Characterization of Human Herpesvirus 8 genomic integration and amplification events in a primary effusion lymphoma cell line
- 1Genomes and Disease, Centre for Research in Molecular Medicine and Chronic Diseases (CiMUS), Universidade de Santiago de Compostela, Santiago de Compostela, Spain
- 2Department of Zoology, Genetics and Physical Anthropology, Universidade de Santiago de Compostela, Santiago de Compostela, Spain
- 3Instituto de Investigaciones Sanitarias de Santiago de Compostela (IDIS), Santiago de Compostela, Spain
- 4Department of Biochemistry, Genetics and Immunology, Universidade de Vigo, Vigo, Spain
- 5Viruses and cancer, Centre for Research in Molecular Medicine and Chronic Diseases (CiMUS), Universidade de Santiago de Compostela, Santiago de Compostela, Spain
In this study, we investigated the integration of Human Herpesvirus 8 (HHV-8) into the human genome using the primary effusion lymphoma (PEL) cell line BC-3. Through next-generation sequencing (NGS) data from multiple independent sequencing runs, we identified two highly supported HHV-8 integrants. These integrants encompassed a region of human chromosome 12 that was amplified approximately 16-fold between the junctions. Significantly, these events could represent the first known instance of HHV-8 integration into a hybrid human-viral extrachromosomal chimeric circular DNA (eccDNA). The amplified fragment contained partial or complete copies of various human genes, including SELPLG and CORO1C. Analysis of long-read Nanopore data indicated that the CpGs at the SELPLG promoter were mostly unmethylated, suggesting that the additional copies of SELPLG within this eccDNA are likely transcriptionally active. Our findings suggest that viral insertion and eccDNA amplification could be crucial mechanisms in the development of HHV-8-related cancers. In conclusion, our study provides valuable insights into the molecular mechanisms involved in HHV-8-induced oncogenesis and emphasizes the importance of investigating viral integration and eccDNAs in cancer development. Furthermore, we highlight the necessity of employing multiple independent sequencing approaches to validate integration events and avoid false positives derived from library construction artifacts.
1 Introduction
Human Herpesvirus 8 (HHV-8) is a well-known pathogen associated with the development of various cancers, including Kaposi’s Sarcoma (KS), multicentric Castleman’s Disease (MCD), and Primary Effusion Lymphoma (PEL) (1, 2). The HHV-8 genome, typically composed of approximately 138 kb of linear dsDNA duplex, undergoes circularization into extrachromosomal episomes upon entering the host nucleus. Remarkably, these episomes adopt a chromatin structure similar to that of the host chromosomes, allowing for the protection and maintenance of viral DNA during latency (3). The latency-associated nuclear antigen 1 protein (LANA-1) physically tethers the viral DNA to the host chromosomes, further ensuring the stability of HHV-8 during this phase (3).
HHV-8 possesses a repertoire of encoded proteins that actively engage various cell cycle regulatory pathways and manipulate the host DNA damage response to facilitate viral DNA replication and packaging (4). Notably, LANA-1 plays a crucial role in this process, as it binds to the tumor suppressor protein p53, promoting its degradation and impeding cell cycle arrest and apoptosis (5). Moreover, HHV-8 has the ability to activate the AKT/mTOR pathway, promoting cell proliferation and survival (6), as well as the ATM/Chk2 pathway, which aids in stabilizing viral DNA replication forks and enhancing genome maintenance (7). Consequently, unraveling the intricate interplay between HHV-8 and the host cell cycle is essential for comprehending the underlying mechanisms of HHV-8-associated cancers and developing effective therapeutic strategies.
Interestingly, the presence of sequence homology between certain viral and cellular proteins has raised intriguing possibilities regarding the origin of the HHV-8 genome through gene acquisition from the host genome (8). However, these claims remain contentious and necessitate further investigation. Our primary objective was to address this hypothesis by conducting an extensive genomic screening of a well-characterized primary effusion lymphoma (PEL) cell line, BC-3. Utilizing a combination of short and long-read sequencing data, we aimed to identify potential chimeric DNAs, serving as indications of host gene capture events by the HHV-8 virus. By employing this approach, we sought to shed light on the potential evolutionary dynamics of HHV-8 and its interaction with the host genome.
2 Methods
2.1 Next-generation sequencing
To gain insight into the genetic landscape of PEL cell lines infected with Kaposi’s sarcoma-associated herpesvirus (KSHV), we performed Illumina whole-genome paired-end sequencing on DNA from the BC-3 cell line. The sequencing reads were generated at 30X coverage with an insert size of 350 bp and a read length of 150 bp. The reads were then aligned to the human reference genome (hg38) using BWA-MEM v0.78.8-r455 and Samtools. To complement this, we conducted whole genome long-read sequencing for BC-3 cell line using MinION R9.4 flowcells (FLO-MIN106 rev-D, Oxford Nanopore Technologies Ltd) controlled by MinKNOW. We used Guppy v2.2.3 to base-call the ONT raw fast5 files into fastqs and reads were mapped to the human reference genome hg38 with minimap2. We also used Megalodon to call methylation at CpG sites from nanopore data with default parameters.
