- 1College of Animal Science and Technology, Northeast Agricultural University, Harbin, China
- 2Tropical Crop Genetic Resource Research Institute, Chinese Academy of Tropical Agricultural Sciences, Haikou, China
- 3Inner Mongolia Ordos City Agricultural and Forestry Technology Extension Center, Ordos, China
- 4Institute of Animal Science & Veterinary, Hainan Academy of Agricultural Science, Haikou, China
The objective of this study was to determine the effect of dietary lycopene supplementation on the growth performance, antioxidant enzyme activity of serum and liver, and gene expressions associated with Kelch-like ech-associated protein-1 (Keap1)/Nuclear Factor E2-related factor 2 (Nrf2) pathway in liver of Arbor Acres broilers. A total of 288 1-day-old male broilers were randomly divided into 4 treatments with 6 replicates and 12 chickens for each replicate. The control group was fed with the basal diet, while the treated groups were fed with the basal diet with 10, 20, and 30 mg/kg lycopene in powder. Feed and water were provided ad libitum for 42 days. Compared with the control group, (a) the average daily gain increased (p = 0.002 vs. p = 0.001) and the feed conversion ratio decreased (p = 0.017 vs. p = 0.023) in groups treated with lycopene in the grower and whole phases, and the average daily feed intake was quadratically affected (p = 0.043) by lycopene in the grower phase; (b) the serum superoxide dismutase content was linearly affected (p = 0.035) by lycopene at 21 days; (c) the serum glutathione peroxidase content, superoxide dismutase content, and total antioxidant capability were higher (p = 0.014, p = 0.003, and p = 0.016, respectively) in the 30 mg/kg lycopene group at 42 days; (d) the liver glutathione peroxidase and superoxide dismutase contents in groups treated with lycopene were higher (p ≤ 0.001 vs. p ≤ 0.001) at 21 days; (e) the liver glutathione peroxidase content was higher (p ≤ 0.001) in the 20 and 30 mg/kg lycopene groups, at 42 days; (f) the mRNA expression levels of Nrf2, superoxide dismutase 2, NAD(P)H quinone dehydrogenase 1, and heme oxygenase 1 genes were higher (21 days: p = 0.042, p = 0.021, p = 0.035, and p = 0.043, respectively; 42 days: p = 0.038, p = 0.025, p = 0.034, and p = 0.043, respectively) in the 20 and 30 mg/kg lycopene groups at 21 and 42 days. The 30 mg/kg lycopene concentration improved the growth performance, antioxidant enzyme activity in serum and liver, and gene expression in the Keap1-Nrf2 signaling pathway of Arbor Acres broilers.
Introduction
Oxidative stress can be caused by moldy feed, poor feeding environment, and imbalance of intestinal flora in poultry (1–3). The phytochemicals, such as oleanolic acid, polyphenols, and carotenoids, could alleviate the adverse effects of the reactive oxygen species in animals (4–6). Lycopene is a polyunsaturated aliphatic hydrocarbon with 11 conjugated double bonds and two non-conjugated double bonds in its chemical structure (7, 8). It is an acyclic isomer of β-carotene that mainly exists in ripe tomatoes and is the most potent antioxidant among most carotenoids (9, 10). As an additive, lycopene is recognized as a class A nutrient by the Food Additives Committee, the Food and Agriculture Organization of the United Nations, and the World Health Organization for its antioxidant and coloring functions (11). In the antioxidant defense network of the poultry, the key antioxidant enzyme system is mainly established by superoxide dismutase, glutathione peroxidase, and catalase (12, 13). It was found that adding dried tomato pomace to the diet could improve the activities of superoxide dismutase and catalase in the plasma of the Lohman Layer (14), the laying rate increased, and malondialdehyde content in serum and eggs decreased (15), indicating that the lycopene has an antioxidant function, but few studies about the antioxidant mechanisms of lycopene are found. The Kelch-like ech-associated protein-1/Nuclear Factor E2-related factor 2 (Keap1-Nrf2) signaling pathway regulates the transcription of antioxidant genes, which is considered to be the most critical pathway in the cellular antioxidant mechanism (16, 17). Wang et al. (18) found that the lycopene degraded intracellular Keap1 and activated Nrf2 could prevent cutaneous tumors. Moreover, it was also found that inhibiting Keap1 expression and enhancing Nrf2 expression in broiler muscles can relieve stress caused by environment (19), and activating Nrf2/heme oxygenase 1 (HO-1) pathway can ameliorate oxidative stress in the aging chicken ovary (7). It is hypothesized that the lycopene improves antioxidative potentials by mediating the Keap 1/Nrf2 signaling pathway.
This study intends to (a) study the effect of lycopene on the growth performance, serum and liver antioxidant enzyme activity, and gene expression in the Keap 1/Nrf2 signaling pathway of Arbor Acres broilers by adding lycopene to the broilers' diets; (b) discuss the feasibility and appropriate contents of lycopene in the production of broilers; and (c) preliminarily explore the impact mechanism of the lycopene on production performance of broilers, to further develop and use the lycopene, and accumulate experience and theoretical basis for safe and reliable additives.
