Synbiotic Effects of Enzyme and Probiotics on Intestinal Health and Growth of Newly Weaned Pigs Challenged With Enterotoxigenic F18+ Escherichia coli
- 1Department of Animal Science, North Carolina State University, Raleigh, NC, United States
- 2BioResource International, Inc., Durham, NC, United States
This study aimed to investigate the effect of dietary supplementation with xylanase and probiotics on growth performance and intestinal health of nursery pigs challenged with enterotoxigenic Escherichia coli (ETEC). Sixty-four newly weaned pigs (32 barrows and 32 gilts with 7.9 ± 0.4 kg BW) were allotted in a randomized complete block design (2 × 2 factorial). Two factors were ETEC challenge (oral inoculation of saline solution or E. coli F18+ at 6 × 109 CFU) and synbiotics (none or a combination of xylanase 10,000 XU/kg and Bacillus sp. 2 × 108 CFU/kg). All pigs were fed experimental diets following NRC (2012) in two phases (P1 for 10 d and P2 for 11 d). The ETEC was orally inoculated on d 7 after weaning. Feed intake and BW were measured on d 7, 10, 15, and 20. On d 20, pigs were euthanized to collect samples to measure gut health parameters and microbiome. Synbiotics increased (P < 0.05) ADG in phase 1 and ETEC reduced (P < 0.05) ADG and G:F in the post-challenge period. ETEC increased (P < 0.05) the fecal score of pigs from d 7 to 13; however, synbiotics reduced (P < 0.05) it at d 9 and 11 in challenged pigs. ETEC increased (P < 0.05) mucosal MDA, IL-6, Ki-67+, and crypt depth, whereas synbiotics tended to reduce TNFα (P = 0.093), protein carbonyl (P = 0.065), and IL-6 (P = 0.064); reduced (P < 0.05) crypt depth and Ki-67+; and increased (P < 0.05) villus height. ETEC reduced (P < 0.05) the relative abundance of Bacteroidetes and Firmicutes and increased (P < 0.05) the relative abundance of Proteobacteria. In conclusion, ETEC challenge reduced growth performance by affecting microbiome, immune response, and oxidative stress in the jejunum. Synbiotics enhanced growth performance by reducing diarrhea, immune response, and oxidative stress in the jejunum.
Introduction
Weaning is a challenging period for nursery pigs especially with their immune and intestinal functions resulting in reduced growth performance (1). During this period, pigs experience environmental, immunological, psychological, and nutritional challenges (2–4). Consequently, pigs at weaning are highly susceptible to pathogenic microorganism, such as enterotoxigenic Escherichia coli (ETEC) causing enteric diseases (5, 6). Moreover, the increasing pressure to ban the use of antibiotics as growth promoter (AGP) around the world due to the concern about microbial resistance has been a big challenge to the swine industry and researchers to maintain the gut health and performance of pigs (7–9).
Among these, newly weaned pigs have a low capacity to digest nutrients from plant-based feed because of the immaturity of the gastrointestinal tract (GIT), the different nutritional composition, compared with milk, and the anti-nutritional content that can damage the intestinal epithelial layers (10). Typical plant-based feeds contains ~2.3–3.8% of xylans, the main non-starch polysaccharides (NSP) (11, 12) increasing digesta viscosity, altering intestinal morphology, and reducing nutrient digestibility (13–16), which can induce a propitious environment for the growing of harmful bacteria, changing the gut associate microbiota in newly weaned pigs (17).
Nutritionally, numerous strategies have been attempted to eliminate or mitigate the effect of these challenges and produce healthy animals. Exogenous enzymes, such as xylanase, have been successfully used to hydrolyze the β-1,4 backbone of xylan, releasing xylan oligosaccharides (XOS) and, consequently, reducing the NSP content and the viscosity of digesta, increasing the digestibility of nutrients (14, 18, 19). Probiotics, such as bacteria from the genus Bacillus, are being largely used as an alternative to promote health and performance in the livestock industry. The genus Bacillus is well-known for its ability to form spores, produce antimicrobial compounds, and produce exogenous enzymes that are related to the ability to utilize different carbohydrate sources including those derived from plants, such as XOS (5, 20).
It is hypothesized that xylanase combined with Bacillus sp. as a synbiotic enhances growth performance of newly weaned pigs challenged with ETEC by enhancing the gut health and modulating the microbiome in the intestine by altering digesta viscosity. Thus, this study aimed to evaluate the supplemental effects of synbiotics on gut health and growth of newly weaned pigs challenged with ETEC.
Materials and Methods
Animals, Experimental Design, Additives, and Diets
The experimental procedures used in this study were reviewed and approved by the Institutional Animal Care and Use Committee at North Carolina State University following the North Carolina State Animal Care and Use Procedures (REG 10.10.01).
Sixty four newly weaned pigs (32 barrows and 32 gilts) at 21 d of age, with an initial body weight at 7.9 ± 0.4 kg, were allotted in a randomized complete block design in a 2 × 2 factorial arrangement, with ETEC challenge (oral inoculation of saline solution or E. coli F18+ at 6 × 109 CFU) and synbiotics (none and a combination of xylanase 10,000 XU/kg and Bacillus sp. 2 × 108 CFU/kg) as two factors. Pigs (PIC 337 × Camborough 22) were purchased from a commercial farm in North Carolina, USA. Sows and piglets used in this study were not vaccinated against E. coli. The symbiotic used in this study was EnzaPro obtained from BioResource International Inc. (Durham, NC). Each factor had 16 pens (n=16; eight pens with barrows and eight pens with gilts; and four body weight blocks within sex) and pigs were housed individually in a pen.
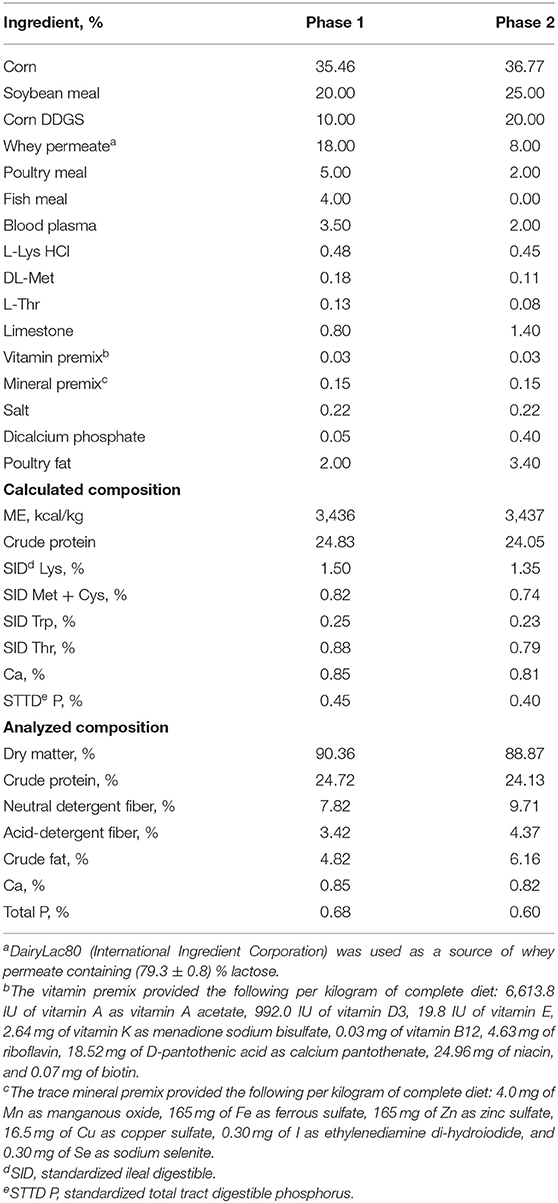
Pigs were fed the assigned experimental diets meeting the nutritional requirements suggested by NRC (21) for 20 d based on two phases (Phase 1: 10 d; and Phase 2: 10 d). The composition of mash basal diets is shown in Table 1.
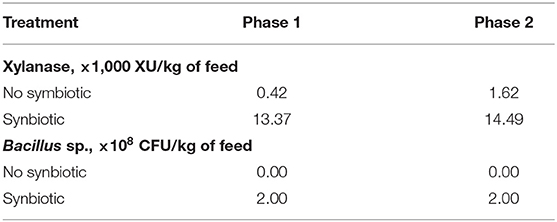
The quantitation of the xylanase (endo-1,4-β-D-xylanase) activity in feeds was performed using modified XylX6 assay (Megazyme, Wicklow, Ireland) as described by Mangan et al. (22). Xylanase enzymatic activity is calculated relative to a reference standard added to 50 mM Trisodium Citrate pH 6.0 buffer measured at A400. One unit of xylanase activity is defined as the amount of enzyme needed for the release of 1 nmol of reducing sugars per second from 0.5% xylan from Beechwood at 50°C in 50 mM Trisodium Citrate pH 6.0. The xylanase enzymatic activity is shown in Table 2. The microbial counting in feeds was conducted at the microbiology lab of BioResource International, Inc.
Experimental Procedures and Sampling
The inoculum of E. coli F18+ was prepared to be nalidixic acid resistant and produce heat-stable toxins A (STa) and heat-stable toxins B (STb), using a strain originally resistant to nalidixic acid following our standard protocol as previously described by Cutler et al. (23). The final concentration was 6 × 109 CFU/mL, orally inoculated and divided into two doses.
