Protective Immunity Elicited by Oral Immunization of Mice with Salmonella enterica Serovar Typhimurium Braun Lipoprotein (Lpp) and Acetyltransferase (MsbB) Mutants
- 1Department of Microbiology and Immunology, University of Texas Medical Branch, Galveston, TX, USA
- 2Department of Veterinary Sciences, University of Texas M. D. Anderson Cancer Center, Bastrop, TX, USA
- 3Institute for Human Infections and Immunity, University of Texas Medical Branch, Galveston, TX, USA
- 4Sealy Center for Vaccine Development and World Health Organisation Collaborating Center for Vaccine Research, University of Texas Medical Branch, Galveston, TX, USA
- 5Center for Biodefense and Emerging Infectious Diseases, University of Texas Medical Branch, Galveston, TX, USA
We evaluated the extent of attenuation and immunogenicity of the ΔlppAB and ΔlppAB ΔmsbB mutants of Salmonella enterica serovar Typhimurium when delivered to mice by the oral route. These mutants were deleted either for the Braun lipoprotein genes (lppA and lppB) or in combination with the msbB gene, which encodes an acetyltransferase required for lipid A modification of lipopolysaccharide. Both the mutants were attenuated (100% animal survival) and triggered robust innate and adaptive immune responses. Comparable levels of IgG and its isotypes were produced in mice infected with wild-type (WT) S. typhimurium or its aforementioned mutant strains. The ΔlppAB ΔmsbB mutant-immunized animals resulted in the production of higher levels of fecal IgA and serum cytokines during later stages of vaccination (adaptive response). A significant production of interleukin-6 from T-cells was also noted in the ΔlppAB ΔmsbB mutant-immunized mice when compared to that of the ΔlppAB mutant. On the other hand, IL-17A production was significantly more in the serum of ΔlppAB mutant-immunized mice (innate response) with a stronger splenic T-cell proliferative and tumor-necrosis factor-α production. Based on 2-dimensional gel analysis, alterations in the levels of several proteins were observed in both the mutant strains when compared to that in WT S. typhimurium and could be associated with the higher immunogenicity of the mutants. Finally, both ΔlppAB and ΔlppAB ΔmsbB mutants provided complete protection to immunized mice against a lethal oral challenge dose of WT S. typhimurium. Thus, these mutants may serve as excellent vaccine candidates and also provide a platform for delivering heterologous antigens.
Introduction
Salmonella enterica serovar Typhimurium is a food-borne pathogen that causes self-limiting gastroenteritis in healthy individuals. The global burden for non-typhoidal salmonellosis (NTS) was estimated at 93 million cases and 155,000 deaths in 2010 (Majowicz et al., 2010). Infection with S. typhimurium in immunologically compromised adults (e.g., HIV+) and children under the age of three, may lead to invasive non-typhoidal salmonellosis (iNTS) characterized by systemic infection and bacteremia, particularly in Africa and parts of south-east Asia, with approximately one million clinical cases per year (Feasey et al., 2012). The case fatality rate for iNTS bacteremia was reported at 25% (Reddy et al., 2010; Gordon, 2011). Antibiotics are the first choice to treat Salmonella infections; however, the rapidly emerging antibiotic resistance among Salmonella serovars has been a significant concern (Anderson, 1975). In the United States, it is estimated that ~7% of NTS infections are invasive, of which about 5% are fatal (Vugia et al., 2004). NTS strains resistant to multiple antibiotics complicate the treatment of iNTS (Anderson, 1975; Varma et al., 2005). In addition, Salmonella can be used as a bioweapon, as occurred in the intentional contamination of restaurant salad bars in Oregon by a religious cult, which resulted in ~1000 cases of gastroenteritis (Greenfield et al., 2002).
Currently, there is no vaccine available for NTS in humans. Several S. typhimurium mutants such as ΔaroA (Hormaeche et al., 1990, 1991), Δcrp Δcdt (Zhang X. et al., 1997; Zhang et al., 1999), ΔphoP (Galán and Curtiss, 1989), ΔrelA ΔspoT (Na et al., 2006), or ΔaroC ΔssaV (designated as WT05; Hindle et al., 2002) have been developed and showed attenuation in mice. These deleted genes have been implicated in a variety of biological functions. For example, the aroA and aeroC are involved in the bacterial aromatic (Aro) pathway (Hormaeche et al., 1991), while the cdt gene product plays an important role in bacterial colonization of deep tissues in the host (Zhang X. et al., 1997; Zhang et al., 1999). Likewise, the crp gene encodes cyclic AMP receptor protein and acts as a global transcriptional regulator (Shimada et al., 2013). A similar regulatory role has also been assigned to PhoP (Groisman et al., 1989). On the other hand, RelA and SpoT are responsible for synthesizing bacterial signal molecule ppGpp (Pizarro-Cerdá and Tedin, 2004), while ssaV encodes a crucial inner membrane structure component of the type III secretion system (T3SS) on the pathogenicity island 2 (SPI-2) of Salmonella (Hindle et al., 2002).
Although, animals vaccinated with the aforementioned mutants were protected against a dose >104-fold above the LD50 of the parental Salmonella strains, these mutants were found to be either reactogenic or had disappointing immunogenicity in human clinical trials (Tennant et al., 2011; Strugnell et al., 2014). In addition, these live attenuated S. typhimurium vaccine strains were shed in the feces for longer periods of time, which is an undesirable trait for any vaccine (Tennant et al., 2011).
To address the rising concerns of iNTS, several new gene targets (e.g., guaBA and clpP or lon, encoding guanine biosynthesis proteins and ATP-dependent protease, respectively) have been deleted singly or in combination with other genes from S. typhimurium and S. enteritidis (Tennant et al., 2011; Matsui et al., 2015). Importantly, the lon and sulA (encoding the suppressor of lon) double deletion mutants of S. typhimurium and S. enteritidis showed cross protection in animal models (Tennant et al., 2011; Matsui et al., 2015). In addition, attenuated Salmonella strains have been used as vehicles to deliver foreign antigens. For example, a novel attenuated S. typhimurium strain SL368 derived from the auxotrophic S. typhimurium aroA strain SL7207 by deleting part of the spiR coding sequence, was used to express hemagglutinin as well as neuraminidase of a highly pathogenic H5N1 influenza virus. This strain provided protection to mice against both H5N1 and H1N1 viral infections (Pei et al., 2015). Likewise, an oral vaccine for type 1 diabetes was based on live attenuated S. typhimurium strain Mvp728 (ΔhtrA ΔpurD) that expressed diabetic autoantigen preproinsulin and transforming growth factor (TGF)-β (Husseiny et al., 2014).
One of the most serious complications of S. typhimurium infection is septic shock in humans and animals, which is mainly mediated by lipopolysaccharide (LPS) (Parrillo, 1993). We previously reported that Braun (murein) lipoprotein (Lpp) also contributed significantly to septic shock induction (Sha et al., 2004; Fadl et al., 2005a,b). Lpp is 5- to 9- kDa in size (Braun and Hantke, 1974; Braun, 1975; Zhang H. et al., 1997; Fenton and Golenbock, 1998) and encoded by two functional copies of the lpp gene (lppA and lppB) which are located in tandem and separated by 82 bp on the chromosome of S. typhimurium 14028 (Sha et al., 2004). Lpp synergizes with LPS to produce pro-inflammatory cytokines/chemokines (Braun, 1975; Sha et al., 2004; Fadl et al., 2005b). While LPS activates cellular responses by binding to CD14 receptor and via Toll-like receptor (TLR)-4 (Ulevitch and Tobias, 1995; Aliprantis et al., 1999; Tobias et al., 1999), Lpp triggers TLR-2 to activate host cell signaling (Aliprantis et al., 1999). In Escherichia coli and S. typhimurium, the msbB (multi-copy suppressor of htrB [high temperature requirement B]) gene encodes an acyltransferase that catalyzes the addition of lauric acid (C12) to the lipid A moiety of LPS, thus increasing its biological potency (Clementz et al., 1996, 1997; Somerville et al., 1996; Rebeil et al., 2006). Mutation in the msbB gene impaired Salmonella's ability to cause lethality in mice and to induce tumor necrosis factor (TNF)-α, interleukin (IL)-1β, and inducible nitric oxide (iNOS) production (Kalupahana et al., 2003).
In our previous studies, we generated various individual (ΔlppA and ΔlppB) and combinatorial lpp and msbB (ΔlppAB, ΔlppA ΔmsbB, ΔlppB ΔmsbB, and ΔlppAB ΔmsbB) gene deletion mutants of S. typhimurium 14028 and characterized them both in vitro and in vivo models of salmonellosis (Sha et al., 2004; Fadl et al., 2005a,b; Liu et al., 2008). We demonstrated that mice intraperitoneally (i.p.) immunized with these various mutant strains were protected from the lethal challenge dose (given by the i.p. route) of wild-type (WT) S. typhimurium. Furthermore, serum IgG1 antibody titers, T-cell proliferation, as well as the expression of T cell activation maker CD44, were substantially higher in mice i.p. immunized with the mutant strains when compared to that of WT S. typhimurium-infected animals (Liu et al., 2008). Both the ΔlppAB and ΔlppAB ΔmsbB mutants were among the most attenuated and immunogenic ones, and, therefore, were considered as excellent vaccine candidates against S. typhimurium infection (Liu et al., 2008).
Oral inoculation is not only the natural route of Salmonella infection in the host but also the easiest and least invasive method of immunization. In the present study, we analyzed the immunological responses, including IgA levels, of inbred (C57BL/6J) mice that were orally immunized with the ΔlppAB or ΔlppAB ΔmsbB mutants. In addition, we studied bacterial protein profiling alterations attributed to the lpp and/or msbB gene deletions by 2-dimensional (2D) gel electrophoresis and analysis. We detected changes in the levels of several potential immunogenic proteins in both the mutant strains, and demonstrated that ΔlppAB and ΔlppAB ΔmsbB mutants induced robust innate and adaptive immune responses in mice and protected them from the lethal oral challenge with WT S. typhimurium. Our presented data further validate the vaccine potential of the mutants and we provide a possible mechanistic basis for the protection provided by these mutants in mice.
Materials and Methods
Bacterial Strains
WT S. enterica serovar Typhimurium 14028 was purchased from American Type Culture Collection (ATCC), Manassas, VA. The ΔlppAB and ΔlppAB ΔmsbB mutants were generated in our laboratory (Sha et al., 2004; Fadl et al., 2005a,b). The Salmonella strains were grown either in Luria-Bertani (LB) or on MSB medium, the latter consisted of LB medium with no NaCl but supplemented with 2 mM MgSO4 and 2 mM CaCl2 (Murray et al., 2001). Bacterial cells from the exponential growth phase at 37°C were harvested and used for both animal studies (immunization and challenge) and 2D gel electrophoresis analysis. All of the Salmonella strains were periodically examined on Salmonella-Shigella (SS) agar plates (Difco, Detroit, MI) for purity.
