Corneal Ulcer in Dogs and Cats: Novel Clinical Application of Regenerative Therapy Using Subconjunctival Injection of Autologous Platelet-Rich Plasma
- 1Department of Surgery, Anaesthesiology and Radiology, Faculty of Veterinary Medicine, Cairo University, Giza, Egypt
- 2Department of Biochemistry and Molecular Biology, Faculty of Veterinary Medicine, Cairo University, Giza, Egypt
- 3Department of Internal Medicine and Infectious Diseases, Faculty of Veterinary Medicine, Cairo University, Giza, Egypt
- 4Department of Pathology, Faculty of Veterinary Medicine, Cairo University, Giza, Egypt
Background: Corneal ulcer could be a major source of distress in small animals, with many contributing agents. In recent years, few studies evaluated the efficacy of platelet-rich plasma (PRP) in healing corneal ulcers.
Aim: This study aimed to assess the ability of subconjunctival injection of autologous PRP in the treatment of corneal ulcers in dogs and cats as well as estimate the expression of matrix metalloproteinase (MMP)-2, MMP-9, and oxidative stress biomarkers in these patients.
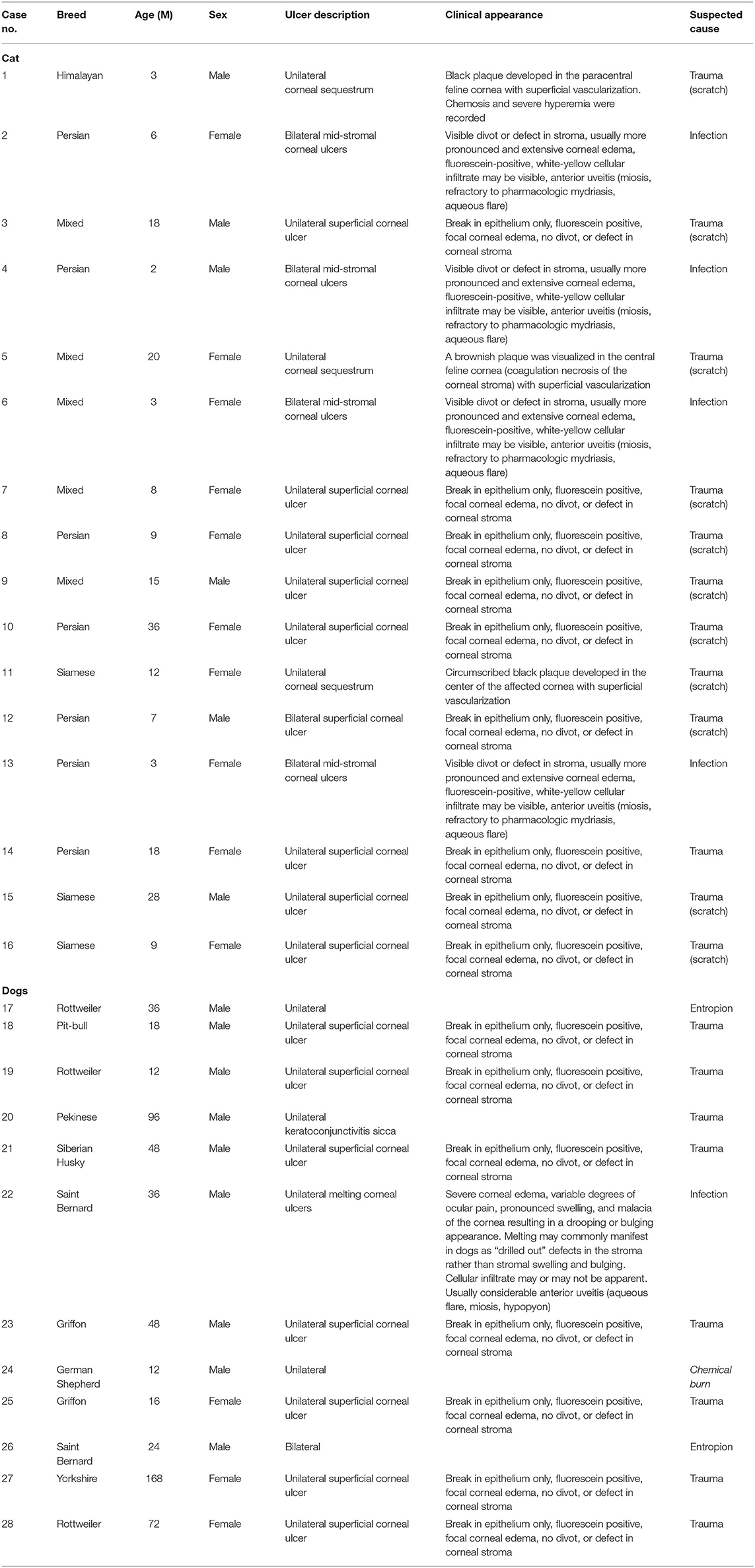
Methods: A total number of 28 animals (16 cats and 12 dogs) were enrolled in this study. Each animal was subjected to clinical, neurologic, and ophthalmic examinations where the type of ulcer was documented. Tear samples were collected for evaluation of oxidative biomarkers and MMPs; conjunctival swabs were taken to identify the involved organism. PRP was prepared from each animal and given as subconjunctival injection; numbers of injections were done according to case response. Clinical follow-up was done and documented for each case.
Results: In cat patients, female and Persian cats were most affected; unilateral and superficial ulcers were most recorded. In male dogs, unilateral, and superficial ulcers were most recorded. FHV-1 was most identified in cats, while Staphylococcus aureus was most identified in dogs. Numbers of injections needed to achieve healing were recorded, with 50% of dogs needing two injections with 1-week intervals and 50% of cats needed three injections with 1-week intervals. Alterations in both oxidative biomarkers and MMPs were recorded in affected animals.
Conclusion: The use of autologous PRP as a subconjunctival injection in treating corneal ulcers in dogs and cats is effective. The number of injections is the case and corneal ulcer type-dependent.
Clinical Significance: Autologous PRP as a subconjunctival injection in treating corneal ulcer is a relatively cheap, safe method and can be done in the clinical setting.
Introduction
Corneal ulceration is defined as a defect in the epithelium with stromal loss and/or inflammation (1). Corneal ulcers can cause a great deal of discomfort in patients, and it was accounted for up to 0.80% of conditions diagnosed in primary care practice in the UK (2). Contributing etiologies for this condition are numerous; trauma, bacterial or fungal infection, and immune-mediated diseases are the most reported causes (3).
In human medicine, extensive researches were conducted to treat corneal ulceration; recently, trials with platelet-rich plasma (PRP) were performed to assess its efficacy in healing corneal ulcers (4). These trials were conducted after promising findings were recorded in experimental animals (1, 5).
PRP, an autologous byproduct of blood that is very rich in platelets, earned wide recognition for its ability to heal various conditions (6, 7). As late as 1990, the term “regenerative medicine” was recognized (8). Platelets contain growth factors, cytokines, and integrins; these factors contribute to the proposed healing ability of PRP (9). The use of PRP is deemed convenient, cost-effective, non-immune provocation, and minimally invasive technique; the ability to administer it shortly after collection and preparation is an added merit (7). PRP can give essential components for the regeneration of tissue as scaffolds and growth factors (9).
PRP is rich in platelets, which are important for wound healing; they are rapidly deployed for injury sites, stick to it, and generate healing via releasing of numerous growth factors and cytokines (10). PRP was used successfully in treating dormant ulcers (11) and corneal epithelium defects following infectious keratitis (10). In human medicine, PRP was used extensively in the field of orthopedics, plastic, oral, and cardiovascular surgeries (11).
Matrix metalloproteinase (MMPs), a group of zinc-reliant extra-cellular endoproteinase, was postulated to play an integral role in corneal ulcer pathogenesis (12). MMP-2 and MMP-9 are thought to be the primary degrading enzymatic byproducts of corneal fibroblast and epithelial tissue (13, 14). MMPs catalyze basement membrane cleavage components (15). MMP-2 and MMP-9 were elevated in tears of patients with corneal diseases (16).
A strong refractive lens is supported by a combination of the corneal and precorneal tear film. In normal wear and tear mechanism, corneal extracellular matrix (ECM) is stabilized by a balance between collagen and ECM synthesis and their degradation by proteinases, which keep surveillance, remodel, and elimination of damaged corneal epithelial cells, and the abnormal component of ECM (15, 17, 18). These proteinases include MMPs, serine proteases, aspartic proteinases, and cysteine proteinases that exist in latent forms in normal conditions followed by activation during inflammation (13, 18–20). Both MMPs and serine proteinases play an important role in the normal and diseased corneal metabolism of human beings and animals (18, 21). MMPs play a vital role in all stages of wound healing and remodeling, and their overexpression results in excessive ECM degradation, which leads to tissue destruction and loss of visual function (22). MMPs are zinc-dependent enzymes that are classified according to their substrate into gelatinases, collagenases, stromelysins, and membrane-type MMPs (18). Gelatinases, such as MMP-2 and MMP-9, represent activity against collagen degradation products as well as against collagen types IV and V (15, 23). These proteases are mostly the predominant proteinases that are overexpressed in corneal ulceration in comparison with antiproteinases; tissue inhibitors of metalloproteinases (TIMPs) result in rapid degradation of collagen and other corneal ECM (15, 21).
Oxidative stress was described in numerous diseases in pet animal practice; both infectious and non-infectious etiologies were associated with damaging effects of oxidative stress mechanisms (24).
The state of elevated oxidant byproduct and reduction of antioxidant counterparts is known as oxidative stress (24). Malondialdehyde (MDA) is a lipid peroxidation byproduct that is usually measured to evaluate the oxidant arm in the body (25), while total antioxidant capacity (TAC) is used to crude estimate the status of the antioxidant system in the body (26).
This study aimed to assess the ability of subconjunctival injection of autologous PRP in the treatment of corneal ulcers in dogs and cats as well as estimate expression of MMP-2 and 9 and oxidative stress biomarkers in these patients.
Materials and Methods
Study Population, Clinical Examination, and Inclusion Criteria
This study was approved by the Animal Use and Care Committee at the Faculty of Veterinary Medicine, Cairo University, Egypt. A total number of 28 animals (16 cats and 12 dogs) were enrolled in this study. Animals were presented to the small animal clinics, faculty of veterinary medicine, Cairo University, and private clinics in the Cairo governorate. The corneal ulcer was documented at the time of admission, and verbal consent was given from animals' owners to participate in this study. Each animal was subjected to clinical examination, and signs were recorded at the time of admission.
The lack of previous medical/surgical interference of ulcer, at least 2 months of age, a clear definition of the morphological type of ulcer, and absence of other local or systemic illness were used as inclusion criteria in this study.