2.2 Detection of viral integrations
We utilized our in-house pipeline, v-TraFiC (9), to detect potential viral integrations in the short read data. The algorithm identifies candidate read pairs uncovering potential virus insertions with either one of the mates mapped on the human genome and the other matching to a virus reference in a non-redundant catalog and/or clipped reads supporting a human-virus junction. Clusters of human-anchored reads mapped closer than 200 bp on the same orientation were collected by v-TraFiC, discarding those supported by less than four reads. Clusters ≤ 350 bp apart with head-to-head orientation were classified as reciprocal, corresponding to both breakpoints of the same integration, while the remaining ones were classified as single-breakpoint events. A consensus sequence for each of the integration breakpoints was then deduced from the non-anchored reads of each cluster using Velvet to assess their viral identity, followed by a BLAST search of all resulting contigs against the RVDB Reference viral database v12.2, keeping only those clusters matching KSHV sequences. Further details are provided in (9). To evaluate whole genome copy number variations (CNVs) affecting large fragments of chromosomes, we used CNVkit and SAMtools depth to assess local ploidy changes potentially linked to KSHV integrations. To gain insight into the configuration of the genomic rearrangements in the BC-3 cell line, we utilized the AmpliconArchitect pipeline, as described by (10). This pipeline allowed for the detection and characterization of genomic rearrangements, construction of junction graphs, and visualization of any cycles present in the data.
2.3 Validation of eccDNA configuration
The detection of eccDNAs in the cell line was carried out using a protocol that involved a miniprep followed by the enzymatic depletion of linear DNA, using pre-established methods (11). In brief, a growing cell culture was harvested and processed with a commercial miniprep kit (Plasmid Mini AX, A&A Biotech), as per the manufacturer’s instructions. After isolation, the DNA was treated with exonuclease V (New England’s Biolab) for 120 hours, an enzyme that specifically digests linear DNA, leaving only the circular DNA forms intact. To verify the presence of specific junctions between HHV-8 and human chromosome 12 (HHV-8-Chr12), PCR amplifications were performed on the resulting DNA samples using primer pairs targeting these junctions. Two primer pairs were used: 12:108959899_A_Fw: GAGTGCATCACCATCACGTC and 12:108959899_A_Rv: CCCTTCTTGGTTGATTCAGG for the left junction, and 12:109083144_B_Fw: TGCTTGAGCCTGGGAGAC and 12:109083144_B_Rv: GCTAGGCCACGCCTACTTTT for the right junction. Additionally, two primer pairs were designed to target the Actin B gene on the linear genome (ACTB_1_Fw: AGCTCAGGCAGGAAAGACAC and ACTB_1_Rv: GGACTTCGAGCAAGAGATGG; ACTB_2_Fw: GAGGGCAGGACTTAGCTTCC and ACTB_2_Rv: TGCTATCCCTGTACGCCTCT, enabling assessment of linear DNA depletion after purification. Additionally, and as positive controls, three sets of primers were used to target circular DNAs, two were specific to the human mitochondrial DNA (MT), consisting of the primer pairs MT_A_Fw: CACTGTCAACCCAACACAGG and MT_A_Rv: AGCATGTACTGCTCGGAGGT, as well as MT_B_Fw: ACACCTATCCCCCATTCTCC and MT_B_Rv: TGGCTCAGTGTCAGTTCGAG. An additional primer pair was designed to target an artificial plasmid, lentiCRISPR v2, which was introduced to the sample prior to purification as a positive control (Amp-R: ATAATACCGCGCCACATAGC and pRS-marker: CGGCATCAGAGCAGATTGTA). Subsequently, the resulting PCR products were separated through agarose gel electrophoresis, and the quantity of the amplified products was determined using ImageJ software (12). This quantification step allowed for the assessment of the abundance of the detected PCR products.
2.4 Cytogenetic analysis
Metaphase spreads were obtained from BC3 cultured cells following standard protocols (13), and pretreated with RNAse and pepsin before dual FISH experiments with commercial probes targeting the whole chromosome 12 paint probe (FWCP-12; Creative Bioarray) and the CORO1C gene (Empire Genomics), respectively labeled with green and red fluorescent dyes and following the provider recommendations. Customized probes for FISH targeting the terminal DNA repeats of HHV-8 genome were produced by PCR using primers HHV-8_tr_F: CTGGACACTACGTGAACACCC and HHV-8_tr_R: GGGAGAAAACGAAAGCAAGCG in a 20 μl mixture containing 50 ng DNA, 1x PCR buffer, 0.5 mM dNTPs, 2.5 mM MgCl2, 1 μM each primer and 1 U BIOTAQ DNA polymerase (Bioline) and supplemented with digoxigenin-11-dUTP (10× DIG Labeling Mix, Roche Applied Science). The PCR reaction included an initial denaturation at 95°C, 30 cycles (95°C, 20 s; 54°C, 20 s; 72°C, 30 s) of amplification and a final extension step of 7 min at 72°C in a GeneAmp PCR system 9700 (Applied Biosystems). Probes were revealed with anti-DIG-mouse monoclonals (Sigma) followed by TRITC-conjugated goat anti-mouse (Sigma) and TRITC-conjugated rabbit anti-goat antibodies as previously indicated (14). The same primers were used for primed in situ labeling following standard procedures (15). After a counterstaining with DAPI (0.14 pg/ml), photographs were taken for each individual color with a Nikon Eclipse-800 fluorescence microscope (Tokyo, Japan) equipped with a DS-Qi1Mc CCD camera (Nikon) and controlled the using NIS-Elements software (Nikon). The resulting images were merged and processed using Adobe Photoshop CS2 (San Jose, CA, USA).
