Hepatitis B virus infection in patients presenting for immunosuppressive cancer therapy with and without underlying HIV infection
- 1HIV and Hepatitis Research Unit, Department of Virology, Sefako Makgatho Health Sciences University and National Health Laboratory Service, Pretoria, South Africa
- 2Cochrane South Africa, South African Medical Research Council, Cape Town, South Africa
- 3Department of Obstetrics and Gynaecology, Dr George Mukhari Academic Hospital, Sefako Makgatho Health Sciences University and National Health Laboratory Service, Pretoria, South Africa
- 4Department of Dermatology, Dr George Mukhari Academic Hospital, Sefako Makgatho Health Sciences University and National Health Laboratory Service, Pretoria, South Africa
- 5Department of Microbiological Pathology, Dr George Mukhari Academic Hospital, Sefako Makgatho Health Sciences University and National Health Laboratory Service, Pretoria, South Africa
Introduction: Reactivation of hepatitis B virus (HBV) infection induced by immunosuppressive cancer therapy is associated with fulminant liver disease and death. While national guidelines recommend HBV screening and antiviral prophylaxis for patients with cancer prior to initiating immunosuppressive therapy, compliance with these measures is unclear. This study characterized the burden of HBV infection among patients diagnosed with gynecological or dermatological cancers, with or without underlying HIV infection, before initiating immunosuppressive therapy.
Methods: Between 2016 – 2018, we recruited study patients from the Dr George Mukhari Academic Hospital in Tshwane, South Africa. Demographic (age, sex) and clinical data (HIV test results, HIV antiviral regimen, type of cancer) were recorded using a standardized data collection form. All participants were tested for HBV surface antigen (HBsAg), and antibodies to the surface (anti-HBs) and core antigens (anti-HBc). For detection of HBV DNA, a nested polymerase chain reaction was used to amplify polymerase gene fragments which were Sanger-sequenced and analyzed using bioinformatics software. All statistical analyses were performed using R version 4.1.0 (2021-05-18) and R studio version 2022.07.2.
Results: Study participants were predominantly female (96.3%, 103/107) with a median (IQR) age of 50 (17.5) years. Cervical cancer was the most frequent cancer diagnosis (72%). Over half (52.3%; 56/107) of the participants were HIV positive and all but four (92.9%) on highly active antiretroviral therapy at the time of enrollment. The prevalence of chronic hepatitis B in the study population was 11.2% [95% CI:6.2-19.1], increasing to 14.3% [95% CI:6.8-26.8] in the HIV positive sub-population. The overall prevalence of occult HBV infection was 20% [95% CI:12.8-29.7], 57.9% [95% CI:33.97-78.9] of whom tested negative for all serological markers. Phylogenetic inference showed that all polymerase gene sequences generated in this study were sub-genotype A2. Mutational analysis did not reveal any drug resistance-associated amino acid variations in this study.
Conclusion: These findings suggest that chronic and occult HBV infections are more prevalent among cancer patients with or without underlying HIV infection compared to what has previously been reported for the general South African population. This underscores the need to scale-up universal HBV serological and molecular screening with timely institution of prophylaxis prior to initiating immunosuppressive cancer therapy.
1 Introduction
Despite the availability of safe and effective vaccines and antiviral therapies, viral hepatitis continues to take a devastating toll on societies, economies, and health systems worldwide. In recognition of this, the World Health Organization in 2016 published its Global Health Sector Strategy on Viral Hepatitis, aiming to provide member states with pragmatic approaches towards eliminating this disease as a global public health threat by 2030 (1). Persistent hepatitis B virus (HBV) infection leads to chronic hepatitis B or occult HBV infection (2, 3). With an estimated 4.1% (316 million) of the world’s population currently living with chronic hepatitis B and 555000 individuals succumbing to HBV-related sequelae in 2019 alone, prevention and control of HBV infection remains a priority within the global health agenda (2). The situation is even more dire in Africa where the prevalence of chronic hepatitis B is estimated at 6.5%, with an annual death count of 71000 individuals (2). The burden of the disease varies across the Western (9.0%), Central (6.4%), Eastern (4.8%), and Southern (4.5%) regions of sub-Saharan Africa. In South Africa, the prevalence of chronic hepatitis B across all age groups is 3.5% (2), driven by a disproportionate residual burden among high risk adult populations like pregnant women (0.4% – 4.5%) (4–6), healthcare workers (1.3% – 5.1%) (7), people who inject drugs (5%) (8), people living with Human Immunodeficiency Virus or HIV (6.4% – 8.5%) (9–12), and persons diagnosed with cancer (14%) (13).
While chronic infection with HBV is associated with a significant lifetime risk of developing hepatocellular carcinoma, HBV infection has also been shown to be prevalent among patients with non-hepatic cancers like lymphoma, breast cancer, skin cancer, and gynecological cancers including cervical, uterine, and ovarian cancers (14–17). Cancer patients undergoing lifesaving cytotoxic or immunosuppressive therapy while living with uncontrolled chronic hepatitis B or occult HBV infection, are at high risk of HBV reactivation (16, 18). Clinical reactivation of HBV infection during prolonged immunosuppression is associated with a worsening prognosis characterized by rapid progression to hepatic sequelae like liver cirrhosis, fulminant liver failure and even death. Fortunately, immunosuppressive cancer therapy induced-HBV reactivation is preventable with timely detection of HBV serological and molecular markers, followed by appropriate institution of vaccination or prophylactic and pre-emptive antiviral treatment with nucleot(s)ide analogues, alongside careful monitoring during cancer therapy and follow-up (19, 20). It is for this reason that the South African national guidelines recommend testing for the HBV surface antigen (HBsAg) and antibodies to the core antigen (anti-HBc) to determine appropriate clinical management prior to initiating immunosuppressive cancer therapy. Measurement of HBV DNA however is only indicated for patients who test positive for HBsAg or anti-HBc, which may exclude patients with seronegative occult HBV infection (negative for all serological markers but positive for HBV DNA) who could still be at risk of reactivation during immunosuppressive therapy (21). It will be important to understand national trends in compliance with these guidelines at the health facility level. Studies conducted elsewhere report inconsistent compliance with screening and administration of prophylaxis prior to patients initiating immunosuppressive cancer therapy in both HBV endemic and low-endemic settings, often citing the need for increasing awareness among clinicians (22–25). Scaling-up implementation and enforcing compliance with national guidelines towards favorable outcomes during immunosuppressive cancer therapy will have to be informed by local evidence of the risk and burden of HBV infection among patients with cancer. Given the co-epidemic of HIV infection in South Africa which has been shown to negatively modify the natural course of chronic hepatitis B and cancer prognosis, it is also important to improve our limited understanding of the dynamics of HBV infection among cancer patients with and without underlying HIV infection (26, 27). In this study, we characterize the burden of HBV infection among patients diagnosed with gynecological or dermatological cancers with or without underlying HIV infection, who are due for immunosuppressive therapy.
2 Methods
2.1 Study site and participants
This was a cross-sectional descriptive study involving patients diagnosed with cancer and due for chemotherapy and/or radiotherapy at the Dr George Mukhari Academic Hospital (DGMAH). The DGMAH is a public academic hospital situated within the North-Western part of the Tshwane region in the Gauteng province of South Africa. The DGMAH is affiliated with the Sefako Makgatho Health Sciences University where the National Health Laboratory Service (NHLS) Department of Virology is based and accredited to provide routine serological and molecular diagnostic services to various provinces across the country.
