Discriminant canonical analysis as a tool for genotype traceability testing based on turkey meat and carcass traits
- 1Department of Genetics, Faculty of Veterinary Sciences, University of Córdoba, Córdoba, Spain
- 2Agropecuary Provincial Centre, Diputación Provincial de Córdoba, Córdoba, Spain
- 3Department of Agriculture and Ecological Husbandry, Area of Agriculture and Environment, Andalusian Institute of Agricultural and Fisheries Research and Training (IFAPA), Alameda del Obispo, Córdoba, Spain
The present study aims to develop a statistical tool for turkey breed traceability testing based on meat and carcass quality characteristics. To this end, a comprehensive meta-analysis was performed, collecting data from a total of 75 studies approaching meat and carcass attributes of 37 turkey strains and landraces since the late 1960s. A total of 22 meat and carcass traits were considered variables, grouped in the following clusters: carcass dressing traits, muscle fiber properties, pH, colorimetry, water-capacity traits, texture-related attributes, and nutritional composition of the meat. Once the multicollinearity analysis allowed the deletion of redundant variables, cold carcass weight, slaughter weight, muscle fiber diameter, sex-female, carcass/piece weight, meat redness, ashes, pH24, meat lightness, moisture, fat, and water-holding capacity showed explanatory properties in the discriminating analysis (p < 0.05). In addition, strong positive and negative correlations were found among those variables studied. Carcass traits were positively associated, particularly slaughter weight and cold carcass weight (+0.561). Among meat physical traits, pH showed positive correlations with drip loss (+0.490) and pH24 (+0.327), and water-holding capacity was positively associated with cholesterol (+0.434) and negatively associated with collagen (−0.398). According to nutritional traits, fat and ash showed a strong correlation (+0.595), and both were negatively associated with moisture (−0.375 and −0.498, respectively). Strong negative correlations were found as well between meat protein and fat (−0.460) and between collagen and cholesterol (−0.654). Finally, the Mahalanobis distance suggested a clustering pattern based on meat and carcass characteristics that report information about interbreeding and variety proximity. This study establishes a departure point in the development of a tool for breed traceability guaranteeing aimed at enhancing distinguished, local breed-based turkey meat.
1 Introduction
The global meat industry has accomplished the goal of providing protein, and meat prices are currently at historic lows (1). Poultry emerges as the meat type with the most efficient production among the current livestock in terms of the use of resources and supply of protein (2), and turkey emerges second in terms of the largest contributor to global poultry meat production (3). Turkey has undergone great selection pressure to target desirable traits such as fast growth and high slaughter weight, which has led to a doubling of its production between 1970 and 2008 (3). However, its negative impact on meat quality (4–6), together with a bad image of their low-sustainable housing systems and welfare conditions, has led to the apparition of alternative, free-range systems using slow-growing strains (1). Provided that the loss of meat quality originates from the growth curve and its physiology, slow-growing strains seem to offer a differential quality product (4). These unconventional systems arise to meet the demand for higher quality meat production that is both sustainable and ethical (1, 7, 8). This trend is exemplified by the ‘Traditional Farmfresh Turkey’ labeling developed in the United Kingdom to distinguish traditional farming turkey meat from commercial products (9).
Those unconventional systems are mainly based on light, slow-growing landraces. The most widespread genotypes involved are heritage breeds such as the Royal Palm (10), Narragansett (11), or Beltsville Small White (12), and other light hybrids from the commercial industry (13, 14). However, those alternative systems represent a great growth opportunity for indigenous, locally adapted genotypes (15). In this respect, native turkeys are more suitable for free-ranging breeding than industrial strains. In harsh and disease-prone environments, a lack of performance and adaptation has been reported in imported genotypes (2). On the other hand, local breeds offer great heat tolerance and immunological competence (16) while preserving ancestral behaviors such as constant food-seeking or anti-predator conduct (16–20).
In addition to their greater suitability for alternative systems, indigenous genotypes are preferred to industrial products by both rural and urban consumers (16, 21). For example, in China, native chicken is preferred to standard broilers because of its better taste and traits, which are well adapted to Chinese cuisine (22). A similar case is found in Italy, where the carcasses from Italian local turkey breeds suit the traditional Italian cuisine (23). These products represent distinguished, gourmet items that are usually associated with specific events, such as traditional festivities (24). In the United States, the consumption of their ‘heritage’ turkey breeds is linked with Thanksgiving, a national holiday (24). In Mexico, the indigenous domestic genotype known as ‘Guajolote’ is highly valued as a ceremonial food, consumed during family festivities, mainly in December (20). In this respect, enhancing the consumption of local breeds’ products could be a crucial strategy to preserve them (25). Most of the native poultry genotypes are threatened due to the rise of highly selected strains in the 20th century, via displacement and genetic erosion (2, 26). This is evidenced by the fact that 6.59% of turkey breeds have undergone extinction, and the status of 70.32% of the populations is still unknown (27). Furthermore, there are no data available about the endangered level of turkey populations worldwide except for some populations from Europe and North America, which are shown in Figure 1.
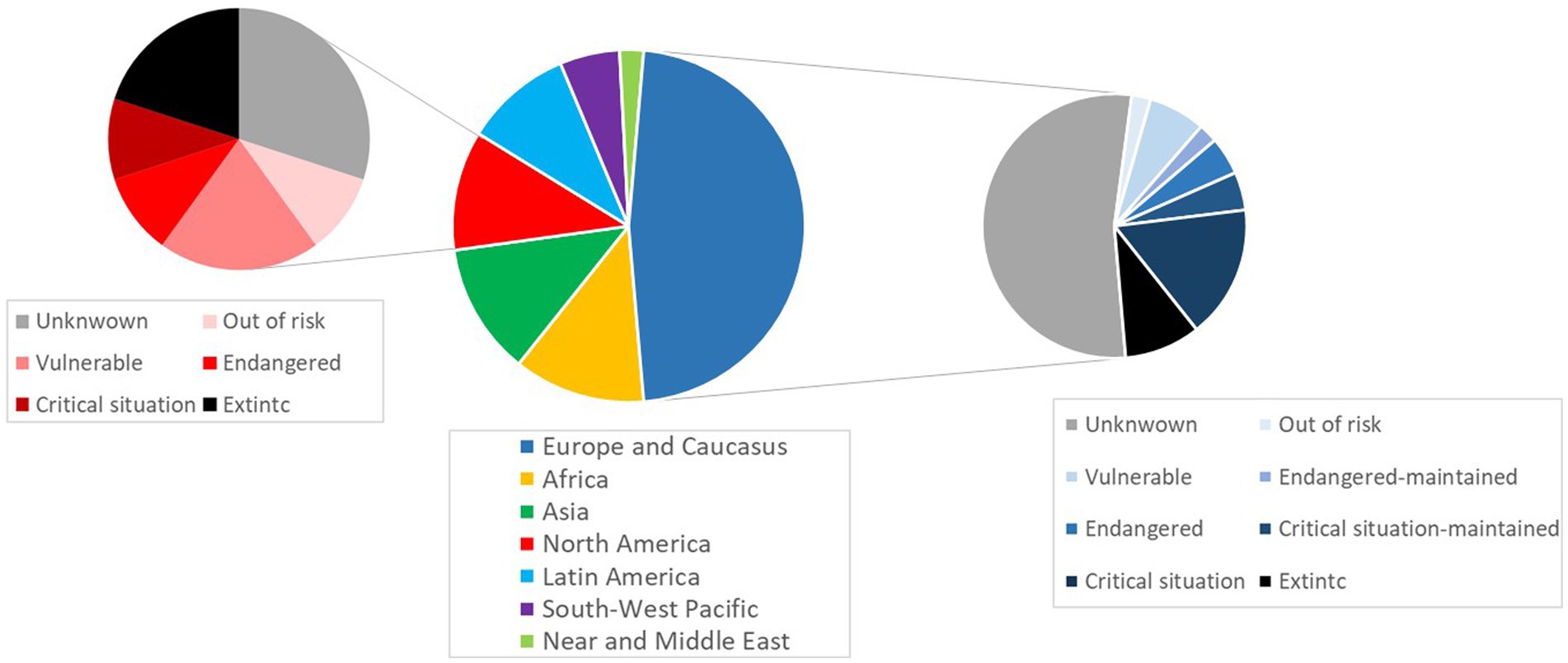
Figure 1. Distribution of worldwide turkey breeds and population status in North America and Europe. Source: Domestic Animal Diversity Information System (DAD-IS) (9).
However, due to the use of higher land proportions and the lower feed efficiency of these alternative systems, those high-quality products, derived from local breeds, must command higher prices to achieve profitability (1). In addition, informed and conscious consumers must be willing to pay the extra price that these premium products, obtained from local breeds, are worth (16, 21, 22). In this respect, breed traceability is a crucial issue for ensuring the origin of traditional and regional foods (28). For example, when marketing carcasses and cuts from local breeds, fraud could occur through crossbreeding or using breeds other than those specified (29). Attempting to define a breed traceability tool, genomic and proteomic approaches have been proposed. However, its technical complexity and high price make molecular traceability unviable for small and low-income productions (28), in which native breeds are usually reared. On the other hand, when fewer resources are available, the status of a native genotype can be approached through phenotypes, since phenotypic traits are a direct consequence of the genotype (24). This approach has been followed in studies comparing meat and carcass quality attributes between turkey breeds and varieties (4–8, 13, 30, 31) and their crossbreeds (32–34).
A recent meta-analysis has proved that meat and carcass quality traits stand as explanatory variables in a discriminant canonical analysis (DCA) to describe clustering patterns among turkey genotypes (35). Authors suggested that, from the cornerstone established in the study, the discriminant function cross-validation analysis could be implemented as a breed traceability tool, which could mean great industrial applications. However, the study included just a few well-differentiated populations and did not deepen the correlation among traits. Describing the correlation of traits is crucial to increasing the efficiency of resource allocation in the method since the reduction of measures is possible by selecting highly explanatory and correlated variables. Thus, correlations between the most common meat and carcass traits have been reported in several poultry species (4, 36–38).
Hence, the present study aims to develop a statistical tool to perform a breed traceability test based on meat and carcass quality attributes. To achieve this goal, a study of the traits acting as differentiating patterns across turkey landraces and varieties within genotypes, together with their correlations, is conducted. Hence, the present study might present a discriminant canonical feature for breed traceability of traditional meat products based on local breeds, as well as to detect breed introgression and hybridization of those genotypes. This tool could be particularly useful for indigenous genotypes and could be employed as a guarantee of their distinguished, breed-based products. Due to the low cost and simplicity of these phenotypical measurements by selecting highly explanatory traits correlated with other significant ones, this tool is particularly suited to low-income and scarce resource systems that are characteristics of local breeds.
2 Materials and methods
2.1 Decision of the systematic review approach
Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines were designed for the healthcare field and do not fit properly in livestock research (39, 40). Furthermore, strict adherence to the PRISMA guidelines failed to detect any alterations in the journal’s level of recommendation and endorsement (41) and exhibited restricted suitability in the context of reviews concerning conservation and environmental management. Instead, the methodology selected in the present study was that described by McLean and Navas Gonzalez (42), which has been reported as an efficient methodology in previous studies (18, 39, 43).
2.2 Data collection
Data collection was executed as previously reported in the literature (18, 39, 43). To this end, the repositories www.scholar.google.es and www.sciencedirect.com (accessed on November 2021) were employed. Other platforms not including data extraction tools for analysis, such as www.ncbi.nlm.gov/pubmed or www.webofscience.com/wos/woscc/basic-search, were excluded, as suggested in the bibliography (18, 43). Restricted documents were accessed through the library service of the University of Córdoba (Córdoba, Spain). Keywords included in the document collection were “meat/carcass quality” and “meat/carcass traits” followed by “turkey”, “Meleagris gallopavo”, and other semantically related terms (18, 44). A total of 75 documents published in English from 1968 to 2021 were found and included in this research.
Documents were recorded in a database where observations were individually registered considering the turkey landrace. In this respect, a total of 37 varieties comprising 9 turkey landraces and well-established populations were obtained and are shown in Table 1. Table 1 also shows the reference from which each variety was collected. The carcass cuts used in the present study were the following: carcass reminder, breast, complete leg, thigh, drumstick, wings, head, neck, feet, shank, back, heart, liver, giblets, kidney, lungs, spleen, pancreas, gallbladder, proventriculus, gizzard (full and empty), stomach, complete intestine, small intestine, cecum, abdominal fat, fat pad, ovary, oviduct, feathers, skin, feather plus skin, blood, and waste. Moreover, the meat and carcass quality traits included in the analysis were as follows: carcass/piece weight, carcass/piece yield, cold carcass weight, slaughter weight, muscle fiber diameter, pH, pH 24 h, L* meat, a* meat, b* meat, drip loss, water-holding capacity, cooking loss, shear force, springiness, fragmentation index, moisture, protein, fat, ash, collagen, and cholesterol. To avoid possible errors when encountering different units of measurement in literature, all units were converted to the most frequently found units across papers. Specific methodologies used in every study for variable determination were not registered as the analysis methods employed were standardized to be accepted in research procedures. This determination is based on the fact that, although distinct techniques may cause differences, as they are standardized methods, these differences may be negligible (18).