3 Results and discussion
Our analysis revealed numerous virus-human junctions distributed across all chromosomes. However, most of these junctions were supported by a relatively low number of reads, indicating the presence of single breakpoints and no concurrent local copy number alterations. While some of these junctions could potentially be attributed to subclonal insertion events or artifacts arising from library preparation, their biological significance remains unclear and warrants further investigation. In contrast, we identified two distinct virus-human junctions on Chromosome 12 (Chr12) that exhibited robust support from NGS data generated from independent sequencing runs and using different technologies, including Hi-C sequencing data reported by reference (16) (Figure 1A). These breakpoints encompassed an approximately 16-fold amplified region spanning 1.2 Mb of Chr12 (GRCh38, Chr12:108566123-108689368). Notably, this region included a segment of the ISCU gene, complete copies of SELPLG and TMEM119, and truncated copies of the CORO1C gene (Figure 1A), consistent with previous findings (17).
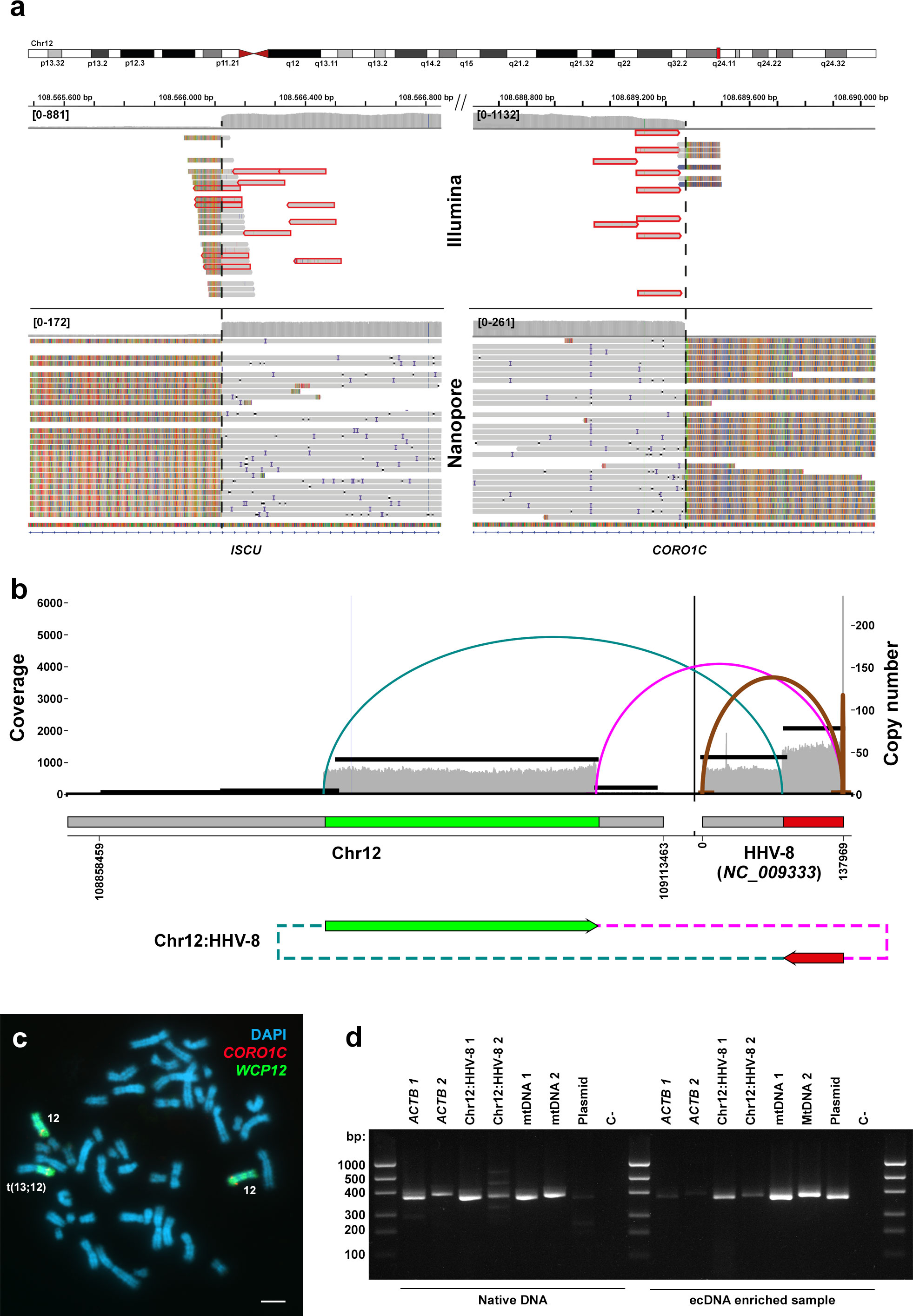
Figure 1 Comprehensive analysis of the genomic rearrangement between HHV-8 and Chr12 in the BC-3 cell line, including assembly, amplicon configuration reconstruction, fluorescent in situ hybridization, and PCR validation. (A) Genome-wide multiplatform sequencing data using short (Illumina paired-ends) and long (Nanopore) reads on this cell line supports two HHV-8-Chr12 junctions coupled to a 16-fold segmental CN gain. (B) An Amplicon Architect reconstruction of the junctions between chr12 and the HHV-8 genome supports either a HHV-8 insertion into Chr12 coupled to a focal amplification or an eccDNA configuration. (C) Despite Fluorescence in situ hybridization (FISH) using CORO1C (red) and whole chromosome 12 (green) probes on metaphase plates of the BC-3 cell line revealed Chr12q aneuploidy, it does not provide conclusive evidence for either alternative scenario. DAPI (blue) was used for counterstaining. The scale bar represents 100 μm. (D) PCR assays targeting a fragment of linear genomic DNA (Actin B gene, ACTb), both junctions between the Chr12 and the HHV-8 genome (Chr12:HHV-8), and two circular DNAs as positive controls: the human mitochondrial DNA (mtDNA) and a lentiCRISPRv2 plasmid (Plasmid) on a native DNA (left panel) and eccDNA enriched (right panel) sample of BC-3 demonstrating its circular configuration.
