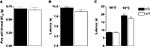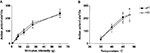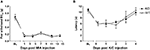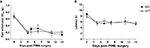1
Neuroscience, Physiology & Pharmacology, University College London, London, UK
2
Department of Pharmacology, The School of Pharmacy, University of London, London, UK
3
Institute for Pharmacy and Molecular Biotechnology, University of Heidelberg, Heidelberg, Germany
GlyR α3 has previously been found to play a critical role in pain hypersensitivity following spinal PGE2 injection, complete Freund’s adjuvant (CFA) and zymosan induced peripheral inflammation. In this study, although all models displayed typical phenotypic behaviours, no significant differences were observed when comparing the pain behaviours of Glra3−/− and wild-type littermates following the injection of capsaicin, carrageenan, kaolin/carrageenan or monosodium iodoacetate, models of rheumatoid and osteoarthritis, respectively. However, clear differences were observed following CFA injection (p < 0.01). No significant differences were observed in the pain behaviours of Glra3−/− and wild-type littermates following experimentally induced neuropathic pain (partial sciatic nerve ligation). Similarly, Glra3−/− and wild-type littermates displayed indistinguishable visceromotor responses to colorectal distension (a model of visceral pain) and in vivo spinal cord dorsal horn electrophysiology revealed no differences in responses to multimodal suprathreshold stimuli, intensities which equate to higher pain scores such as those reported in the clinic. These data suggest that apart from its clear role in CFA- and zymosan-induced pain sensitisation, hypersensitivity associated with other models of inflammation, neuropathy and visceral disturbances involves mechanisms other than the EP2 receptor – GlyR α3 pathway.
Despite recent developments in the understanding of pain mechanisms and analgesia, the relief of pain, particularly that which lasts over long periods, remains a principal concern to healthcare professionals. Chronic pain can be broadly categorised into three types: inflammatory, neuropathic and dysfunctional. Inflammatory pain, arising from tissue damage caused by trauma or infection, is associated with conditions such as arthritis. Neuropathic pain, arising from trauma to, or pathological changes in, the peripheral or central nervous systems can occur as a consequence of stroke, ischaemia, diabetes or nerve infection (e.g. HIV, shingles, etc.). The third type of pain is underpinned by neural dysfunction and is thought to be associated with conditions such as irritable bowel syndrome (IBS), fibromyalgia and migraine which manifest as non-localised diffuse pain unaccompanied by either inflammation or nerve damage.
Tissue damage precipitates the release of multifarious endogenous pro-inflammatory mediators at sites in both the periphery and the CNS (mainly at the level of the spinal cord). Peripheral release of these mediators, namely prostaglandin E2 (PGE2), leads to the sensitization of primary afferents, thereby lowering thresholds for neuronal activation, and increasing nociceptor responsivity. This increased peripheral activity contributes to spinal hypersensitivity (central sensitisation) resulting in increased responsivity to noxious stimulation termed hyperalgesia, and allodynia where normally non-noxious stimuli such as cooling, gentle touch, movement and pressure are now perceived as being painful. Central prostanoid release can induce spinal disinhibition, relieving dorsal horn neurones from inhibitory control via spinal interneurones, thus facilitating the transmission of nociceptive information (Ahmadi et al., 2002
; Reinold et al., 2005
). One such mechanism of spinal disinhibition occurs through the postsynaptic activation of G-protein-coupled EP2 receptors, and subsequent protein kinase A induced inhibition of α3 subunit containing glycine receptors, present in the superficial laminae of the spinal cord (Harvey et al., 2004
).
GlyR α3 subunit knockout mice (Glra3−/−) allow us to probe the function of this ligand-gated ion channel in the absence of any subtype-selective ligands. Previously, a role for GlyR α3 in inflammatory pain has been demonstrated in the CFA and zymosan A models of peripheral inflammation (Harvey et al., 2004
). By contrast, GlyR α3 appears to have little or no role in neuropathic pain and the formalin test (Racz et al., 2005
; Hösl et al., 2006
). In this study, we have investigated the nociceptive phenotypes of GlyR α3 knockout mice in acute models of inflammation and clinically relevant models of persistent inflammatory, neuropathic and visceral pain. Moreover, we investigated the role of GlyR α3 in response to multi-modal suprathreshold stimuli-evoking responses, which equate to higher pain scores experienced by human pain patients.
