Assessing Accuracy of Genomic Predictions for Resistance to Infectious Hematopoietic Necrosis Virus With Progeny Testing of Selection Candidates in a Commercial Rainbow Trout Breeding Population
- 1National Center for Cool and Cold Water Aquaculture, Agricultural Research Service, United States Department of Agriculture, Kearneysville, WV, United States
- 2Department of Animal Science, University of Connecticut, Storrs, CT, United States
- 3Department of Animal Science, University of California, Davis, Davis, CA, United States
- 4Clear Springs Foods Inc., Research Division, Buhl, ID, United States
- 5Gen Tec Consulting, Payette, ID, United States
Infectious hematopoietic necrosis (IHN) is an economically important disease of salmonid fish caused by the IHN virus (IHNV). Under industrial aquaculture settings, IHNV can cause substantial mortality and losses. Actually, there is no confirmed and cost-effective method for IHNV control. Clear Springs Foods, Inc. has been performing family-based selective breeding to increase genetic resistance to IHNV in their rainbow trout breeding program. In an earlier study, we used siblings cross-validation to estimate the accuracy of genomic prediction (GP) for IHNV resistance in this breeding population. In the present report, we used empirical progeny testing data to evaluate whether genomic selection (GS) can improve the accuracy of breeding value predictions over traditional pedigree-based best linear unbiased predictions (PBLUP). We found that the GP accuracy with single-step GBLUP (ssGBLUP) outperformed PBLUP by 15% (from 0.33 to 0.38). Furthermore, we found that ssGBLUP had higher GP accuracy than weighted ssGBLUP (wssGBLUP) and single-step Bayesian multiple regression (ssBMR) models with BayesB and BayesC priors which supports our previous findings that the underlying liability of genetic resistance against IHNV in this breeding population might be polygenic. Our results show that GS can be more effective than either the traditional pedigree-based PBLUP model or the marker-assisted selection approach for improving genetic resistance against IHNV in this commercial rainbow trout population.
Introduction
Infectious hematopoietic necrosis (IHN) is an important disease of salmonid fish that is caused by IHN virus (IHNV), which is a single-stranded negative-sense RNA rhabdovirus (1). IHNV is endemic to the Pacific Northwest in North America (2) and has spread through continental Europe, China, and Japan (3–5). IHNV is infectious to Pacific salmon and trout (Oncorhynchus spp.), as well as to Atlantic salmon (Salmo salar) (2), and can cause significant mortality and losses at nearly all stages of production in commercial aquaculture settings (6–8). Currently, unfortunately, there is no confirmed and cost-effective method for IHNV prevention or treatment. Therefore, the development of rainbow trout strains with genetic resistance to IHNV is needed for improving animal well-being and decreasing economic losses that are inflicted by this infectious viral induced disease to food aquaculture production (8).
The additive genetic basis for IHNV resistance is evident from the moderate estimates of heritability for IHNV binary survival status (h2 = 0.23–0.55) and survival days to death (h2 = 0.02–0.20) in a steelhead trout population (2). Recently, we also found moderate estimates of heritability for IHNV binary survival status (h2 = 0.25–0.28) and survival days to death (h2 = 0.23–0.33) in a commercial rainbow trout breeding population (8). These reports suggest that rainbow trout strains with genetic resistance against IHNV can be developed by means of traditional family-based selective breeding. However, up to now, the development of strains with enhanced resistance to IHNV in rainbow trout has been limited to traditional pedigree-based selection. The process of selecting and developing IHNV-resistant strains is further complicated due to the high genetic variability of the IHN virus as revealed by phylogenetic and nucleotide sequence analyses of 84 IHNV isolates (9). Nevertheless, selective breeding of a rainbow trout strain for resistance to IHNV has been conducted at the Clear Springs Foods Inc. (CSF) breeding program since the year 2000 (10), and the selection differential for resistance to IHNV has been on average 10% for the last eight generations between 2000 and 2016 (8).
Few quantitative trait loci (QTL) mapping research have been previously conducted to detect genetic polymorphisms linked with IHNV resistance and to determine the genetic basis of IHNV resistance in rainbow trout populations. A number of moderate-large-effect QTL associated with IHNV resistance were detected on 12 rainbow trout chromosomes using linkage analysis (3, 11–13) and genome-wide association studies (GWAS) (10). However, these earlier QTL mapping studies had several experimental limitations which we described and discussed elsewhere (8). To this end, we have recently performed GWAS for IHNV resistance using a 57K SNP panel and multiple regression single-step methods and found that the inheritance of resistance to IHNV in the Clear Springs breeding population is controlled by up to 10 small-moderate effect QTL (explained genetic variance = 2.0–8.8%) and large-unknown number of minute effect loci (8).
There is uncertainty on the best computational algorithm when using multiple regression based models in GWAS and genomic selection (GS) studies because the underlying genetic bases of a complex trait and the population structure can have major impact on the statistical power to detect marker effects and on the accuracy of genomic prediction (GP) (8). Therefore, it is recommended to compare the results from the best existing computational GWAS and GP methods when elucidating the genetic architecture of a complex disease and performing GP for the first time in a population (8).
When using multiple regression based GWAS and GP models that fit all single nucleotide polymorphisms (SNPs) with high quality genotypes, the genomic best linear unbiased prediction (GBLUP) method assumes that all SNPs have a non-zero contribution to the variance of the studied trait, with equal variance for each SNP, and that the distribution of the SNP effects follows a normal distribution (14–16). In addition, the single-step GBLUP (ssGBLUP) approach was developed, which combines the pedigree-based (A) and genomic relationship (G) matrices into the H relationship matrix (17, 18). On the other hand, the Bayesian variable selection model assumes that the trait genetic variance is explained by a relatively small number of loci, each with a small-moderate or large effect (14, 19–23). Based on the underlying assumptions of these models, the GBLUP model is expected not to perform as well as the Bayesian variable selection model when the trait genetic architecture is not mainly polygenic. For that reason, the GBLUP method was extended to the weighted ssGBLUP (wssGBLUP) method. The wssGBLUP mimics the Bayesian variable selection model by fitting all SNPs in the multiple regression model but assigning differential weights to the SNPs based on the variance of each SNP effect (8, 24). Lately, Bayesian variable selection models that use a single-step approach have been developed (25–28), including the single-step Bayesian multiple regression (ssBMR) method (8, 25, 28).
Assessment of the accuracy and bias of GP using progeny testing data is preferred over cross-validation (CV) analysis due to these reasons: First and foremost, there is a practical breeding purpose for GS, which is the actual de-facto improvement from selective breeding by passing over genetic variants or alleles associated with superior performance from parents to their offspring. Second, the CV analysis is unreliable because it is highly stochastic, and the GP accuracy is impacted by the magnitude of the estimated heritability. Third, it has been shown that the correlation of mid-parent genomic estimated breeding value (GEBV) with the mean progeny performance for each progeny testing family is a reliable estimator of the accuracy of the predicted breeding value (BV) (29, 30). Fourth, the bias of BV predictions for binary phenotypes (i.e., disease survival STATUS) when using CV analysis is incorrect (8) because all the binary observations within a class of variables are identical (i.e., each animal has one binary survival phenotype record of either 0 or 1) (31). With progeny testing data this drawback is circumvented by calculating the accuracy and bias of BV predictions using progeny performance data per evaluated progeny testing family (e.g., average survival rate) (8, 23, 32).
The development of multiple-regression based GWAS and GS methods, along with the manufacturing of the rainbow trout 57K SNP array (33) have provided the critical tools to perform whole genome-enabled selection for resistance against IHNV in rainbow trout. Recently, we estimated GP accuracies in the range of 0.30–0.39 for IHNV resistance in the CSF rainbow trout breeding population using simulation-based CV analysis (8). In the current study, we aimed to validate those estimated GP accuracies using empirical progeny performance data. Thus, the specific objectives of the study were to (1) evaluate the accuracy and bias of BV predictions for IHNV resistance using empirical data from progeny testing evaluations, (2) compare the accuracy and bias of BV predictions estimated with multiple regression single-step methods (ssGBLUP, wssGBLUP and ssBMR) and traditional pedigree-based PBLUP, (3) determine the extent of linkage disequilibrium (LD) and the effective population size (Ne) in the CSF population, and (4) assess the impact of relatedness between training and testing animals on the GP accuracy in commercial rainbow trout breeding populations.
Materials and Methods
Ethics Statement
This study used rainbow trout fin clips collected after controlled exposure to IHNV as part of a selective breeding program at the CSF research facility. As farm animals used in a commercial breeding program, these fish are exempted from regulation under the U.S. Animal Welfare Act and therefore not subject to oversight by an Institutional Animal Care and Use Committee or other such ethics committee. This exemption is defined in U.S. Code title 7, chapter 54, section 2,132 g. However, experimentation and handling were conducted in accordance with U.S. government principals for the use and care of vertebrate animals used in testing, research, and training, which includes provisions to minimize animal suffering. Specific measures for amelioration of animal suffering during the fish pathogen testing included minimization of handling, maintenance of optimal water temperature, and oxygen saturation, and the fish were fed a standard fish meal diet to satiation daily. Fish near death from severe symptoms of infection during the observation period were removed and terminated (by immersion in a lethal dose of MS222) before collection of fin tissue to minimize suffering. After the 3-week observation period, surviving fish were terminated by immersion in a lethal dose of MS222 before sampling and disposal.