Further analysis involving local reassemblies of both junctions using Nanopore data enabled the recovery of approximately 30 Kb of the HHV-8 reference genome (NC_009333:78.200-107.865) at the 5’ end, accompanied by up to 16 repeats of the viral long terminal repeat (LTR) unit at the 3’ end (Figure 1A). These findings strongly suggest that these two junctions represent compelling evidence of a singular integration event of the virus into the host genome. Furthermore, while HHV-8 infections have been traditionally associated with chromosomal instability, resulting in frequent genomic imbalances observed in PEL cell lines and primary tumors, our findings represent, to the best of our knowledge, the first reported instance of HHV-8 flanking such specific genomic alterations.
Additional analyses were conducted to characterize the nature of this HHV-8 event at Chr12 of the BC-3 cell line. AmpliconArchitect revealed a single bifocal integration connecting this amplified fragment of Chr12 to a segment of the HHV-8 viral genome (NC_009333 78.187-137.969) conforming an ecDNA (Figure 1B). The median copy number of this feature was 39.55, and there was no indication that this ecDNA originated from a breakage-fusion-bridge mechanism. FISH co-hybridizations with CORO1C gene and WC12 paint probes failed to confirm the presence of extrachromosomal circular DNA molecules holding these fragments (Figure 1C), probably attributable to the signal being way below the detection limit of this technique. Traditional FISH and PRINS assays targeting HHV-8 yielded similarly inconclusive results (not shown).
Further analysis based on the purification of extrachromosomal covalently closed circular DNAs (ecDNAs) in the BC-3 cell line verified that these HHV-8-Chr12 chimeric DNAs were actually contained in ecDNAs (Figure 1D). To do so, we designed PCR primers overlapping both virus-human junctions and compared the abundance of their products relative to those targeting two independent DNA amplicons on the linear genome, specifically for the Actin B gene, before and after eccDNA purification. The ratio of PCR band densities on agarose gel electrophoresis for both HHV-8:Chr12 junctions was stable before and after eccDNA purification (0.51). Additionally, the ratio of these PCR products on the alleged eccDNAs relative to both Actin B gene PCRs was actually higher after ecDNA purification (2.54) than without enrichment (1.09), providing evidence of linear genomic DNA depletion and supporting the circular configuration of the HHV-8:Chr12 chimeric DNA. In summary, these results suggest a convoluted genomic reorganization arising from a single HHV-8 insertion event, most likely followed by the subsequent circularization of the resulting virus-human chimeric DNA. eccDNAs are ubiquitous in human cancers, such as glioblastoma, neuroblastoma, and colon and breast cancers (18–20), and have been implicated in the amplification and over-transcription of various oncogenes, including MYC, EGFR, and CDK4, by increasing DNA copy numbers and chromatin accessibility, and enabling ultra-long-range chromatin contacts (20).
