Acute phase proteins levels in horses, after a single carbohydrate overload, associated with cecal alkalinization
- 1Department of Veterinary Internal Medicine and Surgery, School of Agricultural and Veterinarian Sciences, São Paulo State University (Unesp), Jaboticabal, São Paulo, Brazil
- 2Department of Veterinary Medicine, Maringá State University, Umuarama, Paraná, Brazil
- 3Department of Agricultural, Livestock and Environmental Biotechnology, School of Agricultural and Veterinarian Sciences, São Paulo State University (Unesp), Jaboticabal, São Paulo, Brazil
Introduction: Horses submitted to carbohydrate overload can develop laminitis due to changes in cecal pH and microbiota, followed by an increase in transmural absorption of luminal content, including bacterial toxins. In response to acute injury there is hepatic overproduction of several proteins known as acute phase proteins (APP). Few studies have evaluated protein fractionation to characterize the inflammatory response in acute laminitis. The aim of this study was to test the viability of an experimental model to induce acute laminitis, using a single carbohydrate overload, and the influence of a buffering solution on the development of the disease; also, study the kinetics of APP during acute laminitis, as well as the correlation between these proteins and clinical signs associated to this syndrome.
Methods: Ten healthy horses were divided in a factorial and randomized way into four groups (n = 5): control group (CG), starch group (SG), buffer group (BG), and starch C buffer group (SBG). They were evaluated at seven times (T0h, T4h, T8h, T12h, T24h, T48h, and T72h), which included clinical evaluation and blood sample collection. Total serum protein and albumin concentrations were determined by colorimetry and the other APP by polyacrylamide gel electrophoresis containing sodium dodecyl sulfate and commercial ELISA kits. Data were analyzed by two-way ANOVA, followed by Tukey's test (p < 0.05). The correlation between clinical signs and APP were verified using the Pearson's correlation coefficient.
Results and discussion: 40% of the animals from SG and 60% from SBG developed clinical laminitis. A single administration of buffer solution was not able to prevent clinical signs of laminitis. There was no difference between groups on total serum protein, albumin, serum amyloid A and C-reactive protein concentrations (p > 0.05). Transferrin, considered a negative APP, showed a positive response pattern in SG and SBG. Ceruloplasmin had a positive correlation with Obel grade, heart rate on animals from SGB and number of steps on horses submitted to starch overload (SG and SBG). Ceruloplasmin, α-1-antitrypsin and haptoglobin concentrations increased in SBG, suggesting an inflammatory response in animals of this group. Changes in clinical parameters were also more evident in the SBG, corroborating the protein fractionation findings.
1. Introduction
Equine laminitis is a devastating condition characterized, in most cases, by excruciating pain and irreversible changes in the digit, incapacitating animals for their intended use but also resulting in death, with economic or affective losses. Despite all the efforts, treatment is often inadequate, many horses that recover do not return to their original athletic activity (1). For these reasons, establishment of early or preventive treatments is imperative to reduce disease occurrence or severity (2).
Laminitis development due to alimentary factors has been well documented (3). Excessive intake of carbohydrates (CHO) increases cecal fermentation, exceeding buffering capacity, which results in cecal pH acidification, changes in microbiota, accumulation of bacterial byproducts (e.g., endotoxins, exotoxins) that are absorbed, triggering a systemic inflammatory response (4). The single CHO overload model (oligofructose) is a reliable method to experimentally induce laminitis in healthy horses. Furthermore, it has been shown that administration of a buffering solution intracecally can minimize changes in the microbiota, preventing the development of SIRS and associated laminitis (5).
Identification of an inflammatory response preceding initial signs of laminitis is fundamental, since the appearance of these signs generally reflect irreversible changes in the lamellar tissue (2, 6). It has been proposed that protein fractionation can be useful to detect the onset of the inflammatory response, before the appearance of other systemic signs (7). Signals from stressors factors are detected by the hypothalamus, activating the hypothalamic-pituitary-adrenal and sympathoadrenal axes, which lead to the release of glucocorticoids and catecholamines, respectively, that, through the induction of pro-inflammatory cytokines by macrophages and lymphocytes, promote the production of acute phase proteins in hepatocytes and in the circulation in stressed animals. Therefore, serum proteins are involved in the regulation of inflammation and protection against infections, and therefore, serum protein profiling has been used to monitor the severity of inflammatory responses in veterinary medicine (8–12).
Acute phase proteins (APP) such as serum amyloid A and fibrinogen are often routinely used in equine practice to assess infection, severity of infection, but also progression and recovery (13–16). These proteins represent a portion of many other proteins produced by the liver (e.g., ceruloplasmin, haptoglobin, C reactive protein, and α-1-antitrypsin) in response to insults. The rate of production of some APP has been associated with laminitis in horses (7). Considering the recent discoveries about the role of APP in inflammation and laminitis (17), further studies about the establishment of this syndrome and early APP involvement needs to be conducted.
Our main goals were: (1) verify the effectiveness of single carbohydrate overload in promoting laminitis; (2) assess the effect of a buffer solution administered intracecally on the development of laminitis; and (3) study the kinetics of acute phase proteins during acute laminitis, as well as the correlation between these proteins and clinical signs associated to acute laminitis.
2. Materials and methods
2.1. Animals and study design
Ten healthy horses, geldings and females, aged 6 ± 3 years, weighting 350 ± 50 kg, were included in the study. Horses were considered healthy based on physical evaluation, hemogram and biochemistry profile, and were under a regular deworming and vaccination program. Horses had no history of disease for at least 6 months prior to the study, were not lame and had no history or clinical evidence of laminitis. Horses had a body condition score of 6, with no indication of insulin dysregulation, although baseline insulin concentrations were not measured. All horses were submitted to typhlopexy 30 days before the experiment (18). They were housed in the Equinoculture Sector at FCAV/Unesp-Jaboticabal Campus and received daily 2 kg of commercial feed (Selvagem®–Agromix—Jaboticabal, SP, Brazil), 4 kg of coast-cross hay (Cynodon dactylon) and water ad libitum. During the 3 days of experimental period (T0h to T72h), horses were kept in individual stalls, without access to the paddock, and fed the same way as described.
The starch overload consisted in a 17.6 g/kg BW dose of powdered corn starch diluted in 1 kg per liter of water (19). Buffer solution containing 3.5 g of aluminum hydroxide, 65.6 g of magnesium hydroxide and 1.2 g of simethicone, was diluted in half amount of water calculated for the starch overload. Horses were randomly allocated into 4 groups (n = 5) in a factorial 2 × 2 way:
- Control group (CG): water administration via nasogastric tube (NGT) and, after 8 h, intracecal (IC) saline solution.
- Starch group (SG): starch administration via NGT and, after 8 h, IC saline solution.
- Buffer group (BG): water administration via NGT and, after 8 h, IC buffer solution.
- Starch/Buffer group (SBG): starch administration via NGT and, after 8 h, IC buffer solution.
After a 15 days' interval, horses from CG and BG groups received SBG and SG treatments, respectively.
Clinical and behavior parameters were evaluated at seven time points: Immediately before the administration of starch/water via NGT (T0h), and 4 (T4h), 8 (T8h), 12 (T12h), 24 (T24h), 48 (T48h), and 72 h (T72h) after the administration of starch/water via NGT. Clinical parameters included heart rate (bpm), rectal temperature (°C), dehydration (%), oral mucosal aspect (normal or abnormal), plus digital pulse and hoof temperature (normal or elevated). Behavior was assessed by signs of discomfort, movement, food and water intake and feces production. The gait quality and number of steps on a flat cement floor in a six-meter space were visually evaluated and counted. Signs of locomotion impairment and limb pain were categorized according to Obel (20).
