Effect of Supplementation With Selenium-Yeast on Muscle Antioxidant Activity, Meat Quality, Fatty Acids and Amino Acids in Goats
- 1Key Laboratory of Animal Genetics, Breeding and Reproduction in the Plateau Mountainous Region, Ministry of Education, College of Animal Science, Guizhou University, Guiyang, China
- 2Testing Center for Livestock and Poultry Germplasm, Guizhou Agricultural and Rural Affairs Office, Guiyang, China
The objective of this study was to observe the effects of selenium-yeast (SY) on growth performance, muscle antioxidant activity, meat quality, fatty acid and amino acid profiles in growing goats. A total of 18 Qianbei-pockmarked goats were assigned to three groups (six duplicates per group) by body weight (25.75 ± 1.75 kg; mean ± standard deviation) according to a completely randomized design: (1) basal diet (CON); (2) CON with 2.4 mg/kg SY (LS); and (3) CON with 4.8 mg/kg SY (HS). The results indicated that goats receiving SY did not show any differences (P > 0.05) in terms of dry matter intake, growth performance, or muscle chemical composition. In addition, dietary treatment did not affect (P > 0.05) the pH values (pH45min and pH24h), percentage of water loss, drip loss, or cooking loss. The HS group showed a significant increase (P < 0.05) in the dressing percentage, eye muscle area and meat color, as well as muscle total antioxidant capacity, glutathione peroxidase and 2,2-diphenyl-1-picrylhydrazyl scavenging activity levels, whereas it showed a significant drop (P < 0.05) in shear force and muscle malondialdehyde levels relative to the control. Feeding 4.8 mg/kg SY led to a significant (P < 0.05) decrease in the levels of C8:0, C14:0, C15:0, C16:0, C17:0, C18:0, C20:0 and total saturated fatty acids, whereas it led to a significant (P < 0.05) increase in C15:1 in comparison with that of the control group. Goats receiving 2.4 mg/kg SY had significantly (P < 0.05) increased C16:1, C17:1, C18:1n7, C18:2n6, C18:3n3, C20:4n6, C22:1n9, and PUFA relative to the control group. Compared with the control group, the treatment groups had higher (P < 0.05) levels of C18:1n9, C22:4, and monounsaturated fatty acids. The inclusion of 2.4 mg/kg SY induced significant (P < 0.05) increases in 4-aminobutyric acid, glutamic acid and umami amino acid concentrations compared to the control. In addition, the feeding of 4.8 mg/kg SY had significantly higher (P < 0.05) muscle serine, valine, isoleucine, leucine, ornithine hydrochloride, methionine, and tyrosine levels than the control group. Collectively, Se supplementation in the diet did not affect growth performance, muscle chemical composition, whereas it could improve meat quality, muscle antioxidant activity, fatty acid and amino acid profiles in Qianbei-pockmarked goats. This showed that the optimal accession SY level was 4.8 mg/kg under the experimental conditions of this study.
Introduction
When the body suffers from oxidative stress (OS), the amount of reactive oxygen species (ROS) is enhanced and the antioxidant capacity decreases, leading to the initiation of lipid peroxidation, which may cause DNA oxidative damage and abnormal protein expression (1). The development of antioxidants to alleviate OS is popular with many researchers (2). The most important and known biological effect of selenium (Se) is its antioxidative effect. Indeed, Se is an essential trace element that has many biological functions, especially in preventing lung cancer, carcinoma of the prostate and liver cancer. However, excessive or inadequate Se intake will lead to physiological disorders or diseases. Se deficiency or excess can lead to poisoning caused by gastrointestinal disorders, vomiting, nausea, and diarrhea, thereby leading to impaired physiological functions (3).
Supplementation of Se in ruminant feed is achieved with additives such as sodium selenite, sodium selenate and selenium-yeast (SY) Saccharomyces cerevisiae. Antunović et al. (4) showed that organic Se in the feed could be transferred to the muscle and organs, and organic Se had a better bioavailability than inorganic Se in fattening lambs. Notably, SY is a source of organic Se (mainly selenomethionine) because it can be absorbed and retained more readily than inorganic Se (5). The general requirement for Se in the diet of ruminants is 0.1–0.3 mg/kg, and the maximum tolerance of Se is 5 mg/kg (6). Vignola et al. (7) demonstrated that lambs receiving low levels (0.30 and 0.45 mg/kg SY) of SY had no differences in growth performance, feed-to-gain ratio, carcass or meat quality. Goats generally have a strong tolerance of Se, and SY at a high level (4.0 mg/kg) is a relatively safe Se supplement for goats (6).
Polyunsaturated fatty acids (PUFAs) are important for body health. However, ruminant products have been criticized for their saturated fatty acids (SFAs) may have adverse effects on human health, which has contributed to declining consumption (8). Se has been proven to have strong antioxidant activity, delaying the onset of muscle oxidation reactions (9). Hence, ruminant receiving SY had the ability to improve unsaturated fatty acid (UFA) profiles in ruminant products. Moreover, Se has the capability of preventing apoptosis initiated by ROS and it participates in the regulation of amino acid (AA) profiles (10). Therefore, adding Se to the goat diet can not only improve growth performance but also enhance immune responses and antioxidant activity (11). However, research on the influence of a high dose of SY on meat quality in goats is rather limited. Considering the above, we hypothesized that a high dietary dose of SY would increase longissimus dorsi (LD) muscle antioxidant activity, improving meat quality, muscle fatty acids (FAs) and AAs derived from goats. Accordingly, the current research was performed to observe whether supplementation with Se in the diet could affect the meat quality, muscle antioxidant activity, FA and AA of Qianbei-pockmarked goats.
Materials and Methods
Animals, Diets, and Experimental Design
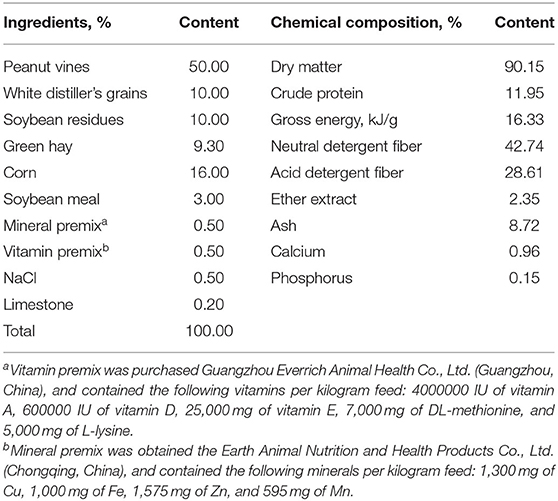
The feeding trial was carried out at a commercial goat farm (Guizhou, China). Signs of Se toxicity usually start to appear at Se levels of 5–8 mg/kg DM in ruminants (12). Therefore, the goats were raised for 74 d, which included a preparation period of 14 d and a formal experimental period of 60 d. Eighteen Qianbei-pockmarked wether goats (a Guizhou native goat breed) were assigned to three groups (six duplicates per group) by body weight (BW, 25.75 ± 1.75 kg; mean ± standard deviation) according to a completely randomized design: (1) basal diet (CON); (2) CON with 2.4 mg/kg SY (LS); and (3) CON with 4.8 mg/kg SY (HS). The SY as part of the basal ration and was mixed in the concentrate, and then the concentrate was mixed with the roughage evenly. SY was obtained from Jiangsu Qianbo Bioengineering Co., Ltd., Jiangsu, China. The information of SY is: food additive production license No. SC20133062401397, the Se content of the SY is 2000 ppm, and the appearance is a yellow powder with a uniform fineness. During the experiment, all animals received water freely and were fed at 08:30 and 16:30 for ad libitum intake. The rations were made according to the National Research Council [(13); NRC, Table 1].
