucella sp. BCCN84.3 WGSA statistics, the metadata of WGSA included in the phylogenetic reconstruction; (iii) the SNPs summary in comparison to B. suis 1330 as reference, (iv) the mutations found in Brucella sp. BCCN84.3 in genes related to virulence as compared to the indicated locus/gene name; (v) the genomic islands the comprises metadata of WGSA included in the analysis and genomic islands reference information; (vi) the metadata of sequences included in the phylogenetic reconstruction using omp2a and omp2b genes, and; (vii) the retention time of the fatty acid methyl ester to construct dendrogram of Figure 2.
References
1. Soler-Lloréns PF, Quance CR, Lawhon SD, Stuber TP, Edwards JF, Ficht TA, et al. A Brucella spp. Isolate from a Pac-Man Frog (Ceratophrys ornata) reveals characteristics departing from classical brucellae. Front Cell Infect Microbiol. (2016) 6:116. doi: 10.3389/fcimb.2016.00116
2. Scholz HC, Mühldorfer K, Shilton C, Benedict S, Whatmore AM, Blom J, et al. The change of a medically important genus: worldwide occurrence of genetically diverse novel Brucella species in exotic frogs. PLoS ONE. (2016) 11:e0168872. doi: 10.1371/journal.pone.0168872
3. Moreno E, Moriyón I. The genus Brucella. In: Dworkin M, Falkow SR, Rosenber E, Schleifer KH, Stackebrant E, editors. The Prokaryotes, Part 1, section 3.1. New York, NY: Springer-Verlag (2006). p. 315–456.
4. Suárez-Esquivel M, Ruiz-Villalobos N, Jiménez-Rojas C, Barquero-Calvo E, Chacón-Díaz C, Víquez-Ruiz E, et al. Brucella neotomae infection in humans, Costa Rica. J Emerg Infect Dis. (2017) 23:997–1000. doi: 10.3201/eid2306.162018
5. Whatmore AM. Current understanding of the genetic diversity of Brucella, an expanding genus of zoonotic pathogens. Infect Genet Evol. (2009) 9:1168–84. doi: 10.1016/j.meegid.2009.07.001
6. Zygmunt MS, Jacques I, Bernardet N, Cloeckaert A. A Lipopolysaccharide heterogeneity in the atypical group of novel emerging Brucella species. Clin Vaccine Immunol. (2012) 19:1370–3. doi: 10.1128/CVI.00300-12
7. Moreno E, Cloeckaert A, Moriyón I. Brucella evolution and taxonomy. Vet Microbiol. (2002) 90:209–27. doi: 10.1016/S0378-1135(02)00210-9
8. Meyer ME. Inter- and intra-strain variants in the genus Brucella. Dev Biol Stand. (1984) 56:73–83.
9. Zheludkov MM, Tsirelson LE. Reservoirs of Brucella infection in nature. Biol Bull. (2010) 37:709–15. doi: 10.1134/S106235901007006X
10. Villalobos-Vindas JM, Amuy E, Barquero-Calvo E, Rojas N, Chacón-Díaz C, Chaves-Olarte E, et al. Brucellosis caused by the wood rat pathogen Brucella neotomae: two case reports. J Med Case Rep. (2017) 11:352. doi: 10.1186/s13256-017-1496-8
11. Sequeira A, Campos E, Mendoza L, San-Román MA, Moreno E. Identificación de especies y biotipos de Brucella aisladas en Costa Rica. Turrialba. (1984) 34:525–6.
12. Verger JM, Grimont F, Grimont PAD, Grayon M. Brucella, a monospecific genus as shown by deoxyribonucleic acid hybridization. Int J Syst Bacteriol. (1985) 35:292–5. doi: 10.1099/00207713-35-3-292
13. Alton GG, Jones LM, Pietz DE. Laboratory techniques in brucellosis. Monogr Ser World Health Organ. (1975) 55:1–163.
14. Carmichael LE, Kenney RM. Canine brucellosis: the clinical disease, pathogenesis, and immune response. J Am Vet Med Assoc. (1970) 156:1726–34.