Blood samples were collected via jugular venipuncture (BD Vacutainer®, Franklin Lakes, NJ, USA) at the same seven time points. The samples were centrifuged for 10 min at 1,000 × g in a temperature-controlled room (21°C) and stored in a −24°C freezer until analyses.
2.2. Serum acute phase proteins and immunoglobulin analysis
Total serum protein and albumin concentrations were determined by spectrophotometry in a semi-automatic biochemical analyzer (Labquest—Labtest Diagnóstica SA, Lagoa Santa, MG, Brazil) by biuret (Total Proteins, Ref. 99-250, Labtest Diagnóstica SA, Lagoa Santa, MG, Brazil / bovine albumin standard 4.0 g/dL) and green bromocresol (Albumin, Ref. 19-1/250, Labtest Diagnóstica SA, Lagoa Santa, MG, Brazil/bovine albumin standard 3.8 g/dL) colorimetric methods, respectively. Identification and quantification of APP were obtained through sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) (21). Quantitative analysis of the SDS-PAGE was performed using a computerized densitometer (model CS-9301PC, Shimadzu Corporation, Tokyo, Japan). Serum concentrations of C-reactive protein (MyBioSource ELISA Horse C-reactive protein, catalog number MBS020917/intra and inter-assay CV is < 15%) and serum amyloid A (MyBioSource ELISA Horse Serum Amyloid A, catalog number MBS281865/intra-assay CV < 8%/inter-assay < 12%) were performed by ELISA.
Five different band fractions of interest were cut from multiple gels generated by the SDS-PAGE technique. The samples were than submitted to trypsin digestion (22) and filtered in a cellulose acetate membrane according to manufacturer's instruction (Spin-X, Corning). Posteriorly, the samples were analyzed by liquid chromatography coupled to mass spectrometry (LC-MS) using a C18 nano-column (15 cm 2 μm, 100 Å) and a chromatographic gradient of 35 min. Mass spectrometer (Q-Exactive, Thermo Scientific) was operated in the data-dependent acquisition mode in a top 20. Protein identification was carried out by spectral correlation in the Patternlab platform (23) and assigned using NCBI predicted protein database from the species Equus caballus. False-discovery rate was adjusted for 1%. This whole analysis was carried out at the Technology Department of the School of Agricultural and Veterinarian Sciences—FCAV/ Unesp-Jaboticabal Campus.
2.3. Statistical analysis
Normality was assessed by the Shapiro–Wilk test and data were normally distributed. Therefore, data were subjected to analysis of variance (ANOVA) and Tukey's test for comparisons. Associations between variables was performed using Pearson's correlation. A p < 0.05 was considered statistically significant. Statistical software included Statistical Analysis System (SAS) program (SAS, 9.1.3 version, SAS Institute, Cary, NC, USA) and GraphPad Prism (Version 7.0).
3. Results
3.1. Experimental model for laminitis induction and effect of treatment using buffer solution
All animals in SG had tachycardia after T4h, and by T24h, 40 (2/5) and 60% (3/5) had dehydration and diarrhea, respectively (Table 1). However, alterations in clinical parameters were more expressive in SBG, especially between T24h and T48h. All horses in SBG had evidence of dehydration at T24h and hyporexia at T48h. Throughout the experimental period, horses from CG and BG maintained their heart rate (HR) and body temperature (°C) within the normal range (24), and presented no alterations of gait, hoof temperature, digital pulse, and food and water intake.
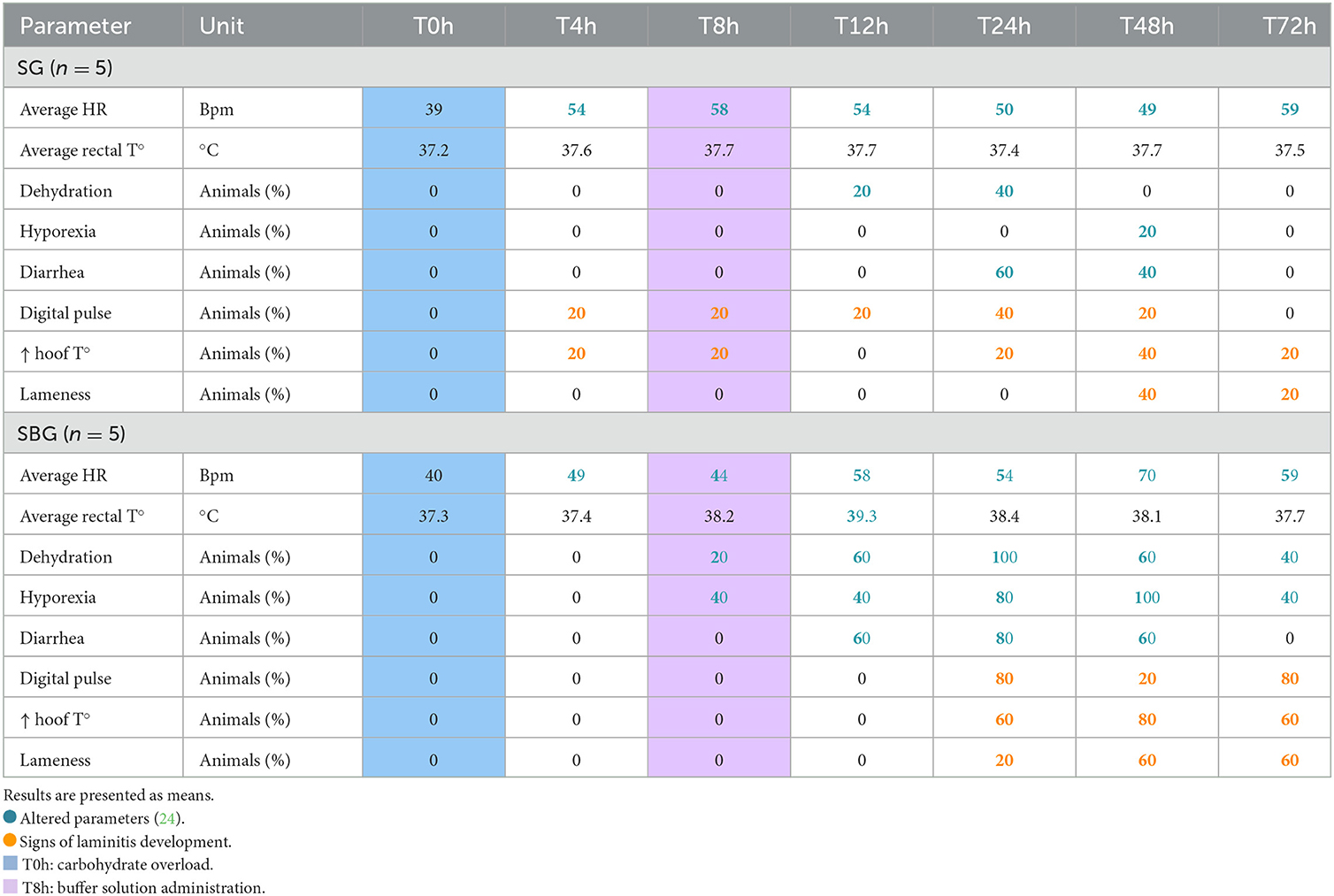
Table 1. Physical examination findings in horses subjected to carbohydrate overload, untreated and treated with intracecal buffer solution (Starch group—SG and Starch/Buffer group—SBG, respectively), in the seven evaluated moments (T0h, T4h, T8h, T12h, T24h, T48h, and T72h).