All pigs were fed the experimental diets for 7 d (pre-challenge period) until ETEC was orally inoculated to 32 pigs on d 7 of the study. The unchallenged group (32 pigs) received an oral administration of sterile physiological saline. The fecal score was recorded by the same trained person from the d 3 to d 7 of the study to analyze the effects of the synbiotic in the pre-challenge period and to confirm that pigs assigned to the challenge group are in normal fecal score before the E. coli F18+ inoculation. The fecal score was also recorded from d 8 to d 20 of the study to analyze the effects of ETEC infection (24, 25). The fecal score was recorded using a 1–5 scale: (1) very firm stool, (2) normal firm stool, (3) moderately loose stool, (4) loose, watery stool, and (5) very watery stool.
After 20 d of feeding, 48 pigs, 12 per treatment, were selected based on initial BW (the heaviest and the lightest pigs within sex were excluded of sampling) and euthanized by exsanguination after the penetration of a captive bolt to head in order to remove the gastrointestinal tract for sample collection. Digesta from mid-jejunum (3 m after the duodenojejunal junction) was collected into a 50-mL tube, placed on ice, and carried to the lab for viscosity measurement. Tissues from mid-jejunum were collected, rinsed with 0.9% saline solution, and placed into a 50-mL tube with 10% buffered formaldehyde to be used for histological evaluation to measure villus height, crypt depth, and the ratio of Ki-67 positive cells to total cells in the crypt, as an indicator of the enterocyte proliferation rate (10, 26).
Segments of mid-jejunum were longitudinally opened and scraped to collect mucosa. Two samples per pig were placed into 2-mL tubes and stored at −80°C, after snap-freezing in liquid nitrogen immediately after collection. Jejunal mucosa samples (500 mg) were suspended in 1 mL of phosphate-buffered saline (PBS) and homogenized on ice using a tissue homogenizer (Tissuemiser; ThermoFisher Scientific Inc., Waltham, MA, USA). After centrifugation at 14,000 × g for 3 min, the supernatant was divided into six sets and stored at −80°C until analysis to measure the concentration of total protein, Tumor necrosis factor-alpha (TNFα), interleukin 6 (IL-6), interleukin 8 (IL-8), protein carbonyl, and malondialdehyde (MDA).
Sample Processing and Analyses
Immediately after collection, digesta from jejunum were divided into two tubes (15 mL) per pig and centrifuged at 1,000 × g at 4°C for 10 min. Then, 2 mL from each tube was centrifuged at 10,000 × g at 4°C for 10 min. The supernatant obtained was transferred to another 1.5-mL tube and kept on ice until measurement. The viscosity was measured using a viscometer (Brookfield Digital Viscometer, Model DV-II Version 2.0, Brookfield Engineering Laboratories Inc., Stoughton, MA), set at 25°C. The viscosity measurement was the average between 45.0/s and 22.5/s shear rates, and the viscosity values were recorded as viscosity in millipascal-seconds (mPa·s) (10, 14).
The concentrations of total protein, TNFα, IL-6, IL-8, MDA, and protein carbonyl were measured by colorimetric methods using commercially available kits according to the instructions of the manufacturers. The absorbance was read using a plate reader (Synergy HT, BioTek Instruments, Winooski, VT) and the Gen5 Data Analysis Software (BioTek Instruments). The concentration was calculated based on the standard curve created from the concentration and absorbance of the respective standard.
Total protein concentration in the mucosa of jejunum was measured using a BCA Protein Assay (23225#, ThermoFisher Scientific Inc. Rockford, IL) following Jang and Kim (26). Before analysis, the samples were diluted (1:60) in PBS to meet the working range for 20–2,000 μg/mL. The absorbance was measured at 562 nm. The total protein concentration was used to normalize the concentrations of TNFα, IL-6, IL-8, MDA, and protein carbonyl.
The concentration of TNFα in the mucosa of the jejunum was measured using the porcine ELISA Kit (PTA00; R&D System Inc. Minneapolis, MN) following Weaver and Kim (27). The working range was 23.4–1,500 pg/mL. Absorbance was read at 450 nm and 540 nm and the TNFα concentration was expressed as pg/mg protein. The concentration of IL-6 in the mucosa of jejunum was determined using the ELISA Kit (P6000B; R&D System Inc.) following Jang and Kim (26). The working range was 18.8–1,200 pg/mL. Absorbance was read at 450 nm and 540 nm, and the IL-6 concentration was expressed as pg/mg protein. The concentration of IL-8 in the mucosa of jejunum was determined using the ELISA Kit (P8000; R&D System Inc.). Before analysis, the samples were diluted (1:6) in PBS to meet the working range of 62.5–4,000 pg/mL. Absorbance was read at 450 and 540 nm, and the IL-8 concentration was expressed as ng/mg protein.
Malondialdehyde concentration in the mucosa of jejunum was measured using the Thiobarbituric Acid Reactive Substance Assay Kit (STA-330, Cell Biolabs, San Diego, CA) following Duarte et al. (10). The working range was 0.98–125 μmol/L. The absorbance was measured at 532 nm, and the MDA concentration mucosa was expressed as μmol/mg of protein.
Protein carbonyl concentration was measured using the ELISA kit (STA-310, Cell Biolabs) following Zhao and Kim (28). Before the analysis, the sample was diluted to reach a protein concentration of 10 μg/mL. The working range was 0.375–7.5 nmol/mL. The absorbance was read at 450 nm, and the protein carbonyl concentration was expressed as nmol/mg of protein.
Two sections of jejunum per pig fixed in 10% buffered formalin were sent to the North Carolina State University Histology Laboratory (College of Veterinary Medicine, Raleigh, NC). The sections were dehydrated, embedded in paraffin, and stained using hematoxylin and eosin dyes for morphological measurement and Ki-67 immunohistochemistry assay to detect Ki-67 positive cells according to Jang and Kim (26).
Villus height, villus width, and crypt depth were measured using a microscope Olympus CX31 (Lumenera Corporation, Ottawa, Canada) with a camera Infinity 2-2 digital CCD following Kim et al. (29). Lengths of ten well-oriented intact villi and their associated crypts were measured in each slide. The villi length was measured from the top of the villi to the villi-crypt junction, the villi width was measured in the middle of the villi, and the crypt depth was measured from the villi-crypt junction to the bottom of the crypt. Then, the villus height to crypt depth (VH:CD) ratio was calculated. Images of 10 intact crypts from each slide were cropped, and the ImageJS software was used for calculating the percentage of Ki-67 positive cells to total cells in the crypt. All analyses of the intestinal morphology were executed by the same person. The averages of the 10 measurements per pig were calculated and reported as one number per pig.
Jejunal mucosa samples were used to extract DNA content to analyze the microbiome. The DNA extraction was performed using the DNA Stool Mini Kit (#51604, Qiagen; Germantown, Maryland, USA) and following the instructions of the manufacturer. Samples were sent to Mako Medical Laboratories (Raleigh, NC, USA) for microbial sequencing using the 16S rRNA technique following Kim et al. (29). Briefly, the Ion Chef instrument was used to prepare the samples for template and sequencing was performed on the Ion S5 system (ThermoFisher, Inc., Wilmington, DE, USA). Variable regions V2, V3, V4, V6, V7, V8, and V9 of the 16S rRNA gene were amplified with the Ion 16S Metagenomics Kit 113 (ThermoFisher Scientific). Sequences were processed using the Torrent Suite Software (version 5.2.2) (ThermoFisher Scientific) to produce raw unaligned sequence data files for further analysis. Sequence data analysis, alignment to GreenGenes and MicroSeq databases, alpha and beta diversity plot generation, and OTU table generation were performed by the Ion Reporter Software Suite (version 5.2.2) of bioinformatics analysis tools (ThermoFisher Scientific). Samples were analyzed using Ion Reporter's Metagenomics 16S workflow powered by Qiime (version w1.1). The OTU data were transformed to relative abundance before statistical analysis. The OTU data with the relative abundance <0.05% within each level were combined as “Others”.
Statistical Analyses
Pigs were allotted in a randomized complete block design using sex and the initial BW as blocks in all measurements to account for the variation of the initial BW and sex dimorphism (30). The experimental unit was the pig, individually housed and fed. The main effects were the factors (ETEC challenge and synbiotics) and their interaction. Factors were handled as fixed effects, and initial BW and sex blocks were handled as random effects. For growth performance and fecal score data, each factor had 16 pigs (n = 16; eight barrows and eight gilts; and four body weight blocks within sex). For other data, each factor had 12 pigs (n=12; six barrows and eight gilts; and three body weight blocks within sex). Data were analyzed using the Mixed procedure in SAS version 9.4 (SAS Inc., Cary, NC, USA). The means were separated using the LSMEANS statement in SAS. When an interaction between the factors was significant or tended to be significant, a pairwise comparison was made using the PDIFF option in SAS. Statistical differences were considered significant with P < 0.05. Tendency was considered when 0.05 ≤ P < 0.10.
Results
Growth Performance
In the pre-challenge period, the synbiotic tended to increase (P = 0.059) the BW of pigs at d 7 after weaning (Table 3). At d 10 of the study, 3 days post-challenge, the oral inoculation of ETEC did not affect the BW, whereas it was increased (P < 0.05) by the dietary use of synbiotics. However, at d 20 of the study, the BW of pigs challenged with ETEC was reduced (P < 0.05), whereas it was not affected by the synbiotic.