Serum Antibodies and Cytokines As Well As Fecal IgA in Mice Orally Infected with S. typhimurium Strains
Six-to-eight week old C57BL/6J female mice were obtained from the Jackson Laboratory (Bar Harbor, ME). Animals were first fasted for at least 4 h and then orally dosed with 100 μl of bacterial suspension with a mouse stomach tube (Sigma, St. Lois, MO). The bacterial doses were prepared in 5% sodium bicarbonate solution to overcome stomach acidity. Briefly, mice (n = 10 per group) were orally infected with 2.0 × 106 colony forming units (CFU)/100 μl of WT S. typhimurium (our calculated LD50 was 2.0 × 105 CFU) or its ΔlppAB or ΔlppAB ΔmsbB mutants. Blood from the surviving animals from four independent experiments was collected by retro-orbital bleeding on days 0, 1, 3, 7, 14, and 21 post infection (p.i.). Total IgG and its isotype antibody titers to the whole bacterial cells on days 14 and 21 p.i. were evaluated by ELISA in serially diluted serum as we previously described (van Lier et al., 2014). Briefly, ELISA plates were first coated with the whole bacterial cells overnight at 4°C. For preparing the bacterial cells, the WT S. typhimurium was grown at 37°C until an OD600nm of 0.8 was reached. The culture was then resuspended to a concentration of 5 × 109 CFU/ml and used to coat the plates treated with poly-L-lysine (10 μg/ml). A serial dilution (1:5) of serum was made to evaluate antibody titers, and a positive antibody titer was defined as the inverse of the highest serum dilution giving an absorbance reading of ≥0.2. Antibody classes and IgG isotypes were also examined by using specific isotype secondary antibodies as we previously described (van Lier et al., 2014).
Likewise, the serum cytokine levels were examined on days 1, 3, 7, 14, and 21 p.i. by using a mouse 6-plex assay kit (Bio-Rad Laboratories Inc., Hercules, CA; Tiner et al., 2015). The fecal matter was collected on day 21 p.i. and suspended in phosphate-buffered saline (PBS) containing 0.15 mg soybean trypsin inhibitor and 25 mM EDTA (ethylenedinitrilo tetraacetic acid) (Sigma-Aldrich, St. Louis, MO). To measure the level of IgA, the ELISA plates were first coated with anti-IgA antibody (without biotinylation), and then the serial dilutions of fecal suspension were added to the plates. After 2 h incubation at room temperature followed by serial washes, the biotinylated anti-IgA antibody with the peroxidase-labeled streptavidin were applied. After further incubation, washes, and addition of the substrate, the plates were read under OD450mm and the values were normalized by their corresponding total protein concentrations (μg/ml) in the fecal suspensions (Cong et al., 1998; Cao et al., 2012). Four independent experiments were performed and the pooled data (each sample was run in duplicate) were presented with statistical analysis. All of the animal studies were performed under an approved Institutional Animal Care and Use Committee protocol.
Oral Immunization, Challenge, and Histopathological Analysis
C57BL/6J mice (n = 10 per group) were orally immunized/infected with 3 × 103 CFU/100 μl of WT S. typhimurium and its ΔlppAB or ΔlppAB ΔmsbB mutants. After 36 days, mice were challenged via the oral route with 2 × 106 CFU/100 μl of WT S. typhimurium. Our goal was to discern efficacy of the vaccine strains at a low dose of 3 × 103 CFU.
In a parallel experiment, mice (n = 5 per group) were orally immunized with a high dose of 1 × 108 CFU (~1000 LD50 of WT S. typhimurium) of ΔlppAB or the ΔlppAB ΔmsbB mutant and all survived. After 21 days, the immunized animals along with naïve control mice (n = 5) were challenged with 1 × 108 CFU of WT S. typhimurium via the oral route. Organs from these challenged animals were excised on either day 7 (for unimmunized mice that were infected with WT S. typhimurium before succumbing to infection) or day 21 (for immunized and challenged animals, all survived). The organs were fixed in 10% neutral buffered formalin (Sha et al., 2008; Agar et al., 2009) and tissues processed and sectioned at 5 μm. The samples were mounted on slides and stained with hematoxylin and eosin (H&E). Tissue lesions were scored on the basis of a severity scale, which correlated with estimates of lesion distribution and the extent of tissue involvement, as we previously described (Sha et al., 2008; Agar et al., 2009). The histopathological evaluation of the tissue sections was performed in a blinded fashion.
T-Cell Proliferative Responses and Cytokine Production
Mice (n = 5) were orally immunized with 1 × 108 CFU of ΔlppAB or the ΔlppAB ΔmsbB mutant, and spleens were harvested on day 21 post immunization. We chose a higher dosage of the mutants for vaccination as Salmonella vaccines are administered at a dosage range of 107-1010 CFU in humans, e.g., S. typhi Ty21a (WHO, 2014) or other typhoid vaccine candidates that have undergone clinical trials (Tacket et al., 2000; Tacket and Levine, 2007). T-cells from the mutant-immunized mice were isolated, and their ability to proliferate as well as to produce cytokines was evaluated upon co-culture with the γ-irradiated and heat-killed WT S. typhimurium-stimulated antigen-presenting cells (APCs; pulsed). T-cells incubated with γ-irradiated naïve APCs (without the bacterial stimulation; un-pulsed) served as controls (Sha et al., 2013; van Lier et al., 2014). After 72 h of incubation, 1μCi of [3H] thymidine was added to a set of each co-culture well, and the cells harvested 16 h later using a semi-automated sample harvester, FilterMate Harvester (PerkinElmer, Waltham, MA), followed by the measurement of radioactive counts (TopCount NXT, PerkinElmer). Likewise, from another set of the T-cell cultures, a portion of the supernatant was collected at 48 and 72 h to measure cytokine/chemokine production by using a mouse 6-plex assay kit (Bio-Rad Laboratories Inc.).
Two-Dimensional (2D) Gel Electrophoresis and Mass Spectrometric Analysis
Bacterial cells (mutants and WT S. typhimurium) were lysed in 8 M urea, 4% CHAPS [3-(3-cholamidopropyl)-dimethylammonio-1-propanesulfonate], and 40 mM Tris-HCl (pH 8.0), followed by 10% (v/v) trichloroacetic acid (TCA) precipitation to remove salts and to concentrate proteins. The TCA precipitated proteins were re-dissolved in the lysis solution and subjected to 2D gel electrophoresis as we previously described (Chopra et al., 2006).
For each vaccine strain, the above prepared samples were run in triplicate gels, and the WT S. typhimurium sample-containing gels were set as a reference during analysis by using Progenesis Workstation (NonlinearDynamics, Durham, NC) at the Protein Chemistry Laboratory, UTMB. The normalized volume (NV) of each spot was calculated by using the total volume normalization method in which each spot on a gel image was expressed relative to the total volume of all spots on that image, and then normalized to the total volume of all spots on the reference gel image (Berth et al., 2007). The detected spots with NVs of ≤60 were filtered out. The NVs of the corresponding spots from gels containing samples of WT S. typhimurium and its two mutants were compared, and a fold-change of ≥2 was considered as differentially expressed/produced proteins. Some of these well separated protein spots (a total of 61) were picked robotically, trypsin-digested, and peptides identified by Matrix-Assisted Laser Desorption Ionization Time-of-Flight Mass Spectrometry (MALDI TOF-MS) at the Protein Chemistry Laboratory, UTMB.
Statistics
Two-way analysis of variance (ANOVA) with the Tukey's post-hoc test or the multiple Student's t-test with the Holm-sidak post-hoc test correction was used for data analysis. We used Kaplan–Meier survival estimates or Fisher exact test for animal studies, and p ≤ 0.05 were considered significant for all of the statistical tests used.
Results
Attenuation of the ΔlppAB and ΔlppAB ΔmsbB Mutants of S. typhimurium in an Oral Mouse Model of Infection
Our previous studies with both ΔlppAB and ΔlppAB ΔmsbB mutants in mice were focused on the i.p. model of infection (Sha et al., 2004; Fadl et al., 2005a,b; Liu et al., 2008). Both the mutant strains displayed significantly decreased virulence phenotype and provided protection to mice upon subsequent WT S. typhimurium lethal challenge. However, their potential as vaccine strains (attenuation and protection) in the oral infection mouse model was not characterized and formed the basis of this study. As shown in Figure 1A, when mice were orally challenged with WT S. typhimurium at the dose of 2.0 × 106 CFU (~10LD50), animals started to die at day 8 p.i., and 90% of them eventually succumbed to infection by day 16 p.i. In sharp contrast, 100% of the mice infected with either the ΔlppAB and or the ΔlppAB ΔmsbB mutant at the same dose of 2.0 × 106 CFU survived up to tested 30 days, and no signs of the disease were observed during the course of infection (Figure 1A).
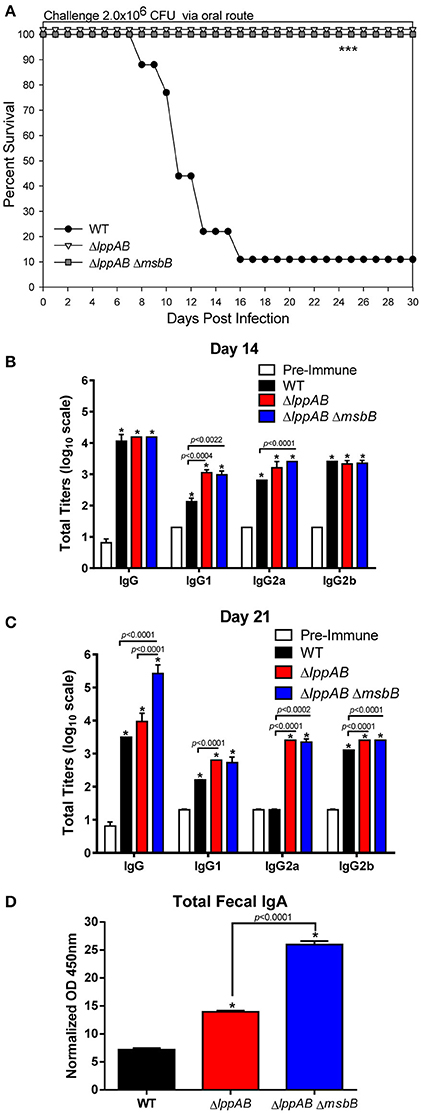
Figure 1. Survival analysis, serum antibodies levels, and fecal IgA production in mice orally infected with WT and mutants of S. typhimurium. Six-to-eight week old C57BL/6J female mice (10 per group) were challenged with 2.0 × 106 CFU/100 μl of WT S. typhimurium or its ΔlppAB and ΔlppAB ΔmsbB mutants via the oral route. Mice were monitored for clinical scores, and the percentage survival in each group was plotted (A). Asterisks indicate statistically significant P-value in comparison to the WT S. typhimurium-infected group and was based on Kaplan–Meier Curve Analysis (0.0027) and Fisher exact test (0.0079). Blood from animals was collected and the titers of IgG and its isotypes (serial dilutions of serum) to the whole bacterial cells on days 14 (B) and 21 (C) post infection (p.i.) were evaluated by ELISA. Serum collected on day 0 before infection served as a pre-immune control. Data were analyzed by using multiple Student's t-test with the Holm–sidak post-hoc test correction. The statistical significances were indicated either by asterisks when comparted to the pre-immune serum or by a line for the compared groups with the P-values. Mice fecal matter was collected on day 21 p.i., and the level of IgA in the fecal suspension was assessed by ELISA and the OD450mm values were normalized by their corresponding total protein concentrations (μg/ml) in the fecal suspensions (D). Two-way ANOVA with the Tukey's post-hoc correction was used to analyze the data, and the statistical significances were indicated either by asterisks when compared to WT S. typhimurium-infected mice or by a line for the compared groups with the P-value. The presented data were pooled from 4 independent experiments representing 4–8 animals/group.
