Results of the META-Health Study Suggest Pathways by Which Vitamin D Affect Obesity and Cardiovascular Risk through Adiponectin Levels may Require Further Characterization in Subgroups
- 1Department of Community Health and Preventive Medicine, Cardiovascular Research Institute, Morehouse School of Medicine, Atlanta, GA, USA
- 2Department of Medicine, Emory School of Medicine, Atlanta, GA, USA
- 3Department of Family Medicine, Morehouse School of Medicine, Atlanta, GA, USA
- 4Department of Epidemiology, Emory University Rollins School of Public Health, Atlanta, GA, USA
- 5Division of Cardiology, Emory Clinical Cardiovascular Research Institute, Emory School of Medicine, Atlanta, GA, USA
- 6National Heart, Lung, and Blood Institute, National Institutes of Health, Bethesda, MD, USA
Background: Low vitamin D and adiponectin levels are both associated with obesity and cardiovascular disease. Previous studies have indicated that vitamin D levels are directly associated with adiponectin, and that this association varies across body mass index (BMI) categories; stronger with increasing BMI. Few studies examined this association in African-Americans (AA), known to have lower levels of vitamin D and adiponectin, and in whites.
Methods: We assessed whether serum vitamin D is associated with serum adiponectin in a biracial population-based sample. Cross-sectional analyses were performed on 426 non-diabetic participants (218 whites and 208 AA) from the META-Health Study, a random sample from the metro Atlanta. Age-adjusted correlations and multivariable linear regression were used for analyses. We investigated the effect modification of the BMI categories of lean, overweight, and obese as defined by standard cut-points (25 and 30 kg/m2).
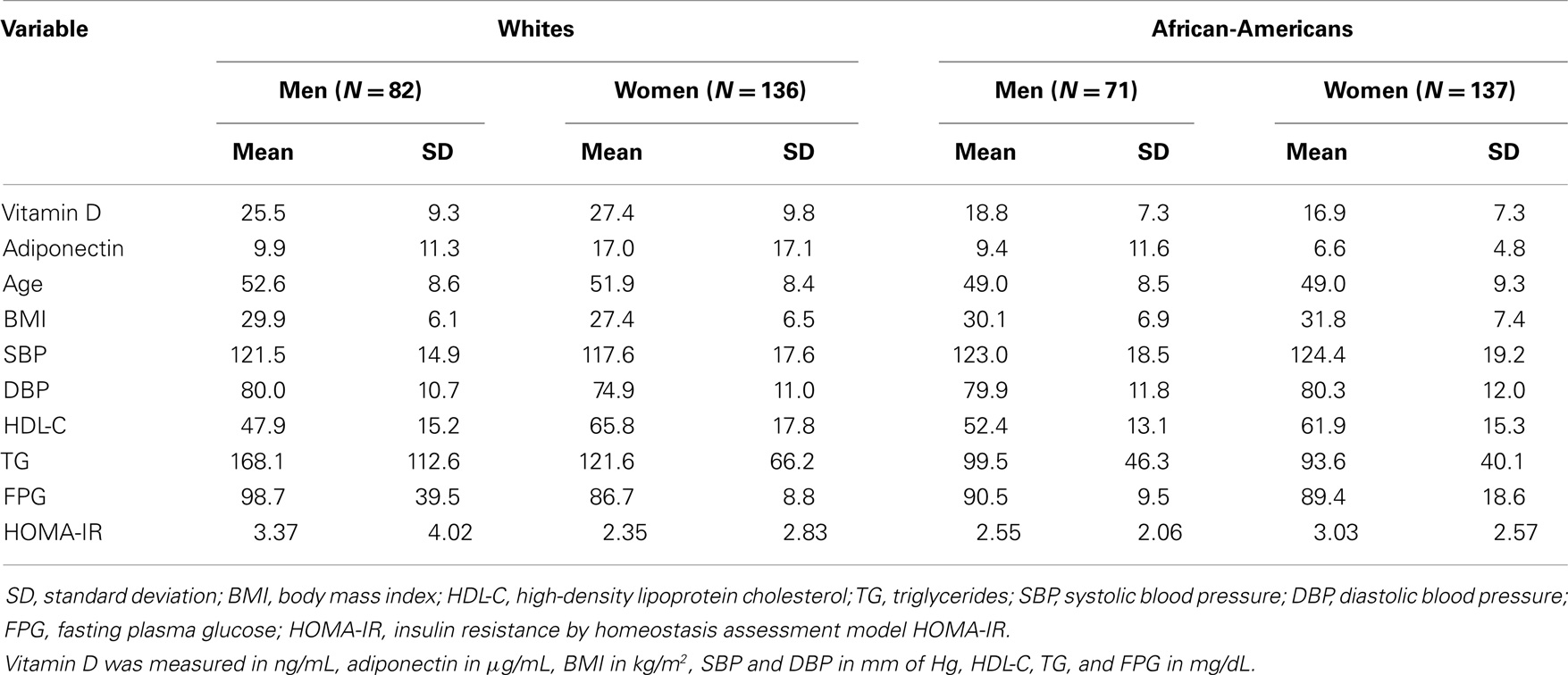
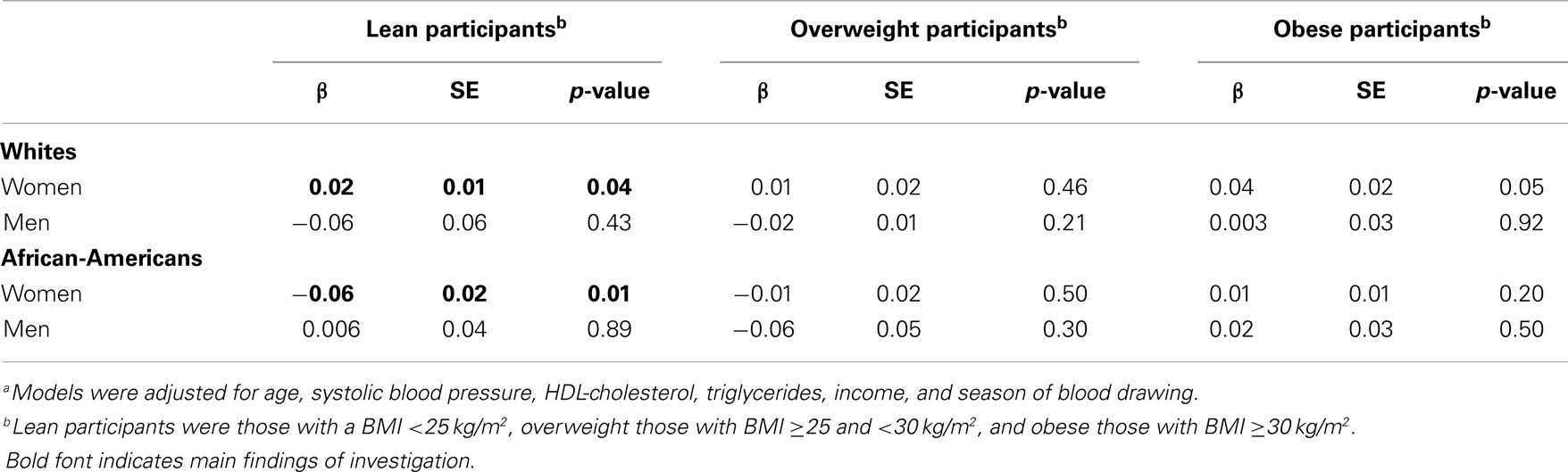
Results: The mean (SD) age of our study sample was 50.5 (9) years. The mean (SD) levels of vitamin D were 27.4 (9.8) ng/mL in white women, 25.5 (9.3) ng/mL in white men, 16.9 (7.3) ng/mL in AA women, and 18.8 (7.3) ng/mL in AA men. The mean (SD) levels of adiponectin were 17.0 (17.1) μg/mL in white women, 9.9 (11.3) μg/mL in white men, 6.6 (4.8) μg/mL in AA women, and 9.4 (11.6) μg/mL in AA men. Among lean white women (n = 63), there was a significant direct association between vitamin D and adiponectin (β = 0.02, p = 0.04) after adjustment for age, systolic blood pressure, HDL-cholesterol, triglycerides, income, and season of blood drawing. On the contrary, in lean AA women (n = 23), there was a significant inverse association (β = −0.06, p = 0.01).
Conclusion: The association of vitamin D and adiponectin is dependent on race, gender, and BMI category. Among lean white women, there was a significant direct association, whereas in lean AA women the association was inverse. No association was present among obese individuals.
