New-onset organ dysfunction as a screening tool for the identification of sepsis and outcome prediction in dogs with systemic inflammation
- Department of Veterinary Medical Sciences, Alma Mater Studiorum – University of Bologna, Bologna, Italy
Introduction: Sepsis in people is defined as a life-threatening organ dysfunction (OD) caused by a dysregulated host response to infection. In veterinary medicine, sepsis is still defined by the presence of systemic inflammation plus the evidence of infection. Based on recent veterinary studies, multiorgan dysfunction syndrome (MODS) has been associated with a worse outcome in sepsis. Thus, the screening for OD is warranted to identify the most critically ill patients. The aim of this study was to investigate the diagnostic value of new-onset OD for the prediction of sepsis and outcome in a population of critically ill dogs with systemic inflammation.
Materials and methods: Dogs admitted to the Emergency Room and/or the Intensive Care Unit with systemic inflammation, defined by a serum C-reactive protein concentration > 1.6 mg/dL, were retrospectively included. Enrolled dogs were categorized according to the presence of sepsis or non-infectious systemic inflammation. The presence of newly diagnosed OD was assessed based on criteria adapted from human literature and previously reported canine criteria.
Results: 275 dogs were included: 128 had sepsis and 147 had non-infectious systemic inflammation. The frequency of new-onset OD was not different between these groups. Only the presence of fluid-refractory hypotension was significantly associated with a diagnosis of sepsis (OR 10.51, 3.08–35.94; p < 0.0001). The frequency of at least two ODs was significantly higher in non-survivors compared to survivors, according to both the human and the veterinary criteria considered for the study (p = 0.0001 and p = 0.0004, respectively). Specifically, the presence of acute kidney injury, stupor or coma, prolonged Prothrombin Time and decreased Base Excess were associated with a higher risk of death in the multivariate binary logistic regression.
Discussion: In this population of critically ill dogs with systemic inflammation, the detection of newly diagnosed ODs was not able to predict sepsis diagnosis, other than the presence of fluid-refractory hypotension. However, given the strong prognostic significance associated with ODs, our results support the early screening for ODs in any severe inflammatory critical care condition to identify high-risk patients and optimize their management.
Introduction
Sepsis in people is defined as life-threatening organ dysfunction caused by a dysregulated host response to infection, and is a leading cause of mortality and critical illness worldwide (1). Clinical criteria best identifying infected patients most likely to have sepsis include existing scores of organ dysfunction, as the Sequential [Sepsis-related] Organ Failure Assessment (SOFA) score (2). Currently, in veterinary medicine, sepsis is still defined as a host’s systemic inflammatory response syndrome (SIRS) to infection, and at least two of four SIRS criteria plus a suspected septic focus are required to confirm the diagnosis (3). Despite still based on the latter criteria for diagnosis, sepsis in dogs and cats is frequently complicated by organ dysfunction (OD) (4). Nonetheless, the value of OD for the prediction of sepsis is not known in veterinary medicine (5, 6). Recent studies in septic dogs and cats highlighted that the presence of severe sepsis or Multiorgan Dysfunction Syndrome (MODS) is associated with worse outcomes compared to uncomplicated sepsis, pointing out that the screening for OD is advocated in order to identify the most critically ill patients (5, 6).
The aims of the current study were: (1) to investigate the value of new-onset OD for the prediction of sepsis in a population of critically ill dogs with systemic inflammation admitted at the Emergency Room (ER) and/or the Intensive Care Unit (ICU); (2) to assess its prognostic potential for outcome prediction in the same population.
We hypothesize that new-onset of OD in critically ill dogs with systemic inflammation is associated with a diagnosis of sepsis, and that the number and types of dysfunctional organ systems has prognostic value in this setting.