Fish Growing and IHNV Disease Challenge
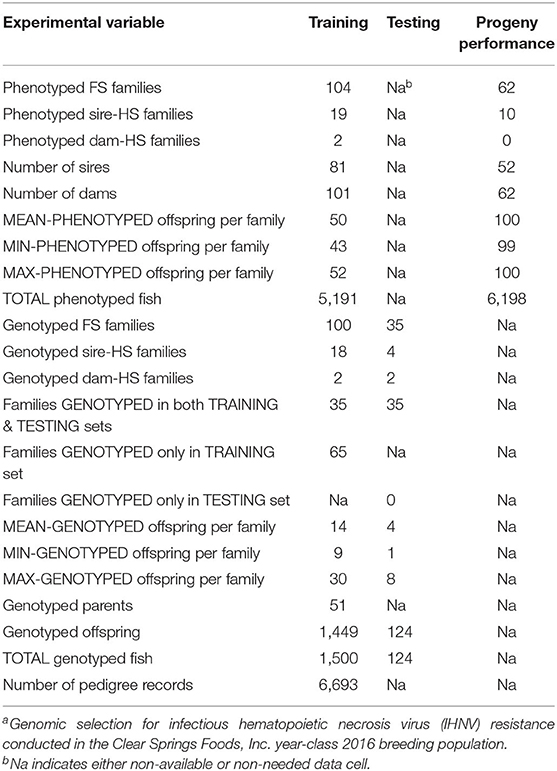
Samples were collected from disease-naïve parents and their disease challenged offspring fish in brood years 2014 and 2016, respectively, by staff at the CSF research facility in Buhl, Idaho, and processed following already described procedures (8). Briefly, healthy fish from the previous generation were artificially spawned to produce fertilized eggs from 104 families of year-class (YC) 2016. Fin tissue samples from each parent fish were collected at the time of spawning. The offspring were grown to ~1 g (62 days post-fertilization) and 50 fish per family were selected randomly for disease challenge and were infected with IHNV by immersion into a volume of water equivalent to 10x the total body weight of the fish in g containing 10,000 plaque-forming units of IHNV per mL for 1 h (IHNV isolate 220-90). After exposure, the fish were moved to 19-L tanks by family (50 fish/family/tank), because young and small fish cannot be labeled individually, and monitored for a 21-day period, with mortality recorded daily. Fin tissue samples were collected from mortalities during the 21-day monitoring period and survivor samples were taken at the end of the challenge. Fin clips from all fish (mortalities and survivors) were individually kept in 95% ethanol until DNA was isolated using published protocols (34).
Training and Testing Sample for GP
The training sample of this GP study comprised 104 pedigreed full-sib (FS) families from YC 2016 of the CSF commercial breeding company. The 104 families included 19 paternal half-sib (HS) families and two maternal HS families, and they were generated using 81 sires and 101 dams (Table 1). Sixty-three families were made by mating each of 63 sires with a single dam. Among the families that were made by mating a sire with multiple dams: 17 sires were mated with two dams, one sire was mated with three dams, and another sire was mated with four dams. Among the families that were produced by mating a dam with multiple sires: two dams were mated with two sires. These YC 2016 families represented a commercial nucleus breeding population that was undergoing intensive selection for growth and IHNV resistance for the past eight generations. The fish were evaluated for IHNV resistance in the laboratory challenge, with one tank per family with an initial stocking of 50 fish per tank. The 104 families were evaluated by groups of 7–10 families at 11 challenge dates. After the IHNV challenge, IHNV resistance phenotype records on N = 5,191 fish were collected. From the total 104 training sample families, 100 FS families were genotyped with an average of 14 offspring fish per family (range 9–30 fish) for a total of N = 1,449 genotyped training offspring fish.
The testing sample of this GP study comprised 35 FS families which were part of the genotyped 100 training FS families. Specifically, from the 100 families that were genotyped as part of the training sample, 35 families were also included in the testing sample. Thus, there were 35 families that contributed offspring fish to both the training and the testing samples. From each testing family, an average of 4 offspring fish were genotyped (range 1–8 fish) for a total of N =124 genotyped testing offspring fish (Table 1).
Progeny Testing Families
In this GS study, 62 progeny testing families (PTF) from the YC 2018 were IHNV challenged to assess the accuracy of the predicted breeding values (BVs) for fish included in the testing sample (Table 1). The 62 PTFs were created using 52 sires and 62 dams; and these 114 breeders were taken from the testing genotyped sample (i.e., these breeders are offspring of the 35 families included in the testing sample). A sample of ~100 fish from each PTF was IHNV challenged to generate IHNV disease survival records from a total of N = 6,198 fish.
IHNV Resistance Phenotypes
We had a binary survival status (STATUS) record for each evaluated fish. The resistance phenotype STATUS had two categories: 1 for fish that died during the 21 days post challenge evaluation period; and 2 for fish that survived for the duration of the challenge. The binary records of disease survival STATUS were analyzed using animal threshold models described below.
SNP Genotyping
The fish sampled from the CSF population were genotyped by a commercial service provider (AKESOgen, Norcross, GA and RUCDR, Rutgers University, Piscataway, NJ) using the Rainbow Trout Axiom 57K SNP array (Chip) following previously described procedures (8, 33). We randomly sampled survivor offspring and early dying offspring per family with an average of 14 fish per family (range of 9–30) from a total of 100 training families (offspring N = 1,449) for SNP genotyping. We also genotyped all the sires from which fin clips were available (N = 51). The dams were not sampled. As part of the testing sample, we randomly sampled an average of four offspring fish per family (range 1–8) from 35 families out of the 100 training families (offspring N = 124) for SNP genotyping (Table 1). The quality control (QC) pipeline procedures applied to the Chip-SNP genotype data was described elsewhere (23). Briefly, the QC pipeline discarded the SNPs that showed a significant distortion from the expected Mendelian segregation in each FS family (Bonferroni adjusted to P < 0.05) and also removed offspring fish that did not have matching genotypes with the parents given in the pedigree (i.e., that did not pass the pedigree check). After this initial data QC, we had genotype data for 42,045 SNPs in the raw Chip genotype dataset.
Before GS analyses, the raw marker genotype dataset was further QC filtered using computer algorithms from the software BLUPF90 (35) and procedures already described (8). Briefly, the QC retained SNPs with a genotype calling rate higher than 0.90, minor allele frequency higher than 0.05, and with departures from Hardy-Weinberg equilibrium lower than 0.15, based on the difference between expected and observed frequency of heterozygotes. Parent-progeny pairs were tested for discrepant homozygous SNPs, and SNPs with a conflict rate higher than 1% were removed from the dataset. Next, we determined the physical map location (GenBank Assembly Accession GCA_002163495.1) (36, 37) of each of the QC filtered SNPs and those that did not have a physical map location were discarded. After this final data QC, we had data on 34,640 genotyped SNPs and 1624 genotyped fish (1,449 training offspring and 124 testing offspring from YC 2016 and 51 sires from YC 2014) for GS analysis (Tables 1, 2).
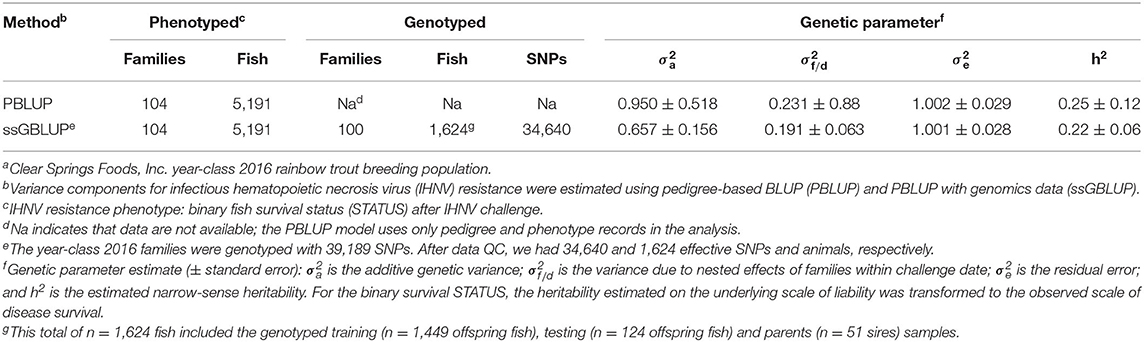
Table 2. Estimated genetic parameters for IHNV resistance in a commercial rainbow trout breeding populationa.
Estimation of Genetic Parameters for IHNV Resistance
The binary survival STATUS records (n = 5,191) were fitted to an animal threshold model to estimate genetic variance parameters for IHNV resistance. The variance components analysis was conducted using pedigree-based BLUP (PBLUP) and PBLUP with genomic information (ssGBLUP) under a Bayesian framework, using computer applications from the software BLUPF90 (35). The binary records of survival STATUS were analyzed using an animal threshold model with the software THRGIBBS1F90. We utilized the same statistical model described below in the section of GS analysis with single-step GBLUP. The Gibbs sampler collected data from a total of one million iterations, of which the first 200,000 iterations were discarded; one sample was saved from every 100 iterations from the remaining 800,000 iterations; thus, results from 8,000 independent samples were used in the analysis. The proper mixing and convergence of the Markov chain Monte Carlo (MCMC) iterations were assessed with an script written using the R package CODA (38).
The heritability for survival STATUS was calculated as: ; where h2 is the narrow-sense heritability; is the additive genetic variance; is the variance due to the nested effect of families within the challenge date; and is the residual error variance. The heritability for the binary survival STATUS estimated on the underlying scale of liability using a threshold model was transformed to the observed scale of disease survival STATUS using methods described elsewhere (23).
GS Using Single-Step GBLUP Methods
The marker genotype data from training fish and pedigree information on all fish included in this GS study were used to estimate GEBV for the genotyped testing fish sample (n = 124) using two methods: (i) ssGBLUP (17, 39); and (ii) wssGBLUP (40). These single-step methods use all available information on sampled fish, including pedigree, genotype, and phenotype records, as well as those offspring fish without genotype data, n = 3,742 (17, 39). The CSF sample used in this GS study included n = 5,191 offspring fish from 104 YC 2016 families that had IHNV resistance data (Table 1). From these 5,191 phenotyped offspring fish, a subset of 1,449 had genotype data from 34,640 effective SNPs (Table 2).
In GS with ssGBLUP, the weight for each SNP is 1 for the first iteration, which means that each SNP has the same weight (i.e., single-step GBLUP). For the following iterations (2nd, 3rd, etc.), the weights are SNP-specific variances that are calculated using the estimate of the SNP allele-substitution effect from the previous iteration and the corresponding SNP allele frequencies (24). Estimates of SNP effects were computed using a weighted relationship matrix, using this equation: , where is the vector of the estimated SNP effects; D is a diagonal matrix of weights for variances of SNP effects; M is a matrix relating genotypes of each SNP to each individual; and is the estimate of the additive genetic effect for genotyped animals. The individual variance of SNP effects, which corresponds to the diagonal elements of D, was estimated as (41): , where: is the square of the effect at SNP i, and pi is the observed allele frequency for the second allele of SNP i. In this GS study, we used results from the 1st (ssGBLUP) and the 2nd iteration (wssGBLUP), because usually the 2nd iteration estimates genomic predictions (32) and SNP effects (24, 42, 43) with the highest accuracy.