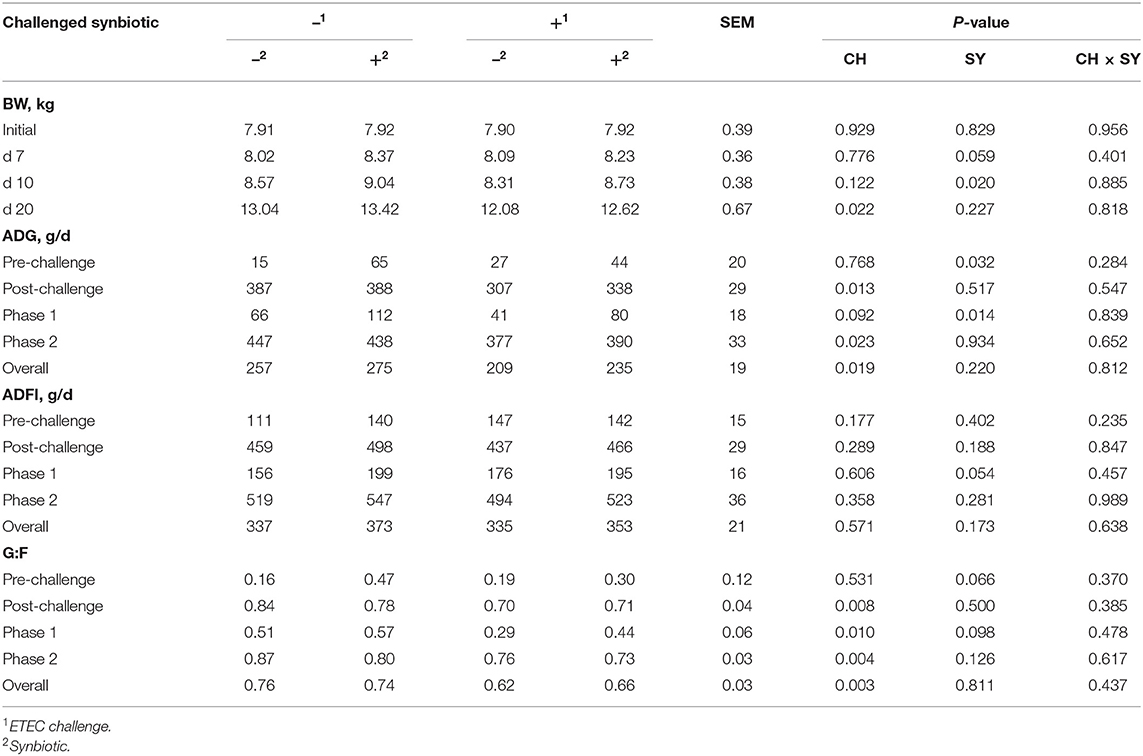
Table 3. Growth performance of pigs challenged with ETEC (CH) on d 7 post-weaning and fed diets supplemented with a synbiotic (SY).
At the pre-challenge period (d 0–7) the synbiotic increased (P < 0.05) the ADG of pigs. On the post-challenge period (d 7–20), the ADG of pigs challenged with ETEC was reduced (P < 0.05). In phase 1 (d 0–10), the ETEC challenge tended to reduce (P = 0.092) the ADG, whereas the use of synbiotics increased (P < 0.05) it, regardless of the challenge. In phase 2 (d 10–20) and overall, the ADG was reduced (P < 0.05) when pigs were challenged with ETEC, whereas it was not affected by the use of synbiotics.
The ADFI was not affected by the use of synbiotics during the pre-challenge period (d 0–7). In the post-challenge period, the ETEC challenge did not affect the ADFI, whereas, the use of synbiotics tended to increase (P = 0.054) the ADFI during phase 1 (d 0–10). The ADFI was not affected by the two factors during phase 2 and overall.
At the pre-challenge period (d 0–7) the use of synbiotics tended to increase (P = 0.066) the G:F ratio of pigs. However, in the post-challenge period (d 7–20), the G:F ratio of pigs challenged with ETEC was reduced (P < 0.05). At phase 1, the G:F ratio was reduced (P < 0.05) when pigs were challenged with ETEC, whereas it tended to increase (P = 0.098) by the use of synbiotics. In phase 2 and overall, the G:F ratio was reduced (P < 0.05) when pigs were challenged with ETEC, whereas it was not affected by the use of synbiotics.
Fecal Score
The fecal score of pigs challenged with ETEC was increased (P < 0.05) at d 7, 9, 11, and 13; however, at d 9 and 11, the use of synbiotics reduced (P < 0.05) the fecal score of pigs challenged with ETEC (Figure 1). The fecal score was not affected by the two factors at d 15, 17, and 20.
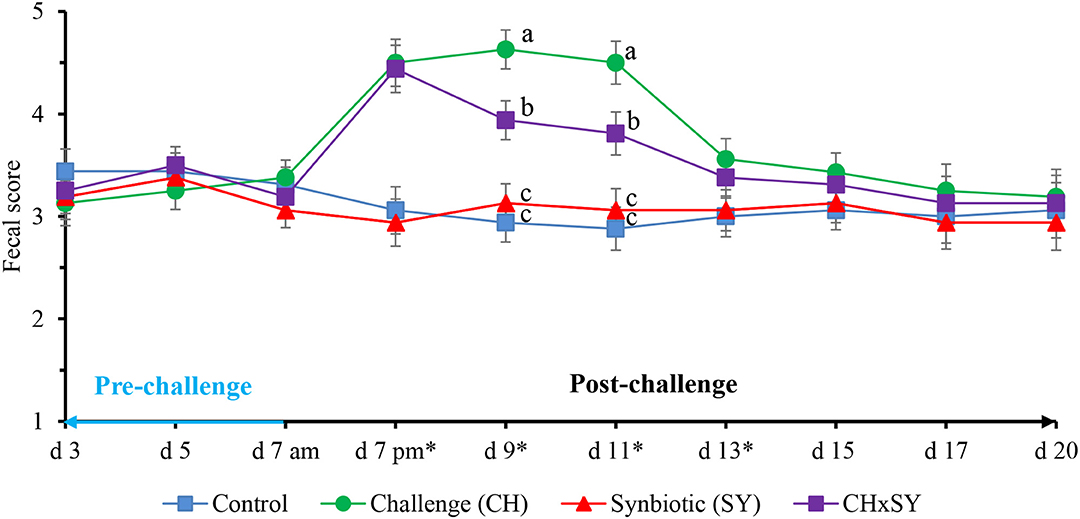
Figure 1. Fecal score of pigs challenged with ETEC (CH) on d 7 post-weaning and fed diets supplemented with a synbiotic (SY). * d 7 pm: CH: (P < 0.001), SY: (P = 0.685), CH × SY: (0.892); d 9: CH: (P < 0.001), SY: (P = 0.124), CH × SY: (P < 0.05); d 11: CH: (P < 0.001), SY: (P = 0.236), CH × SY: (P < 0.05); d 13: CH: (P < 0.05), SY: (P = 0.718), CH × SY: (P = 0.471). a,b Within a column, means without a common superscript letter differ (P < 0.05).
Immune and Oxidative Status
The mucosal concentration of MDA was increased (P < 0.05) when pigs were challenged with ETEC, whereas it was not affected by the use of synbiotics (Table 4). However, the concentration of protein carbonyl was not affected when pigs were challenged with ETEC, whereas it tended to reduce (P = 0.065) with the use of synbiotics. The concentration of TNFα was not affected when pigs were challenged with ETEC, whereas, it tended to reduce (P = 0.093) with the use of synbiotics. The concentration of IL-6 was increased (P < 0.05) when pigs were challenged with ETEC, whereas it tended to decrease (P = 0.064) with the use of synbiotics, regardless of the challenge. The concentration of IL-8 was not affected by the factors.
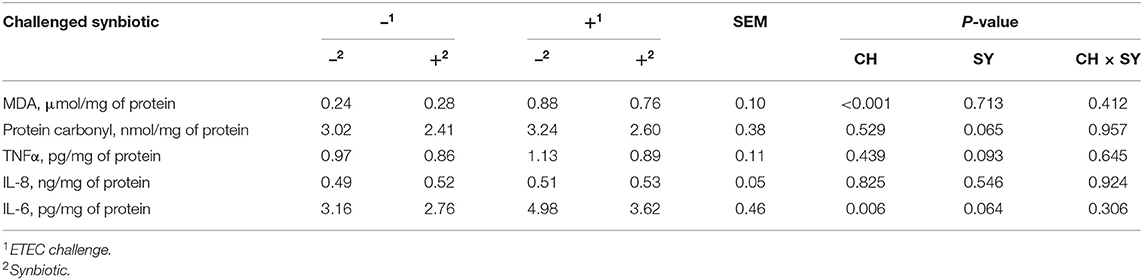
Table 4. Oxidative stress and immune parameters in the jejunal mucosa of pigs challenged with ETEC (CH) on d 7 post-weaning and fed diets supplemented with a synbiotic (SY).
Histomorphology, Immunohistochemistry, and Digesta Viscosity
The enterotoxigenic E. coli challenge at d 7 post-weaning reduced (P < 0.05) the villus height and VH:CD ratio and increased (P < 0.05) the crypt depth and the ratio of Ki-67 positive cells to total cell in the crypt in jejunum of pigs (Table 5), whereas, regardless of the ETEC challenge, the use of synbiotics increased (P < 0.05) the villus height and VH:CD ratio, reduced (P < 0.05) the crypt depth, and tended to reduce (P = 0.053) the ratio of Ki-67 positive cells to total cell in the crypt in the jejunum of pigs. The viscosity of the jejunal digesta was not affected by the factors.