Three horses from SBG and two from SG developed laminitis (6, 20, 25), with increased digital pulse and hoof temperature, and lameness (Table 1). 20% (1/5) of animals from SG showed signs of laminitis before the administration of the buffer solution. In SBG, clinical signs were delayed (8 h after starch overload, matching the exact time of the treatment), when compared to SG.
3.2. APP kinetics and correlation with clinical parameters during the inflammatory process linked to laminitis syndrome
Through SDS-PAGE (Figure 1) and posterior LC-MS confirmation (Table 2), it was possible to identify four APP (followed by its molecular weight in kDa): transferrin (85 kDa), albumin (65 kDa), α-1-antitrypsin (60 kDa), and haptoglobin (45 kDa). Based on ceruloplasmin (Cp) molecular weight, this protein should have been identified in the 130 kDa protein fraction on the SDS-PAGE gel. This section was cut from polyacrylamide gel, submitted to LC-MS (26–28), but it did not bring a reliable identification. Assuming that the location of Cp in the protein fraction matches the literature (26–28), we chose to maintain the results of the alleged ceruloplasmin (aCp) in this study, which was obtained by densitometry analysis from the SDS-PAGE.
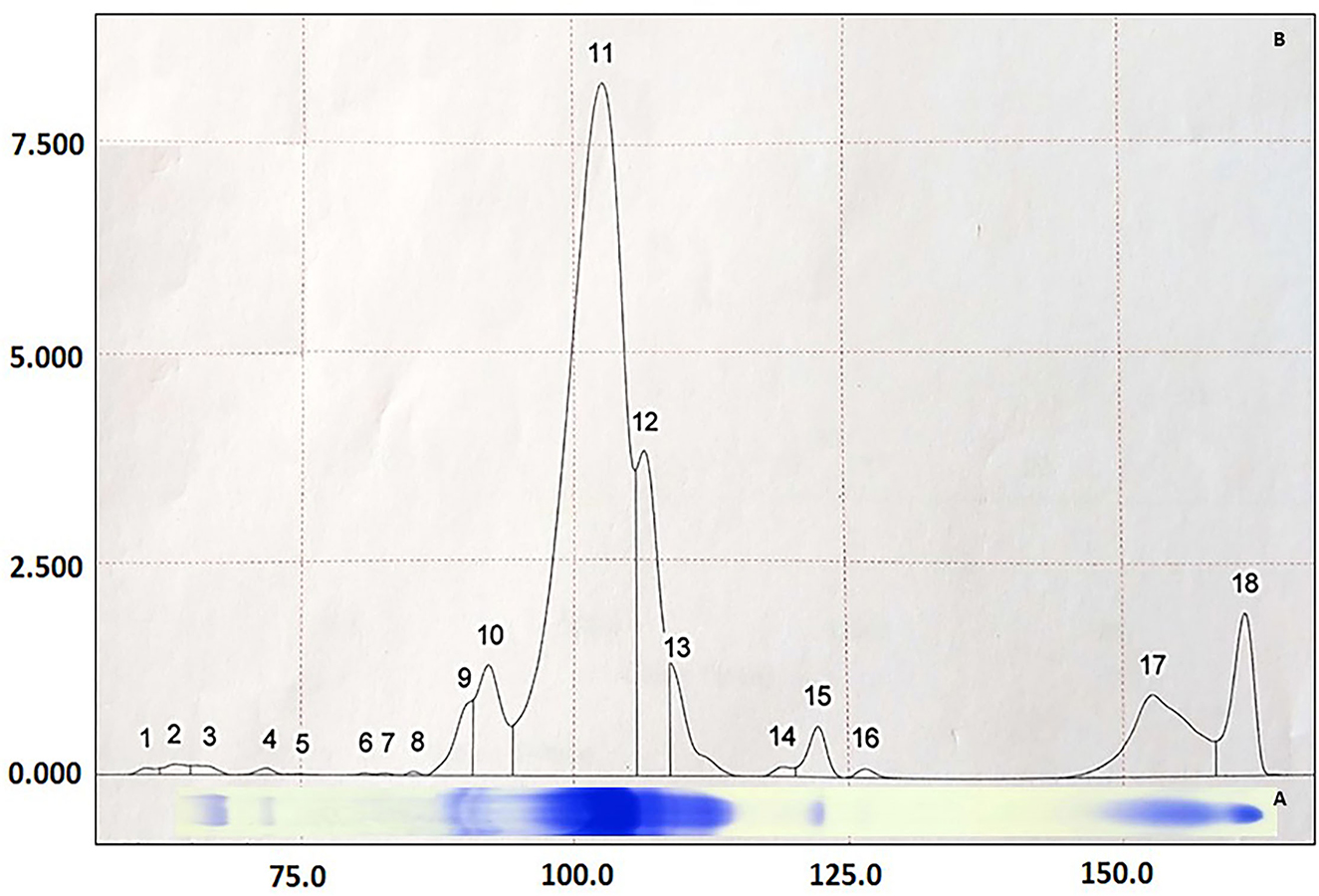
Figure 1. Graphic representation of proteins identified in equine serum by the SDS-PAGE technique (A) and quantified by densitometry (B). 18 proteins were found and 8 identified by their molecular weight (kDa): (3) IgA (175 kDa), (4) ceruloplasmin (130 kDa), (9 and 10) transferrin (85 kDa), (11) albumin (65 kDa), (12) α-1-antitrypsin (60 kDa), (13) IgG-HC (55 kDa), (15) haptoglobin (45 kDa) and (17) IgG-LC (29 kDa).
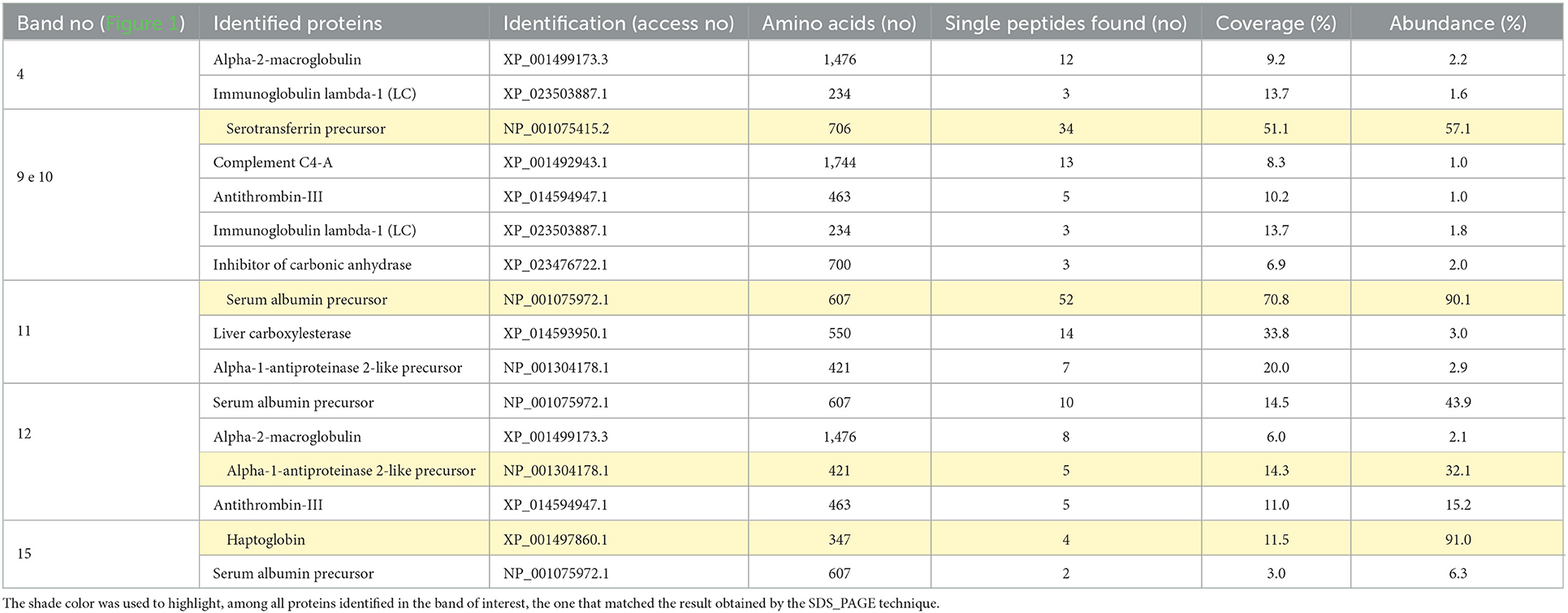
Table 2. Identification of Equus caballus serum proteins by liquid chromatography coupled to mass spectrometry (LC-MS/MS), based on the chemical digestion of five bands of interest in polyacrylamide gels (SDS-PAGE).
