Multi-institutional retrospective study investigating blood culture protocols and test positivity in 701 dogs
- 1Department of Small Animal Clinical Sciences, College of Veterinary Medicine, Michigan State University, East Lansing, MI, United States
- 2Veterinary Diagnostic Laboratory, College of Veterinary Medicine, Michigan State University, Lansing, MI, United States
- 3Department of Medical Sciences, College of Veterinary Medicine, University of Wisconsin-Madison, Madison, WI, United States
- 4Department of Small Animal Clinical Sciences, College of Veterinary Medicine, University of Tennessee, Knoxville, TN, United States
- 5Gastrointestinal Laboratory, Department of Small Animal Clinical Sciences, School of Veterinary Medicine & Biomedical Sciences, Texas A&M University, College Station, TX, United States
- 6Department of Clinical Sciences, Cummings School of Veterinary Medicine, Tufts University, North Grafton, MA, United States
- 7Department of Clinical Sciences, College of Veterinary Medicine & Biomedical Sciences, Colorado State University, Fort Collins, CO, United States
- 8Department of Veterinary Clinical Sciences, College of Veterinary Medicine, Washington State University, Pullman, WA, United States
- 9Department of Small Animal Clinical Sciences, College of Veterinary Medicine, Virginia Tech, Blacksburg, VA, United States
- 10Center for Statistical Training and Consulting, Michigan State University, East Lansing, MI, United States
Objectives: (i) To determine the influence of specimen collection protocol (timing and specimen quantity), primary disease process, and pre-existing antimicrobial or immunosuppressive therapy on blood culture (BC) positivity and (ii) To determine agreement between urine culture and BC results.
Animals: 701 client-owned dogs.
Methods: Multi-institutional retrospective study (2019–2022). Mixed-effect logistic regression was used to determine whether primary disease process, the number of BCs, or the timing of specimen collection was associated with BC positivity. Prediction plots were generated. Associations between urine culture and BC results were performed using logistic regression.
Results: Dogs with a positive urine culture were more likely to have a positive BC (OR: 4.36, 95% CI: 2.12–8.97, p = 0.003). Dogs that had three BC specimens had the greatest odds of obtaining a positive BC result (adjusted predictive value: 0.44, 95% CI: 0.21–0.70), although this was not significant. Isolates from 38.5% of dogs with a positive BC had resistance to ≥3 antimicrobial classes. The timing between specimen collection had no significant association with BC positivity. Pre-existing antibiotic or immunosuppressive therapy had no significant association with BC positivity.
Clinical relevance: Dogs with a positive urine culture were more likely to have a positive BC result.
1 Introduction
Dogs with bacteremia can develop sepsis which is associated with high morbidity and mortality (1–3). Blood cultures (BCs) are considered the gold standard diagnostic technique to identify and document bacteremia; however, they are expensive, and results may take several days. Advanced techniques such as matrix-assisted laser desorption ionization time of flight mass spectrometry (MALDI-TOF-MS) or polymerase chain reaction (PCR) have been proposed to reduce the timeline for identification of bacteremia, but these techniques do not provide information on in vitro antimicrobial susceptibility and do not replace the use of BCs in clinical practice (4, 5). Additionally, conflicting results exist regarding the utility of PCR to detect bacteremia (5, 6). The cost associated with performing serial BCs remains a major challenge and has led to variations in standard operating procedures, such as a reduction in specimens collected to reduce the total diagnostic cost (7, 8). It is unknown whether the number of BC-specimens is associated with the likelihood of obtaining a positive BC result in dogs. Fewer specimens might also make it more challenging to discern if a bacterial isolate is a contaminant or not. Additionally, the use of a single urine culture as a surrogate marker of bacteremia has also been proposed to reduce cost; however, the sensitivity for detection of a bacteremia from urine specimens was found to be poor (30%) in one study (9). An additional variation in BC protocols among clinicians is the timeline between collecting each specimen as part of a series of BCs. The impact of these protocol alterations on test positivity is unknown.
Thus, the objectives of our study were to determine the influence of specimen collection protocol (timing and specimen quantity), primary disease process, and pre-existing antimicrobial or immunosuppressive therapy on blood culture (BC) positivity. An additional objective was to determine agreement between urine culture and BC results.
2 Materials and methods
2.1 Case identification and data collection
Cases were identified by searching the medical record systems at 7 academic institutions for dogs that had BCs performed from Jan 1st, 2019, to December 31st, 2021. Medical and treatment records were analyzed, and the following data was extracted from each patient: BC isolate and in vitro susceptibility data, urine bacterial culture and susceptibility results, number of BCs, time between blood specimen collections, medical treatments (antimicrobials, immunosuppressive agents), and primary disease process. Species specific minimum inhibitory concentration (MIC) breakpoints were used where available, with human MIC breakpoints used if a species-specific MIC breakpoint was unavailable. Antimicrobial agents included any pre-existing treatment within 7 days of BC collection, or within 14 days if cefovecin was administered. The proportion of dogs that remained on antimicrobial treatment within 24 h of specimen collection was also recorded. Topical antimicrobials were not considered as part of pre-existing antimicrobial use. Immunosuppressive agent dosing thresholds were established for prednisone/prednisolone at >1 mg/kg/d (or equivalent dosing of dexamethasone). Chemotherapeutic agents were not considered as pre-existing immunosuppressive use. The time between blood specimen collections as part of a BC series were classified into the following groups: < 30 min, 30–60 min, 61–120 min, or > 120 min. Blood culture specimens were considered part of a single preplanned BC series (e.g., 1 multiple sample BC) when a regular repeating time interval was noted between specimen collections. When aerobic and anaerobic BCs were performed on the same specimen, they were classified as 1 BC-specimen. Contaminants were determined based on either criteria set by the reporting laboratory or review by a board-certified microbiologist, and these results were excluded from subsequent analysis. Primary disease processes were classified into the following groups based on complete medical record review by a single co-author (AJO): cardiac, dermatologic, gastrointestinal, hematology/immunology, hepatobiliary and pancreatic disease, musculoskeletal, neurologic, respiratory, reproductive/urogenital, and other. When animals had disease characteristics affecting multiple body systems, such as a dog with a urinary tract infection (UTI) and discospondylitis, the disease process that was suspected to have developed first (e.g., UTI) (based on medical record review) was listed as the primary disease process. When it was unknown which disease originated first, or when a systemic disease process was noted, the dog’s disease was listed in an alternate category, named “other.”