Animals
Glra3−/− mice on a C57Bl/6 background were produced as described (Harvey et al., 2004
). Glra3−/− mice and their wild-type littermates were group housed on a standard 12-h light/dark cycle with food and water available ad libitum except during behavioural testing. Experiments were performed on animals of at least 8 weeks of age. For all the experiments detailed in this study, the observer was blinded with respect to mouse genotype. All experiments were carried out in accordance with the UK Home Office Animals Scientific procedures Act (1986).
Induction and Behavioural Analysis of Pain States
Glra3−/− and wild-type littermates received either unilateral intraplantar injections of 1 mg/ml complete Freund’s adjuvant (CFA, 20 μl), 2% (w/v) carrageenan (20 μl) or 50 μg/ml capsaicin (5 μl). Models of rheumatoid arthritis and osteoarthritis were induced by unilateral intra-articular injections of 3% (w/v) kaolin/carrageenan (K/C; 15 μl), or 5 mg/ml monosodium iodoacetate (MIA, 10 μl; Harvey and Dickenson, 2009
), respectively. Neuropathic (partial sciatic nerve ligation, PSNL) mice were prepared by unilateral tight ligation of 1/3 to 1/2 of the sciatic nerve using 7-0 silk under isofluorane/O2 anaesthesia (Malmberg and Basbaum, 1998
). Behavioural thermal hypersensitivity (thermal hyperalgesia) was assessed using the Hargreaves’ test and the hot plate, and mechanical hypersensitivity (mechanical allodynia) was assessed using von Frey filaments as described (Harvey and Dickenson, 2009
). Two baseline measurements were recorded prior to the induction of the pain models. Although both contralateral and ipsilateral limbs were tested, only ipsilateral data are shown for clarity since contralateral data were not significantly different from baseline following the induction of any pain model (p > 0.05). Capsaicin-induced acute spontaneous pain behaviours, measured as either biting, flinching, or licking, were measured for 3 min.
Spinal Cord Electrophysiology
Following a laminectomy (L3–L6) in vivo electrophysiology was performed in urethane anesthetised (240 mg/kg) Glra3−/− and wild-type mice using parylene coated tungsten electrodes (A-M Systems, USA), as previously described (Harvey and Dickenson, 2009
). Evoked neuronal responses were recorded from single wide dynamic range dorsal horn neurones receiving inputs from the hindpaw. Transcutaneous electrical stimuli consisted of a train of 16 electrical stimuli (2 ms wide pulses, 0.5 Hz, 3 × C fibre threshold), were delivered by means of pins inserted in to the hindpaw. Post stimulus time histograms were constructed and fibre responses were separated according to the following latencies: A (0–50 ms), C (50–250 ms) and post discharge (250–800 ms). Input (i.e. non-potentiated response) is measured as the number of action potentials in response to the initial stimulus × 16 (total number of stimuli). Wind up values, a measure of the temporal summation of neuronal activity in response to a train of stimuli, was calculated as the total number of action potentials evoked at the end of 16 stimuli minus the input. A wide range of natural stimuli including brush (dynamic mechanical) acetone (innocuous cold), and graded punctate mechanical and thermal stimuli applied using von Frey filaments and a water jet, respectively, were applied to the hindpaw for a period of 10 s. Data were captured and analysed using a CED 1401 interface coupled to a Pentium computer running Spike 2 software (Cambridge Electronic Design).
