Immunopathological comparison of in ovo and post-hatch vaccination techniques for infectious bursal disease vaccine in layer chicks
- 1Department of Pathology, University of Agriculture, Faisalabad, Pakistan
- 2Institute of Animal Science, Guangdong Academy of Agricultural Sciences, Animal Nutrition and Feed Science in South China, Ministry of Agriculture and Rural Affairs, State Key Laboratory of Livestock and Poultry Breeding, Key Laboratory of Guangdong Public Laboratory of Animal Breeding and Nutrition, Guangdong Key Laboratory of Animal Breeding and Nutrition, Guangzhou, China
- 3Department of Animal Science & Technology, Shandong Vocational Animal Sciences and Veterinary College, Weifang, China
- 4Department of Animal Production, Faculty of Agriculture, Benha University, Benha, Egypt
- 5Department of Parasitology, University of Agriculture, Faisalabad, Pakistan
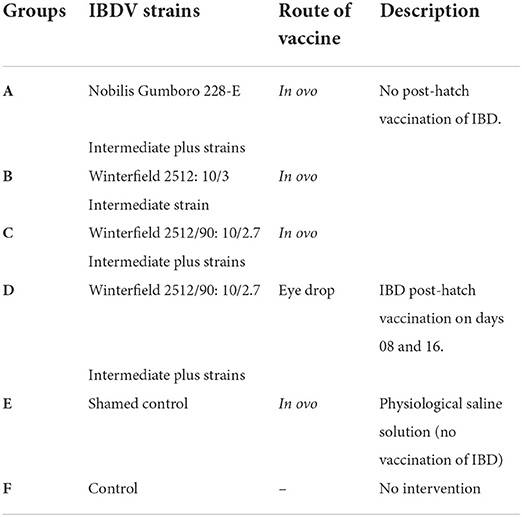
This study was designed to compare immunopathological effects of in ovo vaccination with post-hatch vaccination against IBD in White Leghorn chicks. A total of 189 embryonated eggs were divided into six groups. At day 18 of incubation, groups A–C were administered in ovo with 228E, Winterfield 2512:10/3 and 2512/90:10/2.7, respectively, group D (post-hatch vaccination) and group E as shamed control (for quality evaluation of in ovo vaccination technique), and group F as control. The results showed that antibody titers against IBD detected by ELISA on days 2, 17, and 28 were significantly higher in all in ovo groups as compared to control groups E and F. On day 17, all vaccinated groups (in ovo and post-hatch vaccinated) showed no significant differences in antibody titers among themselves; however, at day 28, only the post-hatch group showed significantly higher antibody titers followed by in ovo vaccinated groups. The cell-mediated immunity determined by PHA-P assay was significantly higher in all vaccinated groups than the non-vaccinated groups. No clinical signs of IBD infection were observed in any of the vaccinated groups. There was only increase in bursa size of groups vaccinated with intermediate plus strains (groups A, C, and D) at day 28. The histopathology showed that all the treatment groups had mild lesions induced by IBD virus in bursa. This study concluded that in ovo vaccination with live IBD vaccines provides protective immunity to the chickens even in the presence of IBD-specific MDA; therefore, the onset of immunity was much earlier than the post-hatch vaccination and in ovo groups also maintained protective immunity against IBD for longer time.
Introduction
Infectious bursal disease virus (IBDV) is a dsRNA virus of birnaviridae family having two serotypes (serotypes I and II), of both only serotype I is responsible for infectious bursal disease (IBD) in chicken also known as “Gumboro diseases.” The virus is difficult to be inactivated by ordinary physical and chemical methods. Therefore, it persists for longer durations in poultry houses. Longer persistence of the diseases in commercial poultry flocks is of prime economic concern that may adversely affect the health and welfare of the flocks (1, 2). Infectious bursal disease is highly contagious in young chicken with acute onset and primarily targets the lymphoid tissue with special affinity to bursa of Fabricius (3, 4). At days 3–4 of post-infection (PI), bursa shows hypertrophy, hyperemia, and edema. At days 5–6 of PI, the bursa regains its normal size and by day 8 gets atrophied to one-third of its normal size. The birds suffer from severe dehydration and exhibit hypertrophy, whitish urate crystal deposits with cell debris in kidneys (5). IBD virus-induced immunosuppression (6) predisposes the young chicks to opportunistic pathogens and prevents the development of adequate immune response to the commonly used vaccines (7). The degree of immunosuppression, mortality rate, clinical signs, and severity of bursal lesions depend upon the age and immune status of the bird and pathogenicity of viral strains (5).
Despite intensive vaccination regimens, IBD still stands as an economically important disease of poultry (8). In field environment, mostly IBD vaccine is administered in drinking water to the chicks. However, in ovo vaccine delivery has shown to induce protective immunity as well (9, 10). The concept of in ovo vaccination evolved by successful experimental administration of Marek's disease virus (MDV) vaccine to chicken embryos which protected the chicks from MDV challenge later in life (11). Following this successful study, this concept of vaccinating the chicken embryos further developed and the chicken embryos were vaccinated with IBD vaccine alone and in combination with MD vaccine, both of which presented the same conclusion as the previous study (12).
In ovo vaccine administration against IBD virus at 18th day of incubation minimizes the susceptibility window, i.e., the duration between the vaccine administration and an early exposure to the IBD virus as compared to routine post-hatch vaccine administration (13). The recent studies have revealed that in comparison with the vaccine administered after hatching, the in ovo vaccination approach stimulated both the innate and adaptive immunity in young chicks (13). The IBD virus exposure induced systemic humoral immunity as well as the cell-mediated immune (CMI) response (14). The local immune response may also play a remarkable role in building protection against IBD virus challenge as the IBD virus via gut-associated tissue enters the circulation and then gets distributed to other organs (15).
Although conventional vaccination strategies are still in use but in ovo vaccination is also being adopted in many parts of the world (16), however, there is less information about the immunopathological sequelae of administering live vaccines by in ovo route as the embryos lack fully developed immune system. Therefore, present experimental study was designed to investigate the pathological changes associated with in ovo administration of IBD live vaccines and comparison of immune response elicited by in ovo vaccination with that of post-hatch vaccination against IBD followed in field conditions.
