- UCT/MRC Research Unit for Exercise Science and Sports Medicine, Department of Human Biology, University of Cape Town, Cape Town, South Africa
An influential book written by A. Mosso in the late nineteenth century proposed that fatigue that “at first sight might appear an imperfection of our body, is on the contrary one of its most marvelous perfections. The fatigue increasing more rapidly than the amount of work done saves us from the injury which lesser sensibility would involve for the organism” so that “muscular fatigue also is at bottom an exhaustion of the nervous system.” It has taken more than a century to confirm Mosso’s idea that both the brain and the muscles alter their function during exercise and that fatigue is predominantly an emotion, part of a complex regulation, the goal of which is to protect the body from harm. Mosso’s ideas were supplanted in the English literature by those of A. V. Hill who believed that fatigue was the result of biochemical changes in the exercising limb muscles – “peripheral fatigue” – to which the central nervous system makes no contribution. The past decade has witnessed the growing realization that this brainless model cannot explain exercise performance. This article traces the evolution of our modern understanding of how the CNS regulates exercise specifically to insure that each exercise bout terminates whilst homeostasis is retained in all bodily systems. The brain uses the symptoms of fatigue as key regulators to insure that the exercise is completed before harm develops. These sensations of fatigue are unique to each individual and are illusionary since their generation is largely independent of the real biological state of the athlete at the time they develop. The model predicts that attempts to understand fatigue and to explain superior human athletic performance purely on the basis of the body’s known physiological and metabolic responses to exercise must fail since subconscious and conscious mental decisions made by winners and losers, in both training and competition, are the ultimate determinants of both fatigue and athletic performance.
Introduction
More modern attempts to understand the factors that determine fatigue and superior athletic performance can be traced to European studies beginning in the late nineteenth century. An influential book (Mosso, 1915) written by Italian physiologist A. Mosso, Professor of Physiology at the University of Turin was one of the first to consider the biological basis for the fatigue that develops during exercise. From his observations of a range of natural performances by animals and birds and of experimental muscle fatigue in human subjects, Mosso concluded that: “In raising a weight we must take account of two factors, both susceptible to fatigue. The first is of central origin and purely nervous in character – namely, the will; the second is peripheral, and is the chemical force which is transformed into mechanical work” (pp. 152–153). He made a number of other observations that were prescient including: “On an examination of what takes place in fatigue, two series of phenomena demand our attention. The first is the diminution of the muscular force. The second is fatigue as a sensation” (p. 154); and “If we regard the brain and the muscles as two telegraph offices, we can understand that the nerves which join them do not suffer from fatigue. But the central or psychical station may influence the peripheral or muscular station, even if the latter is not doing work, seeing that both brain and muscles are irrigated by the blood” (p. 281). He also understood that fatigue that “at first sight might appear an imperfection of our body, is on the contrary one of its most marvelous perfections. The fatigue increasing more rapidly than the amount of work done saves us from the injury which lesser sensibility would involve for the organism” (p. 156). He realized too that the brain is unique as it is the only organ protected from the effects of starvation: “If the brain is the organ in which the most active change of material takes place, how can one explain the fact that it does not diminish in weight when all the rest of the body is wasting?” (p. 282). But he is best remembered for being one of the first to propose that “nervous fatigue is the preponderating phenomenon, and muscular fatigue also is at bottom an exhaustion of the nervous system” (Bainbridge, 1919, p. 177).
It has taken studies of “fatigue” more than a century (Di Giulio et al., 2006) to rediscover what Mosso believed to be obvious – that both the brain (Marcora et al., 2009) and the skeletal muscles (Amann et al., 2006; Amann and Dempsey, 2008) alter their function during exercise; that the change in skeletal muscle function is characterized by a slowing of the force and speed of contraction (Jones et al., 2009); and that fatigue is principally an emotion (St Clair Gibson et al., 2003), part of a complex regulation (Noakes et al., 2004; Noakes, 2011b), the goal of which is to protect the body from harm part. So fatigue is indeed one of the human body’s “most marvelous perfections.”
Interestingly Mosso’s ideas did not gain immediate purchase in the exercise sciences but lay dormant until rediscovered more recently (Di Giulio et al., 2006). Instead they were supplanted after 1923 by a different and more simplistic interpretation promoted by English Nobel Laureate Archibald Vivian Hill.
The studies that would become perhaps the most influential in the history of the exercise sciences were performed by Hill and his colleagues at University College, London between 1923 and 1925 (Hill and Lupton, 1923; Hill et al., 1924a,b,c). But Hill’s personal beliefs of what causes fatigue predetermined his interpretation of the results of his quite simple experiments. Thus his conclusions and, as a result, the intellectual direction down which his ideas channeled the exercise sciences were determined by Hill’s preconceptions even before he undertook his first experiment (Noakes, 1997, 2008a,b). His personal beliefs were fashioned by at least three factors.
Firstly since he was principally a muscle physiologist, it was naturally that Hill’s theories would begin from that perspective.
Secondly were a series of studies performed at Cambridge University by another Nobel Laureate Frederick Gowland Hopkins. The crucial 1907 study (Fletcher and Hopkins, 1907) that influenced Hill’s thinking had been designed to develop a novel method accurately to measure muscle lactate concentrations in recently killed laboratory animals, specifically frogs. By plunging excised frog muscles into ice-cold alcohol, Fletcher and Hopkins were able to show that lactate concentrations were elevated in muscles that had been stimulated to contract until failure. We now know that ice-cold alcohol denatures the glycolytic enzymes activated by ischemia and anoxia and the activation of which cause muscle lactate concentrations to increase in ischemia and hypoxia. Fletcher and Hopkins also showed that skeletal muscle lactate concentrations fell in muscles stored in a high oxygen concentration and conversely rose when stored in nitrogen.
As a result Fletcher and Hopkins concluded that: “Lactic acid is spontaneously developed, under anaerobic conditions, in excised muscle” so that “the accumulation of lactic acid in muscle occurs only in the conditions of anaerobiosis. With a proper oxygen supply it fails to accumulate at all.” They also wrote that: “Fatigue due to contractions is accompanied by an increase of lactic acid.”
But Hill’s interpretation of these results was more doctrinaire specifically (a) that lactic acid is produced only under conditions of muscle anaerobiosis, and (b) that muscle fatigue is caused by increased muscle lactate concentrations. These ideas would form the twin pillars of Hill’s nascent theory of the factors that cause fatigue and determine human athletic performance.
Thirdly were studies published in 1909 and 1910 (Hill and Mackenzie, 1909; Hill and Flack, 1910) apparently showing that the inhalation of oxygen significantly improved performance during exercise. This led to the conclusion that “this limit (to muscular work) is imposed by the supply of oxygen to the muscles and brain rather than by the function of the skeletal muscles” (Bainbridge, 1919, p. 133) so that “the supply of oxygen to the body is the decisive factor in setting the limit to exercise” (Bainbridge, 1919, p. 136).
As a result of studies conducted on himself when he ran at 10, 12, and 16 km/h around an 84.5 m track near the Physiological Laboratory, Manchester, Hill concluded that increasing muscle lactate (lactic acid) concentrations secondary to the development of skeletal muscle anaerobiosis, caused the fatigue he experienced when running at 16 km/h. Accordingly he developed a model of human exercise physiology (Figure 1) that has dominated teaching and research in the exercise sciences ever since (Mitchell et al., 1958; Mitchell and Blomqvist, 1971; Bassett Jr. and Howley, 1997, 2000; Mitchell and Saltin, 2003; Levine, 2008).
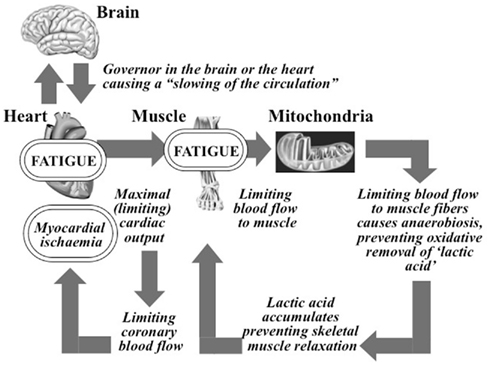
Figure 1. The complete A. V. Hill Cardiovascular/Anaerobic/Catastrophic Model of Human Exercise Performance. The governor component causing a “slowing of the circulation” was lost from the model some time after the 1930s.
Hill’s model predicts that shortly before the termination of maximal exercise the oxygen demands of the exercising muscles exceed the (limiting) capacity of the heart to supply that oxygen. This causes skeletal muscle anaerobiosis with the accumulation of “poisonous” lactate (lactic acid) in the muscles. So Hill believed that the heart’s capacity to pump a large volume of blood to the active skeletal muscles was the single factor determining the human’s ability to perform maximal exercise since the higher the blood supply to muscle, the greater the exercise intensity that could be achieved before the onset of anaerobiosis and fatigue.
Remarkably the most interesting component of Hill’s model is that which has been (conveniently) ignored for the past 90 years. For his model invites the really important question: if the heart’s capacity to produce a maximum cardiac output indeed limits maximum exercise performance, then what limits the maximal cardiac output? This is the key question that has been systematically ignored by all who have favored Hill’s theory for the past 90 years.
Hill believed that the answer was obvious – specifically the development of myocardial ischemia the instant the maximum (limiting) cardiac output was reached. Indeed this would be the modern conclusion since it is established that the development of myocardial ischemia during exercise impairs cardiac function, producing a progressive left ventricular dilatation as a result of impaired myocardial contractility (Rerych et al., 1978).
So Hill’s complete model theorized that maximal exercise is limited by the development of myocardial failure consequent to the development of myocardial ischemia. This model is “catastrophic” since it predicts that exercise is limited by a failure of homeostasis, in this case in the regulation of cardiac function.
This model soon became the standard teaching in the textbooks of the day (Bainbridge, 1931): “The blood supply to the heart, in many men, may be the weak link in the chain of circulatory adjustments during muscular exercise, and as the intensity of muscular exertion increases, a point is probably reached in most individuals at which the supply of oxygen to the heart falls short of its demands, and the continued performance of heavy work becomes difficult or impossible” (pp. 175–176).
