- 1Department of Traumatic Orthopedics, China-Japan Union Hospital of Jilin University, Changchun, China
- 2School of Clinical Medicine, Changchun University of Chinese Medicine, Changchun, China
- 3Department of Physiology and Pathophysiology, College of Medicine, Yanbian University, Yanbian, China
- 4Department of Joint Surgery, Yanbian University Hospital, Yanbian, China
Osteosarcoma (OS), the most prevalent primary malignant bone tumor, disproportionately affects adolescents and is marked by rapid progression and a high rate of pulmonary metastasis. Despite advances in multimodal treatment, outcomes remain dismal for metastatic or relapsed disease, largely due to chemoresistance, immune evasion, and the heterogeneous tumor microenvironment (TME). Increasing evidence underscores the immunoregulatory complexity of osteosarcoma, characterized by immunosuppressive myeloid-derived populations, dysfunctional lymphocyte infiltration, and exosome-mediated immune escape. While immune checkpoint inhibitors have revolutionized treatment in several malignancies, their impact in osteosarcoma remains modest, highlighting the need for combinatorial strategies. Emerging approaches such as adoptive T cell therapies, tumor vaccines, and CAR-T cell interventions are being explored to overcome the “cold” immune milieu. Furthermore, single-cell transcriptomics has shed light on cellular interactions within the osteosarcoma TME, offering insights into resistance mechanisms and potential biomarkers. This review provides a comprehensive overview of the immunological landscape of osteosarcoma and highlights current and emerging immunotherapeutic strategies aimed at improving clinical outcomes in this challenging malignancy.
1 Introduction
Osteosarcoma represents the most prevalent primary malignant tumor of bone, with a marked predilection for adolescents and young adults (1, 2). It arises predominantly within the metaphyseal regions of long bones, notably the distal femur, proximal tibia, and humerus. Clinically, osteosarcoma follows an aggressive trajectory, with subclinical pulmonary micrometastases observed in approximately 80–90% of patients at initial diagnosis. Molecularly, it is characterized by profound heterogeneity across genomic, epigenomic, and transcriptomic layers. Frequent molecular aberrations include inactivation of key tumor suppressors such as TP53 and RB1, disruptions in mesenchymal differentiation pathways, and extensive epigenetic reprogramming mediated by inflammatory signals. These alterations collectively compromise apoptotic control and perturb skeletal tissue homeostasis. Notably, epigenetic modulators like histone modification enzymes and DNA methyltransferases dynamically reshape gene expression profiles, particularly those involving regulatory non-coding RNAs, thereby accelerating tumor progression and correlating with adverse clinical outcomes (3).
The current therapeutic standard comprises radical surgical resection in combination with multi-agent chemotherapy, most commonly employing methotrexate, doxorubicin, and cisplatin (MAP regimen). Although targeted agents such as tyrosine kinase inhibitors (sorafenib, apatinib) have been evaluated, their clinical benefit remains inconsistent (4). Persistent challenges, including chemoresistance, immune evasion, and intratumoral heterogeneity, continue to hinder therapeutic efficacy (5). In response, research has increasingly focused on immunomodulatory strategies. Preclinical and clinical studies underscore the promise of immunotherapy, with evidence including lipopolysaccharide-induced tumor regression and prolonged survival following mifamurtide administration, substantiating the immunogenic nature of osteosarcoma (6–8). Nonetheless, the profoundly immunosuppressive tumor microenvironment poses a significant barrier. Ongoing investigations into immune checkpoint blockade, adoptive T cell therapies, cancer vaccines, and combinatorial immunotherapeutic regimens offer a path forward, with the potential to redefine treatment paradigms for this recalcitrant malignancy.
2 Immune landscape in osteosarcoma
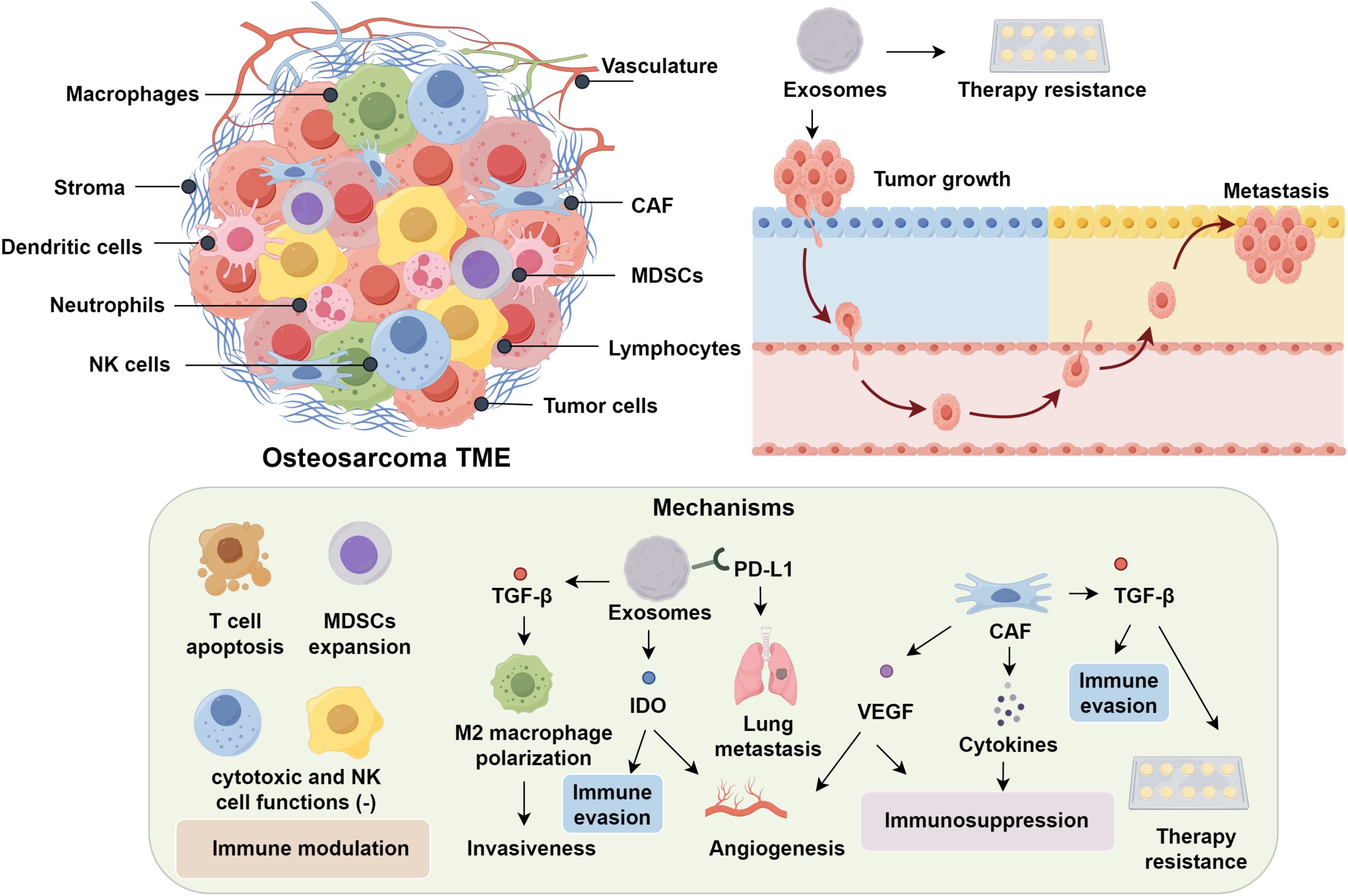