As detailed in Table 3, the only APP that varied through time, when considering each group separately, was haptoglobin (Hp), especially after T48h in SG and SBG (p < 0.05). Between SG and CG, there was no difference (p > 0.05) in the levels of aCp and α-1-antitrypsin (α-1-AT). In contrast, SBG had increased serum concentrations of these three APP, when compared with CG, at least in one of the last three moments (p < 0.05), reinforcing the findings of clinical examination. Only Hp varied between animals of BG and SBG at T24h, T48h, and T72h (p < 0.05). This APP also differed between the BG and SG horses at T72h (p < 0.05). Serum transferrin (Trf) concentration was higher in SG, when compared to CG, at T8h, T24h, and T48h (Table 3). Among the SG and SBG, transferrin elevation was higher at T48h in the group that did not receive intracecal buffer solution (Figure 2). There was no difference in serum concentrations of total protein, albumin (Alb), C-reactive protein (CRP) and serum amyloid A (SAA) among time and groups (p > 0.05).
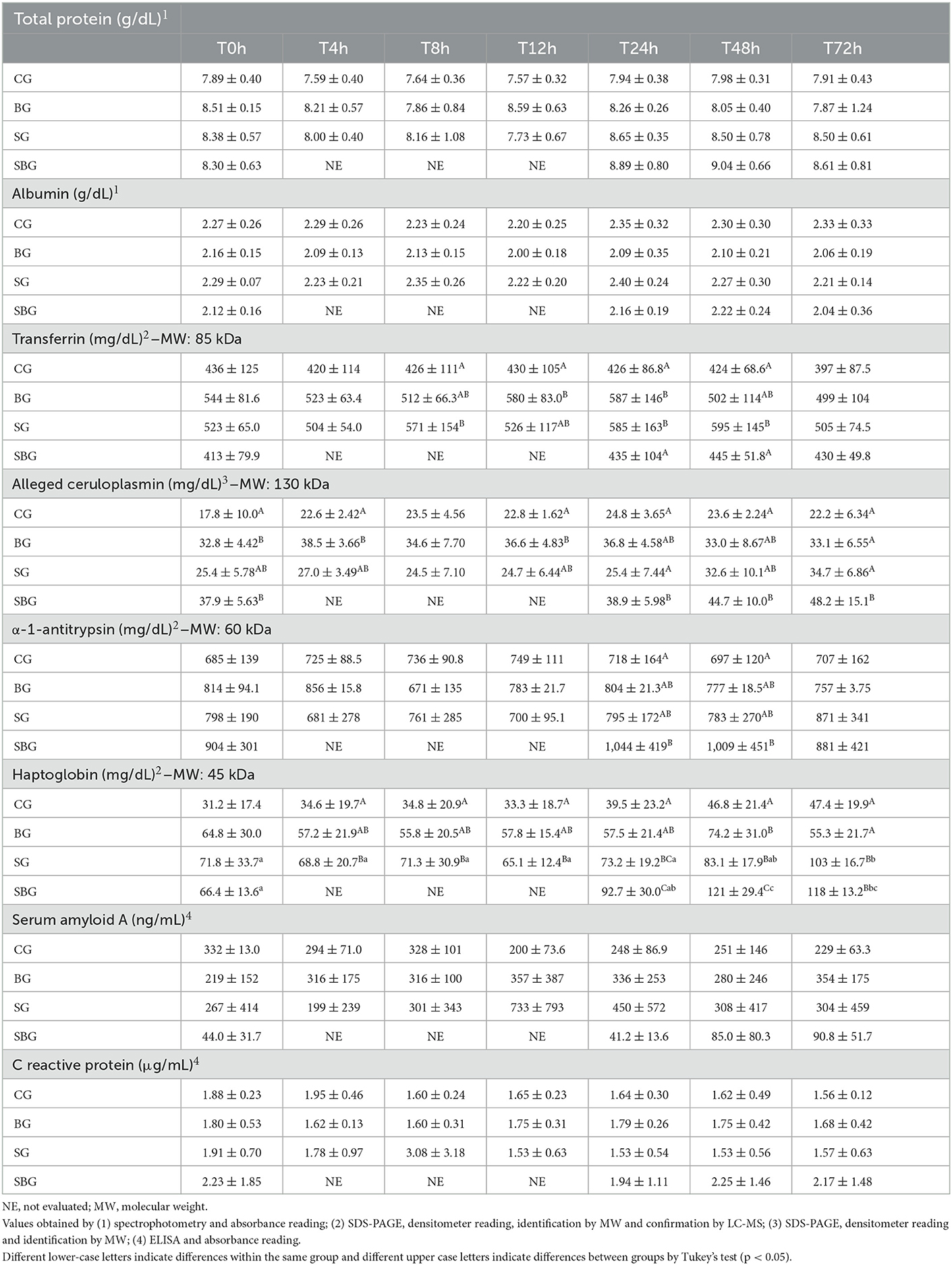
Table 3. Mean ± standard deviation of serum concentrations of total protein, albumin, α-1-antitrypsin, C-reactive protein, transferrin, ceruloplasmin, haptoglobin and serum amyloid A of horses in each experimental group (CG, control; BG, buffer; SG, starch; SBG, starch/buffer); during the seven evaluated moments (T0h, T4h, T8h, T12h, T24h, T48h, and T72h).