Mouse Serum Antibody Titers and Fecal IgA Production
Specific anti-S. typhimurium antibodies were evaluated on days 14 and 21 p.i. from the above infected groups of mice. Compared to the pre-immune serum, animals in all of the infected groups (WT S. typhimurium and the ΔlppAB and ΔlppAB ΔmsbB mutants) developed high levels (~1:10,000) of Salmonella specific IgG antibodies on day 14 p.i. (Figure 1B). More specifically, all infected mice had similar levels of total IgG and IgG2b isotypes; however, slightly higher levels of IgG1 and IgG2a were observed in the mutant-infected groups of animals when compared to mice in the WT S. typhimurium-infected group (Figure 1B).
On day 21 p.i., the total IgG titers remained unchanged in the ΔlppAB mutant-infected mice, while it slightly dropped in animals infected with WT S. typhimurium when compared to that on day 14 p.i. (Figures 1B,C). In contrast, the total IgG titers in the ΔlppAB ΔmsbB mutant-infected mice continued to mount (>1:100,000) on day 21 p.i. (Figure 1C). Likewise, the IgG isotype titers in all of the infected mice were maintained essentially at the similar levels when comparisons were made for days 14 and 21 p.i., with the exception of the level of IgG2a in WT S. typhimurium-infected animals which dropped back to the level detected in the pre-immune serum (Figure 1C).
The intestinal secretory IgA was also monitored during the course of infection, and a significant level of fecal IgA was detected on day 21 p.i. from all of the infected mice, whether with WT S. typhimurium or its ΔlppAB or ΔlppAB ΔmsbB mutants. However, the highest level of IgA was noted in mice infected with the ΔlppAB ΔmsbB mutant followed by the ΔlppAB mutant, and then the WT S. typhimurium-infected group of animals (Figure 1D).
Levels of Cytokines in Mice Serum during the Course of Infection with WT S. typhimurium and Its Mutants
Serum cytokines were monitored periodically by Bioplex during the course of infection. In general, irrespective of whether the mice were infected with WT S. typhimurium or its designated mutants, the levels of examined cytokines, except for IL-6 and interferon (IFN)-γ, were higher in the serum on day 1 p.i. when compared to the pre-immune serum (Figure 2). The levels of all examined cytokines were also maintained at significantly higher levels during the later stages of infection (i.e., days 14 or 21 p.i.), indicating the mounting of both early (innate) and late (adaptive) immune responses in the infected mice (Figure 2).
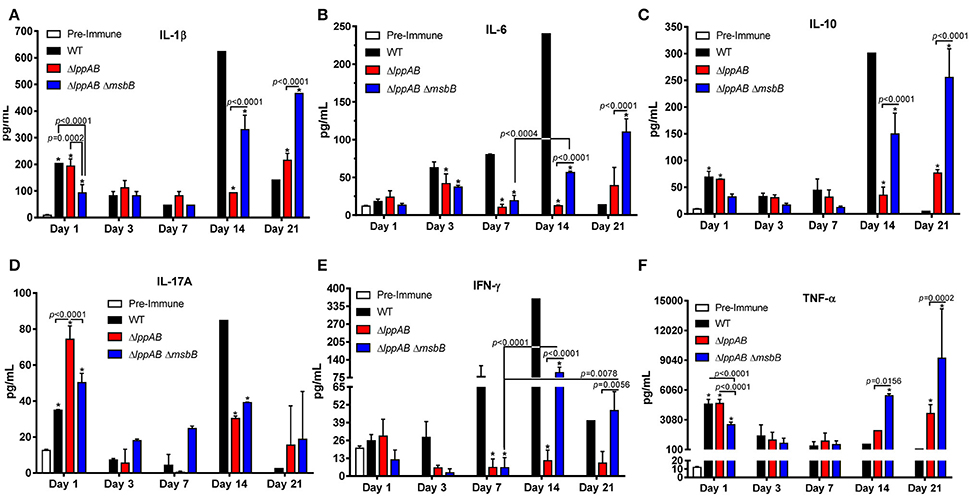
Figure 2. Serum cytokine production in mice orally infected with WT and mutants of S. typhimurium. Six-to-eight week old C57BL/6J female mice (10 per group) were challenged with 2.0 × 106 CFU/100 μl of WT S. typhimurium or its ΔlppAB and ΔlppAB ΔmsbB mutants via the oral route. The serum cytokine levels IL-1β (A), IL-6 (B), IL-10 (C), IL-17A (D), IFN-γ (E), and TNF-α (F) were examined on days 1, 3, 7, 14, and 21 p.i. by using a mouse 6-plex assay kit. Data were analyzed by using Two-way ANOVA with the Tukey's post-hoc correction. The statistical significances were indicated by a line for the compared groups with the P-values or by asterisks when compared to either the pre-immune serum on day 1 or the serum from WT S. typhimurium-infected mice for other days. The presented data were pooled from 4 independent experiments representing 4–8 animals/group.
Interestingly, a similar trend in IL-1β, IL-10, IL-17A, and TNF-α production was observed in all of the infected groups of mice (i.e., WT S. typhimurium or its ΔlppAB or ΔlppAB ΔmsbB mutants), in which their levels were relatively high on day 1 p.i., but subsequently declined by the end of the first week. However, a resurgence in these cytokine production reaching to their highest levels was noted on either day 14 or 21 p.i. (Figures 2A,C,D,F). On the other hand, the trends for IL-6 and IFN-γ production varied among different groups of mice (Figures 2B,E).
IFN-γ levels gradually mounted during the course of infection in the WT S. typhimurium-infected group of mice and reached the peak level on day 14 p.i. (Figure 2E). In contrast, a substantial level of IFN-γ was only detected on days 14 and 21 p.i. in the ΔlppAB ΔmsbB mutant-infected group of mice. Strikingly, minimal IFN-γ was produced in the group of animals infected with the ΔlppAB mutant throughout the infection course (Figure 2E).
Likewise, the level of IL-6 gradually increased during the course of infection in the WT S. typhimurium-infected group of mice and reached the highest level on day 14 p.i. However, the levels of IL-6 in either of the mutant-infected mice increased somewhat on day 3 p.i. and declined on day 7 p.i., followed by its increase on days 14 or 21 p.i. (Figure 2B).
When comparing WT S. typhimurium vs. the mutant-infected mice, the former generally had higher levels of IL-1β, IL-6, IL-10, and IFN-γ on day 14 p.i. (Figures 2A–C,E). These cytokine levels significantly dropped to lower than that of both the mutant-infected groups of mice on day 21 p.i. On the other hand, TNF-α was maintained at generally a similar level across all of the infected mice (with WT S. typhimurium or its ΔlppAB or ΔlppAB ΔmsbB mutants), but were elevated to much higher levels in both of the mutant-infected groups of animals at the later stages of infection (days 14 and 21, Figure 2F).
Interestingly, the production of IL-17A was generally higher in the mutant-infected groups of mice; however, the trend was reversed on day 14 p.i., with significantly higher levels of IL-17A in the WT S. typhimurium -infected group of animals (Figure 2D) compared to that of the mutant-infected groups of mice.
Finally, all of the measured cytokine levels were at the comparable levels in the ΔlppAB mutant-infected group of mice during the first week of infection in comparison to the ΔlppAB ΔmsbB mutant-infected group of animals. However, the trend changed at the later stages of infection (e.g., days 14 and 21 p.i.), during which time significantly higher levels of cytokines were observed in the ΔlppAB ΔmsbB mutant-infected group of mice (Figure 2).
Protection Conferred by Oral Immunization of Mice with the ΔlppAB or the ΔlppAB ΔmsbB Mutant against Subsequent WT. S. typhimurium Challenge
To gauge protection provided by immunization of mice with the mutant strains compared to that of the WT bacterium, animals were initially immunized/infected orally with a relatively low dose (3 × 103 CFU) of the mutant strains (ΔlppAB and ΔlppAB ΔmsbB) or WT S. typhimurium. This low dose ensured animal survival from WT S. typhimurium infection as its LD50 was calculated to be 2.0 × 105 CFU when administered via the oral route. The naïve mice received PBS and served as a control. No animal succumbed to infection during the initial immunization/infection period in all of the groups. On day 36 post immunization, these mice were orally challenged with 2 × 106 CFU of WT S. typhimurium (Figure 3A). All mice vaccinated with either the ΔlppAB or the ΔlppAB ΔmsbB mutant survived, while 60% of the animals were protected in the WT S. typhimurium-vaccinated group. Naïve control mice (80%), on the other hand, succumbed to infection.