Chemical Composition
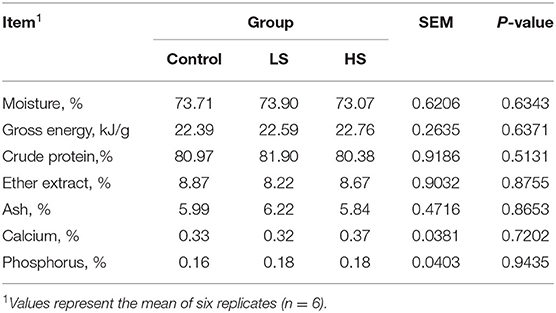
Approximately 100 g of base diet was collected once weekly and mixed after the end of the feeding trial, and 500 g of feed was collected and dried at 65°C in a vacuum oven for 72 h, ground and passed through a 1-mm sieve for further analysis. The chemical composition of dry matter (DM), crude protein (CP), ether extract (EE), ash, calcium (Ca), phosphorus (P) and gross energy (GE) were measured according to the Association of Official Analytical Chemists [(14); AOAC]. Neutral detergent fiber (NDF) and acid detergent fiber (ADF) were determined according to Van Soest et al. (15). Each sample was analyzed in triplicate.
Growth Performance
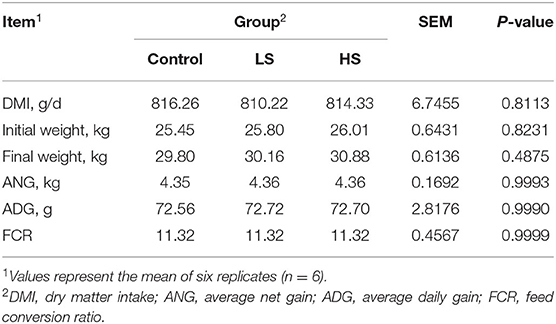
Dry matter intake (DMI) was recorded as the intake of each group of goats every day, and BW was measured on the first day and the last day before the morning feeding to calculate the initial weight (IW) and final weight (FW). The following formulas were used to calculate the average net gain (ANG), average daily gain (ADG), and feed conversion ratio (FCR): ANG (kg) = FW (kg) – IW (kg); ADG (g/d) = ANG/74 × 1000; FCR = DMI/ADG × 100%.
Antioxidant Activity
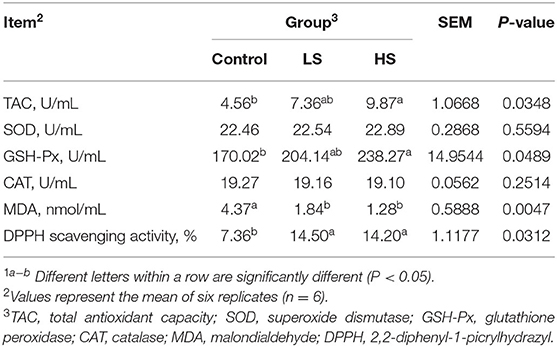
The LD homogenate was prepared as follows: chopped tissue and phosphate buffered saline (PBS) were added to the glass homogenizer (1:9), and the homogenate was broken by ultrasonication using a Bransonic® ultrasonic cleaner (Branson ultrasonics corporation, USA). Next, the supernatant was transferred to a 1.5 mL tube after centrifugation at 4,000 × g for 10 min at 4°C and stored at −80°C until further analysis. The total antioxidant capacity (TAC), superoxide dismutase (SOD), glutathione peroxidase (GSH-Px), catalase (CAT), and malondialdehyde (MDA) were detected by using commercial assay kits (Nanjing Jiangcheng Bioengineering Institute, Nanjing, China) according to the operation guide of the kit. The 2,2-diphenyl-1-picrylhydrazyl (DPPH) scavenging activity was noted according to our previous study (16).
Meat Quality
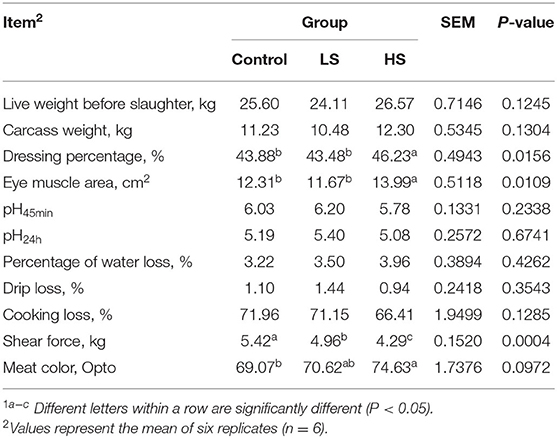
Six goats per group were slaughtered at the end of the experiment for carcass and the left LD muscle was separated for meat quality examination. The parameters as follows: (1) live weight before slaughter (kg): the body weight was measured after stopping feeding for 24 h and water for 2 h before slaughter; (2) carcass weight (kg): after slaughter, blood, fur, head, hoof, and internal organs (the kidney and kidney fat were retained) were removed, weighing the carcass after standing for 20–30 min; (3) dressing percentage (%): dressing percentage = (carcass weight/live weight before slaughter) × 100; (4) eye muscle area (cm2): the cross-sectional area of the LD muscle between the 12th and 13th ribs of the carcass, the outline of the cross section of the eye muscle was drawn with sulfuric acid drawing paper, and then the eye muscle area was calculated by using a digital planimeter KP-90N (No. H18049, made in Japan); (5) pH: the pH value was detected by a pH-star (Matthäus, Eckelsheim, Germany); (6) percentage of water loss (%): a 1 cm thick sample was cut with a 2.5 cm diameter and weighed, then the sample was pressed under 35 kg for 5 min and weighed again, and the percentage of water loss = [(prepressure weight - postpressure weight)/prepressure weight] × 100; (7) drip loss (%): the trimmed meat sample (2.0 × 3.0 × 5.0 cm) was put in a special plastic bag, the bag was inflated and closed, then it was placed in a 4°C refrigerator for 24 h, the juice on the surface of the meat sample was wiped off with filter paper, and the meat was weighed, drip loss = [(initial weight–final weight)/initial weight] × 100%; (8) cooking loss (%): ~100 g of sample (m1) was weighed after removing the fascia, epimysium or fat, and the sample was boiled in water for 30 min, cooled for 30 min and weighed (m2), cooking loss = (m1- m2) × 100; (9) shear force (kg): shear force was observed by a digital display tenderness meter (Xielikeji Co., Ltd., Harbin, China) after drilling the muscle column along the direction of the muscle fiber; and (10) meat color (Opto): meat color was measured using an Opto-Star equipment (Company MATTHÄUS, KLAUSA). Each sample was tested in triplicate.
