Diversity in Habitat Use by White Sturgeon Revealed Using Fin Ray Geochemistry
- 1Cramer Fish Sciences, West Sacramento, CA, United States
- 2Institute of Marine Sciences, University of California, Santa Cruz, Santa Cruz, CA, United States
- 3Southwest Fisheries Science Center, National Marine Fisheries Service, Santa Cruz, CA, United States
- 4Department of Wildlife, Fish, and Conservation Biology, University of California, Davis, Davis, CA, United States
- 5California Department of Fish and Wildlife, Stockton, CA, United States
Understanding life-history diversity in a population is imperative to developing effective fisheries management and conservation practices, particularly in degraded environments with high environmental variability. Here, we examined variation in habitat use and migration patterns of White Sturgeon (Acipenser transmontanus), a long-lived migratory fish that is native to the San Francisco Estuary, CA, United States. Annual increment profiles were combined with respective geochemical (87Sr/86Sr) profiles in sturgeon fin rays to reconstruct annual salinity chronologies for 112 individuals from 5 to 30 years old. Results indicated a complex and diverse amphidromous life history across individuals, characterized largely by estuarine residence, a general ontogenetic trend toward higher-salinity brackish habitats, and high variability in habitat use across all age groups. Hierarchical clustering based on fin ray geochemistry during the first 10 years of life, prior to sexual maturation, indicated at least four distinct migratory phenotypes which differed largely in the timing and duration of juvenile to subadult movements between fresh- and brackish-water habitats. This study provides information regarding habitat use and migration in sub-adult fish that was previously lacking. Different migratory phenotypes vary in exposure to stressors across time and space and populations. Understanding White Sturgeon habitat distributions through space and time at different life stages can help identify areas where habitat restoration would be most effective and develop management actions to reduce stressors associated with specific areas where White Sturgeon are present.
Introduction
Many species have evolved diverse migratory behaviors to persist within dynamic environments (Lundberg et al., 1988; Schindler et al., 2010). For example, populations of fishes composed of both migratory and non-migratory phenotypes (i.e., partial migration) or exhibiting variation in habitat use patterns (i.e., habitat mosaics) can enhance stability and resilience to natural and anthropogenic disturbances (Greene et al., 2010; Schindler et al., 2010). Such phenotypic diversity can serve as a bet-hedging strategy that spreads risk and enhances resilience to environmental variation (Rochet, 2000; Schindler et al., 2010; Moore et al., 2014). Describing and translating such life history diversity into management-relevant tools remains key to developing effective fisheries management and conservation policies. For example, stocks or contingents that express different patterns likely occupy distinct habitats throughout their lives and thus experience different natural and anthropogenic stressors. Quantification and incorporation of complex life histories into management and conservation is needed in order to maximize population stability in the long-term (Greene et al., 2010; Schindler et al., 2010; Brennan et al., 2019).
Migratory fish management is often limited by a lack of information about how environmental variability and management actions intersect with natural complexity in habitat use, movements, and population status of a species (Nelson et al., 2013). This knowledge gap is largely due to the difficulty in reconstructing spatial distributions and habitat associations throughout individuals’ lifespans, especially for long-lived species that make large migrations between habitats (Grande et al., 2009; Blechschmidt et al., 2020). A poor understanding of migration and habitat use patterns for long-lived species makes it difficult to improve or maintain the quality of habitats essential to key life stages and can lead to unanticipated population crashes or extinction events whose causes can only be assessed after the fact (Brainwood et al., 2006; Spencer et al., 2018; Cramer et al., 2020). For example, long-lived species often mature late in life, reproduce infrequently, and are reliant on older-larger females to contribute disproportionately to future populations (Stearns, 1992; Caswell, 2000; Jager et al., 2008). However, spatial and temporal variation in fecundity, density dependence, and survival, can also drive population dynamics (Genovart et al., 2018). Given the speed and extent of anthropogenic environmental change, novel approaches are necessary to improve our understanding regarding complex patterns in habitat use and migration and to apply this knowledge to enhance management and conservation (Heppell et al., 2005; Nowacek et al., 2016; Parsons et al., 2018).
Sturgeons (Acipenseridae) are ancient chondrostean fishes that inhabit aquatic environments throughout the Northern Hemisphere (Birstein et al., 1997; Nelson et al., 2013). Individuals may live over 100 years, mature as late as age 20–25, and reproduce infrequently (Billard and Lecointre, 2001). This longevity, slow growth, and delayed maturation make sturgeons highly vulnerable to overfishing (Rieman and Beamesderfer, 1990; Rochard et al., 1990; Birstein, 1993). Furthermore, sturgeons spawn exclusively in freshwaters of major river systems, migrating across expansive geographic areas and habitats, including estuarine and marine environments. Thus, in addition to fishing, sturgeons are exposed to a broad range of environmental conditions and anthropogenic stressors including habitat loss, degradation, and alteration (Birstein, 1993; Bemis and Kynard, 1997; Heublein et al., 2009; Poletto et al., 2018). This combination of factors make sturgeons both difficult to study and highly vulnerable to extinction (Jager et al., 2008). Due to increasing numbers of imperiled and extirpated sturgeon populations around the globe, sturgeons have become the subject of major conservation efforts in recent decades (Rochard et al., 1990; Birstein, 1993; Bemis and Kynard, 1997; Birstein et al., 1997; Lower Columbia Fish Recovery Board, 2004).
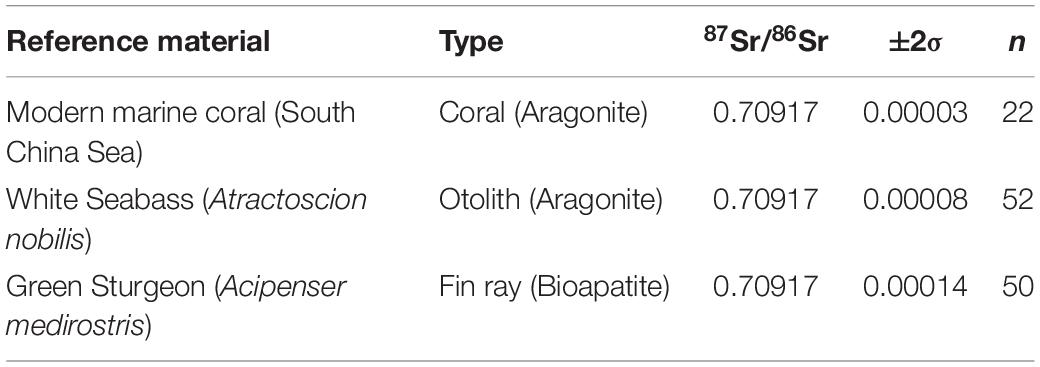
White Sturgeon (A. transmontanus) native to the San Francisco Estuary (SFE), CA, United States (Figure 1A): are believed to spawn in lower stretches of major rivers (e.g., Sacramento River) and spend most of their lives in estuarine habitats of the SFE, with some also moving into coastal waters (Lower Columbia Fish Recovery Board, 2004; Miller et al., 2020). White Sturgeon make spawning migrations every 1–4 years starting at age 10–15, depending on sex and environmental conditions, and very little is known about juvenile habitat use and migratory patterns (Chapman et al., 1996; Miller et al., 2020). White Sturgeon have evolved physiologically and behaviorally to survive and reproduce in a highly dynamic estuary; however, as is occurring globally, anthropogenic alterations to the hydrograph and local climate are reducing the quality and extent of suitable habitats for SFE sturgeons (Cloern et al., 2011; Poletto et al., 2018). Much of historical California freshwater spawning and rearing habitat is now either inaccessible or severely degraded due to impassable barriers, insufficient freshwater flows, agricultural diversions, elevated water temperatures, invasive species, and environmental contaminants such as selenium (Billard and Lecointre, 2001; Mussen et al., 2014; Zeug et al., 2014; Gundersen et al., 2017; National Marine Fisheries Service [NMFS], 2018). SFE White Sturgeon are also exposed to a broad range of direct mortality risks that vary spatially, temporally, and ontogenetically including predation, ship strikes, recreational fishing, and by-catch during commercial fishing (Balazik et al., 2012; Blackburn et al., 2019; Richerson et al., 2019; Baird et al., 2020; Demetras et al., 2020; Doukakis et al., 2020). SFE White Sturgeon are not listed in the federal or state Endangered Species Act but are categorized as a California Species of Special Concern. While White Sturgeon have experienced marked declines over the past century, they continue to support an important recreational fishery throughout the west coast of North America (Moyle et al., 2015; National Marine Fisheries Service [NMFS], 2015). Thus, identifying key environmental stressors and how they intersect spatially or temporally with sturgeon life histories remains a key priority for resource managers (Nelson et al., 2013).
Figure 1. (A) Overview of the four subregions of the San Francisco Estuary (SFE) and fin ray collection sites used in this study. The three lower panels display annual average salinity conditions in the SFE during (B) flood (2011), (C) average (2010), and (D) drought years (2008). Data adapted from MacWilliams et al. (2016). Legend acronyms are California Department of Fish and Wildlife (CDFW) and U.S. Fish and Wildlife Service (USFWS).
Detailed studies of fish movement patterns, such as those using artificial (e.g., acoustic) and natural (e.g., otolith geochemistry) tags, can be used to describe how different life-history phenotypes contribute to specific populations, year-classes, and cohorts, and how this may vary in response to extreme or changing conditions (Hall et al., 2016; Brennan et al., 2019; Hobbs et al., 2019; Sturrock et al., 2020). For example, Rainbow Trout (Oncorhynchus mykiss) exhibit life-history diversity expressed as a range of anadromous and resident behaviors, depending on extrinsic (e.g., environmental) and intrinsic (e.g., body condition) factors (Beakes et al., 2010; Evans et al., 2014; Kendall et al., 2015; Hall et al., 2016). Although several species of Pacific Salmon (Oncorhynchus spp.) and Atlantic Salmon (Salmo salar) exhibit more fixed migratory traits relative to Rainbow Trout (Crozier et al., 2008; Kovach et al., 2012), even these taxa demonstrate diversity in spawning run timing and locations, and dispersal timing from freshwater habitats (Crozier and Hutchings, 2014; Jonsson and Jonsson, 2018). Even smaller-bodied forage fishes such as Delta Smelt (Hypomesus transpacificus) have been found to employ complex resident and migrant behaviors in the SFE (Hobbs et al., 2019). These intraspecific differences have been described as “behavioral syndromes” and have been demonstrated to impact individual reproduction, population structure and distribution, and survival (Cote et al., 2010; Sih et al., 2012; Moiron et al., 2020). Identifying behavioral syndromes in natural populations can help resource managers support diverse life history profiles within a population and determine how resilient populations may be to increased temperatures and changing precipitation conditions predicted by future climate models (Mann and Gleick, 2015; McCabe et al., 2018).
Habitat use research of SFE White Sturgeon has previously been limited to recreational catch data, short-term artificial (acoustic) tags studies, video and eDNA monitoring, and opportunistic data from monitoring programs designed to target other species (Miller, 1970; Schaffter, 1997; Billard and Lecointre, 2001; Pikitch et al., 2005; Heublein et al., 2009; Lindley et al., 2011; Nelson et al., 2013; Poytress et al., 2015; Anderson et al., 2018). Thus, our current understanding is largely based on data that exhibit numerous limitations in extent and scope. Though acoustic tagging studies can provide very detailed information on timing and frequency of individual’s movements through locations where receivers are placed (Johnston et al., 2020; Miller et al., 2020) they are often limited in temporal and spatial scope, thus likely limiting our understanding to a subset of migratory behaviors or habitats for a small portion of an individual’s lifespan.
Unlike these previous methods, natural tags stored in calcified structures of fishes (e.g., fin rays and otoliths) provide a valuable tool for examining the life history of many individuals of a species throughout its entire life (Campana, 1999; Walther et al., 2017). For example, geochemical analysis of calcified structures via laser-ablation inductively coupled plasma mass spectrometry (LA-ICP-MS) has become a valuable tool for reconstructing the migratory histories of individual fish from several species. Strontium isotope ratios (87Sr/86Sr), in particular, have been used extensively in Chinook Salmon, Delta Smelt, and other fishes of the SFE to identify migratory life-history diversity; determine natal origins (e.g., tributaries and hatchery versus wild); and provide information on how habitat use, environmental variability, and growth interact throughout the life history of individual fish (Hobbs et al., 2005; Feyrer et al., 2007; Barnett-Johnson et al., 2008; Sturrock et al., 2015; Phillis et al., 2018; Willmes et al., 2018a).
Fin rays are better suited compared to otoliths for studying sensitive populations because they can be collected non-lethally with undetectable effects on growth, survival, or swimming performance (Collins and Smith, 1996; Nguyen et al., 2016). Fin rays are comprised largely of a calcium-phosphate (hydroxyapatite) and protein matrix, with a large range of elements commonly incorporated as substitutes for calcium, trapped in interstitial spaces, or bound within protein-rich layers of the matrix (Tzadik et al., 2017). Fin rays accrete annual bands and appear reliable for aging sturgeon (Kohlhorst et al., 1980; Rien and Beamesderfer, 1994; Jackson et al., 2007). Chemical composition is controlled by several factors including the chemical composition of the water, ambient water salinity and temperature, diet, and fish physiology, including metabolic rate, growth rate, and reproductive state (Clarke et al., 2007; Kerr and Campana, 2014; Sweeney et al., 2020). Unlike otoliths, fin rays are susceptible to resorption, particularly in the vascularized core of the ray (Beamish, 1981; Campana and Thorrold, 2001; Tzadik et al., 2017). This may reduce precision and accuracy of age and growth estimates and limit the identification of natal origins or detection of short-duration migrations such as freshwater spawning excursions, particularly if individuals are not feeding enough to support fin ray growth during these migrations (Paragamian and Beamesderfer, 2003). However, previous studies have demonstrated that fin rays have minimal tissue turnover and that trace elements and stable isotopes remain stable over time once they are incorporated into the molecular matrix (Lida et al., 2014; Tzadik et al., 2017). Furthermore, recent experiments suggest that environmental signals are archived in White Sturgeon fin rays starting at ∼3 weeks post hatch and that detection of fine-scale freshwater movements (i.e., weeks) is possible in juvenile sturgeon (Sellheim et al., 2017; Sweeney et al., 2020). Sturgeon pectoral fin ray trace elemental ratios (e.g., Sr/Ba, Br/Ca, Sr/Ca) have been successfully used to detect movements between ocean and freshwater habitats (Arai et al., 2002; Allen et al., 2009; Veinott et al., 2011).
Here, we combined annual increment profiles with geochemical (87Sr/86Sr) profiles in White Sturgeon fin rays to examine life-history diversity expressed by this species in the SFE. Salinity chronologies were reconstructed to examine fine-scale and broad-scale ontogenetic patterns in habitat use for individuals ranging from 5 to 30 years old. Furthermore, hierarchical clustering was used to identify unique life history phenotypes from the first 10 years of life before sexual maturation, which was the timeframe that supported a robust statistical analysis given the sample size available. Our aim was to improve our understanding of phenotypic plasticity in managed species, such as White Sturgeon, to better inform predictions of environmental change effects and how specific management actions are likely to influence populations.