15. Bligh EG, Dyer WJ. A rapid method of total lipid extraction and purification. Can J Biochem Physiol. (1959) 37:911–7. doi: 10.1139/y59-099
16. Palacios-Chaves L, Zúñiga-Ripa A, Gutiérrez A, Gil-Ramírez Y, Conde-Álvarez R, Moriyón I, et al. Identification and functional analysis of the cyclopropane fatty acid synthase of Brucella abortus. Microbiology. (2012) 158(Pt 4):1037–44. doi: 10.1099/mic.0.055897-0
17. Isidoro-Ayza M, Ruiz-Villalobos N, Pérez L, Guzmán-Verri C, Muñoz PM, Alegre F, et al. Brucella ceti infection in dolphins from the Western Mediterranean sea. BMC Vet Res. (2014) 10:206. doi: 10.1186/s12917-014-0206-7
18. Garin-Bastuji B, Bowden RA, Dubray G, Limet JN. Sodium dodecyl sulfate-polyacrylamide gel electrophoresis and immunoblotting analysis of smooth-ipopolysaccharide heterogeneity among Brucella biovars related to A and M specificities. Clin Microbiol. (1990) 28:2169–74.
19. Tsai CM, Frasch CE. A sensitive silver stain for detecting lipopolysaccharides in polyacrylamide gels. Anal Biochem. (1982) 119:115–9. doi: 10.1016/0003-2697(82)90673-X
20. Aragón V, Díaz R, Moreno E, Moriyón I. Characterization of Brucella abortus and Brucella melitensis native haptens as outer membrane O-type polysaccharides independent from the smooth lipopolysaccharide. J Bacteriol. (1996) 178:1070–9. doi: 10.1128/jb.178.4.1070-1079.1996
21. Monreal D, Grilló MJ, González D, Marín CM, De Miguel MJ, López-Goñi I, et al. Characterization of Brucella abortus O-polysaccharide and core lipopolysaccharide mutants and demonstration that a complete core is required for rough vaccines to be efficient against Brucella abortus and Brucella ovis in the mouse model. Infect Immun. (2003) 71:3261–71. doi: 10.1128/IAI.71.6.3261-3271.2003
22. Cloeckaert A, Verger JM, Grayon M, Vizcaíno N. Molecular and immunological characterization of the major outer membrane proteins of Brucella. FEMS Microbiol Lett. (1996) 145:1–8. doi: 10.1016/0378-1097(96)00373-4
23. Ramírez P, Bonilla JA, Moreno E, León P. Electrophoretic transfer of viral proteins to nitrocellulose sheets and detection with peroxidase-bound lectins and protein A. J Immunol Methods. (1983) 62:15–22. doi: 10.1016/0022-1759(83)90105-9
24. Freer E, Pizarro-Cerdá J, Weintraub A, Bengoechea JA, Moriyón I, Hultenby K, et al. The outer membrane of Brucella ovis shows increased permeability to hydrophobic probes and is more susceptible to cationic peptides than are the outer membranes of mutant rough Brucella abortus strains. Infect Immun. (1999) 67:6181–6.
25. López-Goñi I, García-Yoldi D, Marín CM, de Miguel MJ, Muñoz PM, Blasco JM, et al. Evaluation of a multiplex PCR assay (Bruce-ladder) or molecular typing of all Brucella species, including the vaccine strains. J Clin Microbiol. (2008) 46:3484–7.
