- 1Department of Neurosurgery, First Hospital of Shanxi Medical University, Taiyuan, China
- 2Cerebrovascular Disease Center, First Hospital of Shanxi Medical University, Taiyuan, China
- 3Center for Cerebrovascular Diseases Research, Shanxi Medical University, Taiyuan, China
- 4MOE Key Laboratory of Coal Environmental Pathogenicity and Prevention, Shanxi Medical University, Taiyuan, China
- 5Department of Emergency, First Hospital of Shanxi Medical University, Taiyuan, China
- 6School of Basic Medicine, Shanxi Medical University, Taiyuan, China
- 7Department of Neurosurgery, Beijing Tiantan Hospital, Capital Medical University, Beijing, China
- 8National Clinical Research Center for Neurological Diseases, Beijing, China
- 9Department of Environmental Health, School of Public Health, Shanxi Medical University, Taiyuan, China
- 10Key Laboratory of Environment and Female Reproductive Health, The Eighth Affiliated Hospital, Sun Yat-sen University, Guangzhou, Shenzhen, China
Background: Moyamoya disease (MMD) is an idiopathic, chronic intracranial vascular stenosis and occlusion disease. However, there is currently a lack of comprehensive analysis on the clinical and radiological course of asymptomatic MMD (AMMD) and hemodynamically stable MMD (HSMMD).
Data source: We conducted a comprehensive literature search using major bibliographic indexing databases, including Embase, Medline, PubMed, Web of Science, and Cochrane Library.
Methods: This systematic review was conducted based on the PRISMA guidelines. The quality of the included studies was accessed using the Methodological Index for Non-Randomized Studies (MINORS). Effect sizes were pooled with a random-effects model. Heterogeneity between studies was estimated via the I2 test. Publication bias was assessed with Egger’s test. The registration code is CRD42023444432.
Result: A total of seven AMMD studies were included in a meta-analysis, involving 393 patients and 649 hemispheres. Three HSMMD studies were all from the same institution. The pooled rate for clinical progression, hemorrhagic stroke, ischemic stroke, transient ischemic attack (TIA), and radiological progress of conservative group was 10% (95% CI 4.9–15.1%), 3.8% (95% CI 0.4–7.2%), 0.7% (95% CI 0–2.3%), 3.6% (95% CI 0.6–6.6%), and 15.6% (95% CI 10.2–22.1%), respectively. The pooled rate for stroke, TIA, and radiological progress of the surgical group was 3.7% (95% CI 0–10.8%), 0.2% (95% CI 0–3.0%), and 4.8% (95% CI 0–10.5%), respectively. Revascularization did not show a protective effect on TIA and radiological progression for AMMD.
Conclusion: AMMD and HSMMD present a concerning risk of clinical and radiological progression over a follow-up period of more than 2 years. Further high-quality studies are needed to optimize treatment strategies.
Systematic review registration: https://www.crd.york.ac.uk/prospero/display_record.php?RecordID=444432, CRD42023444432.
1 Introduction
Moyamoya disease (MMD) is an idiopathic chronic intracranial vascular steno-occlusive disease characterized by progressive stenosis of the terminal segments of the internal carotid arteries bilaterally and/or partial stenosis or occlusion of the proximal portions of the anterior cerebral arteries and the middle cerebral arteries and formation of an abnormal network of vessels at the base of the skull (1, 2). MMD was first reported in Japan in the 1950s and has gradually gained worldwide recognition. The annual morbidity of MMD is 0.5–1.5 per 100,000 in East Asia and 0.1 in other regions (3). Based on hemodynamic stability, MMD can be classified into hemodynamically unstable MMD and hemodynamically stable MMD (HSMMD). Multiple techniques can be used to assess hemodynamic status, including single-photon emission computed tomography (SPECT), perfusion computed tomography (CT), and dynamic susceptibility contrast magnetic resonance imaging (MRI) (4). However, the majority of the studies use normal or slight decrease in basal perfusion and a decrease in the reserve capacity after acetazolamide challenge at less than 50% of the basal perfusion on SPECT to identify HSMMD (5). According to the occurrence of clinical symptoms, MMD can be classified as asymptomatic MMD (AMMD) and symptomatic MMD. Common clinical manifestations include intracranial hemorrhage, cerebral infarction, transient ischemic attack (TIA), and epileptic seizures, which may occur in some patients. Asymptomatic MMD is a special and important one, which refers to patients who have not experienced hemorrhage, ischemia, and other symptoms of neurological deficits before incidental diagnosis (6). At present, the gold standard for the diagnosis of MMD is still digital subtraction angiography (DSA) (4). Considering the implementation conditions and invasiveness of DSA, perfusion and angiography methods based on CT and MRI are the alternative options. With the continuous popularization of diagnostic technology, the detection rate of AMMD continues to increase (7). In a nationwide survey conducted in Japan in 1994, AMMD accounted for 1.5% of all cases of MMD; however, in a detailed survey conducted in Japan in 2008, this proportion rose to 17.8% (8, 9). Therefore, the actual proportion of AMMD may be higher.
Current treatment options for MMD are categorized as non-surgical (conservative observation and antiplatelet agents) and surgical (surgical revascularization with direct or indirect bypasses or a combination of both). The application of endovascular intervention in MMD has not shown satisfactory results, but it serves as the preferred therapy for posterior circulation aneurysms combined with MMD (10). Revascularization has been shown to be effective in improving the prognosis and reducing the risk of adverse events in patients with symptomatic MMD (11–13). In HSMMD and AMMD patients, the natural history of the disease is uncertain, with a variety of factors influencing it (5); the long-term outcome is unclear, and the short- and long-term benefits of surgical treatment have varied from study to study (13). At present, some theories believe that symptomatic MMD should be treated with surgery regardless of the hemodynamic status. However, due to the inconsistency of hemodynamic status assessment methods and standards, the determination and corresponding treatment of patients with HSMMD still need to be explored (4). Treatments of AMMD still require the support of evidence-based medicine. In the natural course of AMMD, it is crucial to balance the risks of stroke or hemorrhage and the risks of complications caused by treatment for the benefit of patients.
However, there is currently a lack of comprehensive analysis of the natural course and post-treatment process of AMMD and HSMMD. This systematic review and meta-analysis will summarize the existing literature, clarify the natural disease course and post-treatment process of AMMD and HSMMD, and help medical workers and health policymakers formulate optimal treatment options.
2 Materials and methods
2.1 Search strategy
The present systematic review is written in accordance with the PRISMA statement (14). The review protocol has been registered in the PROSPERO system (CRD420234444321). The eligible articles in Embase, Medline, PubMed, Web of Science, and Cochrane Library databases from the beginning to November 2023 were systematically searched. We conducted the second search in November 2024 to include new published works of literature from 2023 to 2024. Keywords used to construct search terms include “asymptomatic,” “hemodynamically stable,” and “moyamoya disease.” The complete search queries can be obtained in the Supplementary material.
2.2 Outcome definitions
The outcomes included in this study were clinical progress, radiological progression, TIA, and stroke incidence during follow-up. Clinical progress is defined as neurological symptoms associated with MMD, including TIA, ischemia, and hemorrhagic symptoms. Radiological progress includes progression in the MMD stage or new anomalies on MRI, CT, or perfusion imaging. Given that the mean follow-up time for most included studies was within 2–5 years, the mortality rate of AMMD was extremely low between this time horizon, and almost all studies did not describe mortality, this systematic review did not include mortality as the primary outcome.
2.3 Inclusion and exclusion criteria
The studies included must reach the following criteria: (1) studies reporting the natural or post-treatment clinical course of AMMD or HSMMD; (2) the study should report any of the aforementioned outcomes; (3) either prospective or retrospective study is qualified; (4) if a study reports symptomatic MMD or contains pediatric patients, the data should be represented severally; and (5) the mean or median follow-up time should be more than 12 months.