Materials and Methods
Study Site
Environmental conditions in the SFE are highly dynamic (Kimmerer, 2004), with a salinity gradient from upstream freshwater habitats in the Sacramento-San Joaquin River Delta to marine habitats in San Francisco Bay, and variable brackish conditions through Suisun and San Pablo Bays (Figures 1B–D). The SFE exhibits California’s Mediterranean climate exemplified by warm, dry summers and cool, wet winters. Furthermore, interannual variation in precipitation and subsequent freshwater runoff is high, and similarly, water temperatures and clarity vary spatially and seasonally (Kimmerer, 2004; Cloern, 2019). Native species, such as White Sturgeon, have physiologically and behaviorally evolved to thrive within this dynamic landscape; however, anthropogenic alterations to the local hydrograph and climate are increasingly compressing available habitat quantity and quality (Cloern et al., 2011). For example, as the SFE continues to warm, thermal stress is likely to increasingly impact population dynamics of sensitive species (Brown et al., 2016). Reductions in freshwater outflow, combined with sea level rise, are likely to push salinity gradients further inland, reducing the amount of estuarine habitat, further exacerbating warming trends (Feyrer et al., 2006; Brown et al., 2016). Continuing weather pattern changes, coupled with upstream reservoir sediment capture and expanding aquatic macrophytes, are also likely to impact SFE water turbidity and temperature (Hestir et al., 2016; Bever et al., 2018).
Field Collection and Laboratory Processing
White Sturgeon fin rays were collected opportunistically by the U.S. Fish and Wildlife Service during sturgeon sport fishing derbies from Rio Vista on the Lower Sacramento River to San Pablo Bay, during telemetry studies in the San Joaquin River, and from the California Department of Fish and Wildlife’s trammel net surveys for adult sturgeon in Suisun Bay (Table 1). During field collection, each sturgeon’s fork length was measured to the nearest centimeter (cm). The most anterior fin ray was removed from a pectoral fin of each fish by cutting the ray at the point where the ray articulated with the pectoral girdle (Koch et al., 2008). Fin rays were either collected non-lethally during trammel net surveys and the telemetry study, or were taken from fish that had already been harvested during fishing derbies. Fin rays were stored in separate, labeled bags in a freezer until processed. Fin rays (n = 112) were prepared and processed according to established methods (Koch and Quist, 2007). Briefly, fin ray sections were cleaned of tissue, air dried, mounted in epoxy resin, and thin sectioned from the proximal end of each fin ray using an IsoMet low-speed saw (Buehler, Lake Bluff, IL, United States) with a width from 0.8 to 1.3 millimeter (mm). The saw blade was cleaned between fin rays to prevent contamination. The thin sections were mounted on glass slides and polished until annual increments were visible throughout the section (Figure 2).
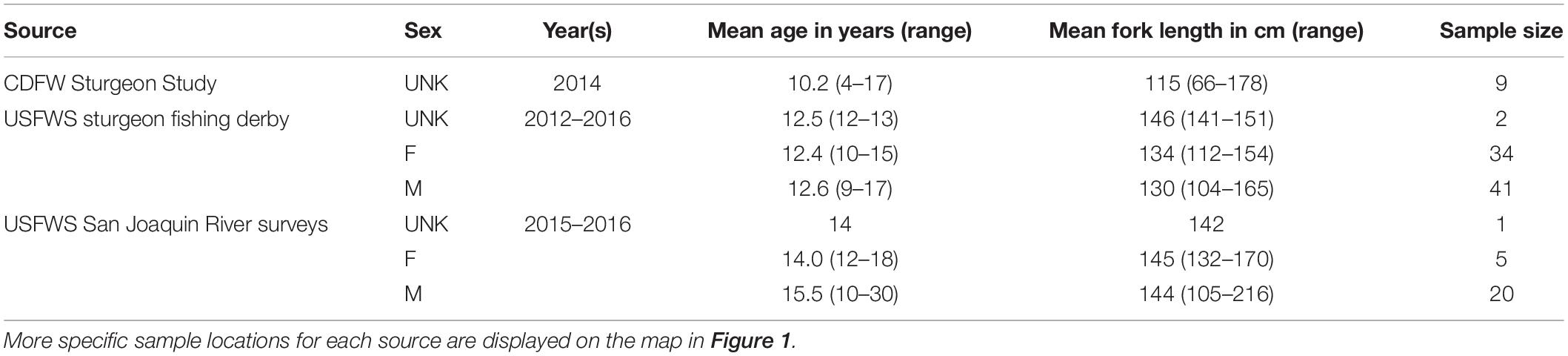
Table 1. White Sturgeon fin ray sample sources, including sample size, years collected, mean and range of fish age and fork length (cm), and sex.
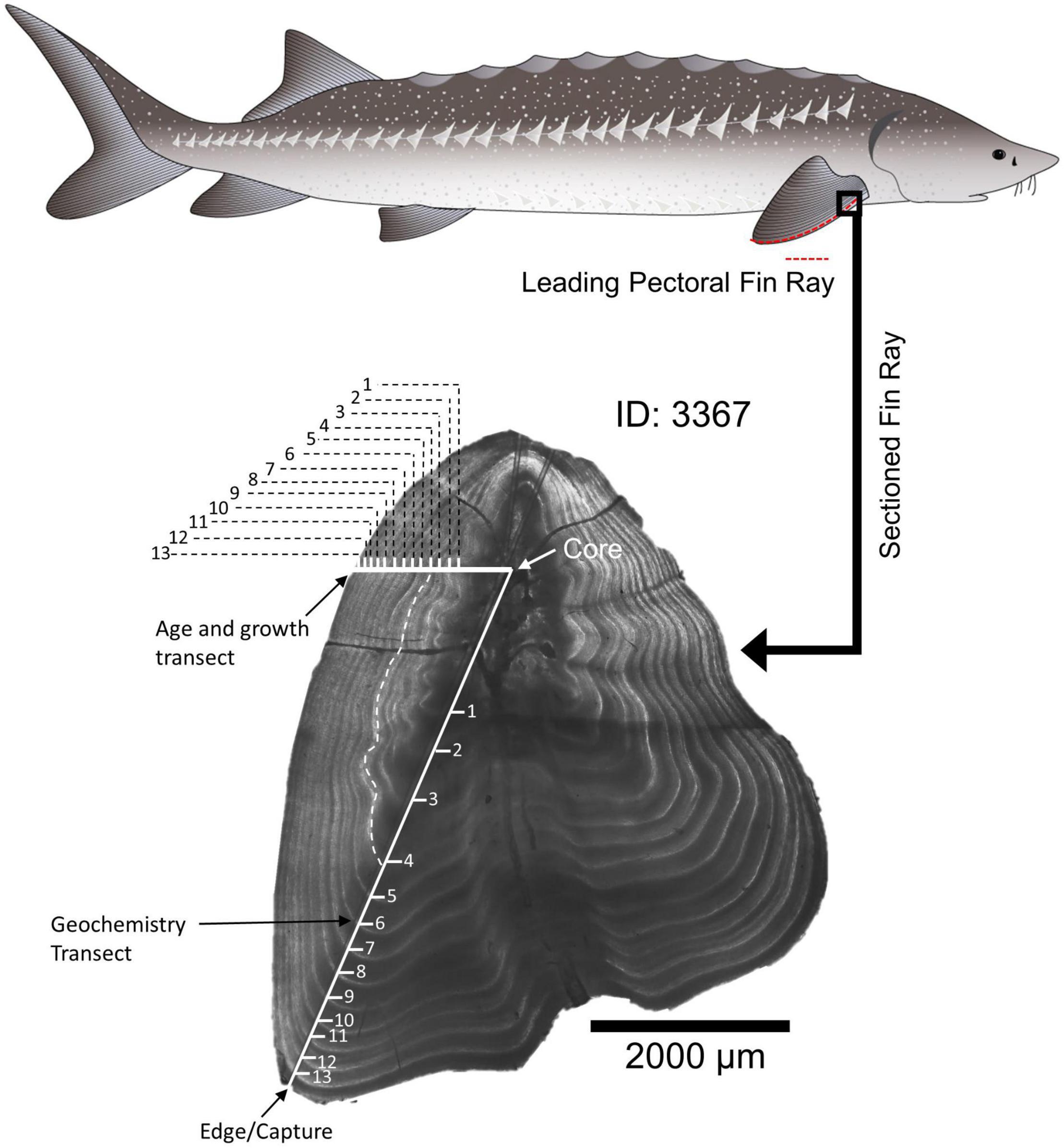
Figure 2. Example fin ray with microstructure and microchemistry analysis trajectories indicated. The white dashed line follows an annulus from the transect used for geochemistry to the transect used for aging. Drawing of White Sturgeon by Adi Khen.
Microstructure Analysis
Sectioned fin rays were imaged before and after microchemistry analysis with Image Pro-Premier® using a Motic BA310 compound microscope with a Motic Cam 5+ camera attached to the trinocular port. Each fin ray was calibrated, photographed, and measured under 40X total magnification. Annual bands were annotated and counted on digital images using Image-Pro Plus software (Media Cybernetics, Rockville, MD, United States) following methods used by Blackburn (2018; Figure 2). The annulus represents the winter, slow growth period prior to reproduction in the late spring (Rien and Beamesderfer, 1994). Therefore, if an individual was captured between September and October (CDFW survey), the edge was not included in the annulus count, as the “annulus” portion of its seasonal growth had not yet occurred. If an individual was captured during the winter or spring (January–April; Derby collections and San Joaquin River surveys), the fin ray edge was counted as an annulus and incorporated into the age estimate. Initial age estimates were completed by the Quist lab, University of Idaho. To assess the precision of sturgeon age estimates, at least three additional independent age reads were conducted by University of California, Davis and Cramer Fish Sciences biologists and evaluated using the FSA package (Ogle, 2016) in R (R Core Team, 2021). If ages did not agree across the three readers, the images were reviewed by all three readers together to determine whether agreement could be achieved or whether the fin ray should be excluded from analysis due to poor sample quality or indistinct annuli (e.g., presence of accessory lobes or other structural features).
Microchemistry Analysis
In situ 87Sr/86Sr values were measured at the University of California, Davis Interdisciplinary Center for Plasma Mass Spectrometry (Table 2). A Nu Plasma HR (Nu032) multi-collector inductively coupled plasma mass spectrometer (MC-ICP-MS) was interfaced with a Nd:YAG 213 nm laser (New Wave Research UP213). A laser beam of 40 μm diameter was traversed across the fin ray from the core to the ventral edge at 5 μm/s, with the laser pulsing at 10-Hz frequency and 5–15 J/cm2 photon output. The instrument was operated in medium resolution mode (∼7500 M/ΔM) to avoid polyatomic spectral interferences, i.e., 40Ca31P16O, or 40Ar31P16O, which can overlap with 87Sr, and consequently cause inaccurate results. Correction and monitoring for potential interferences followed established protocols (Vroon et al., 2008; Lewis et al., 2014; Willmes et al., 2016; Lugli et al., 2017; Griffin et al., 2021). Data reduction was carried out in IsoFishR (Willmes et al., 2018b), and included a background subtraction (30 s, laser off), an exponential mass bias correction, assuming 86Sr/88Sr = 0.1194, and a 87Rb correction, by monitoring the 85Rb signal and applying the same mass bias correction as determined for Sr. A 5-point average was applied to the raw data collected by the mass spectrometer with an integration time of 0.2 s resulting in 1 datapoint per second. Outliers were removed based on 2 IQR outlier criterion using a 40-point moving average window (Hoaglin et al., 1986). Accuracy and reproducibility of the LA-MC-ICP-MS were evaluated using in-house reference materials consisting of a modern marine coral (Acropora sp.) from the South China Sea, a modern marine otolith from a White Seabass (Atractoscion nobilis) collected offshore of Baja California, and a marine Green Sturgeon (Acipenser medirostris) fin ray. Replicate analyses of the different in-house reference materials (Table 3) were in good agreement with the global average 87Sr/86Sr value of modern seawater of 0.70918 (McArthur et al., 2001; Mokadem et al., 2015). 87Sr/86Sr profiles were then smoothed using a thin-plate regression spline (k = 60) and Generalized Cross Validation to optimize the effective degrees of freedom using the mgcv package in R (Wood, 2017).
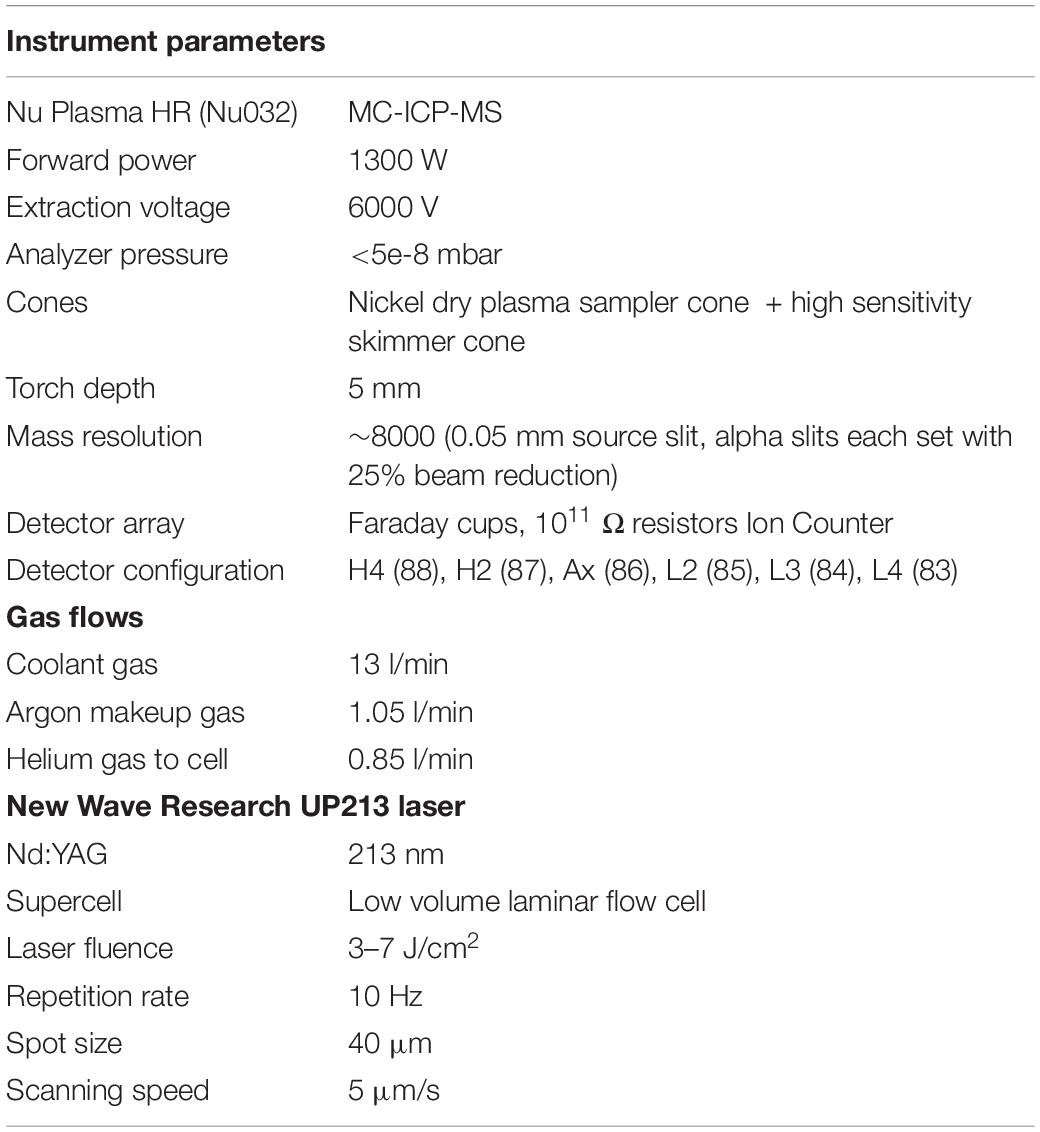
Table 2. Instrument operating conditions of the Nu Plasma HR (Nu032) and New Wave Research UP213 Nd:YAG 213 nm laser.
