- 1Division of General Surgery, Department of Surgery, David Geffen School of Medicine at University of California, Los Angeles, CA, USA
- 2CURE: Digestive Disease Research Center, David Geffen School of Medicine at University of California, Los Angeles, CA, USA
- 3Department of Medicine, David Geffen School of Medicine at University of California, Los Angeles, CA, USA
- 4Department of Medicine at Cedars Sinai Medical Center, Los Angeles, CA, USA
- 5Veterans Affairs, Los Angeles, CA, USA
- 6Materials and Process Simulation Center, California Institute of Technology, Pasadena, CA, USA
- 7Gradalis, Inc., Dallas, TX, USA
- 8Mary Crowley Cancer Research Centers, Dallas, TX, USA
Somatostatin (SST) is a regulatory peptide and acts as an endogenous inhibitory regulator of the secretory and proliferative responses of target cells. SST's actions are mediated by a family of seven transmembrane domain G protein-coupled receptors that comprise five distinct subtypes (SSTR1-5). SSTR5 is one of the major SSTRs in the islets of Langerhans. Homeodomain-containing transcription factor pancreatic and duodenal homeobox-1 (PDX-1) is essential for pancreatic development, β cell differentiation, maintenance of normal β cell functions in adults and tumorigenesis. Recent studies show that SSTR5 acts as a negative regulator for PDX-1 expression and that SSTR5 mediates somatostatin's inhibitory effect on cell proliferation and insulin expression/excretion through down-regulating PDX-1 expression. SSTR5 exerts its inhibitory effect on PDX-1 expression at both the transcriptional level by down-regulating PDX-1 mRNA and the post-translational level by enhancing PDX-1 ubiquitination. Identification of PDX-1 as a transcriptional target for SSTR5 may help in guiding the choice of therapeutic cancer treatments.
Introduction
Somatostatin (SST) is a peptide hormone involved in a wide variety of biological functions including inhibition of endocrine and exocrine secretion; modulation of neurotransmission; motor and cognitive functions; inhibition of intestinal motility; absorption of nutrients and ions; vascular contractility; and regulation of cell proliferation, differentiation, inflammatory and immune responses (Patel, 1999; Wang et al., 2005a; Ballian et al., 2006, 2007; Florio, 2008). SSTs exert their actions through a group of five G protein-coupled transmembrane receptors, termed somatostatin receptor 1-5 (SSTR1-5) (Yamada et al., 1992; Yasuda et al., 1992; Raynor et al., 1993; Reisine and Bell, 1995; Moldovan et al., 1998). Following binding of SST, SSTRs undergo a cell type-specific conformational change, homo/heterodimerization (Rocheville et al., 2000; Pfeiffer et al., 2001; Duran-Prado et al., 2007), internalization (Peverelli et al., 2008), recruiting the G protein family members, and activation of downstream signaling pathways (Patel, 1999; Florio, 2008).
SSTR5 (Moldovan et al., 1998) is one of the major SSTRs in the islets of Langerhans. It is present in 87% of insulin-producing β-cells, 35% of glucagon-producing α-cells, and 75% of somatostatin-producing δ-cells and plays an essential role in mediating the inhibitory effect of somatostatin on insulin expression and secretion (Fagan et al., 1998). SSTR5 gene ablation results in marked increase in insulin secretion, causing basal hypoglycemia and circulating hyperinsulinemia (Wang et al., 2004,2005a,b). Throughout the GI tract, SSTR5 has an anti-proliferative effect by inducing cell cycle arrest at the G1 phase via a mechanism that increases the production of retinoblastoma tumor suppressor protein and p21 (cyclin dependent kinase inhibitor) (Patel, 1999). SSTR5 knockout mice develop islet neoplasia associated with enlarged islets (Wang et al., 2004, 2005a,b). Due to its differential expression, SSTR5 is involved in tumorigenesis and drug responsiveness of a variety of human cancers including pancreatic cancer (Reubi et al., 1988; Li et al., 2011; Zhou et al., 2011a; Kaemmerer et al., 2013), pancreatic endocrine tumors (PETs) (Zhou et al., 2011b, 2012; Kaemmerer et al., 2013), pulmonary neuroendocrine tumors (Tsuta et al., 2012), gastroenteropancreatic neuroendocrine tumors (Kim et al., 2011a; Sclafani et al., 2011), small cell lung cancer (Oddstig et al., 2011), gallbladder cancer (Guo et al., 2013), colon cancer (Wang et al., 2013), endocrine pituitary tumors (Nishioka et al., 2011; Mayr et al., 2013; Chinezu et al., 2014), thyroid cancer (Ocak et al., 2013), corticotroph adenomas (Fleseriu and Petersenn, 2013), prostate cancer (Gu et al., 2010; Mazzucchelli et al., 2011; Lattanzio et al., 2013), and breast cancer (Gu et al., 2010). SSTR5 is also involved in the regulation of angiogenesis (Zatelli et al., 2001) and apoptosis (Qiu et al., 2006; Wang et al., 2013). This is consistent with microarray studies of pancreatic RNA from SSTR5−/− mice in which ablation of SSTR5 results in up-regulation of proliferated and angiogenic genes and suppression of apoptotic genes (Patel et al., 2009).
Pancreatic and duodenal homeobox-1 (PDX-1) is an evolutionally conserved, homeodomain-containing β-cell-specific transcription factor (Leonard et al., 1993; Ohlsson et al., 1993; Olson et al., 1993; Miller et al., 1994; Marshak et al., 1996; Macfarlane et al., 1997). PDX-1 functions as a master regulator for a variety of cellular events including pancreatic development (Jonsson et al., 1994; Stoffers et al., 1997), β cell differentiation (Zhou et al., 2008) and postnatal β-cell function and survival (Ashizawa et al., 2004). PDX-1 expression in adults is essentially restricted to the nuclei of approximately 90% of insulin-producing islet β cells where it binds to the promoters of insulin (Ohlsson et al., 1993), glucose transporter 2 (Ohlsson et al., 1993), islet amyloid polypeptide (Serup et al., 1996; Macfarlane et al., 2000), and glucokinase (Watada et al., 1996). It regulates their expression, thus playing a critical role in maintaining mature β-cell function since all of these genes are critical for glucose sensing and insulin synthesis. As a result, PDX-1 plays an essential role in development of diabetes (Ahlgren et al., 1998; Brissova et al., 2002; Al-Quobaili and Montenarh, 2008; Gauthier et al., 2009). Increasingly, studies show that PDX-1 is also involved in tumorigenesis. PDX-1 is aberrantly overexpressed in a variety of human cancers including pancreatic, gastric, liver, colon, breast, prostate, kidney, lung, and ovarian cancer (Koizumi et al., 2003; Sakai et al., 2004; Wang et al., 2005c; Leys et al., 2006; Miyatsuka et al., 2006; Liu et al., 2007; Jonmarker et al., 2008) and pancreatic neuroendocrine tumor (Liu et al., 2012; Zhou et al., 2012). Moreover, PDX-1 overexpression in patients with cancers is significantly correlated with the pathological parameters (e.g., metastasis and histological grade) (Koizumi et al., 2003; Liu et al., 2007). In addition, PDX-1 is specifically increased in human placentas from intra-uterine growth restriction (IUGR) and preeclampsia (PE) + IUGR pregnancies (Buffat et al., 2007) and represses transcriptional activity of the T-box-containing transcription factor TBX15 in a methylation-dependent manner (Chelbi et al., 2011).
