Dietary supplementation of Chinese herbal medicines enhances the immune response and resistance of rainbow trout (Oncorhynchus mykiss) to infectious hematopoietic necrosis virus
- 1College of Animal Science and Technology, Gansu Agricultural University, Lanzhou, China
- 2College of Science, Gansu Agricultural University, Lanzhou, China
Rainbow trout is a widely farmed economical cold-water fish worldwide, but the prevalence of infectious hematopoietic necrosis virus (IHNV) presents a severe risk to the aquaculture industry, resulting in high mortality and huge economic losses. In this study, the impacts of different concentrations (0, 10, 20, and 30 g/kg) of Chinese herbal medicine mixture (CHMM) on the immune response and resistance of rainbow trout to IHNV infection were evaluated. The results show that CHMM noticeably increased (P < 0.05) T-SOD, CAT, AST, ALT, ACP, and AKP activities and decreased MDA content. NF-κB, TNF-α, IFN-β, IL-1β, JAK1, HSP70, and HSP90 expressions were significantly upregulated (P < 0.05) in all CHMMs, while SOCS2 expression was downregulated (P < 0.05). Following infection with IHNV, feeding rainbow trout with varying amounts of CHMM resulted in noticeably increased (P < 0.05) T-SOD, ACP, and AKP activities and significantly decreased (P < 0.05) MDA content and AST and ALT activities. TNF-α, IFN-β, IL-1β, HSP70, and HSP90 expressions were significantly upregulated (P < 0.05) in all CHMMs, while the expressions of JAK1 and SOCS2 were downregulated. The expression level of the IHNV G protein gene at a dosage of 20 g/kg was notably lower than that of the other CHMM feeding groups. This study provides a solid scientific basis for promoting CHMM as an immunostimulant for boosting antiviral immunity in rainbow trout.
1 Introduction
Aquaculture is a growing industry that provides important proteins and contributes to the production of nutrition (1). Rainbow trout (Oncorhynchus mykiss), belonging to the Salmonidae family, is native to North America and Russia's Pacific coast, and it is widely farmed as a cold-water fish in the global aquaculture industry (2). Intensive farming conditions have contributed to economic growth while also leading to complex aquatic environments that make rainbow trout susceptible to pathogens (3). Infectious hematopoietic necrosis virus (IHNV), which belongs to the genus Novirhabdovirus of the family Rhabdoviridae, is highly pathogenic and widely transmissible, causing not only high mortality in rainbow trout but also brings huge economic losses to the global aquaculture industry (4, 5). Vaccines have long been the main choice for preventing viral diseases, but their efficacy is limited by financial constraints. Relevant studies have shown that immunostimulants enhance immune responses in fish (6). The significance of using immunostimulants in boosting the immune system and assisting in disease prevention in fish is increasing (7).
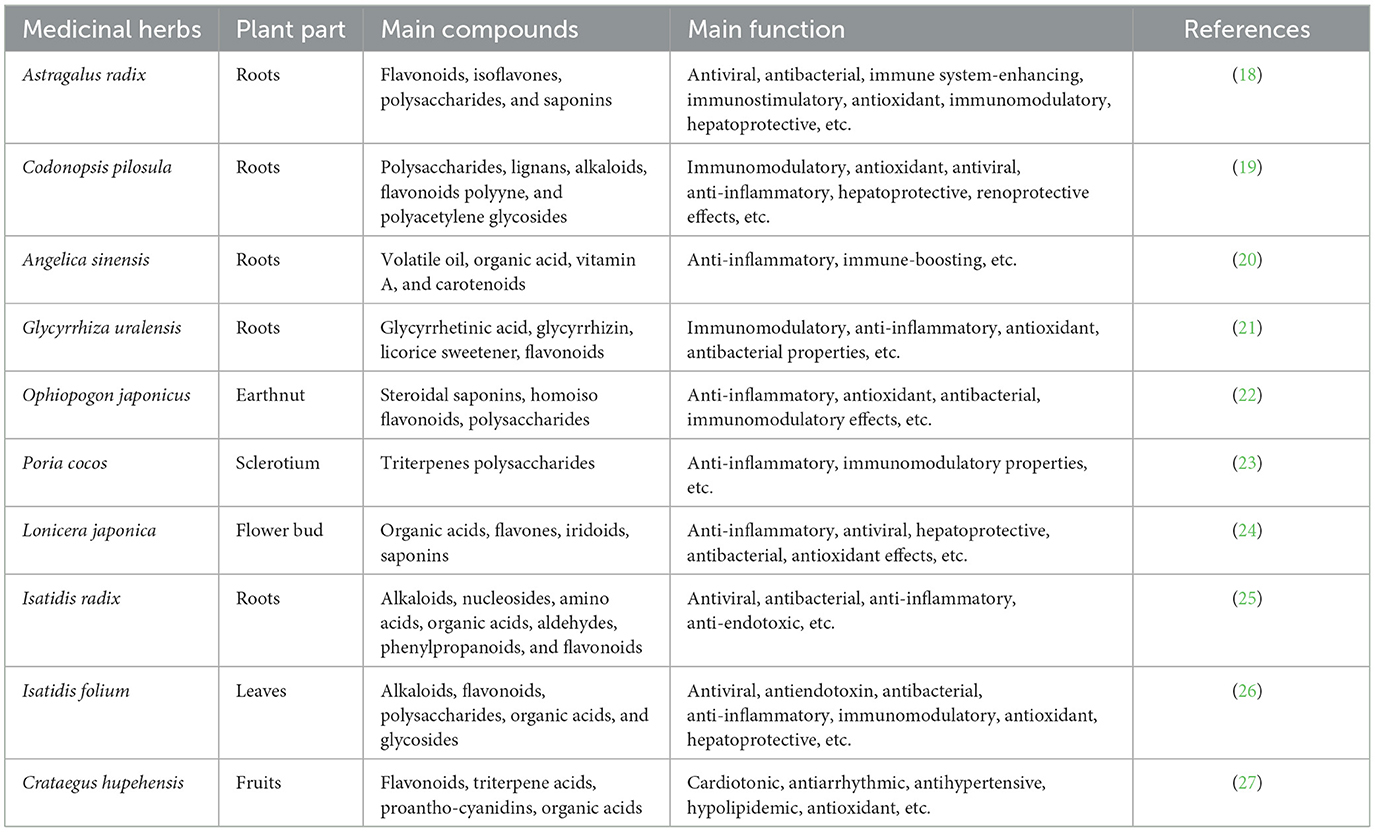
Chinese herbal medicine (CHM) has been utilized as an immunostimulant in China for thousands of years, encompassing a diverse array of organic compounds such as alkaloids, polysaccharides, flavonoids, and organic acids. Additionally, CHM contains essential nutrients that include vitamins, amino acids, and minerals (8). The active ingredients mentioned have been extensively studied for their powerful immune-enhancing, antioxidant, and direct anti-pathogen properties in preventing and controlling viral, bacterial, parasitic, and fungal diseases in fish (9). Moreover, compared with chemical agents, CHM possesses the advantages of low price, fewer side effects, and less environmental pollution. Consequently, CHM is considered a viable alternative to certain synthetic compounds, such as antibiotics and chemicals. The application of CHM in aquatic organisms can significantly improve their feeding capacity, increase their metabolic rate, accelerate protein biosynthesis, enhance antioxidant enzyme activity, and ultimately enhance their immune and disease resistance (10).
The addition of a 1:1:1 mixture of Astragalus membranaceus, Angelica sinensis, and Crataegus hupehens to the feed of Nile tilapia significantly increased the levels of LZM, SOD, and CAT. Furthermore, the survival rate of Nile tilapia in the herbal mixture-administered group was 70% compared to 35% in the control group after Streptococcus lactis-free infestation. These results suggest that Astragalus membranaceus, Angelica sinensis, and Crataegus hupehens act synergistically to improve the immunity and disease resistance in Nile tilapia (11). The addition of an herbal mixture of Astragalus, Angelica, Crataegus pinnatifida, Glycyrrhiza uralensis, and Lonicera japonica to the diet of a hybrid grouper (Epinephelus lanceolatus♂ × Epinephelus fuscoguttatus♀) significantly increased AKP, ACP, T-SOD, and CAT activities in the fish (12). Codonopsis pilosula, Atractylodes macrocephala, Poria cocos, Rehmannia glutinosa, Glycyrrhiza uralensis, Crataegus pinnatifida, Rhus chinensis, Gardenia jasminoides, and Zingiber officinale enhanced non-specific immunity in turbot (13). Similar studies also showed that CHMM, consisting of 12 herbs, enhanced T-SOD activity and LZM content in European eel (14). In addition, diets supplemented with CHMM also significantly increased LZM content and CAT, ACP, and AKP activities in Japanese sea bass (15). Cai et al. found that CHMM of Ziziphus jujuba, Chinese yam, and Astragalus extracts administered to juvenile rainbow trout infected with Vibrio parahaemolyticus for 56 days significantly upregulated the transcript level of HSP90 (16). All these results indicated that the CHMM exerted a beneficial synergistic effect and effectively improved the immunity of fish against diseases. Therefore, it is important to understand the effect of CHMM as an immune booster on the immunity and disease resistance of rainbow trout.