Mosso’s concept that the nervous system could also be the site of fatigue was not entirely abandoned. For the 1931 edition (Bainbridge, 1931) of Bainbridge’s original monograph (Bainbridge, 1919), edited at A. V. Hill’s request by the American physiologists A. V. Bock and D. B. Dill, includes the following statement: “There appear, however, to be two types of fatigue, one arising entirely within the central nervous system, the other in which fatigue of the muscles themselves is superadded to that of the nervous system” (p. 228). But this concept of central fatigue, perhaps borrowed from Mosso, would soon disappear from the teaching of the exercise sciences as the idea became entrenched that peripheral fatigue, situated exclusively in the skeletal muscles, explains all forms of exercise fatigue.
But Hill had not completed his model; he added one final and decisive embellishment to his model. He concluded that some mechanism must exist to protect the ischemic heart from damage whilst it continues to contract until the “poisoning” of the skeletal muscles causes the exercise finally to terminate. So he proposed that a “governor” either in the heart or brain reduces the pumping capacity of the heart immediately this inevitable myocardial ischemia develops. By causing a “slowing of the circulation” (Hill et al., 1924a) this governor would protect the ischemic myocardium from damage in this critical period before the exercise terminated.
But sometime after World War II, Hill’s concept of a “governor” mysteriously disappeared from the next generation of exercise physiology textbooks, perhaps because the introduction of electrographically monitored maximal exercise testing established that the healthy heart does not become ischemic even during maximal exercise (Raskoff et al., 1976). Instead the presence of electrocardiographic evidence of ischemia soon became an important diagnostic tool for the detection of coronary artery disease; the absence of these signs of ischemia was interpreted as evidence that the heart is healthy (Lester et al., 1967).
But instead of concluding that the absence of myocardial ischemia during maximal exercise disproves the Hill model, succeeding generations of exercise physiologists simply removed this inconvenient component from their adopted model. Instead they have continued to preach, as fact, the original Hill hypothesis that a limiting cardiac output is the sole important regulator of human exercise performance.
Indeed the special 2008 Olympic Games edition of the influential Journal of Physiology includes the statement that: “(2) the primary distinguishing characteristic of elite endurance athletes that allows them to run fast over prolonged periods of time is a large, compliant heart with a compliant pericardium that can accommodate a lot of blood, very fast, to take maximal advantage of the Starling mechanism to generate a large stroke volume” (Levine, 2008, p. 31).
Like the Hill model, this explanation continues to interpret fatigue as a “catastrophic” event that occurs only after skeletal muscle function has failed, specifically “severe functional alterations at the local muscle level.” Overlooked is Mosso’s conclusion that fatigue is “one of its (the human body’s) most marvelous perfections.”
But Levine does acknowledge that his description cannot adequately explain why athletes ultimately choose to stop exercising. So he adds that which Hill did not: “(3) athletes stop exercising at because of severe functional alterations at the local muscle level due to what is ultimately a limitation in convective oxygen transport, which activates muscle afferents leading to cessation of central motor drive and voluntary effort” (p. 31). This explanation differs from the original Hill model that hypothesizes that some form of central motor command slows the functioning of the heart not the skeletal muscles. It is however entirely compatible with the action of a central governor (Noakes, 2011b). Paradoxically one aim of Levine’s article was to discredit the concept of such a governor.
Problems with the Traditional A. V. Hill Explanation of How Human Exercise Performance is “Limited”
Hill’s original explanation poses a number of significant problems. First, it seems improbable that human athletic performance can be reduced to a single variable and especially one that allows no role for psychological factors such as motivation and self-belief that most agree clearly play some role in human athletic performance. Although scientists may not believe that such factors are important for performance, this is not a belief shared by many coaches and athletes.
For if exercise is regulated purely by a failure of the cardiac output to provide the muscles with an adequate oxygen supply, then psychological factors cannot play any role in human exercise performance. Yet even those who vigorously defend the Hill model, still acknowledge that by providing “motivation,” the brain is indeed involved in determining a maximal effort. Hence: “There is no doubt that motivation is necessary to achieve ” (Levine, 2008, p. 26). But the Hill model in which the skeletal muscles “limit” the exercise performance, specifically excludes any such interpretation.
For if exercise is regulated purely by a failure of first the heart and then of skeletal muscle function, then there is no need for any special motivation to reach that inevitable state of biological failure; one simply continues to move the legs until they fail. Like the proverbial dead horse, no amount of beating (motivation) can force muscles with “severe functional alterations” to keep working. Nor is any beating required to achieve that catastrophic state. A painful beating will enhance performance only if there is a biological control system that prevents a truly maximal effort (but which can be partially over-ridden or distracted by a “beating”).
Indeed if exercise is “limited” solely by an inevitable catastrophic skeletal muscle failure, then is there no need for the symptoms of fatigue whose principal function must be to forestall homeostatic failure (St Clair Gibson et al., 2003). So the presence of the noxious symptoms of fatigue must indicate that exercise cannot be regulated solely by an inevitable and unavoidable failure of skeletal (and or cardiac) muscle function. Rather fatigue symptoms must play a significant biological role as foreseen by Mosso.
Secondly, according to the Levine interpretation, the best athletes must have the largest hearts and the greatest capacity to transport and consume oxygen. But this has never been shown (Coetzer et al., 1993; Billat et al., 2003). Neither is the – a surrogate measure of peak cardiac function according to this theory – a good predictor of athletic ability (Snell and Mitchell, 1984; Coetzer et al., 1993; Lucia et al., 1998) nor even of the changes in performance that occur with training (Jones, 1998, 2006; Legaz Arrese et al., 2007; Vollaard et al., 2009; Robertson et al., 2010).
Thirdly, if exercise performance is limited solely by the function of the heart, then one would expect the cardiac output always to be maximal during all forms of exercise. But this is clearly not the case.
Improbably, these significant logical arguments have not prevented the global acceptance of this theory as the sole correct explanation (Bassett Jr. and Howley, 1997, 2000; Levine, 2008; Shephard, 2009).
Replacing the Heart Alone “Limitations” Model of Human Exercise Performance
Replacing Hill’s cardiovascular/anaerobic/catastrophic model of exercise performance with a novel model began with the realization that the Hill model is unable to explain two of the most obvious characteristics of human exercise performance. The first is that athletes begin exercise at different intensities or paces depending on the expected duration of the planned exercise bout – a bout of short duration is begun at a much faster pace than is one of longer duration. Furthermore athletes will tend to run harder in competition than in training confirming that physiology alone cannot explain performance. The point is that athletes always show an anticipatory component to their exercise performance and that this anticipatory component can be influenced by neural mechanisms relating to motivation. Since as far as we currently know human skeletal muscle probably does not have the capacity to anticipate what is to happen in the future and especially the demands to which it will be exposed (by the brain), the Hill model of peripheral exercise regulation cannot explain this phenomenon.
The second inexplicable observation is that humans also speed up near the end of exercise, the so-called end spurt. This finding significantly disproves the popular belief that fatigue increases progressively and inexorably during prolonged exercise so that athletes reach their most fatigued state immediately prior to the termination of exercise. Were this so, the end spurt could not occur.
In addition to these two rather obvious logical limitations to the predictions of the Hill model, are also a number of significant problems with certain physiological predictions of this model. These include (Noakes and St Clair Gibson, 2004): an absence of evidence that muscle become “anaerobic” during exercise; the absence of a “plateau” in oxygen consumption or cardiac output at exhaustion during maximal exercise; the failure to identify metabolites that explain why muscles “fatigue” during exercise (Jones, 2010) so that “Metabolic causes for these changes (in fatigued skeletal muscle) are hard to identify” (p. 2985); and the absence of evidence for any catastrophic failure of organ function at exhaustion. Rather exercise always terminates with the maintenance of cellular homeostasis.
But the most compelling evidence is the finding that skeletal muscle is never fully recruited during any form of exercise (Noakes and St Clair Gibson, 2004). For the Hill model predicts that as (peripheral) fatigue develops in the exercising muscle fibers so the brain must compensate by recruiting additional fresh fibers in order to assist those fatiguing fibers to sustain the work rate. This process would continue progressively until all the available motor units in the active muscles had been recruited. Once all recruited fibers had each begun to fail, the work rate would fall and “fatigue” would become apparent.
Yet it is now established that fatigue in all forms of exercise develops before there is complete skeletal muscle recruitment. Indeed only between 35 and 50% of the active muscle mass is recruited during prolonged exercise (Tucker et al., 2004; Amann et al., 2006); during maximal exercise this increases to only about 60% (Sloniger et al., 1997a,b; Albertus, 2008).
These findings suggest that the Hill model is too simple properly to explain how human exercise performance is truly regulated.
The Evolution of a Complex Model of Human Exercise Regulation
Inspired by Hill’s concept of a governor regulating human exercise performance, my colleagues and I have proposed a complex model of human exercise regulation in which human exercise performance is not limited by a failure of homeostasis in key organs like the skeletal muscles but is rather regulated in anticipation specifically to insure that no such biological failure can ever occur, at least in healthy humans. This complex regulation originates within the central nervous system; hence we have termed it the Central Governor Model to honor A. V. Hill’s original concept that a “governor” ultimately protects the body from damage during maximal exercise. This model finally re-integrates a body of evidence provided by the neuroscientists that has largely been ignored by those, principally cardio-respiratory physiologists, who have been responsible for sustaining the Hill model for the past 90 years.
The Contribution of Neuroscientists to the Study of Exercise Fatigue
Whilst most exercise scientists have embraced the “brainless” Hill model as the defining explanation for the factors determining human exercise performance (Bassett Jr. and Howley, 1997, 2000; Bassett Jr., 2002; Joyner and Coyle, 2008; Levine, 2008; Shephard, 2009), a large body of research has been conducted independently by neuroscientists interested in the mechanisms explaining the development of fatigue during exercise. Whilst the original focus was predominantly on sustained isometric contractions, in time the research methodologies advanced to be able to study also voluntary dynamic exercise of different durations and intensities. The most complete review (Gandevia, 2001) of these studies establishes that “muscle fatigue … may arise not only because of peripheral changes at the level of the muscle but also because the central nervous system fails to drive the motoneurons adequately.” As a result “human muscle fatigue does not simply reside in the muscle” (p. 1725).