Fatty Acids
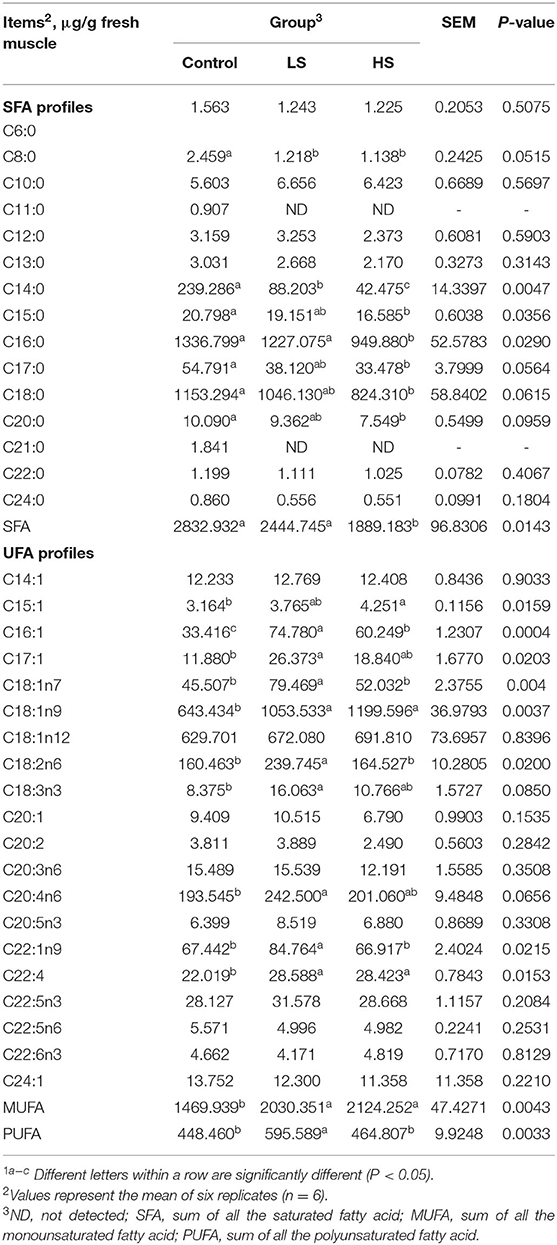
FAs were extracted using a chloroform methanol solution according to Tian et al. (17). Individual FAs were detected using a gas chromatography mass spectrometer (GC–MS; Thermo Fisher Scientific, USA). The GC–MS conditions were as follows: Thermo TG-FAME capillary column (50 m × 0.25 mm × 0.20 μm), 1 μL of injection volume, split ratio of 8:1; injection port temperature of 250°C, ionization temperature of 230°C, transmission line temperature of 250°C, and quadrupole temperature of 150°C. Helium gas was used as the carrier, the flow rate of the carrier gas was 0.63 mL/min, and the energy of ionization was 70 eV.
Amino Acids
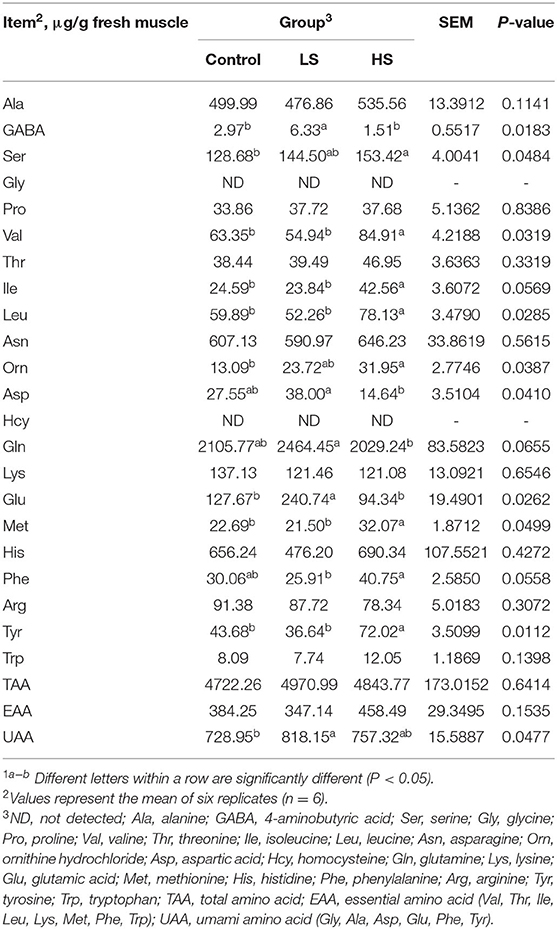
A total 50 mg of meat sample was weighed and transferred to a 2 mL tube. Then, 600 μL of 10% formic acid methanol solution and two steel balls were added and it was run at 60 Hz for 1 min using a high-throughput tissue grinder. Ten microliters of supernatant was added to 490 μL of 10% formic acid methanol solution after centrifugation at 12,000 × g for 5 min at 4°C (Hunan Xiangyi Centrifuge Instrument Co., Ltd., H1850R, Hunan, China) and vortexed at 30 s by a vortex mixer (Haimen Kylin-bell Lab Instruments Co., Ltd., QL866, Jiangsu, China). The 100 μL of diluted sample was mixed with 100 μL of dual isotope internal standard (100 ppb) and vortexed at 30 s. The sample was filtered through a 0.22 μm nylon syringe filter, and the amino acids were detected with a Waters ACQUITY ultra-performance liquid chromatography (UPLC, Waters, Milford, USA) system and tandem mass spectrometry (MS, SCIEX-6500Qtrap; AB Allen-Bradley, Milwaukee, WI) using the internal standard method.