In addition, our analysis of the Nanopore data allowed us to infer the methylation status of the CpG sites and features at the amplified regions and to assess putative differences between the native linear DNA and eccDNAs (Figure 2). We found no major significant differences between reads with and without viral insertions within 14 and 20 Kb from the left and right breakpoints, respectively. Although phasing could not be extended any longer due to constrictions in read size lengths, our methylation analysis revealed that CpGs at the SELPLG promoter were predominantly unmethylated (96.774%), indicating that the additional SELPLG copies within this eccDNA are likely transcriptionally functional. This is consistent with previous qPCR, RNAseq and proteomic assays that showed high and moderate expression levels for SELPLG and CORO1C genes, respectively, in independent subcultures of BC-3 cells (21, 22), likely due to this HHV-8-mediated amplification. It is worth noting that the previously reported overexpression of CORO1C cannot be solely explained by this HHV-8-mediated amplification, as these supernumerary eccDNA copies are 5’ truncated. Recently, a study revealed the presence of oncogenic human-viral hybrid ecDNAs in nearly all tested HPV-mediated oropharyngeal cancers, which also translate into fusion genes from HPV promoters and oncogenes linked to downstream human transcripts, highlighting the impact of ecDNAs in viral-mediated carcinogenesis (23). Although HPV and HHV-8 follow vastly distinct viral cycles —HPV often integrates into the human genome (23)— we wanted to address possible viral-human fusion transcripts derived from the eccDNA described herein. However, after reviewing RNAseq data from previous studies on the BC-3 cell line (21), we found no evidence of fusion transcripts between HHV-8 and any host gene.
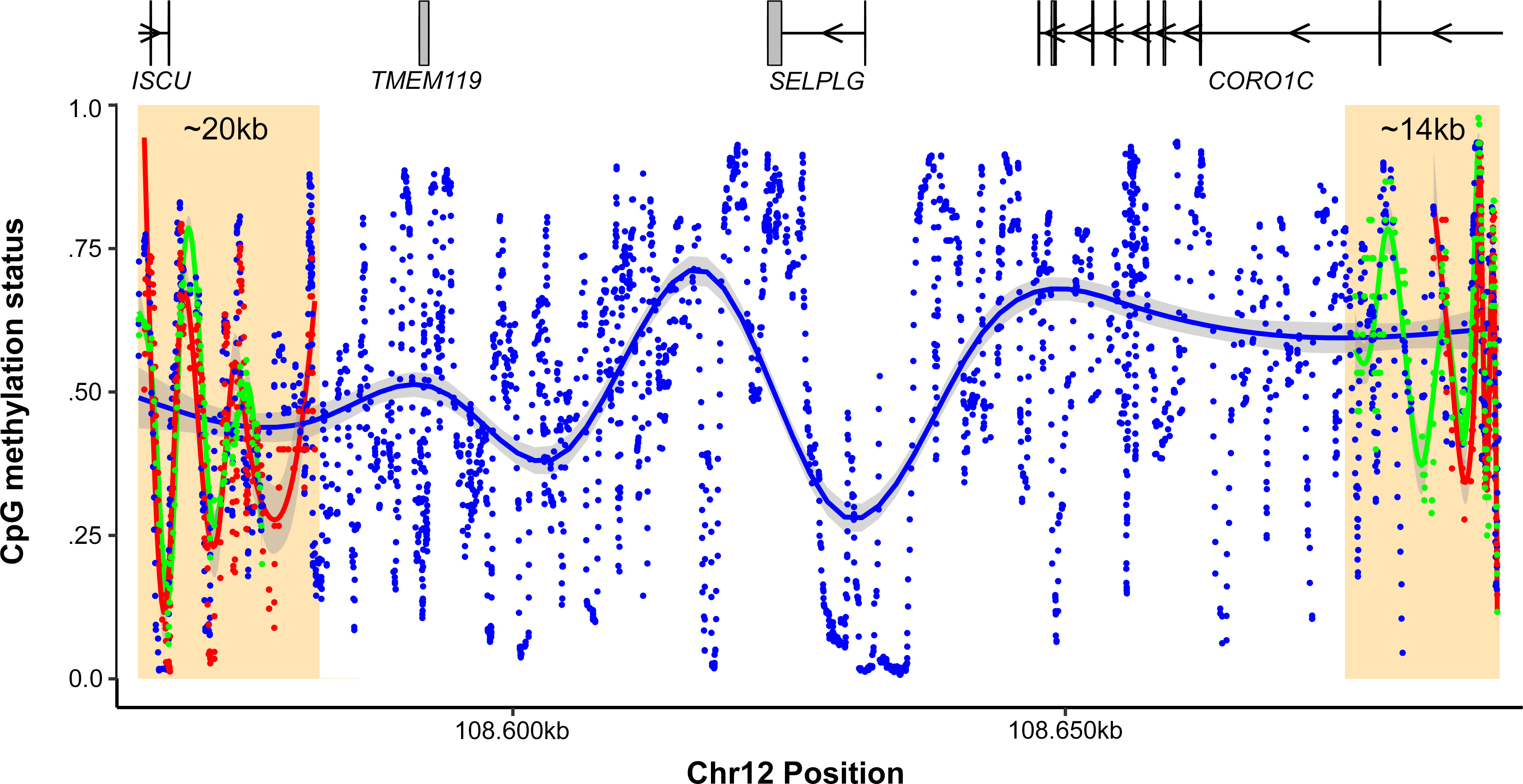
Figure 2 CpG methylation status inference across the amplified 1.2 Mb region of Chr12. The Y-axis shows the methylation status of CpGs, while the X-axis shows genomic coordinates. Regions with pahsed reads with or without the virus over both junctions are highlighted in yellow rectangles. The blue points represent the average methylation status for all CpGs, while green and red dots represent reads with and without the insertion, respectively. To reduce noise, the data was smoothed using rolling average windows of 15 bp.