2.2 Data analysis
Signalment, primary disease process, and the presence or absence of concurrent antimicrobials or immunosuppressive agents were summarized in a descriptive manner. Mixed-effects logistic regression analysis were used to determine the effect of number of BC specimens collected and the timing of BC specimens on the likelihood of a positive BC result. Odds ratios were used to describe the predicted odds of BC positivity associated with each predictor in the model, with odds ratios greater than one indicating an increased odds of a positive result and odds ratios less than one indicating a decreased odds of a positive result. Primary disease process was investigated similarly. Institution was included as a random effect in the model. The reference population to which all others were compared was defined numerically and alphabetically by convention when a single population is not an obvious comparison group. Dogs that had a 1-specimen BC, and dogs with cardiac disease were therefore defined as the reference standards. Prediction plots were also generated to demonstrate the probability of a positive BC result by primary disease process, number of BCs performed, and time between BC specimens. The prediction plots were based on the results from the model and allow for visual comparisons between all groups, rather than only with the reference group. This did not rely on establishment of an arbitrary comparative group. Prediction plots were adjusted for the random effect of institution. Associations between urine culture results and the odds of a positive BC result were assessed using multiple logistic regression. All statistical analyses were performed by NJ using commercially available software (R Statistical Software v4.2.1, R Foundation for Statistical Computing, Vienna, Austria). The “stats” and “pROC” packages of R were utilized. p ≤ 0.05 were considered significant for all comparisons.
2.3 Methods for blood culture and susceptibility
BCs were performed using either VersaTREK (Remel Oxoid, Lenexa, KS) or BACTEC (BD Diagnostics, Sparks, MD) liquid BCs systems according to the manufacturer recommended protocols. In brief, each blood specimen was inoculated separately into an aerobic and anaerobic bottle and incubated at 37°C for 24 h. After the initial 24 h of incubation, the liquid media were analyzed using a Gram stain and subcultured onto standard agar plates (Colombia blood, chocolate, tryptic soy agar with 5% sheep blood, colistin nalidixic agar and MacConkey agar). If the culture remained negative, the BC bottles were incubated for a total of 5–7 days to confirm a lack of bacterial growth on standard agar media. Bacteria grown on agar plates were identified using Microflex LT MALDI ToF (Bruker, Billerica, MA) following manufacturer recommended protocols. Protocols for one study site differed in that separate aerobic and anerobic culture bottles were not used and inoculation to solid media was only performed if a positive signal/pressure change (VersaTREK system, TREK diagnostic Systems, Clevland, OH) was noted for the BC bottle during incubation or when Wampole isolator tubes were used (10). Antimicrobial susceptibility testing was done using broth microdilution method on Sensitive COMPGN1F (for gram negative bacteria) and COMPGP1F (for gram positive bacteria) (ThermoFisher, Cleveland, OH). Minimum inhibitory concentrations were determined, and susceptibility reported using CSLI guidelines (11–16). Antimicrobials evaluated are listed in Supplementary material 1.
2.4 Method of urine culture and susceptibility
Urine samples were inoculated on TSAB (trypticase soy agar with 5% sheep blood, Remel, Lenexa, KS) and two selective plates, MacConkey and CNA agar (Remel, Lenexa, KS). The plates were observed for bacterial growth after 24 h incubation at 37°C with 5% CO2. If no bacterial growth is observed the plates were re-incubated for another 2 days. Bacteria were then identified using Microflex LT MALDI-TOF (Bruker Daltonics, Bremen, Germany) following manufacturer recommended protocols. Antibiotic susceptibility testing was performed on isolated bacteria as described above.
3 Results
3.1 Animals
A total of 701 dogs were included in analysis. The median age of enrolled dogs was 7 years (range, 2 months – 16 years). There were 318 female (43 intact and 276 spayed females) and 380 were male (97 intact and 283 neutered males) dogs included. The sex of 2 dogs was not recorded. The most common dog breed was mixed breed (174 dogs), followed by Labrador retriever (61 dogs), German shepherd dog (55 dogs), golden retriever (38 dogs), boxer (16 dogs), border collie (12 dogs), mastiff (11 dogs), French bulldog (10 dogs), Siberian husky (10 dogs), greyhound (10 dogs), and Great Dane (10 dogs). Other breeds were represented by less than 10 dogs each. Weight was not recorded.
3.2 Bacterial culture and susceptibility results
One hundred and fifty-two dogs had positive BCs (excluding contaminants), yielding a 22% patient positivity rate. The most commonly isolated bacteria were Staphylococcus spp. [Staphyloccus pseudintermedius (34), Staphylococcus aureus (9) other Staphylococcus spp. (9)], Streptococcus spp. [Beta-hemolytic Streptococcus (20) and non-hemolytic Streptococcus (6)], and Escherichia coli (23). Other isolates identified included Enterococcus spp. (12), Pasturella spp. (11), Actinomyces canis (4), Bacillus spp. (4), Pseudomonas aeruginosa (3), and other bacterial species with <3 isolates identified (15). Of the pathogens cultured, 54 (38.5%) were found to have resistance to 3 or more antimicrobial classes. Antimicrobial susceptibility patterns are reported in Supplementary material 1.