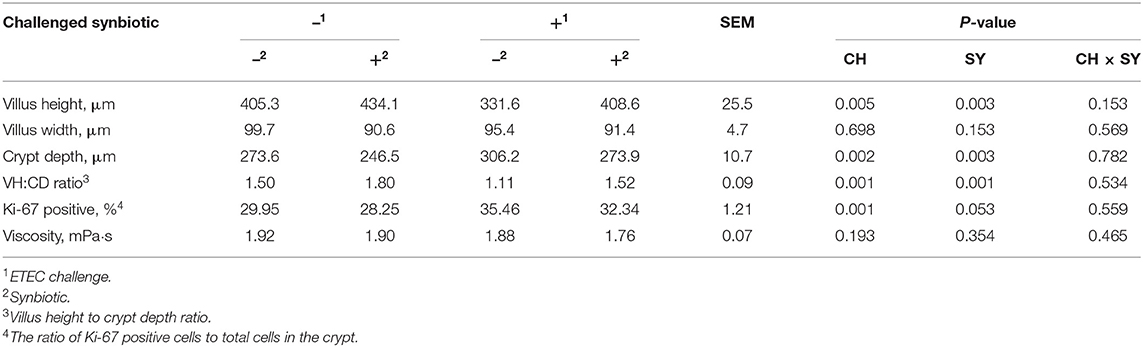
Table 5. Jejunal histomorphology and digesta viscosity of pigs challenged with ETEC (CH) on d 7 post-weaning and fed diets supplemented with a synbiotic (SY).
Microbiome
At the phylum level (Figure 2), the ETEC reduced (P < 0.05) the relative abundance of Bacteroidetes and Firmicute and increased (P < 0.05) the relative abundance of Proteobacteria. The synbiotic did not affect the relative abundance of microbials at the phylum level. At the family level (Table 6), pigs challenged with ETEC tended to reduce the relative abundance of Clostridiaceae (P = 0.067) and Prevotellaceae (P = 0.069), and reduced (P < 0.05) the relative abundance of Veillonellaceae, whereas it tended to increase (P = 0.063) the relative abundance of Helicobacteraceae. The use of synbiotics did not affect the jejunal mucosa-associated microbiota at the family level. In the genus level (Table 7), the ETEC challenge reduced (P < 0.05) the relative abundance of Megasphaera, Mitsuokella, and Selenomonas and tended to reduce (P = 0.060) the relative abundance of Helicobacter, whereas the use of synbiotics did not affect the jejunal mucosa-associated microbiota at the genus level. At the species level (Table 8), the ETEC challenge reduced (P < 0.05) the relative abundance of Acidaminococcus fermentans, Selenomonas bovis, and Selenomonas lipolytica and tended to decrease the relative abundance of Prevotella copri (P = 0.096) and Roseburia faecis (P = 0.079). Pigs fed synbiotics increased (P < 0.05) the relative abundance of Helicobacter_mastomyrinus in unchallenged pigs compared with the control group. Pigs challenged with ETEC and fed a diet with synbiotics increased (P < 0.05) the relative abundance of Campylobacter coli compared with pigs fed synbiotics and not challenged. Pigs challenged with ETEC and fed a diet with a synbiotic tended to increase (P = 0.075) the relative abundance of Campylobacter hyointestinalis compared with pigs fed synbiotics and not challenged.
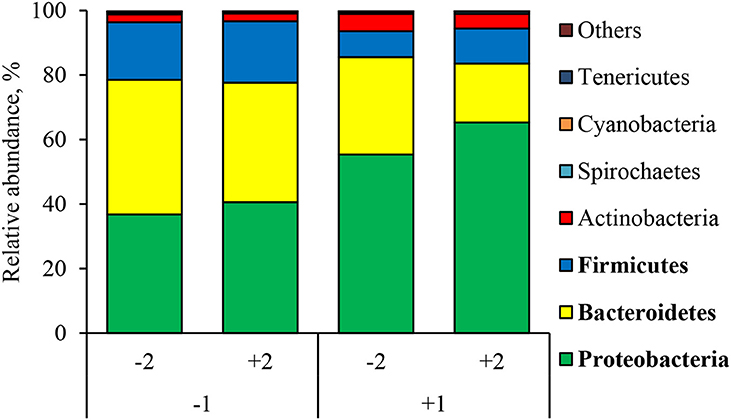
Figure 2. Relative abundance of jejunal mucosa-associated microbiota at the phylum level in pigs challenged with ETEC (CH) on d 7 post-weaning and fed diets supplemented with a synbiotic (SY). Each pattern represents a particular bacterial phylum. Phylum sequences that did not achieve 0.5% within each phylum were combined as “Others.” 1: ETEC challenge (CH); 2: Synbiotic (SY). Proteobacteria: CH: (P < 0.05), SY: (P = 0.339), CH × SY: (P = 0.668). Bacteroidetes: CH: (P < 0.05), SY: (P = 0.162), CH × SY: (P = 0.542); Firmicutes: CH: (P < 0.05), SY: (P = 0.523), CH × SY: (P = 0.803).
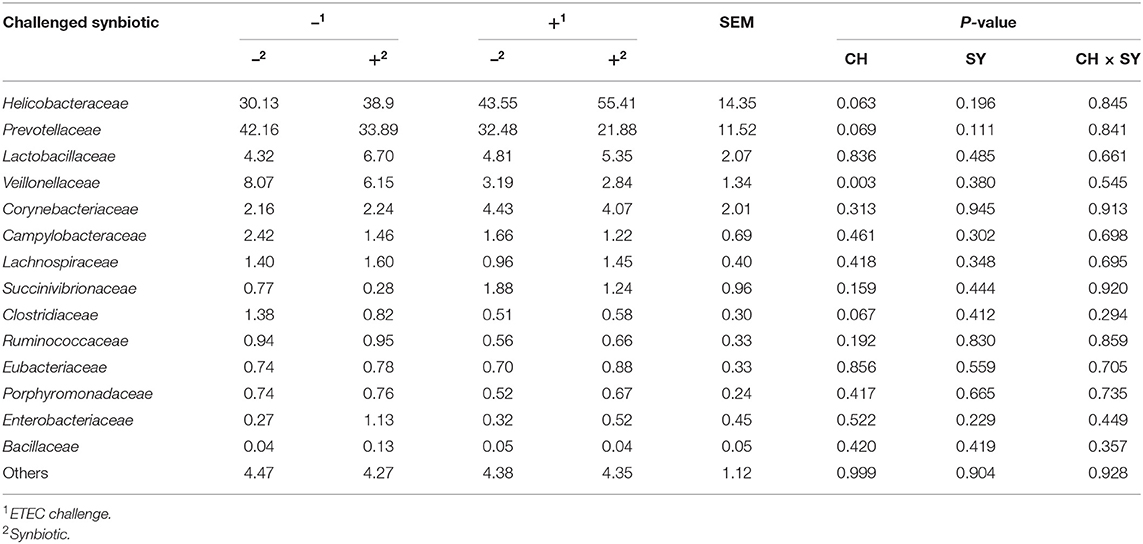
Table 6. Relative abundance of jejunal mucosa-associated microbiota at the family level in pigs challenged with ETEC (CH) on d 7 post-weaning and fed diets supplemented with a synbiotic (SY).
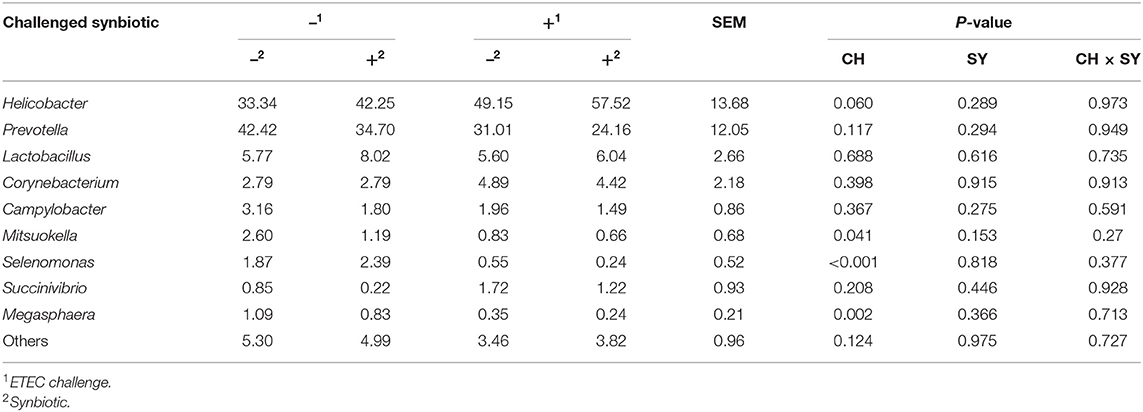
Table 7. Relative abundance of jejunal mucosa-associated microbiota at the genus level in pigs challenged with ETEC (CH) on d 7 post-weaning and fed diets supplemented with a synbiotic (SY).