Neurologic assessments of participants include menace response, palpebral, pupillary light, and dazzle reflexes. Ophthalmic examinations in a dark room with magnification and focal light source were performed. Ophthalmic examinations include direct ophthalmoscopy (Welch Allyn®, Skaneateles Falls, NY), the Schirmer tear test (Color Bar™ instrument; EagleVision, Inc., Memphis, TN), applanation tonometry (Tono-Pen® VET; Medtronic SOLAN, North Jacksonville, FL), and fluorescein dye (Fluorets® Chauvin, France) staining were done to suspected cases (Figure 1).
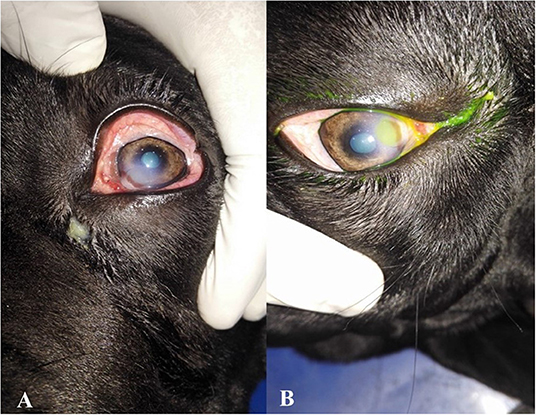
Figure 1. Photographs showing the clinical diagnosis of the ulcer by fluorescent dye. (A) Eye suffered from corneal ulcer, (B) after application of fluorescent dye showing type and depth of corneal ulcers.
Sampling and Laboratory Analysis
Tear fluid (diseased and treated) was collected from the inferior tear meniscus, causing the least irritation possible by the capillary tube method. The tear samples were then stored at −80°C until they were used for gelatin zymography (2).
Tear samples were taken by the method of the Schirmer test I. The sampled test strip was stored at −20°C (19).
Tear samples were used to estimate MDA, catalase (CAT), and TAC using specific test kits (Cat No. MD 25 29, CA 25 17, and TA 25 17, respectively; Bio-diagnostic, Egypt). The activity of MMP-2 and MMP-9 was detected in gelatin zymography by a method described by Hawkes et al. (27). Briefly, tear samples were separated by sodium dodecyl sulfate/polyacrylamide gel electrophoresis (SDS/PAGE) on 7.5% (w/v) gels, containing 1 mg/ml of gelatin under non-reducing conditions. Then, zymogram gels was washed twice for 15 min each in 2.5% (v/v) Triton X-100 and incubated overnight in development buffer (0.05 M of Tris/HCl, pH 8.8, 5 mM of CaCl2, 0.02% NaN3). Gels were stained with 0.1% Coomassie Brilliant Blue R250 in methanol:acetic acid:water (4.5:1:4.5, v/v/v). The zymogram gels were scanned in true color and then analyzed using commercially available software (myImageAnalysis Software; Thermo Scientific™) after conserving to grayscale.
Culture and Molecular Biology
Tears samples and conjunctival swabs of all cases were streaked on mannitol salt agar and Pseudomonas agar base with CN supplement media.
Conjunctival swabs were attained by rotating a sterile cotton swab over the ventral conjunctiva and were deposited in a 2-ml tube containing sterile 0.9% NaCl solution. PCR was conducted to detect Chlamydophila felis and FHV-1 according to methods described before (28, 29); primers and expected amplicons are tabulated in Table 1.
Preparation of the Platelet-Rich Plasma and Injection
PRP was prepared using a double-spin method as a protocol previously described by Kecec et al. (30). Briefly, blood from each animal was collected on 3.8% sodium citrate solution, soft spin at 250 × g/10 min was applied, the top and middle layers were then collected, and hard spin was performed at 2,000 × g/10 min followed by removal of platelet-poor plasma and activation of PRP by 20 mM of CaCl2 and incubation at 37°C/1 h. Centrifugation was then applied at 3,000 × g/20 min for recovering activated PRP.
Identification of Ulcer Types, Treatment Plans, and Complications
For superficial ulcers, a loss of part of the epithelium was the base of categorization. Deep ulcers that spread into/through the stroma and might cause severe scarring; fluorescein stain was taken up by exposed corneal stroma and with green appearance. Fluorescein stain defined the corneal ulcer margin and revealed further details of the surrounding epithelium. Fluorescein dye test was applied in all the cases and used to identify the different sites of the corneal ulcer and their size.
After the ulcer type was identified, two treatment options were done: (1) subconjunctival injection of PRP and, in case of entropion, (2) surgical correction of entropion in affected cases followed by subconjunctival injection of PRP. Surgical correction was done under general anesthesia, as follows: atropine sulfate (1% at 0.05–0.1 mg/kg b.wt.; Adwia Co., S.A.E, Egypt) and xylazine (Xyla-Ject 12% at 1 mg/kg b.wt.; Adwia Co., S.A.E, Egypt) were used as pre-medication, followed by ketamine at 10–20 mg/kg b.wt. (Sigma-Tec, Egypt) for induction and maintenance (31).
Surgical correction of entropion was done following the Hotz–Celsus procedure. Briefly, the removal of a crescent-shaped section of skin from the entropic region of the eyelid was made via a 6400 Beaver blade. At first, a parallel skin incision was done to the rolled-in portion of the eyelid at the eyelid margin by 2–3 mm; a second skin incision was bent away from the eye and commenced at one end of the first incision and end up at the other. The crescent-shaped section of the skin was removed with tenotomy scissors. The surgical gap was sutured via staples, where the first suture joined the epicenter of the first and second incisions. The second and third sutures were placed bilaterally to the first suture. The lingering defects were sutured every 3–4 mm till seamless juxtaposition of skin margins (32).
Subconjunctival injection of autologous PRP was used in each case, and bilaterally if the bilateral ulcer was identified at the time of admission.
Subconjunctival injection of autologous PRP was scheduled and timed for each case; the injection was applied under complete aseptic conditions and repeated till a complete curative clinical response with 1-week interval (Figure 2). Clinical evaluation was conducted via digital photographs of the ulcer at the baseline and at each time of subconjunctival injection (Table 2).
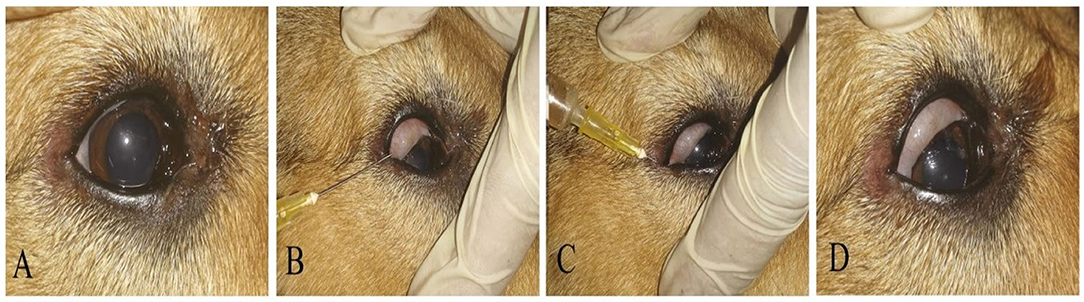
Figure 2. A photography series showing the technique of PRP conjunctival injection in the upper and lower eyelids and conjunctival pouch. (A) Before injection, (B,C) PRP injections in upper and lower eyelids and conjunctival pouch, (D) eye after injection.
Statistical Analysis
Data were compared using the T-test, with p ≤ 0.05 considered significant. Data are represented as mean ± SE, using SPSS for Windows, Version 16.0. Chicago, SPSS Inc. (released 2007).
Results
Data of each animal, description of the lesion, and the number of injections needed are presented in Table 2. In cat patients, females were more likely to be affected than males (62.5 and 37.5%, respectively). Persian cats were more presented compared with mixed-breed and Siamese cats (50, 31.2, and 18.7% respectively). In dog patients, male dogs were predominant than female (75 and 25%, respectively), Rottweiler, Saint Bernard, and Griffon breeds were overpresented in this study.
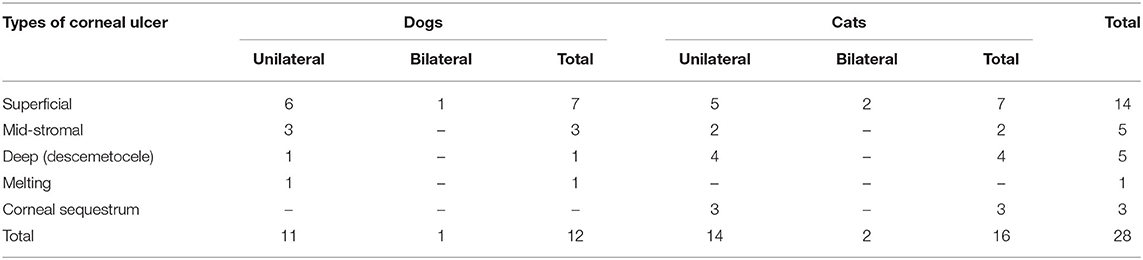
The ulcer morphological type for cats and dogs is shown in Table 3. The superficial corneal ulcer was more predominant in cat patients, followed by deep, corneal sequestration and a mid-stromal corneal ulcer (43.7, 25, 18.75, and 12.5%, respectively). For dogs, superficial corneal ulcer (58.3%) was more predominant followed by mid-stromal ulcer (25%). Unilateral corneal ulcer was prominent in cat patients than bilateral (87.75 and 12.5%, respectively). The unilateral ulcer was most common in dog patients than bilateral (90 and 10%, respectively). Data showed that unilateral corneal ulcer was common in dogs and cats, with similar frequency.
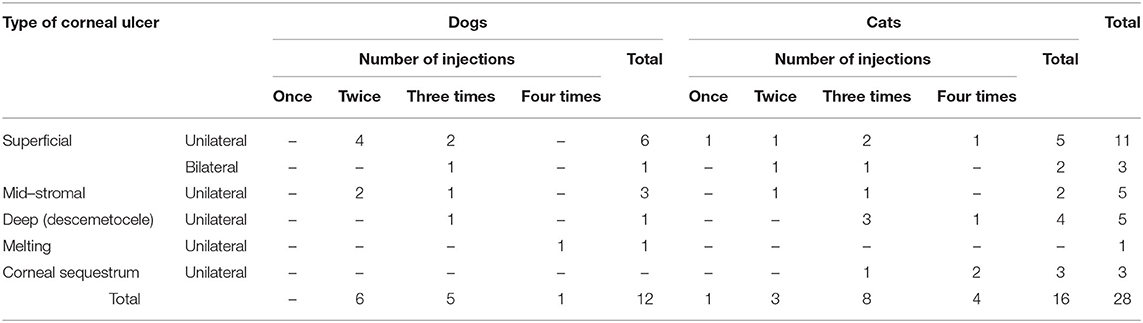
Subconjunctival injection needed for each animal is shown in Table 4. In dog patients, 50% (6/12) of cases needed two injections (1-week interval), while in cats, 50% (8/16) needed three injections.
