- 1Department of Neurology, Barzilai Medical Center, Ashkelon & Faculty of Health Sciences, Ben-Gurion University of the Negev, Beer-Sheva, Israel
- 2Multiple Sclerosis Center and Neuroimmunology Unit, Carmel Medical Center & Rappaport Faculty of Medicine, Technion-Israel Institute of Technology, Haifa, Israel
- 3Unit for Neuroimmunology, Multiple Sclerosis and Cell Therapy, Hadassah Medical Center & Faculty of Medicine, Hebrew University, Jerusalem, Israel
- 4Neuroimmunology and Multiple Sclerosis Unit, Tel Aviv Sourasky Medical Center & Sackler Faculty of Medicine, Sagol School of Neuroscience, Tel Aviv University, Tel Aviv, Israel
- 5Department of Neurology, Rabin Medical Center & Sackler Faculty of Medicine, Tel Aviv University, Tel Aviv, Israel
- 6Department of Biochemistry and Molecular Genetics, Israel Institute for Biological Research, Ness Ziona, Israel
Background: Immunomodulatory/immunosuppressive activity of multiple sclerosis (MS) disease modifying therapies (DMTs) might affect immune responses to SARS-CoV-2 exposure or vaccination in patients with MS (PwMS). We evaluated the effect of DMTs on humoral and cell-mediated immune responses to 2 and 3 vaccinations and the longevity of SARS-Cov-2 IgG levels in PwMS.
Methods: 522 PwMS and 68 healthy controls vaccinated with BNT162b2-Pfizer mRNA vaccine against SARS-CoV-2, or recovering from COVID-19, were recruited in a nation-wide multi-center study. Blood was collected at 3 time-points: 2-16 weeks and ~6 months post 2nd vaccination and 1-16 weeks following 3rd vaccination. Serological responses were measured by quantifying IgG levels against the spike-receptor-binding-domain of SARS-CoV-2, and cellular responses (in a subgroup analysis) by quantifying IFNγ secretion in blood incubated with COVID-19 spike-antigen.
Results: 75% PwMS were seropositive post 2nd or 3rd vaccination. IgG levels decreased by 82% within 6 months from vaccination (p<0.0001), but were boosted 10.3 fold by the 3rd vaccination (p<0.0001), and 1.8 fold compared to ≤3m post 2nd vaccination (p=0.025). Patients treated with most DMTs were seropositive post 2nd and 3rd vaccinations, however only 38% and 44% of ocrelizumab-treated patients and 54% and 46% of fingolimod-treated patients, respectively, were seropositive. Similarly, in COVID-19-recovered patients only 54% of ocrelizumab-treated, 75% of fingolimod-treated and 67% of cladribine-treated patients were seropositive. A time interval of ≥5 months between ocrelizumab infusion and vaccination was associated with higher IgG levels (p=0.039 post-2nd vaccination; p=0.036 post-3rd vaccination), and with higher proportions of seropositive patients. Most fingolimod- and ocrelizumab-treated patients responded similarly to 2nd and 3rd vaccination. IFNγ-T-cell responses were detected in 89% and 63% of PwMS post 2nd and 3rd vaccination, however in only 25% and 0% of fingolimod-treated patients, while in 100% and 86% of ocrelizumab-treated patients, respectively.
Conclusion: PwMS treated with most DMTs developed humoral and T-cell responses following 2 and 3 mRNA SARS-CoV-2 vaccinations. Fingolimod- or ocrelizumab-treated patients had diminished humoral responses, and fingolimod compromised the cellular responses, with no improvement after a 3rd booster. Vaccination following >5 months since ocrelizumab infusion was associated with better sero-positivity. These findings may contribute to the development of treatment-stratified vaccination guidelines for PwMS.
Introduction
With the outbreak of the COVID-19 pandemic and the rapid development of SARS-CoV-2 vaccines worldwide, concern was raised that the immunomodulatory/immunosuppressive effects of multiple sclerosis (MS) disease modifying therapies (DMTs) might affect COVID-19 disease severity as well as the development of humoral and cellular immunity after SARS-CoV-2 exposure or vaccination (1). In Israel, a nationwide COVID-19 vaccination program was rapidly launched starting December 2020, based solely upon the BNT162b2 (Pfizer-BioNTech) mRNA vaccine, offering two doses administered 3 weeks apart, and a third vaccine booster ≥ 5 months after the 2nd dose (2), a vaccination regime associated with a good safety profile (3). All PwMS are recommended by the National Multiple Sclerosis Society of the United States and by the MS International Federation to be vaccinated against SARS-CoV-2 (4, 5). With the variety of available DMTs, that exert their action through diverse immunological mechanisms, it is of high importance to investigate the immune response to SARS-CoV-2 vaccination in PwMS treated with various immunotherapies, in order to develop optimal and treatment-stratified guidelines. The immune response to SARS-CoV-2 consists of both a cellular and a humoral response, but measurement of SARS-CoV-2 IgG is widely used to identify persons who have recovered from COVID-19 infection or as a confirmation of a sufficient vaccine response, based on the fact that the levels of neutralizing antibodies have been shown to be highly predictive of SARS-CoV-2 immune protection (6). Initial reports have demonstrated that while most MS patients show a positive humoral immune response shortly after the 2nd SARS-CoV-2 mRNA vaccination, a considerable proportion of patients treated with fingolimod or ocrelizumab do not develop antibodies (7–12). In light of the immunological effects of the DMTs, it is important to evaluate the effect of the patients’ immune response to the vaccine and to COVID-19 over time and the effects of the third dose of vaccine in patients treated with ocrelizumab and fingolimod. In this nation-wide multi-center prospective study we examined the levels of anti-SARS-CoV-2 antibodies in PwMS treated with most available DMTs and in healthy controls (HC), during a period of at least 6 months following the 2nd SARS-CoV-2 vaccination and at 1-16 weeks following a 3rd vaccine booster. In addition, we evaluated the cell-mediated immune responses against the COVID-19 spike protein in a subgroup of the participants.
Materials and Methods
Participants and Sampling
This observational multi-center prospective study was conducted in the MS centers at 5 major Israeli hospitals: Hadassah Medical Center, Tel Aviv Sourasky Medical Center, Barzilai Medical Center, Rabin Medical Center and Carmel Medical Center, in compliance with the principles of the Declaration of Helsinki, as approved by the Ethics Committees of each hospital. The study included 522 PwMS and 68 HC, that were recently vaccinated or about to be vaccinated against SARS-CoV-2 with the mRNA vaccine BNT162b2 (Pfizer-BioNTech), or who recovered from COVID-19. All participants signed a written informed consent prior to the study procedures. Demographic and clinical data were recorded at recruitment. Serum was collected 2-16 weeks (mean=7.5 weeks, median=6.9 weeks) after the 2nd vaccine dose (time-point 1), ~6 months (17-39 weeks, mean and median=25 weeks.) after the 2nd vaccine dose (time-point 2) and 1-16 weeks (mean=7.2 weeks, median=6.7 weeks) after the 3rd vaccine booster (time-point 3) and kept at -800C until assessed. In a subgroup of 39 PwMS and 8 HC, whole blood was collected at the same time-points and used for assessment of the anti-spike protein T-cell response.
SARS-CoV-2 IgG
The humoral response was measured at a centralized laboratory (the Clinical Virology Laboratory at Hadassah Medical Center, Jerusalem) using the spike receptor-binding domain (RBD) Architect SARS-CoV-2 IgG II Quant Assay (Abbott). Serum positivity was determined in accordance with the definition of The Israeli Health Ministry at: ≥50 arbitrary units per ml in vaccinated and/or COVID-19-recovered persons.
T Cell Reactivity
For evaluation of SARS-CoV-2 spike-specific T-cell responses, a quantitative assay SARS-CoV-2 IGRA stimulation tube set (EuroImmun, Germany) was used according to manufacturer’s protocol. The IFNγ-T cell response assay was carried out at the Israel Institute for Biological Research (Ness Ziona, Israel). Heparinized whole-blood samples were stimulated ex-vivo with the COVID-19 spike protein for 24 hours, then plasma was collected and secreted IFNγ was quantified by ELISA (ELISA DuoSet, R&D Systems, Minneapolis, Minnesota, USA). A similar sample without antigen stimulation was used as control. Values above 25 pg/ml of IFNγ spike-specific response were considered positive.
Statistical Analysis
Data was analyzed using SPSS v24. The antibody levels were transformed on a Log10 scale, to normalize their distribution. A general univariate linear model with Bonferonni adjustment for multiple comparisons was used to compare IgG levels between groups of demographic or clinical variables, adjusted for time from vaccination/infection to blood collection (as samples were collected within a relatively large interval of 2-16 weeks post-vaccine or longer post-COVID-19), age and gender. Since the number of samples from each participant varied, we used a generalized linear model (generalized estimated equation model) to compare IgG levels between time-points, where comparison of 1st and 3rd time-point was adjusted for time from vaccination to blood collection. A multiple linear regression model was used to assess correlation between IgG levels and variables, adjusted for time between vaccination and blood collection, age or gender. P value at <0.05 was considered significant.