SST's effects on cellular functions are at least in part mediated by its ability to regulate transcription factors. For example, octreotide, a somatostatin analog, inhibits mRNA expression of transcription factor c-fos and AP-1 binding activity stimulated by dibutyryl adenosine 3′,5′-cyclic monophosphate, serum and phorbol ester 12-O-tetradecanoylphorbol-13-acetate (TPA) (Todisco et al., 1994, 1995). In this mini review, we summarize recent findings that PDX-1 acts as another transcription factor that is subject to the inhibitory regulation by SSTR5-mediated somatostatin signaling.
Negative Regulation of PDX-1 by SSTR5
SSTR5 Down-Regulates PDX-1
PDX-1 is subject to positive regulation by glucose (21), glucagon-like peptide 1 (GLP-1) (Buteau et al., 1999; Wang et al., 1999; Movassat et al., 2002; Li et al., 2005) and palmitic acid (Arantes et al., 2006). PDX-1 also is negatively regulated by DNA damage (Lebrun et al., 2005), oxidative stress (Boucher et al., 2006), and advanced glycation end-products (AGEs) (Puddu et al., 2010) under different physiological conditions. RPL-1980 is a SSTR5 specific agonist which proves that SSTR5 is the SSTR subtype responsible for inhibiting glucose-stimulated insulin secretion (Fagan et al., 1998; Zhou et al., 2011c). Recent studies show that RPL-1980 abolishes GLP-1-stimulated PDX-1 expression in mouse insulinoma β-TC6 cells (Zhou et al., 2012), indicating that SSTR5-mediated somatostatin signaling is a potential novel negative regulator for PDX-1 expression. Further biochemical and genetic studies confirm the negative regulation of PDX-1 expression by SSTR5 (Zhou et al., 2012). The evidence includes: (1) increased expression of SSTR5 inhibits PDX-1 expression likely due to increased PDX-1 ubiquitination; and (2) knockdown of SSTR5 by SSTR5 shRNA in β-TC6 cells results in increased PDX-1 expression and enhanced insulin secretion in response to a high concentration of glucose. These findings are consistent with the fact that SSTR5 mediates the inhibitory effect of somatostatin on insulin secretion (Fagan et al., 1998; Tirone et al., 2003). A SSTR5 knockdown-induced increase of PDX-1 expression in mouse insulinoma MIN6 cells is accompanied by elevated expression of cyclin E and cdk4 and decreased expression of p21, p27, and p53, supporting previous studies showing that SSTR5 inhibits cell proliferation (Fagan et al., 1998; Feanny et al., 2008) and promotes apoptosis (Qiu et al., 2006). This also is consistent with a previous study showing that SSTR5 contributes to the induction of cyclin-dependent kinase inhibitor p27 (Grant et al., 2008); and (3) genetic ablation of SSTR5 results in increased expression of PDX-1, which is accompanied by increased expression of insulin and proliferating cell nuclear antigen (PCNA) in sstr5−/− islets. Also consistent is the finding that all three SSTR5 knockout mice (general SSTR5−/−, β cell-specific SSTR5−/− and SSTR1/5−/−, SSTR1 and SSTR5 double knockout) develop islet hyperplasia, increased numbers of islets and islet neogenesis compared with littermate wild type controls (Wang et al., 2004, 2005a,b). Given the essential role of PDX-1 in insulin expression (Ashizawa et al., 2004; Kaneto et al., 2007) and cell proliferation (Feanny et al., 2008), these studies support a new concept that SSTR5 may mediate the inhibitory effect of somatostatin on insulin expression and secretion and cell proliferation through a mechanism involving inhibiting PDX-1 expression.
SSTR5 P335l, A Hypofunctional SNP, Causes Up-Regulation of PDX-1
Single nucleotide polymorphisms (SNPs) are the most common type of genetic variations in the human genome, which can occur in all coding, non-coding and regulatory regions of a gene (Botstein and Risch, 2003). A number of SNPs of SSTR5 have been identified, including 20 missense variations (A19T, P34S, G37R, A40T, L48M, A52V, W105R, P109S, V180M, R229K, R234C, R248C, L251S, V267I, R312C, A327V, T333M, P335L, R339K and G357R) (http://www.ncbi.nlm.nih.gov/projects/SNP/snp_ref.cgi?chooseRs=allandgo=GoandlocusId=6755). Genome sequence analysis identified a number of SSTR5 gene mutations in human pancreatic cancer and pancreatic neuroendocrine tumors. Among them, SSTR5 P335L is a non-synonymous SNP resulting from a C to T change at the 1004th nucleotide of the human SSTR5 gene (Zhou et al., 2011c). The SNP widely exists in the human population and in patients with pancreatic cancer (Li et al., 2011; Zhou et al., 2011c) and pancreatic neuroendocrine tumors (Zhou et al., 2011b), which are race-dependent. SSTR5 P335L acts as a hypofunctional SNP since SSTR5 P335L enhances cell proliferation in contrast to wild-type SSTR5 (Zhou et al., 2011c). Moreover, SSTR5 P335L prevents the inhibitory effects of SSTR5 agonist RPL-1980 on cell proliferation of Mia PaCa-2 cells and glucose-stimulated insulin secretion from mouse insulinoma β-TC6 cells, while wild-type SSTR5 enhances the effects (Zhou et al., 2011c). Consistently, SSTR5 P335L enhances PDX-1 expression with an accompanied decreased PDX-1 ubiquitination (Zhou et al., 2012). These studies further confirm that SSTR5 is a negative regulator for PDX-1 expression.
Down-Regulation of PDX-1 by SSTR5 Occurs at Both Transcriptional and Post-Translational Levels
PDX-1 expression is controlled at epigenetic(Park et al., 2008; Ma et al., 2010; Yang et al., 2012), transcriptional (Wu et al., 1997; Gupta et al., 2008; Sun et al., 2008; da Silva Xavier et al., 2010), post-translational [phosphorylation (Lebrun et al., 2005; An et al., 2006, 2010; Boucher et al., 2006; Humphrey et al., 2010), ubiquitination (Lebrun et al., 2005; Boucher et al., 2006; Humphrey et al., 2010; Kim et al., 2011b), and sumoylation (Kishi et al., 2003)] levels. SSTR5 knockdown-induced increase of PDX-1 expression is accompanied by an increased expression of PDX-1 mRNA, while overexpression of SSTR5 inhibits PDX-1 mRNA expression (Zhou et al., 2012), indicating that SSTR5 acts as a negative regulator of PDX-1 at least partially through a mechanism of down-regulating PDX-1 mRNA. In addition, it is also found that wild-type SSTR5 enhances PDX-1 ubiquitiantion, while hypofunctional SSTR5 P335L SNP inhibits PDX-1 ubiquitination (Zhou et al., 2012). Thus, these studies demonstrate that SSTR5 acts as a negative regulator for PDX-1 expression and that SSTR5 exerts its inhibitory effect on PDX-1 expression at both transcriptional level (e.g., down-regulating PDX-1 mRNA) and post-translational level (e.g., enhancing PDX-1 ubiquitination and destabilizing PDX-1).
