Effect of a microencapsulated phyto/phycogenic blend supplementation on growth performance, processing parameters, meat quality, and sensory profile in male broilers
- Department of Poultry Science, University of Arkansas, Fayetteville, AR, United States
Powered by consumer taste, value, and preferences, natural products including phytogenics and algae are increasingly and separately used in the food systems where they have been reported to improve growth performance in poultry and livestock. The present study aimed to determine the effects of a new feed additive, microencapsulated NUQO© NEX, which contains a combination of phytogenic and phycogenic, on broiler growth performance, blood chemistry, bone health, meat quality and sensory profile. Male Cobb500 chicks (n = 1,197) were fed a 3-phase feeding intervals; 1–14d starter, 15–28d grower, and 29–40d finisher. The dietary treatments included a corn-soy basal Control (CON), basal diet supplemented with NUQO© NEX at 100 g/ton from 1 to 28d then 75 g/ton from d 28 to 40 (NEX75), and basal diet supplemented with NUQO© NEX at 100 g/ton from 1 to 40d (NEX100). The NEX100 supplemented birds had 62 g more BWG increase and 2.1-point improvement in FCR compared with CON in the finisher and overall growth phase (p < 0.05), respectively. Day 40 processing body weights and carcass weights were heavier for the NEX100 supplemented birds (p < 0.05). The incidences of muscle myopathies were also higher in NEX treatments, which could be associated with the heavier weights, but the differences were not detected to be significant. The NEX75 breast filets had more yellowness than other dietary treatments (p = 0.003) and the NEX 100 treatment reduced the levels of breast filet TBARS at 7 days-post harvest (p = 0.053). Finally, both NEX treatments reduced the incidence of severe bone (tibia and femur) lesions. In conclusion, the supplementation of the phytogenic NUQO© NEX improved finisher performance parameters, whole phase FCR, processing carcass weights, and breast filet yellowness, at varying inclusion levels.
Introduction
The continuous increase in demand for animal-source protein is anticipated to escalate globally to approximately 152 Megatonne by 2030, and poultry meat is expected to account for a majority (52%) of the projected growth (1). This anticipated increase in poultry production will require alternatives to classical growth and health promotors which meet government regulations and consumer preferences and demand.
Antibiotics have been extensively used as health mediators and growth promotors (AGP) in animal production for over 70 years, but the increased concerns over antimicrobial resistance have led numerous countries to implement legislation to ban or reduce their usage (2). Sweden was the first European country to prohibit antimicrobials in feedstuffs in 1986; a move that set the groundwork for the European Union implementing an antimicrobial monitoring system (ESVAC) in 2005 and eliminating their use as growth promotors in 2006 (3). There have been efforts in the United States to curtail the usage of antimicrobials in animal production dating back to the 1950s, yet there has been less of a proclivity to truly implement the regulations (4, 5). The USDA banned the usage of antibiotics as growth promotors in 2017, which triggered a shift toward their usage as health promotors with identical or nearly identical dosages (5). The term “no antibiotic ever-NAE” or “raised without antibiotics, RWA” are consumer driven and producer implemented guidelines that have rose to prominence in response to consumers wanting a perceivably more natural protein option.
The poultry industry has been successful in reducing its dependency on antimicrobials, due in large part to its implementation of growth promoting alternatives. Numerous strategies to maintain performance have been used, including probiotics, prebiotics, synbiotics, organic acids, enzymes, antimicrobial peptides, hyperimmune egg antibodies, bacteriophages, clay, metals, algae, and phytogenics (6–9). While these alternatives provide tremendous potential to improve the overall health and performance of the bird, there is no panacea to meet at all the needs of a commercial producer (10). Plant extracts or phytogenics are of exceptional interest because they have been shown to display some beneficial effects observed with AGP, while coming from natural sources (6, 11, 12). Phytogenics are plant-derived compounds that are further classified into herbs (flowering nonpersistent plants), spices (herbs with pungent smell and associated taste), essential oils (volatile lipophilic compounds), or oleoresins (extracts from nonaqueous solutions) (13). The bioactivity of the phytogenics can vary widely between products and can depend on the part of plant used, growing conditions, harvesting conditions, processing/extraction process, and storage conditions (13). A large number of botanical options include bioactive compounds such as polyphenols (flavonoids, stilbenes, lignans, and phenolic acids), phenolics (tannins), glycosides, and alkaloids (14–19) provide beneficial effects in chickens through improved growth performance, digestibility, microbiota, immune response, oxidant status, egg quality, and meat quality (20). A growing number of phytogenics options, such as cinnamon, cloves, coriander, cumin, garlic, ginger, green tea, marjoram, mint, oregano, rosemary, sage, thyme, and yarrow have been tested in avian species, with varying levels of success (20). Similarly, algae extracts and compounds (phycogenics) have tremendous potential as animal feed due to the presence of essential biomolecules such as amino acids, poly-unsaturated fatty acids, carotenoids, and vitamins (21). Increasing number of researches have shown that blending feed with algae can positively affect the growth, health, and overall animal physiology and products at quantitative and qualitative levels (22–27).
Although the abovementioned feed additive alternatives were used extensively with evident beneficial effects, the dietary usage of a combined blend of phytogenics and phycogenics are very limited. Recently, using a double micro-encapsulation technology, a new feed additive (NUQO©-NEX, NUQO SAS, France) containing plant extracts and seaweed, has been developed (28) and has been reported to improve growth performance in chickens in Germany and Egypt (29). The objective of the present study was, therefore, to determine the effect of the microencapsulated phyto/phycogenic NUQO©NEX supplementation on broiler growth performance, processing yields, meat quality, and sensory profile under the U.S. experimental conditions.
Materials and methods
Animal care
All animal experiments were approved by the University of Arkansas Institutional Animal Care and Use Committee (IACUC # 21050) and were in accordance with the recommendations in NIH’s Guide for the Care and Use of Laboratory Animals.
Experimental diets and animal husbandry
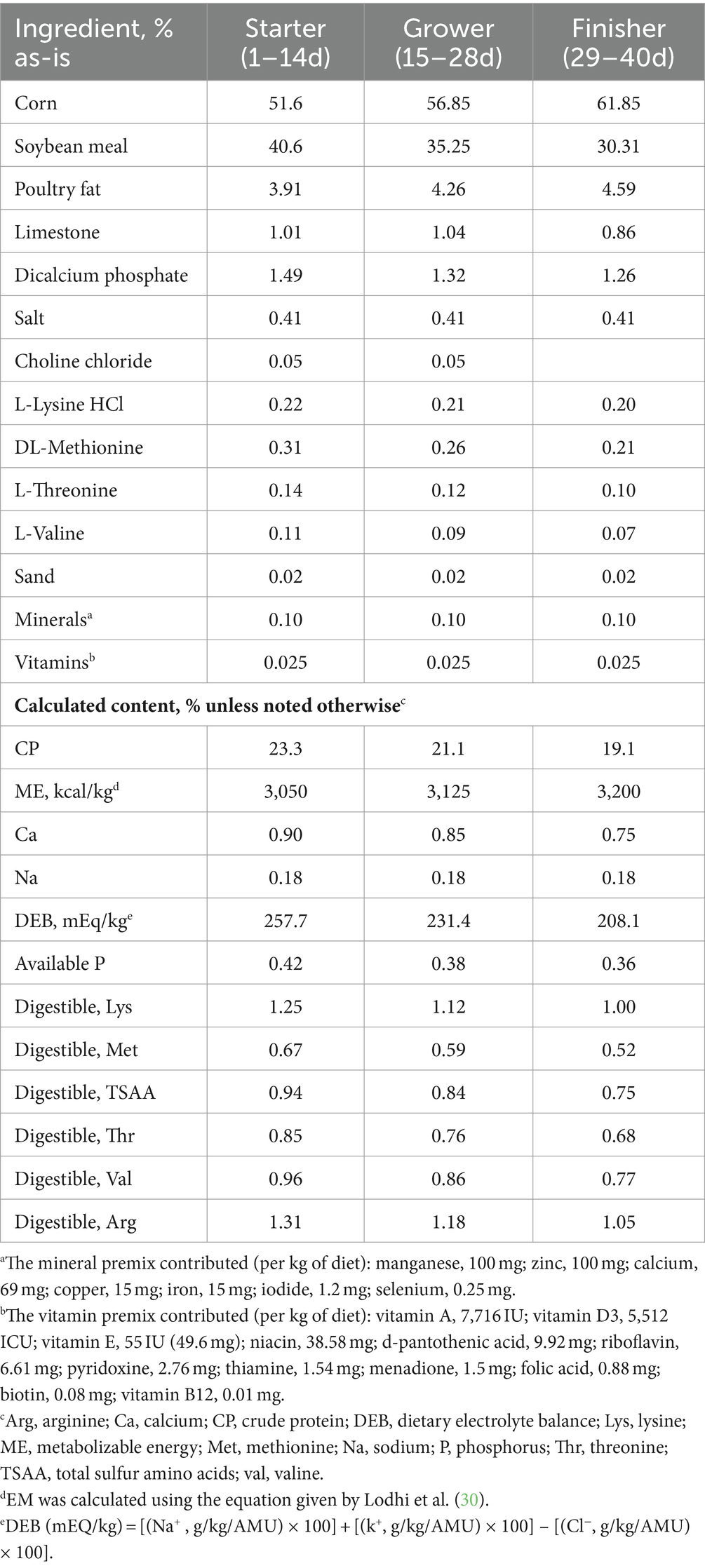
A total of 1,197 male Cobb 500 broiler chicks were obtained from a commercial hatchery (Cobb Vantress, Siloam Spring, Arkansas) and transported to the University of Arkansas broiler research farm. Birds were fed a 3-phase feeding intervals consisting of a crumbled starter (1–14 day), pelleted grower (14–28 day), and pelleted finisher (28–40 day). Birds were randomly allocated into 57 total pens (21 birds/pen, 0.09 m2/bird) and assigned to one of three experimental treatments (Table 1): corn/soybean meal basal diet (CON), basal diet supplemented with NUQO©NEX (NUQO SAS, Annecy, France) phytogenic/phycogenic blend at 100 g/ton from d 1–28 then 75 g/ton from d 29–40 (NEX75), and basal diet supplemented with NUQO©NEX blend at 100 g/ton from d 1–40 (NEX100). The composition of these phytogenic/phycogeni additives are proprietary to NUQO SAS (Annecy, France), but they are polyherbal formulations of pre-standardized and tested herbs (encapsulated micro-particles of dried Kelp, Cinnamaldehyde, Thymol, and Eugenol). Diets were formulated to meet or exceed all nutrient recommendations from the commercial breeder standards (31), and were offered ad libitum. The doses were recommended by the manufacturer. Each pen consisted of litter top-dressed with pine shavings and was equipped with a nipple drinking system and bell feeder. A supplemental paper feed tray was used for the first 7 days. House temperatures were 32 ̊C at d1 and gradually decreased to 20 ̊C at d27. The photoperiod used was 24 L:0D (30 lux) on d 1, 23 L:1D (30 to 10 lux) from d 1 to d 10, and 18 L:6D (10 to 5 lux) for the remainder of the trial.