We fitted a threshold mixed model for the binary data survival STATUS using this animal model: y=1μ+Za+Wc+e, where 1 is a vector of 1s, μ is the overall mean of phenotypic records, a is a vector of random individual animal effects, c is a vector of random common environment effects, e is a vector of residual effects, and Z and W are incidence matrices relating records to random animal and common environment effects in a and c, respectively. The variances of a, c and e are:
where , and are additive genetic, common environment and residual variances, respectively, and H is a matrix that combines pedigree (A) and genomic (G) relationship matrices, as in Aguilar et al. (17), and its inverse as defined elsewhere (17, 39). The fish offspring from each FS family were assigned to one tank for IHNV challenge evaluation, so the tank and family effects were confounded. The 100 tested families were evaluated in 11 challenge dates (date), with 7–10 families per date. This nested random family/date effect was used to account for the common environment effect.
The GS for the binary survival STATUS was conducted using Bayesian methods implemented in the software BLUPF90 (35). Exactly, the GS for STATUS with ssGBLUP and wssGBLUP were conducted using the computer program THRGIBBS1F90. The Gibbs sampling scheme and the methods used to assess the correct mixing and convergence of the MCMC iterations were similar to those described in the section of estimation of genetic parameters for IHNV resistance.
GS Using Single-Step Bayesian Multiple Regression
We conducted GP for the binary IHNV survival STATUS with a single-step Bayesian multiple regression (ssBMR) method using 1-Mb non-overlapping SNP windows (25, 27). The ssBMR method uses the pedigree information and all fish that had phenotype and genotype records, as in the wssGBLUP method. In ssBMR, the genotypes for non-genotyped animals are imputed explicitly given the pedigree data, and corresponding imputation residuals are fitted in the ssBMR model. Thus, the GP with ssBMR model was conducted using the same phenotype and genotype records used for wssGBLUP (Tables 1, 2).
We fitted a threshold mixed model for the binary data STATUS using this animal model: y=1μ+Xb+Zϵϵ+Za+Wc+e; where X is an n×k matrix of observed or imputed genotype covariates for k total number of SNPs across the genome for both genotyped and non-genotyped n individuals; b is a vector of k additive SNP effects; ϵ is the imputation residuals for non-genotyped individuals, Zϵ is the design matrix allocating records to breeding values of non-genotyped individuals; and a is a vector of random polygenic effects. The other elements of the model were already described in the section of GS with ssGBLUP. Scaled inverse chi-squared distributions were used for genetic variance and residual variance as described in (25). In these priors, the degree of freedom was 4, and scaled parameters were estimated by assuming the proportion of variance of the phenotypic data explained by the regression is 0.5. The GS analysis for the binary STATUS was performed with ssBMR using the Bayesian variable selection methods BayesB and BayesC (26) implemented in the software JWAS (44).
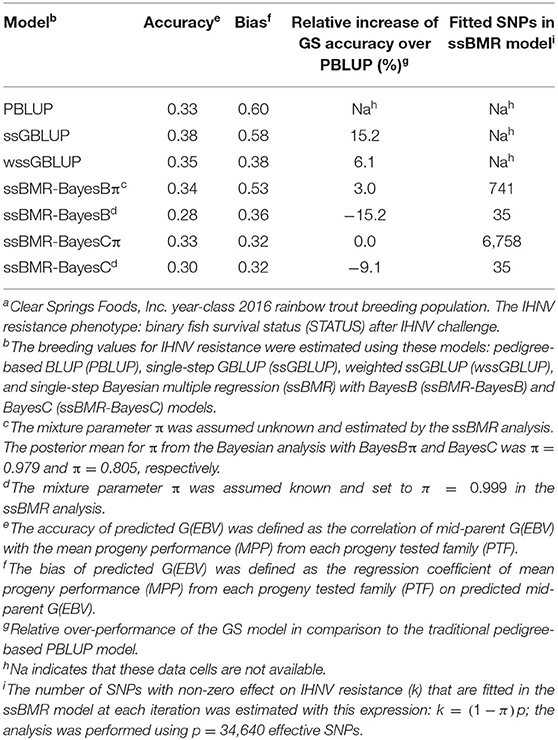
In the BayesB and BayesC, the prior assumption is that the marker effects have identical and independent mixture distributions (21). So, both methods fit a mixture model to estimate marker effects (21), which assume that there are two types of SNPs: a fraction (1−π) of SNPs with non-zero effects and another fraction (π) of SNPs that a-priori have zero effect on the quantitative trait (45). In BayesB, the variance parameter assumed for the random SNP effects is specific to each fitted locus (46). On the contrary, an effect variance that is common to all SNPs is used in BayesC (47). In this study, the parameter π was treated as unknown with a uniform prior and estimated with BayesBπ and BayesCπ models, separately, using the option “estimatePi=true” in the ssBMR analysis. We also tested the impact of treating π as known and setting it to 0.999 on the GP accuracy when performing ssBMR with BayesB and BayesC models. In a previous study with this dataset, we tested few mixture parameters (π = 0.990, 0.995, 0.999) and found that ssBMR-BayesB with π = 0.999 detected the largest number of genomic windows associated with IHNV resistance and with the largest additive genetic variance (8).
The BayesB and BayesC methods use Gibbs sampling in the GS analysis (46). The characteristics of the Gibbs sampler and diagnosis methods to test the proper mixing and convergence of the MCMC iterations were like those used in the section of estimation of genetic parameters for IHNV resistance.
Accuracy and Bias of Breeding Value Predictions
The accuracy of predicted breeding values G(EBV) was estimated as the correlation of the mid-parent G(EBV) with the mean progeny performance (MPP) for each progeny tested family (). After data QC, we had disease challenge data from 62 progeny testing families (n = 6,198 fish).
The bias of the predicted G(EBV) was estimated as the regression coefficient of MPP on the predicted mid-parent G(EBV) for each PTF. A value of 1.0 for the regression of true BV, performance phenotype or MPP on predicted G(EBV) is theoretically expected for unbiased estimates of G(EBV); and a deviation from 1.0 can be interpreted as prediction bias (48, 49). Before estimating the bias or regression coefficient, the predicted G(EBV) for the binary survival STATUS, which was estimated on the underlying scale of liability, was transformed to the observed scale. The categorical data analysis performed with the software BLUPF90 and JWAS uses a probit link function; consequently, the estimated G(EBV) was transformed to the standard normal cumulative distribution function (CDF) to estimate the probability of survival (50, 51).
Relatedness Between Training and Testing Animals in GS Studies
We calculated relatedness between training and testing animals using genomic and pedigree data in the current GS study for IHNV resistance. First, the genomic (G) and pedigree-based relationship (A22) matrices were calculated using the software BLUPF90 (35); the A22 is a pedigree-based relationship matrix only for genotyped animals (52, 53). Then, we calculated summary statistics (e.g., sum, average, variance, standard deviation, minimum, maximum and total number of relationships) for sub-elements of the A22 and G matrices, separately, using a recently developed Fortran90 software which is on request available from the authors.
Likewise, we also computed relatedness between training and testing animals in our already published GS study on resistance to bacterial cold water disease (BCWD) (23) using the above outlined methods. From this published BCWD study, the dataset in which both the training and testing sample sets included siblings from the same 25 families (each with ~40 offspring fish) was used to estimate relatedness between training and testing animals. Subsequently, we compared the relatedness between training and testing animals estimated in the current GS for IHNV resistance study and the previously published GS for BCWD resistance study.
Linkage Disequilibrium and Effective Population Size Estimates
The linkage disequilibrium (LD) analysis comprised 100 unrelated fish from the YC 2016 families from the CSF commercial breeding population. We developed five-replicated samples each with 100 unrelated individuals by randomly sampling, with replacement, one offspring fish from each of the 100 YC 2016 families. Thus, the LD analysis was performed using data from 100 unrelated fish (n = 100) genotyped with 34,640 effective SNPs. It should be noted that the genotype data that we used in the LD analysis was a subset of the training sample that we used for the GP with 100 genotyped YC 2016 families (9–30 offspring per family).
The LD was estimated using described procedures (54). Briefly, the LD was computed as the Pearson's squared correlation coefficient (r2) for each pair of allele counts at two linked loci on a chromosome, and the r2 was estimated for adjacent SNPs within each chromosome. The LD was estimated using the computer program PREGSF90 (55) from the software BLUPF90 (35) using the following expression: ; where D = p11p22 − p12p21 corresponded to the frequency of the genotypes; p and q are the alleles frequencies. The r2 estimates were adjusted for experimental sample size with this expression: , where n is the number of unrelated individuals used in the LD analysis (56).
The LD decay with physical distance was estimated using already described procedures (54). Briefly, the LD decay with distance between markers was estimated with the Sved's equation (57): ; where LDij is the estimated LD for the marker-pair i and j; k is a constant based on the type of chromosome used in the LD analysis (k =4 for autosomes); Net is the effective population size for chromosome t and dij is the distance between markers i and j. The Netwas calculated using the Saura's expression (56): ; where dt is the average length of chromosome t in Morgan units; is the average LD of the chromosome t; (2n)−1 is the adjustment term for experimental sample size; and α is a fixed parameter related to mutation (1= absence of mutation; 2= presence of mutation), and we used α = 2.
Results
Heritability of IHNV Resistance
The estimates of narrow-sense heritability (h2) were 0.22 and 0.25 for ssGBLUP and PBLUP models, respectively (Table 2). The estimate of heritability using genomic data (h2 = 0.22 ± 0.06) was somewhat lower and with smaller standard error than the estimate with the pedigree-based model (h2 = 0.25 ± 0.12).
Accuracy and Bias of BV Predictions for IHNV Resistance
The accuracy of BV predictions was higher for the GP models ssGBLUP (0.38), wssGBLUP (0.35) and ssBMR-BayesBπ (0.34) than for the pedigree-based PBLUP model (0.33), and the PBLUP and ssBMR-BayesCπ models had similar accuracy (Table 3). However, the PBLUP model (0.33) had higher accuracy GP than the ssBMR models BayesB (0.28) and BayesC (0.30) when using a mixture parameter of π = 0.999. Among the GP models, both single-step GBLUP models (ssGBLUP and wssGBLUP) had higher accuracy of GP than the four tested ssBMR models (Figure 1). Among the ssBMR models, the BayesBπ and BayesCπ (0.33–0.34) had higher GP accuracy than the BayesB and BayesC (0.28–0.30) when using a mixture parameter of π = 0.999 (Table 3 and Additional Table S3).