Results
Demographic and Clinical Characteristics of the Participants
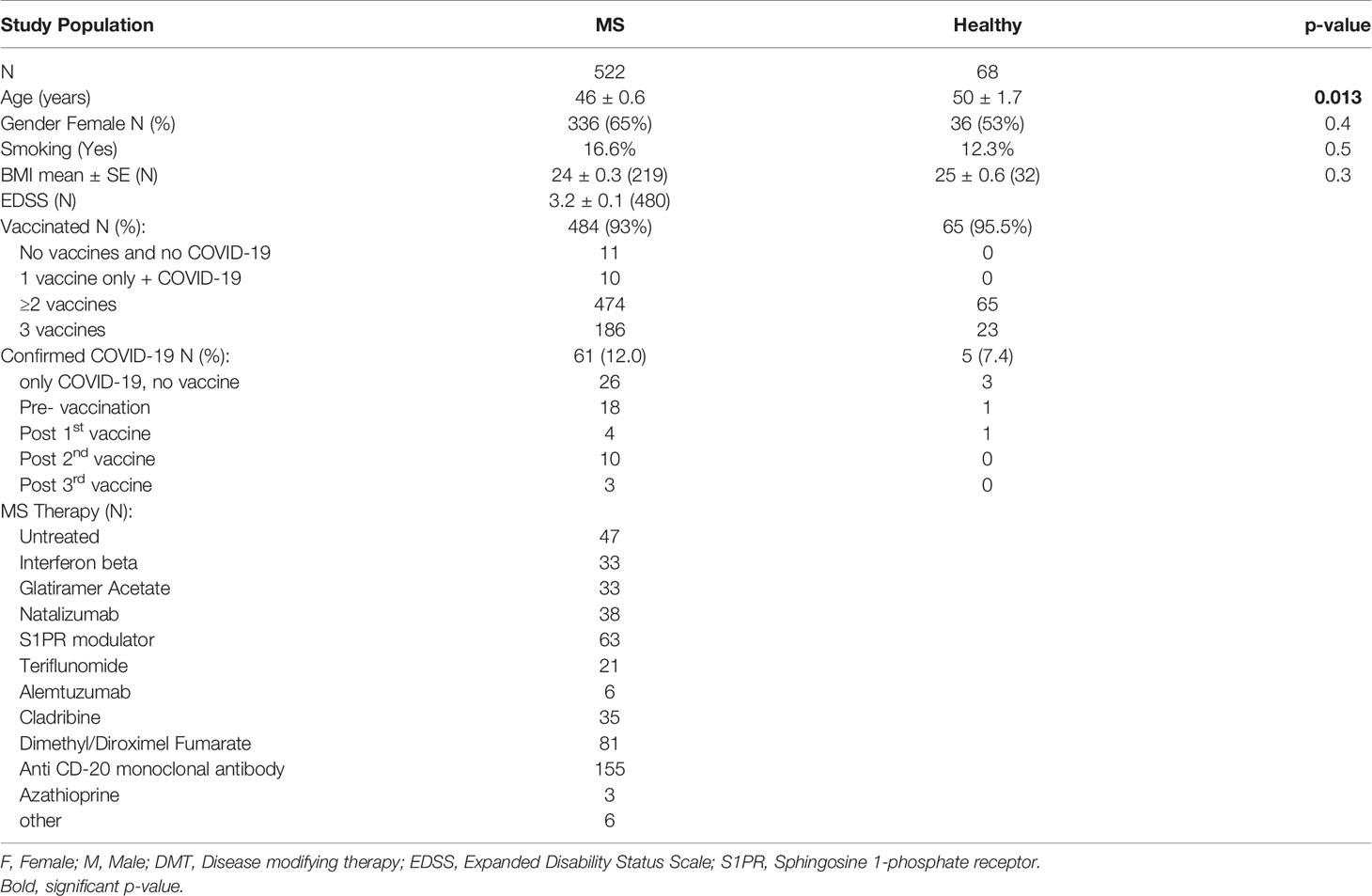
We recruited for this prospective study 522 PwMS and 68 HC, who were vaccinated, about to be vaccinated, or who had been infected with SARS-CoV-2. Table 1 summarizes their demographic and clinical characteristics. There was no significant difference between PwMS and HC regarding gender, BMI and smoking status, with the exception of a small age difference between the healthy participants and the MS patients (50 vs. 46 years, p=0.013). 93% of PwMS and 96% of HC received the COVID-19 vaccine, and 61 PwMS (12%) and 5 HC (7.4%) had a confirmed COVID-19 infection before (44 and 4, respectively) or during the study (17 and 1, respectively). COVID-19 infection was reported in 10 PwMS following the 2nd vaccination and in 3 patients following the 3rd vaccine booster, while in none of the HC after these vaccinations.
Serological Response in Vaccinated and Post-COVID-19 Participants
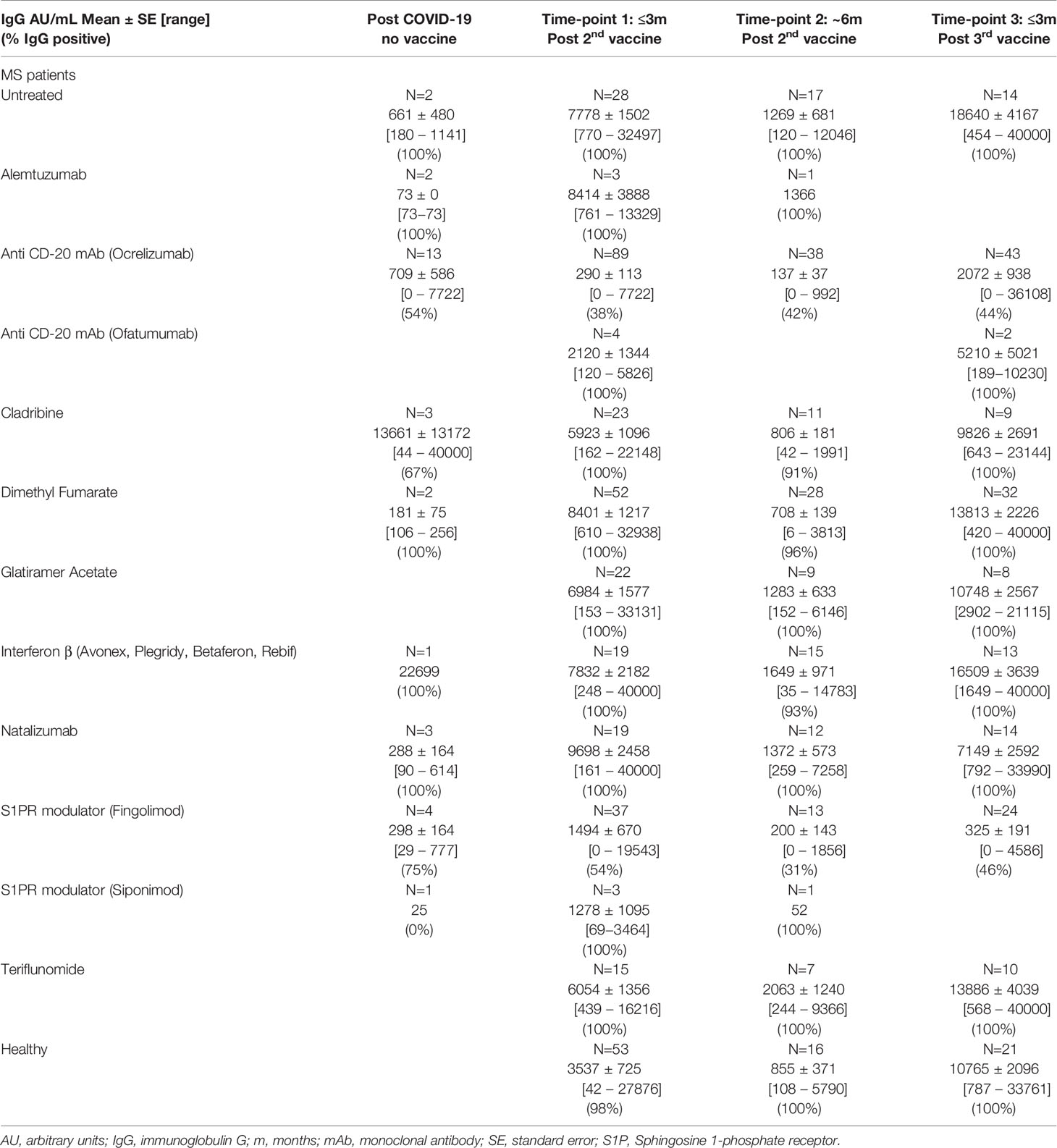
The serological response to SARS-CoV-2 vaccine was measured by detecting the anti-SARS-CoV-2 IgG level at 3 time-points: 2-16 weeks (≤3 months) and at 17-39 weeks (~6 months) after the 2nd vaccination, and at 1-16 weeks (≤3 months) after the 3rd vaccine booster. Table 2 summarizes the IgG levels at each time-point in vaccinated PwMS and HC, as well as the IgG levels post-COVID-19 infection in unvaccinated PwMS. Supplementary data on additional DMTs can be found in Supplementary Table 1. 322 samples were available at ≤3 months from 2nd vaccination (mean=7.5 weeks, median=6.9 weeks), 159 samples at ~6 months from 2nd vaccination (mean=25.1 weeks, median=24.6 weeks) and 172 samples at ≤3 months following the 3rd vaccine booster in PwMS (mean=7.2 weeks, median=6.7 weeks). In total, 75.5% of PwMS and 98% of HC were seropositive after the 2nd vaccination, and 78.5% PwMS and 100% HC were seropositive after the 3rd vaccine booster. After 2 vaccinations, the mean IgG level was slightly lower in PwMS compared to HC (p=0.001) (Figure 1A). 72% of COVID-19-recovered and non-vaccinated patients had positive IgG levels, obtained after an average time of 30 weeks (median=27 weeks) post- infection, not different from the mean IgG level in vaccinated PwMS (p=0.6), when adjusted for time since infection/vaccination (Table 1 and Figure 1A). IgG levels dropped by 82% in MS patients [OR=0.53, 95%CI: (0.47, 0.59), p<0.0001] and by 76% in healthy individuals [OR=0.50, 95%CI: (0.40, 0.63), p<0.0001] at time-point 2, 6 months after the 2nd vaccination (Figures 1B, C). In PwMS the 3rd vaccine booster increased the IgG levels at time-point 3 10 fold compared with the levels at time-point 2 (~6 months post 2nd vaccine) [OR=2.19, 95%CI: (1.75, 2.73), p<0.0001], and 1.8 fold compared with the levels at time-point 1 (≤3 months post 2nd vaccine) [OR=1.21, 95%CI: (1.02, 1.43), p=0.025] (Figure 1B). In the healthy participants the 3rd vaccine booster similarly increased the IgG levels 12.6 fold compared with the levels at time-point 2 [OR=3.58, 95%CI: (2.76, 4.64), p<0.0001], and 3 fold compared with the levels at time-point 1 [OR=1.40, 95%CI: (1.05, 1.87), p=0.023] (Figure 1C). While IgG levels correlated negatively with time since the 2nd vaccination in vaccinated MS patients [B=-0.038 ± 0.019, 95%CI: (-0.075, -0.001), p=0.043] (Figure 1D) and in vaccinated healthy individuals [B=-0.08 ± 0.019, 95%CI: (-0.12, -0.04), p=0.00013] (Figure 1E), no correlation was found between IgG levels and time since COVID-19 infection (COVID-19- recovered, unvaccinated patients), measured for a time interval of 4-72 weeks (mean=30 weeks) [B=0.005 ± 0.012, 95%CI: (-0.02, 0.03),p=0.7] (Figure 1F).