Growth performance, and processing parameters
Birds were weighed by pen at 1, 14, 28, and 40 day-post hatch and feed intake was recorded. Average body weight (BW), body weight gain (BWG), feed intake (FI), and feed conversion ratio (FCR) were calculated for starter, grower, finisher, and overall growth phase. Mortality and dead bird weights were recorded daily and then used to correct FCR, BWG, and FI per bird.
Feed was removed from all pens 10 h prior to processing, while water access was maintained. On day 40, 399 birds (7 birds/pen) were processed at the University of Arkansas Pilot Processing Plant (Fayetteville, AR) via a commercial inline system. Birds were weighed, placed on shackles, and electrically stunned (11 V, 11 mA for 11 s) before being exsanguinated, soft scalded (55°C for 2 min), de-feathered (Foodcraft Model 3; Baker international, MI, United States), and mechanically eviscerated. Carcass or weight without giblets (WOG), and abdominal fat pad weights were recorded immediately post evisceration. Carcasses were hot-deboned, following a brief ice bath, on a section of inline commercial deboning equipment (line speed set to 4″ of run time per second) with birds manually added to the initial cone. Attempting to reduce variability between part yield, trained individuals made identical cuts to debone whole carcasses into subsequent parts of skinless breast, tender, skin-on wing, and skin-on whole leg. Weights were then recorded and used to calculate yields relative to individual live bird weight.
Blood chemistry, gas, and electrolyte parameters
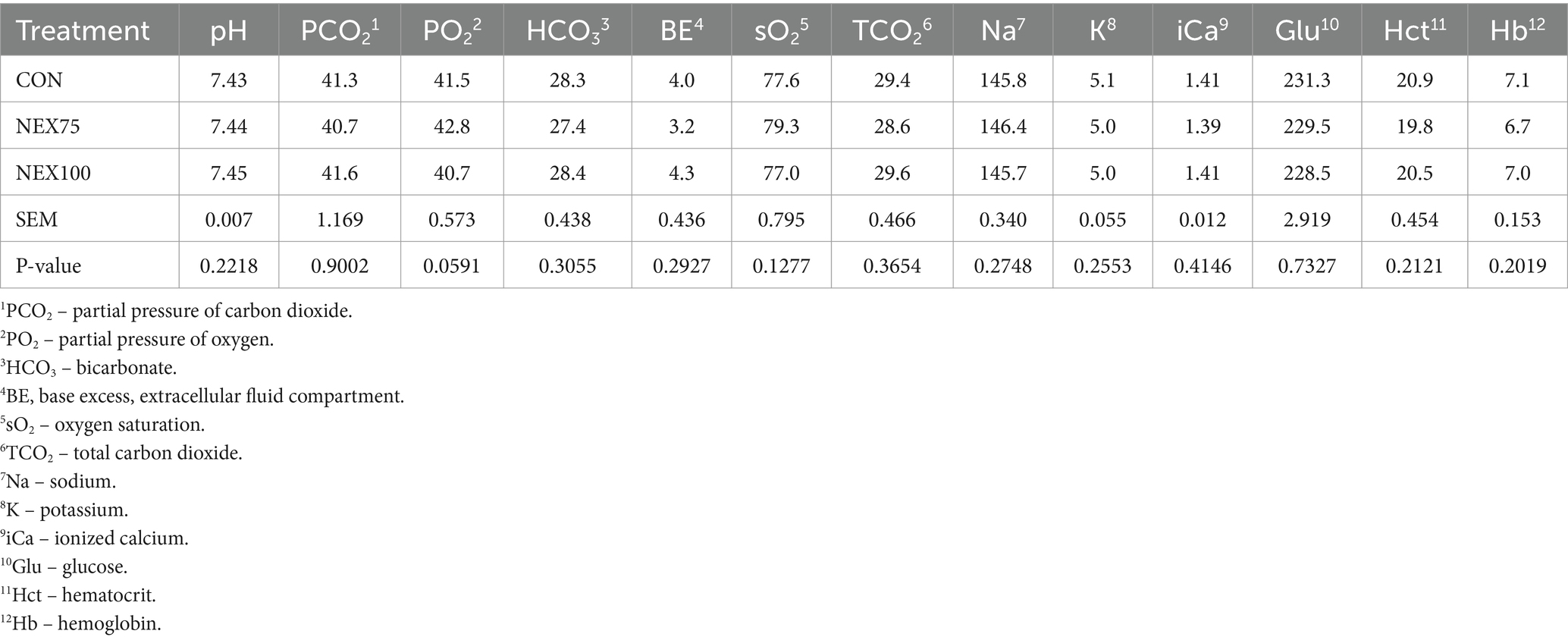
On day 40, approximately 1 mL of whole blood was collected from brachial wing vein (cutaneous ulnar vein) using a 3 mL syringe and a 1-inch 20G needle and placed into K2 EDTA blood collection tubes (Becton Dickinson, Franklin Lakes, NJ) to prevent coagulation. pH, partial pressure of CO2 (pCO2), total CO2 (TCO2), partial pressure of O2 (pO2), bicarbonate (HCO − 3), base excess (BE), O2 saturation (sO2), sodium (Na), potassium (K), ionized calcium (iCa), glucose, hematocrit (Hct), and hemoglobin (HB) analyses were determined via an i-STAT Alinity system (SN:801128; software version JAMS 88.A.1/CLEW D44; Abaxis, Union City, CA, United States) with the i-STAT CG8 + cartridge test (ABBT-03P88-25) according to manufacturer’s recommendation. Analysis was performed at room temperature using the temperature correction function of the i-STAT Alinity system. The i-STAT system has been validated in avian species (32–35).
Femur- and tibia-lesion scoring
On day 40, 114 birds (2 birds/pen, n = 19/treatment) were humanely euthanized and immediately necropsied to determine presence of subclinical lesions in the proximal heads of both the femora and tibiae. Bone was selected macroscopically based on a previously reported scale (36, 37). Briefly, femur scores were classified into the following categories: 0- no sign of lesion and in-tact articular cartilage cap (Normal); 1- separation of the head from the acetabulum, called femur head separation; 2- necrotic lesion encompassing an area larger than approximately 1 cm2 (considered femur head necrosis or bacterial chondronecrosis with osteomyelitis). Tibial lesion severity was also scored on a 0 to 2 scale into the following categories: 0- no abnormalities (Normal); 1- transitional tibial head necrosis, 2- severe tibial head necrosis, characterized by degradation of internal growth plate structure due to infection.
Woody breast and white striping scoring
Boneless breast filets were blind analyzed and macroscopically scored by a well-trained person (who did not know the treatments) for woody breast (WB) and white striping (WS) as previously described (38, 39). Briefly, for WB, a whole number increment scale was used with 0 being flexible throughout (normal), 1 being hard mainly in the cranial region (mild), 2 being mostly hard but some flexibility (moderate), and 3 being extremely hard throughout filet (severe). White striping was scored using 0 (normal), 1 (mild), 2 (moderate), and 3 having striations greater than 3 mm (severe).
24-h pH and color
Breasts filets were collected, placed on trays, covered with plastic overlay, and stored at 4°C for 24 h post-mortem drip loss, pH, and colorimetric analysis. Drip loss was calculated as the difference between hot debone breast weights and chilled breast weights and is expressed as a percentage of hot debone breast weight. Three readings were taken with a colorimeter (CR-400; Konica Minolta Sensing Inc., Sakai Osaka, Japan; size 102(W) X 217 (H) X 63 (D) mm) using illuminant D65 and a 2-degree observer to determine the L* (Lightness), a* (redness), and b* (yellowness) values on the ventral side of the left breast filet. Each left breast lobe was measured 3 times with a temperature-compensating pH meter (Testo 205; Testo Inc., West Chester, PA) to present an averaged breast filet pH.
Sensory analysis and thiobarbituric acid reactive substances assay
At 47 days of age, following a 10 h feed withdrawal, 36 birds (12 birds/treatment) were randomly selected and transported to a commercial processing plant (B&R meat processing, Winslow, AR) to be slaughtered under federal (U.S. Department of Agriculture, Food safety and inspection service) inspection for subsequent sensory and TBARS analyses. After 24 h of air chilling at 4°C, the left breast filet was collected and delivered to the Sensory Science Center at the University of Arkansas Food Science Department for a professional meat descriptive sensory panel (IRB# 13-05-713). The descriptive panel was trained according to the Spectrum method (40) and has extensive experience with poultry and other meat products (41). Each panelist evaluated every sample at random for aroma, flavor, taste, and texture. All sensory attributes and texture characteristics were scored to the nearest 0.5 on a scale ranging from 0 (least intense) to 15 (most intense). TBARS were measured as previously describe (42, 43) with commercially available kits, which were utilized according to manufacturer’s protocol (Cayman Chemical Company, Ann Arbor, MI). Samples were analyzed at 1, 4, and 7 d postmortem and results are presented as mmol of malonaldehyde (MDA)/L.