The exclusion criteria: (1) studies are reviews, letters, meta-analyses, case reports, or comments; (2) the study cohort is all pediatric or adult outcome data in the cohort are inseparable; (3) fewer than 10 patients in the entire cohort; (4) the study contains overlapping cohorts; and (5) non-English published studies. Cohorts from the same institution and overlap period is considered as overlapping cohorts, and only one of them will be included in the meta-analysis.
2.4 Data extraction
Four authors independently conducted the retrieval, screening, and data extraction. The disagreements are resolved by consensus or decided by senior researchers. The extracted data included study basic information (year, journal, author, country of publication, and study type), cohort baseline information (number of patients/hemispheres included, age, gender, concomitant disease, Suzuki stage, and follow-up time), and study outcomes. For the overlapping cohort, we will include the higher-quality study. If any data is not available, we will contact the corresponding author to obtain the complete data for analysis.
2.5 Quality assessment
Four authors independently conducted quality assessment, and the disagreements were resolved by consensus. Studies were appraised by the methodological index for the non-randomized study (MINORS) scale (15). The scale includes eight assessing items for non-comparative studies and four additional items for comparative studies.
2.6 Statistical analysis
Using R and R package “meta” for analysis. Calculate the pooled effect sizes and 95% confidence intervals (CIs) for outcomes. All analyses were performed using a random effect model. Heterogeneity was detected with the I2 and 95% CI. Using Egger’s test to evaluate publication bias for pooling ≥5 studies. Data expressed as mean ± standard deviation, mean (range), or number of events (percentage). Other data formats will be marked.
3 Results
3.1 Screening process
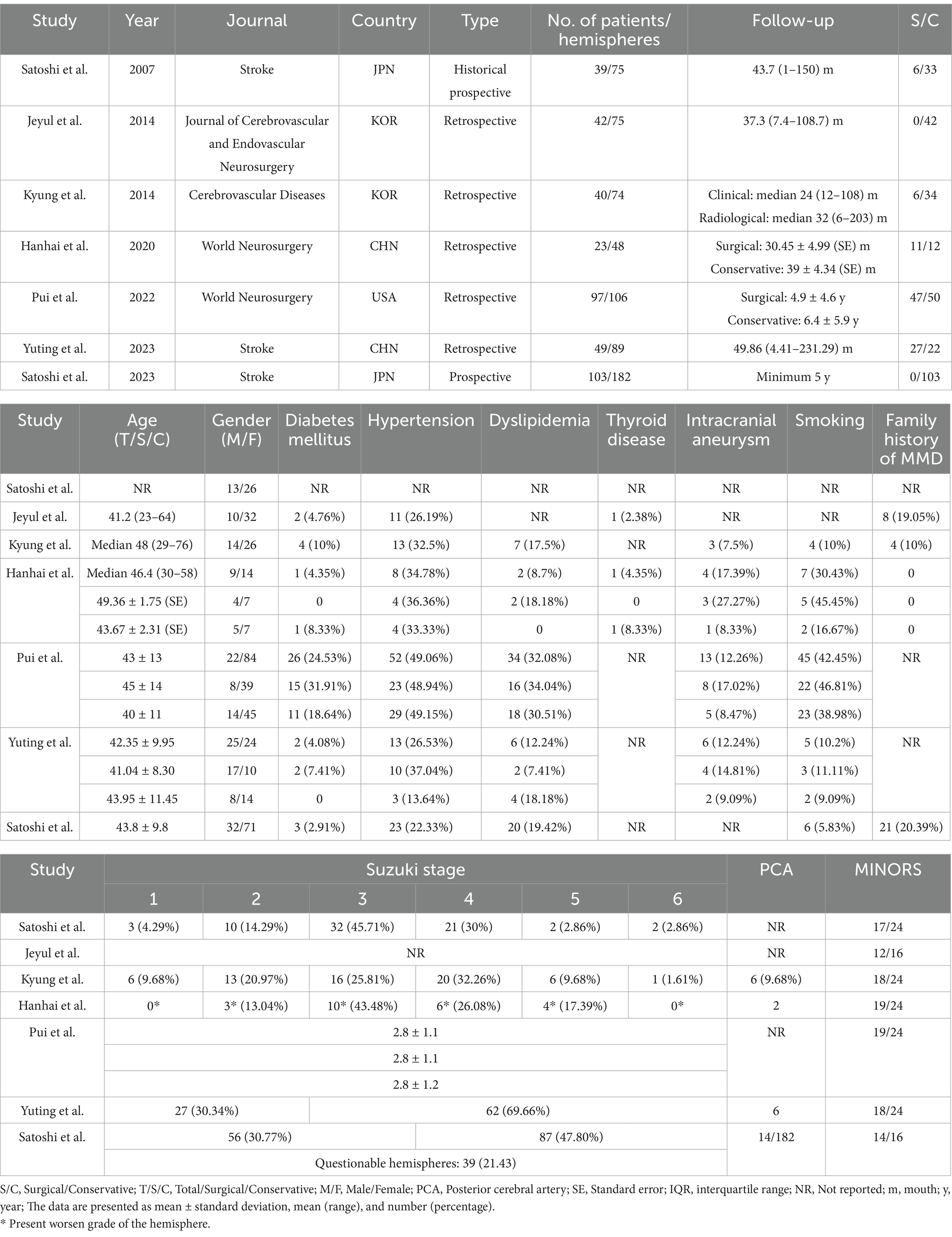
We evaluated a total of 522 unique publications, of which 508 were excluded (Figure 1). We performed data extraction on 14 studies, including six overlapping cohort studies, of which three are HSMMD cohorts (Tables 1, 2) (16). A total of three studies included pediatric patients, with data from one of which is inseparable.
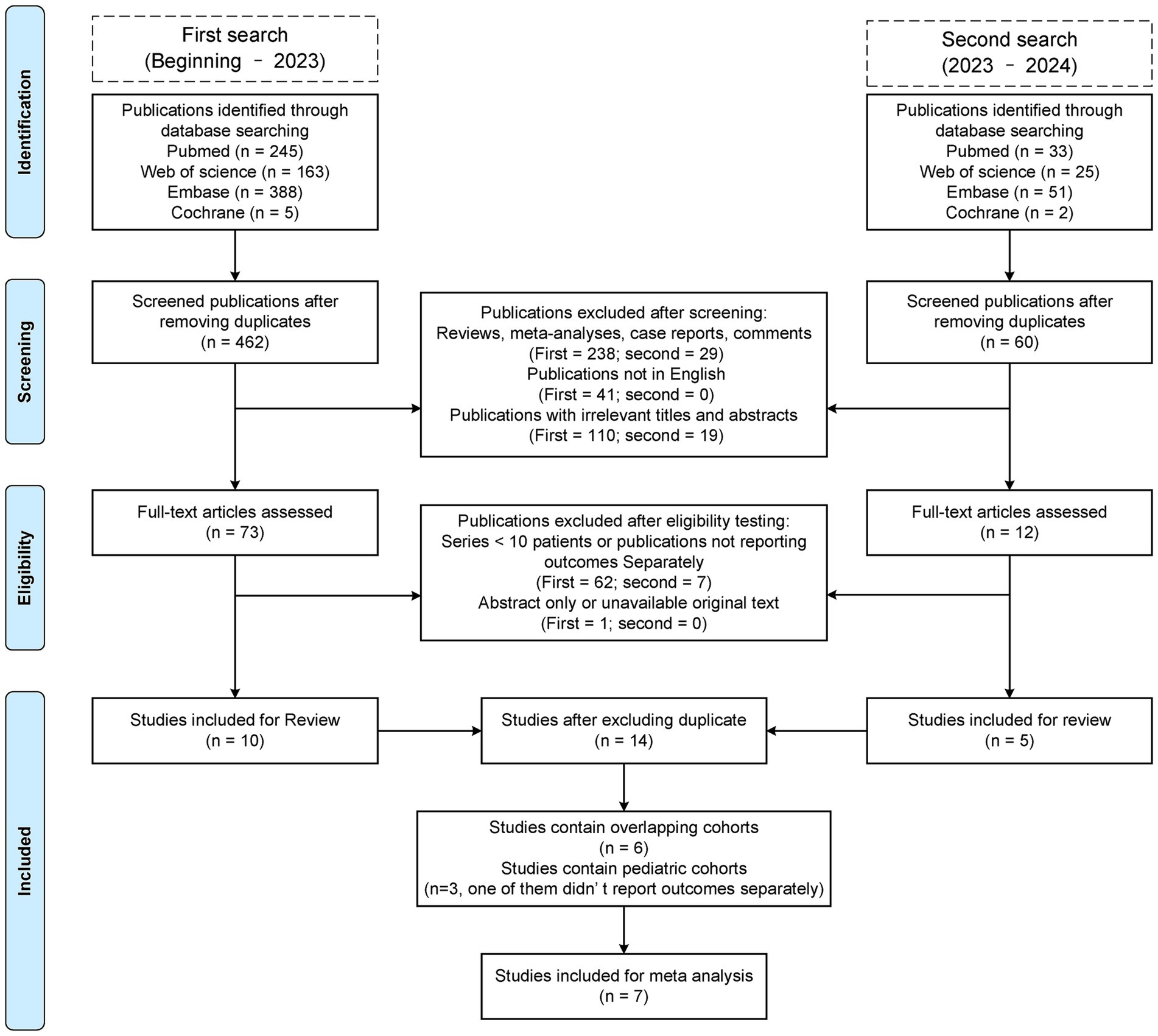
Figure 1. Preferred reporting items for systematic reviews and meta-analysis (PRISMA) flow diagram of this review.