Materials and Methods
Chickens and Management
Newly hatched male Arbor Acres broiler chicks were purchased from Harbin Yinong Poultry Industry Co. Ltd. (Harbin, China) and raised in the Experimental Base of Northeast Agricultural University (Harbin, China). The protocols were approved by the Animal Care and Use Committee of the Animal Nutrition Institute, Northeast Agricultural University (NEAU-2018-0232). In addition, all animals were handled following the Ethics and Animal Welfare Committee of Heilongjiang Province, China. Chickens were reared in battery cages and exposed to 24 h of light with 15-W fluorescent lighting every day throughout the experiment. The average temperature for the first 3 days was 33°C, which reduced 2°C per week until it reached 23°C. The relative humidity in the room was controlled at 70% in the first 3 weeks, and then the relative humidity was maintained at 65%.
Experimental Design and Diets
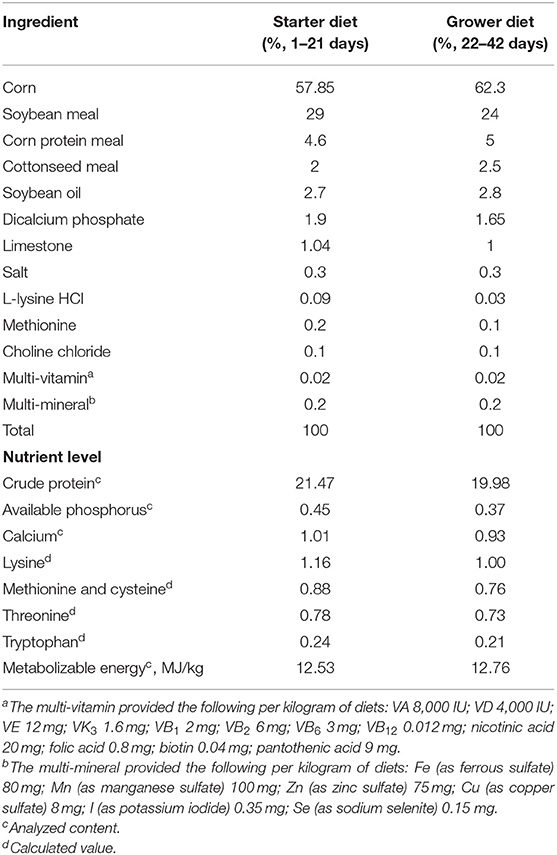
A total of 288 1-day-old male AA broilers were randomly divided into four groups with 6 replicates and 12 chickens for each replicate. The control group was fed the basal diet, while the treated groups were fed the basal diet with 10, 20, and 30 mg/kg lycopene in powder (lycopene concentration ≥96.0%; Nanjing Jingzhu Biotech. Co., Ltd., Nanjing, China). Feed and water were provided ad libitum for 42 days. Each group consisted of six replicates with 12 chickens per replicate. The composition of the basal experiment diets for birds is shown in Table 1. The trial was conducted in 2 phases, namely, a starter phase from 1 to 21 days and a grower phase from 22 to 42 days.
Data and Sample Collections
Body weight was recorded at 1, 21, and 42 days. Average daily gain, average daily feed intake, and feed conversion ratio were calculated by recording body weight and cumulative feed intake. At 21 and 42 days, respectively, the pre-slaughter weight was measured after 12 h of fasting, and 1 broiler with average body weight from each replicate was selected for blood sampling. Blood (10 ml) was collected with disposable vacuum blood collection tubes from the jugular vein and centrifuged at 3,000 × g for 10 min to obtain serum, which was divided into Eppendorf tubes and stored at a −20°C refrigerator for biochemical indicator testing. Ten grams of liver was collected into plastic packaging bags and stored until analyses at −20°C. Two grams of liver was collected into freezing tubes and stored at −80°C refrigerators for further assays.
Antioxidant Enzyme Levels in Serum and Liver
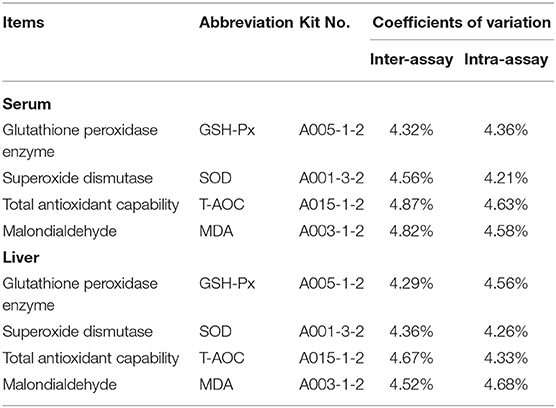
The antioxidant parameters were assayed with an ultraviolet spectrophotometer (UV-2410PC, Shimadzu, Japan). The parameters evaluated in blood serum and liver included superoxide dismutase (SOD), total antioxidant capability (T-AOC), and glutathione peroxidase enzyme (GSH-Px) and malondialdehyde (MDA). The reagent kits were purchased from the Nanjing Jiancheng Biotechnology Co., Ltd. (Jiangsu, China), and the procedures were carried out following the manufacturer's instructions. Information on serum and liver biochemical indexes and the kits used in this study is shown in Table 2.