Acute Visceral Nociception
Visceral nociception was assessed using a method adapted from Kamp et al. (2003)
. Under isofluorane anaesthesia (2.5%, delivered in O2), an electromyographic (EMG) electrode (insulated copper wire, exposed at the ends and 1 cm centrally) was sewn into the external oblique muscle. A latex balloon (20 mm) attached to a pressure transducer via polyethylene tubing, with the distal 15 mm perforated using a 27-gauge needle to facilitate balloon inflation, was inserted intra-anally (25 mm), and secured by surgical tape to the base of the tail. The anaesthesia was gradually reduced to ∼1% isofluorane, and allowed to equilibrate for at least 30 min, such that the animals did not display spontaneous movement but responded to cutaneous hindpaw stimulation. EMG responses were filtered, amplified and recorded in 10 s prior to and during colorectal distensions (10 s) of 10–60 mmHg applied at 4 min intervals. Two consecutive sets of CRD were recorded and averaged. Data are presented relative to the mean wild type maximum response (60 mmHg).
Acute Nociception
Pain thresholds to punctate mechanical stimuli were measured in Glra3−/− mice and wild-type littermates using calibrated von Frey hairs, according to the up–down method (Chaplan et al., 1994
). Paw withdrawal thresholds to mechanical stimuli were not significantly different between groups (p > 0.05; Figure 1
A). Similarly, thermal thresholds assessed using the Hargreaves’ test were indistinguishable between groups (p > 0.05; Figure 1
B). Thermal hypersensitivities were similar across genotypes when measured using the hot plate at either 50°C or 55°C (p > 0.05; Figure 1
C). This absence of an acute phenotype in Glra3−/− mice agrees with previous findings (Harvey et al., 2004
).
Figure 1. Glra3−/− mice show no differences in their acute pain behaviours. (A) Punctate mechanical stimulation using calibrated von Frey (Glra3−/−, n = 16; wild type, n = 10). Thermal stimulation using either (B) the Hargreaves’ apparatus (Glra3−/−, n = 30; wild type, n = 19) or (C) the hot plate at 50°C or 55°C (Glra3−/−, n = 12; wild type, n = 12). Data are presented as mean ± SEM.
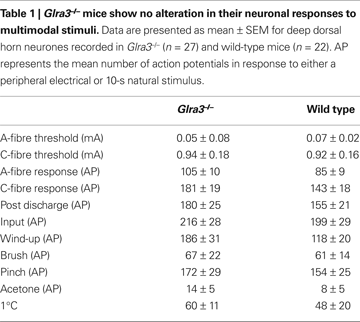
Spinal cord electrophysiology was performed to assess the potential role of GlyR α3 in acute nociception in response to multimodal stimuli. Where behaviour lends information concerning threshold intensities, electrophysiology can be used to inform us about responses to suprathreshold stimuli. Single unit dorsal horn neuronal recordings were performed in Glra3−/− (n = 27) and wild-type littermates (n = 22). The mean depths from the surface of the spinal cord were not significantly different between groups and correspond to the deep laminae of the spinal cord 550 ± 30 μm and 459 ± 38 μm (p > 0.05), respectively ensuring that the population of neurons was similar between the two groups, and hence direct comparisons were possible. Responses to electrical, brush, acetone, noxious cold and pinch (Table 1
); as well as graded mechanical and thermal stimuli (Figures 2
A,B) were indistinguishable between genotypes (p > 0.05).
Figure 2. Glra3−/− mice show no differences in evoked dorsal horn neuronal responses. (A) Graded mechanical stimuli (von Frey); (B) graded thermal stimuli (water jet). Data are presented as mean ± SEM (Glra3−/−, n = 27; wild type, n = 22).
Inflammatory Pain Behaviour
Consistent with previously reported findings (Harvey et al., 2004
) Glra3−/− mice displayed showed no difference in the induction of thermal pain sensitisation following CFA (20 μl) injection, but recovered much more quickly compared with wild-type littermate controls (Figure 3
A). Paw withdrawal latencies to thermal stimulation using the Hargreaves’ apparatus were significant versus contralateral responses for wild-type mice days 1–6 and days 1–3 for Glra3−/− mice (p < 0.001). Consistent with previous findings, Glra3−/− mice (n = 7) displayed significant differences in thermal hypersensitivity at 4, 5 and 6 days following CFA injection compared with wild-type littermates (n = 5, p < 0.01).