This conclusion suggests that any model attempting to explain exercise performance and the development of fatigue purely on the basis of peripheral changes in the exercising muscles as does the “brainless” Hill model (Noakes, 2008c), cannot provide a completely satisfactory explanation of all these complex phenomena (Noakes, 2011b).
The Central Governor Model of Exercise Regulation
The key components of this model and the body of published evidence that it can explain are shown in Figure 2. This model places the brain firmly at the center of this regulation in keeping with the conclusions of the work reviewed by Gandevia (2001).
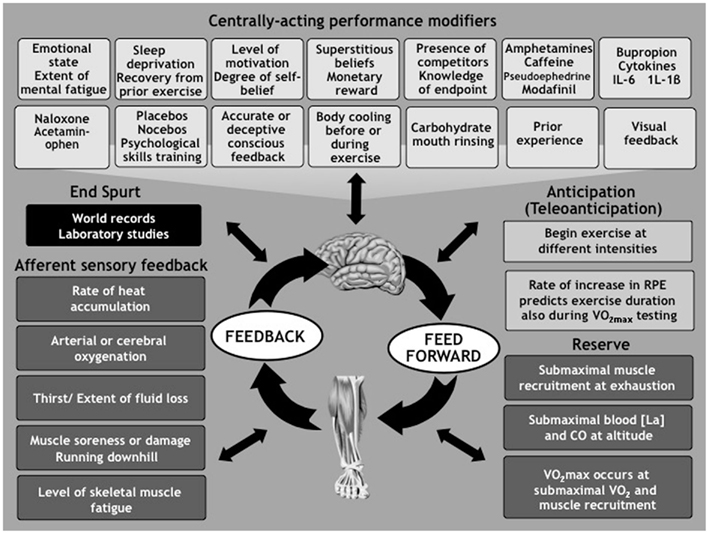
Figure 2. The Central Governor Model of Exercise Regulation proposes that the brain regulates exercise performance by continuously modifying the number of motor units that are recruited in the exercising limbs. This occurs in response to conscious and subconscious factors that are present before and during the exercise, and those which act purely during exercise. The goal of this control is to insure that humans always exercise with reserve and terminate the exercise bout before there is a catastrophic failure of homeostasis. The brain uses the unpleasant (but illusory) sensations of fatigue to insure that the exercise intensity and duration are always within the exerciser’s physiological capacity. This model therefore predicts that the ultimate performances are achieved by athletes who best control the progression of these illusory symptoms during exercise. (For more details see St Clair Gibson et al., 2003; Noakes et al., 2004, 2005; St Clair Gibson and Noakes, 2004; Tucker, 2009; Tucker and Noakes, 2009; Noakes, 2011a,b).
According to this model exercise begins with feedforward motor output to recruit the appropriate number of motor units in the exercising muscles. The extent of this recruitment will be determined by a host of factors including, but not exclusively, the biological state of the athlete at the start of exercise (Hettinga et al., 2011) including the emotional state (Renfree et al., 2011), the extent of mental fatigue (Marcora et al., 2009), or sleep deprivation (Martin, 1981), the state of recovery from a previous exercise bout (Eston et al., 2007), the level of motivation and prior experience (Corbett et al., 2009; Foster et al., 2009; Mauger et al., 2009; Swart et al., 2009a; Micklewright et al., 2010), the degree of self-belief (Micklewright et al., 2010) including superstitious beliefs (Damisch et al., 2010). Factor specific to the event that alter performance include monetary reward (Cabanac, 1986), prior knowledge of the exercise end-point (Ansley et al., 2004a,b; Wittekind et al., 2011), and the presence of competitors (Wilmore, 1968) especially if they are of similar ability (Corbett et al., 2012). A number of chemical agents including the stimulants – amphetamine (Swart et al., 2009b), caffeine (Del et al., 2008; Foad et al., 2008; Hogervorst et al., 2008), pseudoephedrine (Gill et al., 2000; Hodges et al., 2006; Pritchard-Peschek et al., 2010), modafinil (Jacobs and Bell, 2004), and the dopamine/noradrenaline reuptake inhibitor bupropion (Roelands et al., 2008; Roelands and Meeusen, 2010; Watson et al., 2010) – as well as the analgesic, acetaminophen (Mauger et al., 2010), or the analgesic naloxone (Surbey et al., 1984; Sgherza et al., 2002), or the cytokines interleukin-6 (IL-6; Robson-Ansley et al., 2004), or brain IL-1β (Carmichael et al., 2006) have all been shown to alter exercise performance as do placebos (Clark et al., 2000; Benedetti et al., 2007; Pollo et al., 2008; Trojian and Beedie, 2008). Psychological skills training (Barwood et al., 2008) or pre-exercise whole body cooling (Booth et al., 1997) can also improve subsequent exercise performance.
Exercise then begins at an intensity that the brain has determined can be sustained for the expected duration of the exercise bout. As a result all forms of exercise are submaximal since there is always a reserve of motor units in the exercising limbs (Amann et al., 2006; Swart et al., 2009b; Marcora and Staiano, 2010; Ross et al., 2010) that is never fully utilized even during maximal exercise (Sloniger et al., 1997a,b; Albertus, 2008) especially when undertaken at altitude (Kayser et al., 1994; Noakes, 2009). Indeed recent studies show that the conventional testing of the maximum oxygen consumption produce submaximal values for oxygen consumption (Beltrami et al., 2012; Mauger and Sculthorpe, 2012), a finding which seriously challenges the foundation finding on which Hill based his model.
An interesting challenge occurs when exercise is open-ended, that is when the athlete has no idea of the expected duration of the exercise bout in which he or she is participating. This typically occurs during the maximal exercise test used to measure the (Noakes, 2008c) but also occurs when the athlete is unaccustomed to the demands of the specific exercise bout. Under these conditions athletes pace themselves conservatively throughout the exercise bout, increasing their effort only when they are certain how close they are to the finish (Swart et al., 2009a). This uncertainty is associated with a slower rate of rise of the ratings of perceived exertion (RPE).
Once exercise begins, the pace is continuously modified contraction-by-contraction (Tucker et al., 2006a) by continuous feedback from conscious sources including accurate information of the distance covered (Faulkner et al., 2011) and of the end-point (Swart et al., 2009a; Billaut et al., 2011; de Koning et al., 2011). Allowing the pace to change during exercise reduces the physiological effort required to perform a constant amount of work (Lander et al., 2009). Conscious deceptions that improve performance include using the Ramachandran mirror to observe the non-fatigued arm when working with the opposite arm (Tanaka et al., 2011), listening to music (Barwood et al., 2009; Lim et al., 2009; Schneider et al., 2010), the provision of inaccurate information provided by a clock that runs slowly (Morton, 2009) or of the actual distance to be covered (Paterson and Marino, 2004), or of the pace of a prior performance that had been deceptively increased by 2% (Stone et al., 2012), or of the true environmental conditions in which the exercise is being performed and the athlete’s real core body temperature response (Castle et al., 2012). Factors that influence performance and which are likely sensed subconsciously include the degree of arterial (Noakes and Marino, 2007) or cerebral oxygenation (Nybo and Rasmussen, 2007; Rupp and Perrey, 2008, 2009; Johnson et al., 2009; Seifert et al., 2009; Billaut et al., 2010; Rasmussen et al., 2010a,b), the size of the muscle glycogen stores (Rauch et al., 2005; Lima-Silva et al., 2010), the extent of fluid loss or thirst (Edwards et al., 2007; Edwards and Noakes, 2009), and variables relating to the rate of heat accumulation (Marino et al., 2000; Tucker et al., 2004, 2006c; Morante and Brotherhood, 2008; Altareki et al., 2009; Flouris and Cheung, 2009; Schlader et al., 2011). A variety of cooling techniques including to the lower body (Castle et al., 2006; Duffield et al., 2010), the upper body (Arngrimsson et al., 2004), the neck (Tyler et al., 2010; Tyler and Sunderland, 2011a,b), or palms (Kwon et al., 2010) all improve performance presumably by altering the nature of the sensory feedback to the control regions in the brain. Rinsing the mouth with carbohydrate (Rollo et al., 2008, 2010, 2011; Chambers et al., 2009; Gant et al., 2010) improves performance perhaps by acting on specific brain areas. Running downhill (Baron et al., 2009; Townshend et al., 2010) and the presence of muscle damage (Marcora and Bosio, 2007) or muscle soreness (Racinais et al., 2008) are all associated with reduced performance further suggesting the presence of specific sensory pathways subserving these functions. The exercise intensity may also be regulated to insure that a critical level of fatigue is not reached (Amann et al., 2008, 2009, 2010; Amann, 2011). If true this requires a muscle sensor able to detect the level of fatigue in individual motor units.
Finally the presence of the end spurt in which the athlete is able to increase her pace for the last 10% of the exercise bout (Kay et al., 2001; Tucker et al., 2004, 2006b, 2007; Amann et al., 2006; Noakes et al., 2009) confirms the submaximal nature of all exercise performances. More importantly it raises the intriguing questions: Exactly what is fatigue? For how can an athlete speed up near the end of exercise when she is the most tired and should therefore be slowing down according to the traditional definition which describes fatigue as an inability of the contracting muscles to maintain the desired force. According to this definition the athlete who speeds up near the end of exercise cannot be fatigued, regardless of how she feels.
The prediction of this model is that potentially “everything,” not just those factors identified in Figure 2, can potentially affect athletic performance. But that the most important of these effects begin and end in the brain.
The Role of the Sensations of Fatigue in the Regulation of the Exercise Performance in Order to Protect Homeostasis
A key component of the CGM is its proposal that fatigue is not a physical event but rather an emotion (St Clair Gibson et al., 2003) that is used by the brain to regulate the exercise performance (Tucker, 2009). This occurs through changes in the RPE which rise as a linear function of the percentage of the planned exercise bout that has been completed or which remains (Noakes, 2004, 2008b; Tucker, 2009) and which always reach a maximum value at the termination of any truly maximal physical effort. Since the RPE rises as a linear function of the exercise duration, then it must be pre-set either before the exercise bout begins or shortly after its initiation.