Statistical analysis
All data were analyzed using JMP Pro22 fit-model (SAS Institute, 2022, Cary, NC) within a randomized complete block design. Pen (n = 19/treatment) represented the experimental unit for growth performance, processing parameters, meat quality, bone lesion scores, and blood parameters. Bird (n = 12/treatment) represented the experimental unit for sensory and TBARS analyses. Outliers were removed from whole data set when multiple parameters exceeded ±2 SD. Data were analyzed with one-way ANOVA and Tukey as a multiple comparison test. Significance was set at p ≤ 0.05. In the ANOVA of the sensory data, panelist nested within session was included as a random effect in the model. Least square means were computed and separated with the pairwise t-tests (PDIFF option of SAS) when a significant (p < 0.05) F-test was noted.
Results
Dietary supplementation of NUQO©NEX improves growth performances in broilers
The growth performance, feed efficiency, and mortality percent are presented in Table 2. There were no differences observed in the 1–14d starter phase (p > 0.05) for any parameter between treatments. During the grower phase (14–28d), the NEX75 treatment had lower body weight gain (1.297 vs. 1.326 kg, p < 0.001), and feed intake (1.888 vs. 1.923 kg, p < 0.001) compared to the CON group (Table 2). During the finisher phase, the NEX100 treatment had higher body weight gain (1.838 kg vs. 1.776 kg, p = 0.0282) and averaged 3.7 points better FCR (p = 0.0528) compared to the CON group.
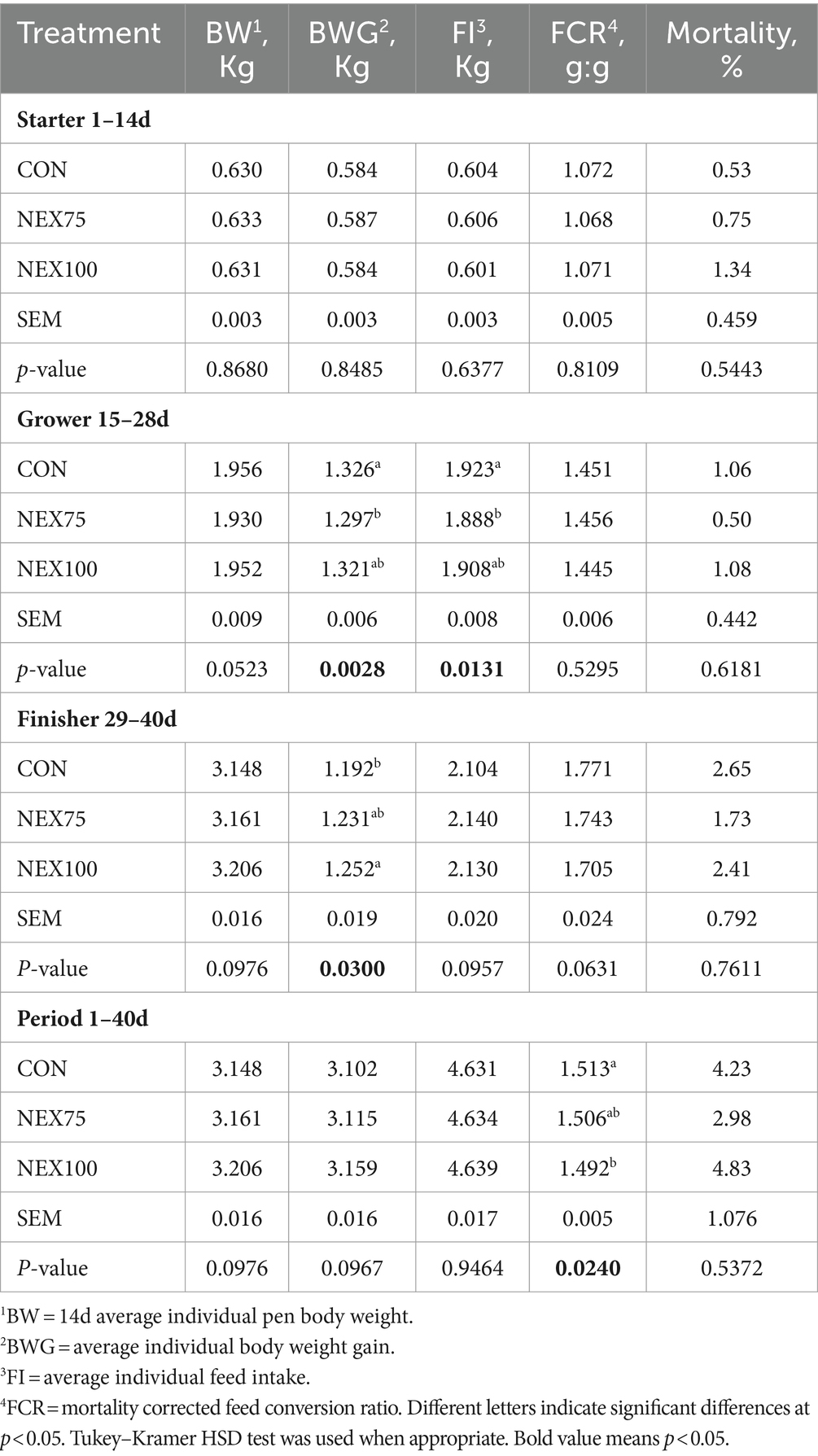
Table 2. Effect of different levels of NUQO© NEX on growth performance and mortality rate of male Cobb 500 chicks during different intervals of ages.
When considering the whole growth phase performance, feed efficiency, and mortality rate, the NEX100 treatment averaged overall 2.1 points better FCR (p = 0.0240) and higher BWG (57 g, p = 0.0967) compared to the CON group. The mortality rate was not impacted by any of the experimental treatment.
As shown in Table 3, NEX supplementation did not affect blood chemistry, gas, and electrolytes.
Effects of NUQO©NEX on the incidence of metabolic (bone and muscle) disorders
Tibia and femur lesion scores are presented in Table 4. The NEX100 treatment reduces the incidence of severe bacterial chondronecrosis with osteomyelitis (BCO, score 2) compared to the CON group (5.6 vs. 8.3%), however the differences were not statistically discernable at p < 0.05.
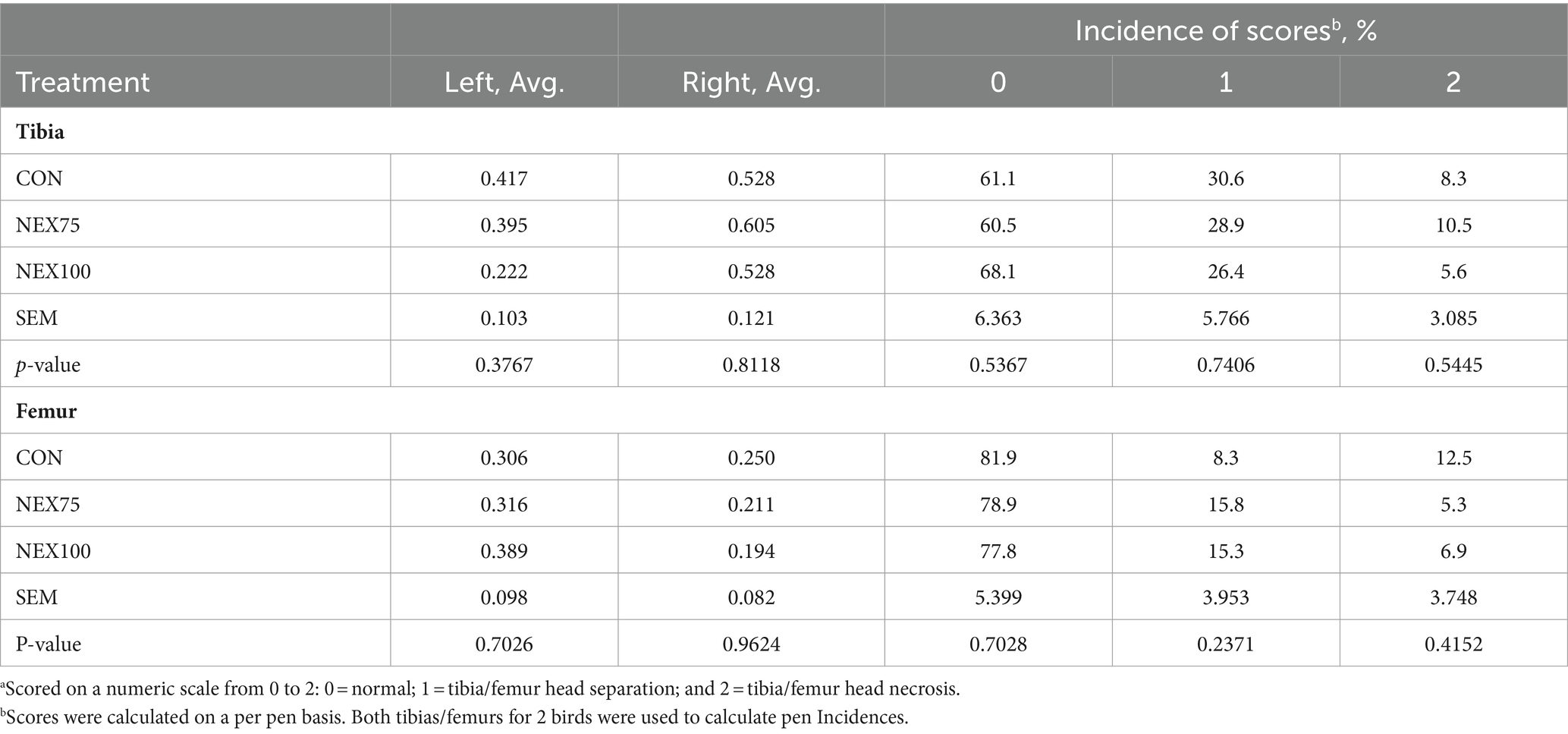
Table 4. Tibia and femur scoresa of 40d Cobb500 male broilers.