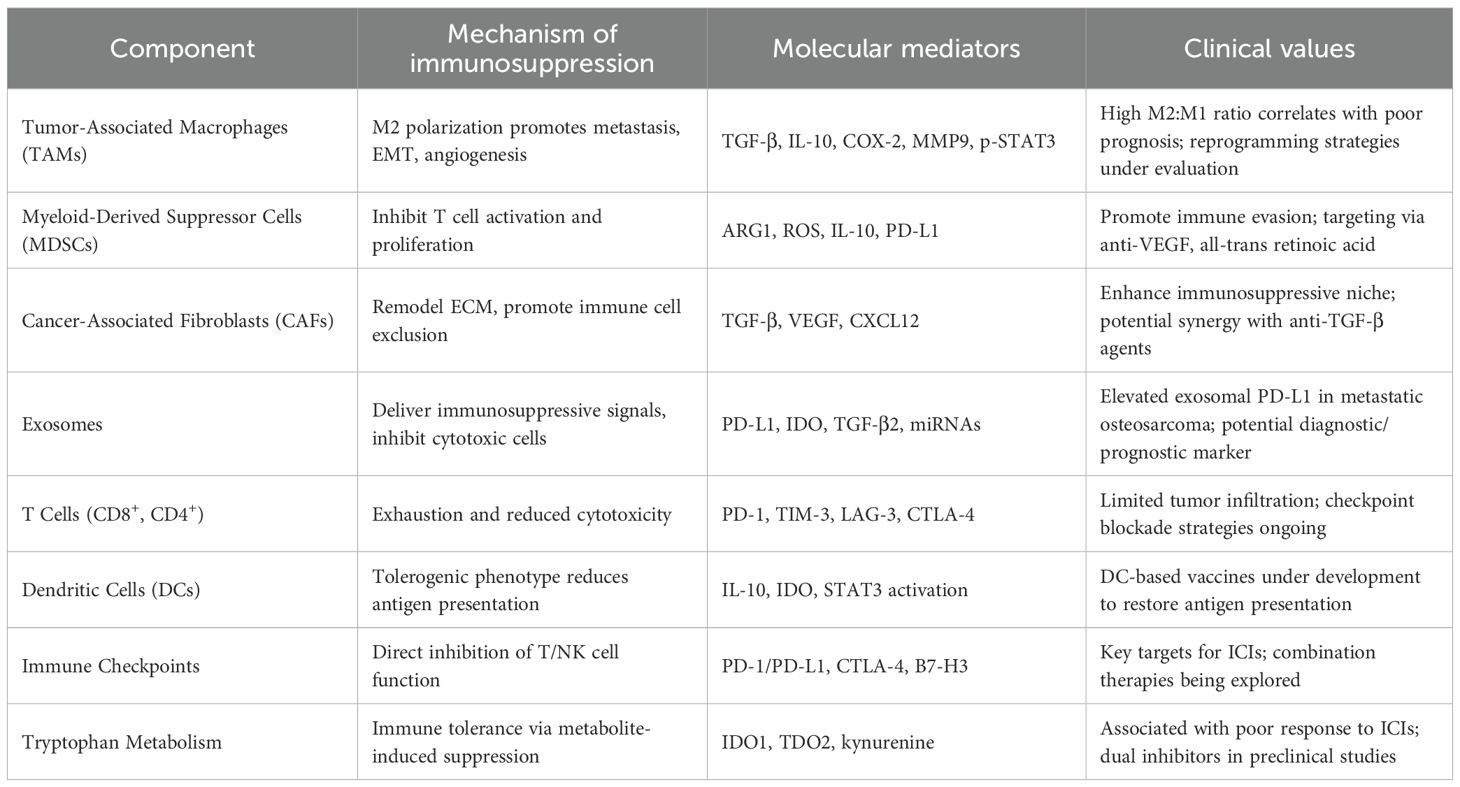
The tumor microenvironment (TME) exerts profound influence over the initiation, progression, and metastatic potential of osteosarcoma (9, 10). Comprising a complex interplay between bone marrow stromal elements and an extensive vascular network, the TME provides a permissive niche that supports tumor cell survival, proliferation, and immune evasion by modulating osteogenic and immunological interactions (11, 12). Notably, osteosarcoma is heavily infiltrated by diverse immune cell subsets, including macrophages, dendritic cells, neutrophils, natural killer (NK) cells, and lymphocytes, reflecting a dynamic immunological landscape (13). Within this complex immunological milieu, exosomes play pivotal roles in promoting tumor growth, metastasis, and therapy resistance via immune modulation (14, 15). Mechanistically, these vesicles promote immunosuppression by inducing T cell apoptosis, impairing cytotoxic T and NK cell function, and expanding populations of myeloid-derived suppressor cells (MDSCs) (16). Analogous mechanisms observed in prostate cancer, wherein tumor-derived exosomes downregulate NKG2D expression on CD8+ T cells and NK cells, underscore the conserved role of exosomes in dampening cytotoxic immunity (17, 18). In osteosarcoma specifically, exosome-associated TGF-β2 has been shown to skew macrophage polarization toward an M2-like phenotype, thereby enhancing tumor invasiveness (19, 20). Furthermore, PD-L1–bearing exosomes correlate with pulmonary metastasis and hold promise as non-invasive prognostic biomarkers (21). Exosomal delivery of indoleamine 2,3-dioxygenase (IDO) further contributes to immune evasion by disrupting tryptophan metabolism and fostering an angiogenic microenvironment (22, 23).
Cancer-associated fibroblasts (CAFs) contribute to the establishment of an immunosuppressive tumor microenvironment through the secretion of tumor-promoting cytokines, thereby supporting immune evasion and disease progression (24, 25). TGF-β has emerged as a pivotal orchestrator of immune escape and therapeutic resistance in osteosarcoma. Dual blockade of TGF-β signaling, particularly when combined with dendritic cell–based immunotherapeutic approaches, demonstrates synergistic antitumor activity in preclinical models (26–28). In parallel, VEGF facilitates neovascularization while concurrently promoting immunosuppression. Pharmacological inhibition of VEGF using multi-kinase inhibitors such as sunitinib not only impairs angiogenesis but also attenuates MDSC accumulation and enhances intratumoral infiltration of CD8+ cytotoxic T lymphocytes (29–31). Beyond its anti-angiogenic capacity, sunitinib has been shown to modulate the immune landscape by depleting MDSCs and enabling effector T cell infiltration (32). Preclinical models have shown that sunitinib-mediated VEGF blockade reconditions the immunosuppressive niche, thereby enhancing CD8+ T cell trafficking and function—effects that provide a compelling rationale for combinatorial strategies with immune checkpoint inhibitors (33). Supporting this, interim results from the NCT03277924 clinical trial revealed that co-administration of sunitinib with the PD-1 inhibitor nivolumab achieved disease stabilization in nearly 50% of patients with advanced sarcomas (34). Tumor-associated macrophages (TAMs), another dominant immune component within the osteosarcoma microenvironment, exhibit marked functional plasticity. While M1-polarized TAMs mediate antitumor immunity, their M2-like counterparts facilitate metastasis through upregulation of COX-2, MMP9, phosphorylated STAT3, and epithelial–mesenchymal transition (EMT)–related markers (35, 36). Transcriptomic analyses reveal that M2-skewed signatures correlate strongly with pulmonary metastatic potential. Pharmacological reprogramming of TAMs toward an M1 phenotype, such as via all-trans retinoic acid, has demonstrated potent antimetastatic effects (20, 37). These findings highlight the therapeutic promise of TAM repolarization, as M2-dominant profiles are consistently linked with heightened metastatic burden, while reprogramming TAMs toward M1 reduces metastatic burden (38) (Figure 1).