Salinity Movement Reconstructions From Strontium Isotopes
To estimate salinity from 87Sr/86Sr values in fin rays, we applied the published salinity mixing model for the SFE (Hobbs et al., 2019; Figure 3A). A two-endmember mixing model was used to describe the freshwater to ocean salinity gradient based on water samples previously analyzed for the SFE (Hobbs et al., 2019). To define the freshwater endmember we used a long-term average of 70% Sacramento and 30% San Joaquin River relative contribution (Chen et al., 2018; Hutton et al., 2019). To assess the sensitivity of our salinity reconstructions to variable Sacramento River and San Joaquin River influences we compared two scenarios, where the freshwater endmember is either only the Sacramento River (100%) or only the San Joaquin River (100%). We found that this introduced only small uncertainties in our ability to reconstruct salinity (Figure 3B).
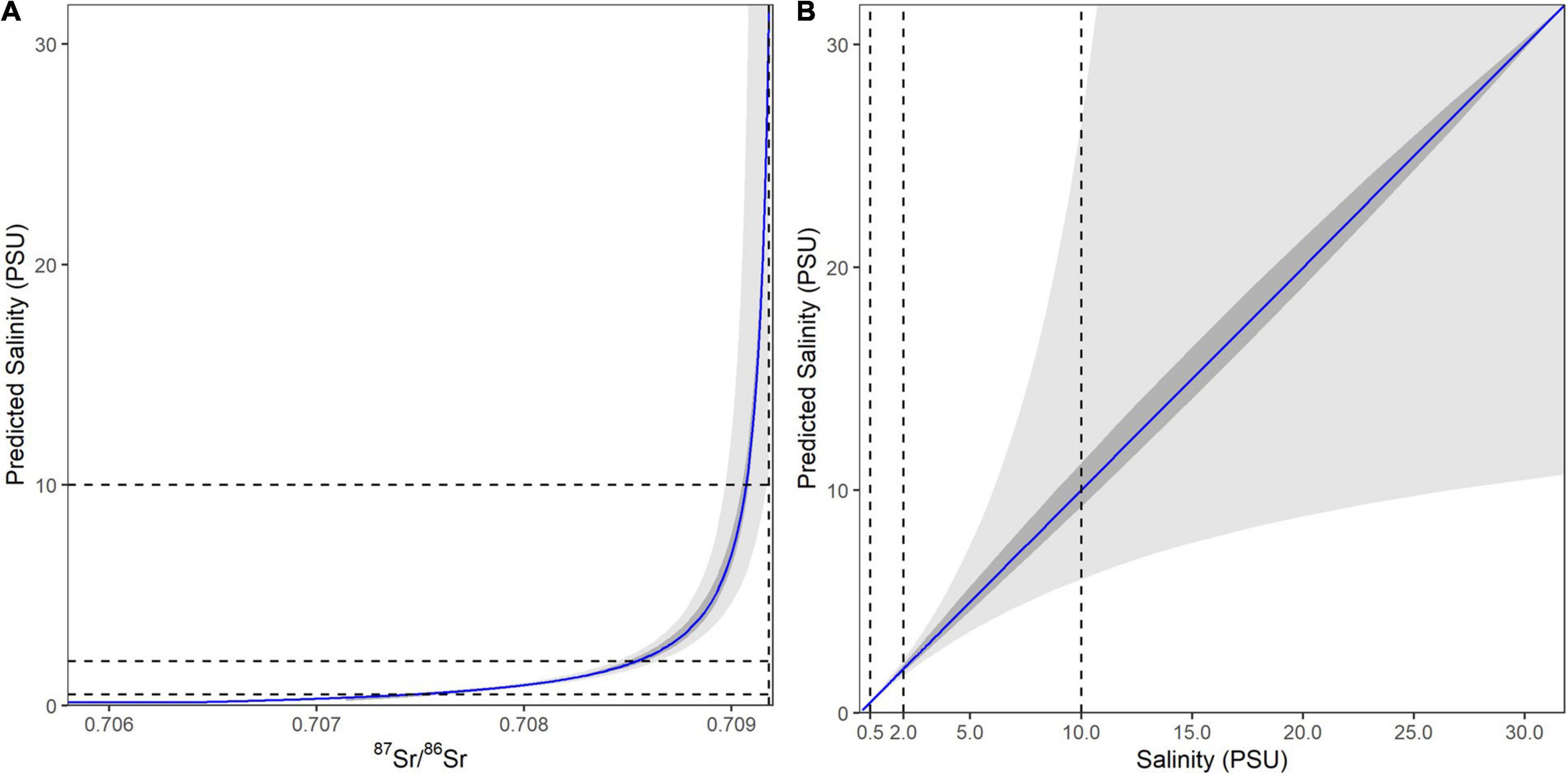
Figure 3. The 87Sr/86Sr to salinity mixing model based on Hobbs et al. (2019). (A) The 87Sr/86Sr to salinity relationship across the 87Sr/86Sr values for the entire SFE. Blue line representing the 70-30 Sacramento to San Joaquin River freshwater endmember and the inner gray band representing the uncertainty introduced by varying between 100% Sacramento and 100% San Joaquin River water. The outer gray band represents the uncertainty introduced into predicted salinities based on the propagated uncertainties from the geochemical analyses. (B) The relationship between predicted versus measured salinity values, showing that prediction uncertainty increases asymmetrically with salinity. Dashed lines show the 0.5, 2, and 10 salinity cutoffs used to identify different habitats. Note that due to these increasing uncertainties salinities >10 psu were treated here as “high salinity” and include salinity values from 10 to 32 (seawater).
Salinity reconstructions using 87Sr/86Sr have high precision at low salinity values, however; uncertainty increases rapidly above 5 psu (practical salinity units) (Figure 3B), due to the low concentration of Sr in freshwater compared to ocean. These uncertainties make it increasingly difficult to distinguish between higher salinities (>10 psu) found in the estuary and those found in the ocean (Figure 1). Based on these uncertainty thresholds and known management and ecological boundaries we divided the SFE into several salinity zones including freshwater (<0.5 psu), low salinity (0.5–2 psu), medium salinity (2–10 psu), and high salinity (>10 psu) which includes ocean values (Allen et al., 2009; MacWilliams et al., 2016).
We then reconstructed temporally resolved salinity histories for each White Sturgeon examined in this study following the approach described by Hobbs et al. (2019). We fit the geochemical data onto the aging transect for each fin ray by aligning the proportional distances (from core to edge) of each age band to the matching proportional distances along the laser-ablation profile. Salinities were then calculated along each merged profile using the 87Sr/86Sr to salinity mixing model.
Annual Average Salinities and Clustering
Salinity values falling within each annual fin ray band were averaged, generating annual mean salinity profiles. We then used these annual averages in an agglomerative hierarchical cluster analysis. The cluster analysis was used to identify dominant ontogenetic patterns in habitat use during the juvenile-subadult life stages (ages 0–10), prior to maturation which typically occurs at 10–15 years of age, depending on sex and environmental conditions (Moyle, 2002; Hildebrand et al., 2016; Miller et al., 2020). We chose this age cutoff because it allowed for a large enough samples size (n = 105) to detect patterns using the hierarchical clustering method, and because relatively little is known about White Sturgeon migratory behavior during the first decade of life prior to spawning. We used the “agnes” function from the cluster package (Maechler et al., 2015) in R (R Core Team, 2021) to compare the different agglomerative methods (“average,” “single,” “complete,” “ward”) and found the “ward” method, which uses the minimum variance method and squared dissimilarities for cluster updating, to provide the strongest internal clustering structure. Next, we used the NbClust package (Charrad et al., 2014) to determine the optimal number of clusters by majority vote and decided to use four clusters though both 3 and 5 could also have been chosen based on similar vote counts.
To test whether sample location introduced bias into our cluster analysis, we used a chi-square test to determine whether the proportion of fish assigned to a given cluster differed significantly across capture locations. Initially we tested for differences across four locations: Delta, San Joaquin River, Suisun Bay, San Pablo Bay; however, sample sizes were too low for a robust statistical analysis. Therefore, we combined the capture locations into “upstream” (Delta and San Joaquin) and “downstream” (San Pablo Bay and Suisun Bay) groups. A threshold of α = 0.05 was used to determine significance, but we also considered α = 0.10 threshold to be conservative in our assessment.
Results
Age and Growth
Fin ray annuli counts provided precise White Sturgeon age estimates. Age estimations between three age readers across all samples, reached an initial agreement of 76% (n = 112, ACV = 1.14, APE = 0.87). Fork lengths ranged from 66 to 216 cm and estimated ages ranged from 4 to 30 years with an average of 12.9 years (Table 1 and Figure 4A). As expected, fork length was positively correlated with fish age (Figure 4B, R2 = 0.77), and growth appeared to be constant at approximately 5.89 (±0.31) cm/year from age 4 to 30. Overall, 61 males and 39 females were collected, and sex could not be determined for 12 fish.
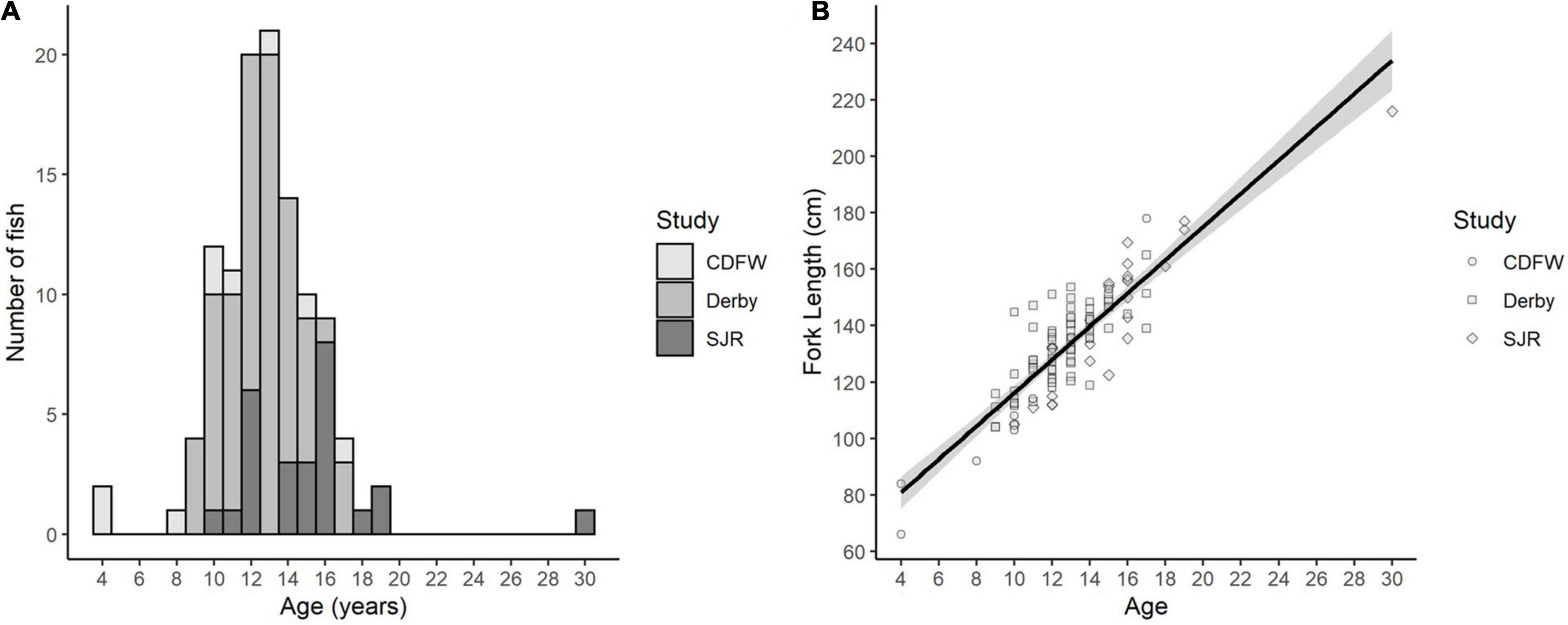
Figure 4. (A) Histogram of number of fish in each age bin, and (B) fork length to fish age relationship (R2 = 0.77). Study designates the origin of the samples collected from the California Department of Fish and Wildlife (CDFW) trawling surveys, sturgeon sport fishing derbies (Derby), or the San Joaquin River (SJR) gill and trammel net surveys.
Interannual Patterns in Salinity Movements
We observed broad diversity in the fine-scale salinity histories of individual White Sturgeon (Figure 5). For example, one of the youngest fish sampled (age 4) demonstrated freshwater habitat residency during most of the first year of life, moving to low and medium salinity at the end of its first year and remaining there through age 4 (Figure 5A). Some individuals spent their entire lives in fresh- or low-salinity water (Figure 5B) while others made short high salinity excursions (Figure 5C) or migrated quickly to higher-salinity brackish water in their first year and remained in these habitats (Figure 5D). The oldest fish sampled (a male, age 30 years) spent the last 14 years of his life in medium salinity habitat and showed no freshwater signature at any point in its life history (Figure 5E). Overall, movements into high salinity (>10 psu) were generally infrequent, short duration, and occurred in 52% of the fish. Most individuals remained in salinities less than 10 psu, and 6% of fin rays never recorded salinities >2 psu. No fin rays appeared to demonstrate prolonged marine residency although, as described above, salinity estimates above 10 psu have high uncertainty (Figure 3).