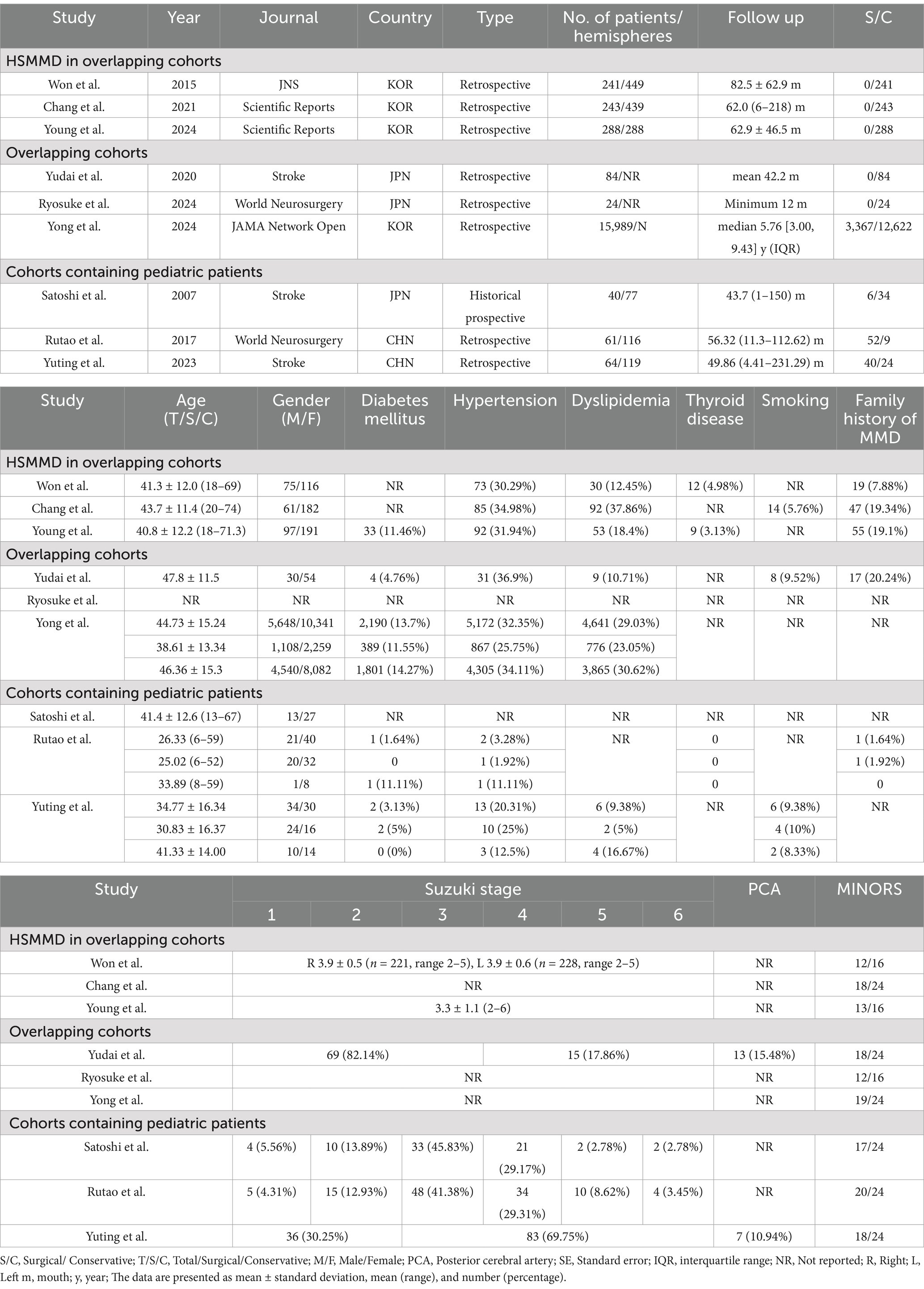
Table 2. Baseline information for overlapping cohorts, HSMMD cohorts, and cohorts containing pediatric patients.
3.2 Baseline information of the studies included in meta-analysis
Following the screening process, a total of seven studies were included in the meta-analysis, involving 393 patients and 649 hemispheres (6, 13, 17–21). All patients are adult AMMD. The study designs included two prospective and five retrospective studies (Table 1). The sample sizes of studies varied between 23 and 103 patients. Except the study by Yuting et al., female patients outnumbered male patients. Most patients had a mean age over 40 years (Table 1). Most studies included both surgical and conservative groups, except for two studies that focused solely on conservative groups. Comorbidities information was available for the vast majority of studies, including diabetes mellitus, hypertension, dyslipidemia, intracranial aneurysm, and smoking, while thyroid disease information was provided in only two studies. Four studies reported information on family history of MMD, and four studies provided information about posterior cerebral artery involvement. All studies described the follow-up duration. Suzuki’s angiographical stage was described in most studies, except for the study by Jeyul et al. A total of five studies were comparative, while two studies were non-comparative. The median scores of non-comparative studies were 13 (12–14), and those of comparative studies were 18 (17–19) (Table 1).