Materials and methods
Experimental design
A total of 189 fertile hatching eggs from White Leghorn (WLH) layer breeder flock were procured from a commercial hatchery. The eggs were clean and shifted to the setter of disinfected incubator in the hatchery at Department of Poultry Science, University of Agriculture Faisalabad. At day 18 of incubation, eggs were candled and only the viable embryos were selected. The egg shell surfaces of all groups except group F (Control) were disinfected with alcohol and groups A, B, C, and E inoculated in ovo via air cell route, using a 2.5-cm 23-gauge needle with individual dose (0.1 ml) of vaccine (17) per egg. Gentamicin sulfate was added at dose rate of 1 mg /ml inoculums (18) to avoid bacterial contamination. The virus particles per dose for group A were (2-3 log10 EID50), B (10 3 EID50), and C (10 2.7 EID50). The eggs of group E served as shamed control and were inoculated with sterilized physiological saline (19). A number of eggs in groups A, B, C, D, E, and F were 33, 32, 32, 32, 30, and 30 eggs, respectively. After that, the eggs were placed in labeled porous bags. These egg-containing bags were then carefully shifted to the hatcher of disinfected incubator till day 21 of incubation. The embryonated eggs of commercial layer were from same batch used for in ovo vaccination and post-hatch study.
After hatching, the chicks were shifted to the experimental poultry house at Department of Clinical Medicine and Surgery, University of Agriculture Faisalabad. Birds of each group were kept in separate cages and were provided drinking water and feed ad libitum. The hatchlings of the in ovo vaccinated groups (A, B, C), group D (used for post-hatch vaccination), shamed control (E), and negative control (F) were used in post-hatch experiment of 28 days. The experimental design of chicks is given in Table 1.
The only group D was administered post-hatch IBD vaccine by intraocular route (20) at days 08 and 16 as per field practice. However, all the groups were vaccinated with live vaccines via eye drop route against Newcastle disease (ND) and infectious bronchitis (IB) at day 5 of age. The duration of experiment was 28 days (because the susceptibility window for IBD infection in layer chicks is at 3–4 weeks of age (21). A total of six birds from each group were bled at days 2 and 28 for collection of blood and visceral organs for evaluation of different parameters. The blood was collected for serum. The bursal tissues were collected at days 2 and 28 and fixed in 10% neutral buffered formalin for histopathology.
Parameters studied
The percent hatchability was determined. Clinical signs were recorded two times daily and body weights weekly. On days 2 and 28, necropsy examination was done to explore any gross changes in the organs. The preserved tissues were processed for histopathological studies (22).
To determine cell-mediated immunity, the cutaneous lymphoproliferative response to phytohemagglutinin (PHA-P) was assessed in vivo at 24, 48, and 72 h as cutaneous basophilic hypersensitivity response = skin thickness in the right foot (PHA-P Ag)—skin thickness in the left foot (control) (23). Humoral immunity was determined using ELISA (Iddex Labs USA). The bursa: body weight index was determined by formula of (24):
Organ weights of immune organs (bursa, thymus, and spleen) were studied by taking average of six replicates, as absolute organ weights and relative organ weights. The formula for relative organ weight was:
The data obtained were subjected to one-way analysis of variance and group means were compared by Duncan's multiple range test (DMR) (p ≤ 0.05) using M Stat-C software package. All the experimental protocols and use of animal were approved by Graduate Studies Research Board (GSRB) of University of Agriculture Faisalabad.
Results
Hatchability
The percent hatchability varied among different groups. The highest percent hatchability was seen in group E followed by groups D, F, A, B, and C. It has been presented in Table 2.
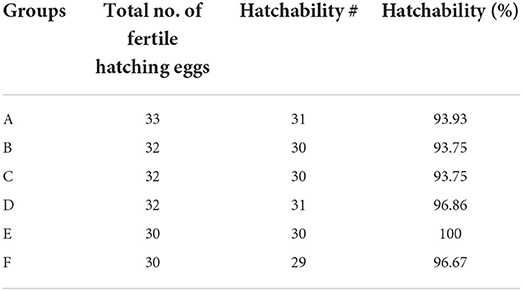
Table 2. Hatchability in different groups of the White Leghorn chicks administered live IBD vaccines in ovo and post-hatch period.
Clinical signs
During the whole period of study, none of the birds from any group presented any clinical sign of disease based upon the parameters of their alertness, hydration status, and fecal consistency.
Body weights
Body weights determined at weekly intervals of different groups have been presented in Table 3. No significant difference was found in the weekly body weights among all groups.
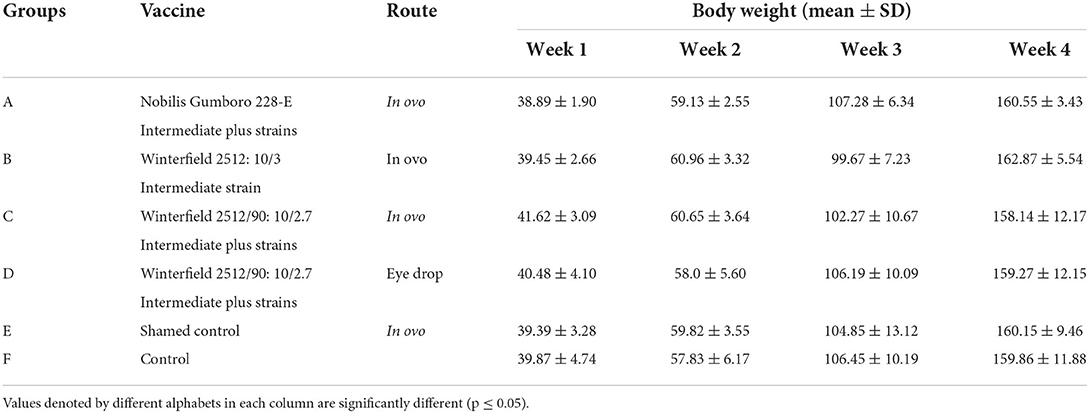
Table 3. Body weights (mean ± SD) of the White Leghorn chicks administered live IBD vaccines in ovo and post-hatch period.
Gross lesions
At day 2 after hatching, all organs including bursa of Fabricius, spleen, kidney, and thymus grossly appeared normal in all groups. At day 28 after hatching, groups E and F showed the normal size of the bursa. However, the bursa of groups A, C, and D was swollen two times their original size followed by those of group B which presented moderate swelling (Figure 1). Other organs such as spleen, kidney, and thymus presented the normal gross appearance.