3.3 Primary disease process
The primary disease process was evaluated in all 701 dogs. The following disease processes were identified: neurologic disease in 142 (20.3%) dogs, hematologic or immunologic disease in 92 (13.1%), gastrointestinal disease in 72 (10.3%), musculoskeletal disease in 65 (9.3%), cardiac disease in 51 (7.3%), respiratory disease in 49 (7.0%), hepatobiliary or pancreatic disease in 45 (6.4%), urogenital disease in 42 (6.0%), and dermatologic disease in 31 (4.4%). One hundred and twelve dogs (16.0%) did not fit into any of the above categories. There was no association between primary disease process and the odds of a positive BC (Table 1). A prediction plot was also generated between primary disease process and odds of positive BC and no significant results were noted (Figure 1).
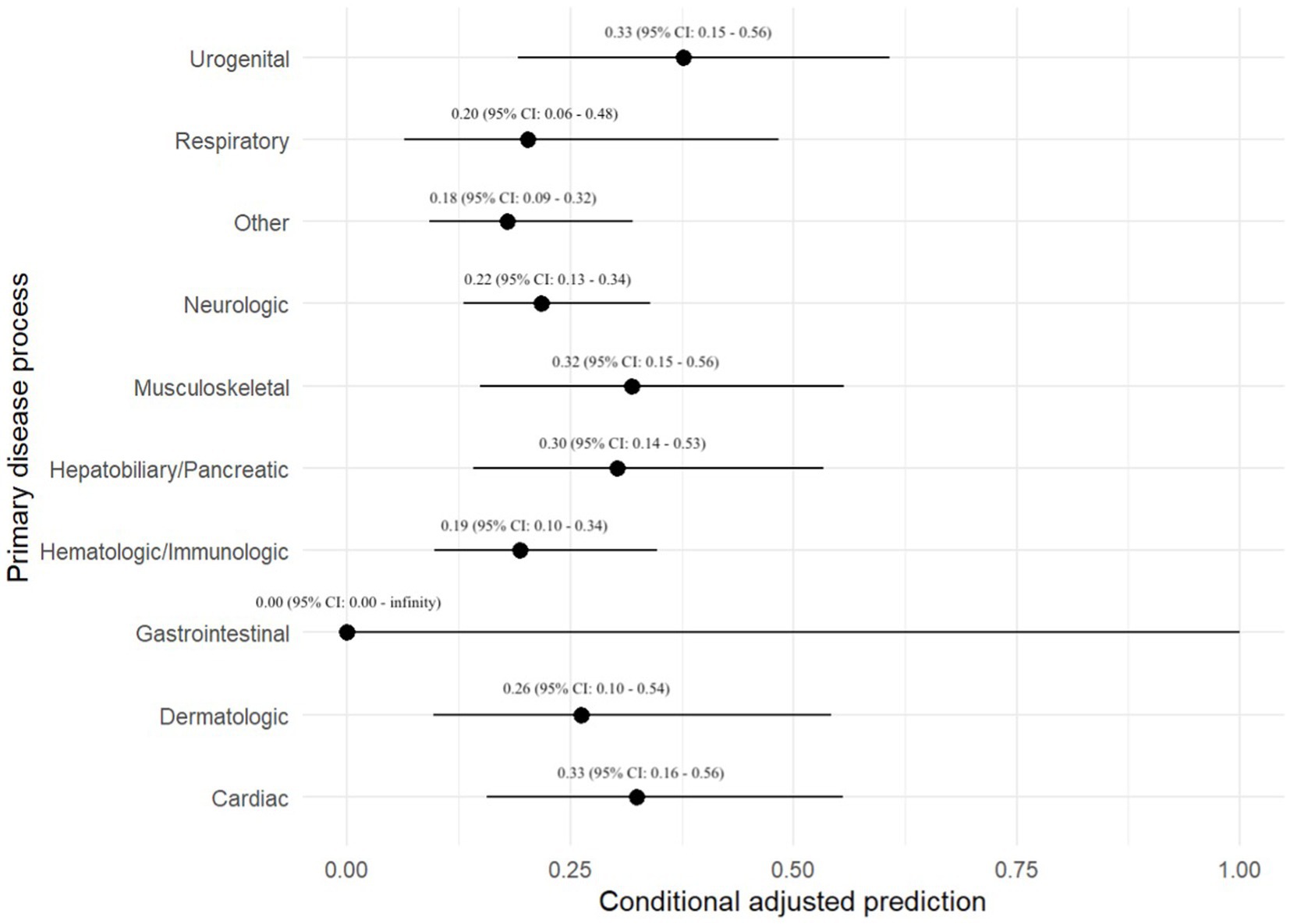
Figure 1. Prediction plot for odds of positive BC by primary disease process. This figure demonstrates a statistically modeled predictive value for BC positivity when compared to primary disease process. The black circle represents the predicted value, while the black line represents the 95% confidence interval.
3.4 Concurrent antimicrobials or immunosuppressive medications
Information on antimicrobial administration was available for 689 (98%) of dogs. Two-hundred and forty-seven (35.2%) dogs received antimicrobials within 7 days of BC collection and 3 dogs (0.4%) received cefovecin (off-label) within 14 days of BC collection. Two-hundred and forty-four (97.6%) of these dogs also received antimicrobials within 24 h of BC collection. Pre-existing antimicrobial therapy yielded an odds ratio of <1 for BC positivity (OR: 0.67, 95% CI: 0.31–1.46), and this relationship was not statistically significant (p = 0.65). One hundred dogs (14.3%) were receiving immunosuppressive medications at the time of specimen collection. There was no significant association between immunosuppressive therapy and odds of a positive BC (p = 0.74).