Suspected causes of ulcers are listed in Table 2. In cat patients, trauma was the leading possible cause at the time of admission in 75% of cases (12/16). In dog patients, trauma was the possible cause at the time of admission in 66.6% of cases.
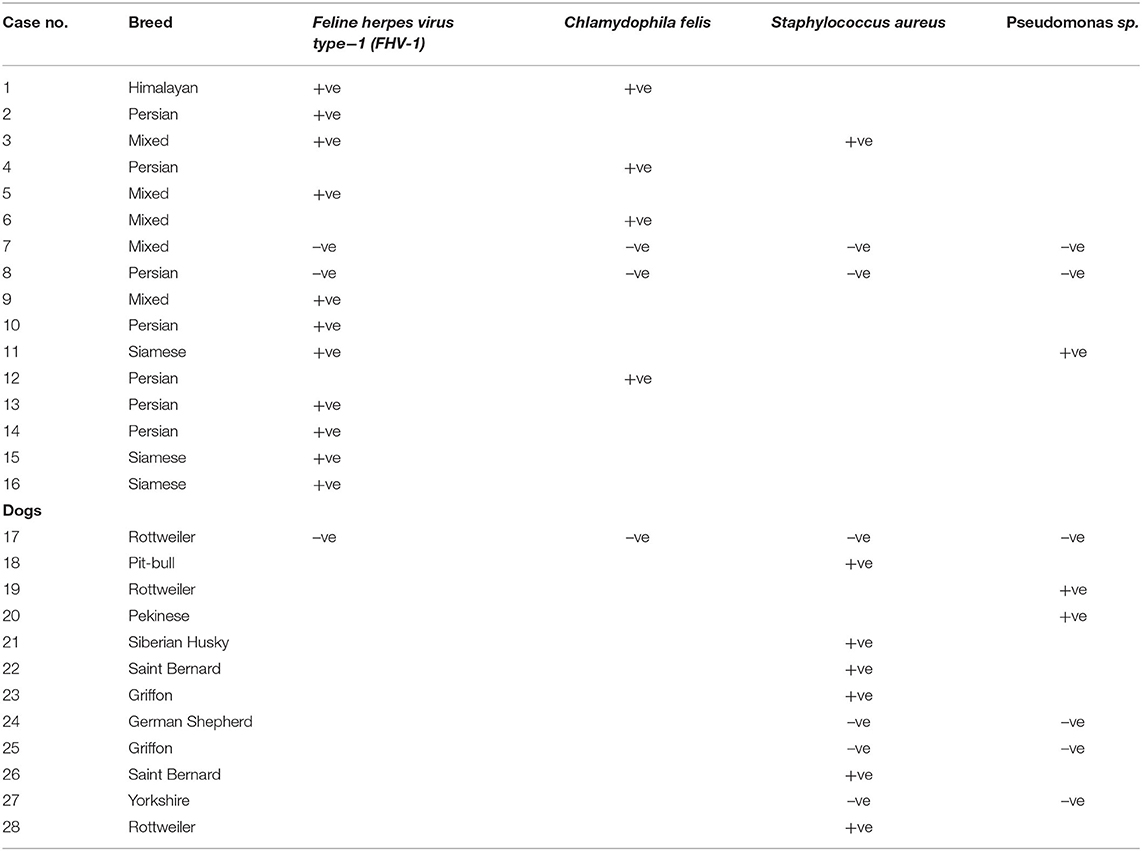
Results of culture and molecular identification are shown in Table 5. In cat patients, FHV-1 was most the predominant organism followed by C. felis. In dogs, Staphylococcus was the most predominant organism.
Clinical description and follow-up are shown in Figures 3–9, Table 2. In the gross examination, the majority of the cases showed mild-to-moderate congestion, lacrimation, and slight corneal opacity. All the reported cases responded to menace reflex and direct pupillary light reflex. There was continuous pawing, itching, irritation, pain, and photophobia in ulcerative cornea, which lead to excessive lacrimation.
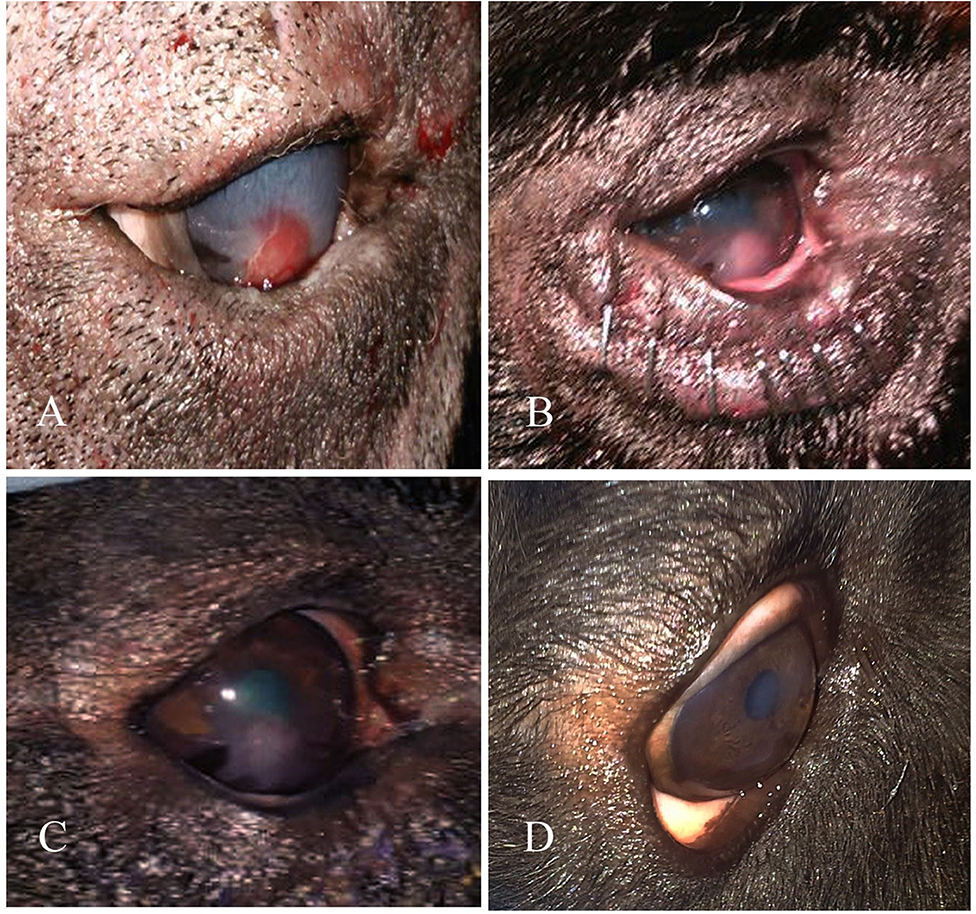
Figure 3. A photography series of Rottweiler dog showing (A) entropion with corneal ulcer (B) post-operative after surgical correction of entropion and injection of PRP (C) reduction of corneal ulcer size after 1-week post-injection (D) complete healing of ulcer after 2 weeks of injection.
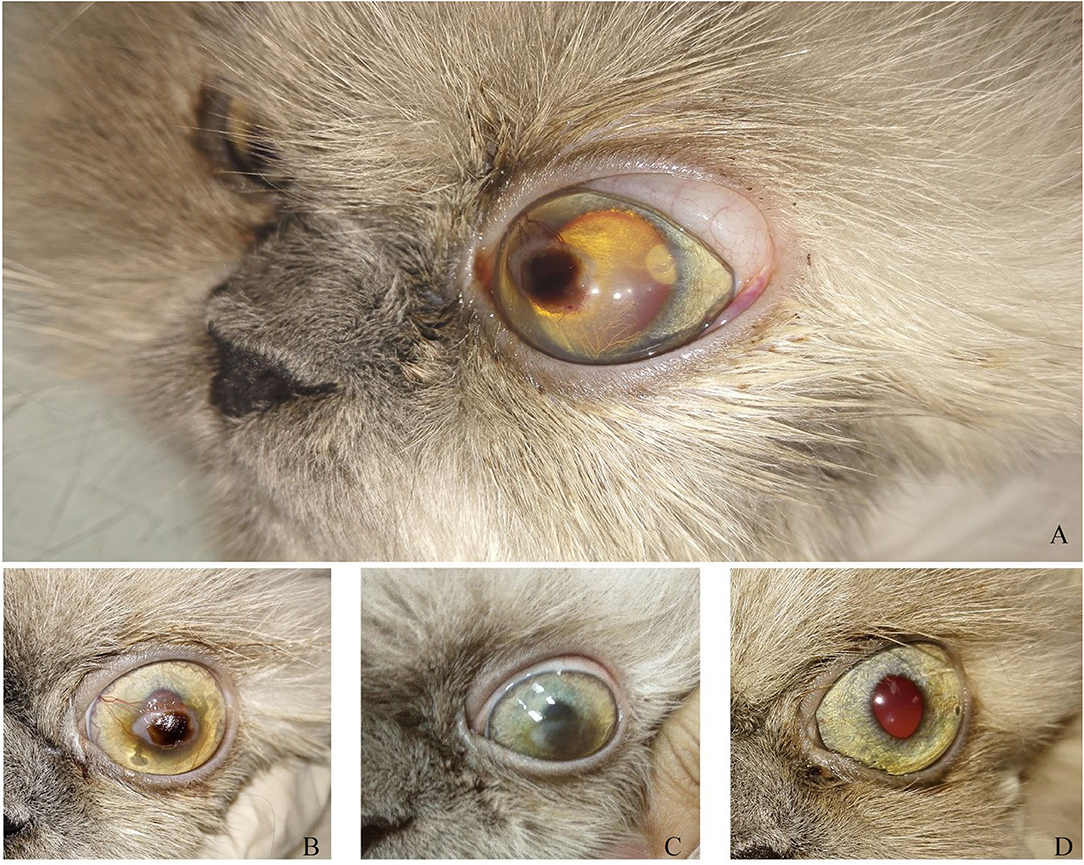
Figure 4. A photography series of Himalayan cat showing (A) superficial ulcer with sequestration (B,C) reduction of corneal ulcer size after 1,2-month post-injection, respectively, (D) complete healing of ulcer after 3 months of injection.