Accordingly Tucker (2009) has proposed a model of exercise regulation which “incorporates anticipatory/feedforward as well as feedback components, using an expectation of exercise duration to set an initial work rate and to generate what has been termed a subconscious ‘template’ for the rate of increase in the RPE. During exercise, afferent feedback from numerous physiological systems is responsible for the generation of the conscious RPE, which is continuously matched with the subconscious template by means of adjustment in power output. The subjective rating is biologically linked, allowing the pacing strategy to be adjusted to prevent catastrophic changes in the monitored physiological variables (homeostats)” (p. 400).
More recently Swart et al. (2012) have advanced our understanding of the manner in which two separate sets of fatigue symptoms interact to determine the exercise performance. These authors wished to distinguish between the symptoms that develop during exercise, specifically the physical sensations produced by exercise as distinct from the sensations produced by the physiological/psychic effort required to continue performing a task at a chosen intensity. They note that in his original description Dr. Gunnar Borg described the RPE as a measure of an “individual’s total physical and psychic reaction to exertion” (Borg, 1962).
Thus they wished to separate the physical sensations produced by the actual performance of the work from those psychic or psychological sensations that represent the neural effort of maintaining a given level of physical work. They loosely defined this later group of sensations – the sense of effort – as the subjective sensations not based on any known physiological changes induced by exercise but which are generated by the brain in response to as yet unidentified specific components of the exercise bout. They further postulated that the sense of effort would serve a biological purpose – in particular the maintenance of homeostasis – so that it would rise only when the exercise was of such an intensity or duration that it threatened homeostasis. A rising sense of effort would then force the subject to reduce the exercise intensity in order to prevent a catastrophic biological failure.
To distinguish changes in the physical symptoms produced by exercise from those measuring the sense of effort, they studied subjects who had been carefully instructed to use the Borg RPE scale to measure only the physical symptoms they experienced during exercise. To quantify their sense of effort –the effort of maintaining the work rate – they were instructed in the use of a novel scale – the task effort and awareness (TEA) scale.
Subjects then completed two 100 km cycling bouts, one at a maximal and the other at a submaximal effort. A series of all-out 1 km sprints were included in both exercise bouts. The key was that subjects were instructed to perform all these sprints with an absolutely maximal effort.
The findings showed that whereas RPE rose progressively during exercise in both trials and was lower in the submaximal trial, it reached a maximal value of 19 only in the final sprint in both trials. In contrast, the TEA score was maximal at the end of each sprint even during the submaximal trial in which each sprint began at a lower TEA (and RPE) score.
Thus this study confirms that the brain uses two distinct and separate sets of fatigue symptoms to insure that homeostasis is maintained during all forms of exercise. The first set are the physical sensations induced by exercise and which are adequately captured by Borg’s original RPE scale. These sensations rise as a linear function of the exercise duration and reach a maximum value only at the point of exercise termination. Maintaining an exercise intensity that produces this linear increase in RPE produces the optimum pacing strategy.
The second group of symptoms measured by the TEA quantifies the psychic effort of sustaining the effort that produces a specific RPE. Provided the rate of increase in RPE matches the predetermined template, the sense of effort remains low and is not consciously perceived. But attempting to maintain a pace that causes an inappropriate increase in the RPE will produce an increase in the conscious sense of effort. Thus: “The direct consequence of the increasing sense of effort will be an altered behavior, specifically a voluntary reduction in the exercise intensity. Conversely, exercise intensities that do not pose a threat to homeostatic control produce no or little sense of effort.” As a result the authors conclude: “the conscious decision of whether to maintain, increase or decrease the current workload or indeed to terminate the exercise altogether may be the outcome of a balance between motivation and affect and the sensation that is defined as the sense of effort.”
It is indeed as Bainbridge wrote in 1919: “…the sense of fatigue is often a very fallacious index of the working capacity of the body…there is not necessarily any correspondence between the subjective feelings of fatigue and the capacity of the muscles to perform work … it is a protective feeling, which tends to restrain a man from continuing to perform muscular work when this would react injuriously upon his whole system” (Bainbridge, 1931, pp. 176–177).
Possible Brain Areas Associated with the Feedback Regulation of the Exercise Response in Humans
A series of early studies have found evidence for activation of the insular cortex, the anterior cingulate cortex (ACC) or medial prefrontal region as well as thalamic regions in the brain in response to increased perception of effort during exercise (Williamson et al., 2006). Williamson and colleagues suggest that different areas in the insular cortex appear to respond to inputs from skeletal muscle afferents and from “central command” whereas the anterior cingulate gyrus “may work in conjunction with portions of the insular cortex as a ‘central command network’ functioning to interpret an individual’s sense of effort and then eliciting appropriate autonomic adjustments to affect cardiovascular responses” (p. 56). Thalamic regions are considered to be involved in the regulation of blood pressure by baroreflex mechanisms. More recent studies have further advanced these ideas.
Thus the brain responses to a form of pedaling exercise studied with fMRI found activation of the medial primary sensory and motor cortices, premotor cortex, supplementary area, and anterior cerebellum associated with the task (Mehta et al., 2009). Studying brain areas involved in the decision to terminate exercise, Hilty et al. (2011a) found activation of the mid/anterior insular region immediately prior to the termination of fatiguing isometric handgrip contractions. Since this area is involved in the evaluation of other homeostatic threats, the authors suggest that activation of this brain region may alert the organism to “urgent homeostatic imbalances.” More recently the same group (Hilty et al., 2011b) found evidence for increased communication between the mid/anterior insular and the motor cortex during fatiguing exercise indicating “a fatigue-induced increase in communication between these regions” (p. 6). They propose that the mid/anterior insular region “might not only integrate and evaluate sensory information from the periphery, but also be in direct communication with the motor cortex” (p. 1). The effect of this could be to act as a central regulator of motor output to the exercising limbs in keeping with the concept of a central governor mechanism responding to afferent sensory feedback.
Studying the response of trained athletes and untrained volunteers to an aversive activity, Paulus et al. (2011) reported “profound” activation of the right and left insula, the dorsolateral prefrontal cortex, and the anterior cingulate gyrus in response to the unpleasant task. But trained athletes showed an attenuated response of the right insular cortex compared to non-athletes suggesting that attenuating the right insular cortex response may be an important adaptation favoring superior athletic performance.
Studies of drugs injected directly into areas of the rat brain show that exposure of the ventromedial hypothalamic (VMH) nuclei to muscarinic blockade substantially reduced exercise performance (Guimaraes et al., 2011). Thus the authors conclude: “muscarinic transmission within the VMH modulates physical performance, even when the effects of the thermoregulatory responses on fatigue are minimal” (p. 9).
Summarizing the current evidence Tanaka and Watanabe (2012) have proposed that physical fatigue is regulated by the balance between inhibitory and facilitatory influences on the motor cortex. Thus “sensory input from the peripheral system to the primary motor cortex (M1) decreases the motor output (supraspinal fatigue), and a neural pathway that interconnects the spinal cord, thalamus (TH), secondary somatosensory cortex, medial insular cortex, posterior insular cortex, ACC, premotor (PM) area, supplementary motor area (SMA), and M1 constitutes the inhibition system. In contrast, a facilitation system … that interconnects the limbic system, basal ganglia (BG), TH, orbitofrontal cortex, prefrontal cortex, ACC, PM, SMA, and M1 constitutes the facilitation system and a motivational input to this facilitation system enhances SMA and then M1 to increase the motor output to the peripheral system” (p. 730).
So is it Really Mind Over Muscle?
For decades physiologists have searched for a single biological variable – a biological silver bullet – that would explain why some athletes are better than all others. Usually this has focused on the heart and circulation (Bassett Jr. and Howley, 2000; Levine, 2008), reflecting the dominance that the Hill model has exerted in this field. But already Bean and Eichna (1943) warned that: “… physical fitness cannot be defined nor can differences be detected by means of a few simple physiological measurements …. obtained during limited tests …. To do so results in focusing attention on some erroneous concept. Man is not a pulse rate, a rectal temperature, but a complex array of many phenomena…. Into performance enters the baffling yet extremely important factor of motivation, the will-to-do. This cannot be measured and remains an uncontrollable, quickly fluctuating, disturbing variable which may at any time completely alter the performance regardless of physical or physiologic state” (p. 157).
Similarly Dr. Roger Bannister, the first man to run the mile in less than 4 min wrote in 1956 (Bannister, 1956) that: “The human body is centuries in advance of the physiologist, and can perform an integration of heart, lungs, and muscles which is too complex for the scientist to analyse” (p. 48). Later he continued: “It is the brain not the heart or lungs, that is the critical organ, it’s the brain” (Entine, 2000, p. 13). Future generations of exercise scientists would be well advised to head the words of these most observant scientists.
Indeed elite athletes, like Sir Roger Bannister, believe that something more complex than the heart is the ultimate determinant of their performances.
Thus Paavo Nuurmi, perhaps the greatest distance runner of all time since he won nine gold and three silver medals in the Olympic Games wrote that: “Mind is everything. Muscles are pieces of rubber. All that I am, I am because of my mind.”
Franz Stampfl who coached Roger Bannister to become the first human to run the mile in less than 4 min also wrote that: “The great barrier is the mental hurdle” (Stampfl, 1955).
One of the greatest mile runners of all time, Australian Herb Elliott has also written that: “To run a world record, you have to have the absolute arrogance to think you can run a mile faster than anyone who’s ever lived; and then you have to have the absolute humility to actually do it” (Elliott, 2011, p. 110). Of Elliott and his coach, a contemporary runner Derek Ibbotson who was unable to beat Elliott wrote admiringly: “Together Cerruty and Elliott have brought athletics to the threshold of a new era. They have proved conclusively that not only the body but also the mind must be conquered” (Ibbotson, 1960). Another Australian, former world marathon record holder Derek Clayton wrote: “The difference between my world record and many world class runners is mental fortitude. I ran believing in mind over matter” (Clayton, 1981).