The UPLC conditions were as follows: individual AAs were separated on an ACQUITY UPLC® BEH C18 column (2.1 × 100 mm × 1.7 μm, Waters, Milford, USA) with a column temperature of 40°C; the injection volume was 5 μL; mobile phase: A = 10% methanol (containing 0.1% formic acid) and B = 50% methanol (containing 0.1% formic acid). The gradient elution conditions were as follows: 0~6.5 min, 10~30% B; 6.5~7 min, 30~100% B; 7~8 min, 100% B; 8~8.5 min, 10~100% B; 8.5~12.5 min, 10% B. The flow rate was as follows: 0~8.5 min, 0.3 mL/min; 8.5~12.5 min, 0.3~0.4 mL/min. The MS conditions were as follows: electrospray ionization source, positive ion ionization mode; ion power temperature was 500°C, ion source voltage was 5,500 V; collision gas pressure of 6 psi, curtain gas pressure of 30 psi; nebulization gas pressure and aux gas pressure were both 50 psi; and multiple-reaction monitoring scan mode. Twenty-two amino acids were detected: alanine (Ala), 4-aminobutyric acid (GABA), serine (Ser), glycine (Gly), proline (Pro), valine (Val), threonine (Thr), isoleucine (Ile), leucine (Leu), asparagine (Asn), ornithine hydrochloride (Orn), aspartic acid (Asp), homocysteine (Hcy), glutamine (Gln), lysine (Lys), glutamic acid (Glu), methionine (Met), histidine (His), phenylalanine (Phe), arginine (Arg), tyrosine (Tyr), and tryptophan (Trp).
Statistical Analysis
The observations were analyzed by using the Statistical Analysis System 9.1.3 software (SAS Institute, Cary, NC, USA) to conduct a one-way analysis of variance model: Yij = μ +τi + εij, where Yij is the observation j (j = 1–6) in the treatment i (i = CON, LS, and HS), μ is the overall mean, τi is the effect of the treatment, εij is the random error with a mean of 0 and variance σ2. The level of significance was set to 0.05.
Results
Growth Performance
Adding SY had no significant influence (P > 0.05) on the DMI, growth performance (ANG, ADG) or the FCR of the goats (Table 2).
Antioxidant Activity
The inclusion of HS resulted in significantly higher (P < 0.05) levels of muscle TAC, GSH-Px and DPPH scavenging activity than that of the control group (Table 3). In contrast, goats receiving HS exhibited a significant decline (P < 0.05) in MDA concentration compared with the control group.
Meat Quality
As shown in Table 4, the muscle chemical composition was not influenced significantly (P > 0.05) by dietary SY supplementation. Similarly, there was no difference (P > 0.05) in live weight before slaughter or carcass weight among the dietary treatment groups (Table 5). However, the dressing percentage of goats fed a high SY diet was higher (P < 0.05) than that of goats fed a basal or low SY diet. Furthermore, adding a high dosage of SY significantly increased (P < 0.05) the eye muscle area of the goats. The lowest shear force was presented by goats fed HS, followed by LS and the control (P < 0.05). Dietary treatment did not affect (P > 0.05) the pH values (pH45min and pH24h), percentage of water loss, drip loss, or cooking loss. Meat color from the goats in the HS group was significantly (P < 0.05) darker than that in the other groups.
Fatty Acids
For the SFA profiles, muscle C11:0 and C21:0 were not detected in either of the two treatments (Table 6). Feeding 4.8 mg/kg SY led to significantly (P < 0.05) decreased levels of C8:0, C14:0, C15:0, C16:0, C17:0, C18:0, C20:0 and total SFAs compared with the control group. For the UFA profiles, goats receiving 2.4 mg/kg SY had significantly (P < 0.05) increased C16:1, C17:1, C18:1n7, C18:2n6, C18:3n3, C20:4n6, C22:1n9, and PUFA relative to the control group. In addition, supplementation with a high dose of SY (4.8 mg/kg) led to a significant (P < 0.05) increase in C15:1 in comparison with that of the control group. Compared to the control group, there were higher (P < 0.05) levels of C18:1n9, C22:4, and monounsaturated FAs (MUFAs) in the treatment groups.
Amino Acids
As shown in Table 7, there were no significant differences (P > 0.05) in muscle Ala, Pro, Thr, Asn, Lys, His, Arg, Trp, total AA (TAA), or essential AA (EAA) values among the three groups. Moreover, Gly and Hcy were unable to be detected in all groups. Compared to the control, feeding with 2.4 mg/kg SY induced significant (P < 0.05) increases in GABA, Glu and umami amino acid (UAA) concentrations. Furthermore, the HS group had significantly higher (P < 0.05) muscle Ser, Val, Ile, Leu, Orn, Met, and Tyr levels than the control group.
Discussion
Small ruminants are more prone to OS status, resulting in reduced growth performance and impaired immunity, leading to depressed husbandry efficiency (18). Hence, finding a suitable method to improve the antioxidation ability of small ruminants and eliminate free radicals (FRs) is a hot subject. Se is not produced by the body and must be obtained through food and water (19). Dietary Se supplementation is the most common method to improve the Se status of ruminants. Se is an essential trace element in biological life activities, playing a biological function in the form of selenoproteins, such as GSH-Px, thioredoxin reductases, deiodinases, and selenoproteins; alternatively, Se exerts its biological function when incorporated into proteins as selenocysteine residues, replacing the sulfur moiety in cysteine (20). Thus, Se, as a component of inactivators of toxic heavy metals, plays an important structural and enzymatic function, able to neutralize ROS and enhance antioxidant activity, protecting against lipid oxidation in the ruminant body (21). Indeed, Se can improve the antioxidant defense system in the body by modulating ROS-generating enzymes and additional synthesis of antioxidant enzymes and promoting inflammation in a cooperative and interactive way, being critically important for the ruminant's adaptation to nutritional stress (22).
Chauhan et al. (23) reported that Se supplementation could improve antioxidant enzymes in the body, protecting against OS and improving ruminant health. Vignola et al. (7) showed that supplementation with Se can enhance muscle Se levels, reducing the lipid oxidation of lambs. Thus, adding SY to the diet could strengthen antioxidant activity in muscle in this study, perhaps because Se is a part of the active center of GSH-Px, which can catalyze the reduction of peroxides (24). This may also be attributed to dioxide Se being a source of oxidant, which can oxidize some groups, such as hydroxides and methyl groups, to aldehyde groups and is reduced to Se itself during the chemical reaction (25). Consistent with our conclusions, Alimohamady et al. (26) showed that dietary Se supplementation did not impact the DMI, ADG, and FCR, whereas it can improve GSH-Px activity in lambs. Similar values of antioxidant activity in the body of fattening lambs receiving Se were obtained by Antunović et al. (4).