In fish, the liver plays a crucial role in numerous metabolic functions and physiological processes, including detoxification, nutrient metabolism, and biosynthesis (17). Additionally, the liver serves as an important immune organ in fish and can be infected with IHNV. To the best of our knowledge, there is currently limited research on how CHMM functions as an immunostimulant and how it affects the immune response and disease resistance in rainbow trout. This study examined the effects of varying the dietary concentrations of CHMM on the immune responses and resistance of rainbow trout to IHNV infection. This study will be the basis for promoting CHMM as an immunostimulant to improve the resistance of rainbow trout to pathogen infections.
2 Materials and methods
2.1 Experimental design
The rainbow trout weighed ~30 ± 0.5 g and were obtained from a trout farm in Yongjing County, Gansu Province, China. The rainbow trout were acclimated at the Aquatic Science Training Center of Gansu Agricultural University for 2 weeks, and all fish were fed a basal diet during rearing. In this study, we used the ratio of the previously studied herbal compound formulations as a reference (14–16). The 10 kinds of CHM used in this experiment (Table 1), all purchased from a local drugstore, were finely ground by using a 100-mesh sieve, and each CHM was uniformly mixed well according to the same mass ratio, dried under sterile control, and then precipitated and stored at 4°C until use. The composition of the feed of rainbow trout is based on the observations from the previous study (28). CHMM was added at a ratio of 0, 10, 20, and 30 g/kg. A total of 480 rainbow trout were placed in four groups in four tanks, with each tank comprising 30 fish. The control group (0 g/kg group) and the feeding groups (10, 20, and 30 g/kg) were set up. After acclimatization, the fish were fed at 3% of their body weight for 35 days. A circulating water system was used, with a 24-h uninterrupted oxygen supply, a water temperature of 12.0 ± 1.0°C, a pH value 7.3 ± 0.3, dissolved oxygen of 8.5 ± 0.5 mg/L, and an ammonia nitrogen concentration of < 0.1 mg/L. This experiment complies with institutional guidelines and protocols approved by the Animal Ethics Committee of Gansu Agricultural University (GSAU-Eth-AST-2021-004).
2.2 Challenge test
After feeding for 35 days, the study tested the impact of CHMM on IHNV-infected rainbow trout. Each rainbow trout received an injection of 100 μL of the cell culture medium containing 500 plaque-forming units of IHNV into the peritoneal cavity. The IHNV G protein gene was relatively quantified after the challenge (29). During the IHNV challenge, the feeding conditions were not changed in all groups.
2.3 Sample collection
On days 7, 21, and 35 of CHMM feeding and day 12 of infection of IHNV in rainbow trout, four rainbow trout were randomly selected from each group, a lethal dose of MS-222 (Sigma Aldrich Co., St. Louis, USA) was administered, and the liver tissue was then excised, put in sterile cryotubes, and quickly transported to liquid nitrogen for storage at −80°C, until further use.
2.4 Determination of antioxidant and immune parameters
First, the liver tissue stored at −80°C was crushed using a freeze mill, then nine times the volume of saline was added according to kit instructions, mixed well, and centrifuged at 4°C at 2,500 r/min for 10 min. Later, the supernatant liquor was collected as a tissue homogenate. According to the commercial kit instructions (Nanjing Jiancheng Institute of Biological Engineering, Nanjing, China), total superoxide dismutase (T-SOD), malondialdehyde (MDA), catalase (CAT), aspartate aminotransferase (AST), alanine aminotransferase (ALT), acid phosphatase (ACP), and alkaline phosphatase (AKP) activities were measured using a spectrophotometer or a microplate reader.
2.5 Detection of liver immune-related gene expression
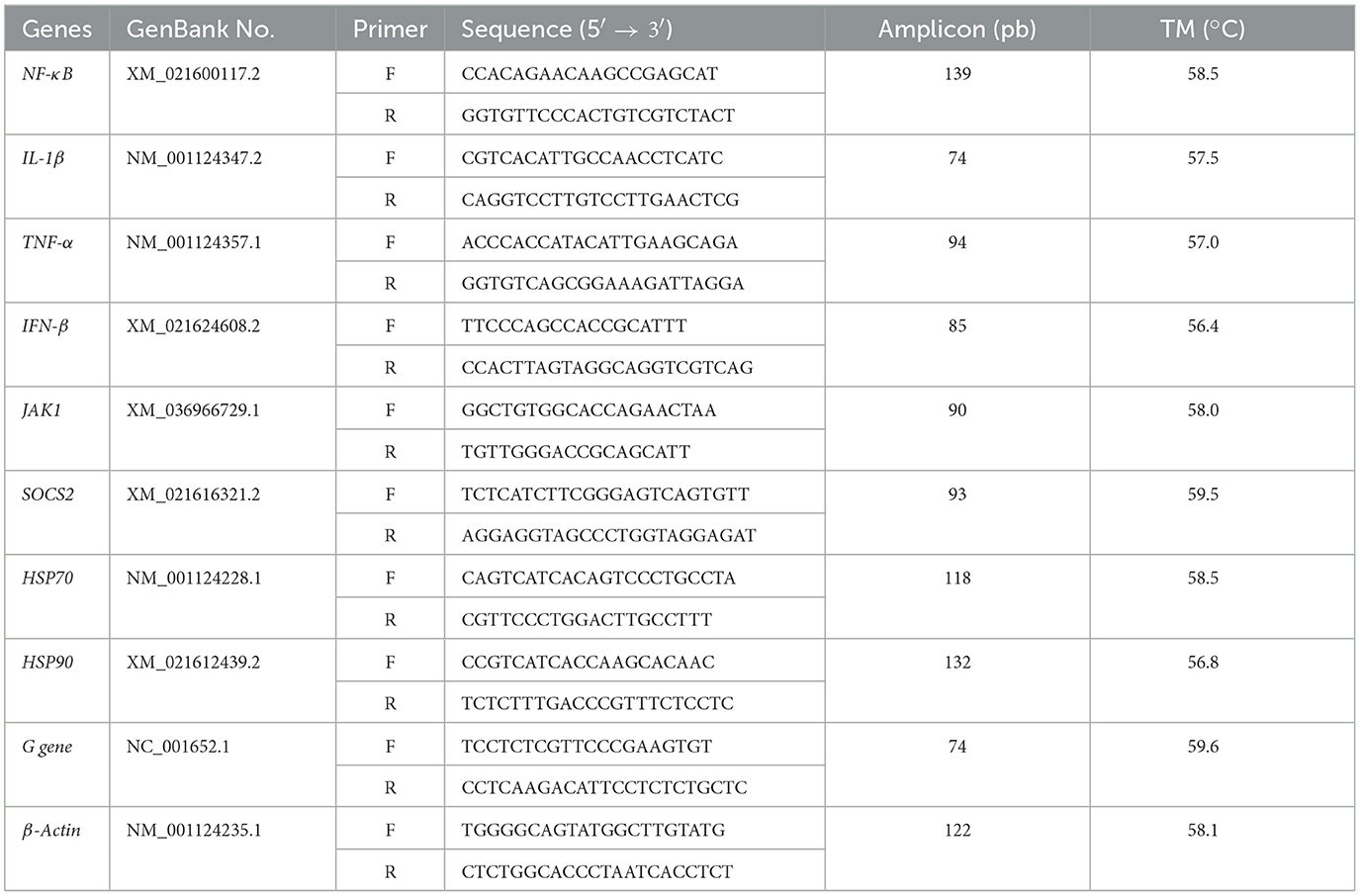
Total RNA was extracted from rainbow trout liver using the RNA extraction kit (TIANGEN, Beijing, China). Electrophoresis by 1.0% agarose gel was used to assess RNA integrity. RNA purity and concentration were measured using a NanoDrop 2000 spectrophotometer (Thermo Scientific, Waltham, USA) with a 260:280 ratio between 1.8 and 2.0. RNA samples were treated with DNase to remove genomic DNA. Subsequently, cDNA was prepared using a reverse transcription (Accurate Biology, Changsha, China). Real-time PCR was performed according to the manufacturer's protocol (n = 3) on a LightCycler®480 II instrument (Roche, Basel, Switzerland). The related target genes include NF-κB, TNF-α, IL-1β, IFN-β, JAK1, SOCS2, HSP70, HSP90, and the IHNV G protein gene. β-actin was selected as an internal reference gene to normalize the mRNA expression. The qRT-PCR reaction system consisted of 20.0 μL of SYBR® Green qPCR Super Mix (2 × ), 10 μmol/L of forward and reverse primers, 7.0 μL of RNase-free water, and 1.0 μL of cDNA template. The real-time PCR conditions were as follows: 95°C for 30 s, followed by 40 cycles at 95°C for 5 s and 60°C for 30 s. The primers for each gene are listed in Table 2. Immunology-related gene expression levels were examined using the 2−ΔΔCt method.