Quantitative Real-Time PCR
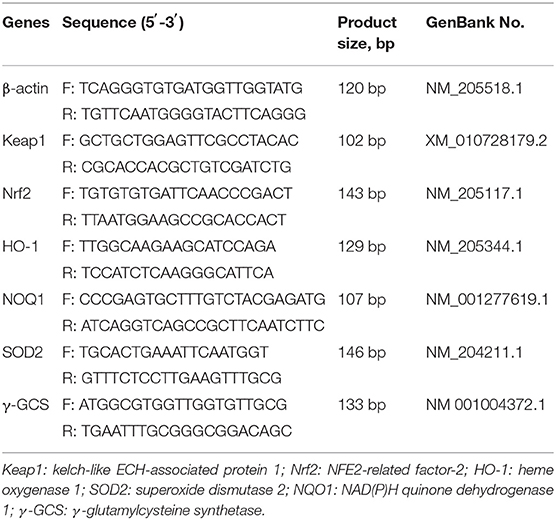
Total RNA of the liver was isolated with TRIzol (Sigma, Saint Louis, MO) according to the procedure of the RNA extraction method. The quality of RNA was determined by 2% agarose gel electrophoresis, and total RNA concentration and purity (A260/A280 ratio) were ascertained with an ultra-micro spectrophotometer (NanoPhotometer, Implen, Germany). The total RNA from each liver was reverse transcribed into cDNA using a Prime Script™ RT reagent kit with gDNA Eraser (TaKaRa, Dalian, China). The obtained cDNA was used for qPCR using a TB Green™ Premix Ex Taq™ PCR kit (TaKaRa, Dalian, China) (20). The primer sequences are shown in Table 3, and the primers corresponding to the chicken gene sequence were synthesized by Sangon (Shanghai, China). Samples were disposed of with an ABI PRISM 7500 SDS thermal cycler (Applied Biosystems, Foster City, CA, USA). The following temperature program was used: one cycle at 95°C for 30 s, 40 cycles of 95°C for 5 s and 60°C for 34 s (20). The relative gene mRNA expression was calculated using the 2−ΔΔCt method and normalized to β-actin expression.
Statistical Analysis
All the data were tested for homogeneity of variance. The data were analyzed by one-way ANOVA and Tukey's multiple-range test in the Statistical Product and Service Solutions Software (SPSS 22.0; IBM-SPSS Inc., Chicago, IL, USA). The linear and quadratic effects of lycopene were assessed using regression analysis. Results were presented as the mean and the standard error of the mean (SEM). p < 0.05 was considered as a significant difference.
Results
Growth Performance
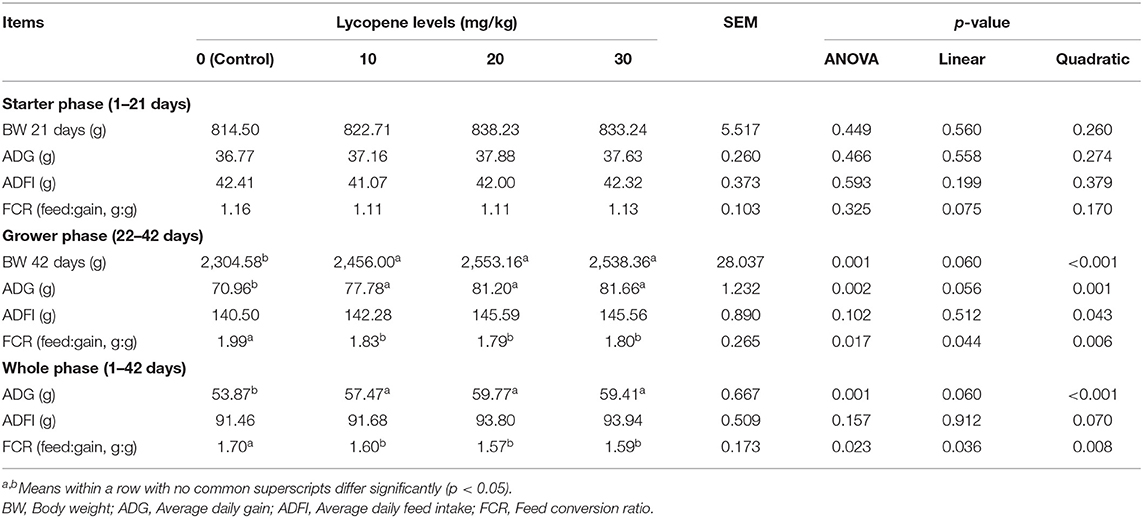
The growth performance data are summarized in Table 4. The ADG increased (p = 0.002 vs. p = 0.001) and the FCR decreased (p = 0.017 vs. p = 0.023) in groups treated with lycopene in the grower (22–42 days) and whole (1–42 days) phases, and the ADFI was quadratically affected (p = 0.043) by lycopene in the grower (22–42 days) phase compared with the control group.
Antioxidant Enzyme Activity in Serum
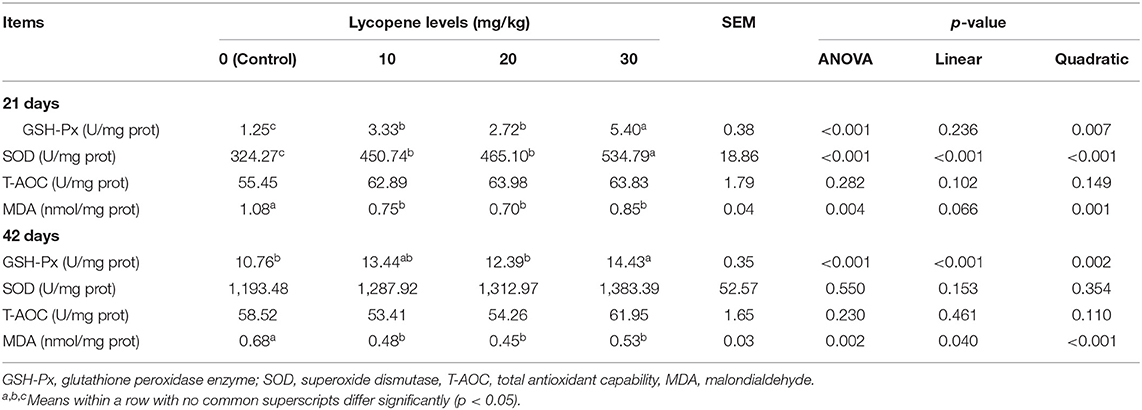
As summarized in Table 5, the MDA contents were lower (p ≤ 0.001) in the groups treated with lycopene than those in the control group, and the MDA contents in the 20 and 30 mg/kg lycopene groups were lower (p < 0.05) than those in the 10 mg/kg lycopene group at 21 days. The SOD contents were linearly affected (p = 0.035) by lycopene at 21 days. The GSH-Px, SOD, and T-AOC levels were higher (p = 0.014, p = 0.003, and p = 0.035, respectively) than those in the control group, and the T-AOC levels in 30 mg/kg lycopene were higher (p < 0.05) than those in the control group at 42 days.