Meat quality traits include color, texture, hardness, tenderness, marble, etc. Among them, the amount of fat deposition and depositional mode are very important. The dressing percentage is an important index to evaluate carcass quality. Generally, the larger the eye muscle area is, the higher the lean meat rate (27). Netto et al. (28) showed that Se supplementation altered lipid metabolism in confined Brangus cattle by reducing the cholesterol concentration in the meat. Similarly, Se intake led to altered mRNA expression of genes associated with cholesterol and lipid metabolism parameters in the liver and LD muscle of growing Polish Merino lambs (29). In the current study, the addition of high SY to the diet improved the dressing percentage and eye muscle area, which may be related to Se regulating fat deposition by regulating lipid metabolism parameters in ruminants. Tenderness is critically important from a sensory viewpoint. Shear force is a measurement that indicates tenderness, mechanically assessed through the force necessary to sever the muscle fibers (30). Meat tenderness is among the important determinants of sensory quality parameters associated with consumer preferences. In the present study, goat meat from the SY diet had a lower shear force value than that from the control diet, indicating a tender texture. Meat is primarily comprised of myoglobin (Mb). The O2 molecule can be bound to Fe2+ and is stabilized through hydrogen binding by the nearby distal His (31). Hence, an important reason for the meat color changing is because the Fe2+ in Mb is unstable, and it is easy to oxidize to Fe3+, resulting in discoloration of the product (32). Therefore, supplementation with SY could improve the meat color of goats, suggesting that Se may prevent the oxidation of Fe2+ to Fe3+, thus improving the meat color.
It is well-known that O2 is vital for all animals. However, some O2 is converted to superoxide () and then converted into hydrogen peroxide (H2O2) and H2O by SOD, GSH-Px, and CAT enzymes (33, 34). FR scavengers are substances that can scavenge FR or block FR involved in oxidative reactions. Additionally, most FR scavengers are antioxidants, which can control the formation of FR by reducing the concentration of active FR intermediates and reducing the efficiency of the expansion stage in the FR chain reaction. UFAs in lipids are active in chemical activities because they contain multiple double bonds, very susceptible to destruction by FR and peroxidation (35). Lipid peroxidation in UFAs is a reaction where FR removes an electron from the lipids because molecular O2 is incorporated into unsaturated lipids to form a lipid hydroperoxide (36). Hence, lipid peroxidation is a natural phenomenon involved in peroxidative loss of unsaturated lipids, thus causing lipid degradation and membrane disorders (37). Ruminant meat is characterized by a high SFA content and a low PUFA content due to biohydrogenation of UFAs in the rumen by microflora (38). Purba et al. (39) did show that SFAs are showed in higher levels compared to the PUFAs in ruminant meat. In biological systems, lipid peroxidation is controlled by antioxidant defense systems that include antioxidant nutrients. Although Se is not an antioxidant, it is necessary for the production of several enzymes that affect the antioxidant network. Additionally, Se exists in the cytoplasm and mitochondrial matrix, which can reduce toxic peroxides to non-toxic hydroxyl compounds and decompose H2O2 into alcohols and water (40). Noticeably, Se also has a synergistic effect with vitamin E, which means that the two nutrients are more powerful than either one alone (19). Kišidayová et al. (41) demonstrated that SY could impact the intake of dietary PUFAs by rumen microbes, especially rumen ciliates, as well as the biohydrogenation of PUFAs to more SFAs. Niedwiedzka et al. (42) suggested that feeding SY can improve the muscle MUFA and PUFA ratios and increase the concentration of EAAs. Thus, Se has a strong antioxidant function, which can improve the content of beneficial FAs in animal byproducts by regulating the hydrogenation of PUFAs in the rumen.
Low intake and poor utilization of feedstuffs could be negative effect on the rumen ecosystem and rumen fermentation (43, 44). Adding of SY did not differ on the DMI and growth performance, while it could improve antioxidant activity in goat meat. Moreover, Ferreira et al. (45) who showed that Se supplementation did not improve rumen fermentation and nutrient utilization, but it tended to increase the concentration of PUFA in omasum flow of FAs. Thus, we observed that SY could improve the meat UFA concentrations of goats in the present study. The mechanism may be related to increasing the antioxidant activity and inhibiting the production of FR, resulting in a reduction in the lipid peroxidation reaction. In addition, feed composition affects ruminal fluid fermentation (46, 47), the affinity of rumen microorganisms (48). Dietary supplementation of SY may improve UFA concentrations by altering rumen fermentation model and ruminal microbiota in goats. Further studies are needed to observe the effect of SY on ruminal microorganisms. This is consistent with the results of Aghwan et al. (49), who demonstrated that supplementation with Se can lead to an increase in UFAs in the supraspinatus, longissimus lumborum, and semitendinosus muscles in Kacang goats. Yong et al. (50) also had a similar observation, that Korean native Hanwoo cattle fed a diet supplemented with organic Se had improved growth performance, carcass traits, and meat quality with an enriched PUFA profile. However, Pereira et al. (51) found that Nellore steers receiving Se did not have any changes in the FA profile of their LD muscle. The reason for the different observations may be as follows: (1) adding different SYs as the source of Se to the daily diet; (2) different species of animals have different metabolic pathways of Se; and (3) different Se compounds and their structural formulas could also be contributing factors (52, 53).
When the body is in a state of OS, it may participate in the AA metabolic process because ROS can also oxidize blood and structural proteins and inhibit the proteolytic system (54). Dai et al. (55) showed that cysteine limitation reduces the biosynthesis of glutathione (GSH) and causes the depletion of GSH, which directly inhibits the activity and stability of GPX4. In addition, cystathionine, cysteine and glycine are important precursors in the synthesis of GSH-Px, which has a rate-limiting role in the synthesis process (56). Indeed, AA has been shown to regulate the upstream glutathione synthesis pathway by reducing OS and the subsequent inflammatory response in ruminants (57). Specifically, most of the Se in the body exists in the active center of specific selenoproteins/selenases in the form of selenocysteine, is non-specifically incorporated into ordinary proteins in the form of selenomethionine, and it exists in the form of methylated Se compounds (58). In this study, Se cleared the FR in goats and upregulated the OS status of the body, thereby improving muscle AAs. Additionally, protein oxidation can cause AA loss or they can be fragmented, leading to alterations of structural proteins or alterations of enzyme functions (54). This is perhaps because Se in the liquid phase binds to protein, followed by microbial cell protein hydrolysis, and then it is absorbed as a Se-containing free AA (59). Of interest, the AA content increased with supplementation with a high dose of SY in this study. One possible reason is that ruminal bacteria are capable of utilizing Se from selenite and selenate for the synthesis of selenoamino acids (60). For young ruminants, Se is closely linked with vitamin E and sulfur-containing AAs (61). Gressley (62) who found that Se is covalently bound to AA to form AA complex trace minerals, with increased bioavailability in ruminants, suggested that allows more AA to be deposited in muscle. This might also be the reason for the lower apparent Se absorption by ruminants than by monogastric animals.