26. López-Goñi I, García-Yoldi D, Marín CM, de Miguel MJ, Barquero-Calvo E, Guzmán-Verri C, et al. New bruce-ladder multiplex PCR assay for the biovar typing of Brucella suis and the discrimination of Brucella suis and Brucella canis. Vet Microbiol. (2011) 154:152–5. doi: 10.1016/j.vetmic.2011.06.035
27. Winchell JM, Wolff BJ, Tiller R, Bowen MD, Hoffmaster AR. Rapid identification and discrimination of Brucella isolates by use of real-time PCR and high-resolution melt analysis. J Clin Microbiol. (2010) 48:697–702. doi: 10.1128/JCM.02021-09
28. Maquart M, Le Flèche P, Foster G, Tryland M, Ramisse F, Djønne B, et al. MLVA-16 typing of 295 marine mammal Brucella isolates from different animal and geographic origins identifies 7 major groups within Brucella ceti and Brucella pinnipedialis. BMC Microbiol. (2009) 9:145. doi: 10.1186/1471-2180-9-145
29. Grissa I, Bouchon P, Pourcel C, Vergnaud G. On-line resources for bacterial microevolution studies using MLVA or CRISPR typing. Biochimie. (2008) 90:660–8. doi: 10.1016/j.biochi.2007.07.014
30. Quail MA, Kozarewa I, Smith F, Scally A, Stephens PJ, Durbin RR, et al. A large genome centre's improvements to the Illumina sequencing system. Nat Method. (2009) 5:1005–10. doi: 10.1038/nmeth.1270
31. Quail MA, Otto TD, Gu Y, Harris SR, Skelly TF, McQuillan JA, et al. Optimal enzymes for amplifying sequencing libraries. Nat Methods. (2011) 9:10–1. doi: 10.1038/nmeth.1814
32. Zerbino DR, Birney E. Velvet: algorithms for de novo short read assembly using de Bruijn graphs. Genome Res. (2008) 18:821–9. doi: 10.1101/gr.074492.107
33. Seemann T. Prokka: rapid prokaryotic genome annotation. Bioinformatics. (2014) 30:2068–9. doi: 10.1093/bioinformatics/btu153
34. Whatmore AM, Koylass MS, Muchowski J, Edwards-Smallbone J, Gopaul KK, Perrett LL. Extended multilocus sequence analysis to describe the global population structure of the genus Brucella: phylogeography and relationship to biovars. Front Microbiol. (2016) 7:2049. doi: 10.3389/fmicb.2016.02049
35. Li H, Handsaker B, Wysoker A, Fennell T, Ruan J, Homer N, et al. The sequence alignment/map format and SAMtools. Bioinformatics. (2009) 25:2078–9. doi: 10.1093/bioinformatics/btp352
36. Page AJ, Taylor B, Delaney AJ, Soares J, Seemann T, Keane JA, et al. SNP-sites: rapid efficient extraction of SNPs from multi- FASTA alignments. Microb Genomics. (2016) 2:e000056. doi: 10.1099/mgen.0.000056
37. Stamatakis A. RAxML-VI-HPC: maximum likelihood-based phylogenetic analyses withthousands of taxa and mixed models. Bioinformatics. (2006) 22:2688–90. doi: 10.1093/bioinformatics/btl446
38. Carver TJ, Rutherford KM, Berriman M, Rajandream MA, Barrell BG, Parkhill J. ACT: the Artemis Comparison Tool. Bioinformatics. (2005) 21:3422–3. doi: 10.1093/bioinformatics/bti553
39. Croucher NJ, Page AJ, Connor TR, Delaney AJ, Keane JA, Bentley SD, et al. Rapid phylogenetic analysis of large samples of recombinant bacterial whole genome sequences using Gubbins. Nucleic Acids Res. (2014) 43:e15. doi: 10.1093/nar/gku1196
40. Hadfield J, Croucher NJ, Goater RJ, Abudahab K, Aanensen DM, Harris SR. Phandango: an interactive viewer for bacterial population genomics. Bioinformatics. (2017) 34:292–3. doi: 10.1093/bioinformatics/btx610
41. Cloeckaert A, Bernardet N, Koylass MS, Whatmore AM, Zygmunt MS. Novel IS711 chromosomal location useful for identification of marine mammal Brucella genotype ST27, which is associated with zoonotic infection. J Clin Microbiol. (2011) 49:3954–9. doi: 10.1128/JCM.05238-11
42. FosterG, Osterman BS, Godfroid J, Jacques I, Cloeckaert A. Brucella ceti sp. nov. and Brucella pinnipedialis sp. nov. for Brucella strains with cetaceans and seals as their preferred hosts. Int J Syst Evol Microbiol. (2007) 57(Pt 11):2688–93. doi: 10.1099/ijs.0.65269-0
43. Kumar S, Stecher G, Tamura K. MEGA7: molecular evolutionary genetics analysis version 7.0 for bigger datasets. Mol Biol Evol. (2016) 33:1870–74. doi: 10.1093/molbev/msw054
44. Tamura K, Nei M. Estimation of the number of nucleotide substitutions in the control region of mitochondrial DNA in humans and chimpanzees. Mol Biol Evol. (1993) 10:512–26.