2.6 Statistical analysis
SPSS 25.0 software was used for all statistical analyses (IBM Corp., Armonk, USA). The Shapiro–Wilk and Levene's tests were used to examine normal distribution (SW > 0.05) and equality of variance (P > 0.05). A one-way ANOVA with Tukey's multiple comparison test was used to analyze multiple comparisons, and differences were considered statistically significant when the p-value was < 0.05. All experimental data are presented as the mean ± standard error (SE).
3 Results
3.1 Changes in immune parameters after feeding the CHMM
During the whole feeding process of CHMM, liver T-SOD, CAT, AST, ALT, and AKP activities in each feeding group were found to noticeably increase (P < 0.05) during the feeding of CHMM as compared to the control group, as shown in Figure 1. At day 7, liver ACP activity did not noticeably increase (P > 0.05); however, all feeding groups showed a reduction in MDA content with an increase in CHMM dosage. At day 21, the MDA content decreased, with the lowest level at the 30 g/kg dosage, and CAT, AST, ALT, and ACP activities increased, reaching a maximum at the 20 g/kg dosage. The MDA content was noticeably decreased (P < 0.05) at day 35. Moreover, AKP activity at 10 g/kg, AST activity at 20 g/kg, and ACP activity at the 30 g/kg dosages were noticeably increased (P < 0.05) in rainbow trout compared to other CHMM feeding groups.
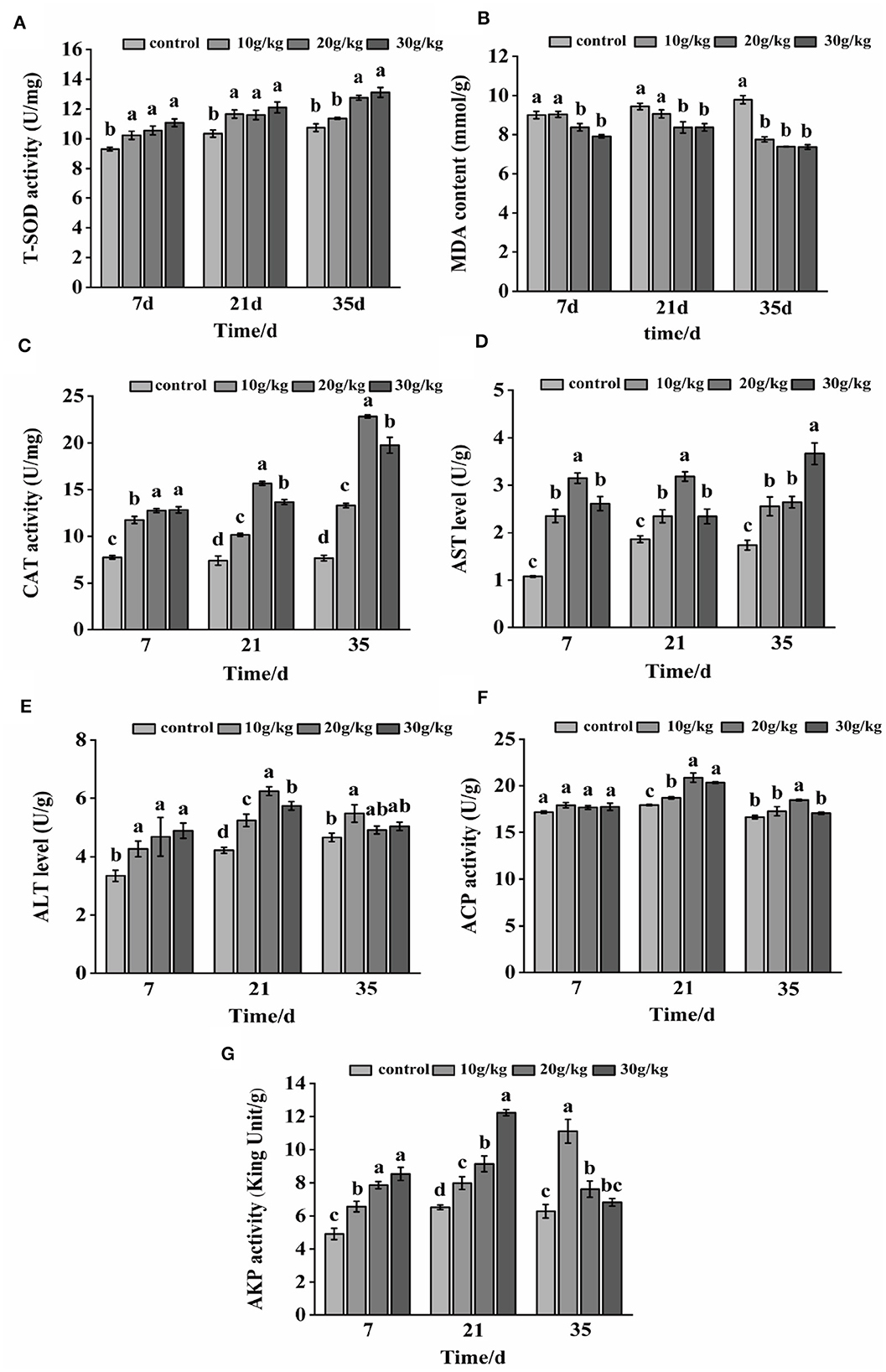
Figure 1. Effects of dietary CHMM on (A) T-SOD, (B) MDA, (C) CAT, (D) AST, (E) ALT, (F) ACP, and (G) AKP activities in the liver of rainbow trout; data are presented as mean ± S.E. with distinct superscript values denoting significance (P < 0.05); normal distribution SW > 0.05, homogeneity of variance P > 0.05.
3.2 Changes in immune-related genes after feeding the CHMM
As shown in Figure 2, at day 35, there was a noticeable upregulation (P < 0.05) of the NF-κB expression level in the 20 and 30 g/kg feeding groups as compared to the control group. At day 35, all feeding groups had significantly higher (P < 0.05) levels of IFN-β and TNF-α, and the highest expression levels were observed in the 20 g/kg group. At day 35, the 20 g/kg group showed a significant upregulation (P < 0.05) of IL-1β, JAK1, HSP70, and HSP90 expression levels. With an increase in dietary CHMM content at 35 days, SOCS2 expression was significantly downregulated (P < 0.05), and the lowest expression was observed in the 30 g/kg group.
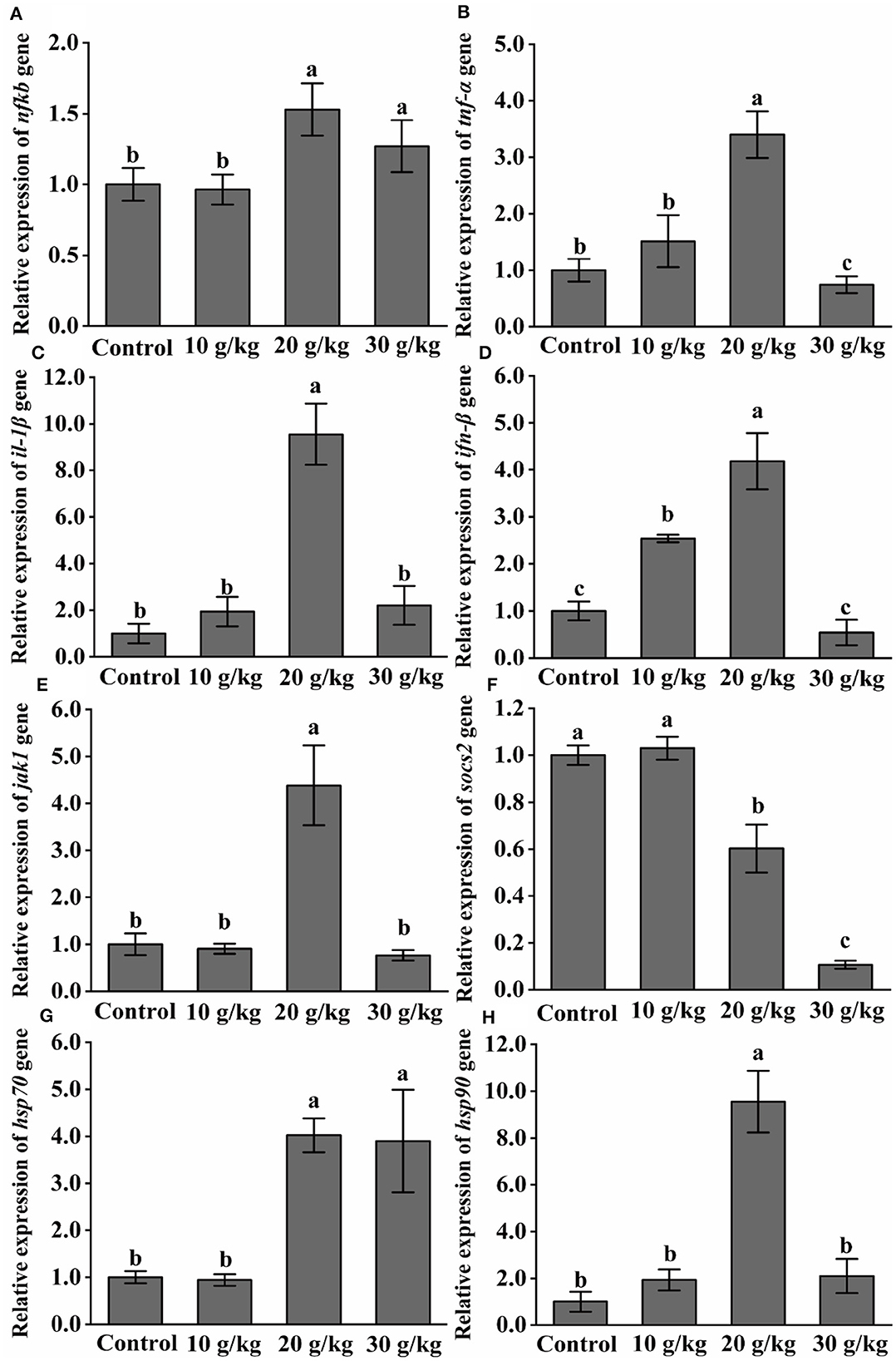
Figure 2. Effects of dietary CHMM on the expression of (A) NF-κB, (B) TNF-α, (C) IL-1β, (D) IFN-β, (E) JAK1, (F) SOCS2, (G) HSP70, and (H) HSP90 genes in the liver of rainbow trout; data are presented as mean ± S.E. with distinct superscript values denoting significance (P < 0.05); normal distribution SW > 0.05, homogeneity of variance P > 0.05.