Materials and methods
Inclusion criteria and data collection
Dogs examined at the Veterinary University Hospital (VUH) between January 2017 and December 2020 were retrospectively included. To be eligible for inclusion, dogs had to fulfill the following criteria: (1) be admitted at the ER and/or the ICU; (2) had evidence of systemic inflammation defined as a serum C-reactive Protein (CRP) > 1.6 mg/dL, as previously reported (7); (3) undergone complete clinical and clinicopathological evaluation for the assessment of OD; (4) had a clear final diagnosis. Based on their final diagnosis, enrolled dogs were divided in two groups: dogs with sepsis and dogs with non-infectious systemic inflammation. Sepsis diagnosis was achieved combining the presence of systemic inflammation with evidence of a septic focus, confirmed by cytology, microbiology, histopathology, or real-time polymerase chain reaction. Clinical records of enrolled dogs were reviewed, and the following variables at the time of ER or ICU admission were recorded: signalment, body weight, clinical findings including rectal temperature, heart rate, respiratory rate, mental status (classified as: normal mentation, depressed mentation, stupor, coma), non-invasive systolic blood pressure measurement (petMAP Graphic, Ramsey Medical, Sydney, Australia; Minidop ES-100 VX, Hadeco, Kawasaki, Japan) and the need to receive oxygen therapy. The SIRS criteria were evaluated for all included dogs: rectal temperature (<38°C, >39°C), heart rate (>120 beats per minute), respiratory rate (>20 breaths per minute), white blood cell (WBC) count (<6 ×103/mm3, >16 ×103/mm3) with percent bands (>3%). SIRS was deemed present if dogs had at least 2/4 criteria (8). Regarding clinicopathological screening, all analyses were performed at the clinical pathology laboratory of our VUH, and samples were obtained according to standard operating procedures. The following clinicopathological data were recorded: blood gas, electrolytes and lactate measurements (ABL800 FLEX, Radiometer Medical ApS, Denmark); complete blood count (ADVIA 2120, Siemens Healthcare Diagnostics, Tarrytown, NY); serum chemistry including CRP concentration (Olympus AU 480, Olympus/Beckman Coulter, Brea; CA); coagulation assays (SEAC Clot 2S and BTF II Siemens, Marburg, Germany). Data collected were used to assess disease severity by calculating the canine Acute Patient Physiologic and Laboratory Evaluation fast (APPLEfast) score, 5-variable score contained glucose (mg/dL), albumin (g/dL), lactate (mg/dL), platelet count (x109/L), mentation score (9). The study was approved by the local Institutional Animal Care and Use Committee (protocol number ID 846).
Organ dysfunction and outcome
At the time of hospital admission, the presence of newly diagnosed ODs was assessed considering two different diagnostic criteria: (1) those adapted from a human study conducted in septic people published from Swenson et al. in 2018 (10); (2) those reported in the available canine literature to address MODS (11) (Table 1). The number of dysfunctional organs according to both the human and canine criteria were also recorded. MODS was defined by the presence of at least two dysfunctional organs, simultaneously. Outcome was defined as survival to hospital discharge, death, or euthanasia for disease severity and perceived guarded prognosis.
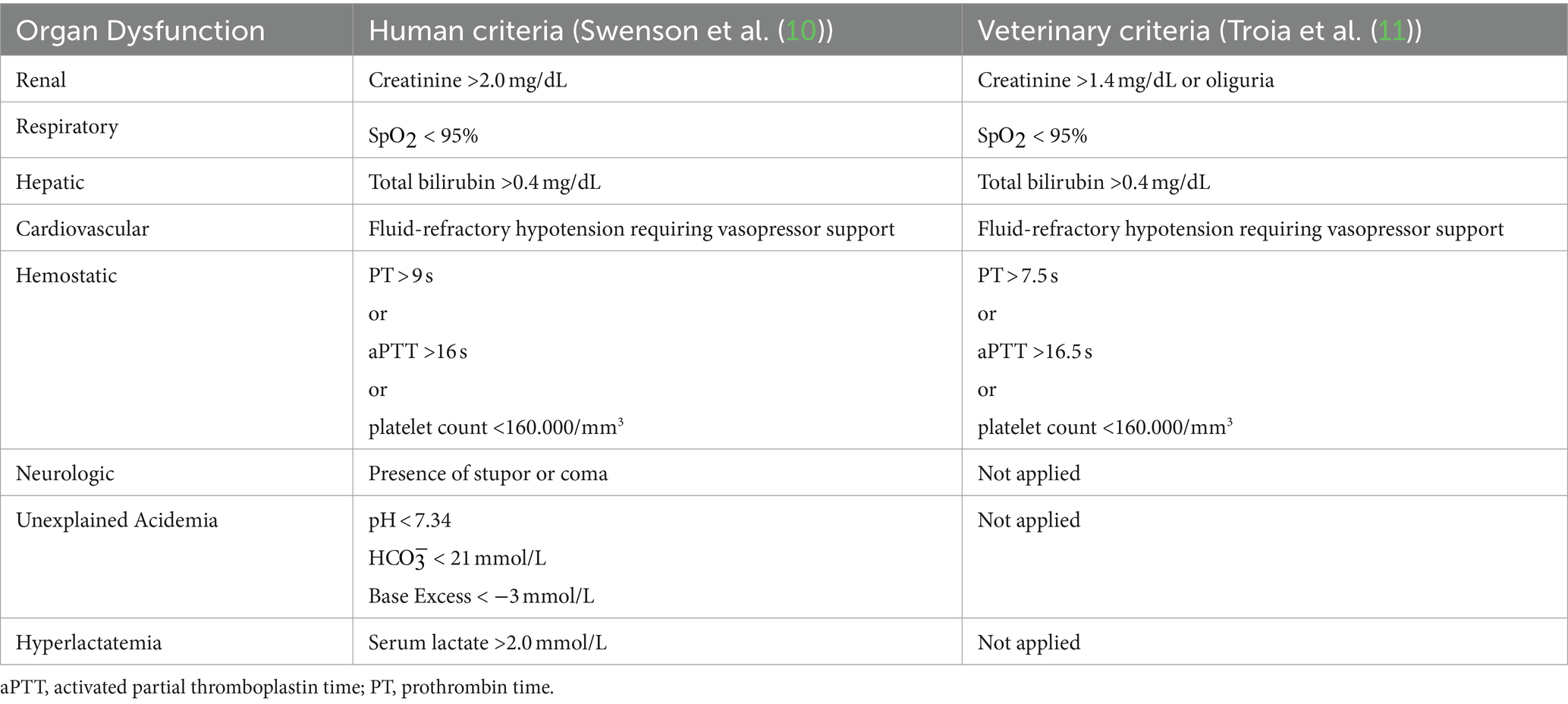
Table 1. Criteria adapted from human and veterinary literature for the identification of organ dysfunction: Swenson et al. (10), Troia et al. (11).