Introduction
Obesity is a global epidemic that is associated with low levels of vitamin D and adiponectin, cardiovascular disease, and death (1–5). Vitamin D deficiency, as reflected by circulating 25-hydroxyvitamin D levels <20 ng/mL, is prevalent in as many as half of middle-aged to elderly adults in developed countries (6). Adiponectin, the most abundant adipokine, has been suggested to play an important favorable role in atherosclerosis, endothelial inflammation, myocardial remodeling, and several of the cardiometabolic risk factors (7, 8). Low vitamin D and adiponectin levels are both associated with obesity and cardiovascular disease (9–11). Besides the favorable effect of both vitamin D and adiponectin on insulin resistance (12), vitamin D is an essential steroid metabolite with multiple metabolic effects that includes a negative regulation of the adipose-tissue renin–angiotensin system supposed to inhibit adiponectin secretion (13).
Previous studies have indicated that vitamin D levels are directly associated with adiponectin, and that this association varies across body mass index (BMI) categories, stronger with increasing BMI (14–17). Because both low vitamin D and low-adiponectin levels are associated with increased obesity, the association vitamin D – adiponectin might represent an explanation for the increased cardiovascular risk in obesity. Few studies examined this association in African-Americans (AA), known to have lower levels of vitamin D and adiponectin, and in whites (17).
The present study was designed to assess whether serum vitamin D is associated with serum adiponectin in a biracial population-based sample of whites and AA. We aimed also to determine if race and gender modify the association between vitamin D and adiponectin. Because a higher adiponectin level may reduce cardiovascular risk, studies to evaluate the association of vitamin D and circulating adiponectin, especially in obesity, are warranted.
Materials and Methods
Study Population
The META-Health Study (Morehouse and Emory Team up to eliminate cardiovascular health disparities) is a two-stage study including a random digit dialing (RDD) interview of self-identified white and AA residents of metro Atlanta (Cobb, DeKalb, Fulton, and Gwinnett counties of Georgia), aged 30–66 years, followed by a clinic visit with detailed testing in a subsample (18). The employed sample design was based mainly on the RDD methodology where sample telephone numbers were selected in 100-series telephone banks containing at least one listed household. Nine calls were placed before deciding a party is “unreachable.” Three day attempts, three night attempts, and three weekend attempts were made. The sampling used for this project follows the behavioral risk factor surveillance system (BRFSS) standard for participating area sample designs. Sample records must be justifiable as a probability sample of all households with telephones.
A total of 3,391 individuals were interviewed by phone. All individuals interviewed were asked to come in for a clinic visit to either the Emory or Morehouse Schools of Medicine. Of these, 219 white and 240 AA men and women were examined at the subsequent clinic visit from December 2005 to October 2009 and had adiponectin and vitamin levels measured, constituting approximately 14% of those interviewed by phone. Pregnant women and subjects with acute illnesses were excluded.
We further excluded individuals with missing or incomplete data in any study variables (n = 33). Therefore, 218 white (71 men and 135 women) and 208 AA (71 men and 137 women) persons were included in the analysis. Compared to those who were included in the analysis, there was a lower number of AA (43 vs. 49%), but similar percentage of women (66 vs. 64%) in the 3,391 participants that were initially phone-interviewed. This study was approved by the Emory and Morehouse institutional review boards and all participants gave informed consent.
Covariates
Age, race, and gender were self-identified. Participants were instructed to fast and to refrain from smoking for 12 h before the study visit. During the clinic visit, height was measured with a Portable Shorr Height Measuring Board. All jewelry and hair dressings were removed and participants were left wearing a disposable lightweight gown and shoes, both provided by the study. Participants were asked to stand straight with their back against the measuring board, Frank line horizontal, and heels close together and legs straight. Height was recorded in centimeters to 0.1 cm. Body weight was measured with the S 6600 High-Capacity Floor Scale and weight was recorded in kilograms rounding to the nearest 0.1 kg. From these data we computed the BMI [BMI = weight (kg)/height(m)2). BMI was categorized utilizing a standard classification of normal weight (BMI ≤24.9 kg/m2), overweight (25.0 ≤ BMI <30), and obesity (BMI ≥30 kg/m2). Blood pressure was determined as the average of three measurements taken after 5 min of silent resting. Lipid variables [high-density lipoprotein cholesterol (HDL-C) and triglycerides (TG)], fasting plasma glucose (FPG), and fasting insulin (FI) were measured using standard laboratory techniques. Insulin resistance status was estimated with the homeostasis model assessment (HOMA-IR), as (FPG × FI)/22.5 (19).