3.3 Changes in immune parameters following IHNV infection
Following IHNV infection, all CHMM groups displayed noticeably higher (P < 0.05) ACP and AKP activities than the control group, as shown in Figure 3. Additionally, all CHMM groups showed a noticeable increase (P < 0.05) in liver T-SOD activity when compared to the control group; the highest level was observed in the 20 g/kg group. Additionally, compared to the control group, liver MDA content decreased and reached the lowest level in the 20 g/kg group. However, CAT activity did not noticeably change (P > 0.05) across all CHMM feeding groups. Furthermore, there was a decrease in liver AST, ALT, ACP, and AKP levels as the dietary CHMM levels increased among the three CHMM groups.
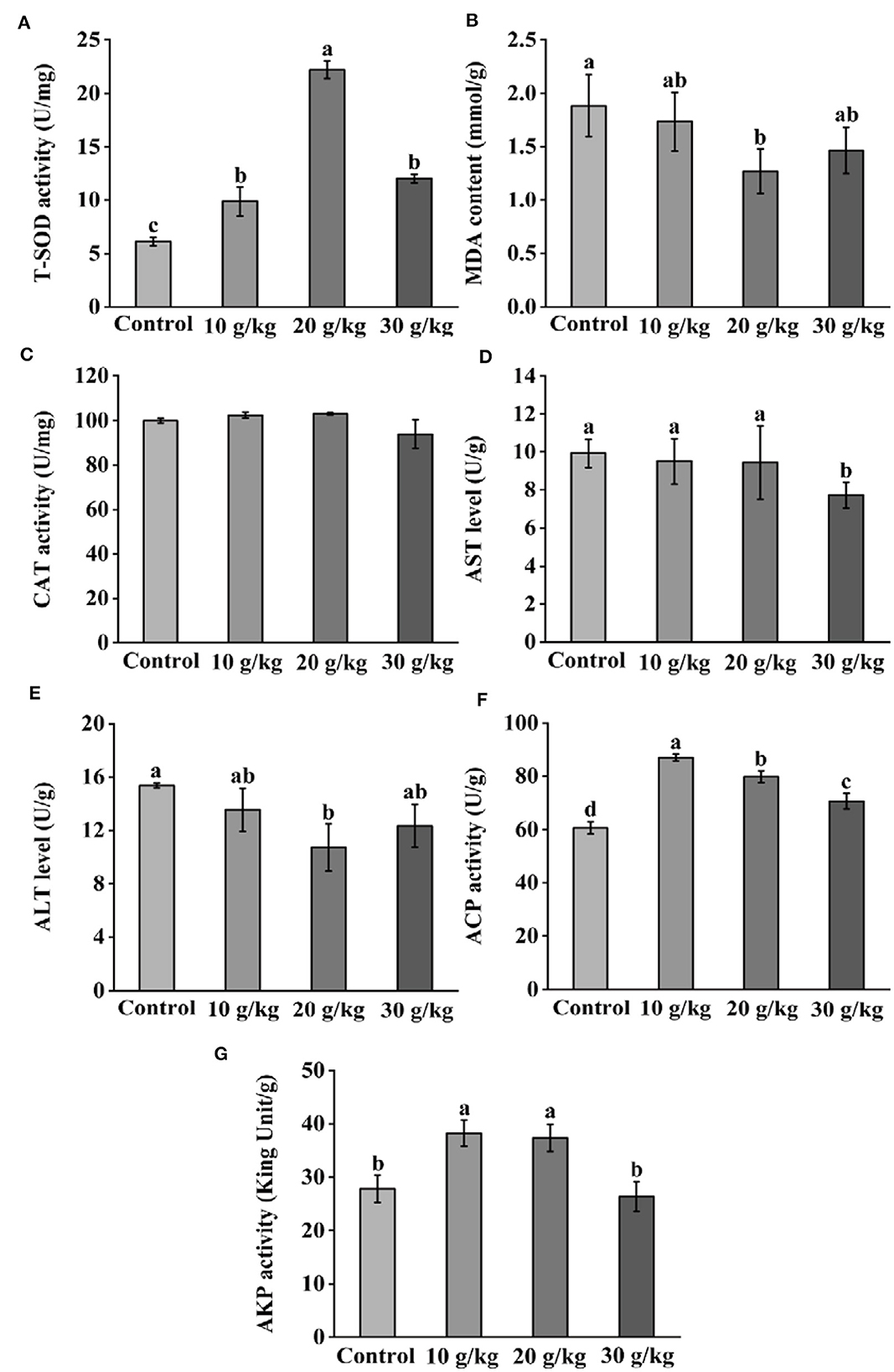
Figure 3. Effects of dietary CHMM on (A) T-SOD, (B) MDA, (C) CAT, (D) AST, (E) ALT, (F) ACP, and (G) AKP activities in the liver of rainbow trout after IHNV infection; data are presented as mean ± S.E. with distinct superscript values denoting significance (P < 0.05); normal distribution SW > 0.05; homogeneity of variance P > 0.05.
3.4 Changes in the expression of immune-related genes following IHNV infection
Considerably higher (P < 0.05) levels of IL-1β, TNF-α, IFN-β, JAK1, HSP70, and HSP90 following IHNV infection in the 10 g/kg group were observed as compared to the control, as shown in Figure 4. In the 20 and 30 g/kg groups, there was a noticeable downregulation (P < 0.05) of the expression of NF-κB, TNF-α, IL-1β, and SOCS2. Additionally as compared to the control group, the 10 g/kg group showed a significant upregulation (P < 0.05) of JAK1 expression, which was then gradually lowered. Following infection, the amount of dietary CHMM was shown to significantly downregulate (P < 0.05) the level of SOCS2, and the lowest expression observed in the 30 g/kg group.