3 Immunotherapeutic approaches in osteosarcoma
3.1 Immune checkpoint inhibition in osteosarcoma
Recent progress in cancer immunotherapy underscores the therapeutic promise of immune checkpoint blockade, particularly targeting CTLA-4, B7-H3, PD-1, and PD-L1. Approximately 23.7% of osteosarcoma specimens exhibit high PD-L1 expression, with moderate expression levels observed in nearly 50% of cases—patterns that are positively associated with metastasis, tumor-infiltrating lymphocytes, and reduced five-year survival rates (39). Notably, PD-L1 expression is more prevalent in metastatic pediatric osteosarcoma than in localized disease (40). A cohort study involving 234 patients further revealed that positivity for PD-L1 and PD-1 significantly correlated with poor prognosis and decreased overall survival (41). Nevertheless, clinical trials evaluating immune checkpoint inhibitors in osteosarcoma have yielded underwhelming results. In the SARC028 trial, pembrolizumab elicited a modest 5% objective response rate (42), a finding corroborated by the NCT03013127 study (43). Similarly, pediatric trials investigating nivolumab (NCT02304458) and atezolizumab (NCT02541604) failed to demonstrate meaningful clinical benefit. These disappointing outcomes are thought to reflect the immunologically inert tumor microenvironment of osteosarcoma, characterized by limited T cell infiltration and effector function (44). In contrast, preclinical models suggest that PD-1 blockade can partially restore T cell functionality under experimental conditions (45). In humanized mouse models, nivolumab has been shown to suppress metastatic spread by enhancing the activation of CD4+ and CD8+ T cells and promoting M1 macrophage polarization. However, such immune activation is often insufficient in isolation, particularly in tumors with low baseline immunogenicity (46). Combination strategies may offer a more promising therapeutic avenue. Dual blockade of PD-1 and CTLA-4 in murine K7M2 models achieved superior tumor control and increased intratumoral CD8+ T cell infiltration compared to monotherapy (46, 47). These preclinical findings have been mirrored in clinical settings. The Alliance A091401 trial demonstrated a 16% objective response rate in patients receiving combined nivolumab–ipilimumab therapy, compared to 5% with nivolumab alone, underscoring the potential of dual checkpoint inhibition in overcoming immunotherapy resistance in osteosarcoma (48) (Table 1).
3.2 Emerging immunotherapeutic targets and resistance mechanisms
B7-H3, an immune checkpoint molecule, is markedly overexpressed in osteosarcoma and various malignancies, while minimally expressed in normal tissues (26, 49). It exerts immunosuppressive functions by delivering inhibitory costimulatory signals that dampen T cell proliferation and cytokine secretion. High B7-H3 levels are associated with increased tumor invasiveness, recurrence, and poor prognosis. Notably, silencing B7-H3 impairs lymphoma progression, and dual blockade of PD-1 and B7-H3 enhances antitumor immunity (50–52). Ongoing clinical trials for B7-H3 chimeric antigen receptor (CAR)-modified T cells therapy in solid tumors including NCT04897321, NCT04483778, NCT04670068 (53). B7-H3-specific CAR-T cells demonstrated significant anti-tumor activity in models of osteosarcoma, as evidenced by both in vitro and in vivo experiments. These findings indicate the potential therapeutic utility of B7-H3-directed CAR-T cell immunotherapy in the management of osteosarcoma (54). Besides, B7-H3-CXCR2 CAR T cells significantly improve the anti-tumor activity in osteosarcoma (55). Another pivotal checkpoint, CTLA-4, a type I transmembrane glycoprotein predominantly expressed on regulatory and memory T lymphocytes, acts by outcompeting CD28 for binding to the B7 ligands CD80 and CD86 on antigen-presenting cells, thereby dampening co-stimulatory activation (56). The CTLA-4-targeted monoclonal antibody ipilimumab is the first second-generation immune checkpoint inhibitor sanctioned for melanoma treatment (57). In osteosarcoma, accumulating data suggest a link between heightened CTLA-4 expression and disease progression (58), potentially mediated through mechanisms involving IDO induction, reduced T cell proliferation, and altered inflammatory cytokine signaling.
CTLA-4 inhibition revitalizes anti-tumor immunity by interrupting the B7–CD28 costimulatory axis and promoting the depletion of immunosuppressive regulatory T cells. In a phase I trial, Merchant et al. reported disease stabilization in approximately 25% of pediatric osteosarcoma cases treated with ipilimumab. Although immune-related toxicities were generally tolerable, gastrointestinal complications were more frequently observed in younger patients (59). Despite these advances, immune checkpoint blockade shows limited efficacy in osteosarcoma. Barriers include low PD-L1 expression, scarcity of tumor-specific neoantigens, inadequate cytotoxic lymphocyte infiltration, and a dense desmoplastic stroma (60). The osteosarcoma extracellular matrix (ECM), composed of collagen I, fibronectin, and hyaluronic acid, restricts T cell penetration, This leads to immune cell sequestration at tumor margins, away from PD-L1-enriched regions (61). Moreover, CAF-derived TGF-β enhances checkpoint molecule expression (PD-1, TIM-3) and suppresses T cell cytotoxicity (62). To overcome these challenges, combinatorial strategies integrating immune checkpoint inhibitors with immunoenhancing modalities, such as anti-angiogenic agents, chemotherapy, or radiotherapy, are being actively explored. Future directions include the identification and targeting of novel checkpoint pathways beyond PD-1 and CTLA-4, expansion of patient eligibility criteria in clinical trials, elucidation of resistance-associated molecular pathways, translation of preclinical insights into human studies, and the development of predictive biomarkers to guide treatment selection. Addressing these priorities is critical for the advancement of safer and more effective immunotherapies.
3.3 Tumor-infiltrating lymphocyte therapy
Tumor-infiltrating lymphocytes (TILs), primarily composed of T cells and NK cells, play a pivotal role in antitumor immunity through granule-mediated cytotoxicity and immune cascade activation (63). Notably, their specificity for tumor-restricted neoantigens permits targeted cytolysis with minimal off-target effects (64). In osteosarcoma, however, TIL presence is highly heterogeneous, ranging from immune-desert to immune-excluded phenotypes (65, 66). Even in infiltrated tumors, chronic antigen stimulation and immunosuppressive cues can drive T cell exhaustion, undermining responses to monotherapies such as PD-1 blockade (67). To overcome these limitations, adoptive TIL therapy, extraction, ex vivo expansion, and reinfusion of autologous lymphocytes, is under clinical exploration. While isolating functional TILs from osteosarcoma tissue is technically challenging (68, 69), preclinical evidence confirms that expanded TILs retain tumor-homing and cytolytic capabilities against allogeneic osteosarcoma cells (70). Encouragingly, the TIL product lifileucel (LN-144) demonstrated durable disease stabilization in 80% of advanced melanoma patients refractory to checkpoint inhibitors (71), prompting clinical investigation of LN-145 in osteosarcoma (NCT03449108). Initial outcomes reveal prolonged disease control and improved survival, reinforcing TILs’ translational potential in osteosarcoma. The efficacy of TIL therapy is further enhanced by combination with immune checkpoint inhibitors. CTLA-4 blockade has been shown to augment HLA binding affinity and stimulate CD8+ T cell proliferation in murine models (72). In osteosarcoma, Wang et al. reported that combining TILs with PD-1 inhibitors significantly improved objective response rates and prolonged both progression-free and overall survival compared to PD-1 monotherapy (73). Similarly, a study involving 60 patients with chemotherapy-refractory metastatic osteosarcoma demonstrated the feasibility and clinical activity of combined TIL/PD-1 therapy (74). Mechanistically, checkpoint blockade revitalizes exhausted CD8+ TILs by disrupting immunosuppressive signaling (75). Persistent antigen exposure induces expression of inhibitory receptors such as PD-1, TIM-3, and LAG-3, impairing cytokine production and effector function (76). PD-1 blockade reactivates T cells by restoring TCR signaling and reversing exhaustion-associated transcriptional programs (TOX, NFAT) (77, 78). Although the optimal treatment paradigm, monotherapy versus combination, is yet to be fully established, accumulating evidence suggests that TILs can circumvent resistance and potentiate antitumor immunity in osteosarcoma.