45. Cloeckaert A, Jacques I, Bowden RA, Dubray G, Limet JN. Monoclonal antibodies to Brucella rough lipopolysaccharide: characterization and evaluation of their protective effect against B. abortus. Res Microbiol. (1993) 144:475–844. doi: 10.1016/0923-2508(93)90055-7
46. Cloeckaert A, Verger JM, Grayon M, Zygmunt MS, Grépinet O. Nucleotide sequence and expression of the gene encoding the major 25-kilodalton outer membrane protein of Brucella ovis: evidence for antigenic shift, compared with other Brucella species, due to a deletion in the gene. Infect Immun. (1996) 64:2047–55.
47. Cloeckaert A, Vizcaíno N, Paquet JY, Bowden RA, Elzer PH. Major outer membrane proteins of Brucella spp.: past, present and future. Vet Microbiol. (2002) 90:229–34. doi: 10.1016/S0378-1135(02)00211-0
48. Le-Flèche P, Jacques I, Grayon M, Al-Dahouk S, Bouchon P, Denoeud F, et al. Evaluation and selection of tandem repeat loci for a Brucella MLVA typing assay. BMC Microbiol. (2006) 6:9. doi: 10.1186/1471-2180-6-9
49. Wahab T, Skarp A, Båverud V, Kaden R. GIFeGSH: a new genomic island might explain the differences in Brucella virulence. Open J Anim Sci. (2017) 7:141–8. doi: 10.4236/ojas.2017.72012
50. Felix C, Kaplan-Türköz B, Ranaldi S, Koelblen T, Terradot L, O'Callaghan D, et al. The Brucella TIR domain containing proteins BtpA and BtpB have a structural WxxxE motif important for protection against microtubule depolymerisation. Cell Commun Signal. (2014) 12:53. doi: 10.1186/s12964-014-0053-y
51. González D, Grilló MJ, De Miguel MJ, Ali T, Arce-Gorvel V, Delrue RM, et al. Brucellosis vaccines: assessment of Brucella melitensis lipopolysaccharide rough mutants defective in core and O-polysaccharide synthesis and export. PLoS ONE. (2008) 3:e2760. doi: 10.1371/journal.pone.0002760
52. Diaz R, Jones LM, Wilson JB. Antigenic relationship of the gram-negative organism causing canine abortion to smooth and rough brucellae. J Bacteriol. (1968) 95:618–24.
53. Moreno E, Jones LM, Berman DT. Immunochemical characterization of rough Brucella lipopolysaccharides. Infect Immun. (1984) 43:779–82.
54. Corrente M, Franchini D, Decaro N, Greco G, D'Abramo M, Greco MF, et al. Detection of Brucella canis in a dog in Italy. New Microbiol. (2010) 33:337–41.
55. Bicknell SR, Bell RA. Brucella abortus in the bitch: subclinical infection ssociated with urinary excretion. J Hyg. (1979) 82:249–54. doi: 10.1017/S0022172400025663