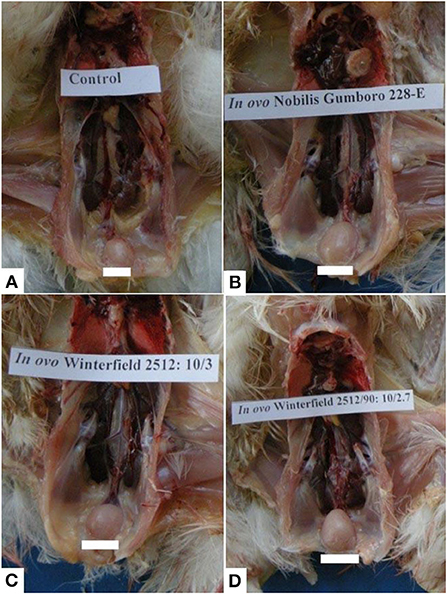
Figure 1. Bursa of Fabricius of layer chicks at day 28. (A) Control group (normal size of bursa) whereas bursal hypertrophy (B–D) in group A (in ovo 228 E), group B (in ovo Winterfield 2512: 10/3) and group C (in ovo Winter field 2512: 10/2.7) in comparison with control group F.
Histopathological lesions
Bursa of Fabricius
Group A (in ovo Nobilis Gumboro 228 E)
At day 2, the medulla of follicles exhibited mild degree of lymphocytic depletion. However, the surface epithelium was intact. There were prominent dark condensed nuclei (pyknotic) depicting the necrosis in few follicles. A thin layer of cells was present between cortex and medulla. Follicles appeared larger in size compared with follicles of other groups. At day 28, the interfollicular connective tissue was minimal. Medulla was not as densely packed as cortex, and it mainly contained small cells with scarce cytoplasm, some larger cells (macrophages), and fibroblasts. Cortex was densely packed with small and large cells with little cytoplasm. Medullae of bursal follicles also showed some scattered cells with pyknotic nuclei. The cortex contained some empty spaces (Figure 2B).
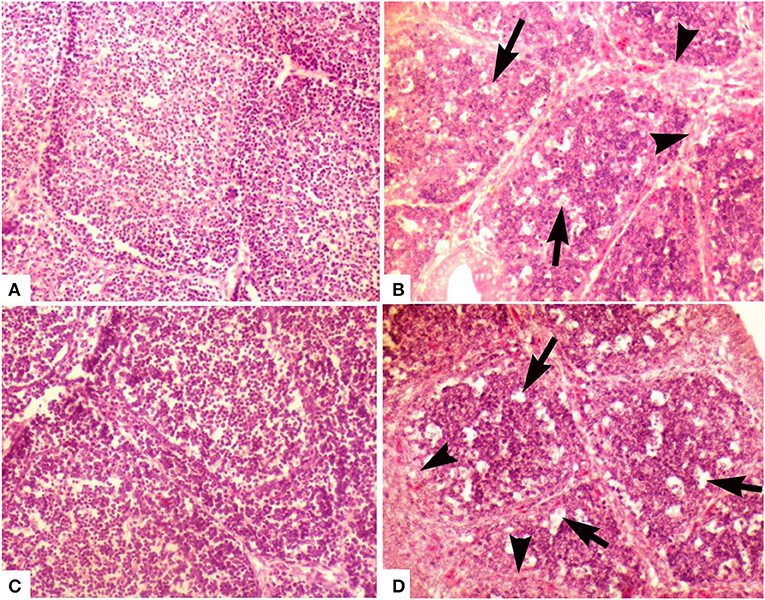
Figure 2. Photomicrograph of bursa of Fabricius of layer chicken on day 28. (A) Developed cortex and medulla (post-hatch vaccination group D), (B) (group A in ovo 228 E) and (C) showing demarcated cortex and medulla (group B) (in ovo Winterfield 2512: 10/3) (H&E staining at 200×) (D) (group C in ovo Winterfield 2512: 10/2.7) showing vacuolar degeneration (arrows), polymorphic cells in the medullary region and interfollicular connective tissue fibrosis (arrow heads).
Group B (in ovo Winterfield 2512: 10/3)
At day 2, the empty spaces and pyknotic nuclei in the medulla of follicles appeared lesser in number as compared to groups A and E while at day 28, surface epithelium was intact and interfollicular connective tissue was thinner than group A. There was clearly demarcated cortex and medulla and the cortical cells were larger than medullary cells. Both cortex and medulla were not as densely packed as that of group A. Some cells with pyknotic nuclei and large cells (macrophages) were present in medulla. Most of the cells present in medulla were small with thin rim of cytoplasm. In medulla, lesser fibroblasts and at some places, segmented cells and macrophages were seen more frequently than in group A (Figure 2C).
Group C (in ovo Winterfield 2512: 10/2.7)
At day 2, follicular size was variable being smaller in some parts of the bursal tissue while normal in other parts. Lesser pyknotic cells were observed as compared to group A suggesting necrosis in a few follicles. Empty spaces were seen in the follicles; however, the surface epithelium of the organ was found intact. Similar pattern was present day 28 (Figure 2D).
Group D (post-hatch Winterfield 2512/90: 10/2.7 eye drop vaccination)
At day 2, empty spaces in the medulla of the follicles were minimum compared with other groups. Medulla and cortex almost indistinguishably separated from each other by a very fine layer of cortical rim around the medulla. The connective tissue was lesser than that observed in group E. The surface epithelium of the bursa of Fabricius was found intact. The interfollicular connective tissue was thin even at day 28. There has been a clear demarcation of cortex and medulla. Medulla was denser than those of groups A, B, and C. Large cells (macrophages) were also present in the medullae; however, there was less fibroblast activity in medulla as compared to groups A and C (Figure 2A).
Group E (in ovo shamed control)
Some empty spaces were observed in the medullae of follicles. Medulla and cortex were almost indistinguishable, and a very fine layer of cortical rim formed around the medulla. The connective tissue was thinner than that observed in group F. The surface epithelium of the organ was intact. There was clearly demarcated cortex and medulla. Cortex and medulla were denser than the groups A, B, C, and D. In medulla, most of the cells were small with thin rim of visible cytoplasm, and some macrophages and fibroblasts were also observed.