3.4 Tumor vaccine
Cancer vaccination strategies aim to elicit tumor-specific immune activation by introducing tumor-associated antigens (TAAs) through platforms such as whole-cell lysates, subcellular fractions, recombinant proteins, or nucleic acid-based vectors. These modalities are often designed to potentiate antigen-specific responses, frequently in conjunction with monoclonal antibodies that recognize tumor surface markers (79). Early work by Marcove et al. (80) demonstrated that immunization using autologous tumor lysates was associated with improved overall survival. Building on this, Mason and colleagues (81) developed DXS31-164, a HER2/neu-targeted vaccine that significantly reduced pulmonary metastatic burden and prolonged lifespan in canine osteosarcoma models, indicating potential for translation to human HER2-positive osteosarcoma. In pediatric osteosarcoma, 71% of patients exhibited immune reactivity to the anti-idiotypic vaccine 105AD7, with an absence of severe toxicities (82). Further clinical evidence by Ullenhag et al. (83) confirmed that 105AD7 vaccination induced robust T cell responses and facilitated recognition of CD55, an antigen structurally akin to the immunogen. Meanwhile, peptide vaccines targeting broadly expressed TAAs, including those associated with papillomaviruses and tumor rejection antigens, are under investigation for osteosarcoma and potentially other malignancies (84). Dendritic cells (DCs), due to their superior antigen presentation capabilities, are integral to initiating cytotoxic T lymphocyte (CTL) responses (85). Among immune-based interventions, DC-centric vaccines have gained attention, especially in refractory tumors such as osteosarcoma. Mackall et al. (86) revealed that metastatic or relapsed Ewing sarcoma patients achieved prolonged survival and minimal toxicity after receiving a combined immunotherapeutic regimen incorporating DC vaccines, autologous T cells, and influenza immunization—even under the constraints of chemotherapy-induced immunosuppression. Nonetheless, the efficacy of tumor-targeted vaccines in solid tumors, including osteosarcoma, remains limited. For example, a phase I trial combining decitabine with DC-based vaccination in patients with sarcoma or neuroblastoma resulted in complete remission in only 10% of cases, while 60% experienced disease progression (86). Despite these challenges, DC-based immunotherapies continue to offer promise, and future studies are warranted to optimize their efficacy, either as standalone treatments or in synergistic combination with other immune-modulating agents. Recent study showed that combining anti-CTLA-4 with CD103+ cDC1 dendritic cell vaccine therapy increased cDC vaccine efficacy against osteosarcoma lung metastases (87).
3.5 CAR-T therapy
Chimeric antigen receptor (CAR)-modified T cells have revolutionized adoptive immunotherapy, particularly in hematologic cancers, where they have demonstrated striking clinical benefits. In a pivotal study, 80% of patients with refractory or relapsed B-cell acute lymphoblastic leukemia achieved remission following infusion with CD22-directed CAR-T cells (88). Likewise, CAR-T therapies targeting CD19 have yielded response rates nearing 90% across various B-cell malignancies, culminating in the first FDA approval for a genetically engineered cell therapy (89). Tandem CD19/CD22 CAR T-cell therapy demonstrates superior efficacy compared to monovalent CD19 CAR T-cell therapy (90). Efforts have also been made to translate CAR-T approaches to osteosarcoma by targeting tumor-specific antigens. For instance, the anti-IGF-1R monoclonal antibody Cixutumumab failed to show clinical efficacy in a phase II trial (91). Additionally, HER2-targeted CAR-T therapy produced only a transient partial response in a patient with metastatic HER2-positive sarcoma (NCT00902044) (92). This diminished effectiveness is likely attributable to the pronounced molecular heterogeneity and complex genomic landscape of osteosarcoma, which undermines the utility of single-target strategies (93). Recently, a Phase I clinical trial (NCT02107963) evaluated the feasibility and safety of administering GD2-targeted CAR-T cells in pediatric and young adult patients with OS and neuroblastoma (94). Restoration of CAR-T cell functionality has been pursued via PD-1 blockade or genetic engineering to render CAR constructs insensitive to PD-1–mediated inhibition (95). Integrating CAR-T therapy with low-dose chemotherapy to attenuate immune suppression and reduce PD-L1 expression is ongoing (NCT04433221). The development of advanced CAR platforms capable of simultaneously targeting multiple antigens aims to mitigate relapse driven by immune escape mechanisms (96). Importantly, co-expression of PD-1 and TIM-3 on CD8 T cells mark a deeper state of exhaustion than PD-1 expression alone, implicating dual immune checkpoint blockade as a potentially more effective approach (97). Moreover, the co-engagement of PD-1 and TIM-3 results in the recruitment of SHP2 phosphatase to their intracellular ITIM and ITSM motifs, which collectively dampens TCR signaling, suppresses granzyme B production, and drives terminal T cell dysfunction (97, 98).
Despite the intrinsically aggressive and metastatic nature of osteosarcoma, rationally designed immunotherapies offer an avenue for clinical advancement. Nevertheless, the low immunogenicity of osteosarcoma, coupled with its elevated mutational burden and immunologically complex TME, necessitates integrative, multi-modal treatment approach (99, 100). Studies at MD Anderson Cancer Center have identified resistance mechanisms, including insufficient effector T cell infiltration, limited neoantigen presentation, and dominance of immunosuppressive pathways (101). Leveraging single-cell RNA sequencing, Cillo et al. (102) revealed enrichment of pro-inflammatory FABP4+ macrophages in pulmonary metastases and a depletion of osteoclasts in recurrent and chondroblastic subtypes. CD8+ T cells in these contexts exhibited elevated inhibitory receptor expression, suggesting potential benefit from checkpoint blockade during relapse. Further integrative transcriptomic analyses have illuminated the immunoregulatory functions of myeloid-derived populations—particularly mature regulatory dendritic cells (mregDCs)—which actively shape TME composition through intricate interactions with stromal and immune constituents (103). Advancing the field necessitates a deeper understanding of resistance mechanisms and the discovery of reliable predictive biomarkers, which will be instrumental in crafting rational, combinatorial immunotherapeutic regimens. While substantial hurdles remain, immune-oriented approaches hold promise for fundamentally altering the therapeutic trajectory of osteosarcoma.
4 Conclusion
Osteosarcoma remains a formidable clinical challenge due to its intrinsic aggressiveness, high metastatic potential, and profoundly immunosuppressive tumor microenvironment. While conventional therapies have plateaued in efficacy, the advent of immunotherapeutic strategies—including immune checkpoint inhibitors, tumor vaccines, CAR-T cells, and adoptive TIL therapies—has catalyzed renewed interest in reshaping the therapeutic landscape. Preclinical and early-phase clinical studies underscore the immunogenic potential of osteosarcoma, yet the limited success of monotherapies highlights the necessity for combinatorial and patient-tailored approaches.
Advancing immunotherapy in osteosarcoma will require a deeper mechanistic understanding of resistance pathways, immune evasion, and intratumoral heterogeneity. Emerging technologies such as single-cell transcriptomics, spatial profiling, and multi-omics integration offer valuable insights into immune cell dysfunction, myeloid cell plasticity, and stromal-immune crosstalk. Future efforts should prioritize the identification of predictive biomarkers, the rational design of synergistic regimens, and the stratification of patients for optimized immunotherapeutic benefit. Ultimately, immune-based interventions hold transformative potential to overcome current therapeutic bottlenecks and improve long-term outcomes in osteosarcoma.