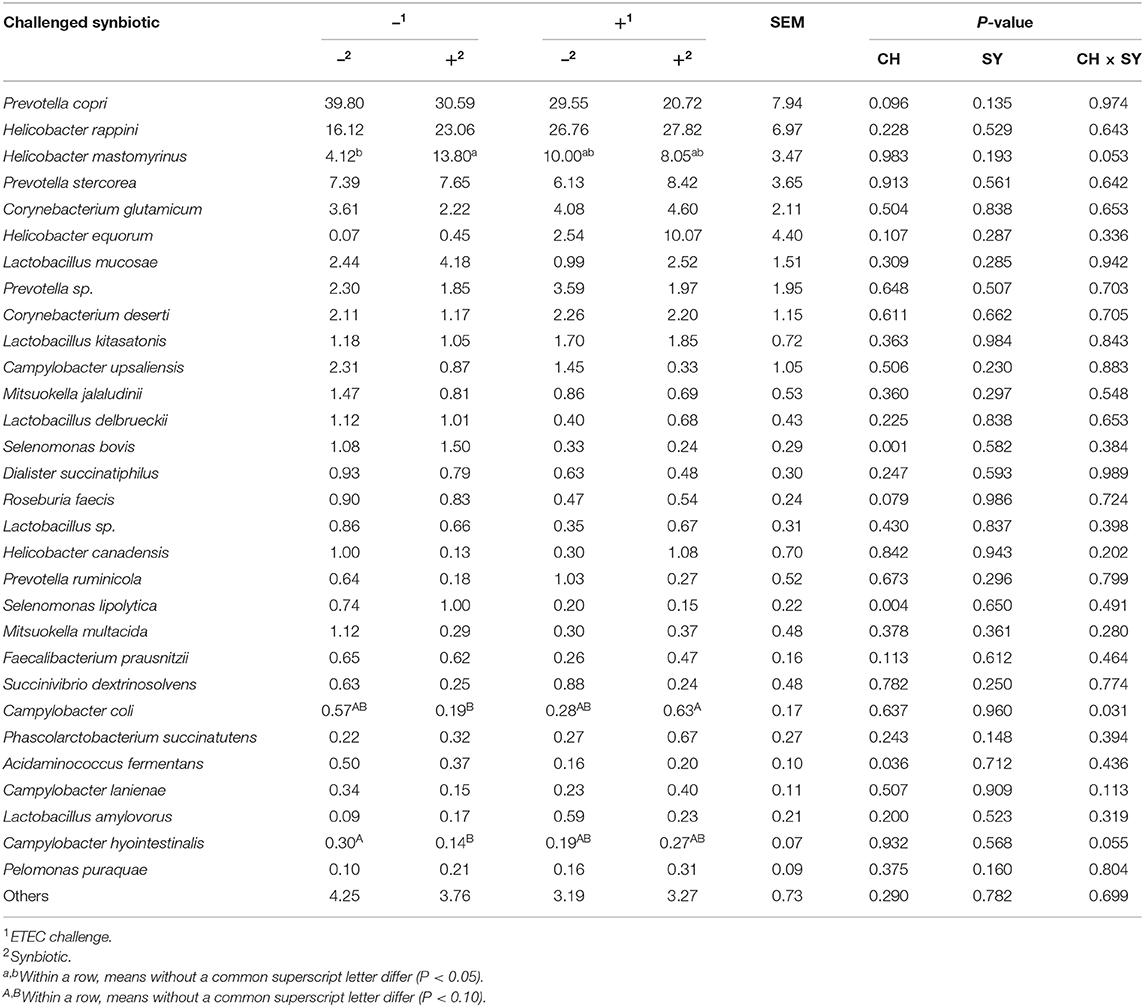
Table 8. Relative abundance of jejunal mucosa-associated microbiota at the species level in pigs challenged (CH) with ETEC on d 7 post-weaning and fed diets supplemented with synbiotics (SY).
There was no effect of the factors on alpha diversity of jejunal mucosa-associated microbiota in pigs estimated with Chao1 richness estimator at the family (Figure 3A) and genus levels (Figure 4A). At the family level, the Shannon diversity index was not affected by the factors (Figure 3B), whereas at the genus level, it tended to be reduced by the ETEC challenge (P = 0.089) and the synbiotic (P = 0.066), regardless of the challenge (Figure 4B). The Simpson diversity index was not affected by the ETEC challenge, whereas it was reduced (P < 0.05) by the synbiotic at the family (Figure 3C) and genus levels (Figure 4C).
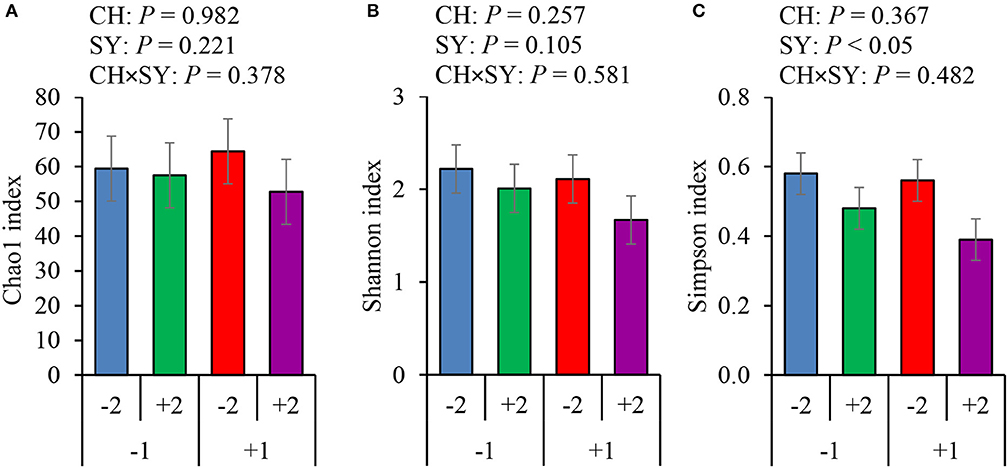
Figure 3. Alpha diversity of jejunal mucosa-associated microbiota at the family level estimated with Chao1 richness (A), Shannon diversity (B), and Simpson diversity (C) in pigs challenged (CH) with ETEC on d 7 post-weaned and fed diets supplemented with a synbiotic (SY). 1: ETEC challenge; 2: Synbiotic; SY: synbiotic; CH: challenge; CH × SY: Challenge and synbiotic.
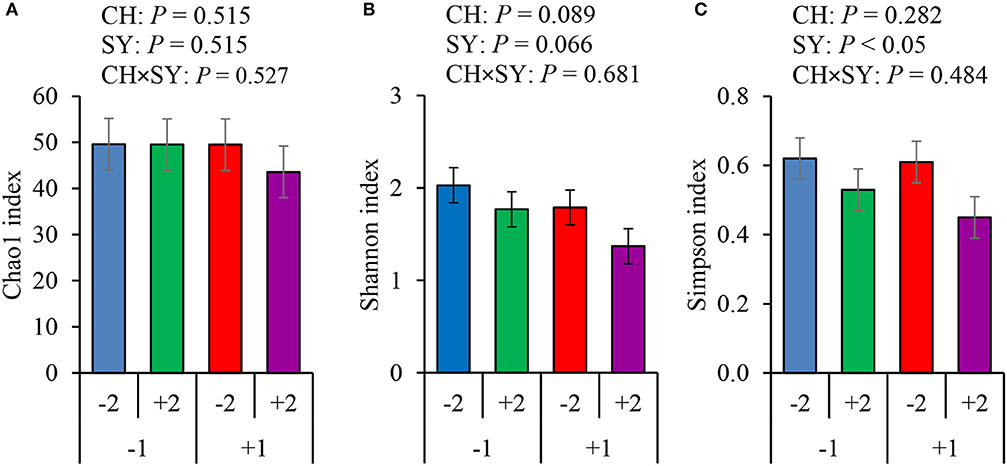
Figure 4. Alpha diversity of jejunal mucosa-associated microbiota at the genus level estimated with Chao1 richness (A), Shannon diversity (B), and Simpson diversity (C) in pigs challenged (CH) with ETEC on d 7 post-weaned and fed diets supplemented with a synbiotic (SY). 1: ETEC challenge; 2: Synbiotic; SY: synbiotic; CH: challenge; CH × SY: Challenge and synbiotics.
Discussion
In this study, pigs were housed individually in order to know the intake of synbiotics affecting intestinal health following procedures previous described (26, 31, 32). The beneficial effects of the synbiotic shown in this study were prominent especially during the period immediately after the weaning when pigs receive the greatest nutritional challenges from plant-based diets, whereas the synbiotic seems to be efficient in enhancing the jejunal histomorphology and reducing the fecal score and the microbial diversity without affecting the growth performance during P2 of this study. Probiotics and prebiotics are shown to be effective to newly weaned pigs because of the immaturity of the intestine and limited digestive capacity of plant-based diets (10, 33). As pigs adapt to plant-based diets, however, pigs develop the intestine to handle fiber and utilize dietary nutrients more efficiently (34–36).
This study confirmed that E. coli F18+ can be associated with post-weaning diarrhea (PWD), reducing the growth, modulating the microbiome, and affecting the gut heath of newly weaned pigs as previously reported (5, 25, 37). Enterotoxins (including STa, and STb) from ETEC are a major cause of increased fecal score as shown in this study. The fimbria of the E. coli bind to glycoproteins in the microvilli of the intestine of newly weaned pigs by a fimbria receptor interaction causing an interference in the electrolytes fluid that leads to diarrhea by enterotoxin interaction (5, 38–40). The predisposition of newly weaned pigs to PWD caused by ETEC have been related to the psychological, environmental, and physiological stress after weaning, as well as sudden transition from sow's milk to plant-based diets that are solid and less digestible. These stressors disrupt the immune system and the intestinal microbiota leading to intestinal inflammation and PWD (41, 42), consequently reducing growth performance (5, 6, 17). As previously reported (31, 43), the challenge with E. coli F18+ in this study reduced growth and feed efficiency without affecting feed intake, which is in agreement with previous studies. Reduced feed efficiency in pigs with E. coli infection is related to impaired nutrient absorption (43, 44) and the activation of immune system partitioning nutrients from growth (45).