3.5 Number and timing of BC specimens
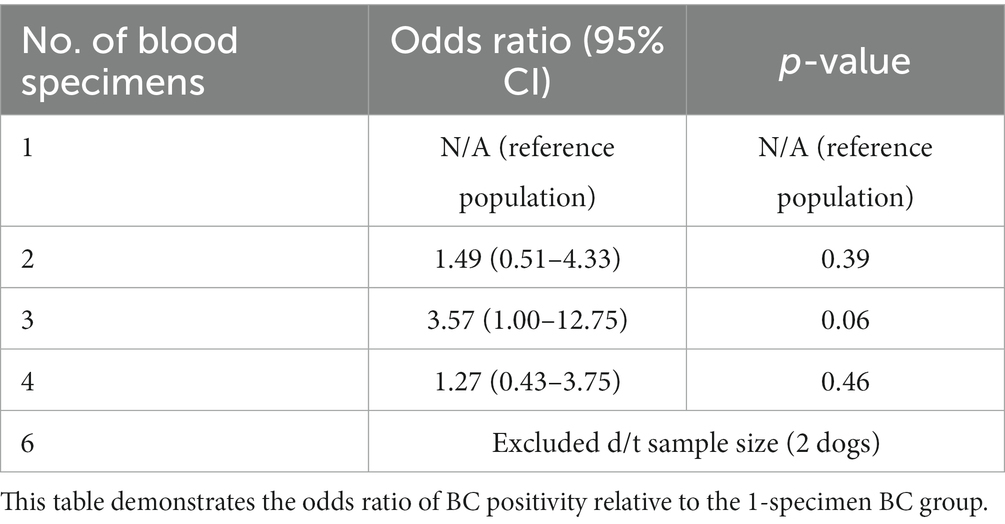
Eighty-nine (12.7%) dogs had a 1-specimen BC submitted, 243 (34.7%) dogs had a 2-specimen BC submitted, 85 (12.1%) dogs had a 3-specimen BC submitted, 256 (36.5%) dogs had a 4-specimen BC submitted, and 2 (< 1%) dogs had a 6-specimen BC submitted. The number of BCs submitted was unavailable for 26 dogs. There was no association between the number of BC specimens collected and the odds of a positive BC (Table 2).
A prediction plot was also generated between number of BC specimens performed and odds of a positive BC result, no significant results were noted (Figure 2). The group with 3-specimen BCs had the highest adjusted predictive value for BC positivity, but this was not significant (OR: 0.44, 95% CI: 0.21–0.70).
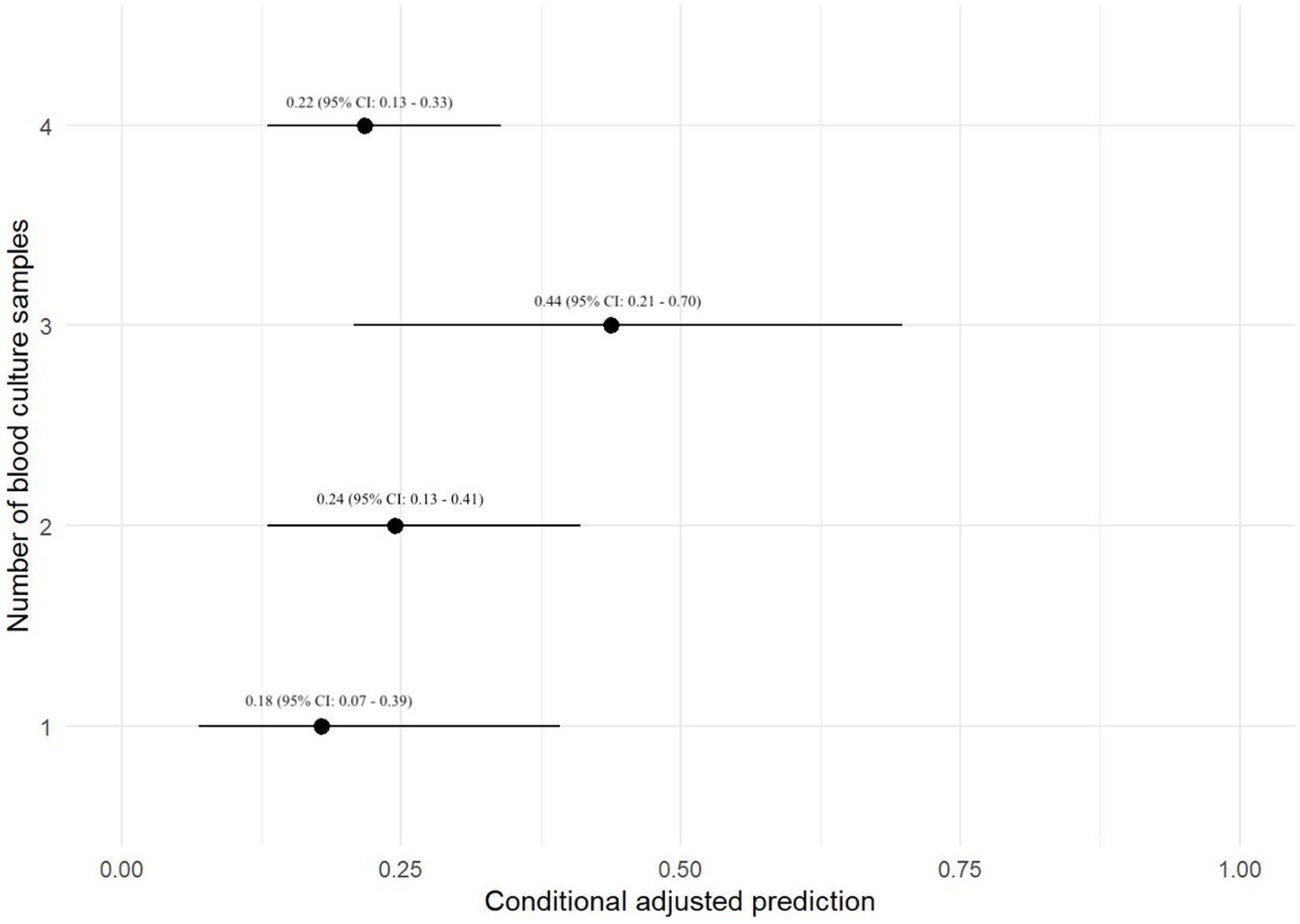
Figure 2. Prediction plot for odds of positive BC by numbers of BCs performed. This figure demonstrates a statistically modeled predictive value for BC positivity when compared to the number of cultures performed in each case. The black circle represents the predicted value, while the black line represents the 95% confidence interval.