A target sample size of 132 participants was estimated using Epi Info™ version 7.1.5 (Centers for Disease Control and Prevention, Atlanta, United States of America), calculated at 80% power and 95% confidence, with a 70% HBV exposure rate (28). Participant recruitment and enrolment took place between June 2016 and April 2018, in the gynecology and dermatology clinics at the DGMAH. Patients diagnosed with cancer and presenting to these clinics to initiate immunosuppressive therapy were informed of the purpose of this study during their scheduled visits. Only those patients who provided written informed consent participated in this study. Participants’ demographic information including age, sex, and province of residence were collected using a standardized data collection form (template in Supplementary File) which was completed by a member of the research team. All participants due for immunosuppressive therapy are routinely tested for HIV infection through the NHLS using the ARCHITECT HIV Ag/Ab Combo assay (ABBOTT Laboratories, Germany, 2010). Clinical information including HIV test results, HIV regimen at the time of the study, type of cancer diagnosis, and planned cancer treatment were also recorded in the data collection form. For HBV serological and molecular assessments, 5ml of blood was collected from each participant prior to initiating immunosuppressive therapy. Serum and plasma fractions were separated by centrifugation and stored at -80˚C until required for testing. Ethics approval to conduct this study was obtained from the Sefako Makgatho University Research Ethics Committee (SMUREC/M/04/2017).
2.2 Detection of HBV serological and molecular markers
Using the Elescys® 2010 Immunoassay Analyzer (Roche, Hitachi, Japan) and Elecsys® test kits (Roche Diagnostics, Penzberg, Germany), the following serological markers were tested for in all serum samples; HBsAg, antibodies to HBsAg (anti-HBs), and total IgG and IgM anti-HBc. For detection of HBV DNA, a nested in-house polymerase chain reaction (PCR) with a lower detection limit of 103 copies/ml was performed (29). Briefly, total nucleic acid was extracted from 200 µl of each plasma sample using the High Pure Viral Nucleic Acid Kit (Roche Diagnostics, Mannheim, Germany). A total of 5 µl of each template (nucleic acid extract or first round PCR product) was then added to a 25 µl PCR reaction mix comprising 2X MyTaq™ Red Mix (Bioline USA Inc. A Meridian Life Science® Company), and 10 pmol/mL of each primer (primers P1 and P2 for the first, and P3 and P4 for the second round PCR) targeting a 645 bp fragment of the HBV polymerase (pol) gene (30, 31), with the final reaction volume made up with PCR grade water (Fermentas, Burlington, Canada). To minimize the risk of cross-contamination, physically separated laboratory rooms were used for nucleic acid extraction, preparation of the PCR reaction mix, and loading of the template. The potential for false positive detection of HBV DNA was excluded by running negative (PCR grade water) and positive (commercially available human serum, ACCURUN™ 325 Series 200 HBV DNA positive control with an HBV DNA concentration of 106 copies/mL from SeraCare™ Life Sciences, Inc. USA) controls concurrently with study samples during each PCR assay. The PCR products were visualized by agarose gel electrophoresis and direct Sanger sequencing carried out with the ABI3500XL Genetic Analyzer (Life Technologies, Carlsbad, CA, USA) using second round primers, P3 and P4.
2.3 Data analysis
Participant demographic, clinical and laboratory data were collated in a database designed in Microsoft Excel. Descriptive analysis was performed with categorical variables reported as frequencies or percentages while continuous variables were presented as median (interquartile range, IQR). In addition, the prevalence of HBV serological and molecular markers within the study population, and stratified across HIV status, were reported as proportions with 95% confidence interval (95% CI). Associations between participant demographic or clinical characteristics and the prevalence of HBV serological and molecular markers could not be determined given the modest sample population studied. Statistical significance level for Chi-square tests was set at a two-tailed p-value <0.05. All statistical analyses were performed using R version 4.1.0 (2021-05-18) and R studio version 2022.07.2 (32).
Phylogenetic analysis of the HBV pol gene sequences was inferred using the Neighbour-Joining method and the Maximum Composite Likelihood substitution model in MEGA version 11 software (33). For investigation of potential inter-genotype and inter-subgenotype recombination, sequences were analyzed in Simplot version 1.3 (34). Prediction of phenotypic drug resistance within the reverse transcriptase domain of the HBV pol gene and escape mutations in the overlapping HBsAg domain were performed by submitting the sequences to the online algorithm, Geno2Pheno [HBV] v2.0 (35). All pol gene sequences generated in this study have been deposited in GenBank under the accession numbers MW322669 – MW322680.
3 Results
3.1 Demographic and clinical characteristics of study participants
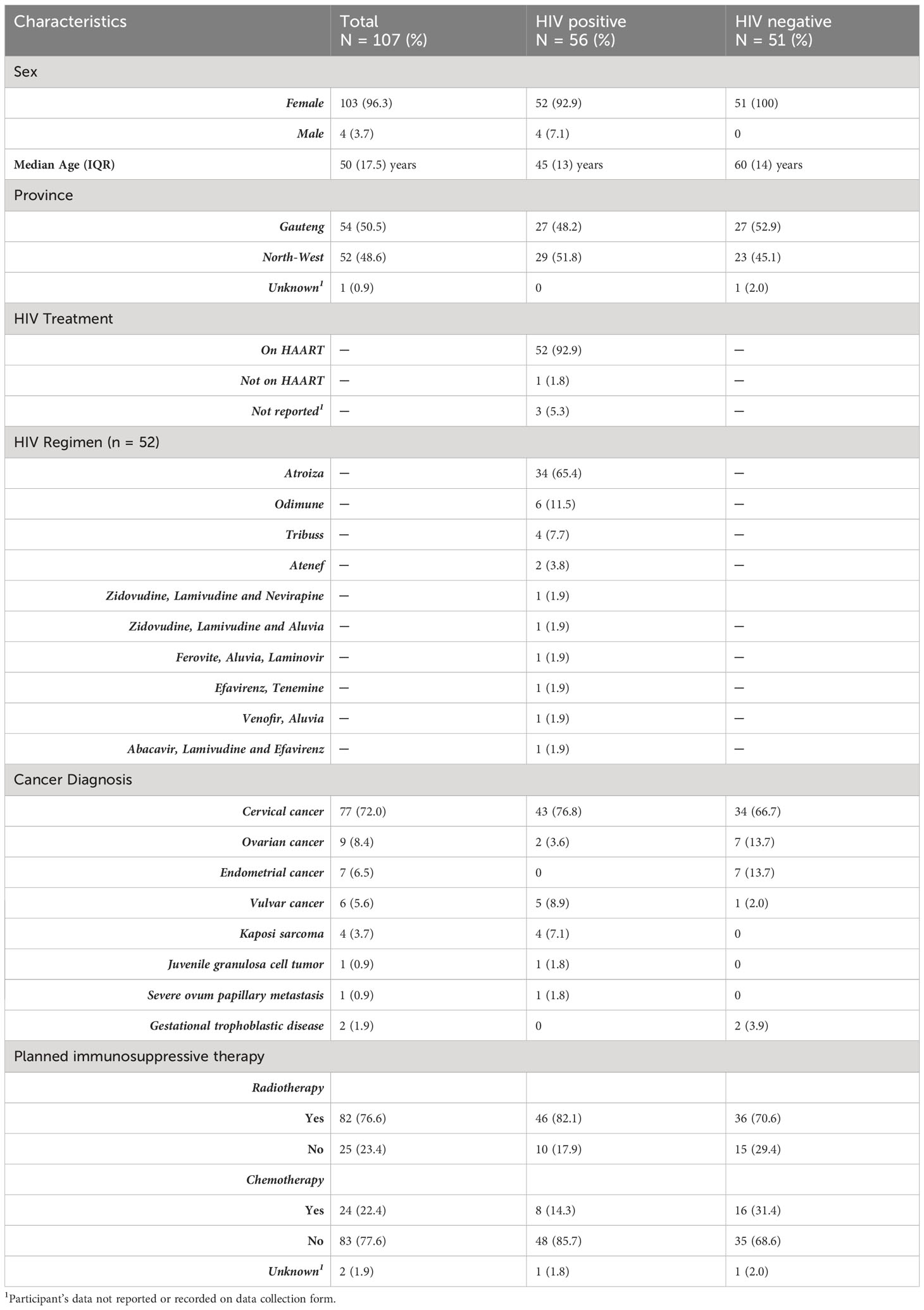
Between June 2016 and April 2018, a total of 107 of the targeted 132 participants were enrolled into the study. The demographic and clinical characteristics of all 107 participants are presented in Table 1. There was an overrepresentation of female participants (96.3%, 103/107), all of whom were recruited from the gynecology clinic. The remainder of the four male participants (3.7%) were recruited from the dermatology clinic. The median (IQR) age of the study population was 50 (17.5) years. Study participants mainly originated from the Gauteng (50.5%, 54/107) and North-West (48.6%, 52/107) provinces. In terms of participants’ clinical history, 52.3% (56/107) were HIV positive while 47.7% (51/107) were HIV negative. All but four (92.9%) of those living with HIV were on highly active antiretroviral therapy (HAART) at the time of the study, with Atroiza being the most frequently used regimen (65.4%, 34/52). Except for two cases, the HAART regimens administered in this study invariably included at least one drug component with co-activity against HBV, such as Lamivudine, Emtricitabine, and Tenofovir Disoproxil Fumarate. The two aforementioned participants were on Ferovite or Venofir in addition to their HAART regimen at the time of the study (Table 1).