Perspective
Identification of PDX-1 as a novel transcriptional target for SSTR5-mediated somatostatin signaling greatly enhances our understanding of the cellular actions of somatostatin. SSTR5 has been shown to regulate a specific set of genes linked to cell proliferation, apoptosis, angiogenesis, immunity and tumorigenesis (Patel et al., 2009). PDX-1 also regulates a group of genes related to cell proliferation, apoptosis, invasion and angiogenesis (Liu et al., 2011). Given the negative regulation of PDX-1 by SSTR5 (Zhou et al., 2012), it is likely that somatostatin exerts its cellular actions through regulating PDX-1-targeted genes with SSTR5. In addition to SSTR5, SSTs also exert their actions through SSTR1, 2, 3, and/or 4 under different cellular context. Therefore, it will be important to further determine if other SSTR-mediated cellular functions of somatostatin also involve PDX-1.
Little is known about the molecular mechanism by which SST/SSTR5 signaling down-regulates PDX-1. The observations that SSTR5 enhances, while SSTR5 P335L inhibits, PDX-1 ubiquitination (Zhou et al., 2012) suggest that regulation of PDX-1 ubiquitination is a key participant in the pathways that connects SSTR5 with PDX-1. It has been shown that PDX-1 ubiquitination is subject to regulation by phosphorylation. DNA-dependent protein kinase (DNA-PK) phosphorylates PDX-1 on Thr 11 and drives PDX-1 degradation by proteasome in response to DNA damage stimulation (Lebrun et al., 2005). Oxidative stress (H2O2)-stimulated, GSK-3-mediated phosphorylation of Ser 61 and Ser 66 also promotes PDX-1 proteasomal degradation (Boucher et al., 2006). ERK MAP kinases have kinase activity toward PDX-1 (Khoo et al., 2003). It has been shown that SST-mediated growth inhibition of human pituitary adenoma and GH3 cells is associated with the down-regulation of pERK and upregulation of p27 (Hubina et al., 2006). In addition, somatostatin exerts its anti-migratory and anti-invasive function through inhibition of ERK 1/2 signaling via the inhibition of small G protein Rac in SHSY5Y cells (Pola et al., 2003). Thus, ERK MAP kinase is one of downstream signaling pathways for somatostatin. It is, thus, reasonable to speculate that somatostatin/SSTR5 signaling may inhibit EGF-stimulated ERK kinase activation, leading to down-regulation of PDX-1 expression through a mechanism involved in the regulation of PDX-1 ubiquitination and stability (Figure 1). PDX-1 is a transcription factor for PDX-1 itself (Ashizawa et al., 2004). Thus, it is possible that down-regulation of PDX-1 mRNA by SSTR5 is due to the down-regulation of PDX-1 expression by SSTR5, which, in turn, results in decreased PDX-1 transcriptional activity. Alternatively, SSTR5 may directly down-regulate PDX-1 mRNA stability, leading to decreased PDX-1 mRNA expression.
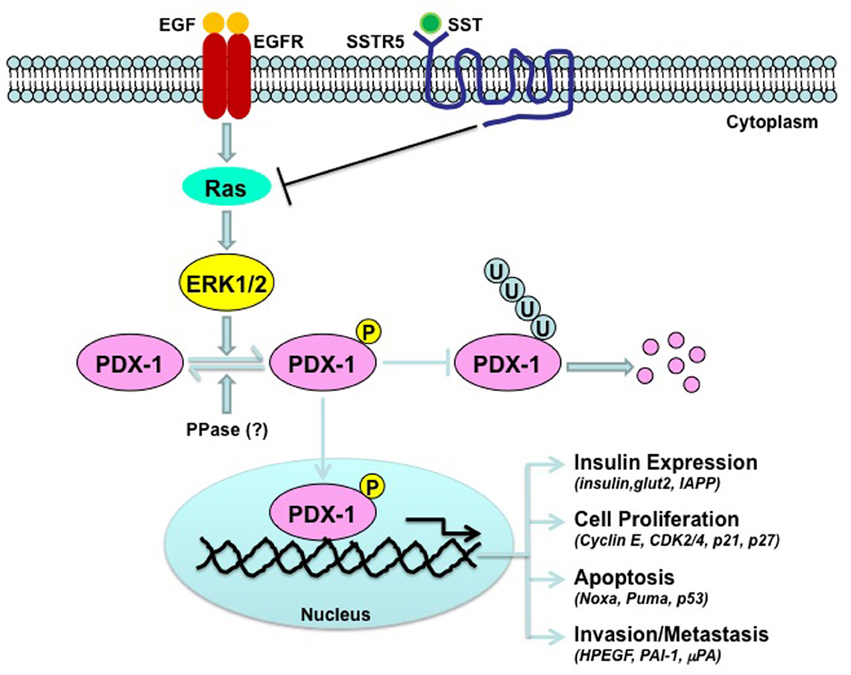
Figure 1. Schematic depiction of the down-regulation of PDX-1 by SSTR5. Epidermal growth factor (EGF) stimulates kinase activation of ERK MAP kinase, which, in turn, interacts with and phosphorylates PDX-1, leading to stabilization of PDX-1 by inhibiting PDX-1 ubiquitination. SSTR5-mediated somatostatin (SST) signaling inhibits EGF signaling by inhibiting small G protein Ras.
SST is an endogenous antiproliferative agent and, thus, a promising antitumor agent (Florio, 2008; Pyronnet et al., 2008; Bodei et al., 2009; Modlin et al., 2009). SSTR5 and other SSTRs are widely and variably expressed in a variety of tumors such as gastroenteropancreatic tumors, pituitary tumors, and carcinoid tumors. Thus, these receptors represent the molecular basis for the clinical use of somatostatin analogs in the treatment of endocrine tumors. However, it has been challenging that only about 50% of patients with insulinoma are responsive to the treatment of somatostatin analog and lack long-term responsiveness. It could be due to the lack of expression of the target SSTRs on the tumor surface, since 10–50% of these tumors do not express SSTRs. Genetic variation(s) within the receptors that affect their cellular functions such as SSTR5 P335L, a hypofunctional SNP (Zhou et al., 2011c), may also contribute to the non-responsiveness to the treatment of somatostatin analogs. Silencing PDX-1 efficiently inhibits tumor cell proliferation in vitro and tumor growth in vivo (Liu et al., 2008, 2011), showing that PDX-1 is a promising therapeutic target in cancer treatment. Thus, it is reasonable to speculate that a combined SSTR5-based tumor therapeutic approach including both somatostatin analog and other inhibiting technology of PDX-1 [for example, RNA interference control of PDX-1 (Liu et al., 2008)] would help improve the traditional treatment with somatostatin analogs. In patients with wild-type SSTR5, knockdown PDX-1 by biPDX-1 shRNA would enhance the therapeutic effect of somatostatin analog. In the case that genetic variations exist such as SSTR5 P335L, efficient knockdown PDX-1 by shRNA would help bypass the hypofunctional effect of SSTR5 P335L. Thus, identification of PDX-1 as a transcriptional target for SSTR5 may help serve to guide the choice of therapeutic treatments.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
This work was supported by The Pilot and Feasibility Study Grant from the CURE: Digestive Disease Research Center (P30DK41301) (to Guisheng Zhou) and the National Institutes of Health (NIH) grants NIDDK R01-DK46441 and NCI R01-CA095731 and Ann and Jerry Moss Foundation (to F. Charles Brunicardi). Gratitude is extended to Katie Elsbury for her editorial assistance and Priscilla Massey and Jacqueline Ismen for their administrative assistance.