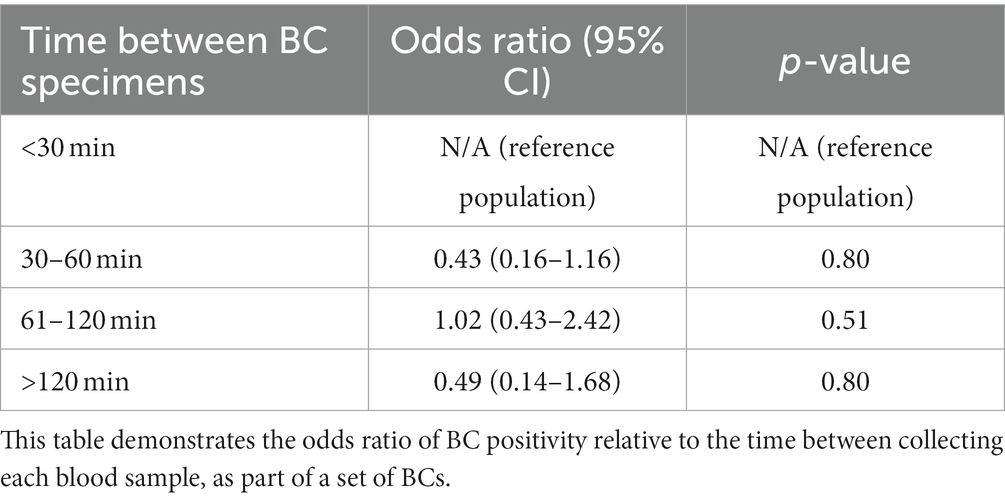
Three hundred and thirty-nine (48.4%) dogs had each of their BC specimens collected <30 min apart, 89 (12.7%) dogs had BCs collected 30–60 min apart, 90 (12.8%) dogs had BCs collected 61–120 min apart, and 38 (5.4%) dogs had BCs collected >120 min apart. The duration between BC collections were unknown in 56 (8.0%) dogs. The remaining 89 dogs (12.7%) only had a 1-specimen BC performed. The maximum duration between specimens was 8 h (n = 2). All other specimens were collected within 2–4 h of each other. There was no association between the timing between BC specimens and the odds of a positive BC (Table 3).
A prediction plot was also generated between timing between BC specimens and odds of a positive BC result (Figure 3). No significant results were found.
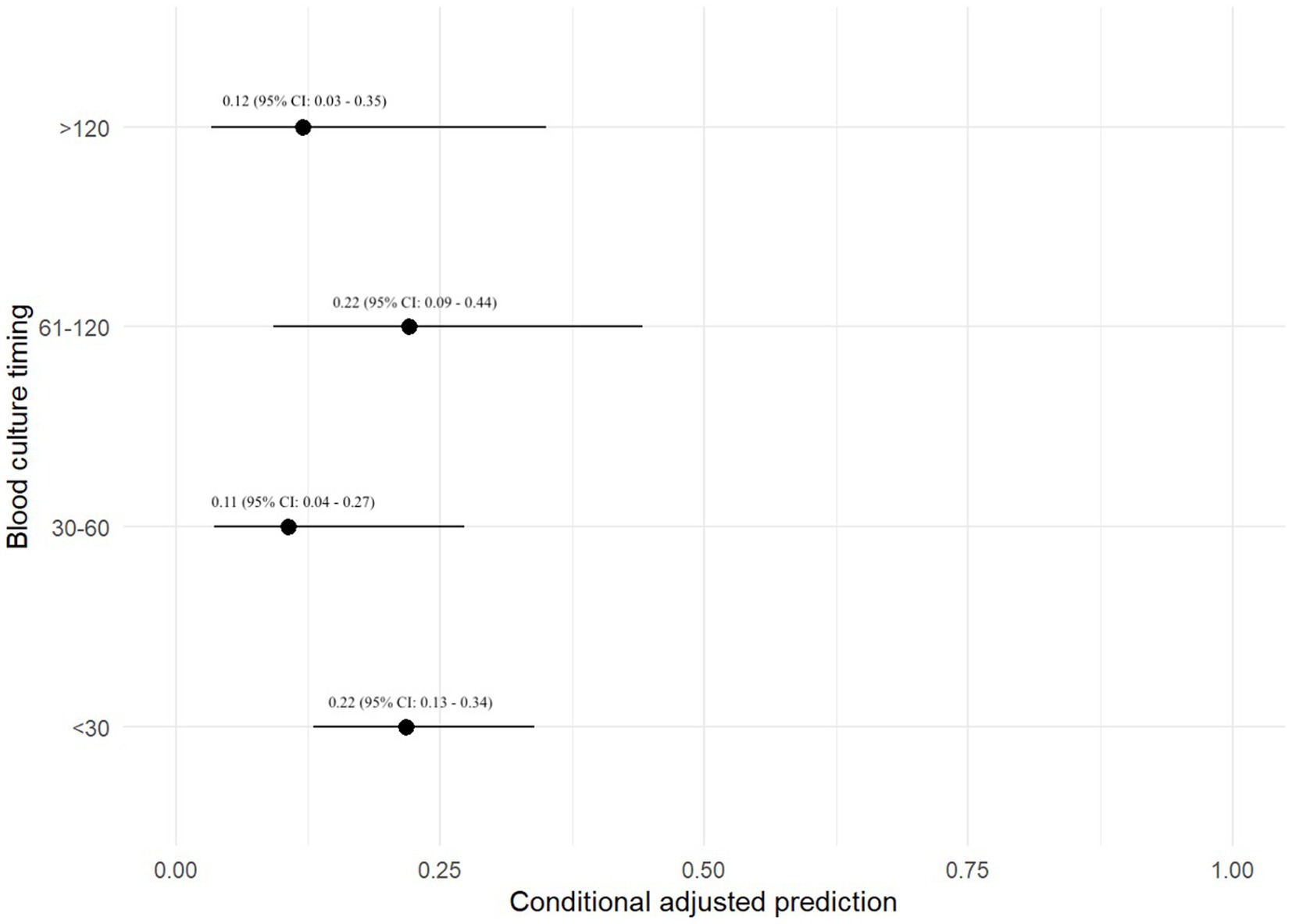
Figure 3. Prediction plot for odds of positive BC by BC timing. This figure demonstrates a statistically modeled predictive value for BC positivity when comparing the timespan between each blood sample as part of a set of BCs. The black circle represents the predicted value, while the black line represents the 95% confidence interval.