2.3 Data analysis
2.3.1 Normality and Bayesian ANOVA tests
To discard alterations of the normality assumption, the Shapiro–Francia W’ test was performed. This test was chosen because of the number of observations collected, which ranged from 50 to 2,500. After obtaining both normally and non-normally distributed variables, a Bayesian ANOVA was used to analyze differences between turkey varieties and strains. The results from the Bayesian ANOVA test reported medians to significantly differ in the majority of possibilities. However, the following variables did not report significant differences: pH (F = 0.747, pv = 0.674), a* meat (F = 0.388, pv = 0.960), b* meat (F = 1.718, pv = 0.101), drip loss (F = 0.487, pv = 0.848), cooking loss (F = 1.849, pv = 0.083), shear force (F = 0.248, pv = 0.983), and collagen (F = 35.764, pv = 0.105). Therefore, the presence of differences in these variables across the turkey varieties justified the implementation of a DCA.
2.3.2 Multicollinearity preliminary testing
To discard linear relationships across predictors and guarantee the variable’s independence, the multicollinearity analyses were run before the statistical analyses per se. The objective of these analyses were to detect noise or redundancy issues in the variables before data manipulation, as the exclusion of unnecessary variables avoids a possible overinflation of the variance’s explanatory potential (39). The variance inflation factor (VIF) is employed as an indicator of multicollinearity, and values above 5 are not recommended (116). VIF is calculated with the following formula:
where R2 represents the coefficient of determination of the regression equation and tolerance (1 – R2) reflects the degree of variability in a specific independent variable that is not explained by the rest, whose recommended values are under 0.20 (117). To perform the multicollinearity test, the multicollinearity statistics routine of the describing data package of XLSTAT software (Addinsoft Pearson Edition 2014, Addinsoft, Paris, France) was employed.
2.3.3 Discriminant canonical analysis
To perform the statistical analysis, turkey landraces and varieties were considered the independent variables to perform the DCA, and the 22 meat and carcass parameters mentioned before were employed as explanatory dependent variables. For each carcass or carcass piece analyzed, the sex of the turkey breed from which it was obtained was included and used as a labeling classification criteria to determine the variability of quality-associated attributes between and within classification clusters and to establish, identify, and outline groupings (118).
A series of discriminant functions were obtained from the statistical analysis and enabled the definition of the clustering patterns described by the sample through a linear combination of meat and carcass quality-related attributes. For the selection of variables, regularized forward stepwise multinomial logistic regression algorithms were used (15). Instead of considering group sizes to be equal, priors were regularized following the group sizes computed from the prior probability option in SPSS version 26.0 software (IBM, Armonk, NY, United States), which prevents groups with different sample sizes from influencing classification quality (119).
2.3.4 DCA efficiency and analysis model reliability
To determine variables significantly contributing to the discriminant function, Wilks’ lambda test was used, as described by González Ariza (118). Values under 0.05 or Wilks’ lambda values can be accepted (120), even though ideal values tend to 0.
For evaluating the assumption of equal covariance matrices in cases of unequal sample sizes, Pillai’s trace criterion is the only acceptable test (121) and was run using the multivariate routine of the general linear model package of the software SPSS, version 26.0. Statistical differences in the dependent variables across the levels of independent variables are suggested to be accepted when significance is under 0.05 (118).
2.3.5 Correlation matrix
A correlation matrix among meat and carcass attributes obtained from the DCA was depicted in a graphical representation. To achieve this goal, a heat map was built through the web server Heatmapper (accessed on 18th April 2023).1 This analysis provides insights into what meat and carcass quality-related traits show a higher correlation between them. With these insights, the knowledge generated in the correlation matrix will allow an optimization of the data collection process.
2.3.6 Variable dimensionality reduction
Overall, variables were narrowed down to the few significant variables that most contributed to the different variations in the different types of birds, which was done through a preliminary principal component analysis (PCA) according to the bibliography (118).
2.3.7 Canonical coefficients and loading interpretation and spatial representation
A discriminant function analysis was employed to determine the degree of assignment of a carcass or a primary cut within its group (which was defined by the turkey variety). Hence, variables exhibiting a discriminant loading of ≥|0.40| were considered to be substantially discriminant, according to the literature (118). In this respect, the discriminant ability was determined considering the absolute coefficients for each particular variable within a series (122). Consecutively, the squared Mahalanobis distance were calculated following this formula:
where D2ij represents the distance between population i and j; Ȳi and Ȳj represent the means of variable x in the ith and jth populations, respectively; and COV−1 represents the inverse of the covariance matrix of measured variable x (120).
The clustering patterns of the observations were visually represented through the squared Mahalanobis distance, which can be defined by the differences in the values for the quality attributes of meat and carcass across the potential classification. Thus, a dendrogram exhibiting the possible clusters within turkey varieties was made using the underweighted pair-group method arithmetic averages (UPGMA) from the Universität Rovira i Virgili (URV), Tarragona, Spain and the phylogeny procedure of MEGA X 10.0.5 (Institute of Molecular Evolutionary Genetics, The Pennsylvania State University, State College, PA, United States).
2.3.8 Discriminant function cross-validation
The leave-one-out cross-validation was used to validate the discriminant function used, aiming for at least 25% higher accuracy than that obtained by chance (118). The Press’ Q significance test was employed to compare the discriminating power using the following formula:
where N represents the number of observations of the sample; n represents the number of correctly classified observations; and K represents the number of groups (variety, in this case). To ensure Press’ Q statistic’s value is significantly better than chance, it was compared with the critical value of 6.64 for χ2 with one degree of freedom with a significance of 0.01 (118).
3 Results
3.1 Reliability of the canonical discriminant analysis model
No multicollinearity issues were reported in the preliminary analysis as all variables exhibited VIF values under 5. Hence, all variables were included in the further analysis of the present study.
Pillai’s trace criterion described significant differences in carcass and meat quality-related traits across turkey varieties and strains (p < 0.05). The values of the Pillai’s trace criterion and the Wilks’ lambda test are shown in Table 2.
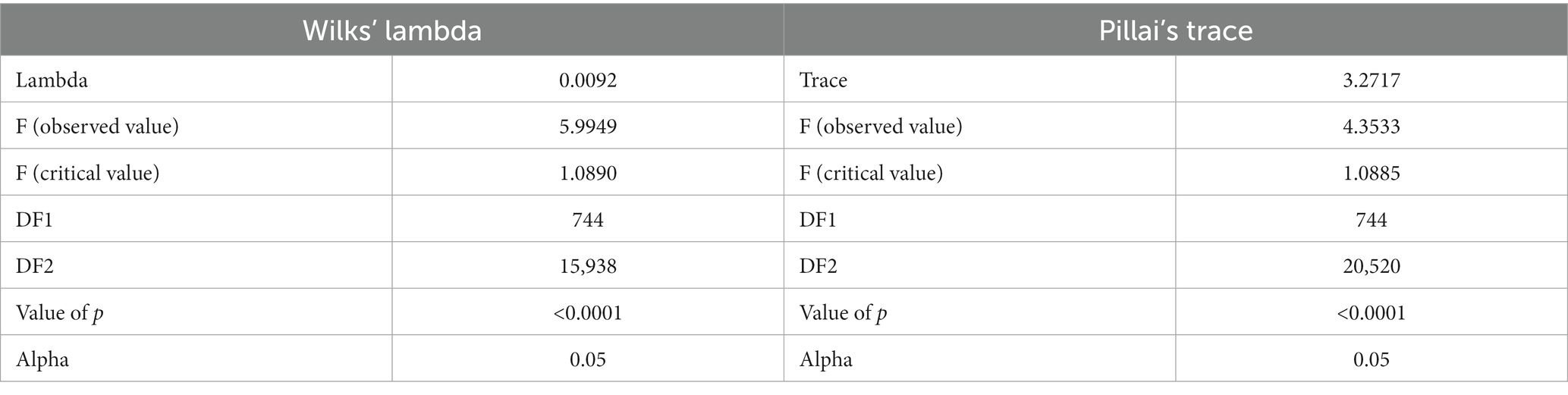
Table 2. Summary of the results of Wilks’ lambda test and Pillai’s trace of equality of covariance matrices of canonical discriminant functions.
3.2 Canonical coefficients, loading interpretation, and spatial representation
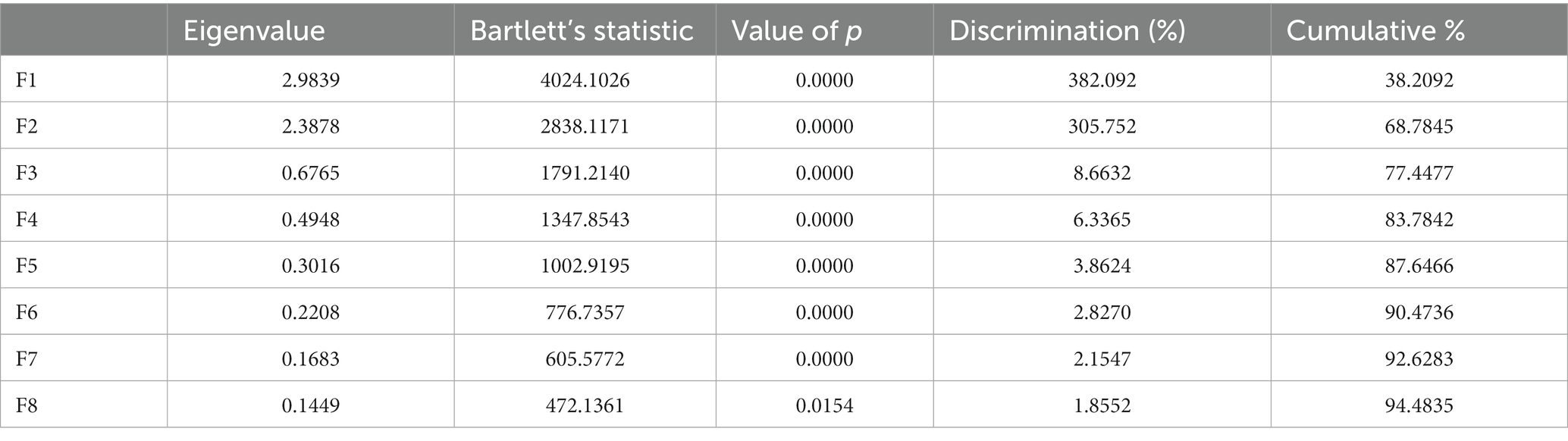
A total of 24 discriminating canonical functions compounded the discriminant analysis. The first eight functions (F1, F2, F3, F4, F5. F6, F7, and F8) were significantly discriminant and contributed 94.48% to the whole variance explanation, as shown in Table 3. By contrast, the rest of the functions were not significantly discriminant (p < 0.05).
Meat and carcass quality-related traits were ranked according to their discriminant capacity through the test of equality of group means (Table 4). Positions in the rank were assigned. A lower value for Wilks’ lambda and a higher value of F indicate the greatest discriminant ability (118).
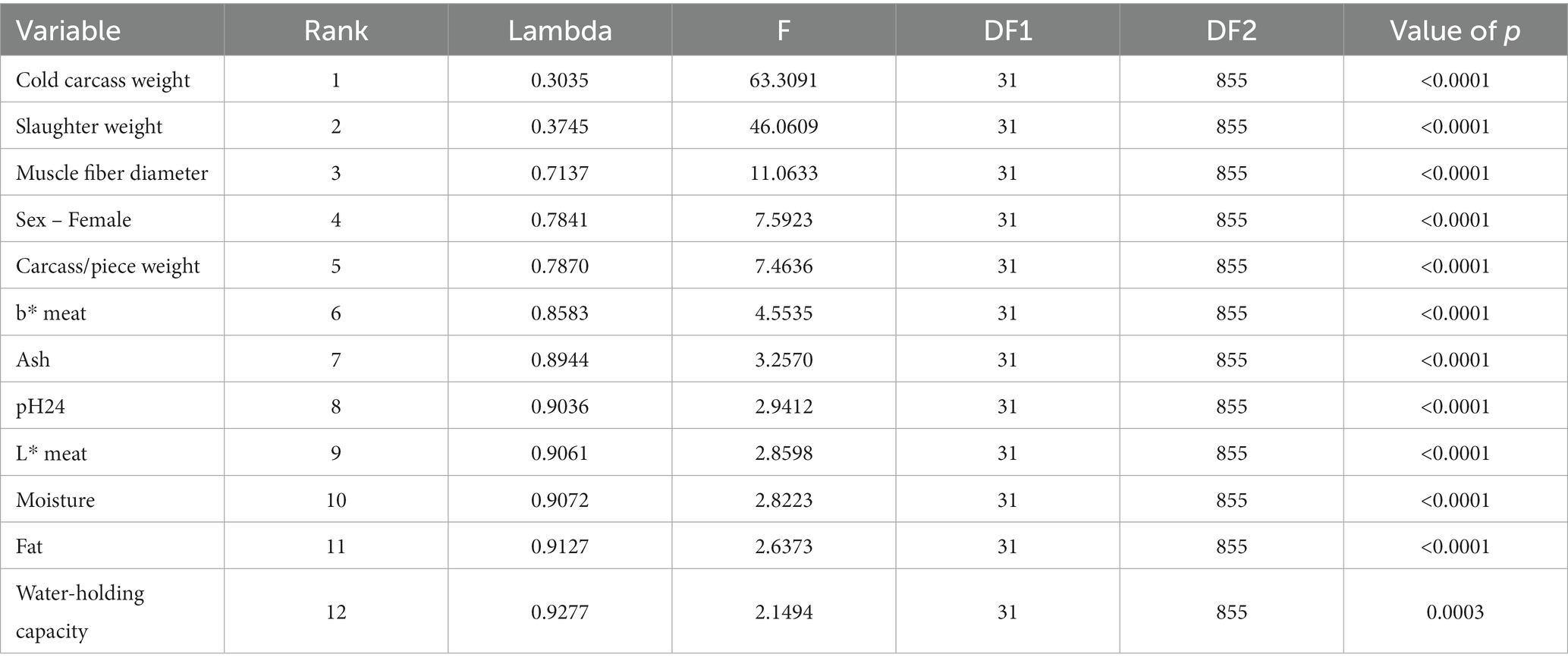
Table 4. The results for the tests of equality of group means to test for difference in the means across meat and carcass quality traits groups once non-significant variables have been removed.