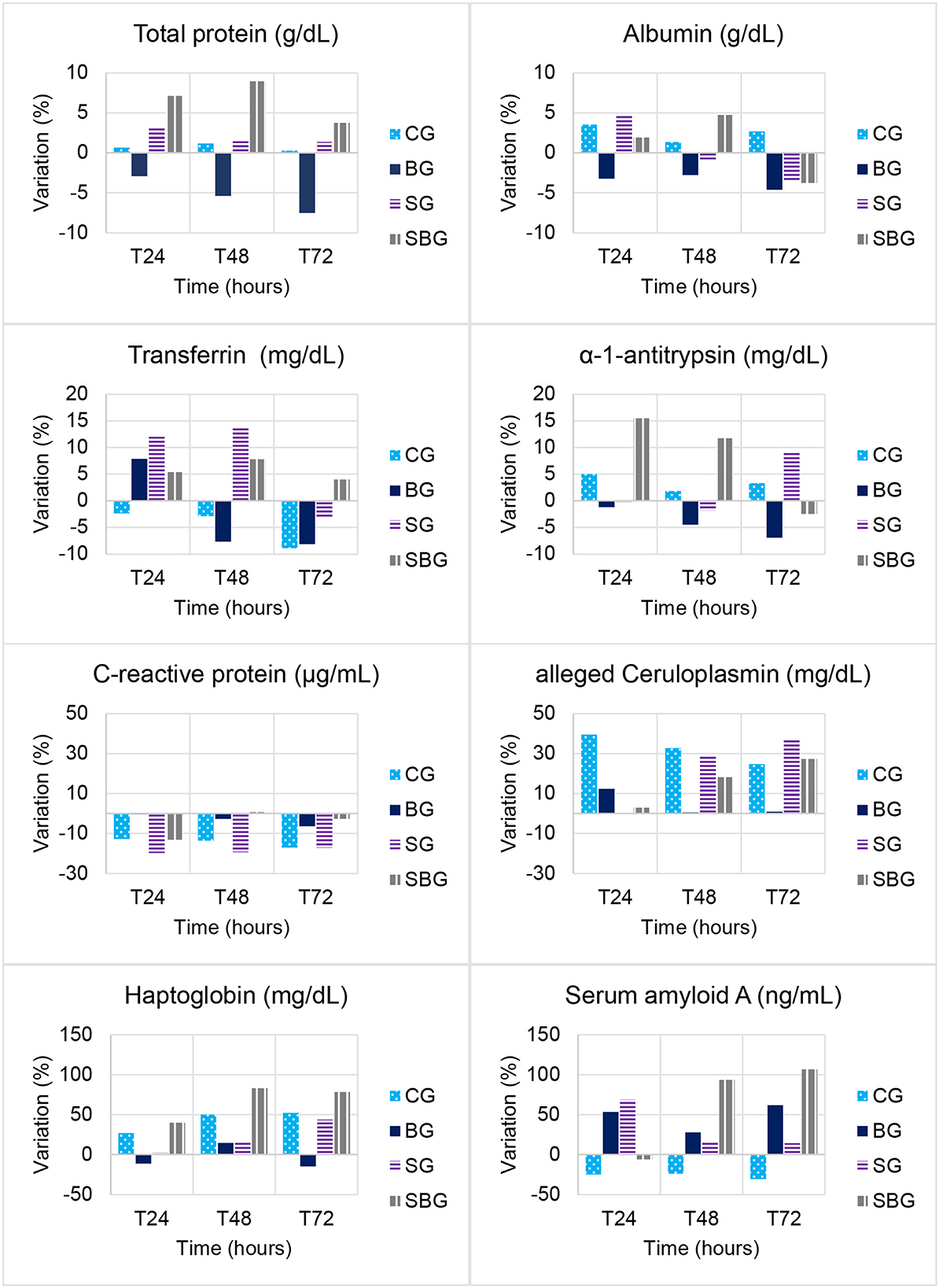
Figure 2. Variation (%) from baseline over time (T0h, T4h, T8h, T12h, T24h, T48h, and T72h) in total serum protein, albumin, transferrin, α-1-antitrypsin, C-reactive protein, alleged ceruloplasmin, haptoglobin and serum amyloid A concentrations in horses submitted to experimental laminitis (CG, control; BG, buffer; SG, starch; SBG, starch/buffer).
As represented in Table 4, aCp showed a positive correlation with number of steps in SG and SBG. In SBG, this protein also correlated positively with Obel grade and HR. In SG, number of steps had a negative correlation with α-1-AT and a positive one with Alb.
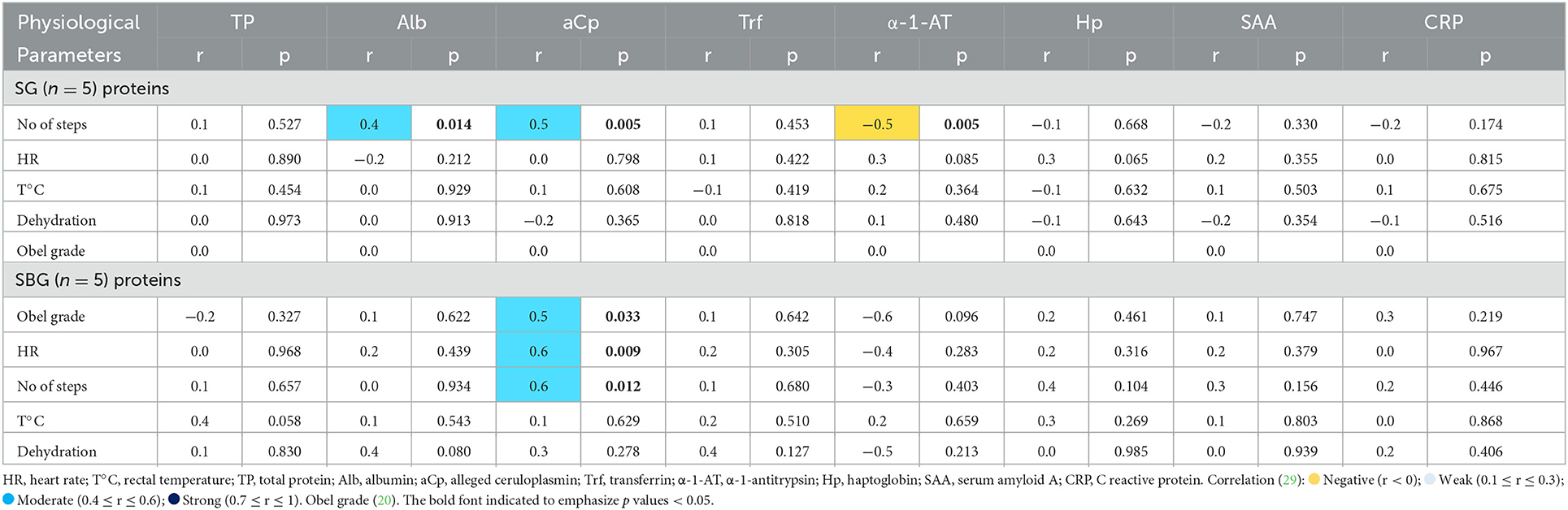
Table 4. Pearson's correlation coefficient (r) and significance (p), from serum concentrations of total protein and acute phase proteins, with the physiological parameters evaluated in horses subjected to carbohydrate overload, untreated and treated with intracecal buffer solution (SG, starch group; SBG, starch/buffer group, respectively).
4. Discussion
4.1. Experimental model for laminitis induction and effect of treatment using buffer solution
Despite the small number of animals per group, we showed that starch overload was effective at inducing laminitis in 50% (5/10) of the animals, regardless of treatment. The partial ineffectiveness of single carbohydrate overload to induce laminitis in the other 50% of the horses can be attributed to individual differences between animals.
Regarding the ineffectiveness of the buffer solution in promoting improvement of the clinical condition in the SBG, some theories can be raised. The buffer solution was composed only of 2 weak bases (aluminum and magnesium hydroxide), without the addition of their respective salts, which may have limited its buffering potential. In a study carried out with healthy horses, the oral supplementation of a solution containing 0.2 g/kg of magnesium hydroxide and aluminum was able to significantly increase the fecal pH, up to 24 h after administration (30). In the present work, the composition of the solution was fixed (3.5 g of aluminum hydroxide and 65.6 g of magnesium hydroxide), regardless of the animal's weight. In this situation, many horses received a dose lower than 0.2 g/kg. In addition, Garner et al. (31) demonstrated that cecal pH usually drops from 7.18 to 5.72 8 h after CHO overload, and to 4.14 after 24 h. Perhaps, SBG would have benefited from a second administration of the buffer solution between T8h and T24h.