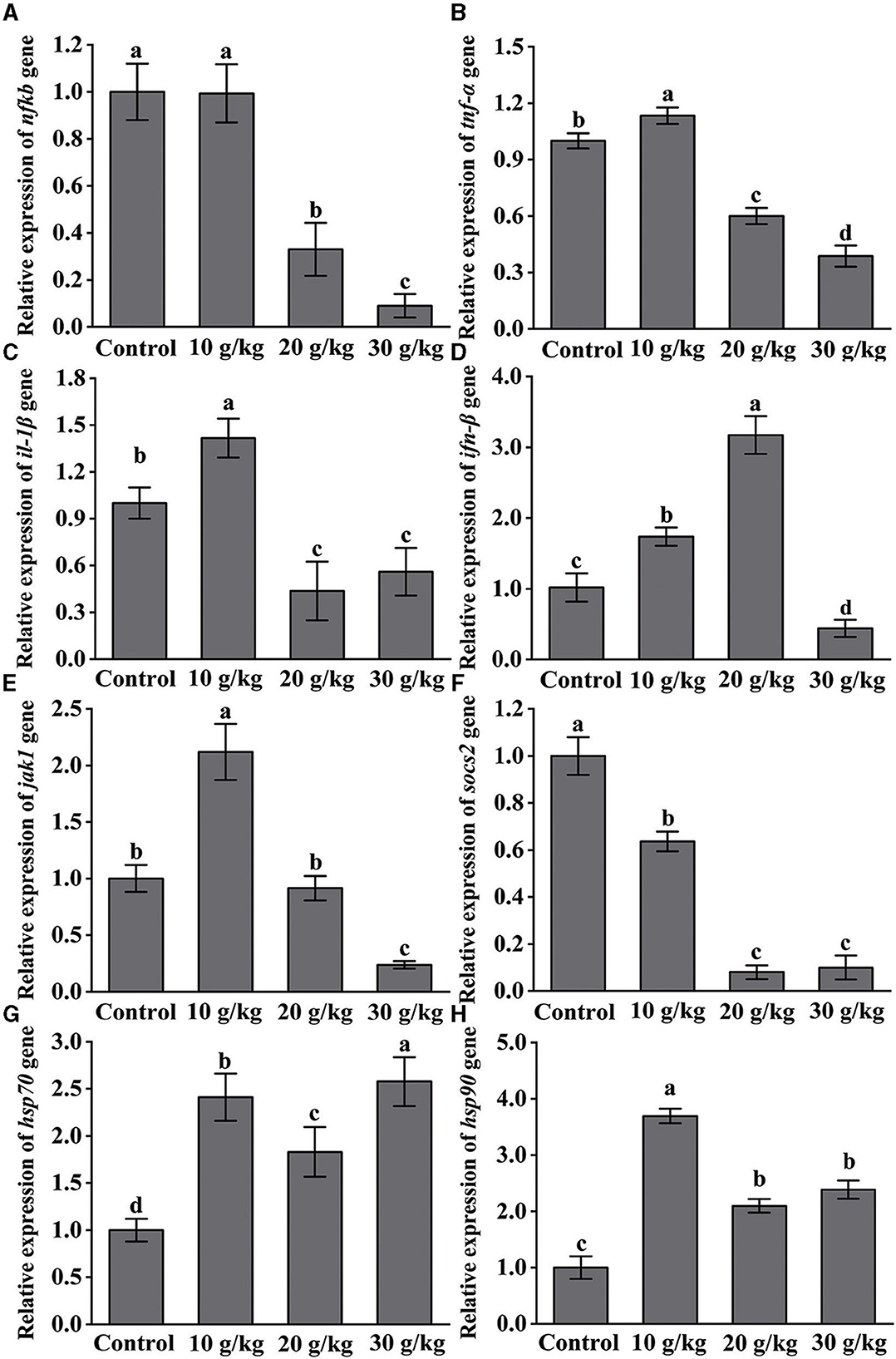
Figure 4. Effects of dietary CHMM on the expression of (A) NF-κB, (B) TNF-α, (C) IL-1β, (D) IFN-β, (E) JAK1, (F) SOCS2, (G) HSP70, and (H) HSP90 genes in the liver of rainbow trout after IHNV infection; data are presented as mean ± S.E. with distinct superscript values denoting significance (P < 0.05); normal distribution SW > 0.05; homogeneity of variance P > 0.05.
3.5 Disease resistance
As shown in Figure 5, the relative expression of the IHNV G protein gene was analyzed compared to the control group, and no significant changes were observed in the 10 g/kg group; the expression level was significantly lower in the 20 g/kg and 30 g/kg groups (P < 0.05), and the 20 g/kg group had the lowest (P < 0.05) relative expression of the G protein gene. This finding further validates the significant role played by the dosage of 20 g/kg CHMM in enhancing immunity and anti-IHNV effects in rainbow trout.
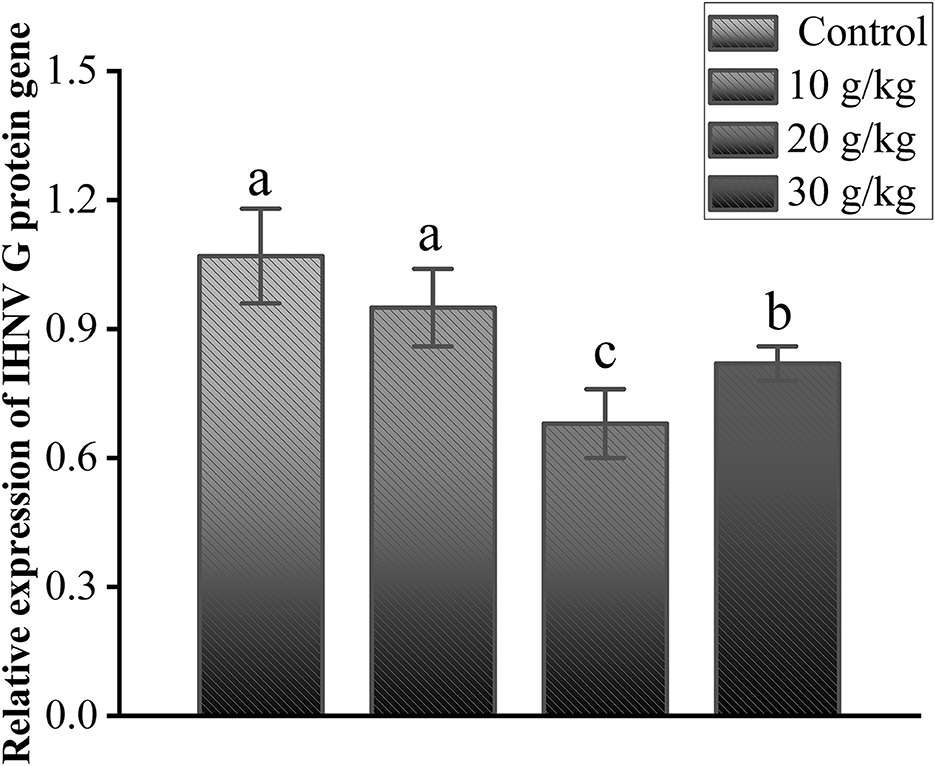
Figure 5. Effects of dietary CHMM on the expression of the IHNV G protein gene in the liver of rainbow trout after IHNV infection; data are presented as mean ± S.E. with distinct superscript values denoting significance (P < 0.05); normal distribution SW > 0.05; homogeneity of variance P > 0.05.
4 Discussion
The outbreak of viral diseases has seriously hindered the sustainable development of rainbow trout farming and caused huge economic losses. Fortunately, CHMM has been demonstrated to have strong antiviral properties and is widely utilized in aquaculture. The impact of CHMM on the immunity and antiviral response in rainbow trout needs to be thoroughly understood. In this study, we examined the impact of feeding rainbow trout with varying levels of CHMM. Our focus was to evaluate antioxidant activity, immune responses, and resistance to IHNV infection in rainbow trout. The findings of this study are expected to provide valuable insights into the potential benefits of CHMM in promoting health and immunity in rainbow trout.
SOD and CAT play a vital role in the removal of reactive oxygen species derivatives and are regularly used as markers of oxidative stress (15, 30). MDA is considered a biomarker for assessing the levels of oxidative stress in various biological systems; MDA is toxic, and excessive levels of MDA can damage cell structure and function (31). CHM has been shown to have powerful antioxidant activity in earlier research (32). In this study, the administration of feeding diets containing CHMM drastically increased the SOD and CAT activities and notably decreased MDA content in rainbow trout. Similarly, the supplementation of Nile tilapia feed with a combination of Chinese herbs, including Astragalus membranaceus, Angelica sinensis, and Crataegus hupehensis, drastically increased SOD and CAT activities (11). Feeding a combination of Chinese herbs, including Codonopsis pilosula, Astragalus, Medicago sativa, Panax notoginseng, and goldenseal, had similar effects on European eel (14). Additionally, other research studies have shown that Lonicera japonica, Radix isatidis, Astragalus radix, Glycyrrhiza uralensis Fisch, Angelica sinensis, and hawthorn can also enhance T-SOD and CAT activities in animals (33). This study showed that CHMM may play a role in increasing the antioxidant capacity of rainbow trout. After IHNV infection, we observed a significant increase in T-SOD activity by feeding CHMM, but CAT activity had no significant change. The results suggest that the activity of liver T-SOD enzymes is relatively increased due to the manifold increase in the antioxidant capacity of rainbow trout in response to pathogen stimulation. However, other antioxidants that are part of the total antioxidant capacity of rainbow trout may inhibit CAT activity. Meanwhile, MDA content decreased, possibly because the body's increased antioxidant capacity reduced the production of lipid peroxidation products, resulting in a decrease in MDA content. In addition, the type of CHMM and the specificity of fish tissue can also lead to changes in antioxidant enzymes (16). In brief, the heightened activity of antioxidant enzymes improved the antioxidant capacity and inhibited the formation of free radicals in rainbow trout.
AST and ALT are important amino acid transferases and important markers of amino acid metabolism in fish (34). Earlier research has demonstrated that the administration of Tridax procumbens leaf extract to Nile tilapia could reduce serum ALT and AST levels (35); the addition of Astragalus propinquus Schischkin polysaccharides to the diet of Channa argus reduced serum AST and ALT levels (36). However, the findings of this study suggest that CHMM exhibits significantly elevated levels of AST and ALT enzymes in rainbow trout, suggesting that the effect of CHMM on the inhibition of amino acid metabolism may contribute to the observed increase in AST and ALT levels (37), and the antagonistic effect of CHMM may affect the normal metabolism of amino acids, but the specific mechanism is needed for further investigation.