Author contributions
HW: Writing – original draft. TZ: Writing – original draft. KY: Writing – original draft. CX: Writing – original draft. ZZ: Writing – original draft. GL: Writing – original draft, Writing – review & editing. HR: Writing – original draft, Writing – review & editing.
Funding
The author(s) declare financial support was received for the research and/or publication of this article. This study was funded by the National Natural Science Foundation of China (82202668).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Shoaib Z, Fan TM, and Irudayaraj JMK. OS mechanobiology and therapeutic targets. Br J Pharmacol. (2022) 179:201–17. doi: 10.1111/bph.15713
2. Wu H, Zheng S, He Q, and Li Y. Recent advances of circular RNAs as biomarkers for OS. Int J Gen Med. (2023) 16:173–83. doi: 10.2147/IJGM.S380834
3. Du X, Wei H, Zhang B, Wang B, Li Z, Pang LK, et al. Molecular mechanisms of OS metastasis and possible treatment opportunities. Front Oncol. (2023) 13:1117867. doi: 10.3389/fonc.2023.1117867
4. Hattinger CM, Salaroglio IC, Fantoni L, Godel M, Casotti C, Kopecka J, et al. Strategies to overcome resistance to immune-based therapies in OS. Int J Mol Sci. (2023) 24:2102. doi: 10.3390/ijms24010799
5. Alfranca A, Martinez-Cruzado L, Tornin J, Abarrategi A, Amaral T, de Alava E, et al. Bone microenvironment signals in OS development. Cell Mol Life Sci. (2015) 72:3097–113. doi: 10.1007/s00018-015-1918-y
6. Oyama R, Nabeshima A, Endo M, Novikov A, Fujiwara T, Phelip C, et al. A detoxified TLR4 agonist inhibits tumour growth and lung metastasis of OS by promoting CD8+ cytotoxic lymphocyte infiltration. BJC Rep. (2025) 3:5. doi: 10.1038/s44276-024-00120-3
7. Yahiro K, Matsumoto Y, Yamada H, Endo M, Setsu N, Fujiwara T, et al. Activation of TLR4 signaling inhibits progression of OS by stimulating CD8-positive cytotoxic lymphocytes. Cancer Immunol Immunother. (2020) 69:745–58. doi: 10.1007/s00262-020-02508-9
8. Johal S, Ralston S, and Knight C. Mifamurtide for high-grade, resectable, nonmetastatic OS following surgical resection: a cost-effectiveness analysis. Val Health. (2013) 16:1123–32. doi: 10.1016/j.jval.2013.08.2294
9. Nirala BK, Yamamichi T, Petrescu DI, Shafin TN, and Yustein JT. Decoding the impact of tumor microenvironment in OS progression and metastasis. Cancers (Basel). (2023) 15:5108. doi: 10.3390/cancers15205108
10. Shi S, Ou X, Liu C, Li R, Zheng Q, and Hu L. NF-κB signaling and the tumor microenvironment in OS: implications for immune evasion and therapeutic resistance. Front Immunol. (2025) 16:1518664. doi: 10.3389/fimmu.2025.1518664
11. Corre I, Verrecchia F, Crenn V, Redini F, and Trichet V. The OS microenvironment: A complex but targetable ecosystem. Cells. (2020) 9:976. doi: 10.3390/cells9040976
12. Yang C, Tian Y, Zhao F, Chen Z, Su P, Li Y, et al. Bone microenvironment and OS metastasis. Int J Mol Sci. (2020) 21:6985. doi: 10.3390/ijms21196985
13. Chen C, Xie L, Ren T, Huang Y, Xu J, and Guo W. Immunotherapy for OS: Fundamental mechanism, rationale, and recent breakthroughs. Cancer Lett. (2021) 500:1–10. doi: 10.1016/j.canlet.2020.12.024
14. De Martino V, Rossi M, Battafarano G, Pepe J, Minisola S, and Del Fattore A. Extracellular vesicles in OS: antagonists or therapeutic agents? Int J Mol Sci. (2021) 22:12586. doi: 10.3390/ijms222212586
15. Fu H, Wu Y, Chen J, Hu X, Wang X, and Xu G. Exosomes and OS drug resistance. Front Oncol. (2023) 13:1133726. doi: 10.3389/fonc.2023.1133726
16. Xie Q-H, Zheng J-Q, Ding J-Y, Wu Y-F, Liu L, Yu Z-L, et al. : Exosome-mediated immunosuppression in tumor microenvironments. Cells (2022) 11:1946. doi: 10.3390/cells11121946
17. Morini M, Vitale C, Ardito M, Dondero A, Cortese K, and Bottino C. Castriconi RJFiI: Exosomes and immune modulation: implications for neuroblastoma immunotherapy. Front Immunol. (2025) 16:1600062. doi: 10.3389/fimmu.2025.1600062
18. Lundholm M, Schröder M, Nagaeva O, Baranov V, Widmark A, Mincheva-Nilsson L, et al. Prostate tumor-derived exosomes down-regulate NKG2D expression on natural killer cells and CD8+ T cells: mechanism of immune evasion. PLoS One (2014) 9:e108925. doi: 10.1371/journal.pone.0108925
19. Zhu T, Han J, Yang L, Cai Z, Sun W, Hua Y, et al. Immune microenvironment in OS: components, therapeutic strategies and clinical applications. Front Immunol. (2022) 13:907550. doi: 10.3389/fimmu.2022.907550
20. Wolf-Dennen K, Gordon N, and Kleinerman ES. Exosomal communication by metastatic OS cells modulates alveolar macrophages to an M2 tumor-promoting phenotype and inhibits tumoricidal functions. Oncoimmunology. (2020) 9:1747677. doi: 10.1080/2162402X.2020.1747677
21. Wang J, Zhang H, Sun X, Wang X, Ren T, Huang Y, et al. Exosomal PD-L1 and N-cadherin predict pulmonary metastasis progression for OS patients. J Nanobiotechnol. (2020) 18:151. doi: 10.1186/s12951-020-00710-6
22. Ying X, Zheng X, Zhang X, Yin Y, and Wang X. Kynurenine in IDO1(high) cancer cell-derived extracellular vesicles promotes angiogenesis by inducing endothelial mitophagy in ovarian cancer. J Transl Med. (2024) 22:267. doi: 10.1186/s12967-024-05054-5
23. Troyer RM, Ruby CE, Goodall CP, Yang L, Maier CS, Albarqi HA, et al. Exosomes from OS and normal osteosarcomateoblast differ in proteomic cargo and immunomodulatory effects on T cells. Exp Cell Res. (2017) 358:369–76. doi: 10.1016/j.yexcr.2017.07.011
24. Pawar JS, Salam MA, Dipto MSU, Al-Amin MY, Salam MT, Sengupta S, et al. Cancer-associated fibroblasts: immunosuppressive crosstalk with tumor-infiltrating immune cells and implications for therapeutic resistance. Cancers (Basel) (2025) 17:2484. doi: 10.3390/cancers17152484
25. Mao X, Xu J, Wang W, Liang C, Hua J, Liu J, et al. Crosstalk between cancer-associated fibroblasts and immune cells in the tumor microenvironment: new findings and future perspectives. Mol Cancer. (2021) 20:131. doi: 10.1186/s12943-021-01428-1
26. Yu S and Yao X. Advances on immunotherapy for OS. Mol Cancer. (2024) 23:192. doi: 10.1186/s12943-024-02105-9