4.2. APP kinetics and correlation with clinical parameters during the inflammatory process linked to laminitis syndrome
α-1-antitrypsin is a major endogenous protease inhibitor, with anti-inflammatory properties that tries to contain tissue damage during acute injury (32). Serum concentration of α-1-AT was significant higher in SBG at T24h and T48h, when comparing to CG. Its 15% increase on SBG at T24h could have contributed to positive modulation of the inflammatory response, event not observed in SG (Figure 2). However, as shown in Table 4, there was a negative correlation between increased number of steps and serum levels of this protein on SG. Bearing in mind that horses that developed clinical laminitis had an increased number of steps, it can be speculated that α-1-AT behaved, in horses within SG, as an anti-inflammatory agent.
Similar elevation patterns of Trf were found by Barros et al. (33) in donkeys submitted to laparoscopic ovariectomy and by Nogueira et al. (26) in the peritoneal fluid of horses with intestinal obstruction. When evaluating the effect of treatment not associated with the inflammatory condition (BG × CG), it is noted an increase in Trf concentration in the animals treated with buffer solution, up to 16 h after its administration (T12h and T24h). Being a negative APP in most mammal species, it was expected decreasing values over time in SG and SBG, due to systemic inflammatory conditions. However, this decrease is associated to Fe2+ reduced absorption to reduce bacterial growth (34). Only one animal from SBG presented suggestive signs of sepsis, 48 h after administration of CHO overload, which reinforces the absence of Trf reduction in SG and SBG.
Correlations among APP, hematological, inflammatory, and systemic parameters were stablished for some diseases in equine species (35–37), including positive correlation between Obel grade and α-1-globulin in clinical laminitis (38). Despite lack of more information on searched data, we were able to demonstrate some positive correlations between APP values and clinical signs in animals subjected to starch overload in this laminitis induction model.
It is known that Cp plays an important role in the metabolism and transport of copper, and its elevation is related to the increase of reactive oxygen species, produced during inflammation (39). Oxidative stress has been directly related with the pathogenesis of laminar lesions, especially during sepsis, or when black walnut extract and hyperinsulinemia models are used to induce laminitis (40–42). In this way, this study showed that aCp was a good bioindicator during the early stages of laminitis, since its positive correlation with number of steps in SG and SBG, and Obel grade and HR in SBG.
The response pattern of serum amyloid A (SAA) in horses has been described in few diseases (13–16, 43–46). SAA concentrations varies considerably according to studies, which may suggest individual differences in the immune response and in the methods used for its quantification. Therefore, it is recommended individual interpretation, comparing SAA baseline values with those after inflammatory stimulus, injury, and treatment (45). The values represented in Table 3 demonstrate high variability (high standard deviation) in SAA concentration, which perhaps justifies the absence of a significant difference over time and between sick groups (treated and untreated). When evaluating percentage of variation (Figure 2), assuming samples collected at T0h as baseline, it is noted significant increased concentration of this protein until T72h in the groups treated with buffer solution (106% in the SBG and 62% in BG). SG showed, on the other hand, an increase of only 14%, demonstrating that the buffer solution had a greater impact in SAA expression than CHO overload. It was demonstrated elevated concentrations of amyloid A isoform 3 in synovial fluid of horses submitted to septic arthritis (47–49). These proteinases are known to be involved in physiological and morbid degradation of the extracellular matrix, being related to development of lamellar lesions in horses with laminitis (50, 51). Therefore, detection and classification of local SAA in injured sites may, in the future, increase the diagnostic potential of this protein, allowing distinction between local and systemic conditions.
The absence of a significant difference in serum CRP values corroborate previously studies (52, 53). In humans, high concentrations of this protein were found in the presence of metabolic syndrome (54, 55) and insulin resistance (56). Although ingestion of high amounts of CHO can contribute with the development of these two syndromes in horses (57), the model used to induce laminitis, in this study, consisted in a single CHO overload administration, which may justify the absence of a uniform response to the development of the syndrome, with little repercussion on the levels of CRP.
As previously described (58), horses with induced laminitis by CHO overload showed no significant changes on total serum protein values at the prodromal stage of this syndrome, corroborating our findings. Alb values, obtained through spectrophotometry, also did not vary over the experimental times (Table 3), nor between groups (p > 0.05). As albumin concentrations tend to decrease from the sixth day after inflammatory stimulus (59), these values were expected, since the last blood sample were collected 72 h after administration of the starch overload, and, also, only 40% (4/10) of horses developed severe systemic inflammatory condition.
5. Conclusions
The single administration of starch overload was able to induce clinical laminitis in 60% of SBG and 40% of SG animals. Also, the single dose of buffer solution was not effective in preventing the development of laminitis, and a booster dose should be considered in further studies. Transferrin, considered a negative APP, showed a positive response pattern in SG and SBG. Serum ceruloplasmin concentrations were positively correlated with heart rate, Obel grade and number of steps in animals from SBG. Ceruloplasmin, α-1-antitrypsin and haptoglobin concentrations increased considerably in SBG, suggesting greater activation of inflammatory response in animals of this group, probably due to the better induction of laminitis. Changes in clinical parameters were also more evident in the SBG, corroborating the protein fractionation findings.
Data availability statement
The original contributions presented in the study are included in the article/supplementary material, further inquiries can be directed to the corresponding author.
Ethics statement
The animal study was reviewed and approved by Ethics Committee on the Use of Animals (CEUA) of the School of Agricultural and Veterinarian Sciences—FCAV/Unesp-Jaboticabal Campus, under the protocol # 23.391/15.
Author contributions
IP, PC, and CA: conception and design of the work. IP, VB, and CC: clinical trial execution and data collection. IP, AS, and DG: data analysis. IP: manuscript elaboration. AS, DG, and CA: manuscript review. AB: LC-MS analysis. All authors contributed to the article and approved the submitted version.
Funding
This work was financially supported by the Fundação de Amparo à Pesquisa do Estado de São Paulo (FAPESP—Process 2018/14720-9) and scholarship provided by the Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES)—Finance Code 001.
Acknowledgments
We would like to express our gratitude the Unesp Research Support Laboratory of Internal Medicine and Veterinary Surgery Department and to the Unesp Plant Proteomics Laboratory.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Belknap JK. Laminitis: an overview. In:Belknap JK, Geor R, , editors. Equine Laminitis. Oxford: Wiley-Blackwell (2016). p. 11–12. doi: 10.1002/9781119169239.ch2
2. Belknap JK, Durham AE. Overview of laminitis prevention. In:Belknap JK, Geor R, , editors. Equine Laminitis. Oxford: Wiley-Blackwell (2016). p. 423–426. doi: 10.1002/9781119169239.ch47
3. Pollitt CC, Visser MB. Carbohydrate alimentary overload laminitis. Vet Clin N Am Equine. (2010) 1:65–78. doi: 10.1016/j.cveq.2010.01.006
4. Eades SC. Sepsis-related laminitis. In:Belknap JK, Geor R, , editors. Equine Laminitis. Oxford: Wiley-Blackwell (2016). p. 191–95. doi: 10.1002/9781119169239.ch22
5. Souza A. Cecal buffering: clinical, physiophatologic and therapeutic aspects in experimental laminitis in equine. Thesis (PhD in Veterinary Surgery)—Unesp, Jaboticabal (2007).