3.3 Pooled outcome of AMMD
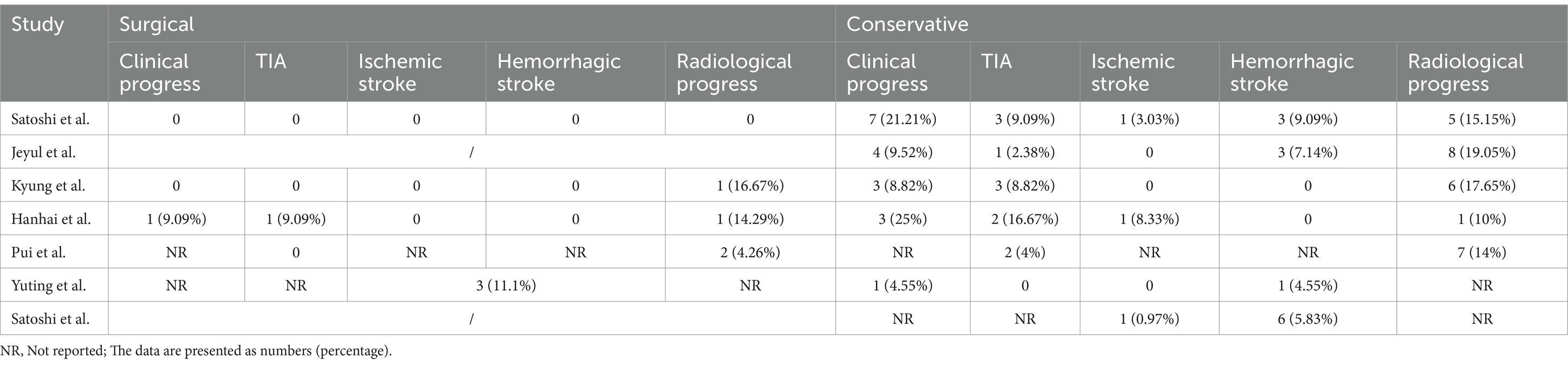
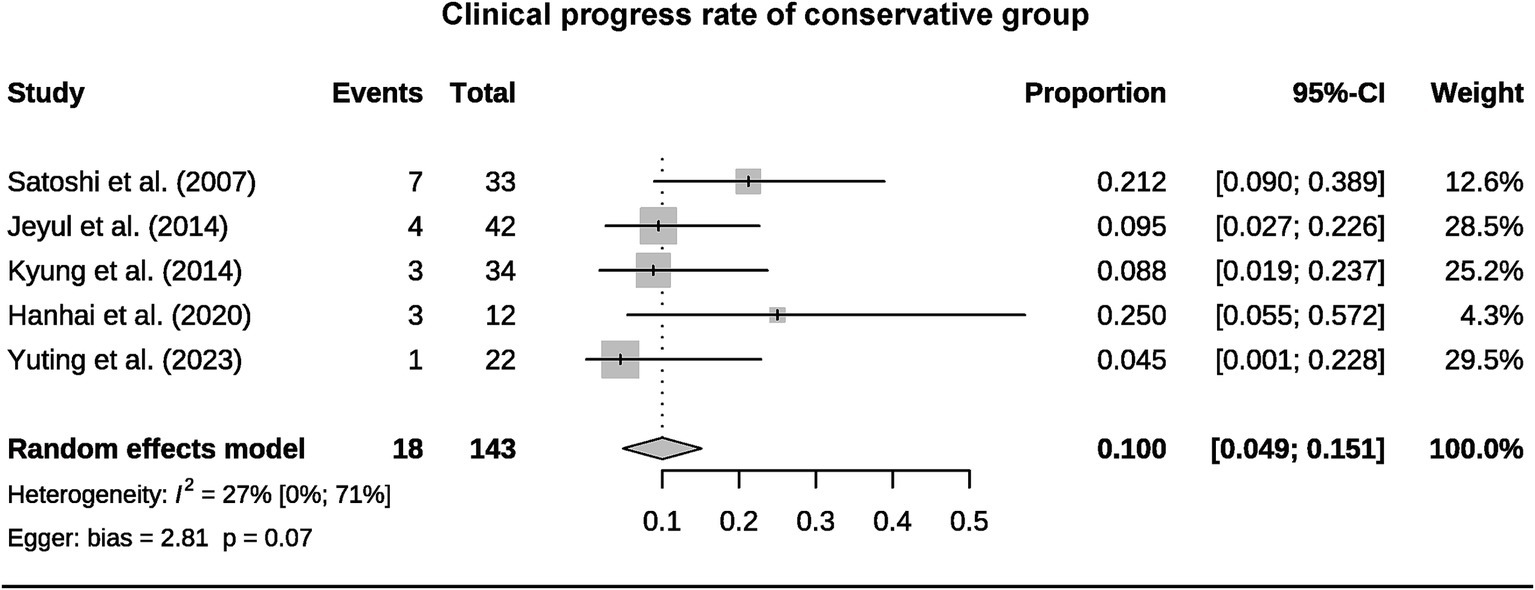
A total of 393 patients were included in the analysis, including 97 cases (25%) that underwent surgical treatment and 296 cases (75%) that underwent conservative treatment (Table 3). Among the conservative group, clinical progression was available for five studies, and the pooled rate was 10% (95% CI 4.9–15.1%) (Figure 2). Six studies provided data on stroke in the conservative group, showing the pooled rate of hemorrhagic stroke of 3.8% (95% CI 0.4–7.2%) and ischemic stroke of 0.7% (95% CI 0–2.3%). The pooled stroke incident rate for the surgical group, based on pooled four studies, was 3.7% (95% CI 0–10.8%) (Figure 3). Four studies compared the incident rate of TIA for the surgical vs. conservative groups, and the rate difference was not significant (OR 0.47, 95% CI 0.11–2.01). The pooled TIA rates were 0.2% in the surgical group and 3.6% in the conservative group (Figure 4). The pooled radiological progress rates were 4.8% in the surgical group and 15.6% in the conservative group. The radiological progress comparison between the surgical and conservative groups showed no significant difference (OR 0.49, 95% CI 0.16–1.52), pooled from four studies (Figure 5). The above-pooled results did not show significant heterogeneity. Publication bias was detected in the TIA pooled rate of the conservative group, which might be caused by small sample effects (22).
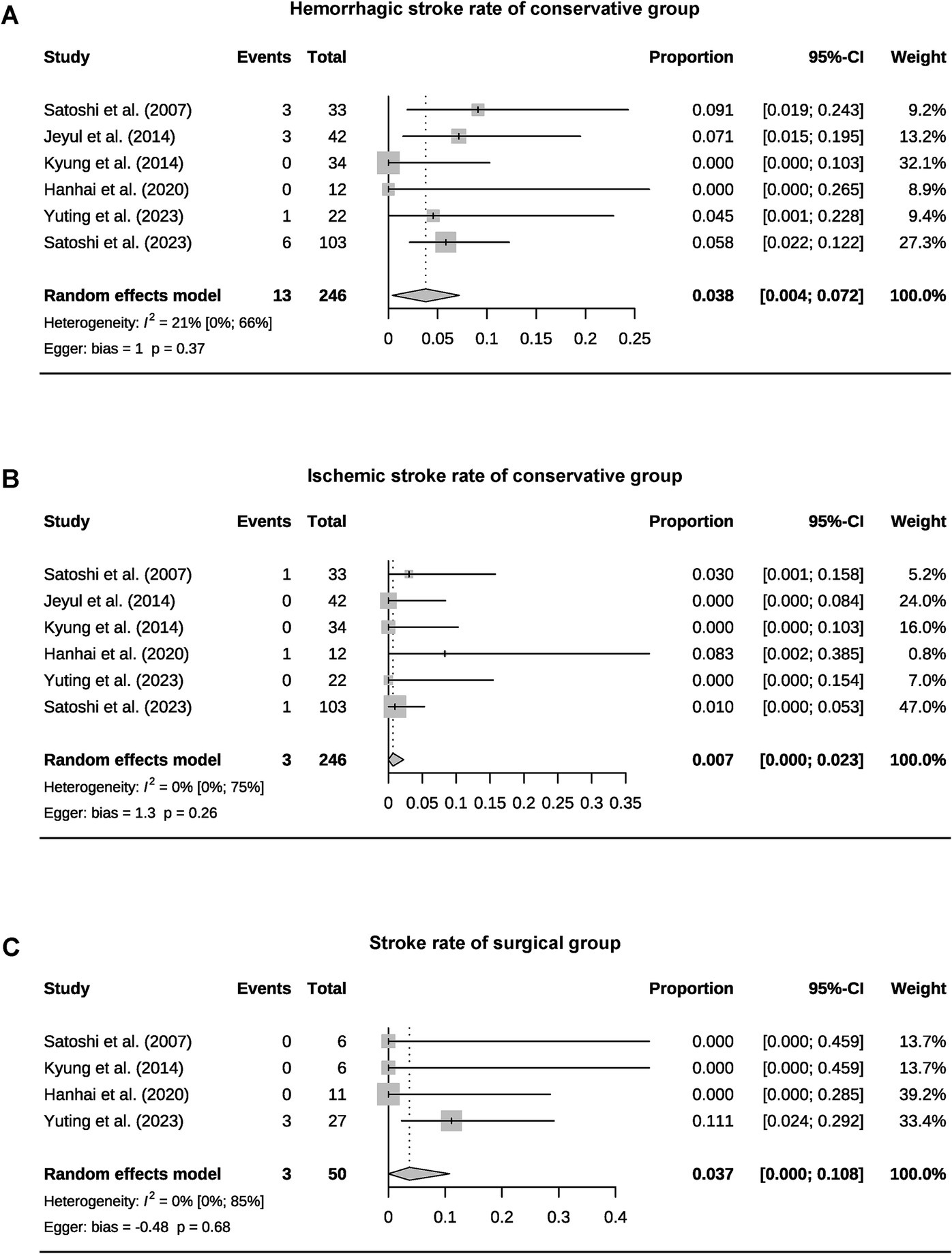
Figure 3. Forest plots for pooled hemorrhagic stroke (A), ischemic stroke rate of the conservative group (B), and stroke rate of surgical group (C).