57. Islamov RZ. Transmission of Brucella melitensis to the offspring of dogs. Veterinarya. (1973) 12:62.
58. Ramamoorthy S, Woldemeskel M, Ligett A, Snider R, Cobb R, Rajeev S. Brucella suis infection in dogs, Georgia, USA. Emerg Infect Dis. (2011) 17:2386–7. doi: 10.3201/eid1712.111127
59. Wareth G, Melzer F, El-Diasty M, Schmoock G, Elbauomy E, Abdel-Hamid N, et al. Isolation of Brucella abortus from a dog and a cat confirms their iological role in re-emergence and dissemination of bovine brucellosis on dairy farms. Transbound Emerg Dis. (2016). 64:e27–30. doi: 10.1111/tbed.12535
60. Corbel MJ, Thomas EL, Garcia-Carillo C. Taxonomic studies on some atypical trains of Brucella suis. Br Vet J. (1984) 140:34–43. doi: 10.1016/0007-1935(84)90055-1
61. Correa WM, Correa CNM, Iamaguti P. Canine brucellosis caused by Brucella suis iotype 1 atypical. Arq Bras Med Vet Zootec. (1984) 36:397–406.
62. Mor SM, Wiethoelter AK, Lee A, Moloney B, James DR, Malik R. Emergence of Brucella suis in dogs in New South Wales, Australia: clinical findings and implications or zoonotic transmission. BMC Vet Res. (2016) 12:199. doi: 10.1186/s12917-016-0835-0
63. Moreno E. Genome evolution within the alpha Proteobacteria: why do some acteria not possess plasmids and others exhibit more than one different chromosome? FEMS Microbiol Rev. (1998) 22:255–75. doi: 10.1016/S0168-6445(98)00016-3
64. Alton GG. Brucella suis. in: Nielsen K, Duncan B,editiors. Animal Brucellosis. Boca Raton. FL: CRC Press, Inc (1990). pp. 244–422.
65. Hernández-Mora G, Bonilla-Montoya R, Barrantes-Granados O, Esquivel-Suárez A, Montero-Caballero D, González-Barrientos R, et al. Brucellosis in mammals of Costa Rica: an epidemiological survey. PLoS ONE. (2017) 12:e0182644. doi: 10.1371/journal.pone.0182644
Keywords: Brucella, Brucella melitensis, Brucella suis, Brucella canis, brucellosis, dog, species, epididymitis
Citation: Guzmán-Verri C, Suárez-Esquivel M, Ruíz-Villalobos N, Zygmunt MS, Gonnet M, Campos E, Víquez-Ruiz E, Chacón-Díaz C, Aragón-Aranda B, Conde-Álvarez R, Moriyón I, Blasco JM, Muñoz PM, Baker KS, Thomson NR, Cloeckaert A and Moreno E (2019) Genetic and Phenotypic Characterization of the Etiological Agent of Canine Orchiepididymitis Smooth Brucella sp. BCCN84.3. Front. Vet. Sci. 6:175. doi: 10.3389/fvets.2019.00175
Received: 23 February 2019; Accepted: 20 May 2019;
Published: 07 June 2019.
Edited by:
Armanda Bastos, University of Pretoria, South AfricaReviewed by:
Sidharath Dev Thakur, Chaudhary Sarwan Kumar Himachal Pradesh Krishi Vishvavidyalaya, IndiaJacques Xavier Godfroid, UiT The Arctic University of Norway, Norway
Copyright © 2019 Guzmán-Verri, Suárez-Esquivel, Ruíz-Villalobos, Zygmunt, Gonnet, Campos, Víquez-Ruiz, Chacón-Díaz, Aragón-Aranda, Conde-Álvarez, Moriyón, Blasco, Muñoz, Baker, Thomson, Cloeckaert and Moreno. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Edgardo Moreno, emoreno@racsa.co.cr
 Caterina Guzmán-Verri
Caterina Guzmán-Verri Marcela Suárez-Esquivel
Marcela Suárez-Esquivel Nazareth Ruíz-Villalobos1
Nazareth Ruíz-Villalobos1  Michel S. Zygmunt
Michel S. Zygmunt Mathieu Gonnet
Mathieu Gonnet Eunice Víquez-Ruiz
Eunice Víquez-Ruiz Raquel Conde-Álvarez
Raquel Conde-Álvarez Ignacio Moriyón
Ignacio Moriyón Pilar M. Muñoz
Pilar M. Muñoz Axel Cloeckaert
Axel Cloeckaert Edgardo Moreno
Edgardo Moreno