Statistical analysis
Statistical analysis was performed using a statistical software package (MedCalc Statistical Software version 19.1.3, Ostend, Belgium 2019). Data were assessed for normality with the D’Agostino-Pearson test and evaluated by descriptive statistics. The Mann–Whitney U-test was used to compare continuous variables between groups. Categorical variables were compared using the Fisher’s exact test. Univariate and multivariate logistic regressions were used to identify variables able to predict diagnosis of sepsis and outcome; results were presented as odds ratio (OR) and 95% confidence interval (CI). Significance was set at p < 0.05.
Results
A total of 403 medical records of critically ill dogs with a CRP > 1.6 mg/dL, admitted at the ER or ICU in the study period were reviewed. Of these, 128 patients were excluded for the lack of complete clinicopathological data or unclear final diagnosis. The final study population included 275 dogs: 128 (47%) had sepsis and 147 (53%) had non-infectious systemic inflammation. In the overall population there were 83/275 (30%) spayed females, 78/275 (28%) intact females, 30/275 (11%) neutered males and 84/275 (31%) intact males. Many breeds were represented, with the most common being Mixed breed (n = 82), Labrador retriever (n = 11) and German Shepherd (n = 11), Golden Retriever (n = 9), English Setter (n = 9), Miniature Poodle (n = 8), and Maltese (n = 8). Median age was 9 years (range 0.1–19), median body weight was 30.1 kg (range 0.4–59.8). Underlying causes for sepsis included reproductive tract infections (27 pyometra, 4 metritis; n = 31), intra-abdominal infections (17 septic peritonitis, 1 penetrating abdominal trauma; n = 18), respiratory tract infections (14 pneumonia, 1 pulmonary abscess, 3 pyothorax; n = 18), complicated urinary tract infections (12 pyelonephritis, 4 prostatic abscess and prostatitis; n = 16), skin and subcutaneous tissue infections (8 bite wounds, 5 necrotizing fasciitis, 1 subcutaneous abscess; n = 14), parvoviral enteritis (n = 13), miscellaneous diseases (6 bacteremia during severe acute hemorrhagic gastroenteritis, 4 leptospirosis, 4 chemotherapy-induced febrile neutropenia, 1 diabetic ketoacidosis complicated with septic shock, 1 rectal laceration, 1 bacterial cholangitis, 1 endocarditis; n = 18). Underlying causes for non-infectious systemic inflammation included immune mediated conditions (21 immune mediated hemolytic anemia, 5 immune mediated thrombocytopenia; n = 26), neoplasia (n = 22), cardio-pulmonary diseases (18 cardiogenic pulmonary edema, 3 pulmonary thromboembolism, n = 21), metabolic diseases such as acute on chronic kidney injury (n = 17), diabetic ketoacidosis (n = 9), seizures (n = 9), enterectomy for intestinal foreign body (n = 7), miscellaneous diseases 8 acute gastroenteritis, 4 gastric dilatation volvulus, 4 snake envenomation, 3 intestinal intussusception, 3 heat stroke, 2 pancreatitis, 2 cholangitis, 1 metaphyseal osteopathy, 1 intoxication by anticoagulant rodenticides, 1 exertional rhabdomyolysis, 1 blunt trauma, 1 hepatic lobe torsion, 1 acute kidney injury caused by non-steroidal anti-inflammatory drug (NSAID), 1 anaphylaxis, 1 Addison’s disease, 1 perineal hernia, 1 traumatic diaphragmatic hernia; (n = 36).
Main clinical and clinicopathologic findings for dogs with sepsis and non-infectious systemic inflammation are reported in Table 2.