3.6 Associations between BC and urine culture results
Four hundred and ten dogs (58.5%) had a urine culture performed, 39 (9.5%) of which were positive (i.e., bacteria were cultured). Dogs that had a positive urine culture (N = 39) were significantly more likely to have a positive BC result than those that had no growth on a urine culture (OR: 4.36, 95% CI: 2.12–8.97, p = 0.003). Of the 701 dogs in the study, 28 had bacterial growth on both blood and urine cultures. Of those, isolates from 21/28 (75%) dogs were the same species of pathogen on both blood and urine culture. This is further summarized in Table 4.
4 Discussion
This study evaluated factors associated with positive BC results in 701 dogs. The top 3 most common bacterial species identified were Staphylococcus pseudintermedius, Escherichia coli, and beta-hemolytic Streptococcus species. There was not a statistically significant relationship between primary disease process and odds of test positivity when utilizing a mixed-effect logistic regression analysis. There was also no statistically significant relationship between the number of BC specimens and odds of BC positivity when utilizing a mixed-effect logistic regression analysis; however, test positivity approached statistical significance when comparing dogs with a 3-specimen BC versus those with a 1-specimen BC. Additionally, the adjusted prediction plot of test positivity in dogs with a 3-specimen BC was 0.44, which was 1.8 times higher than the next closest group at 0.24 (2-specimen BC group). While we suspect that a 3-specimen BC protocol may balance cost and diagnostic yield, this hypothesis would need to be verified in large-scale prospective study. It is unclear why 4 BCs had a lower adjusted prediction for test positivity. In human medicine the number of BCs has been shown to influence the ability to detect bacteremia. Two BCs within 24 h are reported to detect ~90% of bloodstream infections in humans, whereas up to 4 BCs may be required to increase the detection rate to >99% (17). Single BCs have also been shown to be inferior to paired BCs for detection of bacterial pathogens in another study (18). Performing more than one BC is also of value when determining whether a bacterial isolate could be a contaminant. The timespan between each specimen collection as part of a set of BCs was also not significantly associated with BC positivity, and no major findings were detected utilizing predictive plots. The timespan between specimens in a set of BCs is therefore potentially of reduced importance, when compared to other variables such as number of blood specimens in a BC series.
Clinicians may also try to utilize case data to predict the diagnostic yield of BCs. Dogs with a positive urine culture had an increased odds of having a positive BC. In this study, isolates 21 of 28 dogs with concurrent growth on both blood and urine cultures were the same species of pathogen. This supports the findings of a previous study, in which 4 out of 5 dogs with a positive BC had a concurrently positive urine culture (19). However, it is important to note that some studies reveal that urine cultures have a low sensitivity for detection of concurrent bacteremia and a negative urine culture should not preclude a clinician from performing BCs (9). A reverse association may also be of clinical interest, but could not be evaluated in this study due to study design. Further investigation in the reverse direction is indicated in future studies.
In our study, pre-existing antimicrobial therapy did not have a significant effect on BC positivity, reinforcing that prior antimicrobial therapy should not preclude a clinician from performing BCs in a dog where bacteremia is of high clinical suspicion. Previous studies have also demonstrated that pre-existing antimicrobial therapy does not result in a lower percentage of positive BCs in dogs, and 32% of cats with positive BCs had been pre-treated with antimicrobials (20–22). This may be because bacteriostatic antimicrobials slow but fail to eliminate, bacterial growth in blood specimens (23). Antimicrobial resistance, dilution of antimicrobials to sub-therapeutic concentrations within the blood specimen, or clinician sampling biases may also be proposed explanations (20). It is also important to note that several antibiotics were used extra-label (“off label”) in this study including cefovecin. Extra-label drug use is very common in veterinary medicine in the United States as few drugs have been approved by the Federal Drug Administration for use in animals and for the broad range of medical conditions in which the medications are commonly used. The results of this study may however be impacted by population size and this should be considered when interpreting the results. In humans large scale studies have shown that collection of BCs after antimicrobial administration results in false negative BC results (24). The surviving sepsis campaign suggests that BCs should be obtained prior to starting antimicrobial therapy in humans with sepsis unless it results in a substantial delay in the start of antimicrobials (≥ 45 min) (25). Since specimen timing seemed less impactful in this study, the ability to collect cultures quickly (e.g., in less than 30 min) may facilitate collection prior to antimicrobial therapy and avoid delays in treatment for patients where sepsis is suspected. However future prospective studies are needed to confirm or refute this hypothesis. Immunosuppressive therapy had no effect on culture positivity in this study, although this may have been influenced by sample size and the different therapeutic regimes utilized.