The types of AA can be divided into tasteless, bitter, sweet, and umami amino acid according to taste characteristics. The UAAs are represented by Gly, Ala, Asp, Glu, Phe, and Tyr. When the expression of umami substance regulatory genes is high, the content of umami substances in the meat is also increased (63). In addition, adenylosuccinate lyase (ADSL) plays a key role in the formation of umami substances. Interestingly, muscle tenderness can be affected by cellular antioxidants, and ADSL has been previously identified as a putative biomarker of tenderness, being positively correlated with beef tenderness (64). In the current study, SY improved UAA in the LD muscle of goats. Regarding the possible mechanism, it could be assumed that Se can prevent excessive FR, enhance antioxidant enzymes and modulate ADSL-related umami gene expression in the body, thus leading to an increase in UAA content in the muscle of goats, but this needs further verification. Additionally, the meat is rich in Se concentration, the integrity of cell membrane is protected after slaughter, reducing the loss of umami substances, and thus improving UAA content in LD muscle in goats. In short, the experimental results demonstrated that SY supplementation may be useful for growing goats because of its high antioxidant potential.
Conclusion
The findings of the current study suggest that dietary Se supplementation could not only improve the muscle antioxidant activity and meat quality but also improve the amino acid and fatty acid profiles of meat from Qianbei-pockmarked goats. Additional studies are required to clarify the effects of SY on the conjugated PUFA concentrations in the rumen microorganisms of goats from transcriptomics and metagenomics.
Data Availability Statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics Statement
The animal study was reviewed and approved by the Experimental Animal Ethics, Guizhou University (Guiyang, China).
Author Contributions
X-ZT: data curation, writing-original draft preparation, investigation, and data curation. J-XL, Q-YL, XW, and M-MX: investigation and data curation. DZ: supervision. QL: methodology and writing-reviewing and editing. XC: project administration and supervision. All authors have read and approved the final manuscript.
Funding
The research was funded by the Science and Technology Project of Guizhou Province (Qiankehe foundation-ZK[2021] General 164), the National Natural Science Foundation of China (31760652), the Cultivating Project of Guizhou University (2019-33), the Start-up Fund of Guizhou University (2016-76; 2019-26), and the Core Program of Science and Technology Department of Guizhou Province (QKH[2019]2279), respectively.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Valko M, Rhodes CJ, Moncol J, Izakovic M, Mazur M. Free radicals, metals and antioxidants in oxidative stress-induced cancer. Chem Biol Interact. (2006) 160:1–40. doi: 10.1016/j.cbi.2005.12.009
2. Purba RAP, Paengkoum P. Bioanalytical HPLC method of Piper betle L for quantifying phenolic compound, water-soluble vitamin, and essential oil in five different solvent extracts. J Appl Pharm Sci. (2019) 9:033–9. doi: 10.7324/JAPS.2019.90504
3. Zarczyńska K, Sobiech P, Radwińska J, Rekawek W. Effects of selenium on animal health. J Elementol. (2013) 18:329–40. doi: 10.5601/jelem.2013.18.2.12
4. Antunović Z, Novoselec J, Klapec T, Cavar S, Mioč B, Šperanda M. Influence of different selenium sources on performance, blood and meat selenium content of fattening lambs. Ital J Anim Sci. (2009) 8:163–5. doi: 10.4081/ijas.2009.s3.163
5. Sevcikova L, Pechova A, Pavlata L, Antos D, Mala E, Palenik T, et al. The effect of various forms of selenium supplied to pregnant goats on the levels of selenium in the body of their kids at the time of weaning. Biol Trace Elem Res. (2011) 143:882–92. doi: 10.1007/s12011-010-8884-x
6. Shi L, Ren Y, Zhang C, Yue W, Lei F. Effects of organic selenium (se-enriched yeast) supplementation in gestation diet on antioxidant status, hormone profile and haemato-biochemical parameters in Taihang black goats. Anim Feed Sci Tech. (2018) 238:57–65. doi: 10.1016/j.anifeedsci.2018.02.004
7. Vignola G, Lambertini L, Mazzone G, Giammarco M, Tassinari M, Martelli G, et al. Effects of selenium source and level of supplementation on the performance and meat quality of lambs. Meat Sci. (2009) 81:678–85. doi: 10.1016/j.meatsci.2008.11.009
8. Dewhurst RJ, Scollan ND, Lee MRF, Ougham HJ, Humphreys MO. Forage breeding and management to increase the beneficial fatty acid content of ruminant products. Proc Nutr Soc. (2003) 62:329–36. doi: 10.1079/PNS2003241
9. Juniper DT, Phipps RH, Ramosmorales E, Bertin G. Effect of dietary supplementation with selenium-enriched yeast or sodium selenite on selenium tissue distribution and meat quality in beef cattle. J Anim Sci. (2008) 86:3100–9. doi: 10.2527/jas.2007-0595
10. Abdelnour SA, El-Hack MEA, Khafaga AF, Arif M, Taha AE, Noreldin AE. Stress biomarkers and proteomics alteration to thermal stress in ruminants: a review. J Therm Biol. (2019) 79:120–34. doi: 10.1016/j.jtherbio.2018.12.013
11. Aditia M, Sunarso S, Sevilla CC, Angeles AA. Growth performance and mineral status on goats (Caprahircuslinn.) supplemented with zinc proteinate and selenium yeast. Sci Res Essays. (2014) 7:1741–51. doi: 10.12777/ijse.7.2.124-129
12. Arshad MA, Ebeid HM, Hassan F. Revisiting the effects of different dietary sources of selenium on the health and performance of dairy animals: a review. Biol Trace Elem Res. (2021) 199:3319–37. doi: 10.1007/s12011-020-02480-6
13. National Research Council. Nutrient Requirements of Goats: Angora, Dairy, and Meat Goats in Temperate and Tropical Countries. Washington, DC; The National Academies Press (1981).
14. Association of Official Analytical Chemists. Association of Official Analytical Chemists Official Methods of Analysis. 18th ed. Washington, DC; AOAC (2005).