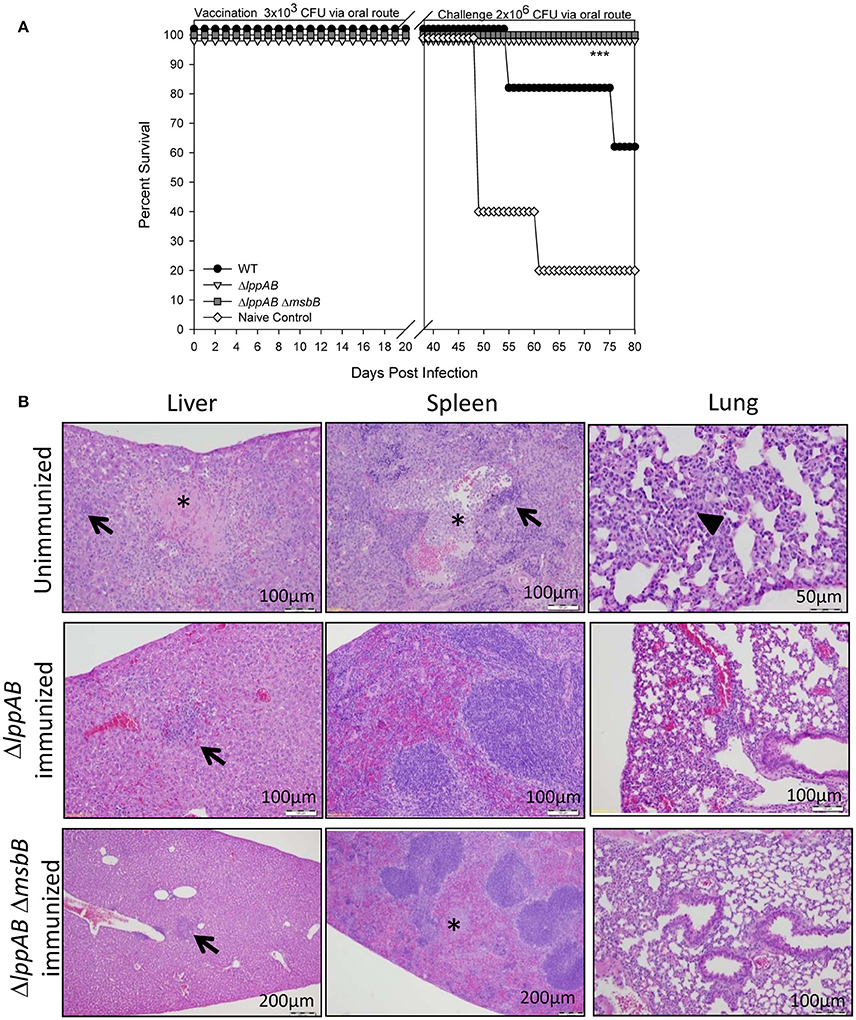
Figure 3. Protection of mice conferred by immunization with the ΔlppAB and ΔlppAB ΔmsbB mutants of S. Typhimurium and histopathological analysis. (A) Six-to-eight week old C57BL/6J female mice (10 per group) were infected with 3 × 103 CFU/100 μl of WT S. typhimurium or its ΔlppAB and ΔlppAB ΔmsbB mutants via the oral route. Naïve mice received PBS and served as a negative control. Thirty-six days after immunization, mice were orally challenged with 2 × 106 CFU/100 μl WT S. typhimurium. Mice were monitored during immunization and subsequent challenge for clinical scores. The percentage survival of mice in each group was plotted. Asterisks indicate statistically significant P-value in comparison to the naïve control group and were based on Kaplan–Meier Curve Analysis (0.013) and Fisher exact test (0.04). (B) Mice (n = 5 per group) were orally immunized with 1 × 108 CFU of ΔlppAB or the ΔlppAB ΔmsbB mutant, and after 21 days, the immunized animals along with unimmunized naïve control mice (n = 5) were challenged with 1 × 108 CFU of WT S. typhimurium via the oral route. Organs were excised from WT S. typhimurium-challenged unimmunized control mice on day 7 and from the mutant-immunized animals on day 21 post challenge with WT S. typhimurium. These organs were stained with H&E and evaluated by light microscopy in a blinded fashion. Asterisks indicate focal necrosis and thrombosis. Solid triangle indicates leukocytic infiltrate, while arrows indicate granulomas. Magnification of the images were also shown. Different magnifications chosen for various panels were to show either detailed histopathologic alterations or a normal tissue architecture over a broader area.
In a parallel experiment, mice were orally immunized with a high dose (1 × 108 CFU) of ΔlppAB or the ΔlppAB ΔmsbB mutant, and no animal succumbed to infection during immunization. After 21 days, the immunized mice were challenged with WT S. typhimurium (1 × 108 CFU) via the oral route and all survived. Mouse organs were excised 21 days post challenge for histopathological analysis. The high doses used for both immunization and challenge were to examine safety of the aforementioned mutants and their protective potential. The unimmunized naïve mice challenged with 1 × 108 CFU of WT S. typhimurium via the oral route were used as a positive control for examining histopathological lesions in organs. These tissues were examined on day 7 post challenge as animals showed severe symptoms such as ruffled fur, lethargy, loss in weight, and diarrhea. As shown in Figure 3B, the WT S. typhimurium-challenged naïve mice had pyogranulomas in various peripheral organs, such as the liver and spleen (arrows), and the lungs had inflammatory cell infiltrate (solid triangle). The splenic tissues revealed lymphoid depletion in white pulps and leukocytosis. The depleted cell types were more likely hematopoietic or stromal cells in the red pulps. In addition, focal necrosis and thrombosis were observed in liver and spleen tissues (asterisks) of mice infected with the WT bacterium. However, intestinal tissue sections, e.g., duodenum, jejunum, and ileum, showed no marked lesions except for inflammation on day 7 p.i. (data not shown).
While analyzing tissues from both of the mutant-immunized groups of mice after WT S. typhimurium challenge, the above observed lesions (e.g., granulomas in the liver, arrows) and focal necrosis/thrombosis (asterisk) in the spleens were much milder in nature with largely intact red and white pulps, when compared to tissues from the WT S. typhimurium-infected naïve mice (Figure 3B).
T-Cell Responses in Mice Immunized with the ΔlppAB or the ΔlppAB ΔmsbB Mutant of S. typhimurium
As shown in Figure 4A, T-cells from both the mutant-immunized groups of mice significantly proliferated in response to the stimulation with WT S. typhimurium antigens. However, the extent of proliferation was much higher for the ΔlppAB mutant-vaccinated mice than that of ΔlppAB ΔmsbB mutant-immunized animals.
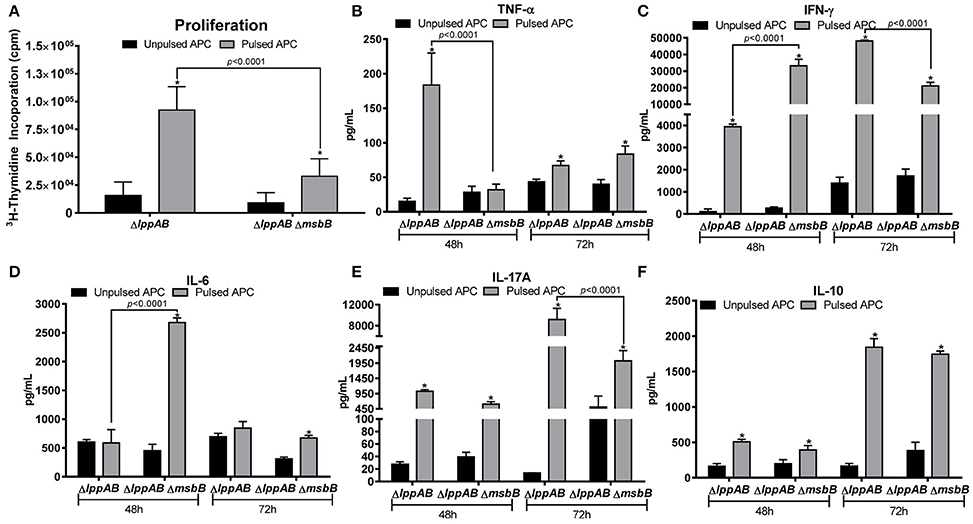
Figure 4. T-cell proliferation in mice immunized with the ΔlppAB and ΔlppAB ΔmsbB mutants of S. typhimurium and cytokine production. (A) Six-to-eight week old C57BL/6J female mice (n = 5) were orally immunized with 1 × 108 CFU/100 μl of ΔlppAB or the ΔlppAB ΔmsbB mutant, and spleens harvested at day 21 post immunization. T-cells were isolated and their ability to proliferate was evaluated upon co-culture with γ-irradiated and heat-killed WT S. typhimurium stimulated APCs (pulsed). T-cells incubated with γ-irradiated naïve APCs without the bacterial stimulation (un-pulsed) served as controls. Two-way ANOVA with the Tukey's post-hoc correction was used to analyze the data, and the statistical significances were indicated either by asterisks when compared to their un-pulsed controls or by a line for the compared groups with the P-values. After 48 and 72 h incubation, the culture supernatants from T-cells were harvested, and the production of cytokine TNF-α (B), IFN-γ (C), IL-6 (D), IL-17A (E), and IL-10 (F) were examined by using a mouse 6-plex assay kit. Two-way ANOVA with the Tukey's post-hoc correction was used to analyze the data, and the statistical significances were indicated either by asterisks when compared to their un-pulsed controls or by a line for the compared groups with P-values.
A robust cytokine production was also observed in T-cells from both the ΔlppAB and ΔlppAB ΔmsbB mutant-immunized mice in response to stimulation with WT S. typhimurium antigens. At 48 h after stimulation, T-cell production of TNF-α in the ΔlppAB-immunized mice was significantly higher when compared to T-cells from the ΔlppAB ΔmsbB mutant-immunized animals (Figure 4B). On the other hand, IFN-γ and IL-6 production from T-cells in the ΔlppAB ΔmsbB mutant-immunized animals (Figures 4C,D) were much higher when compared to the animals immunized with the ΔlppAB mutant.
At 72 h after stimulation, T-cell production of IFN-γ (Figure 4C) and IL-17A (Figure 4E) were much higher in the ΔlppAB mutant-immunized mice when compared to that in the ΔlppAB ΔmsbB mutant-vaccinated animals. The level of IL-10 was maintained at a similar level in both the mutant- immunized mice at both time points of 48 and 72 h (Figure 4F).
Compared to the un-pulsed controls, IFN-γ and IL-10 production were at higher levels in T-cells from both of the mutant infected mice (Figures 4C,F) at both the time points; however, IL-6 levels were specifically induced in T-cells of ΔlppAB ΔmsbB mutant-immunized mice at 48 h time point (Figure 4D), Likewise, TNF-α was significantly produced only from the T-cells of ΔlppAB mutant-immunized mice upon stimulation with the WT S. typhimurium antigens at a 48 h time point, but it was significantly dropped at 72 h (Figure 4B). In contrast, the level of TNF-α in the supernatant of pulsed T-cells from the ΔlppAB ΔmsbB mutant-immunized mice was slightly increased at 72 h time point and reached statistical significance when compared to the un-pulsed control (Figure 4B).