Group F (control)
At day 2, medullary portion of bursal follicles exhibited least empty spaces. Interfollicular connective tissue was prominent. The epithelial folds on the surface of the organ were intact. All follicles showed larger medulla and a thin rim of cortical cells was surrounding it. At day 28, surface epithelium was intact. There was clearly demarcated cortex and medulla. Cortex and medulla were denser than those of groups A, B, C, and D. In medulla, most of the cells were small with thin rim of visible cytoplasm, and some macrophages and fibroblasts were also observed.
Cell-mediated immunity
Lymphoproliferative response to PHA-P as elicited by the thickness of skin at the site of injection has been presented in Table 4. At day 8 of age, it was significantly higher in groups A and C compared with control 24 h post-injection. The response was significantly lower in group B compared with groups A and C. The significantly lowest response was observed in groups D, E, and F. At 48 h post-injection, group B showed significantly lower response and all other groups showed similar trend as it was at 24 h. At 72 h post-injection, significantly higher response was observed in group C followed by groups A and B. At day 21 of age, response to PHA-P at 24 and 48 h post-injection was significantly higher in group D whereas groups A, B, and C showed significantly lower response from group D. The groups E and F showed significantly lower values than all other groups. At 72 h post-injection, significantly higher response was observed in group D followed by group B. The groups A and C showed significantly lower response from group B, whereas the groups E and F showed significantly lower response from all other groups.
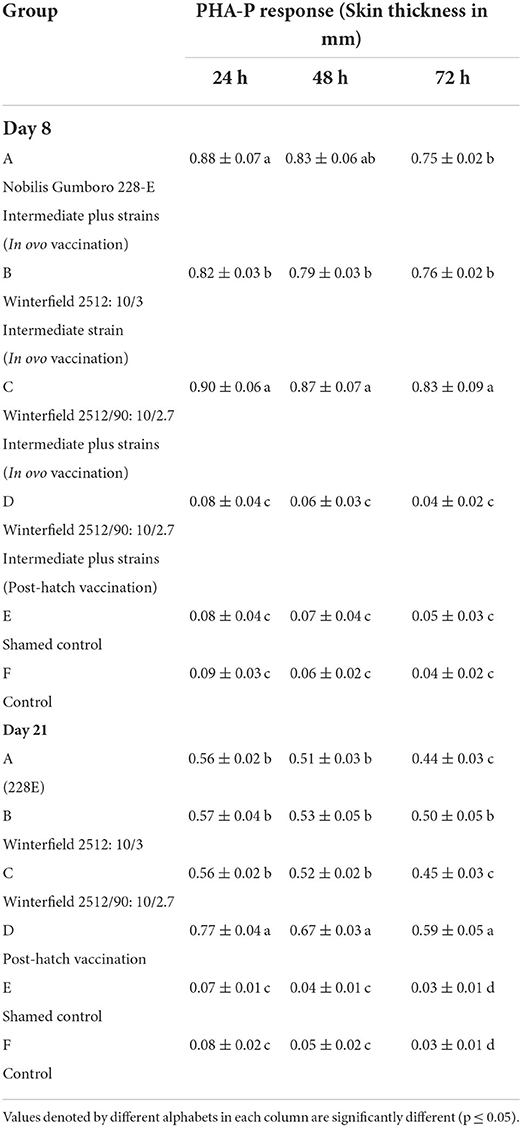
Table 4. Lymphoproliferative response against PHA-P in White Leghorn chicks administered IBD live vaccines by in ovo and post-hatch (mean ± SD).
Humoral immunity
At day 2 of age, the White Leghorn (WLH) chicks showed significantly highest ELISA log2 titers in groups A, B, and C while significantly lower in groups D, E, and F. At day 17 of age, the ELISA titers were significantly highest in groups A, B, C, and D whereas significantly lower titers were observed in groups E and F. At day 28 of age, significantly higher titers were observed in group D followed by groups A, B, and C compared with control (Table 5).
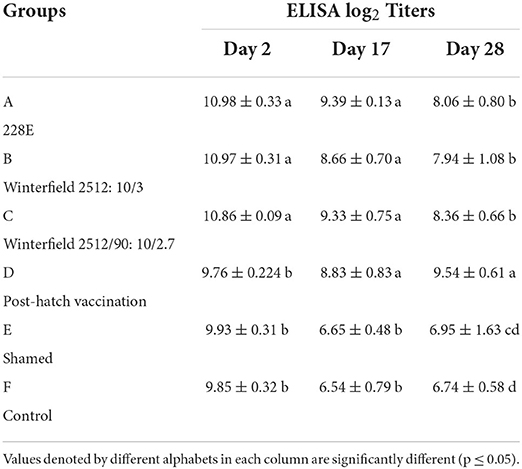
Table 5. ELISA log2 titers of the White Leghorn chicks administered live IBD vaccines in ovo and post-hatch (means ± SD).
Absolute organ weights
The results of absolute organ weights results have been presented in Table 6. At day 2 of age, the White Leghorn chicks of groups A and C showed significantly highest absolute weight of bursa, followed by group B. The significantly lowest bursal weight was observed in groups D, E, and F. The thymus weight was significantly higher in groups A, B, and C whereas significantly lowest thymus weight was observed in groups D, E, and F. The absolute weight of spleen was significantly highest in groups A, B, and C followed by group E and significantly lowest was in groups D and E. At day 28, significantly highest weight of bursa was observed in group D, followed by groups A, B, and C. The significantly lowest bursal weights were observed in groups E and F. The absolute thymic weights were the highest in group D followed by groups B and C which were succeeded by group A. The significantly lowest thymic weight was observed in groups E and F. The absolute weight of spleen was significantly highest in group D followed by groups A, B, and C. The significantly lowest weight of spleen was observed in groups E and F.