ACP and AKP are crucial enzyme markers for immune function in fish. They play a key role in the immune response, helping to combat bacterial and pathogen infections as well as assisting in the removal of microorganisms and other foreign substances (38, 39). Previous research has shown that a Radix Rehmanniae Preparata polysaccharide diet in blunt snout bream significantly boosted ACP and AKP activities (40). Adding Astragalus polysaccharides to the diet of large yellow croakers had a similar effect (41). These results showed that ACP and AKP activities exhibited a significant increase as compared to the control group, which is consistent with previous investigations. After IHNV infection, CHMM had a significant impact on ACP and AKP activities in rainbow trout, suggesting that CHMM may enhance immunity in rainbow trout and similar effects have been observed in other organisms. For example, diets supplemented with Achyranthes aspera significantly increased serum AKP activity in Labeo rohita (42). Under conditions of Aeromonas hydrophila infection, the anthraquinone extract also increased serum AKP activity in Megalobrama amblycephala (43). These results indicated that feed supplementation with CHMM enhanced non-specific immunity in rainbow trout.
NF-κB is a crucial transcription factor involved in the regulation of inflammatory responses. When activated, NF-κB can trigger the expression of inflammatory factors, including TNF-α, IL-1β, and IFN-β (44). In this study, the levels of NF-κB, TNF-α, and IL-1β were significantly altered in rainbow trout after 35 days of administration of different doses of CHMM. The findings suggest that CHMM may enhance the immune responses and disease resistance in rainbow trout. The results are consistent with those of previous studies demonstrating that fish fed with multi-fortified diets (e.g., Ficus carica polysaccharide, Radix Rehmanniae Preparata polysaccharide, and Astragalus radix) displayed higher levels of IL-1β and TNF-α than control fish (40, 45, 46). After IHNV infection, the expressions of NF-κB, TNF-α, and IL-1β were significantly downregulated in all the groups of rainbow trout. Similarly, curcumin-based feed has a similar effect on grass carp with Aeromonas hydrophila infection (47). Thus, the downregulation of NF-κB, TNF-α, and IL-1β expression levels suggests an enhancement of immunity in rainbow trout.
As the main antiviral component of the non-specific immune system with broad-spectrum antiviral effects, interferon (IFN) is essential for immunomodulation and antiviral effects (48). IFN is also a key modulator of the Janus kinase JAK/STAT pathway (49). JAK is a non-receptor tyrosine kinase located inside cells that is essential for several cytokine receptor-mediated signaling processes. The JAK/STAT pathway is considered one of the key pathways to JAK-mediated signaling in living organisms. The JAK/STAT pathway is activated by the binding of cytokines to the cytokine receptor on the surface of cell membranes and ultimately induces specific gene expression, which in turn promotes appropriate cellular responses (50). Furthermore, the JAK/STAT signaling pathway is regulated at different levels by different molecules and approaches, including a family of proteins called suppressor cytokine signaling (SOCS), which plays an essential role in specific and non-specific immunity through the negative feedback of cytokine signaling (51). In this study, CHMM significantly increased JAK1 and IFN-β expressions, while the expression of SOCS2 was decreased in rainbow trout. These results indicated that CHMM could enhance anti-inflammatory immunity in rainbow trout (52). After IHNV infection, in this study, the results showed that adding CHMM to the diet caused a significant upregulation of IFN-β expression, suggesting that feeding CHMM might enhance interferon induction and increase cell resistance to viral interference (53). The expression level of JAK1 was significantly increased and then decreased compared to the control group. The results showed that CHMM may upregulate JAK1 expression, and with an increase in the dose of CHMM, specific cell types and conditions may cause JAK1 to be inhibited (54). Meanwhile, SOCS2 expression was significantly diminished compared to that of the control. These findings showed that CHMM might activate the JAK/STAT pathway and enhance immunity in rainbow trout (55).
Heat shock proteins (HSPs) are a class of chaperone proteins that can be synthesized in large quantities in cells upon exposure to various environmental stressors. Previous studies have demonstrated that these proteins not only act as biomarkers but also play crucial roles in promoting cell survival, enhancing stress resistance, and facilitating tolerance to environmental pressures or injuries (56). HSP70 and HSP90, belonging to the heat stress protein family, have been closely associated with organismal tolerance and survival in challenging environmental conditions. Early research has demonstrated that curcumin upregulates the expression of HSP70 and HSP90 in Puntius sophore (57). In this study, the CHMM noticeably increased HSP70 and HSP90 expression in rainbow trout, similar to previous findings. After IHNV infection, CHMM has also significantly upregulated HSP70 and HSP90 expression in rainbow trout. Similarly, a previous study showed that, under Aeromonas hydrophila infection, Rheum officinale Bail extract upregulated HSP70 expression in Megalobrama amblycephala (58). Thus, these results suggest that upregulation of HSP70 and HSP90 expression might contribute to increased immunity and disease resistance in the organism. However, to elucidate the specific mechanisms involved, further studies are needed.
The IHNV G protein is a glycoprotein that plays an important role in IHNV pathogenicity and virulence, and since IHNV is an RNA virus, the IHNV G protein gene was quantitatively analyzed to determine the transcripts of the viral genes (59, 60). These results suggest that CHMM plays an important role in promoting immunity and protecting rainbow trout from IHNV infection, with the recommended dietary level of CHMM being approximately 20 g/kg.
5 Conclusion
In this study, the utilization of CHMM as an immunostimulant led to significant enhancements in antioxidant enzyme activities and non-specific immune parameters (T-SOD, MDA, CAT, AST, ALT, ACP, and AKP). Furthermore, the expression of immune-related genes (NF-κB, TNF-α, IFNβ, IL-1β, JAK1, HSP70, HSP90, and SOCS2) in rainbow trout were regulated, which ultimately improved the immune status of the organism and resistance to IHNV infection. In addition, the expression of the IHNV G protein gene at 20 g/kg was the lowest compared to that of the other CHMM feeding groups. Combined with the above findings, 20 g/kg CHMM supplemented in the diet had a significant protective effect against IHNV in rainbow trout. The findings of this research show that CHMM has an important function as an immunostimulant in aquaculture, which provides a scientific basis for improving antiviral immunity in rainbow trout.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
The animal study was approved by the Animal Ethics Committee of Gansu Agricultural University. The study was conducted in accordance with the local legislation and institutional requirements.
Author contributions
QW: Writing – review & editing, Data curation, Writing – original draft. YP: Writing – review & editing, Formal analysis, Methodology. JH: Writing – review & editing, Conceptualization, Funding acquisition, Project administration, Resources, Supervision. YL: Conceptualization, Project administration, Writing – review & editing. SW: Writing – review & editing. LZ: Writing – review & editing. TS: Writing – review & editing. YK: Writing – review & editing. ZL: Writing – review & editing.
Funding
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. This work was supported by the Modern Silk Road Cold and Drought Agricultural Science and Technology Support Project (GSLK-2022-11) and the Discipline Team Project of Gansu Agricultural University (GAU-XKTD-2022-23).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Abbreviations
CHM, Chinese herbal medicine; IHNV, infectious hematopoietic necrosis virus; CHMM, Chinese herbal medicine mixture; T-SOD, total superoxide dismutase; MDA, malondialdehyde; CAT, catalase; AST, aspartate aminotransferase; ALT, alanine aminotransferase; ACP, acid phosphatase; AKP, alkaline phosphatase; NF-κB, nuclear factor kappa-B; IL-1β, interleukin-1β; TNF-α, tumor necrosis factor-α; IFN-β, interferon-β; JAK1, Janus kinase 1; SOCS2, suppressor of cytokine signaling 2; HSP70, heat shock protein 70; HSP90, heat shock protein 90; SVCV, spring viremia of carp virus.
References
1. Liu X, Steele JC, Meng XZ. Usage, residue, and human health risk of antibiotics in Chinese aquaculture: a review. Environ Pollut. (2017) 223:161–9. doi: 10.1016/j.envpol.2017.01.003
2. D'Agaro E, Gibertoni P, Esposito S. Recent trends and economic aspects in the rainbow trout (Oncorhynchus mykiss) sector. Appl Sci. (2022) 12:8773. doi: 10.3390/app12178773
3. Wu S, Huang J, Li Y, Lei M, Zhao L, Liu Z. Integrated analysis of immune parameters, miRNA-mRNA interaction, and immune genes expression in the liver of rainbow trout following infectious hematopoietic necrosis virus infection. Front Immunol. (2022) 13:970321. doi: 10.3389/fimmu.2022.970321
4. Kim J, Cho M, Lim J, Choi H, Hong S. Pathogenic mechanism of a highly virulent infectious hematopoietic necrosis virus in head kidney of rainbow trout (Oncorhynchus mykiss) analyzed by RNA-Seq transcriptome profiling. Viruses. (2022) 14:859. doi: 10.3390/v14050859
5. Dixon P, Paley R, Alegria-Moran R, Oidtmann B. Epidemiological characteristics of infectious hematopoietic necrosis virus (IHNV): a review. Vet Res. (2016) 47:63. doi: 10.1186/s13567-016-0341-1
6. Herczeg D, Sipos D, Dán Á, Loy C, Kallert DM, Eszterbauer E. The effect of dietary immunostimulants on the susceptibility of common carp (Cyprinus carpio) to the white spot parasite, Ichthyophthirius multifiliis. Acta Vet Hung. (2017) 65:517–30. doi: 10.1556/004.2017.050
7. Wang F, Liu F, Chen W. Effects of yam (Dioscorea oppositifolia L.) on growth performance, serum biochemical level and liver metabonomics of rainbow trout (Oncorhynchus mykiss). Aquac Rep. (2020) 18:100481. doi: 10.1016/j.aqrep.2020.100481
8. Zhu G, Bai D, Li Y, Ma J, Wu X, Ning B. Preliminary study of 12 Chinese herbs as feed attractants on turbot juvenile (Scophthalmus maximus). Agric Sci Technol. (2010) 11:115–20.