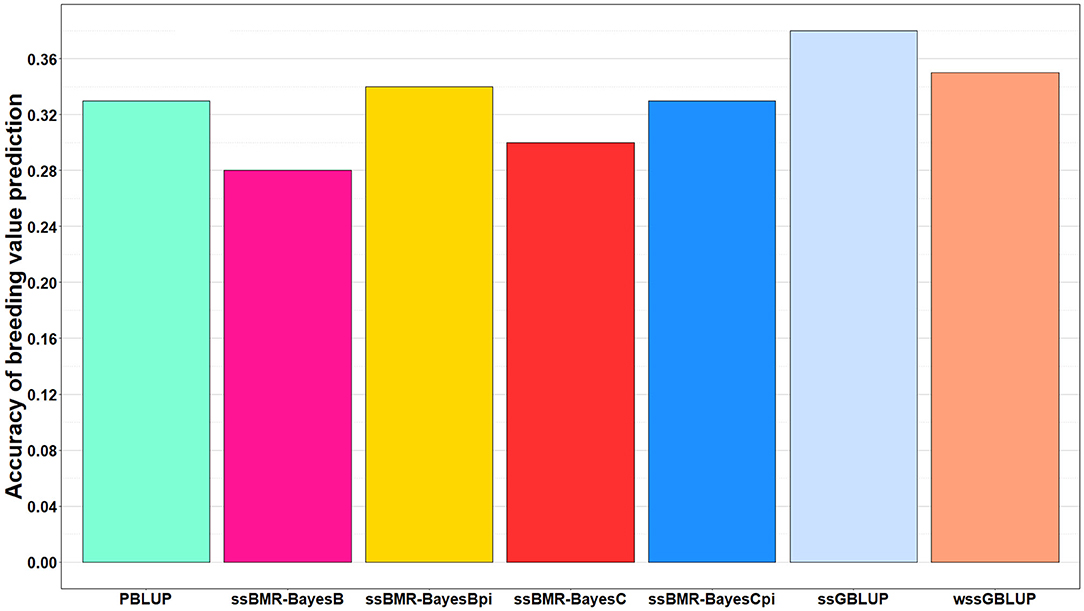
Figure 1. Accuracy of breeding value predictions for IHNV resistance using multiple regression based single-step methods and pedigree-based PBLUP. The genomic breeding value predictions were performed with single-step GBLUP (ssGBLUP), weighted ssGBLUP (wssGBLUP) and four single-step Bayesian multiple regression models (TIFF file).
The relative change in accuracy of GP methods compared to the pedigree-based PBLUP model is shown in Figure 2. The accuracy of predictions with the ssGBLUP, wssGBLUP and ssBMR-BayesBπ models outperformed the PBLUP model, by 15, 6, and 3%, respectively (Table 3). Conversely, the accuracy of predictions with PBLUP outperformed the BayesB and BayesC models when using a mixture parameter of π = 0.999.
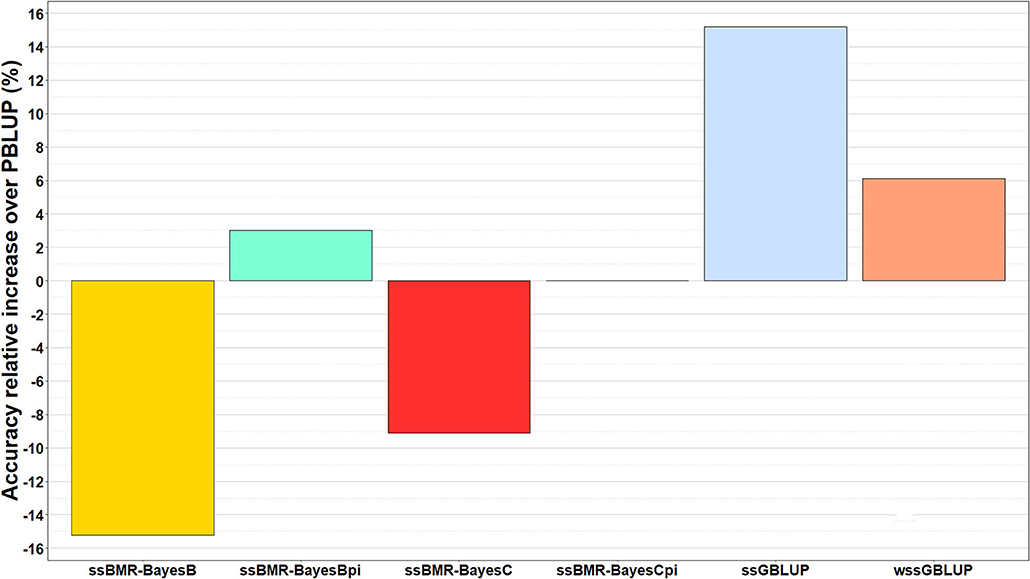
Figure 2. Relative increase in accuracy of genomic predictions over those estimated with pedigree-based PBLUP model. The genomic breeding value predictions were performed with single-step GBLUP (ssGBLUP), weighted ssGBLUP (wssGBLUP) and four single-step Bayesian multiple regression models (TIFF file).
The bias of BV predictions was lower (i.e., closer to 1.0) for PBLUP (0.60), ssGBLUP (0.58) and ssBMR-BayesBπ (0.53) than for wssGBLUP (0.38) and ssBMR-BayesB (0.36) (Table 3). The ssBMR-BayesC and ssBMR-BayesCπ models had the most biased GP (0.32). Overall, the predicted breeding values for IHNV resistance were biased downwards in this study (i.e., average bias of 0.44).
Estimates of Relatedness Between Training and Testing Animals
To assess why the accuracy of genomic BV predictions in this study was lower than in our previous published study on resistance to bacterial cold water disease (BCWD) (23), we quantified the level of relatedness between the training and testing fish in both studies. The summary of the genomic (G) and pedigree-based (A22) measures of relatedness between training and testing animals calculated in the GS studies for IHNV resistance and BCWD resistance are presented in Table 4. Although the number of effective or high quality polymorphic SNPs was similar in both GS studies (~35K SNPs), the number of total genotyped animals (training and testing fish) was higher in the BCWD study (n = 1, 900) (23) than in the current IHNV study (n = 1, 573). However, the number of genotyped training animals with phenotypes was higher in the IHNV study (n = 1, 449) than in the BCWD study (n = 979) (Table 4).
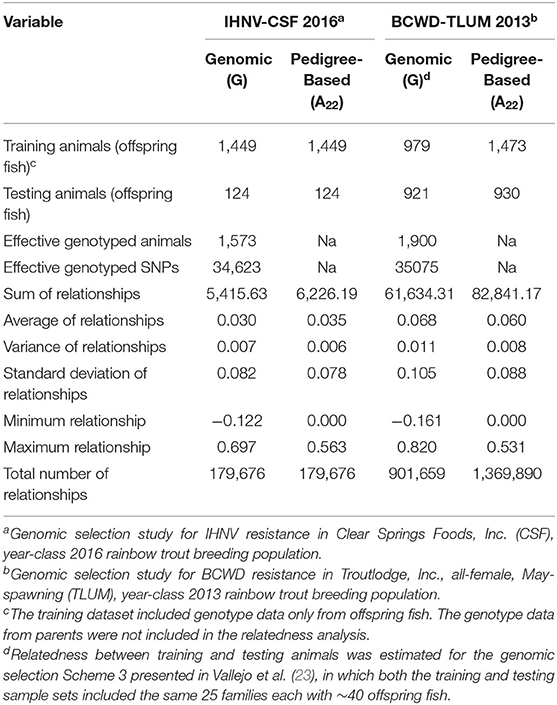
Table 4. Genomic and pedigree-based relationship between training and testing animals in genomic selection studies.
The sum of genomic and pedigree-based relationships between training and testing animals was significatively higher in the GS for BCWD resistance (ΣGij = 61, 634; ΣAij = 82, 841) than in the current GS for IHNV resistance (ΣGij = 5, 416; ΣAij = 6, 226). Likewise, the average of genomic and pedigree-based relationships between training and testing animals was substantially higher in the BCWD resistance study than in this study . The maximum of genomic relationships between training and testing animals was higher in the BCWD study (max Gij = 0.820) than in this study (max Gij = 0.697). However, the maximum of pedigree-based relationships was slightly higher in this study (max Aij = 0.563) than in the BCWD study (max Aij = 0.531).
Estimates of Linkage Disequilibrium and Effective Population Size
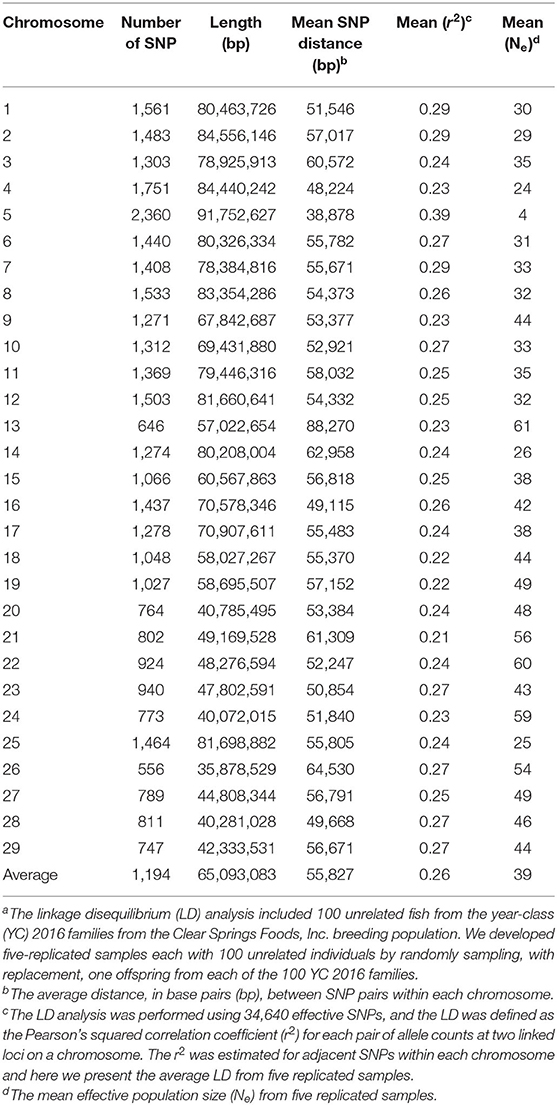
We evaluated the extent of LD and effective population size (Ne) in this rainbow trout commercial population to evaluate the impact of these population genetic parameters on the estimated GP accuracy. The mean LD (r2), distance between analyzed loci-pairs in base pairs (bp), and Ne estimated for each rainbow trout chromosome in the current study for IHNV resistance is presented in Table 5. The estimated whole-genome average LD was r2 = 0.26. The average LD per chromosome ranged from 0.21 to 0.39, with average strong LD of r2 ≥ 0.25 on 17 of the 29 chromosomes.