Antioxidant Enzyme Content in Liver
The antioxidant enzyme contents in the liver are shown in Table 6. The GSH-Px and SOD contents in groups treated with lycopene were higher (p ≤ 0.001 vs. p ≤ 0.001) than those in the control group, and their contents were higher (p < 0.05) in the 30 mg/kg group than those in the 10 and 20 mg/kg groups at 21 days. The MDA contents were lower (p = 0.004) than those in the control group at 21 days. The GSH-Px contents were higher (p ≤ 0.001) in 30 mg/kg lycopene than those in the control and 20 mg/kg lycopene groups at 42 days. The MDA contents were lower (p = 0.002) in the groups treated with lycopene than those in the control group at 42 days.
Quantitative Real-Time PCR of Keap1-Nrf2 Pathway in Liver
As shown in Figure 1, the mRNA expression levels of Nrf2, SOD 2, NQO1, and HO-1 genes were higher (21 days: p = 0.042, p = 0.021, p = 0.035, p = 0.043, respectively; 42 days: p = 0.038, p = 0.025, p = 0.034, p = 0.043, respectively) in the 20 and 30 mg/kg lycopene groups at 21 and 42 days. The mRNA expression levels of Nrf2, NQO1, and HO-1 genes were higher (p <0.05) in the 30 mg/kg lycopene group than those in the 10 mg/kg lycopene group, and the mRNA expression levels of SOD 2 genes were higher (p <0.05) in the 30 mg/kg lycopene group than those in the 10 and 20 mg/kg lycopene groups at 21 days. The mRNA expression levels of Nrf2 and SOD 2 genes were higher (p <0.05) in the 30 mg/kg lycopene group than those in the 10 mg/kg lycopene group, and the mRNA expression levels of NQO1 genes were higher (p <0.05) in the 30 mg/kg lycopene group than those in the 10 and 20 mg/kg lycopene groups at 42 days.
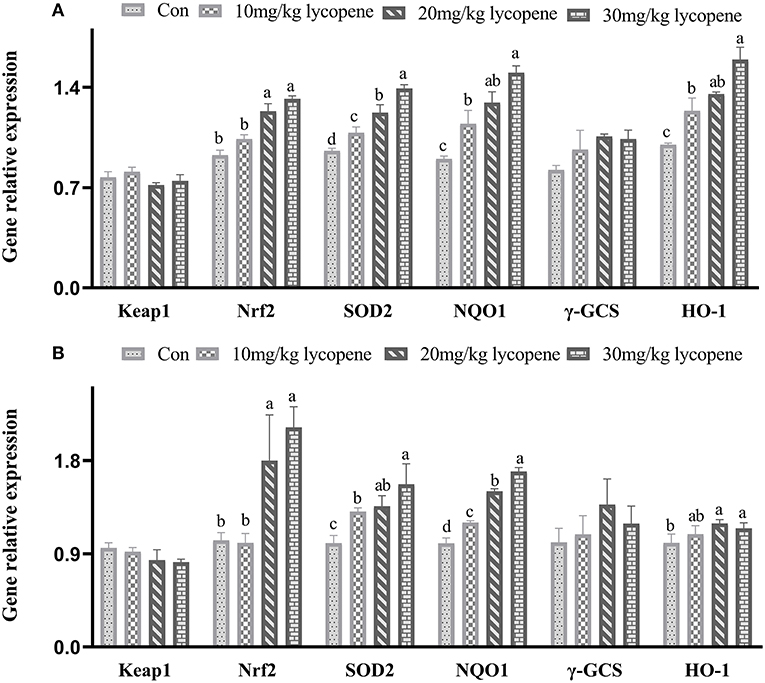
Figure 1. Effect of dietary lycopene levels on the Keap1-Nrf2 regulated gene expression in the liver of AA broilers. (A) The data of Keap1-Nrf2 regulated gene expression in the liver of AA broilers at 21 days in Con, 10, 20, and 30 mg/kg lycopene groups were as follows: Keap 1 gene: 0.77 ± 0.04, 0.81 ± 0.03, 0.72 ± 0.02, and 0.75 ± 0.04, respectively, p = 0.064; Nrf2 gene: 0.93 ± 0.03b, 1.04 ± 0.03b, 1.23 ± 0.05a, and 1.32 ± 0.02a, respectively, p = 0.042; SOD2 gene: 0.96 ± 0.02d, 1.08 ± 0.04c, 1.22 ± 0.05b, and 1.39 ± 0.02a, respectively, p = 0.021; NQO1 gene: 0.90 ± 0.02c, 1.15 ± 0.09b, 1.29 ± 0.08ab, and 1.50 ± 0.04a, respectively, p = 0.035; γ-CGS gene: 0.82 ± 0.03, 0.97 ± 0.13, 1.06 ± 0.02, and 1.35 ± 0.5, respectively, p = 0.072; HO-1 gene: 1.00 ± 0.01c, 1.24 ± 0.09b, 1.35 ± 0.01ab, and 1.59 ± 0.09a, respectively, p = 0.043. (B) The data of Keap1-Nrf2 regulated gene expression in the liver of AA broilers at 42 days in Con, 10, 20, and 30 mg/kg lycopene groups were as follows: Keap 1 gene: 0.96 ± 0.05, 0.92 ± 0.04, 0.84 ± 0.10, and 0.82 ± 0.03, respectively, p = 0.074; Nrf2 gene: 1.03 ± 0.07b, 1.00 ± 0.09b, 1.80 ± 0.44a, and 2.12 ± 0.20a, respectively, p = 0.038; SOD2 gene: 1.00 ± 0.07c, 1.31 ± 0.04b, 1.36 ± 0.10ab, and 1.57 ± 0.20b, respectively, p = 0.025; NQO1 gene: 1.00 ± 0.05d, 1.20 ± 0.02c, 1.50 ± 0.02b, and 1.70 ± 0.04a, respectively, p = 0.034; γ-CGS gene: 1.00 ± 0.14, 1.09 ± 0.18, 1.37 ± 0.25, and 1.19 ± 0.17, respectively, p = 0.059; HO-1 gene: 1.00 ± 0.08b, 1.09 ± 0.08ab, 1.19 ± 0.04a, and 1.15 ± 0.05a, respectively, p = 0.043.
Discussion
Growth performance is an indispensable indicator of poultry growth status under different conditions (21), and the lycopene could improve chicken performance (22, 23). In the present study, the ADG increased, and the FCR decreased in groups treated with lycopene in the grower and whole phases, and the ADFI was quadratically affected by lycopene in the grower phase, which was consistent with Mezbani's study (22), who reported that chicken supplemented with 100 mg/kg lycopene from 21 to 42 days resulted in the increased body weight and decreased feed conversion ratio. However, no differences in growth performance were observed between the groups treated with lycopene and the control group during the starter phase (1–21 days), which might be that the low feed intake before 21 days reduced the lycopene intake. At the same time, the use of vaccines during this period will repeatedly cause immune stresses, and low lycopene dosage may not be sufficient to improve antioxidant capacity. Therefore, it could be considered that the amount of lycopene added can be increased during the early broilers' feeding process.