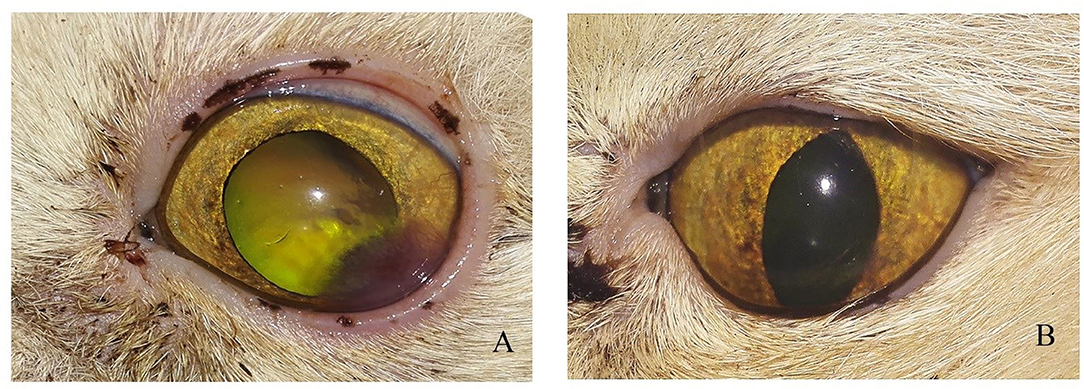
Figure 5. Photographs of a short hair cut showing (A) Stromal ulcer (B) complete healing of ulcer after 1 month of injection.
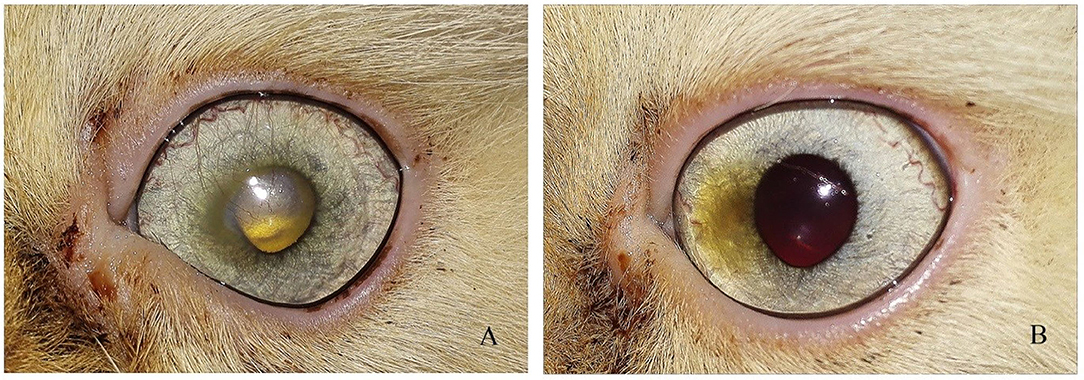
Figure 6. Photograph series of a short hair cut showing (A) keratitis (B) complete healing of ulcer after 1 month of injection.
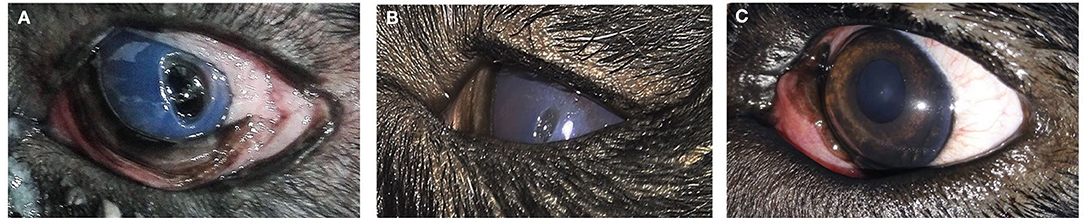
Figure 7. A photography series of Rottweiler dog showing (A) deep ulcer with keratitis (B) reduction of ulcer size after 2-week post-injection (C) complete healing of ulcer after 3 months of injection.
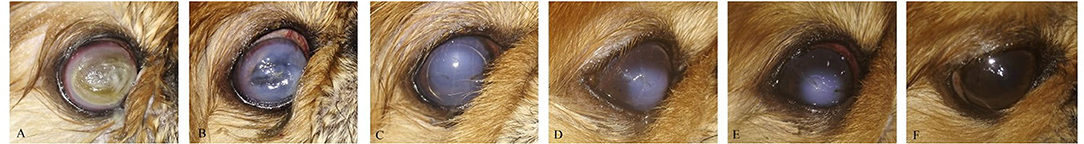
Figure 8. A photography series of Pekingese dog showing (A) melting ulceractive keratitis (B–F) gradual healing and reduction of keratitis after 2-week post-injection till complete healing at 3 months.
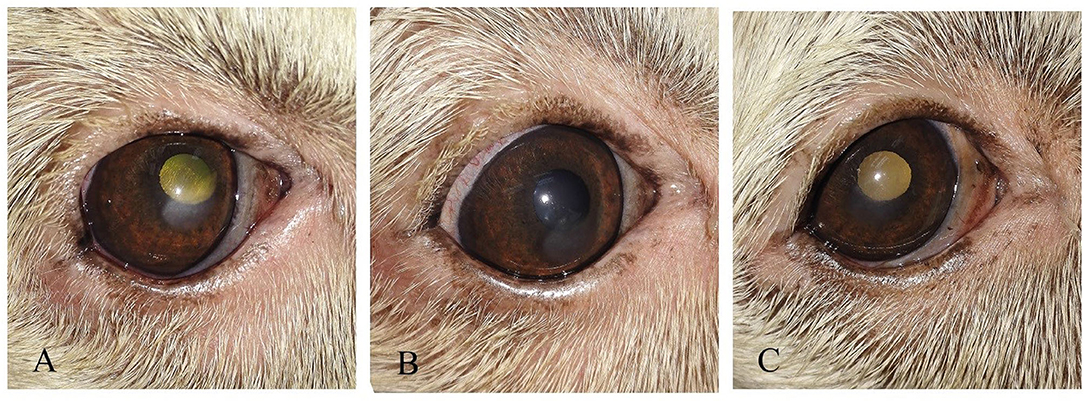
Figure 9. (A) photography series of Griffon dog suffering from keratitis (B) reduction of ulcer size after 1-week post-injection (C) complete healing of ulcer after 2 weeks of injection.
In cats, the majority of cases with superficial unilateral and mid-stromal ulcers needed two injections of PRP till complete healing, the deep (descemetocele) type needed three injections, and the melting type needed four injections, with ~50% of cases receiving three injections. In dogs, two injections of PRP are required for healing in 50% of cases. Some cases needed four injections as in the corneal sequestrum type. The size of ulcer reduced in first 2 weeks in the superficial type and complete healing at 2–4 weeks, while in deep ulcer, reduction of size occurs at 2–4 weeks, and complete healing and transparency of the cornea were maintained at 2 months and reached till 3 months in corneal sequestrum (Table 4).
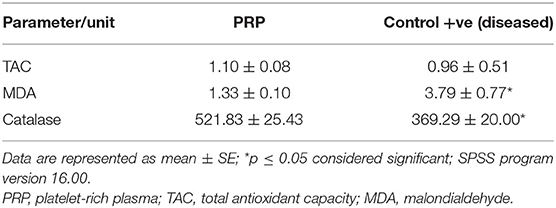
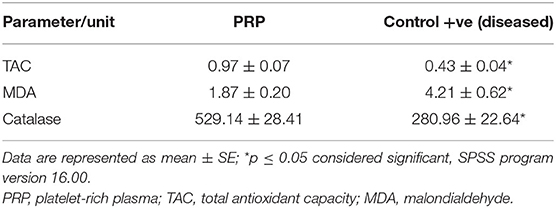
Results of oxidative biomarkers and MMPs are shown in Tables 6–8, Figure 10. In cat patients, a significant increase in MDA associated with a significant decrease in CAT and a non-significant decrease in TAC was reported in tear samples of cats with corneal ulcer as compared with treated cats, while dogs showed a significant decrease in both CAT and TAC associated with the significant increase in MDA in tear samples of affected dogs compared with treated dogs.
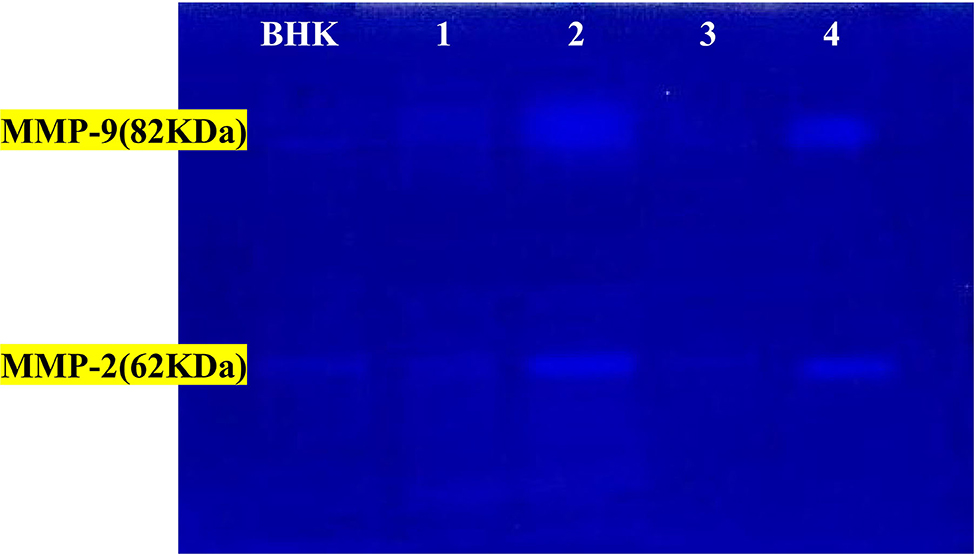
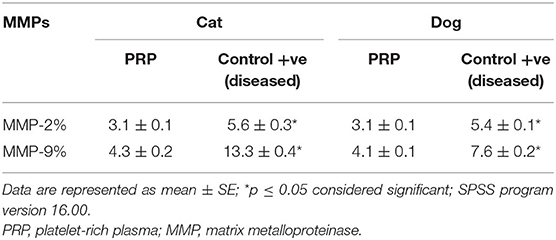
Figure 10. The activity of MMP-2 and MMP-9 by gelatin zymography. Lanes 1 = PRP-group; lanes 2 = control +ve in cat; lanes 3 = PRP groups; lanes 4 = control +ve group in dog. BHK lane is a control marker from baby hamster kidney cells transfected with active MMP-9 (82 kDa) that are indicated by arrows and MMP-2 (62 kDa).
The activity percentages of both MMPs (MMP-2 and MMP-9) were significantly high in the corneal ulcer control positive group (diseased group), which significantly decreased (p ≤ 0.05) after treatment with autologous PRP treated groups in both animals.