Noticeably, chromosome Omy5 had the highest mean LD (r2 = 0.39) followed by Omy1, Omy2 and Omy7 (r2 = 0.29) (Table 5). Chromosomes Omy21 (r2 = 0.21) and Omy18 and Omy19 (r2 = 0.22) had the lowest mean LD estimates. The average distance between two adjacent SNPs ranged from 38,878 to 88,270 bp per chromosome with a genome-wide average distance of 55,827 bp.
The average effective population size was Ne = 39 (Table 5). Chromosomes Omy13 (Ne = 61) and Omy22 (Ne = 60) had the largest effective population size. Chromosome Omy5 had the smallest effective population size (Ne = 4). Relatively small effective population size was also estimated for chromosomes Omy4, Omy14 and Omy25 (Ne = 24 − 26).
The LD decay with physical distance estimated for each of the 29 rainbow trout chromosomes using the ~35K SNP panel are presented in Figure 3. Overall, strong level of LD (r2 ≥ 0.25) extended over 5 Mb on all the 29 chromosomes. Strikingly, the strong level of LD extended over 40 Mb on chromosome Omy5. In addition, the strong level of LD extended over 20 Mb on chromosomes Omy4, Omy14 and Omy25.
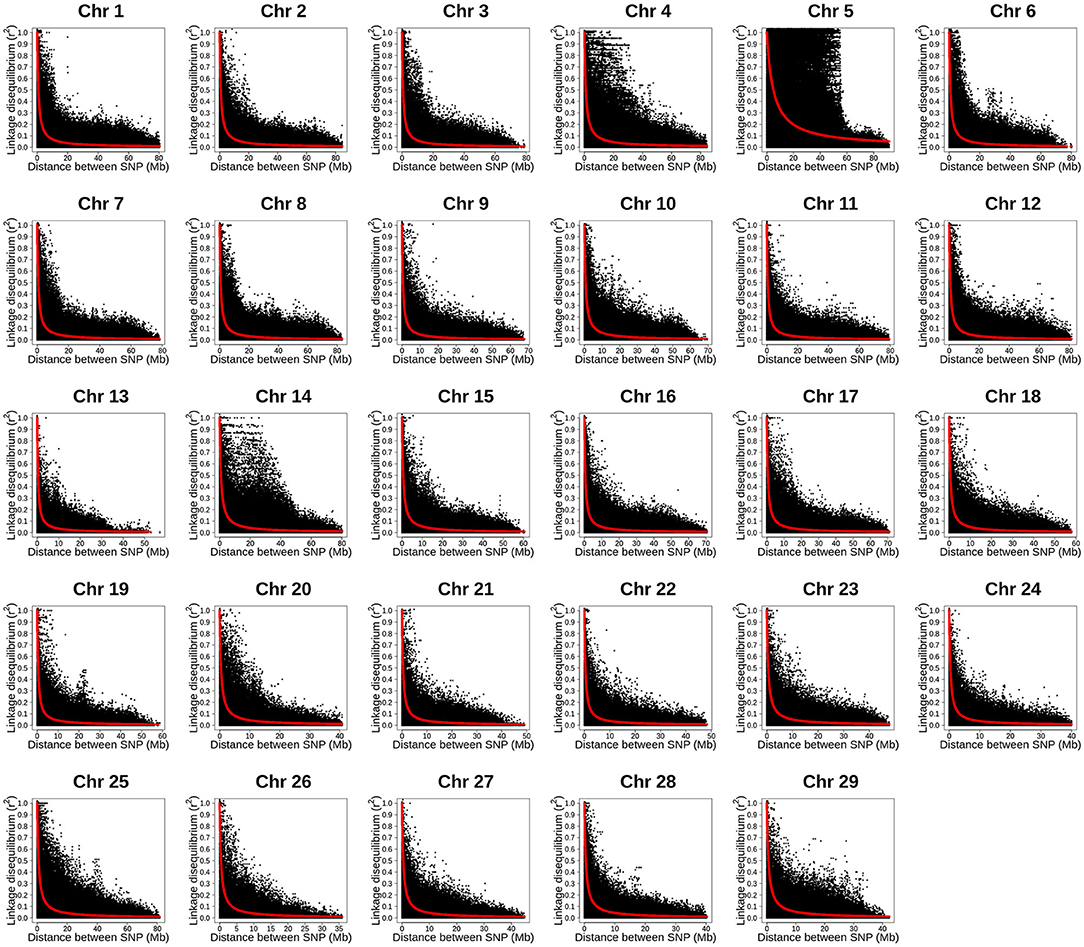
Figure 3. Linkage disequilibrium decay with physical distance estimated by chromosome using 34K SNP panel in year-class 2016 families from Clear Springs Foods, Inc. rainbow trout breeding population. For the LD analysis, in five replicates, one fish from each of the 100 YC 2016 families was randomly sampled with replacement; so, each genotype data set used for LD analysis included 100 unrelated fish. Here, we present results LD decay with physical distance from replication 5 (TIFF file).
Discussion
In this study, for the first time using empirical progeny testing data, we found that the gain in GP accuracy achieved with ssGBLUP and wssGBLUP was higher than traditional pedigree-based PBLUP by 15 and 6%, respectively. Thus, these results highlight the potential for effective genetic improvement of resistance against IHNV using genome-enabled selective breeding methods in this rainbow trout breeding population. In addition, our results confirmed the importance of designing GS studies that warrant a high genomic relationship between training and testing animals to estimate high accuracy GP. Furthermore, we found that ssGBLUP had higher accuracy GP than wssGBLUP and ssBMR which suggest that the underlying liability to IHNV resistance might be polygenic. Taken together, these results indicate that whole genome-enabled BV prediction models will be more effective than traditional pedigree-based prediction model or the marker-assisted selection method for improving genetic resistance against IHNV in this commercial rainbow trout population.
Heritability of IHNV Resistance
The heritability estimates for IHNV resistance in our study were moderate (h2 = 0.22 − 0.25) (Table 2) and much lower than those reported already using pedigree-based model in a different hatchery rainbow trout population that was naïve to IHNV with no previous history of selective breeding for the trait (2). Our estimates of heritability underline the potential for genetic improvement for IHNV resistance through family-based selective breeding in this commercial rainbow trout population. In this study, the heritability estimate based on genomic data (ssGBLUP) was lower (h2 = 0.22 ± 0.06) than those based on PBLUP (h2 = 0.25 ± 0.12). However, the heritability estimated with ssGBLUP had much lower standard error than the heritability estimated with PBLUP.
Strikingly, our estimated heritability for IHNV survival STATUS using genomic data was much lower (h2 = 0.22 ± 0.06) than the heritability reported by Brieuc et al. (2) for IHNV mortality using a pedigree-based model (h2 = 0.38; 95% CI [0.23, 0.55]). Our studied population has been selected for IHNV resistance for eight generations (Richard Towner, unpublished results) and the population used by Brieuc et al. (2) was not under selective breeding pressure for resistance to IHNV, and therefore had larger genetic variation for IHNV resistance than in this study.
Accuracy and Bias of Predicted Animal Breeding Values
Recently, we have shown that the ssGBLUP and wssGBLUP had higher accuracy of BV predictions for resistance to IHNV than PBLUP using an stochastic CV analysis (8) and in this study we confirmed those findings using empirical progeny performance records. The estimated GP accuracy for resistance to IHNV was similar between the two studies. In our previous study, the GP accuracy with wssGBLUP (0.39) was higher than with ssGBLUP (0.34), while in the current study the GP accuracy with ssGBLUP (0.38) was higher than wssGBLUP (0.35) (Additional Table S1). The GP accuracy estimated with progeny performance data in this study is more reliable than that estimated with CV analysis (23, 32) because it has been shown that the correlation of mid-parent GEBV with the mean progeny performance for each PTF is a reliable estimator of the accuracy of the predicted breeding values (29, 30).
The accuracy of BV predictions was higher with PBLUP in the present study using progeny performance data (0.33) than in our previous study using CV analysis (0.24) (8). Due to the increased accuracy of predictions with PBLUP in this study, the relative increase in GP accuracy over the pedigree-based PBLUP in this study (6.1–15.2%) was lower than in our previous study (41.7–62.5%) (8). Interestingly, the accuracy of BV predictions for resistance to IHNV we estimated with PBLUP in this study was similar to the accuracy of PBLUP BV predictions we have previously estimated for bacterial cold water disease (BCWD) resistance in a different rainbow trout population (23). However, the estimates of GP accuracy for resistance to IHNV in this study (0.28–0.38) were lower than the accuracy estimates of genomic predictions for BCWD resistance (0.66–0.71) (Additional Table S1). The lower GP accuracy estimated in this IHNV study was due to the sub-optimal GS study design caused by logistical problems and other limitations that were imposed on the study design by the constraints of the ongoing commercial rainbow trout breeding program. One major disadvantage in the IHNV study was that DNA samples and genotype data could not be obtained from weekend mortalities, which together with other missing samples accounted for ~38% of the fish that were phenotyped as dead or susceptible to the disease. While the phenotype records from those fish were available for the PBLUP model, we were unable to obtain genomic information from them, which has substantially reduced the power of the GS models to estimate SNP effects on disease susceptibility. Another major difference was the higher relatedness between the training and testing fish in the BCWD study, which we quantified and later discuss with more detail.