Antioxidant enzymes in poultry cooperative work to maintain the optimal redox balance in vivo, which regulates various processes, including cell signal transmission, gene expression, stress regulation, and homeostasis maintenance (12). The levels of T-AOC, SOD, GSH-PX, and MDA in liver and serum were usually used to evaluate the antioxidant potentials (24). In this study, the SOD, T-AOC, and GSH-Px levels of serum and liver were significantly increased and MDA levels were decreased, which coincide with the study of Sun et al. (25), who reported that diet with lycopene improved chick antioxidant enzyme activities of the liver, and declined liver MDA. Furthermore, detoxifying oxidative stress factors, GSH-PX and SOD enzyme reduced hydrogen peroxide and superoxide anions to non-toxic hydroxyl compounds (26). The improvement of antioxidant enzyme activity in serum and liver is beneficial to chicken, which contributes to the antioxidant properties of chicken, thereby increasing the daily weight gain (27). Previous studies have shown that supplements with antioxidants, such as selenium, vitamin C, and β-carotene, could improve broilers' antioxidant status and meat quality (27, 28). Similarly, it was proved that certain phytochemicals, such as flavonoids and polyphenols, improved the antioxidant capacity of broilers (29, 30). Lipid and protein oxidation have always been considered the main threats to meat quality except for microorganisms (31).
Nrf2, a key transcription factor, combines with the antioxidant response element (ARE), which activates target gene expression, and regulates the phase II detoxification enzymes and antioxidant enzymes (32). Numerous studies have reported that antioxidants exerted beneficial effects on the broiler by the Keap1-Nrf2 signaling pathway (33, 34). Nevertheless, it was unclear whether lycopene activated the Keap1-Nrf2 signaling pathway to promote the synthesis of the antioxidant enzymes in broilers. The present study showed that the liver Nrf2 expression was upregulated in the 20 and 30 mg/kg lycopene groups, indicating that the Nrf2 pathway was activated by lycopene. Under normal circumstances, Nrf2 combines with Keap1 to form a complex that exists in the cytoplasm. Nrf2 dissociates from Keap1, transfers to the nucleus under oxidative stress, and forms a heterodimer with Maf protein to initiate the transcription of downstream genes (35). However, Keap1 expression was not affected by lycopene at the starter phase. Zhang (36) had demonstrated that the steady-state Nrf2 expression increased significantly after tert-Butylhydroquinone (tBHQ) or sulforaphane treatment, and the tBHQ group significantly reduced the steady-state expression of Keap1. However, the sulforaphane group did not change the steady-state level of Keap1. Nrf2 can bind to the ARE of the phase II genes and accelerates their transcription, including NQO1, HO-1, and SOD2, to maintain the redox balance (37). The results in this study showed that upregulated Nrf2 regulated the antioxidant system to increase NQO1 mRNA expression at the whole phase, indicating that its detoxification capacity was enhanced in the metabolic process. NQO1, a phase II metabolism enzyme, reduces quinone and nitrogen oxide compounds and alleviates oxidative damage (38). Similar to the present study results, Kaspar (39) reported that activating Nrf2 can stimulate the expression of NQO1, which is positively correlated to Nrf2. HO-1 catalyzes heme to convert biliverdin, which has antioxidant and anti-inflammatory effects (40).