15. Van Soest PV, Robertson JB, Lewis BA. Methods for dietary fiber, neutral detergent fiber, and nonstarch polysaccharides in relation to animal nutrition. J Dairy Sci. (1991) 74:3583–97. doi: 10.3168/jds.S0022-0302(91)78551-2
16. Tian XZ, Li JX, Luo QY, Zhou D, Long QM, Wang X, et al. Effects of purple corn anthocyanin on blood biochemical indexes, ruminal fluid fermentation, and rumen microbiota in goats. Front Vet Sci. (2021) 8:715710. doi: 10.3389/fvets.2021.715710
17. Tian XZ, Lu Q, Paengkoum P, Paengkoum S. Short communication: effect of purple corn pigment on change of anthocyanin composition and unsaturated fatty acids during milk storage. J Dairy Sci. (2020) 103:7808–12. doi: 10.3168/jds.2020-18409
18. Tian XZ, Paengkoum P, Paengkoum S, Thongpea S, Ban C. Comparison of forage yield, silage fermentative quality, anthocyanin stability, antioxidant activity, and in vitro rumen fermentation of anthocyanin-rich purple corn (Zea mays L.) stover and sticky corn stover. J Integr Agr. (2018) 17:2082–95. doi: 10.1016/S2095-3119(18)61970-7
20. Chauhan SS, Celi P, Ponnampalam EN, Leury BJ, Liu F, Dunshea FR. Antioxidant dynamics in the live animal and implications for ruminant health and product (meat/milk) quality: role of vitamin E and selenium. Anim Prod Sci. (2014) 54:1525–36. doi: 10.1071/AN14334
21. Kruzhel B, Bakowska M, Vovk S, Nowakowska E, Sergei P. Selenium in the diet of ruminants. Acta Sci Pol Zootechnica. (2014) 13:5–16. Available online at: https://asp.zut.edu.pl//2014/13_4/asp-2014-13-4-256.pdf
22. Surai PF, Kochish II, Fisinin VI, Juniper DT. Revisiting oxidative stress and the use of organic selenium in dairy cow nutrition. Animals. (2019) 9:462. doi: 10.3390/ani9070462
23. Chauhan SS, Liu F, Leury BJ, Cottrell JJ, Celi P, Dunshea FR. Functionality and genomics of selenium and vitamin E supplementation in ruminants. Anim Prod Sci. (2016) 56:1285–1298. doi: 10.1071/AN15263
24. Yu LL, Wang RL, Zhang YZ, Kleemann DO, Zhu XP, Jia ZH. Effects of selenium supplementation on polyunsaturated fatty acid concentrations and antioxidant status in plasma and liver of lambs fed linseed oil or sunflower oil diets. Anim Feed Sci Tech. (2008) 140:39–51. doi: 10.1016/j.anifeedsci.2007.02.003
25. Klein HP. Mechanistic Studies of Selenium-Dioxide Oxidations. [dissertation/Ph.D thesis]. [Tucson]; The University of Arizona (1968).
26. Alimohamady R, Aliarabi H, Bahari A, Dezfoulian AH. Influence of different amounts and sources of selenium supplementation on performance, some blood parameters, and nutrient digestibility in lambs. Biol Trace Elem Res. (2013) 154:45–54. doi: 10.1007/s12011-013-9698-4
27. Mickan FJ, Thomas GW, Spiker SA. A comparison between friesian bulls and steers on pasture for lean meat production. Aust J Exp Agr. (1976) 16:297–301. doi: 10.1071/EA9760297
28. Netto AS, Zanetti MA, Claro G, Melo M, Vilela FG, Corr Ea LB. Effects of copper and selenium supplementation on performance and lipid metabolism in confined Brangus bulls. Asian Austral J Anim. (2014) 27:488–94. doi: 10.5713/ajas.2013.13400
29. Juszczuk-Kubiak E, Bujko K, Cymer M, Wicińska K, Gabryszuk M, Pierzchała M. Effect of inorganic dietary selenium supplementation on selenoprotein and lipid metabolism gene expression patterns in liver and loin muscle of growing lambs. Biol Trace Elem Res. (2016) 172:336–45. doi: 10.1007/s12011-015-0592-0
30. Wheeler TL, Shackelford SD, Johnson LP, Miller MF, Miller RK, Koohmaraie M. A comparison of Warner-Bratzler shear force assessment within and among institutions. J Anim Sci. (1997) 75:2423. doi: 10.2527/1997.7592423x
31. Janani T. Herbs As Antioxidants in Oxidation of Marine Lipids. [dissertation/master's thesis]. [Trondheim]: Norwegian University (2013).
32. Zhang L, Liu Q, Chai Y, Yang F, Cao M, Dai W. In situ growth of g-C3N4 on hexangular flowerlike FeWO4 microcrystals: highly efficient catalyst and the crucial roles of Fe3+/Fe2+ couple in the photoassisted oxidation and reduction reactions. J Phys Chem C. (2018) 122:12900–12. doi: 10.1021/acs.jpcc.8b03575
33. Tian XZ, Xin HL, Paengkoum P, Paengkoum S, Ban C, Sorasak T. Effects of anthocyanin-rich purple corn (Zea mays L.) stover silage on nutrient utilization, rumen fermentation, plasma antioxidant capacity, and mammary gland gene expression in dairy goats. J Anim Sci. (2019) 97:1384–97. doi: 10.1093/jas/sky477
34. Tian XZ, Paengkoum P, Paengkoum S, Chumpawadee S, Ban C, Thongpea S. Short communication: purple corn (Zea mays L.) stover silage with abundant anthocyanins transferring anthocyanin composition to the milk and increasing antioxidant status of lactating dairy goats. J Dairy Sci. (2019) 102:413–8. doi: 10.3168/jds.2018-15423
35. Gardner HW. Oxygen radical chemistry of polyunsaturated fatty acids. Free Radic Biol Med. (1989) 7:65–86. doi: 10.1016/0891-5849(89)90102-0
36. Niki E, Yoshida Y, Saito Y, Nogushi N. Lipid peroxidation: mechanisms, inhibition, and biological effects. Biochem Bioph Res Co. (2005) 338:668–76. doi: 10.1016/j.bbrc.2005.08.072
37. Tian XZ, Lu Q, Zhao SG, Li JX, Luo QY, Wang X, et al. Purple corn anthocyanin affects lipid mechanism, flavor compound profiles, and related gene expression of longissimus thoracis et lumborum muscle in goats. Animals. (2021) 11:2407. doi: 10.3390/ani11082407
38. Doreau M, Ferlay A. Digestion and utilisation of fatty acids by ruminants. Anim Feed Sci Tech. (1994) 45:379–96. doi: 10.1016/0377-8401(94)90039-6
39. Purba RAP, Paengkoum P, Paengkoum S. The links between supplementary tannin levels and conjugated linoleic acid (CLA) formation in ruminants: a systematic review and meta-analysis. PLoS ONE. (2020) 15:e0216187. doi: 10.1371/journal.pone.0216187
40. Bialek M, Czauderna M, Przybylski W, Jaworska D. Selenate and selenite affect ruminal metabolism of C18 unsaturated fatty acids and fatty acid composition of lamb tissues. Livest Sci. (2020) 241:104249. doi: 10.1016/j.livsci.2020.104249
41. Kišidayová S, Mihaliková K, Siroka P, Cobanová K, Váradyová Z. Effects of inorganic and organic selenium on the fatty acid composition of rumen contents of sheep and the rumen bacteria and ciliated protozoa. Anim Feed Sci Tech. (2014) 193:51–7. doi: 10.1016/j.anifeedsci.2014.04.008