The prevalence of dogs with positive BCs (22%) was similar to prior studies, and ~ 70% of isolates had resistance to at least one antimicrobial class. Although all institutions in our study were tertiary referral facilities, which likely impacted this finding, the high number of dogs with organisms resistant to greater than one antimicrobial reinforce the high number of dogs developing multi-drug resistant infections.
This study was limited by its retrospective nature, which inherently includes non-standardized diagnostic and therapeutic approaches. Although some institutions had a standard operating procedure for BCs in dogs, others did not, and it is impossible to determine the degree of compliance to such protocols retrospectively. Therefore, data such as specimen blood volume was not able to be accurately investigated in this study. In humans standard-volume (8.7 mL) BCs have a higher detection rate for bloodstream infection than low-volume (2.7 mL) cultures (26). This requires further investigation in dogs, and blood specimen volume may have influenced the results of this study. We recommend that blood volume is recorded on future BC results to assist in future studies and to act as a quality control. It is possible that sampling bias may have been inadvertently introduced by clinicians when determining BC protocols and this could influence the results of this study. Urine culture techniques were not standardized given the retrospective nature of the study and this could impact the results of the study. Efforts were made during study design and statistical methods to account for confounding variables, but it is possible that they exist and could influence the results of this study. Additionally the study population described here is focused on academic/tertiary referral centers and this may influence the results of the study. There is no standardized criteria for determination of blood culture contaminants in veterinary medicine, and as such our study design mimicked clinical practice by relying on interpretation by the laboratory or a board-certified microbiologist. Development and validation of such criteria would be of significant value but were outside the scope of this manuscript. Statistically non-significant results from this study should be interpreted with caution.
Importantly, this study assessed sampling protocols an BC results for a large population of dogs across multiple U.S. institutions. No significant relationship was found between the number or sample timing of BCs and culture positivity, although a positive urine culture was predictive of a positive BC. Future studies should seek to clarify the optimal specimen volumes and prospectively confirm the number of specimens to optimize BC cost effectiveness.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
Ethical approval was not required for the studies involving animals in accordance with the local legislation and institutional requirements because not required – retrospective study. No patients or clients are identifiable. Written informed consent was not obtained from the owners for the participation of their animals in this study because not required – retrospective study. No patients or clients are identifiable.
Author contributions
AO: Conceptualization, Formal analysis, Investigation, Methodology, Writing – original draft, Writing – review & editing, Data curation. RM: Conceptualization, Data curation, Formal analysis, Investigation, Methodology, Writing – original draft, Writing – review & editing, Supervision. SSc: Conceptualization, Data curation, Formal analysis, Investigation, Methodology, Writing – original draft, Writing – review & editing. EG: Conceptualization, Data curation, Formal analysis, Investigation, Methodology, Writing – original draft, Writing – review & editing. CF: Conceptualization, Data curation, Formal analysis, Investigation, Methodology, Writing – original draft, Writing – review & editing. ID: Conceptualization, Data curation, Formal analysis, Investigation, Methodology, Writing – original draft, Writing – review & editing. SSh: Conceptualization, Data curation, Formal analysis, Investigation, Methodology, Writing – original draft, Writing – review & editing. JH: Conceptualization, Data curation, Formal analysis, Investigation, Methodology, Writing – original draft, Writing – review & editing. TB: Conceptualization, Data curation, Formal analysis, Investigation, Methodology, Writing – original draft, Writing – review & editing. SJ: Conceptualization, Data curation, Formal analysis, Investigation, Methodology, Writing – original draft, Writing – review & editing. NJ: Conceptualization, Formal analysis, Investigation, Methodology, Writing – original draft, Writing – review & editing, Software. HC: Formal analysis, Investigation, Methodology, Writing – original draft, Writing – review & editing, Conceptualization, Supervision.
Funding
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. The open access fee this manuscript was provided by endowments established under the Peter J. and Freda M. Babich fund.
Acknowledgments
The authors would like to thank the microbiology and diagnostic laboratory staff at each institution. The authors would also like to thank Tayna Basqueroto for assistance in review of antimicrobial susceptibility results.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fvets.2023.1301018/full#supplementary-material
References
1. King, L . Postoperative complications and prognostic indicators in dogs and cats with septic peritonitis: 23 cases (1989–1992). J Am Vet Med Assoc. (1994) 204:407–14.