27. Cheng S, Wang H, Kang X, and Zhang H. Immunotherapy innovations in the fight against OS: emerging strategies and promising progress. Pharmaceutics (2024) 16:251. doi: 10.3390/pharmaceutics16020251
28. Kawano M, Itonaga I, Iwasaki T, Tsuchiya H, and Tsumura H. Anti-TGF-β antibody combined with dendritic cells produce antitumor effects in OS. Clin Orthop Relat Res. (2012) 470:2288–94. doi: 10.1007/s11999-012-2299-2
29. Bourhis M, Palle J, Galy-Fauroux I, and Terme M. Direct and indirect modulation of T cells by VEGF-A counteracted by anti-angiogenic treatment. Front Immunol. (2021) 12:616837. doi: 10.3389/fimmu.2021.616837
30. Zhang Y and Brekken RAJ. Direct and indirect regulation of the tumor immune microenvironment by VEGF. J Leukoc Biol. (2022) 111:1269–86. doi: 10.1002/JLB.5RU0222-082R
31. Hu T, Sun W, Jin Y, Dong Y, Liu W, Sun Z, et al. The combination of apatinib and antigen-specific DC-induced T cells exert antitumor effects by potently improving the immune microenvironment of OS. Heliyon (2024) 10:e36016. doi: 10.1016/j.heliyon.2024.e36016
32. Liu J, Lin W-P, Su W, Wu Z-Z, Yang Q-C, Wang S, et al. Sunitinib attenuates reactive MDSCs enhancing anti-tumor immunity in HNSCC. Int Immunopharmacol. (2023) 119:110243. doi: 10.1016/j.intimp.2023.110243
33. Li W, Zhan M, Y-y Q, Wang H, Hua S-n, Li Y, et al. Modulating the tumor immune microenvironment with sunitinib malate supports the rationale for combined treatment with immunotherapy. Int Immunopharmacol (2020) 81:106227. doi: 10.1016/j.intimp.2020.106227
34. Martin-Broto J, Hindi N, Grignani G, Martinez-Trufero J, Redondo A, Valverde C, et al. Nivolumab and sunitinib combination in advanced soft tissue sarcomas: a multicenter, single-arm, phase Ib/II trial. J Immunother Cancer. (2020) 8:e001561. doi: 10.1136/jitc-2020-001561
35. Zhao X, Ren T, Li S, Wang X, Hou R, Guan Z, et al. A new perspective on the therapeutic potential of tumor metastasis: targeting the metabolic interactions between TAMs and tumor cells. Int J Biol Sci. (2024) 20:5109. doi: 10.7150/ijbs.99680
36. Liu J, Geng X, Hou J, and Wu GJCCI. New insights into M1/M2 macrophages: key modulators in cancer progression. Cancer Cell Int. (2021) 21:389. doi: 10.1186/s12935-021-02089-2
37. Zhou Q, Xian M, Xiang S, Xiang D, Shao X, Wang J, et al. All-trans retinoic acid prevents OS metastasis by inhibiting M2 polarization of tumor-associated macrophages. Cancer Immunol Res. (2017) 5:547–59. doi: 10.1158/2326-6066.CIR-16-0259
38. Zhao Y, Zhang B, Zhang Q, Ma X, and Feng H. Tumor-associated macrophages in OS. J Zhejiang Univ Sci B. (2021) 22:885–92. doi: 10.1631/jzus.B2100029
39. Koirala P, Roth ME, Gill J, Piperdi S, Chinai JM, Geller DS, et al. Immune infiltration and PD-L1 expression in the tumor microenvironment are prognostic in OS. Scientific reports (2016) 6:30093. doi: 10.1038/srep30093
40. Huang X, Zhang W, Zhang Z, Shi D, Wu F, Zhong B, et al. Prognostic value of programmed cell death 1 ligand-1 (PD-L1) or PD-1 expression in patients with OS: a meta-analysis. J Cancer (2018) 9:2525. doi: 10.7150/jca.25011
41. Zheng B, Ren T, Huang Y, Sun K, Wang S, Bao X, et al. PD-1 axis expression in musculoskeletal tumors and antitumor effect of nivolumab in OS model of humanized mouse. J Hematol Oncol. (2018) 11:16. doi: 10.1186/s13045-018-0560-1
42. Tawbi HA, Burgess M, Bolejack V, Van Tine BA, Schuetze SM, Hu J, et al. Pembrolizumab in advanced soft-tissue sarcoma and bone sarcoma (SARC028): a multicentre, two-cohort, single-arm, open-label, phase 2 trial. Lancet Oncol. (2017) 18:1493–501. doi: 10.1016/S1470-2045(17)30624-1
43. Boye K, Longhi A, Guren T, Lorenz S, Næss S, Pierini M, et al. Pembrolizumab in advanced OS: results of a single-arm, open-label, phase 2 trial. Cancer Immunol Immunother. (2021) 70:2617–24. doi: 10.1007/s00262-021-02876-w
44. Ma L, Dichwalkar T, Chang JYH, Cossette B, Garafola D, Zhang AQ, et al. Enhanced CAR-T cell activity against solid tumors by vaccine boosting through the chimeric receptor. Science. (2019) 365:162–8. doi: 10.1126/science.aav8692
45. Roberts SS, Chou AJ, and Cheung NK. Immunotherapy of childhood sarcomas. Front Oncol. (2015) 5:181. doi: 10.3389/fonc.2015.00181
46. Lussier DM, Johnson JL, Hingorani P, and Blattman JN. Combination immunotherapy with α-CTLA-4 and α-PD-L1 antibody blockade prevents immune escape and leads to complete control of metastatic OS. J Immunother Cancer. (2015) 3:21. doi: 10.1186/s40425-015-0067-z
47. Helm A, Tinganelli W, Simoniello P, Kurosawa F, Fournier C, Shimokawa T, et al. Reduction of lung metastases in a mouse OS model treated with carbon ions and immune checkpoint inhibitors. Int J Radiat Oncol Biol Phys. (2021) 109:594–602. doi: 10.1016/j.ijrobp.2020.09.041
48. D’Angelo SP, Mahoney MR, Van Tine BA, Atkins J, Milhem MM, Jahagirdar BN, et al. Nivolumab with or without ipilimumab treatment for metastatic sarcoma (Alliance A091401): two open-label, non-comparative, randomised, phase 2 trials. Lancet Oncol. (2018) 19:416–26. doi: 10.1016/S1470-2045(18)30006-8
49. Guo Y, Wang X, Zhang C, Chen W, Fu Y, Yu Y, et al. Therapy: tumor immunotherapy targeting B7-H3: from mechanisms to clinical applications. ImmunoTargets and Therapy (2025) 14:291–320. doi: 10.2147/ITT.S507522
50. Zhou W-T and Jin W-L. B7-H3/CD276: an emerging cancer immunotherapy. Front Immunol. (2021) 12:701006. doi: 10.3389/fimmu.2021.701006
51. Aggarwal C, Prawira A, Antonia S, Rahma O, Tolcher A, Cohen RB, et al. Dual checkpoint targeting of B7-H3 and PD-1 with enoblituzumab and pembrolizumab in advanced solid tumors: interim results from a multicenter phase I/II trial. J Immunother Cancer (2022) 10:e004424. doi: 10.1136/jitc-2021-004424
52. Li H-y, Chen Y-l, Deng X-n, Li H-h, Tan J, Liu G-j, et al. Bispecific antibody targeting both B7-H3 and PD-L1 exhibits superior antitumor activities. Acta Pharmacol Sin. (2023) 44:2322–30. doi: 10.1038/s41401-023-01118-2
53. Talbot LJ, Chabot A, Ross AB, Beckett A, Nguyen P, Fleming A, et al. Redirecting B7-H3.CAR T cells to chemokines expressed in OS enhances homing and antitumor activity in preclinical models. Clin Cancer Res. (2024) 30:4434–49. doi: 10.1158/1078-0432.CCR-23-3298