6. van Eps AW. General clinical aspects of the laminitis case. In:Belknap JK, Geor R, , editors. Equine Laminitis. Oxford: Wiley-Blackwell (2016). p. 183–190. doi: 10.1002/9781119169239.ch21
7. Fagliari JJ, McClenahan D, Evanson OA, Weiss DJ. Changes in plasma protein concentrations in ponies with experimentally induced alimentary laminitis. Am J Vet Res. (1998) 10:1234–7.
8. Casella S, Fazio F, Russo C, Giudice E, Piccione G. Acute phase proteins response in hunting dogs. J Vet Diagn Invest. (2013) 25:577–80. doi: 10.1177/1040638713495851
9. Piccione G, Arfuso F, Faggio C, Casella S, Zumbo A, Panzera M. Serum proteins profile in Comisana lambs during the first month of life. Arch Tierz. (2013) 56:742–50. doi: 10.7482/0003-9438-56-074
10. Fazio F, Casella S, Giannetto C, Giudice E, Piccione G. Characterization of acute phase proteins and oxidative stress response to road transportation in the dog. Exp Anim. (2014) 14:32. doi: 10.1538/expanim.14-0032
11. Gianesella M, Fiore E, Arfuso F, Vecchio D, Curone G, Morgante M, et al. Serum haptoglobin and protein electrophoretic fraction modifications in buffaloes (Bubalus bubalis) around calving and during early lactation. J Dairy Res. (2019) 86:291–5. doi: 10.1017/S0022029919000438
12. Arfuso F, Giannetto C, Fazio F, Panzera F, Piccione G. Training program intensity induces an acute phase response in clinically healthy horses. J Equine Vet Sci. (2020) 88:102986. doi: 10.1016/j.jevs.2020.102986
13. Hultén C, Demmers S. Serum amyloid A (SAA) as an aid in the management of infectious disease in the foal: comparison with total leucocyte count, neutrophil count and fibrinogen. Equine Vet J. (2002) 7:693–8. doi: 10.2746/042516402776250360
14. Duggan VE, Holyoak GR, MacAllister CG, Cooper SR, Confer AW. Amyloid A in equine colostrum and early milk. Vet Immunol Immunopathol. (2008) 1–2, 150–5. doi: 10.1016/j.vetimm.2007.06.030
15. Andersen SA, Petersen HH, Ersbøll AK, Falk-Rønne J, Jacobsen S. Vaccination elicits a prominent acute phase response in horses. Vet J. (2012) 191:199–202. doi: 10.1016/j.tvjl.2011.01.019
16. Casella S, Fazio F, Giannetto C, Giudice E, Piccione G. Influence of transportation on serum concentrations of acute phase proteins in horse. Res Vet Sci. (2012) 93:914–7. doi: 10.1016/j.rvsc.2012.01.004
17. Leise B. The role of neutrophils in equine laminitis. Cell Tissue Res. (2018) 3:541–50. doi: 10.1007/s00441-018-2788-z
18. Diaz ADPU, Santana AE, Valadão CAA, de Souza AH. Cecal cannula in horses. Ciênc Anim Bras. (2010) 11:357–62. doi: 10.526/cab.v11i2.1198
19. Garner HE, Coffman JR, Hahn AW, Hutcheson DP, Tumbleson ME. Equine laminitis of alimentary origin: an experimental model. Am J Vet Res. (1975) 36:441–4.
20. Obel N. Studies on the Histopathology of Acute Laminitis. 1st ed. Uppsala: Almquist and Wiksells Boktrycker Ab (1948).
21. Laemmli UK. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. (1970) 227:680–5. doi: 10.1038/227680a0
22. Shevchenko A, Tomas H, Havlis J, Olsen JV, Mann M. In-gel digestion for mass spectrometric characterization of proteins and proteomes. Nat Protoc. (2007) 1:2856–60. doi: 10.1038/nprot.2006.468
23. Carvalho PC, Lima DB, Leprevost FV, Santos MDM, Fischer JSG, Aquino PF, et al. Integrated analysis of shotgun proteomic data with PatternLab for proteomics 4.0. Nat Protoc. (2016) 11:102–17. doi: 10.1038/nprot.2015.133
25. Hood DM. Laminitis in the horse. Vet Clin North Am Equine Pract. (1999) 15:287–94. doi: 10.1016/S0749-0739(17)30145-1
26. Nogueira AFS, Di Filippo PA, Anai LA, Vieira MC, Simplício MMG, Santana AE. Establishment of peritoneal liquid electrophoretogram from healthy horses and horses submitted to experimentally induced intestinal obstruction. Arq Bras Med Vet Zootec. (2014) 66:665–71. doi: 10.1590/1678-41625993
27. Santana AM, Silva DG, Thomas FC, Bernardes PA, Pizauro LJL, Santana CL, et al. Blood serum acute phase proteins and iron dynamics during acute phase response of Salmonella enterica serotype Dublin experimentally infected buffalo calves. Vet Immunol Immunopathol. (2018) 203:30–9. doi: 10.1016/j.vetimm.2018.07.014
28. Santana AM, Thomas FC, Silva DG, McCulloch E, Vidal AMC, Burchmore RJS, et al. Reference 1D and 2D electrophoresis maps for potential disease related proteins in milk whey from lactating buffaloes and blood serum from buffalo calves (Water buffalo, Bubalus bubalis). Res Vet Sci. (2018) 118:449–65. doi: 10.1016/j.rvsc.2018.04.010
29. Dancey CP, Reidy J. Statistics Without Math for Psychology. 7th ed. Porto Alegre: Artmed (2006).
30. Maia MA, Botteon PTL, Spindola BF, Botteon RCCM. Change of fecal pH of horses by oral administration of alkalizing. Braz J Vet Med. (2017) 1:1–6. doi: 10.29374/2527-2179.bjvm017
31. Garner HE, Moore JN, Johnson JH, Clark L, Amend JF, Tritschler LG. Changes in the caecal flora associated with the onset of laminitis. Equine Vet J. (1978) 4:249–52. doi: 10.1111/j.2042-3306.1978.tb02273.x
32. Reeves EP, Dunlea DM, Mcquillan K, O'Dwyer CA, Carroll TP, Saldova R, et al. Circulating truncated alpha-1 antitrypsin glycoprotein in patient plasma retains anti-inflammatory capacity. J Immunol. (2019) 202:2240–53. doi: 10.4049/jimmunol.1801045
33. Barros IO, Fonseca NBS, Nunes TL, Spagnolo JD, Santos JPA, Neto AS, et al. Peritoneal inflammatory response of the donkeys (Equus asinus) submitted ovariectomy by laparotomy and laparoscopy. Arq Bras Med Vet Zootec. (2018) 70:1468–76. doi: 10.1590/1678-4162-9542
34. Skaar EP. The battle for iron between bacterial pathogens and their vertebrate hosts. PLoS Pathog. (2010) 6:e1000949. doi: 10.1371/journal.ppat.1000949
35. Jacobsen S, Jensen JC, Frei S, Jensen AL, Thoefner MB. Use of serum amyloid A and other acute phase reactants to monitor the inflammatory response after castration in horses: a field study. Equine Vet J. (2010) 37:552–6. doi: 10.2746/042516405775314853
36. Kim PH, Leopold S. Gustilo-anderson classification. Clin Orthop Relat Res. (2012) 470:3270–4. doi: 10.1007/s11999-012-2376-6
37. Leclere M, Lavoie-Lamoureux A, Lavoie JP. Acute phase proteins in racehorses with inflammatory airway disease. J Vet Intern Med. (2015) 29:940–5. doi: 10.1111/jvim.12587
38. Edinger H, Miller I, Stanek C, Gemeiner M. Electrophoretic studies of serum protein fractions in horses with laminitis. Deut Tierarztl Woch. (1992) 99:426–30.