As shown in Table 4, although it is not statistically significant, both NEX (75 and 100) treatments reduced the incidence of severe BCO (score 2) (12.5% vs. 5.3 and 6.9% for CON, NEX75, and NEX100, respectively), but increased that of score 1 compared to the CON group (8.3% vs. 15.8 and 15.3% for CON, NEX75, and NEX100, respectively).
Both NEX treatments increased total incidence of muscle (WB and WS) myopathies, although the differences were not statistically discernable at p < 0.05. Birds fed NEX75 had a higher score for all WB scores, while birds fed NEX100 had lower WB score 2, but an increased WB score 1 and 3 compared to the CON group (Table 5). Furthermore, birds fed NEX75 had a lower WS score 2 compared to the CON, while birds fed NEX100 had a lower WS score 1, but an increased score 3 (Table 5).
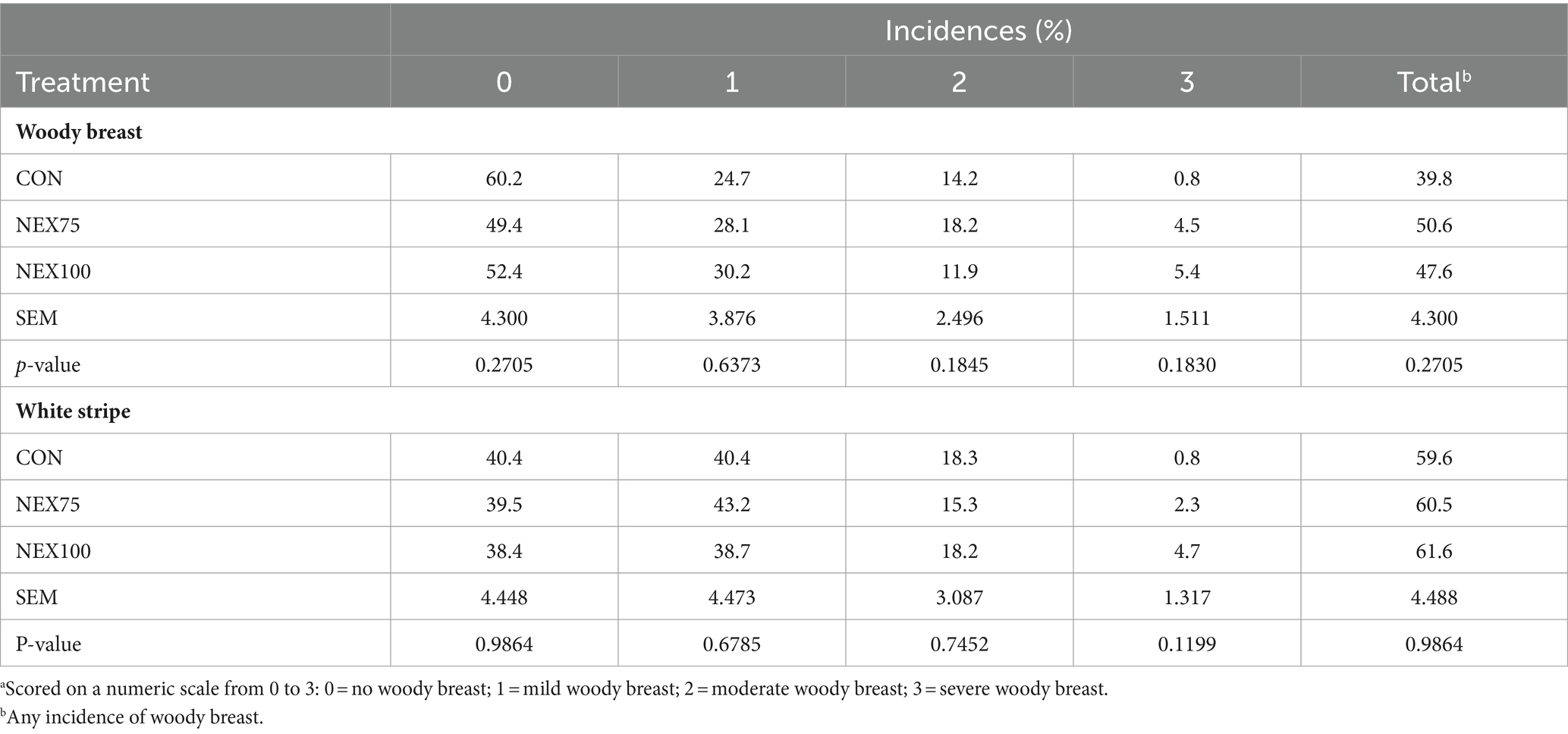
Table 5. Woody breast and white striping scorea of 40d Cobb500 male broilers.
Effects of NUQO©NEX on processing-, meat quality-, and sensory-parameters
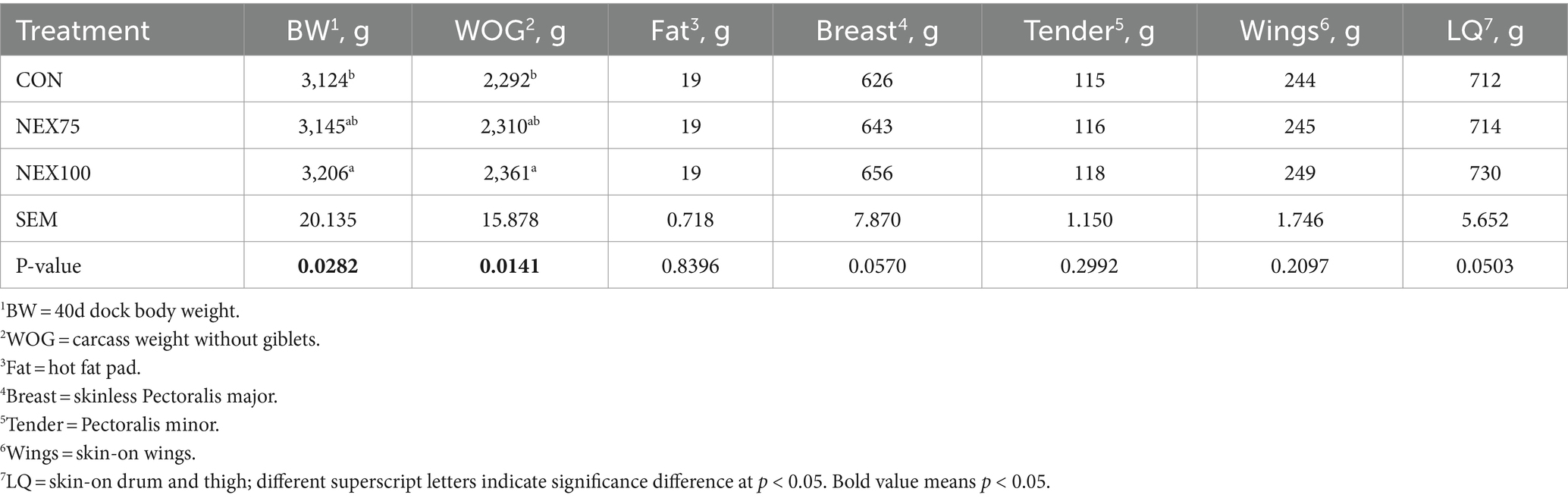
Processing part weights are presented in Table 6. The NEX100 treatment had heavier body weight (3,206 kg vs. 3,124 kg, p = 0.028) and carcass weight (2,361 kg vs. 2,292 kg, p = 0.014) compared to the CON group. The NEX100 treatment also had larger breast (656 g vs. 626 g, p = 0.0570) and heavier leg quarters (730 g vs. 714 g, p = 0.05) than the CON group.
Meat quality data are presented in Table 7. The NEX75 treatment breast filets had more yellowness than the CON and NEX100 treatments (p = 0.003). There were no differences observed in 24-h post-mortem breast filet pH, drip loss, lightness, or redness (p > 0.05).