Figure 3. Glra3−/− mice display attenuated CFA behaviour, but show no differences in other inflammatory models. (A) Paw withdrawal latencies following unilateral intraplantar CFA (20 μl) were significant versus contralateral responses for wild-type mice days 1–6 and days 1–3 for Glra3−/− mice (p < 0.001). Paw withdrawal latencies of Glra3−/− mice were significantly different from wild-type mice at days 4 (p < 0.01, **), 5 and 6 (p < 0.001, ***) (Glra3−/−, n = 7; wild type, n = 5). (B) Total nociceptive behaviour following unilateral intraplantar capsaicin (5 μl, 50 μg/ml) was not significantly different between genotypes (Glra3−/−, n = 12; wild type, n = 12). (C) Significant mechanical hypersensitivity and (D) thermal hypersensitivity was observed at all timepoints following unilateral intraplantar carrageenan (20 μl, 2% w/v) when compared with contralateral controls (p < 0.05 –p < 0.001) but indistinguishable between genotypes (Glra3−/−, n = 5; wild type, n = 7). Data are presented as mean ± SEM and analysed using two-way repeated measures ANOVA, Bonferroni post hoc analysis.
Acute cutaneous neurogenic inflammation induced by intradermal injection of capsaicin (5 μl; 50 μg/ml) elicited spontaneous pain behaviour in both Glra3−/− and wild-type control mice (Figure 3
B). No significant differences were observed between the type of nociceptive behaviour (i.e. licking, biting and flinching) or the duration of such responses (p > 0.05).
Intraplantar injection of carrageenan (20 μl; 2% w/v) elicited significant thermal and mechanical hypersensitivities in both Glra3−/− and wild-type control mice at both acute (2–6 h), and sub-acute phases (24 h) (p < 0.05–p < 0.001; Glra3−/−, n = 5; wild type, n = 7; Figures 3
C,D). Statistical analysis revealed no significant difference in Glra3−/− mice in either the magnitude or duration of thermal and mechanical hypersensitivities following carrageenan injection when compared with wild-type control littermates (p > 0.05).
Clinically relevant models of inflammation
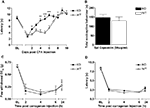
Intra-articular injection of monosodium iodoacetate (MIA, 7 μl; 5 mg/ml) to induce osteoarthritis (OA) evoked significant behavioural mechanical hypersensitivitiy in both Glra3−/− and wild-type control mice when compared with contralateral controls (n = 6; p < 0.001; Figure 4
A). Thermal hypersensitivity was not explored since this has been found to be absent in this model (Harvey and Dickenson, 2009
). Similarly, a model of rheumatoid arthritis (kaolin/carrageenan; 15 μl, 3% w/v) evoked significant thermal hypersensitivity in both Glra3−/− and wild-type control mice when compared with contralateral controls (n = 6; p < 0.05; Figure 4
B). No significant differences were observed between genotypes.
Figure 4. Glra3−/− mice show no differences in behavioural hypersensitivity in models of osteoarthritis or rheumatoid arthritis. (A) Significant mechanical hypersensitivity was observed at all timepoints following unilateral intra-articular MIA (10 μl, 5 mg/ml) in both genotypes when compared with contralateral controls (n = 6; p < 0.001). (B) Significant thermal hypersensitivity was observed at days 1 and 2 following unilateral intra-articular K/C injection (15 μl, 3% w/v) in both genotypes when compared with contralateral controls (n = 6; p < 0.05). No differences were observed between genotypes. Data are presented as mean ± SEM and analysed using two-way repeated measures ANOVA, Bonferroni post hoc analysis.
Neuropathic Pain Behaviour
Following the induction of peripheral nerve injury using the partial sciatic nerve ligation model, both Glra3−/− and wild-type littermates developed robust and persistent mechanical and thermal hypersensitivities from the onset of testing (Figures 5
A,B). Significant mechanical hypersensitivity was observed in Glra3−/− mice at days 3, 5, 7 and 14 (p < 0.01–p < 0.001) and wild-type mice at days 3–14 (p < 0.05–p < 0.01) following surgery compared with contralateral controls (n = 6). Significant thermal hypersensitivity was observed in Glra3−/− mice and wild-type mice at all days following surgery (p < 0.001) compared with contralateral controls (n = 6). Statistical analysis revealed no significant difference in Glra3−/− mice in either the magnitude or duration of thermal and mechanical hypersensitivities following the induction of nerve injury when compared with wild-type control littermates (p > 0.05).