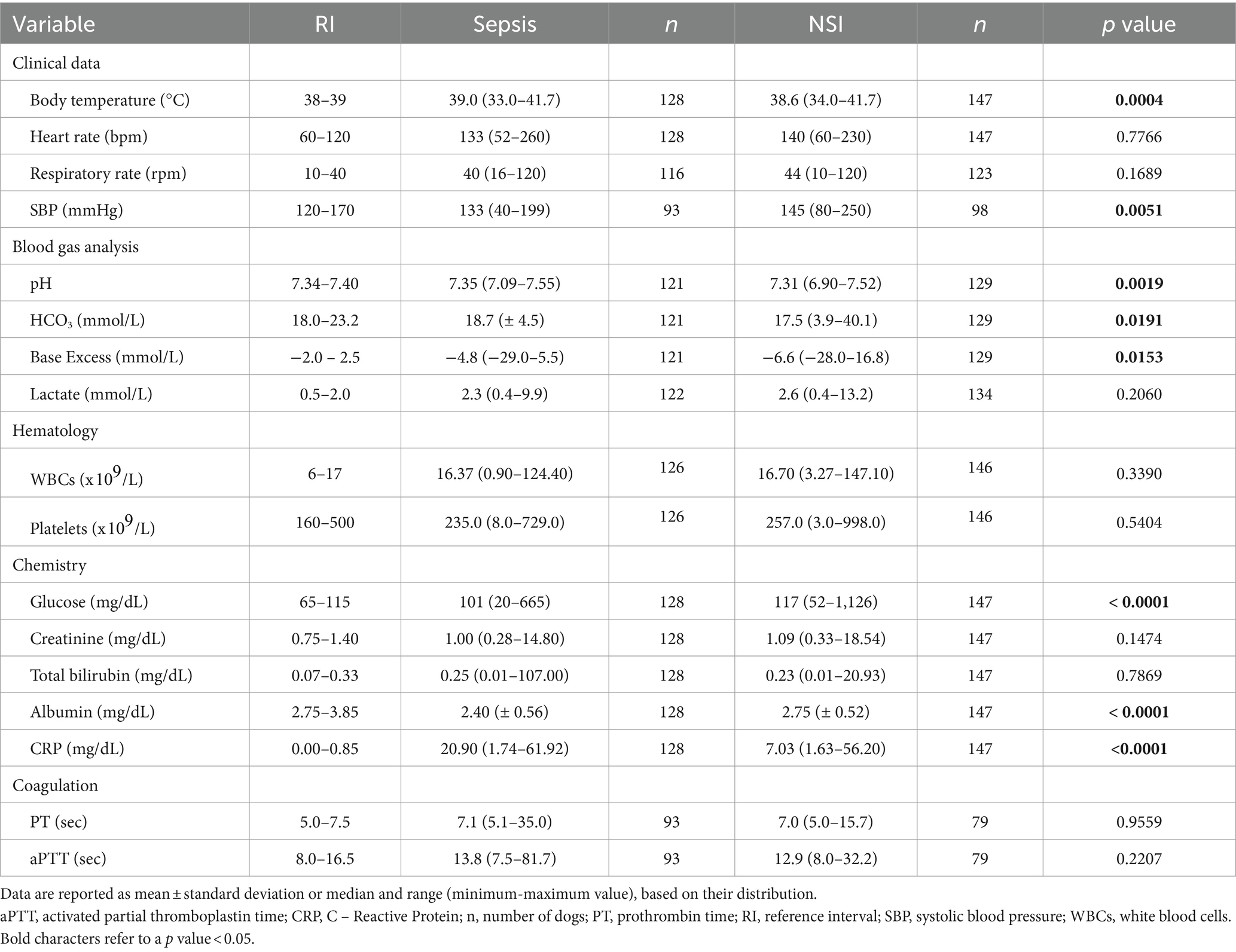
Table 2. Descriptive statistics of selected clinical and clinicopathological variables in dogs with sepsis and dogs with non-infectious systemic inflammation (NSI).
In the overall population of critically ill dogs, 94 (34%) dogs died, and 181 (66%) survived. The frequency of death was similar between dogs with sepsis and those with non-infectious systemic inflammation (34% vs. 34%, p = 1.000).
According to published criteria, the frequency of SIRS was similar between dogs with and without sepsis: 119 (93%) of septic dogs and 128 (87%) dogs with non-infectious systemic inflammation (p = 0.11).
Association between OD and diagnosis of sepsis
In the overall population of critically ill dogs, the number of ODs was distributed as follows: acidemia (n = 202), hyperlactatemia (n = 152), acute kidney injury (n = 96), hyperbilirubinemia (n = 68), prolonged coagulation times (either activated partial thromboplastin time, aPTT, or prothrombin time, PT) (n = 68), thrombocytopenia (n = 64), hypoxemia (n = 41), fluid-refractory hypotension (n = 26), stupor or coma (n = 18).
Overall, the frequency of new-onset ODs was not different between dogs with sepsis and those with non-infectious systemic inflammation. However, based on the univariate logistic regression analysis, the frequency of fluid-refractory hypotension was significantly associated with a diagnosis of sepsis (OR 10.51, 3.08–35.94; p < 0.0001).
No difference in the number of ODs was reported between dogs with sepsis and dogs with non-infectious systemic inflammation. In particular, according to the veterinary criteria, 45/128 (35%) dogs with sepsis and 50/147 (34%) dogs with non-infectious systemic inflammation had at least two or more ODs (p = 0.899).