Alterations in the Protein Profiling of ΔlppAB and ΔlppAB ΔmsbB Mutants in Comparison to WT S. typhimurium
2D gel electrophoresis and analysis were performed to obtain protein profiling of WT S. typhimurium and that of its ΔlppAB and ΔlppAB ΔmsbB mutants. There were a total of 1726, 1899, and 1835 protein spots on the 2D gels for WT bacteria, and its ΔlppAB and ΔlppAB ΔmsbB mutants, respectively. After manual editing and filtering, a total of 1099 spots correlated across the gels of different groups of bacteria (e.g., WT S. typhimurium, ΔlppAB, and ΔlppAB ΔmsbB mutants; Table S1). Compared to the 2D gels for WT S. typhimurium, 22 spots had higher normalized volumes (NVs) and 47 spots had lower NVs in the ΔlppAB mutant (Table S2). These numbers were 46 (higher) and 14 (lower) for the ΔlppAB ΔmsbB mutant. Likewise, 10 spots on the 2D gel for the ΔlppAB mutant and 43 spots for the ΔlppAB ΔmsbB mutant had relatively higher NVs when these two mutants were compared to each other (Table S2).
A total of 61 spots were picked based on their fold changes and NVs for mass spectrometric analysis. Among these identified proteins, the majority of them belonged to bacterial inner and outer membranes such as structural proteins, receptors, and transporters. In addition, some proteases, metabolic enzymes, translation associated components, as well as cell signaling related molecules (synthases and regulatory proteins) were also observed. Interestingly, the levels of two proteins, outer membrane protein A (OmpA) and periplasmic protein TolB were higher in both the ΔlppAB and ΔlppAB ΔmsbB mutants when compared to that in the WT bacterium (Table 1).
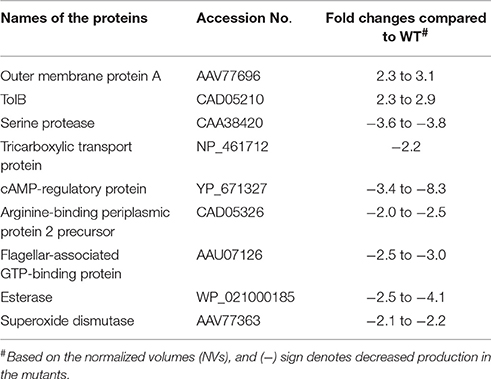
Table 1. Proteins altered in both ΔlppAB and ΔlppAB ΔmsbB mutants compared to WT S. typhimurium based on 2D gel electrophoresis and analysis.
In contrast, the level of seven proteins, including a serine protease, tricarboxylic transport, cAMP-regulatory protein, arginine-binding periplasmic protein 2 precursor, flagellar-associated GTP-binding protein, esterase, and superoxide dismutase, were generally lower in both the ΔlppAB and ΔlppAB ΔmsbB mutants (Table 1). Likewise, when comparison was made between the two mutants, DNA-directed RNA polymerase beta subunit (RpoB) and Rho factor were the two lead proteins produced significantly more with respective 8.1–39.4 and 7.4-fold changes (Table S3), respectively, in the ΔlppAB ΔmsbB mutant. On the other hand, protein chain initiation factor 2 and Lon protease were increased by 7.0 and 4.4-folds, respectively, in the ΔlppAB mutant. A complete list of 61 spots with their protein identification and fold changes are shown in Table S3.
Discussion
Vaccine-based prophylaxis has historically been not only the most significant advances in the healthcare, but also a cost-effective means of public health intervention. We generated S. typhimurium mutants deleted for the lppA and lppB genes alone or in combination with the msbB gene, and studied their attenuation and immunogenicity in mice first in a septicemic mouse model of infection (Sha et al., 2004; Fadl et al., 2005a,b; Liu et al., 2008), and now by the oral route.
Lpp synergizes with LPS to produce pro-inflammatory cytokines/chemokines by activating host cells through TLR-2 and TLR-4 signaling, respectively (Ulevitch and Tobias, 1995; Aliprantis et al., 1999; Tobias et al., 1999). In our previous studies, we showed that both ΔlppAB and ΔlppAB ΔmsbB mutants indeed triggered much less pro-inflammatory cytokines compared to the WT bacterium in an intraperitoneal mouse model of infection during early stages of infection (within a week; Sha et al., 2004; Fadl et al., 2005a,b; Liu et al., 2008). However, this phenomenon was not apparent in mice orally infected with the mutants. For example, IL-17A was detected in higher levels in both the mutant-infected mice when compared to WT S. typhimurium-infected animals during early stages of infection (Figure 2). In addition, a more balanced Th1 and Th2 antibody responses were observed across all orally infected mice, irrespective of whether WT or mutants of S. typhimurium were used (Figures 1B,C). In contrast, IgG1 was the dominant isotype in all mice infected intraperitoneally, accompanied with high levels of T-cell IL-4 production from animals infected with the mutant strains (Liu et al., 2008). These differences may be attributed to different infection routes, bacterial dosages used, and, specifically, C57BL/6J mice are biased to mount a Th1 response (Watanabe et al., 2004).
IL-17 is a signature cytokine of Th17 cells, along with IL-21 and IL-22, and the Th17 response protects animals in an antibody-independent manner (Lin et al., 2011). It has been reported that IL-17 and its associated cytokines (IL-21 and IL-22) are induced in the gastrointestinal tract during Salmonella infection (Ramarathinam et al., 1993; Lee et al., 2012; Kurtz et al., 2014; McSorley, 2014). While Th1 cells are critical for activation of infected macrophages to kill Salmonella in the tissues, Th17 cells are likely essential for recruiting neutrophils to the site of intestinal infection to engulf bacteria (Ramarathinam et al., 1993; Tükel et al., 2009; Broz et al., 2012; Lee et al., 2012). Likewise, fecal IgA, the abundant class of antibodies in the intestinal secretion, serves as the first line of defense against infection (Michetti et al., 1992; Mantis et al., 2011; Gutzeit et al., 2014), and IgA antibodies against a variety of Salmonella antigens are highly effective in preventing salmonellosis (Michetti et al., 1992, 1994; Amarasinghe et al., 2013). Although both Th17 and IgA responses have been shown to provide protection against Salmonella infection (Martinoli et al., 2007; Ko et al., 2009; Mayuzumi et al., 2010; Geddes et al., 2011; Keestra et al., 2011), it is still unclear whether both the ΔlppAB and ΔlppAB ΔmsbB mutant-induced Th17 and IgA responses in mice are directly linked to their protection against subsequent WT S. typhimurium challenge.
Nevertheless, the serum levels of IL-17A were not only generally maintained at higher levels in the mutant-infected mice when compared to WT bacterium-infected animals at an early infection stage (e.g., day 1), but were also sustained longer in the mutant-infected mice at the later infection stages (Figure 2D). More importantly, these IL-17A levels correlated with more fecal IgA production on day 21 p.i. in mice infected with the ΔlppAB and ΔlppAB ΔmsbB mutants (Figure 1D). As we demonstrated recently that Th17 cell production of IL-17 promoted intestinal IgA responses (Cao et al., 2012), it is very likely that the increased IL-17 mediated the high fecal IgA response in mice immunized with the mutants.
In addition to higher IL-17A and fecal IgA production, increased levels of Salmonella- specific antibodies as well as other examined serum cytokines, which were generally sustained for longer periods (up to day 21 p.i.; Figures 1, 2) in mice orally infected with the mutants, might have contributed to full protection of immunized mice to WT bacterial challenge. This protection was 60% in WT S. typhimurium -infected mice during the subsequent re-challenge with WT bacterium (Figure 3A). Interestingly, each of the two mutants displayed some unique immunological aspects. For example, the ΔlppAB mutant-infected mice had relatively higher serum cytokines at the early infection stage (Figure 2), and elicited stronger T-cell proliferation which was associated with the production of TNF-α in comparison to animals infected with the ΔlppAB ΔmsbB mutant (Figure 4).
On the other hand, the ΔlppAB ΔmsbB mutant-infected mice generally displayed higher levels of serum cytokines during the later stages of infection (Figure 2) and IL-6 production from T-cells (Figure 4), as well as more fecal IgA production (Figure 1). However, it is unclear whether all of these differences are related to the compromised TLR-4 signaling as a result of the msbB deletion, which needs to be further investigated. Interestingly, LPS, a sero-dominant and protective antigen in most gram-negative bacteria, has undergone limited analysis as a human Salmonella vaccinogen (MacLennan et al., 2010). This is being re-addressed in new exploratory programs to develop Salmonella vaccines using LPS from S. enteritidis (Simon et al., 2011) and S. typhimurium (Simon et al., 2013) as part of efforts to combat iNTS (Strugnell et al., 2014). Most importantly, a vaccine using GMMA (generalized modules for membrane antigens) from msbB and pagP (encoding lipid A palmitoyltransferase) deletion mutants of S. typhimurium and S. enteritidis with reduced potential for in vivo reactogenicity showed a slightly higher stimulatory potential than WT S. typhimurium harboring WT lipid A (Rossi et al., 2016). Therefore, in this regard, the ΔlppAB ΔmsbB mutant with modified LPS may have some advantages over the ΔlppAB mutant.
Based on 2D gel analysis (Table 1 and Table S3), the production of several proteins such as OmpA, OmpX, TolB, and superoxide dismutase were found to be altered in the ΔlppAB and/or ΔlppAB ΔmsbB mutants compared to WT S. typhimurium. More specifically, the production of first three proteins was up-regulated while the production of superoxide dismutase was down-regulated in the mutants. OmpA binds to and activates APCs, resulting in protective cytotoxic T-lymphocyte (CTL) responses in Klebsiella pneumoniae. It also augments cytokine production (IL-1, -10, and -12) by dendritic cells (DCs) and their migration across the polarized human intestinal epithelial cells in E. coli O157:H7 (Jeannin et al., 2000; Maisnier-Patin et al., 2003; Torres et al., 2006). While OmpX, also referred to as Ail (attachment-invasion locus), is a major contributor to serum resistance and complement evasion in Yersinia pestis (Bartra et al., 2008; Tiner et al., 2015).