Vitamin D and Adiponectin Measurements
Venous blood samples were withdrawn from each subject at baseline examination after more than 8 h of fasting as described elsewhere (18). Serum vitamin D (25-hydroxy-vitamin D) was measured by a liquid chromatography, tandem mass spectrometry (LC/MS/MS) procedure (20). The extraction was done via protein precipitation, and the separation via high-performance liquid chromatography (HPLC). Adiponectin concentration was measured as total adiponectin by an ELISA system (R&D Systems, Minneapolis, MN, USA) (21). The inter-assay coefficient of variation was 8.8%. No biological degradation has been described using stored specimens, indicating a high validity for our measurements.
Statistical Analysis
All analyses were stratified by gender to explore heterogeneities in the independent associations of vitamin D with adiponectin. Descriptive analyses stratified by gender and race/ethnicity, age-adjusted correlations, and multivariable linear regression were used to analyze this association. We investigated the effect modification of the BMI categories of lean, overweight, and obese as defined above.
Linear regression models were used to assess the association of vitamin D with adiponectin with adjustment for age, gender, BMI, systolic blood pressure, HDL-cholesterol, triglycerides, FPG, insulin resistance estimated with the HOMA-IR, income, and season of blood drawing.
The dietary intake of vitamin D, available in a subsample of participants (n = 167, including 68 AA), was used for correlations with serum vitamin D.
All computations were performed using the SAS software version 9.2 (SAS® Institute, Inc., Cary, NC, USA).
Results
Among our 426 study participants, the mean (SD) age of the study sample was 50.5 (9) years (Table 1). The mean (SD) levels of vitamin D were 27.4 (9.8) ng/mL in white women, 25.5 (9.3) ng/mL in white men, 16.9 (7.3) ng/mL in AA (AA) women, and 18.8 (7.3) ng/mL in AA men (Table 1). Thus, the average vitamin D levels among AA were in the deficiency category. The mean (SD) levels of adiponectin were 17.0 (17.1) μg/mL in white women, 9.9 (11.3) μg/mL in white men, 6.6 (4.8) μg/mL in AA women, and 9.4 (11.6) μg/mL in AA men (Table 1). Therefore, the AA women had extremely low-average adiponectin levels, due probably to high-BMI levels. AA participants had a higher BMI average, but a lower mean for triglycerides in both genders (Table 1). HDL-cholesterol was higher in AA men, but lower in AA women (Table 1).
The age-adjusted correlations between vitamin D and adiponectin were r = 0.38 (p < 0.0001) in white women but not significant among white men and among AA. Among whites, vitamin D was statistical significantly correlated with BMI (r = −0.28) in both genders, with HDL-cholesterol (r = 0.29) in women. Among AA, vitamin D was significantly correlated in women with HDL-cholesterol (r = 0.20) and HOMA-IR (r = −0.23). Among white men participants, adiponectin was age-adjusted correlated with HDL-cholesterol (r = 0.34) and triglycerides (r = −0.25), whereas in white women participants adiponectin was correlated with BMI (r = −0.36), HDL-cholesterol (r = 0.48), triglycerides (r = −0.26), and HOMA-IR (r = −0.19). Among AA men participants, adiponectin was significantly correlated with HDL-cholesterol (r = 0.32) and HOMA-IR (r = −0.30), whereas in AA women, adiponectin was correlated with HDL-cholesterol (r = 0.41), triglycerides (r = −0.25), and HOMA-IR (r = −0.28).
There was a borderline effect modification of the association vitamin D – adiponectin by BMI among AA participants by gender subgroups (p = 0.05), but not among white participants. Among lean white women (n = 63), there was a significant direct association between vitamin D and adiponectin (β = 0.02, p = 0.04) after adjustment for age, systolic blood pressure, HDL-cholesterol, triglycerides, income, and season of blood drawing (Table 2). On the contrary, in lean AA women (n = 23), there was a significant inverse association (β = −0.06, p = 0.01) after the same adjustment (Table 2). These estimates remained unchanged (identical) when additionally adjusting for insulin resistance (HOMA-IR).
Dietary vitamin D was significantly associated with serum vitamin D in both white women (r = 0.30) and white men (r = 0.52), but among AA only in women (r = 0.43).
Discussion
Principal Findings
The association of vitamin D and adiponectin is dependent on race, gender, and BMI category. Among lean white women, there was a significant direct association, whereas in lean AA women the association was inverse. No association was present among obese individuals.