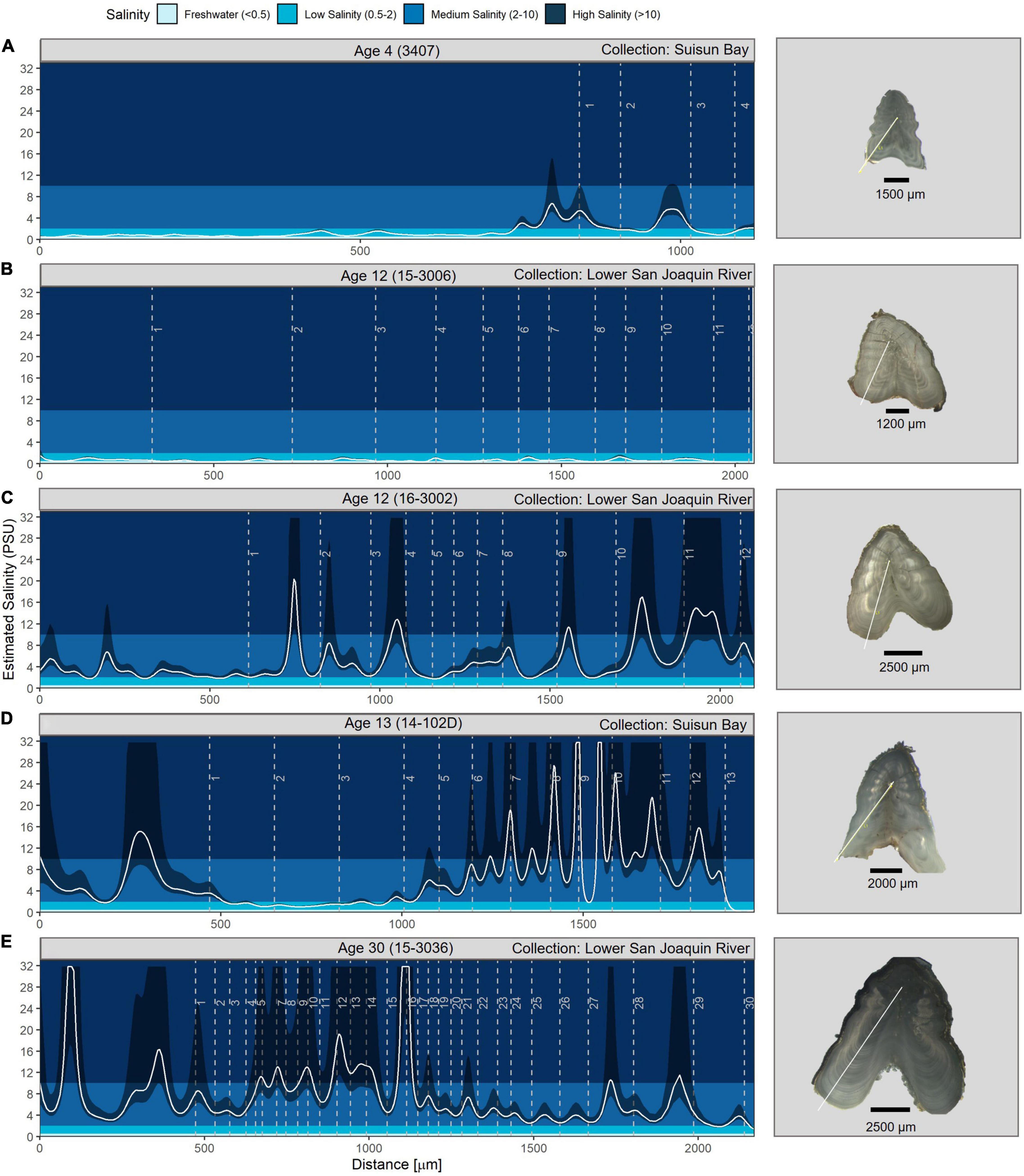
Figure 5. Salinity profile examples showing variability in use of different salinity habitats across a range of ages. The white line is the salinity spline, and the dark shaded area is the associated estimated uncertainty. Note patterns at higher salinity values are less certain due to the non-linear nature of the mixing curve (Figure 3). Dashed vertical age lines represent the opaque (summer) portion of the annuli. Note the x-axis range varies among different plots because of fish age and because fin ray sections were cut from different fin ray regions across individual fish. The fish ID is shown at the top of each graph and links to the Supplementary Table 1. Profiles for all fish are shown in Supplementary Figure 1 and annual average salinities for each fish are in Supplementary Figure 2. An image of each fin ray section is included to the right of each figure to provide a representation of size variability. The white line on the fin rays shows the location of the geochemistry transect. Fin ray images were taken by Shannon Blackburn. (A) is the youngest fish sampled, (B) resided in predominantly fresh- or low-salinity water (B), (C) made short high salinity excursions, (D) exhibited early migration to higher-salinity brackish water, and (E) is the oldest fish sampled.
Of 112 fin rays analyzed, most demonstrated brief excursions among different salinity habitats, which reflects their amphidromous migratory behavior (Figure 5). Annual salinity ranges, defined as the maximum-minimum salinity experienced by individual sturgeon at a given age, were highly variable and did not show an age trend (Figure 6).
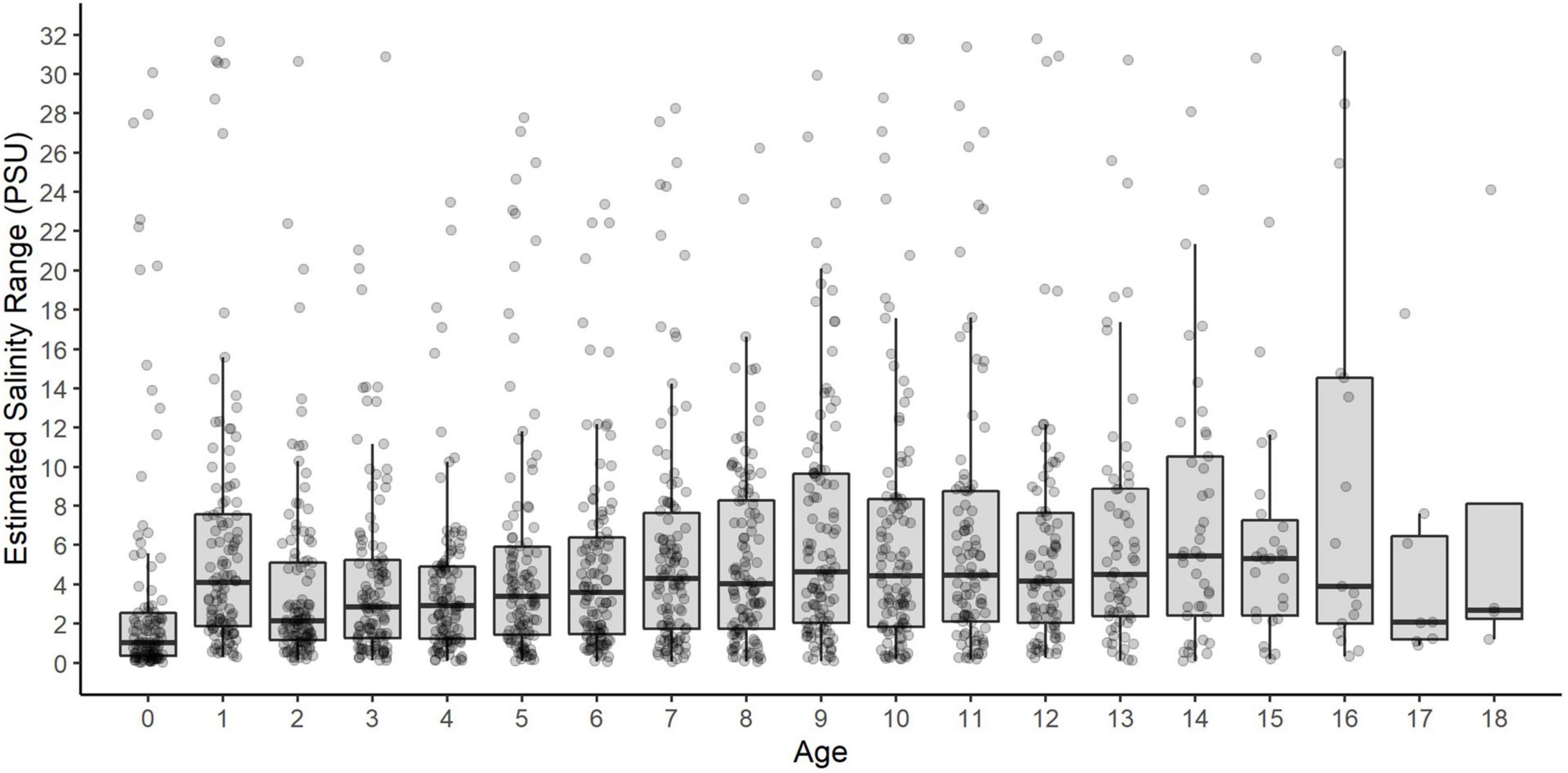
Figure 6. Intra-annual variation (range) in salinity by age for each White Sturgeon. Points represent the salinity range for each year of each fish’s life (maximum-minimum salinity at that age). Box plots show the salinity ranges across all fish within each age class.
Annual Mean Salinity Habitat Use
Adequate samples were available to assess annual salinity exposure to 18 years of age. Medium salinities were most common across all annuli for all fish (48–75%, depending on age), followed by low salinity (7–48%), and high salinity (2–29%). Freshwater only accounted for 1–12% of salinity habitat use, and there was no general trend with age. However, there were trends in the dominant salinity value by age group. For age 0–3, for example, an average of 42% (range 32–48%) of fish exhibited low-salinity values, while only 4% (range 0–6%) exhibited high salinity values (Figure 7). Beyond age 3, there was a trend toward a greater proportion of fish inhabiting higher salinity waters (19%, range 2–29%), with only 17% (range 7–33%) in low salinity (Figure 7).
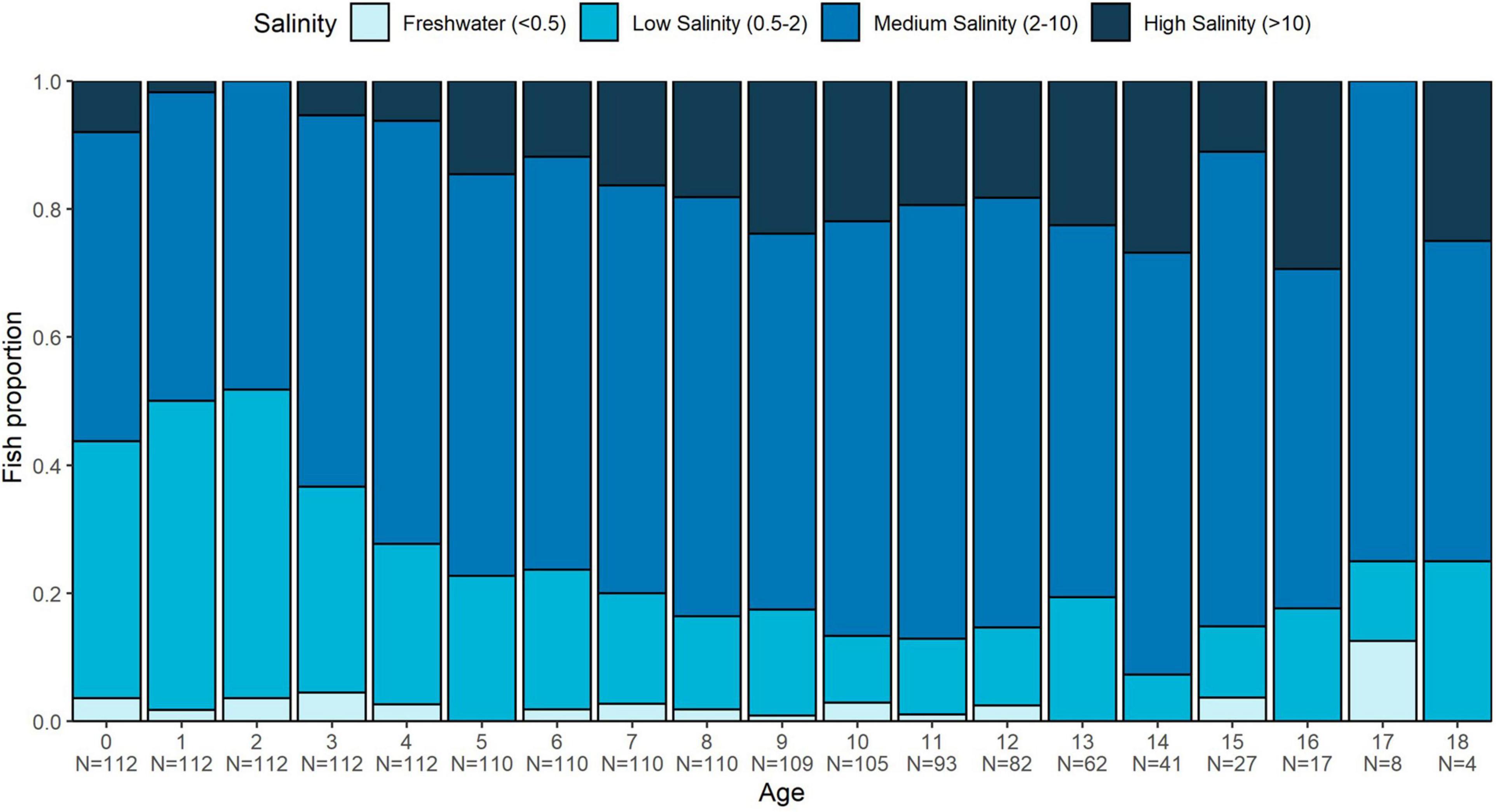
Figure 7. Proportion of White Sturgeon ≤18 years of age observed in each of four salinity zones by year-post-hatch. Salinity zones were assigned to each year for each fish by converting the mean annual 87Sr/86Sr value into salinity estimates using the mixing model (Figure 3).
Sub-Adult Habitat Clusters
Mean annual salinity values revealed a diversity of ontogenetic patterns in White Sturgeon habitat use. Several distinct statistical clusters were identified based on mean annual salinity values across the first 10 years for each individual (Figure 8). Individuals within Cluster 1 generally remained in fresh- to low-salinity (<2) habitats throughout their lives, with a few individuals demonstrating occasional medium-salinity migrations but no ontogenetic movements into higher salinities. Individuals in Cluster 2 spent most of their lives in medium salinity (∼2–5 psu), with ontogenetic movements to higher salinities after age 7. In contrast, individuals in Clusters 3 and 4 exhibited ontogenetic movements from lower to higher salinities, with Cluster 3 inhabiting lower salinities until around year 2, when they migrated to medium and high salinity, and Cluster 4 exhibiting rapid early movements toward higher salinity values (generally >5 psu) with little or no evidence of low salinity residence across individuals (Figure 8).