References
Ahlgren, U., Jonsson, J., Jonsson, L., Simu, K., and Edlund, H. (1998). beta-cell-specific inactivation of the mouse Ipf1/Pdx1 gene results in loss of the beta-cell phenotype and maturity onset diabetes. Genes Dev. 12, 1763–1768. doi: 10.1101/gad.12.12.1763
Al-Quobaili, F., and Montenarh, M. (2008). Pancreatic duodenal homeobox factor-1 and diabetes mellitus type 2 (review). Int. J. Mol. Med. 21, 399–404. doi: 10.3892/ijmm.21.4.399
An, R., da Silva Xavier, G., Hao, H. X., Semplici, F., Rutter, J., and Rutter, G. A. (2006). Regulation by Per-Arnt-Sim (PAS) kinase of pancreatic duodenal homeobox-1 nuclear import in pancreatic beta-cells. Biochem. Soc. Trans. 34, 791–793. doi: 10.1042/BST0340791
An, R., da Silva Xavier, G., Semplici, F., Vakhshouri, S., Hao, H. X., Rutter, J., et al. (2010). Pancreatic and duodenal homeobox 1 (PDX1) phosphorylation at serine-269 is HIPK2-dependent and affects PDX1 subnuclear localization. Biochem. Biophys. Res. Commun. 399, 155–161. doi: 10.1016/j.bbrc.2010.07.035
Arantes, V. C., Reis, M. A., Latorraca, M. Q., Ferreira, F., Stoppiglia, L. F., Carneiro, E. M., et al. (2006). Palmitic acid increase levels of pancreatic duodenal homeobox-1 and p38/stress-activated protein kinase in islets from rats maintained on a low protein diet. Br. J. Nutr. 96, 1006–1012. doi: 10.1017/BJN20061950
Ashizawa, S., Brunicardi, F. C., and Wang, X. P. (2004). PDX-1 and the pancreas. Pancreas 28, 109–120. doi: 10.1097/00006676-200403000-00001
Ballian, N., Brunicardi, F. C., and Wang, X. P. (2006). Somatostatin and its receptors in the development of the endocrine pancreas. Pancreas 33, 1–12. doi: 10.1097/01.mpa.0000226894.16817.e8
Ballian, N., Hu, M., Liu, S. H., and Brunicardi, F. C. (2007). Proliferation, hyperplasia, neogenesis, and neoplasia in the islets of Langerhans. Pancreas 35, 199–206. doi: 10.1097/mpa.0b013e318074c6ed
Bodei, L., Ferone, D., Grana, C. M., Cremonesi, M., Signore, A., Dierckx, R. A., et al. (2009). Peptide receptor therapies in neuroendocrine tumors. J. Endocrinol. Invest. 32, 360–369. doi: 10.1007/BF03345728
Botstein, D., and Risch, N. (2003). Discovering genotypes underlying human phenotypes: past successes for mendelian disease, future approaches for complex disease. Nat. Genet. 33, 228–237. doi: 10.1038/ng1090
Boucher, M. J., Selander, L., Carlsson, L., and Edlund, H. (2006). Phosphorylation marks IPF1/PDX1 protein for degradation by glycogen synthase kinase 3-dependent mechanisms. J. Biol. Chem. 281, 6395–6403. doi: 10.1074/jbc.M511597200
Brissova, M., Shiota, M., Nicholson, W. E., Gannon, M., Knobel, S. M., Piston, D. W., et al. (2002). Reduction in pancreatic transcription factor PDX-1 impairs glucose-stimulated insulin secretion. J. Biol. Chem. 277, 11225–11232. doi: 10.1074/jbc.M111272200
Buffat, C., Mondon, F., Rigourd, V., Boubred, F., Bessieres, B., Fayol, L., et al. (2007). A hierarchical analysis of transcriptome alterations in intrauterine growth restriction (IUGR) reveals common pathophysiological pathways in mammals. J. Pathol. 213, 337–346. doi: 10.1002/path.2233
Buteau, J., Roduit, R., Susini, S., and Prentki, M. (1999). Glucagon-like peptide-1 promotes DNA synthesis, activates phosphatidylinositol 3-kinase and increases transcription factor pancreatic and duodenal homeobox gene 1 (PDX-1) DNA binding activity in beta (INS-1)-cells. Diabetologia 42, 856–864. doi: 10.1007/s001250051238
Chelbi, S. T., Doridot, L., Mondon, F., Dussour, C., Rebourcet, R., Busato, F., et al. (2011). Combination of promoter hypomethylation and PDX1 overexpression leads to TBX15 decrease in vascular IUGR placentas. Epigenetics 6, 247–255. doi: 10.4161/epi.6.2.13791
Chinezu, L., Vasiljevic, A., Jouanneau, E., Francois, P., Borda, A., Trouillas, J., et al. (2014). Expression of somatostatin receptors, SSTR2A and SSTR5, in 108 endocrine pituitary tumors using immunohistochemical detection with new specific monoclonal antibodies. Hum. Pathol. 45, 71–77. doi: 10.1016/j.humpath.2013.08.007
da Silva Xavier, G., Sun, G., Qian, Q., Rutter, G. A., and Leclerc, I. (2010). ChREBP regulates Pdx-1 and other glucose-sensitive genes in pancreatic beta-cells. Biochem. Biophys. Res. Commun. 402, 252–257. doi: 10.1016/j.bbrc.2010.10.010
Duran-Prado, M., Bucharles, C., Gonzalez, B. J., Vazquez-Martinez, R., Martinez-Fuentes, A. J., Garcia-Navarro, S., et al. (2007). Porcine somatostatin receptor 2 displays typical pharmacological sst2 features but unique dynamics of homodimerization and internalization. Endocrinology 148, 411–421. doi: 10.1210/en.2006-0920
Fagan, S. P., Azizzadeh, A., Moldovan, S., Ray, M., Adrian, T., Ding, X., et al. (1998). Insulin secretion is inhibited by subtype five somatostatin receptor in the mouse. Surgery 124, 254–259. doi: 10.1016/S0039-6060(98)70128-X
Feanny, M. A., Fagan, S. P., Ballian, N., Liu, S. H., Li, Z., Wang, X., et al. (2008). PDX-1 expression is associated with islet proliferation in vitro and in vivo. J. Surg. Res. 144, 8–16. doi: 10.1016/j.jss.2007.04.018