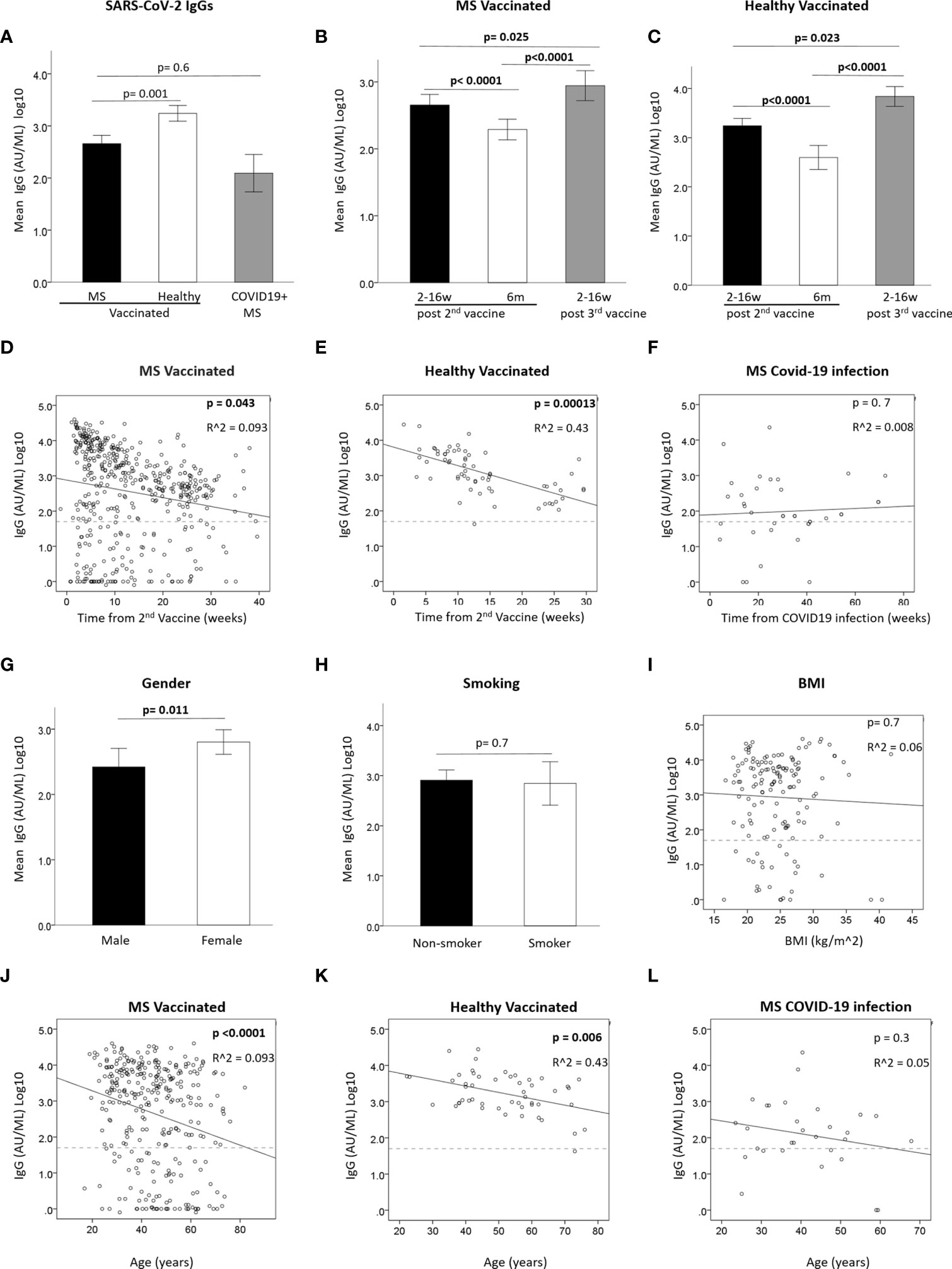
Figure 1 (A) Mean IgG levels after 2nd vaccine in vaccinated PwMS (N=309) and healthy (N=48) and in unvaccinated COVID-19-recovered PwMS (N=29). (B, C) Mean IgG level in vaccinated PwMS (N=479) (B) or healthy (N=65) (C) at 3 time-points: <3months and 6 months from 2nd vaccine and <3 months from 3rd vaccine. (D, E) Correlation between IgG and time since 2nd vaccine in vaccinated PwMS (N=311) (D) or healthy) (N=46) (E). (F) Correlation between IgG and time since COVID-19 infection in unvaccinated PwMS. (G) mean IgG difference between male (N=107) and female (N=204) PwMS after 2nd vaccine. (H) mean IgG difference between non-smokers (N=166) and smokers (N=37) PwMS after 2nd vaccine. (I) Correlation between IgG post 2nd vaccine and BMI in PwMS (N=144). (J, K) Correlation between IgG after 2nd vaccine and age in vaccinated PwMS (N=311) (J) or healthy (N=46) (K). (L) Correlation between IgG and age in unvaccinated PwMS recovered from COVID-19 (N=38). Dashed horizontal grey line represents minimum seropositive border (log10(50AU/ml).
Effect of Demographic Factors on the Serological Response
We assessed whether the serological response after the 2nd vaccination was affected by demographic parameters (Figures 1G–L). Mean IgG levels were higher in female than in male PwMS (F=6.49, p=0.011) (Figure 1G). No gender-related difference was found in the small cohort of HC (data not shown). No difference was found in PwMS between smokers and non-smokers (F=0.1, P=0.7) (Figure 1H), and IgG levels did not correlate with body mass index (BMI) [B=-0.009 ± 0.024, 95%CI: (-0.056, -0.038), p=0.7] (Figure 1I). Age negatively correlated with IgG levels, both in vaccinated MS patients [B= -0.026 ± 0.006, 95%CI: (-0.038,-0.015), p<0.0001] (Figure 1J) and in vaccinated healthy individuals [B=-0.015 ± 0.005, 95%CI: (-0.025, -0.004), p=0.006] (Figure 1K), but not in MS patients post-COVID-19 infection [B=-0.018± 0.016, 95%CI: (-0.05, 0.014), p=0.3] (Figure 1L).