Cervical cancer was the most common cancer diagnosis (72%, 77/107) within the study population. Overall, 76.6% (82/107) and 22.4% (24/107) of the study participants were due for radiotherapy and/or chemotherapy, respectively.
3.2 Burden of chronic and occult HBV infection within the study population
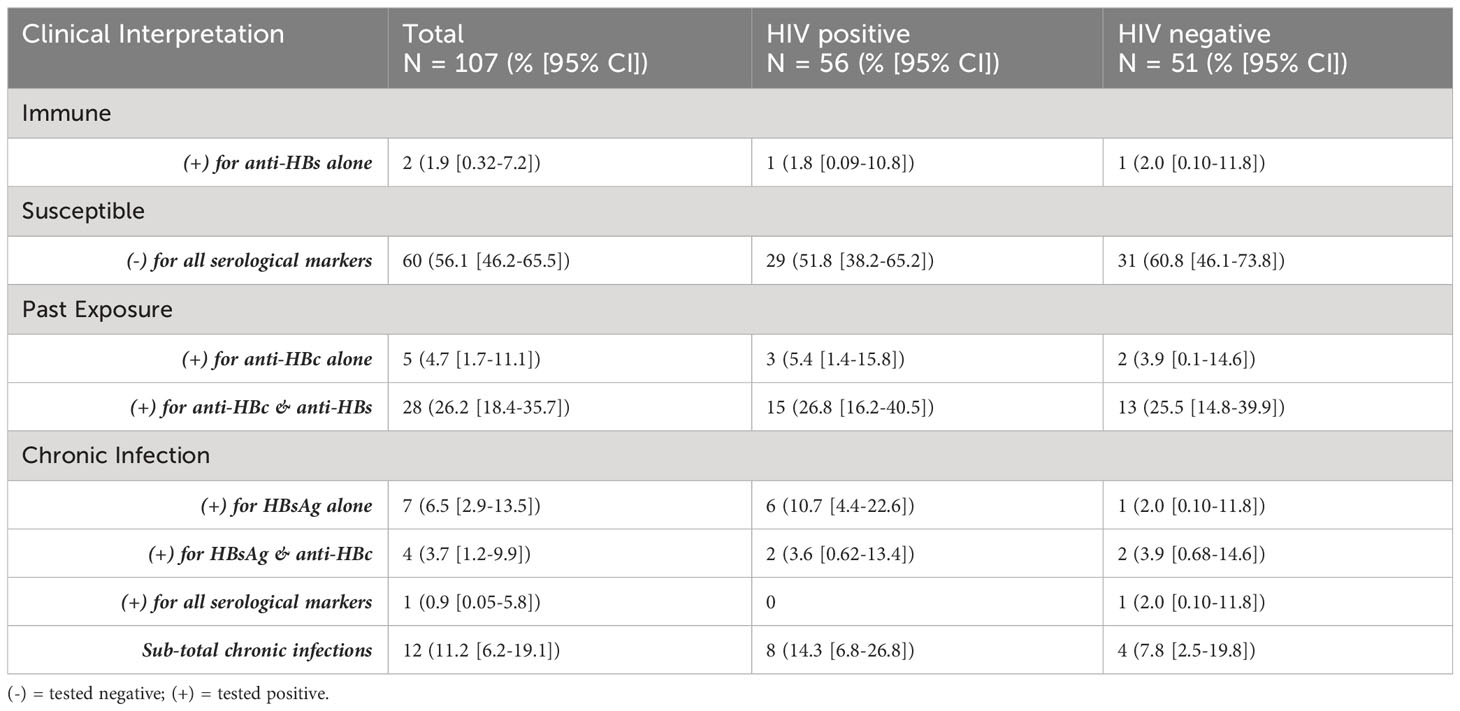
As shown in Table 2, more than half of the study population (56.1% [95% CI:46.2-65.5], 60/107) tested negative for all three serological markers and were susceptible to HBV infection. Only 1.9% ([95% CI:0.32-7.2], 2/107) tested positive for the marker of immunity, anti-HBs, although one of these participants also tested positive for HBV DNA which is indicative of potential occult HBV infection. Notably, there was a high burden of chronic HBV infection (11.2% [95% CI:6.2-19.1], 12/107) within the study population, with the HBsAg prevalence found in the HIV positive sub-population (14.3% [95% CI:6.8-26.8], 8/56) being twice as high as that in the HIV negative group (7.8% [95% CI:2.5-19.8], 4/51). However, this was not a statistically significant difference (p = 0.4544). All HIV positive participants found to be living with chronic hepatitis B were on HAART regimens (Atroiza [n=7] or Odimune [n=1]) containing at least one HBV active drug component.