But how might the CGM help us to understand their meaning. I am particularly interested in what the CGM predicts about the athlete who finishes second in a close event.
According to the traditional Hill model the athlete who finished a close second in any event must have had either higher muscle lactate concentrations or lower muscle glycogen concentrations so that his “poisoned” or “depleted” muscles were simply unable to close that 3-s gap. But simple logic exposes the error in this explanation.
For in the final stages of any race, perhaps as many as 65% of the muscle fibers in both the leading athletes’ legs are inactive and do not contribute to the physical effort. Surely the second runner could have activated just a few more of those fibers in order to achieve everlasting sporting glory? What prevented that choice?
The CGM predicts that brain-generated sensations of fatigue unique to each individual and influenced by a host of currently unknown individual factors (Figure 2), insure that athletes will complete all exercise bouts without risking a catastrophic failure. In the case of a close finish the CGM was clearly successful – neither athlete died. But if the second runner did not die, why did he not run just a little faster and so approach death a little closer? For surely he could have sped up by just a fraction without dying? Yet he did not. Why not?
My unproven hypothesis is that in the case of a close finish, physiology does not determine who wins. Rather somewhere in the final section of the race, the brains of the second, and lower placed finishers accept their respective finishing positions and no longer choose to challenge for a higher finish. Once each runner consciously accepts his or her finishing position, the outcome of the race is decided. So just as a single athlete must “decide” to win, so too must the rest of the top finishers decide the opposite – specifically that they are not going to win.
Furthermore the CGM suggests that this outcome will be strongly influenced by the manner in which the brains of the respective runners generate the sensations of fatigue during exercise. Recall that these symptoms of fatigue are entirely self-generated by each athlete’s brain and so are unique to each individual. As such they are illusionary.
According to this model, the winning athlete is the one whose illusionary symptoms interfere the least with the actual performance – in much the same way that the most successful golfer is the one who does not consciously think when playing any shot.
In contrast athletes who finish behind the winner may make the conscious decision not to win, perhaps even before the race begins. Their deceptive symptoms of “fatigue” may then be used to justify that decision. So the winner is the athlete for whom defeat is the least acceptable rationalization.
How athletes and coaches achieve this winning mental attitude is the great unknown. But if the study of the purely physiological basis of fatigue has taught us anything, it is that such studies will never provide an adequate answer.
Rather that future lies in identifying the manner in which the brains of different athletes generate these illusory symptoms. Especially interesting would be studies of the performance of athletes competing in events in which they do not have any experience nor any knowledge of the quality of the opposition. In a close finish under these conditions, how does each athlete decide where she or he will finish? For surely under those specific conditions the uncertain mind will be an even more important determinant of the outcome?
And why they are suppressed in the winning athletes even as they exercise more vigorously than all others.
“The fight,” wrote Muhammad Ali “is won or lost far away from witnesses, behind the lines, in the gym, out there on the road, long before I dance under the lights” (De Rond, 2009, p. 154).
Vince Lombardi, the great American football coach, once wrote that: “Fatigue makes cowards of us all.” But he was wrong. For his arrow of causation points in the wrong direction.
It is cowardice that exacerbates the sensations of fatigue, not the reverse.
Conflict of Interest Statement
The author declares that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
This article is an expanded version of an original outline to be published in Dialogues in Cardiovascular Medicine. The author’s work on which this review is based is funded by Discovery Health, the Medical Research Council, the National Research Foundation and the University of Cape Town.
References
Albertus, Y. (2008). Critical Analysis of Techniques for Normalising Electromyographic Data. Ph.D. thesis, University of Cape Town, Cape Town, 1–219.
Altareki, N., Drust, B., Atkinson, G., Cable, T., and Gregson, W. (2009). Effects of environmental heat stress (35 degrees C) with simulated air movement on the thermoregulatory responses during a 4-km cycling time trial. Int. J. Sports Med. 30, 9–15.
Amann, M. (2011). Central and peripheral fatigue: interaction during cycling exercise in humans. Med. Sci. Sports Exerc. 43, 2039–2045.
Amann, M., Blain, G. M., Proctor, L. T., Sebranek, J. J., Pegelow, D. F., and Dempsey, J. A. (2010). Group III and IV muscle afferents contribute to ventilatory and cardiovascular response to rhythmic exercise in humans. J. Appl. Physiol. 109, 966–976.
Amann, M., and Dempsey, J. A. (2008). Locomotor muscle fatigue modifies central motor drive in healthy humans and imposes a limitation to exercise performance. J. Physiol. (Lond.) 586, 161–173.
Amann, M., Eldridge, M. W., Lovering, A. T., Stickland, M. K., Pegelow, D. F., and Dempsey, J. A. (2006). Arterial oxygenation influences central motor output and exercise performance via effects on peripheral locomotor muscle fatigue in humans. J. Physiol. (Lond.) 575(Pt 3), 937–952.
Amann, M., Proctor, L. T., Sebranek, J. J., Eldridge, M. W., Pegelow, D. F., and Dempsey, J. A. (2008). Somatosensory feedback from the limbs exerts inhibitory influences on central neural drive during whole body endurance exercise. J. Appl. Physiol. 105, 1714–1724.
Amann, M., Proctor, L. T., Sebranek, J. J., Pegelow, D. F., and Dempsey, J. A. (2009). Opioid-mediated muscle afferents inhibit central motor drive and limit peripheral muscle fatigue development in humans. J. Physiol. (Lond.) 587(Pt 1), 271–283.
Ansley, L., Robson, P. J., St Clair, G. A., and Noakes, T. D. (2004a). Anticipatory pacing strategies during supramaximal exercise lasting longer than 30 s. Med. Sci. Sports Exerc. 36, 309–314.
Ansley, L., Schabort, E., St Clair, G. A., Lambert, M. I., and Noakes, T. D. (2004b). Regulation of pacing strategies during successive 4-km time trials. Med. Sci. Sports Exerc. 36, 1819–1825.
Arngrimsson, S. A., Petitt, D. S., Stueck, M. G., Jorgensen, D. K., and Cureton, K. J. (2004). Cooling vest worn during active warm-up improves 5-km run performance in the heat. J. Appl. Physiol. 96, 1867–1874.
Bainbridge, F. A. (1919). The Physiology of Muscular Exercise, 1st Edn. London: Longmans, Green and Co.
Bainbridge, F. A. (1931). The Physiology of Muscular Exercise, 3rd Edn. London: Longmans, Green and Co.
Baron, B., Deruelle, F., Moullan, F., Dalleau, G., Verkindt, C., and Noakes, T. D. (2009). The eccentric muscle loading influences the pacing strategies during repeated downhill sprint intervals. Eur. J. Appl. Physiol. 105, 749–757.
Barwood, M. J., Thelwell, R. C., and Tipton, M. J. (2008). Psychological skills training improves exercise performance in the heat. Med. Sci. Sports Exerc. 40, 387–396.
Barwood, M. J., Weston, N. J. V., Thelwell, R., and Page, J. (2009). A motivational music and video intervention improves high-intensity exercise performance. J. Sports Sci. Med. 8, 422–442.
Bassett, D. R. Jr. (2002). Scientific contributions of A. V. Hill: exercise physiology pioneer. J. Appl. Physiol. 93, 1567–1582.
Bassett, D. R. Jr., and Howley, E. T. (1997). Maximal oxygen uptake: “classical” versus “contemporary” viewpoints. Med. Sci. Sports Exerc. 29, 591–603.
Bassett, D. R. Jr., and Howley, E. T. (2000). Limiting factors for maximum oxygen uptake and determinants of endurance performance. Med. Sci. Sports Exerc. 32, 70–84.
Bean, W. B., and Eichna, L. W. (1943). Performance in relation to environmental temperature. Fed. Proc. 2, 144–158.
Beltrami, F. G., Froyd, C., Mauger, A. R., Metcalfe, A. J., Marino, F., and Noakes, T. D. (2012). Conventional testing methods produce submaximal values of maximum oxygen consumption. Br. J. Sports Med. 46, 23–29.
Benedetti, F., Pollo, A., and Colloca, L. (2007). Opioid-mediated placebo responses boost pain endurance and physical performance: is it doping in sport competitions? J. Neurosci. 27, 11934–11939.
Billat, V., Lepretre, P. M., Heugas, A. M., Laurence, M. H., Salim, D., and Koralsztein, J. P. (2003). Training and bioenergetic characteristics in elite male and female Kenyan runners. Med. Sci. Sports Exerc. 35, 297–304.
Billaut, F., Bishop, D. J., Schaerz, S., and Noakes, T. D. (2011). Influence of knowledge of sprint number on pacing during repeated-sprint exercise. Med. Sci. Sports Exerc. 43, 665–672.
Billaut, F., Davis, J. M., Smith, K. J., Marino, F. E., and Noakes, T. D. (2010). Cerebral oxygenation decreases but does not impair performance during self-paced, strenuous exercise. Acta Physiol. (Oxf.) 198, 477–486.
Booth, J., Marino, F., and Ward, J. J. (1997). Improved running performance in hot humid conditions following whole body precooling. Med. Sci. Sports Exerc. 29, 943–949.
Borg, G. (1962). A simple rating scale for use in physical work tests. Kungliga Fysiografi ska Sallskapets I Lund Forhandigne 32, 7–15.
Cabanac, M. (1986). Money versus pain: experimental study of a conflict in humans. J. Exp. Anal. Behav. 46, 37–44.
Carmichael, M. D., Davis, J. M., Murphy, A., Brown, A. S., Carson, J. A., Mayer, E. P., and Ghaffar, A. (2006). Role of brain IL – 1beta on fatigue after exercise-induced muscle damage. Am. J. Physiol. 291, R1344–R1348.
Castle, P. C., Macdonald, A. L., Philp, A., Webborn, A., Watt, P. W., and Maxwell, N. S. (2006). Precooling leg muscle improves intermittent sprint exercise performance in hot, humid conditions. J. Appl. Physiol. 100, 1377–1384.