Effect of DMTs on the Serological Response
Table 2 and Supplementary Table 1 present the IgG levels according to the DMT that patients were receiving. Patients treated with most DMTs had a positive IgG response both after the 2nd and the 3rd vaccination, with IgG levels similar to untreated MS patients. However patients treated with the S1PR1 modulator fingolimod and the anti-CD20 therapy ocrelizumab had lower IgG levels after both the 2nd and 3rd vaccination (both p<0.0001), with only 38% of ocrelizumab-treated patients and 54% of fingolimod-treated patients being seropositive after the 2nd vaccine dose, and 44% and 46% respectively being seropositive after the 3rd vaccine booster (Table 2). Patients receiving other S1PR-modulators ponesimod or siponimod were seropositive both after the 2nd and the 3rd vaccination, but with relatively low IgG levels compared to untreated patients. Interestingly, patients receiving the anti-CD-20 therapy ofatumumab were all seropositive both after the 2nd and the 3rd vaccination, with relatively normal IgG levels (Table 2 and Supplementary Table 1). In contrast, two patients receiving rituximab (a first generation anti-CD20 therapy) had very low IgG levels, only one being borderline seropositive (Supplementary Table 1). 100% of cladribine-treated patients were seropositive after the 2nd and the 3rd vaccination, and their IgG levels were comparable to untreated patients (Table 2). Although the number of unvaccinated patients who recovered from COVID-19 infection was small (N=32), a similar trend was observed with a positive IgG response in patients under treatment with most DMTs, with the exception of ocrelizumab [7/13 (54%) positivity], fingolimod [3/4 (75%) positivity], siponimod (0/1 positivity) and cladribine [2/3 (67%) positivity] at time of sample collection (Table 2).
Relation Between Time Since Last Ocrelizumab Infusion and IgG Response
Since anti-CD20 therapies are expected to have a lowering effect on antibody production after vaccination, and since ocrelizumab is administered periodically every 6 months, it is important to appreciate the preferred timing for immunization between treatment doses. We evaluated the anti SARS-CoV-2 spike protein IgG levels according to the time interval between last ocrelizumab infusion and vaccine administration (Figure 2, Supplementary Figure 1AB and Supplementary Table 2). Only a very weak, although significant, correlation was found between the time since previous ocrelizumab infusion and IgG levels after the 2nd vaccination [B=0.02 ± 0.01, 95%CI: (0.0, 0.04), p=0.048] (Supplementary Figure 1A) or after the 3rd vaccine booster [B=0.048 ± 0.022, 95% CI (0.00, 0.09), p=0.041] (Supplementary Figure 1B). Mean IgG levels were significantly higher in patients with a ≥ 5 months interval between last infusion and the 1st vaccine dose (Figure 2A and Supplementary Table 2) or the 3rd vaccine booster (Figure 2B and Supplementary Table 2), compared to patients with an interval of < 5 months (F=4.43, p=0.039 and F=4.76, p=0.036, respectively). Similarly, patients with a ≥6 months interval had significantly higher IgG levels than patients with < 6 months between last infusion and 1st (Figure 2A) or 3rd vaccine (Figure 2B) (F= 5.19, p=0.026 and F=4.43, p=0.042, respectively) (Supplementary Table 2). Furthermore, the percentage of seropositive patients was higher in patients with ≥5 months interval than in those with <5 months interval between last infusion and 1st or 3rd vaccine (44% vs. 26% and 62% vs. 30%, respectively), and higher in patients with ≥6 months interval than in those with <6 months interval (60% vs. 25% and 75% vs. 36%, respectively) (Supplementary Table 2). Comparing the IgG levels in ocrelizumab-treated patients after the 2nd and 3rd vaccinations revealed a similar pattern for most patients, e.g. those who had a positive response after the first 2 vaccine doses were most likely to be also seropositive after the 3rd vaccination, while most patients with an insufficient response after the 2nd vaccination remained mostly seronegative after the 3rd vaccination, although some patients did benefit from the booster (Figure 2C). Using a generalized linear model to compare IgG levels between the 3 time-points we found no differences between time-point 1 and 2 post 2nd vaccination [OR1.00, 95%CI: (0.73, 1.39), p=0.98], between time-point 2 post 2nd vaccine and time-point 3 post 3rd vaccination [OR1.25, 95%CI: (0.70, 2.2), p=0.5], or between time-point 1 post-2nd vaccination and time-point 3 post-3rd vaccination [OR1.38, 95%CI: (0.89, 2.1), p=0.2] (Figure 2D).
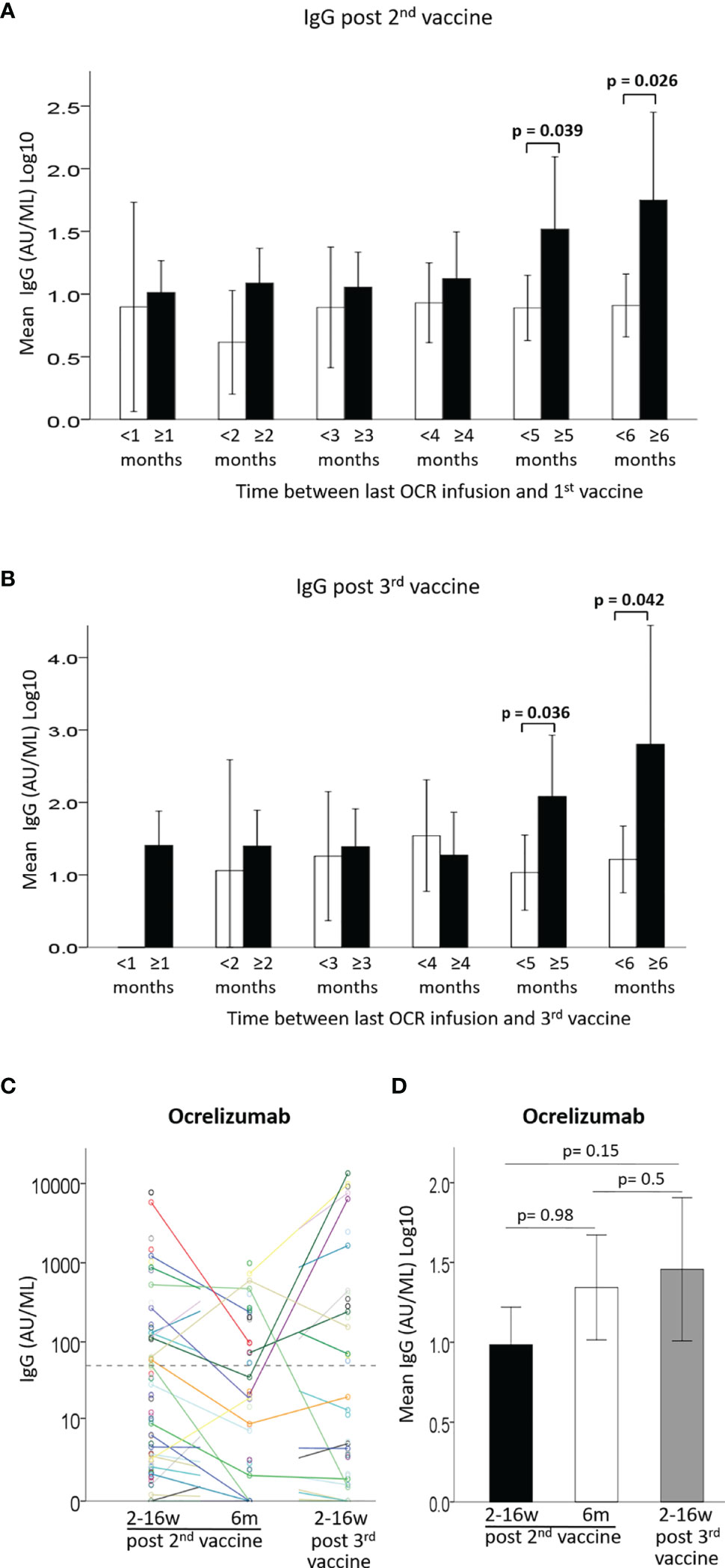
Figure 2 (A) Mean IgG levels after 2nd vaccine in patients with < (white) or ≥ (black) 1-6 months between last Ocrelizumab infusion and 1st vaccine. (B) Mean IgG levels after 3rd vaccine in patients with < (white) or ≥ (black) 1-6 months between last Ocrelizumab infusion and 3rd vaccine. (C) IgG levels of Ocrelizumab-treated patients at 3 time-points: ≤3months and 6 months from 2nd vaccine and ≤3 months from 3rd vaccine. (D) Comparison of IgG levels in Ocrelizumab-treated patients at 3 time-points: ≤3months (N=89) and 6 months (N=38) from 2nd vaccine and ≤3 months from 3rd vaccine (N=43). Dashed horizontal grey line represents minimum seropositive border (log10(50AU/ml).
IgG Levels in Fingolimod-Treated Patients
IgG levels did not correlate with absolute lymphocyte counts at time of vaccination in fingolimod-treated patients [B=0.537 ± 0.64, 95%CI: (-0.77, 1.85), p=0.4] (Figure 3A). Comparison of the IgG response after the 2nd and 3rd vaccination showed a similar pattern for most fingolimod-treated patients, with seronegative patients remaining negative also after the 3rd vaccination (Figure 3B). A generalized linear model comparing IgG levels between the 3 time-points revealed that the IgG levels were reduced between time-point 1 and 2 after the 2nd vaccination [OR=0.53, 95%CI: (0.36, 0.78), p=0.001], and increased between 2nd and 3rd time-point following the vaccine booster [OR=1.88, 95%CI: (1.42, 2.49), p<0.0001]; however, the 3rd vaccine booster did not increase the IgG levels further than at time-point 1 (post 2nd vaccination) [OR=0.76, 95%CI: (0.48, 1.21), p=0.3] (Figure 3C).