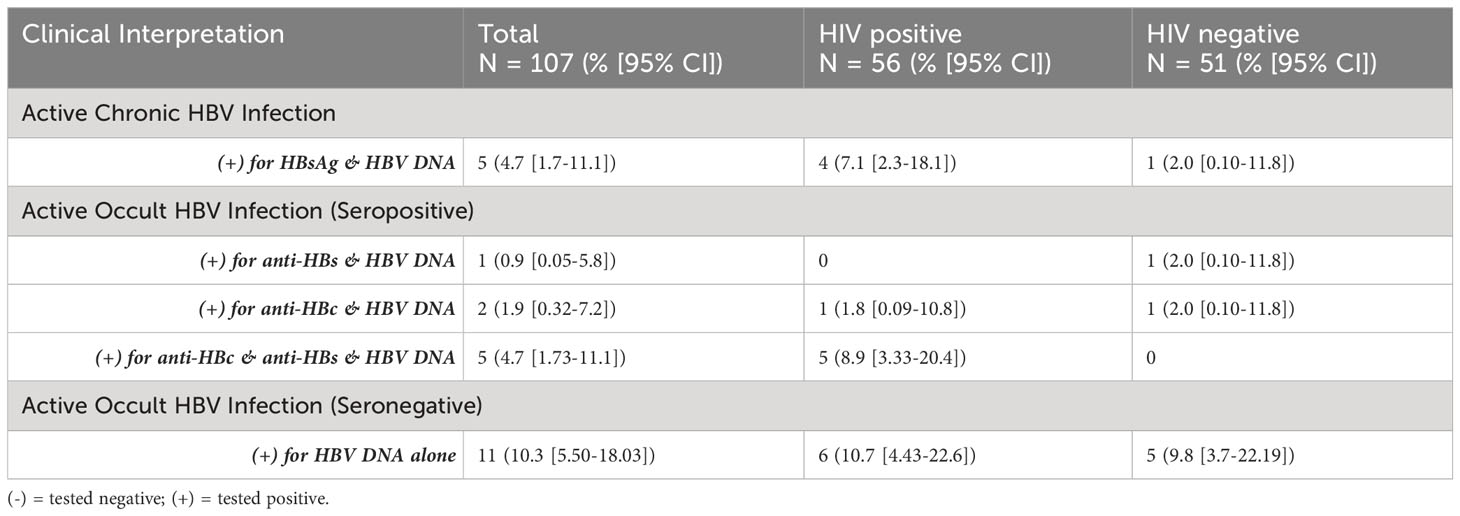
Among those testing negative for HBsAg (n = 95), the overall prevalence of occult HBV infection based on the detection of HBV DNA was 20% ([95% CI:12.8-29.7],19/95), with a higher burden in the HIV positive (25% [95% CI:14.1-39.9],12/48) compared to the HIV negative (14.9% [95% CI: 6.69-28.9], 7/47) sub-populations (p = 0.3297). Furthermore, 57.9% ([95% CI:33.97-78.9], 11/19) of participants with occult HBV infection were also seronegative for the other diagnostic markers, namely, anti-HBs and anti-HBc. Table 3 further presents the prevalence of active chronic and occult (seropositive vs seronegative) HBV infection within the study population.
Among those with seropositive occult HBV infection (n=8), five tested positive for both anti-HBs and anti-HBc, and were all HIV positive (Table 3). The difference in seronegative occult HBV infection between HIV positive (10.7% [4.43-22.6]) and HIV negative (9.8% [3.7-22.19]) sub-populations was not significant.
Figure 1 shows the prevalence of hepatitis B immunity and exposure as well as chronic and occult HBV infection stratified across the different types of cancers diagnosed within the study population. Overall, chronic HBV infection was more prevalent among those diagnosed with vulvar (33.3% [95% CI:6.00-75.90], 2/6) and endometrial (28.6% [95% CI:5.11-69.7], 2/7) cancers. In comparison, the prevalence of chronic HBV infection among those diagnosed with ovarian and cervical cancers was found to be 11.1% ([95% CI:0.58-49.3], 1/9) and 9.1% ([95% CI:4.04-18.4], 7/77), respectively. Each of the participants diagnosed with juvenile granulosa cell tumor and severe ovum papillary metastasis were found to have underlying occult HBV infection, as were 25% ([95% CI:1.3-78.1], 1/4), 19.5% ([95% CI:11.7-30.4], 15/77), and 11.1% ([95% CI:0.6-49.3], 1/9) of those diagnosed with Kaposi sarcoma, cervical cancer, and ovarian cancer, respectively. None of the two participants diagnosed with gestational trophoblastic disease had any indication of ever being exposed to hepatitis B, testing negative for all serological and molecular markers (Figure 1).
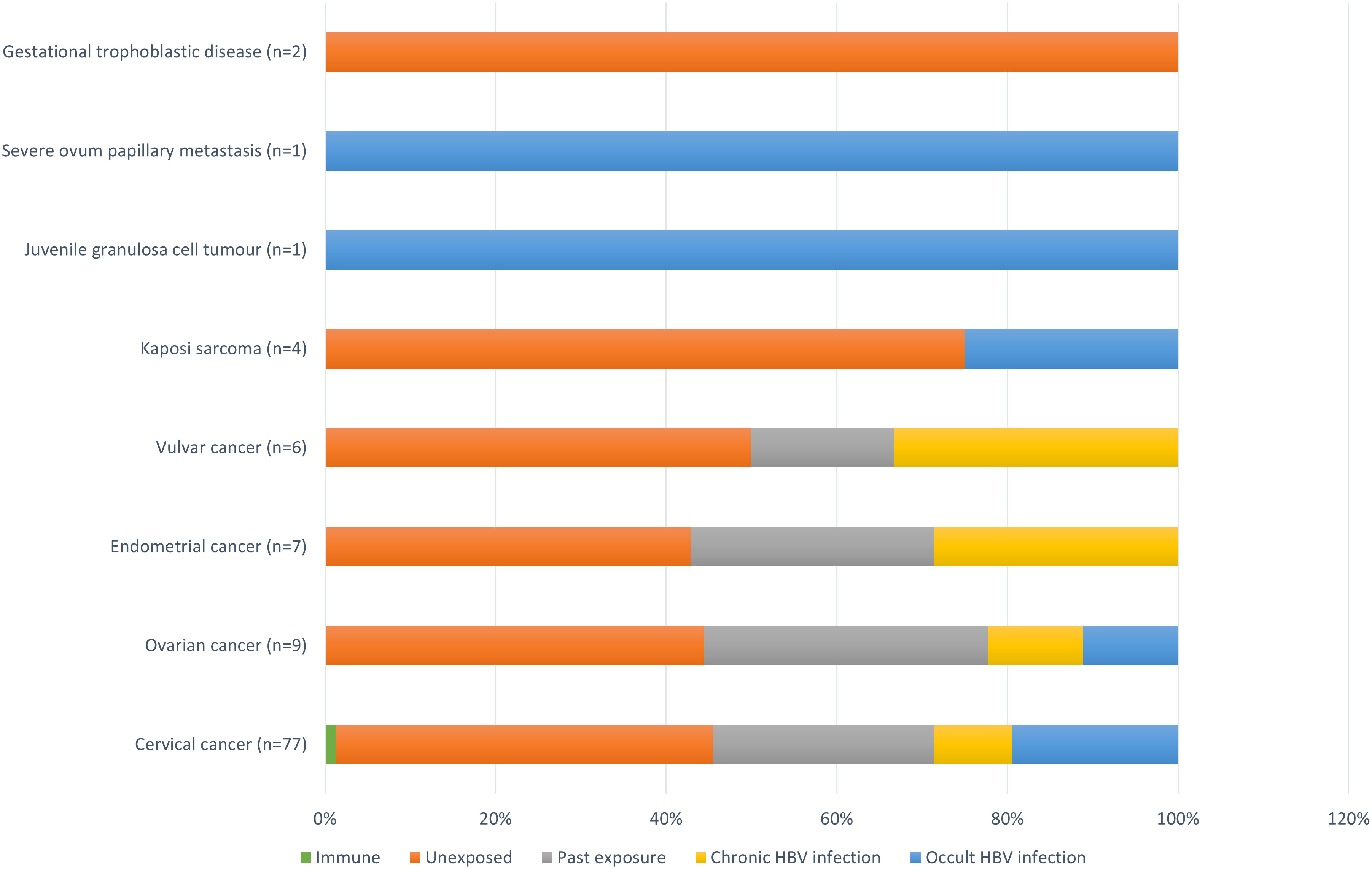
Figure 1 Prevalence of hepatitis B immunity and exposure, and chronic and occult HBV infection stratified across the different types of cancers diagnosed within the study population. Immune = anti-HBs alone; Unexposed = negative for both serological and molecular markers; Past exposure = positive for anti-HBs and anti-HBc alone; Chronic HBV infection = positive for HBsAg; Occult HBV infection = negative for HBsAg and positive for HBV DNA, with or without anti-HBs and/or anti-HBc.