McLamb et al. (24) previously reported that pigs challenged with ETEC have the immune response activated. According to Loos et al. (46), the secretion of IL-6 in the lumen of the small intestine is stimulated by STa produced by E. coli F18+. In this study, the ETEC challenge increased the concentration of IL-6 as previous reported (46, 47). High levels of IL-6 reduce the secretion of growth hormone (48) and damage the intestinal epithelium (49, 50). The challenge, however, did not affect the concentration of IL-8 and TNFα in this study. According to Loos et al. (51), the IL-8 has low expression in response to ETEC. The activation of the immune system in response to the ETEC infection may lead to an exhaustion of the antioxidant mechanism causing the oxidation of cellular protein, lipids, and DNA (52). The results of this study show, on challenged pigs, an increasing level of MDA, a final product of the lipid oxidation, and an indicator of oxidative stress (53, 54). The metabolites from oxidative stress can directly affect the enterocytes' cell wall components, such as lipids and proteins, causing apoptosis and, consequently, reduction of villi length (54, 55). The villi reduction in the challenged pigs leads to increasing the crypt cell proliferation rate, and consequently, increasing the crypt depth which is in accordance with previous studies (3, 43, 56). The increased oxidative stress due to the activated immune system may also redirect energy and nutrients from growth to immune response, and to repair the epithelium.
The use of synbiotics, however, was effective to reduce the jejunal mucosal levels of IL-6, TNFα, and protein carbonyl regardless of the ETEC challenge. The reduction of the immune and oxidative stress indicators reduces the epithelial damage and the cell proliferation rate by reducing the deleterious effect of the ETEC on nursery pigs (43, 57–59). These results showed a potential benefit of dietary xylanase and Bacillus sp. as a synbiotic on enhancing the gut health and further reducing the jejunal mucosal protein carbonyl concentration. Therefore, this study targeted the investigation of the combinational effects of xylanase and Bacillus sp. The synbiotic had beneficial outcomes because xylanase successfully hydrolyzed xylans to XOS in feeds (10, 31, 60, 61), reducing the viscosity of digesta (10, 19) releasing nutrients for digestion (14, 18). Passos et al. (14) reported that dietary supplementation with xylanase showed a linear increase in the ileal digestibility of NDF, indicating the hydrolysis of NSP-releasing oligosaccharides, such as XOS. In addition, Bacillus sp. effectively utilizes XOS released by xylanase hydrolysis, further exerting synergetic effects (20) including their antibacterial properties (43, 62, 63).
The synbiotic can selectively affect the growth of microorganisms in the intestine, including those directly added in the diet (64, 65), targeting some metabolic processes and possibly changing the physical-chemical properties of the digesta (66). This mechanism can promote gut health benefits, such as modulation of gut microbiota by competition and antimicrobial property (67), and consequently, affect the immune system, reduce the oxidative stress, and increase the growth performance of newly weaned pigs (31).
Pigs from the challenge group-fed diets with the synbiotic had reduced diarrhea occurrence earlier than those without synbiotic supplementation. This outcome shows that the dietary supplementation of synbiotics may prevent ETEC from damaging the intestinal epithelium. Although the jejunal digesta viscosity was not affected by either factors in this study, the viscosity can be affected by the ingredient in the diet (19, 31) and the ratio of insoluble to soluble NSP (68). The viscosity observed in this study ranged from 1.8 to 1.9 mPa·s, which is lower than previously reported by Duarte et al. (10) and Passos et al. (14) due to differences in dietary compositions.
The ETEC slightly reduced the microbial diversity index but caused an imbalance in jejunal mucosa-associated microbiota by increasing the relative abundance of Proteobacteria by increasing the family Helicobacteraceae and the genus Helicobacter, consequently reducing the relative abundance of Bacteroidetes and Firmicutes, Prevotellaceae, and Mitsukella and Selenomonas, as previously reported by Bin et al. (69) and Pollock et al. (70). The adherence of the ETEC and the production of enterotoxins with the subsequent secretion of fluid to the intestinal lumen (41, 51) create a propitious environment to the growth of proteobacteria (17). The high abundance of Helicobacteraceae which belong to the Proteobacteria has been reported to cause a reduction of the mucous layer protection (65), which explains the impact of the challenge on the villus height, immune response, and the oxidative stress status, whereas, Prevotellaceae, which belongs to the Bacteroidetes has been related to intestinal mucosa of healthy pigs fed plant-based diets (71, 72).
The synbiotic reduced the diversity of the microbiome without affecting the relative abundance of microbials. Bacillus spores, Lactobacillus acidophilus and Pediococcus acidilactici used as probiotics has been reported to reduce the microbial diversity in pigs (33, 73). According to Poulsen et al. (33), Bacillus spores are able to adhere to intestinal epithelium and competitively affect the colonization pattern. Reduction on mucosa-associated microbial diversity have been related to increased inflammatory response (74), even though this was not observed in this study. These results may suggest that the type of the dominant microbials in the jejunal mucosa is more important to affect the intestinal immune response than the microbial diversity. According to Wang et al. (73), the ability of probiotics to reduce the diversity or richness of microbiome can positively affect the growth performance by reducing the deleterious effects of harmful microbes that can affect the immune system, oxidative stress, and intestinal histomorphology. It was confirmed in this study that the synbiotic supplementation increased growth performance, and villus height, reducing diarrhea, immune response, and oxidative stress in nursery pigs.
In conclusion, the ETEC challenge reduced the growth performance of newly weaned pigs by increasing the relative abundance of harmful bacteria, intestinal immune response, intestinal oxidative stress, and crypt depth while reducing the villus height in the small intestine. Dietary supplementation of xylanase and Bacillus sp. as a synbiotic enhanced growth performance by increasing the relative abundance of beneficial bacteria in the small intestine, reducing diarrhea, reducing the oxidative stress, and increasing the villus height in the small intestine regardless of the challenge. The synbiotic showed potential benefits on growth performance, reducing diarrhea, immune response, and the oxidative stress status in the small intestine, leading to a protective function on the intestinal epithelium. Therefore, it was demonstrated that the E. coli F18+ greatly affects the gut health and growth performance of pigs, whereas the novel synbiotic showed a potential to mitigate the effects of E. coli F18+ infection in an AGP-free diet.
Data Availability Statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics Statement
The experimental procedures used in this study were reviewed and approved by the Institutional Animal Care and Use Committee (IACUC) at North Carolina State University.
Author Contributions
SK conceptualized the study and secured the funding. SK and JT designed the study. MD performed the experiments. MD and SK analyzed the data. The manuscript was written and reviewed by MD and SK. SK, MD, and JT discussed the results and approved the final manuscript. All authors contributed to the article and approved the submitted version.
Funding
This study was selected for a research award and funded from North Carolina Biotechnology Center (CFG-8003).