In the Context of Previous Literature
Our results are consistent with the observation that AA have lower plasma levels of vitamin D when compared with their white counterparts. As a recent study indicated, AA in the Southeastern U.S. are four times as likely to have a sub-optimal vitamin D plasma concentration (<15 ng/mL) than their white counterparts (22). A direct association between plasma vitamin D and circulating adiponectin has been suggested by a series of previous studies (15, 16, 23, 24). Some studies showed that the association was increased among participants with higher BMI (15, 24). Nevertheless, other studies failed to identify the same trend, probably due to relatively lean participants in some of the previous studies (16). Our metro Atlanta participants had a relatively high BMI and therefore are well suited to contrast the association vitamin D – adiponectin among those relatively lean and those obese.
Contrary to some previous studies, we did not find a direct association between vitamin D and adiponectin among those participants that are obese. In a recent study with Korean participants (25), after adjusting for age, sex, BMI, smoking, and alcohol intake, serum vitamin D levels showed a significant correlation with adiponectin (p < 0.05) level among overweight and obese people (BMI >23) who are likely to be at a risk for cardiovascular disease. In a clinical trial in which 19 vitamin D-deficient peritoneal dialysis patients were treated with cholecalciferol, despite a mean 25(OH)D significantly increase, no change in serum adiponectin after vitamin D replacement was detected (26). Similarly, serum levels of adiponectin were not related with vitamin D and calcium levels in patients with primary hyperparathyroidism (27). Similarly, to our findings in white lean participants, controlling for age, sex, race, sexual maturation, season, physical activity, and percent body fat, 25(OH)D concentrations were significantly correlated with adiponectin (r = 0.06, p = 0.05) among healthy black and white adolescents residing at southern U.S. latitudes with a year-round sunny climate (28).
Potential Mechanisms
Vitamin D negatively regulates the expression of renin, and thus, the activity of the renin–angiotensin system (29, 30). It appears that adipocytes produce all the components of a local adipose-tissue renin–angiotensin system whose activity in turn inhibits adiponectin secretion (31, 32). Since the activity of the adipose-tissue renin–angiotensin system increases with higher adiposity (33), it was previously hypothesized that increased adipose-tissue renin–angiotensin system activity may represent a potential mechanism for the relative low-adiponectin levels seen in obesity (23). The direct association between vitamin D and adiponectin may be mediated by the negative regulation of the adipose-tissue renin–angiotensin system by vitamin D metabolites. As our results showed a direct association vitamin D – adiponectin only among lean white participants, other mechanisms appear to play a more important role. Among those is the observation that vitamin D insufficiency is related to glucose intolerance and type 2 diabetes (34, 35). It has been shown previously that vitamin D supplementation improves insulin resistance as estimated by HOMA-IR, the homeostasis model assessment for insulin resistance (12). As adiponectin is also generally inversely associated with HOMA-IR, the inverse association between vitamin D and adiponectin observed among our AA participants that were lean could not be attributed to such mechanism. Moreover, as mentioned there was no significant correlation between vitamin D and HOMA-IR, and the magnitude of the association vitamin D – adiponectin estimates among our participants did not change after adjustment for HOMA-IR. Therefore, other characteristics of our sample might play an important role. Among those are the relatively low levels of vitamin D among our AA participants that can be explained by their relatively high-BMI average. One of the clinical characteristics associated with vitamin D deficiency is obesity (36, 37). The association between obesity and vitamin D deficiency is either indirect, as obese individuals tend to have less outdoor activity and thus less sunlight exposure, or direct, as vitamin D is fat soluble and may be sequestered and stored in fat tissues (38), which decreases the bioavailability of vitamin D in the circulation.
Therefore, despite a relatively high-average BMI, among our non-lean women participants and among men participants, no direct association between vitamin D and adiponectin was detected. The lower vitamin D due to higher BMI may also explain the higher adiponectin level. If the adipose tissue is absorbing/metabolizing the vitamin D, then this may lead to adiponectin synthesis and release. Thus, the inverse relationship in lean AA women might be a compensatory response to the “normal,” physiological vitamin D, and adiponectin interaction/interrelationship. Failure to detect this in Caucasians may be a consequence of lower vitamin D stored within the adipose tissue.
Strengths and Limitations
The main strength of our investigation is that this study is among the first on the topic with inclusion of both white and AA participants that have a wide range of BMI values. On the other hand, our study sample is localized to one geographical area, so generalizability inherently cannot be inferred. Another important limitation is the fact that we have a limited sample size. To this should be added the inherent fact that the first stage of the META-Health Study (as well as the clinic visit) has a number of epidemiological limitations (e.g., sampling challenges, non-response bias, social response bias, other general selection bias, etc.) that are not fully accounted for.