Discussion
Results and Key Findings
Corneal ulceration is defined as a defect in the epithelium with stromal loss and/or inflammation (1). The conventional treatment of corneal ulcer with different types of antibiotics (chloramphenicol, gentamicin sulfate, neomycin sulfate, tobramycin, ciprofloxacin, gatifloxacin, and ofloxacin) was sometimes associated with harmful effects on corneal re-epithelialization during healing (33).
This study examined the effect of subconjunctival injection of autologous PRP in different types of corneal ulcers in dogs and cats. The main findings of this study are that autologous PRP heals different types of corneal ulcers in both dogs and cats and the number of injections needed is case-dependent; however, most of the dogs needed two injections for complete healing, while the cat counterpart needed three injections. The most common microorganism implicated in corneal ulcers in cats is FHV-1, while in dogs, Staphylococcus. Corneal ulcers in dogs and cats are associated with oxidative process and alterations in MMPs.
The secondary finding of this study is that superficial corneal ulcer is the more predominant type in dogs and cats. Female Persian cats appeared to be most affected, while male dogs were overpresented compared with females.
Findings and Critics
Patient Data
Female Persian cats were overpresented, with corneal sequestration mainly seen in Siamese, Persian, and Himalayan in this study. All breeds, gender, and age group are susceptible to developing corneal ulcers (34, 35), though owners' preference to raise female cats may be implicated. In this study, male dogs were more presented than female. Rottweiler, Saint Bernard, and Griffon dogs were most likely to be affected; these findings contradict those reported by O'Neill et al. (2), as they reported that Boxer and Pug were most likely to be affected. The difference in breed presentation could be related to breeding preference and popularity.
Ulcer Morphological Type
In cat patients, superficial ulcer followed by corneal sequestration was the most predominant morphological type. The superficial ulcer is associated with defective epithelium with the baring of stroma, while descemetocele is usually associated with the absence of stroma at the ulcer base (34). The revival of latent FHV-1 is linked to conjunctivitis with superficial ulceration as a sequel, which may be unilateral or bilateral (36). Corneal sequestration usually occurs secondary to ulcerative keratitis of chronic nature (37). In dogs, superficial corneal ulcer with unilateral involvement was the most common morphological type. Other reports showed that unilateral ulcer was overrepresented (38). In the study conducted by Kim et al. (39), they found that superficial corneal ulcer represented 44% of cases followed by a deep ulcer that entails about 2/3 of the stroma.
Etiological Agents
FHV-1 was implicated in most of the cat patients in this study. It is postulated that many cases of corneal ulcer in cats originated mainly from FHV-1 infection (40–43). Other causes of ulceration are trauma, entropion, and chemical trauma (34). In dogs, Staphylococcus aureus was isolated from 50% of patients; in one report performed on 19 dogs with corneal ulcers, Staphylococcus was the most prominent isolate (38). A canine corneal ulcer usually arises from canine herpes virus or spontaneous chronic ulcer as primary causes with entropion, trauma, and corneal degeneration as secondary causes (28, 44–48).
Subconjunctival Injection of Autologous Platelet-Rich Plasma
PRP, especially E-PRP, was advocated for usage in human medicine for its cost-effectiveness, ability to heal the corneal wound as sole/adjuvant therapy, and relative safety (49); it was used successfully in the treatment of dormant corneal ulcer and was proven to help reduce inflammation and pain in these patients (11). The rationale for deploying such methods was described extensively in the literature, with the absence of clotting factors and the presence of many growth factors as the main advocate for its usage (49). In one human study, PRP was used as eye drops, the healing was improved significantly, and complete healing was possible in most of the participant cases (11).
The eyes' position in the body makes them continuously exposed to numerous agents and traumatic events (50). These circumstances make them react to any insult constrained and may lead to vision loss (51). In recent years, PRP was used in the regeneration and reconstruction of tissues, plastic, and cardiovascular surgeries as well as it aids in corneal lesion healing (1). A subconjunctival route was chosen to administer PRP in both dogs and cats. In two reports dealing with rabbits and dogs, the subconjunctival injection was used instead of drops and can be given as a single shot contrary to the drop form (5, 52).
PRP is a powerful, effective, and safe cure for various wound-healing processes. In this study, half of the dog patients required two injections at 1 week apart, regardless of the morphological type, while approximately half of the cat patients required three injections at 1 week apart regardless of the morphological type of ulcer. Compared with subconjunctival injection, PRP drops were used in clinical practice twice daily for 15 days in dogs after defrosting PRP every time it was used (53).
In one study conducted in rabbits, PRP contains growth factors that were directly applied on the injured surface of the cornea and in turn modulate repair and reduce formation of scars (50). As PRP is not categorized as a drug agent by the Food and Drug Administration (FDA), a protocol of administration is yet to be defined (54). PRP administration improves the recovery of muscle and tendon injuries (55). Cytokines and growth factors within platelets initiate and boost tissue healing via stimulation of cell proliferation, migration, and angiogenesis (6). In a human study dealt with a non-healing ulcer, PRP drops were employed, and they concluded that it is an efficient and cost-effective way to treat neurotrophic keratitis (56). PRP rushes healing and enhances epithelium (57). PRP is inexpensive, easy to obtain, and possesses the merit of being a non-surgical alternative with lesser side effects (1).
Clinical and Photographic Follow-Up
The utilization of PRP as a subconjunctival injection is a recent approach in human and veterinary medicine. In the superficial type, the ulcer size is reduced in the first 2 weeks, and complete healing occurs at 2–4 weeks. In deep ulcer, reduction of size occurs at 2–4 weeks with complete healing, and transparency of the cornea was maintained at 2 months and till 3 months in cases of the corneal sequestrum. Conventional treatment usually employs antibiotics; for instance, galifloxacin 0.3% might heal the cornea within 15 days; however, 78% of indolent ulcers could be healed with the combination of chondroitin and tobramycin in up to 4 weeks (33). The expected action of PRP based on the extraordinary concentration of epithelia-trophic growth factors might lead to faster healing (53). In the study conducted by Ferrone Neto et al. (33), they concluded that the addition of PRP to conventional therapy improved clinical cure.
Corneal Ulcer and Oxidative Stress
When a balance between reactive oxygen species (ROS) production and the ability of an antioxidant to remove them is disrupted, the oxidative process will ensue. Cellular damage and peroxidation of lipid in the cellular membrane are expected (58, 59). In the present study, corneal ulcers in both dogs and cats showed oxidative stress in the form of elevated MDA and reduction in TAC and CAT in tear samples, which normalized to normal levels after therapy. Abundance in ROS overwhelms the antioxidant system, and the eye tissue became overloaded with ROS (60). The increase in MDA level could be attributed to increasing ROS output or as compensation for the reduction in antioxidants (24). Alterations in the hydration of the cornea are factored in resultant oxidative stress (61). MDA elevation is a good indicator for lipid peroxidation (27). In ulcerative keratitis, the elevation in MDA and reduction in CAT and superoxide dismutase (SOD) were recorded and attributed to inflammation (62).
The enzymatic antioxidant as CAT plays a role in protecting ocular tissue; CAT is known to convert H2O2 to water; both CAT and SOD act together as frontrunners in antioxidant effort to remove ROS (63, 64). TAC showed a significant reduction in corneal ulcers regardless of their morphological status. TAC represents a collective act of all antioxidants, and it could give a crude assessment of antioxidant levels in the body (25). As the frontrunners act as a first-line defense in the antioxidant system (65), a reduction in TAC is expected. TAC could be a simple tool to assess antioxidant systems regardless of system antioxidant modus operandi (66). Experimental keratitis was associated with oxidative stress in the cornea (62). Oxidative stress in corneal affections is linked to many factors, and this process can disrupt the cornea, which ranged from a reduction in visual perception to vision loss (58).
Corneal Ulcer and Matrix Metalloproteinases
MMPs are expressed in low levels in normal non-injured corneal tissues, as MMP-2 exists in an inactive form and MMP-9 is undetectable with induction and activated or upregulated in response to cytokines and growth factors (13, 18). MMP-9 is incorporated in the early stages of corneal epithelial wound healing, while MMP-2 is associated with the remodeling in the later stages of corneal wound healing (67). Gelatin zymography is a method used for easy detection of latent and active MMPs. Because the active forms of MMPs are water-soluble enzymes, it is difficult to detect by immunohistochemical or molecular biological method (68).
In the present study, the activity of gelatinases (MMP-2 and MMP-9) was significantly high in the corneal ulcer control positive group, which significantly decreased after treatment with autologous PRP in both dogs and cats. These results agreed with those in (21, 69), which stated that MMPs and serine proteinases were predominant in horse and dog corneal ulceration that released into the microenvironment of injured corneal tissue from leukocyte infiltration. The precorneal tear film serine proteinase levels were significantly higher in dogs with indolent ulcers vs. normal controls (70).
Singh et al. (68) demonstrated that the active forms of MMP-9 are present in tears of severe ulcerative and ocular surface disorder patients with no detection in the control group. Also, an injury without bacterial infection resulted in an increase in both MMP-2 and−9 and a slight but significant downregulation of TIMP-1 in mouse corneal ulcer (19). In the administration of anti-interleukin (IL)-1 antibodies before infection, it showed a significant decrease in MMP levels and a significant change in the time course of TIMP induction. This reduction in MMP-9 may contribute to reduced corneal damage.
There is evidence that in ulcerative corneal disease, alteration of the ratio of MMPs to TIMPs plays a role in progressive stromal degradation (71). Furthermore, the expression of collagenolytic and gelatinolytic MMPs is increased in bacterial corneal ulcers of mice and rabbits (19). The levels of MMP-9 and MMP-2 were found to be significantly elevated in climatic droplet keratopathy patients (72). These data suggest that MMPs produced by resident corneal cells and polymorphonuclear (PMN) leukocytes may play a role in early epithelial keratitis and the ulcerative process in the late phase after corneal HSV-1 infection in BALB/c mice (73). Mulholland et al. (74) showed that MMP-2 and−9 were overexpressed in anterior keratectomy (AK) and lamellar keratectomy (LK) wounds in rabbits and then rapidly fell to low levels after epithelial closure.
MMP-2 is produced by corneal epithelial cells, stromal keratocytes/fibroblasts, and PMN leukocytes and acts as housekeeping proteinase in the normal cornea by degrading occasionally damaged collagen fibers (75, 76). However, MMP-9 is secreted by corneal epithelial and stromal cells; it is responsible for destroying the adhesive structure of the epithelial cell basement membrane before overt stromal ulceration and delays the re-epithelialization of the ulcerated cornea (15, 18).