Figure 5. Glra3−/− mice show no differences in behavioural hypersensitivitiy following experimentally induced peripheral neuropathy. (A) Significant mechanical hypersensitivity was observed in Glra3−/− mice at days 3, 5, 7 and 14 (p < 0.01–p < 0.001) and wild-type mice at days 3–14 (p < 0.05–p < 0.01) following surgery compared with contralateral controls (n = 6). (B) Significant thermal hypersensitivity was observed in Glra3−/− mice and wild-type mice at all days following surgery (p < 0.001) compared with contralateral controls (n = 6). No differences were observed between genotypes. Data are presented as mean ± SEM and analysed using two-way repeated measures ANOVA, Bonferroni post hoc analysis.
Visceral Pain
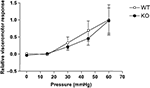
The visceromotor response (VMR), evoked by the distension of the colon by a balloon inserted intra-colonically, provides a quantifiable measure of visceral pain (visceral hyperalgesia). In rodents, pressures which elicit pain in humans evoke graded abdominal muscle contractions (measured as the VMR), tachycardia and the activation of primary afferent visceral nerves (e.g. pelvic nerve) and subsequent activation of second order projection neurones (Kamp et al., 2003
). The response measured at 0 mmHg (with the balloon deflated) was considered as the baseline response; 30 mmHg was considered mildly noxious and 60 mmHg as significantly noxious. Both wild-type and Glra3−/− mice displayed coded responses to the graded stimuli (Figure 6
), however, the responses were not significantly different between the groups (p > 0.05).
Figure 6. Glra3−/− mice show no differences in visceromotor responses to colorectal distension. Isoflurane anesthetised mice display graded visceromotor responses to increasing colorectal pressures. No differences were observed between genotypes (Glra3−/−, n = 5; wild type, n = 7). Data are presented as mean (normalised to maximum wild-type control) ± SEM.
This study confirms, consistent with previous findings, that GlyR α3 has a significant role in certain inflammatory pain states. In this study, we have used knockout mice to investigate the role of GlyR α3 in a variety of acute, persistent and clinically relevant behavioural pain models as well as neuropathic and visceral pain models. Moreover, in vivo electrophysiology has been used to investigate the potential role of these channels in response to suprathreshold stimuli, which equate to intensities that produce high pain scores such as those seen in the clinic.
Acute Pain Thresholds
Consistent with previous findings, acute behavioural thermal and mechanical thresholds in Glra3−/− mice were unaltered (Harvey et al., 2004
). These behavioural findings are re-enforced by the electrophysiological characterisation of Glra3−/− mice to an array of different modalities ranging from threshold to suprathreshold intensities, suggesting that GlyR α3 is not implicated in acute nociception. These characteristics are advantageous when investigating potential therapeutic targets since ideal analgesic targets should be pain-state specific, leaving normal nociceptive processing and thus normal sensation unaltered.
Inflammatory Pain Models
Although the role of GlyR α3 in inflammatory pain has been previously investigated in selected models, this study expands on the current work by investigating its potential role in other inflammatory pain states including capsaicin and carrageenan inflammation and clinically relevant models such as the kaolin/carrageenan (K/C) model of rheumatoid arthritis and the monosodium iodoacetate model of osteoarthritis. CFA-induced behavioural hypersensitivity was reconfirmed in this study, where Glra3−/− mice displayed an accelerated rate of recovery, in line with previous findings (Harvey et al., 2004
). Since GlyR α3 displayed this inflammatory phenotype, its potential role in other models of inflammation was investigated.