Conflict of Interest
JT was employed by the company BioResources International.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
1. Campbell JM, Crenshaw JD, Polo J. The biological stress of early weaned piglets. J Anim Sci Biotechnol. (2013) 4:2–5. doi: 10.1186/2049-1891-4-19
2. Kim SW, Hansen JA. Diet formulation and feeding programs. In: Chiba LI, editor. Sustainable Swine Nutrition. Oxford: Blackwell Publishing Ltd. (2012). p. 215–27. doi: 10.1002/9781118491454.ch9
3. Pluske JR, Turpin DL, Kim JC. Gastrointestinal tract (gut) health in the young pig. Anim Nutr. (2018) 4:187–96. doi: 10.1016/j.aninu.2017.12.004
4. Moeser AJ, Pohl CS, Rajput M. Weaning stress and gastrointestinal barrier development: implications for lifelong gut health in pigs. Anim Nutr. (2017) 3:313–21. doi: 10.1016/j.aninu.2017.06.003
5. Sun Y, Kim SW. Intestinal challenge with enterotoxigenic Escherichia coli in pigs, and nutritional intervention to prevent postweaning diarrhea. Anim Nutr. (2017) 3:322–30. doi: 10.1016/j.aninu.2017.10.001
6. Guevarra RB, Lee JH, Lee SH, Seok MJ, Kim DW, Kang BN, et al. Piglet gut microbial shifts early in life: causes and effects. J Anim Sci Biotechnol. (2019) 10:1–10. doi: 10.1186/s40104-018-0308-3
7. Weber N, Nielsen JP, Jakobsen AS, Pedersen LL, Hansen CF, Pedersen KS. Occurrence of diarrhoea and intestinal pathogens in non-medicated nursery pigs. Acta Vet Scand. (2015) 57:1–6. doi: 10.1186/s13028-015-0156-5
8. Li P, Niu Q, Wei Q, Zhang Y, Ma X, Kim SW, et al. Microbial shifts in the porcine distal gut in response to diets supplemented with Enterococcus faecalis as alternatives to antibiotics. Sci Rep. (2017) 7:1–10. doi: 10.1038/srep41395
9. Diana A, Boyle LA, Leonard FC, Carroll C, Sheehan E, Murphy D, et al. Removing prophylactic antibiotics from pig feed: how does it affect their performance and health? BMC Vet Res. (2019) 15:67. doi: 10.1186/s12917-019-1808-x
10. Duarte ME, Zhou FX, Dutra WM, Kim SW. Dietary supplementation of xylanase and protease on growth performance, digesta viscosity, nutrient digestibility, immune and oxidative stress status, and gut health of newly weaned pigs. Anim Nutr. (2019) 5:351–8. doi: 10.1016/j.aninu.2019.04.005
11. Knudsen KEB. Fiber and nonstarch polysaccharide content and variation in common crops used in broiler diets. Poult Sci. (2014) 93:2380–93. doi: 10.3382/ps.2014-03902
12. Jaworski NW, Lærke HN, Knudsen KEB, Stein HH. Carbohydrate composition and in vitro digestibility of dry matter and nonstarch polysaccharides in corn, sorghum, and wheat and coproducts from these grains. J Anim Sci. (2015) 93:1103–13. doi: 10.2527/jas2014-8147
13. Lindberg JE. Fiber effects in nutrition and gut health in pigs. J Anim Sci Biotechnol. (2014) 5:1–7. doi: 10.1186/2049-1891-5-15
14. Passos AA, Park I, Ferket P, von Heimendahl E, Kim SW. Effect of dietary supplementation of xylanase on apparent ileal digestibility of nutrients, viscosity of digesta, and intestinal morphology of growing pigs fed corn and soybean meal based diet. Anim Nutr. (2015) 1:19–23. doi: 10.1016/j.aninu.2015.02.006
15. Tiwari UP, Chen H, Kim SW, Jha R. Supplemental effect of xylanase and mannanase on nutrient digestibility and gut health of nursery pigs studied using both in vivo and in vitro models. Anim Feed Sci Technol. (2018) 245:77–90. doi: 10.1016/j.anifeedsci.2018.07.002
16. Chen H, Zhang S, Park I, Kim SW. Impacts of energy feeds and supplemental protease on growth performance, nutrient digestibility, and gut health of pigs from 18 to 45 kg body weight. Anim Nutr. (2017) 3:359–65. doi: 10.1016/j.aninu.2017.09.005
17. Gresse R, Chaucheyras-Durand F, Fleury MA, Van de Wiele T, Forano E, Blanquet-Diot S. Gut microbiota dysbiosis in postweaning piglets: understanding the keys to health. Trends Microbiol. (2017) 25:851–73. doi: 10.1016/j.tim.2017.05.004
18. Cadogan DJ, Choct M. Pattern of non-starch polysaccharide digestion along the gut of the pig: contribution to available energy. Anim Nutr. (2015) 1:160–5. doi: 10.1016/j.aninu.2015.08.011
19. Lærke HN, Arent S, Dalsgaard S, Bach Knudsen KE. Effect of xylanases on ileal viscosity, intestinal fiber modification, and apparent ileal fiber and nutrient digestibility of rye and wheat in growing pigs. J Anim Sci. (2015) 93:4323–35. doi: 10.2527/jas2015-9096
20. Mingmongkolchai S, Panbangred W. Bacillus probiotics: an alternative to antibiotics for livestock production. J Appl Microbiol. (2018) 124:1334–46. doi: 10.1111/jam.13690
21. NRC. Nutrient Requirements of Swine. 11th revision. Washington, DC: National Academies Press (2012).
22. Mangan D, Cornaggia C, Liadova A, McCormack N, Ivory R, McKie VA, et al. Novel substrates for the automated and manual assay of endo-1,4-β-xylanase. Carbohyd Res. (2017) 445:14–22. doi: 10.1016/j.carres.2017.02.009
23. Cutler SA, Lonergan SM, Cornick N, Johnson AK, Stahl CH. Dietary inclusion of colicin E1 is effective in preventing postweaning diarrhea caused by F18-positive Escherichia coli in pigs. Antimicrob Agents Chemother. (2007) 51:3830–5. doi: 10.1128/AAC.00360-07
24. McLamb BL, Gibson AJ, Overman EL, Stahl C, Moeser AJ. Early weaning stress in pigs impairs innate mucosal immune responses to enterotoxigenic E. coli challenge and exacerbates intestinal injury and clinical disease. PLoS ONE. (2013) 8:e0059838. doi: 10.1371/journal.pone.0059838
25. Luise D, Lauridsen C, Bosi P, Trevisi P. Methodology and application of Escherichia coli F4 and F18 encoding infection models in post-weaning pigs. J Anim Sci Biotechnol. (2019) 10:1–20. doi: 10.1186/s40104-019-0352-7
26. Jang K, Kim SW. Supplemental effects of dietary nucleotides on intestinal health and growth performance of newly weaned pigs. J Anim Sci. (2019) 96:157–8. doi: 10.1093/jas/skz334
27. Weaver AC, Kim SW. Supplemental nucleotides high in inosine 5′-monophosphate to improve the growth and health of nursery pigs. J Anim Sci. (2014) 92:645–51. doi: 10.2527/jas.2013-6564
28. Zhao Y, Kim SW. Oxidative stress status and reproductive performance of sows during gestation and lactation under different thermal environments. Asian Australas J Anim Sci. (2020) 33:722–31. doi: 10.5713/ajas.19.0334
29. Kim SW, Holanda DM, Gao X, Park I, Yiannikouris A. Efficacy of a yeast cell wall extract to mitigate the effect of naturally co-occurring mycotoxins contaminating feed ingredients fed to young pigs: impact on gut health, microbiome, and growth. Toxins (Basel). (2019) 11:633. doi: 10.3390/toxins11110633
30. Christoforidou Z, Mora Ortiz M, Poveda C, Abbas M, Walton G, Bailey M, et al. Sexual dimorphism in immune development and in response to nutritional intervention in neonatal piglets. Front Immunol. (2019) 10:2705. doi: 10.3389/fimmu.2019.02705
31. Li Q, Burrough ER, Gabler NK, Loving CL, Sahin O, Gould SA, et al. A soluble and highly fermentable dietary fiber with carbohydrases improved gut barrier integrity markers and growth performance in F18 ETEC challenged pigs1. J Anim Sci. (2019) 97:2139–53. doi: 10.1093/jas/skz093
32. Liu P, Piao XS, Kim SW, Wang L, Shen YB, Lee HS, et al. Effects of chito-oligosaccharide supplementation on the growth performance, nutrient digestibility, intestinal morphology, and fecal shedding of Escherichia coli and Lactobacillus in weaning pigs. J Anim Sci. (2008) 86:2609–18. doi: 10.2527/jas.2007-0668
33. Poulsen ASR, de Jonge N, Nielsen JL, Højberg O, Lauridsen C, Cutting SM, et al. Impact of Bacillus spp. spores and gentamicin on the gastrointestinal microbiota of suckling and newly weaned piglets. PLoS ONE. (2018) 13:e0207382. doi: 10.1371/journal.pone.0207382
34. Barba-vidal E, Martín-orúe SM, Castillejos L. Practical aspects of the use of probiotics in pig production: a review. Livest Sci. (2019) 223:84–96. doi: 10.1016/j.livsci.2019.02.017
35. Zimmermann JA, Fusari ML, Rossler E, Blajman JE, Romero-Scharpen A, Astesana DM, et al. Effects of probiotics in swines growth performance: a meta-analysis of randomised controlled trials. Anim Feed Sci Technol. (2016) 219:280–93. doi: 10.1016/j.anifeedsci.2016.06.021
36. Niu Q, Li P, Hao S, Zhang Y, Kim SW, Li H, et al. Dynamic distribution of the gut microbiota and the relationship with apparent crude fiber digestibility and growth stages in pigs. Sci Rep. (2015) 5:1–7. doi: 10.1038/srep09938
37. Luppi A, Gibellini M, Gin T, Vangroenweghe F, Vandenbroucke V, Bauerfeind R, et al. Prevalence of virulence factors in enterotoxigenic Escherichia coli isolated from pigs with post-weaning diarrhoea in Europe. Porc Heal Manag. (2016) 2:20. doi: 10.1186/s40813-016-0039-9
38. Nagy B, Fekete PZ. Enterotoxigenic Escherichia coli in veterinary medicine. Int J Med Microbiol. (2005) 295:443–54. doi: 10.1016/j.ijmm.2005.07.003