9. Zhang W, Zhao J, Ma Y, Li J, Chen X. The effective components of herbal medicines used for prevention and control of fish diseases. Fish Shellfish Immunol. (2022) 126:73–83. doi: 10.1016/j.fsi.2022.05.036
10. Harikrishnan R, Balasundaram C, Heo MS. Impact of plant products on innate and adaptive immune system of cultured finfish and shellfish. Aquaculture. (2011) 317:1–15. doi: 10.1016/j.aquaculture.2011.03.039
11. Abarike ED, Jian J, Tang J, Cai J, Yu H, Chen L. Traditional Chinese medicine enhances growth, immune response, and resistance to Streptococcus agalactiae in Nile tilapia. J Aquat Anim Health. (2019) 31:46–55. doi: 10.1002/aah.10049
12. Cai J, Yang ZH, Huang Y, Jian JC, Tang JF. Effects of Chinese herbal medicines on growth performance, intestinal flora, immunity and serum metabolites of hybrid grouper (Epinephelus fuscoguttatus♀ × Epinephelus lanceolatu♂). Fish Shellfish Immunol. (2023) 140:108946. doi: 10.1016/j.fsi.2023.108946
13. Gao XZ, Zhang PY, Shi XY, Ben LZ, Guo QQ, Liu HY. Effects of different levels of Chinese herbal medicine on the growth performance, feed utilization and non-specific immunity of juvenile turbot (Scophthalmus maximus l). Acta Hydrobiol Sin. (2022) 46:257–64. doi: 10.7541/2021.2020.174
14. Huang Z, Lu J, Ye Y, Xu A, Li Z. Effects of dietary Chinese herbal medicines mixture on growth performance, digestive enzyme activity and serum biochemical parameters of European eel, Anguilla. Aquac Rep. (2020) 18:100510. doi: 10.1016/j.aqrep.2020.100510
15. Wang CY, Li ZB, Sun YZ, Chen Q, Li WJ, Huang YC, et al. Effects of Chinese herbal medicines mixture on growth performance digestive enzyme activity immune response of juvenile Japanese seabass, Lateolabrax japonicus. Aquac Nutr. (2018) 24:683–93. doi: 10.1111/anu.12597
16. Cai B, Chen WG, Wang F, Zou X. Effects of compound Chinese herbal medicine on expression of immune-related genes in rainbow trout under Vibrio infection. Fish Sci. (2020) 39:727–33.
17. Jia R, Li Y, Cao L, Du J, Zheng T, Qian H, et al. Antioxidative, anti-inflammatory and hepatoprotective effects of resveratrol on oxidative stress-induced liver damage in tilapia (Oreochromis niloticus). Comp Biochem Physiol C Toxicol Pharmacol. (2019) 215:56–66. doi: 10.1016/j.cbpc.2018.10.002
18. Shahrajabian MH, Sun W, Cheng Q. A review of Astragalus species as foodstuffs, dietary supplements, a traditional Chinese medicine and a part of modern pharmaceutical science. Appl Ecol Env Res. (2019) 17:13371–82. doi: 10.15666/aeer/1706_1337113382
19. Luan F, Ji Y, Peng L, Liu Q, Cao H, Yang Y, et al. Extraction, purification, structural characteristics, and biological properties of the polysaccharides from Codonopsis pilosula: a review. Carbohydr Polym. (2021) 261:117863. doi: 10.1016/j.carbpol.2021.117863
20. Fang L, Xiao X, Liu C, He X. Recent advance in studies on Angelica sinensis. Chinese Herb Med. (2012) 4:12–25. doi: 10.3969/j.issn.1674-6384.2012.01.004
21. Wang Y, Li Y, Ma X, Ren H, Fan W, Leng F, et al. Extraction, purification, and bioactivities analyses of polysaccharides from Glycyrrhiza uralensis. Ind Crops Prod. (2018) 122:596–608. doi: 10.1016/j.indcrop.2018.06.011
22. Chen MH, Chen XJ, Wang M, Lin LG, Wang YT. Ophiopogon japonicus–A phytochemical, ethnomedicinal and pharmacological review. J Ethnopharmacol. (2016) 181:193–213. doi: 10.1016/j.jep.2016.01.037
23. Ríos JL. Chemical constituents and pharmacological properties of Poria cocos. Planta Med. (2011) 77:681–91. doi: 10.1055/s-0030-1270823
24. Shang X, Pan H, Li M, Miao X, Ding H. Lonicera japonica Thunb.: ethnopharmacology, phytochemistry and pharmacology of an important traditional Chinese medicine. J Ethnopharmacol. (2011) 138:1–21. doi: 10.1016/j.jep.2011.08.016
25. Zhou W, Zhang XY. Research progress of Chinese herbal medicine Radix isatidis (banlangen). Am J Chin Med. (2013) 41:743–64. doi: 10.1142/S0192415X1350050X
26. Chen J, Zhu Z, Gao T, Chen Y, Yang Q, Fu C, et al. Isatidis Radix and Isatidis Folium: a systematic review on ethnopharmacology, phytochemistry and pharmacology. J Ethnopharmacol. (2022) 283:114648. doi: 10.1016/j.jep.2021.114648
27. Nazhand A, Lucarini M, Durazzo A, Zaccardelli M, Cristarella S, Souto SB, et al. Hawthorn (Crataegus spp.): an updated overview on its beneficial properties. Forests. (2020) 11:564. doi: 10.3390/f11050564
28. Wang Q, Huang J, Li Y, Wu S, Zhao L, Pan Y, et al. Chinese herbal medicines mixture improved antioxidant enzymes, immunity and disease resistance to infectious hematopoietic necrosis virus infection in rainbow trout (Oncorhynchus mykiss). Aquac. Int. (2023) 1–16. doi: 10.1007/s10499-023-01319-w
29. Ballesteros NA, Alonso M, Saint-Jean SR, Perez-Prieto SI. An oral DNA vaccine against infectious haematopoietic necrosis virus (IHNV) encapsulated in alginate microspheres induces dose-dependent immune responses and significant protection in rainbow trout (Oncorrhynchus mykiss). Fish Shellfish Immunol. (2015) 45:877–88. doi: 10.1016/j.fsi.2015.05.045
30. Hoseinifar SH, Shakouri M, Doan HV, Shafiei S, Yousefi M, Raeisi M, et al. Dietary supplementation of lemon verbena (Aloysia citrodora) improved immunity, immune-related genes expression and antioxidant enzymes in rainbow trout (Oncorrhyncus mykiss). Fish Shellfish Immunol. (2020) 99:379–85. doi: 10.1016/j.fsi.2020.02.006
31. Liu F, Qu YK, Geng C, Wang AM, Zhang JH, Chen KJ, et al. Effects of hesperidin on the growth performance, antioxidant capacity, immune responses and disease resistance of red swamp crayfish (Procambarus clarkii). Fish Shellfish Immunol. (2020) 99:154–66. doi: 10.1016/j.fsi.2020.02.014
32. Pu H, Li X, Du Q, Cui H, Xu Y. Research progress in the application of Chinese herbal medicines in aquaculture: a review. Engineering. (2017) 3:731–7. doi: 10.1016/J.ENG.2017.03.017
33. Lan R, Park J, Lee D, Kim I. Effects of Astragalus membranaceus, Codonopsis pilosula and allicin mixture on growth performance, nutrient digestibility, faecal microbial shedding, immune response and meat quality in finishing pigs. J Anim Physiol Anim Nutr. (2017) 101:1122–9. doi: 10.1111/jpn.12625
34. Shi Y, Zhong L, Zhong H, Liu Y, Zhang J, Wei Z, et al. Effects of andrographolide on lipopolysaccharide-induced serum biochemical indices, immune responses and intestinal inflammation related to gene expression of Monopterus albus. Aquac Res. (2021) 52:4670–80. doi: 10.1111/are.15301
35. Adeshina I, Abdel-Tawwab M, Tijjani ZA, Tiamiyu LO, Jahanbakhshi A. Dietary Tridax procumbens leaves extract stimulated growth, antioxidants, immunity, and resistance of Nile tilapia, Oreochromis niloticus, to monogenean parasitic infection. Aquaculture. (2021) 532:736047. doi: 10.1016/j.aquaculture.2020.736047
36. Zhu XM, Liu XY, Xia CG, Li MY, Niu XT, Wang GQ, et al. Effects of dietary Astragalus Propinquus Schischkin polysaccharides on growth performance, immunological parameters, antioxidants responses and inflammation-related gene expression in Channa argus. Comp Biochem Physiol C Toxicol Pharmacol. (2021) 249:109121. doi: 10.1016/j.cbpc.2021.109121