The correlation matrix values among meat and carcass attributes ranged from +0.595 to −0.654 and are represented in Figure 2.
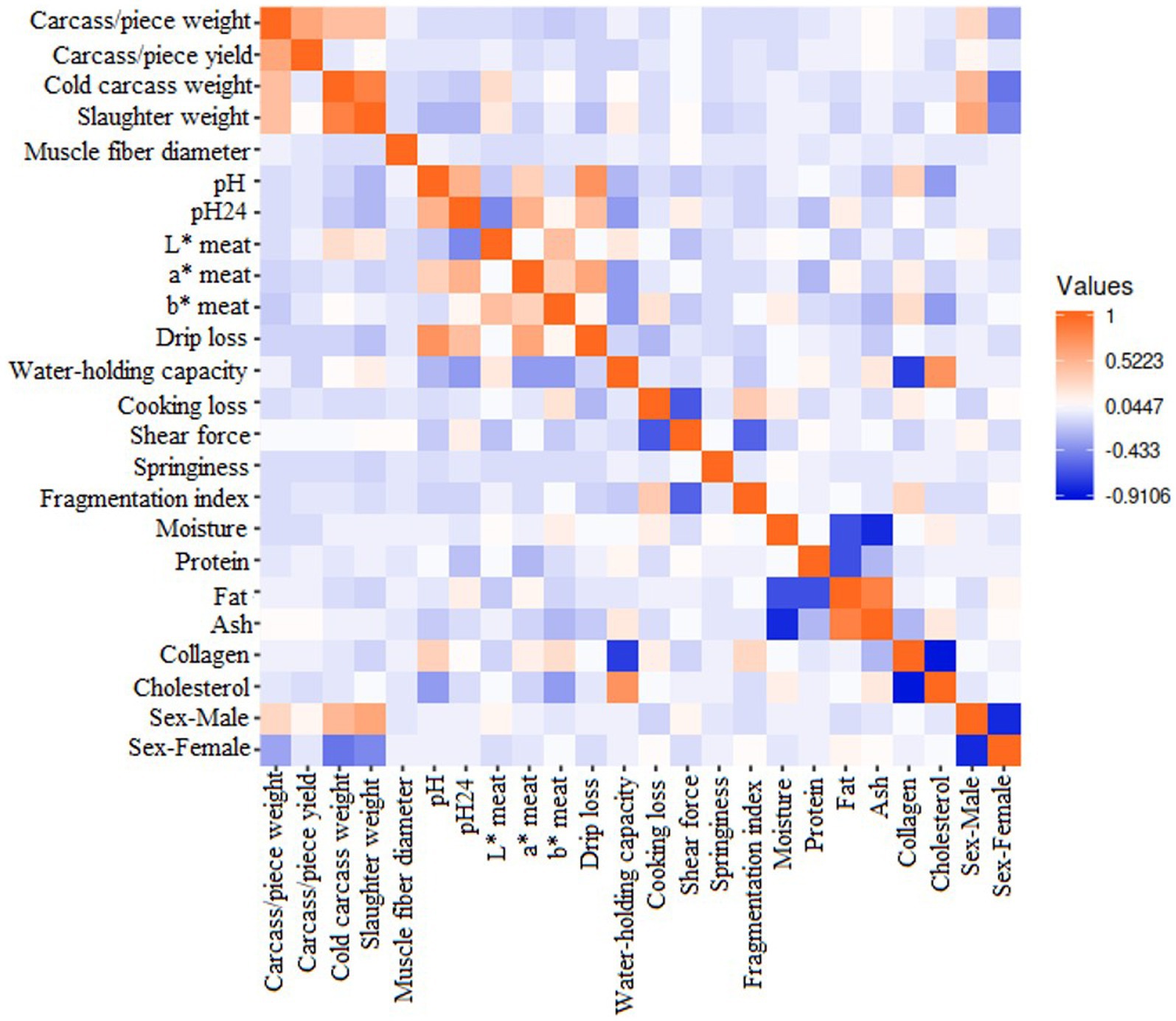
Figure 2. The correlation matrix between meat and carcass quality-related attributes included in the present study.
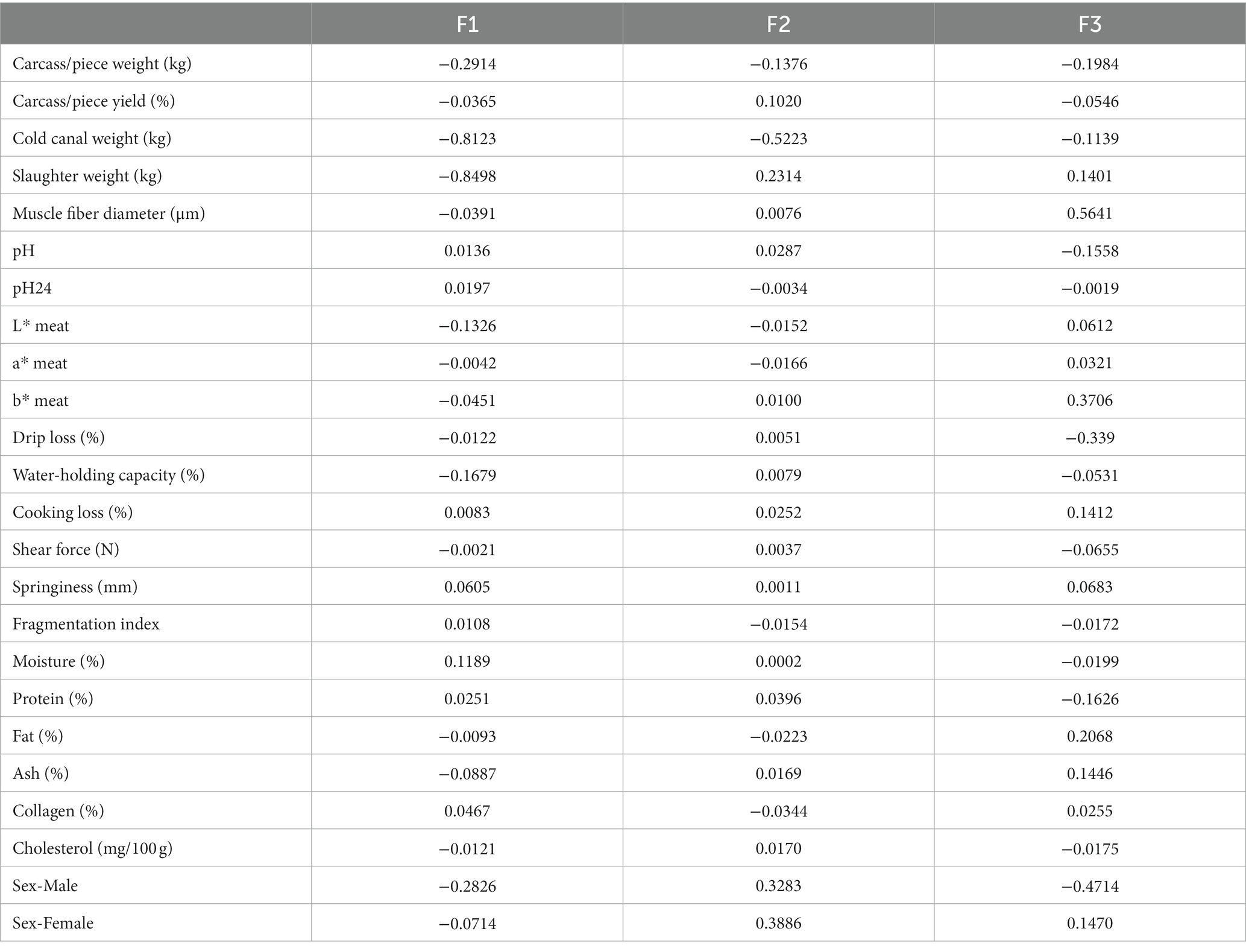
Standardized discriminant coefficients are shown in Table 5. By evaluating these coefficients, possible reductions in the discriminant power of the variables can be detected as a consequence of multicollinearity between pairs. Moreover, the relative weight of each meat and carcass trait across the discriminant functions has been represented in Figure 3.
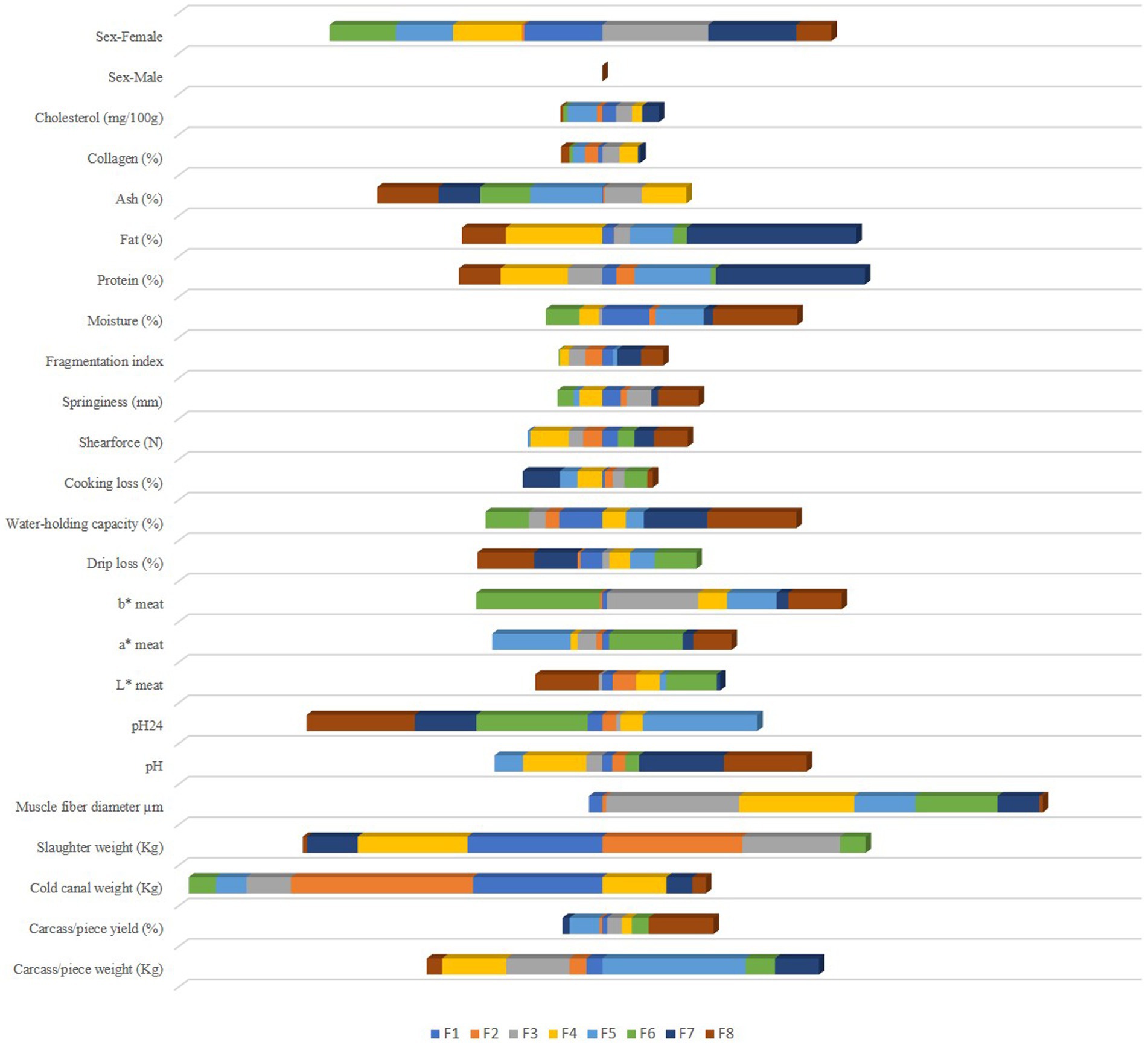
Figure 3. Discriminant coefficients for turkey meat and carcass quality attributes on each discriminant function.