Importantly, active immunization with either OmpA or Ail (OmpX) provided protection to mice and rats against developing bubonic and/or pneumonic plague (Erova et al., 2013). The periplasmic protein TolB belongs to the gram-negative bacterial Tol system which comprises five proteins (TolQ, TolR, TolA, TolB, and Pal), and are involved in maintaining bacterial outer membrane stability (Lazzaroni et al., 2002). A study has shown that Groupers, e.g., Epinephelus awoara, a fish belonging to the family Serranidae, immunized with the recombinant TolB of Vibrio alginolyticus generated high antibody responses and protected the immunized fish from V. alginolyticus infection (Pang et al., 2015). The superoxide dismutase protects Salmonella from products of phagocyte NADPH-oxidase and nitric oxide synthase (De Groote et al., 1997). Consequently, the reduced production of superoxide dismutase in the ΔlppAB ΔmsbB mutant compared to WT S. typhimurium might result in efficient killing of the mutant by phagocytic cells as we previously demonstrated (Fadl et al., 2005b), a feature desirable for any vaccine candidate (Golubeva and Slauch, 2006).
Interestingly, the level of flagellar-associated GTP-binding protein decreased in both ΔlppAB and ΔlppAB ΔmsbB mutant strains based on our 2D gel analysis (Table 1), which was in agreement with the reduced transcription level of flagellar structure protein-encoding genes detected by our previous microarray analysis (Fadl et al., 2006), thus adding credibility to our data obtained by both 2D and microarray analyses. Flagellin, a TLR-5 agonist, has been recognized and used as an adjuvant for many vaccines (Hayashi et al., 2001; Yin et al., 2013; Gupta et al., 2014). However, a recent study showed that flagellin was not a major factor for GMMA-mediated immune stimulation against salmonellosis (Rossi et al., 2016). Therefore, the immunogenicity of our mutants either should not be significantly influenced by the decreased flagellar-associated proteins or can be further enhanced in conjunction with the flagellin based adjuvants.
In addition, a study with Y. pestis EV ΔlpxM (msbB) mutant also revealed pleiotropic effects of LpxM in altering synthesis of major immunoreactive antigens (Feodorova et al., 2009). Therefore, it will be interesting to further discern the role of the altered potential immunogens in triggering better immune responses by the mutants than the WT S. typhimurium, and explore the possibility of developing a subunit vaccine(s) against salmonellosis.
Nevertheless, the two attenuated S. typhimurium mutant strains, ΔlppAB and ΔlppAB ΔmsbB, elicited robust innate and adapt immune responses in mice as measured by sera cytokines and specific Salmonella antibody levels, as well as T cell responses. Thus, both ΔlppAB and ΔlppAB ΔmsbB mutants showed potential as viable vaccine candidates against S. typhimurium infection. In addition, the immunological differences observed between these mutants may provide unique perspective for specific applications. Our future direction will include studying the potential cross-protection conferred by the immunization of animals with ΔlppAB and ΔlppAB ΔmsbB mutants of S. typhimurium against other S. enterica serovars (e.g., S. enteritidis).
Author Contributions
TE, MK, and JS planned as well as executed all of the experiments described above. EF and JA helped with animal experiments. DP performed the histopathological animal experiments and WB analyzed the histopathological data. MK, EF, and BT helped in data analysis and formatting the figures. YC and AC helped in the planning of all experiments and discussion of the acquired results. AC and JS also contributed to the writing and editing of the manuscript. AC is the guarantor.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
The funds available through the Robert E. Shope MD and John S. Dunn Distinguished Chair in Global Health, UTMB, to AC allowed us to perform these studies. EF was supported in part by the T32 biodefense training grant, DP by the James W. McLaughlin postdoctoral fellowship, JA by the Sealy Center for Vaccine Development and T32 biodefense training grant, and BT by the J.B. Kempner predoctoral fellowship awards, UTMB. We thank Dr. Linsey A. Yeager for editing the manuscript.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/article/10.3389/fcimb.2016.00148/full#supplementary-material
References
Agar, S. L., Sha, J., Baze, W. B., Erova, T. E., Foltz, S. M., Suarez, G., et al. (2009). Deletion of Braun lipoprotein gene (lpp) and curing of plasmid pPCP1 dramatically alter the virulence of Yersinia pestis CO92 in a mouse model of pneumonic plague. Microbiology 155(Pt 10), 3247–3259. doi: 10.1099/mic.0.029124-0
Aliprantis, A. O., Yang, R. B., Mark, M. R., Suggett, S., Devaux, B., Radolf, J. D., et al. (1999). Cell activation and apoptosis by bacterial lipoproteins through toll-like receptor-2. Science 285, 736–739. doi: 10.1126/science.285.5428.736
Amarasinghe, J. J., D'Hondt, R. E., Waters, C. M., and Mantis, N. J. (2013). Exposure of Salmonella enterica Serovar typhimurium to a protective monoclonal IgA triggers exopolysaccharide production via a diguanylate cyclase-dependent pathway. Infect. Immun. 81, 653–664. doi: 10.1128/IAI.00813-12
Anderson, E. S. (1975). The problem and implications of chloramphenicol resistance in the typhoid bacillus. J. Hyg. (Lond). 74, 289–299. doi: 10.1017/S0022172400024360
Bartra, S. S., Styer, K. L., O'Bryant, D. M., Nilles, M. L., Hinnebusch, B. J., Aballay, A., et al. (2008). Resistance of Yersinia pestis to complement-dependent killing is mediated by the Ail outer membrane protein. Infect. Immun. 76, 612–622. doi: 10.1128/IAI.01125-07
Berth, M., Moser, F. M., Kolbe, M., and Bernhardt, J. (2007). The state of the art in the analysis of two-dimensional gel electrophoresis images. Appl. Microbiol. Biotechnol. 76, 1223–1243. doi: 10.1007/s00253-007-1128-0
Braun, V. (1975). Covalent lipoprotein from the outer membrane of Escherichia coli. Biochim. Biophys. Acta 415, 335–377. doi: 10.1016/0304-4157(75)90013-1
Braun, V., and Hantke, K. (1974). Biochemistry of bacterial cell envelopes. Annu. Rev. Biochem. 43, 89–121. doi: 10.1146/annurev.bi.43.070174.000513
Broz, P., Ohlson, M. B., and Monack, D. M. (2012). Innate immune response to Salmonella typhimurium, a model enteric pathogen. Gut Microbes 3, 62–70. doi: 10.4161/gmic.19141
Cao, A. T., Yao, S., Gong, B., Elson, C. O., and Cong, Y. (2012). Th17 cells upregulate polymeric Ig receptor and intestinal IgA and contribute to intestinal homeostasis. J. Immunol. 189, 4666–4673. doi: 10.4049/jimmunol.1200955
Chopra, V., Fadl, A. A., Sha, J., Chopra, S., Galindo, C. L., and Chopra, A. K. (2006). Alterations in the virulence potential of enteric pathogens and bacterial-host cell interactions under simulated microgravity conditions. J. Toxicol. Environ. Health Part A 69, 1345–1370. doi: 10.1080/15287390500361792
Clementz, T., Bednarski, J. J., and Raetz, C. R. (1996). Function of the htrB high temperature requirement gene of Escherchia coli in the acylation of lipid A: HtrB catalyzed incorporation of laurate. J. Biol. Chem. 271, 12095–12102. doi: 10.1074/jbc.271.20.12095
Clementz, T., Zhou, Z., and Raetz, C. R. (1997). Function of the Escherichia coli msbB gene, a multicopy suppressor of htrB knockouts, in the acylation of lipid A. Acylation by MsbB follows laurate incorporation by HtrB. J. Biol. Chem. 272, 10353–10360. doi: 10.1074/jbc.272.16.10353
Cong, Y., Brandwein, S. L., McCabe, R. P., Lazenby, A., Birkenmeier, E. H., Sundberg, J. P., et al. (1998). CD4+ T cells reactive to enteric bacterial antigens in spontaneously colitic C3H/HeJBir mice: increased T helper cell type 1 response and ability to transfer disease. J. Exp. Med. 187, 855–864. doi: 10.1084/jem.187.6.855
De Groote, M. A., Ochsner, U. A., Shiloh, M. U., Nathan, C., McCord, J. M., Dinauer, M. C., et al. (1997). Periplasmic superoxide dismutase protects Salmonella from products of phagocyte NADPH-oxidase and nitric oxide synthase. Proc. Natl. Acad. Sci. U.S.A. 94, 13997–14001. doi: 10.1073/pnas.94.25.13997
Erova, T. E., Rosenzweig, J. A., Sha, J., Suarez, G., Sierra, J. C., Kirtley, M. L., et al. (2013). Evaluation of protective potential of Yersinia pestis outer membrane protein antigens as possible candidates for a new-generation recombinant plague vaccine. Clin. Vaccine Immunol. 20, 227–238. doi: 10.1128/CVI.00597-12
Fadl, A. A., Galindo, C. L., Sha, J., Klimpel, G. R., Popov, V. L., and Chopra, A. K. (2006). Global gene expression of a murein (Braun) lipoprotein mutant of Salmonella enterica serovar Typhimurium by microarray analysis. Gene 374, 121–127. doi: 10.1016/j.gene.2006.01.034
Fadl, A. A., Sha, J., Klimpel, G. R., Olano, J. P., Galindo, C. L., and Chopra, A. K. (2005a). Attenuation of Salmonella enterica Serovar Typhimurium by altering biological functions of murein lipoprotein and lipopolysaccharide. Infect. Immun. 73, 8433–8436. doi: 10.1128/IAI.73.12.8433-8436.2005
Fadl, A. A., Sha, J., Klimpel, G. R., Olano, J. P., Niesel, D. W., and Chopra, A. K. (2005b). Murein lipoprotein is a critical outer membrane component involved in Salmonella enterica serovar typhimurium systemic infection. Infect. Immun. 73, 1081–1096. doi: 10.1128/IAI.73.2.1081-1096.2005
Feasey, N. A., Dougan, G., Kingsley, R. A., Heyderman, R. S., and Gordon, M. A. (2012). Invasive non-typhoidal salmonella disease: an emerging and neglected tropical disease in Africa. Lancet 379, 2489–2499. doi: 10.1016/S0140-6736(11)61752-2
Fenton, M. J., and Golenbock, D. T. (1998). LPS-binding proteins and receptors. J. Leukoc. Biol. 64, 25–32.