3.3 Phylogenetic analysis and genetic variations within HBV pol gene sequences
Of the 107 samples tested, 24 (22.4%) had detectable HBV viral loads (≥103 copies/ml as per the lower limit of detection of the PCR assay). The HBV pol gene was successfully sequenced for 12 of the 24 PCR positive samples, with the remaining half failing the sequencing process potentially as a results of poor sample quality or quantity. Phylogenetic analysis showed that all 12 sequences clustered with HBV genotype A and sub-genotype A2 reference sequences from countries like Japan, Belgium, Cuba, and South Africa (Figure 2). All pol gene sequences were further investigated for inter-genotypic and sub-genotypic recombination events. Results showed that all 12 study sequences were pure genotypes and did not contain genetic recombination within the pol gene fragment analyzed. Although amino acid variations were observed in the reverse transcriptase domain of the pol gene sequences, none of them were predicted to be drug resistance-associated mutations. Instead, sub-genotype A2-specific variations like the L217R and L209V were commonly observed within the reverse transcriptase and overlapping HBsAg domains of all study sequences, respectively. An N131S secondary mutation with the potential for allowing viral escape from neutralizing antibodies from the administration of hepatitis B immunoglobulin therapy was predicted within the overlapping HBsAg domain in sequence MW322672. A review of the serological profile of the participant from whom this MW322672 sequence was isolated revealed that they also tested negative for HBsAg.
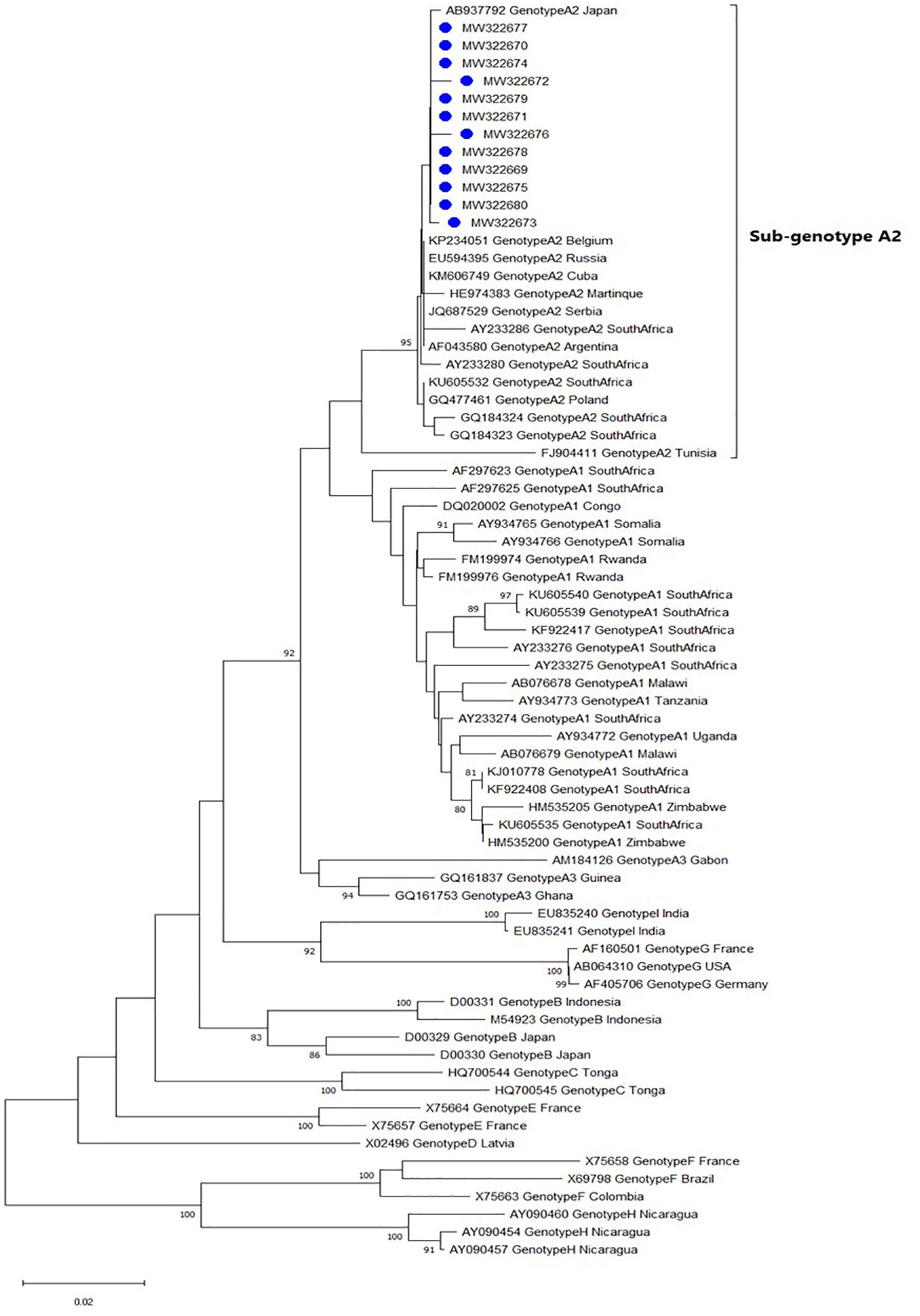
Figure 2 Phylogenetic inference tree showing the evolutionary history of HBV pol gene sequences. Study sequences (labelled with blue circles) and reference sequences can be identified by their accession numbers as they appear in GenBank. The percentage of replicate trees >75% in which the associated taxa clustered in the bootstrap test (1000 replicates) are shown next to the branch nodes.
4 Discussion
This cross-sectional study characterized the burden of HBV infection among patients presenting for immunosuppressive therapy for various gynecological and dermatological cancers. Among patients diagnosed with cancer who are undergoing cytotoxic and immunosuppressive chemotherapy or radiotherapy, HBsAg negative HBV infection may reactivate while those with chronic active HBV infection may experience a progressively worsening prognosis, both leading to varying degrees of potentially fatal liver disease (36, 37). It is for this reason that HBV screening and timely prophylaxis prior to initiation of immunosuppressive cancer therapy is widely recommended by global advisory groups (38–40). In South Africa where the burden of chronic hepatitis B remains high, the national guidelines for the management of viral hepatitis recommend hepatitis B vaccination for individuals receiving immunosuppressive cancer therapy (21). Further to this, the national guidelines make provision for antiviral treatment for all individuals receiving chemotherapy, rituximab or immunosuppressive therapy regardless of the phase of HBV infection (21). Despite these guidelines, inadequacies in screening practices and access to care significantly hamper timely management of cancer patients with undetected HBV infection.