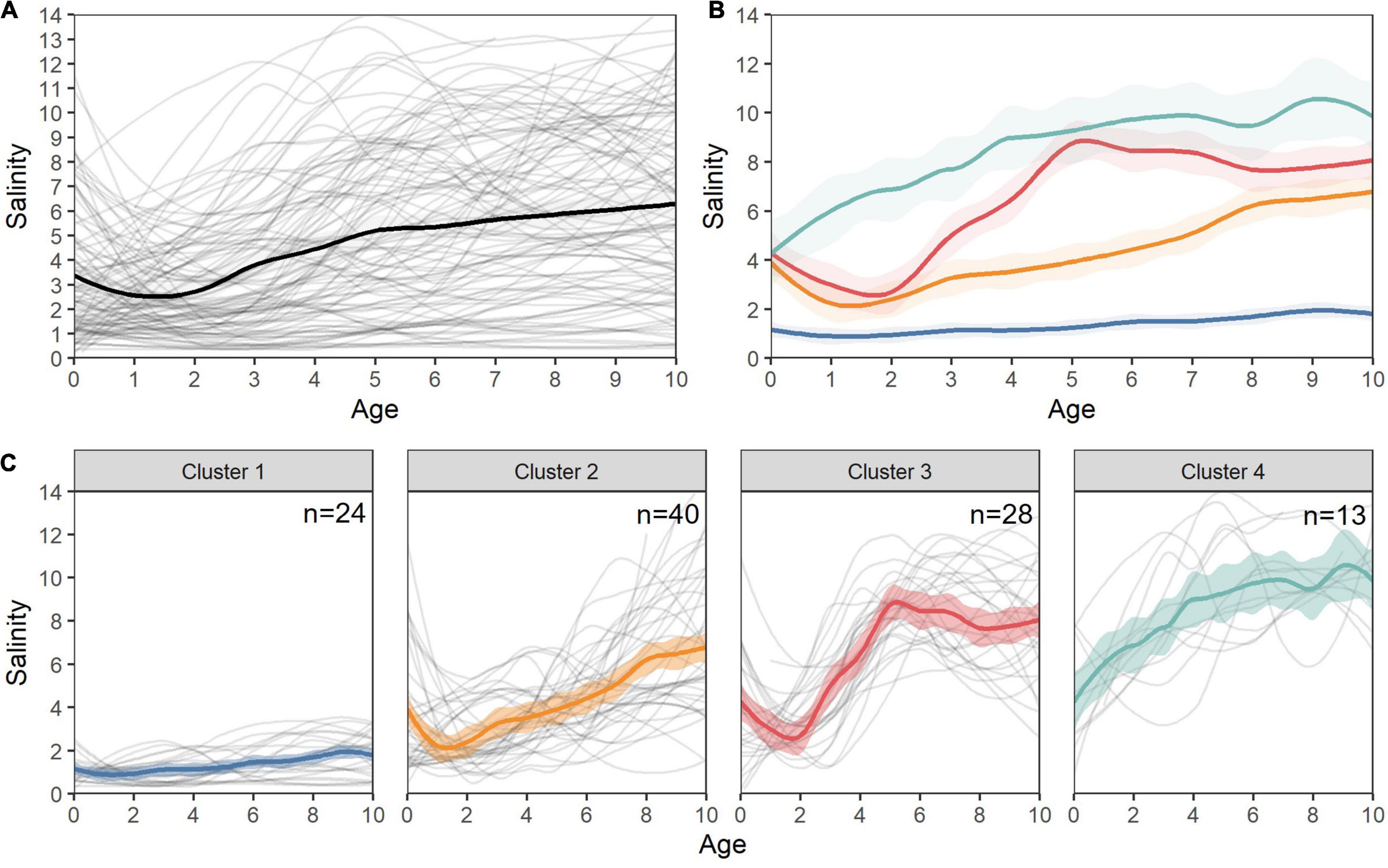
Figure 8. Individual salinity chronologies and ontogenetic salinity clusters for San Francisco Estuary White Sturgeon. (A) Individual (gray) and mean (black) salinity splines for all fish, (B) overlayed mean loess smooths (span = 0.3) for each of the four statistical life history clusters, (C) overlayed mean loess smooths (span = 0.3) and individual (gray) salinity splines for all fish in each cluster.
Proportion of Cluster Across Capture Locations and by Sex
We observed some evidence for salinity cluster composition differences across sample collection locations (Figure 9). When locations were combined into “downstream” (San Pablo and Suisun Bay) and “upstream” (Delta and Lower San Joaquin River) locations, the chi-square was not significant at α = 0.05 but was significant at α = 0.10 (χ2 = 7.15, df = 3, p = 0.07). There were no significant differences in cluster assignment between males and females (χ2 = 3.87, df = 3, p = 0.28).
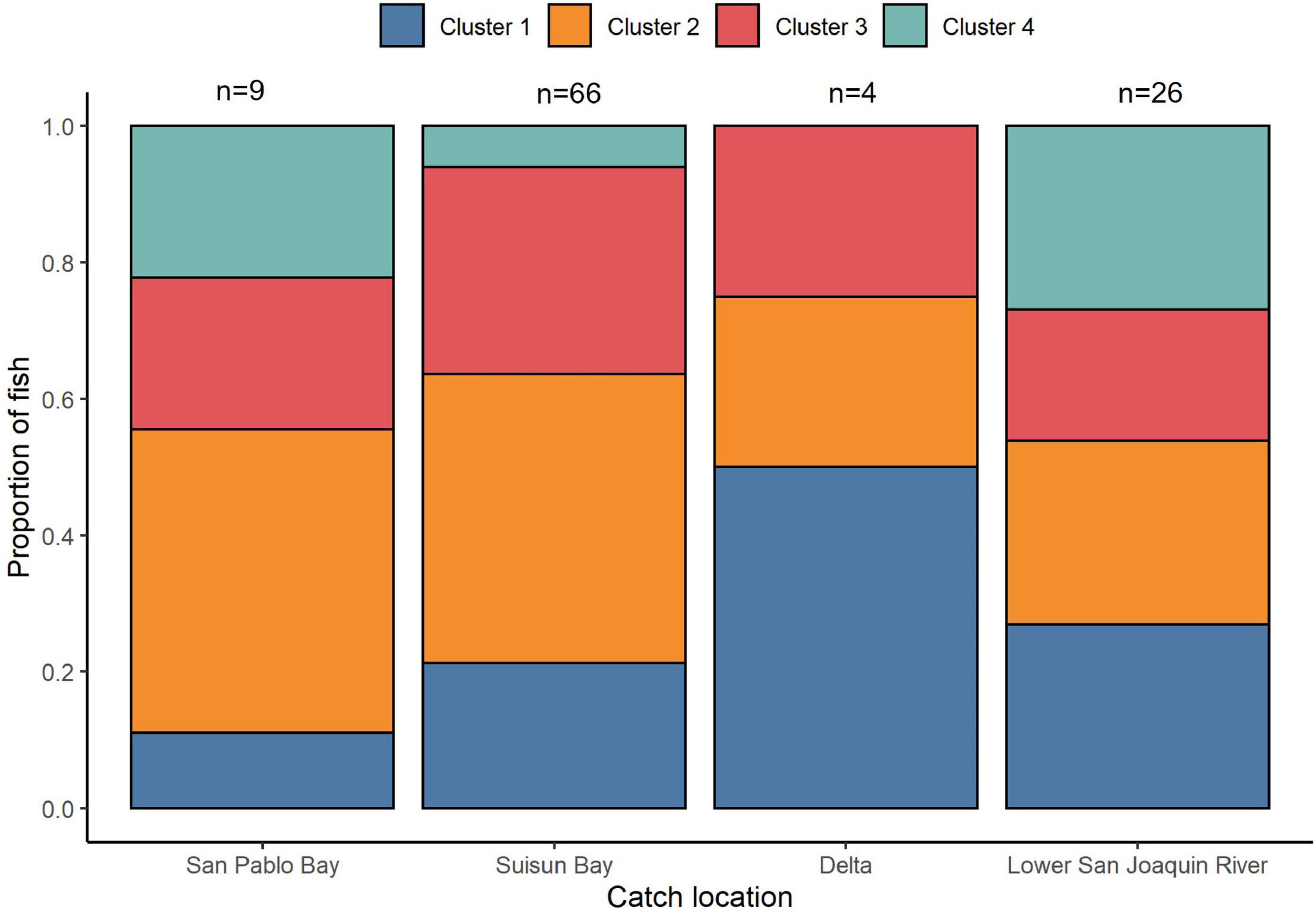
Figure 9. Regional variation in migratory cluster composition of San Francisco Estuary White Sturgeon. The proportion of White Sturgeon belonging to each of the four sub-adult life-history clusters is shown for each region. Regions are defined in Figure 1 and clusters are defined in Figure 8.
Discussion
We quantified life-long variation in exposure to salinity concentrations in White Sturgeon to reconstruct movements and habitat use in the SFE using fin ray Sr isotope (87Sr/86Sr) geochemistry. Although previous studies have described habitat use of White Sturgeon in the SFE using baited hooks, telemetry, and mark recapture techniques (Nelson et al., 2013; Miller et al., 2020; Patton et al., 2020), our study is the first to employ a natural geochemical tracer to examine the ontogenetic movements and habitat use patterns of over 100 individual White Sturgeon over their lifespan prior to capture. Our results largely corroborated prior observations of amphidromous migratory behaviors (Bemis and Kynard, 1997; Patton et al., 2020) with prolonged periods of estuarine residence (DeVore et al., 1999; Miller et al., 2020). However, we also observed broad variation among individual fish. The fin ray geochemistry migratory profiles supported identification of at least four distinct habitat use patterns expressed by White Sturgeon in the SFE. Such diversity in “behavioral syndromes” is likely to influence species distribution and abundance patterns, species interactions, population dynamics, responses to anthropogenic impacts, and ecological invasions (Sih et al., 2012). Given these considerations, it is imperative that future studies quantify variation in the habitat use of managed species to improve population model accuracy and support effective management decisions.
Life History Diversity and Population Resilience
The salinity clusters observed in this study suggest that, although subadult amphidromous movements were quite diverse across individuals, there were statistically distinct behavioral types, ranging from those that primarily inhabited low salinity waters to those who resided in high salinity water following a few years in low or medium salinity. Environmental stressors vary spatially and temporally within the SFE, and the relative population impacts of different stressors are poorly understood (Light and Marchetti, 2007; Null and Viers, 2013; Gundersen et al., 2017). Different ontogenetic stages and habitat use strategies may expose individuals to distinct types and degrees of risk. For example, sturgeon inhabiting primarily freshwater habitats may be more exposed to summer high temperatures or poor water quality due to agricultural runoff and entrainment (Grimaldo et al., 2009; Mussen et al., 2014). Conversely, sturgeon inhabiting higher salinity waters may be disproportionately exposed to shipping channel impacts including boat strikes and channel dredging, particularly with their preference of deep, open water habitats (Demetras et al., 2020; Patton et al., 2020). Because these risks vary across annual or seasonal time scales and are influenced by anthropogenic activities and climate change, some behavioral types may be more resilient than others.
Sturgeon Fin Ray Microchemistry vs. Other Microchemistry Studies
Our study contributes to a growing body of literature that leverages microchemistry data in bony parts to document life history diversity and migratory behavior in anadromous fish. Most prior studies have reconstructed migratory histories for anadromous species with a more limited life span than White Sturgeon or focused on a particular life stage. For example, Hermann et al. (2016) used otolith microchemistry to compare migratory behaviors in two long-distance migratory catfishes (Brachyplatystoma rousseauxii and Brachyplatystoma filamentosum), and found that B. rousseauxii resided in the Amazon estuary for its first few years, while B. filamentosum did not enter the estuary during its entire life history. Yokouchi et al. (2012) used otolith Sr:Ca ratios to document a high degree of phenotypic plasticity in fresh and estuarine habitat use in Japanese eels (Anguilla japonica) from the Hamana Lake system of central Japan, but also found that early salinity experience influenced individual migratory trajectories. Feutry et al. (2012) used otolith microchemistry and microstructure to compare juvenile migratory histories of three Kuhlia species (Teleostei) from fresh and brackish environments, providing basic biological information about the similarities and differences in habitat use across the three species. Like White Sturgeon, the focal species of each of these studies were commercially exploited populations exposed to a range of stressors. Understanding basic migratory patterns and life history diversity has global fisheries management implications, in particular if these patterns can be translated into specific management tools and approaches (Schindler et al., 2010).
Several previous studies of anadromous fish that migrate through the SFE have used microchemistry studies to underscore the importance of maintaining life history diversity portfolios to enhance population resilience under a range of future environmental conditions. For example, Hobbs et al. (2019) used laser-ablation otolith strontium isotope microchemistry to uncover three distinct life-history phenotypes for an endangered Delta Smelt, including freshwater resident, brackish-water resident, and semi-anadromous fish, and that the semi-anadromous phenotype could be categorized into at least four additional life-history phenotypes that varied by natal origin, dispersal age, and adult salinity history. Several Chinook Salmon microchemistry studies have documented diverse out-migration strategies in juveniles and have highlighted the importance of maintaining high life history diversity in order to support viable populations under a variable and changing climate (Phillis et al., 2018; Sturrock et al., 2020; Cordoleani et al., 2021; Willmes et al., 2021). Our study suggests that sturgeon may be more behaviorally plastic and have broader life history diversity compared with fish species in these previous microchemistry studies which tend to have shorter lifespans, smaller body size, and more defined ontogenetic migratory patterns that vary on a smaller spatial and temporal scale. This complicates sturgeon population management, as highly plastic behavior reduces predictability of sturgeon distributional patterns over space and time and makes it more difficult to determine how the population will react to a particular environmental change, stressor, flow management decision, or restoration action. Structuring future population-level studies specifically to include all life-history strategies and determining the relative success of each under varying environmental conditions would aid in understanding life-history expressions and better inform management of these iconic fish.
Evidence for the Use of Coastal Marine Habitats
Based on mark-recapture data, White Sturgeon have been known to migrate across thousands of kilometers of ocean habitat (DeVore et al., 1999), and specimens have been found as far south as the Pacific coast of Mexico (Ruiz-Campos et al., 2011). These observations have been supported by estimates of genetic isolation and gene flow (Anders, 2002). Nevertheless, little remains known about the ocean migratory behavior of this species. Here, we used the salinity chronologies reconstructed from fin ray Sr isotope profiles to assess the use of coastal marine habitats by White Sturgeon in the SFE. Though several profiles indicated short excursions into relatively high salinity, such excursions were generally brief and rare, and never resulted in a mean annual signal indicating year-round marine residence. This, in part, could be due to the limitations of the Sr isotope-salinity mixing model, where confidence decreases rapidly and non-linearly with increasing salinity values. However, though confidence might decrease with increasing salinity, Sr isotope ratios of 0.70918 (the global ocean value) are commonly observed in calcified structures of other species (e.g., Chinook Salmon) that are known to migrate to the coastal ocean (Kennedy et al., 2002; Johnson et al., 2016). Our results suggest that none of the 112 White Sturgeon examined in this study spent a significant portion of their life in fully marine habitats, and all largely remained within fresh- to brackish-water habitats within the SFE. Limited ocean migration was also observed in previous studies of White Sturgeon in the Columbia River. For example, of the 69,609 White Sturgeon originally tagged in the unimpounded Columbia River below the Bonneville Dam from 1976 to 1997, only 471 were recaptured outside of the Columbia River Basin (DeVore et al., 1999). Furthermore, statistical comparisons of mitochondria DNA (mtDNA) haplotype frequency distributions indicated significant isolation between populations separated by 1,000 km of ocean habitat (Anders, 2002). Together, these results further suggest White Sturgeon are mostly estuarine residents throughout much of their lives with limited use of coastal marine habitats.