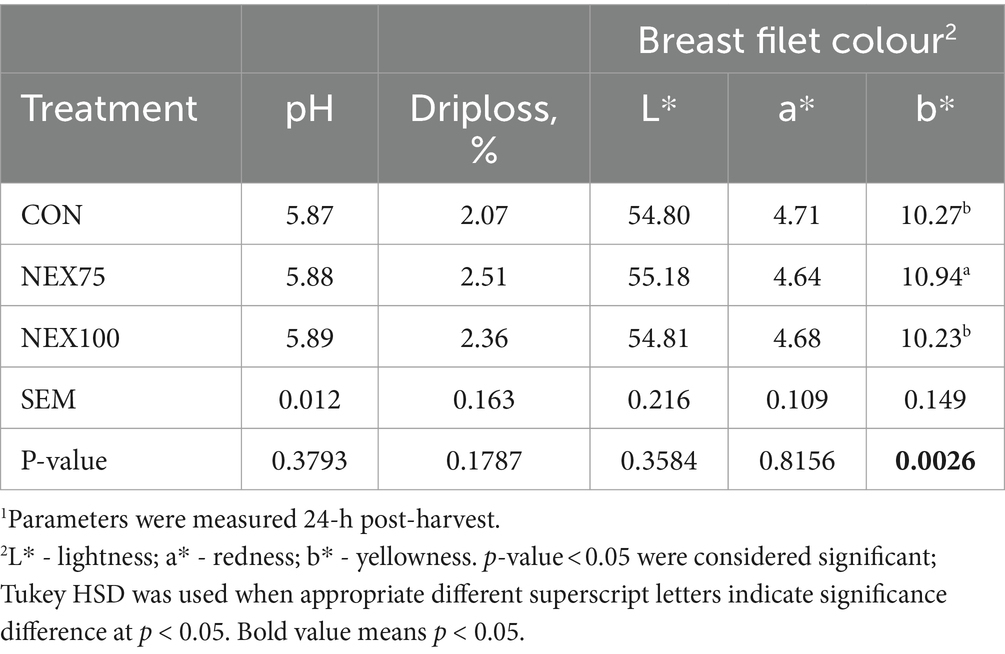
Table 7. Meat quality of 40d Cobb500 male broilers1.
Sensory profile data are presented in Figure 1. There were no differences in any aroma (Figure 1A), flavor (Figure 1B), or taste/texture (Figure 1C) of the breast filets (p > 0.05).
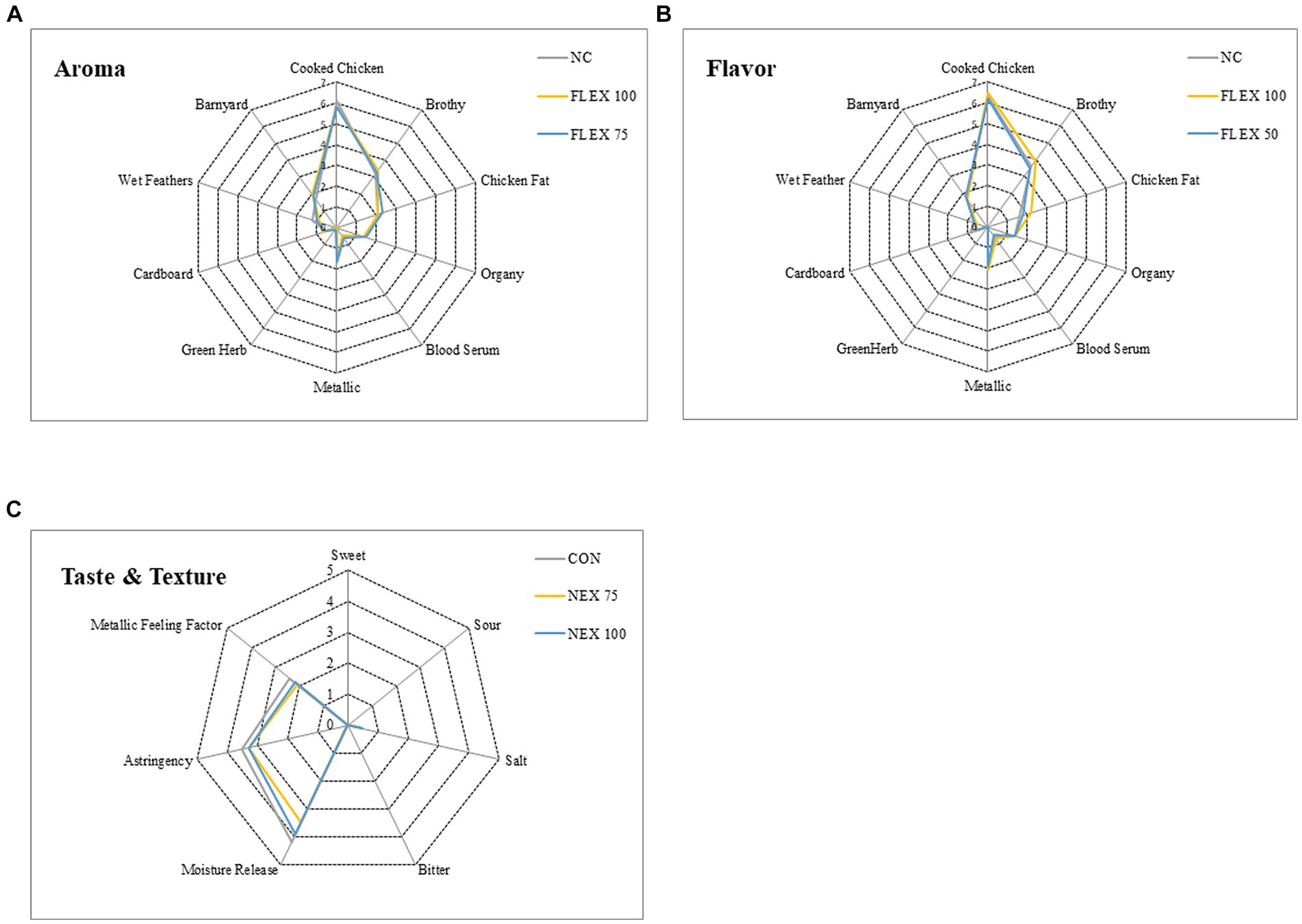
Figure 1. Effects of NUQO©NEX supplementation on broiler breast meat sensory profile. After 24 h of air chilling at 4°C, the left breast filet was collected and delivered to the Sensory Science Center at the University of Arkansas Food Science Department for a professional meat descriptive sensory panel. Each panelist evaluated every sample at random for aroma (A), flavor (B), and taste and texture (C). All sensory attributes and texture characteristics were scored to the nearest 0.5 on a scale ranging from 0 (least intense) to 15 (most intense).
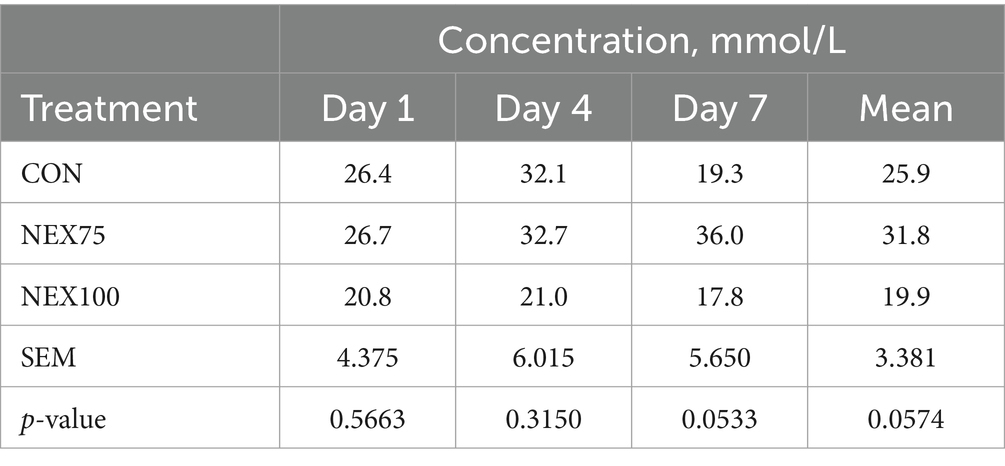
TBARS data are presented in Table 8. Although the differences were not statistically discernable, NEX100 treatment reduced the levels of breast filet TBARS at 1, 4, or 7 days-post harvest (p = 0.5663, p = 0.3150, and p = 0.053, respectively).
Discussion
With an anticipated annual market growth of 7.2% from 2022 to 2027, blended phytogenic feed additives have tremendous potential to promote health, performance, and uniformity in animal production (44). Overall, performance results of phytogenic inclusion in poultry diet have been inconsistent, which is accredited to the wide range of compositions of the active ingredients being used (6). The exact underlying mechanism for every phytogenic is complex and remains to be fully elucidated, yet recent emerging scientific findings are starting to provide a better mechanistic understanding of the mode of action of these natural feed additives.
The composition of the new microencapsulated NUQO©NEX feed additive is a proprietary of NUQO SAS (Annecy, France), but is a polyherbal formulation consisted of a combination of metabolites from plants and from marine algae (phytogenic and phycogenic). It contains dried kelp and the essential oils thymol, cinnamaldehyde and eugenol (derived from thyme, cinnamon and cloves, respectively) encapsulated in a fat matrix. Correctly identified, validated, and blended phytogenics can exhibit synergistic effects that can enhance their antimicrobial and antioxidant bioactivity, while being included at much lower concentrations (20). Phytogenics also need to be stabilized to survive processing conditions and delivered at the right place in the digestive tract to exert their activity in the animal (45).
In our experimental conditions, all treatments exceeded breeder’s growth guide (31). This optimization of broiler genetic potential was the result of optimal environmental conditions, adequate nutrient supply, and the absence of any extrinsic health challenges, which was supported by the lack of any significant differences in blood chemistry, gas, and electrolytes, as well as bone attrition. It is worth noting that although the differences were not statistically significant, the supplementation of NUQO©NEX reduce the severity of both tibia and femur BCO, which merits further investigations.