37. Ye Q, Feng Y, Wang Z, Zhou A, Xie S, Zhang Y, et al. Effects of dietary Gelsemium elegans alkaloids on growth performance, immune responses and disease resistance of Megalobrama amblycephala. Fish Shellfish Immunol. (2019) 91:29–39. doi: 10.1016/j.fsi.2019.05.026
38. Liu J, Zhang P, Wang B, Lu Y, Li L, Li Y, et al. Evaluation of the effects of Astragalus polysaccharides as immunostimulants on the immune response of crucian carp and against SVCV in vitro and in vivo. Comp Biochem Physiol C Toxicol Pharmacol. (2022) 253:109249. doi: 10.1016/j.cbpc.2021.109249
39. Liu F, Geng C, Qu YK, Cheng BX, Zhang Y, Wang AM, et al. The feeding of dietary Codonopsis pilosula polysaccharide enhances the immune responses, the expression of immune-related genes and the growth performance of red swamp crayfish (Procambarus clarkii). Fish Shellfish Immunol. (2020) 103:321–31. doi: 10.1016/j.fsi.2020.05.034
40. Wu C, Shan J, Feng J, Wang J, Qin C, Nie G, et al. Effects of dietary Radix Rehmanniae Preparata polysaccharides on the growth performance, immune response, and disease resistance of Luciobarbus capito. Fish Shellfish Immunol. (2019) 89:641–6. doi: 10.1016/j.fsi.2019.04.027
41. Zhang W, Zhang M, Cheng A, Hao E, Huang X, Chen X. Immunomodulatory and antioxidant effects of Astragalus polysaccharide liposome in large yellow croaker (Larimichthys crocea). Fish Shellfish Immunol. (2020) 100:126–36. doi: 10.1016/j.fsi.2020.03.004
42. Vasudeva Rao Y, Das BK, Jyotyrmayee P, Chakrabarti R. Effect of Achyranthes aspera on the immunity and survival of Labeo rohita infected with Aeromonas hydrophila. Fish Shellfish Immunol. (2006) 20:263–73. doi: 10.1016/j.fsi.2005.04.006
43. Liu B, Ge X, Xie J, Xu P, He Y, Cui Y, et al. Effects of anthraquinone extract from Rheum officinale Bail on the physiological responses and HSP70 gene expression of Megalobrama amblycephala under Aeromonas hydrophila infection. Fish Shellfish Immunol. (2012) 32:1–7. doi: 10.1016/j.fsi.2011.02.015
44. Yu Z, Quan YN, Huang ZQ, Wang HH, Wu LF. Monitoring oxidative stress, immune response, Nrf2/NF-κB signaling molecules of Rhynchocypris lagowski living in BFT system and exposed to waterborne ammonia. Ecotoxicol. (2020) 205:111161. doi: 10.1016/j.ecoenv.2020.111161
45. Yang X, Guo JL, Ye JY, Zhang YX, Wang W. The effects of Ficus carica polysaccharide on immune response and expression of some immune-related genes in grass carp (Ctenopharyngodon idella). Fish Shellfish Immunol. (2015) 42:132–7. doi: 10.1016/j.fsi.2014.10.037
46. Yuan C, Pan X, Gong Y, Xia A, Wu G, Tang J, et al. Effects of Astragalus polysaccharides (APS) on the expression of immune response genes in head kidney, gill and spleen of the common carp, Cyprinus carpio L. Int Immunopharmacol. (2008) 8:51–8. doi: 10.1016/j.intimp.2007.10.009
47. Ming J, Ye J, Zhang Y, Xu Q, Yang X, Shao X, et al. Optimal dietary curcumin improved growth performance, and modulated innate immunity, antioxidant capacity and related genes expression of NF-κB and Nrf2 signaling pathways in grass carp (Ctenopharyngodon idella) after infection with Aeromonas hydrophila. Fish Shellfish Immunol. (2020) 97:540–53. doi: 10.1016/j.fsi.2019.12.074
48. Malmgaard L. Induction and regulation of IFNs during viral infections. J Interferon Cytokine Res. (2004) 24:439–54. doi: 10.1089/1079990041689665
49. Schmeisser H, Bekisz J, Zoon KC. New function of type I IFN: induction of autophagy. Autophagy. (2014) 34:71–8. doi: 10.1089/jir.2013.0128
50. Liongue C, O'Sullivan LA, Trengove MC, Ward AC. Evolution of JAK-STAT pathway components: mechanisms and role in immune system development. PLoS ONE. (2012) 7:e32777. doi: 10.1371/journal.pone.0032777
51. Skjesol A, Liebe T, Iliev DB, Thomassen EI, Tollersrud LG, Sobhkhez M, et al. Functional conservation of suppressors of cytokine signaling proteins between teleosts and mammals: Atlantic salmon SOCS1 binds to JAK/STAT family members and suppresses type I and II IFN signaling. Dev Comp Immunol. (2014) 45:177–89. doi: 10.1016/j.dci.2014.02.009
52. Robertsen B, Bergan V, Røkenes T, Larsen R, Albuquerque A, Robertsen B, et al. Atlantic salmon interferon genes: cloning, sequence analysis, expression, and biological activity. J Interferon Cytokine Res. (2003) 23:601–12. doi: 10.1089/107999003322485107
53. González-Navajas JM, Lee J, David M, Raz E. Immunomodulatory functions of type I interferons. Nat Rev Immunol. (2012) 12:125–35. doi: 10.1038/nri3133
54. Song Z, Jiao C, Chen B, Xu W, Wang M, Zou J, et al. Dietary Acanthopanax senticosus extracts modulated the inflammatory and apoptotic responses of yellow catfish to protect against Edwardsiella ictaluri infection. Aquac Res. (2021) 52:5078–92. doi: 10.1111/are.15379
55. Huang S, Liu K, Cheng A, Wang M, Cui M, Huang J, et al. SOCS proteins participate in the regulation of innate immune response caused by viruses. Front Immunol. (2020) 11:558341. doi: 10.3389/fimmu.2020.558341
56. Tan X, Sun Z, Huang Z, Zhou C, Lin H, Tan L, et al. Effects of dietary hawthorn extract on growth performance, immune responses, growth- and immune-related genes expression of juvenile golden pompano (Trachinotus ovatus) and its susceptibility to Vibrio harveyi infection. Fish Shellfish Immunol. (2017) 70:656–64. doi: 10.1016/j.fsi.2017.09.041
57. Mahanty A, Mohanty S, Mohanty BP. Dietary supplementation of curcumin augments heat stress tolerance through upregulation of Nrf-2-mediated antioxidative enzymes and hsps in Puntius sophore. Fish Physiol Biochem. (2017) 43:1131–41. doi: 10.1007/s10695-017-0358-z
58. Huda N, Liu H, Jin J, Zhu X, Han D, Yang Y, et al. Dietary supplementation of Geotrichum candidum improves growth, gut microbiota, immune-related gene expression and disease resistance in gibel carp CAS III (Carassius auratus gibelio). Fish Shellfish Immunol. (2020) 99:144–53. doi: 10.1016/j.fsi.2020.02.001
59. Yong C, Ong H, Tang H, Yeap S, Omar A, Ho K, et al. Infectious hematopoietic necrosis virus: advances in diagnosis and vaccine development. PeerJ. (2019) 7:e7151. doi: 10.7717/peerj.7151
Keywords: rainbow trout, Chinese herbal medicine mixture, antioxidant, immune response, IHNV
Citation: Wang Q, Pan Y, Huang J, Li Y, Wu S, Zhao L, Sun T, Kang Y and Liu Z (2024) Dietary supplementation of Chinese herbal medicines enhances the immune response and resistance of rainbow trout (Oncorhynchus mykiss) to infectious hematopoietic necrosis virus. Front. Vet. Sci. 11:1341920. doi: 10.3389/fvets.2024.1341920
Received: 21 November 2023; Accepted: 14 March 2024;
Published: 17 April 2024.
Edited by:
Hetron M. Munang'andu, Nord University, NorwayReviewed by:
Seyed Pezhman Hosseini Shekarabi, Agricultural Research, Education and Extension Organization (AREEO), IranXu-Jie Zhang, Huazhong Agricultural University, China
Copyright © 2024 Wang, Pan, Huang, Li, Wu, Zhao, Sun, Kang and Liu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Jinqiang Huang, huangjinq@163.com
 Qi Wang1
Qi Wang1  Yucai Pan
Yucai Pan Jinqiang Huang
Jinqiang Huang Yujun Kang
Yujun Kang Zhe Liu
Zhe Liu