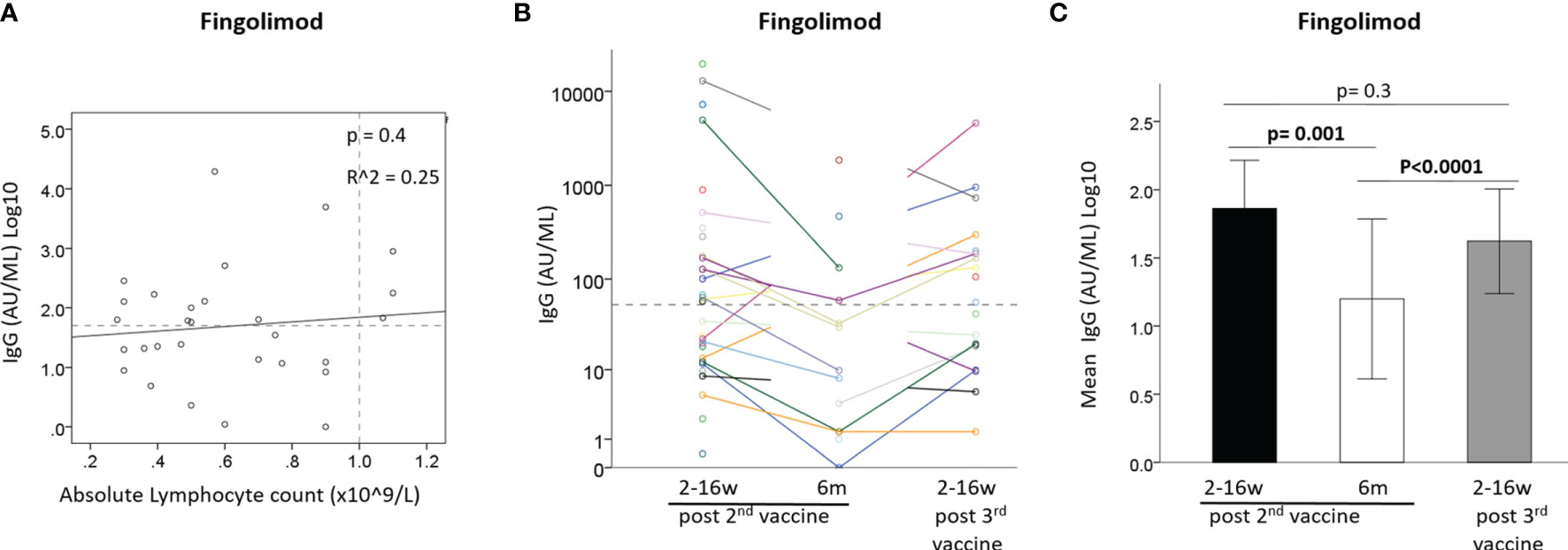
Figure 3 (A) Correlation between IgG after 2nd vaccine and absolute lymphocyte count in Fingolimod-treated patients (N=29). (B) IgG levels of Fingolimod-treated patients at 3 time-points: ≤3months and 6 months from 2nd vaccine and ≤3 months from 3rd vaccine. (C) Mean IgG levels in Fingolimod-treated patients at 3 time-points: ≤3months and 6 months from 2nd vaccine and ≤3 months from 3rd vaccine. Dashed horizontal grey line represents minimum seropositive border (log10(50AU/ml), dashed vertical line represent normal lymphocyte count border.
INFγ Immune Cell Response
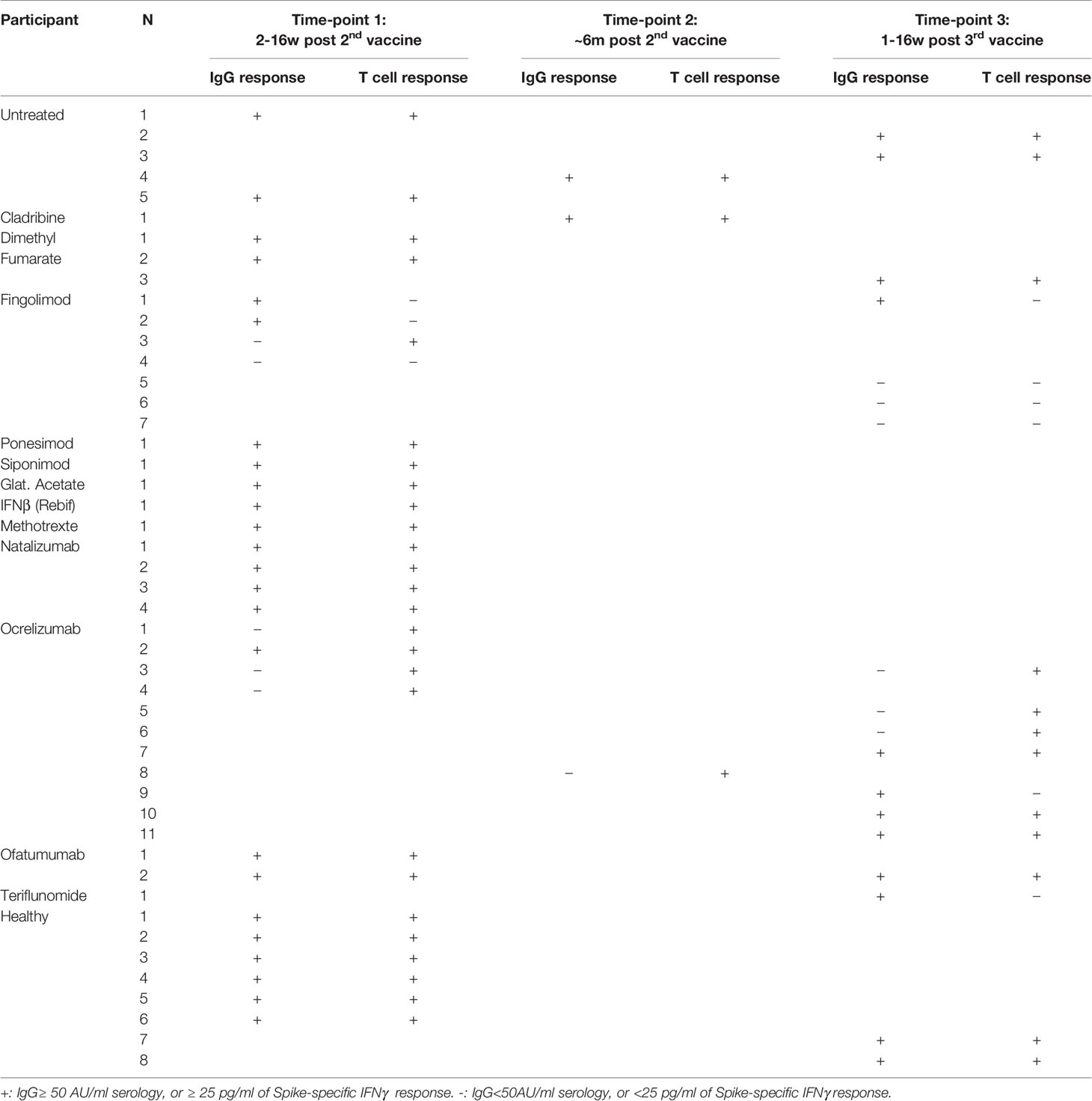
T-cell immune response to the vaccine was assessed by measuring IFNγ secretion in response to incubation of whole blood with the SARS-CoV-2 spike protein. Data were available from 26 patients and 6 healthy participants after 2nd vaccine, along with their serological assay, and from 16 patients and 2 healthy participants after the 3rd vaccine booster (Table 3 and Supplementary Table 3). There was no significant difference in IFNγ-T-cell response levels between PwMS and healthy participants after the 2nd vaccination (p=0.8) or after the 3rd vaccine booster (p=0.2). 100% of healthy participants and 88.5% of MS patients had a positive IFNγ-T cell response after the 2nd vaccination, with 100% and 63% positivity after the 3rd vaccine booster, respectively. Only 25% (1/4) of fingolimod-treated patients had a positive T cell response after the 2nd vaccination and 0% (0/4) after the 3rd vaccination, including patients who were seropositive. In contrast, all ocrelizumab-treated patients (5/5) had a positive T cell response after the 2nd vaccine, despite 58% being seronegative, and 86% (6/7) showed a T cell response after the 3rd vaccine (Table 3). One teriflunomide-treated patient did not develop an IFNγ-T cell response post 3rd vaccination, while all of the remaining patients treated with various other DMTs had a positive T cell response.