Of particular interest, the in-feed supplementation of microencapsulated NUQO©NEX at higher dose, resulted in an average 57 g greater BWG, 2.1 better FCR, and 30 g heavier breast weight compared to their counterpart control birds that were already at or close to genetic potential. The rule of thumb, as the 2021-global broiler production was ~71 billion birds, is that NUQO©NEX supplementation would increase the worldwide broiler meat production by 4 million tones, with a 2.1 million tones improvement in breast weight. The observed improvement here in growth performance was not surprising as it has been reported that NUQO©NEX ameliorate nutrient and mineral digestibility and thereby enhance performance at 21 days with better gain (+2.2%) and better FCR (−0.6%) (29). Furthermore, phytogenic combinations such as thymol, cinnamaldehyde, eugenol, and various others have had positive impacts through increased BWG and decreased FCR (46–52). Bravo and Ionescu concluded in a 13 trial meta-analysis that blended products (carvacrol, cinnamaldehyde and capsicum oleoresin) increased BWG, while reducing FCR and mortality (53). Contrarily, Najafi and Torki found that only thymol positively impacted BWG and FCR, while cinnamaldehyde and eugenol showed no effects on performance (54). In another 124 trial meta-analysis, it was concluded that a blend of thymol, carvacrol, and cinnamaldehyde displayed a higher inclination for improved performance and feed efficiency (55). The differences observed in the beneficial effects of the aforesaid phytogenics might due to their compositions, dosage, intestinal stability of their active compounds, chicken strains, diet composition, and/or experimental conditions and duration.
Although it was not studied in combination with phytogenics, increasing number of investigations have shown that dietary administration of algae alone improved also growth performances in poultry (2, 25, 56–58). Here, it is clear that the encapsulated phytogenic-phycogenic combination synergistically improved growth performance in broilers, and therefore its underlying mechanism warrants further in-depth investigations.
Although its mode of action warrants future studies, it is possible that the microencapsulated NUQO©NEX has a good protection of its active ingredients, a better stability, and an optimal release in the gut. This, in turn, would improve macronutrient and mineral digestibility and boost broiler performances. It is also possible that NUQO©NEX exerts a cytoprotective effect via its antioxidant, immunomodulatory, and/or anti-inflammatory property (28). Furthermore, Dridi’s group has recently shown that in feed- or in water-phytogenics administration improved growth performances via modulation of feeding-related hypothalamic neuropeptides and peripheral intermediary (lipogenic and lipolytic program) metabolisms (59–62). According to recent reports, there is increasing evidence that phytogenics stabilize and improve gut microbiota, which in turn enhance intestinal integrity and health leading to better growth (63–66). Although these speculations for NUQO©NEX’s mode of action are scientifically sound, they need to be supported and proven by mechanism-based studies.
Regarding processing weights and meat yield, in contrast to CON birds that lost an average of 24 g/bird after 10 h feed withdraw, birds fed NUQO©NEX at higher dose maintained the same body weight. This could be a result of a better gut integrity and health and improved resilience to the nutritional withdraw stress. Furthermore, NUQO©NEX birds had higher numerical breast meat yield (+0.42%) compared to the CON group, which could have a significant economic impact on the industry, especially in regions such as the U.S.A. where more than 90% of the consumers strongly prefer breast meat to the alternative dark meat (67). Although the effect was not statistically significant, the flip side of the coin is that this improvement tended to increase the incidence of muscle (WB and WS) myopathies. This is not surprising as body weight has shown to have a direct positive correlation with incidence of severity of WB and WS (68). The comparison to the current control is, however, unfair as it has a lower body weight. Essentially, the effect of NUQO© NEX on muscle myopathies should be studied versus a control at iso-weight.
The breast filet quality (pH, drip loss, and color) from the current trial are in close alignment with other experiments conducted with the same equipment (69). The postmortem pH can have large impacts on meat quality through altered functionality, color, drip loss, and shelf life (70). The observed values (~5.8) here are considered in the “normal” range of 5.7–6.1 (71–73). The breast filets from the NEX75 treatment had more yellowness than both other treatments. Broiler meat color can be impacted by a variety of factors including sarcoplasmic protein (myoglobin, hemoglobin, cytochrome, catalases, etc.), pH, age, sex, breed, management, processing techniques, and/or diet (67, 74). Phytogenic feed additives have displayed tremendous potential to improve pigmentation. A study by Reis et al. found that breast filet yellowness increased when broilers were fed a phytogenic blend of carvacrol, thymol, and cinnamic aldehyde (75). Similar results were observed from another group, which found that thymol, cinnamaldehyde and carvacrol increased yellowness with and without curcumin (76). On the other hand, Dridi’s group and Alfaia and co-workers have shown that algae treatment increased meat yellowness (b*), which is probably due to high levels of carotenoids and retinol (77).
Conclusion
In conclusion, although meat quality was not altered, the supplementation of the phyto/phycogenic NUQO©NEX improved finisher performance parameters, whole phase FCR, processing carcass weights, and breast filet yellowness, at varying inclusion levels. Further titration and mechanistic studies are warranted to identify the most beneficial dose of NUQO©NEX and to define its mode action.
Data availability statement
The original contributions presented in the study are included in the article/supplementary material, further inquiries can be directed to the corresponding author.
Ethics statement
All animal experiments were approved by the University of Arkansas Institutional Animal Care and Use Committee (IACUC # 21050) and were in accordance with the recommendations in NIH’s Guide for the Care and Use of Laboratory Animals. The study was conducted in accordance with the local legislation and institutional requirements.
Author contributions
GM: Data curation, Formal analysis, Writing – original draft. EG: Data curation, Writing – review & editing. AR: Data curation, Writing – original draft. CM: Data curation, Formal analysis, Writing – original draft. SD: Conceptualization, Funding acquisition, Visualization, Writing – original draft, Writing – review & editing.
Funding
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. This study was supported by a grant from NUQO SAS, France (FY2023 to SD). NUQO had no role in conducting the research, generating the data, interpreting the results or writing the manuscript.
Acknowledgments
The authors would like to thank Dr. Mike Kidd, Dr. Sara Orlowski, Maryam Ardakani, Wafa Youssfi, Karim Taouis, Blake Nelson, Savannah Crafton, Matheus Costa, Benjamin Angel, Maricela Maqueda, Kirsten Shaffer, Travis Tabler, Devon Jackson, and the crew at the University of Arkansas poultry research farm and processing plant.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The author(s) declared that they were an editorial board member of Frontiers, at the time of submission. This had no impact on the peer review process and the final decision.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
2. Alwaleed, EA, El-Sheekh, M, Abdel-Daim, MM, and Saber, H. Effects of Spirulina platensis and Amphora Coffeaeformis as dietary supplements on blood biochemical parameters, intestinal microbial population, and productive Performance in broiler chickens. Environ Sci Pollut Res Int. (2021) 28:1801–11. doi: 10.1007/s11356-020-10597-3
3. Mulchandani, R, Wang, Y, Gilbert, M, and Van Boeckel, TP. Global trends in antimicrobial use in food-producing animals: 2020 to 2030. PLOS Glob Public Health. (2023) 3:e0001305. doi: 10.1371/journal.pgph.0001305