Conclusion
While our study adds to the evidence base regarding the relationship between vitamin D and adiponectin levels in non-white populations, the results raised a number of unintended questions about how vitamin D and adiponectin interact in circumstances when they are not related to weight status. Further research is clearly needed to evaluate the effects of vitamin D therapy on circulating adiponectin in relationship with normal weight and obesity. The design of the present study, unfortunately, lacks the scope to inform if and how randomized clinical trials (RCT) on vitamin D supplementation should be conducted. RCT investigators should weigh in on these and other emerging data that describe the relationships among vitamin D, adiponectin, and individual characteristics such as race, gender, and BMI category.
The inverse association between vitamin D and adiponectin among lean AA women might explain the lower levels of adiponectin among AA due to a possible reduced negative regulation (e.g., of the adipose-tissue renin–angiotensin system) by vitamin D metabolites.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
The authors thank the other investigators, the staff, and the participants of the META-Health Study for their valuable contributions. The authors also thank Emory General Clinical Research Center (GCRC) and Morehouse Clinical Research Center (CRC) staff for their important contributions. This study was supported by PHS award NIH/NHLBI 1 U01 HL079156-01 (Emory) and 1 U01 HL79214-01 (Morehouse) from the NIH/National Heart Lung and Blood Institute, by the NIH/National Center for Research Resources award M01-RR00039 for the Emory GCRC, by award NIH/NCRR 5P20RR11104 for the Morehouse CRC and by the award NIH/NCRR 5U54RR022814 for the Morehouse CRC. The study’s contents are solely the responsibility of the authors and do not necessarily represent the official views of National Institutes of Health (NIH). The authors would like to acknowledge posthumously the contribution of Dorothy L. Coverson. The results described in this article have been presented in part during the American Heart Association Scientific Sessions Conference, March 2013 in New Orleans, LA, USA.
References
1. Oda E. Obesity-related risk factors of cardiovascular disease. Circ J (2009) 73:2204–5. doi: 10.1253/circj.CJ-09-0653
2. Ogden CL, Carroll MD, McDowell MA, Flegal KM. Obesity among adults in the United States – no change since 2003–2004. NCHS data brief no 1. Hyattsville, MD: National Center for Health Statistics (2007).
3. Perez Perez A, Ybarra Munoz J, Blay Cortes V, de Pablos Velasco P. Obesity and cardiovascular disease. Public Health Nutr (2007) 10:1156–63. doi:10.1017/S1368980007000651
4. Pischon T, Nothlings U, Boeing H. Obesity and cancer. Proc Nutr Soc (2008) 67:128–45. doi:10.1017/S0029665108006976
5. Zalesin KC, Franklin BA, Miller WM, Peterson ED, McCullough PA. Impact of obesity on cardiovascular disease. Endocrinol Metab Clin North Am (2008) 37:663–84. doi:10.1016/j.ecl.2008.06.004
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
6. Holick MF. Vitamin D status: measurement, interpretation, and clinical application. Ann Epidemiol (2009) 19:73–8. doi:10.1016/j.annepidem.2007.12.001
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
7. Ouchi N, Kihara S, Arita Y, Okamoto Y, Maeda K, Kuriyama H, et al. Adiponectin, an adipocyte-derived plasma protein, inhibits endothelial NF-kappaB signaling through a cAMP-dependent pathway. Circulation (2000) 102:1296–301. doi:10.1161/01.CIR.102.11.1296
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
8. Ouchi N, Shibata R, Walsh K. Cardioprotection by adiponectin. Trends Cardiovasc Med (2006) 16:141–6. doi:10.1016/j.tcm.2006.03.001
9. Giovannucci E, Liu Y, Hollis BW, Rimm EB. 25-hydroxyvitamin D and risk of myocardial infarction in men: a prospective study. Arch Intern Med (2008) 168:1174–80. doi:10.1001/archinte.168.11.1174
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
10. Wang TJ, Pencina MJ, Booth SL, Jacques PF, Ingelsson E, Lanier K, et al. Vitamin D deficiency and risk of cardiovascular disease. Circulation (2008) 117:503–11. doi:10.1161/CIRCULATIONAHA.107.706127
11. Walsh K. Adipokines, myokines and cardiovascular disease. Circ J (2009) 73:13–8. doi:10.1253/circj.CJ-08-0961