54. Zhang Q, Zhang Z, Liu G, Li D, Gu Z, Zhang L, et al. B7-H3 targeted CAR-T cells show highly efficient anti-tumor function against OS both. Vitro vivo BMC Cancer. (2022) 22:1124. doi: 10.1186/s12885-022-10229-8
55. Cao JW, Lake J, Impastato R, Chow L, Perez L, Chubb L, et al. Targeting OS with canine B7-H3 CAR T cells and impact of CXCR2 Co-expression on functional activity. Cancer Immunol Immunother. (2024) 73:77. doi: 10.1007/s00262-024-03642-4
56. Van Coillie S, Wiernicki B, and Xu J. Molecular and Cellular Functions of CTLA-4. Adv Exp Med Biol. (2020) 1248:7–32. doi: 10.1007/978-981-15-3266-5_2
57. Wolchok JD, Neyns B, Linette G, Negrier S, Lutzky J, Thomas L, et al. Ipilimumab monotherapy in patients with pretreated advanced melanoma: a randomised, double-blind, multicentre, phase 2, dose-ranging study. Lancet Oncol. (2010) 11:155–64. doi: 10.1016/S1470-2045(09)70334-1
58. Hingorani P, Maas ML, Gustafson MP, Dickman P, Adams RH, Watanabe M, et al. Increased CTLA-4(+) T cells and an increased ratio of monocytes with loss of class II (CD14(+) HLA-DR(lo/neg)) found in aggressive pediatric sarcoma patients. J Immunother Cancer. (2015) 3:35. doi: 10.1186/s40425-015-0082-0
59. Merchant MS, Wright M, Baird K, Wexler LH, Rodriguez-Galindo C, Bernstein D, et al. Phase I clinical trial of ipilimumab in pediatric patients with advanced solid tumors. Clin Cancer Res. (2016) 22:1364–70. doi: 10.1158/1078-0432.CCR-15-0491
60. Wen Y, Tang F, Tu C, Hornicek F, Duan Z, and Min L. Immune checkpoints in OS: Recent advances and therapeutic potential. Cancer Lett. (2022) 547:215887. doi: 10.1016/j.canlet.2022.215887
61. Hu J, Lazar AJ, Ingram D, Wang W-L, Zhang W, Jia Z, et al. Cell membrane-anchored and tumor-targeted IL-12 T-cell therapy destroys cancer-associated fibroblasts and disrupts extracellular matrix in heterogenous OS xenograft models. J Immunother Cancer (2024) 12:e006991. doi: 10.1136/jitc-2023-006991
62. Koppensteiner L, Mathieson L, O’Connor RA, and Akram AR. Cancer associated fibroblasts-an impediment to effective anti-cancer T cell immunity. Front Immunol. (2022) 13:887380. doi: 10.3389/fimmu.2022.887380
63. Guo J-H, Afzal A, Ahmad S, Saeed G, Rehman A, Saddozai UAK, et al. Novel strategies to overcome tumor immunotherapy resistance using CAR NK cells. Front Immunol. (2025) 16:1550652. doi: 10.3389/fimmu.2025.1550652
64. Junker N, Kvistborg P, Køllgaard T, Straten P, Andersen MH, and Svane IM. Tumor associated antigen specific T-cell populations identified in ex vivo expanded TIL cultures. Cell Immunol. (2012) 273:1–9. doi: 10.1016/j.cellimm.2011.12.004
65. Zheng S, Wang W, Shen L, Yao Y, Xia W, and Ni CJEH. Oncology: Tumor battlefield within inflamed, excluded or desert immune phenotypes: the mechanisms and strategies. Exp Hematol Oncol. (2024) 13:80. doi: 10.1186/s40164-024-00543-1
66. Ligon JA, Choi W, Cojocaru G, Fu W, Hsiue EH-C, Oke TF, et al. Pathways of immune exclusion in metastatic OS are associated with inferior patient outcomes. J Immunother Cancer (2021) 9:e001772. doi: 10.1136/jitc-2020-001772
67. Baessler A and Vignali DA. T cell exhaustion. Annual review of immunology (2024) 42:179–206. doi: 10.1146/annurev-immunol-090222-110914
68. Hinrichs CS and Rosenberg SA. Exploiting the curative potential of adoptive T-cell therapy for cancer. Immunol Rev. (2014) 257:56–71. doi: 10.1111/imr.12132
69. Kazemi MH, Sadri M, Najafi A, Rahimi A, Baghernejadan Z, Khorramdelazad H, et al. Tumor-infiltrating lymphocytes for treatment of solid tumors: It takes two to tango? Front Immunol. (2022) 13:1018962. doi: 10.3389/fimmu.2022.1018962
70. Théoleyre S, Mori K, Cherrier B, Passuti N, Gouin F, Rédini F, et al. Phenotypic and functional analysis of lymphocytes infiltrating osteosarcomateolytic tumors: use as a possible therapeutic approach of OS. BMC Cancer. (2005) 5:123. doi: 10.1186/1471-2407-5-123
71. Sarnaik AA, Hamid O, Khushalani NI, Lewis KD, Medina T, Kluger HM, et al. Lifileucel, a tumor-Infiltrating lymphocyte therapy, in metastatic melanoma. J Clin Oncol. (2021) 39:2656–66. doi: 10.1200/JCO.21.00612
72. Tsukahara T, Emori M, Murata K, Mizushima E, Shibayama Y, Kubo T, et al. The future of immunotherapy for sarcoma. Expert Opin Biol Ther. (2016) 16:1049–57. doi: 10.1080/14712598.2016.1188075
73. Wang C, Li M, Wei R, and Wu J. Adoptive transfer of TILs plus anti-PD1 therapy: An alternative combination therapy for treating metastatic OS. J Bone Oncol. (2020) 25:100332. doi: 10.1016/j.jbo.2020.100332
74. Zhou X, Wu J, Duan C, and Liu Y. Retrospective analysis of adoptive TIL therapy plus anti-PD1 therapy in patients with chemotherapy-resistant metastatic OS. J Immunol Res. (2020) 2020:7890985. doi: 10.1155/2020/7890985
75. Hossain MA, Liu G, Dai B, Si Y, Yang Q, Wazir J, et al. Reinvigorating exhausted CD8+ cytotoxic T lymphocytes in the tumor microenvironment and current strategies in cancer immunotherapy. Med Res Rev. (2021) 41:156–201. doi: 10.1002/med.21727
76. Joller N, Anderson AC, and Kuchroo VK. LAG-3, TIM-3, and TIGIT: Distinct functions in immune regulation. Immunity (2024) 57:206–22. doi: 10.1016/j.immuni.2024.01.010
77. Chow A, Perica K, Klebanoff CA, and Wolchok JD. Clinical implications of T cell exhaustion for cancer immunotherapy. Nat Rev Clin Oncol. (2022) 19:775–90. doi: 10.1038/s41571-022-00689-z
78. Mognol GP, Spreafico R, Wong V, Scott-Browne JP, Togher S, Hoffmann A, et al. Exhaustion-associated regulatory regions in CD8+ tumor-infiltrating T cells. Proc Natl Acad Sci U S A (2017) 114:E2776–85. doi: 10.1073/pnas.1620498114
79. Pollack SM, Ingham M, Spraker MB, and Schwartz GK. Emerging targeted and immune-based therapies in sarcoma. J Clin Oncol. (2018) 36:125–35. doi: 10.1200/JCO.2017.75.1610
80. Marcove RC, Miké V, Huvos AG, Southam CM, and Levin AG. Vaccine trials for osteosarcomateogenic sarcoma. A prelim Rep CA Cancer J Clin. (1973) 23:74–80. doi: 10.3322/canjclin.23.2.74