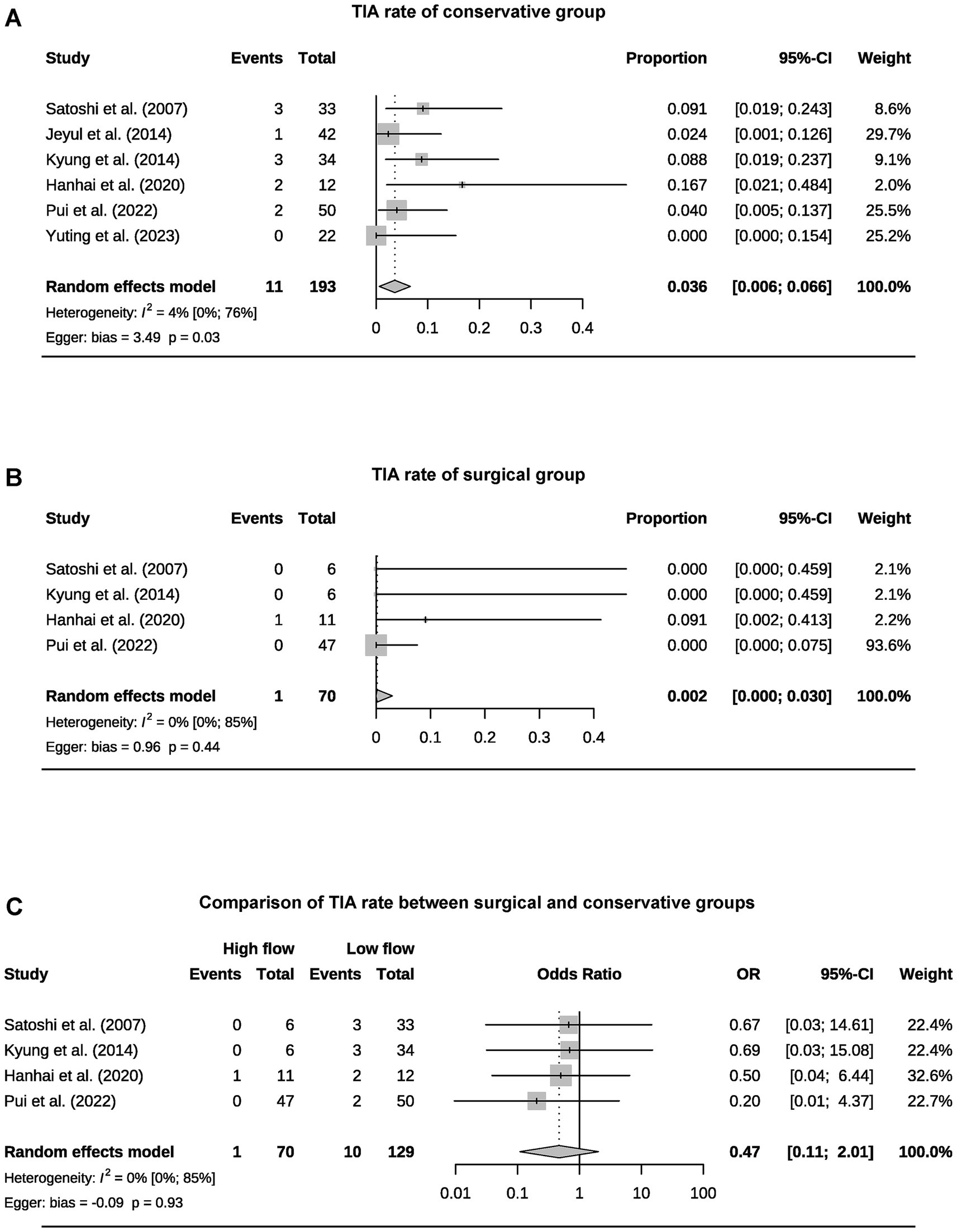
Figure 4. Forest plots for pooled TIA rate of conservative (A) and surgical group (B), and comparison of TIA rate between two groups (C).
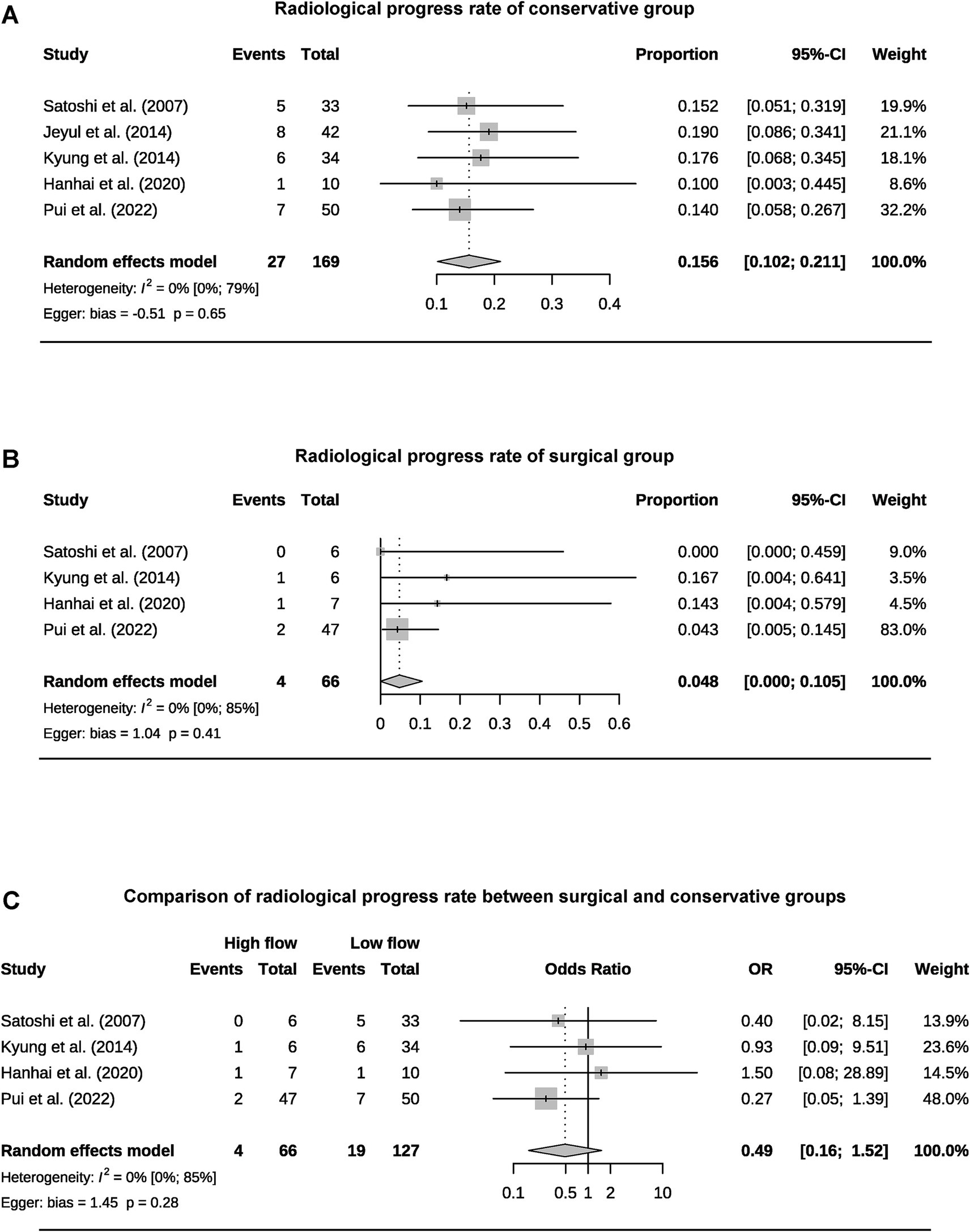
Figure 5. Forest plots for pooled radiological progress rate of conservative (A) and surgical group (B), and comparison of radiological progress rate between two groups (C).