Feodorova, V. A., Pan'kina, L. N., Savostina, E. P., Kuznetsov, O. S., Konnov, N. P., Sayapina, L. V., et al. (2009). Pleiotropic effects of the lpxM mutation in Yersinia pestis resulting in modification of the biosynthesis of major immunoreactive antigens. Vaccine 27, 2240–2250. doi: 10.1016/j.vaccine.2009.02.020
Galán, J. E., and Curtiss, R. III. (1989). Virulence and vaccine potential of phoP mutants of Salmonella typhimurium. Microb. Pathog. 6, 433–443. doi: 10.1016/0882-4010(89)90085-5
Geddes, K., Rubino, S. J., Magalhaes, J. G., Streutker, C., Le Bourhis, L., Cho, J. H., et al. (2011). Identification of an innate T helper type 17 response to intestinal bacterial pathogens. Nat. Med. 17, 837–844. doi: 10.1038/nm.2391
Golubeva, Y. A., and Slauch, J. M. (2006). Salmonella enterica serovar Typhimurium periplasmic superoxide dismutase SodCI is a member of the PhoPQ regulon and is induced in macrophages. J. Bacteriol. 188, 7853–7861. doi: 10.1128/JB.00706-06
Gordon, M. A. (2011). Invasive nontyphoidal Salmonella disease: epidemiology, pathogenesis and diagnosis. Curr. Opin. Infect. Dis. 24, 484–489. doi: 10.1097/QCO.0b013e32834a9980
Greenfield, R. A., Drevets, D. A., MacHado, L. J., Voskuhl, G. W., Cornea, P., and Bronze, M. S. (2002). Bacterial pathogens as biological weapons and agents of bioterrorism. Am. J. Med. Sci. 323, 299–315. doi: 10.1097/00000441-200206000-00003
Groisman, E. A., Chiao, E., Lipps, C. J., and Heffron, F. (1989). Salmonella typhimurium phoP virulence gene is a transcriptional regulator. Proc. Natl. Acad. Sci. U.S.A. 86, 7077–7081. doi: 10.1073/pnas.86.18.7077
Gupta, S. K., Bajwa, P., Deb, R., Chellappa, M. M., and Dey, S. (2014). Flagellin a toll-like receptor 5 agonist as an adjuvant in chicken vaccines. Clin. Vaccine Immunol. 21, 261–270. doi: 10.1128/CVI.00669-13
Gutzeit, C., Magri, G., and Cerutti, A. (2014). Intestinal IgA production and its role in host-microbe interaction. Immunol. Rev. 260, 76–85. doi: 10.1111/imr.12189
Hayashi, F., Smith, K. D., Ozinsky, A., Hawn, T. R., Yi, E. C., Goodlett, D. R., et al. (2001). The innate immune response to bacterial flagellin is mediated by Toll-like receptor 5. Nature 410, 1099–1103. doi: 10.1038/35074106
Hindle, Z., Chatfield, S. N., Phillimore, J., Bentley, M., Johnson, J., Cosgrove, C. A., et al. (2002). Characterization of Salmonella enterica derivatives harboring defined aroC and Salmonella pathogenicity island 2 type III secretion system (ssaV) mutations by immunization of healthy volunteers. Infect. Immun. 70, 3457–3467. doi: 10.1128/IAI.70.7.3457-3467.2002
Hormaeche, C. E., Joysey, H. S., Desilva, L., Izhar, M., and Stocker, B. A. (1990). Immunity induced by live attenuated Salmonella vaccines. Res. Microbiol. 141, 757–764. doi: 10.1016/0923-2508(90)90107-2
Hormaeche, C. E., Joysey, H. S., Desilva, L., Izhar, M., and Stocker, B. A. (1991). Immunity conferred by Aro- Salmonella live vaccines. Microb. Pathog. 10, 149–158. doi: 10.1016/0882-4010(91)90075-L
Husseiny, M. I., Rawson, J., Kaye, A., Nair, I., Todorov, I., Hensel, M., et al. (2014). An oral vaccine for type 1 diabetes based on live attenuated Salmonella. Vaccine 32, 2300–2307. doi: 10.1016/j.vaccine.2014.02.070
Jeannin, P., Renno, T., Goetsch, L., Miconnet, I., Aubry, J. P., Delneste, Y., et al. (2000). OmpA targets dendritic cells, induces their maturation and delivers antigen into the MHC class I presentation pathway. Nat. Immunol. 1, 502–509. doi: 10.1038/82751
Kalupahana, R., Emilianus, A. R., Maskell, D., and Blacklaws, B. (2003). Salmonella enterica serovar Typhimurium expressing mutant lipid A with decreased endotoxicity causes maturation of murine dendritic cells. Infect. Immun. 71, 6132–6140. doi: 10.1128/IAI.71.11.6132-6140.2003
Keestra, A. M., Godinez, I., Xavier, M. N., Winter, M. G., Winter, S. E., Tsolis, R. M., et al. (2011). Early MyD88-dependent induction of interleukin-17A expression during Salmonella colitis. Infect. Immun. 79, 3131–3140. doi: 10.1128/IAI.00018-11
Ko, H. J., Yang, J. Y., Shim, D. H., Yang, H., Park, S. M., Curtiss, R. III., et al. (2009). Innate immunity mediated by MyD88 signal is not essential for induction of lipopolysaccharide-specific B cell responses but is indispensable for protection against Salmonella enterica serovar Typhimurium infection. J. Immunol. 182, 2305–2312. doi: 10.4049/jimmunol.0801980
Kurtz, J. R., Petersen, H. E., Frederick, D. R., Morici, L. A., and McLachlan, J. B. (2014). Vaccination with a single CD4 T cell peptide epitope from a Salmonella type III-secreted effector protein provides protection against lethal infection. Infect. Immun. 82, 2424–2433. doi: 10.1128/IAI.00052-14
Lazzaroni, J. C., Dubuisson, J. F., and Vianney, A. (2002). The Tol proteins of Escherichia coli and their involvement in the translocation of group A colicins. Biochimie 84, 391–397. doi: 10.1016/S0300-9084(02)01419-0
Lee, S. J., McLachlan, J. B., Kurtz, J. R., Fan, D., Winter, S. E., Baumler, A. J., et al. (2012). Temporal expression of bacterial proteins instructs host CD4 T cell expansion and Th17 development. PLoS Pathog. 8:e1002499. doi: 10.1371/journal.ppat.1002499
Lin, J. S., Kummer, L. W., Szaba, F. M., and Smiley, S. T. (2011). IL-17 contributes to cell-mediated defense against pulmonary Yersinia pestis infection. J. Immunol. 186, 1675–1684. doi: 10.4049/jimmunol.1003303
Liu, T., König, R., Sha, J., Agar, S. L., Tseng, C. T., Klimpel, G. R., et al. (2008). Immunological responses against Salmonella enterica serovar Typhimurium Braun lipoprotein and lipid A mutant strains in Swiss-Webster mice: potential use as live-attenuated vaccines. Microb. Pathog. 44, 224–237. doi: 10.1016/j.micpath.2007.09.005
MacLennan, C. A., Gilchrist, J. J., Gordon, M. A., Cunningham, A. F., Cobbold, M., Goodall, M., et al. (2010). Dysregulated humoral immunity to nontyphoidal Salmonella in HIV-infected African adults. Science 328, 508–512. doi: 10.1126/science.1180346
Maisnier-Patin, K., Malissard, M., Jeannin, P., Haeuw, J. F., Corbière, J. C., Hoeffel, G., et al. (2003). The outer membrane protein X from Escherichia coli exhibits immune properties. Vaccine 21, 3765–3774. doi: 10.1016/S0264-410X(03)00316-5
Majowicz, S. E., Musto, J., Scallan, E., Angulo, F. J., Kirk, M., O'Brien, S. J., et al. (2010). The global burden of nontyphoidal Salmonella gastroenteritis. Clin. Infect. Dis. 50, 882–889. doi: 10.1086/650733
Mantis, N. J., Rol, N., and Corthésy, B. (2011). Secretory IgA's complex roles in immunity and mucosal homeostasis in the gut. Mucosal Immunol. 4, 603–611. doi: 10.1038/mi.2011.41
Martinoli, C., Chiavelli, A., and Rescigno, M. (2007). Entry route of Salmonella typhimurium directs the type of induced immune response. Immunity 27, 975–984. doi: 10.1016/j.immuni.2007.10.011
Matsui, H., Fukiya, S., Kodama-Akaboshi, C., Eguchi, M., and Yamamoto, T. (2015). Mouse models for assessing the cross-protective efficacy of oral non-typhoidal Salmonella vaccine candidates harboring in-frame deletions of the ATP-dependent protease lon and other genes. J. Med. Microbiol. 64, 295–302. doi: 10.1099/jmm.0.000014
Mayuzumi, H., Inagaki-Ohara, K., Uyttenhove, C., Okamoto, Y., and Matsuzaki, G. (2010). Interleukin-17A is required to suppress invasion of Salmonella enterica serovar Typhimurium to enteric mucosa. Immunology 131, 377–385. doi: 10.1111/j.1365-2567.2010.03310.x
McSorley, S. J. (2014). Immunity to intestinal pathogens: lessons learned from Salmonella. Immunol. Rev. 260, 168–182. doi: 10.1111/imr.12184
Michetti, P., Mahan, M. J., Slauch, J. M., Mekalanos, J. J., and Neutra, M. R. (1992). Monoclonal secretory immunoglobulin A protects mice against oral challenge with the invasive pathogen Salmonella typhimurium. Infect. Immun. 60, 1786–1792.
Michetti, P., Porta, N., Mahan, M. J., Slauch, J. M., Mekalanos, J. J., Blum, A. L., et al. (1994). Monoclonal immunoglobulin A prevents adherence and invasion of polarized epithelial cell monolayers by Salmonella typhimurium. Gastroenterology 107, 915–923. doi: 10.1016/0016-5085(94)90214-3
Murray, S. R., Bermudes, D., de Felipe, K. S., and Low, K. B. (2001). Extragenic suppressors of growth defects in msbB Salmonella. J. Bacteriol. 183, 5554–5561. doi: 10.1128/JB.183.19.5554-5561.2001
Na, H. S., Kim, H. J., Lee, H. C., Hong, Y., Rhee, J. H., and Choy, H. E. (2006). Immune response induced by Salmonella typhimurium defective in ppGpp synthesis. Vaccine 24, 2027–2034. doi: 10.1016/j.vaccine.2005.11.031
Pang, H., Zhou, Z., Zhang, Y., Li, J., Qiu, M., Ding, Y., et al. (2015). Immunogenicity and Immunoprotection of Translocation Protein B(TolB) in Vibrio alginolyticus. Biotechnol. Bull. 31, 143–149. doi: 10.13560/j.cnki.biotech.bull.1985.2015.07.021
Parrillo, J. E. (1993). Pathogenetic mechanisms of septic shock. N. Engl. J. Med. 328, 1471–1477. doi: 10.1056/NEJM199305203282008
Pei, Z., Jiang, X., Yang, Z., Ren, X., Gong, H., Reeves, M., et al. (2015). Oral delivery of a novel attenuated Salmonella vaccine expressing influenza A virus proteins protects mice against H5N1 and H1N1 viral infection. PLoS ONE 10:e0129276. doi: 10.1371/journal.pone.0129276
Pizarro-Cerda, J., and Tedin, K. (2004). The bacterial signal molecule, ppGpp, regulates Salmonella virulence gene expression. Mol. Microbiol. 52, 1827–1844. doi: 10.1111/j.1365-2958.2004.04122.x
Ramarathinam, L., Niesel, D. W., and Klimpel, G. R. (1993). Salmonella typhimurium induces IFN-gamma production in murine splenocytes. Role of natural killer cells and macrophages. J. Immunol. 150, 3973–3981.