Castle, P. C., Maxwell, N., Allchorn, A., Mauger, A. R., and White, D. K. (2012). Deception of ambient and body core temperature improves self paced cycling in hot, humid conditions. Eur. J. Appl. Physiol. 112, 377–385.
Chambers, E. S., Bridge, M. W., and Jones, D. A. (2009). Carbohydrate sensing in the human mouth: effects on exercise performance and brain activity. J. Physiol. 587(Pt 8), 1779–1794.
Clark, V. R., Hopkins, W. G., Hawley, J. A., and Burke, L. M. (2000). Placebo effect of carbohydrate feedings during a 40-km cycling time trial. Med. Sci. Sports Exerc. 32, 1642–1647.
Coetzer, P., Noakes, T. D., Sanders, B., Lambert, M. I., Bosch, A. N., Wiggins, T., and Dennis, S. C. (1993). Superior fatigue resistance of elite black South African distance runners. J. Appl. Physiol. 75, 1822–1827.
Corbett, J., Barwood, M. J., Ouzounoglou, A., Thelwell, R., and Dicks, M. (2012). Influence of competition on performance and pacing during cycling exercise. Med. Sci. Sports Exerc. 44, 509–515.
Corbett, J., Barwood, M. J., and Parkhouse, K. (2009). Effect of task familiarisation on distribution of energy during a 2000 m cycling time trial. Br. J. Sports Med. 43, 770–774.
Damisch, L., Stoberock, B., and Mussweiler, T. (2010). Keep your fingers crossed!: how superstition improves performance. Psychol. Sci. 21, 1014–1020.
de Koning, J. J., Foster, C., Bakkum, A., Kloppenburg, S., Thiel, C., Joseph, T., Cohen, J., and Porcari, J. P. (2011). Regulation of pacing strategy during athletic competition. PLoS ONE 6, e15863. doi:10.1371/journal.pone.0015863
De Rond, M. (2009). The Last Amateurs: To Hell and Back with the Cambridge Boat Race Crew. London: Icon Books Ltd.
Del, C. J., Estevez, E., and Mora-Rodriguez, R. (2008). Caffeine effects on short-term performance during prolonged exercise in the heat. Med. Sci. Sports Exerc. 40, 744–751.
Di Giulio, C., Daniele, F., and Tipton, C. M. (2006). Angelo Mosso and muscular fatigue: 116 years after the first Congress of Physiologists: IUPS commemoration. Adv. Physiol. Educ. 30, 51–57.
Duffield, R., Green, R., Castle, P., and Maxwell, N. (2010). Precooling can prevent the reduction of self-paced exercise intensity in the heat. Med. Sci. Sports Exerc. 42, 577–584.
Edwards, A. M., Mann, M. E., Marfell-Jones, M. J., Rankin, D. M., Noakes, T. D., and Shillington, D. P. (2007). The influence of moderate dehydration on soccer performance: physiological responses to 45-min of outdoors match-play and the immediate subsequent performance of sport-specific and mental concentration tests. Br. J. Sports Med. 41, 385–391.
Edwards, A. M., and Noakes, T. D. (2009). Dehydration: cause of fatigue or sign of pacing in elite soccer? Sports Med. 39, 1–13.
Elliott, R. (2011). Runners on Running: The Best Nonfiction of Distance Running. Champaign, IL: Human Kinetics.
Entine, J. (2000). Taboo: Why Black Athletes Dominate Sports and Why We’re Afraid to Talk About it. New York: Public Affairs.
Eston, R., Faulkner, J., St Clair Gibson, A., Noakes, T., and Parfitt, G. (2007). The effect of antecedent fatiguing activity on the relationship between perceived exertion and physiological activity during a constant load exercise task. Psychophysiology 44, 779–786.
Faulkner, J., Arnold, T., and Eston, R. (2011). Effect of accurate and inaccurate distance feedback on performance markers and pacing strategies during running. Scand. J. Med. Sci. Sports 21, e176–e183.
Fletcher, W. M., and Hopkins, W. G. (1907). Lactic acid in amphibian muscle. J. Physiol. (Lond.) 35, 247–309.
Flouris, A. D., and Cheung, S. S. (2009). Human conscious response to thermal input is adjusted to changes in mean body temperature. Br. J. Sports Med. 43, 199–203.
Foad, A. J., Beedie, C. J., and Coleman, D. A. (2008). Pharmacological and psychological effects of caffeine ingestion in 40-km cycling performance. Med. Sci. Sports Exerc. 40, 158–165.
Foster, C., Hendrickson, K. J., Peyer, K., Reiner, B., deKoning, J. J., Lucia, A., Battista, R. A., Hettinga, F. J., Porcari, J. P., and Wright, G. (2009). Pattern of developing the performance template. Br. J. Sports Med. 43, 765–769.
Gandevia, S. C. (2001). Spinal and supraspinal factors in human muscle fatigue. Physiol. Rev. 81, 1725–1789.
Gant, N., Stinear, C. M., and Byblow, W. D. (2010). Carbohydrate in the mouth immediately facilitates motor output. Brain Res. 1350, 151–158.
Gill, N. D., Shield, A., Blazevich, A. J., Zhou, S., and Weatherby, R. P. (2000). Muscular and cardiorespiratory effects of pseudoephedrine in human athletes. Br. J. Clin. Pharmacol. 50, 205–213.
Guimaraes, J. B., Wanner, S. P., Machado, S. C., Lima, M. R., Cordeiro, L. M., Pires, W., La Guardia, R. B., Silami-Garcia, E., Rodrigues, L. O., and Lima, N. R. (2011). Fatigue is mediated by cholinoceptors within the ventromedial hypothalamus independent of changes in core temperature. Scand. J. Med. Sci. Sports. doi: 10.1111/j.1600-0838.2011.01350.x. [Epub ahead of print].
Hettinga, F. J., de Koning, J. J., Schmidt, L. J., Wind, N. A., MacIntosh, B. R., and Foster, C. (2011). Optimal pacing strategy: from theoretical modelling to reality in 1500-m speed skating. Br. J. Sports Med. 45, 30–35.
Hill, A. V., Long, C. H. N., and Lupton, H. (1924a). Muscular exercise, lactic acid and the supply and utilisation of oxygen: parts VII-VIII. Proc. R. Soc. Lond. B Biol. Sci. 97, 155–176.
Hill, A. V., Long, C. H. N., and Lupton, H. (1924b). Muscular exercise, lactic acid, and the supply utilization of oxygen: parts I-III. Proc. R. Soc. Lond. B Biol. Sci. 96, 438–475.
Hill, A. V., Long, C. H. N., and Lupton, H. (1924c). Muscular exercise, lactic acid, and the supply utilization of oxygen: parts IV-VI. Proc. R. Soc. Lond. B Biol. Sci. 97, 84–138.
Hill, A. V., and Lupton, H. (1923). Muscular exercise, lactic acid, and the supply and utilization of oxygen. Q. J. Med. 16, 135–171.
Hill, L., and Flack, M. (1910). The influence of oxygen inhalations on muscular work. J. Physiol. (Lond.) 40, 347.
Hill, L., and Mackenzie, J. (1909). The effects of oxygen inhalation on muscular exertion. J. Physiol. (Lond.) 39, xxxiii.
Hilty, L., Jancke, L., Luechinger, R., Boutellier, U., and Lutz, K. (2011a). Limitation of physical performance in a muscle fatiguing handgrip exercise is mediated by thalamo-insular activity. Hum. Brain Mapp. 32, 2151–2160.
Hilty, L., Langer, N., Pascual-Marqui, R., Boutellier, U., and Lutz, K. (2011b). Fatigue-induced increase in intracortical communication between mid/anterior insular and motor cortex during cycling exercise. Eur. J. Neurosci. 34, 2035–2042.
Hodges, K., Hancock, S., Currell, K., Hamilton, B., and Jeukendrup, A. E. (2006). Pseudoephedrine enhances performance in 1500-m runners. Med. Sci. Sports Exerc. 38, 329–333.
Hogervorst, E., Bandelow, S., Schmitt, J., Jentjens, R., Oliveira, M., Allgrove, J., Carter, T., and Gleeson, M. (2008). Caffeine improves physical and cognitive performance during exhaustive exercise. Med. Sci. Sports Exerc. 40, 1841–1851.
Jacobs, I., and Bell, D. G. (2004). Effects of acute modafinil ingestion on exercise time to exhaustion. Med. Sci. Sports Exerc. 36, 1078–1082.
Johnson, B. D., Joseph, T., Wright, G., Battista, R. A., Dodge, C., Balweg, A., de Koning, J. J., and Foster, C. (2009). Rapidity of responding to a hypoxic challenge during exercise. Eur. J. Appl. Physiol. 106, 493–499.
Jones, A. M. (1998). A five year physiological case study of an Olympic runner. Br. J. Sports Med. 32, 39–43.
Jones, A. M. (2006). The physiology of the world record holder for the women’s marathon. Int. J. Sports Sci. Coach. 1, 101–116.
Jones, D. A. (2010). Changes in the force-velocity relationship of fatigued muscle: implications for power production and possible causes. J. Physiol. (Lond.) 588(Pt 16), 2977–2986.
Jones, D. A., Turner, D. L., McIntyre, D. B., and Newham, D. J. (2009). Energy turnover in relation to slowing of contractile properties during fatiguing contractions of the human anterior tibialis muscle. J. Physiol. (Lond.) 587(Pt 17), 4329–4338.
Joyner, M. J., and Coyle, E. F. (2008). Endurance exercise performance: the physiology of champions. J. Physiol. (Lond.) 586, 35–44.
Kay, D., Marino, F. E., Cannon, J., St Clair, G. A., Lambert, M. I., and Noakes, T. D. (2001). Evidence for neuromuscular fatigue during high-intensity cycling in warm, humid conditions. Eur. J. Appl. Physiol. 84, 115–121.
Kayser, B., Narici, M., Binzoni, T., Grassi, B., and Cerretelli, P. (1994). Fatigue and exhaustion in chronic hypobaric hypoxia: influence of exercising muscle mass. J. Appl. Physiol. 76, 634–640.