Additionally, SOD2 is a metalloenzyme composed of metal coenzymes and proteins, which can dismutase superoxide radicals into molecular oxygen and hydrogen peroxide (41). The present results showed that the gene expression levels of SOD2 and HO-1 in the liver were significantly increased after lycopene treatment. Several studies have shown that Nrf2 dissociates from Keap1 and into the nucleus, upregulating Nrf2 expression and its downstream related genes, such as HO-1, NQO1, and SOD (42, 43). Nrf2-regulated is of great importance for antioxidant defense. Apart from that, whether changes in Nrf2-regulated gene expression in the liver activated by lycopene are affected by other signaling pathways remains to be further explored. The expression of NQO1, HO-1, and SOD2 was upregulated, indicating the improved antioxidant potentials in broilers. Activating the Keap1-Nrf2 signaling pathway by lycopene might contribute to redox status stability in broilers.
Conclusions
The effects of lycopene on the growth performance, antioxidant enzyme activity in serum and liver, and gene expression in the Keap1/Nrf2 signaling pathway of Arbor Acres broilers in the different treatment groups were assessed. The average daily gain, feed conversion ratio, average daily feed intake, serum and liver glutathione peroxidase and superoxide dismutase contents, mRNAs of Nuclear Factor E2-related factor 2, superoxide dismutase 2, NAD(P)H quinone dehydrogenase 1, and heme oxygenase 1 gene expression levels were affected by lycopene in Arbor Acres broilers. The lycopene concentration of 30 mg/kg improved the growth performance, antioxidant enzyme activity in serum and liver, and gene expression in the Keap1-Nrf2 signaling pathway of Arbor Acres broilers. In the early stages of broiler growth, the amount of lycopene added should be increased appropriately.
Data Availability Statement
The original contributions presented in the study are included in the article/supplementary material, further inquiries can be directed to the corresponding author.
Ethics Statement
The animal study was reviewed and approved by Animal Care and Use Committee of the Animal Nutrition Institute, Northeast Agricultural University (NEAU-2018-0232).
Author Contributions
SW and HW prepared the manuscript and collected some data. YZ, HCu, JY, ML, HCh, LG, and TX collected the samples. LX was responsible for the design and direction of the experiment. All authors have read and agreed to the published version of the manuscript.
Funding
This study was supported by the National Natural Science Foundation of China (32072749) and China Scholarship Council (CSC) National Public Graduate Program for Building Highly Qualified Universities.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Tan BL, Norhaizan ME, Liew WP. Nutrients and oxidative stress: friend or foe? Oxid Med Cell Longev. (2018) 2018:9719584. doi: 10.1155/2018/9719584
2. Luo H, Chiang HH, Louw M, Susanto A, Chen D. Nutrient sensing and the oxidative stress response. Trends Endocrinol Metab. (2017) 28:449–60. doi: 10.1016/j.tem.2017.02.008
3. Chen Z, Xing T, Li J, Zhang L, Jiang Y, Gao F. Hydrogen peroxide–induced oxidative stress impairs redox status and damages aerobic metabolism of breast muscle in broilers. Poult Sci. (2021) 100:918–25. doi: 10.1016/j.psj.2020.11.029
4. Lillehoj H, Liu Y, Calsamiglia S, Fernandez-Miyakawa ME, Chi F, Cravens RL, et al. Phytochemicals as antibiotic alternatives to promote growth and enhance host health. Vet Res. (2018) 49:76. doi: 10.1186/s13567-018-0562-6
5. Guan R, Van Le Q, Yang H, Zhang D, Gu H, Yang Y, et al. A review of dietary phytochemicals and their relation to oxidative stress and human diseases. Chemosphere. (2021) 271:129499. doi: 10.1016/j.chemosphere.2020.129499
6. Hao L, Li Y, Xue C, Dong N, Bi C, Shan A. Aquaporin: targets for dietary nutrients to regulate intestinal health. J Anim Physiol An N. (2022) 106:167–80. doi: 10.1111/jpn.13539
7. Liu X, Lin X, Zhang S, Guo C, Li J, Mi Y, et al. Lycopene ameliorates oxidative stress in the aging chicken ovary via activation of Nrf2/HO-1 pathway. Aging. (2018) 10:2016–36. doi: 10.18632/aging.101526
8. Li N, Wu X, Zhuang W, Xia L, Chen Y, Wu C, et al. Tomato and lycopene and multiple health outcomes: umbrella review. Food Chem. (2021) 343:128396. doi: 10.1016/j.foodchem.2020.128396
9. Arain MA, Mei Z, Hassan FU, Saeed M, Rajput IR. Lycopene: a natural antioxidant for prevention of heat-induced oxidative stress in poultry. World Poult Sci J. (2018) 74:89–100. doi: 10.1017/S0043933917001040