Association between OD and outcome
According to the human criteria, the frequency of at least 2 ODs was significantly higher in non-survivors compared to survivors (88% vs. 68%, p = 0.0001). Similarly, when the veterinary criteria were considered, the frequency of at least 2 ODs was significantly higher in non-survivors compared to survivors (49% vs. 27%, p = 0.0004).
The presence of acute kidney injury, stupor or coma, hyperbilirubinemia, hyperlactatemia, fluid-refractory hypotension, prolonged PT and decreased Base Excess (BE) as a marker of acidemia were independently associated with a higher risk of death by univariate logistic regression analysis. When the multivariate binary logistic regression analysis was performed, the presence of acute kidney injury, stupor or coma, prolonged PT and decreased BE were the variables retained by the model (Table 3).
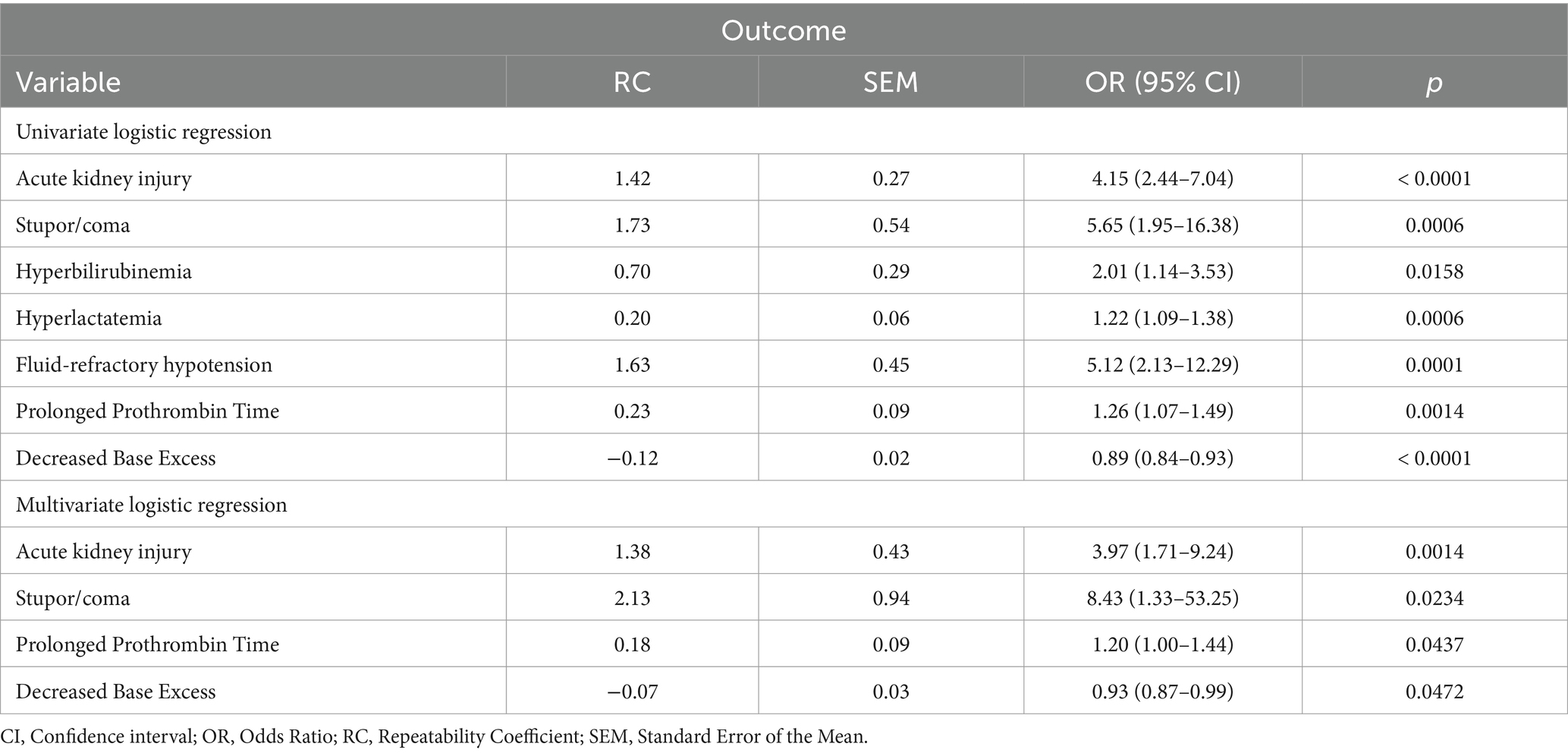
Table 3. Significant variables based on univariate and multivariate analysis for outcome prediction.
Discussion
In our population of critically ill dogs, presence of new-onset OD was not related to a diagnosis of sepsis. Indeed, only fluid-refractory hypotension was specifically linked to the presence of sepsis. Thus, the presence of fluid-refractory shock has to spur clinicians to screen for sepsis and treat patients according to the sepsis bundle, even if the septic focus is not obvious or remains undetected (1).