12. Pittas AG, Harris SS, Stark PC, Dawson-Hughes B. The effects of calcium and vitamin D supplementation on blood glucose and markers of inflammation in nondiabetic adults. Diabetes Care (2007) 30:980–6. doi:10.2337/dc07-0745
13. Vaidya A, Forman JP, Hopkins PN, Seely EW, Williams JS. 25-Hydroxyvitamin D is associated with plasma renin activity and the pressor response to dietary sodium intake in Caucasians. J Renin Angiotensin Aldosterone Syst (2011) 12:311–9. doi:10.1177/1470320310391922
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
14. Young KA, Engelman CD, Langefeld CD, Hairston KG, Haffner SM, Bryer-Ash M, et al. Association of plasma vitamin D levels with adiposity in Hispanic and African Americans. J Clin Endocrinol Metab (2009) 94:3306–13. doi:10.1210/jc.2009-0079
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
15. Gannage-Yared MH, Chedid R, Khalife S, Azzi E, Zoghbi F, Halaby G. Vitamin D in relation to metabolic risk factors, insulin sensitivity and adiponectin in a young Middle-Eastern population. Eur J Endocrinol (2009) 160:965–71. doi:10.1530/EJE-08-0952
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
16. Nimitphong H, Chanprasertyothin S, Jongjaroenprasert W, Ongphiphadhanakul B. The association between vitamin D status and circulating adiponectin independent of adiposity in subjects with abnormal glucose tolerance. Endocrine (2009) 36:205–10. doi:10.1007/s12020-009-9216-9
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
17. Vaidya A. Vitamin D and cardio-metabolic disease. Metabolism (2013) 62:1697–9. doi:10.1016/j.metabol.2013.08.009
18. Morris AA, Zhao L, Patel RS, Jones DP, Ahmed Y, Stoyanova N, et al. Differences in systemic oxidative stress based on race and the metabolic syndrome: the Morehouse and Emory team up to eliminate health disparities (META-Health) study. Metab Syndr Relat Disord (2012) 10:252–9. doi:10.1089/met.2011.0117
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
19. Matthews DR, Hosker JP, Rudenski AS, Naylor BA, Treacher DF, Turner RC. Homeostasis model assessment: insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia (1985) 28:412–9. doi:10.1007/BF00280883
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
20. Maunsell Z, Wright DJ, Rainbow SJ. Routine isotope-dilution liquid chromatography-tandem mass spectrometry assay for simultaneous measurement of the 25-hydroxy metabolites of vitamins D2 and D3. Clin Chem (2005) 51:1683–90. doi:10.1373/clinchem.2005.052936
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
21. Bidulescu A, Liu J, Musani SK, Fox ER, Samdarshi TE, Sarpong DF, et al. Association of adiponectin with left ventricular mass in blacks: the Jackson Heart Study. Circ Heart Fail (2011) 4:747–53. doi:10.1161/CIRCHEARTFAILURE.110.959742
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
22. Egan KM, Signorello LB, Munro HM, Hargreaves MK, Hollis BW, Blot WJ. Vitamin D insufficiency among African-Americans in the southeastern United States: implications for cancer disparities (United States). Cancer Causes Control (2008) 19:527–35. doi:10.1007/s10552-008-9115-z
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
23. Vaidya A, Forman JP, Underwood PC, Hopkins PN, Williams GH, Pojoga LH, et al. The influence of body mass index and renin-angiotensin-aldosterone system activity on the relationship between 25-hydroxyvitamin D and adiponectin in Caucasian men. Eur J Endocrinol (2011) 164:995–1002. doi:10.1530/EJE-11-0025
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
24. Vaidya A, Williams JS, Forman JP. The independent association between 25-hydroxyvitamin D and adiponectin and its relation with BMI in two large cohorts: the NHS and the HPFS. Obesity (Silver Spring) (2012) 20:186–91. doi:10.1038/oby.2011.210
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
25. Kim M, Na W, Sohn C. Correlation between vitamin D and cardiovascular disease predictors in overweight and obese Koreans. J Clin Biochem Nutr (2013) 52:167–71. doi:10.3164/jcbn.12-81
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
26. Ulutas O, Taskapan H, Taskapan MC, Temel I. Vitamin D deficiency, insulin resistance, serum adipokine, and leptin levels in peritoneal dialysis patients. Int Urol Nephrol (2013) 45:879–84. doi:10.1007/s11255-012-0308-8