Fleseriu, M., and Petersenn, S. (2013). New avenues in the medical treatment of Cushing's disease: corticotroph tumor targeted therapy. J. Neurooncol. 114, 1–11. doi: 10.1007/s11060-013-1151-1
Florio, T. (2008). Molecular mechanisms of the antiproliferative activity of somatostatin receptors (SSTRs) in neuroendocrine tumors. Front. Biosci. 13, 822–840. doi: 10.2741/2722
Gauthier, B. R., Wiederkehr, A., Baquie, M., Dai, C., Powers, A. C., Kerr-Conte, J., et al. (2009). PDX1 deficiency causes mitochondrial dysfunction and defective insulin secretion through TFAM suppression. Cell Metab. 10, 110–118. doi: 10.1016/j.cmet.2009.07.002
Grant, M., Alturaihi, H., Jaquet, P., Collier, B., and Kumar, U. (2008). Cell growth inhibition and functioning of human somatostatin receptor type 2 are modulated by receptor heterodimerization. Mol. Endocrinol. 22, 2278–2292. doi: 10.1210/me.2007-0334
Gu, F., Schumacher, F. R., Canzian, F., Allen, N. E., Albanes, D., Berg, C. D., et al. (2010). Eighteen insulin-like growth factor pathway genes, circulating levels of IGF-I and its binding protein, and risk of prostate and breast cancer. Cancer Epidemiol. Biomarkers Prev. 19, 2877–2887. doi: 10.1158/1055-9965.EPI-10-0507
Guo, R. S., Shi, P. D., Zhou, J., and Chen, Y. Y. (2013). Somatostatin receptors 3, 4 and 5 play important roles in gallbladder cancer. Asian Pac. J. Cancer Prev. 14, 4071–4075. doi: 10.7314/APJCP.2013.14.7.4071
Gupta, D., Jetton, T. L., Mortensen, R. M., Duan, S. Z., Peshavaria, M., and Leahy, J. L. (2008). In vivo and in vitro studies of a functional peroxisome proliferator-activated receptor gamma response element in the mouse pdx-1 promoter. J. Biol. Chem. 283, 32462–32470. doi: 10.1074/jbc.M801813200
Hubina, E., Nanzer, A. M., Hanson, M. R., Ciccarelli, E., Losa, M., Gaia, D., et al. (2006). Somatostatin analogues stimulate p27 expression and inhibit the MAP kinase pathway in pituitary tumours. Eur. J. Endocrinol. 155, 371–379. doi: 10.1530/eje.1.02213
Humphrey, R. K., Yu, S. M., Flores, L. E., and Jhala, U. S. (2010). Glucose regulates steady-state levels of PDX1 via the reciprocal actions of GSK3 and AKT kinases. J. Biol. Chem. 285, 3406–3416. doi: 10.1074/jbc.M109.006734
Jonmarker, S., Glaessgen, A., Culp, W. D., Pisa, P., Lewensohn, R., Ekman, P., et al. (2008). Expression of PDX-1 in prostate cancer, prostatic intraepithelial neoplasia and benign prostatic tissue. APMIS 116, 491–498. doi: 10.1111/j.1600-0463.2008.01020.x
Jonsson, J., Carlsson, L., Edlund, T., and Edlund, H. (1994). Insulin-promoter-factor 1 is required for pancreas development in mice. Nature 371, 606–609. doi: 10.1038/371606a0
Kaemmerer, D., Lupp, A., Peter, L., Fischer, E., Schulz, S., Kloppel, G., et al. (2013). Correlation of monoclonal and polyclonal somatostatin receptor 5 antibodies in pancreatic neuroendocrine tumors. Int. J. Clin. Exp. Pathol. 6, 49–54.
Kaneto, H., Miyatsuka, T., Shiraiwa, T., Yamamoto, K., Kato, K., Fujitani, Y., et al. (2007). Crucial role of PDX-1 in pancreas development, beta-cell differentiation, and induction of surrogate beta-cells. Curr. Med. Chem. 14, 1745–1752. doi: 10.2174/092986707781058887
Khoo, S., Griffen, S. C., Xia, Y., Baer, R. J., German, M. S., and Cobb, M. H. (2003). Regulation of insulin gene transcription by ERK1 and ERK2 in pancreatic beta cells. J. Biol. Chem. 278, 32969–32977. doi: 10.1074/jbc.M301198200
Kim, H. S., Lee, H. S., and Kim, W. H. (2011a). Clinical significance of protein expression of cyclooxygenase-2 and somatostatin receptors in gastroenteropancreatic neuroendocrine tumors. Cancer Res. Treat. 43, 181–188. doi: 10.4143/crt.2011.43.3.181
Kim, Y. C., Kim, S. Y., Mellado-Gil, J. M., Yadav, H., Neidermyer, W., Kamaraju, A. K., et al. (2011b). RB regulates pancreas development by stabilizing Pdx1. EMBO J. 30, 1563–1576. doi: 10.1038/emboj.2011.57
Kishi, A., Nakamura, T., Nishio, Y., Maegawa, H., and Kashiwagi, A. (2003). Sumoylation of Pdx1 is associated with its nuclear localization and insulin gene activation. Am. J. Physiol. Endocrinol. Metab. 284, E830–E840. doi: 10.1152/ajpendo.00390.2002
Koizumi, M., Doi, R., Toyoda, E., Masui, T., Tulachan, S. S., Kawaguchi, Y., et al. (2003). Increased PDX-1 expression is associated with outcome in patients with pancreatic cancer. Surgery 134, 260–266. doi: 10.1067/msy.2003.231
Lattanzio, L., Tonissi, F., Monteverde, M., Milano, G., Merlano, M. C., and Lo Nigro, C. (2013). Differential molecular mechanism of docetaxel-octreotide combined treatment according to the docetaxel-resistance status in PC3 prostate cancer cells. Anticancer Drugs 24, 120–130. doi: 10.1097/CAD.0b013e328358d1dc
Lebrun, P., Montminy, M. R., and Van Obberghen, E. (2005). Regulation of the pancreatic duodenal homeobox-1 protein by DNA-dependent protein kinase. J. Biol. Chem. 280, 38203–38210. doi: 10.1074/jbc.M504842200
Leonard, J., Peers, B., Johnson, T., Ferreri, K., Lee, S., and Montminy, M. R. (1993). Characterization of somatostatin transactivating factor-1, a novel homeobox factor that stimulates somatostatin expression in pancreatic islet cells. Mol. Endocrinol. 7, 1275–1283.