10. Carvalho GC, de Camargo BAF, de Araújo JTC, Chorilli M. Lycopene: from tomato to its nutraceutical use and its association with nanotechnology. Trends Food Sci Technol. (2021) 118:447–58. doi: 10.1016/j.tifs.2021.10.015
11. Jiang LN, Liu YB Li BH. Lycopene exerts anti-inflammatory effect to inhibit prostate cancer progression. Asian J Androl. (2018) 21:80–5. doi: 10.4103/aja.aja_70_18
12. Surai PF, Fisinin VI. Vitagenes in poultry production: part 3. Vitagene concept development. World Poult Sci J. (2016) 72:793–804. doi: 10.1017/S0043933916000751
13. Abd El-Hack ME, El-Saadony MT, Saad AM, Salem HM, Ashry NM, Ghanima MMA, et al. Essential oils and their nanoemulsions as green alternatives to antibiotics in poultry nutrition: a comprehensive review. Poul Sci. (2021) 101:101584. doi: 10.1016/j.psj.2021.101584
14. Mohammed TT, Farhan SM, Majid AA, Mohammed ZJ, Abdulateef FM. Effect of vitamin C and natural antioxidants on the production performance and antioxidant status of laying hens through heat Original Research. J Res Ecol. (2018) 6:2364–75. doi: 10.13140/RG.2.2.23253.58080
15. Byoung-Ki A, Won-Don C, Chang-Won K, Jienny L. Effects of dietary lycopene or tomato paste on laying performance and serum lipids in laying hens and on malondialdehyde content in egg yolk upon storage. J Poul Sci. (2019) 56:52–7. doi: 10.2141/jpsa.0170118
16. Taguchi K, Motohashi H, Yamamoto M. Molecular mechanisms of the Keap1–Nrf2 pathway in stress response and cancer evolution. Genes Cells. (2011) 16:123–40. doi: 10.1111/j.1365-2443.2010.01473.x
17. Kaur G, Sharma A, Bhatnagar A. Role of oxidative stress in pathophysiology of rheumatoid arthritis: insights into NRF2-KEAP1 signalling. Autoimmunity. (2021) 54:385–97. doi: 10.1080/08916934.2021.1963959
18. Wang S, Wu YY, Wang X, Shen P. Lycopene prevents carcinogen-induced cutaneous tumor by enhancing activation of the Nrf2 pathway through p62-triggered autophagic Keap1 degradation. Aging. (2020) 12:8167–90. doi: 10.18632/aging.103132
19. Sahin K, Orhan C, Tuzcu M, Sahin N, Hayirli A. Lycopene activates antioxidant enzymes and nuclear transcription factor systems in heat-stressed broilers. Poult Sci. (2016) 95:1088–95. doi: 10.3382/ps/pew012
20. Wu H, Sun H, Ma C, Lian L, Lu L, Xu L, et al. Effects of maternal dietary energy restriction on breast muscle fibre development in the offspring of broiler breeders. Asian-australas J Anim Sci. (2021) 34:1829–38. doi: 10.5713/ab.20.0712
21. Wu H, Yang J, Wang S, Zhang X, Hou J, Xu F, et al. Effects of soybean isoflavone and astragalus polysaccharide mixture on colostrum components, serum antioxidant, immune and hormone levels of lactating sows. Animals. (2021) 11:132. doi: 10.3390/ani11010132
22. Mezbani A, Kavan BP, Kiani A, Masouri B. Effect of dietary lycopene supplementation on growth performance, blood parameters and antioxidant enzymes status in broiler chickens. Livest Res Rural Dev. (2019) 31:3101–12. Available online at: http://www.lrrd.org/lrrd31/1/bahma31012.html
23. Pozzo L, Tarantola M, Biasibetti E, Teresa Capucchio M, Pagella M, Mellia E, et al. Adverse effects in broiler chickens fed a high lycopene concentration supplemented diet. Canadian J Anim Sci. (2013) 93:231–41. doi: 10.4141/cjas2012-081
24. Rashmi R, Magesh SB, Ramkumar KM, Suryanarayanan S, SubbaRao MV. Antioxidant potential of naringenin helps to protect liver tissue from streptozotocin-induced damage. Rep Biochem Mol Biol. (2018) 7:76. Available online at: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC6175592/
25. Sun B, Chen C, Wang W. Effects of lycopene supplementation in both maternal and offspring diets on growth performance, antioxidant capacity and biochemical parameters in chicks. J Anim Physiol Anim Nutr. (2015) 99:42–7. doi: 10.1111/jpn.12196
26. Li C, Tan F, Yang J, Yang Y, Gou Y, Li S, et al. Antioxidant effects of Apocynum venetum tea extracts on d-galactose-induced aging model in mice. Antioxidants. (2019) 8:381. doi: 10.3390/antiox8090381
27. Surai PF. Effect of selenium and vitamin E content of the maternal diet on the antioxidant system of the yolk and the developing chick. Br Poult Sci. (2000) 41:235–43. doi: 10.1080/713654909
28. Carreras I, Guerrero L, Guàrdia MD, Esteve Garcia E, García Regueiro JA, Sárraga C. Vitamin E levels, thiobarbituric acid test and sensory evaluation of breast muscles from broilers fed α tocopheryl acetate and β carotene supplemented diets. J Sci Food Agr. (2004) 84:313–7. doi: 10.1002/jsfa.1639