81. Mason NJ, Gnanandarajah JS, Engiles JB, Gray F, Laughlin D, Gaurnier-Hausser A, et al. Immunotherapy with a HER2-targeting listeria induces HER2-specific immunity and demonstrates potential therapeutic effects in a phase I trial in canine OS. Clin Cancer Res. (2016) 22:4380–90. doi: 10.1158/1078-0432.CCR-16-0088
82. Pritchard-Jones K, Spendlove I, Wilton C, Whelan J, Weeden S, Lewis I, et al. Immune responses to the 105AD7 human anti-idiotypic vaccine after intensive chemotherapy, for OS. Br J Cancer. (2005) 92:1358–65. doi: 10.1038/sj.bjc.6602500
83. Ullenhag GJ, Spendlove I, Watson NF, Kallmeyer C, Pritchard-Jones K, and Durrant LG. T-cell responses in OS patients vaccinated with an anti-idiotypic antibody, 105AD7, mimicking CD55. Clin Immunol. (2008) 128:148–54. doi: 10.1016/j.clim.2008.03.512
84. Tsukahara T, Nabeta Y, Kawaguchi S, Ikeda H, Sato Y, Shimozawa K, et al. Identification of human autologous cytotoxic T-lymphocyte-defined OS gene that encodes a transcriptional regulator, papillomavirus binding factor. Cancer Res. (2004) 64:5442–8. doi: 10.1158/0008-5472.CAN-04-0522
85. Nava S, Lisini D, Frigerio S, and Bersano A. Dendritic cells and cancer immunotherapy: the adjuvant effect. Int J Mol Sci. (2021) 22:12339. doi: 10.3390/ijms222212339
86. Krishnadas DK, Shusterman S, Bai F, Diller L, Sullivan JE, Cheerva AC, et al. A phase I trial combining decitabine/dendritic cell vaccine targeting MAGE-A1, MAGE-A3 and NY-ESO-1 for children with relapsed or therapy-refractory neuroblastoma and sarcoma. Cancer Immunol Immunother. (2015) 64:1251–60. doi: 10.1007/s00262-015-1731-3
87. Yang Y, Zhou Y, Wang J, Zhou Y, Watowich SS, and Kleinerman ES. CD103(+) cDC1 dendritic cell vaccine therapy for OS lung metastases. Cancers (Basel). (2024) 16:3251. doi: 10.3390/cancers16193251
88. Pan J, Niu Q, Deng B, Liu S, Wu T, Gao Z, et al. CD22 CAR T-cell therapy in refractory or relapsed B acute lymphoblastic leukemia. Leukemia. (2019) 33:2854–66. doi: 10.1038/s41375-019-0488-7
89. Hong M, Clubb JD, and Chen YY. Engineering CAR-T cells for next-generation cancer therapy. Cancer Cell. (2020) 38:473–88. doi: 10.1016/j.ccell.2020.07.005
90. Liu S, Zhang X, Dai H, Cui W, Yin J, Li Z, et al. Which one is better for refractory/relapsed acute B-cell lymphoblastic leukemia: Single-target (CD19) or dual-target (tandem or sequential CD19/CD22) CAR T-cell therapy? Blood Cancer J. (2023) 13:60. doi: 10.1038/s41408-023-00819-5
91. Weigel B, Malempati S, Reid JM, Voss SD, Cho SY, Chen HX, et al. Phase 2 trial of cixutumumab in children, adolescents, and young adults with refractory solid tumors: a report from the Children’s Oncology Group. Pediatr Blood Cancer. (2014) 61:452–6. doi: 10.1002/pbc.24605
92. Ahmed N, Brawley VS, Hegde M, Robertson C, Ghazi A, Gerken C, et al. Human epidermal growth factor receptor 2 (HER2) -Specific chimeric antigen receptor-Modified T cells for the immunotherapy of HER2-Positive sarcoma. J Clin Oncol. (2015) 33:1688–96. doi: 10.1200/JCO.2014.58.0225
93. Zhou Y, Yang D, Yang Q, Lv X, Huang W, Zhou Z, et al. Single-cell RNA landscape of intratumoral heterogeneity and immunosuppressive microenvironment in advanced OS. Nat Commun. (2020) 11:6322. doi: 10.1038/s41467-020-20059-6
94. Kaczanowska S, Murty T, Alimadadi A, Contreras CF, Duault C, Subrahmanyam PB, et al. Immune determinants of CAR-T cell expansion in solid tumor patients receiving GD2 CAR-T cell therapy. Cancer Cell. (2024) 42:35–51.e38. doi: 10.1016/j.ccell.2023.11.011
95. Chen N, Morello A, Tano Z, and Adusumilli PS. CAR T-cell intrinsic PD-1 checkpoint blockade: A two-in-one approach for solid tumor immunotherapy. Oncoimmunology. (2017) 6:e1273302. doi: 10.1080/2162402X.2016.1273302
96. Tian Y, Li Y, Shao Y, and Zhang Y. Gene modification strategies for next-generation CAR T cells against solid cancers. J Hematol Oncol. (2020) 13:54. doi: 10.1186/s13045-020-00890-6
97. Yan Z, Wang C, Wu J, Wang J, and Ma T. TIM-3 teams up with PD-1 in cancer immunotherapy: mechanisms and perspectives. Mol BioMed. (2025) 6:27. doi: 10.1186/s43556-025-00267-6
98. Marasco M, Berteotti A, Weyershaeuser J, Thorausch N, Sikorska J, Krausze J, et al. Molecular mechanism of SHP2 activation by PD-1 stimulation. Sci Adv. (2020) 6:eaay4458. doi: 10.1126/sciadv.aay4458
99. Kansara M, Teng MW, Smyth MJ, and Thomas DM. Translational biology of OS. Nat Rev Cancer. (2014) 14:722–35. doi: 10.1038/nrc3838
100. Kovac M, Blattmann C, Ribi S, Smida J, Mueller NS, Engert F, et al. Exome sequencing of OS reveals mutation signatures reminiscent of BRCA deficiency. Nat Commun. (2015) 6:8940. doi: 10.1038/ncomms9940
101. Wu CC, Beird HC, Andrew Livingston J, Advani S, Mitra A, Cao S, et al. Immuno-genomic landscape of OS. Nat Commun. (2020) 11:1008. doi: 10.1038/s41467-020-14646-w
102. Cillo AR, Mukherjee E, Bailey NG, Onkar S, Daley J, Salgado C, et al. Ewing sarcoma and OS have distinct immune signatures and intercellular communication networks. Clin Cancer Res. (2022) 28:4968–82. doi: 10.1158/1078-0432.CCR-22-1471
Keywords: osteosarcoma, tumor microenvironment, immunosuppression, immune checkpoint inhibitors, immunotherapy, combinatorial approaches
Citation: Wang H, Zhu T, Yang K, Xu C, Zhang Z, Liu G and Ren H (2025) Decoding the immune microenvironment in osteosarcoma: new insights into checkpoints, vaccines, and CAR-T cells. Front. Oncol. 15:1702219. doi: 10.3389/fonc.2025.1702219
Received: 09 September 2025; Accepted: 16 October 2025;
Published: 31 October 2025.
Edited by:
Shangke Huang, Southwest Medical University, ChinaReviewed by:
Daqiang Song, First Affiliated Hospital of Chongqing Medical University, ChinaCopyright © 2025 Wang, Zhu, Yang, Xu, Zhang, Liu and Ren. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Guangyao Liu, Z3lsaXVAamx1LmVkdS5jbg==; Hongge Ren, MjU2MzgzNTU0NkBxcS5jb20=
 Haitian Wang
Haitian Wang Tongtong Zhu
Tongtong Zhu Kunpeng Yang
Kunpeng Yang Chenkai Xu
Chenkai Xu Zhaoyi Zhang
Zhaoyi Zhang Guangyao Liu
Guangyao Liu Hongge Ren4*
Hongge Ren4*