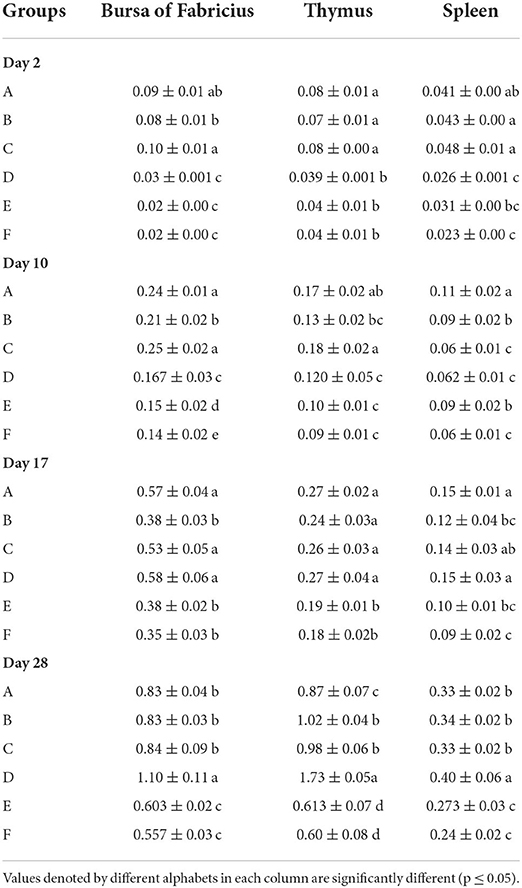
Table 6. Absolute organ weights (mean ± SD) of the White Leghorn chicks administered live IBD vaccines in ovo and post-hatch.
Relative organ weight
The results of relative organ weights results have been presented in Table 7. The relative organ weights of WL chicks calculated at days 2, 10, and 17 of age showed that the relative weight of bursa and thymus was significantly higher in groups A and C followed by group B which was succeeded by significantly lowest groups D, E, and F. The relative weight of spleen was significantly the highest in groups A, B, and C compared with F. At day 28, the relative weight of bursa and thymus was significantly highest in group D, followed by groups A, B, and C in comparison with E and F. The relative weight of thymus was significantly the higher in group D, followed by groups B and C compared with groups E and F. The relative weight of spleen was significantly highest in group A followed by groups A, B, and C, which were succeeded by group E. The significantly lower relative splenic weights were observed in group F.
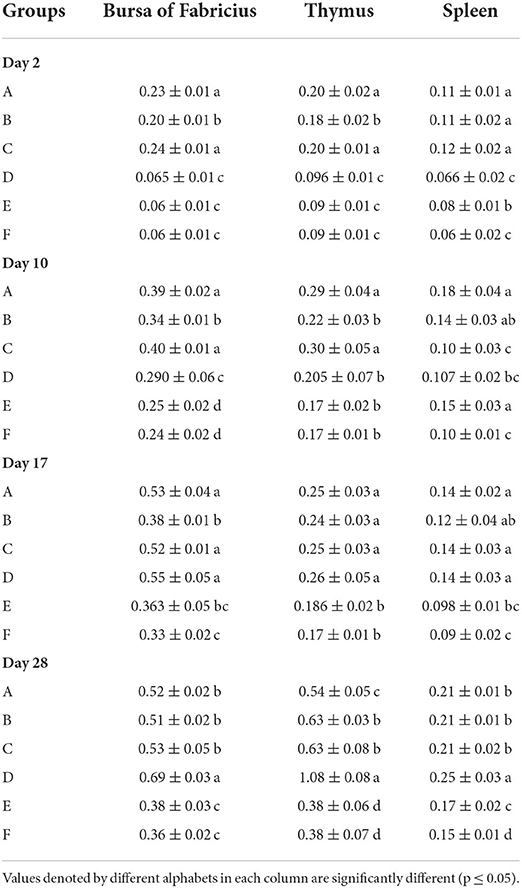
Table 7. Relative organ weights (mean ± SD) of the White Leghorn chicks administered live IBD vaccines in ovo and post-hatch.
Bursa: Body weight index (BB index)
The results are presented in Table 8. The BB index of WL chicks at day 2 of age was significantly highest in groups A and C followed by group B whereas significantly lowest BB index was recorded in group D. At day 10 of age, BB index was significantly highest in groups A and C succeeded by group B followed by group E while significantly lowest BB index was recorded in group D. At day 17 of age, groups B and E showed significantly lower BB index whereas other groups showed significantly higher BB index which was non-significantly different among themselves. At day 28, group D showed significantly highest BB index followed by groups A, B, and C whereas group E showed significantly lowest BB index.
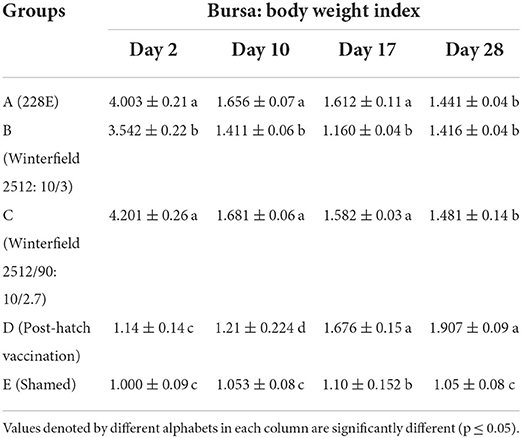
Table 8. Bursa: Body weight index of the White Leghorn chicks administered live IBD vaccines in ovo and post-hatch period at days 2, 10, 17, and 28.
Histomorphometry of bursa of Fabricius
The histomorphometry of bursa of Fabricius at 2 day of age of birds showed significantly highest follicular diameter in group B, followed by groups D, E, and F, whereas significantly lowest follicular diameter was observed in groups A and C. The cortex diameter was significantly higher in group B and C compared with control group F. However, there was a non-significant difference among groups B and D, E, and F. The diameter of medulla was significantly higher in groups B and D, succeeded by groups E and F. The interfollicular tissue was significantly highest in groups A, C, D, and E as compared to control whereas significantly lower interfollicular connective tissue was observed in group B compared with control (Table 9).