Kwon, Y. S., Robergs, R. A., Kravitz, L. R., Gurney, B. A., Mermier, C. M., and Schneider, S. M. (2010). Palm cooling delays fatigue during high-intensity bench press exercise. Med. Sci. Sports Exerc. 42, 1557–1565.
Lander, P. J., Butterly, R. J., and Edwards, A. M. (2009). Self-paced exercise is less physically challenging than enforced constant pace exercise of the same intensity: influence of complex central metabolic control. Br. J. Sports Med. 43, 789–795.
Legaz Arrese, A., Izquierdo, D., Nuviala, A., Serveto-Galindo, O., Urdiales, D., and Masia, J. (2007). Averag VO2max as a function of running performances on different distances. Sci. Sports 22, 43–49.
Lester, F. M., Sheffield, L. T., and Reeves, T. J. (1967). Electrocardiographic changes in clinically normal older men following near maximal and maximal exercise. Circulation 36, 5–14.
Levine, B. D. (2008). VO2max: what do we know, and what do we still need to know? J. Physiol. (Lond.) 586, 25–34.
Lim, H. B., Atkinson, G., Karageorghis, C. I., and Eubank, M. R. (2009). Effects of differentiated music on cycling time trial. Int. J. Sports Med. 30, 435–442.
Lima-Silva, A. E., Pires, F. O., Bertuzzi, R. C., Lira, F. S., Casarini, D., and Kiss, M. A. (2010). Low carbohydrate diet affects the oxygen uptake on-kinetics and rating of perceived exertion in high intensity exercise. Psychophysiology. [Epub ahead of print].
Lucia, A., Pardo, J., Durantez, A., Hoyos, J., and Chicharro, J. L. (1998). Physiological differences between professional and elite road cyclists. Int. J. Sports Med. 19, 342–348.
Marcora, S. M., and Bosio, A. (2007). Effect of exercise-induced muscle damage on endurance running performance in humans. Scand. J. Med. Sci. Sports 17, 662–671.
Marcora, S. M., and Staiano, W. (2010). The limit to exercise tolerance in humans: mind over muscle? Eur. J. Appl. Physiol. 109, 763–770.
Marcora, S. M., Staiano, W., and Manning, V. (2009). Mental fatigue impairs physical performance in humans. J. Appl. Physiol. 106, 857–864.
Marino, F. E., Mbambo, Z., Kortekaas, E., Wilson, G., Lambert, M. I., Noakes, T. D., and Dennis, S. C. (2000). Advantages of smaller body mass during distance running in warm, humid environments. Pflugers Arch. 441, 359–367.
Martin, B. J. (1981). Effect of sleep deprivation on tolerance of prolonged exercise. Eur. J. Appl. Physiol. Occup. Physiol. 47, 345–354.
Mauger, A. R., Jones, A. M., and Williams, C. A. (2009). Influence of feedback and prior experience on pacing during a 4-km cycle time trial. Med. Sci. Sports Exerc. 41, 451–458.
Mauger, A. R., Jones, A. M., and Williams, C. A. (2010). Influence of acetaminophen on performance during time trial cycling. J. Appl. Physiol. 108, 98–104.
Mauger, A. R., and Sculthorpe, N. (2012). A new VO2max protocol allowing self-pacing in maximal incremental exercise. Br. J. Sports Med. 46, 59–63.
Mehta, J. P., Verber, M. D., Wieser, J. A., Schmit, B. D., and Schindler-Ivens, S. M. (2009). A novel technique for examining human brain activity associated with pedaling using fMRI. J. Neurosci. Methods 179, 230–239.
Micklewright, D., Papadopoulou, E., Swart, J., and Noakes, T. (2010). Previous experience influences pacing during 20 km time trial cycling. Br. J. Sports Med. 44, 952–960.
Mitchell, J. H., and Saltin, B. (2003). “The oxygen transport system and maximal oxygen uptake,” in Exercise Physiology, ed. C. M. Tipton (Oxford: Oxford University Press), 255–291.
Mitchell, J. H., Sproule, B. J., and Chapman, C. B. (1958). The physiological meaning of the maximal oxygen intake test. J. Clin. Invest. 37, 538–546.
Morante, S. M., and Brotherhood, J. R. (2008). Autonomic and behavioural thermoregulation in tennis. Br. J. Sports Med. 42, 679–685.
Morton, R. H. (2009). Deception by manipulating the clock calibration influences cycle ergometer endurance time in males. J. Sci. Med. Sport. 12, 332–337.
Noakes, T. D. (1997). Challenging beliefs: ex Africa semper aliquid novi: 1996 J. B. Wolffe Memorial Lecture. Med. Sci. Sports Exerc. 29, 571–590.
Noakes, T. D. (2004). Linear relationship between the perception of effort and the duration of constant load exercise that remains. J. Appl. Physiol. 96, 1571–1572.
Noakes, T. D. (2008a). How did A V Hill understand the VO2max and the “plateau phenomenon?” Still no clarity? Br. J. Sports Med. 42, 574–580.
Noakes, T. D. (2008b). Rating of perceived exertion as a predictor of the duration of exercise that remains until exhaustion. Br. J. Sports Med. 42, 623–624.
Noakes, T. D. (2008c). Testing for maximum oxygen consumption has produced a brainless model of human exercise performance. Br. J. Sports Med. 42, 551–555.
Noakes, T. D. (2009). Evidence that reduced skeletal muscle recruitment explains the lactate paradox during exercise at high altitude. J. Appl. Physiol. 106, 737–738.
Noakes, T. D. (2011a). Is it time to retire the A.V. Hill Model? A rebuttal to the article by Professor Roy Shephard. Sports Med. 41, 263–277.
Noakes, T. D. (2011b). Time to move beyond a brainless exercise physiology: the evidence for complex regulation of human exercise performance. Appl. Physiol. Nutr. Metab. 36, 23–35.
Noakes, T. D., Lambert, M., and Human, R. (2009). Which lap is the slowest? An analysis of 32 world record performances. Br. J. Sports Med. 43, 760–764.
Noakes, T. D., and Marino, F. E. (2007). Arterial oxygenation, central motor output and exercise performance in humans. J. Physiol. (Lond.) 585(Pt 3), 919–921.
Noakes, T. D., and St Clair Gibson, A. (2004). Logical limitations to the “catastrophe” models of fatigue during exercise in humans. Br. J. Sports Med. 38, 648–649.
Noakes, T. D., St Clair Gibson, A., and Lambert, E. V. (2004). From catastrophe to complexity: a novel model of integrative central neural regulation of effort and fatigue during exercise in humans. Br. J. Sports Med. 38, 511–514.
Noakes, T. D., St Clair Gibson, A., and Lambert, E. V. (2005). From catastrophe to complexity: a novel model of integrative central neural regulation of effort and fatigue during exercise in humans: summary and conclusions. Br. J. Sports Med. 39, 120–124.
Nybo, L., and Rasmussen, P. (2007). Inadequate cerebral oxygen delivery and central fatigue during strenuous exercise. Exerc. Sport Sci. Rev. 35, 110–118.
Paterson, S., and Marino, F. E. (2004). Effect of deception of distance on prolonged cycling performance. Percept. Mot. Skills 98(Pt 1), 1017–1026.
Paulus, M. P., Flagan, T., Simmons, A. N., Gillis, K., Kotturi, S., Thom, N., Johnson, D. C., Van Orden, K. F., Davenport, P. W., and Swain, J. L. (2011). Subjecting elite athletes to inspiratory breathing load reveals behavioral and neural signatures of optimal performers in extreme environments. PLoS ONE 7, e29394. doi:10.1371/journal.pone.0029394
Pollo, A., Carlino, E., and Benedetti, F. (2008). The top-down influence of ergogenic placebos on muscle work and fatigue. Eur. J. Neurosci. 28, 379–388.
Pritchard-Peschek, K. R., Jenkins, D. G., Osborne, M. A., and Slater, G. J. (2010). Pseudoephedrine ingestion and cycling time-trial performance. Int. J. Sport Nutr. Exerc. Metab. 20, 132–138.
Racinais, S., Bringard, A., Puchaux, K., Noakes, T. D., and Perrey, S. (2008). Modulation in voluntary neural drive in relation to muscle soreness. Eur. J. Appl. Physiol. 102, 439–446.
Raskoff, W. J., Goldman, S., and Cohn, K. (1976). The “athletic heart.” Prevalence and physiological significance of left ventricular enlargement in distance runners. JAMA 236, 158–162.
Rasmussen, P., Nielsen, J., Overgaard, M., Krogh-Madsen, R., Gjedde, A., Secher, N. H., and Petersen, N. C. (2010a). Reduced muscle activation during exercise related to brain oxygenation and metabolism in humans. J. Physiol. (Lond.) 588(Pt 11), 1985–1995.
Rasmussen, P., Nybo, L., Volianitis, S., Moller, K., Secher, N. H., and Gjedde, A. (2010b). Cerebral oxygenation is reduced during hyperthermic exercise in humans. Acta Physiol. (Oxf.) 199, 63–70.
Rauch, H. G., St Clair Gibson, A., Lambert, E. V., and Noakes, T. D. (2005). A signalling role for muscle glycogen in the regulation of pace during prolonged exercise. Br. J. Sports Med. 39, 34–38.
Renfree, A., West, J., Corbett, M., Rhoden, C., and St Clair, G. A. (2011). Complex interplay between determinants of pacing and performance during 20 km cycle time trials. Int. J. Sports Physiol. Perform. [Epub ahead of print].
Rerych, S. K., Scholz, P. M., Newman, G. E., Sabiston, D. C. Jr., and Jones, R. H. (1978). Cardiac function at rest and during exercise in normals and in patients with coronary heart disease: evaluation by radionuclide angiocardiography. Ann. Surg. 187, 449–464.
Robertson, E. Y., Saunders, P. U., Pyne, D. B., Aughey, R. J., Anson, J. M., and Gore, C. J. (2010). Reproducibility of performance changes to simulated live high/train low altitude. Med. Sci. Sports Exerc. 42, 394–401.