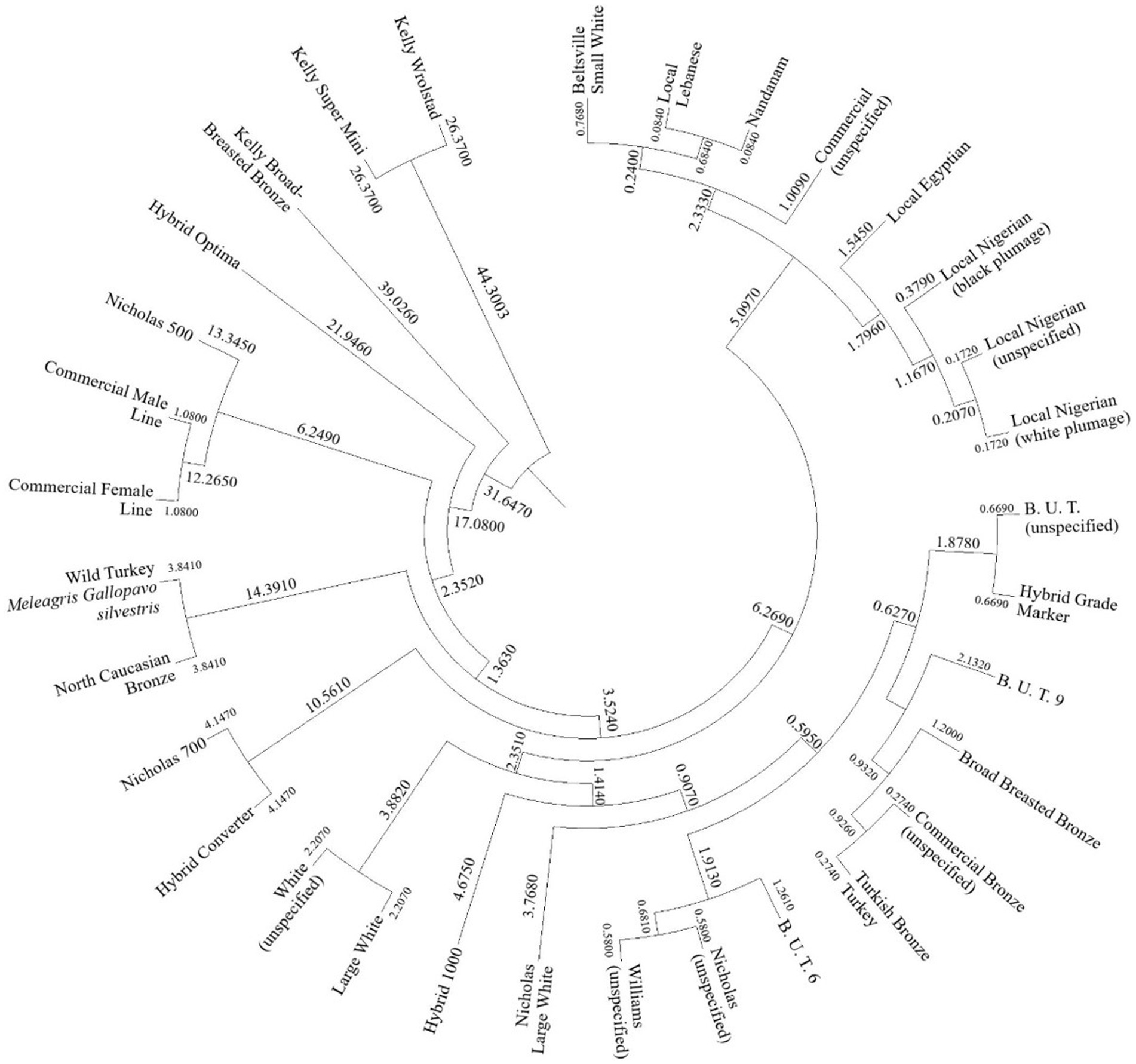
The Mahalanobis distance were used to cluster turkey varieties and strains attending to carcass and meat quality traits. In this respect, the Mahalanobis distance represent the hit rate of matching an unknown observation into a particular classification group, which are turkey varieties, attending to its intrinsic carcass and meat characteristics. Hence, the likelihood of matching an observation into a group was estimated according to the literature (123). The Mahalanobis distance obtained after the evaluation of the discriminant analysis matrix were graphically expressed in a cladogram after their transference to squared Euclidean distance (Figure 4).
4 Discussion
4.1 Background
The domestication and migration processes have resulted in a great variety of livestock breeds (3), which have evolved and specialized to fit specific environments and human purposes (124). This is exemplified in the greater phenotypic diversity among breeds than that observed across wild populations (3). However, phenotypic variability between turkey breeds is much more limited compared with the fowl breeds (3), which could be attributed to a smaller effective turkey population size and a narrower effective population size before specialized strains appeared in the 20th century (124). Such specialization was translated into the development of a few highly selected strains used worldwide (125, 126), which, on the other hand, caused a reduction of the overall genetic diversity. This situation becomes even more worrying when considering the already low rates of diversity of the ‘heritage landraces’ that gave origin to these modern hybrids (3).
In addition to this limited phenotypic variability, a remarkable lack of literature addressing local breeds compared with industrial hybrids hinders the research of meat and carcass attributes. This disequilibrium is a direct consequence of the lack of resources allocated to the study of native genotypes (18), which in turn are mainly carried out in developing countries. It can be evidenced in the present study, as the only articles approaching local breeds were performed in Nigeria, Egypt, Lebanon, Bulgaria, and Turkey. However, meat and product characterization studies are essential for achieving the official recognition of a local breed (20), which is crucial for implementing further conservancy proceedings. Moreover, the knowledge of the attributes of their products allows the enhancement of their value by those consumers demanding for extra-quality and recognizable products (39).
4.2 Normality assumption and differences between turkey landraces and varieties
The Shapiro–Francia W’ test reported a few variables not showing statistical differences, which are as follows: pH, a* meat, b* meat, drip loss, cooking loss, shear force, and collagen. These attributes have previously reported controversial and inconsistent results in the literature comparing turkey genetic lines (4, 6, 8, 13). Thus, they could be suggested not to be approached in the breed traceability and differentiation of turkey meat products. Those traits are generally correlated, as pH directly influences meat color (4, 8, 30) and other textural traits such as drip loss (4, 8, 13, 30), cooking loss (4, 13), and shear force (4, 13). Those attributes might be expected to differ across turkey genotypes, as they are a consequence of the rate of pH decline (13) and muscle fiber diameter (8), which have been described as highly breed-dependent traits (4, 7, 8, 13, 30). However, the lack of variability found in the present study could be attributed to other influencing factors such as the rearing system (8), pre-slaughter wing flapping, or a different time of parameter measurement among studies (13). The controversial results are also found on meat collagen differences across strains. Some authors describe a lack of variability across two heavy commercial strains (6), while others found differences comparing slow- and fast-growing lines (30).
4.3 Meat and carcass quality-related traits correlation
The correlation matrix among variables depicted in Figure 2 describes a high positive correlation between carcass/piece weight to carcass/piece yield (+0.393), cold carcass weight (+0.266), and slaughter weight (+0.252). In this respect, factors influencing carcass/piece weights include slaughter age, rearing system, sex, and genotype (67, 127). Genotype plays an important role, as the exhaustive selection for meat production in specialized strains has been derived not only in the rise of body weight and growth but also in the enhancement of edible components (128), which is the consequence of the high value that certain parts of the carcass, especially the breasts, can achieve since most of the commercial turkey production is destined for further processing (127, 129). As the weight of a carcass or its cuts is employed in the estimation of its yielding (33, 106), those parameters were expected to be highly associated. The positive association between both cold and slaughter carcass weights with its components suggests a continuous development of body components as the animal grows. However, the yielding of the carcass or its cuts reported no correlations with the weight at slaughter or of the cold carcass. This finding could be contradictory when applying the allometric growth principles. Allometric growth holds great bone development at the hatching moment, which then leads to rapid muscle development and, in later stages of growth, to fat deposition (130). Hence, a greater yielding of muscular cuts (such as breasts and thighs) could be expected with increased live weight. This lack of association could be due to differences in the allometric growth pattern across breeds (127) or to a different age at slaughter between genotypes (67). In this respect, genotypes might differ in the time needed to reach slaughter weight or are subjected to different consumption preferences and cultural habits (131).
pH and pH24 showed a moderate positive correlation between them (+0.327), which has been previously reported in the literature (4). Muscle transformation into meat is caused by post-mortem anaerobic glycogen metabolism in the muscle (30), which causes tissue acidification. Hence, this process can be monitored by measuring pH at different stages post-mortem, usually shortly after the slaughter and a few hours later (4, 8, 13). This meat acidification process is particularly quick in poultry compared with other species, accounting for a few hours to its achievement (132). The positive correlation among these variables could suggest that, due to the speed of the process, pH could serve as an indication of the following meat acidification (measured as pH24). However, this association might not be linear, as pH did not show explanatory properties in the test of equality of groups, while pH24 did.
Both pH parameters showed a strong positive correlation with drip loss (pH: +0.490; pH24: +0.237), possibly due to the influence of the meat acidification process on water captivity attributes such as drip loss (8, 30, 47, 111). However, while the literature describes positive associations between drip loss and pH (4, 133), a negative correlation with pH24 is generally reported (4, 38, 101). This is because intense pH declines worsen water captivity attributes (4). Meat redness (a* meat) was also positively associated with acidification parameters (pH: +0.157; pH24: +0.301), as reported in the bibliography (7, 54, 75, 133, 134). In this respect, meat redness (a*) reflects the myoglobin content of the muscle fiber, which is directly associated with pH (101) and rapid, harmful pH decreases in meat due to quick glycolysis, resulting in redder meat (54).
The results obtained in the present study support the correlation among all colorimetry traits (L* meat-a* meat: +0.087; L* meat-b* meat: +0.316; a* meat -b* meat: +0.213), which has been previously reported by some authors (4, 8). However, the most widespread assumption holds that, when lightness (L*) increases, lower values of redness (a*) and greater yellowness (b*) could be expected (7, 36). Hence, no conclusive results could be achieved in this respect, as reported in the bibliography (7). This inconclusiveness could be due to the great variety of factors influencing colorimetry, such as genotype, diet, rearing system, season of slaughter, type of muscle, slaughter method, and time of measures post-mortem (6, 7, 13). These complex interactions between meat color traits highlight the need for further studies focused on their correlations.
Water-holding capacity showed negative correlations with a* meat (−0.157) and b* meat (−0.163). By contrast, b* meat showed positive associations with cooking loss (+0.149). This finding could be attached to the correlation of meat redness and yellowness to the pH decrease rate. Thus, the a*/b*ratio has been employed as an indicator of myoglobin oxidation, and a correlation with the rate of post-mortem fall was found (30). Rapid decreases in pH fall led to a more intense red and yellow color (54), which also could promote the denaturation of myofibrillar proteins, reducing water-holding capacity (36).
pH showed a moderate positive association with collagen (+0.194). Association among meat collagen and pH could be attached to the fact that both variables are influenced by characteristics of muscle fiber structure (37) and its connective tissue (6). However, a negative correlation between those traits might be expected according to described in the literature (73). Authors found meat collagen to be negatively correlated with pH and positively associated with muscle glycogen. Thus, fast-growing birds, which are usually early slaughtered, develop immature muscle collagen (6) and show lower meat acidification (higher meat pH) (4).
pH24 showed a moderate negative correlation with meat lightness (L* meat) (−0.223). This negative genetic correlation has been described before in turkey (54, 73), as well as in chicken (135, 136) and Japanese quail (38). pH24 reported a negative association with water-holding capacity (−0.172) as well, which was also described in chicken (136) and Japanese quail (38). Therefore, as pH24 increases (and meat lightness decreases), the water-holding capacity attributes of the meat are improved. This finding is reinforced by the slight positive correlation between lightness and WHC (+0.119) obtained in the present study. Hence, paler meat with a lower pH is usually associated with greater cooking losses and, hence, a reduced water-holding capacity (73).
The fragmentation index is moderately positively associated with cooking loss (+0.174). The fragmentation index is used as an indicator of meat textural attributes (137), describing post-mortem muscle protein degradation (138). This protein denaturation plays an important role in the cooking loss variable (137, 139), as denatured protein is less soluble than physiological protein (139), leading to greater humidity wastes during culinary processes (140). Furthermore, the fragmentation index showed a moderate positive association with meat collagen (+0.194), which is another meat textural indicator (13, 134, 141). In this respect, meat collagen has been described to influence the fragmentation index in woody-breast filets in chicken (142, 143).
Fat is strongly and positively associated with ash (+0.595). Regarding these traits, contradictory results are found in the bibliography. A positive association between traits has been described (144), and parallel tendencies of variables were reported when comparing turkey genotypes (8) and rearing systems (7). On the other hand, a negative correlation (144) and no correlation (145) among traits have been reported as well. This lack of consistency could be due to the great variability of factors influencing meat chemical composition, as the ash proportion is influenced by genotype, sex, age, rearing system, and nutrition (146). The aforementioned variables (fat and ash) showed negative correlations with moisture (−0.375 and −0.498, respectively), which is supported by literature (144, 147, 148), which could be attached to the fact that greater fatty muscles repulse moisture due to their low solubility (147). Fat was also found to be negatively associated with protein (−0.460), which is reinforced by previous authors (149). Moreover, muscle type (146) and rearing system (7) should be considered among those variables influencing meat composition. An inverse behavior has been described for these macronutrients in outdoor systems. Free-access systems are suggested to facilitate muscle (protein) development due to exercise and prevent fat deposition due to greater energy losses allocated to thermoregulation (7).