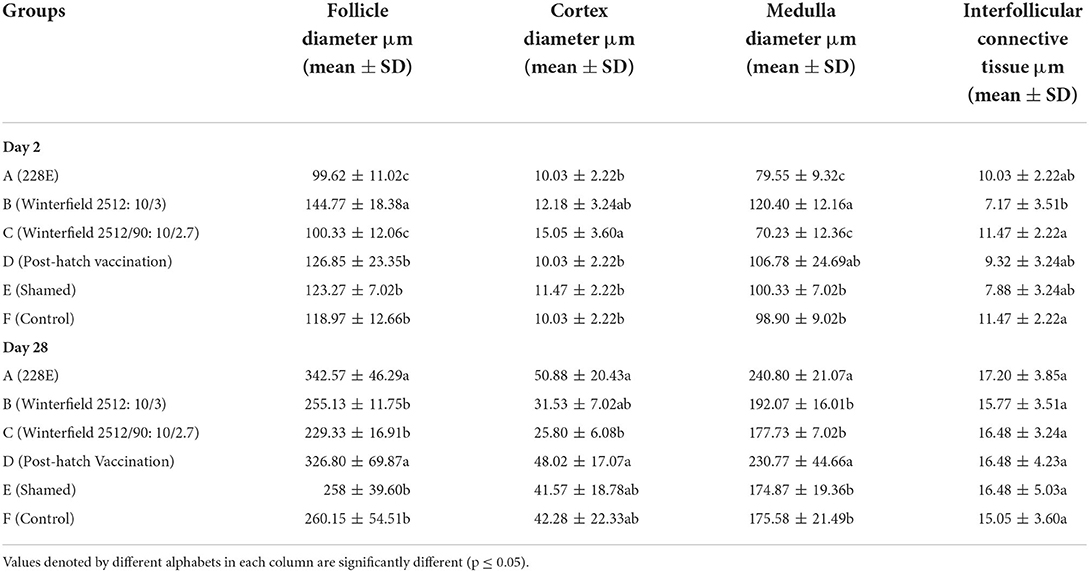
Table 9. Histomorphometery of bursa of Fabricius of the White Leghorn chicks administered live IBD vaccines in ovo and post-hatch period at days 2 and 28 of age.
At day 28, the follicular diameter was significantly higher in groups A and D whereas it was compared with control group F. The diameter of the cortex was significantly higher in groups A, B, D, and E as compared to control group whereas it was significantly lower in group C. The diameter of medulla was significantly higher in groups A and D in comparison with group F. The interfollicular connective tissue showed no significant difference among all groups.
Discussion
Infectious bursal disease is a highly contagious and immunosuppressive disease of economic importance for commercial poultry (8). Chickens of all breeds are susceptible to IBD; however, White Leghorn shows higher morbidity and mortality rate. Vaccination is the principle control strategy for IBD (3) and contributes to minimize the IBD-related losses. However, there is no vaccine and vaccination technique which could provide 100% protection. Hence, a rational and economically viable control strategy is required to culminate the disease (6).
In ovo vaccination of chick embryos is among contemporary vaccination strategies. The concept of this vaccination protocol is to stimulate the developing immune system in late embryonic life against probable IBDV challenge in post-hatch life. The in ovo vaccination reduces the cost of labor and can initiate primary immune response even in the presence of maternal antibodies (7, 25). Some studies suggested that there are few live vaccines used in post-hatch vaccination regimes which can be considered for in ovo administration without any detrimental effects on the survival of chicken embryos (26).
The results of this study revealed that all groups administered physiological saline or live vaccines via in ovo route produced hatchability percentage of 90.91, 93.75, 90.63, and 100% in groups A (intermediate plus strain; Nobilis Gumboro 228 E), B (intermediate strain; Winterfield 2512: 10/3), C (intermediate plus strain; Winterfield 2512/90:10/2.7), and E (shamed; physiological saline), respectively. This finding indicates that neither in ovo administration technique nor the post-hatch delivery of live IBD vaccines negatively affected the hatchability percentage or the survival of newly hatched chicks. Our findings are in line with the suggestions of Romao et al. (19) and Saeed et al. (27). However, the hatchability percentage in this experiment was higher than that of commercial hatcheries because all non-viable eggs were discarded at day 18 of incubation after candling as suggested by Moura et al. (28).
None of the vaccine treatment groups irrespective of vaccination method (whether in ovo or post-hatch vaccination) showed clinical presentation of disease. The results suggested that the birds, of which their embryos subjected to in ovo vaccination, did not undergo much stress to yield post-hatch clinical IBD. Similarly, Hedayati et al. (29) and De Wit et al. (25) reported no clinical signs or ailment in chicks administered in ovo IBD vaccines.
The body weights of the layer type chick vaccinated (in ovo or post-hatch vaccinated) with intermediate vaccinal strain (Winterfield 2512: 10/3), and intermediate plus strains (Nobilis Gumboro 228 E and Winterfield 2512/90:10/2.7) showed no significant differences from the control negative groups. Otsyina et al. (30) demonstrated no deleterious effect of intermediate plus vaccination of White Leghorn chicks upon body weights. Similarly, Ashash et al. (7) and De Wit et al. (25) have shown that in ovo vaccination against IBD with live vaccines does not interfere with the body weights of the chickens.
The absolute and relative bursal weight in this study was significantly higher in chicks administered in ovo intermediate plus strains (Nobilis Gumboro 228 E and Winterfield 2512/90:10/2.7) than all other groups at day 2 and 10. On day 17, the highest bursal weight was recorded in chicks administered in ovo and intermediate plus strains (Nobilis Gumboro 228 E and Winterfield 2512/90:10/2.7) and the post-hatch vaccinated group. On day 28, the significantly highest bursal weight was recorded in chicks of post-hatch vaccinated group. These findings were contrary to the findings of Rautenschlein et al. (31) who reported a decrease in bursal weight of broiler chicks administered intermediate plus vaccine. A similar trend was observed in the relative weight of the broiler chicks administered in ovo intermediate plus vaccines. Different authors have shown decreased bursal weights in White Leghorn chicks administered intermediate plus vaccines (30). An absence of decrease in bursal weight of chicks administered intermediate plus vaccine by in ovo technique indicated that the negative effect of intermediate plus vaccine upon bursal weight was prevented and it could be helpful in the prevention of possible immunosuppression caused by these stronger vaccines.
The bursa showed minor histopathological changes in in ovo vaccinated groups. Similarly, the bursa of post-hatch vaccinated group showed mild lesions as investigated by Rautenschlein et al. (31) and Giambrone et al. (32).
The humoral immunity determined by ELISA showed that the IBD-specific antibody titers were significantly higher for in ovo vaccinated groups than control and shamed group at day 2 post-hatching, and similar observations have been reported by Corley et al. (9) and Coletti et al. (33), whereas at day 17, the antibody titers of all vaccinated groups (in ovo and post-hatch vaccinated) were showed significantly higher titers than control negative and shammed control groups; however, all vaccinated groups showed non-significant difference in titers among themselves. At day 28, the post-hatch vaccination group showed significantly highest antibody titers. In some recent studies, it has been determined that live vaccines of IBD do not interfere with the maternal antibodies of the commercial chicken in embryonic life or at early post-hatch vaccination; rather, the multiplication of live vaccine virus slows down due to unknown mechanisms (7, 25). Moreover, the mean titers in breeder flock were 9.5 (ELISA log2 titers). Despite the higher levels of maternal antibodies transferred to the experimental/ commercial flock, none of the vaccination method of vaccine strain's antibody titers seemed to be affected by breeders' antibody level in this study.