Furthermore, the IHNV and BCWD GS studies differed in these experimental design variables (Additional Table S2): (i) number of phenotyped fish in the training sample (BCWD: n = 7,893; IHNV: n = 5,191); (ii) sibship size of the training families (BCWD: mean genotyped offspring per family = 29, range 19–40; IHNV: mean genotyped offspring per family = 14; range 9–30); (iii) sibship size of the testing families (BCWD: mean genotyped offspring per family = 31, range 1–44; IHNV: mean genotyped offspring per family = 4, range 1–8); and (iv) number of genotyped fish in testing sample (BCWD: n = 930; IHNV: n = 124). Consequently, the GS for BCWD resistance afforded higher statistical power to detect marker effects than the GS for IHNV resistance. In addition, another major factor which might have determined the noticeable difference on GP accuracy between these two GS studies was the genetic architecture of the studied traits. The genetic resistance against IHNV might be polygenic in the CSF 2016 population, and thus may require larger size of training sample for high accuracy GP (Daniela Lourenco, personal communication), whereas the genetic resistance against BCWD had an oligogenic architecture in the studied population. Previously, we have found that oligogenic trait architecture is advantageous for estimating high accuracy GP using relatively small-moderate training sample size (23).
The bias of BV predictions for the binary survival STATUS (i.e., IHNV resistance) estimated with CV analysis is incorrect and, consequently, was the most biased (i.e., bias farther from 1.0) (Additional Table S1) (8) due to extreme-phenotype problems in which all binary observations within class variables are identical (i.e., each individual has one binary survival phenotype record of either 0 or 1) (31). In this study, the above problem was avoided by estimating the accuracy and bias of BV predictions using empirical progeny testing data (i.e., offspring survival rate per IHNV challenged progeny testing family) (23, 32). Thus, the BV predictions for IHNV resistance in this study had much lower bias (i.e., bias closer to 1.0) than those estimated with CV analysis (i.e., bias farther from 1.0). However, the bias estimates of predictions in this study were higher than those in a GS study for BCWD resistance (i.e., bias closer to 1.0) conducted in a different rainbow trout population (Additional Table S1) (23).
Overall, the gain in GP accuracy obtained with ssGBLUP and wssGBLUP was 15 and 6%, respectively, higher than using traditional pedigree-based PBLUP. Thus, these results highlight the potential for effective genetic improvement of IHNV genetic resistance in this rainbow trout breeding population using genome-enabled selective breeding method.
Comparison of Multiple Regression Single-Step GS Methods
The usage of accurate statistical methods and computer algorithms is key for elucidating the genetic basis of resistance to complex diseases and GP using GWAS and GS, respectively. In this study, genomic predictions for IHNV resistance were computed using two multiple regression single-step GS methods that calculate the effect of all markers simultaneously, thus accounting for LD between neighboring loci (21, 46, 58). A unique feature of these multiple regression single-step based methods is that they use all available pedigreed animals with genotype and/or phenotype records in the GWAS and GS analysis. Thus, they have higher power of marker effect detection than those methods that do not use a single-step method and test for association using one-marker at a time without accounting for LD between neighboring loci and without using phenotypes on non-genotyped relatives.
Among the GS models, ssGBLUP had the highest GP accuracy for IHNV resistance (Table 3) followed by GS models (wssGBLUP and ssBMR models) expected to be powerful when analyzing traits that are controlled by few moderate-large effect QTL (i.e., oligogenic inheritance trait). In addition, among the ssBMR models, we found that models that fit a higher number of SNPs (BayesBπ and BayesCπ) had higher prediction accuracy than those that fit a lower number of SNPs (BayesB and BayesC) (Additional Table S3). These results suggest that the genetic resistance against IHNV must be more polygenic than oligogenic. In a previous study, we found that IHNV resistance was controlled by few loci with moderate effects (EGV = 2.0–8.8%) and a large-unknown number of minute-effect loci (8), which further support the hypothesis of polygenic inheritance for IHNV resistance in rainbow trout.
The observed difference in GP accuracy between the two single-step based GS methods (ssGBLUP/wssGBLUP and ssBMR) is due to differences in the underlying model assumptions and the genetic architecture of the analyzed trait. In this study, we evaluated GP models that assume a purely polygenic inheritance for IHNV resistance and a normal distribution of the marker effects such as ssGBLUP, and we also evaluated GS methods such as ssBMR and wssGBLUP, which assume that the trait genetic variance is explained by a reduced number of moderate-large effect QTL, instead of purely polygenic inheritance (25, 59, 60). Indeed, the Bayesian variable selection models run with ssBMR was shown more powerful than standard mixed linear GBLUP-based models when the trait under study is controlled by few loci with moderate-large effect and many minute-effect loci, i.e., oligogenic inheritance trait (23, 60, 61). In this study, ssGBLUP had higher GP accuracy than wssGBLUP and Bayesian variable selection models run with ssBMR. In addition, ssBMR models that fit a high number of non-null effect SNPs (BayesBπ with 741 SNP, and BayesCπ with 6,758 SNP) had higher GP accuracy than ssBMR models that fit a low number of SNPs (BayesB and BayesC each with 35 SNP). Thus, these results suggest that the underlying liability of genetic resistance against IHNV might be polygenic, which imply that the heritable component of IHNV resistance is due to thousands of loci each having a minor effect on liability to IHNV resistance.
Impact of Relatedness Between Training and Testing Animals on GP Accuracy
The most critical factors for estimating high accuracy GP when performing intra-population GS are (i) the extent of LD between markers and QTL; (ii) the training population sample size; and (ii) the degree of relatedness between the training and testing animals (14, 62, 63). In this study, we computed pedigree-based (A22) and genomic (G) relationship between training and testing individuals for two GS studies (Table 4), separately, and found that the average and sum of genomic and pedigree-based relationships in the current GS for IHNV resistance was substantially lower than in the GS for BCWD resistance (23). Intuitively, the level of relatedness between training and testing individuals is defined by the GS study design; and clearly, the design features of the GS for BCWD resistance ensured a higher level of relatedness between the training and testing animals than in the GS for IHNV resistance (Additional Table S2). Thus, the lower level of relatedness between the training and testing fish in the current IHNV resistance study likely contributed to the lower GP accuracy estimates in comparison with the BCWD study.
With simulation-based studies using empirical data from two of our GS studies for BCWD resistance using 100 and 138 families from YC 2013 and YC 2015, respectively, from the TLUM population, we determined that we need to evaluate 20–40 FS offspring per training (and testing) family to compute GP with high accuracy (unpublished results). Thus, when performing intra-population GS with rainbow trout, if the breeding goal is to make population-wide inferences on GP, then we should aim to include in the training and testing sample a large collection of families each with small family sibship size, i.e., 150 families each with ~10 offspring. However, if the breeding goal is to make high accuracy GP in a subset of the population (i.e., pre-selected top performance families with candidate breeders for the next generation), then we should include in the training and testing sample a reduced number of families each with a reasonably large family sibship size, i.e., 30–50 families each with 20–40 offspring. Furthermore, we should include full-sibs from the same families in the training and testing sets to maximize the degree of relationship between the training and testing animals, and thus estimate genomic predictions with high accuracy.
Linkage Disequilibrium and Effective Population Size
In this study, we estimated for the first time genome-wide distribution of LD and its decay with physical distance using a dense 34K SNP panel in the CSF YC 2016 rainbow trout breeding population. The mean LD across the rainbow trout chromosomes was high (r2 = 0.26). The average LD estimated in this population was high and similar to the average LD estimated in the TLUM population (r2 = 0.27) (54). Chromosome Omy5 had the highest average LD (r2 = 0.39) and also the lowest effective population size (Ne = 4). Similarly, Omy5 had also the highest LD (r2 = 0.44) and lowest effective population size (Ne = 14) in the TLUM population (54). In Omy5, the higher than average LD is probably caused by large chromosomal inversions detected in other studies, which prevented recombination in fish that are heterozygous to the inversion (37). Overall, this CSF rainbow trout population had an average effective population size of Ne = 39 which was smaller than the estimates of Ne = 145 and Ne = 155 in the NCCCWA (64) and TLUM (54) rainbow trout populations, respectively.
We found that the strong LD level (r2 ≥ 0.25) extended over 5 Mb in all the rainbow trout chromosomes, over 40 Mb on Omy5 and over 20 Mb on Omy4, Omy14, and Omy25 in the CSF YC 2016 rainbow trout population. In the past, we found that the level of strong LD decayed rapidly at distances >2 cM which is equivalent to ~1.2 Mb in the NCCCWA rainbow trout breeding population (64). Recently, we found strong level of LD extending over 1 Mb on all the chromosomes and over 25 Mb on Omy5 in the TLUM rainbow trout population using 34K SNP panel (54). Therefore, our results confirmed that the extent of long-range LD in this commercial rainbow trout population was as high as those observed in two other rainbow trout breeding populations.
Taken together, we found that the current GS for IHNV resistance and the GS for BCWD resistance (23) studies had similar levels of genome-wide average LD (0.26 vs. 0.27) and total samples size of training genotyped fish (1,500 vs. 1,570), although a different samples size of phenotyped fish (5,191 vs. 7,893) (Additional Table S2). However, the average relatedness between the training and testing animals was substantially lower in the current GS for IHNV resistance than in the GS for BCWD resistance with genetic/pedigree relatedness of 0.035 vs. 0.060 and genomic relatedness of 0.030 vs. 0.068 for the IHNV and BCWD studies, respectively (Table 4). Clearly, the level of relatedness between training and testing animals on each GS study was imposed by the study design (Additional Table S2). Therefore, as already suggested for terrestrial livestock species (14, 62, 63), our results confirm the importance of designing optimal GS studies which warrant a high relatedness between training and testing animals to estimate genomic predictions with high accuracy in rainbow trout.
Conclusion
Our current study on genomic selection for improving resistance to IHNV in rainbow trout using empirical progeny testing data and multiple regression single-step methods found that genomic selection models improved the accuracy of breeding value predictions by 3–15% over the pedigree-based PBLUP. Furthermore, we found that ssGBLUP had higher GP accuracy than wssGBLUP and ssBMR models which suggest that the underlying liability of genetic resistance against IHNV in this rainbow trout breeding population might be polygenic. Therefore, our results highlight that the GP models will be more effective than either the traditional pedigree-based PBLUP model or the marker-assisted selection method for improving genetic resistance against IHNV in rainbow trout aquaculture.