We present evidence to support the urgent scale-up of HBV screening and prophylaxis for patients with cancer as part of routine clinical practice in South Africa. Our findings suggest a chronic hepatitis B prevalence rate of 11.2% among patients diagnosed with gynecological and dermatological cancers which is higher than the 3.5% reported in the general South African population (2). Previous studies conducted among patients with cancer have reported chronic hepatitis B prevalence rates of 0.3% in France (41), 4.6% in the Republic of Korea (14), 6.6% in Iran (16), 7.78% in Taiwan (42), 8.2% in China (15), 14% in South Africa (13) and 29.1% in Kenya (17). These prevalence rates vary across country-specific hepatitis B endemicity levels, types of cancers studied and individual risk factors. Unfortunately, robust evidence on the burden of chronic hepatitis B among African patients with non-hepatic cancers is less forthcoming and may reflect the insufficiencies of routine screening services and disease surveillance efforts within the region. We further stratified the outcomes of this study across HIV status. Among those with underlying HIV infection, the prevalence of chronic hepatitis B was found to be twice as high as that in the HIV negative sub-population (14.3% vs 7.8%). The 14.3% prevalence of HIV-HBV co-infection found in this study is comparable to that (17.4%) reported by a study conducted among a Kenyan cohort of patients with ovarian cancer (17). In a previous study conducted by our group within the same setting but among a smaller population (N=34) of patients diagnosed with cancer and living with HIV, we found that only 2.9% were chronically infected with HBV (29). It might be that the expansion of the population studied increased the likelihood of detecting HIV-HBV co-infections in the current study. Ultimately, it is well established that the brunt of chronic hepatitis B in South Africa is borne by the HIV positive adult population with prevalence rates ranging from 3.4% to as high as 22.9% in some studies (12, 43, 44).
In addition to the high prevalence of chronic hepatitis B, it is notable that a further 20% of the study population were found to have occult HBV infection which is within the range of previous estimates from studies conducted in South Africa and elsewhere among various risk groups (18, 29, 45–47). Of concern is the fact that over half (57.9%) of those with occult HBV infection in the current study were negative for all other serological markers and will typically go undiagnosed if molecular assays for detection of HBV DNA are not included in routine screening programs as per the current national guidelines (21). It is possible that the use of a nested PCR, rather than the more sensitive quantitative real-time PCR assay, may have led to an underestimation of occult HBV infection within the study population. It is equally possible that the prevalence of chronic and occult HBV infections reported for those with underlying HIV infection is an underestimation of the true burden given that 89% of them were on a HAART regimen with co-activity against HBV at the time of enrolment. While HBV infected patients with underlying HIV infection may benefit from HBV-active HAART regimens, HIV negative patients who are unaware of their hepatitis B status will remain at high risk for adverse outcomes during immunosuppressive cancer therapy without appropriate screening and timely linkage to care. Evidently, these findings underscore the importance of universal serological and molecular screening, followed by linkage to appropriate prophylaxis and care for patients with cancer in hepatitis B endemic settings like South Africa. Such prophylaxis should include hepatitis B vaccination or re-vaccination for eligible persons. In our study, nearly half (45.8%) of the participants were negative for all serological and molecular markers and thus would be excellent candidates for vaccination against hepatitis B prior to initiating immunosuppressive cancer therapy (48).
The identification of genotype A strains within the study population is consistent with the geographical distribution of HBV genotypes in South Africa (49, 50). Although sub-genotype A1 is the predominant strain in South Africa, all sequences generated in this study clustered with a large dataset of A2 reference sequences retrieved from GeneBank. Of the 12 HBV pol gene sequences generated in this study, 10 were from participants with underlying HIV infection, nine of whom were on HBV active HAART at the time of enrolment (Atanef = 1, Atrioza = 3, Tribuss = 2, Odimune = 2, and Zidovudine, Lamivudine and Nevirapine = 1). Mutational analysis did not yield any HBV drug resistance variants. While we did not have records of the duration of HAART for each study participant, long term combination therapy with nucleot(s)ide analogues such as Emtricitabine and Tenofovir Disoproxil Fumarate (both part of Atanef, Atrioza, Tribuss, and Odimune regimens) is widely recommended given their relatively superior genetic barrier to resistance compared to Lamivudine monotherapy (21, 38, 39). Without access to antiviral prophylaxis, patients like the treatment naïve HIV negative population in this study, are at significant risk of chemotherapy- and/or radiotherapy- induced HBV reactivation with potential adverse outcomes (36, 37, 51).
The findings of this study should be carefully considered in light of some limitations. This was a single center study with a modest population size. Despite recruiting from two separate clinics within the DGMAH, all but four of our study participants were enrolled from the gynecology clinic which introduced selection bias, and thereby limits the generalizability of the study outcomes. There is a need for further studies from the African setting, with a similar research focus. Such studies will benefit from population-based longitudinal designs, investigating additional serological markers like the hepatitis B e antigen (HBeAg) and antibodies to HBeAg, in order to better guide reforms and implementation of national guidelines for the management of hepatitis B among patients due for immunosuppressive cancer therapy. Studies with age- and sex- matched control groups may provide further understanding of the burden of chronic and occult HBV infections among patients with cancer compared to the general South African population. Further to this, an improved understanding of predictors (including socio-demographic and economic determinants) of HBV infection within this population will be integral to a robust public health response and as such, should form part of the future research agenda.
In summary, chronic and occult HBV infections are prevalent among patients with gynecological and dermatological cancers. Furthermore, a substantial proportion of patients with cancer do not have adequate immune protection against hepatitis B prior to initiating cytotoxic or immunosuppressive chemotherapy and radiotherapy. Given the significant risk of immunosuppressive therapy-induced reactivation of HBV and the potential for adverse outcomes within this population, there is a need to expand access to serological and molecular screening services, vaccination, and pre-emptive and prophylactic antiviral therapy as part of routine clinical practice.
Data availability statement
The datasets presented in this study can be found in online repositories. The name of the repository and accession numbers can be found in the article.
Ethics statement
This study involving humans was approved by Sefako Makgatho Health Sciences University Human Research Ethics Committee. The study was conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study.
Author contributions
This research was conceptualized by MN, EA-D, SS and AM. MN, EA-D and AM were involved in data curation and formal analysis. MN and EA-D drafted the original manuscript. NB and AM validated the data. NB, SS, TM, MM and AM reviewed and edited the final draft of the manuscript. All authors contributed to the article and approved the submitted version.
Funding
The current project was funded by grants from the Poliomyelitis Research Foundation (PRF) [grant number: 18/59]; National Research Foundation (NRF) [Grant number: SFH150727131644]; Services SETA Bursary [Reference: SSETA 2018 – 013992].
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fviro.2023.1160078/full#supplementary-material
References
1. World Health Organization. Global Health Sector Strategy on Viral Hepatitis 2016-2021. Towards Ending Viral Hepatitis. (2016). World Health Organization. Available at: https://apps.who.int/iris/handle/10665/246177 (Accessed January 20, 2023).