4. Love, DC, Davis, MF, Bassett, A, Gunther, A, and Nachman, KE. Dose imprecision and resistance: free-choice medicated feeds in industrial food animal production in the United States. Environ Health Perspect. (2011) 119:279–83. doi: 10.1289/ehp.1002625
5. Wallinga, D, Smit, LAM, Davis, MF, Casey, JA, and Nachman, KE. A review of the effectiveness of current us policies on antimicrobial use in meat and poultry production. Curr Environ Health Rep. (2022) 9:339–54. doi: 10.1007/s40572-022-00351-x
6. Gadde, U, Kim, WH, Oh, ST, and Lillehoj, HS. Alternatives to antibiotics for maximizing growth Performance and feed efficiency in poultry: a review. Anim Health Res Rev. (2017) 18:26–45. doi: 10.1017/S1466252316000207
7. Callaway, TR, Lillehoj, H, Chuanchuen, R, and Gay, CG. Alternatives to antibiotics: a symposium on the challenges and solutions for animal health and production. Antibiotics (Basel). (2021) 10:471. doi: 10.3390/antibiotics10050471
8. Cheng, G, Hao, H, Xie, S, Wang, X, Dai, M, Huang, L, et al. Antibiotic alternatives: the substitution of antibiotics in animal husbandry? Front Microbiol. (2014) 5:217. doi: 10.3389/fmicb.2014.00217
9. Rahman, MRT, Fliss, I, and Biron, E. Insights in the development and uses of alternatives to antibiotic growth promoters in poultry and swine production. Antibiotics (Basel). (2022) 11:766. doi: 10.3390/antibiotics11060766
10. Tellez-Isaias, G, and Latorre, JD. Editorial: alternatives to antimicrobial growth promoters and their impact in gut microbiota, health and disease: volume ii. Front Vet Sci. (2022) 9:857583. doi: 10.3389/fvets.2022.857583
11. Kikusato, M. Phytobiotics to improve health and production of broiler chickens: functions beyond the antioxidant activity. Anim Biosci. (2021) 34:345–53. doi: 10.5713/ab.20.0842
12. Amad, AA, Manner, K, Wendler, KR, Neumann, K, and Zentek, J. Effects of a phytogenic feed additive on growth Performance and Ileal nutrient digestibility in broiler chickens. Poult Sci. (2011) 90:2811–6. doi: 10.3382/ps.2011-01515
13. Windisch, W, Schedle, K, Plitzner, C, and Kroismayr, A. Use of phytogenic products as feed additives for swine and poultry. J Anim Sci. (2008) 86:E140–8. doi: 10.2527/jas.2007-0459
14. Surai, PF. Polyphenol compounds in the chicken/animal diet: from the past to the future. J Anim Physiol Anim Nutr (Berl). (2014) 98:19–31. doi: 10.1111/jpn.12070
15. Mazur-Kusnirek, M, Antoszkiewicz, Z, Lipinski, K, Kaliniewicz, J, Kotlarczyk, S, and Zukowski, P. The effect of polyphenols and vitamin E on the antioxidant status and meat quality of broiler chickens exposed to high temperature. Arch Anim Nutr. (2019) 73:111–26. doi: 10.1080/1745039X.2019.1572342
16. Hidayat, C, Irawan, A, Jayanegara, A, Sholikin, MM, Prihambodo, TR, Yanza, YR, et al. Effect of dietary tannins on the Performance, lymphoid organ weight, and amino acid Ileal digestibility of broiler chickens: a Meta-analysis. Vet World. (2021) 14:1405–11. doi: 10.14202/vetworld.2021.1405-1411
17. Rupasinghe, HP, Ronalds, CM, Rathgeber, B, and Robinson, RA. Absorption and tissue distribution of dietary quercetin and quercetin glycosides of Apple skin in broiler chickens. J Sci Food Agric. (2010) 90:1172–8. doi: 10.1002/jsfa.3944
18. Kikusato, M, Xue, G, Pastor, A, Niewold, TA, and Toyomizu, M. Effects of plant-derived Isoquinoline alkaloids on growth Performance and intestinal function of broiler chickens under heat stress. Poult Sci. (2021) 100:957–63. doi: 10.1016/j.psj.2020.11.050
19. Liu, Y, Li, Y, Niu, J, Liu, H, Jiao, N, Huang, L, et al. Effects of dietary Macleaya Cordata extract containing Isoquinoline alkaloids supplementation as an alternative to antibiotics in the diets on growth Performance and liver health of broiler chickens. Front Vet Sci. (2022) 9:950174. doi: 10.3389/fvets.2022.950174
20. Abdelli, N, Sola-Oriol, D, and Perez, JF. Phytogenic feed additives in poultry: achievements, prospective and challenges. Animals (Basel). (2021) 11:3471. doi: 10.3390/ani11123471
21. Saadaoui, I, Rasheed, R, Aguilar, A, Cherif, M, Al Jabri, H, Sayadi, S, et al. Microalgal-based feed: promising alternative feedstocks for livestock and poultry production. J Anim Sci Biotechnol. (2021) 12:76. doi: 10.1186/s40104-021-00593-z
22. Navarro, F, Forjan, E, Vazquez, M, Montero, Z, Bermejo, E, Castano, MA, et al. Microalgae as a safe food source for animals: nutritional characteristics of the acidophilic microalga Coccomyxa Onubensis. Food Nutr Res. (2016) 60:30472. doi: 10.3402/fnr.v60.30472
23. Tavernari, FC, Roza, LF, Surek, D, Sordi, C, Silva, M, Albino, LFT, et al. Apparent Metabolisable energy and amino acid digestibility of microalgae Spirulina platensis as an ingredient in broiler chicken diets. Br Poult Sci. (2018) 59:562–7. Epub 2018/07/05. doi: 10.1080/00071668.2018.1496401
24. Sasso, S, Pohnert, G, Lohr, M, Mittag, M, and Hertweck, C. Microalgae in the postgenomic era: a blooming reservoir for new natural products. FEMS Microbiol Rev. (2012) 36:761–85. doi: 10.1111/j.1574-6976.2011.00304.x
25. El-Bahr, S, Shousha, S, Shehab, A, Khattab, W, Ahmed-Farid, O, Sabike, I, et al. Effect of dietary microalgae on growth Performance, profiles of amino and fatty acids, antioxidant status, and meat quality of broiler chickens. Animals (Basel). (2020) 10:761. doi: 10.3390/ani10050761
26. Martins, CF, Ribeiro, DM, Costa, M, Coelho, D, Alfaia, CM, Lordelo, M, et al. Using microalgae as a sustainable feed resource to enhance quality and nutritional value of pork and poultry meat. Food Secur. (2021) 10:2933. doi: 10.3390/foods10122933
27. Martins, CF, Pestana, JM, Alfaia, CM, Costa, M, Ribeiro, DM, Coelho, D, et al. Effects of Chlorella Vulgaris as a feed ingredient on the quality and nutritional value of weaned Piglets' meat. Food Secur. (2021) 10:1155. doi: 10.3390/foods10061155
28. Ladirat, S In: S Muirhead, editor Controlling gut health safely with new double-Encapsulateion technology : Informa markets: Farm Progress (2022). 3.Feedstuffs 365
29. Ladirat, S. Solid & Science-Based Technology to optimize feed costs In: Feed Additive, International Magazine for Animal Feed $ Additive Industry (2023)
30. Lodhi, GN, Singh, D, and Ichhponani, JS. Variation in nutrient content of Feedingstuffs rich in protein and reassessment of the chemical method for Metabolizable energy estimation for poultry. J Agric Sci. (1976) 86:293–303. doi: 10.1017/S0021859600054757
31. Cobb-Vantress. Cobb500 broiler Performance and nutrition supplement Siloam. Cobb Vantress (2022) Available at: https://cobbgenetics.com/. (Accessed July 29, 2023).
32. Martin, MP, Wineland, M, and Barnes, HJ. Selected blood chemistry and gas reference ranges for broiler breeders using the I-Stat handheld clinical analyzer. Avian Dis. (2010) 54:1016–20. doi: 10.1637/9223-122209-Reg.1
33. Schaal, TP, Arango, J, Wolc, A, Brady, JV, Fulton, JE, Rubinoff, I, et al. Commercial Hy-line W-36 pullet and laying hen venous blood gas and chemistry profiles utilizing the portable I-Stat(R)1 analyzer. Poult Sci. (2016) 95:466–71. doi: 10.3382/ps/pev350
34. Steinmetz, HW, Vogt, R, Kastner, S, Riond, B, and Hatt, JM. Evaluation of the I-Stat portable clinical analyzer in chickens (Gallus Gallus). J Vet Diagn Invest. (2007) 19:382–8. doi: 10.1177/104063870701900407
35. Ruiz-Jimenez, F, Gruber, E, Correa, M, and Crespo, R. Comparison of portable and conventional laboratory analyzers for biochemical tests in chickens. Poult Sci. (2021) 100:746–54. Epub 2021/02/02. doi: 10.1016/j.psj.2020.11.060
36. Wideman, RF Jr, Hamal, KR, Stark, JM, Blankenship, J, Lester, H, Mitchell, KN, et al. A wire-flooring model for inducing lameness in broilers: evaluation of probiotics as a prophylactic treatment. Poult Sci. (2012) 91:870–83. doi: 10.3382/ps.2011-01907
37. Ramser, A, Greene, E, Wideman, R, and Dridi, S. Local and systemic cytokine, chemokine, and Fgf profile in bacterial Chondronecrosis with osteomyelitis (Bco)-affected broilers. Cells. (2021) 10:3174. doi: 10.3390/cells10113174
38. Greene, E, Flees, J, Dadgar, S, Mallmann, B, Orlowski, S, Dhamad, A, et al. Quantum blue reduces the severity of Woody breast myopathy via modulation of oxygen homeostasis-related genes in broiler chickens. Front Physiol. (2019) 10:1251. doi: 10.3389/fphys.2019.01251
39. Cauble, RN, Greene, ES, Orlowski, S, Walk, C, Bedford, M, Apple, J, et al. Research note: dietary Phytase reduces broiler Woody breast severity via potential modulation of breast muscle fatty acid profiles. Poult Sci. (2020) 99:4009–15. doi: 10.1016/j.psj.2020.05.005