Data Availability Statement
The original contributions presented in the study are included in the article/Supplementary Materials, further inquiries can be directed to the corresponding author/s.
Ethics Statement
Ethical review and approval was not required for the animal study because this study used rainbow trout fin clips collected after controlled exposure to IHNV as part of a selective breeding program at the CSF research facility. As farm animals used in a commercial breeding program, these fish are exempted from regulation under the U.S. Animal Welfare Act and therefore not subject to oversight by an Institutional Animal Care and Use Committee or other such ethics committee. This exemption is defined in U.S. Code title 7, chapter 54, section 2,132 g.
Author Contributions
YP, RV, JM, and RT conceived and planned the study. JM and RT coordinated and supervised the disease challenges and samples collection. RT provided the pedigree and phenotype records and KS and RL processed the samples and identified the fish for genotyping based on pedigree and phenotype records. YP coordinated and supervised samples processing and SNP chip genotyping. GG performed genotype data quality control and bioinformatics filtering and developed a database pipeline to assemble genotype and phenotype records. RV planned and executed the statistical analyses for the genetic and GS data, and wrote the first draft of this manuscript. BF provided support in performing GS with ssGBLUP and wssGBLUP models, and develop a Fortran software (that uses routines from the BLUPF90 software) to compute relatedness between training and testing animals. HC provided support in performing GS with ssBMR models. All authors read and approved the final manuscript.
Funding
This research project was supported by funds from the USDA Agricultural Research Service in house project number 8082-31000-012. HC's work was funded by the USDA, Agriculture and Food Research Initiative National Institute of Food and Agriculture Competitive Grant No. 2018-67015-27957.
Conflict of Interest
RT and JM were employed by the company Clear Springs Foods, Inc.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
We thank Bill Shewmaker, Robin Burkhart and Amanda Smith for fish rearing, phenotyping, and sampling. The authors are very grateful to Andre L. S. Garcia, Rafael M. O. Silva, Daniela Lourenco, Shogo Tsuruta, and Ignacy Misztal for their excellent support on performing GP with single-step GBLUP models and LD analysis with software BLUPF90. We gratefully acknowledge the Frontiers in Genetics Associate Editor and Reviewers for their constructive critique and outstanding suggestions to improve this manuscript. Mention of trade names or commercial products in this publication is solely for the purpose of providing specific information and does not imply recommendation or endorsement by the U.S. Department of Agriculture. USDA is an equal opportunity provider and employer.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fvets.2020.590048/full#supplementary-material
References
2. Brieuc MS, Purcell MK, Palmer AD, Naish KA. Genetic variation underlying resistance to infectious hematopoietic necrosis virus in a steelhead trout (Oncorhynchus mykiss) population. Dis Aquat Organ. (2015) 117:77–83. doi: 10.3354/dao02933
3. Khoo SK, Ozaki A, Nakamura F, Arakawa T, Ishimoto S, Nickolov R, et al. Identification of a novel chromosomal region associated with infectious hematopoietic necrosis (IHN) resistance in rainbow trout Oncorhynchus mykiss. Fish Pathol. (2004) 39:95–101. doi: 10.3147/jsfp.39.95
4. Yu ZH, Deng ML, Geng Y, Zhou Y, Wang KY, Chen DF, et al. An outbreak of infectious haematopoietic necrosis virus (IHNV) infection in cultured rainbow trout (Oncorhynchus mykiss) in Southwest China. Aquaculture Res. (2016) 47:2355–62. doi: 10.1111/are.12680
5. Jia P, Breyta RB, Li Q, Qian X, Wu B, Zheng W, et al. Insight into infectious hematopoietic necrosis virus (IHNV) in Chinese rainbow trout aquaculture from virus isolated from 7 provinces in 2010-2014. Aquaculture. (2018) 496:239–46. doi: 10.1016/j.aquaculture.2018.06.062
6. Busch RA. Viral Disease Considerations in the Com Mercial Trout Industry in Idaho. Portland, OR: Bouneville Power Administration Special Publication (1983).
7. Brudeseth BE, Castric J, Evensen O. Studies on pathogenesis following single and double infection with viral hemorrhagic septicemia virus and infectious hematopoietic necrosis virus in rainbow trout (Oncorhynchus mykiss). Vet Pathol. (2002) 39:180–9. doi: 10.1354/vp.39-2-180
8. Vallejo RL, Cheng H, Fragomeni BO, Shewbridge KL, Gao G, MacMillan JR, et al. Genome-wide association analysis and accuracy of genome-enabled breeding value predictions for resistance to infectious hematopoietic necrosis virus in a commercial rainbow trout breeding population. Genet Sel Evol. (2019) 51:47. doi: 10.1186/s12711-019-0489-z
9. Troyer RM, LaPatra SE, Kurath G. Genetic analyses reveal unusually high diversity of infectious haematopoietic necrosis virus in rainbow trout aquaculture. J Gen Virol. (2000) 81:2823–32. doi: 10.1099/0022-1317-81-12-2823
10. Campbell NR, LaPatra SE, Overturf K, Towner R, Narum SR. Association mapping of disease resistance traits in rainbow trout using restriction site associated DNA sequencing. G3. (2014) 4:2473–81. doi: 10.1534/g3.114.014621
11. Palti Y, Nichols KM, Waller KI, Parsons JE, Thorgaard GH. Association between DNA polymorphisms tightly linked to MHC class II genes and IHN virus resistance in backcrosses of rainbow and cutthroat trout. Aquaculture. (2001) 194:283–9. doi: 10.1016/S0044-8486(00)00526-3
12. Rodriguez MF, LaPatra S, Williams S, Famula T, May B. Genetic markers associated with resistance to infectious hematopoietic necrosis in rainbow and steelhead trout (Oncorhynchus mykiss) backcrosses. Aquaculture. (2004) 241:93–115. doi: 10.1016/j.aquaculture.2004.08.003
13. Barroso RM, Wheeler PA, LaPatra SE, Drew RE, Thorgaard GH. QTL for IHNV resistance and growth identified in a rainbow (Oncorhynchus mykiss) x Yellowstone cutthroat (Oncorhynchus clarki bouvieri) trout cross. Aquaculture. (2008) 277:156–63. doi: 10.1016/j.aquaculture.2008.03.001
14. Habier D, Fernando RL, Dekkers JC. The impact of genetic relationship information on genome-assisted breeding values. Genetics. (2007) 177:2389–97. doi: 10.1534/genetics.107.081190
15. VanRaden PM. Efficient methods to compute genomic predictions. J Dairy Sci. (2008) 91:4414–23. doi: 10.3168/jds.2007-0980
16. Goddard ME, Hayes BJ, Meuwissen THE. Using the genomic relationship matrix to predict the accuracy of genomic selection. J Animal Breed Genet. (2011) 128:409–21. doi: 10.1111/j.1439-0388.2011.00964.x
17. Aguilar I, Misztal I, Johnson DL, Legarra A, Tsuruta S, Lawlor TJ. Hot topic: A unified approach to utilize phenotypic, full pedigree, and genomic information for genetic evaluation of Holstein final score. J Dairy Sci. (2010) 93:743–52. doi: 10.3168/jds.2009-2730
18. Legarra A, Christensen OF, Aguilar I, Misztal I. Single Step, a general approach for genomic selection. Livestock Sci. (2014) 166:54–65. doi: 10.1016/j.livsci.2014.04.029
19. Hayes BJ, Visscher PM, Goddard ME. Increased accuracy of artificial selection by using the realized relationship matrix. Genetics Res. (2009) 91:47–60. doi: 10.1017/S0016672308009981
20. de los Campos G, Vazquez AI, Fernando R, Klimentidis YC, Sorensen D. Prediction of complex human traits using the genomic best linear unbiased predictor. PLoS Genet. (2013) 9:1003608. doi: 10.1371/journal.pgen.1003608
21. Fernando RL, Garrick DJ. Bayesian methods applied to GWAS. In: C Gondro, Jvd Werf, and B Hayes, editors. Genome-Wide Association Studies and Genomic Prediction. New York, NY: Humana Press (2013). p. 237–74. doi: 10.1007/978-1-62703-447-0_10
22. Howard JT, Jiao S, Tiezzi F, Huang Y, Gray KA, Maltecca C. Genome-wide association study on legendre random regression coefficients for the growth and feed intake trajectory on Duroc Boars. BMC Genet. (2015) 16:59. doi: 10.1186/s12863-015-0218-8
23. Vallejo RL, Leeds TD, Gao G, Parsons JE, Martin KE, Evenhuis JP, et al. Genomic selection models double the accuracy of predicted breeding values for bacterial cold water disease resistance compared to a traditional pedigree-based model in rainbow trout aquaculture. Genet Sel Evol. (2017) 49:17. doi: 10.1186/s12711-017-0293-6
24. Lourenco DAL, Tsuruta S, Fragomeni BO, Masuda Y, Aguilar I, Legarra A, et al. Genetic evaluation using single-step genomic best linear unbiased predictor in American Angus. J Animal Sci. (2015) 93:2653–62. doi: 10.2527/jas.2014-8836
25. Fernando RL, Dekkers JCM, Garrick DJ. A class of Bayesian methods to combine large numbers of genotyped and non-genotyped animals for whole-genome analyses. Genetics Select Evol. (2014) 46:50. doi: 10.1186/1297-9686-46-50
26. Cheng H, Qu L, Garrick DJ, Fernando RL. A fast and efficient Gibbs sampler for BayesB in whole-genome analyses. Genet Sel Evol. (2015) 47:80. doi: 10.1186/s12711-015-0157-x
27. Fernando RL, Cheng H, Golden BL, Garrick DJ. Computational strategies for alternative single-step Bayesian regression models with large numbers of genotyped and non-genotyped animals. Genet Sel Evol. (2016) 48:96. doi: 10.1186/s12711-016-0273-2
28. Toosi A, Fernando RL, Dekkers JCM. Genome-wide mapping of quantitative trait loci in admixed populations using mixed linear model and Bayesian multiple regression analysis. Genet Sel Evol. (2018) 50:32. doi: 10.1186/s12711-018-0402-1
29. Odegard J, Olesen I, Gjerde B, Klemetsdal G. Evaluation of statistical models for genetic analysis of challenge-test data on ISA resistance in Atlantic salmon (Salmo salar): Prediction of progeny survival. Aquaculture. (2007) 266:70–6. doi: 10.1016/j.aquaculture.2007.02.012
30. Cheng HH, Perumbakkam S, Pyrkosz AB, Dunn JR, Legarra A, Muir WM. Fine mapping of QTL and genomic prediction using allele-specific expression SNPs demonstrates that the complex trait of genetic resistance to Marek's disease is predominantly determined by transcriptional regulation. BMC Genom. (2015) 16:816. doi: 10.1186/s12864-015-2016-0
31. Odegard J, Meuwissen TH, Heringstad B, Madsen P. A simple algorithm to estimate genetic variance in an animal threshold model using Bayesian inference. Genet Sel Evol. (2010) 42:29. doi: 10.1186/1297-9686-42-29
32. Vallejo RL, Leeds TD, Fragomeni BO, Gao G, Hernandez AG, Misztal I, et al. Evaluation of genome-enabled selection for bacterial cold water disease resistance using progeny performance data in rainbow trout: insights on genotyping methods and genomic prediction models. Front Genet. (2016) 7:96. doi: 10.3389/fgene.2016.00096
33. Palti Y, Gao G, Liu S, Kent MP, Lien S, Miller MR, et al. The development and characterization of a 57K single nucleotide polymorphism array for rainbow trout. Mol Ecol Resour. (2015) 15:662–72. doi: 10.1111/1755-0998.12337
34. Palti Y, Silverstein JT, Wieman H, Phillips JG, Barrows FT, Parsons JE. Evaluation of family growth response to fishmeal and gluten-based diets in rainbow trout (Oncorhynchus mykiss). Aquaculture. (2006) 255:548–56. doi: 10.1016/j.aquaculture.2005.11.029
35. Misztal I, Tsuruta S, Lourenco D, Aguilar I, Legarra A, Vitezica Z. Manual for BLUPF90 Family of Programs. (2015). Available online at: http://nce.ads.uga.edu/wiki/lib/exe/fetch.php?media=blupf90_all2.pdf (accessed August 10, 2020).