Leys, C. M., Nomura, S., Rudzinski, E., Kaminishi, M., Montgomery, E., Washington, M., et al. (2006). Expression of PDX-1 in human gastric metaplasia and gastric adenocarcinoma. Hum. Pathol. 37, 1162–1168. doi: 10.1016/j.humpath.2006.04.011
Li, D., Tanaka, M., Brunicardi, F. C., Fisher, W. E., Gibbs, R. A., and Gingras, M. C. (2011). Association between somatostatin receptor 5 gene polymorphisms and pancreatic cancer risk and survival. Cancer 117, 2863–2872. doi: 10.1002/cncr.25858
Li, Y., Cao, X., Li, L. X., Brubaker, P. L., Edlund, H., and Drucker, D. J. (2005). beta-Cell Pdx1 expression is essential for the glucoregulatory, proliferative, and cytoprotective actions of glucagon-like peptide-1. Diabetes 54, 482–491. doi: 10.2337/diabetes.54.2.482
Liu, S., Ballian, N., Belaguli, N. S., Patel, S., Li, M., Templeton, N. S., et al. (2008). PDX-1 acts as a potential molecular target for treatment of human pancreatic cancer. Pancreas 37, 210–220. doi: 10.1097/MPA.0b013e31816a4a33
Liu, S. H., Patel, S., Gingras, M. C., Nemunaitis, J., Zhou, G., Chen, C., et al. (2011). PDX-1: demonstration of oncogenic properties in pancreatic cancer. Cancer 117, 723–733. doi: 10.1002/cncr.25629
Liu, S. H., Rao, D. D., Nemunaitis, J., Senzer, N., Zhou, G., Dawson, D., et al. (2012). PDX-1 is a therapeutic target for pancreatic cancer, insulinoma and islet neoplasia using a novel RNA interference platform. PLoS ONE 7:e40452. doi: 10.1371/journal.pone.0040452
Liu, T., Gou, S. M., Wang, C. Y., Wu, H. S., Xiong, J. X., and Zhou, F. (2007). Pancreas duodenal homeobox-1 expression and significance in pancreatic cancer. World J. Gastroenterol. 13, 2615–2618.
Ma, J., Wang, J. D., Zhang, W. J., Zou, B., Chen, W. J., Lam, C. S., et al. (2010). Promoter hypermethylation and histone hypoacetylation contribute to pancreatic-duodenal homeobox 1 silencing in gastric cancer. Carcinogenesis 31, 1552–1560. doi: 10.1093/carcin/bgq140
Macfarlane, W. M., Campbell, S. C., Elrick, L. J., Oates, V., Bermano, G., Lindley, K. J., et al. (2000). Glucose regulates islet amyloid polypeptide gene transcription in a PDX1- and calcium-dependent manner. J. Biol. Chem. 275, 15330–15335. doi: 10.1074/jbc.M908045199
Macfarlane, W. M., Smith, S. B., James, R. F., Clifton, A. D., Doza, Y. N., Cohen, P., et al. (1997). The p38/reactivating kinase mitogen-activated protein kinase cascade mediates the activation of the transcription factor insulin upstream factor 1 and insulin gene transcription by high glucose in pancreatic beta-cells. J. Biol. Chem. 272, 20936–20944. doi: 10.1074/jbc.272.33.20936
Marshak, S., Totary, H., Cerasi, E., and Melloul, D. (1996). Purification of the beta-cell glucose-sensitive factor that transactivates the insulin gene differentially in normal and transformed islet cells. Proc. Natl. Acad. Sci. U.S.A. 93, 15057–15062. doi: 10.1073/pnas.93.26.15057
Mayr, B., Buslei, R., Theodoropoulou, M., Stalla, G. K., Buchfelder, M., and Schofl, C. (2013). Molecular and functional properties of densely and sparsely granulated GH-producing pituitary adenomas. Eur. J. Endocrinol. 169, 391–400. doi: 10.1530/EJE-13-0134
Mazzucchelli, R., Morichetti, D., Santinelli, A., Scarpelli, M., Bono, A. V., Lopez-Beltran, A., et al. (2011). Immunohistochemical expression and localization of somatostatin receptor subtypes in androgen ablated prostate cancer. Cell. Oncol. (Dordr). 34, 235–243. doi: 10.1007/s13402-011-0031-y
Miller, C. P., McGehee, R. E. Jr., and Habener, J. F. (1994). IDX-1: a new homeodomain transcription factor expressed in rat pancreatic islets and duodenum that transactivates the somatostatin gene. EMBO J. 13, 1145–1156.
Miyatsuka, T., Kaneto, H., Shiraiwa, T., Matsuoka, T. A., and Yamamoto, K. (2006). Persistent expression of PDX-1 in the pancreas causes acinar-to-ductal metaplasia through Stat3 activation. Genes Dev. 20, 1435–1440. doi: 10.1101/gad.1412806
Modlin, I. M., Pavel, M., Kidd, M., and Gustafsson, B. I. (2009). Review article: somatostatin analogues in the treatment of gastroenteropancreatic neuroendocrine (carcinoid) tumours. Aliment. Pharmacol. Ther. 31, 169–188. doi: 10.1111/j.1365-2036.2009.04174.x
Moldovan, S., DeMayo, F., and Brunicardi, F. C. (1998). Cloning of the mouse SSTR5 gene. J. Surg. Res. 76, 57–60. doi: 10.1006/jsre.1998.5286
Movassat, J., Beattie, G. M., Lopez, A. D., and Hayek, A. (2002). Exendin 4 up-regulates expression of PDX 1 and hastens differentiation and maturation of human fetal pancreatic cells. J. Clin. Endocrinol. Metab. 87, 4775–4781. doi: 10.1210/jc.2002-020137
Nishioka, H., Tamura, K., Iida, H., Kutsukake, M., Endo, A., Ikeda, Y., et al. (2011). Co-expression of somatostatin receptor subtypes and estrogen receptor-alpha mRNAs by non-functioning pituitary adenomas in young patients. Mol. Cell. Endocrinol. 331, 73–78. doi: 10.1016/j.mce.2010.08.011
Ocak, M., Demirci, E., Kabasakal, L., Aygun, A., Tutar, R. O., Araman, A., et al. (2013). Evaluation and comparison of Ga-68 DOTA-TATE and Ga-68 DOTA-NOC PET/CT imaging in well-differentiated thyroid cancer. Nucl. Med. Commun. 34, 1084–1089. doi: 10.1097/MNM.0b013e328364eaab
Oddstig, J., Bernhardt, P., Nilsson, O., Ahlman, H., and Forssell-Aronsson, E. (2011). Radiation induces up-regulation of somatostatin receptors 1, 2, and 5 in small cell lung cancer in vitro also at low absorbed doses. Cancer Biother. Radiopharm. 26, 759–765. doi: 10.1089/cbr.2010.0921
Ohlsson, H., Karlsson, K., and Edlund, T. (1993). IPF1, a homeodomain-containing transactivator of the insulin gene. EMBO J. 12, 4251–4259.