Discussion
To the best of our knowledge, this is the first comprehensive prospective study on the magnitude and durability of SARS-CoV-2 IgG levels combined with T cell responses in PwMS, treated with various DMTs, for over 6 months from vaccination and following a 3rd vaccine booster. Vaccination is the major available tool to control and fight the pandemic of COVID-19. Several vaccines have been developed worldwide, using different strategies and platforms, including the novel strategy of vaccination with the SARS-CoV-2 spike protein mRNA as in the case of the BNT162b2 (Pfizer/BioNTech,Inc) and the mRNA-1273 (Moderna Tx,Inc) vaccines. These mRNA vaccines appear to be safe and have not been associated with increased incidence of MS disease activation (13–16). In general, the use of DMTs in PwMS has not been found to significantly affect COVID-19 disease course (17), although anti-CD20 therapies have been associated with higher incidence of COVID-19 infection (18) and with a possibly increased risk of severe disease course (19).
An effective, long-term, memory immune response is driven by the adaptive immune system, consisting of both a humoral response mediated by B cells, producing antigen-specific antibodies, and a T cell-mediated response, causing destruction of virus-infected cells and necessary for the development of plasma cells and memory B cells. Thus, measurement of both the humoral and the cell-mediated responses is required to precisely estimate the immune response to SARS-CoV-2 vaccine. However, the relatively easily accessible measurement of anti SARS-CoV-2 antibody levels in the serum is the common and mostly used method to identify individuals who recovered from COVID-19 infection or to confirm a sufficient vaccine response, especially since SARS-CoV-2 neutralizing antibodies were shown to be highly predictive of SARS-CoV-2 immune protection (6). Evidence for positive humoral immune responses to the SARS-CoV-2 vaccine in MS patients is accumulating (9, 10, 12, 20, 21). Our current study provides longitudinal accumulating data regarding the IgG serum levels following >6 months follow-up and also after a 3rd vaccine booster. Our findings show that IgG levels to the SARS-CoV-2 spike protein in MS patients were slightly lower than in healthy individuals, declined by >80% within 6 months from the 2nd vaccination, and were significantly increased following a 3rd vaccine booster. A similar decline in IgG levels at 6 months from vaccination and a beneficial effect of the 3rd vaccine booster was also demonstrated in the healthy cohort. Interestingly, we did not detect a negative correlation between IgG levels after COVID-19 infection and time, suggesting a more robust and long-lasting immune response to the SARS-CoV-2 virus following natural infection vs vaccination. However, the median time between COVID-19-infection and blood collection in our cohort was 27 weeks, thus may not being able to capture the major, initial decline in IgG levels. Antibody levels following COVID-19 infection were shown to be associated with COVID-19 disease severity (a parameter that was not registered in our study), and were reported to start declining after 60 days, but still to be detectable for at least 120 days (22). For the COVID-19-recovered patients in our study, the follow up data were limited, as the majority of these patients received the recommended post–infection vaccination. Interestingly, we found higher IgG levels in vaccinated female than in male patients, not reported in previous reports (20, 23). While we did not detect a similar difference in IgG levels between genders in HC, probably due to the relatively small cohort, both levels of IgGs and of neutralizing antibodies have been shown to be higher in females than in males receiving the BNT162b2 Covid-19 Vaccine, especially in association with age (24), a difference that at least in part is thought to be hormone-mediated (25). The negative correlation between IgG levels and age in vaccinated PwMS or healthy participants presented in our study has also been suggested elsewhere (11, 26), and confirms the potential elevated risk of COVID-19 infection in elderly people.
Our data show, similarly to previous reports, that MS patients treated with most DMTs develop a positive humoral and cell-mediated immune response to the mRNA vaccine, which in general does not differ significantly from that observed in untreated patients or healthy individuals. The reduced humoral response and low frequency of seropositive patients that was observed in PwMS treated with fingolimod and ocrelizumab are in line with several recent studies (7, 8, 10–12, 20, 21, 27–30). In addition, we could show that a similar humoral response and low frequency of seropositive patients is also observed after the 3rd vaccine booster in the patients treated with fingolimod or ocrelizumab, and that in those patients (in contrast to healthy individuals and PwMS in general), the 3rd vaccination did not boost the IgG levels further than the first 2 vaccine doses. One recent study reported similarly that only 1 out of 16 ocrelizumab-treated patients was seropositive after the 3rd BNT162b2 vaccine booster (29). It seems thus, that while some PwMS treated with S1PR-modulators or anti-CD20 therapies do benefit from the additional vaccine dose, an optional vaccine strategy for these patients should be considered. On the other hand, we found that the vast majority of ocrelizumab-treated patients developed a positive anti-spike protein IFNγ-T-cell immune response (both after the 2nd and the 3rd vaccinations) and thus, despite the lack of a sufficient humoral response, they may carry a relative protection against COVID-19 infection or severe disease. Similar results were recently reported by other investigators: Gadani et al. found that 96.9% of ocrelizumab patients developed a T-cell response (30), Tortorella et al. reported a 92% positive T-cell response rate (21) and Brill et al. found a response rate of 89.7% (11). Aposolidis et al. reported positive CD-4 and CD-8 T-cell responses to vaccination in all anti CD-20-treated patients, but these responses were somewhat skewed, with reduced follicular helper T (TFH) cell development and elevated CD8 T-cell responses, while Th1 responses were preserved, especially in patients who were seronegative (31). Although these findings of relatively preserved T-cell-mediated anti-COVID-19 responses are encouraging, studies with larger patient cohorts, including follow-up on the risk and outcome of COVID-19 infection are needed to accurately estimate the vaccine-induced protection in anti-CD20- treated patients. Interestingly, we observed both positive humoral and cellular immune responses to the vaccine in patients treated with another anti-CD20 mAB - ofatumumab. We are not aware of published data on the response to vaccination in these patients, however, one study found that COVID-19- recovered ofatumumab-treated, B-cell depleted patients were seronegative for anti SARS-Cov-2 antibodies, but developed a positive cellular response (32), while another study reported a positive anti-SARS-CoV-2 antibody response after COVID-19 in a B-cell depleted ofatumumab-treated patient (33). Confirmation of this finding in a larger group of ofatumumab-treated patients will be of high interest.
Anti-CD20 therapies such as ocrelizumab reduce the number of circulating B cells, thus preventing a sufficient B cell response to antigens and the development of antibodies, an effect which is likely to persist until sufficient B cell reconstitution occurs. It would therefore be of interest to determine the optimal time interval between the infusion and vaccination that would allow for sufficient reconstitution of B cells to enable an effective humoral immune response. This issue has been studied by a few groups but remains debatable; while in some reports there was a significant correlation between time from last treatment to vaccination and the IgG levels (11, 20, 30), others could not confirm this finding (21). In our study, IgG levels correlated only very weakly with the time between infusion and vaccination, both after the 2nd and the 3rd vaccine administration. One study found that 143 days following ocrelizumab infusion is the time-point when IgG starts to increase (20). We found that a time interval of more than 5 months between ocrelizumab infusion and vaccination allows for higher IgG levels than a time interval of <5 months, and a similar difference was also observed in patients vaccinated ≥6 months vs. those vaccinated <6 months after ocrelizumab infusion. Our data also showed that a higher proportion of ocrelizumab-treated patients were seropositive if they were at least 5 months from last infusion at time of vaccine, and in patients with ≥ 6 months time interval, sero-positivity increased to 75% post the 3rd vaccination. Based on our data, and since the humoral response to the vaccine is effective about 14 days after vaccination (34), we recommend that immunization should be timed to the window of 5 months after the last ocrelizumab dose and two weeks before the next dose of ocrelizumab. It was recently suggested that measuring the association between IgG response and B cell reconstitution, rather than the time interval since infusion, is more useful for determining the optimal timing of vaccination administration in ocrelizumab-treated patients (31). A recent study found that the mRNA-1273 vaccine, containing 100 micrograms of the spike protein mRNA, induced higher IgG levels in MS patients compared to the BNT162b2 mRNA vaccine which contains only 30 micrograms of mRNA (20). Based on this finding, the investigators suggested that the mRNA-1273 vaccine would be preferable as vaccination booster in PwMS on anti-CD20 therapy or fingolimod, who did not develop efficient humoral responses following BNT162b2 vaccination.
Our findings indicate that the vast majority of patients treated with fingolimod fail to mount an IFNγ-T-cell immune response. Tortorella et al. similarly found a T-cell response in only 14.3% of fingolimod-treated patients (21). With both reduced or insufficient humoral and cell-mediated immune responses, these patients may be at increased risk of COVID-19 infection and severe disease. Interestingly, it has been suggested that the immunosuppressive effects of fingolimod could be beneficial for prevention of acute respiratory distress syndrome in patients with severe COVID-19, by reducing the cytokine storm (35), and in a recent study fingolimod-treated PwMS were not found to be at increased risk of severe COVID-19 (19). Our data did not confirm a previous report of correlation between IgG response and lymphocyte count in fingolimod-treated patients (20).