42. Niedwiedzka KM, Kowalczyk J, Czauderna M. Influence of selenate and linseed oil on fatty-acid and amino-acid profiles in the liver, muscles, fat tissues and blood plasma of sheep. J Anim Feed Sci. (2008) 17:328–43. doi: 10.22358/jafs/66612/2008
43. Paengkoum P, Liang JB, Jelan ZA, Baserry M. Utilization of steam-treated oil palm fronds in growing Saanen goats: II supplementation with energy and urea. Asian Austral J Anim. (2006) 19:1623–31. doi: 10.5713/ajas.2006.1623
44. Wanapat M, Chumpawadee S, Paengkoum P. Utilization of urea-treated rice straw and whole sugar cane crop as roughage sources for dairy cattle during the dry season. Asian Austral J Anim. (2000) 13:474–7. doi: 10.5713/ajas.2000.474
45. Ferreira AVDP, Cominotte A, Ladeira MM, Casagrande DR, Teixeira PD, van Cleef E, et al. Feedlot diets with soybean oil, selenium and vitamin E alters rumen metabolism and fatty acids content in steers. Anim Feed Sci Tech. (2020) 260:114362. doi: 10.1016/j.anifeedsci.2019.114362
46. Kaewwongsa W, Traiyakun S, Yuangklang C, Wachirapakorn C, Paengkoum P. Protein enrichment of cassava pulp fermentation by saccharomyces cerevisiae. J Anim Vet Adv. (2011) 10:2434–40. doi: 10.3923/javaa.2011.2434.2440
47. Paengkoum P. Effects of neem (Azadirachta indica) and leucaena (Leucaena leucocephala) fodders on digestibility, rumen fermentation and nitrogen balance of goats fed corn silage. J Anim Vet Adv. (2010) 9:883–6. doi: 10.3923/javaa.2010.883.886
48. Purba R, Yuangklang C, Paengkoum S, Paengkoum P. Milk fatty acid composition, rumen microbial population and animal performance in response to diets rich in linoleic acid supplemented with Piper betle L leaves in saanen goats. Anim Prod Sci. (2020) doi: 10.1071/AN20182
49. Aghwan ZA, Alimon AR, Goh YM, Nakyinsige K, Sazili AQ. Fatty acid profiles of supraspinatus, longissimus lumborum and semitendinosus muscles and serum in Kacang goats supplemented with inorganic selenium and iodine. Asian Austral J Anim. (2014) 27:543–50. doi: 10.5713/ajas.2013.13545
50. Yong GS, Rathnayake D, Hong SM, Dilawar MA, Yang CJ. Sensory attributes, microbial activity, fatty acid composition and meat quality traits of Hanwoo cattle fed a diet supplemented with stevioside and organic selenium. Foods. (2011) 10:129. doi: 10.3390/foods10010129
51. Pereira ASC, Santos MV, Aferri G, Corte RRPDS, Silva S, Freitas JED Jr, et al. Lipid and selenium sources on fatty acid composition of intramuscular fat and muscle selenium concentration of Nellore steers. Rev Bras Zootecn. (2012) 41:2357–63. doi: 10.1590/S1516-35982012001100009
52. Givens DI, Allison R, Cottrill B, Blake JS. Enhancing selenium content of bovine milk through alteration of the form and concentration of selenium in the diet of dairy cows. J Sci Food Agr. (2004) 84:811–7. doi: 10.1002/jsfa.1737
53. Dumont E, Vanhaecke F, Cornelis R. Selenium speciation from food source to metabolites: a critical review. Anal Bioanal Chem. (2006) 385:1304–23. doi: 10.1007/s00216-006-0529-8
54. Finaud J, Lac G, Filaire E. Oxidative stress: relationship with exercise and training. Sports Med. (2006) 36:327–58. doi: 10.2165/00007256-200636040-00004
55. Dai T, Li J, Lu X, Ye L, Yu H, Zhang L, et al. Prognostic role and potential mechanisms of the ferroptosis-related metabolic gene signature in hepatocellular carcinoma. Pharmacogen Pers Med. (2021) 14:927–45. doi: 10.2147/PGPM.S319524
56. Moreno P, Jiménez-Jiménez C, Garrido-Rodríguez M, Calderón-Santiago M, Molina S, Lara-Chica M, et al. Metabolomic profiling of human lung tumor tissues: nucleotide metabolism as a candidate for therapeutic interventions and biomarkers. Mol Oncol. (2018) 12:1778–96. doi: 10.1002/1878-0261.12369
57. Halsted CH. B-vitamin dependent methionine metabolism and alcoholic liver disease. Clin Chem Lab Med. (2013) 51:457–65. doi: 10.1515/cclm-2012-0308
58. Gresakova L, Cobanova K, Faix S. Selenium retention in lambs fed diets supplemented with selenium from inorganic or organic sources. Small Ruminant Res. (2013) 111:76–82. doi: 10.1016/j.smallrumres.2012.10.009
59. Ammerman CB, Miller SM. Selenium in ruminant nutrition: a review. J Dairy Sci. (1975) 58:1561–77. doi: 10.3168/jds.S0022-0302(75)84752-7
61. Radwinska J, Zarczynska K. Effects of mineral deficiency on the health of young ruminants. J Elementol. (2014) 19:915–28. doi: 10.5601/jelem.2014.19.2.620
62. Gressley TF. Zinc, copper, manganese, and selenium in dairy cattle rations. In Proceedings of the 7th Annual Mid-Atlantic Nutrition Conference. College Park: University of Maryland (2009).
63. Kenzo K. Umami the fifth basic taste: history of studies on receptor mechanisms and role as a food flavor. Biomed Res Int. (2015) 6:189402. doi: 10.1155/2015/189402
64. Zhu Y, Gagaoua M, Mullen AM, Kelly AL, Sweeney T, Cafferky J, et al. A proteomic study for the discovery of beef tenderness biomarkers and prediction of warner–bratzler shear force measured on longissimus thoracis muscles of young Limousin-Sired bulls. Foods. (2021) 10:952. doi: 10.3390/foods10050952
Keywords: selenium-yeast, antioxidant activity, meat quality, fatty acid, amino acid
Citation: Tian X-Z, Li J-X, Luo Q-Y, Wang X, Xiao M-M, Zhou D, Lu Q and Chen X (2022) Effect of Supplementation With Selenium-Yeast on Muscle Antioxidant Activity, Meat Quality, Fatty Acids and Amino Acids in Goats. Front. Vet. Sci. 8:813672. doi: 10.3389/fvets.2021.813672
Received: 12 November 2021; Accepted: 28 December 2021;
Published: 25 January 2022.
Edited by:
Dan Wan, Chinese Academy of Sciences (CAS), ChinaReviewed by:
Pramote Paengkoum, Suranaree University of Technology, ThailandDuanqin Wu, Chinese Academy of Agricultural Sciences (CAAS), China
Copyright © 2022 Tian, Li, Luo, Wang, Xiao, Zhou, Lu and Chen. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Qi Lu, luqi2556728@163.com; Xiang Chen, xchen2@gzu.edu.cn
 Xing-Zhou Tian
Xing-Zhou Tian Jia-Xuan Li1
Jia-Xuan Li1  Xiang Chen
Xiang Chen