2. Sheena BS, Hiebert L, Han H, Ippolito H, Abbasi-Kangevar M, Abbasi-Kangevari Z, et al. Global, regional, and national burden of hepatitis B, 1990–2019: a systematic analysis for the Global Burden of Disease Study 2019. Lancet Gastroenterol Hepatol (2022) 7(9):796–829. doi: 10.1016/S2468-1253(22)00124-8
3. Saitta C, Pollicino T, Raimondo G. Occult hepatitis B virus infection: an update. Viruses (2022) 14(7):1504. doi: 10.3390/v14071504
4. Joseph Davey D, Hsiao Ny, Spearman CW, Sonderup M, Hu NC, Mashele N, et al. Low prevalence of hepatitis B virus infection in HIV-uninfected pregnant women in Cape Town, South Africa: implications for oral pre-exposure prophylaxis roll out. BMC Infect Dis (2022) 22(1):719. doi: 10.1186/s12879-022-07697-5
5. Chotun N, Preiser W, van Rensburg CJ, Fernandez P, Theron GB, Glebe D, et al. Point-of-care screening for hepatitis B virus infection in pregnant women at an antenatal clinic: A South African experience. PloS One (2017) 12(7):e0181267. doi: 10.1371/journal.pone.0181267
6. Diale Q, Pattinson R, Chokoe R, Masenyetse L, Mayaphi S. Antenatal screening for hepatitis B virus in HIV-infected and uninfected pregnant women in the Tshwane district of South Africa. South Afr Med J (2015) 106(1):97–100. doi: 10.7196/SAMJ.2016.v106i1.9932
7. Burnett RJ, Dramowski A, Amponsah-Dacosta E, Meyer JC. Increasing hepatitis B vaccination coverage of healthcare workers — global lessons for South Africa. Curr Opin Immunol (2021) 71:6–12. doi: 10.1016/j.coi.2021.03.010
8. Scheibe A, Young K, Moses L, Basson RL, Versfeld A, Spearman CW, et al. Understanding hepatitis B, hepatitis C and HIV among people who inject drugs in South Africa: findings from a three-city cross-sectional survey. Harm Reduction J (2019) 16(1):28. doi: 10.1186/s12954-019-0298-2
9. Samsunder N, Ngcapu S, Lewis L, Baxter C, Cawood C, Khanyile D, et al. Seroprevalence of hepatitis B virus: Findings from a population-based household survey in KwaZulu-Natal, South Africa. Int J Infect Dis (2019) 85:150–7. doi: 10.1016/j.ijid.2019.06.005
10. King J, Hagemeister DT. Hepatitis B co-infection in HIV-infected patients receiving antiretroviral therapy at the TC Newman Anti Retroviral Treatment Clinic in Paarl, Western Cape. South Afr J HIV Med (2016) 17(1):336. doi: 10.4102/sajhivmed.v17i1.336
11. Chonco F, Rangiah S. Susceptibility to hepatitis B infection, hepatitis B/HIV co-infections and hepatitis B immunity in HIV-positive patients starting HAART in Durban, South Africa. South Afr Family Practice (2019) 61(2):65–8. doi: 10.1080/20786190.2018.1518023
12. Msomi N, Naidoo K, Yende-Zuma N, Padayatchi N, Govender K, Singh JA, et al. High incidence and persistence of hepatitis B virus infection in individuals receiving HIV care in KwaZulu-Natal, South Africa. BMC Infect Dis (2020) 20(1):847. doi: 10.1186/s12879-020-05575-6
13. Mak D, de Villiers CB, Chasela C, Urban MI, Kramvis A. Analysis of risk factors associated with hepatocellular carcinoma in black South Africans: 2000–2012. PloS One (2018) 13(5):e0196057. doi: 10.1371/journal.pone.0196057
14. An J, Kim JW, Shim JH, Han S, Yu CS, Choe J, et al. Chronic hepatitis B infection and non-hepatocellular cancers: A hospital registry-based, case-control study. PloS One (2018) 13(3):e0193232. doi: 10.1371/journal.pone.0193232
15. Lu LJ, Adhikari VP, Zhao CX, Wu H, Dai W, Li X, et al. Clinical study on the relationship between hepatitis B virus infection and risk of breast cancer: a large sized case-control and single center study in southwest of China. Oncotarget (2017) 8(42):72044–53. doi: 10.18632/oncotarget.19132
16. Mahmoudvand S, Shokri S, Mirzaei H, Ramezani A, Makvandi M, Neisi N, et al. Frequency of Hepatitis B Virus infection among Patients before Chemotherapy Treatment. Asian Pacific J Cancer Prev (2021) 22(9):2939–44. doi: 10.31557/APJCP.2021.22.9.2939
17. Wanyama FM, Tauber R, Mokomba A, Nyongesa C, Blanchard V. The burden of hepatitis B, hepatitis C, and human immunodeficiency viruses in ovarian cancer patients in Nairobi, Kenya. Infect Dis Rep (2022) 14(3):433–45. doi: 10.3390/idr14030047
18. Elbedewy TA, Elashtokhy HEA, Rabee ES, Kheder GE. Prevalence and chemotherapy-induced reactivation of occult hepatitis B virus among hepatitis B surface antigen negative patients with diffuse large B-cell lymphoma: Significance of hepatitis B core antibodies screening. J Egypt Natl Cancer Instit (2015) 27(1):11–8. doi: 10.1016/j.jnci.2015.01.004
19. Pattullo V. Hepatitis B reactivation in the setting of chemotherapy and immunosuppression - prevention is better than cure. World J Hepatol (2015) 7(7):954–67. doi: 10.4254/wjh.v7.i7.954
20. Pattullo V. Prevention of Hepatitis B reactivation in the setting of immunosuppression. Clin Mol Hepatol (2016) 22(2):219–37. doi: 10.3350/cmh.2016.0024
21. South African National Department of Health. National Guidelines for the Management of Viral Hepatitis. Available at: https://www.knowledgehub.org.za/elibrary/national-guidelines-management-viral-hepatitis (Accessed January 12, 2023).
22. Sun WC, Hsu PI, Yu HC, Lin KH, Tsay FW, Wang HM, et al. The compliance of doctors with viral hepatitis B screening and antiviral prophylaxis in cancer patients receiving cytotoxic chemotherapy using a hospital-based screening reminder system. PloS One (2015) 10(2):e0116978. doi: 10.1371/journal.pone.0116978
23. Sun WC, Tang PL, Chen WC, Tsay FW, Wang HM, Tsai TJ, et al. Hepatitis B virus screening before cancer chemotherapy in Taiwan: A nationwide population-based study. Front Med (2021) 8:657109. doi: 10.3389/fmed.2021.657109
24. Leber K, Otten HMJMMB, Brandjes DPM, Claassen MAA, Lauw FN. Clinical practice of hepatitis B screening in patients starting with chemotherapy: A survey among Dutch oncologists. Eur J Cancer Care (2021) 30(6):e13495. doi: 10.1111/ecc.13495
25. Hwang JP, Fisch MJ, Lok ASF, Zhang H, Vierling JM, Suarez-Almazor ME. Trends in hepatitis B virus screening at the onset of chemotherapy in a large US cancer center. BMC Cancer (2013) 13(1):534. doi: 10.1186/1471-2407-13-534
26. Platt L, French CE, McGowan CR, Sabin K, Gower E, Trickey A, et al. Prevalence and burden of HBV co-infection among people living with HIV: A global systematic review and meta-analysis. J Viral Hepatitis (2020) 27(3):294–315. doi: 10.1111/jvh.13217
27. Yuan T, Hu Y, Zhou X, Yang L, Wang H, Li L, et al. Incidence and mortality of non-AIDS-defining cancers among people living with HIV: A systematic review and meta-analysis. eClinicalMedicine (2022) 52. doi: 10.1016/j.eclinm.2022.101613
28. Kew MC. Hepatitis B virus infection: the burden of disease in South Africa. South Afr J Epidemiol Infect (2008) 23(1):4–8. doi: 10.1080/10158782.2008.11441293
29. Musyoki AM, Msibi TL, Motswaledi MH, Selabe SG, Monokoane TS, Mphahlele MJ. Active co-infection with HBV and/or HCV in South African HIV positive patients due for cancer therapy. J Med Virol (2015) 87(2):213–21. doi: 10.1002/jmv.24055
30. Gutfreund KS, Williams M, George R, Bain VG, Ma MM, Yoshida EM, et al. Genotypic succession of mutations of the hepatitis B virus polymerase associated with lamivudine resistance. J Hepatol (2000) 33(3):469–75. doi: 10.1016/S0168-8278(00)80284-6
31. Selabe SG, Lukhwareni A, Song E, Leeuw YGM, Burnett RJ, Mphahlele MJ. Mutations associated with lamivudine-resistance in therapy-naïve hepatitis B virus (HBV) infected patients with and without HIV co-infection: Implications for antiretroviral therapy in HBV and HIV co-infected South African patients. J Med Virol (2007) 79(11):1650–4. doi: 10.1002/jmv.20974
32. R Core Team. R: A language and environment for statistical computing. The R Project for Statistical Computing. Available at: https://www.r-project.org/ (Accessed January 12, 2023).