39. Smith, B. P. (2014). Large Animal Internal Medicine. 6th ed. St. Louis: Elsevier Health Sciences.
40. Laskoski LM, Dittrich RL, Valadão CAA, Brum JS, Brandão Y, Brito HFV, et al. Oxidative stress in hoof laminar tissue of horses with lethal gastrointestinal diseases. Vet Immunol Immunopathol. (2016) 171:66–72. doi: 10.1016/j.vetimm.2016.02.008
41. McGowan C, Patterson-Kane J. Experimental models of laminitis: Hyperinsulinemia. In:Belknap JK, Geor R, , editors. Equine Laminitis. Oxford: Wiley-Blackwell (2016). p. 68–74. doi: 10.1002/9781119169239.ch10
42. Peroni JF. Experimental models of laminitis: black walnut extract. In:Belknap JK, Geor R, , editors. Equine Laminitis. Oxford: Wiley-Blackwell (2016). p. 64–67. doi: 10.1002/9781119169239.ch9
43. Hobo S, Niwa H, Anzai T. Evaluation of serum amyloid A and surfactant protein d in sera for identification of the clinical condition of horses with bacterial pneumonia. J Vet Med Sci. (2007) 8:827–30. doi: 10.1292/jvms.69.827
44. Jacobsen S, Nielsen JV, Kjelgaard-Hansen M, Toelboell T, Fjeldborg J, Halling-Thomsen M, et al. Acute phase response to surgery of varying intensity in horses: a preliminary study. Vet Surg. (2009) 38:762–9. doi: 10.1111/j.1532-950X.2009.00564.x
45. Witkowska-Piłaszewicz OD, Zmigrodzka M, Winnicka A, Miśkiewicz A, Strzelec K, Cywińska A. Serum amyloid A in equine health and disease. Equine Vet J. (2019) 3:293–8. doi: 10.1111/evj.13062
46. Witkowska-Piłaszewicz OD, Baska P, Czopowicz M, Zmigrodzka M, Szczepaniak J, Szarska E, et al. Changes in serum amyloid A (SAA) concentration in Arabian endurance horses during first training season. Animals. (2019) 6:1–9. doi: 10.3390/ani9060330
47. Jacobsen S, Niewold TA, Halling-Thomsen M, Nanni S, Olsen E, Lindegaard C, et al. Serum amyloid A isoforms in serum and synovial fluid in horses with lipopolysaccharide-induced arthritis. Vet Immunol Immunopathol. (2006) 3–4:325–30. doi: 10.1016/j.vetimm.2005.10.012
48. Jacobsen S, Halling-Thomsen M, Nanni S. Concentrations of serum amyloid A in serum and synovial fluid from healthy horses and horses with joint disease. Am J Vet Res. (2006) 10:1738–42. doi: 10.2460/ajvr.67.10.1738
49. Ludwig EK, Wiese RB, Graham MR, Tyler AJ, Settlage JM, Were SR, et al. Serum and synovial fluid serum amyloid A response in equine models of synovitis and septic arthritis. Vet Surg. (2016) 45:859–67. doi: 10.1111/vsu.12531
50. Kyaw-Tanner M, Pollitt CC. Equine laminitis: increased transcription of matrix metalloproteinase-2 (MMP-2) occurs during the developmental phase. Equine Vet J. (2004) 36:221–5. doi: 10.2746/0425164044877242
51. Kyaw-Tanner MT, Wattle O, van Eps AW, Pollitt CC. Equine laminitis: membrane type matrix metalloproteinase-1 (MMP-14) is involved in acute phase onset. Equine Vet J. (2008) 5:482–7. doi: 10.2746/042516408X270353
52. Pollock PJ, Prendergast M, Schumacher J, Bellenger CR. Effects of surgery on the acute phase response in clinically normal and diseased horses. Vet Rec. (2005) 17:538. doi: 10.1136/vr.156.17.538
53. Lavoie-Lamoureux A, Leclere M, Lemos K, Wagner B, Lavoie JP. Markers of systemic inflammation in horses with heaves. J Vet Intern Med. (2012) 6:1419–26. doi: 10.1111/j.1939-1676.2012.00993.x
54. Ryu SY, Kim KS, Park J, Kang MG, Han MA. The association between circulating inflammatory markers and metabolic syndrome in Korean rural adults. J Prev Med Public Health. (2008) 41:413–8. doi: 10.3961/jpmph.2008.41.6.413
55. Maleki A, Rashidi N, Meybodi HA, Montazeri M, Montazeri M, Falsafi F, et al. Metabolic syndrome and inflammatory biomarkers in adults: a population-based survey in western region of Iran. Int Cardiovasc Res J. (2014) 8:156–60.
56. Ingelsson E, Hulthe J, Lind L. Inflammatory markers in relation to insulin resistance and the metabolic syndrome. Eur J Clin Invest. (2008) 38:502–9. doi: 10.1111/j.1365-2362.2008.01962.x
57. Burns TA, Toribio RE. Metabolic syndrome in humans and horses: the relationship between obesity and insulin resistance. In:Belknap JK, Geor R, , editors. Equine Laminitis. Oxford: Wiley-Blackwell (2016). p. 49–166. doi: 10.1002/9781119169239.ch18
58. Martins Filho LP, Fagliari JJ, Moraes JRE, Sampaio RC, Oliveira JA, Lacerda Neto JC. Clinical and laboratorial evaluation in prodromal stage of carbohydrate overload-induced equine laminitis. Ars Vet. (2007) 23:32–9.
Keywords: electrophoresis, gastrointestinal buffering, protein fractionation, SIRS, acute phase response
Citation: Peixoto Rabelo I, Barroco de Paula V, Carvalho Bustamante C, Santana AM, Gomes da Silva D, Baldassi AC, Canola PA and Araújo Valadão CA (2023) Acute phase proteins levels in horses, after a single carbohydrate overload, associated with cecal alkalinization. Front. Vet. Sci. 10:1043656. doi: 10.3389/fvets.2023.1043656
Received: 13 September 2022; Accepted: 16 January 2023;
Published: 02 February 2023.
Edited by:
Patrizia Licata, University of Messina, ItalyReviewed by:
Małgorzata Pomorska-Mól, Poznan University of Life Sciences, PolandArash Omidi, Shiraz University, Iran
Copyright © 2023 Peixoto Rabelo, Barroco de Paula, Carvalho Bustamante, Santana, Gomes da Silva, Baldassi, Canola and Araújo Valadão. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Isabela Peixoto Rabelo,  isabela.peixoto@unesp.br
isabela.peixoto@unesp.br
 Isabela Peixoto Rabelo
Isabela Peixoto Rabelo Vanessa Barroco de Paula
Vanessa Barroco de Paula Caio Carvalho Bustamante
Caio Carvalho Bustamante André Marcos Santana
André Marcos Santana Daniela Gomes da Silva
Daniela Gomes da Silva Amanda Cristina Baldassi
Amanda Cristina Baldassi Paulo Aléscio Canola
Paulo Aléscio Canola Carlos Augusto Araújo Valadão
Carlos Augusto Araújo Valadão