29. Kamboh AA, Memon AM, Mughal MJ, Memon J, Bakhetgul M. Dietary effects of soy and citrus flavonoid on antioxidation and microbial quality of meat in broilers. J Anim Physiol Anim Nutr. (2018) 102:235–40. doi: 10.1111/jpn.12683
30. Zhang C, Wang L, Zhao XH, Chen XY, Yang L, Geng ZY. Dietary resveratrol supplementation prevents transport-stress-impaired meat quality of broilers through maintaining muscle energy metabolism and antioxidant status. Poult Sci. (2017) 96:2219–25. doi: 10.3382/ps/pex004
31. Sabow AB, Sazili AQ, Aghwan ZA. Changes of microbial spoilage, lipid-protein oxidation physicochemical properties during post mortem refrigerated storage of goat meat. Anim Sci J. (2016) 87:816–26. doi: 10.1111/asj.12496
32. Li N, Alam J, Venkatesan MI, Eiguren-Fernandez A, Schmitz D, Di Stefano E, et al. Nrf2 is a key transcription factor that regulates antioxidant defense in macrophages and epithelial cells: protecting against the proinflammatory and oxidizing effects of diesel exhaust chemicals. J Immunol. (2004) 173:3467–81. doi: 10.4049/jimmunol.173.5.3467
33. Zhang C, Chen K, Zhao XH, Geng ZY. Protective effects of resveratrol against high ambient temperature-induced spleen dysplasia in broilers through modulating splenic redox status and apoptosis. J Sci Food Agr. (2018) 98:5409–17. doi: 10.1002/jsfa.9084
34. Zhuang L, He XF, Ma BB, Zhang L, Li JL. Dietary taurine supplementation improves breast meat quality in chronic heat-stressed broilers via activating Nrf2 pathway and protecting mitochondria from oxidative attack. J Sci Food Agr. (2018) 99:1066–72. doi: 10.1002/jsfa.9273
35. Zhang DD, Hannink M. Distinct cysteine residues in Keap1 are required for Keap1-dependent ubiquitination of Nrf2 and for stabilization of Nrf2 by chemopreventive agents and oxidative stress. Mol Cell Biol. (2003) 23:8137–51. doi: 10.1128/MCB.23.22.8137-8151.2003
36. Zhang DD, Lo SC, Sun Z, Habib GM. Ubiquitination of Keap1, a BTB-Kelch Substrate Adaptor Protein for Cul3, Targets Keap1 for Degradation by a Proteasome-independent Pathway. J Bio Chem. (2005) 280:30091–99. doi: 10.1074/jbc.M501279200
37. Li J, Ichikawa T, Janicki JS, Cui T. Targeting the Nrf2 pathway against cardiovascular disease. Expert Opin Ther Targets. (2009) 13:785–94. doi: 10.1517/14728220903025762
38. Tang XP, Liu B, Wang XG Yu QF, Fang RJ. Epidermal growth factor, through alleviating oxidative stress, protect IPEC-J2 cells from lipopolysaccharides-induced apoptosis. Int J Mol Sci. (2018) 19:848. doi: 10.3390/ijms19030848
39. Kaspar JW, Jaiswal AK. Antioxidant-induced phosphorylation of tyrosine 486 leads to rapid nuclear export of Bach1 that allows Nrf2 to bind to the antioxidant response element and activate defensive gene expression. J Biol Chem. (2010) 285:153–62. doi: 10.1074/jbc.M109.040022
40. González-Mañán D, D'Espessailles A, Dossi CG, Martín MS. Rosa mosqueta oil prevents oxidative stress and inflammation through the upregulation of PPAR-α and NRF2 in C57BL/6J mice fed a high fat diet. J Nutr. (2017) 147:579–88. doi: 10.3945/jn.116.243261
41. Cheng SP, Shan G, Lee GH, Kim DS, Kim SH. Sulforaphane protects ischemic injury of hearts through antioxidant pathway and mitochondrial KATP channels. Pharmacol Res. (2009) 61:342–8. doi: 10.1016/j.phrs.2009.11.009
42. Xing HY, Cai YQ, Wang XF, Wang LL. The cytoprotective effect of hyperoside against oxidative stress is mediated by the Nrf2-ARE signaling pathway through GSK-3β inactivation. PLoS ONE. (2015) 10:e0145183. doi: 10.1371/journal.pone.0145183
Keywords: lycopene, growth performance, antioxidant enzyme activity, Keap1-Nrf2, broilers
Citation: Wang S, Wu H, Zhu Y, Cui H, Yang J, Lu M, Cheng H, Gu L, Xu T and Xu L (2022) Effect of Lycopene on the Growth Performance, Antioxidant Enzyme Activity, and Expression of Gene in the Keap1-Nrf2 Signaling Pathway of Arbor Acres Broilers. Front. Vet. Sci. 9:833346. doi: 10.3389/fvets.2022.833346
Received: 11 December 2021; Accepted: 31 January 2022;
Published: 14 March 2022.
Edited by:
Asghar Kamboh, Sindh Agriculture University, PakistanReviewed by:
Ilias Giannenas, Aristotle University of Thessaloniki, GreeceLv Zengpeng, China Agricultural University, China
Muhammad Saeed, Northwest A&F University, China
Copyright © 2022 Wang, Wu, Zhu, Cui, Yang, Lu, Cheng, Gu, Xu and Xu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Li Xu, eHVsaV8xOTYyMTk5MUAxNjMuY29t
†These authors have contributed equally to this work
 Sibo Wang1†
Sibo Wang1† Hongzhi Wu
Hongzhi Wu