Our study has certain limitations. Due to the rapid vaccination program in Israel, the serum samples after both the 2nd and the 3rd vaccines were collected during a variable and rather long time period (2-16 weeks, median ~7 weeks) when patients visited the outpatient clinics, and thus the antibody measurement does not represent the peak of post-vaccination humoral response in every patient. Since there is a natural reduction in IgG levels with time, adjustment for time between vaccination and blood collection was integrated in the statistical analysis. The number of patients treated with other than fingolimod or ocrelizumab S1PR-modulators or anti-CD20 therapies was small, thus limiting any conclusion-making on their effects on the vaccine response. The number of patients assessed for T-cell mediated responses (assessed only in a sub-group of patients) was also low and not sufficiently representative for each DMT, but still our findings add to information emerging from other sites. Data on COVID-19 infection before and during the follow-up period were based only on reports by the participants attending the clinics, and was not intended for interpretation regarding the efficacy of vaccination and the risk of infection in our study population. Since our samples were collected before the outbreak of the latest Omicron variant in Israel, which seems to have the capability to escape immune-surveillance, the impact of the vaccination program on Omicron and future variants is yet to be determined. However, the information on the immune responses of PwMS after the first 3 mRNA vaccines is likely to be relevant for future vaccine development.
The merit of this study is the longitudinal follow-up of IgG levels and cell-mediated responses after 2 vaccinations and the response to a 3rd vaccine booster in PwMS treated with a wide variety of DMTs. While for most DMTs, the humoral and cell-mediated responses appear to be similar to those of untreated PwMS, the finding of sero-negativity in >50% of patients treated with the S1PR-modulator fingolimod and the anti-CD20 therapy ocrelizumab even after a 3 vaccine booster, along with the lack of cell-mediated immune response in the vast majority of fingolimod-treated patients, may suggest that the strategy of boosting the immune system with additional vaccine doses is not effective enough for these patients and that other vaccination strategies should be considered. Such considerations could include optimization of the timing between vaccine administrations, specifically in the context of MS immunotherapies, use of combinational vaccines based on different development platforms and targets, optimization of the vaccine dose and choice of appropriate vaccine in relation to its ability to induce higher antibody levels. In general, efforts should be focused on development of optimal vaccine strategies aiming at improving immunogenicity and long-lasting immunity, tailored for each PwMS under treatment with a specific immunotherapy. Based on the cumulative data until today, updated recommendations about the type and timing of SARS-CoV-2 vaccinations of MS patients are needed.
Data Availability Statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics Statement
The studies involving human participants were reviewed and approved by Helsinki Committee, Barzilai Medical Center; Helsinki Committee, Carmel Medical Center; Helsinki Committee, Hadassah Medical Center; Helsinki Committee, Tel Aviv Sourasky Medical Center; Helsinki Committee, Rabin Medical Center;. The patients/participants provided their written informed consent to participate in this study.
The Israeli Neuroimmunology Study Group on COVID-19 vaccination in Multiple Sclerosis
The Israeli Neuroimmunology Study Group on COVID-19 vaccination in Multiple Sclerosis:
Lea Glass-Marmor, Carmel Medical Center; Anat Volkovitz, Carmel Medical Center; Sara Dishon, Carmel Medical Center; Zeev Dishon, Carmel Medical Center; Netta Kugelman, Carmel Medical Center; Zeev Nitsan, Barzilai Medical Center; Marwan Alkrenawi, Barzilai Medical Center; Nurit Hovel, Barzilai Medical Center; Nir Michal, Barzilai Medical Center; Vered Loew-Shavit, Barzilai Medical Center; Lital Mizrahi, Barzilai Medical Center; Marsel Zafrani, Barzilai Medical Center; Panayiota Petrou, Hadassah Medical Center; Nour Eddine-Yaghmour, Hadassah Medical Center; Ariel Ginzberg, Hadassah Medical Center; Ibrahim Kassis, Hadassah Medical Center; Michelle Halimi, Hadassah Medical Center; Keren Regev, Tel Aviv Sourasky Medical Center; Hadar Kolb, Tel Aviv Sourasky Medical Center; Ifat Vigiser, Tel Aviv Sourasky Medical Center; Yoav Piora, Tel Aviv Sourasky Medical Center; Irina Komarov, Tel Aviv Sourasky Medical Center; Avigail Hindi, Tel Aviv Sourasky Medical Center; Adi Wilf-Yarkoni, Rabin Medical Center; Itay Lotan, Rabin Medical Center; Elia Uri, Israel Institute for Biological Research; Shaher Rotem, Israel Institute for Biological Research; Hila Cohen, Israel Institute for Biological Research.
Author Contributions
AM conceptualized and designed the study, contributed to acquisition and interpretation of the data and revised the manuscript for intellectual content. ES-R designed the study, analyzed and interpreted the data, drafted the manuscript. RM, DK and AK contributed to the design of the study, acquisition and interpretation of the data, and revised the manuscript for intellectual content. MH contributed to acquisition and interpretation of the data and revised the manuscript for intellectual content. EB-H contributed to the acquisition and analysis of the data. The Israeli Neuroimmunology Study Group on COVID-19 vaccination in Multiple Sclerosis contributed to acquisition of the data. All authors contributed to the article and approved the submitted version.
Funding
This study was coordinated and funded by the Israel Neuroimmunological Society through grants received by Merck, Roche and Novartis companies. The funding sources were not involved in the study design, collection, analysis, interpretation of data, the writing of this article or the decision to submit for publication.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Acknowledgments
We thank the patients and healthy volunteers for participation in this study. We gratefully thank Idit Lavy, Department of Community Medicine & Epidemiology, Carmel Medical Center, Haifa, Israel, for statistical consultation.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fimmu.2022.868915/full#supplementary-material
Supplementary Figure 1 | (A) Correlation between IgG after 2nd vaccine and time between Ocrelizumab infusion and 1st vaccine (N=81). (B) Correlation between IgG post 3rd vaccine and time between Ocrelizumab infusion and 3rd vaccine (N=40). OCR- ocrelizumab. Dashed horizontal grey line represents minimum seropositive border (log10(50AU/ml).
References
1. Sellner J, Rommer PS. Multiple Sclerosis and SARS-CoV-2 Vaccination: Considerations for Immune-Depleting Therapies. Vaccines (2021) 9:1–12. doi: 10.3390/vaccines9020099
2. Bar-On YM, Goldberg Y, Mandel M, Bodenheimer O, Freedman L, Kalkstein N, et al. Protection of BNT162b2 Vaccine Booster Against Covid-19 in Israel. N Engl J Med (2021) 385:1393–400. doi: 10.1056/nejmoa2114255
3. Barda N, Dagan N, Ben-Shlomo Y, Kepten E, Waxman J, Ohana R, et al. Safety of the BNT162b2 mRNA Covid-19 Vaccine in a Nationwide Setting. N Engl J Med (2021) 385:1078–90. doi: 10.1056/nejmoa2110475
4. The National Multiple Sclerosis Society. Available at: https://www.nationalmssociety.org/coronavirus-covid-19-information/multiple-sclerosis-and-coronavirus/covid-19-vaccine-guidance. (Accessed January 18.2022).
5. https://www.msif.org/news/2020/02/10/the-coronavirus-and-ms-what-you-need-to-know/ (Accessed January 28, 2022).