Cholesterol is positively associated with water-holding capacity (+0.434) and moisture (+0.189). However, the results showed no association between meat fat and cholesterol inclusion. This finding has been widely described before (150–152), as great cholesterol inclusion is not dependent on meat fat content. The low inclusion of cholesterol is a desirable trait in poultry, even though it serves as a great meat quality index (83). Caponization of birds seeks to increase meat lipids and cholesterol, which is translated into improved quality traits such as tenderness (153, 154). A possible explanation underlying this increase could be the important structural function cholesterol performs on cell membranes (155) and, hence, could prevent moisture losses from the destruction of cell walls (156). Moreover, descriptions of reduced moisture losses have been attributed to greater fat losses during cooking (157). Cholesterol also showed a strong negative correlation with collagen (−0.654). Meat collagen has been reported to mature with age (158), while cholesterol deposition is known to decrease (159).
4.4 Differences across turkey landraces and varieties
The results of the test of equality of groups show variables statistically explaining differences across turkey varieties. In the present study, we developed a tool to assess the improvement of meat differentiation and product traceability. A strong influence of weight-related traits is evidenced, particularly in cold carcass and slaughter weight variables. Due to the intense selection for growth that turkey species have experienced over the last few years (160), great phenotypic variability across breeds could be expected in live weight. Moreover, body weight is one of the most differentiating traits among breeds of poultry, which has been shown in unselected poultry breeds such as Brianzolo and Nero d’Italia turkeys (161) or Utrerana avian breed (15). On the other hand, live weight is a main classification criterion in the hybrid commercial industry (4, 7, 13).
Muscle fiber diameter showed strong explanatory properties as well, in parallel with the descriptions of a study on worldwide native chicken populations (18). Muscle fiber size varies among genotypes in poultry and other domestic species, as muscle growth is caused by an increase in fiber diameter instead of cell hyperplasia (8). The most widespread assumption among livestock species is that the fast-growing genotypes have larger muscle fibers than slow-growing lines (162). However, differences can be observed even within fast- and slow-growing strains (8).
The female sex resulted as a great differentiating variable, which might suggest a great variability across turkey breeds in the effect of sexual dimorphism on meat and carcass attributes. Turkey species show a great sexual dimorphism in morphometric traits (45), which is particularly evident for body weight traits. However, a different effect of sexual dimorphism on body weight is exhibited. For example, males from native genotypes tend to double or triple female body weight (17, 163), while this difference has been described to be lower in commercial turkeys (164).
Some of the technological properties (yellowness, lightness, pH24, and WHC) were also discriminating among turkey varieties. Those are traits mainly depending on muscle post-mortem metabolism, particularly the meat acidification process (30). Hence, differences among breeds and varieties on factors influencing muscle metabolism (such as glycogen fiber reserves, and pre-slaughter stress predisposition) could explain these results (4, 7, 13, 30). Meat nutritional traits (ash, moisture, fat) showed explanatory properties as well. However, inconsistent and no conclusive results are found in the literature considering breed differences in meat chemical composition (6–8, 30, 31).
4.5 Mahalanobis distance between landraces and varieties
Finally, the cladogram reflects the Mahalanobis distance performed by the discriminant analysis across turkey varieties. Interesting results are found attending to the clustering pattern. Some of the turkey landraces showed unspecified designations in their respective studies. However, they have been clustered close to each other, which is the case of the “female line” and “male line” nomenclature used (70), which were clustered close to the ‘Nicholas 500’ strain. A similar situation was found in the “White” strain, in Turkey (104), which was grouped near “Large White,” according to the developed discriminating tool.
Another interesting result is the clustering of bronze strains together (Broad Breasted Bronze, unspecified Commercial Bronze, and Turkish Bronze). This fact suggests their proximity. According to previous authors, this Turkish Bronze strain is widespread in Turkish rural areas and is reared under extensive systems. However, some authors also suggest that the origin of this breed in the American bronze. Therefore, its origin would be the same as the strain Broad Breasted Turkey (104).
Moreover, the cladogram clusters African breeds together. Additionally, within the Nigerian turkey, the unspecified feathered animals (107, 108) were clustered close to the white variety (106), which can be understood as those unspecified belonging to the white feather variety.
Finally, there was a final cluster with Beltsville Small White, Nandanam, and Local Lebanese. The Nandanam line descends from the Beltsville Small White breed, as this strain is obtained by crossing with the Indian “Desi” native breed (165), which could explain their similarities in carcass and meat characteristics, as they are grouped closely. Additionally, the “Local Lebanese” strain was clustered close to the Nandanam line, which evidences the closeness of these two genotypes. Due to the lack of literature on this landrace, a possible importation of Nandanam turkeys to Lebanon could be suggested (165).
5 Conclusion
In the present study, a DCA based on turkey meat and carcass quality attributes has been developed as a tool for addressing breed traceability trials. These trials lie in the description of highly differential traits across turkey strains and breeds, as well as the descriptions of strong associations among variables. Carcass weights and yields showed the greatest explanatory power, especially cold carcass weight and slaughter weight. Sex described good explanatory properties as well, possibly due to a different effect of sexual dimorphism across genotypes. Among other explanatory variables are some of the meat’s physical (colorimetry and pH traits) and nutritional quality traits. According to the correlations of attributes analyzed, strong positive and negative associations have been described between and within physical and nutritional traits. Thus, positive correlations were found among carcass yield variables, particularly those comprising carcass/piece weight. Muscle post-mortem metabolism produced strong associations between pH, colorimetry, water captivity, and meat chemical composition traits. Finally, the analysis of the Mahalanobis distance suggested the feasibility of this tool as a breed-clustering feature due to the aggrupation of known genetically close populations. Hence, the statistical tool developed in the present study could be employed in the post-mortem phase in turkey slaughterhouses, as it allows breed discrimination of both carcasses and cuts. This tool could be employed to protect breed-based products to avoid fraud or hybridization. Moreover, the selection of the most highly explanatory traits and those more representative in terms of greater correlations allows for the simplification and cost reduction of this tool, which can benefit those low-income smallholder farmers growing indigenous genotypes. However, contradictory results found in the bibliography and the lack of consensus among authors about some aspects highlight the need for further studies, especially for local breeds, which have not been deeply studied.
Data availability statement
The original contributions presented in the study are included in the article, further inquiries can be directed to the corresponding authors.
Author contributions
JS: Conceptualization, Investigation, Software, Writing – original draft, Writing – review & editing. AG: Conceptualization, Data curation, Formal analysis, Investigation, Methodology, Resources, Software, Supervision, Validation, Visualization, Writing – original draft, Writing – review & editing. FN: Conceptualization, Data curation, Formal analysis, Investigation, Methodology, Software, Supervision, Validation, Writing – review & editing. JL: Investigation, Resources, Software, Writing – review & editing. ED: Investigation, Writing – review & editing. JD: Project administration, Resources, Supervision, Visualization, Writing – review & editing. MC: Funding acquisition, Project administration, Resources, Supervision, Visualization, Writing – review & editing.
Funding
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. This research was funded by FEDER project P20_00893 and during the covering period of a predoctoral contract (FPU Fellowship) funded by the Spanish Ministry of Science and Innovation. Moreover, the present research was carried out during the covering period of a Ramón y Cajal Post-Doctoral fellowship financially supported by MCIN/AEI/10.13039/501100011033 and European Union Europea “NextGenerationEU”/PRTR” (Recovery, Transformation, and Resilience Plan-Funded by the European Union-NextGenerationEU).
Acknowledgments
This research would not have been possible without the assistance of ACPA (Asociación de Criadores del Pavo Andaluz), IFAPA (Instituto de Investigación y Formación Agraria y Pesquera), Diputación de Córdoba, and PAIDI AGR 218 research group.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Footnotes
References
1. Kleyn, F, and Ciacciariello, M. Future demands of the poultry industry: will we meet our commitments sustainably in developed and developing economies? Worlds Poult Sci J. (2021) 77:267–78. doi: 10.1080/00439339.2021.1904314
2. Mottet, A, and Tempio, G. Global poultry production: current state and future outlook and challenges. Worlds Poult Sci J. (2017) 73:245–56. doi: 10.1017/S0043933917000071
3. Aslam, ML, Bastiaansen, JW, Elferink, MG, Megens, H-J, Crooijmans, RP, Blomberg, LA, et al. Whole genome SNP discovery and analysis of genetic diversity in Turkey (Meleagris gallopavo). BMC Genomics. (2012) 13:391–14. doi: 10.1186/1471-2164-13-391
4. Hiscock, HM, Leishman, EM, Vanderhout, RJ, Adams, SM, Mohr, J, Wood, BJ, et al. Describing the relationships among meat quality traits in domestic Turkey (Meleagris gallopavo) populations. Poult Sci. (2022) 101:102055. doi: 10.1016/j.psj.2022.102055
5. Updike, M, Zerby, H, Sawdy, J, Lilburn, M, Kaletunc, G, and Wick, M. Turkey breast meat functionality differences among turkeys selected for body weight and/or breast yield. Meat Sci. (2005) 71:706–12. doi: 10.1016/j.meatsci.2005.05.014
6. Zampiga, M, Tavaniello, S, Soglia, F, Petracci, M, Mazzoni, M, Maiorano, G, et al. Comparison of 2 commercial Turkey hybrids: productivity, occurrence of breast myopathies, and meat quality properties. Poult Sci. (2019) 98:2305–15. doi: 10.3382/ps/pey607
7. Sarica, M, Ocak, N, Turhan, S, Kop, C, and Yamak, U. Evaluation of meat quality from 3 Turkey genotypes reared with or without outdoor access. Poult Sci. (2011) 90:1313–23. doi: 10.3382/ps.2009-00600
8. Werner, C, Riegel, J, and Wicke, M. Slaughter performance of four different Turkey strains, with special focus on the muscle fiber structure and the meat quality of the breast muscle. Poult Sci. (2008) 87:1849–59. doi: 10.3382/ps.2007-00188
9. Tosato, A. The protection of traditional foods in the EU: traditional specialities guaranteed. Eur Law J. (2013) 19:545–76. doi: 10.1111/eulj.12040
10. Gyenai, KB. An Assessment of the Effects of Oxidative Stress and Dietary Antioxidants on Toxin-Induced Dilated Cardiomyopathy in the Turkey (Meleagris gallopavo). Doctoral dissertation, Virginia Tech (2009).
11. Chrzan, J. Slow food: what, why, and to where? Food Cult Soc. (2004) 7:117–32. doi: 10.2752/155280104786577798
12. Martin, J. The commercialisation of British Turkey production. Rural Hist. (2009) 20:209–28. doi: 10.1017/S0956793309990057
13. Leishman, EM, Vanderhout, RJ, van Staaveren, N, Barbut, S, Mohr, J, Wood, BJ, et al. Influence of post mortem muscle activity on Turkey meat quality. Front Vet Sci. (2022) 9:822447. doi: 10.3389/fvets.2022.822447
14. Olschewsky, A, Riehn, K, and Knierim, U. Suitability of slower growing commercial Turkey strains for organic husbandry in terms of animal welfare and performance. Front Vet Sci. (2021) 7:600846. doi: 10.3389/fvets.2020.600846
15. González Ariza, A, Arando Arbulu, A, León Jurado, JM, Navas González, FJ, Delgado Bermejo, JV, and Camacho Vallejo, ME. Discriminant canonical tool for differential biometric characterization of multivariety endangered hen breeds. Animals. (2021) 11:2211. doi: 10.3390/ani11082211
16. Pym, R. Poultry genetics and breeding in developing countries. Poult Dev Rev FAO. (2013) 1:80–3.
17. Arando, A, González-Ariza, A, Lupi, T, Nogales, S, León, J, Navas-González, F, et al. Comparison of non-linear models to describe the growth in the Andalusian Turkey breed. Ital J Anim Sci. (2021) 20:1156–67. doi: 10.1080/1828051X.2021.1950054
18. González Ariza, A, Navas González, FJ, Arando Arbulu, A, León Jurado, JM, Delgado Bermejo, JV, and Camacho Vallejo, ME. Variability of meat and carcass quality from worldwide native chicken breeds. Food Secur. (2022) 11:1700. doi: 10.3390/foods11121700
19. Pardo, JS, González, FJN, Ariza, AG, Arbulu, AA, Jurado, JL, Bermejo, JD, et al. Traditional sexing methods and external egg characteristics combination allow highly accurate early sex determination in an endangered native Turkey breed. Front Vet Sci. (2022) 9:948502. doi: 10.3389/fvets.2022.948502
20. Portillo-Salgado, R, Herrera Haro, J, Bautista-Ortega, J, Chay-Canul, A, and Cigarroa, VF. Guajolote–a poultry genetic resource native to Mexico. Worlds Poult Sci J. (2022) 78:467–82. doi: 10.1080/00439339.2022.2028217
21. González Ariza, A, Arando Arbulu, A, Navas González, FJ, Ruíz Morales, FA, León Jurado, JM, Barba Capote, CJ, et al. Sensory preference and professional profile affinity definition of endangered native breed eggs compared to commercial laying lineages’ eggs. Animals. (2019) 9:920. doi: 10.3390/ani9110920
22. Yin, H, Gilbert, ER, Chen, S, Wang, Y, Zhang, Z, Zhao, X, et al. Effect of hybridization on carcass traits and meat quality of erlang mountainous chickens. Asian Australas J Anim Sci. (2013) 26:1504–10. doi: 10.5713/ajas.2013.13097
23. Bernini, F, Bagnato, A, Marelli, SP, Zaniboni, L, Cerolini, S, and Strillacci, MG. Genetic diversity and identification of homozygosity-rich genomic regions in seven Italian heritage Turkey (Meleagris gallopavo) breeds. Genes. (2021) 12:1342. doi: 10.3390/genes12091342
24. Sponenberg, DP, Martin, A, Couch, C, and Beranger, J. Conservation strategies for local breed biodiversity. Diversity. (2019) 11:177. doi: 10.3390/d11100177
25. González Ariza, A, Navas González, FJ, Arando Arbulu, A, León Jurado, JM, Delgado Bermejo, JV, and Camacho Vallejo, ME. Discriminant canonical analysis as a tool to determine traces of endangered native hen breed introgression through egg hatchability phenomics. Anim Biosci. (2022):1–13. doi: 10.5713/ab.22.0163
26. González Ariza, A, Navas González, FJ, Arando Arbulu, A, León Jurado, JM, Barba Capote, CJ, and Camacho Vallejo, ME. Non-parametrical canonical analysis of quality-related characteristics of eggs of different varieties of native hens compared to laying lineage. Animals. (2019) 9:153. doi: 10.3390/ani9040153
27. FAO. Domestic Animal Diversity Information System (DAD-IS). (2023). Available at: https://www.fao.org/dad-is/risk-status-of-animal-genetic-resources/en/ (Accessed April 18, 2023).