The cell-mediated immune response to PHA-P antigen at day 8 was statistically non-significant between both in ovo administered intermediate plus strains but were significantly higher than intermediate strain. While at day 21, the post-hatch vaccinated group showed significantly higher response to PHA-P antigen followed by a non-significant difference among in ovo vaccinated groups (irrespective of different levels of virulence of vaccinal strains). However, all vaccinated groups showed significantly higher responses than control group. Sharma et al. (34), however, reported opposite results using the field isolates of IBDV. A reason for the difference in the present result and those of Sharma et al. (34) could be that they used field isolates of IBDV, which might be high in virulence compared to vaccinal strains used in the present study. The purpose of monitoring CMI using PHA-P assay was to assess whether there had been any kind of immunosuppression (in peripheral t-cell activity) in response to live IBD vaccines (especially intermediate and intermediate plus vaccines), which was not observed in this study.
Conclusion
From the results of this study, it might be concluded that in ovo vaccinating approach reduces the susceptibility period of chickens to field IBDV challenge and the birds' immune organs start getting functional even before hatching. In comparison with the post-hatch vaccination strategy, the in ovo vaccinated chickens not only develop humoral and cell-mediated immunity much earlier, but also the antibody induction is higher in birds vaccinated via in ovo route.
Data availability statement
The datasets presented in this article are not readily available because not applicable. Requests to access the datasets should be directed to dr.iqzaheer@gmail.com.
Ethics statement
The animal study was reviewed and approved by Institutional Bioethics Committee, University of Agriculture, Faisalabad.
Author contributions
IZ, AK, MS, and MK contributed to conception and design of the study. IZ and TZ organized the database. IZ performed the statistical analysis. IZ and MK wrote the first draft of the manuscript. IZ, MS, TZ, AE, and WC wrote sections of the manuscript. WC and AE were partial funding collaborators in this project. All authors contributed to manuscript revision, read, and approved the submitted version.
Funding
This work was supported by the National Key Research and Development Program (Grant Nos. 2018YFE0128200 and 2021YFD1300405), Fund for China Agricultural Research System (CARS-42-13), Modern Agricultural Industry Technology System Innovation Team of Guangdong Province (2019KJ137), Key Project of the Science and Technology Program of Guangzhou City (Grant No. 2019A050505007), Foreign Expert Project (QNL20200130001), the Science and Technology Program of Guangdong Province (2021A0505050003), Special Fund for Scientific Innovation Strategy-Construction of High Level Academy of Agriculture Science (R2020PY-JX008), and the Science and Technology Program of Guangdong Academy of Agricultural Sciences (202106TD), opening project for State Key Laboratory of Livestock and Poultry Breeding (2021GZ06).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Lin X, Mohsin M, Abbas RZ, Li L, Chen H, Huang C, et al. Evaluation of immunogenicity and protective efficacy of Eimeria maxima immune mapped protein 1 with EDA adjuvant in chicken. Pak Vet J. (2020) 40:209–13. doi: 10.29261/pakvetj/2020.043
2. Ali M, Khan MUR, Aslam A, Rehman HU, Masood S, Masood A, et al. Comparative molecular characterization and pathogenicity of H9N2 avian influenza virus in commercial poultry flocks in Pakistan. Pak Vet J. (2021) 41:451–5. doi: 10.29261/pakvetj/2021.047
3. Eterradossi YMS. Infectious bursal disease. In: Swayne DE, editor. Diseases of poultry. 14th ed. New York: Wiley Blackwell (2020). p. 257–83. doi: 10.1002/9781119371199.ch7
4. Ma J, Yu Y, Zhang H, Mo H, Ou C, Wang X, et al. Over-expression of Rab1 gene during infectious bursal disease virus infection in layer chicken. Pak Vet J. (2016) 36:73–6.
5. Van Den Berg TP, Eterradossi N, Toquin D, Meulemans G. Infectious bursal disease (Gumboro disease). Rev Sci Tech OIE. (2000) 19:527–43. doi: 10.20506/rst.19.2.1227
6. Van den Berg TP. Acute infectious bursal disease in poultry: a review. Avian Pathol. (2000) 29:175–94. doi: 10.1080/03079450050045431
7. Ashash U, Noach C, Perelman B, Costello C, Sansalone P, Brazil T, et al. In ovo and day of hatch application of a live infectious bursal disease virus vaccine to commercial broilers. Avian Dis. (2019) 63:713–20. doi: 10.1637/aviandiseases-D-19-00087
8. Saeed NM. Sequence analysis and comparison of infectious bursal disease virus affecting indigenous kurdish breed and broiler chickens in Sulaymaniyah, Kurdistan Region of Iraq. Pak Vet J. (2021) 41:249–53. doi: 10.29261/pakvetj/2021.017
9. Corley MM, Giambrone JJ, Dormitorio TV. Detection of infectious bursal disease vaccine viruses in lymphoid tissues after in ovo vaccination of specific-pathogen-free embryos. Avian Dis. (2001) 45:897–905. doi: 10.2307/1592869
10. Shah MU, Aslam A, Mustafa G, Zahid B, Imran MS. Effect of mentofin and asimirus on humoral immune response and tissue changes in infectious bursal disease vaccinated broiler birds. Pak Vet J. (2018) 38:56–60. doi: 10.29261/pakvetj/2018.011
11. Sharma JM, Burmester BR. Resistance of Marek's disease at hatching in chickens vaccinated as embryos with the turkey herpesvirus. Avian Dis. (1982) 26:134–49. doi: 10.2307/1590032
12. Sharma JM. Embryo Vaccination with infectious bursal disease virus alone or in combination with marek's disease vaccine. Avian Dis. (1985) 29:1155–69. doi: 10.2307/1590469
13. Negash T, Al-Garib SO, Gruys E. Comparison of in ovo and post-hatch vaccination with particular reference to infectious bursal disease, a review. Vet Q. (2004) 26:76–87. doi: 10.1080/01652176.2004.9695170
14. Yeh HY, Rautenschlein S, Sharma JM. Protective immunity against infectious bursal disease virus in chickens in the absence of virus-specific antibodies. Vet Immunol Immunopathol. (2002) 89:149–58. doi: 10.1016/S0165-2427(02)00206-4
15. Muller R, Weiss IK, Reinacher M, Weiss E. Immunofluorescence studies of early virus propagation after oral infection with infectious bursal disease virus (IBDV). Zentralbl Veterinarmed B. (1979) 26:345–52. doi: 10.1111/j.1439-0450.1979.tb00823.x
16. Khatri M, Sharma JM. Response of embryonic chicken lymphoid cells to infectious bursal disease virus. Vet Immunol Immunopathol. (2009) 127:316–24. doi: 10.1016/j.vetimm.2008.10.327
17. McCarty JE, Brown TP, Giambrone JJ. Delay of infectious bursal disease virus infection by in ovo vaccination of antibody-positive chicken eggs. J Appl Poult Res. (2005) 14:136–40. doi: 10.1093/japr/14.1.136
18. Senne DA. Virus propagation inembryonating eggs. In: Swayne DE, Glisson JR, Jackwood MW, Pearson JE, Reed WM, editor. Laboratory Manual for The Isolation and Identification of Avian Pathogens. 4th Ed. American association of avian pathologists. Pennsylvannia: Kennett Square (1998). p. 235–40.