The findings presented in this study provide compelling evidence of a hijacking event by HHV-8, leading to subsequent amplification of functional genes from the host genome. Importantly, this is the first report documenting such a phenomenon for HHV-8, underscoring the significance of investigating the intricate interplay between HHV-8 and the host cell cycle in order to gain insights into the pathogenesis of HHV-8-associated cancers. This raises the pertinent question of whether this integration stemmed from a sporadic event during the course of the cell line’s culturing or rather priorly along the original patient, in which case it could’ve played a role during the tumoral initiation or its evolution, highlighting the need for further investigations to clarify this question. Indeed, the abundance and perpetuation of this amplification through successive independent cultures since its first initial observation in 2010 (17) could imply selective pressures promoting the maintenance of this event and by which the virus could take advantage from. In these regards, recent studies indicate that overexpression of CORO1C concurs with increased cell proliferation, invasiveness and metastasis in various cancers (24–27) and acts as a bad prognostic marker in liver cancer (28, 29), while overexpression of SELPLG has been associated to unfavorable prognosis in renal cancer (28, 30) and osteosarcoma (31). Understanding the specific roles and mechanisms by which these chimeras contribute to the pathogenesis of HHV-8-associated cancers will provide crucial insights for the development of effective therapeutic strategies.
Moreover, this study contributes to the expanding body of knowledge on the role of extrachromosomal covalently closed circular DNAs (eccDNAs) in both cancer and virology. By elucidating the involvement of eccDNAs in the amplification and rearrangement of functional genes, our findings shed light on the complex genomic alterations associated with HHV-8 infection. Overall, this work advances our understanding of the intricate interactions between HHV-8 and the host genome, and their implications in the development of HHV-8-associated cancers. Further investigations are warranted to uncover the full extent of these HHV-8-human chimeras in a larger dataset including cell lines and primary tumors, as well as the possible mechanisms that originated them.
Data availability statement
The datasets presented in this study can be found in online repositories. The names of the repository/repositories and accession number(s) can be found below: BioProject, PRJNA998843.
Ethics statement
Ethical approval was not required for the studies on humans in accordance with the local legislation and institutional requirements because only commercially available established cell lines were used.
Author contributions
EÁ: Conceptualization, Data curation, Formal Analysis, Investigation, Software, Writing – original draft, Writing – review & editing. PO: Conceptualization, Data curation, Formal Analysis, Investigation, Methodology, Software, Supervision, Writing – original draft, Writing – review & editing. BR-M: Conceptualization, Formal Analysis, Investigation, Software, Writing – review & editing. AP-V: Methodology, Resources, Supervision, Validation, Writing – review & editing. AV-C: Formal Analysis, Investigation, Methodology, Resources, Writing – original draft, Writing – review & editing. IO: Writing – review & editing, Data curation, Software. JR-C: Investigation, Methodology, Resources, Supervision, Validation, Writing – review & editing. JP: Investigation, Methodology, Resources, Validation, Writing – review & editing. CR: Investigation, Methodology, Resources, Validation, Writing – review & editing. JT: Conceptualization, Funding acquisition, Investigation, Project administration, Resources, Writing – review & editing. DG-S: Supervision, Validation, Visualization, Writing – original draft, Writing – review & editing, Conceptualization, Data curation, Formal Analysis, Investigation, Methodology, Project administration.
Funding
EÁ acknowledges support from the Ministerio de Ciencia e Innovación through the FPU17/05396 grant. PO acknowledges funding from the Ministerio de Ciencia e Innovación through the FPU18/03421 grant. DG-S acknowledges support from the Consellería de Cultura, Educación e Ordenación Universitaria, Xunta de Galicia, under the grant ED481D-2022-001. JT acknowledges funding from the Ministerio de Ciencia e Innovación through the PID2021-126493OB-I00 grant.