Sepsis definition has changed over the last few decades. The first proposed definition and diagnosis of sepsis was based on the SIRS criteria, which is referred to as Sepsis-1 (12, 13). Because of the low specificity of the SIRS criteria and due to the common occurrence of sepsis-induced OD that is strongly related to worse outcomes, the current definition of sepsis in humans emphasizes the presence of organ system failure or dysfunction. Indeed, according to the new consensus definition Sepsis-3, sepsis is now defined as the life-threatening organ dysfunction caused by a dysregulated host response to infection (1).
In veterinary medicine, sepsis definition is still based on the SIRS criteria, and eventually on the presence of systemic inflammation detected by a rise in major acute phase proteins, like CRP in dogs (7, 14). For this reason, we enrolled critically ill dogs with a serum CRP at admission in ER above 1.6 mg/dL, as previously suggested (7), with the purpose of evaluating the diagnostic value of new-onset OD for sepsis diagnosis, as well as its prognostic potential.
None of the additional ODs considered for this study, based on both the human and veterinary criteria used, was able to identify sepsis in our population. There are multiple possible explanations that could be hypothesized behind this result, and partially linked to our study design: first of all, enrolled dogs were all characterized by the presence of systemic inflammation with a high frequency of OD. It is well known that OD is not unique for patients with sepsis, as many non-infectious critical care conditions, such as trauma and pancreatitis, are indeed accompanied by the acute onset of organ failure (13, 15). According to the retrospective nature of our study, we were not able to record potential septic complications developed during hospitalization in the latter patients. Similarly, the retrospective identification of septic dogs poses a significant challenge in the enrollment of our critically ill dogs, as the final diagnosis of sepsis could have been questionable in some cases and, on the other hand, might not have been definitely excluded in some patients classified with non-infectious systemic inflammation.
The new onset of specific ODs was able to predict outcome in our population of critically ill dogs admitted to ER and/or ICU. Specifically, patients with acute kidney injury, presence of stupor or coma, a prolonged Prothrombin time and an unexplained acidemia, showed higher risk of non-survival. Acute kidney injury in ICU is a heterogeneous syndrome with regard to exposure (eg, sepsis, surgery, toxicity), pathophysiology (eg, inflammation, hypoperfusion) and clinical presentation. The prognostic importance of AKI has been well demonstrated in critically ill humans, acting both as an indicator of disease severity and an independent predictor of mortality (16, 17). Due to the retrospective design of our study, we were not able to further characterize AKI etiologies and phenotypes (transient vs. intrinsic AKI), as previously suggested (18). Nonetheless, the results of the present study are in line with previous findings in dogs, overall corroborating the need for AKI screening in critically ill patients (18). With regard to neurological dysfunction, this appears to be one of the most important risk factors for sepsis-related mortality in humans, although only a low prevalence is normally reported (19). Neurological dysfunction is poorly defined in small animals, being not even considered in some of the available criteria for MODS in dogs (11, 20). Our results highlight the prognostic significance of the presence of stupor and coma in the current study population, underlining the need for a standardized neurological assessment in critically ill dogs. Coagulopathy in the course of critical care diseases results from interactions between inflammation, endotheliopathy and coagulation leading to microthrombi formation, consumption of coagulation factors, and finally in the development of disseminated intravascular coagulation (21). A reduced activity of endogenous anticoagulants and a possible imbalance of homeostasis with a tendency to a procoagulant state has been demonstrated in dogs with sepsis (22). Furthermore, selected hemostatic variables acted as predictors of survival in critically ill dogs (5, 23). Although our results do not allow for characterization of the phenotype of the hemostatic derangement, these results corroborate its prognostic significance in critical dogs. Finally, also the presence of unexplained acidemia was associated with a worse outcome in our study. In septic people, both lactate and base deficit have been proposed as endpoints for resuscitation and outcome predictors (24). Data in veterinary literature are limited to selected studies pointing out the prognostic role of acidemia and base deficit in critically ill dogs with sepsis and different emergency conditions (25, 26).
Overall, our results support the association between the development of OD and a poor prognosis (11). Therefore, given the lack of specific treatment for MODS, the early recognition of ODs is imperative to identify the most critical ICU subpopulation and to improve its intensive management, aiming to ameliorate outcomes.
The present study has several limitations. Due to the retrospective design, the diagnostic and therapeutic protocol of patients included was not standardized, but chosen by attending clinicians, and for this reason some clinicopathological data were missing for some cases, potentially affecting a complete evaluation of organ dysfunction. In addition, despite the inclusion of critically ill dogs with a clear final diagnosis, diagnosing sepsis is always a challenge: potential septic patients might have been erroneously diagnosed as non-septic, and at the same time, dogs that were not septic at the time of ER/ICU arrival could have then developed sepsis during hospitalization, causing unpredictable overlap in the two subpopulations considered in the study, as previously discussed. Finally, for some of the enrolled dogs there were no baseline laboratory data; hence, the diagnosis of the new-onset OD based on laboratory criteria (eg, acute kidney injury, hyperbilirubinemia) could have been potentially confounded by the possibility of previous disease or comorbidity.