19. Romao JM, de Moraes TV, Salles RR, Cardoso WM, Buxade CC. Effect of in ovo vaccination procedures on japanese quail embryos (Coturnix japonica) and incubation performance. Ci Anim Bras. (2001) 12:584–92. doi: 10.5216/cab.v12i4.5234
20. Kim IJ, Gagic M, Sharma JM. Recovery of antibody producing ability and lymphocyte repopulation of bursal follicles in chickens exposed to infectious bursal disease virus. Avian Dis. (1999) 43:401–13. doi: 10.2307/1592637
21. Sultana R, Hussain SA, Ilyas S, Ch A, Anjum R, Zaidi FH. Epidemiology of infectious bursal disease in broiler and layer flocks in and around Lahore, Pakistan. Punjab Univ J Zool. (2008) 23:067–72.
22. Bancroft JD, Gamble M. Theory and Practise of Histological Techniques. 5th ed. London: Churchill Livingstone (2008).
23. Ul-Hassan Z, Zargham Khan M, Khan A, Javed I. Immunological status of the progeny of breeder hens kept on ochratoxin A (OTA)- contaminated diet. J Immunotoxicol. (2011) 8:122–30. doi: 10.3109/1547691X.2010.547886
24. Thangavelu A, Raj AGD, Elankumaran S, Manohar BM, Koteeswaran A, Venugopalan AT, et al. Pathogenicity and immunosuppressive properties of infectious bursal disease virus field isolates and commercial vaccines In India. Trop Anim Health Prod. (1998) 30:167–76. doi: 10.1023/A:1005059619825
25. de Wit JJ, Jorna I, Finger A, Loeb V, Dijkman R, Ashash U, et al. In ovo application of a live infectious bursal disease vaccine to commercial broilers confers proper immunity. Avian Pathol. (2021) 50:531–9. doi: 10.1080/03079457.2021.1986618
26. Mast J, Meulemans G. Attenuated mutant Newcastle disease virus strains for in ovo vaccination, method for preparing and their use. European Patent WO 03/030932 Al (2003).
27. Saeed M, Babazadeh D, Naveed M, Alagawany M, Abd El-Hack ME, Arain MA, et al. In ovo delivery of various biological supplements, vaccines and drugs in poultry: current knowledge. J Sci Food Agric. (2019) 99:3727–39. doi: 10.1002/jsfa.9593
28. Moura L, Vakharia V, Liu M, Song H. In ovo vaccine against infectious bursal disease. Int J Poult Sci. (2007) 6:770–5. doi: 10.3923/ijps.2007.770.775
29. Hedayati A, Nili H, Bahonar A. Comparison of pathogenicity and serologic response of four commercial infectious bursal disease live vaccines. Arch Razi Inst. (2005) 59:65–73. doi: 10.22092/ARI.2005.103814
30. Otsyina HR, Anim JA, Aning KG. Protective efficacy of commercial live vaccines against very virulent infectious Bursal disease virus (vvIBDV) in Ghana. Vet Med Anim Health. (2009) 1:023–7.
31. Rautenschlein S, Kraemer CH, Vanmarcke J, Montiel E. Protective efficacy of intermediate and intermediate plus infectious bursal disease virus (IBDV) vaccines against very virulent IBDV in commercial broilers. Avian Dis. (2005) 49:231–7. doi: 10.1637/7310-112204R
32. Giambrone JJ, Dormitorio T, Brown T. Safety and efficacy of in ovo administration of infectious bursal disease viral vaccines. Avian Dis. (2001) 45:144–8. doi: 10.2307/1593021
33. Coletti M, Del Rossi E, Franciosini MP, Passamonti F, Tacconi G, Marini C. Efficacy and safety of an infectious bursal disease virus intermediate vaccine in ovo. Avian Dis. (2001) 45:1036–43. doi: 10.2307/1592885
Keywords: IBD, in-ovo, vaccine, immunopathology, layers
Citation: Zaheer I, Chen W, Khan A, Elokil A, Saleemi MK, Zaheer T and Khan MZ (2022) Immunopathological comparison of in ovo and post-hatch vaccination techniques for infectious bursal disease vaccine in layer chicks. Front. Vet. Sci. 9:947522. doi: 10.3389/fvets.2022.947522
Received: 18 May 2022; Accepted: 30 June 2022;
Published: 26 July 2022.
Edited by:
Kun Li, Nanjing Agricultural University, ChinaReviewed by:
Awais Ghaffar, University of Calgary, CanadaAbdul Rahman Omar, Putra Malaysia University, Malaysia
Copyright © 2022 Zaheer, Chen, Khan, Elokil, Saleemi, Zaheer and Khan. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Iqra Zaheer, dr.iqzaheer@gmail.com; Wei Chen, cwei010230@163.com
 Iqra Zaheer
Iqra Zaheer Wei Chen2*
Wei Chen2*  Ahrar Khan
Ahrar Khan Muhammad Kashif Saleemi
Muhammad Kashif Saleemi Tean Zaheer
Tean Zaheer