Capture Location Effect on Migration Strategy
Many sturgeon sampling efforts and long-term tagging studies target sampling within Suisun and San Pablo bays (Miller et al., 2020; Patton et al., 2020). For example, Miller et al. (2020) used acoustic telemetry arrays to track large juvenile, subadult, and adult sturgeon over 5–7 years and found that juveniles and sub-adults were detected almost exclusively in San Pablo Bay, Suisun Bay, and the Delta, with only very rare incidences of ocean movements (1 of 160 fish). Patton et al. (2020) used baited hooks (set lines) to examine habitat use patterns in eastern Suisun Bay, finding that fish were present year-round in shoal and channel habitats, but not shallow wetland habitats. The geographically limited nature of sampling, although often necessary due to funding or time limitations, introduces potential bias into studies attempting to document habitat utilization. Our study examined sturgeon collected from throughout the central Delta and San Joaquin River, filling in a key gap in sampling range for sturgeon movement and habitat use studies. We observed weak differences in the distribution of clusters between individuals collected in San Pablo and Suisun bays and those collected in the Delta and San Joaquin River, which may have been due to low sample size in particular locations. Even so, our study did not collect individuals from the Sacramento River or its tributaries, which is a key spawning reach for White Sturgeon (Kohlhorst and Cech, 2001; Miller et al., 2020). Further, our sample size from the Delta was also relatively low (<4%). These areas should be sampled in future studies to further expand on the distribution of migratory strategies present in the SFE. In more extensively studied anadromous fishes such as Rainbow Trout, as many as 32 different life-history strategies have been described (Thorpe, 1998; Moore et al., 2014). As White Sturgeon samples are collected from a broader geographic area, additional strategies may be documented.
Migratory History Versus Salinity History
We used 87Sr/86Sr in fin rays to generate time-resolved salinity histories for individual White Sturgeon. Given that White Sturgeon are known to move across broad geographic salinity gradients, we assumed that fin ray salinity histories reflect geographic movements for each fish. At finer scales, however, reconstructed salinity patterns in fin rays may not reflect geographic movements. For example, SFE salinity gradients are dynamic, moving tidally, seasonally, and interannually, depending on the balance between freshwater outflow and tidal conditions. It is possible, therefore, for a fish to remain at a single geographic location and exhibit a variable salinity history in its fin rays. In our study, we used the annual mean salinity value to estimate the average position of each fish across the salinity gradient for each year of its life; therefore, the patterns we report herein are integrated across the full year and are not sensitive to seasonal variation in geography. The mean values, however, could reflect different geographic locations in different years, depending on the water year type and total freshwater outflow. At smaller scales, this could be important; however, at larger scales, the mean salinity gradient across wet and dry years remains relatively stable in the SFE. To improve confidence in inferred geographic positions based on geochemically reconstructed salinity profiles, future efforts could aim to contrast finer-scale spatiotemporal patterns in salinity gradients with higher-resolution salinity profiles in fin rays. Further, finer-scale spatio-temporal environmental variability could be overlaid with fin ray growth increments to examine how environmental conditions influence individual growth.
Temporal Resolution
This study focused on categorizing dominant movement patterns for the sub adult (<10 years) component of sturgeon life history, as the sample size of adult spawning-aged sturgeon was not large enough to support a hierarchical clustering analysis. For the adult sturgeon in the dataset, which are expected to make spawning runs into freshwater, we observed little evidence of freshwater excursions. Similarly, we observed little evidence of freshwater residence in age-0 fish, which presumably are exposed to freshwater habitats for at least a short period during the larval stage (Bemis and Kynard, 1997). These results suggest that salinity reconstructions from fin rays are time-averaged measurements that can represent mean patterns in habitat use across months or seasons throughout an individual’s life but may not always capture very fine scale movement patterns. For example, freshwater rearing or spawning (Miller et al., 2020) for only 1 month would represent <10% of the annual mean fin ray chemistry value, and even less if growth is reduced during these periods. Thus, short-term movements may not be detectible in fin rays using the techniques defined here including biologically important but low frequency or duration events such as early freshwater residency, freshwater spawning migrations, or occasional excursions into coastal marine habitats. Other methods, such as acoustic tagging studies, may be better suited to capturing spawning migrations or sturgeon movements through particular locations of interest (e.g., habitat restoration sites, reaches with high mortality risk such as shipping channels). These methods, however, only capture a small portion of an individual’s life history (Balazik et al., 2012). In contrast, fin ray geochemistry may be the best tool for examining the life history of White Sturgeon over broader temporal and spatial scales.
Future Opportunities
The SFE is a highly altered system with impassable barriers blocking most historic habitat and widespread degradation of remaining accessible habitat, and it is likely that the current population represents a small subset of historical life history and genetic diversity. Therefore, it is imperative that we improve understanding of habitat use, stressors impacting the remaining population, and the genes driving life history expression to prevent further loss of diversity, and subsequently, population resilience in the face of changing climatic conditions and increased environmental variability (Greene et al., 2010; Moore et al., 2014; Rundio et al., 2021). Future studies linking ontogenetic migratory patterns to inter-annual variation in temperature, stream flow, or other environmental factors that reflect climate regimes (flood/drought) or other temporally variable stressors would elucidate whether specific annual behavioral movements between salinity zones are correlated with shifts in environmental conditions. Incorporating growth using fin ray increments into these analyses could improve understanding of what specific environmental factors drive growth patterns, and how these vary spatially and temporally (Rundio et al., 2021). Ultimately, understanding how individual life history types interact spatially and temporally with specific habitats and stressors will support management decisions meant to benefit SFE White Sturgeon, including prioritizing habitat restoration efforts and developing policies to remedy stressors in areas where sturgeon are likely to occur.
Conclusion
This study demonstrated that White Sturgeon exhibit a wide range of migratory behavior during the first decade of life and that most individuals exhibit an amphidromous life history, moving between fresh and brackish water. We revealed four distinct behavioral types that utilized brackish habitats for different portions of their first 10 years. These data fill an important gap in basic biological knowledge of subadult sturgeon migratory behavior and habitat utilization. Individuals or groups that express different migratory behaviors will be exposed to distinct environmental stressors over time and space, and White Sturgeon populations will be more resilient if their diverse ontogenetic migratory behaviors are incorporated into habitat conservation and management strategies.
Data Availability Statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics Statement
Ethical review and approval was not required for the animal study because the samples used in this study were either taken during previous surveys by state or federal entities, or were taken from fish already harvested by recreational anglers.
Author Contributions
KS: conceptualization, project administration, funding acquisition, supervision, and writing and revision. MW: conceptualization, methodology, formal analysis, investigation, data curation, writing and revision, and visualization. LL: conceptualization, formal analysis, writing and revision, and visualization. JS: investigation, data curation, visualization, and revision. JM: conceptualization, funding acquisition, supervision, and revision. JH: conceptualization and revision. All authors contributed to the article and approved the submitted version.
Funding
This research was funded by the U.S. Fish and Wildlife Service grant # F16AC01081-03.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Acknowledgments
California Department of Fish and Wildlife and U.S. Fish and Wildlife Service provided fin rays and metadata for this study. Zachary Jackson, Laura Heironimus, and Cramer Fish Sciences technical staff supported field collections. Zachary Jackson also supported early concept development. Shannon Blackburn and Michael Quist from University of Idaho supported fin ray aging. Michael MacWilliams provided support for spatial salinity analysis and visualization. Justin Glessner from the University of California, Davis Interdisciplinary Center for Plasma Mass Spectrometry lab provided analytical support. Numerous California sturgeon anglers donated fin rays and supported collection of metadata on the sturgeon analyzed in this study.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fmars.2022.859038/full#supplementary-material
References
Allen, P. J., Hobbs, J. A., Cech, J. J., Van Eenennaam, J. P., and Doroshov, S. I. (2009). Using Trace Elements in Pectoral Fin Rays to Assess Life History Movements in Sturgeon: estimating Age at Initial Seawater Entry in Klamath River Green Sturgeon. Trans. Am. Fish. Soc. 138, 240–250. doi: 10.1577/t08-061.1
Anderson, J., Schumer, G., Anders, P., Horvath, K., and Merz, J. (2018). Confirmed Observation: a North American Green Sturgeon Acipenser medirostris Recorded in the Stanislaus River, California. J. Fish Wildl. Manag. 9, 624–630.
Arai, T., Levin, A. V., Boltunov, A. N., and Miyazaki, N. (2002). Migratory history of the Russian sturgeon Acipenser guldenstadti in the Caspian Sea, as revealed by pectoral fin spine Sr:Ca ratios. Mar. Biol. 141, 315–319. doi: 10.1007/s00227-002-0820-y
Baird, S. E., Steel, A. E., Cocherell, D. E., Poletto, J. B., Follenfant, R., and Fangue, N. A. (2020). Experimental assessment of predation risk for juvenile green sturgeon, Acipenser medirostris, by two predatory fishes. J. Appl. Ichthyol. 36, 14–24. doi: 10.1111/jai.13990
Balazik, M. T., Reine, K. J., Spells, A. J., Fredrickson, C. A., Fine, M. L., Garman, G. C., et al. (2012). The potential for vessel interactions with adult Atlantic sturgeon in the James River. Virginia. North Am. J. Fish. Manag. 32, 1062–1069. doi: 10.1080/02755947.2012.716016
Barnett-Johnson, R., Pearson, T. E., Ramos, F. C., Grimes, C. B., and MacFarlane, R. B. (2008). Tracking natal origins of salmon using isotopes, otoliths, and landscape geology. Limnol. Oceanogr. 53, 1633–1642. doi: 10.4319/lo.2008.53.4.1633
Beakes, M. P., Satterthwaite, W. H., Collins, E. M., Swank, D. R., Merz, J. E., Titus, R. G., et al. (2010). Smolt Transformation in Two California Steelhead Populations: effects of Temporal Variability in Growth. Trans. Am. Fish. Soc. 139, 1263–1275. doi: 10.1577/t09-146.1
Beamish, R. J. (1981). Use of fin-ray sections to age walleye pollock, pacific cod, and albacore and the importance of this method. Trans. Am. Fish. Soc. 110, 287–299.
Bemis, W. E., and Kynard, B. (1997). Sturgeon rivers?: an introduction to acipenseriform biogeography and life. Environ. Biol. Fishes 48, 167–183. doi: 10.1023/A:1007312524792
Bever, A. J., MacWilliams, M. L., and Fullerton, D. K. (2018). Influence of an Observed Decadal Decline in Wind Speed on Turbidity in the San Francisco Estuary. Estuaries and Coasts 41, 1943–1967. doi: 10.1007/s12237-018-0403-x
Billard, R., and Lecointre, G. (2001). Biology and conservation of sturgeon and paddlefish. Rev. Fish Biol. Fish. 10, 355–392.
Birstein, V. J. (1993). Sturgeons and Paddlefishes: threatened Fishes in Need of Conservation. Conserv. Biol. 7, 773–787. doi: 10.1046/j.1523-1739.1993.740773.x
Birstein, V. J., Waldman, J. R., and Bemis, W. E. (1997). Sturgeon Biodiversity and Conservation. Netherlands: Springer. doi: 10.1007/0-306-46854-9
Blackburn, S. E. (2018). Population Dynamics and Management for White Sturgeon in the Sacramento-San Joaquin River Basin, California. Moscow: University of Idaho.
Blackburn, S. E., Gingras, M. L., DuBois, J., Jackson, Z. J., and Quist, M. C. (2019). Population Dynamics and Evaluation of Management Scenarios for White Sturgeon in the Sacramento–San Joaquin River Basin. North Am. J. Fish. Manag. 39, 896–912. doi: 10.1002/nafm.10316
Blechschmidt, J., Wittmann, M., and Bluml, C. (2020). Climate Change and Green Sea Turtle Sex Ratio—Preventing Possible Extinction. Genes 11:588. doi: 10.3390/genes11050588
Brainwood, M., Burgin, S., and Byrne, M. (2006). Is the decline of freshwater mussel populations in a regulated coastal river in south-eastern Australia linked with human modification of habitat? Aquat. Conserv. 16, 501–516.
Brennan, S. R., Schindler, D. E., Cline, T. J., Walsworth, T. E., Buck, G., and Fernandez, D. P. (2019). Shifting habitat mosaics and fish production across river basins. Science 364, 783–786. doi: 10.1126/science.aav4313
Brown, L. R., Komoroske, L. M., Wagner, R. W., Morgan-King, T., May, T., Connon, R. E., et al. (2016). Coupled downscaled climate models and ecophysiological metrics forecast habitat compression for an endangered estuarine fish. PLoS One 11:e0146724. doi: 10.1371/journal.pone.0146724
Campana, S. E. (1999). Chemistry and composition of fish otoliths: pathways, mechanisms and applications. Mar. Ecol. Prog. Ser. 188, 263–297. doi: 10.3354/meps188263
Campana, S. E., and Thorrold, S. R. (2001). Otoliths, increments, and elements: keys to a comprehensive understanding of fish populations? Can. J. Fish. Aquat. Sci. 58, 30–38. doi: 10.1139/f00-177
Caswell, H. (2000). Prospective and retrospective perturbation analysis: their roles in conservation biology. Ecology 81, 619–627.
Chapman, F. A., Van Eenennaam, J. P., and Doroshov, S. I. (1996). The reproductive condition of white sturgeon, Acipenser transmontanus, in San Francisco Bay, California. Fish. Bull. 94, 628–634.