During the corneal injury, wound healing is started by recruitment of leukocytes, fibroblasts, and vascular endothelial cells to begin healing phases, including inflammation, angiogenesis, re-epithelialization, granulation tissue formation, and ECM deposition in response to MMPs and other proteinases (18). In severely damaged corneas, corneal stromal ulceration propagates through epithelial basement membrane degeneration because of overexpression of MMPs, elevated plasmin activity from inflammatory cells, and pro-inflammatory cytokines such as tumor necrotic factor-alpha and transforming growth factor-beta (12, 69, 77).
In the case of corneal infection, these proteinases are oversecreted by infectious organisms that simultaneously enhance corneal damage (19, 21, 78). In context, Pseudomonas produces two types of MMPs, alkaline and elastase proteases, that are responsible for aggressive ulcerative keratitis associated with this microbe (77). Also, bacterial and fungal infections induce recruitment of corneal epithelial cells, corneal stromal fibroblasts, and PMN leukocytes in the tear film that upregulate cytokines (IL-1, IL-6, and IL-8), leukocyte infiltration, and angiogenic factors that induce explosive production of MMPs to lead to stromal scarring and loss of corneal transparency (12, 18, 19, 78).
The treatment for severe ocular surface disorders has been a long-standing challenge and has been advocated by a variety of medical techniques and surgical procedures. As a result of the elevation of MMP levels, tear film parallels the severity of the corneal disease. These levels diminish when treatment is initiated and as the ulcer heals (69). Thus, PRP rich with their growth factors has been recommended for the treatment of corneal ulcer and ocular surface disorders to reduce the progression of stromal ulcer and to minimize corneal scarring through reduction of tear film proteolytic activity (68). Normalizing proteolytic activity in the tear film is an objective sign of effectiveness in the treatment of corneal ulcers and ocular surface disorder.
Comparison With Human Literatures
In this study, PRP was useful in treating corneal ulcers in small animal patients. It was used in many human studies for treating different types of ulcers (10, 56, 79–81). This study optioned for PRP usage as subconjunctival injection that could be administered weekly. In human patients, it was given mostly as eye drops (56, 79). A unique way developed by Alio et al. (80) combined E-PRP clot with fibrin membrane for corneal perforation closure. PRP was used as a solid application in ulcer in another report (81). It appears that subconjunctival injection of PRP in human patient is not widely used as compared with eye drops. This study opted to utilize subconjunctival administration instead of eye drops; this method, in the authors' opinion, albeit gives incredible results in human patients (82), has many hindrances for pet patients, as the need for repeated daily administration of product is the biggest hindrance. In one case report that dealt with refractory corneal ulcer, the patient was advised to administer PRP every 2 h for 15 days (79). As owner's compliance could not be ensured, subconjunctival method seems more appropriate for small animal patients.
In this study, most of dog patients required at least two injections with 1-week interval, while cats needed at least three injections with 1-week injection. In human literature, an average of three to eight drop application/day was reported (4, 82–85); the follow-up differs according to case, 2 months in dry eye diseases (11) and 6 months in recurrent corneal erosion (86).
The cause of ulcer in this study was often multifactorial, with infectious agent involvement in some cases. PRP was able to heal different types of corneal ulcers. In human literatures, PRP was used to cure ulcers with various etiologies (11, 80, 83, 87). PRP was efficiently used in treating persistent corneal abnormalities following an infectious condition (10).
This study lacked a control group to act as comparison arm. In human literature, there was a split between studies that deployed comparison arm (88–90); other reports did not deploy a comparison arm (11, 84, 91, 92). In a recent systematic review conducted by You et al. (93), they analyzed the results of 35 clinical studies that dealt with different ocular diseases and utilized platelet products as line of therapy, under corneal ulcer in their table description. They reported 16 studies that deployed PRP in treating different corneal ulcers: six studies used comparison arms and 10 studies did not utilize/report involvement of comparison group.
Limitations and Strength
This study dealt with a novel way of treating different types of corneal ulcers in dogs and cats. The study uses PRP, a cheap and applicable method, as the main therapy. However, there was no comparison group using the conventional way of treatment to compare its efficacy and ability to improve healing. Comparing the results of subconjunctival injection of PRP with those of traditional therapy should be investigated in both dogs and cats in further studies.
Conclusion
Subconjunctival injection of autologous PRP is cost-effective, easy to apply, and improves the healing of corneal ulceration in both dogs and cats regardless of their morphological type. The number of injections needed to reach complete healing is case-dependent rather than morphological type-dependent. A corneal ulcer is associated with local oxidative stress manifested by elevation in MDA and reduction in TAC and CAT; this oxidative process is present independent of the morphological type. MMP-2 and MMP-9 increased with corneal injury, though they normalize after PRP administration.
Data Availability Statement
The original contributions presented in the study are included in the article/supplementary material, further inquiries can be directed to the corresponding author/s.
Ethics Statement
The animal study was reviewed and approved by the Institutional Animal Care and Use Committee, Cairo University (Vet CU 20022020127). Written informed consent for participation was not obtained from the owners because the owners were informed orally about the steps of the study and all contact data of the owners are available in the archive of the small animal clinic, faculty of veterinary medicine, Cairo University, and participated private clinics in Cairo governorate.
Author Contributions
HF established the research hypothesis and designed the study. HF, NA, and IE collected the data and applied the subconjunctival PRP injections. HA, ER, and NS prepared the PRP and performed the biochemical laboratory analysis. MK prepared the PRP and performed the statistical analysis. All authors participated in writing and approved the final manuscript.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
The authors would also like to thank the team of Alpha Vet Pet Clinic, Office Building at Al-Rehab Cairo, Cairo Governorate, Egypt, for their assistance in data collection and clinical follow-up. The authors would like to thank Department of Surgery, Anesthesiology and Radiology, Faculty of veterinary medicine for facilitating this work.
References
1. Acosta L, Castro M, Fernandez M, Oliveres E, Gomez-Demmel E, Tartara L. Treatment of corneal ulcers with platelet rich plasma. Arch la Soc Española Oftalmol. (2014) 89:48–52. doi: 10.1016/j.oftale.2014.04.012
2. O'Neill DG, Lee MM, Brodbelt DC, Church DB, Sanchez RF. Corneal ulcerative disease in dogs under primary veterinary care in England: epidemiology and clinical management. Canine Genet Epidemiol. (2017) 4:1–12. doi: 10.1186/s40575-017-0045-5
3. Chidambaram J. Recent advances in the diagnosis and management of bacterial keratitis. Int Ophthalmol Clin. (2007) 47:1–6. doi: 10.1097/IIO.0b013e318074e797
4. Alizadeh S, Balagholi S, Baradaran-Rafii A, Delfaza-Baher S, Safi S, Safi H, et al. Autologous platelet-rich plasma eye drops accelerate re-epithelialization of post-keratoplasty persistent corneal epithelial defects. J Ophthalmic Vis Res. (2019) 14:131–5. doi: 10.4103/jovr.jovr_279_17
5. Tanidir ST, Yuksel N, Altintas O, Yildiz DK, Sener E, Caglar Y. The effect of subconjunctival platelet-rich plasma on corneal epithelial wound healing. Cornea. (2010) 29:664–9. doi: 10.1097/ICO.0b013e3181c29633
6. Pündük Z, Oral O, Özkayin N, Rahman K. Single dose of intra-muscular platlet rich plasma reverses the increase in plasma iron levels in exercise induced muscle damage: a pilot study. Orthop J Sport Med. (2014) 2:109–14. doi: 10.1016/j.jshs.2014.11.005
7. Everts PA, Hoogbergen MM, Weber TA, Devilee RJ, van Monftort G, de Hingh IH. Is the use of autologous platelet-rich plasma gels in gynecologic, cardiac, and general, reconstructive surgery beneficial? Curr Pharm Biotechnol. (2012) 13:1163–72. doi: 10.2174/138920112800624346
8. Haseltine WA. Interview: commercial translation of cell-based therapies and regenerative medicine: learning by experience. interview by Emily Culme-Seymour. Regenerative med. (2011) 6:431–5. doi: 10.2217/rme.11.40
9. Sanchez-Gonzalez DJ, Mendez-Bolaina E, Trejo-Bahena NI. Platelet-rich plasma peptides: key for regeneration. Int J Pept. (2012) 2012:532519. doi: 10.1155/2012/532519
10. Kim KM, Shin YT, Kim HK. Effect of autologous platelet-rich plasma on persistent corneal epithelial defect after infectious keratitis. Jpn J Ophthalmol. (2012) 56:544–50. doi: 10.1007/s10384-012-0175-y
11. Alio JL, Abad M, Artola A, Rodriguez-Prats JL, Pastor S, Ruiz-Colecha J. Use of autologous platelet-rich plasma in the treatment of dormant corneal ulcers. Ophthalmology. (2007) 114:1286–93.e1. doi: 10.1016/j.ophtha.2006.10.044
12. Li DQ, Shang TY, Kim HS, Solomon A, Lokeshwar BL, Pflugfelder SC. Regulated expression of collagenases MMP-1,−8, and−13 and stromelysins MMP-3,−10, and−11 by human corneal epithelial cells. Investig Ophthalmol Vis Sci. (2003) 44:2928–36. doi: 10.1167/iovs.02-0874
13. Fini ME, Girard MT, Matsubara M, Bartlett JD. Unique regulation of the matrix metalloproteinase, gelatinase B. Invest Ophthalmol Vis Sci. (1995) 36:622–33.
14. Meller D, Li DQ, Tseng SC. Regulation of collagenase, stromelysin, and gelatinase B in human conjunctival and conjunctivochalasis fibroblasts by interleukin-1beta and tumor necrosis factor-alpha. Invest Ophthalmol Vis Sci. (2000) 41:2922–9.