Conclusion
To conclude, our results support the importance of the early identification of ODs in the veterinary critical care setting, and highlight the prognostic role of specific ODs like neurological, renal, hemostatic dysfunction and unexplained acidemia in dogs, early at the time of ER or ICU arrival. In this population, the detection of ODs was not helpful for diagnosing sepsis, and only the finding of fluid-refractory hypotension was clearly associated with sepsis. However, searching for a septic process in a patient showing new-onset ODs has always to be pursued. Finally, the value of the current results has to be investigated in larger population of critically ill patients, ideally considering a multi-centric prospective study design.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
The study was approved by the local Institutional Animal Care and Use Committee (protocol number ID 846). The studies were conducted in accordance with the local legislation and institutional requirements. Written informed consent was obtained from the owners for the participation of their animals in this study.
Author contributions
EC: Writing – original draft, Writing – review & editing. RT: Writing – original draft, Writing – review & editing. CB: Writing – review & editing. AP: Writing – review & editing. FB: Writing – review & editing. MG: Writing – original draft, Writing – review & editing.
Funding
The author(s) declare that no financial support was received for the research, authorship, and/or publication of this article.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The author(s) declared that they were an editorial board member of Frontiers, at the time of submission. This had no impact on the peer review process and the final decision.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Singer, M, Deutschman, CS, Seymour, CW, Shankar-Hari, M, Annane, D, Bauer, M, et al. The third international consensus definitions for Sepsis and septic shock (Sepsis-3). JAMA. (2016) 315:801–10. doi: 10.1001/jama.2016.0287
2. Seymour, CW, Liu, VX, Iwashyna, TJ, Brunkhorst, FM, Rea, TD, Scherag, A, et al. Assessment of clinical criteria for Sepsis: for the third international consensus definitions for Sepsis and septic shock (Sepsis-3). JAMA. (2016) 315:762–74. doi: 10.1001/jama.2016.0288
3. Alves, F, Prata, S, Nunes, T, Gomes, J, Aguiar, S, Aires da Silva, F, et al. Canine parvovirus: a predicting canine model for sepsis. BMC Vet Res. (2020) 16:199. doi: 10.1186/s12917-020-02417-0
4. Keir, I, and Kellum, JA. Acute kidney injury in severe sepsis: pathophysiology, diagnosis, and treatment recommendations. J Vet Emerg Crit Care. (2015) 25:200–9. doi: 10.1111/vec.12297
5. Kenney, EM, Rozanski, EA, Rush, JE, deLaforcade-Buress, AM, Berg, JR, Silverstein, DC, et al. Association between outcome and organ system dysfunction in dogs with sepsis: 114 cases (2003–2007). J Am Vet Med Assoc. (2010) 236:83–7. doi: 10.2460/javma.236.1.83
6. Troia, R, Mascalzoni, G, Calipa, S, Magagnoli, I, Dondi, F, and Giunti, M. Multiorgan dysfunction syndrome in feline sepsis: prevalence and prognostic implication. J Feline Med Surg. (2019) 21:559–65. doi: 10.1177/1098612X18792106
7. Christensen, MB, Langhorn, R, Goddard, A, Andreasen, EB, Moldal, E, Tvarijonaviciute, A, et al. Comparison of serum amyloid a and C-reactive protein as diagnostic markers of systemic inflammation in dogs. Can Vet J. (2014) 55:161–8.