Acknowledgments
The cell line present in this study was purchased from DSMZ, the German Collection of Microorganisms and Cell Cultures GmbH.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The authors DG-S and JP declared that they were editorial board members of Frontiers, at the time of submission. This had no impact on the peer review process and the final decision.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Soulier J, Grollet L, Oksenhendler E, Cacoub P, Cazals-Hatem D, Babinet P, et al. Kaposi’s sarcoma-associated herpesvirus-like DNA sequences in multicentric Castleman’s disease. Blood (1995) 86(4):1276–80. doi: 10.1182/blood.V86.4.1276.bloodjournal8641276
2. Cesarman E, Chang Y, Moore PS, Said JW, Knowles DM. Kaposi’s sarcoma-associated herpesvirus-like DNA sequences in AIDS-related body-cavity-based lymphomas. N Engl J Med (1995) 332(18):1186–91. doi: 10.1056/NEJM199505043321802
3. Shinohara H, Fukushi M, Higuchi M, Oie M, Hoshi O, Ushiki T, et al. Chromosome binding site of latency-associated nuclear antigen of Kaposi’s sarcoma-associated herpesvirus is essential for persistent episome maintenance and is functionally replaced by histone H1. J Virol (2002) 76(24):12917–24. doi: 10.1128/jvi.76.24.12917-12924.2002
4. Zhang H, Ni G, Damania B. ADAR1 facilitates KSHV lytic reactivation by modulating the RLR-dependent signaling pathway. Cell Rep (2020) 31(4):107564. doi: 10.1016/j.celrep.2020.107564
5. Friborg J Jr, Kong W, Hottiger MO, Nabel GJ. p53 inhibition by the LANA protein of KSHV protects against cell death. Nature (1999) 402(6764):889–94. doi: 10.1038/47266
6. Surviladze Z, Sterk RT, DeHaro SA, Ozbun MA. Cellular entry of human papillomavirus type 16 involves activation of the phosphatidylinositol 3-kinase/Akt/mTOR pathway and inhibition of autophagy. J Virol (2013) 87(5):2508–17. doi: 10.1128/JVI.02319-12
7. Gillespie KA, Mehta KP, Laimins LA, Moody CA. Human papillomaviruses recruit cellular DNA repair and homologous recombination factors to viral replication centers. J Virol (2012) 86(17):9520–6. doi: 10.1128/JVI.00247-12
8. Shackelton LA, Holmes EC. The evolution of large DNA viruses: combining genomic information of viruses and their hosts. Trends Microbiol (2004) 12(10):458–65. doi: 10.1016/j.tim.2004.08.005
9. Álvarez EG, Demeulemeester J, Otero P, Jolly C, García-Souto D, Pequeño-Valtierra A, et al. Aberrant integration of Hepatitis B virus DNA promotes major restructuring of human hepatocellular carcinoma genome architecture. Nat Commun (2021) 12(1):6910. doi: 10.1038/s41467-021-26805-8
10. Deshpande V, Luebeck J, Nguyen ND, Bakhtiari M, Turner KM, Schwab R, et al. Exploring the landscape of focal amplifications in cancer using AmpliconArchitect. Nat Commun (2019) 10(1):392. doi: 10.1038/s41467-018-08200-y
11. Møller HD, Bojsen RK, Tachibana C, Parsons L, Botstein D, Regenberg B. Genome-wide purification of extrachromosomal circular DNA from eukaryotic cells. J Vis Exp (2016) 110:e54239. doi: 10.3791/54239
12. Schneider CA, Rasband WS, Eliceiri KW. NIH Image to ImageJ: 25 years of image analysis. Nat Methods (2012) 9(7):671–5. doi: 10.1038/nmeth.2089
13. Schempp W, Meer B. Cytologic evidence for three human X-chromosomal segments escaping inactivation. Hum Genet (1983) 63:171–4. doi: 10.1007/BF00291539
14. Liehr T, Kreskowski K, Ziegler M, Piaszinski K, Rittscher K. standard FISH procedure. In: Liehr T, editor. Fluorescence in situ hybridization (FISH). Springer protocols handbooks. Berlin, Heidelberg: Springer (2017).