3.4 HSMMD and pediatric cohorts
Three HSMMD studies were conducted at the Seoul National University Hospital, and all patients received conservative treatment. All hemodynamically stable hemispheres included in Young’s study were post-revascularized on the contralateral hemisphere. All three studies were followed up for more than 5 years on average, with an average age of more than 40 years. Moreover, female patients were more than male patients (Table 2). Won et al. reported the highest incidence of stroke, with an ischemic stroke rate of 13.28% and a hemorrhagic stroke rate of 14.11% (Table 4). The other two studies reported a relatively low incidence of stroke during the follow-up. None of the three studies reported a radiological progress rate. Rutao et al. reported 61 patients with AMMD aged 6–59 years (Table 3). Fifty-two received revascularization, while nine received conservative treatment. No stroke occurred during the follow-up of the surgical group, while one hemorrhagic stroke occurred in the conservative group. Satoshi et al.’s study included only one pediatric patient. Yuting et al.’s study included 15 pediatric patients, 40 patients underwent revascularization, and 24 patients underwent conservative treatment. A total of three patients developed stroke during the follow-up of the surgical group. One hemorrhagic stroke occurred in the conservative group.
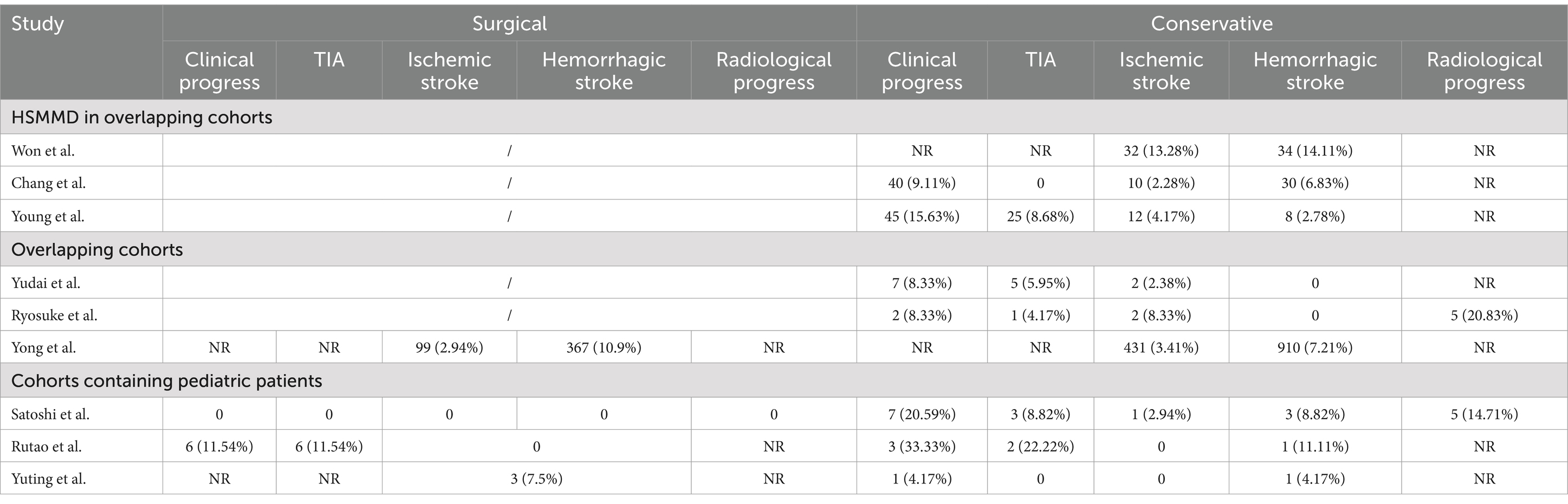
Table 4. Outcomes for overlapping cohorts, HSMMD cohorts, and cohorts containing pediatric patients.
4 Discussion
Due to the limitations imposed by its incidence, research on AMMD is quite limited and primarily concentrated in East Asia, which is associated with the RNF213 coding gene carried by East Asian populations (4). This meta-analysis included seven studies, encompassing a total of 393 patients and 649 hemispheres. The number of female patients exceeded that of male patients, which is consistent with previous research findings (23). The Suzuki stage at the time of diagnosis may have a significant impact on the clinical progress of patients during follow-up (24). This may explain why cohorts with a higher average Suzuki stage tend to have a higher incidence of stroke, as observed in the studies by Yuting et al. (5, 19, 21). Comorbidities can affect the prognosis of patients with MMD. For example, hypertension and diabetes increase the risk of postoperative complications and stroke (25, 26). Moreover, smoking and dyslipidemia are independent risk factors for ischemic stroke in MMD patients (27). Therefore, controlling comorbidities is crucial for patients with MMD, and treatment options for these patients should be more cautious.
Currently, the benefits of antiplatelet therapy (APT) in ischemic MMD remain controversial. However, APT did not increase the risk of hemorrhagic complications. Further high-level evidence is still required to support this conclusion (28, 29). Cilostazol has demonstrated unique advantages compared to other antiplatelet agents (30). The studies by Yuting and Won are the only ones that specifically analyzed the efficacy of APT (5, 21). Unfortunately, APT failed to show a stroke protective effect on either AMMD or HSMMD.
Given the preventive nature of revascularization, the treatment selection for AMMD should be undertaken with greater caution. Currently, evidence-based medical research only supports the benefits of revascularization in symptomatic MMD patients (4, 31). The studies included in the meta-analysis all had an average follow-up period of more than 2 years. The pooled clinical progress rate for the conservative group was 10%, with a hemorrhagic stroke rate of 3.8%, an ischemic stroke rate of 0.7%, and a TIA rate of 3.6%. The stroke rate in the surgical group was 3.7%, while the TIA rate was 0.2%. Revascularization has not demonstrated a significant protective effect on TIA. The clinical progress rate of AMMD without surgical intervention is concerning. Due to the inability to standardize the stroke incidence within a specific follow-up period, this review can only provide a rough estimate of the risk over 2–5 years. The Asymptomatic Moyamoya Registry (AMORE) trial is the only long-term, multicenter, prospective cohort study on the natural course of AMMD. Its interim results indicate an annual stroke risk of 1.4% per person and 0.8% per hemisphere (20). Microbleeds and Grade-2 choroidal anastomosis are predictors of stroke. These results may help optimize treatment choices for AMMD. Yong’s study is the largest retrospective study on AMMD to date. However, due to heterogeneity and differences in the definition of adult age, it was not included in the meta-analysis (32). The study indicates that, compared to conservative treatment, revascularization can reduce mortality in AMMD patients but increase the risk of hemorrhagic stroke and does not provide protection against ischemic stroke. Surgical treatment for AMMD should be approached with caution. Considering that most AMMD patients are middle-aged at the time of diagnosis, their clinical progression rate over their lifetime may be quite significant (33). This suggests that treatment choices based on age can be more specific, with a preference for conservative management in elderly patients (34, 35). Meanwhile, the stroke-protective effect of surgical treatment in younger patients may become more apparent over time. By definition, AMMD will continue to progress even if the patient remains asymptomatic (36). In this review, the pooled radiological progress rate was 15.6% in the conservative group and 4.8% in the surgical group. However, the surgical group did not demonstrate a significant protective effect. This further highlights the importance of developing stroke risk prediction models and establishing regular follow-up guides for AMMD patients.