Olson, L. K., Redmon, J. B., Towle, H. C., and Robertson, R. P. (1993). Chronic exposure of HIT cells to high glucose concentrations paradoxically decreases insulin gene transcription and alters binding of insulin gene regulatory protein. J. Clin. Invest. 92, 514–519. doi: 10.1172/JCI116596
Park, J. H., Stoffers, D. A., Nicholls, R. D., and Simmons, R. A. (2008). Development of type 2 diabetes following intrauterine growth retardation in rats is associated with progressive epigenetic silencing of Pdx1. J. Clin. Invest. 118, 2316–2324. doi: 10.1172/JCI33655
Patel, S. G., Zhou, G., Liu, S. H., Li, M., Jeong, J. W., Demayo, F. J., et al. (2009). Microarray analysis of somatostatin receptor 5-regulated gene expression profiles in murine pancreas. World J. Surg. 33, 630–637. doi: 10.1007/s00268-008-9893-1
Patel, Y. C. (1999). Somatostatin and its receptor family. Front. Neuroendocrinol. 20, 157–198. doi: 10.1006/frne.1999.0183
Peverelli, E., Mantovani, G., Calebiro, D., Doni, A., Bondioni, S., Lania, A., et al. (2008). The third intracellular loop of the human somatostatin receptor 5 is crucial for arrestin binding and receptor internalization after somatostatin stimulation. Mol. Endocrinol. 22, 676–688. doi: 10.1210/me.2007-0068
Pfeiffer, M., Koch, T., Schroder, H., Klutzny, M., Kirscht, S., Kreienkamp, H. J., et al. (2001). Homo- and heterodimerization of somatostatin receptor subtypes. Inactivation of sst(3) receptor function by heterodimerization with sst(2A). J. Biol. Chem. 276, 14027–14036. doi: 10.1074/jbc.M006084200
Pola, S., Cattaneo, M. G., and Vicentini, L. M. (2003). Anti-migratory and anti-invasive effect of somatostatin in human neuroblastoma cells: involvement of Rac and MAP kinase activity. J. Biol. Chem. 278, 40601–40606. doi: 10.1074/jbc.M306510200
Puddu, A., Storace, D., Odetti, P., and Viviani, G. L. (2010). Advanced glycation end-products affect transcription factors regulating insulin gene expression. Biochem. Biophys. Res. Commun. 395, 122–125. doi: 10.1016/j.bbrc.2010.03.152
Pyronnet, S., Bousquet, C., Najib, S., Azar, R., Laklai, H., and Susini, C. (2008). Antitumor effects of somatostatin. Mol. Cell. Endocrinol. 286, 230–237. doi: 10.1016/j.mce.2008.02.002
Qiu, C. Z., Wang, C., Huang, Z. X., Zhu, S. Z., Wu, Y. Y., and Qiu, J. L. (2006). Relationship between somatostatin receptor subtype expression and clinicopathology, Ki-67, Bcl-2 and p53 in colorectal cancer. World J. Gastroenterol. 12, 2011–2015. doi: 10.3748/wjg.v12.i13.2011
Raynor, K., O'Carroll, A. M., Kong, H., Yasuda, K., Mahan, L. C., Bell, G. I., et al. (1993). Characterization of cloned somatostatin receptors SSTR4 and SSTR5. Mol. Pharmacol. 44, 385–392.
Reisine, T., and Bell, G. I. (1995). Molecular biology of somatostatin receptors. Endocr. Rev. 16, 427–442.
Reubi, J. C., Horisberger, U., Essed, C. E., Jeekel, J., Klijn, J. G., and Lamberts, S. W. (1988). Absence of somatostatin receptors in human exocrine pancreatic adenocarcinomas. Gastroenterology 95, 760–763.
Rocheville, M., Lange, D. C., Kumar, U., Sasi, R., Patel, R. C., and Patel, Y. C. (2000). Subtypes of the somatostatin receptor assemble as functional homo- and heterodimers. J. Biol. Chem. 275, 7862–7869. doi: 10.1074/jbc.275.11.7862
Sakai, H., Eishi, Y., Li, X. L., Akiyama, Y., Miyake, S., Takizawa, T., et al. (2004). PDX-1 homeobox protein expression in pseudopyloric glands and gastric carcinomas. Gut 53, 323–330. doi: 10.1136/gut.2003.026609
Sclafani, F., Carnaghi, C., Di Tommaso, L., Rodari, M., Destro, A., Rimassa, L., et al. (2011). Detection of somatostatin receptor subtypes 2 and 5 by somatostatin receptor scintigraphy and immunohistochemistry: clinical implications in the diagnostic and therapeutic management of gastroenteropancreatic neuroendocrine tumors. Tumori 97, 620–628. doi: 10.1700/989.10722
Serup, P., Jensen, J., Andersen, F. G., Jorgensen, M. C., Blume, N., Holst, J. J., et al. (1996). Induction of insulin and islet amyloid polypeptide production in pancreatic islet glucagonoma cells by insulin promoter factor 1. Proc. Natl. Acad. Sci. U.S.A. 93, 9015–9020. doi: 10.1073/pnas.93.17.9015
Stoffers, D. A., Zinkin, N. T., Stanojevic, V., Clarke, W. L., and Habener, J. F. (1997). Pancreatic agenesis attributable to a single nucleotide deletion in the human IPF1 gene coding sequence. Nat. Genet. 15, 106–110. doi: 10.1038/ng0197-106
Sun, Y., Zhang, L., Gu, H. F., Han, W., Ren, M., Wang, F., et al. (2008). Peroxisome proliferator-activated receptor-alpha regulates the expression of pancreatic/duodenal homeobox-1 in rat insulinoma (INS-1) cells and ameliorates glucose-induced insulin secretion impaired by palmitate. Endocrinology 149, 662–671. doi: 10.1210/en.2007-1275
Tirone, T. A., Norman, M. A., Moldovan, S., DeMayo, F. J., Wang, X. P., and Brunicardi, F. C. (2003). Pancreatic somatostatin inhibits insulin secretion via SSTR-5 in the isolated perfused mouse pancreas model. Pancreas 26, e67–e73. doi: 10.1097/00006676-200304000-00025
Todisco, A., Campbell, V., Dickinson, C. J., DelValle, J., and Yamada, T. (1994). Molecular basis for somatostatin action: inhibition of c-fos expression and AP-1 binding. Am. J. Physiol. 267, G245–G253.
Todisco, A., Seva, C., Takeuchi, Y., Dickinson, C. J., and Yamada, T. (1995). Somatostatin inhibits AP-1 function via multiple protein phosphatases. Am. J. Physiol. 269, G160–G166.
Tsuta, K., Wistuba, I. I., and Moran, C. A. (2012). Differential expression of somatostatin receptors 1-5 in neuroendocrine carcinoma of the lung. Pathol. Res. Pract. 208, 470–474. doi: 10.1016/j.prp.2012.05.014
Wang, S., Bao, Z., Liang, Q. M., Long, J. W., Xiao, Z. S., Jiang, Z. J., et al. (2013). Octreotide stimulates somatostatin receptor-induced apoptosis of SW480 colon cancer cells by activation of glycogen synthase kinase-3beta, A Wnt/beta-catenin pathway modulator. Hepatogastroenterology 60, 1639–1646.