15. Matsubara M, Girard MT, Kublin CL, Cintron C, Fini ME. Differential roles for two gelatinolytic enzymes of the matrix metalloproteinase family in the remodelling cornea. Dev Biol. (1991) 147:425–39. doi: 10.1016/0012-1606(91)90300-R
16. Smith VA, Rishmawi H, Hussein H, Easty DL. Tear film MMP accumulation and corneal disease. Br J Ophthalmol. (2001) 85:147–53. doi: 10.1136/bjo.85.2.147
17. Arican M, Ceylan C. Metalloproteinases in canine experimental traumatic keratoconjunctivitis. J Vet Med. (1999) 46:527–32. doi: 10.1046/j.1439-0442.1999.00238.x
18. Wong TTL, Sethi C, Daniels JT, Limb GA, Murphy G, Khaw PT. Matrix metalloproteinases in disease and repair processes in the anterior segment. Surv Ophthalmol. (2002) 47:239–56. doi: 10.1016/S0039-6257(02)00287-4
19. Xue M, Wakefield D, Willcox M, Lloyd AR, Di Girolamo N, Cole N, et al. Regulation of MMPs and TIMPs by IL-1b during corneal ulceration and infection. Invest Ophthalmol Vis Sci. (2003) 44:2020–5. doi: 10.1167/iovs.02-0565
20. Hibbits K, Hines B, Williams D. An overview of proteinase inhibitors. J Vet Intern Med. (1999) 13:302–8. doi: 10.1111/j.1939-1676.1999.tb02185.x
21. Strubbe DT, Brooks DE, Schultz GS, Willis-Goulet H, Gelatt KN, Andrew SE, et al. Evaluation of tear film proteinases in horses with ulcerative keratitis. Vet Ophthalmol. (2000) 3:111–9. doi: 10.1046/j.1463-5224.2000.00093.x
22. Birkedal-Hansen H. From tadpole collagenase to a family of matrix metalloproteinases. J Oral Pathol. (1988) 17:445–51. doi: 10.1111/j.1600-0714.1988.tb01313.x
23. Watanabe H, Hattori S, Katsuda S, Nakanishi I, Nagai Y. Human neutrophil elastase: degradation of basement membrane components and immunolocalization in the tissues. J Biochem. (1990) 108:753–9. doi: 10.1093/oxfordjournals.jbchem.a123277
24. Salem NY, Abdel-Saeed H, Farag HS, Ghandour RA. Canine demodicosis: hematological and biochemical alterations. Veterinary World. (2020) 13:68–72. doi: 10.14202/vetworld.2020.68-72
25. Abdel-Saeed H, Salem NY. Evaluation of total antioxidant capacity, malondialdehyde, catalase, proteins, zinc, copper and IgE response in ovine verminous pneumonia. Inter J Vet Sci. (2019) 8:255–8.
26. Elsayed NM, Kubesy AA, Salem NY. Altered blood oxidative stress biomarkers in association with canine parvovirus enteritis. Comp Clin Pathol. (2020) 29:355–9. doi: 10.1007/s00580-019-03067-x
27. Hawkes SP, Li H, Taniguchi GT. Zymography and reverse zymography for detecting MMPs, and TIMPs. In: Clark I. editor. Matrix Metalloproteinases Protocols. Totowa: Humana Press (2001) p. 399–410. doi: 10.1385/1-59259-046-2:399
28. Rampazzo A, Appino S, Pregel P, Tarducci A, Zini E, Biolatti B. Prevalence of Chlamydophila felis and feline herpesvirus 1 in cats with conjunctivitis in northern Italy. J Veterinary Internal Med. (2003) 17:799–807. doi: 10.1111/j.1939-1676.2003.tb02517.x
29. Suchy A, Bauder B, Gelbmann W, Löhr CV, Teifke JP, Weissenböck H. Diagnosis of feline herpesvirus infection by immunohistochemistry, polymerase chain reaction, and in situ hybridisation. J Vet Diagn Invest. (2000) 12:186–91. doi: 10.1177/104063870001200220
30. Kececi Y, Ozsu S, Bilgir O. A cost-effective method for obtaining standard platelet-rich plasma. Wounds. (2014) 26:232–8.
31. Abd Elkader NA, Emam IA, Farghali HA, M DS, Salem NY. Oesophageal foreign bodies in cats: clinical and anatomic findings. PLoS ONE. (2020) 15:e0233983. doi: 10.1371/journal.pone.0233983
32. Westermeyer HD, Hendrix DVH. Basic Ophthalmic Surgical Procedures. Veterinary Surgery: Small Animal Expert 2nd edition. Canada: Saunders (2018).
33. Ferrone Neto RL, Veras de Mello ML, de Mello Bobany D. Treatment of corneal ulcer complicated in domestic canine, with the complementary use of homeopathy, moxabustion and autologous serum. IOSR J Agric Veterinary Sci. (2018) 11:38–41.
34. Hartley C. Aetiology of corneal ulcers assume FHV-1 unless proven otherwise. J Feline Med Surg. (2010) 12:24–35. doi: 10.1016/j.jfms.2009.12.004
35. Dalla F, Pisoni L, Masetti L. Feline corneal sequestration: a review of medical treatment in 37 cases. Veterinary Res Commun. (2007) 31 (Suppl. 1):285–8. doi: 10.1007/s11259-007-0098-0
36. Nasisse MP. Manifestations, diagnosis, and treatment of ocular herpes - virus infection in the cat. Compend Cont Educ Pract Vet. (1982) 4:962–70.
37. Stiles J, McDermott M, Bigsby D, Willis M, Martin C, Roberts W, et al. Use of nested polymerase chain reaction to identify feline herpesvirus in ocular tissue from clinically normal cats and cats with corneal sequestra or conjunctivitis. Am J Vet Res. (1997) 58:338–42.
38. Prado MR, Brito EHS, Girão MD, Sidrim JJC, Rocha MFG. Identification and antimicrobial susceptibility of bacteria isolated from corneal ulcers of dogs. Arquivo Brasileiro de Medicina Veterinária e Zootecnia. (2006) 58:1024–9. doi: 10.1590/S0102-09352006000600008
39. Kim JY, Won H-J, Jeong S-W. A retrospective study of ulcerative keratitis in 32 dogs. Intern J Appl Res Vet Med. (2009) 7:27–31.
40. Low HC, Powell CC, Veir JK, Hawley JR, Lappin MR. Prevalence of feline herpesvirus 1, Chlamydophila felis, and Mycoplasma spp DNA in conjunctival cells collected from cats with and without conjunctivitis. Am J Vet Res. (2007) 68:643–48. doi: 10.2460/ajvr.68.6.643
41. Di Martino B, Di Francesco CE, Meridiani I, Marsilio F. Aetiological investigation of multiple respiratory infections in cats. New Microbiol. (2007) 30:455–61.
42. Kartashov S, Kartashova E, Butenkov A, Rakityanskaya A, Petrova M, Oboeva M, et al. Corneal ulcers associated with FHV-1 in cats. In: IOP Conference Series: Earth and Environmental Science. (2019) Vol. 403. XII International Scientific Conference on Agricultural Machinery Industry 10–13 September 2019, Don State Technical University, Russian Federation. doi: 10.1088/1755-1315/403/1/012024
43. Maggs DJ. Update on pathogenesis, diagnosis and treatment of feline herpesvirus type 1. Clin Tech Small Anim Pract. (2005) 20:94–101. doi: 10.1053/j.ctsap.2004.12.013
45. Bentley E, Abrams GA, Covitz D, Cook CS, Fischer CA, Hacker D, et al. Morphology and immunohistochemistry of spontaneous chronic corneal epithelial defects (scced) in dogs. Investig Ophthalmol Vis Sci. (2001) 42:2262–9.
46. Van Der Woerdt A. Adnexal surgery in dogs and cats. Vet Ophthalmol. (2004) 7:284–90. doi: 10.1111/j.1463-5224.2004.04044.x
47. Sansom J, Blunden T. Calcareous degeneration of the canine cornea. Vet Ophthalmol. (2010) 13:238–43. doi: 10.1111/j.1463-5224.2010.00791.x
48. Gervais KJ, Pirie CG, Ledbetter EC, Pizzirani S. Acute primary canine herpesvirus-1 dendritic ulcerative keratitis in an adult dog. Vet Ophthalmol. (2012) 15:133–8. doi: 10.1111/j.1463-5224.2011.00952.x
49. Arnalich F, Rodriguez AE, Luque-Rio A, Alio JL. Solid platelet rich plasma in corneal surgery. Ophthalmol Ther. (2016) 5:31–45. doi: 10.1007/s40123-016-0051-9
50. Donatti C, Brandão CVS, Ranzani JJT, Perches CS, Padovani CR, et al. Uso do plasma rico em plaquetas no reparo de úlceras de córnea profundas induzidas em coelhos. Avaliação clínica e histomorfométrica. Arquivo Brasileiro de Medicina Veterinária e Zootecnia. (2013) 65:809–18. doi: 10.1590/S0102-09352013000300029
51. Cremonini DN, Ranzani JJT, Marques MEA, Rodrigues GN, Brandão CVS. Cryopreserved amniotic membrane transplantation for corneal healing with limbic cell deficiency in rabbits. Arq Bras Med Vet Zootec. (2007) 59:1462–7. doi: 10.1590/S0102-09352007000600017
52. Yang S-F, Yang H-L, Liang S-L, Wang P-C. Case report: subconjunctival injection with autologous platelet-rich plasma for refractory corneal ulcers in a dog. Taiwan Veterinary J. (2018) 44:1–6. doi: 10.1142/S1682648518720034
53. Simona DP, Chiara C, Francesca A, Rosario P, Giusi V, Elisabetta G. Platelet rich plasma eye drops: preparation, storage and clinical use in dogs and cats.preliminary results. Arch Vet Sci Technol. (2017) 104. doi: 10.29011/2637-9988/100004
54. Textor J. Platelet-Rich Plasma (PRP) as a therapeutic agent: platelet biology, growth factors and a review of the literature. In: Lana JFSD, Andrade Santana MH, Dias W, Belangero AC, Malheiros Luzo, editors. Platelet-Rich Plasma: Regenerative Medicine: Sports Medicine, Orthopedic, and Recovery of Musculoskeletal Injuries. Berlin, Heidelberg: Springer Berlin Heidelberg (2014) p. 61–94.