15. Yan J, Gadji M, Krabchi K, Drouin R. New rapid multicolor PRINS protocol. Methods Mol Biol (2006) 334:3–13. doi: 10.1385/1-59745-068-5:3
16. Kumar A, Lyu Y, Yanagihashi Y, Chantarasrivong C, Majerciak V, Salemi M, et al. KSHV episome tethering sites on host chromosomes and regulation of latency-lytic switch by CHD4. Cell Rep (2022) 39(6):110788. doi: 10.1016/j.celrep.2022.110788
17. Luan SL, Boulanger E, Ye H, Chanudet E, Johnson N, Hamoudi RA, et al. Primary effusion lymphoma: genomic profiling revealed amplification of SELPLG and CORO1C encoding for proteins important for cell migration. J Pathol (2010) 222(2):166–79. doi: 10.1002/path.2752
18. Wang Y, Wang M, Djekidel MN, Chen H, Liu D, Alt FW, et al. eccDNAs are apoptotic products with high innate immunostimulatory activity. Nature (2011) 599(7884):308–14. doi: 10.1038/s41586-021-04009-w
19. Chen Y, Qiu Q, She J, Yu J. Extrachromosomal circular DNA in colorectal cancer: biogenesis, function and potential as therapeutic target. Oncogene (2023) 42(13):941–51. doi: 10.1038/s41388-023-02640-7
20. Peng L, Zhou N, Zhang CY, Li GC, Yuan XQ. eccDNAdb: a database of extrachromosomal circular DNA profiles in human cancers. Oncogene (2022) 41(19):2696–705. doi: 10.1038/s41388-022-02286-x
21. Wong JP, Stuhlmiller TJ, Giffin LC, Lin C, Bigi R, Zhao J, et al. Kinome profiling of non-Hodgkin lymphoma identifies Tyro3 as a therapeutic target in primary effusion lymphoma. Proc Natl Acad Sci USA (2019) 116(33):16541–50. doi: 10.1073/pnas.1903991116
22. Gonçalves E, Poulos RC, Cai Z, Barthorpe S, Manda SS, Lucas N, et al. Pan-cancer proteomic map of 949 human cell lines. Cancer Cell (2022) 40(8):835–49. doi: 10.1016/j.ccell.2022.06.010
23. Pang J, Nguyen N, Luebeck J, Ball L, Finegersh A, Ren S, et al. Extrachromosomal DNA in HPV-mediated oropharyngeal cancer drives diverse oncogene transcription. Clin Cancer Res (2021) 27(24):6772–86. doi: 10.1158/1078-0432.CCR-21-2484
24. Wu L, Hou JX, Peng CW, Zhang YH, Chen C, Chen LD, et al. Increased coronin-1C expression is related to hepatocellular carcinoma invasion and metastasis. Zhonghua Gan Zang Bing Za Zhi (2010) 18:516–9. doi: 10.3760/CMA.J.ISSN.1007-3418.2010.07.011
25. Han S, Ding X, Wang S, Xu L, Li W, Sun W. Mir-133a-3p regulates hepatocellular carcinoma progression through targeting coro1c. Cmar (2020) 12:8685–93. doi: 10.2147/CMAR.S254617
26. Liao M, Peng L. MiR-206 may suppress non-small lung cancer metastasis by targeting CORO1C. Cell Mol Biol Lett (2020) 25:22. doi: 10.1186/s11658-020-00216-x
27. Wang Z, Jia L, Sun Y, Li C, Zhang L, Wang X, et al. CORO1C is associated with poor prognosis and promotes metastasis through PI3K/AKT pathway in colorectal cancer. Front Mol Biosci (2021) 8:682594. doi: 10.3389/fmolb.2021.682594
28. Uhlen U, Zhang Z, Lee L, Sjöstedt S, Fagerberg F, Bidkhori B, et al. A pathology atlas of the human cancer transcriptome. Science (2017) 357(6352):eaan2507. doi: 10.1126/science.aan2507
29. Coro1c protein expression summary - the human protein atlas. Available at: https://www.proteinatlas.org/ENSG00000110880-CORO1C (Accessed August 28, 2023).
30. Selplg protein expression summary - the human protein atlas. Available at: https://www.proteinatlas.org/ENSG00000110876-SELPLG (Accessed August 28, 2023).
Keywords: HHV-8, extrachromosomal chimeric circular DNA, primary effusion lymphoma, long-read sequencing, cancer
Citation: Álvarez EG, Otero P, Rodríguez-Martín B, Pequeño-Valtierra A, Otero I, Vidal-Capón A, Rodríguez-Castro J, Pasantes JJ, Rivas C, Tubío JMC and García-Souto D (2023) Characterization of Human Herpesvirus 8 genomic integration and amplification events in a primary effusion lymphoma cell line. Front. Virol. 3:1253416. doi: 10.3389/fviro.2023.1253416
Received: 05 July 2023; Accepted: 14 September 2023;
Published: 02 October 2023.
Edited by:
Diego Forni, Scientific Institute for Research, Hospitalization and Healthcare (IRCCS), ItalyReviewed by:
Sunantha Sethuraman, Repare Therapeutics Inc., United StatesKaterina Strati, University of Cyprus, Cyprus
Lei Yang, Pacific Northwest Research Institute (PNRI), United States
Copyright © 2023 Álvarez, Otero, Rodríguez-Martín, Pequeño-Valtierra, Otero, Vidal-Capón, Rodríguez-Castro, Pasantes, Rivas, Tubío and García-Souto. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Jose M.C. Tubío, jose.mc.tubio@usc.es; Daniel García-Souto, daniel.garcia.souto@usc.es
†These authors have contributed equally to this work and share first authorship
‡These authors have contributed equally to this work and share last authorship
 Eva G. Álvarez1,2,3†
Eva G. Álvarez1,2,3†  Paula Otero
Paula Otero Bernardo Rodríguez-Martín
Bernardo Rodríguez-Martín André Vidal-Capón
André Vidal-Capón Jorge Rodríguez-Castro
Jorge Rodríguez-Castro Juan J. Pasantes
Juan J. Pasantes Carmen Rivas
Carmen Rivas Daniel García-Souto
Daniel García-Souto