Charrad, M., Ghazzali, N., Boiteau, V., and Niknafs, A. (2014). Nbclust: an R package for determining the relevant number of clusters in a data set. J. Stat. Softw. 61, 1–36. doi: 10.18637/jss.v061.i06
Chen, L., Roy, S. B., and Hutton, P. H. (2018). Emulation of a process-based estuarine hydrodynamic model. Hydrol. Sci. J. 63, 783–802. doi: 10.1080/02626667.2018.1447112
Clarke, A. D., Telmer, K. H., and Mark Shrimpton, J. (2007). Elemental analysis of otoliths, fin rays and scales: a comparison of bony structures to provide population and life-history information for the Arctic grayling (Thymallus arcticus). Ecol. Freshw. Fish 16, 354–361. doi: 10.1111/j.1600-0633.2007.00232.x
Cloern, J. E. (2019). Patterns, pace, and processes of water-quality variability in a long-studied estuary. Limnol. Oceanogr. 64, 192–208. doi: 10.1002/lno.10958
Cloern, J. E., Knowles, N., Brown, L. R., Cayan, D., Dettinger, M. D., Morgan, T. L., et al. (2011). Projected evolution of California’s San Francisco bay-delta-river system in a century of climate change. PLoS One 6:e24465. doi: 10.1371/journal.pone.0024465
Collins, M. R., and Smith, T. I. J. (1996). Sturgeon Fin Ray Removal is Nondeleterious. North Am. J. Fish. Manag. 16, 939–941. doi: 10.1577/1548-86751996016<0939:SFRRIN>2.3.CO;2
Cordoleani, F., Phillis, C. C., Sturrock, A. M., FitzGerald, A. M., Malkassian, A., Whitman, G. E., et al. (2021). Threatened salmon rely on a rare life history strategy in a warming landscape. Nat. Clim. Chang. 11, 982–988. doi: 10.1038/s41558-021-01186-4
Cote, J., Clobert, J., Brodin, T., Fogarty, S., and Sih, A. (2010). Personality-dependent dispersal: characterization, ontogeny and consequences for spatially structured populations. Philos. Trans. R. Soc. B Biol. Sci. 365, 4065–4076. doi: 10.1098/RSTB.2010.0176
Cramer, K. L., Jackson, J. B. C., Donovan, M. K., Greenstein, B. J., Korpanty, C. A., Cook, G. M., et al. (2020). Widespread loss of Caribbean acroporid corals was underway before coral bleaching and disease outbreaks. Sci. Adv. 6:eaax9395. doi: 10.1126/sciadv.aax9395
Crozier, L. G., Hendry, A. P., Lawson, P. W., Quinn, T. P., Mantua, N. J., Battin, J., et al. (2008). Potential responses to climate change in organisms with complex life histories: evolution and plasticity in Pacific salmon. Evol. Appl. 1, 252–270. doi: 10.1111/j.1752-4571.2008.00033.x
Crozier, L. G., and Hutchings, J. A. (2014). Plastic and evolutionary responses to climate change in fish. Evol. Appl. 7, 68–87. doi: 10.1111/eva.12135
Demetras, N. J., Helwig, B. A., and McHuron, A. S. (2020). Reported vessel strike as a source of mortality of White Sturgeon in San Francisco Bay. Calif. Fish Game 106, 59–65.
DeVore, J., James, B., and Beamesderfer, R. C. (1999). Lower Columbia River White Sturgeon Current Stock Status and Management Implications. United States: Washington Department of Fish and Wildlife Southwest Regional Office. 1999.
Doukakis, P., Mora, E. A., Wang, S., Reilly, P., Bellmer, R., Lesyna, K., et al. (2020). Postrelease survival of green sturgeon (Acipenser medirostris) encountered as bycatch in the trawl fishery that targets california halibut (Paralichthys californicus), estimated by using pop-up satellite archival tags. Fish. Bull. 118, 63–73. doi: 10.7755/FB.118.1.6
Evans, A. F., Hostetter, N. J., Collis, K., Roby, D. D., and Loge, F. J. (2014). Relationship between Juvenile Fish Condition and Survival to Adulthood in Steelhead. Trans. Am. Fish. Soc. 143, 899–909. doi: 10.1080/00028487.2014.901248
Feutry, P., Tabouret, H., Maeda, K. Pécheyran, C., and Keith, P. (2012). Diadromous life cycle and behavioural plasticity in freshwater and estuarine Kuhliidae species (Teleostei) revealed by otolith microchemistry. Aquat. Biol. 15, 195–204.
Feyrer, F., Sommer, T., and Harrell, W. (2006). Importance of Flood Dynamics versus Intrinsic Physical Habitat in Structuring Fish Communities: evidence from Two Adjacent Engineered Floodplains on the Sacramento River. California. North Am. J. Fish. Manag. 26, 408–417. doi: 10.1577/M05-113.1
Feyrer, F., Sommer, T., and Hobbs, J. (2007). Living in a Dynamic Environment: variability in Life History Traits of Age-0 Splittail in Tributaries of San Francisco Bay. Trans. Am. Fish. Soc. 136, 1393–1405. doi: 10.1577/T06-253.1
Genovart, M., Oro, D., and Tenan, S. (2018). Immature survival, fertility, and density dependence drive global population dynamics in a long-lived species. Ecology 99, 2823–2832. doi: 10.1002/ecy.2515
Grande, J. M., Serrano, D., Tavecchia, G., Carrete, M., Ceballos, O., Díaz-Delgado, R., et al. (2009). Survival in a long-lived territorial migrant: effects of life-history traits and ecological conditions in wintering and breeding areas. Oikos 118, 580–590. doi: 10.1111/j.1600-0706.2009.17218.x
Greene, C. M., Hall, J. E., Guilbault, K. R., and Quinn, T. P. (2010). Improved viability of populations with diverse life-history portfolios. Biol. Lett. 6, 382–386. doi: 10.1098/rsbl.2009.0780
Griffin, J. M., Montañez, I. P., Glessner, J. J. G., Chen, J., and Willmes, M. (2021). Geologic variability of conodont strontium isotopic composition quantified by laser ablation multiple collection inductively coupled plasma mass spectrometry. Palaeogeogr. Palaeoclimatol. Palaeoecol. 568:110308. doi: 10.1016/j.palaeo.2021.110308
Grimaldo, L. F., Sommer, T., Van Ark, N., Jones, G., Holland, E., Moyle, P. B., et al. (2009). Factors Affecting Fish Entrainment into Massive Water Diversions in a Tidal Freshwater Estuary: can Fish Losses be Managed? North Am. J. Fish. Manag. 29, 1253–1270. doi: 10.1577/m08-062.1
Gundersen, D. T., Zeug, S. C., Bringolf, R. B., Merz, J., Jackson, Z., and Webb, M. A. H. (2017). Tissue Contaminant Burdens in San Francisco Estuary White Sturgeon (Acipenser transmontanus): implications for Population Recovery. Arch. Environ. Contam. Toxicol. 73, 334–347. doi: 10.1007/s00244-017-0378-9
Hall, J., Roni, P., Bennett, T., McMillan, J., Hanson, K., Moses, R., et al. (2016). Life History Diversity of Steelhead in Two Coastal Washington Watersheds. Trans. Am. Fish. Soc. 145, 990–1005. doi: 10.1080/00028487.2016.1194893
Heppell, S., Heppell, S., Read, A., and Crowder, L. (2005). Effects of Fishing on Long-lived Marine Organisms. Washington: Island Press. 211–231.
Hermann, T. W., Stewart, D. J., Limburg, K. E., and Castello, L. (2016). Unravelling the life history of Amazonian fishes through otolith microchemistry. R. Soc. Open Sci. 3:160206. doi: 10.1098/rsos.160206
Hestir, E. L., Schoellhamer, D. H., Greenberg, J., Morgan-King, T., and Ustin, S. L. (2016). The Effect of Submerged Aquatic Vegetation Expansion on a Declining Turbidity Trend in the Sacramento-San Joaquin River Delta. Estuar. Coasts 39, 1100–1112. doi: 10.1007/s12237-015-0055-z
Heublein, J. C., Kelly, J. T., Crocker, C. E., Klimley, A. P., and Lindley, S. T. (2009). Migration of green sturgeon, Acipenser medirostris, in the Sacramento River. Environ. Biol. Fishes 84, 245–258. doi: 10.1007/s10641-008-9432-9
Hildebrand, L. R., Drauch Schreier, A., Lepla, K., McAdam, S. O., McLellan, J., Parsley, M. J., et al. (2016). Status of White Sturgeon (Acipenser transmontanus Richardson, 1863) throughout the species range, threats to survival, and prognosis for the future. J. Appl. Ichthyol. 32, 261–312. doi: 10.1111/jai.13243
Hoaglin, D. C., Iglewicz, B., and Tukey, J. W. (1986). Performance of some resistant rules for outlier labeling. J. Am. Stat. Assoc. 81, 991–999.
Hobbs, J. A., Lewis, L. S., Willmes, M., Denney, C., and Bush, E. (2019). Complex life histories discovered in a critically endangered fish. Sci. Rep. 9:16772. doi: 10.1038/s41598-019-52273-8
Hobbs, J. A., Yin, Q., Burton, J., and Bennett, W. A. (2005). Retrospective determination of natal habitats for an estuarine fish with otolith strontium isotope ratios. Mar. Freshw. Res. 56:655. doi: 10.1071/MF04136
Hutton, P. H., Chen, L., Rath, J. S., and Roy, S. B. (2019). Tidally-averaged flows in the interior Sacramento–San Joaquin River Delta: trends and change attribution. Hydrol. Process. 33, 230–243. doi: 10.1002/hyp.13320
Jackson, N. D., Garvey, J. E., and Colombo, R. E. (2007). Comparing aging precision of calcified structures in shovelnose sturgeon. J. Appl. Ichthyol. 23, 525–528. doi: 10.1111/j.1439-0426.2007.00875.x
Jager, H. I., Rose, K. A., and Vila-Gispert, A. (2008). Life History Correlates and Extinction Risk of Capital-breeding Fishes. Netherlands: Springer. 15–25. doi: 10.1007/978-1-4020-8548-2_2
Johnson, R. C., Garza, J. C., MacFarlane, R. B., Grimes, C. B., Phillis, C. C., and Koch, P. L. (2016). Isotopes and genes reveal freshwater origins of Chinook salmon Oncorhynchus tshawytscha aggregations in California’s coastal ocean. Mar. Ecol. Prog. Ser. 548, 181–196. doi: 10.3354/meps11623
Johnston, M., Frantzich, J., Espe, M. B., Goertler, P., Singer, G., Sommer, T., et al. (2020). Contrasting the migratory behavior and stranding risk of White Sturgeon and Chinook Salmon in a modified floodplain of California. Environ. Biol. Fishes 103, 481–493. doi: 10.1007/s10641-020-00974-9
Jonsson, B., and Jonsson, N. (2018). Egg incubation temperature affects the timing of the Atlantic salmon Salmo salar homing migration. J. Fish Biol. 93, 1016–1020. doi: 10.1111/jfb.13817
Kendall, N. W., McMillan, J. R., Sloat, M. R., Buehrens, T. W., Quinn, T. P., Pess, G. R., et al. (2015). Anadromy and residency in steelhead and rainbow trout (Oncorhynchus mykiss): a review of the Processes and Patterns. Can. J. Fish. Aquat. Sci. 72, 319–342. doi: 10.1139/cjfas-2014-0192
Kennedy, B. P., Klaue, A., Blum, J. D., Folt, C. L., and Nislow, K. H. (2002). Reconstructing the lives of fish using Sr isotopes in otoliths. Can. J. Fish. Aquat. Sci. 59, 925–929. doi: 10.1139/F02-070
Kerr, L. A., and Campana, S. E. (2014). Chemical Composition of Fish Hard Parts as a Natural Marker of Fish Stocks. San Diego: Academic Press. 205–234. doi: 10.1016/B978-0-12-397003-9.00011-4
Kimmerer, W. (2004). Open Water Processes of the San Francisco Estuary: from Physical Forcing to Biological Responses Journal. San Francis. Estuar. Watershed Sci. 2. doi: 10.5811/westjem.2011.5.6700
Koch, J. D., and Quist, M. C. (2007). A technique for preparing fin rays and spines for age and growth analysis. North Am. J. Fish. Manag. 27, 782–784. doi: 10.1577/M06-224.1
Koch, J. D., Schreck, W. J., and Quist, M. C. (2008). Standardised removal and sectioning locations for shovelnose sturgeon fin rays. Fish. Manag. Ecol. 15, 139–145. doi: 10.1111/j.1365-2400.2008.00594.x
Kohlhorst, D., Miller, L., and Orsi, J. (1980). Age and growth of white sturgeon collected in the Sacramento-San Joaquin Estuary, California: 1965-1970 and 1973-1976. Calif. Fish Game 66, 83–95.
Kohlhorst, D. W., and Cech, J. (2001). “White Sturgeon” in California’s Living Marine Resources: a Status Report. United States: California Department of Fish and Game. 467–469.
Kovach, R. P., Gharrett, A. J., and Tallmon, D. A. (2012). Genetic change for earlier migration timing in a pink salmon population. Proc. R. Soc. B Biol. Sci. 279, 3870–3878. doi: 10.1098/rspb.2012.1158
Lewis, J., Coath, C. D., and Pike, A. W. G. (2014). An improved protocol for 87Sr/86Sr by laser ablation multi-collector inductively coupled plasma mass spectrometry using oxide reduction and a customised plasma interface. Chem. Geol. 390, 173–181. doi: 10.1016/j.chemgeo.2014.10.021
Lida, Y., Hibiya, K., Inohaya, K., and Kudo, A. (2014). Eda/Edar signaling guides fin ray formation with preceding osteoblast differentiation, as revealed by analyses of the medaka all-fin less mutant afl. Dev. Dyn. 243, 765–777. doi: 10.1002/dvdy.24120
Light, T., and Marchetti, M. P. (2007). Distinguishing between invasions and habitat changes as drivers of diversity loss among California’s freshwater fishes. Conserv. Biol. 21, 434–446. doi: 10.1111/J.1523-1739.2006.00643.X
Lindley, S. T., Erickson, D. L., Moser, M. L., Williams, G., Langness, O. P., Belchik, M., et al. (2011). Electronic tagging of green sturgeon reveals population structure and movement among estuaries. Trans. Am. Fish. Soc. 140, 108–122. doi: 10.1080/00028487.2011.557017
Lower Columbia Fish Recovery Board (2004). Columbia Salmon and Steelhead Recovery and Subbasin Plan. United States: Northwest Power and Conservation Council.
Lugli, F., Cipriani, A., Peretto, C., Mazzucchelli, M., and Brunelli, D. (2017). In situ high spatial resolution 87 Sr/86 Sr ratio determination of two Middle Pleistocene (c.a. 580 ka) Stephanorhinus hundsheimensis teeth by LA–MC–ICP–MS. Int. J. Mass Spectrom. 412, 38–48. doi: 10.1016/j.ijms.2016.12.012
MacWilliams, M., Bever, A., and Foresman, E. (2016). 3-D Simulations of the San Francisco Estuary with Subgrid Bathymetry to Explore Long-Term Trends in Salinity Distribution and Fish Abundance. San. Fr. Estuary Watershed Sci. 14, doi: 10.15447/sfews.2016v14iss2art3
Maechler, M., Rousseeuw, P., Struyf, A., Hubert, M., and Hornik, K. (2015). cluster: cluster Analysis Basics and Extensions. R package version 2.0.3.