39. Dubreuil JD, Isaacson RE, Schifferli DM. Animal enterotoxigenic Escherichia coli. EcoSal Plus. (2016) 7:1. doi: 10.1128/ecosalplus.ESP-0006-2016
40. Kaper JB, Nataro JP, Mobley HLT. Pathogenic Escherichia coli. Nat Rev Microbiol. (2004) 2:123–40. doi: 10.1038/nrmicro818
41. Wang H, Zhong Z, Luo Y, Cox E, Devriendt B. Heat-stable enterotoxins of enterotoxigenic escherichia coli and their impact on host immunity. Toxins (Basel). (2019) 11:1–12. doi: 10.3390/toxins11010024
42. Becker SL, Li Q, Burrough ER, Kenne D, Sahin O, Gould SA, et al. Effects of an F18 enterotoxigenic Escherichia coli challenge on growth performance, immunological status and gastrointestinal structure of weaned pigs and the potential protective effect of direct-fed microbial blends. J Anim Sci. (2020) 98:1–10. doi: 10.1093/jas/skaa113
43. Kim K, He Y, Xiong X, Ehrlich A, Li X, Raybould H, et al. Dietary supplementation of Bacillus subtilis influenced intestinal health of weaned pigs experimentally infected with a pathogenic E. coli. J Anim Sci Biotechnol. (2019) 10:1–12. doi: 10.1186/s40104-019-0364-3
44. Bomba L, Minuti A, Moisá SJ, Trevisi E, Eufemi E, Lizier M, et al. Gut response induced by weaning in piglet features marked changes in immune and inflammatory response. Funct Integr Genomics. (2014) 14:657–71. doi: 10.1007/s10142-014-0396-x
45. Huntley NF, Nyachoti CM, Patience JF. Lipopolysaccharide immune stimulation but not β-mannanase supplementation affects maintenance energy requirements in young weaned pigs. J Anim Sci Biotechnol. (2018) 9:1–16. doi: 10.1186/s40104-018-0264-y
46. Loos M, Hellemans A, Cox E. Optimization of a small intestinal segment perfusion model for heat-stable enterotoxin A induced secretion in pigs. Vet Immunol Immunopathol. (2013) 152:82–6. doi: 10.1016/j.vetimm.2012.09.014
47. Devriendt B, Stuyven E, Verdonck F, Goddeeris BM, Cox E. Enterotoxigenic Escherichia coli (K88) induce proinflammatory responses in porcine intestinal epithelial cells. Dev Comp Immunol. (2010) 34:1175–82. doi: 10.1016/j.dci.2010.06.009
48. Gong FY, Shi YF, Deng JY. The regulatory mechanism by which interleukin-6 stimulates GH-gene expression in rat GH3 cells. J Endocrinol. (2006) 190:397–406. doi: 10.1677/joe.1.06736
49. Tanaka T, Narazaki M, Kishimoto T. IL-6 in inflammation, immunity, and disease. Cold Spring Harb Perspect Biol. (2014) 6:a016295. doi: 10.1101/cshperspect.a016295
50. Tanaka T, Kishimoto T. The biology and medical implications of interleukin-6. Cancer Immunol Res. (2014) 2:288–94. doi: 10.1158/2326-6066.CIR-14-0022
51. Loos M, Geens M, Schauvliege S, Gasthuys F, van der Meulen J, Dubreuil JD, et al. Role of heat-stable enterotoxins in the induction of early immune responses in piglets after infection with enterotoxigenic escherichia coli. PLoS ONE. (2012) 7:e0041041. doi: 10.1371/journal.pone.0041041
52. Gagné F, editor. Oxidative stress. In: Biochemical Ecotoxicology. Oxford: Elsevier (2014). p. 103–15. doi: 10.1016/B978-0-12-411604-7.00006-4
53. Celi P, Cowieson AJ, Fru-Nji F, Steinert RE, Kluenter A-M, Verlhac V. Gastrointestinal functionality in animal nutrition and health: new opportunities for sustainable animal production. Anim Feed Sci Technol. (2017) 234:88–100. doi: 10.1016/j.anifeedsci.2017.09.012
54. Sido A, Radhakrishnan S, Kim SW, Eriksson E, Shen F, Li Q, et al. A food-based approach that targets interleukin-6, a key regulator of chronic intestinal inflammation and colon carcinogenesis. J Nutr Biochem. (2017) 43:11–7. doi: 10.1016/j.jnutbio.2017.01.012
55. Assimakopoulos SF, Tsamandas AC, Louvros E, Vagianos CE, Nikolopoulou VN, Thomopoulos KC, et al. Intestinal epithelial cell proliferation, apoptosis and expression of tight junction proteins in patients with obstructive jaundice. Eur J Clin Invest. (2011) 41:117–25. doi: 10.1111/j.1365-2362.2010.02379.x
56. Xiong X, Tan B, Song M, Ji P, Kim K, Yin Y, et al. Nutritional intervention for the intestinal development and health of weaned pigs. Front Vet Sci. (2019) 6:46. doi: 10.3389/fvets.2019.00046
57. Liao SF, Nyachoti M. Using probiotics to improve swine gut health and nutrient utilization. Anim Nutr. (2017) 3:331–43. doi: 10.1016/j.aninu.2017.06.007
58. Zhang Z, Tun HM, Li R, Gonzalez BJM, Keenes HC, Nyachoti CM, et al. Impact of xylanases on gut microbiota of growing pigs fed corn- or wheat-based diets. Anim Nutr. (2018) 4:339–50. doi: 10.1016/j.aninu.2018.06.007
59. Celi P, Gabai G. Oxidant/antioxidant balance in animal nutrition and health: the role of protein oxidation. Front Vet Sci. (2015) 2:48. doi: 10.3389/fvets.2015.00048
60. Stein HH, Kil DY. Reduced use of antibiotic growth promoters in diets fed to weanling pigs: dietary tools, part 2. Anim Biotechnol. (2006) 17:217–31. doi: 10.1080/10495390600957191
61. Casas GA, Stein HH. Effects of microbial xylanase on digestibility of dry matter, organic matter, neutral detergent fiber, and energy and the concentrations of digestible and metabolizable energy in rice coproducts fed to weanling pigs. J Anim Sci. (2016) 94:1933–9. doi: 10.2527/jas.2015-0064
62. Dubreuil JD. Enterotoxigenic Escherichia coli and probiotics in swine: what the bleep do we know? Biosci Microbiota Food Heal. (2017) 36:75–90. doi: 10.12938/bmfh.16-030
63. Yang YY, Fan YF, Cao YH, Guo PP, Dong B, Ma YX. Effects of exogenous phytase and xylanase, individually or in combination, and pelleting on nutrient digestibility, available energy content of wheat and performance of growing pigs fed wheat-based diets. Asian Australas J Anim Sci. (2017) 30:57–63. doi: 10.5713/ajas.15.0876
64. Singh RD, Banerjee J, Arora A. Prebiotic potential of oligosaccharides: a focus on xylan derived oligosaccharides. Bioact Carbohyd Diet Fibre. (2015) 5:19–30. doi: 10.1016/j.bcdf.2014.11.003
65. Zhang W, Zhu Y-H, Zhou D, Wu Q, Song D, Dicksved J, et al. Oral administration of a select mixture of bacillus probiotics affects the gut microbiota and goblet cell function following Escherichia coli challenge in newly weaned pigs of genotype MUC4 that are supposed to be enterotoxigenic E. coli F4ab/ac receptor. Appl Environ Microbiol. (2017) 83:1–18. doi: 10.1128/AEM.02747-16
66. Markowiak P, Slizewska K. The role of probiotics, prebiotics and synbiotics in animal nutrition. Gut Pathog. (2018) 10:1–20. doi: 10.1186/s13099-018-0250-0
67. Molist F, van Oostrum M, Pérez JF, Mateos GG, Nyachoti CM, van der Aar PJ. Relevance of functional properties of dietary fibre in diets for weanling pigs. Anim Feed Sci Technol. (2014) 189:1–10. doi: 10.1016/j.anifeedsci.2013.12.013
68. Knudsen KEB, Hedemann MS, Lærke HN. The role of carbohydrates in intestinal health of pigs. Anim Feed Sci Technol. (2012) 173:41–53. doi: 10.1016/j.anifeedsci.2011.12.020
69. Bin P, Tang Z, Liu S, Chen S, Xia Y, Liu J, et al. Intestinal microbiota mediates enterotoxigenic Escherichia coli-induced diarrhea in piglets. BMC Vet Res. (2018) 14:385. doi: 10.1186/s12917-018-1704-9
70. Pollock J, Gally DL, Glendinning L, Tiwari R, Hutchings MR, Houdijk JGM. Analysis of temporal fecal microbiota dynamics in weaner pigs with and without exposure to enterotoxigenic Escherichia coli. J Anim Sci. (2018) 96:3777–90. doi: 10.1093/jas/sky260
71. Crespo-Piazuelo D, Estellé J, Revilla M, Criado-Mesas L, Ramayo-Caldas Y, Óvilo C, et al. Characterization of bacterial microbiota compositions along the intestinal tract in pigs and their interactions and functions. Sci Rep. (2018) 8:1–12. doi: 10.1038/s41598-018-30932-6
72. Adhikari B, Kim S, Kwon Y. Characterization of microbiota associated with digesta and mucosa in different regions of gastrointestinal tract of nursery pigs. Int J Mol Sci. (2019) 20:1630. doi: 10.3390/ijms20071630
73. Wang JQ, Yin FG, Zhu C, Yu H, Niven SJ, de Lange CFM, et al. Evaluation of probiotic bacteria for their effects on the growth performance and intestinal microbiota of newly-weaned pigs fed fermented high-moisture maize. Livest Sci. (2012) 145:79–86. doi: 10.1016/j.livsci.2011.12.024
Keywords: Escherichia coli, growth performance, intestinal health, newly weaned pigs, probiotics, synbiotics, xylanase
Citation: Duarte ME, Tyus J and Kim SW (2020) Synbiotic Effects of Enzyme and Probiotics on Intestinal Health and Growth of Newly Weaned Pigs Challenged With Enterotoxigenic F18+ Escherichia coli. Front. Vet. Sci. 7:573. doi: 10.3389/fvets.2020.00573
Received: 05 May 2020; Accepted: 17 July 2020;
Published: 09 September 2020.
Edited by:
Pietro Celi, DSM, United StatesReviewed by:
Elijah G. Kiarie, University of Guelph, CanadaCormac John O'Shea, University of Nottingham, United Kingdom
Copyright © 2020 Duarte, Tyus and Kim. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Sung Woo Kim, sungwoo_kim@ncsu.edu
 Marcos Elias Duarte
Marcos Elias Duarte James Tyus2
James Tyus2  Sung Woo Kim
Sung Woo Kim