Intraplantar capsaicin elicits immediate spontaneous pain behaviour in rodents through the activation of the vanilloid receptor 1 (VR1/TRPV1). This evoked nocifensive behaviour is thought to arise from the direct activation of C-fibres rather than as a consequence of local inflammation per se. Although capsaicin-induced C fibre activation has previously been found to elicit prostaglandin E2 release in spinal cord slices (Malmberg and Yaksh, 1994
), the exact spinal mechanisms have not been fully established. In this study, no behavioural phenotype was observed following intraplantar capsaicin injection in Glra3−/− mice.
Intraplantar injection of carrageenan evokes both mechanical and thermal hypersensitivity in rodents. These behaviours can be subdivided into an acute phase (2–6 h) and sub-acute phase (24 h) (Kolhekar et al., 1997
). Prostaglandin synthesis occurs both at peripheral and spinal sites since both systemic and intrathecal cyclo-oxygenase (COX) inhibition attenuates nocifensive behaviour. However, intrathecal COX inhibitors were only effective when given prior to carrageenan injection (Dirig et al., 1998
). A later study found that spinal CSF levels of PGE2 peaked in the acute phase but remained significantly elevated up to 24 h (Guay et al., 2004
). Taken together, this suggests that there is potentially only a limited time frame where spinal PGE2 is contributing to the pain behaviours, whereas peripheral or supraspinal sites are involved in both the induction and maintenance of carrageenan-evoked pain behaviours. In this study, no phenotype was observed in either the acute or sub-acute phase of carrageenan behavioural hypersensitivity in Glra3−/− mice. Therefore, although spinal prostaglandins are involved in the induction, and to a lesser extent the maintenance of this model, the EP2 receptor – GlyR α3 signalling pathway is unlikely to represent the downstream substrate.
Arthritic Pain Models
Arthritic pain, as a result of either rheumatoid arthritis or osteoarthritis, represents a major chronic healthcare problem escalating with our aging and obese population (Neugebauer et al., 2007
). Although different models exist for their study, the chemical models of MIA, for osteoarthritis, and K/C for rheumatoid arthritis, are popular among pain researchers. In this study, mice displayed significant behavioural hypersensitivity in the paw of the affected limb in both arthritic models, consistent with other literature (Yang et al., 1996
; Harvey and Dickenson, 2009
). Similar to other peripheral inflammatory models, spinal prostanoids are thought to contribute largely to the observed pain behaviours. Infrapatellar K/C injection leads to a robust and persistent (up to 72 h) increase in spinal PGE2 levels in rats (Dirig and Yaksh, 1999
). In the MIA model of osteoarthritis, NSAIDs are only effective at the early stages of the model, suggesting a switch in the underlying mechanisms at later time points although these have not yet been fully established (Bove et al., 2003
; Pomonis et al., 2005
; Vonsy et al., 2008
). In this study, it would appear that despite good evidence for central prostanoid release, GlyR α3 is not implicated in the underlying pain pathophysiology.
Neuropathic Pain
The benefits of using NSAIDs to treat neuropathic pain remains controversial, and at best they may only be effective at very early stages following the nerve insult (Ma et al., 2002
; Schafers et al., 2004
). In this study, analysis of the pain behaviours following the induction of peripheral nerve injury using the partial sciatic nerve injury model revealed that Glra3−/− mice developed profound mechanical and thermal hypersensitivities indistinguishable from wild-type littermates. This is in accordance with previous studies investigating the role of GlyR α3 in the chronic constriction injury model of neuropathic pain (Racz et al., 2005
; Hösl et al., 2006
). It is apparent, therefore, that unlike its clear role in certain types of inflammatory pain, GlyR α3 is not critical for the induction or maintenance of neuropathic pain.