Robson-Ansley, P. J., de Milander, L., Collins, M., and Noakes, T. D. (2004). Acute interleukin-6 administration impairs athletic performance in healthy, trained male runners. Can. J. Appl. Physiol. 29, 411–418.
Roelands, B., Hasegawa, H., Watson, P., Piacentini, M. F., Buyse, L., De Schutter, G., and Meeusen, R. (2008). The effects of acute dopamine reuptake inhibition on performance. Med. Sci. Sports Exerc. 40, 879–885.
Roelands, B., and Meeusen, R. (2010). Alterations in central fatigue by pharmacological manipulations of neurotransmitters in normal and high ambient temperature. Sports Med. 40, 229–246.
Rollo, I., Cole, M., Miller, R., and Williams, C. (2010). Influence of mouth rinsing a carbohydrate solution on 1-h running performance. Med. Sci. Sports Exerc. 42, 798–804.
Rollo, I., Williams, C., Gant, N., and Nute, M. (2008). The influence of carbohydrate mouth rinse on self-selected speeds during a 30-min treadmill run. Int. J. Sport Nutr. Exerc. Metab. 18, 585–600.
Rollo, I., Williams, C., and Nevill, M. (2011). Influence of ingesting versus mouth rinsing a carbohydrate solution during a 1-h run. Med. Sci. Sports Exerc. 43, 468–475.
Ross, E. Z., Goodall, S., Stevens, A., and Harris, I. (2010). Time course of neuromuscular changes during running in well-trained subjects. Med. Sci. Sports Exerc. 42, 1184–1190.
Rupp, T., and Perrey, S. (2008). Prefrontal cortex oxygenation and neuromuscular responses to exhaustive exercise. Eur. J. Appl. Physiol. 102, 153–163.
Rupp, T., and Perrey, S. (2009). Effect of severe hypoxia on prefrontal cortex and muscle oxygenation responses at rest and during exhaustive exercise. Adv. Exp. Med. Biol. 645, 329–334.
Schlader, Z. J., Simmons, S. E., Stannard, S. R., and Mundel, T. (2011). The independent roles of temperature and thermal perception in the control of human thermoregulatory behavior. Physiol. Behav. 103, 217–224.
Schneider, S., Askew, C. D., Abel, T., and Struder, H. K. (2010). Exercise, music, and the brain: is there a central pattern generator? J. Sports Sci. 28, 1337–1343.
Seifert, T., Rasmussen, P., Secher, N. H., and Nielsen, H. B. (2009). Cerebral oxygenation decreases during exercise in humans with beta-adrenergic blockade. Acta Physiol. (Oxf.) 196, 295–302.
Sgherza, A. L., Axen, K., Fain, R., Hoffman, R. S., Dunbar, C. C., and Haas, F. (2002). Effect of naloxone on perceived exertion and exercise capacity during maximal cycle ergometry. J. Appl. Physiol. 93, 2023–2028.
Sloniger, M. A., Cureton, K. J., Prior, B. M., and Evans, E. M. (1997a). Anaerobic capacity and muscle activation during horizontal and uphill running. J. Appl. Physiol. 83, 262–269.
Sloniger, M. A., Cureton, K. J., Prior, B. M., and Evans, E. M. (1997b). Lower extremity muscle activation during horizontal and uphill running. J. Appl. Physiol. 83, 2073–2079.
Snell, P. G., and Mitchell, J. H. (1984). The role of maximal oxygen uptake in exercise performance. Clin. Chest Med. 5, 51–62.
St Clair Gibson, A., Baden, D. A., Lambert, M. I., Lambert, E. V., Harley, Y. X., Hampson, D., Russell, V. A., and Noakes, T. D. (2003). The conscious perception of the sensation of fatigue. Sports Med. 33, 167–176.
St Clair Gibson, A., and Noakes, T. D. (2004). Evidence for complex system integration and dynamic neural regulation of skeletal muscle recruitment during exercise in humans. Br. J. Sports Med. 38, 797–806.
Stone, M. R., Thomas, K., Wilkinson, M., Jones, A. M., Gibson, A. S., and Thompson, K. G. (2012). Effects of deception on exercise performance: implications for determinants of fatigue in humans. Med. Sci. Sports Exerc. 44, 534–541.
Surbey, G. D., Andrew, G. M., Cervenko, F. W., and Hamilton, P. P. (1984). Effects of naloxone on exercise performance. J. Appl. Physiol. 57, 674–679.
Swart, J., Lamberts, R. P., Lambert, M. I., Lambert, E. V., Woolrich, R. W., Johnston, S., and Noakes, T. D. (2009a). Exercising with reserve: exercise regulation by perceived exertion in relation to duration of exercise and knowledge of endpoint. Br. J. Sports Med. 43, 775–781.
Swart, J., Lamberts, R. P., Lambert, M. I., St Clair, G. A., Lambert, E. V., Skowno, J., and Noakes, T. D. (2009b). Exercising with reserve: evidence that the central nervous system regulates prolonged exercise performance. Br. J. Sports Med. 43, 782–788.
Swart, J., Lindsay, T. R., Lambert, M. I., Brown, J. C., and Noakes, T. D. (2012). Perceptual cues in the regulation of exercise performance – physical sensations of exercise and awareness of effort interact as separate cues. Br. J. Sports Med. 46, 42–48.
Tanaka, M., Shigihara, Y., and Watanabe, Y. (2011). Central inhibition regulates motor output during physical fatigue. Brain Res. 1412, 37–43.
Tanaka, M., and Watanabe, Y. (2012). Supraspinal regulation of physical fatigue. Neurosci. Biobehav. Rev. 36, 727–734.
Townshend, A. D., Worringham, C. J., and Stewart, I. B. (2010). Spontaneous pacing during overground hill running. Med. Sci. Sports Exerc. 42, 160–169.
Trojian, T. H., and Beedie, C. J. (2008). Placebo effect and athletes. Curr. Sports Med. Rep. 7, 214–217.
Tucker, R. (2009). The anticipatory regulation of performance: the physiological basis for pacing strategies and the development of a perception-based model for exercise performance. Br. J. Sports Med. 43, 392–400.
Tucker, R., Bester, A., Lambert, E. V., Noakes, T. D., Vaughan, C. L., and St Clair, G. A. (2006a). Non-random fluctuations in power output during self-paced exercise. Br. J. Sports Med. 40, 912–917.
Tucker, R., Lambert, M. I., and Noakes, T. D. (2006b). An analysis of pacing strategies during men’s world record performances in track athletics. Int. J. Sports Physiol. Perform. 1, 233–245.
Tucker, R., Marle, T., Lambert, E. V., and Noakes, T. D. (2006c). The rate of heat storage mediates an anticipatory reduction in exercise intensity during cycling at a fixed rating of perceived exertion. J. Physiol. (Lond.) 574(Pt 3), 905–915.
Tucker, R., Kayser, B., Rae, E., Raunch, L., Bosch, A., and Noakes, T. (2007). Hyperoxia improves 20 km cycling time trial performance by increasing muscle activation levels while perceived exertion stays the same. Eur. J. Appl. Physiol. 101, 771–781.
Tucker, R., and Noakes, T. D. (2009). The physiological regulation of pacing strategy during exercise: a critical review. Br. J. Sports Med. 43, e1.
Tucker, R., Rauch, L., Harley, Y. X., and Noakes, T. D. (2004). Impaired exercise performance in the heat is associated with an anticipatory reduction in skeletal muscle recruitment. Pflugers Arch. 448, 422–430.
Tyler, C. J., and Sunderland, C. (2011a). Cooling the neck region during exercise in the heat. J. Athl. Train. 46, 61–68.
Tyler, C. J., and Sunderland, C. (2011b). Neck cooling and running performance in the heat: single versus repeated application. Med. Sci. Sports Exerc. 43, 2388–2395.
Tyler, C. J., Wild, P., and Sunderland, C. (2010). Practical neck cooling and time-trial running performance in a hot environment. Eur. J. Appl. Physiol. 110, 1063–1074.
Vollaard, N. B., Constantin-Teodosiu, D., Fredriksson, K., Rooyackers, O., Jansson, E., Greenhaff, P. L., Timmons, J. A., and Sundberg, C. J. (2009). Systematic analysis of adaptations in aerobic capacity and submaximal energy metabolism provides a unique insight into determinants of human aerobic performance. J. Appl. Physiol. 106, 1479–1486.
Watson, P., Hasegawa, H., Roelands, B., Piacentini, M. F., Looverie, R., and Meeusen, R. (2010). Acute dopamine/noradrenaline reuptake inhibition enhances human exercise performance in warm, but not temperate conditions. J. Physiol. (Lond.) 565, 873–883.
Williamson, J. W., Fadel, P. J., and Mitchell, J. H. (2006). New insights into central cardiovascular control during exercise in humans: a central command update. Exp. Physiol. 91, 51–58.
Wilmore, J. (1968). Influence of motivation on physical work capacity and performance. J. Appl. Physiol. 24, 459–463.
Keywords: fatigue, central nervous system, central governor model, anticipation, feedback, feedforward, brain, skeletal muscle
Citation: Noakes TD (2012) Fatigue is a brain-derived emotion that regulates the exercise behavior to ensure the protection of whole body homeostasis. Front. Physio. 3:82. doi: 10.3389/fphys.2012.00082
Received: 19 December 2011; Paper pending published: 09 January 2012;
Accepted: 20 March 2012; Published online: 11 April 2012.
Edited by:
Christina Karatzaferi, University of Thessaly, GreeceReviewed by:
Henriette Van Praag, National Institutes of Health, USAGiorgos K. Sakkas, Center for Research and Technology Thessaly, Greece
Copyright: © 2012 Noakes. This is an open-access article distributed under the terms of the Creative Commons Attribution Non Commercial License, which permits non-commercial use, distribution, and reproduction in other forums, provided the original authors and source are credited.
*Correspondence: Timothy David Noakes, UCT/MRC Research Unit for Exercise Science and Sports Medicine, Department of Human Biology, University of Cape Town, Boundary Road, Newlands, 7700 Cape Town, South Africa. e-mail: timothy.noakes@uct.ac.za