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
27. de Luis DA, Soto GD, Conde R, Izaola O, de la Fuente B. Relation of leptin and adiponectin with cardiovascular risk factors, intact parathormone, and vitamin D levels in patients with primary hyperparathyroidism. J Clin Lab Anal (2012) 26:398–402. doi:10.1002/jcla.21541
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
28. Parikh S, Guo DH, Pollock NK, Petty K, Bhagatwala J, Gutin B, et al. Circulating 25-hydroxyvitamin D concentrations are correlated with cardiometabolic risk among American black and white adolescents living in a year-round sunny climate. Diabetes Care (2012) 35:1133–8. doi:10.2337/dc11-1944
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
29. Li YC, Kong J, Wei M, Chen ZF, Liu SQ, Cao LP. 1,25-Dihydroxyvitamin D(3) is a negative endocrine regulator of the renin-angiotensin system. J Clin Invest (2002) 110:229–38. doi:10.1172/JCI15219
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
30. Lee KW, Lip GY. Insulin resistance and vascular remodelling, in relation to left ventricular mass, geometry and function: an answer to LIFE? J Hum Hypertens (2003) 17:299–304. doi:10.1038/sj.jhh.1001561
31. Engeli S, Negrel R, Sharma AM. Physiology and pathophysiology of the adipose tissue renin-angiotensin system. Hypertension (2000) 35:1270–7. doi:10.1161/01.HYP.35.6.1270
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
32. Kim S, Soltani-Bejnood M, Quignard-Boulange A, Massiera F, Teboul M, Ailhaud G, et al. The adipose renin-angiotensin system modulates systemic markers of insulin sensitivity and activates the intrarenal renin-angiotensin system. J Biomed Biotechnol (2006) 2006:27012. doi:10.1155/JBB/2006/27012
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
33. Yasue S, Masuzaki H, Okada S, Ishii T, Kozuka C, Tanaka T, et al. Adipose tissue-specific regulation of angiotensinogen in obese humans and mice: impact of nutritional status and adipocyte hypertrophy. Am J Hypertens (2010) 23:425–31. doi:10.1038/ajh.2009.263
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
34. Scragg R, Sowers M, Bell C. Serum 25-hydroxyvitamin D, diabetes, and ethnicity in the Third National Health and Nutrition Examination Survey. Diabetes Care (2004) 27:2813–8. doi:10.2337/diacare.27.12.2813
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
35. Chiu KC, Chu A, Go VL, Saad MF. Hypovitaminosis D is associated with insulin resistance and beta cell dysfunction. Am J Clin Nutr (2004) 79:820–5.
36. Konradsen S, Ag H, Lindberg F, Hexeberg S, Jorde R. Serum 1,25-dihydroxy vitamin D is inversely associated with body mass index. Eur J Nutr (2008) 47:87–91. doi:10.1007/s00394-008-0700-4
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
37. Parikh SJ, Edelman M, Uwaifo GI, Freedman RJ, Semega-Janneh M, Reynolds J, et al. The relationship between obesity and serum 1,25-dihydroxy vitamin D concentrations in healthy adults. J Clin Endocrinol Metab (2004) 89:1196–9. doi:10.1210/jc.2003-031398
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Keywords: vitamin D, adiponectin, obesity, minorities, African-Americans, obesity
Citation: Bidulescu A, Morris AA, Stoyanova N, Meng Y-X, Vaccarino V, Quyyumi AA and Gibbons GH (2014) Association between vitamin D and adiponectin and its relationship with body mass index: the META-Health Study. Front. Public Health 2:193. doi: 10.3389/fpubh.2014.00193
Received: 29 July 2014; Paper pending published: 19 September 2014;
Accepted: 29 September 2014; Published online: 14 October 2014.
Edited by:
Hilda Toth Maibach, Social & Scientific Systems, Inc., USAReviewed by:
Mildred Audrey Pointer, North Carolina Central University, USATony Kuo, Los Angeles County Department of Public Health, USA
Copyright: © 2014 Bidulescu, Morris, Stoyanova, Meng, Vaccarino, Quyyumi and Gibbons. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) or licensor are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Aurelian Bidulescu, Department of Epidemiology and Biostatistics, Indiana University School of Public Health – Bloomington, 1025 E 7th Street, Bloomington, IN 47405, USA e-mail: abidules@indiana.edu
 Aurelian Bidulescu
Aurelian Bidulescu Alanna A. Morris2
Alanna A. Morris2 Yuan-Xiang Meng
Yuan-Xiang Meng Viola Vaccarino
Viola Vaccarino Arshed A. Quyyumi
Arshed A. Quyyumi