6. Khoury DS, Cromer D, Reynaldi A, Schlub TE, Wheatley AK, Juno JA, et al. Neutralizing Antibody Levels are Highly Predictive of Immune Protection From Symptomatic SARS-CoV-2 Infection. Nat Med (2021) 27:1205–11. doi: 10.1038/s41591-021-01377-8
7. Guerrieri S, Lazzarin S, Zanetta C, Nozzolillo A, Filippi M, Moiola L. Serological Response to SARS-CoV-2 Vaccination in Multiple Sclerosis Patients Treated With Fingolimod or Ocrelizumab: An Initial Real-Life Experience. J Neurol (2021) 269(1):39–43. doi: 10.1007/s00415-021-10663-x
8. Gadani SP, Reyes-mantilla M, Jank L, Harris S, Douglas M, Smith MD, et al. Discordant Humoral and T Cell Immune Responses to SARS-CoV-2 Vaccination in People With Multiple Sclerosis on Anti-CD20 Therapy Sachin. EBioMedicine (2021) 73:103636. doi: 10.1016/j.ebiom.2021.103636
9. Boekel L, Steenhuis M, Hooijberg F, Besten YR, van Kempen ZLE, Kummer LY, et al. Antibody Development After COVID-19 Vaccination in Patients With Autoimmune Diseases in the Netherlands: A Substudy of Data From Two Prospective Cohort Studies. Lancet Rheumatol (2021) 3:e778–88. doi: 10.1016/s2665-9913(21)00222-8
10. Bigaut K, Kremer L, Fleury M, Lanotte L, Collongues N, de Seze J. Impact of Disease-Modifying Treatments on Humoral Response After COVID-19 Vaccination: A Mirror of the Response After SARS-CoV-2 Infection. Rev Neurol (Paris) (2021). 177(10):1237–40. doi: 10.1016/j.neurol.2021.05.001
11. Brill L, Ariel R, Omri Z, Nitzan H, Esther O-D, WD G, et al. Humural and T-Cell Response to SARS-CoV-2 Vaccination in Patients With MS Treated With Ocrelizumab. JAMA Neurol (2021) 23:e213599. doi: 10.1001/jamaneurol.2021.3599
12. Achiron A, Mandel M, Dreyer-Alster S, Harari G, Magalashvili D, Sonis P, et al. Humoral Immune Response to COVID-19 mRNA Vaccine in Patients With Multiple Sclerosis Treated With High-Efficacy Disease-Modifying Therapies. Ther Adv Neurol Disord (2021) 14:1–8. doi: 10.1177/17562864211012835
13. Bellucci G, Rinaldi V, Buscarinu MC, Reniè R, Bigi R, Pellicciari G, et al. Multiple Sclerosis and SARS-CoV-2: Has the Interplay Started? Front Immunol (2021) 12:755333. doi: 10.3389/fimmu.2021.755333
14. Lotan I, Wilf-Yarkoni A, Friedman Y, Stiebel-Kalish H, Steiner I, Hellmann MA. Safety of the BNT162b2 COVID-19 Vaccine in Multiple Sclerosis (MS): Early Experience From a Tertiary MS Center in Israel. Eur J Neurol (2021) 28:3742–8. doi: 10.1111/ene.15028
15. Achiron A, Dolev M, Menascu S, Zohar DN, Dreyer-Alster S, Miron S, et al. COVID-19 Vaccination in Patients With Multiple Sclerosis: What We Have Learnt by February 2021. Mult Scler J (2021) 27:864–70. doi: 10.1177/13524585211003476
16. Briggs FBS, Mateen FJ, Schmidt H, Currie KM, Siefers HM, Crouthamel S, et al. COVID-19 Vaccination Reactogenicity in Persons With Multiple Sclerosis. Neurol Neuroimmunol Neuroinflamm (2022) 9:e1104. doi: 10.1212/nxi.0000000000001104
17. Chaudhury F, Bulka H, Rathnam AS, Said OM, Lin J, Lorigan H, et al. COVID-19 in Multiple Sclerosis Patients and Risk Factors for Severe Infection. J Neurol Sci (2020) 418:117147. doi: 10.1016/j.jns.2020.117147
18. Reder AT, Centonze D, Naylor ML, Nagpal A, Rajbhandari R, Altincatal A, et al. COVID-19 in Patients With Multiple Sclerosis: Associations With Disease-Modifying Therapies. CNS Drugs (2021) 35:317–30. doi: 10.1007/s40263-021-00804-1
19. Sormani MP, De Rossi N, Schiavetti I, Carmisciano L, Cordioli C, Moiola L, et al. Disease-Modifying Therapies and Coronavirus Disease 2019 Severity in Multiple Sclerosis. Ann Neurol (2021) 89:780–9. doi: 10.1002/ana.26028
20. Sormani MP, Inglese M, Schiavetti I, Carmisciano L, Laroni A, Lapucci C, et al. Effect of SARS-CoV-2 mRNA Vaccination in MS Patients Treated With Disease Modifying Therapies. EBioMedicine (2021) 72:103581. doi: 10.1016/j.ebiom.2021.103581
21. Tortorella C, Aiello A, Gasperini C, Agrati C, Castilletti C, Ruggieri S, et al. Humoral- and T-Cell–Specific Immune Responses to SARS-CoV-2 mRNA Vaccination in Patients With MS Using Different Disease-Modifying Therapies. Neurology (2022) 98(5):e541–54 doi: 10.1212/wnl.0000000000013108.
22. Arkhipova-Jenkins I, Helfand M, Armstrong C, Gean E, Anderson J, Paynter RA, et al. Antibody Response After SARS-CoV-2 Infection and Implications for Immunity: A Rapid Living Review. Ann Intern Med (2021) 174:811–21. doi: 10.7326/M20-7547
23. Wall EC, Wu M, Harvey R, Kelly G, Warchal S, Sawyer C, et al. Neutralising Antibody Activity Against SARS-CoV-2 VOCs B.1.617.2 and B.1.351 by BNT162b2 Vaccination. Lancet (2021) 397:2331–3. doi: 10.1016/S0140-6736(21)01290-3
24. Levin EG, Lustig Y, Cohen C, Fluss R, Indenbaum V, Amit S, et al. Waning Immune Humoral Response to BNT162b2 Covid-19 Vaccine Over 6 Months. N Engl J Med (2021) 385:e84. doi: 10.1056/nejmoa2114583
25. Ciarambino T, Para O, Giordano M. Immune System and COVID-19 by Sex Differences and Age. Women’s Heal (2021) 17:17455065211022262. doi: 10.1177/17455065211022262
26. Chvatal-Medina M, Mendez-Cortina Y, Patiño PJ, Velilla PA, Rugeles MT. Antibody Responses in COVID-19: A Review. Front Immunol (2021) 12:633184. doi: 10.3389/fimmu.2021.633184
27. Novak F, Christine A, Nielsen C, Holm DK, Kamilla Ø, Bystrup A, et al. Humoral Immune Response Following SARS-CoV-2 mRNA Vaccination Concomitant to Anti-CD20 Therapy in Multiple Sclerosis ☆. Mult Scler Relat Disord (2021) 56:103251. doi: 10.1016/j.msard.2021.103251
28. Gallo A, Capuano R, Donnarumma G, Bisecco A, Grimaldi E, Conte M, et al. Preliminary Evidence of Blunted Humoral Response to SARS-CoV-2 mRNA Vaccine in Multiple Sclerosis Patients Treated With Ocrelizumab. Neurol Sci (2021) 42:3523–6. doi: 10.1007/s10072-021-05397-7
29. Achtnichts L, Jakopp B, Oberle M, Nedeltchev K, Fux CA, Sellner J, et al. Humoral Immune Response After the Third SARS-CoV-2 mRNA Vaccination in CD20 Depleted People With Multiple Sclerosis. Vaccines (2021) 9:1470. doi: 10.3390/vaccines9121470
30. Gadani SP, Reyes-Mantilla M, Jank L, Harris S, Douglas M, Smith MD, et al. Discordant Humoral and T Cell Immune Responses to SARS-CoV-2 Vaccination in People With Multiple Sclerosis on Anti-CD20 Therapy. EBioMedicine (2021) 73:34462762. doi: 10.1016/j.ebiom.2021.103636
31. Apostolidis SA, Kakara M, Painter MM, Goel RR, Mathew D, Lenzi K, et al. Cellular and Humoral Immune Responses Following SARS-CoV-2 mRNA Vaccination in Patients With Multiple Sclerosis on Anti-CD20 Therapy. Nat Med (2021) 27:1990–2001. doi: 10.1038/s41591-021-01507-2
32. Adamec I, Rogi D. Humoral and Cellular Immunity in Convalescent COVID-19 People With Multiple Sclerosis Treated With Ofatumumab. J Neuroimmunol (2022) 362:577788. doi: 10.1016/j.jneuroim.2021.577788
33. Flores-Gonzalez RE, Hernandez J, Tornes L, Rammohan K, Delgado S. Development of SARS-CoV-2 IgM and IgG Antibodies in a Relapsing Multiple Sclerosis Patient on Ofatumumab. Mult Scler Relat Disord (2021) 49:2020–2. doi: 10.1016/j.msard.2021.102777
34. Doria-Rose N, Suthar MS, Makowski M, O’Connell S, McDermott AB, Flach B, et al. Antibody Persistence Through 6 Months After the Second Dose of mRNA-1273 Vaccine for Covid-19. N Engl J Med (2021) 384:2259–61. doi: 10.1056/nejmc2103916
Keywords: autoimmunity, COVID-19, humoral response, IgG, multiple sclerosis, SARS-CoV-2, T-cell immune response, disease modifying therapies (DMTs)
Citation: Milo R, Staun-Ram E, Karussis D, Karni A, Hellmann MA, Bar-Haim E, Miller A and The Israeli Neuroimmunology Study Group on COVID-19 Vaccination in Multiple Sclerosis (2022) Humoral and Cellular Immune Responses to SARS-CoV-2 mRNA Vaccination in Patients with Multiple Sclerosis: An Israeli Multi-Center Experience Following 3 Vaccine Doses. Front. Immunol. 13:868915. doi: 10.3389/fimmu.2022.868915
Received: 03 February 2022; Accepted: 04 March 2022;
Published: 01 April 2022.
Edited by:
Martina Prelog, University of Wuerzburg, GermanyReviewed by:
Domizia Vecchio, University of Eastern Piedmont, ItalyBenjamin Knier, Technical University of Munich, Germany
Copyright © 2022 Milo, Staun-Ram, Karussis, Karni, Hellmann, Bar-Haim, Miller and The Israeli Neuroimmunology Study Group on COVID-19 Vaccination in Multiple Sclerosis. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Ariel Miller, ariel_miller@clalit.org.il; millera@technion.ac.il
†These authors have contributed equally to this work
 Ron Milo
Ron Milo Elsebeth Staun-Ram
Elsebeth Staun-Ram Dimitrios Karussis
Dimitrios Karussis Arnon Karni
Arnon Karni Erez Bar-Haim6
Erez Bar-Haim6 Ariel Miller
Ariel Miller