2. Hauptman, J , Walshaw, R , and Olivier, N . Evaluation of the sensitivity and specificity of diagnostic criteria for sepsis in dogs. Vet Surg. (1997) 26:393–7. doi: 10.1111/j.1532-950X.1997.tb01699.x
3. de Laforcade, A , Freeman, L , Shaw, S , Brooks, MB , Rozanski, EA , and Rush, JE . Hemostatic changes in dogs with naturally occurring sepsis. J Vet Intern Med. (2003) 17:674–9. doi: 10.1111/j.1939-1676.2003.tb02499.x
4. Ulrich, S , Gottschalk, C , Straubinger, R , Schwaiger, K , and Dörfelt, R . Acceleration of the identification of sepsis-inducing bacteria in cultures of dog and cat blood. J Small Anim Pract. (2020) 61:42–5. doi: 10.1111/jsap.13056
5. da Silveira, M , Cândido, SL , dos Santos, K , Maia, MO , Souza, RL , Sousa, VRF, et al. Polymerase chain reaction and blood culture for diagnosis of canine sepsis. Cienc Rural. (2018) 48:e20170871. doi: 10.1590/0103-8478cr20170871
6. Heilmann, R , Xenoulis, P , Barr, JW , Dowd, SE , Lawhon, SD , Suchodolski, JS, et al. Comparison of PCR and conventional blood culture to analyze blood from dogs with suspected sepsis. Vet J. (2013) 198:714–6. doi: 10.1016/j.tvjl.2013.10.001
7. Saarenkari, HK , Sharp, CR , and Smart, L . Retrospective evaluation of the utility of blood cultures in dogs (2009–2018): 45 cases. J Vet Emerg Crit Care (San Antonio). (2022) 32:141–5. doi: 10.1111/vec.13144
8. Reagan, KL , Visser, LC , Epstein, SE , Stern, JA , and Johnson, LR . Outcome and prognostic factors in infective endocarditis in dogs: 113 cases (2005–2020). J Vet Intern Med. (2022) 36:429–40. doi: 10.1111/jvim.16380
9. Barash, N , Birkenheuer, A , Vaden, S , and Jacob, ME . Agreement between parallel canine blood and urine cultures: is urine culture the poor man’s blood culture? J Clin Microbiol. (2018) 56:e00506–18. doi: 10.1128/JCM.00506-18
10. Mirrett, S , Hanson, KE , and Reller, LB . Controlled clinical comparison of VersaTREK and BacT/ALERT blood culture systems. J Clin Microbiol. (2007) 45:299–302. doi: 10.1128/JCM.01697-06
11. Methods for antimicrobial susceptibility testing of infrequently isolated fastidious bacteria. 3rd ed. Malvern, PA: CLSI document M45: Clinical and Laboratory Standards Institute. (2015).
12. Performance standards for antimicrobial disk and dilution susceptibility testing. 32nd ed. Malvern, PA: CLSI document M100: Clinical and Laboratory Standards Institute. (2023).
13. Understanding susceptibility test data as a component of antimicrobial stewardship in veterinary setting. 1st ed. Malvern, PA: CLSI document VET09: Clinical and Laboratory Standards Institute. (2019).
14. Methods for antimicrobial susceptibility testing of infrequently isolated fastidious bacteria from animals. 1st ed. Malvern, PA: CLSI supplement VET06: Clinical and Laboratory Standards Institute. (2017).
15. Performance standards for antimicrobial disk and dilution susceptibility tests for bacteria isolated from animals. 4th ed. Malvern, PA: CLSI supplement VET08: Clinical and Laboratory Standards Institute. (2018).
16. Performance standards for antimicrobial disk and dilution susceptibility tests for bacteria isolated from animals. 5th ed. Malvern, PA: CLSI supplement VET01S: Clinical and Laboratory Standards Institute. (2018).
17. Lee, A , Mirrett, S , Reller, LB , and Weinstein, MP . Detection of bloodstream infections in adults: how many blood cultures are needed? J Clin Microbiol. (2007) 45:3546–8. doi: 10.1128/JCM.01555-07
18. Tarai, B , Jain, D , das, P , and Budhiraja, S . Paired blood cultures increase the sensitivity for detecting pathogens in both inpatients and outpatients. Eur J Clin Microbiol Infect Dis. (2018) 37:435–41. doi: 10.1007/s10096-018-3188-8
19. Perry, KM , Lynch, AM , Caudill, A , Vigani, A , Roberston, JB , and Vaden, S . Clinical features, outcome, and illness severity scoring in 32 dogs with urosepsis (2017–2018). J Vet Emerg Crit Care (San Antonio). (2022) 32:236–42. doi: 10.1111/vec.13158
20. Dow, S , Curtis, C , and Jones, R . Bacterial culture of blood from critically ill dogs and cats: 100 cases (1985–1987). J Am Vet Med Assoc. (1989) 195:113–7.
21. Greiner, M , Wolf, G , and Hartmann, K . Bacteraemia in 66 cats and antimicrobial susceptibility of the isolates (1995–2004). J Feline Med Surg. (2007) 9:404–10. doi: 10.1016/j.jfms.2007.04.004
22. Calvert, CA . Valvular bacterial endocarditis in the dog. J Am Vet Med Assoc. (1982) 180:1080–4.
23. Reller, L , Murray, P , and MacLowry, J . Blood cultures II In: IA C , editor. American society for microbiology. Washington. (1982). 1–30.
24. Cheng, M , Stenstrom, R , Paquette, K , Stabler, SN , Akhter, M , Davidson, AC, et al. Blood culture results before and after antimicrobial administration in patients with severe manifestations of sepsis: a diagnostic study. Ann Intern Med. (2019) 171:547–54. doi: 10.7326/M19-1696
25. Evans, L , Rhodes, A , Alhazzani, W , Antonelli, M , Coopersmith, CM , French, C, et al. Surviving sepsis campaign: international guidelines for management of sepsis and septic shock 2021. Intensive Care Med. (2021) 47:1181–247. doi: 10.1007/s00134-021-06506-y
Keywords: bacteria, fungi, septicemia, susceptibility, MIC
Citation: Ogrodny AJ, Mani R, Schmid SM, Gould EN, Fellman CL, DeStefano I, Shropshire S, Haines JM, Bolton TA, Jablonski SA, Jess N and Cridge H (2023) Multi-institutional retrospective study investigating blood culture protocols and test positivity in 701 dogs. Front. Vet. Sci. 10:1301018. doi: 10.3389/fvets.2023.1301018
Edited by:
Valentina Virginia Ebani, University of Pisa, ItalyReviewed by:
Fabrizio Bertelloni, University of Pisa, ItalyClaudia Pollera, University of Milan, Italy
Copyright © 2023 Ogrodny, Mani, Schmid, Gould, Fellman, DeStefano, Shropshire, Haines, Bolton, Jablonski, Jess and Cridge. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence:Harry Cridge, harry.cridge@gmail.com
 Andrzej J. Ogrodny1
Andrzej J. Ogrodny1  Timothy A. Bolton
Timothy A. Bolton Harry Cridge
Harry Cridge