36. Gao G, Nome T, Pearse DE, Moen T, Naish KA, Thorgaard GH, et al. A new single nucleotide polymorphism database for rainbow trout generated through whole genome resequencing. Front Genet. (2018) 9:147. doi: 10.3389/fgene.2018.00147
37. Pearse DE, Barson NJ, Nome T, Gao G, Campbell MA, Abadia-Cardoso A, et al. Sex-dependent dominance maintains migration supergene in rainbow trout. Nat Ecol Evol. (2019) 3:1731–42. doi: 10.1038/s41559-019-1044-6
38. Plummer M, Best N, Cowles K, Vines K. CODA: convergence diagnosis and output analysis for MCMC. R News. (2006) 6:7–11.
39. Christensen OF, Lund MS. Genomic prediction when some animals are not genotyped. Genet Select Evol. (2010) 42:2. doi: 10.1186/1297-9686-42-2
40. Wang H, Misztal I, Aguilar I, Legarra A, Muir WM. Genome-wide association mapping including phenotypes from relatives without genotypes. Genet Res. (2012) 94:73–83. doi: 10.1017/S0016672312000274
41. Zhang Z, Liu J, Ding X, Bijma P, de Koning DJ, Zhang Q. Best linear unbiased prediction of genomic breeding values using a trait-specific marker-derived relationship matrix. PLoS ONE. (2010) 5:12648. doi: 10.1371/journal.pone.0012648
42. Irano N, de Camargo GM, Costa RB, Terakado AP, Magalhaes AF, Silva RM, et al. Genome-wide association study for indicator traits of sexual precocity in nellore cattle. PLoS ONE. (2016) 11:e0159502. doi: 10.1371/journal.pone.0159502
43. Melo TP, Takada L, Baldi F, Oliveira HN, Dias MM, Neves HH, et al. Assessing the value of phenotypic information from non-genotyped animals for QTL mapping of complex traits in real and simulated populations. BMC Genet. (2016) 17:89. doi: 10.1186/s12863-016-0394-1
44. Cheng H, Fernando R, Garrick D. JWAS: julia implementation of whole-genome analysis software. In: Proceedings, 11th World Congress on Genetics Applied to Livestock Production (2018).
45. Meuwissen THE, Hayes BJ, Goddard ME. Prediction of total genetic value using genome-wide dense marker maps. Genetics. (2001) 157:1819–29.
46. Garrick DJ, Fernando RL. Implementing a QTL detection study (GWAS) using genomic prediction methodology. In: C Gondro, Jvd Werf and B Hayes, editors. Genome-Wide Association Studies and Genomic Prediction. New York, NY: Humana Press. (2013). p. 275–98. doi: 10.1007/978-1-62703-447-0_11
47. Habier D, Fernando RL, Kizilkaya K, Garrick DJ. Extension of the bayesian alphabet for genomic selection. BMC Bioinformatics. (2011) 12:186. doi: 10.1186/1471-2105-12-186
48. Saatchi M, McClure MC, McKay SD, Rolf MM, Kim J, Decker JE, et al. Accuracies of genomic breeding values in American Angus beef cattle using K-means clustering for cross-validation. Genetics Select Evol. (2011) 43:40. doi: 10.1186/1297-9686-43-40
49. Saatchi M, Ward J, Garrick DJ. Accuracies of direct genomic breeding values in Hereford beef cattle using national or international training populations. J Animal Sci. (2013) 91:1538–51. doi: 10.2527/jas.2012-5593
51. Van Melis MH, Eler JP, Oliveira HN, Rosa GJM, Silva JAIIV, Ferraz JBS, et al. Study of stayability in Nellore cows using a threshold model. J Anim Sci. (2007) 85:1780–6. doi: 10.2527/jas.2005-608
52. Legarra A, Aguilar I, Misztal I. A relationship matrix including full pedigree and genomic information. J Dairy Sci. (2009) 92:4656–63. doi: 10.3168/jds.2009-2061
53. Misztal I, Legarra A, Aguilar I. Computing procedures for genetic evaluation including phenotypic, full pedigree, and genomic information. J Dairy Sci. (2009) 92:4648–55. doi: 10.3168/jds.2009-2064
54. Vallejo RL, Silva RMO, Evenhuis JP, Gao G, Liu S, Parsons JE, et al. Accurate genomic predictions for BCWD resistance in rainbow trout are achieved using low-density SNP panels: Evidence that long-range LD is a major contributing factor. J Anim Breed Genet. (2018) 135:263–74. doi: 10.1111/jbg.12335
55. Aguilar I, Misztal I, Tsuruta S, Legarra A, Wang H. PREGSF90 - POSTGSF90: Computational Tools for the Implementation of Single-step Genomic Selection and Genome-wide Association with Ungenotyped Individuals in BLUPF90 Programs. In: Proceedings, 10th World Congress of Genetics Applied to Livestock Production (2014).
56. Saura M, Tenesa A, Woolliams JA, Fernandez A, Villanueva B. Evaluation of the linkage-disequilibrium method for the estimation of effective population size when generations overlap: an empirical case. BMC Genom. (2015) 16:922. doi: 10.1186/s12864-015-2167-z
57. Sved JA. Linkage disequilibrium and homozygosity of chromosome segments in finite populations. Theor Popul Biol. (1971) 2:125–41. doi: 10.1016/0040-5809(71)90011-6
58. Misztal I, Wang H, Aguilar I, Legarra A, Tsuruta S, Lourenco DAL, et al. GWAS using ssGBLUP. In: Proceedings, 10th World Congress of Genetics Applied to Livestock Production (2014).
59. Rolf MM, Garrick DJ, Fountain T, Ramey HR, Weaber RL, Decker JE, et al. Comparison of Bayesian models to estimate direct genomic values in multi-breed commercial beef cattle. Genetics Selection Evolution. (2015) 47:23. doi: 10.1186/s12711-015-0106-8
60. Meuwissen THE, Hayes B, Goddard M. Genomic selection: A paradigm shift in animal breeding. Animal Front. (2016) 6:6–14. doi: 10.2527/af.2016-0002
61. Fernando RL, Toosi A, Garrick DJ, Dekkers JCM. Application of whole-genome prediction methods for genome-wide association studies: bayesian approach. In: Proceedings, 10th World Congress of Genetics Applied to Livestock Production (2014).
62. Pszczola M, Strabel T, Mulder HA, Calus MPL. Reliability of direct genomic values for animals with different relationships within and to the reference population. J Dairy Sci. (2012) 95:389–400. doi: 10.3168/jds.2011-4338
63. Pszczola M, Calus MPL. Updating the reference population to achieve constant genomic prediction reliability across generations. Animal 10:1018–24. doi: 10.1017/S1751731115002785
Keywords: bayesian multiple regression, disease resistance, genomic selection, infectious hematopoietic necrosis virus, rainbow trout, single-step GBLUP
Citation: Vallejo RL, Fragomeni BO, Cheng H, Gao G, Long RL, Shewbridge KL, MacMillan JR, Towner R and Palti Y (2020) Assessing Accuracy of Genomic Predictions for Resistance to Infectious Hematopoietic Necrosis Virus With Progeny Testing of Selection Candidates in a Commercial Rainbow Trout Breeding Population. Front. Vet. Sci. 7:590048. doi: 10.3389/fvets.2020.590048
Received: 31 July 2020; Accepted: 19 October 2020;
Published: 05 November 2020.
Edited by:
Nguyen Hong Nguyen, University of the Sunshine Coast, AustraliaReviewed by:
Matthew L. Spangler, University of Nebraska-Lincoln, United StatesRoss Houston, University of Edinburgh, United Kingdom
Copyright © 2020 Vallejo, Fragomeni, Cheng, Gao, Long, Shewbridge, MacMillan, Towner and Palti. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Roger L. Vallejo, roger.vallejo@usda.gov
 Roger L. Vallejo
Roger L. Vallejo Breno O. Fragomeni
Breno O. Fragomeni Hao Cheng
Hao Cheng Guangtu Gao1
Guangtu Gao1  Kristy L. Shewbridge
Kristy L. Shewbridge