8. Hauptman, JG, Walshaw, R, and Olivier, NB. Evaluation of the sensitivity and specificity of diagnostic criteria for sepsis in dogs. Vet Surg. (1997) 26:393–7. doi: 10.1111/j.1532-950x.1997.tb01699.x
9. Hayes, G, Mathews, K, Doig, G, Kruth, S, Boston, S, Nykamp, S, et al. The acute patient physiologic and laboratory evaluation (APPLE) score: a severity of illness stratification system for hospitalized dogs. J Vet Intern Med. (2010) 24:1034–47. doi: 10.1111/j.1939-1676.2010.0552.x
10. Swenson, KE, Dziura, JD, Aydin, A, Reynolds, J, and Wira, CR. Evaluation of a novel 5-group classification system of sepsis by vasopressor use and initial serum lactate in the emergency department. Intern Emerg Med. (2018) 13:257–68. doi: 10.1007/s11739-017-1607-y
11. Troia, R, Giunti, M, and Goggs, R. Plasma procalcitonin concentrations predict organ dysfunction and outcome in dogs with sepsis. BMC Vet Res. (2018) 14:111–9. doi: 10.1186/s12917-018-1427-y
12. Bone, RC, Balk, RA, Cerra, FB, Dellinger, RP, Fein, AM, Knaus, WA, et al. Definitions for sepsis and organ failure and guidelines for the use of innovative therapies in sepsis. Chest. (1992) 101:1644–55. doi: 10.1378/chest.101.6.1644
13. Fang, X, Wang, Z, Yang, J, Cai, H, Yao, Z, Li, K, et al. Clinical evaluation of Sepsis-1 and Sepsis-3 in the ICU. Chest. (2018) 153:1169–76. doi: 10.1016/j.chest.2017.06.037
14. Bulgarelli, C, Ciuffoli, E, Troia, R, Goggs, R, Dondi, F, and Giunti, M. Apolipoprotein A1 and serum amyloid a in dogs with sepsis and septic shock. Front Vet Sci. (2023) 10:1098322. doi: 10.3389/fvets.2023.1098322
15. Abraham, E. New definitions for sepsis and septic shock: continuing evolution but with much still to be done. JAMA. (2016) 315:757–9. doi: 10.1001/jama.2016.0290
16. Druml, W. Long term prognosis of patients with acute renal failure: is intensive care worth it? Intensive Care Med. (2005) 31:1145–7. doi: 10.1007/s00134-005-2682-5
17. Pickkers, P, Darmon, M, Hoste, E, Joannidis, M, Legrand, M, Ostermann, M, et al. Acute kidney injury in the critically ill: an updated review on pathophysiology and management. Intensive Care Med. (2021) 47:835–50. doi: 10.1007/s00134-021-06454-7
18. Troia, R, Gruarin, M, Grisetti, C, Serafini, F, Magna, L, Monari, E, et al. Fractional excretion of electrolytes in volume-responsive and intrinsic acute kidney injury in dogs: diagnostic and prognostic implications. J Vet Intern Med. (2018) 32:1372–82. doi: 10.1111/jvim.15146
19. Mayr, VD, Dünser, MW, Greil, V, Jochberger, S, Luckner, G, Ulmer, H, et al. Causes of death and determinants of outcome in critically ill patients. Crit Care. (2006) 10:R154–13. doi: 10.1186/cc5086
20. Ripanti, D, Dino, G, Piovano, G, and Farca, AM. Application of the sequential organ failure assessment score to predict outcome in critically ill dogs: preliminary results. Schweiz Arch Tierheilkd. (2012) 154:325–30. doi: 10.1024/0036-7281/a000356
21. Gourd, NM, and Nikitas, N. Multiple organ dysfunction syndrome. J Intensive Care Med. (2020) 35:1564–75. doi: 10.1177/0885066619871452
22. de Laforcade, AM, Freeman, LM, Shaw, SP, Brooks, MB, Rozanski, EA, and Rush, JE. Hemostatic changes in dogs with naturally occurring sepsis. J Vet Intern Med. (2003) 17:674–9. doi: 10.1111/j.1939-1676.2003.tb02499.x
23. Bentley, AM, Mayhew, PD, Culp, WT, and Otto, CM. Alterations in the hemostatic profiles of dogs with naturally occurring septic peritonitis. J Vet Emerg Crit Care. (2013) 23:14–22. doi: 10.1111/vec.12013
24. Rivers, E, Nguyen, B, Havstad, S, Ressler, J, Muzzin, A, Knoblich, B, et al. Early goal-directed therapy in the treatment of severe sepsis and septic shock. N Engl J Med. (2001) 345:1368–77. doi: 10.1056/NEJMoa010307
25. Conti-Patara, A, de Araújo Caldeira, J, de Mattos-Junior, E, de Carvalho, HDS, Reinoldes, A, Pedron, BG, et al. Changes in tissue perfusion parameters in dogs with severe sepsis/septic shock in response to goal-directed hemodynamic optimization at admission to ICU and the relation to outcome. J Vet Emerg Crit Care. (2012) 22:409–18. doi: 10.1111/j.1476-4431.2012.00769.x
Keywords: canine, base excess, stupor, coma, acute kidney injury, hemostatic dysfunction
Citation: Ciuffoli E, Troìa R, Bulgarelli C, Pontiero A, Buzzurra F and Giunti M (2024) New-onset organ dysfunction as a screening tool for the identification of sepsis and outcome prediction in dogs with systemic inflammation. Front. Vet. Sci. 11:1369533. doi: 10.3389/fvets.2024.1369533
Edited by:
Katja-Nicole Adamik, University of Bern, SwitzerlandReviewed by:
Esther Hassdenteufel, University of Giessen, GermanyThomas H. Edwards, United States Army Institute of Surgical Research, United States
Copyright © 2024 Ciuffoli, Troìa, Bulgarelli, Pontiero, Buzzurra and Giunti. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Elena Ciuffoli, elena.ciuffoli3@unibo.it
 Elena Ciuffoli
Elena Ciuffoli Roberta Troìa
Roberta Troìa Cecilia Bulgarelli
Cecilia Bulgarelli  Alessandra Pontiero
Alessandra Pontiero Massimo Giunti
Massimo Giunti