28. Sentandreu, MÁ, and Sentandreu, E. Authenticity of meat products: tools against fraud. Food Res Int. (2014) 60:19–29. doi: 10.1016/j.foodres.2014.03.030
29. Montowska, M, and Pospiech, E. Is authentication of regional and traditional food made of meat possible? Crit Rev Food Sci Nutr. (2012) 52:475–87. doi: 10.1080/10408398.2010.501408
30. Fernandez, X, Sante, V, Baéza, E, Lebihan-Duval, E, Berri, C, Rémignon, H, et al. Post mortem muscle metabolism and meat quality in three genetic types of turkey. Br Poult Sci. (2001) 42:462–9. doi: 10.1080/00071660120070604
31. Roberson, K, Rahn, A, Balander, R, Orth, M, Smith, D, Booren, B, et al. Evaluation of the growth potential, carcass components and meat quality characteristics of three commercial strains of tom turkeys. J Appl Poult Res. (2003) 12:229–36. doi: 10.1093/japr/12.2.229
32. Abdel-Kafy, EM, Zayed, S, Behiry, FM, Gorgy, M, Ahmed, MA, and Ibraheim, SE. Assessment of growth, carcass traits, and some physiological parameters of bronze, and white turkeys (meleagris gallopavo), and their crosses. Egypt Poult Sci J. (2022) 42:355–72. doi: 10.21608/epsj.2022.264656
33. Damaziak, K, Michalczuk, M, Zdanowska-Sąsiadek, Ż, Niemiec, J, and Gozdowski, D. Variation in growth performance and carcass yield of pure and reciprocal crossbred turkeys. Ann Anim Sci. (2015) 15:51–66. doi: 10.2478/aoas-2014-0058
34. El-Naggar, N, Abdel-Ghany, A, Dowidar, Y, and Qota, E. Influence of crossing white Holland and broad breasted bronze turkeys on meat and serum characteristics. Egypt Poult Sci J. (2000) 20:441–65.
35. Salgado Pardo, JI, Navas González, FJ, González Ariza, A, León Jurado, JM, Galán Luque, I, Delgado Bermejo, JV, et al. Study of meat and carcass quality-related traits in Turkey populations through discriminant canonical analysis. Food Secur. (2023) 12:3828. doi: 10.3390/foods12203828
36. Fraqueza, M, Cardoso, A, Ferreira, M, and Barreto, A. Incidence of pectoralis major Turkey muscles with light and dark color in a Portuguese slaughterhouse. Poult Sci. (2006) 85:1992–2000. doi: 10.1093/ps/85.11.1992
37. Huo, W, Weng, K, Gu, T, Zhang, Y, Zhang, Y, Chen, G, et al. Effect of muscle fiber characteristics on meat quality in fast-and slow-growing ducks. Poult Sci. (2021) 100:101264. doi: 10.1016/j.psj.2021.101264
38. Narinc, D, Aksoy, T, Karaman, E, Aygun, A, Firat, MZ, and Uslu, MK. Japanese quail meat quality: characteristics, heritabilities, and genetic correlations with some slaughter traits. Poult Sci. (2013) 92:1735–44. doi: 10.3382/ps.2013-03075
39. González Ariza, A, Navas González, FJ, León Jurado, JM, Arando Arbulu, A, Delgado Bermejo, JV, and Camacho Vallejo, ME. Data mining as a tool to infer chicken carcass and meat cut quality from autochthonous genotypes. Animals. (2022) 12:2702. doi: 10.3390/ani12192702
40. Page, MJ, Moher, D, and McKenzie, JE. Introduction to PRISMA 2020 and implications for research synthesis methodologists. Res Synth Methods. (2022) 13:156–63. doi: 10.1002/jrsm.1535
41. Tam, WW, Lo, KK, and Khalechelvam, P. Endorsement of PRISMA statement and quality of systematic reviews and meta-analyses published in nursing journals: a cross-sectional study. BMJ Open. (2017) 7:e013905. doi: 10.1136/bmjopen-2016-013905
42. McLean, AK, and Gonzalez, FJN. Can scientists influence donkey welfare? Historical perspective and a contemporary view. J Equine Vet. (2018) 65:25–32. doi: 10.1016/j.jevs.2018.03.008
43. Iglesias Pastrana, C, Navas González, FJ, Ciani, E, Barba Capote, CJ, and Delgado Bermejo, JV. Effect of research impact on emerging camel husbandry, welfare and social-related awareness. Animals. (2020) 10:780. doi: 10.3390/ani10050780
44. Schlosser, RW, Wendt, O, Bhavnani, S, and Nail-Chiwetalu, B. Use of information-seeking strategies for developing systematic reviews and engaging in evidence-based practice: the application of traditional and comprehensive pearl growing. A review. Int J Lang Commun Disord. (2006) 41:567–82. doi: 10.1080/13682820600742190
45. MA Anandh, Jagatheesan, PNR. (Eds.). Effect of Sex on Slaughter and Carcass Traits of Beltsville Small White Turkey (Meleagris gallopavo) under Indian Hot Humid Climatic Condition. Vet. World (2017) 5:226–9.
46. Anandh, MA. Slaughter and carcass characteristics of Beltsville small white and broad breasted bronze turkeys (Meleagris gallopavo). Int J Environ Sci Technol. (2018) 7:577–83.
47. Anandh, MA, and Jagatheesan, PR. Meat quality characteristics of Beltsville small white broiler and spent hen turkeys (Meleagris gallopavo). Int J Chem Stud. (2020) 8:393–7. doi: 10.22271/chemi.2020.v8.i5f.10324
48. Anna, AM. Effect of rearing systems on slaughter and carcass characteristics of Turkey (Meleagris gallopavo). Res J Anim Husbandry Dairy Sci. (2017) 8:46–50. doi: 10.15740/HAS/RJAHDS/8.1/46-50
49. Sirohi, R, Shukla, PK, Bhattacharyya, A, Singh, Y, Singh, DN, and Kumar, A. Effect of photoperiod on the production performance and carcass quality traits of Turkey Poults. J Anim Res. (2018) 8:1059–63. doi: 10.30954/2277-940X.12.2018.18
50. Blacha, I, Krischek, C, and Klein, G. Influence of modified atmosphere packaging on meat quality parameters of Turkey breast muscles. J Food Prot. (2014) 77:127–32. doi: 10.4315/0362-028X.JFP-13-242
51. Anandh, MA. Effect of sex on slaughter and carcass characteristics of broad breasted bronze turkeys (Meleagris gallopavo). Asian J Anim Sci. (2019) 14:6–9. doi: 10.15740/HAS/TAJAS/14.1/6-9
52. Dorra, TI, Ibrahim, SE, and Zayed, SM. Effect of dietary betaine supplementation on growth performance and carcass traits of growing Turkey. J Anim Poult Prod. (2012) 3:365–77. doi: 10.21608/jappmu.2012.82940
53. Fernandez, X, Santé, V, Baéza, E, Lebihan-Duval, E, Berri, C, Rémignon, H, et al. Effects of the rate of muscle post mortem pH fall on the technological quality of Turkey meat. Br Poult Sci. (2002) 43:245–52. doi: 10.1080/00071660120121463
54. Le Bihan-Duval, É, Berri, C, Baéza, É, Santé, V, Astruc, T, Rémignon, H, et al. Genetic parameters of meat technological quality traits in a grand-parental commercial line of Turkey. Genet Sel Evol. (2003) 35:623–35. doi: 10.1186/1297-9686-35-7-623
55. Oke, FO, Onasanya, GO, Adedire, AO, Oduguwa, OO, Obadire, SO, and Osofowora, AO. Effects of feed probiotics on serum biochemistry and carcass characteristics of tropically bred exotic Turkey. IOSR J Agric Vet Sci (IOSR-JAVS). (2014) 7:53–9. doi: 10.9790/2380-071115359
56. Moran, E Jr, Leeson, S, and Summers, J. Large Turkey commercial strain comparisons of performance, carcass quality and meat yield during 1969 and 1977. Can J Anim Sci. (1978) 58:291–302. doi: 10.4141/cjas78-039
57. Abdel-Ghany, A. The effect of Origanum majorana supplementation on growth performance, blood parameters and meat quality in BUT9 commercial turkeys. J Anim Poult Fish Prod. (2015) 3:17–29. doi: 10.21608/japfp.2015.7428
58. Mikulski, D, Jankowski, J, Zdunczyk, Z, Wróblewska, M, Sartowska, K, and Majewska, T. The effect of selenium source on performance, carcass traits, oxidative status of the organism, and meat quality of turkeys. J Anim Feed Sci. (2009) 18:518–30. doi: 10.22358/jafs/66427/2009
59. Sante, V, Le Pottier, G, Astruc, T, Mouchoniere, M, and Fernandez, X. Effect of stunning current frequency on carcass downgrading and meat quality of Turkey. Poult Sci. (2000) 79:1208–14. doi: 10.1093/ps/79.8.1208
60. Taha, N, and Farran, M. Comparative study of thigh muscles and bones conformation and some carcass traits of local vs. imported Turkey strain. Int. J Poult Sci. (2009) 8:368–72. doi: 10.3923/ijps.2009.368.372
61. Wojtysiak, D, and Górska, M. Effect of aging time on meat quality and rate of desmin and dystrophin degradation of pale, soft, exudative (PSE) and normal Turkey breast muscle. Folia Biologica (Kraków). (2018) 66:63–72. doi: 10.3409/fb_66-2.07
62. Bianchi, M, Capozzi, F, Cremonini, MA, Laghi, L, Petracci, M, Placucci, G, et al. Influence of the season on the relationships between NMR transverse relaxation data and water-holding capacity of Turkey breast meat. J Sci Food Agric. (2004) 84:1535–40. doi: 10.1002/jsfa.1808
63. Gibril, S, Hassan, HA, Yassin, OE, and Shamseldin, RM. Growth performance and carcass characteristics of turkeys (Meleagris gallopavo) under semi intensive system in the Sudan. Univ Khartoum J Agric Sci. (2013) 21:99–115.
64. Heincinger, M, Balogh, K, Mézes, M, and Fébel, H. Effects of distillers dried grain with soluble (DDGS) on meat quality, lipid peroxide and some of antioxidant status parameters of fattening Turkey. J Poult Sci. (2012) 49:268–72. doi: 10.2141/jpsa.0120004
65. Lemme, A, Frackenpohl, U, Petri, A, and Meyer, H. Effects of reduced dietary protein concentrations with amino acid supplementation on performance and carcass quality in Turkey toms 14 to 140 days of age. Int J Poult Sci. (2004) 3:391–9. doi: 10.3923/ijps.2004.391.399
66. Mikulski, D, Jankowski, J, Zdunczyk, Z, Juskiewicz, J, and Slominski, B. The effect of different dietary levels of rapeseed meal on growth performance, carcass traits, and meat quality in turkeys. Poult Sci. (2012) 91:215–23. doi: 10.3382/ps.2011-01587
67. Shamseldin, R, Gibril, S, Atta, M, Yassin, O, and Hassan, A. Effect of rearing system, slaughter age and sex on Turkey (Meleagris gallopavo) carcass components percentages. Res Opinions Anim Vet Sci. (2014) 4:198–202.