Mann, M. E., and Gleick, P. H. (2015). Climate change and California drought in the 21st century. Proc. Natl. Acad. Sci. U. S. A. 112, 3858–3859. doi: 10.1073/pnas.1503667112
McArthur, J. M., Howarth, R. J., and Bailey, T. R. (2001). Strontium Isotope Stratigraphy: lOWESS Version 3: best Fit to the Marine Sr-Isotope Curve for 0–509 Ma and Accompanying Look-up Table for Deriving Numerical Age. J. Geol. 109, 155–170. doi: 10.1086/319243
McCabe, G. J., Wolock, D. M., and Valentin, M. (2018). Warming is driving decreases in snow fractions while runoff efficiency remains mostly unchanged in snow-covered areas of the western United States. J. Hydrometeorol. 19, 803–814. doi: 10.1175/JHM-D-17-0227.1
Miller, E. A., Singer, G. P., Peterson, M. L., Chapman, E. D., Johnston, M. E., Thomas, M. J., et al. (2020). Spatio-temporal distribution of Green Sturgeon (Acipenser medirostris) and White Sturgeon (A. transmontanus) in the San Francisco Estuary and Sacramento River. California. Environ. Biol. Fishes 103, 577–603. doi: 10.1007/s10641-020-00972-x
Miller, L. W. (1970). Migrations of sturgeon tagged in the Sacramento-San Joaquin Estuary. Calif. Fish Game 58, 102–106.
Moiron, M., Laskowski, K. L., and Niemelä, P. T. (2020). Individual differences in behaviour explain variation in survival: a meta-analysis. Ecol. Lett. 23, 399–408. doi: 10.1111/ELE.13438
Mokadem, F., Parkinson, I. J., Hathorne, E. C., Anand, P., Allen, J. T., and Burton, K. W. (2015). High-precision radiogenic strontium isotope measurements of the modern and glacial ocean: limits on glacial–interglacial variations in continental weathering. Earth Planet. Sci. Lett. 415, 111–120. doi: 10.1016/j.epsl.2015.01.036
Moore, J. W., Yeakel, J. D., Peard, D., Lough, J., and Beere, M. (2014). Life-history diversity and its importance to population stability and persistence of a migratory fish: steelhead in two large North American watersheds. J. Anim. Ecol. 83, 1035–1046. doi: 10.1111/1365-2656.12212
Moyle, P., Quiñones, R., Katz, J., and Weaver, J. (2015). Fish Species of Special Concern in California 3rd Edn. Sacramento: California Department of Fish and Wildlife.
Mussen, T. D., Cocherell, D., Poletto, J. B., Reardon, J. S., Hockett, Z., Ercan, A., et al. (2014). Unscreened water-diversion pipes pose an entrainment risk to the threatened green sturgeon, Acipenser medirostris. PLoS One 9:e86321. doi: 10.1371/journal.pone.0086321
National Marine Fisheries Service [NMFS] (2015). Southern Distinct Population Segment of the North American Green Sturgeon Acipenser medirostris: 5-Year Review. United States: NAOO.
Nelson, T. C., Doukakis, P., Lindley, S. T., Schreier, A. D., Hightower, J. E., Hildebrand, L. R., et al. (2013). Research Tools to Investigate Movements, Migrations, and Life History of Sturgeons (Acipenseridae), with an Emphasis on Marine-Oriented Populations. PLoS One 8:e71552. doi: 10.1371/journal.pone.0071552
Nguyen, P. L., Jackson, Z. J., and Peterson, D. L. (2016). Comparison of fin ray sampling methods on white sturgeon Acipenser transmontanus growth and swimming performance. J. Fish Biol. 88, 655–667. doi: 10.1111/jfb.12866
National Marine Fisheries Service [NMFS] (2018). Recovery Plan for the Southern Distinct Population Segment of North American Green Sturgeon (Acipenser medirostris). United States: NAOO.
Nowacek, D. P., Christiansen, F., Bejder, L., Goldbogen, J. A., and Friedlaender, A. S. (2016). Studying cetacean behaviour: new technological approaches and conservation applications. Anim. Behav. 120, 235–244. doi: 10.1016/j.anbehav.2016.07.019
Null, S. E., and Viers, J. H. (2013). In bad waters: water year classification in nonstationary climates. Water Resour. Res. 49, 1137–1148. doi: 10.1002/WRCR.20097
Paragamian, V. L., and Beamesderfer, R. C. P. (2003). Growth Estimates from Tagged White Sturgeon Suggest That Ages from Fin Rays Underestimate True Age in the Kootenai River, USA and Canada. Trans. Am. Fish. Soc. 132, 895–903. doi: 10.1577/t02-120
Parsons, K. M., Everett, M., Dahlheim, M., and Park, L. (2018). Water, water everywhere: environmental DNA can unlock population structure in elusive marine species. R. Soc. Open Sci. 5:180537. doi: 10.1098/rsos.180537
Patton, O., Larwood, V., and Young, M. (2020). Estuarine Habitat Use by White Sturgeon (Acipenser transmontanus). San Fr. Estuary Watershed Sci. 18, 1–10. doi: 10.15447/sfews.2020v18iss4art4
Phillis, C. C., Sturrock, A. M., Johnson, R. C., and Weber, P. K. (2018). Endangered winter-run Chinook salmon rely on diverse rearing habitats in a highly altered landscape. Biol. Conserv. 217, 358–362. doi: 10.1016/j.biocon.2017.10.023
Pikitch, E. K., Doukakis, P., Lauck, L., Chakrabarty, P., and Erickson, D. L. (2005). Status, trends and management of sturgeon and paddlefish fisheries. Fish Fish. 6, 233–265. doi: 10.1111/j.1467-2979.2005.00190.x
Poletto, J. B., Martin, B., Danner, E., Baird, S. E., Cocherell, D. E., Hamda, N., et al. (2018). Assessment of multiple stressors on the growth of larval green sturgeon Acipenser medirostris: implications for recruitment of early life-history stages. J. Fish Biol. 93, 952–960. doi: 10.1111/jfb.13805
Poytress, W. R., Gruber, J. J., Van Eenennaam, J. P., and Gard, M. (2015). Spatial and temporal distribution of spawning events and habitat characteristics of Sacramento River green sturgeon. Trans. Am. Fish. Soc. 144, 1129–1142. doi: 10.1080/00028487.2015.1069213
R Core Team (2021). R: a Language and Environment for Statistical Computing. Vienna: R Foundation for Statistical Computing.
Richerson, K., Jannot, J. E., McVeigh, J., Somers, K., Tuttle, V., and Wang, S. (2019). Observed and Estimated Bycatch of Green Sturgeon in 2002-2017 Us West Coast Groundfish Fisheries. United States: NAOO.
Rieman, B. E., and Beamesderfer, R. C. (1990). White sturgeon in the lower Columbia River: is the stock overexploited. N. Am. J. Fish. Manage. 10, 388–396.
Rien, T. A., and Beamesderfer, R. C. (1994). Accuracy and Precision of White Sturgeon Age Estimates from Pectoral Fin Rays. Trans. Am. Fish. Soc. 123, 255–265. doi: 10.1577/1548-86591994123<0255:AAPOWS>2.3.CO;2
Rochard, E., Castelnaud, G., and Lepage, M. (1990). Sturgeons (Pisces: acipenseridae); threats and prospects. J. Fish Biol. 37, 123–132. doi: 10.1111/j.1095-8649.1990.tb05028.x
Rochet, M. J. (2000). A comparative approach to life-history strategies and tactics among four orders of teleost fish. ICES J. Mar. Sci. J. du Cons. 57, 228–239. doi: 10.1006/jmsc.2000.0641
Ruiz-Campos, G., Castro-Aguirre, J., and Garcia-DeLeon, F. (2011). First specimen of the white sturgeon (Acipenser transmontanus Richardson, 1836) in coastal waters of Mexico with data on its genetic identity. Calif. Fish Game 97, 36–43.
Rundio, D. E., Garza, J. C., Lindley, S. T., Williams, T. H., and Pearse, D. E. (2021). Differences in growth and condition of juvenile Oncorhynchus mykiss related to sex and a migration-associated genomic region. Can. J. Fish. Aquat. Sci. 78, 322–331. doi: 10.1139/CJFAS-2020-0073
Schaffter, R. G. (1997). White sturgeon spawning migrations and location of spawning habitat in the Sacramento River. California. Calif. Fish Game 83, 1–20.
Schindler, D. E., Hilborn, R., Chasco, B., Boatright, C. P., Quinn, T. P., Rogers, L. A., et al. (2010). Population diversity and the portfolio effect in an exploited species. Nature 465, 609–612. doi: 10.1038/nature09060
Sellheim, K., Willmes, M., Hobbs, J. A., Glessner, J. J. G., Jackson, Z. J., and Merz, J. E. (2017). Validating Fin Ray Microchemistry as a Tool to Reconstruct the Migratory History of White Sturgeon. Trans. Am. Fish. Soc. 146, 844–857. doi: 10.1080/00028487.2017.1320305
Sih, A., Cote, J., Evans, M., Fogarty, S., and Pruitt, J. (2012). Ecological implications of behavioural syndromes. Ecol. Lett. 15, 278–289. doi: 10.1111/j.1461-0248.2011.01731.x
Spencer, R. J., Van Dyke, J., Petrov, K., Ferronato, B., McDougall, F., Austin, M., et al. (2018). Profiling a possible rapid extinction event in a long-lived species. Biol. Conserv. 221, 190–197.
Sturrock, A. M., Carlson, S. M., Wikert, J. D., Heyne, T., Nusslé, S., Merz, J. E., et al. (2020). Unnatural selection of salmon life histories in a modified riverscape. Glob. Chang. Biol. 26, 1235–1247. doi: 10.1111/gcb.14896
Sturrock, A. M., Wikert, J. D., Heyne, T., Mesick, C., Hubbard, A. E., Hinkelman, T. M., et al. (2015). Reconstructing the Migratory Behavior and Long-Term Survivorship of Juvenile Chinook Salmon under Contrasting Hydrologic Regimes. PLoS One 10:e0122380. doi: 10.5061/dryad.c56rk
Sweeney, J. K., Willmes, M., Sellheim, K., Lewis, L. S., Hobbs, J. A., Fangue, N. A., et al. (2020). Ontogenetic patterns in the calcification and element incorporation in fin rays of age-0 White Sturgeon. Environ. Biol. Fishes 103, 1401–1418. doi: 10.1007/s10641-020-01031-1
Thorpe, J. (1998). Salmonid life-history evolution as a constraint on marine stock enhancement. Bull. Mar. Sci. 62, 465–475.
Tzadik, O. E., Curtis, J. S., Granneman, J. E., Kurth, B. N., Pusack, T. J., Wallace, A. A., et al. (2017). Chemical archives in fishes beyond otoliths: a review on the use of other body parts as chronological recorders of microchemical constituents for expanding interpretations of environmental, ecological, and life-history changes. Limnol. Oceanogr. Methods 15, 238–263. doi: 10.1002/lom3.10153
Veinott, G., Northcote, T., Rosenau, M., and Evans, R. D. (2011). Concentrations of strontium in the pectoral fin rays of the white sturgeon (Acipenser transmontanus) by laser ablation sampling - inductively coupled plasma - mass spectrometry as an indicator of marine migrations. Can. J. Fish. Aquat. Sci. 56, 1981–1990. doi: 10.1139/f99-120
Vroon, P. Z., van der Wagt, B., Koornneef, J. M., and Davies, G. R. (2008). Problems in obtaining precise and accurate Sr isotope analysis from geological materials using laser ablation MC-ICPMS. Anal. Bioanal. Chem. 390, 465–476. doi: 10.1007/s00216-007-1742-9
Walther, B. D., Limburg, K. E., Jones, C. M., and Schaffler, J. J. (2017). Frontiers in otolith chemistry: insights, advances and applications. J. Fish Biol. 90, 473–479. doi: 10.1111/jfb.13266
Willmes, M., Hobbs, J. A., Sturrock, A. M., Bess, Z., Lewis, L. S., Glessner, J. J. G., et al. (2018a). Fishery collapse, recovery, and the cryptic decline of wild salmon on a major California river. Can. J. Fish. Aquat. Sci. 75, 1836–1848. doi: 10.1139/cjfas-2017-0273
Willmes, M., Ransom, K. M., Lewis, L. S., Denney, C. T., Glessner, J. J. G., and Hobbs, J. A. (2018b). IsoFishR: an application for reproducible data reduction and analysis of strontium isotope ratios (87Sr/86Sr) obtained via laser-ablation MC-ICP-MS. PLoS One 13:e0204519. doi: 10.1371/journal.pone.0204519
Willmes, M., Jacinto, E. E., Lewis, L. S., Fichman, R. A., Bess, Z., Singer, G., et al. (2021). Geochemical Tools Identify the Origins of Chinook Salmon Returning to a Restored Creek. Fisheries 46, 22–32. doi: 10.1002/fsh.10516
Willmes, M., Kinsley, L., Moncel, M.-H., Armstrong, R. A., Aubert, M., Eggins, S., et al. (2016). Improvement of laser ablation in situ micro-analysis to identify diagenetic alteration and measure strontium isotope ratios in fossil human teeth. J. Archaeol. Sci. 70, 102–116. doi: 10.1016/j.jas.2016.04.017
Wood, S. N. (2017). Generalized Additive Models: an Introduction with R. United States: CRC Press Inc.
Yokouchi, K., Fukuda, N., Miller, M. J., Aoyama, J., Daverat, F., and Tsukamoto, K. (2012). Influences of early habitat use on the migratory plasticity and demography of Japanese eels in central Japan. Estuar. Coast. Shelf Sci. 107, 132–140.
Keywords: White Sturgeon, life history diversity, microchemistry, habitat use, migration, San Francisco Estuary
Citation: Sellheim K, Willmes M, Lewis LS, Sweeney J, Merz J and Hobbs JA (2022) Diversity in Habitat Use by White Sturgeon Revealed Using Fin Ray Geochemistry. Front. Mar. Sci. 9:859038. doi: 10.3389/fmars.2022.859038
Received: 20 January 2022; Accepted: 22 February 2022;
Published: 17 March 2022.
Edited by:
Patrick Reis-Santos, University of Adelaide, AustraliaReviewed by:
John Austin Mohan, University of New England, United StatesMatthew McMillan, Department of Agriculture and Fisheries, Queensland Government, Australia
Copyright © 2022 Sellheim, Willmes, Lewis, Sweeney, Merz and Hobbs. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Kirsten Sellheim, kirstens@fishsciences.net
†These authors have contributed equally to this work and share first authorship
 Kirsten Sellheim
Kirsten Sellheim Malte Willmes
Malte Willmes Levi S. Lewis
Levi S. Lewis Jamie Sweeney1
Jamie Sweeney1  Joseph Merz
Joseph Merz James A. Hobbs
James A. Hobbs