55. Peerbooms JC, Sluimer J, Bruijn DJ, Gosens T. Positive effect of an autologous platelet concentrate in lateral epicondylitis in a doubleblind randomized controlled trial: platelet-rich plasma versus corticosteroid injection with a 1-year follow-up. Am J Sports Med. (2010) 38:255–62. doi: 10.1177/0363546509355445
56. Wróbel-Dudzińska D, Alio J, Rodriguez A, Suchodoła-Ratajewicz E, Kosior-Jarecka E, Rymgayłło-Jankowska B, et al. Clinical efficacy of platelet-rich plasma in the treatment of neurotrophic corneal ulcer. J Ophthalmol. (2018) 2018:3538764. doi: 10.1155/2018/3538764
57. Matsumoto Y, Dogru M, Goto E, Ohashi Y, Kojima T, Ishida R, et al. Autologous serum application in the treatment of neurotrophic keratopathy. Ophthalmology. (2004) 111:1115–20. doi: 10.1016/j.ophtha.2003.10.019
58. Cejka C, Cejkova J. Oxidative stress to the cornea, changes in corneal optical properties, and advances in treatment of corneal oxidative injuries. Oxid Med Cell Longev. (2015) 2015:591530. doi: 10.1155/2015/591530
59. Salem NY, Yehia SG, Farag HS, Elkhiat MA. Clinical, hemato-biochemical alterations and oxidant–antioxidant biomarkers in Babesia-infected calves. Int J Veterinary Sci Med. (2016) 4:17–22. doi: 10.1016/j.ijvsm.2016.10.003
60. Ankamah E, Sebag J, Ng E, Nolan J. Vitreous antioxidants, degeneration, and vitreo-retinopathy: exploring the links. Antioxidants. (2020) 9:7. doi: 10.3390/antiox9010007
61. Kubota M, Shimmura S, Kubota S, Miyashita H, Kato N, Noda K, et al. Hydrogen and Nacetyl- L-cysteine rescue oxidative stress-induced angiogenesis in a mouse corneal alkali-burn model. Invest Ophthalmol Vis Sci. (2011) 52:427–33. doi: 10.1167/iovs.10-6167
62. Ruban VV, Anbukkarasi M, Anand T, Thomas PA, Geraldine P. Oxidative stress in corneal tissue in experimental keratitis due to Aspergillus flavus: effect of topical voriconazole therapy. Biocatal Agric Biotechnol. (2019) 21:101323. doi: 10.1016/j.bcab.2019.101323
63. Scandalios JG. Oxidative stress: molecular perception and transduction of signals triggering antioxidant gene defenses. Braz J Med Biol Res. (2005) 38:995–4. doi: 10.1590/S0100-879X2005000700003
64. Varma SD. Scientific basis for medical therapy of cataracts by antioxidants. Am J Clin Nutr. (1991) 53:335S−45S. doi: 10.1093/ajcn/53.1.335S
65. Abdulaziz AR, Almuzaini AA, Hassan AA. Evaluation of the anti-oxidative activity and trace elements concentrations in Demodex canis infected dogs. Peer Res Nest. (2019) 1:1–6.
66. Mancino R, Di Pierro N, Varesi C, Cerulli A, Feraco A, Cedrone, et al. Lipid peroxidation and total antioxidant capacity in vitreous, aqueous humor, and blood samplesfrom patients with diabetic retinopathy. Mol Vis. (2011) 17:1298–304.
67. Ye HQ, Azar DT. Expression of gelatinase A and B, and TIMPS 1 and 2 during corneal wound healing. Invest Ophthalmol Vis Sci. (1998) 39:913–21.
68. Singh A, Maurya OP, Jagannadhan MV, Patel A. Matrix metalloproteinases (MMP-2 and MMP-9) activity in corneal ulcer and ocular surface disorders determined by gelatin zymography. J Ocul Biol Dis Inform. (2012) 5:31–5. doi: 10.1007/s12177-012-9096-8
69. Ollivier FJ, Brooks DE, Kallberg ME, Komaromy AM, Lassaline ME, Andrew SE, et al. Evaluation of various compounds to inhibit activity of matrix metalloproteinase activities in the tear film of horses with ulcerative keratitis. Am J Vet Res. (2003) 64:1081–7. doi: 10.2460/ajvr.2003.64.1081
70. Willeford KO, Miller WM, Abrams KL, Vaughn BM. Modulation of proteolytic activity associated with persistent corneal ulcers in dogs. Vet Ophthalmol. (1998) 1:5–8. doi: 10.1046/j.1463-5224.1998.00001.x
71. Oliver GW, Leferson JD, Stetler-Stevenson WG, Kleiner DE. Quantitative reverse zymography: analysis of picogram amounts of metalloproteinase inhibitors using gelatinase A and B reverse zymograms. Anal Biochem. (1997) 244:161–6. doi: 10.1006/abio.1996.9895
72. Holopainen JM, Serra HM, Sanchez MC, Sorsa T, Zalentein WN, Barcelona PF, et al. Altered expression of matrix metalloproteinases and their tissue inhibitors as possible contributors to corneal droplet formation in climatic droplet keratopathy. Acta Ophthalmol. (2011) 89:569–74. doi: 10.1111/j.1755-3768.2009.01764.x
73. Yanga Y-N, Bauera D, Wasmutha S, Steuhl K-P, Heiligenhau A. Matrix metalloproteinases (MMP-2 and 9) and tissue inhibitors of matrix metalloproteinases (TIMP-1 and 2) during the course of experimental necrotizing herpetic keratitis. Exp Eye Res. (2003) 77:227–37 doi: 10.1016/S0014-4835(03)00112-X
74. Mulholland B, Tuft SJ, Khaw PT. Matrix metalloproteinase distribution during early corneal wound healing. Eye. (2005) 19:584–8. doi: 10.1038/sj.eye.6701557
75. Twining SS. Regulation of proteolytic activity in tissues. Crit Rev Biochem Mol Biol. (1994) 29:315–83. doi: 10.3109/10409239409083484
76. Azar DT, Pluznik D, Jain S, Khoury JM. Gelatinase B and A expression after laser in situ keratomileusis and photorefractive keratectomy. Arch Ophthalmol. (1998) 116:1206–8. doi: 10.1001/archopht.116.9.1206
77. Barletta JP, Angella G, Balch KC, Domova HG, Stern GA, Moser MT, et al. Inhibition of pseudomonal ulceration in rabbit corneas by a synthetic matrix metalloproteinase inhibitor. Invest Ophthalmol Vis Sci. (1996) 37:20–8.
78. Sotozono C. Second injury in the cornea. the role of inflammatory cytokines in corneal damage and repair. Cornea. (2000) 19(Suppl. 3):S155–9. doi: 10.1097/00003226-200000003-00004
79. Rechichi M, Ferrise M, Romano F, Gallelli L, Toschi V, Dominijanni A, et al. Autologous platelet-rich plasma in the treatment of refractory corneal ulcers: a case report. Am J Ophthalmol Case Rep. (2020) 20:100838. doi: 10.1016/j.ajoc.2020.100838
80. Alio JL, Rodriguez AE, Martinez LM, Rio AL. Autologous fibrin membrane combined with solid platelet-rich plasma in the management of perforated corneal ulcers: a pilot study. JAMA Ophthalmol. (2013) 131:745–51. doi: 10.1001/jamaophthalmol.2013.2474
81. Ortuño-Prados VJ, Alio JL. Tratamiento de úlcera corneal neurotrófica con plasma rico en plaquetas y Tutopatch® [Treatment of a neurotrophic corneal ulcer with solid platelet-rich plasma and Tutopatch®]. Arch Soc Esp Oftalmol. (2011) 86:121–3. Spanish. doi: 10.1016/j.oftal.2010.11.006
82. Ronci C, Ferraro AS, Lanti A, Missiroli F, Sinopoli S, Del Proposto G, et al. Platelet-rich plasma as treatment for persistent ocular epithelial defects. Transfus Apher Sci. (2015) 52:300–4. doi: 10.1016/j.transci.2014.12.027
83. Alio JL, Rodriguez AE, De Arriba P, Gisbert S, Abdelghany AA. Treatment with platelet-rich plasma of surgically related dormant corneal ulcers. Eur J Ophthalmol. (2018) 28:515–20. doi: 10.1177/1120672117747042
84. Alio JL, Rodriguez AE, Ferreira-Oliveira R, Wrobel-Dudzinska D, Abdelghany AA. Treatment of dry eye disease with autologous platelet-rich plasma: a prospective, interventional, non-randomized study. Ophthalmol Ther. (2017) 6:285–93. doi: 10.1007/s40123-017-0100-z
85. Wu TE, Chen CJ, Hu CC, Cheng CK. Easy-to-prepare autologous platelet-rich plasma in the treatment of refractory corneal ulcers. Taiwan J Ophthalmol. (2015) 5:132–5.26. doi: 10.1016/j.tjo.2014.09.001
86. Lee JH, Kim MJ, Ha SW, Kim HK. Autologous platelet-rich plasma eye drops in the treatment of recurrent corneal erosions. Korean J Ophthalmol. (2016) 30:101–7.69 doi: 10.3341/kjo.2016.30.2.101
87. Alio JL, Pastor S, Ruiz-Colecha J, Rodriguez A, Artola A. Treatment of ocular surface syndrome after LASIK with autologous platelet-rich plasma. J Refract Surg. (2007) 23:617–9. doi: 10.3928/1081-597X-20070601-13
88. Lopez-Plandolit S, Morales MC, Freire V, Etxebarria J, Duran JA. Plasma rich in growth factors as a therapeutic agent for persistent corneal epithelial defects. Cornea. (2010) 29:843–8. doi: 10.1097/ICO.0b013e3181a81820
89. Marquez De Aracena Del Cid R, Montero De Espinosa Escoriaza I. Subconjunctival application of regenerative factor-rich plasma for the treatment of ocular alkali burns. Eur J Ophthalmol. (2009) 19:909–15. doi: 10.1177/112067210901900603
90. Alio JL, Toprak I, Rodriguez AE. Treatment of severe keratoconus hydrops with intracameral platelet-rich plasma injection. Cornea. (2019) 1:254. doi: 10.1097/ICO.0000000000002070
91. Geremicca W, Fonte C, Vecchio S. Blood components for topical use in tissue regeneration: evaluation of corneal lesions treated with platelet lysate and considerations on repair mechanisms. Blood Transfusion. (2010) 8:107–12. doi: 10.2450/2009.0091-09
92. Can ME, Dereli Can G, Cagil N, Cakmak HB, Sungu N. Urgent Therapeutic grafting of platelet-rich fibrin membrane in descemetocele. Cornea. (2016) 35:1245–9. doi: 10.1097/ICO.0000000000000917
Keywords: corneal ulcer, autologous PRP, subconjunctival injections, dogs, cats, oxidative stress, MMPs
Citation: Farghali HA, AbdElKader NA, AbuBakr HO, Ramadan ES, Khattab MS, Salem NY and Emam IA (2021) Corneal Ulcer in Dogs and Cats: Novel Clinical Application of Regenerative Therapy Using Subconjunctival Injection of Autologous Platelet-Rich Plasma. Front. Vet. Sci. 8:641265. doi: 10.3389/fvets.2021.641265
Received: 14 December 2020; Accepted: 28 January 2021;
Published: 18 March 2021.
Edited by:
Roberta Perego, University of Milan, ItalyReviewed by:
Jorge L. Alio, Miguel Hernández University, SpainElena De Felice, University of Camerino, Italy
Copyright © 2021 Farghali, AbdElKader, AbuBakr, Ramadan, Khattab, Salem and Emam. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Haithem A. Farghali, dr_haithem0@yahoo.com; orcid.org/0000-0002-3311-1335
 Haithem A. Farghali
Haithem A. Farghali Naglaa A. AbdElKader1
Naglaa A. AbdElKader1  Huda O. AbuBakr
Huda O. AbuBakr Marwa S. Khattab
Marwa S. Khattab Noha Y. Salem
Noha Y. Salem Ibrahim A. Emam
Ibrahim A. Emam