Visceral Pain
Bowel disorders such as irritable bowel syndrome (IBS) are characterised by poorly localised abdominal pain and sufferers display visceral hypersensitivity to experimental colorectal distension compared with healthy volunteers (Verne et al., 2001
). To investigate the potential role of GlyR α3 in such bowel complaints we used a method of colorectal distension which produces a quantifiable visceromotor response in lightly anaesthetised mice. In this study, although the magnitude of the VMR was dependent on the applied pressure, no significant differences were observed between genotypes. This contrasts with other studies using the acetic acid writhing test, which reported a significant attenuation in the writhing responses in Glra3−/− mice (Racz et al., 2005
). This model, however, is often difficult to interpret as little is known about the pathophysiology following acetic acid injection. The acute testing regimen utilised in this study, however, may not be sufficiently long to involve the proposed EP2 receptor – GlyR α3 signalling pathway. A phenotype using the CRD model might be uncovered by future experiments involving colonic hypersensitivity where VMR are recorded in response to an irritant injected intra-colonically which enhance the VMR to both noxious and non-noxious intensities in rats (Coutinho et al., 1996
), although care must be taken to avoid general systemic effects (Deitch et al., 1992
).
Conclusion
Since the contribution of a particular molecular substrate to the nociceptive phenotype will most likely depend on the origin of the pain (e.g. inflammatory, neuropathic or dysfunctional), the temporal characteristics (i.e. acute or chronic), and the underlying sensitivity (peripheral or central) it is likely that different substrates underlie different pain states.
A selective role of GlyR α3 can be observed based on clear effects in the CFA model, yet there is no clear role in other inflammatory, neuropathic or visceral pain models. This study, therefore, suggests that GlyR α3 may play an important role in mediating PGE2-induced sensitisation, but only in certain pain states. Previously, GlyR α3 has been found not to contribute to behavioural hypersensitivity following formalin injection (Hösl et al., 2006
). Although this model, like most models of peripheral injury, evokes spinal PGE2 release and is attenuated by COX inhibitors, it most likely reflects a different downstream target since EP2 receptor knockout mice, but not Glra3−/− mice, displayed attenuated nocifensive behaviour. This could also hold true for the other inflammatory models investigated in this study and may point to the differential involvement of other prostanoids and EP receptors, located either centrally or peripherally, that may activate different molecular effectors (e.g. Matsumoto et al., 2005
; Hutchinson et al., 2009
). What is clear, however, is that different inflammatory pain states are underpinned by different molecular substrates such that their investigation will help to dissect out pain-state specific mechanisms. These observations may be reflected in different clinical phenotypes and, as such, a more mechanistic based approach may be required in the clinic.
The authors declare that the research was conducted in the absence of any commercial or financial relationship that could be construed as a potential conflict of interest.
Robert J. Harvey and Anthony H. Dickenson conceived the study, Victoria L. Harvey and Anthony H. Dickenson designed and performed the experiments, analysed data and wrote the manuscript with input from all authors. Alex Caley provided mouse genotyping; Robert J. Harvey and Ulrike C. Müller generated and supplied the Glra3−/− mice. This work was supported by MRC Project Grant (G0500833) to Robert J. Harvey and Anthony H. Dickenson.
Bove, S. E., Calcaterra, S. L., Brooker, R. M., Huber, C. M., Guzman, R. E., Juneau, P. L., Schrier, D. J., and Kilgore, K. S. (2003). Weight bearing as a measure of disease progression and efficacy of anti-inflammatory compounds in a model of monosodium iodoacetate-induced osteoarthritis. Osteoarthr. Cartil. 11, 821–830.
Harvey, R. J., Depner, U. B., Wässle, H., Ahmadi, S., Heindl, C., Reinold, H., Smart, T. G., Harvey, K., Schutz, B., Abo-Salem, O. M., Zimmer, A., Poisbeau, P., Welzl, H., Wolfer, D. P., Betz, H., Zeilhofer, H. U., and Müller, U. (2004). GlyR α3: an essential target for spinal PGE2-mediated inflammatory pain sensitization. Science 304, 884–887.
Hösl, K., Reinold, H., Harvey, R. J., Müller, U., Narumiya, S., and Zeilhofer, H. U. (2006). Spinal prostaglandin E receptors of the EP2 subtype and the glycine receptor α3 subunit, which mediate central inflammatory hyperalgesia, do not contribute to pain after peripheral nerve injury or formalin injection. Pain 126, 46–53.