40. Meilgaard, M, G. V. Civile, and B. T. Carr. Sensory evaluation techniques. 4 Florida: CRC press (2007). 464.
41. Orlowski, S, Flees, J, Greene, ES, Ashley, D, Lee, SO, Yang, FL, et al. Effects of phytogenic additives on meat quality traits in broiler Chickens1. J Anim Sci. (2018) 96:3757–67. doi: 10.1093/jas/sky238
42. Greene, ES, Emami, NK, and Dridi, S. Research note: Phytobiotics modulate the expression profile of circulating Inflammasome and Cyto(chemo)Kine in whole blood of broilers exposed to cyclic heat stress. Poult Sci. (2021) 100:100801. doi: 10.1016/j.psj.2020.10.055
43. Baxter, MFA, Latorre, JD, Dridi, S, Merino-Guzman, R, Hernandez-Velasco, X, Hargis, BM, et al. Identification of serum biomarkers for intestinal integrity in a broiler chicken malabsorption model. Front Vet Sci. (2019) 6:144. Epub 2019/05/31. doi: 10.3389/fvets.2019.00144
44. Stevanovic, ZD, Bosnjak-Neumuller, J, Pajic-Lijakovic, I, Raj, J, and Vasiljevic, M. Essential oils as feed additives-future perspectives. Molecules. (2018) 23:1717. doi: 10.3390/molecules23071717
45. Moharreri, M, Vakili, R, Oskoueian, E, and Rajabzadeh, G. Phytobiotic role of essential oil-loaded microcapsules in improving the health parameters in Clostridium Perfringens-infected broiler chickens. Ital J Anim Sci. (2021) 20:2075–85. doi: 10.1080/1828051X.2021.1993093
46. Liu, S, Song, M, Yun, W, Lee, J, Lee, C, Kwak, W, et al. Effects of Oral Administration of Different Dosages of Carvacrol essential oils on intestinal barrier function in broilers. J Anim Physiol Anim Nutr (Berl). (2018) 102:1257–65. doi: 10.1111/jpn.12944
47. Du, E, Wang, W, Gan, L, Li, Z, Guo, S, and Guo, Y. Effects of thymol and Carvacrol supplementation on intestinal integrity and immune responses of broiler chickens challenged with Clostridium Perfringens. J Anim Sci Biotechnol. (2016) 7:19. doi: 10.1186/s40104-016-0079-7
48. Pirgozliev, V, Mansbridge, SC, Rose, SP, Lillehoj, HS, and Bravo, D. Immune modulation, growth Performance, and nutrient retention in broiler chickens fed a blend of phytogenic feed additives. Poult Sci. (2019) 98:3443–9. doi: 10.3382/ps/pey472
49. Ibrahim, D, Eldemery, F, Metwally, AS, Abd-Allah, EM, Mohamed, DT, Ismail, TA, et al. Dietary eugenol Nanoemulsion potentiated Performance of broiler chickens: orchestration of digestive enzymes, intestinal barrier functions and cytokines related gene expression with a consequence of attenuating the severity of E. coli O78 infection. Front Vet Sci. (2022) 9:847580. doi: 10.3389/fvets.2022.847580
50. Yang, C, Diarra, MS, Choi, J, Rodas-Gonzalez, A, Lepp, D, Liu, S, et al. Effects of encapsulated Cinnamaldehyde on growth Performance, intestinal digestive and absorptive functions, meat quality and gut microbiota in broiler chickens. Transl Anim Sci. (2021) 5:txab099. doi: 10.1093/tas/txab099
51. Noruzi, S, Torki, M, and Mohammadi, H. Effects of supplementing diet with thyme (Thymuas vulgaris L.) essential oil and/or selenium yeast on production Performance and blood variables of broiler chickens. Vet Med Sci. (2022) 8:1137–45. doi: 10.1002/vms3.736
52. Gholami-Ahangaran, M, Ahmadi-Dastgerdi, A, Azizi, S, Basiratpour, A, Zokaei, M, and Derakhshan, M. Thymol and Carvacrol supplementation in poultry health and Performance. Vet Med Sci. (2022) 8:267–88. doi: 10.1002/vms3.663
53. Bravo, D, Pirgozliev, V, and Rose, SP. Meta-analysis of the effect of a mixture of Carvacrol, Cinnamaldehyde and Capsicum oleoresin in broilers. Poult Sci. (2008) 87:75. doi: 10.3920/9789086865789_300
54. Najafi, PT, and Performance, M. Blood metabolites and Immunocompetaence of broiler chicks fed diets included Essentioal oils of medicinal herbs. J Anim Vet Adv. (2010) 9:1164–8. doi: 10.3923/javaa.2010.1164.1168
55. Irawan, A, Hidayat, C, Jayanegara, A, and Ratriyanto, A. Essential oils as growth-promoting additives on Performance, nutrient digestibility, Cecal microbes, and serum metabolites of broiler chickens: a Meta-analysis. Anim Biosci. (2021) 34:1499–513. doi: 10.5713/ab.20.0668
56. Sefcova, MA, Santacruz, F, Larrea-Alvarez, CM, Vinueza-Burgos, C, Ortega-Paredes, D, Molina-Cuasapaz, G, et al. Administration of Dietary Microalgae Ameliorates Intestinal Parameters, improves body weight, and reduces thawing loss of fillets in broiler chickens: a pilot study. Animals (Basel). (2021) 11:3601. doi: 10.3390/ani11123601
57. El-Shall, NA, Jiang, S, Farag, MR, Azzam, M, Al-Abdullatif, AA, Alhotan, R, et al. Potential of Spirulina platensis as a feed supplement for poultry to enhance growth Performance and immune modulation. Front Immunol. (2023) 14:1072787. doi: 10.3389/fimmu.2023.1072787
58. Evans, AM, Smith, DL, and Moritz, JS. Effects of algae incorporation into broiler starter diet formulations on nutrient digestibility and 3 to 21 D bird Performance. J Appl Poult Res. (2015) 24:206–14. doi: 10.3382/japr/pfv027
59. Flees, JJ, Ganguly, B, and Dridi, S. Phytogenic feed additives improve broiler feed efficiency via modulation of intermediary lipid and protein metabolism-related signaling pathways. Poult Sci. (2021) 100:100963. doi: 10.1016/j.psj.2020.12.060
60. Flees, J, Greene, E, Ganguly, B, and Dridi, S. Phytogenic feed- and water-additives improve feed efficiency in broilers via modulation of (an)Orexigenic hypothalamic neuropeptide expression. Neuropeptides. (2020) 81:102005. doi: 10.1016/j.npep.2020.102005
61. Flees, JJ, Emami, NK, Greene, E, Ganguly, B, and Dridi, S. Phytogenic water additives improve broiler growth Performance via modulation of intermediary metabolism-related signaling pathways. Animals (Basel). (2021) 11:750. doi: 10.3390/ani11030750
62. Greene, ES, Cauble, R, Kadhim, H, de Almeida, MB, Gu, I, Lee, SO, et al. Protective effects of the phytogenic feed additive "comfort" on growth Performance via modulation of hypothalamic feeding- and drinking-related neuropeptides in cyclic heat-stressed broilers. Domest Anim Endocrinol. (2021) 74:106487. doi: 10.1016/j.domaniend.2020.106487
63. Cross, DE, McDevitt, RM, Hillman, K, and Acamovic, T. The effect of herbs and their associated essential oils on Performance, dietary digestibility and gut microflora in chickens from 7 to 28 days of age. Br Poult Sci. (2007) 48:496–506. doi: 10.1080/00071660701463221
64. Giannenas, I, Papaneophytou, CP, Tsalie, E, Pappas, I, Triantafillou, E, Tontis, D, et al. Dietary supplementation of benzoic acid and essential oil compounds affects buffering capacity of the feeds, Performance of Turkey Poults and their antioxidant status, Ph in the digestive tract, intestinal microbiota and morphology. Asian Australas J Anim Sci. (2014) 27:225–36. doi: 10.5713/ajas.2013.13376
65. Khalaji, S, Zaghari, M, Hatami, K, Hedari-Dastjerdi, S, Lotfi, L, and Nazarian, H. Black cumin seeds, Artemisia leaves (Artemisia Sieberi), and Camellia L. plant extract as phytogenic products in broiler diets and their effects on Performance, blood constituents, immunity, and Cecal microbial population. Poult Sci. (2011) 90:2500–10. Epub 2011/10/20. doi: 10.3382/ps.2011-01393
66. Leusink, G, Rempel, H, Skura, B, Berkyto, M, White, W, Yang, Y, et al. Growth Performance, meat quality, and gut microflora of broiler chickens fed with cranberry extract. Poult Sci. (2010) 89:1514–23. doi: 10.3382/ps.2009-00364
67. Wideman, N, O’Bryan, C, and Crandall, P. Factors affecting poultry meat colour and consumer preferences - a review. Worlds Poult Sci J. (2016) 72:353–66. doi: 10.1017/S0043933916000015
68. Lorenzi, MMS, Cavani, C, and Petracci, M. Incidence of White striping under commercial conditions in medium and heavy broiler chickens in Italy. J Appl Poult Res. (2014) 23:754–8. doi: 10.3382/japr.2014-00968
69. Maynard, CJ, Jackson, AR, Caldas-Cueva, JP, Mauromoustakos, A, Kidd, MT, Rochell, SJ, et al. Meat quality attributes of male and female broilers from 4 commercial strains processed for 2 market programs. Poult Sci. (2023) 102:102570. doi: 10.1016/j.psj.2023.102570
70. Allen, CD, Russell, SM, and Fletcher, DL. The relationship of broiler breast meat color and Ph to shelf-life and odor development. Poult Sci. (1997) 76:1042–6. doi: 10.1093/ps/76.7.1042
71. Barbut, S. Problem of pale soft exudative meat in broiler chickens. Br Poult Sci. (1997) 38:355–8. doi: 10.1080/00071669708418002
72. Fletcher, DL, Qiao, M, and Smith, DP. The relationship of raw broiler breast meat color and Ph to cooked meat color and Ph. Poult Sci. (2000) 79:784–8. doi: 10.1093/ps/79.5.784
73. Qiao, M, Fletcher, DL, Smith, DP, and Northcutt, JK. The effect of broiler breast meat color on Ph, moisture, water-holding capacity, and emulsification capacity. Poult Sci. (2001) 80:676–80. doi: 10.1093/ps/80.5.676
74. Suman, SP, and Joseph, P. Myoglobin chemistry and meat color. Annu Rev Food Sci Technol. (2013) 4:79–99. doi: 10.1146/annurev-food-030212-182623
75. Reis, JH, Gebert, RR, Barreta, M, Baldissera, MD, Dos Santos, ID, Wagner, R, et al. Effects of phytogenic feed additive based on thymol, Carvacrol and Cinnamic aldehyde on body weight, blood parameters and environmental Bacteria in broilers chickens. Microb Pathog. (2018) 125:168–76. doi: 10.1016/j.micpath.2018.09.015
76. Galli, GM, Gerbet, RR, Griss, LG, Fortuoso, BF, Petrolli, TG, Boiago, MM, et al. Combination of herbal components (curcumin, Carvacrol, thymol, Cinnamaldehyde) in broiler chicken feed: impacts on response parameters, Performance, fatty acid profiles, meat quality and control of Coccidia and Bacteria. Microb Pathog. (2020) 139:103916. doi: 10.1016/j.micpath.2019.103916
Keywords: feed additive, NUQO-NEX, body weight, feed intake, meat color
Citation: Mullenix GJ, Greene ES, Ramser A, Maynard C and Dridi S (2024) Effect of a microencapsulated phyto/phycogenic blend supplementation on growth performance, processing parameters, meat quality, and sensory profile in male broilers. Front. Vet. Sci. 11:1382535. doi: 10.3389/fvets.2024.1382535
Edited by:
Jingjing Du, Max Planck Institute for Heart and Lund Research, GermanyReviewed by:
Sabreen Ezzat Fadl, Matrouh University, EgyptElwy Ashour, Poultry Research Institute, Türkiye
Copyright © 2024 Mullenix, Greene, Ramser, Maynard and Dridi. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Sami Dridi, dridi@uark.edu
†Present addresses: Garrett J. Mullenix, Cargill Inc., Minneapolis, MN, United StatesClay Maynard, Tyson Foods, Springdale, AR, United States
 Garrett J. Mullenix†
Garrett J. Mullenix†  Elizabeth S. Greene
Elizabeth S. Greene Alison Ramser
Alison Ramser Sami Dridi
Sami Dridi