68. Śmiecińska, K, Hnatyk, N, Daszkiewicz, T, Kubiak, D, and Matusevičiu, P. The effect of frozen storage on the quality of vacuum-packaged Turkey meat. Veterinarija ir Zootechnika. (2015) 71
69. Leeson, S, and Caston, L. Response of two strains of Turkey hens to various protein and energy feeding programs. Poult Sci. (1991) 70:1739–47. doi: 10.3382/ps.0701739
70. Melnychuk, V, Robinson, F, Renema, R, Hardin, R, Emmerson, D, and Bagley, L. Carcass traits and reproductive development at the onset of lay in two lines of female turkeys. Poult Sci. (1997) 76:1197–204. doi: 10.1093/ps/76.9.1197
71. Salmon, R. Effect of grower and finisher protein on performance, carcass grade, and meat yield of Turkey broilers. Poult Sci. (1984) 63:1980–6. doi: 10.3382/ps.0631980
72. Lee, H, Erasmus, M, Swanson, J, Hong, H, and Kang, I. Improvement of Turkey breast meat quality and cooked gel functionality using hot-boning, quarter sectioning, crust-freeze-air-chilling and cold-batter-mincing technologies. Poult Sci. (2016) 95:138–43. doi: 10.3382/ps/pev313
73. Przywitowski, M, Mikulski, D, Zdunczyk, Z, Rogiewicz, A, and Jankowski, J. The effect of dietary high-tannin and low-tannin faba bean (Vicia faba L.) on the growth performance, carcass traits and breast meat characteristics of finisher turkeys. Anim Feed Sci Technol. (2016) 221:124–36. doi: 10.1016/j.anifeedsci.2016.08.027
74. Ciurescu, G, Vasilachi, A, and Grosu, H. Efficacy of microbial phytase on growth performance, carcass traits, bone mineralization, and blood biochemistry parameters in broiler turkeys fed raw chickpea (Cicer arietinum L., cv. Burnas) diets. J Appl Poult Res. (2020) 29:171–84. doi: 10.1016/j.japr.2019.10.004
75. Gálvez, F, Domínguez, R, Pateiro, M, Carballo, J, Tomasevic, I, and Lorenzo, JM. Effect of gender on breast and thigh Turkey meat quality. Br Poult Sci. (2018) 59:408–15. doi: 10.1080/00071668.2018.1465177
76. Case, L, Wood, B, and Miller, S. The investigation of ultrasound technology to measure breast muscle depth as a correlated trait to breast meat yield in Turkey (Meleagris gallopavo). J Anim Sci. (2012) 90:3410–7. doi: 10.2527/jas.2011-4822
77. Darshana, B, Bhaisare, DT, Churchil, RR, and Punniamurthy, N. Effect of dietary supplementation of herbal seeds on carcass traits of Turkey poults. Vet World. (2014) 7:938–42. doi: 10.14202/vetworld.2014.938-942
78. Barbut, S. Colour measurements for evaluating the pale soft exudative (PSE) occurrence in Turkey meat. Food Res Int. (1993) 26:39–43. doi: 10.1016/0963-9969(93)90103-P
79. Cavalcanti, ÉNF, Giampietro-Ganeco, A, Mello, JL, Fidelis, HA, Oliveira, RF, Pereira, MR, et al. Breast meat quality of Turkey breeder hens at disposal age affected by deep pectoral myopathy. Poult Sci. (2021) 100:101259. doi: 10.1016/j.psj.2021.101259
80. Owens, C, and Sams, A. The influence of transportation on Turkey meat quality. Poult Sci. (2000) 79:1204–7. doi: 10.1093/ps/79.8.1204
81. Parteca, S, Tonial, IB, do Prado, NV, and da Trindade Alfaro, A. Electrical stunning parameters: impact on the quality of Turkey meat (Meleagris gallopavo). J Food Sci Technol. (2020) 57:2612–8. doi: 10.1007/s13197-020-04297-6
82. Sell, J, Ferket, P, Angel, C, Scheideler, S, Escribano, F, and Zatari, I. Performance and carcass characteristics of Turkey toms as influenced by dietary protein and metabolizable energy. Nut Rep Int. (1989) 40:979–92.
83. Laudadio, V, Introna, M, Lastella, NM, and Tufarelli, V. Feeding of low-fibre sunflower (Helianthus annus L.) meal as substitute of soybean meal in Turkey rations: effects on growth performance and meat quality. J Poult Sci. (2013) 51:185–90. doi: 10.2141/jpsa.0130132
84. Carvalho, RH, Soares, AL, Guarnieri, PD, Oba, A, Ida, EI, and Shimokomaki, M. Turkey meat. Seasonal effect on meat quality and on dead on arrival index in a commercial plant. Braz Arch Biol Technol. (2018) 61:61. doi: 10.1590/1678-4324-2018180106
85. Northcutt, J, Buhr, R, and Young, L. Influence of preslaughter stunning on Turkey breast muscle quality. Poult Sci. (1998) 77:487–92. doi: 10.1093/ps/77.3.487
86. Laudadio, V, Tufarelli, V, Dario, M, D’emilio, F, and Vicenti, A. Growth performance and carcass characteristics of female turkeys as affected by feeding programs. Poult Sci. (2009) 88:805–10. doi: 10.3382/ps.2008-00082
87. Biswas, A, Divya, S, Mandal, A, Majumdar, S, and Singh, R. Effects of dietary supplementation of organic chromium (picolinate) on physical and biochemical characteristics of semen and carcass traits of male turkeys. Anim Reprod Sci. (2014) 151:237–43. doi: 10.1016/j.anireprosci.2014.10.007
88. Boukhris, H, Damergi, C, Najar, T, and Samet, A. Transport stress impact on postmortem metabolisms of Turkey meat quality. J New Sci. (2017) 26:37.
89. Deus, D, Kehrenberg, C, Schaudien, D, Klein, G, and Krischek, C. Effect of a nano-silver coating on the quality of fresh Turkey meat during storage after modified atmosphere or vacuum packaging. Poult Sci. (2017) 96:449–57. doi: 10.3382/ps/pew308
90. Drażbo, A, Kozłowski, K, Ognik, K, Zaworska, A, and Jankowski, J. The effect of raw and fermented rapeseed cake on growth performance, carcass traits, and breast meat quality in Turkey. Poult Sci. (2019) 98:6161–9. doi: 10.3382/ps/pez322
91. Feng, X, Moon, SH, Lee, HY, and Ahn, DU. Effect of irradiation on the parameters that influence quality characteristics of raw Turkey breast meat. Radiat Phys Chem. (2017) 130:40–6. doi: 10.1016/j.radphyschem.2016.07.015
92. Ferket, P, Malheiros, R, Moraes, V, Ayoola, A, Barasch, I, Toomer, O, et al. Effects of functional oils on the growth, carcass and meat characteristics, and intestinal morphology of commercial Turkey toms. Poult Sci. (2020) 99:3752–60. doi: 10.1016/j.psj.2020.03.050
93. Hristakieva, P, Oblakova, M, Mincheva, N, Ivanova, I, Lalev, M, Ivanov, N, et al. Effect of dry herbal feed additive on the performance and meat quality of turkeys broilers. J Hyg Eng. (2021) 35:22–30.
94. Javid, A, Hussain, A, Ashraf, M, Mahmud, A, Altaf, M, Hussain, SM, et al. Variations in carcass yield and meat sensory quality attributes between turkeys (meleagris gallopavo) reared in free-range and confinement rearing systems. Indian J Anim Res. (2019) 53:1543–7.
95. Majumdar, S, Bhanja, S, Singh, R, and Agarwal, S. Effect of age on the carcass traits and meat quality of Turkey poults. J Appl Anim Res. (2005) 27:85–8. doi: 10.1080/09712119.2005.9706546
96. Maki, A, and Froning, G. Effect of post-mortem electrical stimulation on quality of Turkey meat. Poult Sci. (1987) 66:1155–7. doi: 10.3382/ps.0661155
97. Moran, E Jr, Summers, J, and Orr, H. The effect of absolute alterations in energy concentration of developing and finishing diets for the large white Turkey on performance and carcass quality with a note on the correlation of back skin fat and grade of finish. Br Poult Sci. (1969) 10:127–38. doi: 10.1080/00071666908415751
98. Ngoka, DA, Froning, G, Lowry, S, and Babji, A. Effects of sex, age, preslaughter factors, and holding conditions on the quality characteristics and chemical composition of Turkey breast muscles. Poult Sci. (1982) 61:1996–2003. doi: 10.3382/ps.0611996
99. Obanor, F, Morton, J, Geesink, G, and Bickerstaffe, R. Effect of processing on Turkey meat quality and proteolysis. Poult Sci. (2005) 84:1123–8. doi: 10.1093/ps/84.7.1123
100. Oblakova, M, Hristakieva, P, Mincheva, N, Ivanova, I, Lalev, M, Ivanov, N, et al. Effect of dietary herbal essential oils on the performance and meat quality of female turkeys broilers. Trakia J Sci. (2021) 19:130–8. doi: 10.15547/tjs.2021.02.003
101. Oblakova, M, Ribarski, S, Oblakov, N, and Hristakieva, P. Chemical composition and quality of Turkey-broiler meat from crosses of layer light (ll) and meat heavy (mh) Turkey. Trakia J Sci. (2016) 14:142–7. doi: 10.15547/tjs.2016.02.004
102. Patterson, B, Matarneh, S, Stufft, K, Preisser, R, Shi, H, Gerrard, D, et al. Pectoralis major muscle of Turkey displays divergent function as correlated with meat quality. Poult Sci. (2017) 96:1492–503. doi: 10.3382/ps/pew410
103. Ylä-Ajos, M, Tuominen, S, Hänninen, L, Ruusunen, M, Puolanne, E, and Valros, A. Gas composition in controlled atmosphere stunning affects Turkey meat quality traits. Br Poult Sci. (2012) 53:47–56. doi: 10.1080/00071668.2012.658025
104. Işgüzar, E. Growth, carcass traits and meat quality of bronze and white turkeys in Isparta province of Turkey. Arch Anim Breed. (2003) 46:471–81. doi: 10.5194/aab-46-471-2003
105. Farghly, M, and Abou-Kassem, D. Impacts of feed color and form on growth performance of local Turkey. Egypt J Nut Feeds. (2014) 17:537–47.
106. Safiyu, KK, Sogunle, OM, Egbeyale, LT, and Shittu, TA. An exploratory study on the effects of rearing system and plumage colour on performance, carcass characteristics and meat quality of local turkeys. Int J Health Anim Sci Food Saf. (2019) 6:1–15. doi: 10.13130/2283-3927/10794
107. Ojewola, G, Abasiekong, S, and Nwachukwu, C. Methionine supplementation in the productive efficiency, carcass characteristics and economics of growing indigenous Turkey. Niger J Anim Sci. (2001) 4:49949. doi: 10.4314/tjas.v4i2.49949
108. Ojewola, G, Ukachukwu, S, and Onyenucheya, F. Comparative carcass characteristics of indigenous Turkey poults fed different agro-industrial by-products. Niger J Anim Sci. (2000) 3:159–14.
109. Ribarski, S, Oblakova, M, Miteva, D, and Oblakov, N. Comparative study of carcass characteristics and chemical composition of meat in north caucasian bronze and wild Turkey (Meleagris gallopavo silvestris vieillot). Sci Technol. (2015) 5:94–9.
110. Ribarski, S, and Oblakova, M. Slaughter yield and quality of meat from wild Turkey (Meleagris gallopavo silvestris Vieillot) reared in hunting reserve in South Bulgaria. Trakia J Sci. (2016) 14:135–41. doi: 10.15547/tjs.2016.02.003
111. Babji, A, Froning, G, and Ngoka, D. The effect of preslaughter environmental temperature in the presence of electrolyte treatment on Turkey meat quality. Poult Sci. (1982) 61:2385–9. doi: 10.3382/ps.0612385
112. Babji, A, Froning, G, and Ngoka, D. The effect of preslaughter dietary electrolyte treatment on carcass yield and Turkey meat quality characteristics. Poult Sci. (1982) 61:1972–5. doi: 10.3382/ps.0611972
113. Biswas, A, Mandal, A, and Singh, R. Effect of dietary supplementation of chromium picolinate on productive performance, egg quality and carcass traits in laying turkeys. Anim Nutr Feed Technol. (2015) 15:59–66. doi: 10.5958/0974-181X.2015.00007.4
114. Farghly, M, Alagawany, M, and Abd, E-HM. Feeding time can alleviate negative effects of heat stress on performance, meat quality and health status of Turkey. Br Poult Sci. (2018) 59:205–10. doi: 10.1080/00071668.2017.1413233
115. Sell, J. Influence of metabolizable energy feeding sequence and dietary protein on performance and selected carcass traits of tom turkeys. Poult Sci. (1993) 72:521–34. doi: 10.3382/ps.0720521
116. Rogerson, PA. Data reduction: factor analysis and cluster analysis. Stat Methods Geogr. (2001) 2001:192–7. doi: 10.4135/9781849209953.n10
117. Nanda, MA, Seminar, KB, Nandika, D, and Maddu, A. Discriminant analysis as a tool for detecting the acoustic signals of termites Coptotermes curvignathus (Isoptera: Rhinotermitidae). Int J Technol. (2018) 9:840–51. doi: 10.14716/ijtech.v9i4.455
118. González Ariza, A, Arando Arbulu, A, Navas González, FJ, Delgado Bermejo, JV, and Camacho Vallejo, ME. Discriminant canonical analysis as a validation tool for multivariety native breed egg commercial quality classification. Food Secur. (2021) 10:632. doi: 10.3390/foods10030632
119. Tai, F, and Pan, W. Incorporating prior knowledge of gene functional groups into regularized discriminant analysis of microarray data. Bioinformatics. (2007) 23:3170–7. doi: 10.1093/bioinformatics/btm488
120. Anuthama, K, Shankar, S, Ilayaraja, V, Kumar, GS, and Rajmohan, M. Determining dental sex dimorphism in south Indians using discriminant function analysis. Forensic Sci Int. (2011) 212:86–9. doi: 10.1016/j.forsciint.2011.05.018
121. Zhang, Q, Hu, J, and Bai, Z. Modified Pillai’s trace statistics for two high-dimensional sample covariance matrices. J Statist Plan Inference. (2020) 207:255–75. doi: 10.1016/j.jspi.2020.01.002
122. Marín Navas, C, Delgado Bermejo, JV, McLean, AK, León Jurado, JM, de la Borbolla, R, Ruiberriz de Torres, A, et al. Discriminant canonical analysis of the contribution of Spanish and Arabian purebred horses to the genetic diversity and population structure of Hispano-Arabian horses. Animals. (2021) 11:269. doi: 10.3390/ani11020269
123. Hair, JF, Black, W, Babin, BJ, Anderson, R, and Tatham, R. Canonical correlation analysis: a supplement to multivariate data analysis. Multivariate Data Analy. (2010) 7:1–43.