Wang, X., Cahill, C. M., Pineyro, M. A., Zhou, J., Doyle, M. E., and Egan, J. M. (1999). Glucagon-like peptide-1 regulates the beta cell transcription factor, PDX-1, in insulinoma cells. Endocrinology 140, 4904–4907. doi: 10.1210/endo.140.10.7158
Wang, X. P., Li, Z. J., Magnusson, J., and Brunicardi, F. C. (2005c). Tissue MicroArray analyses of pancreatic duodenal homeobox-1 in human cancers. World J. Surg. 29, 334–338. doi: 10.1007/s00268-004-7823-4
Wang, X. P., Norman, M., Yang, J., Liu, S. H., Magnusson, J., DeMayo, F. J., et al. (2005a). The effect of global SSTR5 gene ablation on the endocrine pancreas and glucose regulation in aging mice. J. Surg. Res. 129, 64–72. doi: 10.1016/j.jss.2005.05.024
Wang, X. P., Norman, M. A., Yang, J., Cheung, A., Moldovan, S., Demayo, F. J., et al. (2004). Double-gene ablation of SSTR1 and SSTR5 results in hyperinsulinemia and improved glucose tolerance in mice. Surgery 136, 585–592. doi: 10.1016/j.surg.2004.05.042
Wang, X. P., Young, J., Norman, M. A., Magnusson, J., DeMayo, F. J., and Brunicardi, F. C. (2005b). SSTR5 ablation in islet results in alterations in glucose homeostasis in mice. FEBS Lett. 579, 3107–3114. doi: 10.1016/j.febslet.2005.04.069
Watada, H., Kajimoto, Y., Miyagawa, J., Hanafusa, T., Hamaguchi, K., Matsuoka, T., et al. (1996). PDX-1 induces insulin and glucokinase gene expressions in alphaTC1 clone 6 cells in the presence of betacellulin. Diabetes 45, 1826–1831. doi: 10.2337/diab.45.12.1826
Wu, K. L., Gannon, M., Peshavaria, M., Offield, M. F., Henderson, E., Ray, M., et al. (1997). Hepatocyte nuclear factor 3beta is involved in pancreatic beta-cell-specific transcription of the pdx-1 gene. Mol. Cell. Biol. 17, 6002–6013.
Yamada, Y., Post, S. R., Wang, K., Tager, H. S., Bell, G. I., and Seino, S. (1992). Cloning and functional characterization of a family of human and mouse somatostatin receptors expressed in brain, gastrointestinal tract, and kidney. Proc. Natl. Acad. Sci. U.S.A. 89, 251–255. doi: 10.1073/pnas.89.1.251
Yang, B. T., Dayeh, T. A., Volkov, P. A., Kirkpatrick, C. L., Malmgren, S., Jing, X., et al. (2012). Increased DNA methylation and decreased expression of PDX-1 in pancreatic islets from patients with type 2 diabetes. Mol. Endocrinol. 26, 1203–1212. doi: 10.1210/me.2012-1004
Yasuda, K., Rens-Domiano, S., Breder, C. D., Law, S. F., Saper, C. B., Reisine, T., et al. (1992). Cloning of a novel somatostatin receptor, SSTR3, coupled to adenylylcyclase. J. Biol. Chem. 267, 20422–20428.
Zatelli, M. C., Tagliati, F., Taylor, J. E., Rossi, R., Culler, M. D., and degli Uberti, E. C. (2001). Somatostatin receptor subtypes 2 and 5 differentially affect proliferation in vitro of the human medullary thyroid carcinoma cell line tt. J. Clin. Endocrinol. Metab. 86, 2161–2169. doi: 10.1210/jc.86.5.2161
Zhou, G., Gingras, M., Liu, S. H., Li, D., Li, Z., Catania, R. L., et al. (2011c). The hypofunctional effect of P335L single nucleotide polymorphism on SSTR5 function. World J. Surg. 35, 1715–1724. doi: 10.1007/s00268-010-0939-9
Zhou, G., Gingras, M. C., Liu, S. H., Li, D., Li, Z., Catania, R. L., et al. (2011a). The hypofunctional effect of P335L single nucleotide polymorphism on SSTR5 function. World J. Surg. 35, 1715–1724. doi: 10.1007/s00268-010-0939-9
Zhou, G., Gingras, M. C., Liu, S. H., Sanchez, R., Edwards, D., Dawson, D., et al. (2011b). SSTR5 P335L monoclonal antibody differentiates pancreatic neuroendocrine neuroplasms with different SSTR5 genotypes. Surgery 150, 1136–1142. doi: 10.1016/j.surg.2011.09.044
Zhou, G., Liu, S. H., Shahi, K. M., Wang, H., Duan, X., Lin, X., et al. (2012). Negative regulation of pancreatic and duodenal homeobox-1 by somatostatin receptor subtype 5. Mol. Endocrinol. 26, 1225–1234. doi: 10.1210/me.2012-1095
Keywords: G protein-coupled receptors, pancreatic and duodenal homeobox-1, single nucleotide polymorphisms, somatostatin, somatostatin receptor
Citation: Zhou G, Sinnett-Smith J, Liu S-H, Yu J, Wu J, Sanchez R, Pandol SJ, Abrol R, Nemunaitis J, Rozengurt E and Brunicardi FC (2014) Down-regulation of pancreatic and duodenal homeobox-1 by somatostatin receptor subtype 5: a novel mechanism for inhibition of cellular proliferation and insulin secretion by somatostatin. Front. Physiol. 5:226. doi: 10.3389/fphys.2014.00226
Received: 25 April 2014; Accepted: 31 May 2014;
Published online: 25 June 2014.
Edited by:
Joseph Pisegna, Department of Veterans Affairs, USAReviewed by:
Robert T. Jensen, The National Institute of Diabetes and Digestive and Kidney Diseases, USAWilliam Robert Gower, James A Haley VA Hospital, USA
Copyright © 2014 Zhou, Sinnett-Smith, Liu, Yu, Wu, Sanchez, Pandol, Abrol, Nemunaitis, Rozengurt and Brunicardi. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) or licensor are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: F. Charles Brunicardi, Division of General Surgery, Department of Surgery, David Geffen School of Medicine at University of California, Los Angeles, 1304 15th Street, Suite 102, Santa Monica, CA 90404, USA e-mail: cbrunicardi@mednet.ucla.edu
 Guisheng Zhou
Guisheng Zhou Jim Sinnett-Smith2,3
Jim Sinnett-Smith2,3 James Wu
James Wu Robbi Sanchez
Robbi Sanchez Stephen J. Pandol
Stephen J. Pandol Ravinder Abrol
Ravinder Abrol Enrique Rozengurt
Enrique Rozengurt F. Charles Brunicardi
F. Charles Brunicardi