Currently, only three studies have investigated HSMMD, all of which are from the same institution (5, 37, 38). Won et al. reported an annual stroke incidence of 4.5% per person, with rates of 3.4% in the asymptomatic group, 2.5% for hemorrhagic stroke, and 0.8% for ischemic stroke. Young et al. reported an overall annual stroke risk of 3.0% per person, with ischemic and hemorrhagic stroke rates of 2.5 and 0.5%, respectively. Both studies focused on the natural course of the disease, underscoring the need for future research on the potential effects of APT and revascularization. Pediatric MMD is not within the scope of this systematic review, and a total of three studies, including pediatric AMMD, were included with very limited sample sizes (17, 21, 39). Yuting’s study included 15 pediatric AMMDs, of whom two received conservative treatment, 13 underwent surgical treatment, and all did not experience stroke events (21). More research on pediatric AMMD is needed in the future to guide its treatment options.
This study has several limitations. The majority of the included studies were retrospective and had relatively small sample sizes, particularly in the surgical group. Due to the incidence of AMMD and patients’ concerns regarding preventive surgical treatment, conducting prospective studies remains challenging. Additionally, follow-up durations were not sufficiently long, and standardizing the annual stroke risk was difficult. Studies that mainly focused on HSMMD are all from the Seoul National University College of Medicine, which implies potential numerous overlapping in the cohort, severely limiting the generalizability of results. Given the temporary stability and progressive nature of AMMD and HSMMD, studies with follow-up periods exceeding 10 years may provide more meaningful insights. Furthermore, the majority of studies did not report details of conservative treatment strategies or corresponding clinical outcomes.
In conclusion, AMMD and HSMMD present a concerning risk of clinical and radiological progression over a follow-up period of more than 2 years. However, revascularization has not demonstrated significant benefits within this timeframe. Further high-quality studies are needed to optimize treatment strategies.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary material, further inquiries can be directed to the corresponding author.
Author contributions
YaC: Conceptualization, Data curation, Software, Validation, Writing – original draft, Writing – review & editing. ZZ: Conceptualization, Methodology, Writing – original draft, Writing – review & editing. BQ: Conceptualization, Software, Visualization, Writing – original draft, Writing – review & editing. YL: Validation, Writing – review & editing. PL: Data curation, Writing – review & editing. ZL: Data curation, Writing – review & editing. YuC: Data curation, Writing – review & editing. RL: Methodology, Writing – review & editing. BY: Methodology, Writing – review & editing. XW: Visualization, Writing – review & editing. YW: Visualization, Writing – review & editing. XG: Validation, Writing – review & editing. HZ: Software, Writing – review & editing. GG: Conceptualization, Funding acquisition, Supervision, Writing – review & editing.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. This work was supported by the Scientific Research Foundation of Shanxi Intelligence Institute of Big Data Technology and Innovation (SIBD-2020-YL0052), Shanxi Province Higher Education “Billion Project” Science and Technology Guidance Project, Shanxi Province Science Popularization Special Project (202304091004018) and National Science and Technology Innovation 2030—Research on the Prevention and Treatment of Cancer, Cardiovascular and Cerebrovascular Diseases, Respiratory Diseases, and Metabolic Diseases (2023ZD0505106).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The reviewer ZS declared a past co-authorship with the author YC to the handling editor.
The reviewer XY declared a shared parent affiliation with the author YC to the handling editor at the time of review.
Generative AI statement
The authors declare that no Gen AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fneur.2025.1626817/full#supplementary-material
Footnotes
References
1. Kuroda, S, and Houkin, K. Moyamoya disease: current concepts and future perspectives. Lancet Neurol. (2008) 7:1056–66. doi: 10.1016/S1474-4422(08)70240-0
2. Burke, GM, Burke, AM, Sherma, AK, Hurley, MC, Batjer, HH, and Bendok, BR. Moyamoya disease: a summary. Neurosurg Focus. (2009) 26:E11. doi: 10.3171/2009.1.FOCUS08310
3. Uchino, K, Johnston, SC, Becker, KJ, and Tirschwell, DL. Moyamoya disease in Washington state and California. Neurology. (2005) 65:956–8. doi: 10.1212/01.wnl.0000176066.33797.82
4. Ihara, M, Yamamoto, Y, Hattori, Y, Liu, W, Kobayashi, H, Ishiyama, H, et al. Moyamoya disease: diagnosis and interventions. Lancet Neurol. (2022) 21:747–58. doi: 10.1016/S1474-4422(22)00165-X
5. Cho, WS, Chung, YS, Kim, JE, Jeon, JP, Son, YJ, Bang, JS, et al. The natural clinical course of hemodynamically stable adult moyamoya disease. J Neurosurg. (2015) 122:82–9. doi: 10.3171/2014.9.JNS132281
6. Zeng, H, Guo, Y, Li, Y, Yu, X, Yan, F, Tan, X, et al. Comparison of operative and conservative treatment for Asymptomatic Moyamoya Disease: preliminary experience in small retrospective series. World Neurosurg. (2021) 146:e955–60. doi: 10.1016/j.wneu.2020.11.045
7. Asymptomatic, KS, and Disease, M In: S Kuroda, editor. Moyamoya Disease: Current knowledge and future perspectives. Springer Singapore: Singapore (2021). 143–52.
8. Baba, T, Houkin, K, and Kuroda, S. Novel epidemiological features of moyamoya disease. J Neurol Neurosurg Psychiatry. (2008) 79:900–4. doi: 10.1136/jnnp.2007.130666
9. Yamada, M, Fujii, K, and Fukui, M. Clinical features and outcomes in patients with asymptomatic moyamoya disease--from the results of nation-wide questionnaire survey. No Shinkei Geka. (2005) 33:337–42.