33. Tamura K, Stecher G, Kumar S. MEGA11: molecular evolutionary genetics analysis version 11. Mol Biol Evol (2021) 38(7):3022–7. doi: 10.1093/molbev/msab120
34. Lole KS, Bollinger RC, Paranjape RS, Gadkari D, Kulkarni SS, Novak NG, et al. Full-length human immunodeficiency virus type 1 genomes from subtype C-infected seroconverters in India, with evidence of intersubtype recombination. J Virol (1999) 73(1):152–60. doi: 10.1128/JVI.73.1.152-160.1999
35. Neumann-Fraune M, Beggel B, Kaiser R, Obermeier M. Hepatitis B virus drug resistance tools: one sequence, two predictions. Intervirology (2014) 57(3-4):232–6. doi: 10.1159/000361076
36. Ding ZN, Meng GX, Xue JS, Yan LJ, Liu H, Yan YC, et al. Hepatitis B virus reactivation in patients undergoing immune checkpoint inhibition: systematic review with meta-analysis. J Cancer Res Clin Oncol (2023) 149(5):1993–2008. doi: 10.1007/s00432-022-04133-8
37. Paul S, Saxena A, Terrin N, Viveiros K, Balk EM, Wong JB. Hepatitis B virus reactivation and prophylaxis during solid tumor chemotherapy. Ann Internal Med (2016) 164(1):30–40. doi: 10.7326/M15-1121
38. Terrault NA, Lok ASF, McMahon BJ, Chang KM, Hwang JP, Jonas MM, et al. Update on prevention, diagnosis, and treatment of chronic hepatitis B: AASLD 2018 hepatitis B guidance. Hepatology (2018) 67(4):1560–99. doi: 10.1002/hep.29800
39. Hwang JP, Feld JJ, Hammond SP, Wang SH, Alston-Johnson DE, Cryer DR, et al. Hepatitis B virus screening and management for patients with cancer prior to therapy: ASCO provisional clinical opinion update. J Clin Oncol (2020) 38(31):3698–715. doi: 10.1200/JCO.20.01757
40. European Association for the Study of the Liver. EASL Clinical Practice Guidelines: Management of chronic hepatitis B virus infection. J Hepatol (2012) 57(1):167–85. doi: 10.1016/j.jhep.2012.02.010
41. Brasseur M, Heurgué-Berlot A, Barbe C, Brami C, Rey JB, Vella-Boucaud J, et al. Prevalence of hepatitis B and C and sensibility of a selective screening questionnaire in patients receiving chemotherapy for solid tumors. BMC Cancer (2015) 15(1):999. doi: 10.1186/s12885-015-2033-z
42. Chen CH, Hsieh HH, Wu TY. Real-world prevalence of hepatitis B virus reactivation in cancer patients in Taiwan. J Oncol Pharm Practice (2021) 27(1):63–70. doi: 10.1177/1078155220913095
43. Matthews PC, Geretti AM, Goulder PJR, Klenerman P. Epidemiology and impact of HIV coinfection with Hepatitis B and Hepatitis C viruses in Sub-Saharan Africa. J Clin Virol (2014) 61(1):20–33. doi: 10.1016/j.jcv.2014.05.018
44. Amponsah-Dacosta E, Lebelo RL, Rakgole JN, Burnett RJ, Selabe SG, Mphahlele MJ. Evidence for a change in the epidemiology of hepatitis B virus infection after nearly two decades of universal hepatitis B vaccination in South Africa. J Med Virol (2014) 86(6):918–24. doi: 10.1002/jmv.23910
45. Amponsah-Dacosta E, Lebelo RL, Rakgole JN, Selabe SG, Gededzha MP, Mayaphi SH, et al. Hepatitis B virus infection in post-vaccination South Africa: Occult HBV infection and circulating surface gene variants. J Clin Virol (2015) 63:12–7. doi: 10.1016/j.jcv.2014.11.032
46. Ondigui JLN, Kenmoe S, Kengne-Ndé C, Ebogo-Belobo JT, Takuissu GR, Kenfack-Momo R, et al. Epidemiology of occult hepatitis B and C in Africa: A systematic review and meta-analysis. J Infect Public Health (2022) 15(12):1436–45. doi: 10.1016/j.jiph.2022.11.008
47. Baghbanian M, Halvani M, Roghani HS, Lotfi MH, Yazdi MF, Vahedian-Ardakani HA. Prevalence of occult hepatitis b infection in iranian cancer patients before chemotherapy treatment. Arquivos Gastroenterol (2016) 53:175–9. doi: 10.1590/S0004-28032016000300010
48. Ariza-Heredia EJ, Chemaly RF. Practical review of immunizations in adult patients with cancer. Hum Vaccines Immunother (2015) 11(11):2606–14. doi: 10.1080/21645515.2015.1062189
49. Kramvis A. Molecular characteristics and clinical relevance of African genotypes and subgenotypes of hepatitis B virus. SAMJ: South Afr Med J (2018) 108(8):17–21. doi: 10.7196/samj.2018.v108i8.13495
50. Mak D, Kramvis A. Molecular characterization of hepatitis B virus isolated from Black South African cancer patients, with and without hepatocellular carcinoma. Arch Virol (2020) 165(8):1815–25. doi: 10.1007/s00705-020-04686-4
Keywords: hepatitis B, occult HBV infection, HIV, immunosuppression, cancer
Citation: Ngoato MH, Amponsah-Dacosta E, Blose N, Selabe SG, Msibi TL, Motswaledi MH and Musyoki AM (2023) Hepatitis B virus infection in patients presenting for immunosuppressive cancer therapy with and without underlying HIV infection. Front. Virol. 3:1160078. doi: 10.3389/fviro.2023.1160078
Received: 06 February 2023; Accepted: 28 August 2023;
Published: 26 September 2023.
Edited by:
Kristina M. Adams Waldorf, University of Washington, United StatesReviewed by:
Stefania Varchetta, San Matteo Hospital Foundation (IRCCS), ItalyNishi Prabdial-Sing, National Institute for Communicable Diseases, South Africa
Copyright © 2023 Ngoato, Amponsah-Dacosta, Blose, Selabe, Msibi, Motswaledi and Musyoki. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Malowane H. Ngoato, malowanehoward@gmail.com
 Malowane H. Ngoato
Malowane H. Ngoato Edina Amponsah-Dacosta
Edina Amponsah-Dacosta Ntombifuthi Blose2
Ntombifuthi Blose2  Mojakgomo H. Motswaledi
Mojakgomo H. Motswaledi Andrew M. Musyoki
Andrew M. Musyoki