124. Andersson, L. Genetic dissection of phenotypic diversity in farm animals. Nat Rev Genet. (2001) 2:130–8. doi: 10.1038/35052563
125. Kamara, D, Gyenai, K, Geng, T, Hammade, H, and Smith, E. Microsatellite marker-based genetic analysis of relatedness between commercial and heritage turkeys (Meleagris gallopavo). Poult Sci. (2007) 86:46–9. doi: 10.1093/ps/86.1.46
126. Strillacci, MG, Marelli, SP, Milanesi, R, Zaniboni, L, Punturiero, C, and Cerolini, S. Copy number variants in four Italian Turkey breeds. Animals. (2021) 11:391. doi: 10.3390/ani11020391
127. Brenoe, U, and Kolstad, K. Body composition and development measured repeatedly by computer tomography during growth in two types of turkeys. Poult Sci. (2000) 79:546–52. doi: 10.1093/ps/79.4.546
128. Murawska, D. The effect of age on growth performance and carcass quality parameters in different poultry species. Poult Sci. (2017) 1:33–50. doi: 10.5772/64860
129. Brake, J, Havenstein, G, Ferket, P, Rives, D, and Giesbrecht, F. Relationship of sex, strain, and body weight to carcass yield and offal production in turkeys. Poult Sci. (1995) 74:161–8. doi: 10.3382/ps.0740161
130. Peng, I, Adams, R, Furumoto, E, Hester, P, Larsen, J, Pike, O, et al. Allometric growth patterns and meat yields of carcass parts of Turkey toms as influenced by lighting programs and age. Poult Sci. (1985) 64:871–6. doi: 10.3382/ps.0640871
131. Sow, T, and Grongnet, J-F. Sensory characteristics and consumer preference for chicken meat in Guinea. Poult Sci. (2010) 89:2281–92. doi: 10.3382/ps.2010-00679
132. Grashorn, M. Research into poultry meat quality. Br Poult Sci. (2010) 51:60–7. doi: 10.1080/00071668.2010.506761
133. Nkukwana, T, Muchenje, V, Masika, P, Pieterse, E, Hoffman, L, and Dzama, K. Proximate composition and variation in colour, drip loss and pH of breast meat from broilers supplemented with Moringa oleifera leaf meal over time. Anim Prod Sci. (2015) 56:1208–16. doi: 10.1071/AN14055
134. Saláková, A, Straková, E, Válková, V, Buchtová, H, and Steinhauserová, I. Quality indicators of chicken broiler raw and cooked meat depending on their sex. Acta Vet Brno. (2009) 78:497–504. doi: 10.2754/avb200978030497
135. Bihan-Duval, L, Debut, M, Berri, CM, Sellier, N, Santé-Lhoutellier, V, Jégo, Y, et al. Chicken meat quality: genetic variability and relationship with growth and muscle characteristics. BMC Genet. (2008) 9:1–6. doi: 10.1186/1471-2156-9-53
136. Le Bihan-Duval, E, Berri, C, Baéza, E, Millet, N, and Beaumont, C. Estimation of the genetic parameters of meat characteristics and of their genetic correlations with growth and body composition in an experimental broiler line. Poult Sci. (2001) 80:839–43. doi: 10.1093/ps/80.7.839
137. Chumngoen, W, Chen, C, Chen, H, and Tan, F. Influences of end-point heating temperature on the quality attributes of chicken meat. Br Poult Sci. (2016) 57:740–50. doi: 10.1080/00071668.2016.1216942
138. Mello, J, Souza, R, Paschoalin, G, Ferrari, F, Berton, M, Giampietro-Ganeco, A, et al. Physical and chemical characteristics of spent hen breast meat aged for 7 days. Anim Prod Sci. (2016) 57:2133–40. doi: 10.1071/AN16195
139. Bowker, B, and Zhuang, H. Relationship between water-holding capacity and protein denaturation in broiler breast meat. Poult Sci. (2015) 94:1657–64. doi: 10.3382/ps/pev120
140. Li, X, Wang, Y, Sun, Y, Pan, D, and Cao, J. The effect of ultrasound treatments on the tenderizing pathway of goose meat during conditioning. Poult Sci. (2018) 97:2957–65. doi: 10.3382/ps/pey143
141. Culler, R, Smith, G, and Cross, H. Relationship of myofibril fragmentation index to certain chemical, physical and sensory characteristics of bovine longissimus muscle. J Food Sci. (1978) 43:1177–80. doi: 10.1111/j.1365-2621.1978.tb15263.x
142. Sun, X, Koltes, D, Coon, C, Chen, K, and Owens, C. Instrumental compression force and meat attribute changes in woody broiler breast fillets during short-term storage. Poult Sci. (2018) 97:2600–6. doi: 10.3382/ps/pey107
143. Tijare, VV, Yang, F, Kuttappan, V, Alvarado, C, Coon, C, and Owens, C. Meat quality of broiler breast fillets with white striping and woody breast muscle myopathies. Poult Sci. (2016) 95:2167–73. doi: 10.3382/ps/pew129
144. Hailemariam, A, Esatu, W, Abegaz, S, Urge, M, Assefa, G, and Dessie, T. Nutritional composition and sensory characteristics of breast meat from different chickens. Appl Food Res. (2022) 2:100233. doi: 10.1016/j.afres.2022.100233
145. Molapo, S, and Webb, E. Influence of feed restriction method and season on the chemical composition of meat in Koekoek chickens. Online J Anim Feed Res. (2020) 10:290–6. doi: 10.51227/ojafr.2020.39
146. Faustin-Evaris, E, Sarmiento-Franco, LA, Capetillo-Leal, CM, and Sandoval-Castro, CA. Composition of slow-growing male Chicken’s meat and bone quality as affected by dietary Moringa oleifera lam. Meal Animals. (2022) 12:3482. doi: 10.3390/ani12243482
147. Lonergan, S, Deeb, N, Fedler, C, and Lamont, S. Breast meat quality and composition in unique chicken populations. Poult Sci. (2003) 82:1990–4. doi: 10.1093/ps/82.12.1990
148. Renema, R, Robinson, F, Newcombe, M, and McKay, R. Effects of body weight and feed allocation during sexual maturation in broiler breeder hens. 1. Growth and carcass characteristics. Poult Sci. (1999) 78:619–28. doi: 10.1093/ps/78.5.619
149. Tougan, P, Dahouda, M, Salifou, C, Ahounou, G, Kossou, D, Amenou, C, et al. Relationships between technological and nutritional meat quality parameters in local poultry populations (Gallus gallus) of Benin. Int J Biol Chem Sci. (2013) 7:2450–67. doi: 10.4314/ijbcs.v7i6.22
150. Lewis, P Jr, Rakes, L, Brown, H, Brown, A Jr, Johnson, Z, and Brown, C. Effects of maturation rate, marbling and cooking on the fat, cholesterol and lipid phosphorus composition of beef muscle 1. J Muscle Foods. (1993) 4:41–56. doi: 10.1111/j.1745-4573.1993.tb00490.x
151. Milićević, D, Vranić, D, Mašić, Z, Parunović, N, Trbović, D, Nedeljković-Trailović, J, et al. The role of total fats, saturated/unsaturated fatty acids and cholesterol content in chicken meat as cardiovascular risk factors. Lipids Health Dis. (2014) 13:1–12. doi: 10.1186/1476-511X-13-42
152. Rhee, K, Smith, G, and Dutson, T. Relationships of ether-extractable fat vs Folch fat and fat content vs cholesterol content for beef longissimus muscles: a research note. J Food Sci. (1988) 53:969–70. doi: 10.1111/j.1365-2621.1988.tb08999.x
153. Ponte, P, Mendes, I, Quaresma, M, Aguiar, M, Lemos, J, Ferreira, L, et al. Cholesterol levels and sensory characteristics of meat from broilers consuming moderate to high levels of alfalfa. Poult Sci. (2004) 83:810–4. doi: 10.1093/ps/83.5.810
154. Sirri, F, Bianchi, M, Petracci, M, and Meluzzi, A. Influence of partial and complete caponization on chicken meat quality. Poult Sci. (2009) 88:1466–73. doi: 10.3382/ps.2008-00405
155. Chizzolini, R, Zanardi, E, Dorigoni, V, and Ghidini, S. Calorific value and cholesterol content of normal and low-fat meat and meat products. Trends Food Sci Technol. (1999) 10:119–28. doi: 10.1016/S0924-2244(99)00034-5
156. Aksoy, Y, Çiçek, Ü, Şen, U, Şirin, E, Uğurlu, M, Önenç, A, et al. Meat production characteristics of Turkish native breeds: II. Meat quality, fatty acid, and cholesterol profile of lambs. Arch Animal Breed. (2019) 62:41–8. doi: 10.5194/aab-62-41-2019
157. Reitmeier, C, and Prusa, K. Cholesterol content and sensory analysis of ground pork as influenced by fat level and heating. J Food Sci. (1987) 52:916–8. doi: 10.1111/j.1365-2621.1987.tb14241.x
158. Coró, FA, Youssef, EY, and Shimokomaki, M. Age related changes in poultry breast meat collagen pyridinoline and texture. J Food Biochem. (2002) 26:533–41. doi: 10.1111/j.1745-4514.2002.tb00771.x
159. Komprda, T, Sarmonova, I, Zelenka, J, Bakaj, P, Fialova, M, and Fajmonova, E. Effect of sex and age on cholesterol and fatty acid content in Turkey meat. Archiv fur Geflugelkunde. (2002) 66:263–73.
160. Dransfield, E, and Sosnicki, A. Relationship between muscle growth and poultry meat quality. Poult Sci. (1999) 78:743–6. doi: 10.1093/ps/78.5.743
161. Marelli, SP, Zaniboni, L, Strillacci, MG, Madeddu, M, and Cerolini, S. Morphological characterization of two light Italian Turkey breeds. Animals. (2022) 12:571. doi: 10.3390/ani12050571
162. Tang, H, Gong, Y, Wu, C, Jiang, J, Wang, Y, and Li, K. Variation of meat quality traits among five genotypes of chicken. Poult Sci. (2009) 88:2212–8. doi: 10.3382/ps.2008-00036
163. Pérez-Lara, E, Camacho-Escobar, M, García-López, J, Machorro-Samano, S, Ávila-Serrano, N, and Arroyo-Ledezma, J. Mathematical modeling of the native Mexican turkey's growth. Open J Animal Sci. (2013) 3:305–10. doi: 10.4236/ojas.2013.34045
164. Segura-Correa, J, Santos-Ricalde, R, and Palma-Avila, I. Non-linear model to describe growth curves of commercial Turkey in the tropics of Mexico. Brazilian J Poult Sci. (2017) 19:27–32. doi: 10.1590/1806-9061-2016-0246
Keywords: breed traceability, weight-related traits, histological properties, sexual dimorphism, color-related traits, pH, gross nutrient, water-holding capacity
Citation: Salgado Pardo JI, González Ariza A, Navas González FJ, León Jurado JM, Díaz Ruiz E, Delgado Bermejo JV and Camacho Vallejo ME (2024) Discriminant canonical analysis as a tool for genotype traceability testing based on turkey meat and carcass traits. Front. Vet. Sci. 11:1326519. doi: 10.3389/fvets.2024.1326519
Edited by:
Şenol Çelik, Bingöl University, TürkiyeReviewed by:
Georgios Arsenos, Aristotle University of Thessaloniki, GreeceMartina Bordini, University of Bologna, Italy
Achille Schiavone, University of Turin, Italy
Copyright © 2024 Salgado Pardo, González Ariza, Navas González, León Jurado, Díaz Ruiz, Delgado Bermejo and Camacho Vallejo. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Antonio González Ariza, angoarvet@outlook.es; Francisco Javier Navas González, fjng87@hotmail.com
 José Ignacio Salgado Pardo
José Ignacio Salgado Pardo Antonio González Ariza
Antonio González Ariza Francisco Javier Navas González
Francisco Javier Navas González José Manuel León Jurado2
José Manuel León Jurado2  Esther Díaz Ruiz
Esther Díaz Ruiz Juan Vicente Delgado Bermejo
Juan Vicente Delgado Bermejo