10. He, S, Zhou, Z, Cheng, MY, Hao, X, Chiang, T, Wang, Y, et al. Advances in moyamoya disease: pathogenesis, diagnosis, and therapeutic interventions. Med Comm. (2020) 6:2025:e70054. doi: 10.1002/mco2.70054
11. Kawaguchi, S, Okuno, S, and Sakaki, T. Effect of direct arterial bypass on the prevention of future stroke in patients with the hemorrhagic variety of moyamoya disease. J Neurosurg. (2000) 93:397–401. doi: 10.3171/jns.2000.93.3.0397
12. Kuroda, S, Houkin, K, Ishikawa, T, Nakayama, N, and Iwasaki, Y. Novel bypass surgery for moyamoya disease using pericranial flap: its impacts on cerebral hemodynamics and long-term outcome. Neurosurgery. (2010) 66:1093–101. doi: 10.1227/01.NEU.0000369606.00861.91
13. Lai, PMR, Gomez-Paz, S, Patel, NJ, Frerichs, KU, Thomas, AJ, Aziz-Sultan, MA, et al. Asymptomatic Moyamoya Disease in a north American adult cohort. World Neurosurg. (2022) 161:e146–53. doi: 10.1016/j.wneu.2022.01.076
14. Liberati, A, Altman, DG, Tetzlaff, J, Mulrow, C, Gøtzsche, PC, Ioannidis, JP, et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate healthcare interventions: explanation and elaboration. BMJ. (2009) 339:b2700. doi: 10.1136/bmj.b2700
15. Slim, K, Nini, E, Forestier, D, Kwiatkowski, F, Panis, Y, and Chipponi, J. Methodological index for non-randomized studies (minors): development and validation of a new instrument. ANZ J Surg. (2003) 73:712–6. doi: 10.1046/j.1445-2197.2003.02748.x
16. Sakai, R, Hara, S, Inaji, M, Tanaka, Y, Nariai, T, and Maehara, T. Stroke and Disease Progression During Long-Term Follow-Up of Patients with Moyamoya Disease Older Than 50 Years. World Neurosurg. (2024) 187:e898–e907. doi: 10.1016/j.wneu.2024.05.008
17. Kuroda, S, Hashimoto, N, Yoshimoto, T, and Iwasaki, Y. Radiological findings, clinical course, and outcome in asymptomatic moyamoya disease: results of multicenter survey in Japan. Stroke. (2007) 38:1430–5. doi: 10.1161/STROKEAHA.106.478297
18. Jo, KI, Yeon, JY, Hong, SC, and Kim, JS. Clinical course of asymptomatic adult moyamoya disease. Cerebrovasc Dis. (2014) 37:94–101. doi: 10.1159/000356350
19. Yang, J, Hong, JC, Oh, CW, Kwon, OK, Hwang, G, Kim, JE, et al. Clinicoepidemiological features of asymptomatic moyamoya disease in adult patients. J Cerebrovasc Endovasc Neurosurg. (2014) 16:241–6. doi: 10.7461/jcen.2014.16.3.241
20. Kuroda, S, Yamamoto, S, Funaki, T, Fujimura, M, Kataoka, H, Hishikawa, T, et al. Five-year stroke risk and its predictors in Asymptomatic Moyamoya Disease: Asymptomatic Moyamoya registry (AMORE). Stroke. (2023) 54:1494–504. doi: 10.1161/STROKEAHA.122.041932
21. Luo, Y, Cao, Z, Ye, H, Wu, S, and Sun, X. Antiplatelet therapy may improve the prognosis of patients with moyamoya disease: a 12-year retrospective study. J Neurol. (2023) 270:3876–84. doi: 10.1007/s00415-023-11702-5
22. Sterne, JA, Sutton, AJ, Ioannidis, JP, Terrin, N, Jones, DR, Lau, J, et al. Recommendations for examining and interpreting funnel plot asymmetry in meta-analyses of randomised controlled trials. BMJ. (2011) 343:d4002. doi: 10.1136/bmj.d4002
23. Kappel, AD, Feroze, AH, Torio, E, Sukumaran, M, and Du, R. Management of moyamoya disease: a review of current and future therapeutic strategies. J Neurosurg. (2024) 141:975–82. doi: 10.3171/2024.1.JNS221977
24. Zhao, M, Zhang, D, Wang, S, Zhang, Y, Wang, R, and Zhao, J. Transient ischemic attack in pediatric patients with Moyamoya Disease: clinical features, natural history, and predictors of stroke. Pediatr Neurol. (2017) 75:48–54. doi: 10.1016/j.pediatrneurol.2017.06.020
25. Ma, Y, Zhao, M, Deng, X, Zhang, D, Wang, S, Zeng, Z, et al. Comparison of clinical outcomes and characteristics between patients with and without hypertension in moyamoya disease. J Clin Neurosci. (2020) 75:163–7. doi: 10.1016/j.jocn.2019.12.016
26. Wei, W, Chen, X, Yu, J, and Li, XQ. Risk factors for postoperative stroke in adults patients with moyamoya disease: a systematic review with meta-analysis. BMC Neurol. (2019) 19:98. doi: 10.1186/s12883-019-1327-1
27. Unda, SR, Antoniazzi, AM, Miller, R, Klyde, D, Javed, K, Fluss, R, et al. Moyamoya Disease and syndrome: a National Inpatient Study of ischemic stroke predictors. J Stroke Cerebrovasc Dis. (2021) 30:105965. doi: 10.1016/j.jstrokecerebrovasdis.2021.105965
28. Gonzalez, NR, Amin-Hanjani, S, Bang, OY, Coffey, C, Du, R, Fierstra, J, et al. Adult Moyamoya Disease and syndrome: current perspectives and future directions: a scientific statement from the American Heart Association/American Stroke Association. Stroke. (2023) 54:e465–79. doi: 10.1161/STR.0000000000000443
29. Ye, F, Li, J, Wang, T, Lan, K, Li, H, Yin, H, et al. Efficacy and safety of antiplatelet agents for adult patients with ischemic Moyamoya disease. Front Neurol. (2020) 11:608000. doi: 10.3389/fneur.2020.608000
30. Seo, WK, Kim, JY, Choi, EH, Kim, YS, Chung, JW, Saver, JL, et al. Association of Antiplatelet Therapy, including Cilostazol, with improved survival in patients with Moyamoya Disease in a Nationwide study. J Am Heart Assoc. (2021) 10:e017701. doi: 10.1161/JAHA.120.017701
31. Kuroda, S. Asymptomatic moyamoya disease: literature review and ongoing AMORE study. Neurol Med Chir (Tokyo). (2015) 55:194–8. doi: 10.2176/nmc.ra.2014-0305
32. Lim, YC, Lee, E, and Song, J. Outcomes of bypass surgery in adult Moyamoya Disease by onset type. JAMA Netw Open. (2024) 7:e2415102. doi: 10.1001/jamanetworkopen.2024.15102
33. Hirano, Y, Miyawaki, S, Imai, H, Hongo, H, Ohara, K, Dofuku, S, et al. Association Between the Onset Pattern of Adult Moyamoya Disease and Risk Factors for Stroke. Stroke. (2020) 51:3124–8. doi: 10.1161/strokeaha.120.030653
34. Vetrano, IG, Bersano, A, Canavero, I, Restelli, F, Raccuia, G, Ciceri, EF, et al. Characteristics of Moyamoya Disease in the older population: is it possible to define a typical presentation and optimal Therapeutical management? J Clin Med. (2021) 10:2287. doi: 10.3390/jcm10112287
35. Navandhar, PS, Gharde, P, Shinde, RK, and Nagtode, T. Moyamoya disease: advances in diagnosis, treatment, and surgical interventions. Cureus. (2024) 16:e59826. doi: 10.7759/cureus.59826
36. Kuroda, S, Fujimura, M, Takahashi, J, Kataoka, H, Ogasawara, K, Iwama, T, et al. Diagnostic criteria for Moyamoya Disease −2021 revised version. Neurol Med Chir (Tokyo). (2022) 62:307–12. doi: 10.2176/jns-nmc.2022-0072
37. Pang, CH, Cho, WS, Kang, HS, and Kim, JE. Benefits and risks of antiplatelet medication in hemodynamically stable adult moyamoya disease. Sci Rep. (2021) 11:19367. doi: 10.1038/s41598-021-99009-1
38. Kang, YS, Cho, WS, Nam, SM, Chung, Y, Lee, SH, Kim, K, et al. Natural course of hemodynamically stable hemispheres contralateral to operated hemispheres in adult patients with ischemic moyamoya diseases. Sci Rep. (2024) 14:8358. doi: 10.1038/s41598-024-59141-0
Keywords: moyamoya disease, asymptomatic, hemodynamically stable, clinical course, radiological course
Citation: Chen Y, Zhou Z, Qin B, Liang Y, Li P, Li Z, Chen Y, Li R, Yang B, Wang X, Wu Y, Guo X, Zhang H and Guo G (2025) Clinical and radiological course of asymptomatic and hemodynamically stable moyamoya disease: a systematic review and meta-analysis. Front. Neurol. 16:1626817. doi: 10.3389/fneur.2025.1626817
Edited by:
Shihao He, Peking Union Medical College Hospital (CAMS), ChinaReviewed by:
Zhiyong Shi, Nanjing Drum Tower Hospital, ChinaXiaofan Yu, Capital Medical University, China
Copyright © 2025 Chen, Zhou, Qin, Liang, Li, Li, Chen, Li, Yang, Wang, Wu, Guo, Zhang and Guo. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Geng Guo, Z3VvZ2VuZzk3M0AxNjMuY29t
†These authors have contributed equally to this work
 Yang Chen
Yang Chen Zixuan Zhou
Zixuan Zhou Bingyang Qin
Bingyang Qin Yan Liang6
Yan Liang6 Ziao Li
Ziao Li Yu Chen
Yu Chen Ren Li
Ren Li Biao Yang
Biao Yang Geng Guo
Geng Guo