Rebeil, R., Ernst, R. K., Jarrett, C. O., Adams, K. N., Miller, S. I., and Hinnebusch, B. J. (2006). Characterization of late acyltransferase genes of Yersinia pestis and their role in temperature-dependent lipid A variation. J. Bacteriol. 188, 1381–1388. doi: 10.1128/JB.188.4.1381-1388.2006
Reddy, E. A., Shaw, A. V., and Crump, J. A. (2010). Community-acquired bloodstream infections in Africa: a systematic review and meta-analysis. Lancet Infect. Dis. 10, 417–432. doi: 10.1016/S1473-3099(10)70072-4
Rossi, O., Caboni, M., Negrea, A., Necchi, F., Alfini, R., Micoli, F., et al. (2016). Toll-like receptor activation by generalized modules for membrane antigens from lipid A mutants of Salmonella enterica Serovars Typhimurium and Enteritidis. Clin. Vaccine Immunol. 23, 304–314. doi: 10.1128/CVI.00023-16
Sha, J., Agar, S. L., Baze, W. B., Olano, J. P., Fadl, A. A., Erova, T. E., et al. (2008). Braun lipoprotein (Lpp) contributes to virulence of yersiniae: potential role of Lpp in inducing bubonic and pneumonic plague. Infect. Immun. 76, 1390–1409. doi: 10.1128/IAI.01529-07
Sha, J., Fadl, A. A., Klimpel, G. R., Niesel, D. W., Popov, V. L., and Chopra, A. K. (2004). The two murein lipoproteins of Salmonella enterica serovar Typhimurium contribute to the virulence of the organism. Infect. Immun. 72, 3987–4003. doi: 10.1128/IAI.72.7.3987-4003.2004
Sha, J., Kirtley, M. L., van Lier, C. J., Wang, S., Erova, T. E., Kozlova, E. V., et al. (2013). Deletion of the Braun lipoprotein-encoding gene and altering the function of lipopolysaccharide attenuate the plague bacterium. Infect. Immun. 81, 815–828. doi: 10.1128/IAI.01067-12
Shimada, T., Yoshida, H., and Ishihama, A. (2013). Involvement of cyclic AMP receptor protein in regulation of the rmf gene encoding the ribosome modulation factor in Escherichia coli. J. Bacteriol. 195, 2212–2219. doi: 10.1128/JB.02279-12
Simon, R., Tennant, S. M., Wang, J. Y., Schmidlein, P. J., Lees, A., Ernst, R. K., et al. (2011). Salmonella enterica serovar Enteritidis core O polysaccharide conjugated to H:g,m flagellin as a candidate vaccine for protection against invasive infection with S. enteritidis. Infect. Immun. 79, 4240–4249. doi: 10.1128/IAI.05484-11
Simon, R., Wang, J. Y., Boyd, M. A., Tulapurkar, M. E., Ramachandran, G., Tennant, S. M., et al. (2013). Sustained protection in mice immunized with fractional doses of Salmonella Enteritidis core and O polysaccharide-flagellin glycoconjugates. PLoS ONE 8:e64680. doi: 10.1371/journal.pone.0064680
Somerville, J. E. Jr., Cassiano, L., Bainbridge, B., Cunningham, M. D., and Darveau, R. P. (1996). A novel Escherichia coli lipid A mutant that produces an antiinflammatory lipopolysaccharide. J. Clin. Invest. 97, 359–365. doi: 10.1172/JCI118423
Strugnell, R. A., Scott, T. A., Wang, N., Yang, C., Peres, N., Bedoui, S., et al. (2014). Salmonella vaccines: lessons from the mouse model or bad teaching? Curr. Opin. Microbiol. 17, 99–105. doi: 10.1016/j.mib.2013.12.004
Tacket, C. O., and Levine, M. M. (2007). CVD 908, CVD 908-htrA, and CVD 909 live oral typhoid vaccines: a logical progression. Clin. Infect. Dis. 45(Suppl. 1), S20–S23. doi: 10.1086/518135
Tacket, C. O., Sztein, M. B., Wasserman, S. S., Losonsky, G., Kotloff, K. L., Wyant, T. L., et al. (2000). Phase 2 clinical trial of attenuated Salmonella enterica serovar Typhi oral live vector vaccine CVD 908-htrA in U.S. volunteers. Infect. Immun. 68, 1196–1201. doi: 10.1128/IAI.68.3.1196-1201.2000
Tennant, S. M., Wang, J. Y., Galen, J. E., Simon, R., Pasetti, M. F., Gat, O., et al. (2011). Engineering and preclinical evaluation of attenuated nontyphoidal Salmonella strains serving as live oral vaccines and as reagent strains. Infect. Immun. 79, 4175–4185. doi: 10.1128/IAI.05278-11
Tiner, B. L., Sha, J., Kirtley, M. L., Erova, T. E., Popov, V. L., Baze, W. B., et al. (2015). Combinational deletion of three membrane protein-encoding genes highly attenuates Yersinia pestis while retaining immunogenicity in a mouse model of pneumonic plague. Infect. Immun. 83, 1318–1338. doi: 10.1128/IAI.02778-14
Tobias, P. S., Tapping, R. I., and Gegner, J. A. (1999). Endotoxin interactions with lipopolysaccharide-responsive cells. Clin. Infect. Dis. 28, 476–481. doi: 10.1086/515163
Torres, A. G., Li, Y., Tutt, C. B., Xin, L., Eaves-Pyles, T., and Soong, L. (2006). Outer membrane protein A of Escherichia coli O157:H7 stimulates dendritic cell activation. Infect. Immun. 74, 2676–2685. doi: 10.1128/IAI.74.5.2676-2685.2006
Tükel, C., Wilson, R. P., Nishimori, J. H., Pezeshki, M., Chromy, B. A., and Bäumler, A. J. (2009). Responses to amyloids of microbial and host origin are mediated through toll-like receptor 2. Cell Host Microbe 6, 45–53. doi: 10.1016/j.chom.2009.05.020
Ulevitch, R. J., and Tobias, P. S. (1995). Receptor-dependent mechanisms of cell stimulation by bacterial endotoxin. Annu. Rev. Immunol. 13, 437–457. doi: 10.1146/annurev.iy.13.040195.002253
van Lier, C. J., Sha, J., Kirtley, M. L., Cao, A., Tiner, B. L., Erova, T. E., et al. (2014). Deletion of Braun lipoprotein and plasminogen-activating protease-encoding genes attenuates Yersinia pestis in mouse models of bubonic and pneumonic plague. Infect. Immun. 82, 2485–2503. doi: 10.1128/IAI.01595-13
Varma, J. K., Molbak, K., Barrett, T. J., Beebe, J. L., Jones, T. F., Rabatsky-Ehr, T., et al. (2005). Antimicrobial-resistant nontyphoidal Salmonella is associated with excess bloodstream infections and hospitalizations. J. Infect. Dis. 191, 554–561. doi: 10.1086/427263
Vugia, D. J., Samuel, M., Farley, M. M., Marcus, R., Shiferaw, B., Shallow, S., et al. (2004). Invasive Salmonella infections in the United States, FoodNet, 1996-1999: incidence, serotype distribution, and outcome. Clin. Infect. Dis. 38(Suppl. 3), S149–S156. doi: 10.1086/381581
Watanabe, H., Numata, K., Ito, T., Takagi, K., and Matsukawa, A. (2004). Innate immune response in Th1- and Th2-dominant mouse strains. Shock 22, 460–466. doi: 10.1097/01.shk.0000142249.08135.e9
WHO (2014). Information Sheet Observed Rate of Vaccine Reactions Typhoid Vaccine. Available online at: http://www.who.int/vaccine_safety/initiative/tools/Typhoid_vaccine_rates_information_sheet.pdf [Accessed April, 2014].
Yin, G., Qin, M., Liu, X., Suo, J., Tang, X., Tao, G., et al. (2013). An Eimeria vaccine candidate based on Eimeria tenella immune mapped protein 1 and the TLR-5 against Salmonella typhimurium FliC flagellin. Biochem. Biophys. Res. Commun. 440, 437–442. doi: 10.1016/j.bbrc.2013.09.088
Zhang, H., Peterson, J. W., Niesel, D. W., and Klimpel, G. R. (1997). Bacterial lipoprotein and lipopolysaccharide act synergistically to induce lethal shock and proinflammatory cytokine production. J. Immunol. 159, 4868–4878.
Zhang, X., Kelly, S. M., Bollen, W., and Curtiss, R. III. (1999). Protection and immune responses induced by attenuated Salmonella typhimurium UK-1 strains. Microb. Pathog. 26, 121–130. doi: 10.1006/mpat.1998.0245
Keywords: Salmonella enterica serovar Typhimurium, mouse model of salmonellosis, braun or murein lipoprotein (Lpp), lipopolysaccharide (LPS), acetyltransferase (MsbB), innate and adaptive immune responses, oral live-attenuated vaccine, 2-dimensional gel electrophoresis and analysis
Citation: Erova TE, Kirtley ML, Fitts EC, Ponnusamy D, Baze WB, Andersson JA, Cong Y, Tiner BL, Sha J and Chopra AK (2016) Protective Immunity Elicited by Oral Immunization of Mice with Salmonella enterica Serovar Typhimurium Braun Lipoprotein (Lpp) and Acetyltransferase (MsbB) Mutants. Front. Cell. Infect. Microbiol. 6:148. doi: 10.3389/fcimb.2016.00148
Received: 17 August 2016; Accepted: 27 October 2016;
Published: 10 November 2016.
Edited by:
Lorenza Putignani, Bambino Gesù Children's Hospital (IRCCS), ItalyReviewed by:
William D. Picking, University of Kansas, USAEdward Shaw, Oklahoma State University–Stillwater, USA
Copyright © 2016 Erova, Kirtley, Fitts, Ponnusamy, Baze, Andersson, Cong, Tiner, Sha and Chopra. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) or licensor are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Jian Sha, jisha@utmb.edu
Ashok K. Chopra, achopra@utmb.edu
†These authors have contributed equally to this work.
 Tatiana E. Erova1†
Tatiana E. Erova1†  Duraisamy Ponnusamy
Duraisamy Ponnusamy Yingzi Cong
Yingzi Cong Jian Sha
Jian Sha Ashok K. Chopra
Ashok K. Chopra